Abstract
The growing production of polyurethane foam (PUF) and increasing global PUF waste generation urges the development of a circular economy strategy to promote the recovery of its raw materials, namely polyether polyols, in a sustainable and economically feasible way. This work assesses the promising microwave-assisted PUF aminolysis technology from three different perspectives: (a) evaluating the experimental feasibility and characteristics of the recycled products; (b) modeling an industrially relevant holistic process based on experimental findings to assess energy requirements and c) comparing the environmental impacts ascribed to the production of virgin vs. recycled polyols. The most relevant findings are as follows: (1) the recycled polyols out of MW-aminolysis are indistinguishable from virgin polyols; (2) the potential energy consumption of the overall process (including post-processing steps) for a continuous PUF depolymerization process with a 14.8 kg/h RP production capacity is as low as 1.9 kWh/kg RP and (3) recycled polyols have a substantially lower environmental footprint than virgin polyols in all selected impact categories, ranging from a reduction in CO2 emissions (38% decrease) to water consumption (74% decrease). These results and analyses pave the way for enhancing material circularity in the PU sector.
1. Introduction
Polyurethane (PU) is one of the most widely produced and utilized polymeric materials worldwide, with applications spanning across construction, automotive, furniture and packaging industries. Global PU production has experienced significant growth, exceeding 25 million metric tons annually [1], a trend driven by increasing demand for durable and versatile materials. However, the widespread use of PU has led to an escalating accumulation of PU waste, posing severe environmental and economic challenges due to its inherent resistance to degradation and conventional recycling processes [2]. Landfilling and incineration remain the predominant disposal methods, leading to substantial carbon emissions and resource depletion. In light of these issues, the development of circular economy strategies for PU waste recycling is imperative to mitigate environmental impacts and promote sustainable resource utilization [2,3]. A promising avenue to achieve this goal is thermochemical recycling, which enables the conversion of PU waste into valuable secondary raw materials, such as polyether polyols, thereby closing the material loop in PU production [2,3,4].
Several recycling technologies have been explored to manage PU waste, with mechanical and chemical recycling being the most commonly implemented. Mechanical recycling involves grinding PU waste into fine particles for use as fillers or in composite materials, being the easiest and most basic way to recycle PUs [3], but this approach does not allow for the recovery of high-value raw materials. Chemical recycling, on the other hand, involves breaking down PU into its original building blocks, enabling the production of recycled polyols [4,5]. Among chemical recycling methods, various solvolysis routes have been addressed in the literature, including glycolysis [6,7,8], hydrogenolysis [9], acidolysis [10,11,12] and aminolysis [13,14], each offering different advantages depending on process objectives. Some routes minimize CAPEX and OPEX costs by requiring simpler equipment, fewer and/or less demanding purification steps or inexpensive reactants, while others focus on achieving higher-quality recycled polyols at the expense of increased process complexity and costs (e.g., reactor configurations and separation processes).
For instance, it was proven that the recycled polyol resulting from aminolysis is fully hydroxyl-functionalized analogue of the corresponding virgin polyol [14], whereas acidolysis-derived recycled polyols show different end-group functionalities, e.g., hydroxyl, aromatic amine originating from incomplete urethane group degradation and carboxyl as a consequence of an esterification side reaction between the carboxyl groups of adipic acid and the hydroxyl groups of the polyol [11,15]. Depending on the end use of such polyols, these functionalities may play a critical role. In case the recycled polyols aim to be employed as raw materials for the production of PU foam (PUF), it was found that these carboxyl functional groups may influence the relative kinetic of cross-linking and foaming reactions, potentially resulting in closed-cell morphology and deteriorated properties of PUFs synthesized from acidolysis-derived recycled polyol [15].
As counterpart, the cost of aminolysis reagents and/or catalysts such as tris(2-aminoethyl)amine, i.e., TREN, may represent an order of magnitude higher than that of conventional acidolysis ones (e.g., adipic acid), thus, leading to significantly increased operating costs in the case that the catalysts are not efficiently recovered along the downstream processing.
In addition, regardless of the employed route, the thermochemical degradation of PU waste via solvolysis requires a very relevant heat supply [2,3,4]. Typically, the heat requirements are satisfied either by fuel combustion or resistive heating. The former leads to a significant carbon footprint, while the latter is quite energy consuming, provides typically slow heating rates and low energy efficiency derived from large energy losses.
To counteract both heating source limitations, microwave-assisted heating (MW) emerges as an alternative heat source to decarbonize and electrify such thermochemical processes at mid and industrial scales [16,17,18]. Among the specific benefits ascribed to microwave heating for polymers recycling, this technology has been endorsed to enhance depolymerization reaction kinetics as well as to reduce energy consumption and overall reaction times by enabling selective heating of polar components in polymer matrices [16,19,20]. Specifically, the application of microwave energy in PU recycling accelerates bond cleavage and enhances process control, while decreasing operational temperatures from a certain polymer valorization degree [10,15], leading to increased efficiency and lower environmental impact. Additionally, as an electrification technology, microwave heating aligns with decarbonization strategies by reducing reliance on fossil-fuel-based heating systems, further improving the sustainability of PU waste recycling.
Despite its promising prospects, the industrialization of this technology is fraught with uncertainties due to the absence of benchmarking microwave-solvolysis industrial prototypes in operation for plastics recycling and the demanding process requirements in terms of equipment, materials and heat distribution along the reactor system [21,22,23,24].
The motivation of this work is, thus, to assess its potentials and gain insight into the feasibility and benefits of the MW-assisted thermochemical PUF recycling technology at an industrially relevant scale, aiming to pave the way for enhancing material circularity in the PU sector.
Based on the previous arguments and balancing process complexity, recycled products features and economic feasibility, this paper presents an integrated approach to the electrified and decarbonized thermochemical aminolysis of PU foams assisted by microwave heating for the production of recycled polyol. The study covers three main aspects: (a) the experimental assessment of MW-assisted PU aminolysis, validating the proof of concept at a mid-scale level (1 L stirred-tank capacity); (b) a modeling approach for process scalability, considering the integration of pre-processing and post-processing steps to optimize energy and material consumption efficiency and (c) a comprehensive life cycle assessment (LCA) to compare the environmental impact, carbon footprint and resource savings between recycled and virgin polyol production.
