Recycling Industrial Waste: Ferritization Products for Zn2+ Removal from Wastewater
Abstract
1. Introduction
- − to study the structure of precipitates from ferritization treatment of etching solutions to confirm possibility of their use as sorbents;
- − to study experimentally the sorption capacity of the Fe-rich materials obtained by the improved ferritization method to sorb Zn2+ ions;
- − to determine the influence of sorption conditions on efficiency of zinc ion removal by these sorbents;
- − to study structures of exhausted sorbents.
2. Materials and Methods
3. Results and Discussion
3.1. Results of Structural Analysis of Fe-Rich Materials Obtained by Ferritization of Etching Solutions
3.2. Study of the Ability to Adsorb Zn2+ Ions by the Studied Fe-Rich Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vujanović, M.; Wang, Q.; Mohsen, M.; Duić, N.; Yan, J. Recent Progress in Sustainable Energy-Efficient Technologies and Environmental Impacts on Energy Systems. Appl. Energy 2021, 283, 116280. [Google Scholar] [CrossRef]
- Crouch, M.L.; Jacobs, H.E.; Speight, V.L. Defining Domestic Water Consumption Based on Personal Water Use Activities. J. Water Supply Res. Technol. Aqua 2021, 70, jws2021056. [Google Scholar] [CrossRef]
- Van Vliet, M.T.H.; Jones, E.R.; Flörke, M.; Franssen, W.H.P.; Hanasaki, N.; Wada, Y.; Yearsley, J.R. Global Water Scarcity Including Surface Water Quality and Expansions of Clean Water Technologies. Environ. Res. Lett. 2021, 16, 024020. [Google Scholar] [CrossRef]
- Behera, M.; Nayak, J.; Banerjee, S.; Chakrabortty, S.; Tripathy, S.K. A Review on the Treatment of Textile Industry Waste Effluents towards the Development of Efficient Mitigation Strategy: An Integrated System Design Approach. J. Environ. Chem. Eng. 2021, 9, 105277. [Google Scholar] [CrossRef]
- Kowalik-Klimczak, A.; Gajewska-Midziałek, A.; Buczko, Z.; Łożyńska, M.; Życki, M.; Barszcz, W.; Ciciszwili, T.; Dąbrowski, A.; Kasierot, S.; Charasińska, J.; et al. Circular Economy Approach in Treatment of Galvanic Wastewater Employing Membrane Processes. Membranes 2023, 13, 325. [Google Scholar] [CrossRef]
- Rajoria, S.; Vashishtha, M.; Sangal, V.K. Treatment of Electroplating Industry Wastewater: A Review on the Various Techniques. Environ. Sci. Pollut. Res. 2022, 29, 72196–72246. [Google Scholar] [CrossRef]
- Gugua, E.C.; Ujah, C.O.; Asadu, C.O.; Von Kallon, D.V.; Ekwueme, B.N. Electroplating in the Modern Era, Improvements and Challenges: A Review. Hybrid Adv. 2024, 7, 100286. [Google Scholar] [CrossRef]
- Kamar, M.T.; Elattar, H.; Mahmoud, A.S.; Peters, R.W.; Mostafa, M.K. A Critical Review of State-of-the-Art Technologies for Electroplating Wastewater Treatment. Int. J. Environ. Anal. Chem. 2024, 104, 4143–4176. [Google Scholar] [CrossRef]
- Liu, Q.; Cao, Y.; Chen, S.; Xu, X.; Yao, M.; Fang, J.; Lei, K.; Liu, G. Hot-Dip Galvanizing Process and the Influence of Metallic Elements on Composite Coatings. J. Compos. Sci. 2024, 8, 160. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Guerrero–Barajas, C.; Ahmad, A.; Ibrahim, M.N.M.; Alshammari, M.B. Advanced Technologies for Wastewater Treatment. In Green Chemistry for Sustainable Water Purification; Wiley: Hoboken, NJ, USA, 2023; pp. 179–202. [Google Scholar]
- Xiao, Y.; Li, L.; Huang, M.; Liu, Y.; Xu, J.; Xu, Z.; Lei, Y. Treating Waste with Waste: Metals Recovery from Electroplating Sludge Using Spent Cathode Carbon Combustion Dust and Copper Refining Slag. Sci. Total Environ. 2022, 838, 156453. [Google Scholar] [CrossRef]
