Alternative Leaching Agents for Selective Recovery of Gold and Copper from Computer Waste Printed Circuit Boards
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Selection
2.2. Sample Preparation
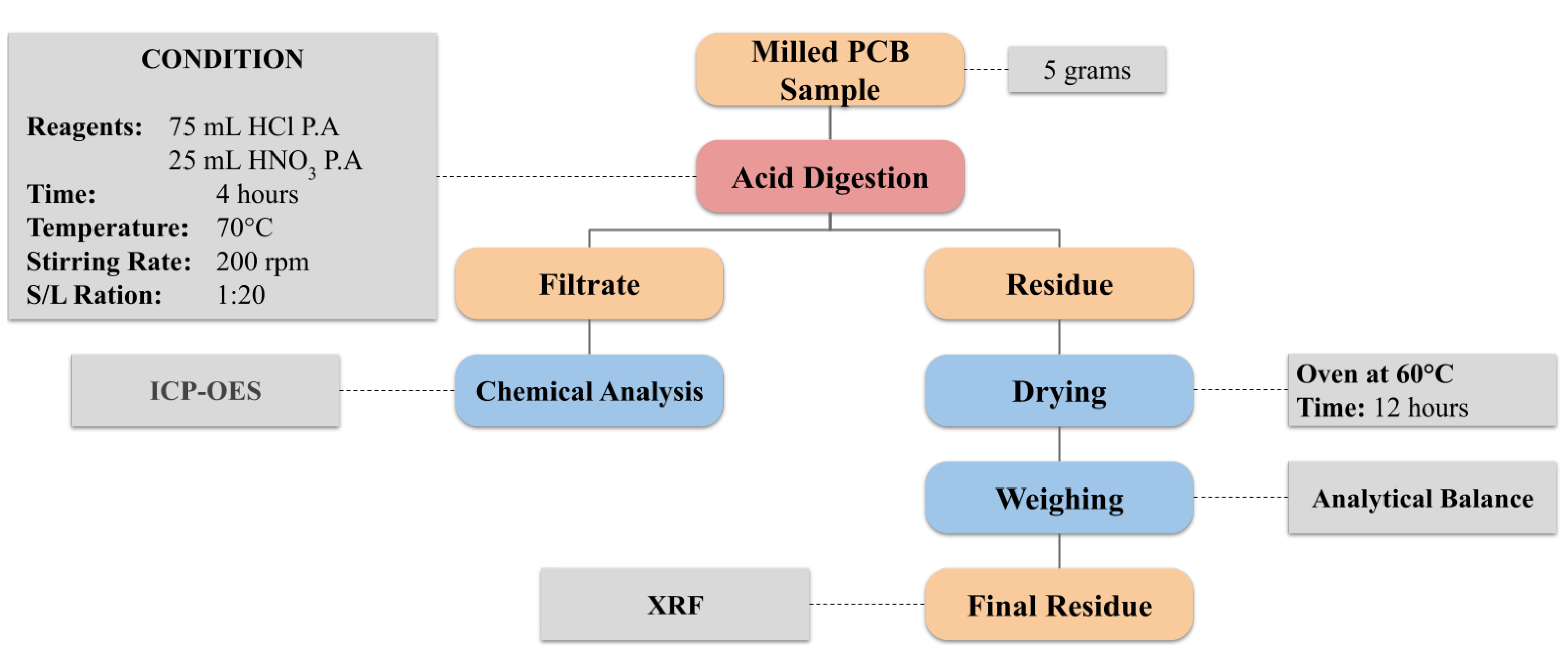
2.3. Acid Digestion for Sample Characterization
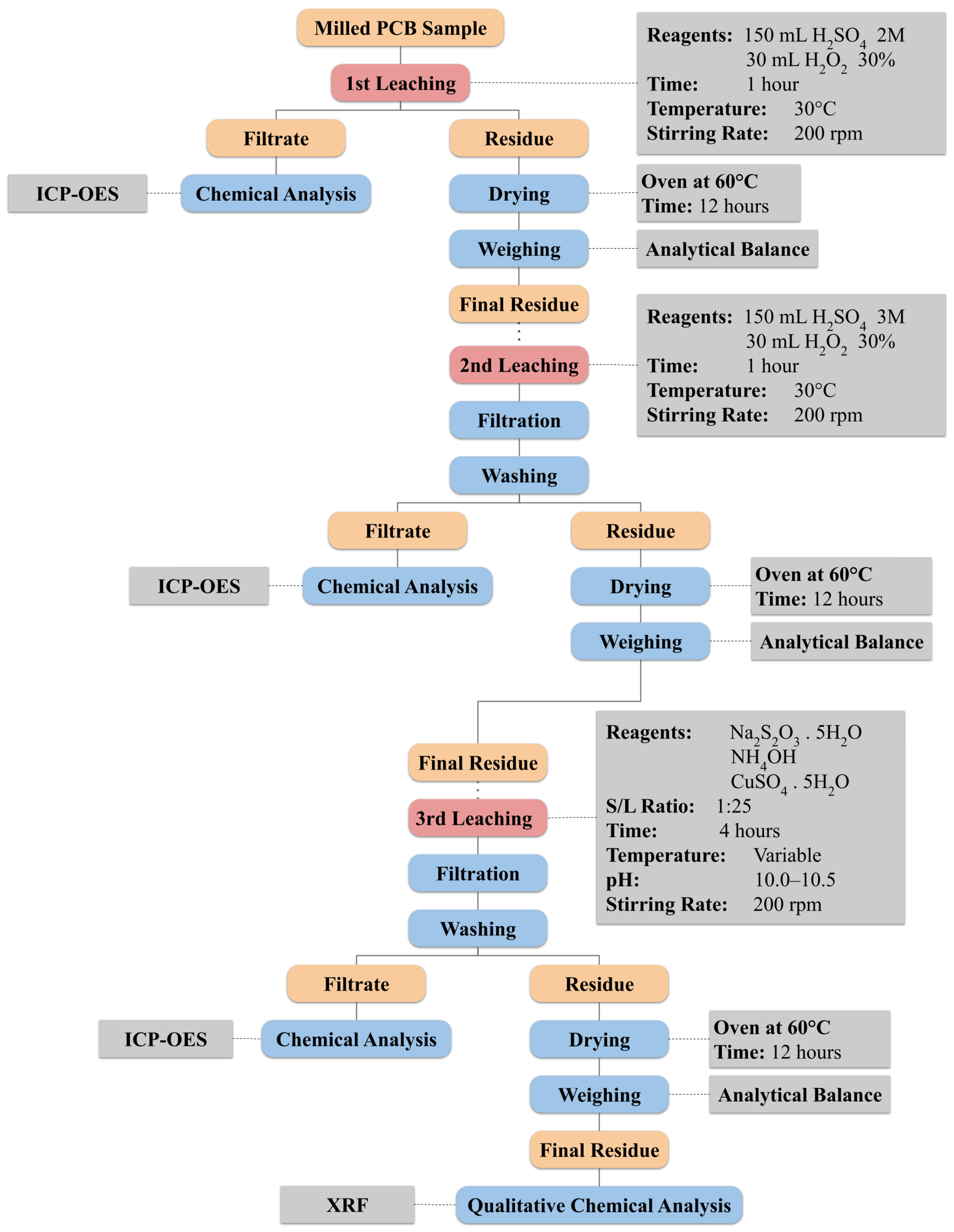
2.4. Leaching of Base Metals
2.5. Gold Leaching
3. Results and Discussion
3.1. Evaluation of the Physical-Mechanical Processing of PCBs
3.2. Chemical Composition Analysis of PCBs
3.2.1. Characterization of Ground PCB via XRF
3.2.2. The Effectiveness of Acid Digestion in Determining the Composition of PCBs
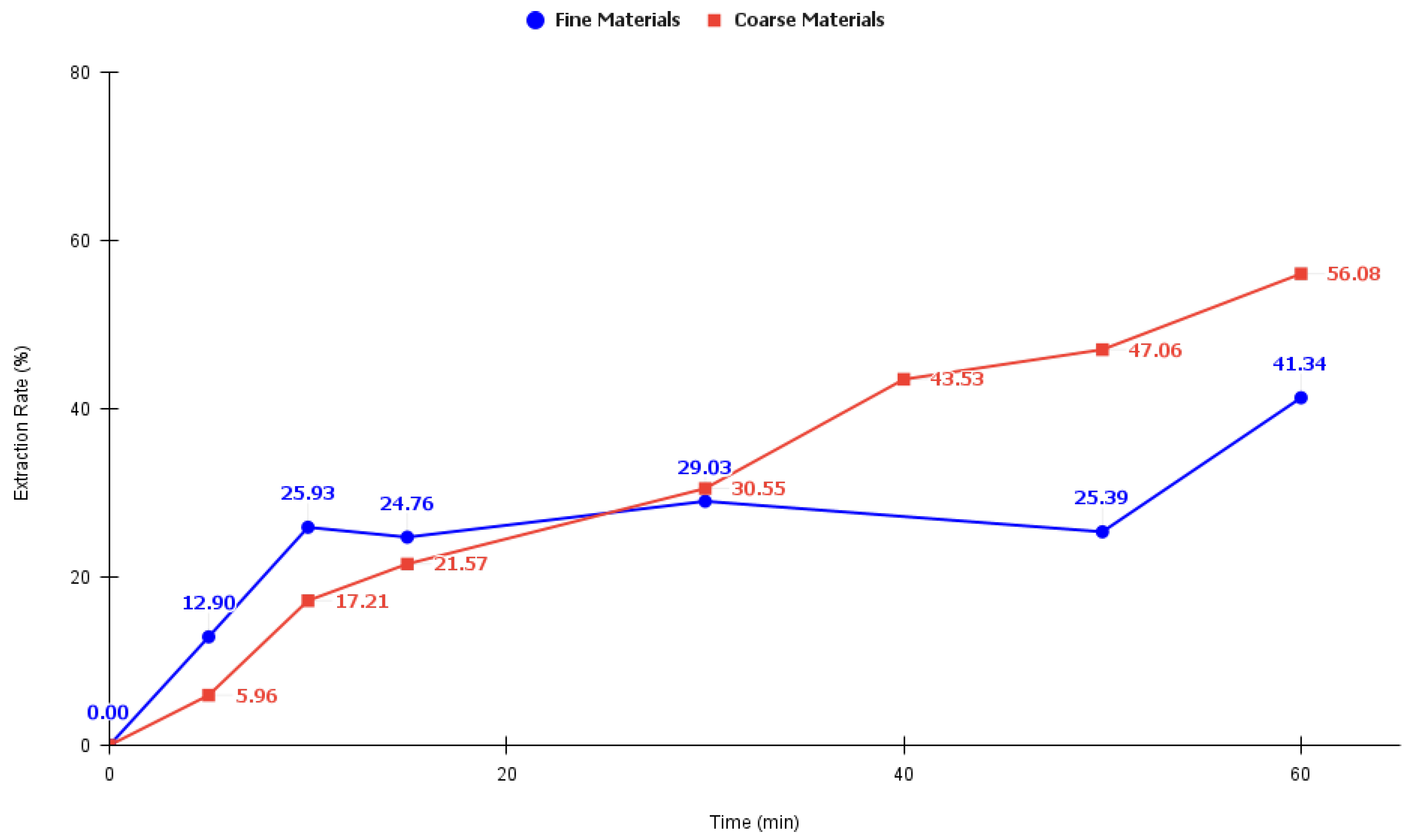
3.2.3. Efficiency of Sequential Leaching Stages with Sulfuric Acid and Hydrogen Peroxide for Base Metals Extraction
3.2.4. Effects of Different Experimental Conditions for Gold Leaching in PCBs
Evaluation of the Effect of Sample Granulometry
Evaluation of the Effect of Temperature on Gold Extraction
Evaluation of the Effect of Copper Sulfate and Ammonium Hydroxide Concentration on Gold Extraction
3.2.5. The Optimal Experimental Condition for Gold Leaching in PCBs
4. Final Considerations
- Characterization of PCB samples through chemical analyses (XRF and ICP-OES) confirmed significant concentrations of valuable metals, primarily copper and gold, aligning with previous literature.
