Using Cellulose Nanofibril from Sugarcane Bagasse as an Eco-Friendly Ductile Reinforcement in Starch Films for Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
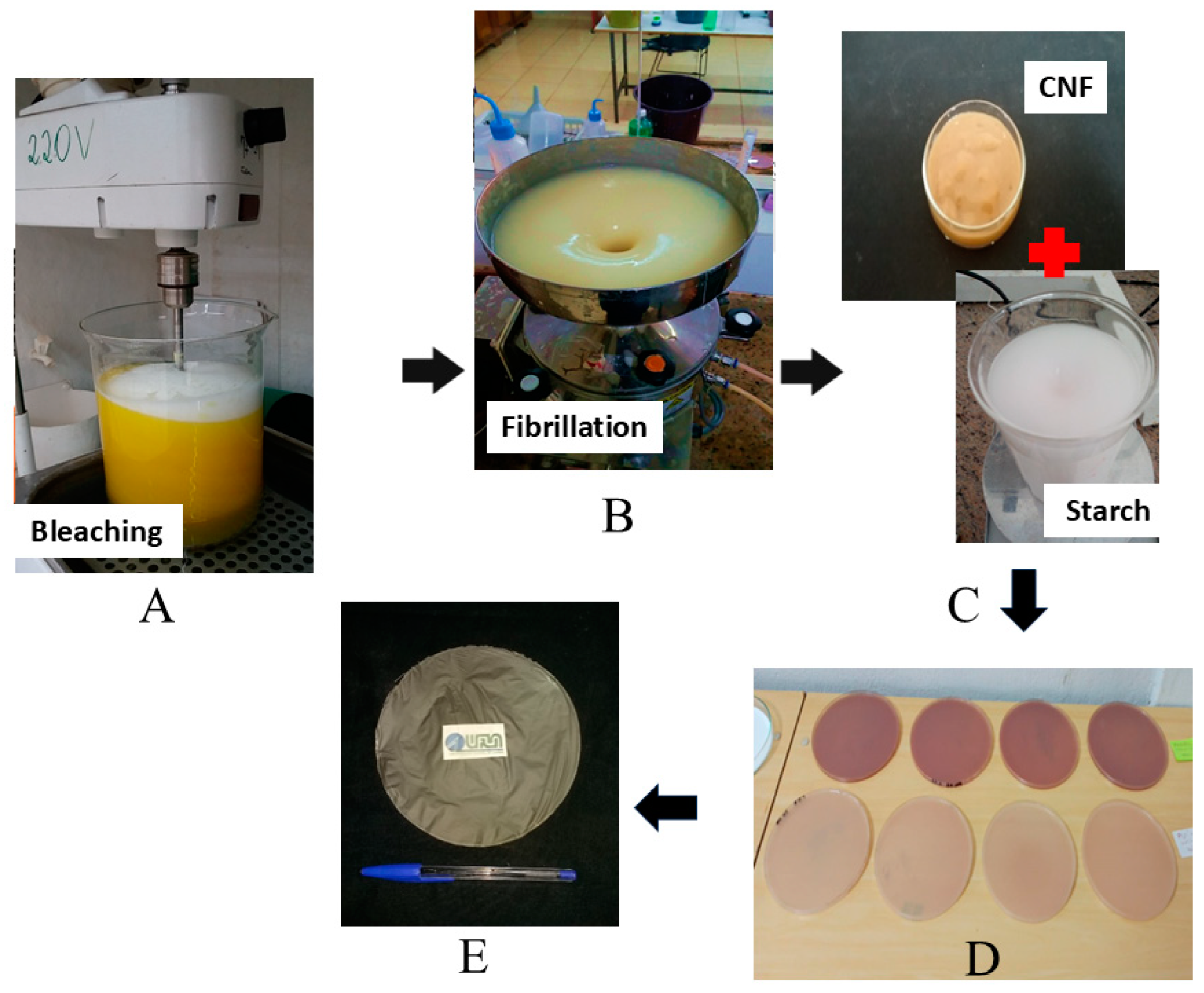
2.2. Alkaline and Bleaching Treatments
2.3. Mechanical Fibrillation
2.4. Film Preparation
2.5. Chemical Characterization of Sugarcane Bagasse
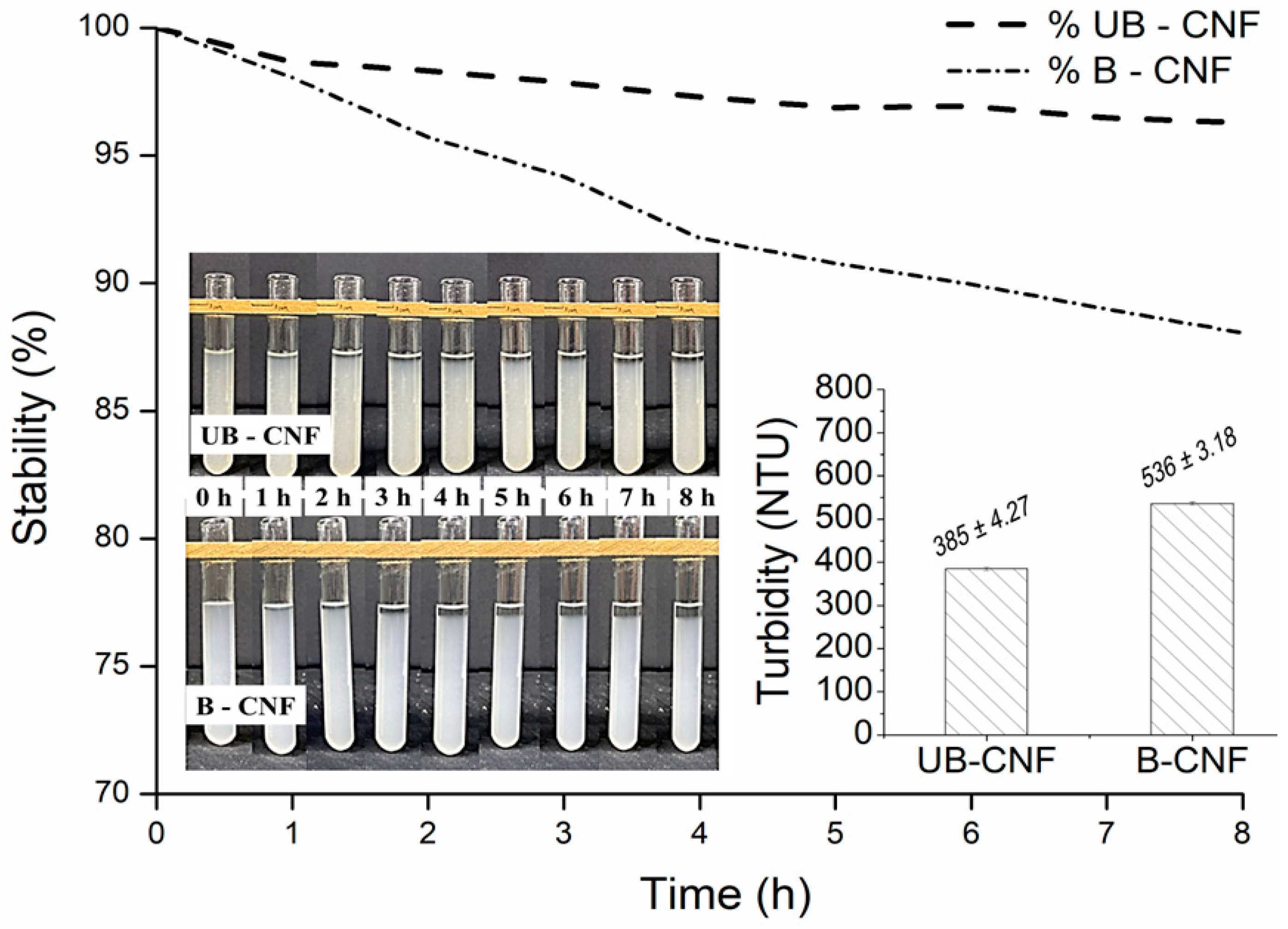
2.6. Turbidity and Stability of Suspensions
2.7. Water Vapor Transmission Rate (WVTR) and Water Vapor Permeability (WVP)
2.8. Contact Angle and Wettability
2.9. Mechanical Properties
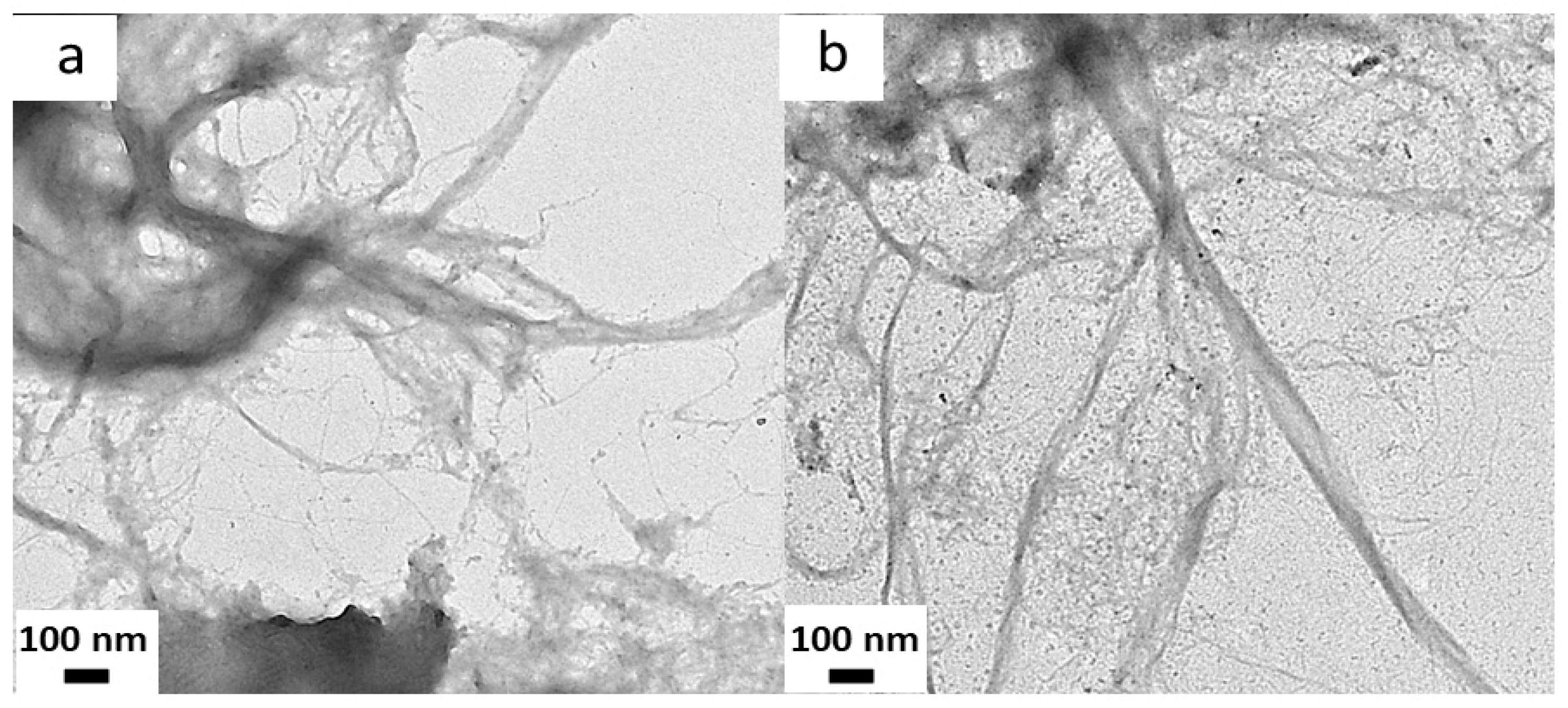
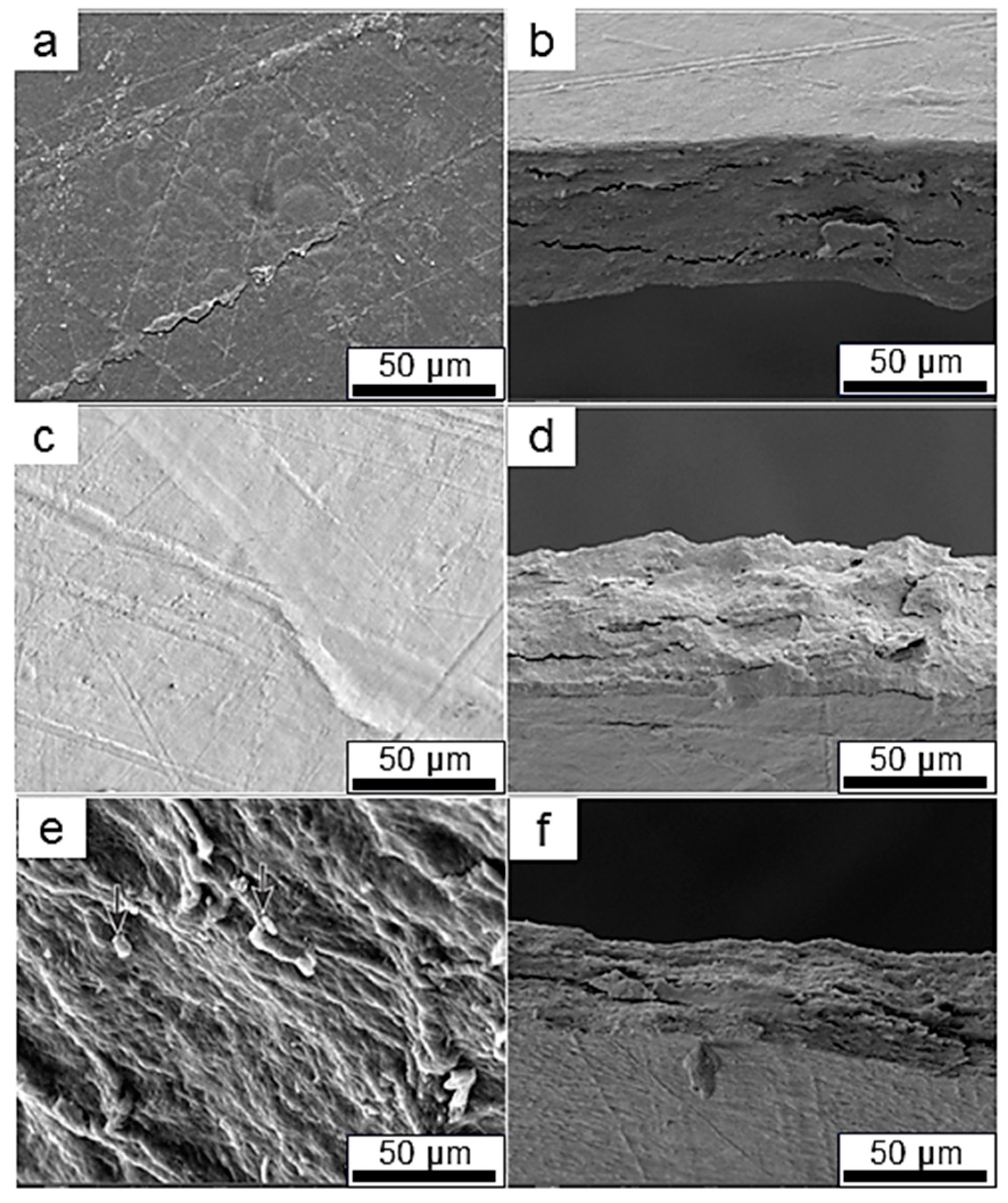
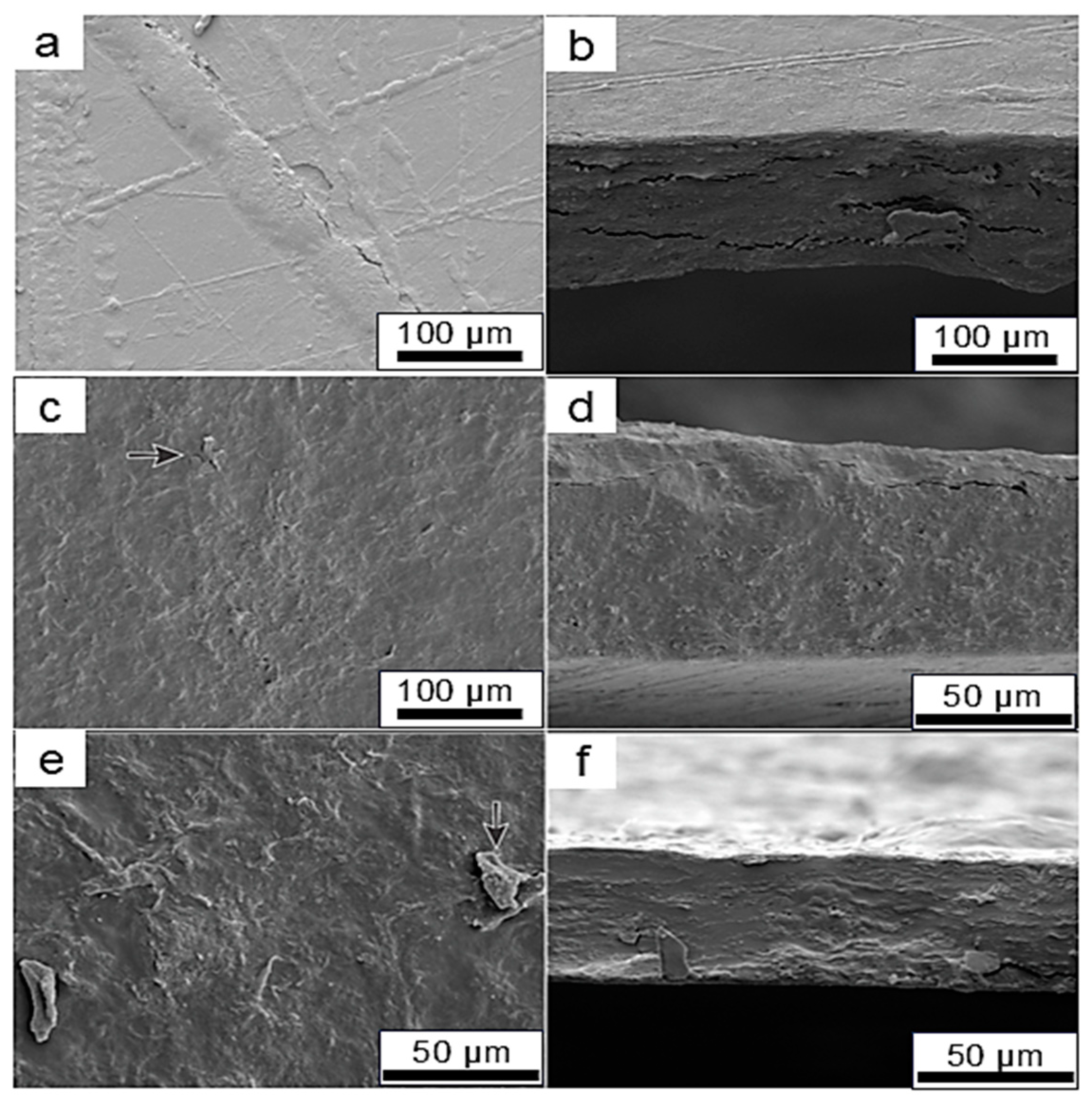
2.10. Microstructural Analysis of CNFs and Films
3. Results and Discussion
3.1. Chemical Characterization of Sugarcane Bagasse
3.2. Stability and Turbidity of Suspensions
3.3. Barrier Properties and Wettability
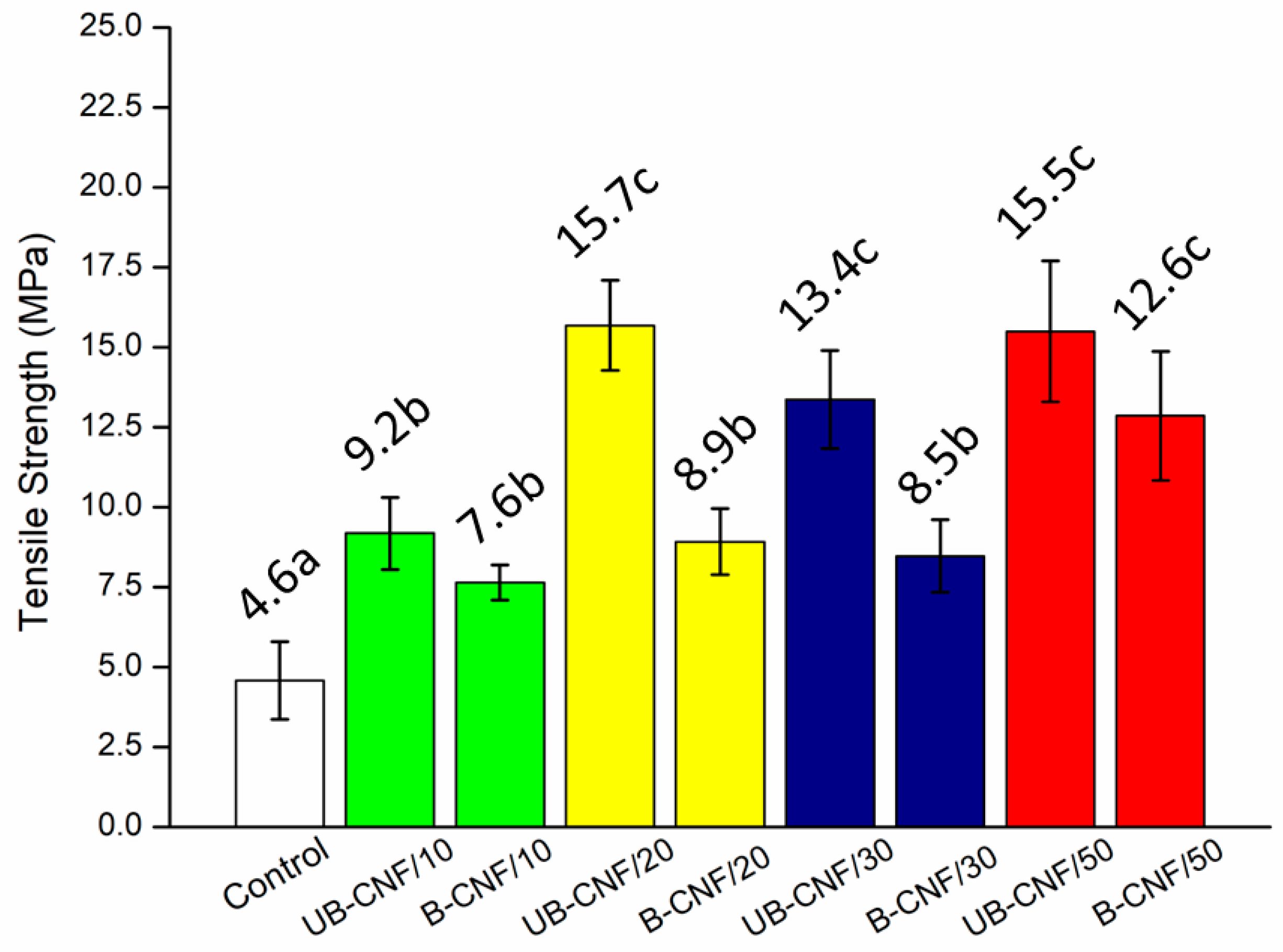
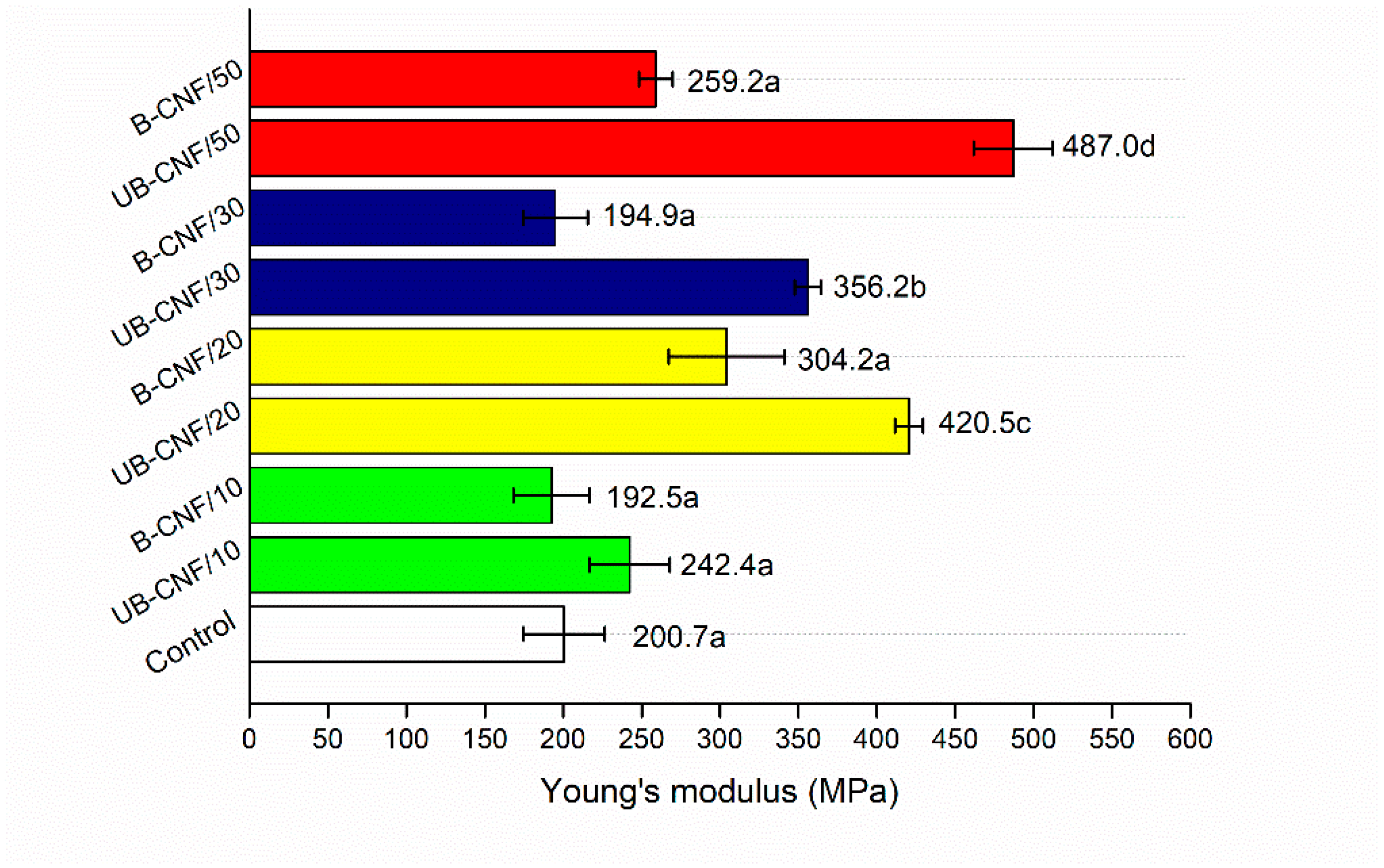
3.4. The Mechanical Properties of the Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. An overview of plastic waste generation and management in food packaging industries. Recycling 2021, 6, 12. [Google Scholar] [CrossRef]