- Chen, X.; Ma, R.; Luo, J.; Huang, W.; Fang, L.; Sun, S.; Lin, J. Co-Microwave Pyrolysis of Electroplating Sludge and Municipal Sewage Sludge to Synergistically Improve the Immobilization of High-Concentration Heavy Metals and an Analysis of the Mechanism. J. Hazard. Mater. 2021, 417, 126099. [Google Scholar] [CrossRef] [PubMed]
- Scarazzato, T.; Panossian, Z.; Tenório, J.A.S.; Pérez-Herranz, V.; Espinosa, D.C.R. A Review of Cleaner Production in Electroplating Industries Using Electrodialysis. J. Clean. Prod. 2017, 168, 1590–1602. [Google Scholar] [CrossRef]
- Meng, S.; Wen, S.; Han, G.; Wang, X.; Feng, Q. Wastewater Treatment in Mineral Processing of Non-Ferrous Metal Resources: A Review. Water 2022, 14, 726. [Google Scholar] [CrossRef]
- Trach, Y.; Chernyshev, D.; Biedunkova, O.; Moshynskyi, V.; Trach, R.; Statnyk, I. Modeling of Water Quality in West Ukrainian Rivers Based on Fluctuating Asymmetry of the Fish Population. Water 2022, 14, 3511. [Google Scholar] [CrossRef]
- Saleh, T.A.; Mustaqeem, M.; Khaled, M. Water Treatment Technologies in Removing Heavy Metal Ions from Wastewater: A Review. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100617. [Google Scholar] [CrossRef]
- Aziz, H.A.; Adlan, M.N.; Ariffin, K.S. Heavy Metals (Cd, Pb, Zn, Ni, Cu and Cr(III)) Removal from Water in Malaysia: Post Treatment by High Quality Limestone. Bioresour. Technol. 2008, 99, 1578–1583. [Google Scholar] [CrossRef]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B. Adsorption of Heavy Metal Ions by Various Low-Cost Adsorbents: A Review. Int. J. Environ. Anal. Chem. 2022, 102, 342–379. [Google Scholar] [CrossRef]
- Hussain, S.; Ali, S. Removal of Heavy Metal by Ion Exchange Using Bentonite Clay. J. Ecol. Eng. 2021, 22, 104–111. [Google Scholar] [CrossRef]
- Trach, Y.; Melnychuk, V.; Michel, M.M.; Reczek, L.; Siwiec, T.; Trach, R. The Characterization of Ukrainian Volcanic Tuffs from the Khmelnytsky Region with the Theoretical Analysis of Their Application in Construction and Environmental Technologies. Materials 2021, 14, 7723. [Google Scholar] [CrossRef]
- Hannachi, Y. Utilization of Tunisian Bentonite as Ion-Exchange and Sorbent Material in the Removal of Lead from Aqueous Solutions. Holist. Approach Environ. 2013, 3, 123–140. [Google Scholar]
- Liu, L.; Li, C.; Bao, C.; Jia, Q.; Xiao, P.; Liu, X.; Zhang, Q. Preparation and Characterization of Chitosan/Graphene Oxide Composites for the Adsorption of Au(III) and Pd(II). Talanta 2012, 93, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, W.; Zhao, Y.; Bai, H.; Wen, T.; Kang, S.; Song, G.; Song, S.; Komarneni, S. Removal of Heavy Metals and Dyes by Clay-Based Adsorbents: From Natural Clays to 1D and 2D Nano-Composites. Chem. Eng. J. 2021, 420, 127574. [Google Scholar] [CrossRef]
- Renu; Sithole, T. A Review on Regeneration of Adsorbent and Recovery of Metals: Adsorbent Disposal and Regeneration Mechanism. S. Afr. J. Chem. Eng. 2024, 50, 39–50. [Google Scholar] [CrossRef]
- Trach, Y.; Melnychuk, V.; Melnychuk, G.; Mazur, Ł.; Podlasek, A.; Vaverková, M.; Koda, E. Using Local Mineral Materials for the Rehabilitation of the Ustya River—A Case Study. Desalin. Water Treat. 2021, 232, 346–356. [Google Scholar] [CrossRef]
- Shi, C.; He, F.; Fernández-Jiménez, A.; Pavel Krivenko, V.; Palomo, A. Classification and Characteristics of Alkali-Activated Cements. Kuei Suan Jen Hsueh Pao J. Chin. Ceram. Soc. 2012, 40, 69–75. [Google Scholar]
- Alonso, M.M.; Pasko, A.; Gascó, C.; Suarez, J.A.; Kovalchuk, O.; Krivenko, P.; Puertas, F. Radioactivity and Pb and Ni Immobilization in SCM-Bearing Alkali-Activated Matrices. Constr. Build. Mater. 2018, 159, 745–754. [Google Scholar] [CrossRef]
- Hu, B.; He, M.; Chen, B. 9—Magnetic Nanoparticle Sorbents. In Solid-Phase Extraction; Poole, C.F., Ed.; Handbooks in Separation Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 235–284. ISBN 978-0-12-816906-3. [Google Scholar]