- Elevated palladium concentrations were detected, likely due to specific components soldered to the PCB base.
- Sodium thiosulfate was used as an alternative lixiviant to cyanide, achieving gold recovery after two preceding copper leaching stages. Although the gold recovery (~15%) is still limited, the process demonstrates the potential for environmentally friendlier practices. Further optimization is required to improve efficiency.
- Optimal conditions for gold recovery were identified as higher copper sulfate and ammonium hydroxide concentrations at 40 C.
- The presented hydrometallurgical method proved environmentally safer and economically viable compared to cyanide-based processes, supporting sustainable recycling of WEEE.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shittu, O.S.; Williams, I.D.; Shaw, P.J. The ‘WEEE’ challenge: Is reuse the “new recycling”? Resour. Conserv. Recycl. 2021, 174, 105817. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, X.; Zi, F.; Yang, P.; Chen, Y.; Chen, S. Environmentally friendly extraction of gold from refractory concentrate using a copper–ethylenediamine–thiosulfate solution. J. Clean. Prod. 2019, 214, 860–872. [Google Scholar] [CrossRef]
- Cottes, M.; Mainardis, M.; Simeoni, P. Assessing the techno-economic feasibility of waste electric and electronic equipment treatment plant: A multi-decisional modeling approach. Sustainability 2023, 15, 16248. [Google Scholar] [CrossRef]
- Azizi, D.D.S.; Hanafiah, M.M.; Woon, K.S. Material flow analysis in WEEE management for circular economy: A content review on applications, limitations, and future outlook. Sustainability 2023, 15, 3505. [Google Scholar] [CrossRef]
- Shams, H.; Molla, A.H.; Ab Rahman, M.N.; Hishamuddin, H.; Harun, Z.; Kumar, N.M. Exploring industry-specific research themes on E-waste: A literature review. Sustainability 2023, 15, 12244. [Google Scholar] [CrossRef]
- Alzate, A.; López, M.E.; Serna, C. Recovery of gold from waste electrical and electronic equipment (WEEE) using ammonium persulfate. Waste Manag. 2016, 57, 113–120. [Google Scholar] [CrossRef]
- Zhang, W.; Ren, J.; Liu, S.; Yuan, Z. Mechanism and clean procedure to extract gold from printed circuit board. Procedia Environ. Sci. 2016, 31, 171–177. [Google Scholar] [CrossRef]
- Zeng, X.; Xiao, T.; Xu, G.; Albalghiti, E.; Shan, G.; Li, J. Comparing the costs and benefits of virgin and urban mining. J. Manag. Sci. Eng. 2022, 7, 98–106. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2020; Technical report; U.S. Geological Survey: Reston, VA, USA, 2020.
