Recent Advances in Plant-Based Green Synthesis of Nanoparticles: A Sustainable Approach for Combating Plant-Parasitic Nematodes
Abstract
1. Introduction
2. Overview of Nanoparticle Synthesis
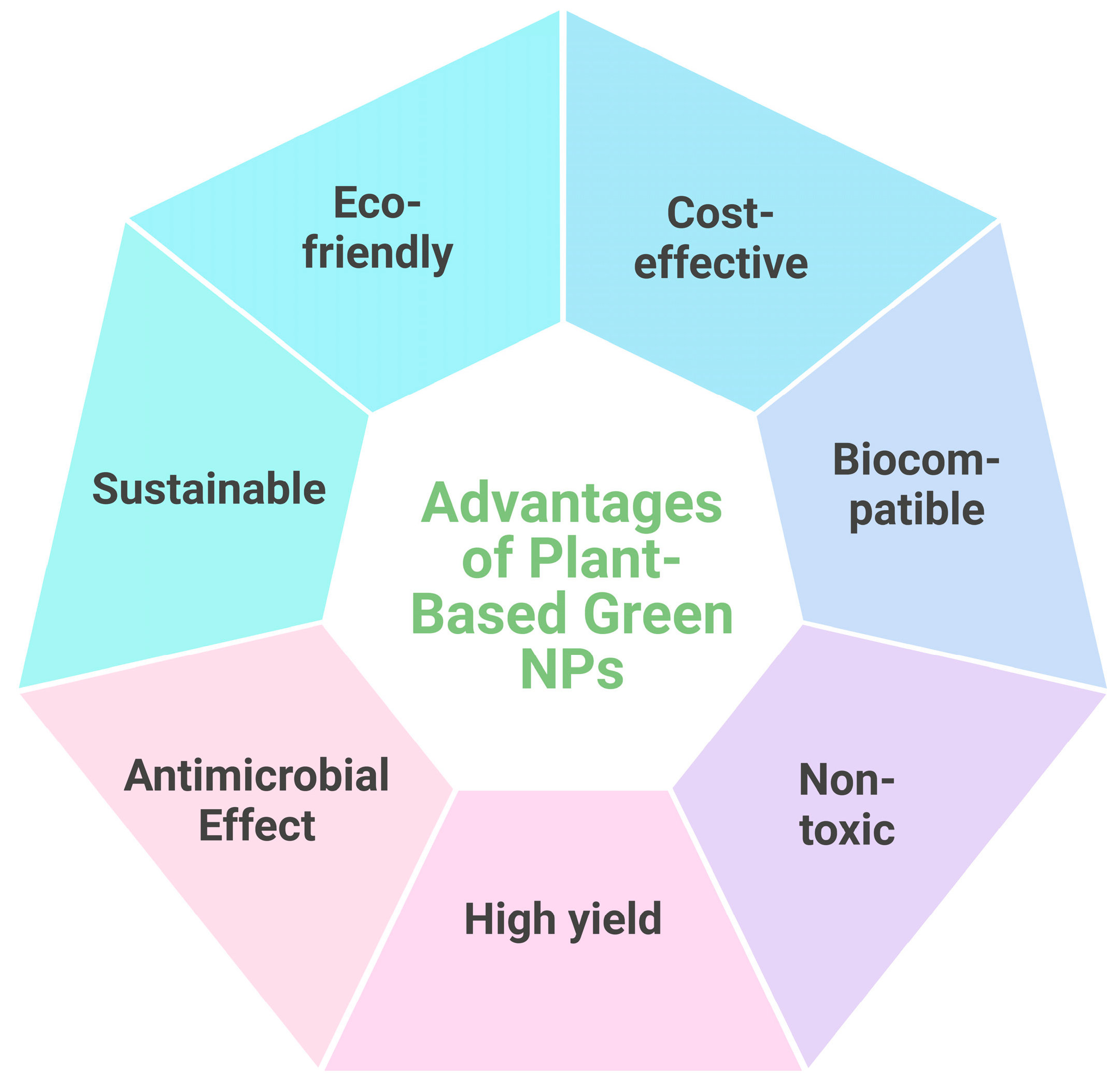
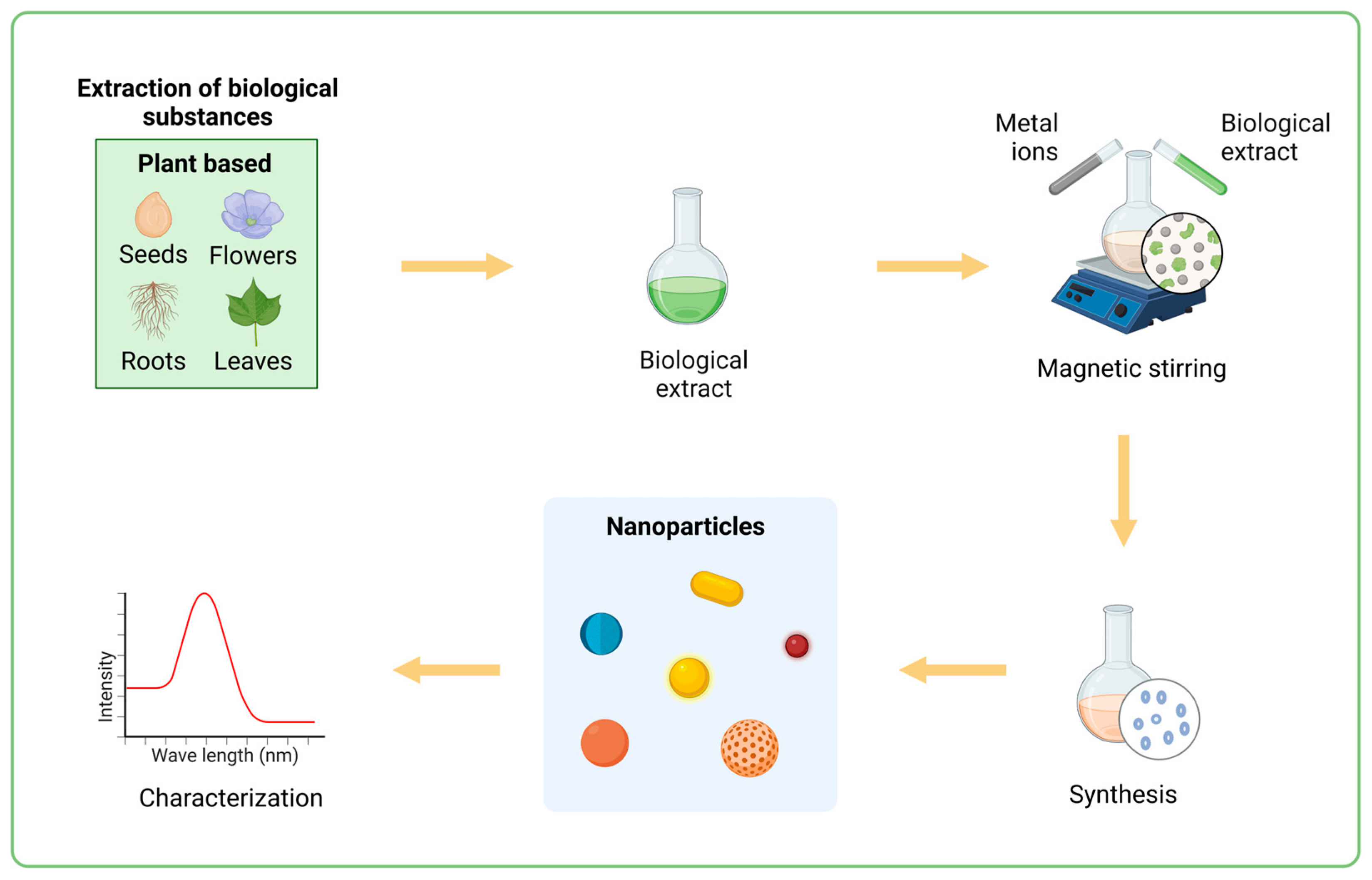
3. Green Synthesis of Nanoparticles
4. Plant-Based Green Synthesis of Nanoparticles
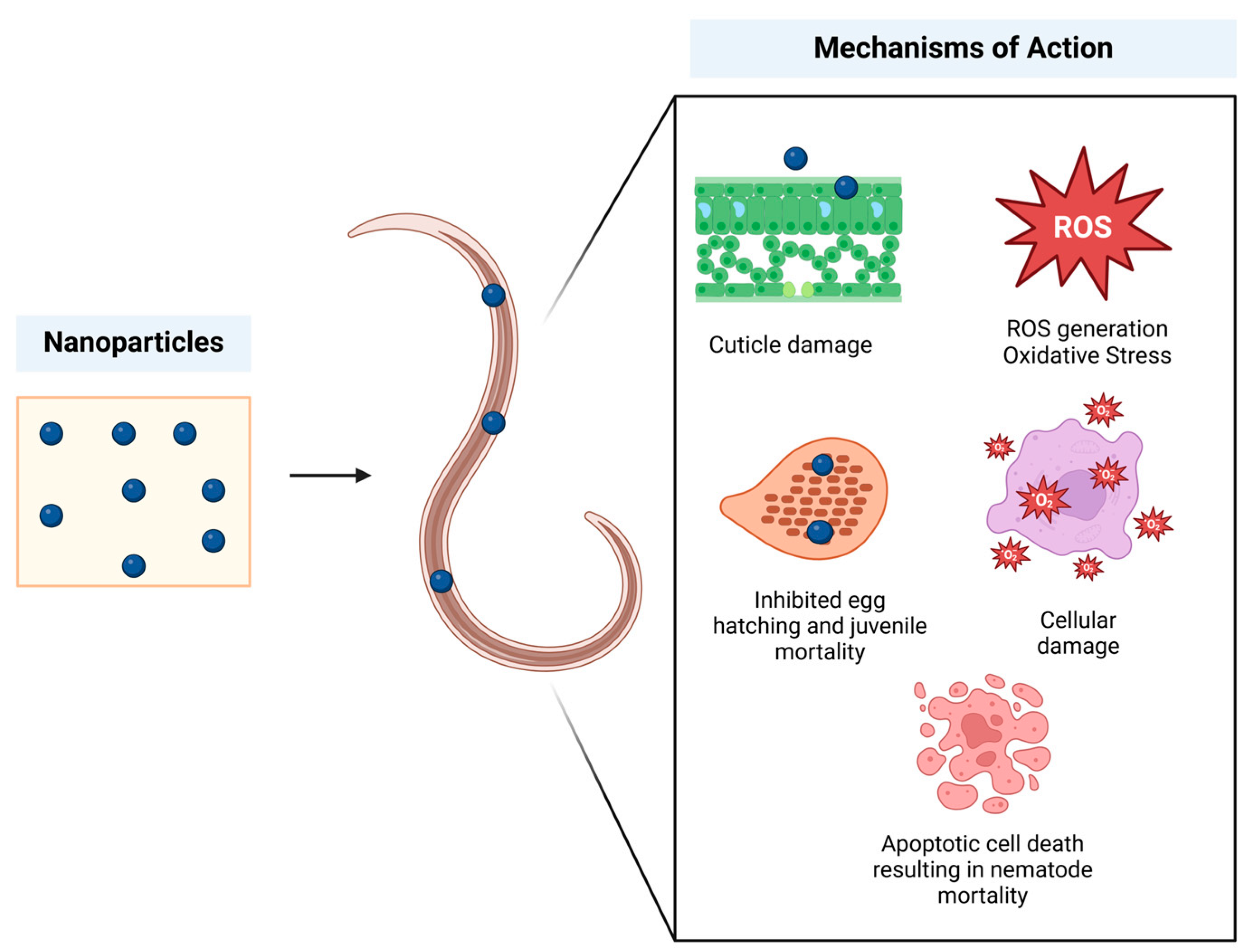
5. Applications of Plant-Based Green Synthesis of Nanoparticles Against Plant-Parasitic Nematodes
6. Challenges and Limitations
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PPNs | Plant-parasitic nematodes |
| RKNs | Root-knot nematodes |
| J2 | Second-stage juveniles |
| NPs | Nanoparticles |
References
- Singh, M.; Srivastava, M.; Kumar, A.; Pandey, K.D. Biosynthesis of nanoparticles and applications in agriculture. In Role of Plant Growth Promoting Microorganisms in Sustainable Agriculture and Nanotechnology; Kumar, A., Singh, A.K., Choudhary, K.K., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 199–217. [Google Scholar]
- Strange, R.N.; Scott, P.R. Plant disease: A threat to global food security. Annu. Rev. Phytopathol. 2005, 43, 83–116. [Google Scholar] [CrossRef]
- O’Brien, P.A. Biological control of plant diseases. Australas. Plant Pathol. 2017, 46, 293–304. [Google Scholar] [CrossRef]
- Savary, S.; Bregaglio, S.; Willocquet, L.; Gustafson, D.; Mason D’Croz, D.; Sparks, A.; Garrett, K. Crop health and its global impacts on the components of food security. Food Secur. 2017, 9, 311–327. [Google Scholar] [CrossRef]
- Rizzo, D.M.; Lichtveld, M.; Mazet, J.A.; Togami, E.; Miller, S.A. Plant health and its effects on food safety and security in a One Health framework: Four case studies. One Health Outlook 2021, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Bird, D.M.; Kaloshian, I. Are roots special? Nematodes have their say. Physiol. Mol. Plant Pathol. 2003, 62, 115–123. [Google Scholar] [CrossRef]
- Chitwood, D.J. Research on plant-parasitic nematode biology conducted by the United States Department of Agriculture–Agricultural Research Service. Pest Manag. Sci. 2003, 59, 748–753. [Google Scholar] [CrossRef]
- Singh, S.; Singh, B.; Singh, A.P. Nematodes: A threat to sustainability of agriculture. Procedia Environ. Sci. 2015, 29, 215–216. [Google Scholar] [CrossRef]
- D’addabbo, T.; Carbonara, T.; Leonetti, P.; Radicci, V.; Tava, A.; Avato, P. Control of plant parasitic nematodes with active saponins and biomass from Medicago sativa. Phytochem. Rev. 2011, 10, 503–519. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Bhaumik, A.; Rani, P.U.; Mandal, S.; Epidi, T.T. Nano-particles-A recent approach to insect pest control. Afr. J. Biotechnol. 2010, 9, 3489–3493. [Google Scholar]
- Rai, M.; Yadav, A.; Gade, A. CRC 675—Current trends in phytosynthesis of metal nanoparticles. Crit. Rev. Biotechnol. 2008, 28, 277–284. [Google Scholar] [CrossRef]
- Singaravelan, R.; Bangaru Sudarsan Alwar, S. Electrochemical synthesis, characterisation and phytogenic properties of silver nanoparticles. Appl. Nanosci. 2015, 5, 983–991. [Google Scholar] [CrossRef]
