Enhancing Biogas Production: Pre-Treatment of Lignocellulosic Biomass Using Biogas Plant Digestate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- Substrates
- Inoculum
- Digestate
2.2. Analytical Methods
2.3. Tests of Biogas Potential
- -
- Soaking in digestate at room temperature for 1, 2, or 5 days, using either the cut or ground fraction of maize waste.
- -
- Soaking in water at room temperature for 1, 2, or 5 days, using the cut fraction of maize waste.
2.4. Long-Term Reactor Operation
3. Results and Discussion
3.1. Tests of Biogas Potential
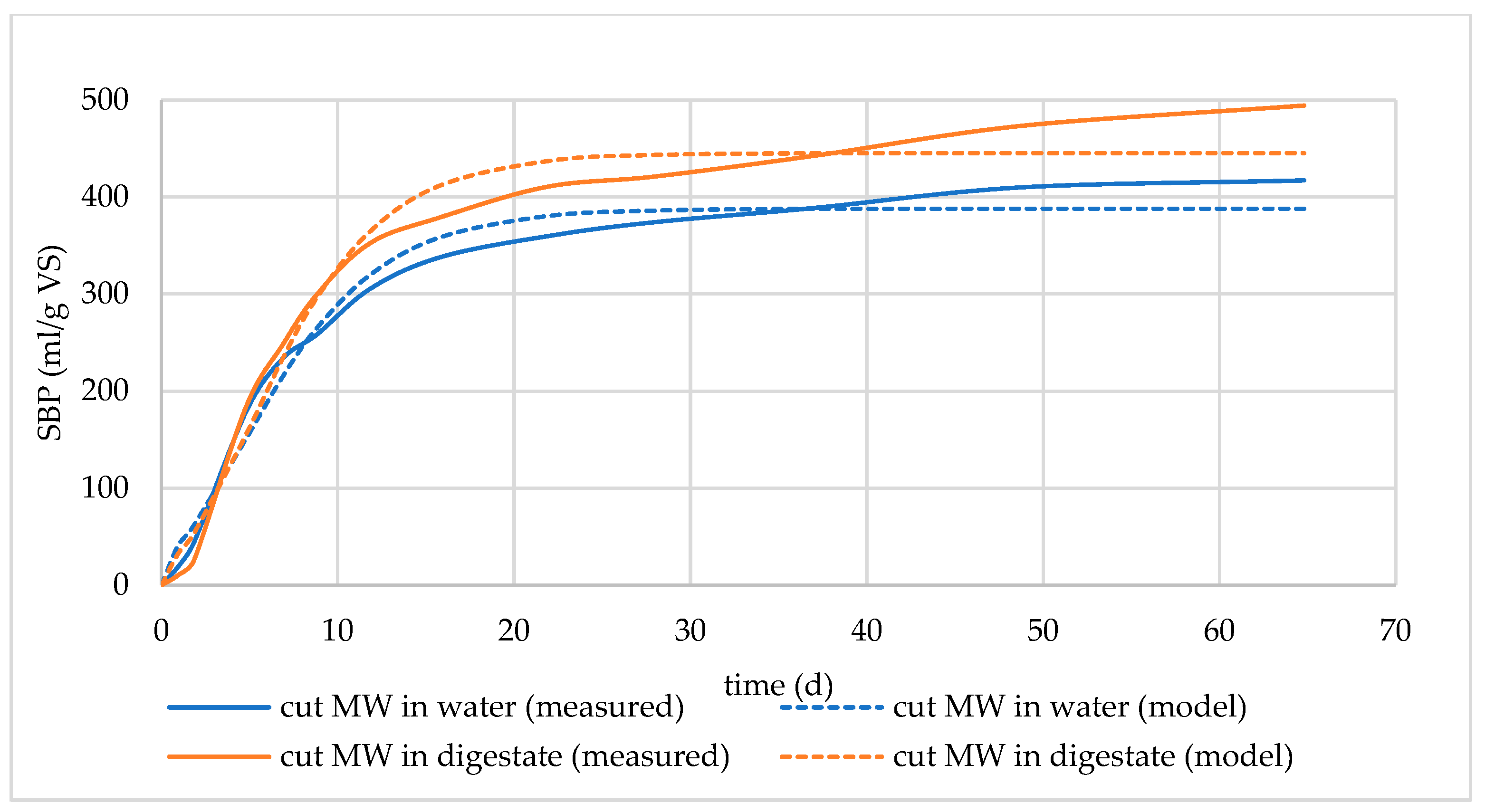
3.1.1. Effect of Using Digestate Versus Water for Pre-Treatment
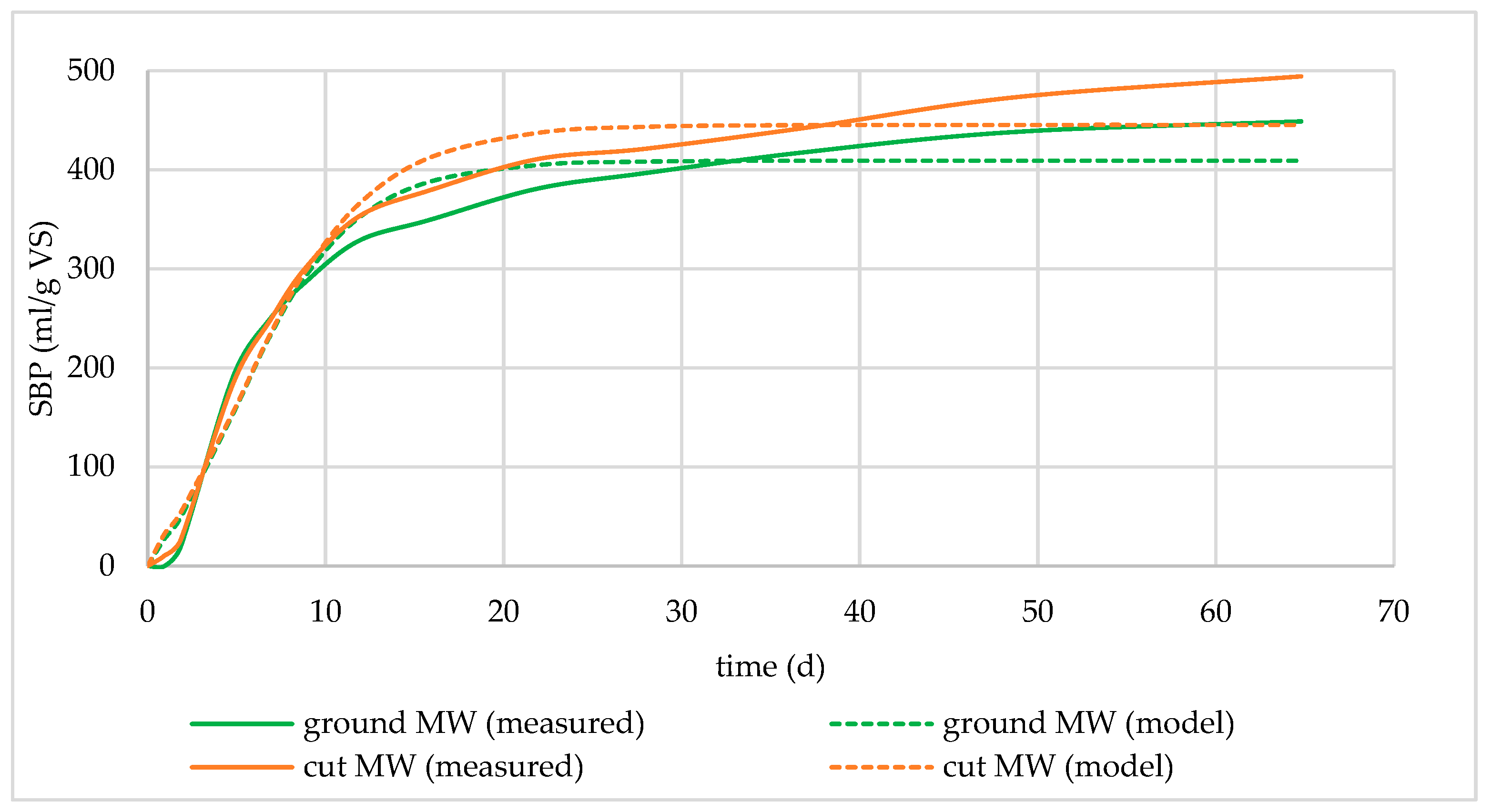
3.1.2. Effect of Substrate Particle Size
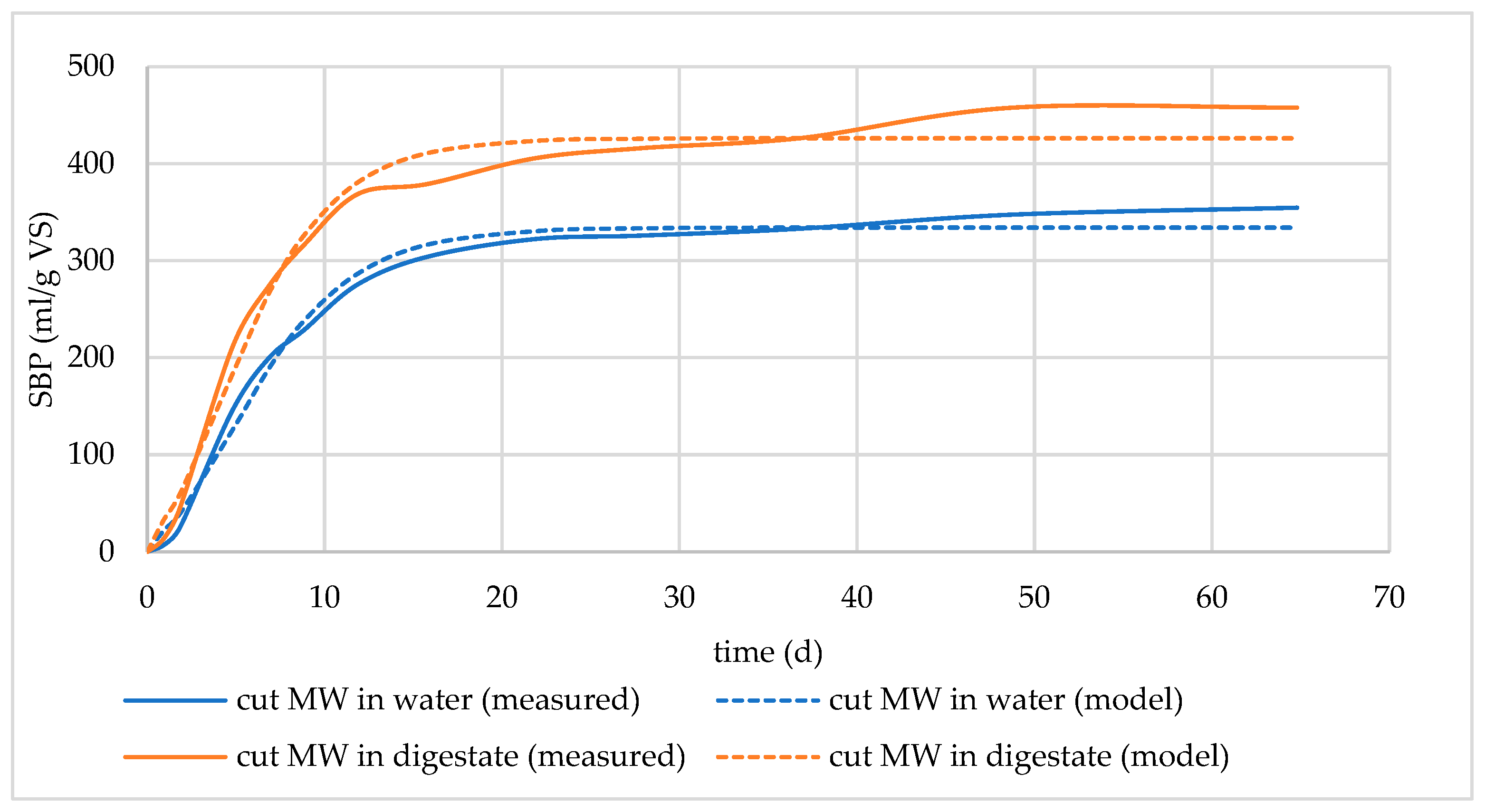
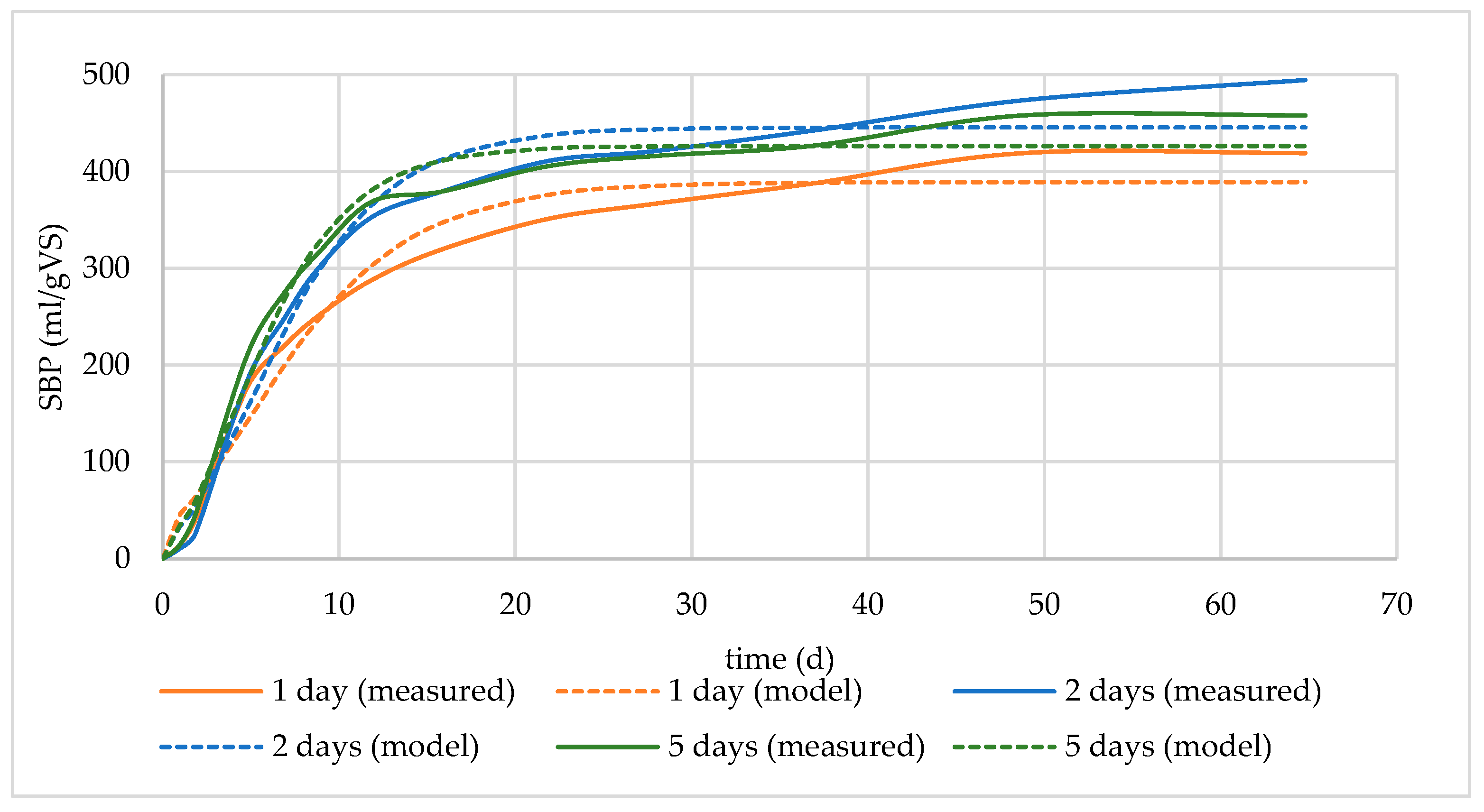
3.1.3. Effect of Soaking Time in Digestate
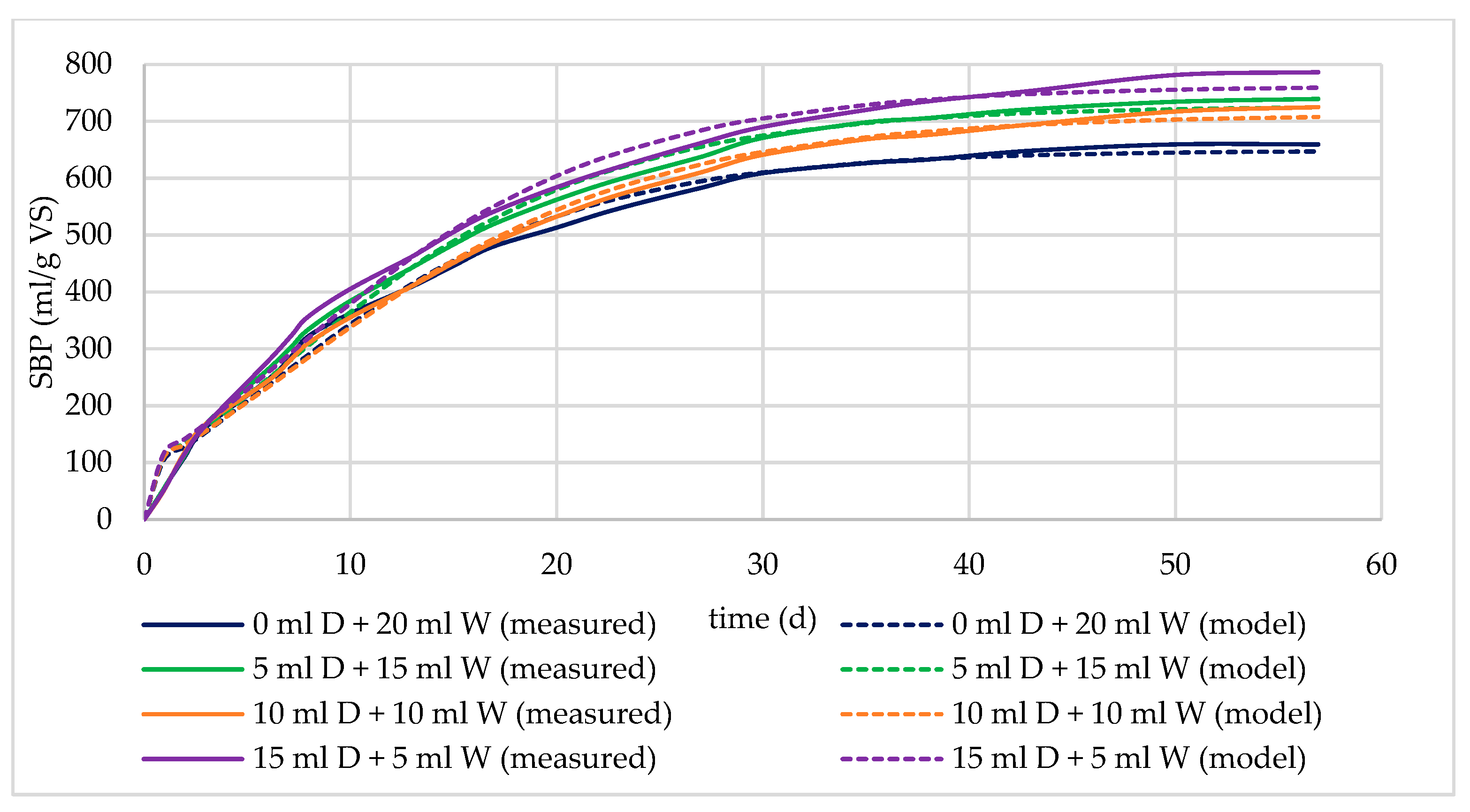
3.1.4. Effect of the Amount of Digestate
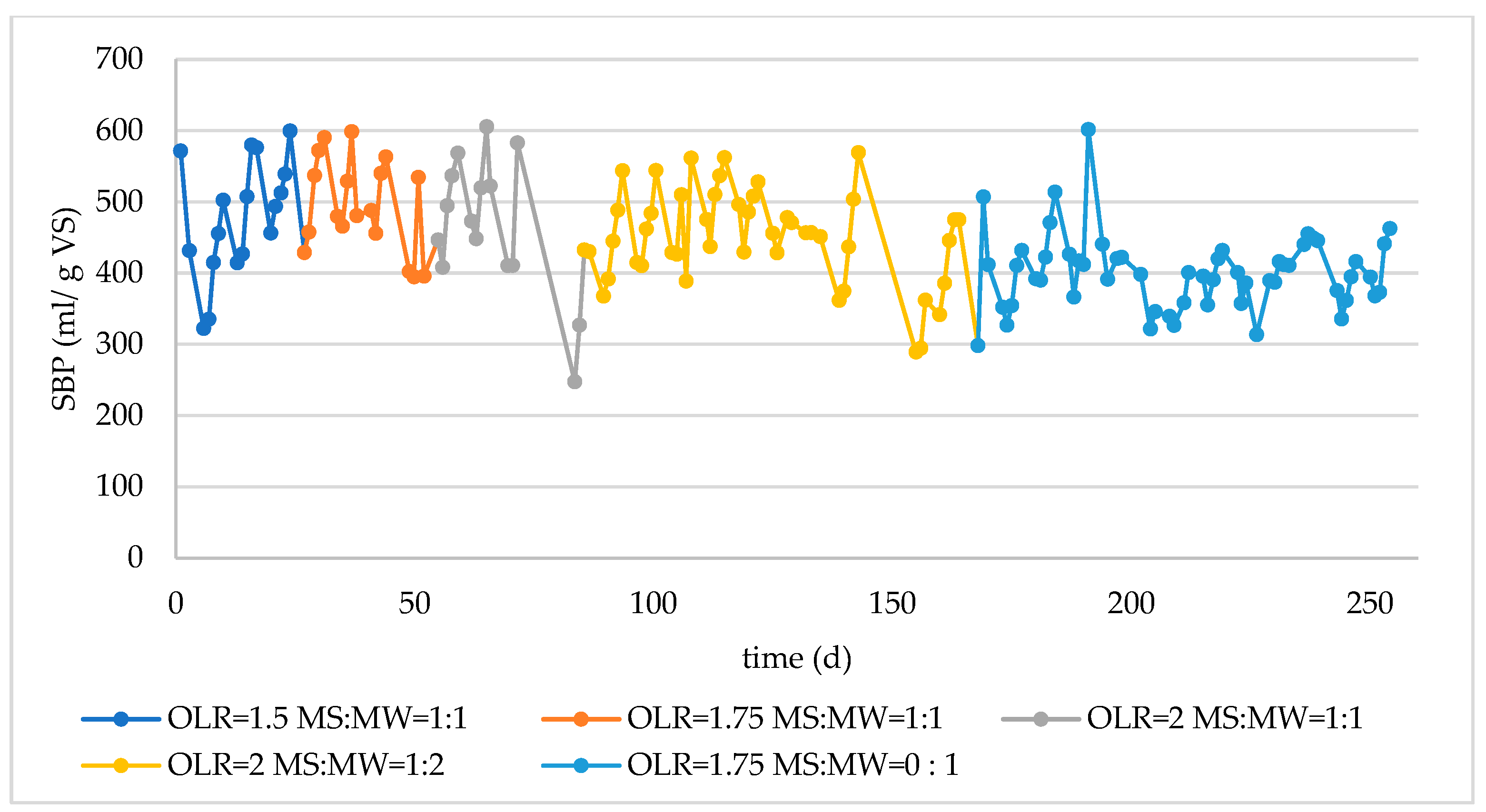
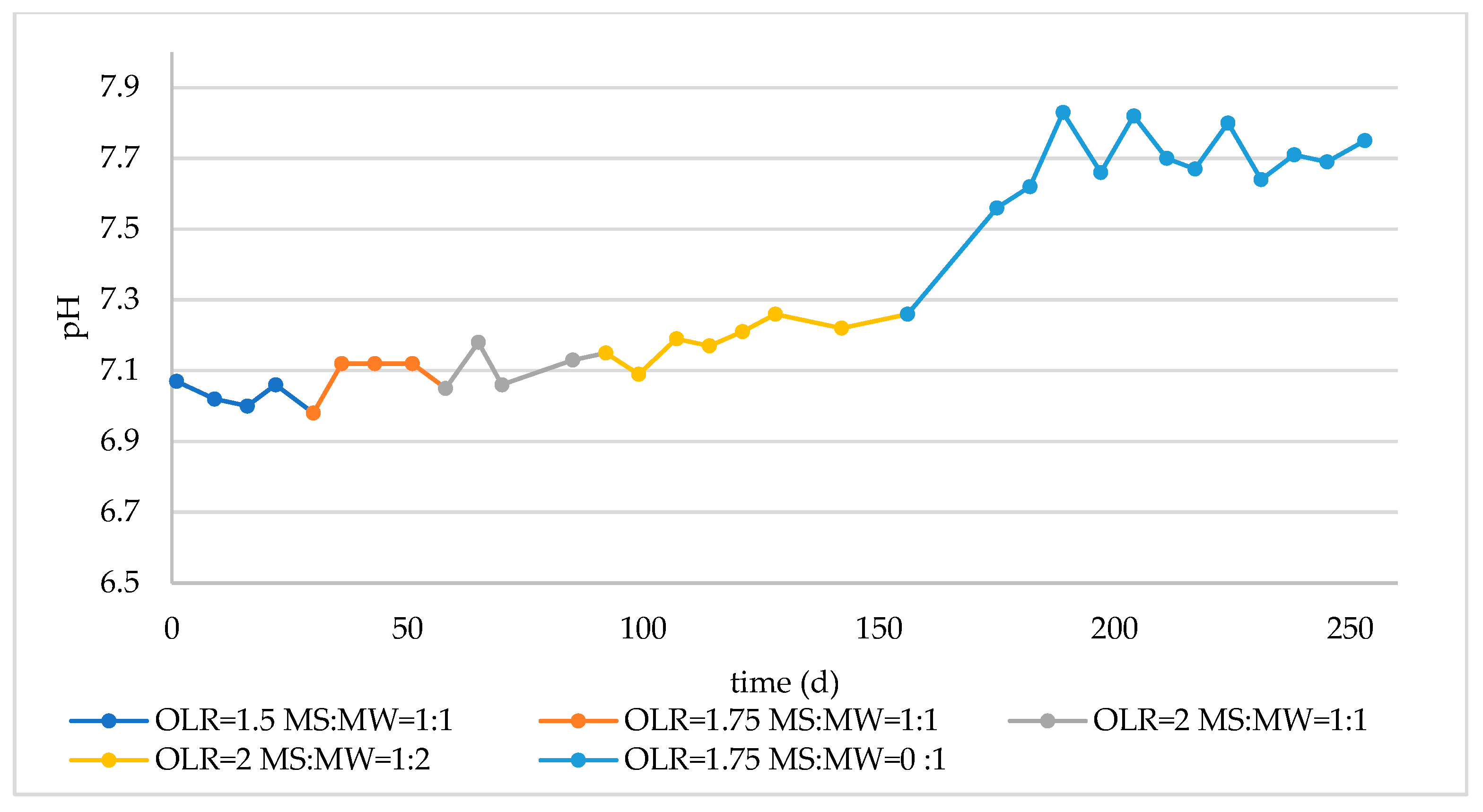
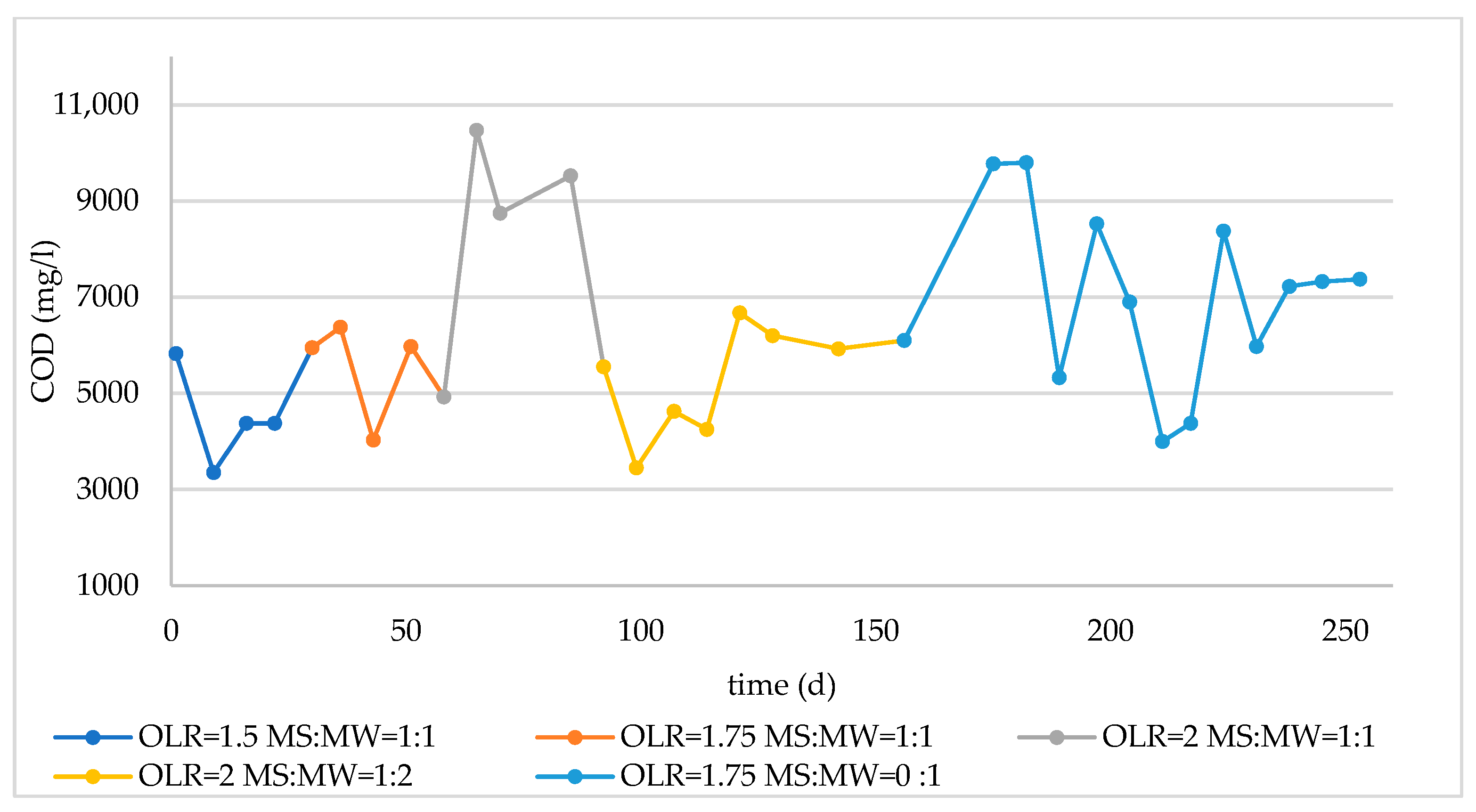
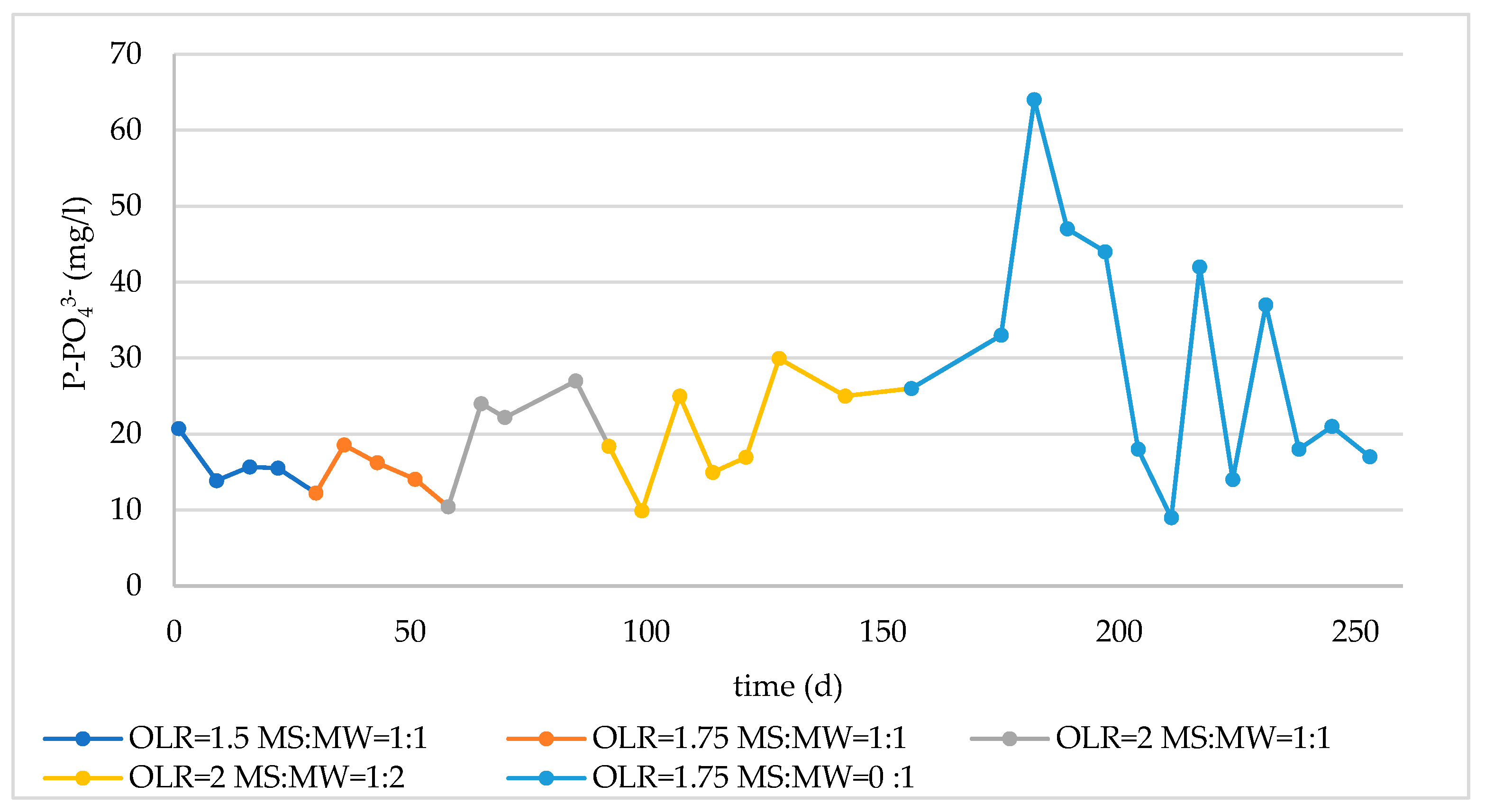
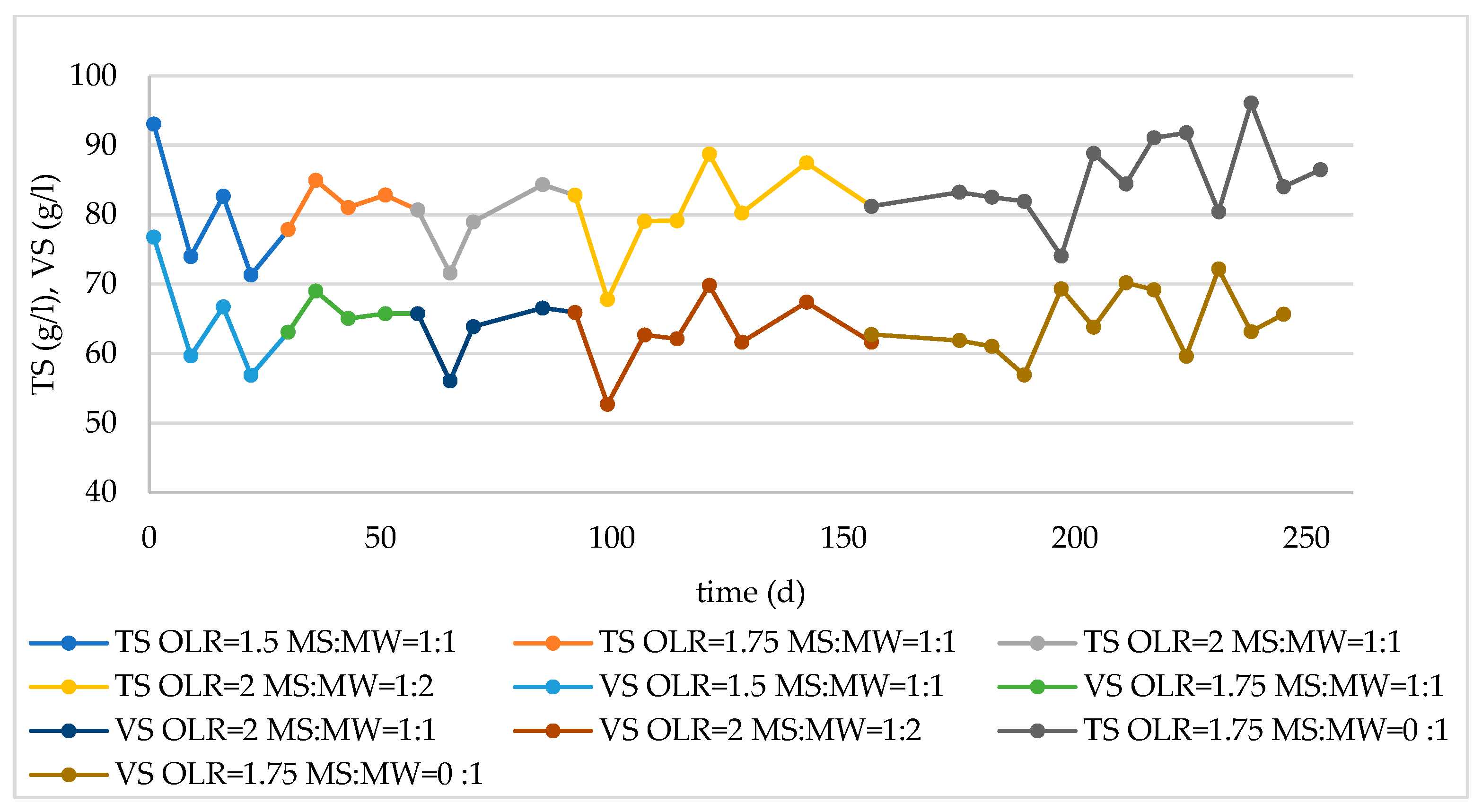
3.2. Monitoring of Long-Term Reactor Operation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ang, T.Z.; Salem, M.; Kamarol, M.; Das, H.S.; Nazari, M.A.; Prabaharan, N. A comprehensive study of renewable energy sources: Classifications, challenges and suggestions. Energy Strategy Rev. 2022, 43, 100939. [Google Scholar] [CrossRef]
- Janiszewska, D.; Ossowska, L. The role of agricultural biomass as a renewable energy source in European Union countries. Energies 2022, 15, 6756. [Google Scholar] [CrossRef]