- Bian, H.; Chen, L.; Dong, M.; Wang, L.; Wang, R.; Zhou, X.; Dai, H. Natural lignocellulosic nanofibril film with excellent ultraviolet blocking performance and robust environment resistance. Int. J. Biol. Macromol. 2021, 166, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Song, B.; Zeng, G.; Zhang, Y.; Huang, W.; Wen, X.; Tang, W. Are biodegradable plastics a promising solution to solve the global plastic pollution? Environ. Pollut. 2020, 263, 114469. [Google Scholar] [CrossRef] [PubMed]
- Kirchherr, J.; Reike, D.; Hekkert, M. Conceptualizing the circular economy: An analysis of 114 definitions. Resour. Conserv. Recycl. 2017, 127, 221–232. [Google Scholar] [CrossRef]
- Acquavia, M.A.; Pascale, R.; Martelli, G.; Bondoni, M.; Bianco, G. Natural Polymeric Materials: A Solution to Plastic Pollution from the Agro-Food Sector. Polymers 2021, 13, 158. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Ashfaq, J.; Sohail, Z.; Channa, I.A.; Sánchez-Ferrer, A.; Ali, S.N.; Chandio, A.D. Lignocellulosic bioplastics in sustainable packaging–Recent developments in materials design and processing: A comprehensive review. Sustain. Mat. Technol. 2024, 41, e01077. [Google Scholar] [CrossRef]