- Adeel, M.; Shakoor, N.; Hussain, T.; Azeem, I.; Zhou, P.; Zhang, P.; Hao, Y.; Rinklebe, J.; Rui, Y. Bio-interaction of nano and bulk lanthanum and ytterbium oxides in soil system: Biochemical, genetic, and histopathological effects on Eisenia fetida. J. Hazard. Mater. 2021, 415, 125574. [Google Scholar] [CrossRef]
- Adeel, M.; Shakoor, N.; Shafiq, M.; Pavlicek, A.; Part, F.; Zafiu, C.; Raza, A.; Ahmad, M.A.; Jilani, G.; White, J.C.; et al. A critical review of the environmental impacts of manufactured nano-objects on earthworm species. Environ. Pollut. 2021, 290, 118041. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, J.D.; Ranjan, S.; Dasgupta, N.; Saha, P. Nanotechnology for tissue engineering: Need, techniques and applications. J. Pharm. Res. 2013, 7, 200–204. [Google Scholar] [CrossRef]
- Bogunia-Kubik, K.; Sugisaka, M. From molecular biology to nanotechnology and nanomedicine. Biosystems 2002, 65, 123–138. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomedicine 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; ur Rehman, H.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef]
- Jaskulski, D.; Jaskulska, I.; Majewska, J.; Radziemska, M.; Bilgin, A.; Brtnicky, M. Silver nanoparticles (AgNPs) in urea solution in laboratory tests and field experiments with crops and vegetables. Materials 2022, 15, 870. [Google Scholar] [CrossRef] [PubMed]
- Beig, B.; Niazi, M.B.K.; Sher, F.; Jahan, Z.; Malik, U.S.; Khan, M.D.; Vo, D.V.N. Nanotechnology-based controlled release of sustainable fertilizers. A review. Environ. Chem. Lett. 2022, 20, 2709–2726. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.J.; Wang, C.; Mathiyalagan, R.; El-Agamy Farh, M.; Yang, D.C. Biogenic silver and gold nanoparticles synthesized using red ginseng root extract, and their applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 811–816. [Google Scholar] [CrossRef]
- Patil, S.; Chandrasekaran, R. Biogenic nanoparticles: A comprehensive perspective in synthesis, characterization, application and its challenges. J. Genet. Eng. Biotechnol. 2020, 18, 67. [Google Scholar] [CrossRef] [PubMed]
- Li, S.N.; Wang, R.; Ho, S.H. Algae-mediated biosystems for metallic nanoparticle production: From synthetic mechanisms to aquatic environmental applications. J. Hazard. Mater. 2021, 420, 126625. [Google Scholar] [CrossRef]
- Bhattarai, B.; Zaker, Y.; Bigioni, T.P. Green synthesis of gold and silver nanoparticles: Challenges and opportunities. Curr. Opin. Green Sustain. Chem. 2018, 12, 91–100. [Google Scholar] [CrossRef]
- Bhuyan, T.; Mishra, K.; Khanuja, M.; Prasad, R.; Varma, A. Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater. Sci. Semicond. Process. 2015, 32, 55–61. [Google Scholar] [CrossRef]
- Hano, C.; Abbasi, B.H. Plant-based green synthesis of nanoparticles: Production, characterization and applications. Biomolecules 2022, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Sepeur, S. Nanotechnology: Technical Basics and Applications; Vincentz Network GmbH & Co. KG: Hannover, Germany, 2008. [Google Scholar]