- Teseletso, L.S.; Adachi, T. Future availability of mineral resources: Ultimate reserves and total material requirement. Miner. Econ. 2023, 36, 189–206. [Google Scholar] [CrossRef]
- Achillas, C.; Vlachokostas, C.; Moussiopoulos, N.; Perkoulidis, G.; Banias, G.; Mastropavlos, M. Electronic waste management cost: A scenario-based analysis for greece. Waste Manag. Res. J. A Sustain. Circ. Econ. 2011, 29, 963–972. [Google Scholar] [CrossRef]
- Cucchiella, F.; D’Adamo, I.; Rosa, P.; Terzi, S. Automotive printed circuit boards recycling: An economic analysis. J. Clean. Prod. 2016, 121, 130–141. [Google Scholar] [CrossRef]
- Meem, T.T.; Khan, M.S.; Hassan, M.M.; Mamtaz, R. Assessment of hazardous and precious metal content in E-waste. In Advances in Civil Engineering; Arthur, S., Saitoh, M., Pal, S.K., Eds.; Series Title: Lecture Notes in Civil Engineering; Springer: Singapore, 2022; Volume 184, pp. 65–72. [Google Scholar] [CrossRef]
- Birloaga, I.; Vegliò, F. Study of multi-step hydrometallurgical methods to extract the valuable content of gold, silver and copper from waste printed circuit boards. J. Environ. Chem. Eng. 2016, 4, 20–29. [Google Scholar] [CrossRef]
- Li, H.; Eksteen, J.; Oraby, E. Hydrometallurgical recovery of metals from waste printed circuit boards (WPCBs): Current status and perspectives—A review. Resour. Conserv. Recycl. 2018, 139, 122–139. [Google Scholar] [CrossRef]
- Ribeiro, P.P.M.; Santos, I.D.d.; Dutra, A.J.B. Copper and metals concentration from printed circuit boards using a zig-zag classifier. J. Mater. Res. Technol. 2019, 8, 513–520. [Google Scholar] [CrossRef]
- Tunsu, C.; Petranikova, M.; Gergorić, M.; Ekberg, C.; Retegan, T. Reclaiming rare earth elements from end-of-life products: A review of the perspectives for urban mining using hydrometallurgical unit operations. Hydrometallurgy 2015, 156, 239–258. [Google Scholar] [CrossRef]
- Choubey, S.; Goswami, P.; Gautam, S. Recovery of copper from waste PCB boards using electrolysis. Mater. Today Proc. 2021, 42, 2656–2659. [Google Scholar] [CrossRef]
- Fogarasi, S.; Imre-Lucaci, F.; Imre-Lucaci, Á.; Ilea, P. Copper recovery and gold enrichment from waste printed circuit boards by mediated electrochemical oxidation. J. Hazard. Mater. 2014, 273, 215–221. [Google Scholar] [CrossRef]
- Qiu, R.; Lin, M.; Ruan, J.; Fu, Y.; Hu, J.; Deng, M.; Tang, Y.; Qiu, R. Recovering full metallic resources from waste printed circuit boards: A refined review. J. Clean. Prod. 2020, 244, 118690. [Google Scholar] [CrossRef]
- Rao, M.D.; Singh, K.K.; Morrison, C.A.; Love, J.B. Challenges and opportunities in the recovery of gold from electronic waste. RSC Adv. 2020, 10, 4300–4309. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, Z. Precious metals recovery from waste printed circuit boards: A review for current status and perspective. Resour. Conserv. Recycl. 2016, 113, 28–39. [Google Scholar] [CrossRef]
- Birloaga, I.; De Michelis, I.; Ferella, F.; Buzatu, M.; Vegliò, F. Study on the influence of various factors in the hydrometallurgical processing of waste printed circuit boards for copper and gold recovery. Waste Manag. 2013, 33, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Birloaga, I.; Coman, V.; Kopacek, B.; Vegliò, F. An advanced study on the hydrometallurgical processing of waste computer printed circuit boards to extract their valuable content of metals. Waste Manag. 2014, 34, 2581–2586. [Google Scholar] [CrossRef] [PubMed]