- Haniffa, M.A.C.M.; Ching, Y.C.; Illias, H.A.; Munawar, K.; Ibrahim, S.; Nguyen, D.H.; Chuah, C.H. Cellulose Supported Promising Magnetic Sorbents for Magnetic Solid-Phase Extraction: A Review. Carbohydr. Polym. 2021, 253, 117245. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Estrella-Nuñez, J.; Arcentales-Vera, B.; Chichande-Proaño, E.; Bucio, E. Polymeric Composite of Magnetite Iron Oxide Nanoparticles and Their Application in Biomedicine: A Review. Polymers 2022, 14, 752. [Google Scholar] [CrossRef]
- Trach, Y.; Bujakowski, F.; Koda, E.; Mazur, Ł.; Nejbert, K.; Podlasek, A.; Vaverková, M.D. Characterization of Adsorbents from Ukrainian Kaolinite Clay for the Sorption of Nickel: Insight and Practical Application for Water Treatment in Conditions of Economic Constraints. Desalin. Water Treat. 2022, 278, 1–12. [Google Scholar] [CrossRef]
- Roy, A.; Bhattacharya, J. Removal of Cu(II), Zn(II) and Pb(II) from Water Using Microwave-Assisted Synthesized Maghemite Nanotubes. Chem. Eng. J. 2012, 211–212, 493–500. [Google Scholar] [CrossRef]
- Karami, H. Heavy Metal Removal from Water by Magnetite Nanorods. Chem. Eng. J. 2013, 219, 209–216. [Google Scholar] [CrossRef]
- Ren, Y.; Abbood, H.A.; He, F.; Peng, H.; Huang, K. Magnetic EDTA-Modified Chitosan/SiO2/Fe3O4 Adsorbent: Preparation, Characterization, and Application in Heavy Metal Adsorption. Chem. Eng. J. 2013, 226, 300–311. [Google Scholar] [CrossRef]
- Ahmadi, A.; Heidarzadeh, S.; Mokhtari, A.R.; Darezereshki, E.; Harouni, H.A. Optimization of Heavy Metal Removal from Aqueous Solutions by Maghemite (γ-Fe2O3) Nanoparticles Using Response Surface Methodology. J. Geochem. Explor. 2014, 147, 151–158. [Google Scholar] [CrossRef]
- Habila, M.A.; ALOthman, Z.A.; El-Toni, A.M.; Labis, J.P.; Soylak, M. Synthesis and Application of Fe3O4@SiO2@TiO2 for Photocatalytic Decomposition of Organic Matrix Simultaneously with Magnetic Solid Phase Extraction of Heavy Metals Prior to ICP-MS Analysis. Talanta 2016, 154, 539–547. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, J.; Li, N.; Wang, W.; Nan, J.; Zhao, Z.; Cui, F. Highly Efficient Removal of Bivalent Heavy Metals from Aqueous Systems by Magnetic Porous Fe3O4-MnO2: Adsorption Behavior and Process Study. Chem. Eng. J. 2016, 304, 737–746. [Google Scholar] [CrossRef]
- Asadi, R.; Abdollahi, H.; Gharabaghi, M.; Boroumand, Z. Effective Removal of Zn (II) Ions from Aqueous Solution by the Magnetic MnFe2O4 and CoFe2O4 Spinel Ferrite Nanoparticles with Focuses on Synthesis, Characterization, Adsorption, and Desorption. Adv. Powder Technol. 2020, 31, 1480–1489. [Google Scholar] [CrossRef]
- Ali, A.; Zafar, H.; Zia, M.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, Characterization, Applications, and Challenges of Iron Oxide Nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef]
- Heuss-Aßbichler, S.; John, M.; Klapper, D.; Bläß, U.W.; Kochetov, G. Recovery of Copper as Zero-Valent Phase and/or Copper Oxide Nanoparticles from Wastewater by Ferritization. J. Environ. Manag. 2016, 181, 1–7. [Google Scholar] [CrossRef]
- Teremova, M.I.; Petrakovskaya, E.A.; Romanchenko, A.S.; Tuzikov, F.V.; Gurevich, Y.L.; Tsibina, O.V.; Yakubailik, E.K. Abhilash Ferritization of Industrial Waste Water and Microbial Synthesis of Iron-Based Magnetic Nanomaterials from Sediments. Environ. Prog. Sustain. Energy 2016, 35, 1407–1414. [Google Scholar] [CrossRef]
- Kochetov, G.; Prikhna, T.; Kovalchuk, O.; Samchenko, D. Research of the Treatment of Depleted Nickel-Plating Electrolytes by the Ferritization Method. East. Eur. J. Enterp. Technol. 2018, 3, 52–60. [Google Scholar]