- Xu, C.; De, S.; Balu, A.M.; Ojeda, M.; Luque, R. Mechanochemical synthesis of advanced nanomaterials for catalytic applications. Chem. Commun. 2015, 51, 6698–6713. [Google Scholar] [CrossRef]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Sastry, M. Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: A novel biological approach to nanoparticle synthesis. Nano Lett. 2001, 1, 515–519. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Ali, A.; Aasim, M.; Çelik, K.; Nadeem, M.A.; Baloch, F.S. Frontiers in bacterial-based green synthesized nanoparticles (nps): A sustainable strategy for combating infectious plant pathogens. Biocatal. Agric. Biotechnol. 2024, 60, 103293. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Shavandi, A.; Raie, D.S.; Sangeetha, J.; Soleimani, M.; Hajibehzad, S.S.; Varma, R.S. Plant molecular farming: Production of metallic nanoparticles and therapeutic proteins using green factories. Green Chem. 2019, 21, 1845–1865. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Abd-Al-Aleem, A.H.; Abu-Saied, M.A.; Youssef, A.M. Synthesis of environmentally benign antimicrobial dressing nanofibers based on polycaprolactone blended with gold nanoparticles and spearmint oil nanoemulsion. J. Mater. Res. Technol. 2021, 15, 3447–3460. [Google Scholar] [CrossRef]
- Sarip, N.A.; Aminudin, N.I.; Danial, W.H. Green synthesis of metal nanoparticles using Garcinia extracts: A review. Environ. Chem. Lett. 2022, 20, 469–493. [Google Scholar] [CrossRef]
- Malik, A.Q.; Mir, T.U.G.; Kumar, D.; Mir, I.A.; Rashid, A.; Ayoub, M.; Shukla, S. A review on the green synthesis of nanoparticles, their biological applications, and photocatalytic efficiency against environmental toxins. Environ. Sci. Pollut. Res. 2023, 30, 69796–69823. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, M.; Sung, J.S.; Koduru, J.R.; Nile, S.H.; Syed, A.; Ghodake, G.S. Extracellular synthesis of silver nanoparticle using yeast extracts: Antibacterial and seed priming applications. Appl. Microbiol. Biotechnol. 2024, 108, 150. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.L.; Kim, H.; Pan, S.Y.; Tseng, P.C.; Lin, Y.P.; Chiang, P.C. Implementation of green chemistry principles in circular economy system towards sustainable development goals: Challenges and perspectives. Sci. Total Environ. 2020, 716, 136998. [Google Scholar] [CrossRef]
- Nadeem, M.; Tungmunnithum, D.; Hano, C.; Abbasi, B.H.; Hashmi, S.S.; Ahmad, W.; Zahir, A. The current trends in the green syntheses of titanium oxide nanoparticles and their applications. Green Chem. Lett. Rev. 2018, 11, 492–502. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Green synthesis of biogenic metal nanoparticles by terrestrial and aquatic phototrophic and heterotrophic eukaryotes and biocompatible agents. Adv. Colloid Interface Sci. 2011, 169, 59–79. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications—An updated report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef]
- Ali, Z.A.; Roslan, M.A.; Yahiya, R.; Sulaiman, W.Y.W.; Puteh, R. Eco-friendly synthesis of silver nanoparticles and its larvicidal property against fourth instar larvae of Aedes aegypti. IET Nanobiotechnol. 2017, 11, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Gautam, P.K.; Verma, A.; Singh, V.; Shivapriya, P.M.; Shivalkar, S.; Samanta, S.K. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnol. Rep. 2020, 25, e00427. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, H.; Nakara, A.; Shanmugam, V.K. Anti-inflammatory mechanism of various metal and metal oxide nanoparticles synthesized using plant extracts: A review. Biomed. Pharmacother. 2019, 109, 2561–2572. [Google Scholar] [CrossRef] [PubMed]