- Mascarenhas, A.R.P.; Setter, C.; Scatolino, M.V.; do Lago, R.C.D.; Batista, F.G.; Medeiros, D.T.D.; Tonoli, G.H.D. Valorization of Coffea arabica Wood Waste to Obtain Suspensions of Lignocellulose Microfibrils and Lignocellulose Nanofibrils (LCMF/LCNF) and Production of Eco-Friendly Films for Packaging. Forests 2024, 15, 1834. [Google Scholar] [CrossRef]
- Martins, C.C.N.; Mendonça, M.C.; Mascarenhas, A.R.P.; Moulin, J.C.; Mulin, L.B.; Bufalino, L.; Tonoli, G.H.D. Papers coated with crosslinked natural rubber latex and phosphorylated cellulose microfibrils for industrial packaging applications. Ind. Crops Prod. 2024, 218, 118917. [Google Scholar] [CrossRef]
- Farooq, M.; Zou, T.; Riviere, G.; Sipponen, M.H.; Osterberg, M. Strong, Ductile, and Waterproof Cellulose Nanofibril Composite Films with Colloidal Lignin Particles. Biomacromolecules 2018, 20, 693–704. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Ragauskas, A. Lignin as a UV Light blocker-a review. Polymers 2020, 12, 1134. [Google Scholar] [CrossRef]
