Exploring New Applications of Municipal Solid Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analysis of MSW
2.3. MSW Composites and Testing
3. Results
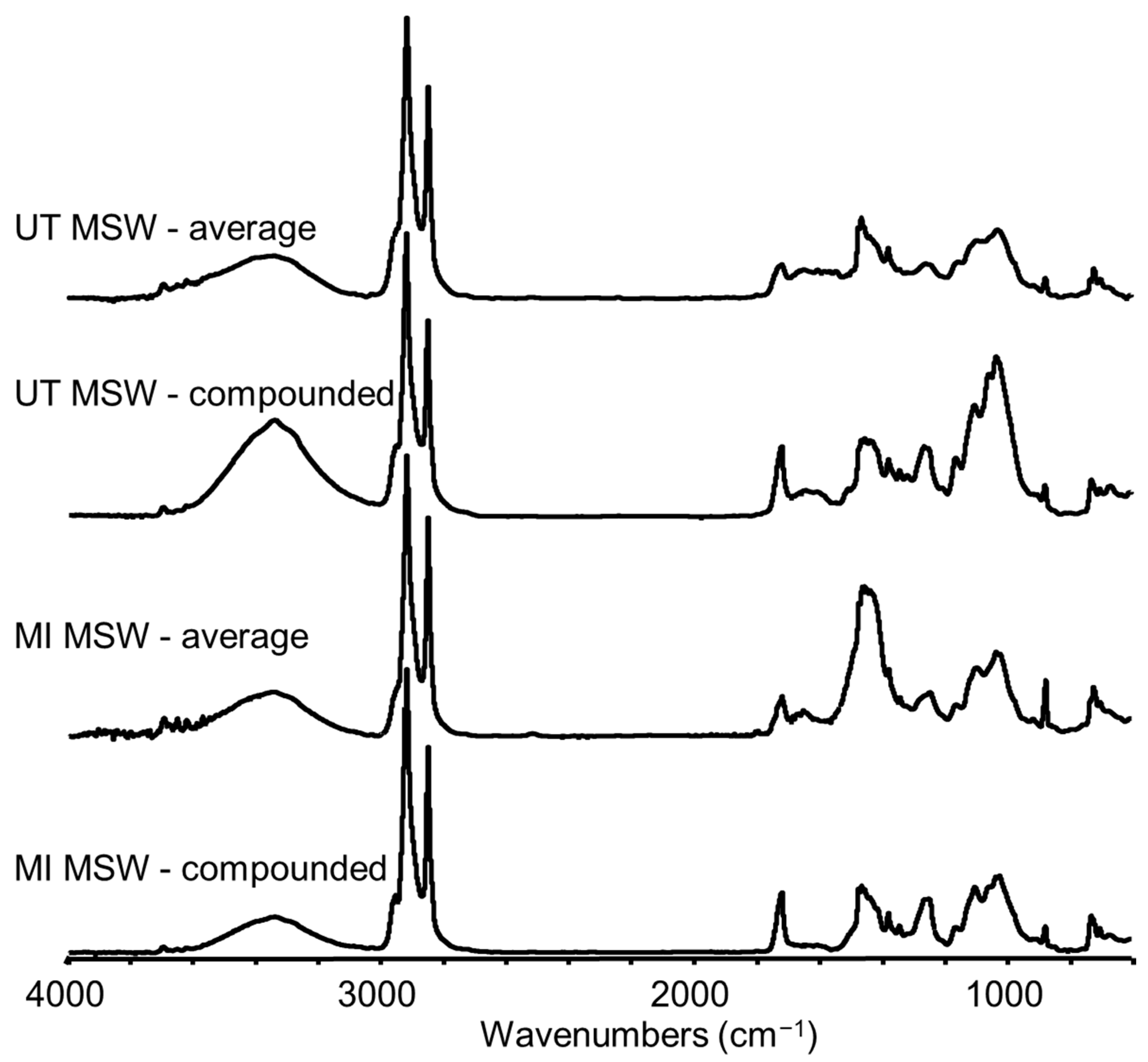
3.1. Characterization of MSW
3.2. Compounding of MSW
3.3. Characterization of Extruded MSW Composites
4. Technical Challenges and Future Direction of MSW Valorization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASTM | American Society of Standards and Testing |
| HDPE | High-density polyethylene |
| LDPE | Low-density polyethylene |
| GCMS | Gas chromatography–mass spectrometry |
| HPLC | High-pressure liquid chromatography |
| FTIR | Fourier-transform infrared spectroscopy |
| MPa | Mega Pascal |
| GPa | Giga Pascal |
| PET | Poly (ethylene terephthalate) |
| PBD | Polybutadiene |
| XRD | X-ray diffraction |
| MSW | Municipal solid waste |
| LAB | Lignin acetyl-bromide |
| PS | Polystyrene |
| PE | Polyethylene |
| MC | Moisture content |
| VM | Volatile matter |
| FC | Fixed carbon |
| MI | Michigan |
| UT | Utah |
| KL | Klasson lignin |
| HI | Hydroxyl index |
| CI | Carbonyl index |
| Tm | Melt temperature |
| Tg | Glass transition |
| Xc | Degree of crystallinity |
| PP | Polypropylene |
References
- Bahukhandi, K.D.; Ollemman, S. A Review of Municipal Solid Waste: Its Generation, Composition, Impacts, Management and Challenges in Urban Areas with Special Focus on India. In Springer Proceedings in Earth and Environmental Sciences; Springer: Berlin/Heidelberg, Germany, 2022; pp. 273–307. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. Solid waste issue: Sources, composition, disposal, recycling, and valorization. Egypt. J. Pet. 2018, 27, 1275–1290. [Google Scholar] [CrossRef]
- Kibria, M.G.; Masuk, N.I.; Safayet, R.; Nguyen, H.Q.; Mourshed, M. Plastic Waste: Challenges and Opportunities to Mitigate Pollution and Effective Management. Int. J. Environ. Res. 2023, 17, 20. [Google Scholar] [CrossRef]
- Abubakar, I.R.; Maniruzzaman, K.M.; Dano, U.L.; AlShihri, F.S.; AlShammari, M.S.; Ahmed, S.M.S.; Al-Gehlani, W.A.G.; Alrawaf, T.I. Environmental Sustainability Impacts of Solid Waste Management Practices in the Global South. Int. J. Environ. Res. Public Health 2022, 19, 12717. [Google Scholar] [CrossRef]
- Yang, M.; Chen, L.; Wang, J.; Msigwa, G.; Osman, A.I.; Fawzy, S.; Rooney, D.W.; Yap, P.-S. Circular economy strategies for combating climate change and other environmental issues. Environ. Chem. Lett. 2022, 21, 55–80. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.; Chen, Y.; Zheng, G.; Chen, Y. Source separation, transportation, pretreatment, and valorization of municipal solid waste: A critical review. Environ. Dev. Sustain. 2021, 24, 11471–11513. [Google Scholar] [CrossRef]