- Basavegowda, N.; Baek, K.H. Multimetallic nanoparticles as alternative antimicrobial agents: Challenges and perspectives. Molecules 2021, 26, 912. [Google Scholar] [CrossRef]
- Kalaiselvi, D.; Mohankumar, A.; Shanmugam, G.; Nivitha, S.; Sundararaj, P. Green synthesis of silver nanoparticles using latex extract of Euphorbia tirucalli: A novel approach for the management of root knot nematode, Meloidogyne incognita. Crop Prot. 2019, 117, 108–114. [Google Scholar] [CrossRef]
- Nasr, A.; Yousef, A.F.; Hegazy, M.G.; Abdel-Mageed, M.A.; Elshazly, E.H.; Gad, M.; Seleim, M.A. Biosynthesized silver nanoparticles mitigate charcoal rot and root-knot nematode disease complex in faba bean. Physiol. Mol. Plant Pathol. 2025, 136, 102610. [Google Scholar] [CrossRef]
- Heflish, A.A.; Hanfy, A.E.; Ansari, M.J.; Dessoky, E.S.; Attia, A.O.; Elshaer, M.M.; Behiry, S.I. Green biosynthesized silver nanoparticles using Acalypha wilkesiana extract control root-knot nematode. J. King Saud Univ. Sci. 2021, 33, 101516. [Google Scholar] [CrossRef]
- Daramola, F.; Lewu, N.; Nkiko, J.; Lewu, F. Nematicidal effects of silver nanoparticles (Ag-NPs) on the root-knot nematode, Meloidogyne javanica associated with Swiss chard (Beta vulgaris L.). Helminthologia 2023, 60, 189. [Google Scholar] [CrossRef]
- Oluwatoyin, F.; Olatunji, G.; Atolani, O.; Olawuyi, O. Preparation of bio-nematicidal nanoparticles of Eucalyptus officinalis for the control of cyst nematode (Heterodera sacchari). J. Anim. Plant Sci. 2020, 30, 1172–1177. [Google Scholar]
- Ahmad, S.; Ahmad, M.A.; Umar, F.; Munir, I.; Iqbal, N.; Ahmad, N.; Rehman, A.U. Green nano-synthesis: Salix alba bark-derived zinc oxide nanoparticle and their nematicidal efficacy against root-knot nematode Meloidogyne incognita. Adv. Life Sci. 2024, 10, 675–681. [Google Scholar] [CrossRef]
- Soliman, M.M.; Abdallah, A.M.; Hafez, E.E.; Kadous, A.E.; Kassem, F.A. Nematicidal activity of chemical and green biosynthesis of copper nanoparticles against root-knot nematode, Meloidogyne incognita. Alex. Sci. Exch. J. 2022, 43, 583–591. [Google Scholar] [CrossRef]
- Khan, A.U.; Khan, M.; Khan, A.A.; Parveen, A.; Ansari, S.; Alam, M. Effect of phyto-assisted synthesis of magnesium oxide nanoparticles (MgO-NPs) on bacteria and the root-knot nematode. Bioinorg. Chem. Appl. 2022, 2022, 3973841. [Google Scholar] [CrossRef]
- Thiruvenkataswamy, S.; Paulpandi, S.; Narayanasamy, M. Biosynthesis, characterization, and nematicidal efficacy of silver nanoparticles synthesized using Solanum nigrum fruit against root-knot nematode Meloidogyne incognita. Nano Tech. Nano Sci. Ind. J. 2022, 16, 140. [Google Scholar]
- Danish, M.; Altaf, M.; Robab, M.I.; Shahid, M.; Manoharadas, S.; Hussain, S.A.; Shaikh, H. Green synthesized silver nanoparticles mitigate biotic stress induced by Meloidogyne incognita in Trachyspermum ammi (L.) by improving growth, biochemical, and antioxidant enzyme activities. ACS Omega 2021, 6, 11389–11403. [Google Scholar] [CrossRef] [PubMed]