- Kasper, A.C. Utilização de Técnicas Hidrometalúrgicas e Eletrometalúrgicas na Recuperação de ouro Proveniente de Sucatas de Telefones Celulares. Ph.D. Thesis, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, 2016. [Google Scholar]
- Kasper, A.C.; Veit, H.M. Gold recovery from printed circuit boards of mobile phones scraps using a leaching solution alternative to cyanide. Braz. J. Chem. Eng. 2018, 35, 931–942. [Google Scholar] [CrossRef]
- Zupanc, A.; Install, J.; Jereb, M.; Repo, T. Sustainable and selective modern methods of noble metal recycling. Angew. Chem. Int. Ed. 2023, 62, e202214453. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, H.; Li, X.; He, W.; Ma, J.; Xu, Y.; Xu, Y.; Ming, W. Recent advances in bioleaching and biosorption of metals from waste printed circuit boards: A review. J. Environ. Manag. 2024, 371, 123008. [Google Scholar] [CrossRef]
- Baniasadi, M.; Graves, J.E.; Ray, D.A.; De Silva, A.L.; Renshaw, D.; Farnaud, S. Closed-loop recycling of copper from waste printed circuit boards using bioleaching and electrowinning processes. Waste Biomass Valoriz. 2021, 12, 3125–3136. [Google Scholar] [CrossRef]
- Aylmore, M.; Muir, D. Thiosulfate leaching of gold—A review. Miner. Eng. 2001, 14, 135–174. [Google Scholar] [CrossRef]
- Petter, P.M.H.; Veit, H.M.; Bernardes, A.M. Evaluation of gold and silver leaching from printed circuit board of cellphones. Waste Manag. 2014, 34, 475–482. [Google Scholar] [CrossRef]
- Veit, H.M.; De Pereira, C.C.; Bernardes, A.M. Using mechanical processing in recycling printed wiring boards. JOM 2002, 54, 45–47. [Google Scholar] [CrossRef]
- Buchert, M.; Manhart, A.; Bleher, D.; Pingel, D. Recycling Critical Raw Materials from Waste Electronic Equipment; Technical Report; North RhineWestphalia State Agency for Nature, Environment and Consumer Protection: Darmstadt, Germany, 2012. [Google Scholar]
- Chen, B.; He, J.; Xi, Y.; Zeng, X.; Kaban, I.; Zhao, J.; Hao, H. Liquid-liquid hierarchical separation and metal recycling of waste printed circuit boards. J. Hazard. Mater. 2019, 364, 388–395. [Google Scholar] [CrossRef]
- Hao, J.; Wang, Y.; Wu, Y.; Guo, F. Metal recovery from waste printed circuit boards: A review for current status and perspectives. Resour. Conserv. Recycl. 2020, 157, 104787. [Google Scholar] [CrossRef]
- Li, H.; Oraby, E.; Eksteen, J.; Mali, T. Extraction of gold and copper from flotation tailings using glycine-ammonia solutions in the presence of permanganate. Minerals 2022, 12, 612. [Google Scholar] [CrossRef]
- Magoda, K.; Mekuto, L. Biohydrometallurgical recovery of metals from waste electronic equipment: Current status and proposed process. Recycling 2022, 7, 67. [Google Scholar] [CrossRef]
- Xu, B.; Kong, W.; Li, Q.; Yang, Y.; Jiang, T.; Liu, X. A review of thiosulfate leaching of gold: Focus on thiosulfate consumption and gold recovery from pregnant solution. Metals 2017, 7, 222. [Google Scholar] [CrossRef]
- Kaya, M. Electronic Waste and Printed Circuit Board Recycling Technologies; The Minerals, Metals & Materials Series; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, C.; Zhao, Y.; Zhou, Y.; Ma, E.; Bai, J.; Wang, J. Recycling gold from printed circuit boards gold-plated layer of waste mobile phones in “mild aqua regia” system. J. Clean. Prod. 2021, 278, 123597. [Google Scholar] [CrossRef]