- Assis, M.B.D.S.; Werneck, I.H.S.R.; De Moraes, G.N.; Semaan, F.S.; Pacheco Pereira, R. Citrate-Capped Iron Oxide Nanoparticles: Ultrasound-Assisted Synthesis, Structure and Thermal Properties. Mater. Res. Express 2019, 6, 045064. [Google Scholar] [CrossRef]
- Velusamy, S.; Roy, A.; Sundaram, S.; Kumar Mallick, T. A Review on Heavy Metal Ions and Containing Dyes Removal Through Graphene Oxide-Based Adsorption Strategies for Textile Wastewater Treatment. Chem. Rec. 2021, 21, 1570–1610. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Deng, L.; Fan, X.; Li, K.; Lu, H.; Li, W. Removal of Heavy Metal Ion Cobalt (II) from Wastewater via Adsorption Method Using Microcrystalline Cellulose–Magnesium Hydroxide. Int. J. Biol. Macromol. 2021, 189, 607–617. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Hamza, M.F.; Guibal, E. Effect of Agitation Mode (Mechanical, Ultrasound and Microwave) on Uranium Sorption Using Amine- and Dithizone-Functionalized Magnetic Chitosan Hybrid Materials. Chem. Eng. J. 2021, 411, 128553. [Google Scholar] [CrossRef]
- Samchenko, D.M.; Bagliuk, G.A.; Kochetov, G.M.; Lastivka, O.V.; Derecha, D.O.; Prikhna, T.O. Mechanical and Functional Properties of Composite Coatings with Fine Reinforcements Produced from Galvanic Processing Waste. Powder Metall. Met. Ceram. 2023, 62, 233–240. [Google Scholar] [CrossRef]
- Kochetov, G.; Glyva, V.; Malyshev, V.; Gots, V.; Samchenko, D.; Lastivka, O. Application of innovative electromagnetic screens for reconstruction and restoration of buildings. Int. J. Conserv. Sci. 2024, 15, 63–72. [Google Scholar] [CrossRef]
- Kovalchuk, O.; Grabovchak, V.; Govdun, Y. Alkali Activated Cements Mix Design for Concretes Application in High Corrosive Conditions. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2018; Volume 230. [Google Scholar]
- Davidovits, J. Environmentally Driven Geopolymer Cement Applications. In Proceedings of the 2002 Geopolymer Conference, Melbourne, Australia, 28–29 October 2002. [Google Scholar]
- Samchenko, D.; Kochetov, G.; Derecha, D.O.; Skirta, Y.B. Sustainable Approach for Galvanic Waste Processing by Energy-Saving Ferritization with AC-Magnetic Field Activation. Cogent Eng. 2022, 9, 2143072. [Google Scholar] [CrossRef]
- Kochetov, G.; Samchenko, D.; Arhatenko, T. Determination of influence of ph on reaction mixture of ferritation process with electromagnetic pulse activation on the processing of galvanic sludge. East. Eur. J. Enterp. Technol. 2021, 4, 24–30. [Google Scholar] [CrossRef]
- McIllece, J.J. On Generalized Variance Functions for Sample Means; US Bureau of Labor Statistics: Washington, DC, USA, 2018. [Google Scholar]
- Lu, T.; Gilfedder, B.S.; Peng, H.; Peiffer, S.; Papastavrou, G.; Ottermann, K.; Frei, S. Relevance of Iron Oxyhydroxide and Pore Water Chemistry on the Mobility of Nanoplastic Particles in Water-Saturated Porous Media Environments. Water Air Soil Pollut. 2021, 232, 168. [Google Scholar] [CrossRef]
- Samchenko, D.; Kochetov, G.; Trach, Y.; Chernyshev, D.; Kravchuk, A. Influence of Technological Factors on the Formation and Transformation of Iron-Containing Phases in the Process of Ferritization of Exhausted Etching Solutions. Water 2024, 16, 1085. [Google Scholar] [CrossRef]
- Guo, B.; Huo, H.; Zhuang, Q.; Ren, X.; Wen, X.; Yang, B.; Huang, X.; Chang, Q.; Li, S. Iron Oxyhydroxide: Structure and Applications in Electrocatalytic Oxygen Evolution Reaction. Adv. Funct. Mater. 2023, 33, 2300557. [Google Scholar] [CrossRef]
- DSTU GOST 9.314:2009; Unified System of Corrosion and Ageing Protection. Paint Coatings. Methods of Accelerated Tests for Resistance. Derzhspozhyvstandart of Ukraine: Kyiv, Ukrine, 2010; 23p.