- Hernández-Beltrán, J.U.; Hernández-De Lira, I.O.; Cruz-Santos, M.M.; Saucedo-Luevanos, A.; Hernández-Terán, F.; Balagurusamy, N. Insight into pretreatment methods of lignocellulosic biomass to increase biogas yield: Current state, challenges, and opportunities. Appl. Sci. 2019, 9, 3721. [Google Scholar] [CrossRef]
- Olatunji, K.O.; Ahmed, N.A.; Ogunkunle, O. Optimization of biogas yield from lignocellulosic materials with different pretreatment methods: A review. Biotechnol. Biofuels 2021, 14, 159. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.; Usman, M.; Ashraf, M.A.; Dutta, N.; Luo, G.; Zhang, S. A review of recent advancements in pretreatment techniques of lignocellulosic materials for biogas production: Opportunities and Limitations. Chem. Eng. J. Adv. 2022, 10, 100263. [Google Scholar] [CrossRef]
- Poddar, B.J.; Nakhate, S.P.; Gupta, R.K.; Chavan, A.R.; Singh, A.K.; Khardenavis, A.A.; Purohit, H.J. A comprehensive review on the pretreatment of lignocellulosic wastes for improved biogas production by anaerobic digestion. Int. J. Environ. Sci. Technol. 2022, 19, 3429–3456. [Google Scholar] [CrossRef]
- Stanley, J.T.; Thanarasu, A.; Kumar, P.S.; Periyasamy, K.; Raghunandhakumar, S.; Periyaraman, P.; Devaraj, K.; Dhanasekaran, A.; Subramanian, S. Potential pre-treatment of lignocellulosic biomass for the enhancement of biomethane production through anaerobic digestion—A review. Fuel 2022, 318, 123593. [Google Scholar] [CrossRef]
- Kasinath, A.; Fudala-Ksiazek, S.; Szopinska, M.; Bylinski, H.; Artichowicz, W.; Remiszewska-Skwarek, A.; Luczkiewicz, A. Biomass in biogas production: Pretreatment and codigestion. Renew. Sustain. Energy Rev. 2021, 150, 111509. [Google Scholar] [CrossRef]