- Ippolito, N.M.; Passadoro, M.; Ferella, F.; Pellei, G.; Vegliò, F. Recovery of metals from printed circuit boards by gold-REC 1 hydrometallurgical process. Sustainability 2023, 15, 7348. [Google Scholar] [CrossRef]
- Rao, M.D.; Singh, K.K.; Morrison, C.A.; Love, J.B. Recycling copper and gold from e-waste by a two-stage leaching and solvent extraction process. Sep. Purif. Technol. 2021, 263, 118400. [Google Scholar] [CrossRef]
- Boro, B.; Tiwari, P. Material recovery from waste printed circuit board using pyrolysis and metal extraction. In Sustainable Environment; Deka, D., Majumder, S.K., Purkait, M.K., Eds.; Springer Nature: Singapore, 2023; pp. 199–210. [Google Scholar] [CrossRef]
- Behnamfard, A.; Salarirad, M.M.; Veglio, F. Process development for recovery of copper and precious metals from waste printed circuit boards with emphasize on palladium and gold leaching and precipitation. Waste Manag. 2013, 33, 2354–2363. [Google Scholar] [CrossRef]
- Wath, S.B.; Katariya, M.N.; Bansiwal, A.K.; Shinde, V.M.; Vaidya, A.N. Evaluation of the effect of size reduction and thermal treatment on metal extraction from PCBs of mother board and digital video drive of desktop PC. Curr. Sci. 2016, 110, 800–807. [Google Scholar]
- Oraby, E.A.; Eksteen, J.J.; O’Connor, G.M. Gold leaching from oxide ores in alkaline glycine solutions in the presence of permanganate. Hydrometallurgy 2020, 198, 105527. [Google Scholar] [CrossRef]
- Ha, V.H.; Lee, J.c.; Huynh, T.H.; Jeong, J.; Pandey, B.D. Optimizing the thiosulfate leaching of gold from printed circuit boards of discarded mobile phone. Hydrometallurgy 2014, 149, 118–126. [Google Scholar] [CrossRef]
- Sohaili, J.; Muniyandi, S.K.; Mohamad, S.S. A review on printed circuit boards waste recycling technologies and reuse of recovered nonmetallic materials. Int. J. Sci. Eng. Res. 2012, 3, 1–7. [Google Scholar]
- Peres, A.E.C.; Chaves, A.P.; Lins, F.A.F.; Torem, M.L. Beneficiamento de Minérios de Ouro. In Extração de Ouro: Princípios, Tecnologia e Meio Ambiente; CETEM/MCT: Rio de Janeiro, Brazil, 2002; pp. 23–58. [Google Scholar]
- Silva, M.L.L.D.; Oliveira, C.E.A.D.; Ferreira, R.D.P.; Cardoso, A.M. Risco ambiental e biotecnologia na recuperação de metais da placa de circuito impresso (PCI). Braz. Appl. Sci. Rev. 2020, 4, 2494–2505. [Google Scholar] [CrossRef]
- Jing-ying, L.; Xiu-li, X.; Wen-quan, L. Thiourea leaching gold and silver from the printed circuit boards of waste mobile phones. Waste Manag. 2012, 32, 1209–1212. [Google Scholar] [CrossRef]
- Gámez, S.; Garcés, K.; de la Torre, E.; Guevara, A. Precious metals recovery from waste printed circuit boards using thiosulfate leaching and ion exchange resin. Hydrometallurgy 2019, 186, 1–11. [Google Scholar] [CrossRef]
- Jadhav, U.; Hocheng, H. Hydrometallurgical recovery of metals from large printed circuit board pieces. Sci. Rep. 2015, 5, 14574. [Google Scholar] [CrossRef]

| Experimental Conditions | Analyzed Variables | |||
|---|---|---|---|---|
| Concentration of Reagents | Temperature (C) | |||
| Na2S2O3 (M) | NH4OH (M) | CuSO4 (mM) | ||
| 0.12 | 0.2 | 20 | 30 | Temperature |
| 40 | ||||
| 50 | ||||