- Szpilko, D.; Gallegos, A.d.l.T.; Naharro, F.J.; Rzepka, A.; Remiszewska, A. Waste Management in the Smart City: Current Practices and Future Directions. Resources 2023, 12, 115. [Google Scholar] [CrossRef]
- Lange, J.P. Managing Plastic Waste-Sorting, Recycling, Disposal, and Product Redesign. ACS Sustain. Chem. Eng. 2021, 9, 15722–15738. [Google Scholar] [CrossRef]
- Durak, H. Comprehensive Assessment of Thermochemical Processes for Sustainable Waste Management and Resource Recovery. Processes 2023, 11, 2092. [Google Scholar] [CrossRef]
- Khan, S.; Anjum, R.; Raza, S.T.; Bazai, N.A.; Ihtisham, M. Technologies for municipal solid waste management: Current status, challenges, and future perspectives. Chemosphere 2022, 288, 132403. [Google Scholar] [CrossRef]
- Liu, J.; Li, Q.; Gu, W.; Wang, C. The Impact of Consumption Patterns on the Generation of Municipal Solid Waste in China: Evidences from Provincial Data. Int. J. Environ. Res. Public Health 2019, 16, 1717. [Google Scholar] [CrossRef]
- Fontaine, L.; Legros, R.; Frayret, J.M. Solid waste generation prediction model framework using socioeconomic and demographic factors with real-time MSW collection data. Waste Manag. Res. 2024, 43, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Ayilara, M.S.; Olanrewaju, O.S.; Babalola, O.O.; Odeyemi, O. Waste Management through Composting: Challenges and Potentials. Sustainability 2020, 12, 4456. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Kumar, R.; Kumar, A. Environmental waste management strategies and vermi transformation for sustainable development. Environ. Chall. 2023, 13, 100747. [Google Scholar] [CrossRef]
- Maitlo, G.; Ali, I.; Maitlo, H.A.; Ali, S.; Unar, I.N.; Ahmad, M.B.; Bhutto, D.K.; Karmani, R.K.; Naich, S.U.R.; Sajjad, R.U.; et al. Plastic Waste Recycling, Applications, and Future Prospects for a Sustainable Environment. Sustainability 2022, 14, 11637. [Google Scholar] [CrossRef]
- Pilapitiya, P.G.C.N.T.; Ratnayake, A.S. The world of plastic waste: A review. Clean. Mater. 2024, 11, 100220. [Google Scholar] [CrossRef]
- Fan, Q.; Duan, H.; Xing, X. A review of composite materials for enhancing support, flexibility and strength in exercise. Alex. Eng. J. 2024, 94, 90–103. [Google Scholar] [CrossRef]
- Popescu, C.M. Wood as bio-based building material. Perform. Bio-Based Build. Mater. 2017, 21–96. [Google Scholar] [CrossRef]
- Wang, X.; Sotoudehniakarani, F.; Yu, Z.; Morrell, J.J.; Cappellazzi, J.; McDonald, A.G. Evaluation of corrugated cardboard biochar as reinforcing fiber on properties, biodegradability and weatherability of wood-plastic composites. Polym. Degrad. Stab. 2019, 168, 108955. [Google Scholar] [CrossRef]