2. Materials and Methods
2.1. Experimental Microwave-Assisted Aminolysis Set-Up
The microwave-assisted aminolysis set-up consists of a multimodal microwave cavity prototype (BP-211, Microwave Research and Applications, Inc., Carol Stream, IL, USA) [25] powered by four 800 W microwave power magnetrons working at 2.45 GHz, four WR340 waveguides excited with the fundamental mode TE10 and their corresponding mode stirrers distributed at the lateral applicator walls in order to provide a time-average homogeneous electromagnetic field distribution within the cavity. The inner applicator dimensions are 110 × 45 × 60 cm (h × w × d).
The heated sample, including shredded post-industrial PUF, virgin polyol and tris(2-aminoethyl)amine (TREN) as reagent, is placed in a 1 L quartz vessel, having an internal diameter of 4.8 cm (Figure 1a). This vessel is arranged within a PEEK chamber designed to withstand temperatures up to 300 °C and pressures up to 30 bar (Figure 1b). The top end of the chamber has two ports for mechanical stirring and temperature measurement via K-type thermocouple, respectively. The top end is attached to the chamber through a 12 PEEK screws corona (Figure 1b–d). A quartz sheath is employed to prevent the thermocouple from being damaged in the presence of the caustic environment (Figure 1c). The top end of the thermocouple is tightly screwed to the applicator choke to ground it and avoid the generation of antenna effects in the presence of a strong electromagnetic field (Figure 1f). Figure 1e shows the pressurized vessel within the microwave resonator. It is placed on a thermal insulator (muffle) to avoid overheating the microwave-transparent plastic support.

Figure 1.
(a) Post-industrial shredded PU foam; (b) front: PEEK chamber; (c) guiding chokes; (d) thermocouple inserted; (e) PEEK chamber placed within the microwave; (f) threaded attachment of the thermocouple.
A rotor with adaptive stirring velocity provides the required power to overcome the torque of the viscous sample upon PUF degradation. The cap immediately above the cross fitting holds a dynamic seal to allow the rotation of the stirrer shaft while keeping the system pressurized and gas tight. The arrangement of the PEEK-based dynamic seal is critical and must be carefully aligned with the stirring shaft to avoid gas and/or liquid leaking. The pressurized line is made of corrosion-resistant 316 L stainless steel, and it holds an on–off valve at one end, a pressure meter and a relief valve at the other end. The diameter and length of the different chokes at the external walls of the applicator were carefully dimensioned to avoid microwave irradiation leaking. MW-leaking tests were conducted using an MW-oven leakage detector specifically calibrated for the 2.45 GHz frequency band. It was found that the maximum leaking 5 cm away from any of the potential leaking sources does not exceed 5 mW/cm2, which is the maximum allowed power density for such equipment in this range of microwave frequencies [26].
2.2. MW-Assisted Aminolysis Procedure
The MW-assisted PUF aminolysis tests were conducted using a constant PUF to virgin polyol (VP) medium weight ratio of 2 and an amount of TREN to accomplish a 3.5 amino-per-urethane group equivalent. The employed VP is ALCUPOL® F-4811 supplied by Repsol. The post-industrial PUF consisted of a mixture of the said VP and toluene diisocyanate (TDI), with their weight proportions being 80/20. The isocyanate index, i.e., TDI amount/stochiometric TDI amount, was 107%. The PUF sample contains 4.5 parts-per-polyol (ppp) of water and up to 0.35 ppp of other components (Tegoamin 33, Tegoamin BDE, Kosmos 29 catalyst and silicone B2370), besides 54.9 ppp of TDI. As a result, the calculated theoretical amount of polyol in the PUF is ca. 66 wt%. The experimental trials were conducted mixing 72 g of PUF with 36 g of VP and 8 g of TREN. The reaction mixture was microwave-heated up to 225 °C and maintained at this temperature for 30 min. The maximum operating pressure at these conditions remained below 8 bar since CO2 was previously outgassed. The depolymerization product consists of a mixture of solid residue and a dark yellowish recycled polyol. The residue, which is composed of PUF hard segments terminated with amino groups, is separated from recycled polyol by centrifugation. The crude polyol contains small amounts of soluble side products, such as toluene diamine isomers (TDA). TDA is a carcinogenic product, so its generation is not desired [27]. A subsequent liquid–liquid (L–L) separation step using ethyl acetate (EA) and diluted HCl as organic solvent and aqueous media, respectively, is conducted to remove TDA and urea from the polyol since both migrate to the aqueous phase [14,15]. The polyol is finally isolated by EA distillation. Tentatively, six consecutive liquid–liquid extractions are sufficient to remove the impurities from the polyol. The final steps consist of washing the crude polyol dissolved in ethyl acetate with deionized water to remove HCl, followed by vacuum distillation of ethyl acetate at 40 °C and 50 mbar. Figure 2 shows the stepwise post-processing procedure adopted to bring the reaction mixture to the segregation of hard segments, extraction of TDA and urea fractions and final distillation of EA to obtain pure recycled polyol (RP). Eight cleaning cycles with a slightly acidic HCl solution plus three washing cycles with Milli-Q water were required in order to eliminate the TDA and urea and to remove the traces of HCl from the organic phase, respectively. Figure 3 summarizes the stepwise PUF recycling block diagram.
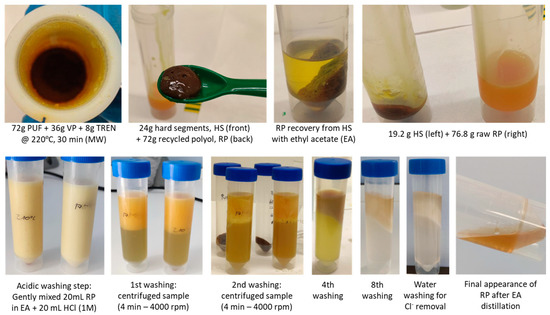
Figure 2.