- Vasylkivskyi, I.; Ishchenko, V.; Sakalova, H.; Ullianodt, G.C.H.; Polyvanyi, S. Municipal Wastewater Management in Ukraine. Desalin. Water Treat. 2023, 288, 159–164. [Google Scholar] [CrossRef]
- Jain, R. Recent Advances of Magnetite Nanomaterials to Remove Arsenic from Water. RSC Adv. 2022, 12, 32197–32209. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Chen, J.; Huang, G. Process Optimization of Steel Pickling Waste Liquor Treated by Electrochemical Synthesis of Fe3O4. Water Air Soil Pollut. 2022, 233, 365. [Google Scholar] [CrossRef]
- Mansour, C.; Lefèvre, G.; Pavageau, E.M.; Catalette, H.; Fédoroff, M.; Zanna, S. Sorption of Sulfate Ions onto Magnetite. J. Colloid Interface Sci. 2009, 331, 77–82. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Singh, V.K.; Alexandre-Franco, M.; Pittman, C.U. Development of Magnetic Activated Carbon from Almond Shells for Trinitrophenol Removal from Water. Chem. Eng. J. 2011, 172, 1111–1125. [Google Scholar] [CrossRef]
- Kumari, M.; Pittman, C.U.; Mohan, D. Heavy Metals [Chromium (VI) and Lead (II)] Removal from Water Using Mesoporous Magnetite (Fe3O4) Nanospheres. J. Colloid Interface Sci. 2015, 442, 120–132. [Google Scholar] [CrossRef]
- Darezereshki, E.; khodadadi Darban, A.; Abdollahy, M.; Jamshidi-Zanjani, A. Influence of Heavy Metals on the Adsorption of Arsenate by Magnetite Nanoparticles: Kinetics and Thermodynamic. Environ. Nanotechnol. Monit. Manag. 2018, 10, 51–62. [Google Scholar] [CrossRef]
- Illés, E.; Tombácz, E. The Role of Variable Surface Charge and Surface Complexation in the Adsorption of Humic Acid on Magnetite. Colloids Surf. A Physicochem. Eng. Asp. 2003, 230, 99–109. [Google Scholar] [CrossRef]