- Al Banna, L.S.; Salem, N.M.; Jaleel, G.A.; Awwad, A.M. Green synthesis of sulfur nanoparticles using Rosmarinus officinalis leaves extract and nematicidal activity against Meloidogyne javanica. Chem. Int. 2020, 6, 137–143. [Google Scholar]
- Soliman, B.; Abbassy, M.; Abdel-Rasoul, M.; Nassar, A. Efficacy of silver nanoparticles of extractives of Artemisia judaica against root-knot nematode. J. Environ. Stud. Res. 2017, 7, 832–844. [Google Scholar] [CrossRef]
- Sherin, S.; Rose Rizvi, N.F.; Parveen, M.; Jaseem, K.P.; Favas, A.; Mubeena, E.S. Innovative nano-solution: Bio-synthesized nickel oxide nanoparticles (NPs) protect carrot roots from root knot nematode, Meloidogyne incognita infestation. Int. J. Environ. Agric. Biotechnol. 2024, 9, 28–40. [Google Scholar] [CrossRef]
- Khan, M.; Khan, A.U.; Bogdanchikova, N.; Garibo, D. Antibacterial and antifungal studies of biosynthesized silver nanoparticles against plant-parasitic nematode Meloidogyne incognita, plant pathogens Ralstonia solanacearum and Fusarium oxysporum. Molecules 2021, 26, 2462. [Google Scholar] [CrossRef]
- Rani, K.; Devi, N.; Banakar, P.; Kharb, P.; Kaushik, P. Nematicidal potential of green silver nanoparticles synthesized using aqueous root extract of Glycyrrhiza glabra. Nanomaterials 2022, 12, 2966. [Google Scholar] [CrossRef]
- Oliveira, L.S.D.; Furtado, L.L.; Diniz, F.D.A.D.S.; Mendes, B.L.; Araújo, T.R.D.; Silva, L.P.; Santiago, T.R. Eco-friendly silver nanoparticles synthesized from a soybean by-product with nematicidal efficacy against Pratylenchus brachyurus. Nanomaterials 2023, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Abbassy, M.A.; Abdel-Rasoul, M.A.; Nassar, A.M.; Soliman, B.S. Nematicidal activity of silver nanoparticles of botanical products against root-knot nematode, Meloidogyne incognita. Arch. Phytopathol. Plant Protect. 2017, 50, 909–926. [Google Scholar] [CrossRef]
- Fabiyi, O.; Lateef, A.; Gueguim-Kana, E.B.; Beukes, L.S.; Matyumza, N.; Bello, T.; Olatunji, G. Characterization and nematicidal potential of copper, iron, and zinc nanoparticles synthesized from Tridax procumbens L. extract on Meloidogyne incognita infected cabbage plants. Eur. J. Plant Pathol. 2024, 168, 683–695. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Khader, A.; Mahmoud, E. Green iron oxide nanoparticles and magnetic nanobiochar: Enhancing tomato performance, phytochemicals, and root-knot nematode resistance. BMC Plant Biol. 2024, 24, 469. [Google Scholar] [CrossRef]
- Akhter, G.; Khan, A.; Ali, S.G.; Khan, T.A.; Siddiqi, K.S.; Khan, H.M. Antibacterial and nematicidal properties of biosynthesized Cu nanoparticles using extract of holoparasitic plant. SN Appl. Sci. 2020, 2, 1268. [Google Scholar] [CrossRef]
- Fabiyi, O.A. Sustainable management of Meloidogyne incognita infecting carrot (Daucus carota): Green synthesis of silver nanoparticles with Cnidoscolus aconitifolius. Vegetos 2021, 34, 277–285. [Google Scholar] [CrossRef]
- Khan, A.; Bani Mfarrej, M.F.; Danish, M.; Shariq, M.; Khan, M.F.; Ansari, M.S.; Ahmad, F. Synthesized copper oxide nanoparticles via the green route act as antagonists to pathogenic root-knot nematode, Meloidogyne incognita. Green Chem. Lett. Rev. 2022, 15, 491–507. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A.; Behiry, S.I. Bacillus licheniformis strain POT1 mediated polyphenol biosynthetic pathways genes activation and systemic resistance in potato plants against Alfalfa mosaic virus. Sci. Rep. 2020, 10, 16120. [Google Scholar] [CrossRef]