- Baksi, S.; Saha, D.; Saha, S.; Sarkar, U.; Basu, D.; Kuniyal, J.C. Pre-treatment of lignocellulosic biomass: Review of various physico-chemical and biological methods influencing the extent of biomass depolymerization. Int. J. Environ. Sci. Technol. 2023, 20, 13895–13922. [Google Scholar] [CrossRef]
- Cai, Y.; Zheng, Z.; Schäfer, F.; Stinner, W.; Yuan, X.; Wang, H.; Cui, Z.; Wang, X. A review about pretreatment of lignocellulosic biomass in anaerobic digestion: Achievement and challenge in Germany and China. J. Clean. Prod. 2021, 299, 126885. [Google Scholar] [CrossRef]
- Jankovičová, B.; Hutňan, M.; Czölderová, M.N.; Hencelová, K.; Imreová, Z. Comparison of acid and alkaline pre-treatment of lignocellulosic materials for biogas production. Plant Soil. Environ. 2022, 68, 195–204. [Google Scholar] [CrossRef]
- Jankovičová, B.; Hutňan, M.; Imreová, Z.; Zakhar, R. Increased biogas production from lignocellulosic biomass by soaking in water. Agron. Res. 2023, 21, 120–134. [Google Scholar] [CrossRef]
- Czekała, W.; Jasiński, T.; Grzelak, M.; Witaszek, K.; Dach, J. Biogas plant operation: Digestate as the valuable product. Energies 2022, 15, 8275. [Google Scholar] [CrossRef]
- Czekała, W. Digestate as a source of nutrients: Nitrogen and its fractions. Water 2022, 14, 4067. [Google Scholar] [CrossRef]
- Mazurkiewicz, J.; Marczuk, A.; Pochwatka, P.; Kujawa, S. Maize straw as a valuable energetic material for biogas plant feeding. Materials 2019, 12, 3848. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2017; ISBN 978-0-87553-287-5. [Google Scholar]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef]
- Hu, Y.; Pang, Y.; Yuan, H.; Zou, D.; Liu, Y.; Zhu, B.; Chufo, W.A.; Jaffar, M.; Li, X. Promoting anaerobic biogasification of corn stover through biological pretreatment by liquid fraction of digestate (LFD). Bioresour. Technol. 2015, 175, 167–173. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, X.; Li, Z.; Wang, X.; Sun, J. Effects of liquid digestate pretreatment on biogas production for anaerobic digestion of wheat straw. Bioresour. Technol. 2019, 280, 345–351. [Google Scholar] [CrossRef]