Stepwise post-processing procedure to obtain pure recycled polyol from PUF waste: (1) appearance of reaction mixture after MW-aminolysis at 225 °C, (2) solid hard segments (HS) and recycled polyol-rich liquid phase (RP), (3) RP leaching from HS with ethyl acetate (EA), (4) HS and RP isolated fractions, (5) emulsion resulting from the acidic washing of the organic phase with HCl 0.1 M, (6) centrifugation and L–L extraction, (7) final washing step with water to remove any hydrochloric traces from the organic phase, (8) distillation of EA and recovery of pure RP.
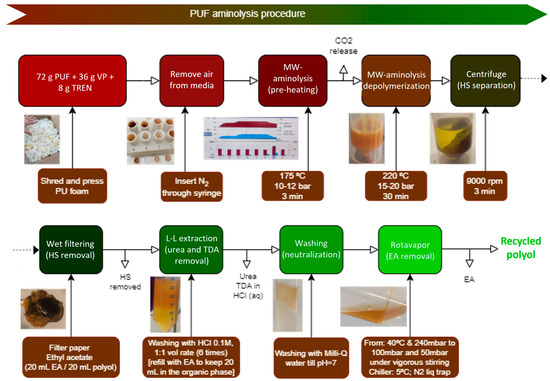
Figure 3.
Stepwise procedure of MW-assisted PUF aminolysis.
2.3. Materials Characterization Techniques
The isolated recycled polyol from MW-assisted PUF aminolysis was characterized by both 1H NMR and size-exclusion chromatography combined with ultraviolet, multiangle laser light scattering and refractive index (SEC/UV-MALS-RI) detectors. 1H NMR spectra were recorded at room temperature in deuterated dimethyl sulfoxide (DMSO-d6) with and without the addition of trifluoracetic acid (TFA) using a Bruker AVANCE NEO 600 MHz instrument (Bruker Corporation, Billerica, MA, USA) [14,15,20]. Regarding the SEC/UV-MALS-RI measurements, the molar mass characteristics (Mw, Mn, dispersity: Đ = Mw/Mn) and purity of RPs were determined using SEC coupled to a UV detector operating at a wavelength of 280 nm (Agilent Technologies, Santa Clara, CA, USA), a DAWN multiangle light-scattering photometer (Wyatt Technology Corp., Santa Barbara, California, USA), and an Optilab interferometric refractometer (RI) (Wyatt Technology Corp., USA) [14,15,20]. Separations were performed at room temperature using a TSKgel Alpha-2500 SEC column (7.8 mm ID × 30.0 cm L, pore size 7 µm and exclusion limit 10 kDa) with a precolumn (Tosoh Bioscience GmbH, Griesheim, Germany). Methanol (MeOH) was used as the solvent and mobile phase at a flow rate of 0.7 mL/min. The typical RP concentration in MeOH was 1.0 × 10−2 g/mL, while the injection volume was typically 100 µL. The specific refractive index increment (dn/dc) required to calculate the molar masses of RPs was determined from VP, assuming 100% mass recovery of the samples from the column. Astra 8 software was used for data acquisition and analysis (Wyatt Technology Corp., USA) [14,15,20].
3. Conceptual PUF Recycling Scale-Up Design
This section presents the conceptual scale-up design of the microwave-assisted post-industrial polyurethane foam (PUF) depolymerization, providing the first insight to evaluate whether the technology has industrial deployment potential. The proposed scale-up design focuses on process optimization through energy and mass balance analyses in the main equipment, prioritizing the overall process efficiency and resource management. The aim was to model a process capable of reclaiming up to 15 kg of recycled polyol per batch, which is an industrially relevant production capacity. According to the bench experimental MW-aminolysis process performance and RP yield, the calculated stirred-tank reactor capacity was roughly 300 L. In this preliminary assessment, geometry aspects, material and equipment technical specifications are beyond the scope. These will be addressed in future work.
The assumptions considered for the scaling-up of the process are described as follows:
- Semi-continuous stirred-tank reactor capacity: 300 L;
- Same PUF/solvent/reagent weight ratio as that used in bench tests (PUF:VP:TREN = 10:5:1 wt%);
- The energy for pre-squeezing shredded PUF and loading the reactor was considered negligible;
- 100% conversion of PUF was assumed;
- Same reaction time and yield as in bench tests were adopted;
- An approach to keep the scaled tank stirred was to scale it as follows the proportion of shredded PUF/1 L reactor → 22.5 kg PUF/300 L (steady-state reactor loading);
- Mixer–settlers were assumed to be used for liquid–liquid extraction, i.e., extraction of TDA from the organic phase (recycled polyol + ethyl acetate) using an aqueous HCl solution;
- The distillation processes were modeled as evaporating units;
- The recirculation of solvents (ethyl acetate and HCl) and part of the recycled polyol are envisioned to reduce the consumption of raw materials and environmental footprint;
- The hard segments and TDA are the only waste flows;
- The analysis excludes the mechanical PUF waste feeding and product removal systems.
Figure 4 shows the conceptual scale-up design of the depolymerization process of PUF based on the laboratory procedure. The process starts with pouring the recycled polyol and PUF into the reactor. Magnetrons generate and deliver microwave irradiation into the reactor, leading to target sample heating and PUF depolymerization. The reaction products, namely a mixture of polyol, hard segments, toluene diamine (TDA) and urea leave the reactor tank, flowing towards a filter. The hard segments are here separated, while the remaining mixture flows to a mixer–settler, where diluted HCl and ethyl acetate (EA) are added to the mixture. Two streams leave the mixer to settle: one containing EA and polyol and the other composed of diluted HCl, TDA and urea. The polyol/EA stream flows to an evaporator where the EA is evaporated, then condensed and returned to the mixer–settler. The stream of the HCl/TDA flows to another evaporator where the HCl is evaporated and then condensed and returned to the settler–mixer where the process occurs over again. The cooled fluid from an industrial process is used in the heat exchanger to cool the mixture leaving the reactor. The heat recovered from this process is used to evaporate EA from polyol. In the model, both energy inputs related to PUF feeding and the reactor engine were considered as a single energy consumption source under the reactor engine item. Moreover, it was assumed that the initial amount of VP required to start up the process is negligible against the materials flow under continuous operation.