- Sun, Z.-X.; Su, F.-W.; Forsling, W.; Samskog, P.-O. Surface Characteristics of Magnetite in Aqueous Suspension. J. Colloid Interface Sci. 1998, 197, 151–159. [Google Scholar] [CrossRef]
- Marmier, N.; Delisée, A.; Fromage, F. Surface Complexation Modeling of Yb(III), Ni(II), and Cs(I) Sorption on Magnetite. J. Colloid Interface Sci. 1999, 211, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Arabi, M.; Ghaedi, M.; Ostovan, A.; Tashkhourian, J.; Asadallahzadeh, H. Synthesis and Application of Molecularly Imprinted Nanoparticles Combined Ultrasonic Assisted for Highly Selective Solid Phase Extraction Trace Amount of Celecoxib from Human Plasma Samples Using Design Expert (DXB) Software. Ultrason. Sonochem. 2016, 33, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Khoee, S.; Saadatinia, A.; Bafkary, R. Ultrasound-Assisted Synthesis of pH-Responsive Nanovector Based on PEG/Chitosan Coated Magnetite Nanoparticles for 5-FU Delivery. Ultrason. Sonochem. 2017, 39, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Asfaram, A.; Ghaedi, M.; Hajati, S.; Goudarzi, A.; Dil, E.A. Screening and Optimization of Highly Effective Ultrasound-Assisted Simultaneous Adsorption of Cationic Dyes onto Mn-Doped Fe3O4-Nanoparticle-Loaded Activated Carbon. Ultrason. Sonochem. 2017, 34, 1–12. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Chiha, M.; Naffrechoux, E. Ultrasound-Assisted Removal of Malachite Green from Aqueous Solution by Dead Pine Needles. Ultrason. Sonochem. 2008, 15, 799–807. [Google Scholar] [CrossRef]
- Karadirek, Ş.; Okkay, H. Ultrasound Assisted Green Synthesis of Silver Nanoparticle Attached Activated Carbon for Levofloxacin Adsorption. J. Taiwan Inst. Chem. Eng. 2019, 105, 39–49. [Google Scholar] [CrossRef]