- Kaur, M. Effect of particle size on enhancement of biogas production from crop residue. Mater. Today Proc. 2022, 57, 1950–1954. [Google Scholar] [CrossRef]
- Victorin, M.; Davidsson, Å.; Wallberg, O. Characterization of mechanically pretreated wheat straw for biogas production. Bioenergy Res. 2020, 13, 833–844. [Google Scholar] [CrossRef]
- Menardo, S.; Airoldi, G.; Balsari, P. The effect of particle size and thermal pre-treatment on the methane yield of four agricultural by-products. Bioresour. Technol. 2012, 104, 708–714. [Google Scholar] [CrossRef]
- De la Rubia, M.A.; Fernández-Cegrí, V.; Raposo, F.; Borja, R. Influence of particle size and chemical composition on the performance and kinetics of anaerobic digestion process of sunflower oil cake in batch mode. Biochem. Eng. J. 2011, 58, 162–167. [Google Scholar] [CrossRef]
- Zhang, Y.; Banks, C.J. Impact of different particle size distributions on anaerobic digestion of the organic fraction of municipal solid waste. Waste Manag. 2013, 33, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Tang, F.; Lin, Y.; Xu, Z.; Xie, Z.; Tian, J. Solid-state anaerobic digestion of rice straw pretreated with swine manure digested effluent. J. Clean. Prod. 2022, 348, 131252. [Google Scholar] [CrossRef]
- Zhong, Y.; Ragauskas, A.J.; Zheng, Y.; Meng, X.; Zhou, Y.; Lin, Y. A review on the pretreatment of straw biomass by using biogas slurry. Process Saf. Environ. Prot. 2025, 195, 106843. [Google Scholar] [CrossRef]