- Abo-Zaid, G.A.; Matar, S.M.; Abdelkhalek, A. Induction of plant resistance against tobacco mosaic virus using the biocontrol agent Streptomyces cellulosae isolate Actino 48. Agronomy 2020, 10, 1620. [Google Scholar] [CrossRef]
- Sikora, R.A.; Fernandez, E. Nematode parasites of vegetables. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture; CABI Publishing: Wallingford, UK, 2005; pp. 319–392. [Google Scholar]
- Masry, S.H.D.; Taha, T.H.; Botros, W.A.; Mahfouz, H.; Al-Kahtani, S.N.; Ansari, M.J.; Hafez, E.E. Antimicrobial activity of camphor tree silver nanoparticles against foulbrood diseases and finding out new strain of Serratia marcescens via DGGE-PCR, as a secondary infection on honeybee larvae. Saudi J. Biol. Sci. 2021, 28, 2067–2075. [Google Scholar] [CrossRef]
- Nassar, A.M. Effectiveness of silver nanoparticles of extracts of Urtica urens (Urticaceae) against root-knot nematode Meloidogyne incognita. Asian J. Nematol. 2016, 5, 14–19. [Google Scholar] [CrossRef]
- Furmanczyk, E.M.; Kozacki, D.; Hyk, W.; Muszyńska, M.; Sekrecka, M.; Skwiercz, A.T. In vitro study on nematicidal effect of silver nanoparticles against Meloidogyne incognita. Molecules 2025, 30, 1132. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Liu, S.; Yang, J.; Meng, Z.; Lei, L.; Zhang, K. Comparison of homology models and crystal structures of cuticle-degrading proteases from nematophagous fungi: Structural basis of nematicidal activity. FASEB J. 2011, 25, 1894–1902. [Google Scholar] [CrossRef]
- Girma, A. Alternative mechanisms of action of metallic nanoparticles to mitigate the global spread of antibiotic-resistant bacteria. Cell Surf. 2023, 10, 100112. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, V.; Bhat, A.H.; Savarirayan, I.K.; Ataya, F.S.; Fouad, D. Root-knot nematode suppression through biogenic silver nanoparticles: A promising path for sustainable agriculture. J. Nanopart. Res. 2024, 26, 249. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Jha, A.K.; Prasad, K.; Prasad, K.; Kulkarni, A.R. Plant system: Nature’s nanofactory. Colloids Surf. B Biointerfaces 2009, 73, 219–223. [Google Scholar] [CrossRef]
- Mohammadzadeh, V.; Barani, M.; Amiri, M.S.; Yazdi, M.E.T.; Hassanisaadi, M.; Rahdar, A.; Varma, R.S. Applications of plant-based nanoparticles in nanomedicine: A review. Sustain. Chem. Pharm. 2022, 25, 100606. [Google Scholar] [CrossRef]
| Nanoparticle Type | Plant Species Used | Characterization Techniques/Tools | Particle Size Range (nm) | Morphology | Stability and Surface Charge | Reference |
|---|---|---|---|---|---|---|
| Ag-NPs | Euphorbia tirucalli | UV–vis spectroscopy, FTIR | 20–30 | Spherical and cubic | Not mentioned | [48] |
| Ag-NPs | Citrus limon L. | UV–vis spectroscopy, SEM, TEM, XRD, FTIR | 16.56–83.09 average size of 38.31 | Face-centered cubic (fcc) | Not mentioned | [49] |
| Ag-NPs | Acalypha wilkesiana | UV–vis spectroscopy, SEM, FTIR, XRD | 10 to 30 | Spherical | Zeta value: −16.1 mV | [50] |
| Ag-NPs | Eichhornia crassipes | TEM | 15.71 | Spherical | Not mentioned | [51] |