- Xu, Z.; Kolapkar, S.S.; Zinchik, S.; Bar-Ziv, E.; Ewurum, L.; McDonald, A.G.; Klinger, J.; Fillerup, E.; Schaller, K.; Pilgrim, C. Bypassing Energy Barriers in Fiber-Polymer Torrefaction. Front. Energy Res. 2021, 9, 643371. [Google Scholar] [CrossRef]
- Saleem, J.; Moghal, Z.K.B.; Sun, L.; McKay, G. Valorization of mixed plastics waste for the synthesis of flexible superhydrophobic films. Adv. Compos. Hybrid. Mater. 2024, 7, 1–20. [Google Scholar] [CrossRef]
- Yaqoob, L.; Noor, T.; Iqbal, N. Conversion of Plastic Waste to Carbon-Based Compounds and Application in Energy Storage Devices. ACS Omega 2022, 7, 13403. [Google Scholar] [CrossRef]
- Saha, N.; Klinger, J.; Islam, M.T.; Reza, T. Advanced biorefinery feedstock from non-recyclable municipal solid waste by mechanical preprocessing. Front. Fuels 2023, 1, 1105637. [Google Scholar] [CrossRef]
- ASTM D1102-84; Standard Test Method for Ash in Wood. Book of Standards Volume: 04.10. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM E872-24; Standard Test Method for Volatile Matter in the Analysis of Particulate Wood Fuels. Book of Standards Volume: 05.06. ASTM International: West Conshohocken, PA, USA, 2024.
- ASTM 5865; Test Method for Gross Calorific Value of Coal and Coke. Book of Standards Volume: 05.06. ASTM International: West Conshohocken, PA, USA, 2019.
- Zinchik, S.; Xu, Z.; Kolapkar, S.S.; Bar-Ziv, E.; McDonald, A.G. Properties of pellets of torrefied U.S. waste blends. Waste Manag. 2020, 104, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Database of Raman Spectroscopy. X-Ray Diffraction and Chemistry of Minerals. Available online: https://rruff.info/ (accessed on 16 April 2025).
- ASTM D1108; Standard Test Method for Dichloromethane Solubles in Wood. Book of Standards Volume: 04.10. ASTM International: West Conshohocken, PA, USA, 2021.
- Sotoudehnia, F.; McDonald, A.G. Upgrading Mixed Agricultural Plastic and Lignocellulosic Waste to Liquid Fuels by Catalytic Pyrolysis. Catalysts 2022, 12, 1381. [Google Scholar] [CrossRef]
- ASTM D1106-21; Standard Test Method for Acid-Insoluble Lignin in Wood. Volume: 04.10. ASTM International: West Conshohocken, PA, USA, 2021.
- Schoning, A. Absorptiometric determination of acid-soluble lignin in semichemical bisulfite pulps and in some woods and plants. Sven. Papperstidn.-Nord. Cellul. 1965, 68, 607–613. [Google Scholar]
- ASTM E1758-24; Standard Test Method for Determination of Carbohydrates in Biomass by High Performance Liquid Chromatography. Volume: 05.06. ASTM International: West Conshohocken, PA, USA, 2024.