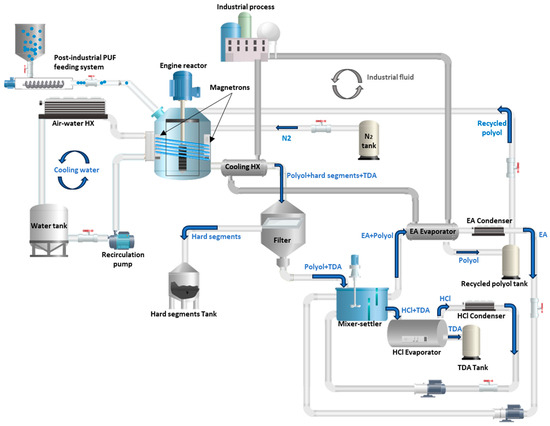
Figure 4.
Conceptual scale-up design of the depolymerization process of PUF.
The overall process has a semi-continuous nature, in which the different process steps have varying time durations. The process design as a fully continuous operation was discarded due to the relatively large residence times required for the complete solvolysis of PUF waste (ca. 30 min) and the operation under pressurized conditions to avoid reaction mixture evaporation (ca. 8 bar). The former would require the implementation of comparatively larger reactor volumes and/or very low flow rates, while the latter would require the adoption of demanding pumps to pressurize and drive the multiphase reaction mixture into the reactor vessel at pressures higher than operating process ones in order to avoid reverse flows.
Mathematical Modeling
Table 1 presents the thermodynamic properties of the components present in the depolymerization process of the PUF using a microwave-assisted continuous stirred-tank reactor, similar to the conceptual design of Frisa-Rubio et al. [28]

Table 1.
Thermodynamic properties of the components present in the depolymerization process of PUF.
The mathematical modeling and the assumptions considered to calculate the power consumption of each individual equipment used to scale up are presented in Table 2.

Table 2.
Mathematical modeling of the equipment used in the scale up process.
4. Life Cycle Assessment (LCA)
The environmental impacts of the recycled polyol (RP) obtained from the PUF depolymerization process have been calculated by applying the LCA methodology under a cradle-to-gate perspective. LCA is a powerful tool able to assess the environmental impact of a process or service [29,30] with an important framework provided by ISO 14040:2006 [31] and ISO 14044:2006 [32] standards. This tool collects all energy and material flows data of the different stages and quantifies the environmental impact of a product. The process considered in this study is the production of a kilogram of recycled polyol from PUF depolymerization. Detailed information about materials and energy sources involved in the process can be consulted in Table 3.

Table 3.
Life cycle inventory of scale-up process of PUF depolymerization per kg of RP.
The life cycle has been modeled using SimaPro 9.3 software and Ecoinvent 3.8 as the background database. For the study, five environmental indicators identified as relevant for the plastic sector have been selected from the ReCiPe 2016 v1.1 midpoint method, Hierarchist version:
- Global warming (kg CO2 eq) measures the contribution of an activity or product to climate change, expressed in CO2 equivalents. It includes other greenhouse gases like methane or nitrogen oxides, which are converted to CO2 based on their global warming potential;
- Fine particulate matter formation (kg PM2.5-eq) assesses the formation of fine particulate matter (PM2.5) in the air, which can have negative effects on human health and the environment. These particles can cause respiratory issues and contribute to air pollution;
- Terrestrial acidification (kg SO2-eq) measures the impact of acidic emissions (such as sulfur dioxide, SO2) that can acidify the soil and harm ecosystems. Acidification lowers soil quality and can affect biodiversity;
- Fossil resource scarcity (kg oil eq) estimates the amount of non-renewable fossil resources, like oil, that are depleted due to extraction and use. This category reflects the availability and sustainability of these resources over time;
- Water consumption (m3) measures the total volume of water used throughout the lifecycle of a product or activity, including water consumed in industrial processes, agricultural irrigation and other uses. Water scarcity is a key concern in many regions.
The environmental impacts of virgin polyether polyol production were calculated by applying the LCA methodology to a polyol manufacturing process reported in the literature [33] under a cradle-to-gate approach. The process considered in this study is the production (in USA) of 1 kg of polyether polyol from propylene oxide, limestone, electricity generated by residual fuel oil and natural gas combusted in the boilers. Alternatively, the environmental impacts of recycled polyol production were calculated on the basis of the experimental procedure described in Section 2.2 and process yields reported in Section 5.1.
5. Results and Discussion
This Section describes the results from (a) the experimental MW-assisted PUF depolymerization assessment, including process yield and recycled polyol characterization; (b) the estimated energy consumption of a modeled scaled-up PUF depolymerization process assisted by MW-heating via aminolysis; (c) the LCA of both recycled and virgin polyols as raw materials for the production of PUF.
5.1. Experimental PUF Recycling: Materials Characterization and Process Yield
Under the experimental conditions explored, the overall MW-assisted aminolysis yield into the liquid phase is slightly above 70% wt., whereas the undesired productivity of hard segments (HS) peaks up to nearly 18% wt. The liquid phase includes TDA and derivatives, urea and polyol, the latter being the targeting product. The typical purified RP production per batch is ca. 30 g, starting from 72 g of post-industrial PUF foam. Taking into account the theoretical composition of the post-industrial PUF, which was estimated to be ca. 66 wt%, the process yield peaks up to ca. 67%. Its features are discussed below, based on both 1H NMR and SEC/UV-MALS-RI.