| Ag-NPs | Eucalyptus officinalis | UV–vis spectroscopy, FTIR, SEM | 100 | Spherical | Not mentioned | [52] |
| ZnO-NPs | Salix alba | UV–vis spectroscopy, SEM, FTIR, XRD | 7.14 | Irregular shapes | Not mentioned | [53] |
| Cu-NPs | Haplophyllum tuberculatum | SEM | 85 | Not mentioned | Not mentioned | [54] |
| MgO-NPs | Fragaria × ananassa | UV–vis spectroscopy, EDX, TEM, FTIR, XRD | 100 | Spherical | Zeta potential: −34.5 mV, | [55] |
| Ag-NPs | Solanum nigrum | UV–vis spectroscopy, EDS, TEM, FTIR, XRD | 30 | Spherical | Not mentioned | [56] |
| Ag-NPs | Senna seamia | UV–vis spectroscopy, EDX, SEM, TEM, FTIR, XRD | 05–60 | Polydisperse | Not mentioned | [57] |
| S-NPs | Rosmarinus officinalis | UV–vis spectroscopy, SEM, TEM, FTIR | 40 | Spherical | Not mentioned | [58] |
| Ag-NPs | Artemisia judaica | UV–vis spectroscopy, SEM | 50–150 | Not mentioned | Not mentioned | [59] |
| NiO-NPs | Ocimum sanctum | UV–vis spectroscopy, FTIR, SEM | 15–40 | Uniformly | Not mentioned | [60] |
| Ag-NPs | Fragaria × ananassa | UV–vis spectroscopy, SEM, TEM, FTIR, XRD | 55–70 | Rectangular | Not mentioned | [61] |
| Ag-NPs | Glycyrrhiza glabra | UV–vis spectroscopy, EDX, SEM, TEM, FTIR | 9–20 | Spherical | Zeta potential: −35.7 mV | [62] |
| Ag-NPs | Glycine max | UV–vis spectroscopy, SPR, DLS, FTIR | 11 | Spherical | Zeta potential: −23 to 25 | [63] |
| Ag-NPs | Cynanchum dioscoridis, Melia azedarach, Moringa oleifera | UV–vis spectroscopy, SEM | 30–100 | Spherical | Not mentioned | [64] |
| Cu-NPs, Fe-NPs, Zn-NPs | Tridax procumbens | UV–vis spectroscopy, EDX, TEM, FTIR, SAED | 2–100 | Rod-shaped | Not mentioned | [65] |
| Fe-NPs | Camellia sinensis | TEM, FTIR, XRD | 36–55 | Spherical | Not mentioned | [66] |
| Cu-NPs | Orobanche aegyptiaca | UV–vis spectroscopy, XRD, TEM, SEM | 50 | Spherical | Not mentioned | [67] |
| Ag-NPs | Cnidoscolus aconitifolius | UV–vis spectroscopy, FTIR, SEM | 2–20 | Spherical | Not mentioned | [68] |
| CuO-NPs | Jatropha curcas | UV–vis DRS spectroscopy, FTIR, TEM, XRD | 5–15 | Spherical, pure | Not mentioned | [69] |
| Plant Name | Prepared Nanoparticle Name/Type | Nematode Species | Host Plant | Condition/Method (In Vitro or İn Vivo) | Efficacy | References |
|---|---|---|---|---|---|---|
| Euphorbia tirucalli | Ag-NPs | Meloidogyne incognita | Tomato | In vitro and In vivo | 96–100% J2 mortality in 6 h, 46.95% infectivity reduction at 100 ng/mL and 100% at 1000 ng/mL. | [48] |
| Citrus limon L. | Ag-NPs | Meloidogyne javanica | Faba bean | In vitro and In vivo | 100% J2 mortality at 100 ppm, reduced disease severity by 66.67%, nematode populations by 99.27%. | [49] |
| Acalypha wilkesiana | Ag-NPs | Meloidogyne incognita | - | In vitro | 53.3% nematode mortality after 48 h at 100 mg/mL. | [50] |
| Eichhornia crassipes | Ag-NPs | Meloidogyne javanica | Swiss chard | In vivo | 115.14 egg masses, 2909 juveniles, RF 0.92 with 3 μg/mL Ag-NPs. | [51] |
| Eucalyptus officinalis | Ag-NPs | Heterodera sacchari | Rice | In vivo | 11.01 kg seed weight, 10 cysts (carbofuran: 11.20 kg seed weight, 7 cysts). | [52] |
| Salix alba | ZnO-NPs | Meloidogyne incognita | - | In vitro | 92.2% mortality at 1000 µg/mL after 48 h. | [53] |