| 0 | 40 | Concentration CuSO4 e NH4OH | ||
| 0.15 | 20 | Concentration de NH4OH | ||
| 0.10 | ||||
| 0.06 | 10 | Concentration de CuSO4 | ||
| 0 | ||||
| Sample | Mesh | Granulometry (mm) | Mass (g) | Percentage (%) |
|---|---|---|---|---|
| Fines | 1500 | 39.0 | ||
| Coarses | 2350 | 61.0 | ||
| Total | 3850 | 10.0 |
| Metal | Sample Concentration | |
|---|---|---|
| Fines | Coarses | |
| Au (ppm) | 1154.78 | 487.31 |
| Ag (ppm) | 628.79 | 22.36 |
| Pd (ppm) | ≈0 | ≈0 |
| Cu (%) | 13.21 | 12.47 |
| Fe (%) | 8.84 | 0.44 |
| Ni (%) | 0.36 | 0.05 |
| Pb (%) | 0.04 | 0.02 |
| Zn (%) | 0.40 | 0.18 |
| Al (%) | 6.41 | 10.20 |
| Cr (%) | 0.10 | 0.02 |
| Si (%) | 8.25 | 5.92 |
| Sn (%) | 1.91 | 0.15 |
| Metal | Sample | Average Concentration (%) | Relative Standard Deviation (%) | Total Concentration (%) | Literature Concentration (%) | |
|---|---|---|---|---|---|---|
| Sample 01 | Sample 02 | |||||
| Cu | Fines | 0.233 | 0.219 | 0.010 | 22.57 | 13.70 |
| Coarses | 0.265 | 0.262 | 0.033 | 24.19 | ||
| Fe | Fines | 0.139 | 0.130 | 0.006 | 13.45 | 4.90 |
| Coarses | 0.123 | 0.166 | 0.030 | 14.47 | ||
| Ni | Fines | 0.007 | 0.007 | 0.000 | 0.73 | 1.10 |
| Coarses | 0.008 | 0.010 | 0.001 | 0.99 | ||
| Pb | Fines | 0.001 | 0.001 | 0.000 | 0.10 | 2.20 |
| Coarses | 0.002 | 0.001 | 0.004 | 0.14 | ||
| Zn | Fines | 0.017 | 0.022 | 0.004 | 1.92 | 4.70 |
| Coarses | 0.038 | 0.035 | 0.002 | 3.69 | ||
| Al | Fines | 0.035 | 0.034 | 0.001 | 3.42 | 4.60 |
| Coarses | 0.043 | 0.037 | 0.005 | 4.00 | ||
| Cr | Fines | 0.002 | 0.002 | 0.000 | 0.18 | 1.20 |
| Coarses | 0.003 | 0.003 | 0.000 | 0.30 | ||
| Sn | Fines | 0.044 | 0.048 | 0.003 | 4.60 | 2.30 |
| Coarses | 0.066 | 0.075 | 0.006 | 7.07 | ||
| Metal | Sample | Average Concentration (ppm) | Relative Standard Deviation (ppm) | Total Concentration (ppm) | Literature Concentration (ppm) | |
|---|---|---|---|---|---|---|
| Sample 01 | Sample 02 | |||||
| Au | Fines | 0.53 | 0.96 | 0.30 | 74.50 | 150 |
| Coarses | 0.59 | 1.02 | 0.30 | 80.50 | ||
| Ag | Fines | 3.09 | 1.94 | 0.81 | 251.50 | 300 |
| Coarses | 2.92 | 2.73 | 0.13 | 282.50 | ||
| Pd | Fines | 40.69 | 38.06 | 1.86 | 3937.50 | 124 |
| Coarses | 78.56 | 43.15 | 25.04 | 6085.50 | ||
| Metal | Estimated Average Remaining Mass (mg) | |
|---|---|---|
| Fines | Coarses | |
| Au | 2.35 | 3.48 |
| Ag | 0.04 | 0.10 |
| Pd | ≈0 | ≈0 |
| Cu | 3.27 | 1.82 |
| Fe | 70.86 | 25.51 |
| Ni | 3.73 | 1.17 |
| Pb | 0.82 | 0.35 |
| Zn | 7.35 | 3.03 |
| Al | 70.86 | 61.67 |
| Cr | 1.04 | ≈0 |
| Sn | 3.39 | 0.27 |
| Concentration (mg/L) | Relative Standard Deviation (mg/L) | Average Extraction Percentage (%) | |||
|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Average | |||
| Fines | 6033.82 | 6005.26 | 6019.54 | 20.19 | 53.33 |
| 5378.07 | 5427.34 | 5402.70 | 34.84 | 47.87 | |
| Coarses | 6213.07 | 6083.48 | 6148.27 | 91.63 | 46.66 |
| 3780.75 | 3999.84 | 3890.29 | 154.92 | 49.20 | |
| Concentration (mg/L) | Relative Standard Deviation (mg/L) | Average Extraction Percentage (%) | |||||
|---|---|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Average | 2nd Leaching | 1st + 2nd Leaching | Accumulated | ||
| Fines | 2790.39 | 1514.34 | 2152.36 | 902.30 | 31.78 | 85.12 | 77.95 |
| 1493.26 | 1608.74 | 1551.00 | 81.66 | 22.90 | 70.77 | ||
| Coarses | 1351.92 | 1020.77 | 1186.34 | 234.16 | 15.00 | 61.66 | 72.57 |