- Lv, A.; Hu, C.; Nie, Y.; Qu, J. Catalytic Ozonation of Toxic Pollutants over Magnetic Cobalt-Doped Fe3O4 Suspensions. Appl. Catal. B Environ. 2012, 117–118, 246–252. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, H.; Wang, L.; Chen, L. Ultrasound-Enhanced Magnetite Catalytic Ozonation of Tetracycline in Water. Chem. Eng. J. 2013, 229, 577–584. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, J.; Zhai, X. Enhanced Mechanism of Catalytic Ozonation by Ultrasound with Orthogonal Dual Frequencies for the Degradation of Nitrobenzene in Aqueous Solution. Ultrason. Sonochem. 2010, 17, 84–91. [Google Scholar] [CrossRef]

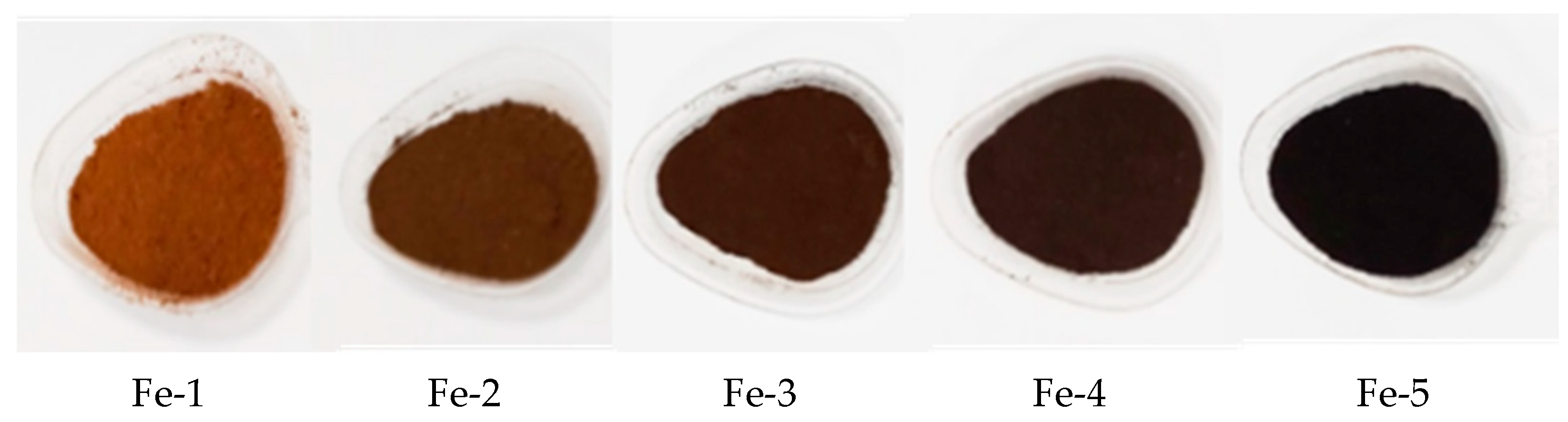
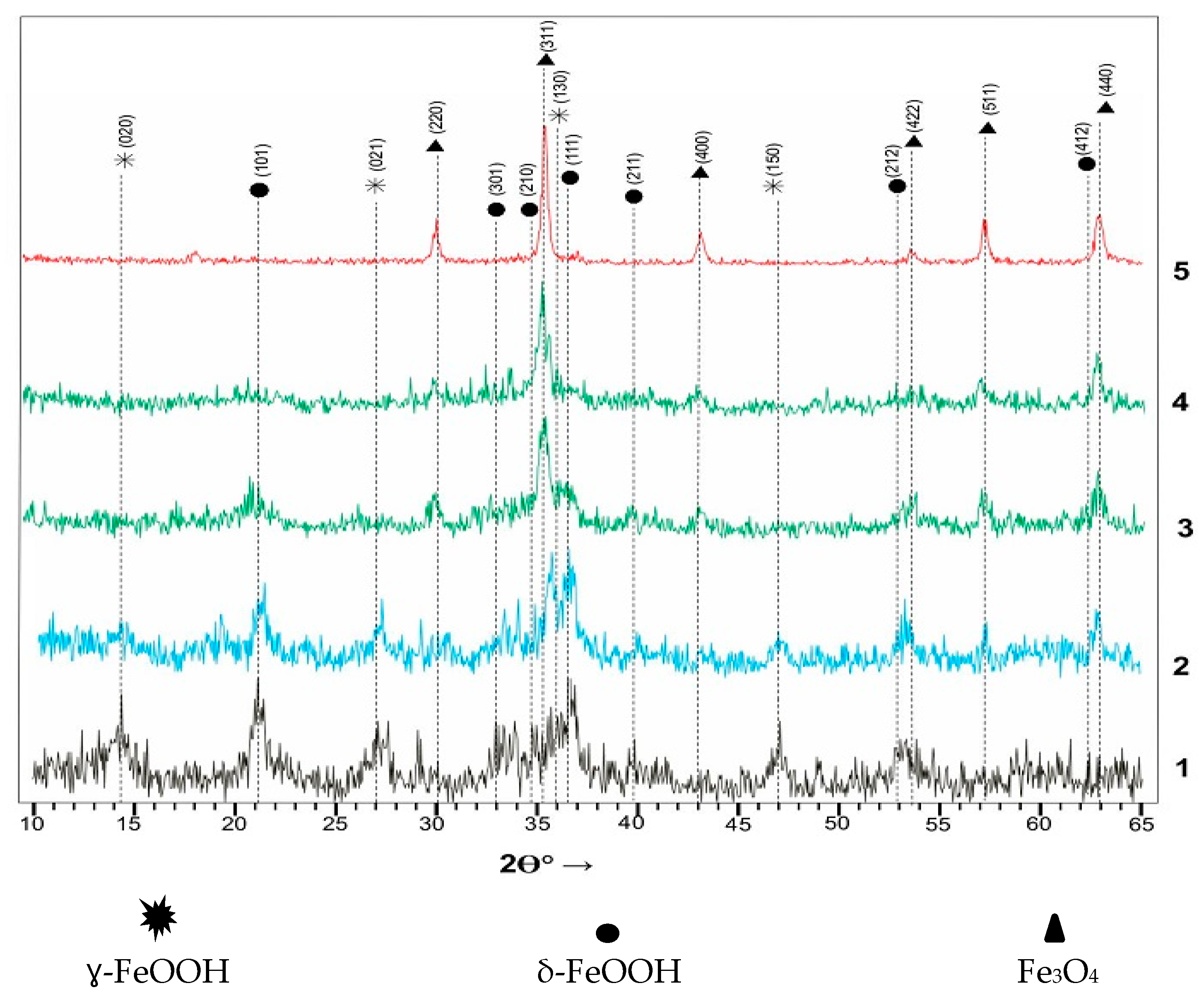


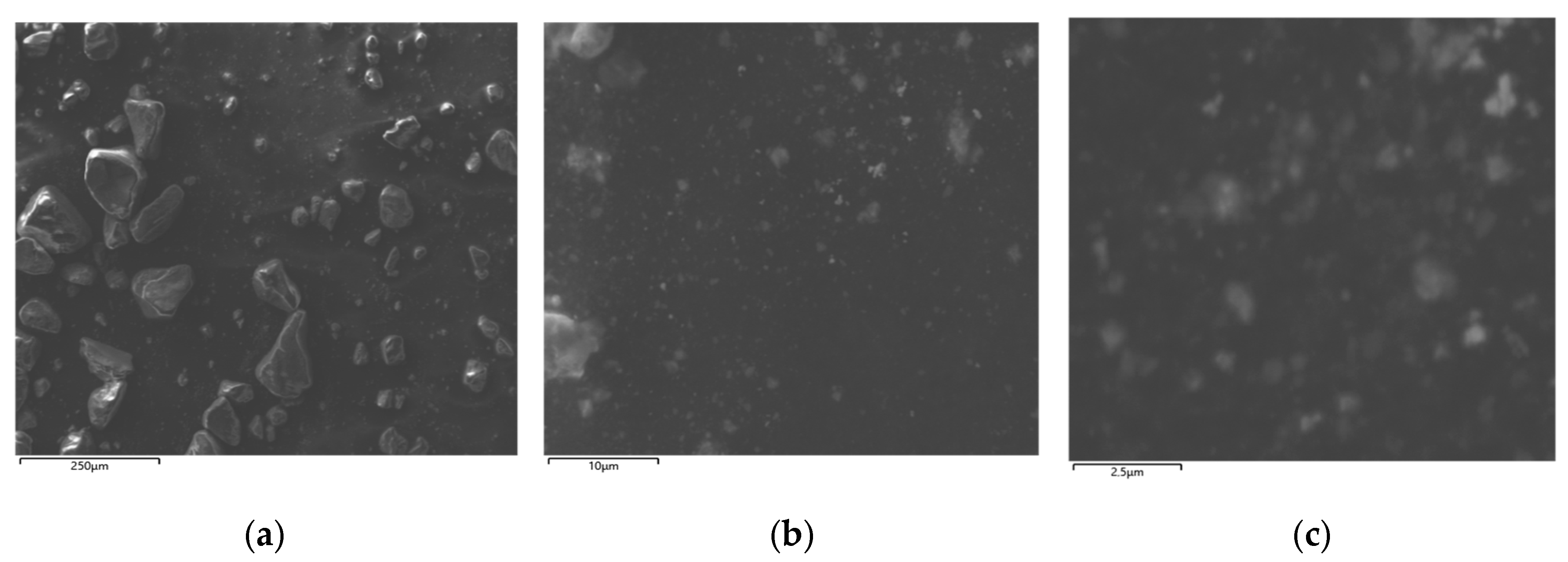
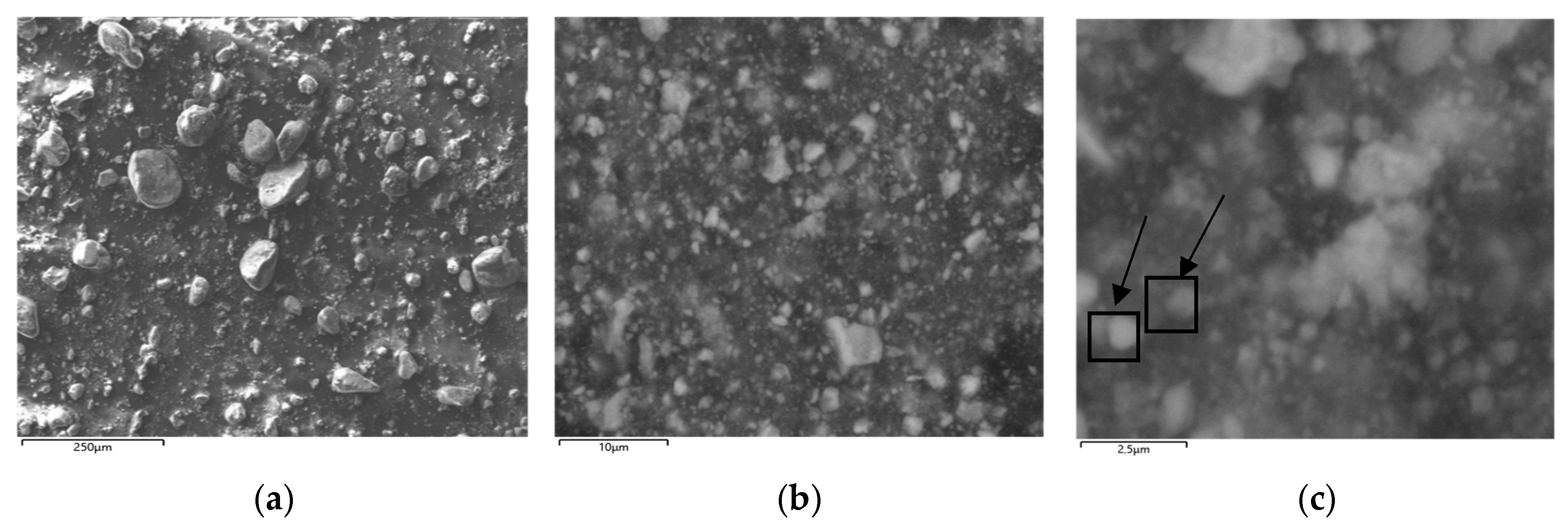
| № | Magnetic Sorbents | Adsorption Capacity, mg/g | Adsorption Duration, min | pH | Temperature °C | Literature |
|---|---|---|---|---|---|---|
| 1 | γ-Fe2O3 | 111.10 | - | 6 | 25 | [32] |
| 2 | nano-tubes Fe3O4 | 107.27 | 60 | 6 | 25 | [33] |
| 3 | nano-tubes γ-Fe2O3 | 86.95 | - | - | - | [34] |
| 4 | Fe3O4-SiO2 | 81.60 | 60 | 5 | - | [35] |
| 5 | Fe3O4-SiO2-TiO2 | 137.0 | 30 | - | - | [36] |
| 6 | Fe3O4-MnO2 | 100.24 | - | - | 25 | [37] |
| 7 | MnFe2O4 | 454.40 | 120 | 6 | 25 | [38] |
| 8 | CoFe2O4 | 384.60 | 120 | 6 | 25 | [38] |
| ID | Method of Activating the Mix in the Ferritization Process | Air Oxygen Aeration Rate, dm3/s | pH of Liquid | Concentration Fe2+ in Liquid, g/dm3 | Duration of the Ferritization Process, min |
|---|---|---|---|---|---|
| Fe-1 | Temperature (20 °C) | 0.02 | 10.5 | 14.5 | 30 |
| Fe-2 | Ultrasonic | 0.02 | |||
| Fe-3 | Electromagnetic pulse | 0.04 | |||
| Fe-4 | Electromagnetic pulse | 0.05 | |||
| Fe-5 | Thermal (75 °C) | 0.06 |
| Study Sampls | Phase Content, % | ||
|---|---|---|---|
| ɣ-FeOOH | δ-FeOOH | Fe3O4 | |