| Haplophyllum tuberculatum | Cu-NPs | Meloidogyne incognita | - | In vitro | Cu-NPs caused 63.59% mortality, Nemaprope®10G showed the lowest mortality at 6.14%. | [54] |
| Fragaria × ananassa | MgO-NPs | Meloidogyne incognita | - | In vitro | 71 ± 2 juveniles at 24 h, 106 ± 2 at 48 h; 14 dead juveniles at 48 h. | [55] |
| Solanum nigrum | Ag-NPs | Meloidogyne incognita | - | In vitro | 100% mortality at 2.5 µg/mL, 48% mortality at 0.5 µg/mL. | [56] |
| Senna Siemia | Cu-NPs | Meloidogyne incognita | Ajwain | In vivo | 100 ppm AgNPs reduced gall formation and egg mass production compared to inoculated control. | [57] |
| Rosmarinus officinalis | S-NPs | Meloidogyne javanica | - | In vitro | 100% mortality at 30 ppm and 60 ppm after 4 days. | [58] |
| Fragaria × ananassa | Ag-NPs | Meloidogyne incognita | - | In vitro | Reduced hatching, 9 dead juveniles compared to 4 in control after 48 h. | [61] |
| Jatropha curcas | CuO-NPs | Meloidogyne incognita | Chickpea | In vitro and In vivo | 80% inhibition at 200 ppm, dose-dependent reduction in J2 hatching. | [69] |
| Glycyrrhiza glabra | Ag-NPs | Meloidogyne incognita | - | In vitro | 100% egg-hatching inhibition at 10.0 ppm, 100% mortality at 6.0 ppm, LC-50 = 0.805 ± 0.177 ppm. | [62] |
| Glycine max | Ag-NPs | Pratylenchus brachyurus | Soybean | In vitro and In vivo | Inhibited P. brachyurus mobility at 25 µmol L−1 or higher after 48 h, IC50 and IC90 values calculated. | [63] |
| Tridax procumbens | Cu-NPs, Fe-NPs and Zn-NPs | Meloidogyne incognita | Cabbage | In vivo | Reduced Meloidogyne incognita population, egg count, and gall index at 100 ppm. | [65] |
| Camellia sinensis | Fe-NPs | Meloidogyne incognita | Tomato | In vivo | Reduced root galls by 22.44% at 3 mg/kg, 17.76% at 6 mg/kg. | [66] |
| Orobanche aegyptiaca | Cu-NPs | Meloidogyne incognita | - | In vitro | Inhibited juvenile emergence by 83% at 800 μg/mL, 68%, 53%, 39%, and 26% at lower concentrations. | [67] |
| Cnidoscolus aconitifolius | Ag-NPs | Meloidogyne incognita | Carrot | In vivo | 67 soil nematodes ± 0.5, 58 root nematodes ± 1.3 (control: 3107 soil and 928 root nematodes). | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulaş, F.; Yüksel, E.; Dinçer, D.; Dababat, A.; İmren, M. Recent Advances in Plant-Based Green Synthesis of Nanoparticles: A Sustainable Approach for Combating Plant-Parasitic Nematodes. Sustainability 2025, 17, 4152. https://doi.org/10.3390/su17094152
Ulaş F, Yüksel E, Dinçer D, Dababat A, İmren M. Recent Advances in Plant-Based Green Synthesis of Nanoparticles: A Sustainable Approach for Combating Plant-Parasitic Nematodes. Sustainability. 2025; 17(9):4152. https://doi.org/10.3390/su17094152
Chicago/Turabian StyleUlaş, Furkan, Ebubekir Yüksel, Dilek Dinçer, Abdelfattah Dababat, and Mustafa İmren. 2025. "Recent Advances in Plant-Based Green Synthesis of Nanoparticles: A Sustainable Approach for Combating Plant-Parasitic Nematodes" Sustainability 17, no. 9: 4152. https://doi.org/10.3390/su17094152
APA StyleUlaş, F., Yüksel, E., Dinçer, D., Dababat, A., & İmren, M. (2025). Recent Advances in Plant-Based Green Synthesis of Nanoparticles: A Sustainable Approach for Combating Plant-Parasitic Nematodes. Sustainability, 17(9), 4152. https://doi.org/10.3390/su17094152