- Sotoudehnia, F.; Mengistie, E.; Alayat, A.; McDonald, A.G. Valorization of waste waxed corrugated cardboard via pyrolysis for recovering wax. Environ. Prog Sustain. Energy 2021, 40, e13566. [Google Scholar] [CrossRef]
- Green, T.R.; Popa, R. A Simple Assay for Monitoring Cellulose in Paper-Spiked Soil. J. Polym. Environ. 2010, 18, 634–637. [Google Scholar] [CrossRef]
- ASTM D790-17; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. Volume: 08.01. ASTM International: West Conshohocken, PA, USA, 2017.
- Ewurum, L.I.; Jokic, D.; Bar-Ziv, E.; McDonald, A.G. Evaluation of the Rheological and Mechanical Properties of Mixed Plastic Waste-Based Composites. Waste Biomass Valoriz. 2022, 13, 4625–4637. [Google Scholar] [CrossRef]
- Appiah, H.; Dawson-Andoh, B.; Oginni, O.; McDonald, A.G. Evaluation of Deep Eutectic Solvents for Xylan-Based Furfural Synthesis. ACS Sustain. Resour. Manag. 2025, 2, 536–545. [Google Scholar] [CrossRef]
- Blaine, R.L. Thermal Applications Note Polymer Heats of Fusion. Available online: https://www.tainstruments.com/pdf/literature/TN048.pdf (accessed on 17 April 2025).
- ASTM D570-22; Standard Test Method for Water Absorption of Plastics. Volume: 08.01. ASTM International: West Conshohocken, PA, USA, 2022.
- Mengistie, E.; Alayat, A.M.; Sotoudehnia, F.; Bokros, N.; DeBolt, S.; McDonald, A.G. Evaluation of Cell Wall Chemistry of della and Its Mutant Sweet Sorghum Stalks. J. Agric. Food Chem. 2022, 70, 1689–1703. [Google Scholar] [CrossRef]
- Alfè, M.; Gargiulo, V.; Porto, M.; Migliaccio, R.; Le Pera, A.; Sellaro, M.; Pellegrino, C.; Abe, A.A.; Urciuolo, M.; Caputo, P.; et al. Pyrolysis and Gasification of a Real Refuse-Derived Fuel (RDF): The Potential Use of the Products under a Circular Economy Vision. Molecules 2022, 27, 8114. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, E.; Pateraki, C.; Ladakis, D.; Cruz-Fernández, M.; Latorre-Sánchez, M.; Coll, C.; Koutinas, A. Evaluation of organic fractions of municipal solid waste as renewable feedstock for succinic acid production. Biotechnol. Biofuels 2020, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, J.J.; Greetham, D. Optimizing Cellulase Production from Municipal Solid Waste (MSW) using Solid State Fermentation (SSF). J. Fundam. Renew. Energy Appl. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Bekier, J.; Drozd, J.; Jamroz, E.; Jarosz, B.; Kocowicz, A.; Walenczak, K.; Weber, J. Changes in selected hydrophobic components during composting of municipal solid wastes. J. Soils Sediments 2014, 14, 305–311. [Google Scholar] [CrossRef]
- Price, G.A.; Barlaz, M.A.; Hater, G.R. Nitrogen management in bioreactor landfills. Waste Manag. 2003, 23, 675–688. [Google Scholar] [CrossRef]
- Feng, C.; Li, Z.; Wang, Z.; Wang, B.; Wang, Z. Optimizing torque rheometry parameters for assessing the rheological characteristics and extrusion processability of wood plastic composites. J. Thermoplast. Compos. Mater. 2019, 32, 123–140. [Google Scholar] [CrossRef]
- Miller, F.A.; Mayo, D.W.; Hannah, R.W. Course Notes on the Interpretation of Infrared and Raman Spectra. 2003, p. 567. Available online: https://www.wiley.com/en-us/Course+Notes+on+the+Interpretation+of+Infrared+and+Raman+Spectra-p-9780471248231 (accessed on 28 April 2024).