| Fe-1 | 25.9 | 74.1 | - |
| Fe-2 | 30.2 | 40.1 | 29.6 |
| Fe-3 | - | 61.3 | 38.7 |
| Fe-4 | - | 31.7 | 68.3 |
| Fe-5 | - | - | 100 |
| Study Samples | Residual Concentration of Total Iron in Solution, mg/dm3 | Removal of Total Iron, % |
|---|---|---|
| Fe-1 | 5.47 | 99.96 |
| Fe-2 | 3.58 | 99.97 |
| Fe-3 | 2.82 | 99.98 |
| Fe-4 | 1.91 | 99.98 |
| Fe-5 | 1.40 | 99.99 |
| Adsorption Conditions | pH of Solution | Residual Concentration Zn2+ in Solution, mg/dm3 | Efficiency of Zn2+Adsorption, % | |
|---|---|---|---|---|
| Before Adsorption | After Adsorption | |||
| Mechanical mixing | 5.6 | 5.6 | 16.84 | 43.8 |
| 8.0 | 7.8 | 14.52 | 51.6 | |
| 10.0 | 9.6 | 3.78 | 87.4 | |
| Ultrasound | 5.6 | 5.5 | 15.43 | 48.5 |
| 8.0 | 6.2 | 5.31 | 82.3 | |
| 10.0 | 7.5 | 0.31 | 98.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samchenko, D.; Kochetov, G.; Hao, S.; Trach, Y.; Trach, R.; Hnes, O. Recycling Industrial Waste: Ferritization Products for Zn2+ Removal from Wastewater. Sustainability 2025, 17, 4008. https://doi.org/10.3390/su17094008
Samchenko D, Kochetov G, Hao S, Trach Y, Trach R, Hnes O. Recycling Industrial Waste: Ferritization Products for Zn2+ Removal from Wastewater. Sustainability. 2025; 17(9):4008. https://doi.org/10.3390/su17094008
Chicago/Turabian StyleSamchenko, Dmitry, Gennadii Kochetov, Shuwei Hao, Yuliia Trach, Roman Trach, and Olena Hnes. 2025. "Recycling Industrial Waste: Ferritization Products for Zn2+ Removal from Wastewater" Sustainability 17, no. 9: 4008. https://doi.org/10.3390/su17094008
APA StyleSamchenko, D., Kochetov, G., Hao, S., Trach, Y., Trach, R., & Hnes, O. (2025). Recycling Industrial Waste: Ferritization Products for Zn2+ Removal from Wastewater. Sustainability, 17(9), 4008. https://doi.org/10.3390/su17094008