Figure 5 shows 1H NMR spectra of samples including virgin polyol and purified recycled polyol pre- and post-drying steps. In this figure, the chemical shifts (δ) are given in ppm relative to a DMSO-d6 residual peak. As can be observed, the bands corresponding to polyol methyl, methylene and methyne protons are found at 1.04 and 3.15–3.70 ppm, while the –OH group is found at 4.40 ppm. 1H NMR spectra of purified RP (blue) showed traces of solvent ethyl acetate (EA), the characteristic peaks of which are found at 1.17 (triplet), 1.99 (singlet) and 4.03 ppm (quartet). As previously discussed, EA was used as the organic medium along the liquid–liquid RP purification procedure. The peak of EA at 2.00 ppm is overlapping with the signals of methyl protons of amino end groups, which are found at 1.87, 1.96 and 2.00 ppm [14,15]. The dried RP (magenta) resulting from an additional rotary evaporation of the purified RP at 50 °C and low pressure (around 20 mbar) allowed for obtaining the information about the remaining end groups.
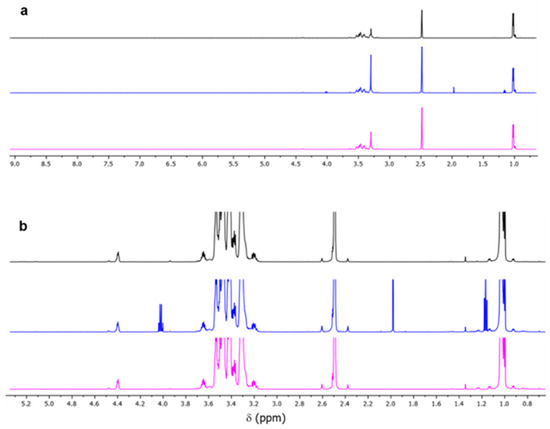
Figure 5.
(a) 1H NMR spectra and (b) enlarged 1H NMR spectra of (1) VP ALCUPOL® F-4811 (black), (2) RP by MW-aminolysis prior (blue) and after drying (magenta).
The degradation degree of the original PUF sample can also be estimated based on the 1H NMR spectra. The complete degradation was confirmed with the absence of a peak at 4.88 ppm (in DMSO-d6 with added TFA), which belongs to the polyol methyne proton next to the remaining urethane groups. Analogously, the complete degradation is also confirmed as indicated by the disappearance of the methyl proton signals of the end amino groups (1.87, 1.96 and 2.00 ppm) of TDA isomers bound via the urethane group [14,15].
Regarding the SEC/UV-MALS-RI measurements, Figure 6a shows the overlay of the chromatograms corresponding to the above-mentioned virgin (black) and recycled samples (blue). For the sake of analysis completion, these spectra were also compared to those of RP obtained under MW-assisted acidolysis (orange) and aminolysis (green) at 10× downscaled lab equipment. As can be observed, the absence of any high-molecular-weight species and the presence of polyol at 9.4 min confirm a high degree of PUF network degradation [14,15]. Since all recycled polyols are purified using liquid–liquid extraction, low-molecular residues of hard segments, mainly isomers of toluene diamine (TDA; 2,4-TDA and 2,6-TDA), which elute at longer elution times (22–37 min), are not present/detected by RI (solid line) nor UV (dashed line) detector.
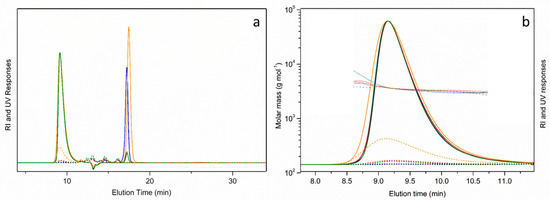
Figure 6.
Overlay (a) and enlarged overlay (b) of SEC/UV-MALS-RI chromatograms of (1) VP ALCUPOL® F-4811 (black), (2) bench (100 mL) RP by MW-aminolysis (green), (3) bench (100 mL) RP by MW-acidolysis (orange), (4) scaled (1L) RP by MW-aminolysis (blue).
Zooming the SEC/UV-MALS-RI chromatogram regions to elution times between 8 and 11 min (Figure 6b), it can be observed that the molecular weight characteristics, i.e., weight and number average molecular weights and dispersity of the RPs are comparable to those of the virgin polyol. The resulting molecular weight of all samples is 3500 (Da), with their being dispersity Đ = 1.01.
To summarize the previous findings, both characterization techniques suggest that (1) PUF degradation was completed successfully under the employed MW-assisted aminolysis procedure and (2) the chemical composition and structure of the recycled polyol cannot be distinguished from that of the virgin polyol, thus confirming the success of the recycling process and the validity of RP as raw material to produce second-generation polyurethane foams.
Microwave-assisted heating was previously claimed to potentially intensify the PUF depolymerization process by shortening the reaction time for full degradation, the transient heating time or the overall process temperature. The next section quantifies the potential energy consumption of a scaled-up MW-assisted PUF aminolysis process based on the modeling study described in Section 3.
5.2. Energy Consumption Assessment of an Upscaled MW-Driven PUF Depolymerization Process
The energy consumption results of an upscaled (300 L reactor capacity) MW-assisted continuous PUF depolymerization process are presented in Table 4. The modeled process allows for producing up to 14.8 kg/h RP. The overall consumption is estimated to be 28.4 [kWh], with the normalized energy requirement per kg of recycled polyol being: 1.9 [kWh/kg]. The magnetrons are the highest contributors to the overall energy consumption (11 kWh), followed by the engine mixer–settler and the water-cooling system. To illustrate the encouraging energy savings of the proposed upscaled process against the experimentally validated batch PUF aminolysis process carried out in a 1 L MW-assisted stirred tank reactor, the same calculation was conducted for such a lab-scale batch process. The resulting normalized energy requirements for this process are 9.61 kWh/kg RP. This suggests that the potential energy savings of the upscaled process may eventually reach ca. 80.2%.

Table 4.
Energy consumption in the upscale design of MW-assisted PUF depolymerization process.