- Pandey, K.K. A Study of Chemical Structure of Soft and Hardwood and Wood Polymers by FTIR Spectroscopy. J. Appl. Polym. Sci. 1999, 71, 2101–2120. [Google Scholar] [CrossRef]
- Kazemi, Y.; Cloutier, A.; Rodrigue, D. Mechanical and morphological properties of wood plastic composites based on municipal plastic waste. Polym. Compos. 2013, 34, 487–493. [Google Scholar] [CrossRef]
- Albor, G.; Mirkouei, A.; McDonald, A.G.; Struhs, E.; Sotoudehnia, F. Fixed Bed Batch Slow Pyrolysis Process for Polystyrene Waste Recycling. Processes 2023, 11, 1126. [Google Scholar] [CrossRef]
- Marcovich, N.E.; Reboredo, M.M.; Kenny, J.; Aranguren, M.I. Rheology of particle suspensions in viscoelastic media. Wood flour-polypropylene melt. Rheol. Acta 2004, 43, 293–303. [Google Scholar] [CrossRef]
- Mazzanti, V.; Malagutti, L.; Santoni, A.; Sbardella, F.; Calzolari, A.; Sarasini, F.; Mollica, F. Correlation between Mechanical Properties and Processing Conditions in Rubber-Toughened Wood Polymer Composites. Polymers 2020, 12, 1170. [Google Scholar] [CrossRef]
- Li, T.Q.; Wolcott, M.P. Rheology of HDPE–wood composites. I. Steady state shear and extensional flow. Compos. Part. A Appl. Sci. Manuf. 2004, 35, 303–311. [Google Scholar] [CrossRef]
- Lv, X.; Hao, X.; Ou, R.; Liu, T.; Guo, C.; Wang, Q.; Yi, X.; Sun, L. Rheological properties of wood–plastic composites by 3d numerical simulations: Different components. Forests 2021, 12, 417. [Google Scholar] [CrossRef]
- Murray, G.; White, C.V.; Weise, W. Introduction to Engineering Materials. In Introduction to Engineering Materials; Taylor & Francis: London, UK, 2007. [Google Scholar] [CrossRef]
- Shah, V. Handbook of Plastics Testing Technology, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1998. [Google Scholar]
- Trex. Trex-Physical-Mechanical-Properties-Transcend-Enhance; Trex: Winchester, VA, USA, 2025; p. 1. [Google Scholar]
- Cai, Z.; Senalik, C.A.; Ross, R. Mechanical properties of wood-based composite materials. In General Technical Report FPL-GTR-282; U.S. Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 2021; Chapter 12; pp. 1–12. Available online: https://research.fs.usda.gov/treesearch/62260 (accessed on 16 April 2025).
- Talcott, S.; Uptmor, B.; McDonald, A.G. Evaluation of the Mechanical, Thermal and Rheological Properties of Hop, Hemp and Wood Fiber Plastic Composites. Materials 2023, 16, 4187. [Google Scholar] [CrossRef]
- Srinivas, G.R. Sisal/Coconut Coir Natural Fibers-Epoxy Composites: Water Absorption and Mechanical Properties. Certif. Int. J. Eng. Innov. Technol. (IJEIT) 2008, 9001, 2277–3754. [Google Scholar]
- Espert, A.; Vilaplana, F.; Karlsson, S. Comparison of water absorption in natural cellulosic fibres from wood and one-year crops in polypropylene composites and its influence on their mechanical properties. Compos. Part. A Appl. Sci. Manuf. 2004, 35, 1267–1276. [Google Scholar] [CrossRef]
- Clemons, C. Wood-plastic composites in the United States: The interfacing of two industries. For. Prod. J. 2002, 52, 10–18. Available online: https://www.fs.usda.gov/research/treesearch/8778 (accessed on 3 June 2024).