- Bascón-Villegas, I.; Pereira, M.; Espinosa, E.; Sánchez-Gutiérrez, M.; Rodríguez, A.; Pérez-Rodríguez, F. A new eco-friendly packaging system incorporating lignocellulose nanofibres from agri-food residues applied to fresh-cut lettuce. J. Clean. Prod. 2022, 372, 133597. [Google Scholar] [CrossRef]
- Pascoli, D.U.; Dichiara, A.; Roumeli, E.; Gustafson, R.; Bura, R. Lignocellulosic nanomaterials production from wheat straw via peracetic acid pretreatment and their application in plastic composites. Carbohydr. Polym. 2022, 295, 119857. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, M.; Dias, M.C.; Martins, C.C.N.; Durães, A.F.S.; Damásio, R.A.P.; Tonoli, G.H.D. Alkaline pretreatment facilitate mechanical fibrillation of unbleached cellulose pulps for obtaining of cellulose micro/nanofibrils (MFC). J. Nat. Fibers 2022, 19, 13385–13400. [Google Scholar] [CrossRef]
- Gilfillan, W.N.; Moghaddam, L.; Doherty, W.O.S. Preparation and characterization of composites from starch with sugarcane bagasse nanofibres. Cellulose 2014, 21, 2695–2712. [Google Scholar] [CrossRef]