- Dong, L.; Cao, G.; Tian, Y.; Wu, J.; Zhou, C.; Liu, B.; Zhao, L.; Fan, J.; Ren, N. Improvement of biogas production in plug flow reactor using biogas slurry pretreated cornstalk. Bioresour. Technol. Rep. 2020, 9, 100378. [Google Scholar] [CrossRef]
- Björnsson, L.; Murto, M.; Mattiasson, B. Evaluation of parameters for monitoring an anaerobic co-digestion process. Appl. Microbiol. Biotechnol. 2000, 54, 844–849. [Google Scholar] [CrossRef]
- Azizan, N.A.Z.; Yuzir, A.; Abdullah, N. Pharmaceutical compounds in anaerobic digestion: A review on the removals and effect to the process performance. J. Environ. Chem. Eng. 2021, 9, 105926. [Google Scholar] [CrossRef]
- Gil, A.; Siles, J.A.; Serrano, A.; Chica, A.F.; Martín, M.A. Effect of variation in the C/[N+ P] ratio on anaerobic digestion. Environ. Prog. Sustain. Energy 2019, 38, 228–236. [Google Scholar] [CrossRef]
- Patowary, D.; Baruah, D.C. Effect of combined chemical and thermal pretreatments on biogas production from lignocellulosic biomasses. Ind. Crops Prod. 2018, 124, 735–746. [Google Scholar] [CrossRef]
- Sumardiono, S.; Matin, H.H.A.; Hartono, I.I.; Choiruly, L. Biogas production from corn stalk as agricultural waste containing high cellulose material by anaerobic process. Mater. Today Proc. 2022, 63, S477–S483. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, H.; Mi, X.; Wang, L.; Zhang, L.; Ai, Y. Research on anaerobic digestion of corn stover enhanced by dilute acid pretreatment: Mechanism study and potential utilization in practical application. J. Renew. Sustain. Energy 2016, 8, 023103. [Google Scholar] [CrossRef]