Furthermore, the modeled upscaled process has certain room for energy management optimization. For instance, a standard magnetron efficiency was considered in the model. Potential optimizations in magnetron design, operation or coupling with the heated sample may play a crucial role in reducing energy consumption, as magnetrons are major energy consumers in microwave-assisted reactor systems. Alternatively, the implementation of heat recovery systems could further enhance the overall energy efficiency by recovering waste heat from certain process steps. In addition, the engine of the mixer–settler and the water-cooling system also contribute notably to the overall energy requirements in the current scenario and, thus, these represent potential areas for further optimization by appropriate design and arrangement. In addition, improvements in the microwave system (e.g., cavity design, power delivery) and process parameters (e.g., temperature, time, solvent ratio) could substantially impact energy efficiency. Nonetheless, the precise control over microwave power and temperature profiles are key to maximizing energy transfer to the reactants while minimizing losses.
In any case, the achieved reduction in normalized energy consumption encourages further research towards the development and implantation of the proposed technology on a larger scale as a sustainable, decarbonized and energy-efficient PUF recycling solution.
5.3. Life Cycle Analysis (LCA)
The environmental impact of the microwave MW-assisted PUF depolymerization to obtain RP was evaluated. The LCA assessment results highlight that electricity consumption, tris(2-aminoethyl)amine (TREN) and PUF waste are the most significant contributors across all impact categories selected for the analysis, namely global warming potential, fine particulate matter formation, terrestrial acidification, fossil fuel scarcity and water consumption (Figure 7). Analogously, Table 5 showcases the quantified environmental impacts of PUF depolymerization per ton of recycled polyol. The presented values are indicative and rely on the experimental process yields reported in Section 5.1 as well as on the benchmarking cradle-to-gate process for polyol production from raw materials [33].
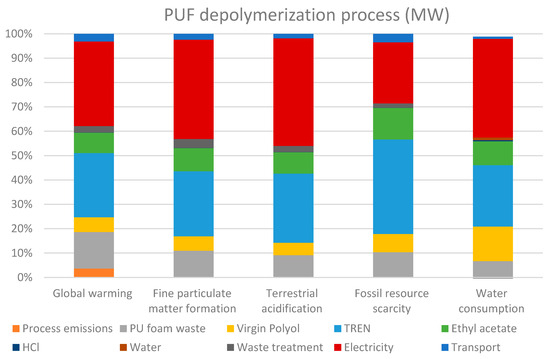
Figure 7.
Percentage distribution of the impacts attributable to the production of 1 tonne of recycled polyol thought MW-assisted depolymerization of PUF.

Table 5.
Environmental impacts of PUF depolymerization per ton of recycled polyol.
As can be observed, electricity consumption is the primary driver of environmental impact in the recycling process, accounting for between 25% to 44% of the impact on the different indicators. While the use of MW heating offers advantages in reaction kinetics and process efficiency over conventional heating [34,35], its reliance on electricity remains a major factor influencing environmental performance.
TREN, a relevant reactant in the recycling process, is the second-most impactful input, contributing significantly (between 26% and 39%) to the overall environmental burden, particularly in fossil resource scarcity, where it is the most impactful material. The main impact of TREN is attributed to its production, which involves resource-intensive chemical synthesis and high energy requirements. Incoming PUF waste, which serves as the primary feedstock for polyol recovery, also has a considerable impact, primarily because it is the main input of the process. It is very important to highlight that, as waste material, the impact of raw material extraction is eliminated, and only the impact of its collection, separation and transport is attributed to it. In the analysis, its transport is considered as a separate variable in the results. Additionally, ethyl acetate, a solvent used in the depolymerization process, contributes notably to fossil resource scarcity and global warming potential.
Despite these environmental burdens, the MW-assisted process presents notable advantages compared to conventional virgin polyol (VP) production. Impacts of polyol production from virgin raw materials were calculated through the modeling of an inventory found in the literature [33], and their results are presented in Table 6.

Table 6.
Impacts of virgin polyol vs. recycled polyols (per ton of material).
The comparison between RP obtained via MW-assisted depolymerization of PUF and VP production highlights substantial environmental benefits associated with recycling process. In all selected impact categories, RP demonstrates a lower environmental footprint than VP, with reductions ranging from 26% to 74%. In terms of global warming potential, RP production achieves a 38% reduction in CO2 emissions. This improvement is largely due to the avoidance of energy-intensive petrochemical synthesis required for propylene oxide, the main precursor in VP production. The fine particulate matter formation impact is also significantly reduced by 36% in RP production. This is primarily attributed to the lower emissions associated with the recycling process compared to the combustion of fossil fuels required for the synthesis of VP. Similarly, terrestrial acidification is reduced by 26%, reflecting the lower nitrogen oxide (NOx) and sulfur oxide (SOx) emissions in RP production. The most significant reductions are observed in fossil resource scarcity (50%) and water consumption (74%). VP production relies heavily on fossil-based raw materials, particularly propylene oxide, which accounts for a substantial portion of the total impact in this category. By contrast, RP production utilizes post-consumer polyurethane waste, significantly lowering demand for virgin fossil resources. Likewise, water consumption is drastically reduced due to the elimination of water-intensive petrochemical processing steps required for VP synthesis.
6. Conclusions
This work presented a holistic assessment of PUF recycling via microwave-assisted aminolysis through three different perspectives: (1) experimental technology validation at an intermediate 1 L reactor-scale level; (2) overall process modeling of an industrially relevant-sized continuous PUF depolymerization plant (300× increased capacity with respect to the lab-scale validated process) and (3) LCA analysis of virgin- and recycled-polyol raw materials for polyurethane production, showcasing the impacts of both polyol production processes.
The experimental process assessment demonstrated the technical viability of the MW-aminolysis for full polyurethane degradation at mild temperatures (225 °C) and very short reaction times (<30 min) in the presence of tris(2-aminoethyl)amine. These represent optimum process conditions for this type of PUF waste, as reported elsewhere. The process yield towards liquids was beyond 70%, with the production of hard segments being below 18%. The obtained recycled polyol represents ca. 42% wt. of the initial weight of the treated PUF waste and ca. 67 wt% of the virgin polyol contained in its formulation.
The overall energy requirements of the MW-assisted PUF depolymerization process were assessed for a full pilot set-up containing a set of post-processing separation and purification units as well as a continuous 300 L stirred MW-reactor tank. The energy consumption was estimated to be ca. 1.9 kWh/kg RP for the upscaled process, in contrast with the 9.61 kWh/kg RP calculated for the benchmarking experimental batch process. The encouraging 80% reduction in the normalized energy consumption poses excellent prospects for further research and development of the technology scaling.