- do Lago, R.C.; de Oliveira, A.L.M.; dos Santos, A.D.A.; Zitha, E.Z.M.; Carvalho, E.E.N.; Tonoli, G.H.D.; Boas, E.V.D.B.V. Addition of wheat straw nanofibrils to improve the mechanical and barrier properties of cassava starch–based bionanocomposites. Ind. Crop. Prod. 2021, 170, 113816. [Google Scholar] [CrossRef]
- TAPPI Technical Association of the Pulp and Paper Industry. T204 cm-17, Solvent Extractives of Wood and Pulp; TAPPI Technical Association of the Pulp and Paper Industry: Atlanta, GA, USA, 2017. [Google Scholar]
- TAPPI Technical Association of the Pulp and Paper Industry. T222 om-02, Acid Insoluble Lignin in Wood and Pulp; TAPPI Technical Association of the Pulp and Paper Industry: Atlanta, GA, USA, 2002. [Google Scholar]
- TAPPI Technical Association of the Pulp and Paper Industry. T 211 om-12, Ash in Wood, Pulp, Paper, and Paperboard: Combustion at 525 °C; TAPPI Technical Association of the Pulp and Paper Industry: Atlanta, GA, USA, 2012. [Google Scholar]
- Browning, B.L. The Chemistry of Wood; Interscience: Warrenville, IL, USA, 1963; p. 689. [Google Scholar]
- Kennedy, F.; Phillips, G.O.; Williams, E.P.A. Wood and Cellulosic: Industrial Utilization, Biotechnology, Structure and Properties; Halsted: New York, NY, USA, 1987; p. 1130. [Google Scholar]
- Desmaisons, J.; Boutonnet, E.; Rueff, M.; Dufresne, A.; Bras, J. A new quality index for benchmarking of different cellulose nanofibrils. Carbohydr. Polym. 2017, 174, 318–329. [Google Scholar] [CrossRef]
- Guimarães Jr, M.; Botaro, V.R.; Novack, K.M.; Flauzino Neto, W.P.; Mendes, L.M.; Tonoli, G.H. Preparation of cellulose nanofibrils from bamboo pulp by mechanical defibrillation for their applications in biodegradable composites. J. Nanosci. Nanotechnol. 2015, 15, 6751–6768. [Google Scholar] [CrossRef]
- E96M-16; Standard Test Methods for Water Vapor Transmission of Materials. American Society for Testing and Materials: West Conshohocken, PA, USA, 2016; Volume 14, pp. 1–10.
- D724–99 (Reapproved 2003); Standard Test Method for Surface Wettability of Paper (Angle-of-Contact Method). American Society for Testing and Materials: West Conshohocken, PA, USA, 2003; Volume 14, pp. 1–10.
- D 882-02; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. American Society for Testing and Materials: West Conshohocken, PA, USA, 2002; Volume 14, pp. 1–10.