- Venturin, B.; Camargo, A.F.; Scapini, T.; Mulinari, J.; Bonatto, C.; Bazoti, S.; Siqueira, D.P.; Colla, L.M.; Alves, S.L., Jr.; Bender, J.P.; et al. Effect of pretreatments on corn stalk chemical properties for biogas production purposes. Bioresour. Technol. 2018, 266, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dang, F.; Zhang, Y.T.; Zou, D.; Yuan, H. Anaerobic digestion performance and mechanism of ammoniation pretreatment of corn stover. Bioresources 2015, 10, 5777–5790. [Google Scholar] [CrossRef]
- Lizasoain, J.; Trulea, A.; Gittinger, J.; Kral, I.; Piringer, G.; Schedl, A.; Nilsen, P.J.; Potthast, A.; Gronauer, A.; Bauer, A. Corn stover for biogas production: Effect of steam explosion pretreatment on the gas yields and on the biodegradation kinetics of the primary structural compounds. Bioresour. Technol. 2017, 244, 949–956. [Google Scholar] [CrossRef]








| Substrate | TS (g/g) | VS (g/g) |
|---|---|---|
| maize waste | 0.940 ± 0.022 | 0.866 ± 0.031 |
| maize silage | 0.441 ± 0.058 | 0.427 ± 0.059 |
| Inoculum | TS (g/L) | VS (g/L) |
|---|---|---|
| tests of biogas potential—impact of digestate | 23.63 ± 0.21 | 17.26 ± 0.23 |
| tests of biogas potential—impact of amount of digestate | 17.30 ± 0.21 | 12.36 ± 0.22 |
| long-term reactor operation | 30.54 ± 0.18 | 20.37 ± 0.19 |
| Parameter | |
|---|---|
| TS (g/L) | 62.39 ± 0.71 |
| VS (g/L) | 47.45 ± 0.69 |
| pH | 7.91 |
| COD (mg/L) | 6300 ± 200 |
| N-NH4+ (mg/L) | 1372 ± 6 |
| P-PO43− (mg/L) | 22 ± 0.6 |
| VFA (mg/L) | 1805 ± 135 |
| Inoculum (mL) | Water (mL) | Digestate (mL) | Cut MW (g) | Ground MW (g) | |
|---|---|---|---|---|---|
| blank | 140 | 20 | - | - | - |
| blank + digestate | 140 | 18 | 2 | - | - |
| cut MW soaked in digestate for 1 day | 140 | 18 | 2 | 0.238 | - |
| cut MW soaked in digestate for 2 days | 140 | 18 | 2 | 0.238 | - |
| cut MW soaked in digestate for 5 days | 140 | 18 | 2 | 0.238 | - |
| cut MW soaked in water for 1 day | 140 | 20 | - | 0.238 | - |