Furthermore, the LCA analysis demonstrated that MW-assisted PUF depolymerization is an environmentally favorable alternative to conventional polyol production. The process not only reduces reliance on fossil resources but also minimizes emissions and resource depletion, supporting circular economy principles in polyurethane manufacturing. Further improvements in energy efficiency, particularly in electricity consumption, for example, the adoption of renewable energy sources and alternative reactants with lower environmental burdens, could enhance the sustainability of RP production even further. This approach needs to be explored together with the proper development of an energy management system relying on waste heat recovery, magnetron design optimization as well as on greener reagents than TREN and operating process conditions optimization.
Author Contributions
Conceptualization, D.M.-H., M.F.-M. and I.J.; experimental methodology, I.J.; modeling methodology, M.F.-M. and LCA methodology, D.M.-H.; formal analysis, D.M.-H., M.F.-M. and I.J.; data curation, D.M.-H., M.F.-M. and I.J.; writing—original draft preparation, D.M.-H., M.F.-M. and I.J.; writing—review and editing, I.J.; supervision, I.J. All authors have read and agreed to the published version of the manuscript.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No. 820665 (polynSPIRE).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request.
Acknowledgments
The authors thank David Pahovnic and Ema Žagar from the National Institute of Chemistry (Slovenia) for the VP and RP materials characterization. The authors also thank Repsol S.A. for providing the PUF samples and corresponding virgin polyol ALCUPOL® F-4811.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CAPEX | Capital expenditure |
| EA | Ethyl acetate |
| LCA | Life cycle assessment |
| MW | Microwave-assisted |
| NMR | Nuclear magnetic resonance |
| OPEX | Operating costs |
| PEEK | Polyether–ether–ketone |
| PUF | Polyurethane foam |
| RP | Recycled polyol |
| SEC/UV-MALS-RI | Size-exclusion chromatography combined with ultraviolet, multiangle laser light scattering and refractive index |
| TDA | Toluene diamine |
| TREN | Tris(2-aminoethyl)amine |
| VP | Virgin polyol |
References
- Statista Research Department. Global Polyurethane Market Volume 2015–2030; Statista: Hamburg, Germany, 2024. [Google Scholar]
- Rossignolo, G.; Malucelli, G.; Lorenzetti, A. Recycling of polyurethanes: Where we are and where we are going. Green Chem. 2024, 26, 1132–1152. [Google Scholar] [CrossRef]
- Kemona, A.; Piotrowska, M. Polyurethane recycling and disposal: Methods and prospects. Polymers 2020, 12, 1752. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Dewil, R.; Appels, L.; Ansart, R.; Baeyens, J.; Kang, Q. Reviewing the thermo-chemical recycling of waste polyurethane foam. J. Environ. Manag. 2021, 278, 111527. [Google Scholar] [CrossRef] [PubMed]
- Simón, D.; Borreguero, A.M.; de Lucas, A.; Rodríguez, J.F. Recycling of polyurethanes from laboratory to industry, a journey towards the sustainability. Waste Manag. 2018, 76, 147–171. [Google Scholar] [CrossRef]
- Wu, C.H.; Chang, C.Y.; Cheng, C.M.; Huang, H.C. Glycolysis of waste flexible polyurethane foam. Polym. Degrad. Stab. 2003, 80, 103–111. [Google Scholar] [CrossRef]
- Wieczorek, K.; Bukowski, P.; Stawiński, K.; Ryłko, I. Recycling of Polyurethane Foams via Glycolysis: A Review. Materials 2024, 17, 4617. [Google Scholar] [CrossRef]
- Heiran, R.; Ghaderian, A.; Reghunadhan, A.; Sedaghati, F.; Thomas, S.; Haghighi, A.H. Glycolysis: An efficient route for recycling of end-of-life polyurethane foams. J. Polym. Res. 2021, 28, 22. [Google Scholar] [CrossRef]
- Zhou, W.; Neumann, P.; Al Batal, M.; Rominger, F.; Hashmi, A.S.K.; Schaub, T. Depolymerization of Technical-Grade Polyamide 66 and Polyurethane Materials through Hydrogenation. Chem. Sus. Chem. 2021, 14, 4176–4180. [Google Scholar] [CrossRef]
- Gama, N.; Godinho, B.; Madureira, P.; Marques, G.; Barros-Timmons, A.; Ferreira, A. Polyurethane Recycling Through Acidolysis: Current Status and Prospects for the Future. J. Polym. Environ. 2024, 32, 4777–4793. [Google Scholar] [CrossRef]
- Gama, N.; Godinho, B.; Marques, G.; Silva, R.; Barros-Timmons, A.; Ferreira, A. Recycling of polyurethane by acidolysis: The effect of reaction conditions on the properties of the recovered polyol. Polymer 2021, 219, 123561. [Google Scholar] [CrossRef]
- Gama, N.; Godinho, B.; Marques, G.; Silva, R.; Barros-Timmons, A.; Ferreira, A. Recycling of polyurethane scraps via acidolysis. Chem. Eng. J. 2020, 395, 125102. [Google Scholar] [CrossRef]
- Bhandari, S.; Gupta, P. Chemical Depolymerization of Polyurethane Foam via Ammonolysis and Aminolysis. In Recycling of Polyurethane Foams; William Andrew: Norwich, NY, USA, 2018; pp. 77–87. [Google Scholar] [CrossRef]
- Grdadolnik, M.; Zdovc, B.; Drinčić, A.; Onder, O.C.; Utroša, P.; Ramos, S.G.; Ramos, E.D.; Pahovnik, D.; Žagar, E. Chemical Recycling of Flexible Polyurethane Foams by Aminolysis to Recover High-Quality Polyols. ACS Sust. Chem. Eng. 2023, 11, 10864–10873. [Google Scholar] [CrossRef]