- Liu, X.; Li, Y.; Ewulonu, C.M.; Ralph, J.; Xu, F.; Zhang, Q.; Huang, Y. Mild Alkaline Pretreatment for Isolation of Native-Like Lignin and Lignin-Containing Cellulose Nanofibers (LCNF) from Crop Waste. ACS Sust. Chem. Eng. 2019, 7, 14135–14142. [Google Scholar] [CrossRef]
- Zhang, L.; Tsuzuki, T.; Wang, X. Preparation of cellulose nanofiber from softwood pulp by ball milling. Cellulose 2015, 22, 1729–1741. [Google Scholar] [CrossRef]
- Asem, M.; Jimat, D.N.; Jafri, N.H.S.; Nawawi, W.M.F.W.; Azmin, N.F.M.; Abd Wahab, M.F. Entangled cellulose nanofibers produced from sugarcane bagasse via alkaline treatment, mild acid hydrolysis assisted with ultrasonication. J. King Saud. Univ.-Eng. Sci. 2023, 35, 24–31. [Google Scholar] [CrossRef]
- Jonoobi, M.J.; Jalaludin Harun, J.H.; Shakeri, A.; Misra, M.; Oksman, K. Chemical composition, crystallinity, and thermal degradation of bleached and unbleached kenaf bast (Hibiscus cannabinus) pulp and nanofibers. BioResources 2009, 4, 626–639. [Google Scholar] [CrossRef]
- Banvillet, G.; Depres, G.; Belgacem, N.; Bras, J. Alkaline treatment combined with enzymatic hydrolysis for efficient cellulose nanofibrils production. Carbohydr. Polym. 2021, 255, 117383. [Google Scholar] [CrossRef]
- Espinosa, E.; Rol, F.; Bras, J.; Rodríguez, A. Use of multi-factorial analysis to determine the quality of cellulose nanofibers: Effect of nanofibrillation treatment and residual lignin content. Cellulose 2020, 27, 10689–10705. [Google Scholar] [CrossRef]
- Solala, I.; Iglesias, M.C.; Peresin, M.S. On the potential of lignin-containing cellulose nanofibrils (LCNFs): A review on properties and applications. Cellulose 2019, 27, 1853–1877. [Google Scholar] [CrossRef]
- Chaker, A.; Alila, S.; Mutjé, P.; Vilar, M.R.; Boufi, S. Key role of the hemicellulose content and the cell morphology on the nanofibrillation effectiveness of cellulose pulps. Cellulose 2013, 20, 2863–2875. [Google Scholar] [CrossRef]
- Menzel, C.; González-Martínez, C.; Vilaplana, F.; Diretto, G.; Chiralt, A. Incorporation of natural antioxidants from rice straw into renewable starch films. Int. J. Biol. Macromol. 2020, 146, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Monga, S.; Bansal, M.; Sharma, A. Bionanocomposites reinforced with cellulose nanofibers derived from sugarcane bagasse. Polym. Comp. 2018, 39, E55–E64. [Google Scholar] [CrossRef]
- Luchese, C.L.; Spada, J.C.; Tessaro, I.C. Starch content affects physicochemical properties of corn and cassava starch-based films. Ind. Crop. Prod. 2017, 109, 619–626. [Google Scholar] [CrossRef]
- Marques, G.S.; de Carvalho, G.R.; Marinho, N.P.; de Muniz, G.I.B.; de Matos Jorge, L.M.; Jorge, R.M.M. Production and characterization of starch-based films reinforced by ramie nanofibers (Boehmeria nivea). J. Appl. Polym. Sci. 2019, 136, 47919. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, T.R. Contact Angle and Wetting Properties. In Surface Science Techniques; Springer Series in Surface Sciences; Bracco, G., Holst, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 51, pp. 3–34. [Google Scholar]
- Li, M.; Tian, X.; Jin, R.; Li, D. Preparation and characterization of nanocomposite films containing starch and cellulose nanofibers. Ind. Crop. Prod. 2018, 123, 654–660. [Google Scholar] [CrossRef]
- Ma, L.; Zhu, Y.; Huang, Y.; Zhang, L.; Wang, Z. Strong water-resistant, UV-blocking cellulose/glucomannan/lignin composite films inspired by natural LCC bonds. Carbohydr. Polym. 2022, 281, 119083. [Google Scholar] [CrossRef] [PubMed]
- Białopiotrowicz, T. Wettability of starch gel films. Food Hydrocol. 2003, 17, 141–147. [Google Scholar] [CrossRef]
- Spence, K.L.; Venditti, R.A.; Habibi, Y.; Rojas, O.J.; Pawlak, J.J. The effect of chemical composition on microfibrillar cellulose films from wood pulps: Mechanical processing and physical properties. Bioresour. Technol. 2010, 101, 5961–5968. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Tanpichai, S.; Biswas, S.K.; Witayakran, S.; Yano, H. Water hyacinth: A sustainable lignin-poor cellulose source for the production of cellulose nanofibers. ACS Sustain. Chem. Eng. 2019, 7, 18884–18893. [Google Scholar] [CrossRef]