| cut MW soaked in water for 2 days | 140 | 20 | - | 0.238 | - |
| cut MW soaked in water for 5 days | 140 | 20 | - | 0.238 | - |
| ground MW soaked in digestate for 1 day | 140 | 18 | 2 | - | 0.238 |
| ground MW soaked in digestate for 2 days | 140 | 18 | 2 | - | 0.238 |
| ground MW soaked in digestate for 5 days | 140 | 18 | 2 | - | 0.238 |
| Inoculum (mL) | Water (mL) | Digestate (mL) | Cut MW (g) | |
|---|---|---|---|---|
| blank | 140 | 20 | - | - |
| blank + digestate | 140 | - | 20 | - |
| cut MW soaked in 5 mL of digestate | 140 | 15 | 5 | 0.993 |
| cut MW soaked in 10 mL of digestate | 140 | 10 | 10 | 0.993 |
| cut MW soaked in 15 mL of digestate | 140 | 5 | 15 | 0.993 |
| cut MW soaked in water | 140 | 20 | - | 0.993 |
| SBP (Measured) (mL/g VS) | SBP (Model) (mL/g VS) | Rmax (mL/g VS/d) | λ (d) | R2 | |
|---|---|---|---|---|---|
| blank | 147.88 | 142.29 | 4.21 | −4.2823 | 0.9800 |
| blank + digestate | 148.54 | 143.22 | 4.56 | −3.9645 | 0.9834 |
| cut MW soaked in digestate for 1 day | 419.13 | 389.14 | 28.29 | −0.2127 | 0.9743 |
| cut MW soaked in digestate for 2 days | 494.51 | 445.55 | 38.74 | 0.7839 | 0.9801 |
| cut MW soaked in digestate for 5 days | 457.96 | 426.38 | 44.77 | 0.7143 | 0.9856 |
| cut MW soaked in water for 1 day | 358.26 | 338.36 | 24.01 | −0.0583 | 0.9809 |
| cut MW soaked in water for 2 days | 417.31 | 388.24 | 32.19 | 0.0992 | 0.9832 |
| cut MW soaked in water for 5 days | 354.70 | 334.26 | 32.38 | 0.9316 | 0.9910 |
| SBP (Measured) (mL/g VS) | SBP (Model) (mL/g VS) | Rmax (mL/g VS/d) | λ (d) | R2 | |
|---|---|---|---|---|---|
| cut MW soaked in digestate for 1 day | 419.13 | 389.14 | 28.29 | −0.2127 | 0.9743 |
| cut MW soaked in digestate for 2 days | 494.51 | 445.55 | 38.74 | 0.7839 | 0.9801 |