- Grdadolnik, M.; Drinčić, A.; Oreški, A.; Onder, O.C.; Utroša, P.; Pahovnik, D.; Žagar, E. Insight into Chemical Recycling of Flexible Polyurethane Foams by Acidolysis. ACS Sust. Chem. Eng. 2022, 10, 1323–1332. [Google Scholar] [CrossRef]
- Stefanidis, G.D.; Muñoz, A.N.; Sturm, G.S.J.; Stankiewicz, A. A helicopter view of microwave application to chemical processes: Reactions, separations, and equipment concepts. Rev. Chem. Eng. 2014, 30, 233–259. [Google Scholar] [CrossRef]
- Goyal, H.; Chen, T.Y.; Chen, W.; Vlachos, D.G. A review of microwave-assisted process intensified multiphase reactors. Chem. Eng. J. 2022, 430, 133183. [Google Scholar] [CrossRef]
- Stankiewicz, A.I.; Nigar, H. Beyond electrolysis: Old challenges and new concepts of electricity-driven chemical reactors. React. Chem. Eng. 2020, 5, 1005–1016. [Google Scholar] [CrossRef]
- Frisa-Rubio, A.; González-Niño, C.; Royo, P.; García-Polanco, N.; Martínez-Hernández, D.; Royo-Pascual, L.; Fiesser, S.; Žagar, E.; García-Armingol, T. Chemical recycling of plastics assisted by microwave multi-frequency heating. Clean Eng. Technol. 2021, 5, 100297. [Google Scholar] [CrossRef]
- Češarek, U.; Pahovnik, D.; Žagar, E. Chemical recycling of aliphatic polyamides by microwave-assisted hydrolysis for efficient monomer recovery. ACS Sustain. Chem. Eng. 2020, 8, 16274–16282. [Google Scholar] [CrossRef]
- De La Hoz, A.; Carrillo, J.; Herrero, M.A.; Prieto, P.; Al Díaz-Ortiz, Á. 7—Reproducibility and Scalability of Microwave-Assisted Reactions. In Microwave Heating; InTech Open: London, UK, 2011. [Google Scholar] [CrossRef]
- Julian, I.; Pedersen, C.M.; Jensen, A.B.; Baden, A.K.; Hueso, J.L.; Friderichsen, A.V.; Birkedal, H.; Mallada, R.; Santamaria, J. From bench scale to pilot plant: A 150x scaled-up configuration of a microwave-driven structured reactor for methane dehydroaromatization. Catal. Today 2022, 383, 21–30. [Google Scholar] [CrossRef]
- Julian, I.; García-Jiménez, A.; Aguado, A.; Arenal, C.; Calero, A.; Campos, V.; Escobar, G.; López-Buendía, A.M.; Romero, D.; Verdejo, E.; et al. Advances in the circularity of end-of-life fibre-reinforced polymers by microwave intensification. Chem. Eng. Proc.—Proc. Intensif. 2022, 178, 109015. [Google Scholar] [CrossRef]
- Goyal, H.; Mehdad, A.; Lobo, R.F.; Stefanidis, G.D.; Vlachos, D.G. Scaleup of a Single-Mode Microwave Reactor. Ind. Eng. Chem. Res. 2020, 59, 2516–2523. [Google Scholar] [CrossRef]
- Fresneda-Cruz, A.; Murillo-Ciordia, G.; Figueirêdo, M.B.; Tovar-Lasheras, F.; Farra, A.A.L.; Arauzo, J.; Julian, I. Microwave-assisted pyrolysis of waste LDPE: Unveiling the role of induced gas-solid thermal gradients on pyrolysis oil product distribution. J. Anal. Appl. Pyrolysis 2025, 187, 106984. [Google Scholar] [CrossRef]
- US Food and Drug Administration (FDA). Microwave Oven Radiation. Available online: https://www.fda.gov/radiation-emitting-products/resources-you-radiation-emitting-products/microwave-oven-radiation (accessed on 27 April 2025).
- Shanmugam, K.; Subrahmanyam, S.; Tarakad, S.V.; Kodandapani, N.; Stanly, D.F. 2,4-Toluene Diamines—Their Carcinogenicity, Biodegradation, Analytical Techniques and an Approach towards Development of Biosensors. Anal. Sci. 2001, 17, 1369–1374. [Google Scholar] [CrossRef]
- Frisa-Rubio, A.; Campo-Valera, M.; Mallah, M.; Murillo-Ciordia, G.; Rodriguez-Rodriguez, I. A Novel Combined Design of Vessel and Resonant Cavity for Microwave Multi-Frequency Heating Chemical Reactor Using Antennas as Applicators. IEEE Access 2023, 11, 39448–39456. [Google Scholar] [CrossRef]
- Jensen, A.A.; Elkington, J.; Christiansen, K.; Hoffmann, L.; Møller, B.T.; Schmidt, A.; van Dijk, F. Life Cycle Assessment: A Guide to Approaches, Experiences and Information Sources; European Environment Agency: Copenhaguen, Denmark, 1998. [Google Scholar]
- Ibáñez-Forés, V.; Bovea, M.D.; Pérez-Belis, V. A holistic review of applied methodologies for assessing and selecting the optimal technological alternative from a sustainability perspective. J. Clean Prod. 2014, 70, 259–281. [Google Scholar] [CrossRef]
- UNE-EN ISO 14040:2006; Environmental Management—Life Cycle Assessment—Principles and Framework. International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 14044:2006; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. International Organization for Standardization: Geneva, Switzerland, 2006.
- Ghannadzadeh, A. Assessment of power generation from natural gas and biomass to enhance environmental sustainability of a polyol ether production process for rigid foam polyurethane synthesis. Renew. Energ. 2018, 115, 846–858. [Google Scholar] [CrossRef]
- Larhed, M.; Moberg, C.; Hallberg, A. Microwave-accelerated homogeneous catalysis in organic chemistry. Acc. Chem. Res. 2002, 35, 717–727. [Google Scholar] [CrossRef]
- Hayes, B.L. Recent Advances in Microwave-Assisted Synthesis. Aldrichim. Acta 2004, 37, 66–76. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).