- de Souza Fonseca, A.; Panthapulakkal, S.; Konar, S.K.; Sain, M.; Bufalino, L.; Raabe, J.; Tonoli, G.H.D. Improving cellulose nanofibrillation of non-wood fiber using alkaline and bleaching pre-treatments. Ind. Crop. Prod. 2019, 131, 203–212. [Google Scholar] [CrossRef]
- Zhang, M.; Jeong, S.; Cho, W.; Ryu, J.; Zhang, B.; Crovella, P.; Yoo, C.G. Green co-solvent-assisted one-pot synthesis of high-performance flexible lignin polyurethane foam. Chem. Eng. J. 2024, 499, 156142. [Google Scholar] [CrossRef]
- Österberg, M.; Sipponen, M.H.; Mattos, B.D.; Rojas, O.J. Spherical lignin particles: A review on their sustainability and applications. Green. Chem. 2020, 22, 2712–2733. [Google Scholar] [CrossRef]
- Zhang, C.W.; Nair, S.S.; Chen, H.; Yan, N.; Farnood, R.; Li, F.Y. Thermally stable, enhanced water barrier, high strength starch bio-composite reinforced with lignin containing cellulose nanofibrils. Carbohydr. Polym. 2020, 230, 115626. [Google Scholar] [CrossRef]
- Amini, E.; Hafez, I.; Tajvidi, M.; Bousfield, D.W. Cellulose and lignocellulose nanofibril suspensions and films: A comparison. Carbohydr. Polym. 2020, 250, 117011. [Google Scholar] [CrossRef]
- Shapi’i, R.A.; Othman, S.H. Effect of concentration of chitosan on the mechanical, morphological and optical properties of tapioca starch film. Int. Food Res. J. 2016, 23, 187–193. [Google Scholar]
- Maniglia, B.C.; Tessaro, L.; Ramos, A.P.; Tapia-Blácido, D.R. Which plasticizer is suitable for films based on babassu starch isolated by different methods? Food Hydrocoll. 2019, 89, 143–152. [Google Scholar] [CrossRef]


| CNF (% m/m) * | Starch (% m/m) |
|---|---|
| Control ** | 100 |
| B-CNF/10 | 90 |
| B-CNF/20 | 80 |
| B-CNF/30 | 70 |
| B-CNF/50 | 50 |
| UB-CNF/10 | 90 |
| UB-CNF/20 | 80 |
| UB-CNF/30 | 70 |
| UB-CNF/50 | 50 |
| Initial Material | Total Extractives | Ashes | Insoluble Lignin | Hemicelluloses | Cellulose |
|---|---|---|---|---|---|
| ------------------------------------------%-------------------------------------------- | |||||
| Sugarcane bagasse pulp (in natura) | 7.76 ± 0.04 * | 1.66 ± 0.07 | 3.21 ± 0.45 | 36.28 ± 0.10 | 61.46 ± 0.17 |
| Unbleached pulp | 4.93 ± 0.12 | 1.32 ± 0.08 | 1.27 ± 0.31 | 15.23 ± 0.06 | 80.48 ± 0.28 |
| Bleached pulp | 3.66 ± 0.42 | 1.41 ± 0.07 | 0.83 ± 0.38 | 7.67 ± 0.37 | 90.73 ± 1.33 |
| WVP (g mm/kPa−1 day−1 m2) | Contact Angle | Wettability (°s−1) | |
|---|---|---|---|
| Control * | 0.352 ± 0.07 **c | 82.75 ± 2.06 b | 0.11 ± 0.02 a |
| UB-CNF/10 | 3.085 ± 0.81 a | 91.16 ± 4.09 a | 0.10 ± 0.03 a |
| B-CNF/10 | 3.212 ± 0.31 a | 93.85 ± 3.24 a | 0.05 ± 0.02 b |
| UB-CNF/20 | 2.788 ± 0.29 a | 92.36 ± 2.23 a | 0.09 ± 0.07 a |
| B-CNF/20 | 2.621 ± 0.07 a | 96.42 ± 7.18 a | 0.07 ± 0.005 b |
| UB-CNF/30 | 2.862 ± 0.63 a | 86.16 ± 0.10 b | 0.07 ± 0.02 a |
| B-CNF/30 | 2.467 ± 0.21 b | 97.28 ± 5.10 a | 0.04 ± 0.005 b |
| UB-CNF/50 | 2.050 ± 0.18 b | 83.68 ± 4.08 b | 0.06 ± 0.002 b |
| B-CNF/50 | 2.178 ± 0.08 b | 93.08 ± 2.08 a | 0.02 ± 0.002 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, T.S.M.; Martins, C.C.N.; Scatolino, M.V.; Dias, M.C.; Mascarenhas, A.R.P.; Ferreira, C.B.; Bianchi, M.L.; Tonoli, G.H.D. Using Cellulose Nanofibril from Sugarcane Bagasse as an Eco-Friendly Ductile Reinforcement in Starch Films for Packaging. Sustainability 2025, 17, 4128. https://doi.org/10.3390/su17094128
Ribeiro TSM, Martins CCN, Scatolino MV, Dias MC, Mascarenhas ARP, Ferreira CB, Bianchi ML, Tonoli GHD. Using Cellulose Nanofibril from Sugarcane Bagasse as an Eco-Friendly Ductile Reinforcement in Starch Films for Packaging. Sustainability. 2025; 17(9):4128. https://doi.org/10.3390/su17094128
Chicago/Turabian StyleRibeiro, Thayrine Silva Matos, Caio Cesar Nemer Martins, Mário Vanoli Scatolino, Matheus Cordazzo Dias, Adriano Reis Prazeres Mascarenhas, Cecilia Baldoino Ferreira, Maria Lucia Bianchi, and Gustavo Henrique Denzin Tonoli. 2025. "Using Cellulose Nanofibril from Sugarcane Bagasse as an Eco-Friendly Ductile Reinforcement in Starch Films for Packaging" Sustainability 17, no. 9: 4128. https://doi.org/10.3390/su17094128
APA StyleRibeiro, T. S. M., Martins, C. C. N., Scatolino, M. V., Dias, M. C., Mascarenhas, A. R. P., Ferreira, C. B., Bianchi, M. L., & Tonoli, G. H. D. (2025). Using Cellulose Nanofibril from Sugarcane Bagasse as an Eco-Friendly Ductile Reinforcement in Starch Films for Packaging. Sustainability, 17(9), 4128. https://doi.org/10.3390/su17094128