| cut MW soaked in digestate for 5 days | 457.96 | 426.38 | 44.77 | 0.7143 | 0.9856 |
| ground MW soaked in digestate for 1 day | 498.25 | 449.69 | 41.86 | 0.6135 | 0.9716 |
| ground MW soaked in digestate for 2 days | 449.03 | 409.35 | 39.67 | 0.9160 | 0.9755 |
| ground MW soaked in digestate for 5 days | 428.20 | 408.35 | 40.46 | 0.9291 | 0.9846 |
| SBP (Measured) (mL/g VS) | SBP (Model) (mL/g VS) | Rmax (mL/g VS/d) | λ (d) | R2 | |
|---|---|---|---|---|---|
| cut MW soaked in water | 659.44 | 649.11 | 27.89 | −2.4512 | 0.9912 |
| cut MW soaked in 5 mL of digestate | 739.69 | 727.36 | 29.77 | −2.3241 | 0.9921 |
| cut MW soaked in 10 mL of digestate | 725.12 | 712.13 | 26.69 | −2.7209 | 0.9925 |
| cut MW soaked in 15 mL of digestate | 786.74 | 762.38 | 30.75 | −2.4189 | 0.9884 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jankovičová, B.; Hutňan, M.; Sammarah, M. Enhancing Biogas Production: Pre-Treatment of Lignocellulosic Biomass Using Biogas Plant Digestate. Sustainability 2025, 17, 3898. https://doi.org/10.3390/su17093898
Jankovičová B, Hutňan M, Sammarah M. Enhancing Biogas Production: Pre-Treatment of Lignocellulosic Biomass Using Biogas Plant Digestate. Sustainability. 2025; 17(9):3898. https://doi.org/10.3390/su17093898
Chicago/Turabian StyleJankovičová, Barbora, Miroslav Hutňan, and Mikhael Sammarah. 2025. "Enhancing Biogas Production: Pre-Treatment of Lignocellulosic Biomass Using Biogas Plant Digestate" Sustainability 17, no. 9: 3898. https://doi.org/10.3390/su17093898
APA StyleJankovičová, B., Hutňan, M., & Sammarah, M. (2025). Enhancing Biogas Production: Pre-Treatment of Lignocellulosic Biomass Using Biogas Plant Digestate. Sustainability, 17(9), 3898. https://doi.org/10.3390/su17093898