| 2684.62 | 2736.99 | 2710.80 | 37.03 | 34.28 | 83.49 | ||
| Experimental Conditions | Time (h) | Sample | Concentration Au (ppm) | Average Mass Extracted (mg) | Average Extraction Percentage (%) | ||
|---|---|---|---|---|---|---|---|
| Sample 1 | Sample 2 | Average | |||||
| 0.12 M Na2S2O3 + 0.2 M NH4OH + 20 mM CuSO4 − 30 C | 4 | Fines | 0.050 | 0.060 | 0.055 | 0.017 | 2.46 |
| 4 | Coarses | 0.000 | 0.600 | 0.300 | 0.074 | 6.09 | |
| 0.12 M Na2S2O3 + 0.2 M NH4OH + 20 mM CuSO4 − 40 C | 4 | Fines | 0.620 | 0.040 | 0.330 | 0.165 | 14.77 |
| 4 | Coarses | 0.570 | 0.190 | 0.380 | 0.190 | 15.73 | |
| 0.12 M Na2S2O3 + 0.2 M NH4OH + 20 mM CuSO4 − 50 C | 4 | Fines | 0.445 | 0.530 | 0.488 | 0.174 | 15.54 |
| 4 | Coarses | 0.220 | 0.350 | 0.285 | 0.143 | 11.80 | |
| 0.12 M Na2S2O3 + 0.06 M NH4OH − 40 C | 4 | Fines | 0.330 | 0.230 | 0.280 | 0.046 | 4.22 |
| 4F * | 0.040 | 0.160 | 0.100 | 0.058 | 4.13 | ||
| 4 | Coarses | 0.040 | 0.030 | 0.035 | 0.008 | 0.64 | |
| 4F * | 0.020 | 0.530 | 0.020 | 0.010 | 0.83 | ||
| 0.12 M Na2S2O3 + 0.2 M NH4OH − 40 C | 4 | Fines | 0.320 | 0.050 | 0.185 | 0.033 | 2.67 |
| 4F * | 0.040 | 0.030 | 0.035 | 0.018 | 1.57 | ||
| 4 | Coarses | 0.030 | 0.040 | 0.035 | 0.007 | 0.57 | |
| 4F * | 0.040 | 0.050 | 0.045 | 0.023 | 1.86 | ||
| 0.12 M Na2S2O3 + 0.06 M NH4OH + 10 mM CuSO4 − 40 C | 4 | Fines | 0.250 | 0.000 | 0.250 | 0.042 | 3.72 |
| 4F * | 0.070 | 0.060 | 0.065 | 0.033 | 2.91 | ||
| 4 | Coarses | 0.120 | 0.210 | 0.165 | 0.036 | 2.94 | |
| 4F * | 0.060 | 0.080 | 0.070 | 0.035 | 2.90 | ||
| 0.12 M Na2S2O3 + 0.10 M NH4OH + 20 mM CuSO4 − 40 C | 4 | Coarses | 0.500 | 0.000 | 0.500 | 0.101 | 8.33 |
| 0.12 M Na2S2O3 + 0.15 M NH4OH + 20 mM CuSO4 − 40 C | 4 | Coarses | 0.250 | 0.470 | 0.360 | 0.072 | 6.00 |
| Experimental Conditions | Estimated Average Remaining Mass | ||||
|---|---|---|---|---|---|
| Concentration of Reagents |
Temperature ( C) | Au (mg) | |||
| Na2S2O3 (M) | NH4OH (M) | CuSO4 (mM) | Fines | Coarses | |
| 0.12 | 0.20 | 20 | 30 | 8.35 | 11.01 |
| 40 | 6.34 | 8.68 | |||
| 50 | 7.29 | 11.17 | |||
| 0 | 40 | 7.94 | 8.82 | ||
| 0.15 | 20 | 7.24 | 12.24 | ||
| 0.10 | 8.11 | 9.20 | |||
| 0.06 | 10 | 7.59 | 7.43 | ||
| 0 | 10.03 | 10.73 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magalhães, M.C.; Cavalcante, D.C.; Veloso, T.C.; Benvenuti, T. Alternative Leaching Agents for Selective Recovery of Gold and Copper from Computer Waste Printed Circuit Boards. Sustainability 2025, 17, 3886. https://doi.org/10.3390/su17093886
Magalhães MC, Cavalcante DC, Veloso TC, Benvenuti T. Alternative Leaching Agents for Selective Recovery of Gold and Copper from Computer Waste Printed Circuit Boards. Sustainability. 2025; 17(9):3886. https://doi.org/10.3390/su17093886
Chicago/Turabian StyleMagalhães, Mariana Cordeiro, Danielly Cardoso Cavalcante, Tácia Costa Veloso, and Tatiane Benvenuti. 2025. "Alternative Leaching Agents for Selective Recovery of Gold and Copper from Computer Waste Printed Circuit Boards" Sustainability 17, no. 9: 3886. https://doi.org/10.3390/su17093886
APA StyleMagalhães, M. C., Cavalcante, D. C., Veloso, T. C., & Benvenuti, T. (2025). Alternative Leaching Agents for Selective Recovery of Gold and Copper from Computer Waste Printed Circuit Boards. Sustainability, 17(9), 3886. https://doi.org/10.3390/su17093886