- Kihila, J.M.; Wernsted, K.; Kaseva, M. Waste segregation and potential for recycling—A case study in Dar es Salaam City, Tanzania. Sustain. Environ. 2021, 7, 1935532. [Google Scholar] [CrossRef]
- Waste Management and the Circular Economy in Selected OECD Countries. In OECD Environmental Performance Reviews; OECD: Paris, France, 2019. [CrossRef]
- Sharma, A.; Ganguly, R.; Gupta, A.K. Life cycle assessment of municipal solid waste generated from hilly cities in India—A case study. Heliyon 2023, 9, e21575. [Google Scholar] [CrossRef]
- Tsalis, T.; Amarantidou, S.; Calabró, P.; Nikolaou, I.; Komilis, D. Door-to-door recyclables collection programmes: Willingness to participate and influential factors with a case study in the city of Xanthi (Greece). Waste Manag. Res. 2018, 36, 760–766. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Gori, M.; Lubello, C. European trends in greenhouse gases emissions from integrated solid waste management. Environ. Technol. 2015, 36, 2125–2137. [Google Scholar] [CrossRef] [PubMed]
- Boey, J.Y.; Lee, C.K.; Tay, G.S. Factors Affecting Mechanical Properties of Reinforced Bioplastics: A Review. Polymers 2022, 14, 3737. [Google Scholar] [CrossRef] [PubMed]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef]





| UT MSW | MI MSW | |
|---|---|---|
| Fixed carbon (%) | 12.2 ± 1.2 | 9.3 ± 1.2 |
| Volatile matter (%) | 77.2 ± 0.5 | 80.3 ± 0.8 |
| Ash (%) | 10.6 ± 1.2 | 10.4 ± 1.1 |
| Calorific value (kJ/g) | 27.0 ± 0.7 | 29.6 ± 0.2 |
| CH2Cl2 extractives (%) | 8.6 ± 0.5 | 9.0 ± 0.5 |
| Carbohydrates by phenol–sulfuric acid method (%) | 54.6 ± 5.0 | 47.7 ± 3.5 |
| Lignin (LAB) (%) | 30.7 ± 2.7 | 28.8 ± 0.9 |
| KL (%) | 38.2 ± 0.7 | 14.6 ± 0.1 |
| ASL (%) | 0.9 ± 0.05 | 0.5 ± 0.1 |
| Total lignin (%) | 39.1 | 15.1 |
| Glucan (%) | 22.5 ± 0.9 | 34.2 ± 0.6 |
| Xylan (%) | 3.3 ± 0.3 | 7.2 ± 0.9 |
| Galactan (%) | 0.6 ± 0.7 | 0.7 ± 0.1 |
| Mannan (%) | 1.8 ± 0.3 | 4.13 ± 0.3 |
| Total carbohydrate (%) | 28.2 | 46.3 |
| Fatty acid composition | ||
| Myristic acid (µg/g MSW) | 2.7 | 1.8 |
| Palmitic acid (µg/g MSW) | 11.8 | 10.2 |
| Linoleic acid (µg/g MSW) | 3.1 | 66.3 |
| Oleic acid (µg/g MSW) | 39.9 | 3.9 |
| Stearic acid (µg/g MSW) | 55.6 | 5.2 |
| Tm1 | Tm2 | Tm3 | Xc PE in MSW | Xc PP in MSW | Xc PET in MSW | Tg | TC1 | TC2 | |
|---|---|---|---|---|---|---|---|---|---|
| (°C) | (°C) | (°C) | (%) | (%) | (%) | (°C) | (°C) | (°C) | |
| UT | 124.6 | 158.4 | 249.5 | 2.3 | 1.0 | 0.43 | 89.3 | 214.6 | 111.5 |
| MI | 132.7 | 160.8 | 247.8 | 10.8 | 2.7 | 1.0 | 94.1 | 209.1 | 115.3 |
| Formulation | η* (Pa.s) | Power Law Fit Model | |||
|---|---|---|---|---|---|
| 1 Hz | Equation | K (Pa.s) | n | R2 | |
| UT-MSW | 27,800 | y = 30971x−0.885 | 30,971 | 0.115 | 0.992 |
| MI-MSW | 42,580 | y = 45070x−0.758 | 45,070 | 0.242 | 0.976 |
| HDPE | 4465 | y = 4247x−0.542 | 4247 | 0.458 | 0.979 |
| Tonset1 (°C) | Tonset2 (°C) | Tonset3 (°C) | Tonset4 (°C) | Tonset5 (°C) | DTGm1 (°C) | DTGm2 (°C) | DTGm3 (°C) | DTGm4 (°C) | DTGm5 (°C) | Residual (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| UT | 48 | 291 | 484 | 590 | 688 | 71 | 361 | 510 | 625 | 733 | 20.8 |
| MI | 48 | 318 | 481 | 601 | 689 | 73 | 365 | 513 | 626 | 726 | 17.3 |
| Cellulose | 360 | 385 | |||||||||
| PET | 440 | 470 | |||||||||
| Nylon 66 | 443 | 473 | |||||||||
| PP | 469 | 498 | |||||||||
| HDPE | 499 | 523 | |||||||||
| Silicone | 473 | 679 | 560 | 727 | |||||||
| CaCO3 | 716 | 778 |
| Flexural Strength (MPa) | Flexural Modulus (GPa) | |
|---|---|---|
| MI—Compression-molded | 18.3 ± 0.3 | 0.200 ± 0.001 |
| UT—Compression-molded | 10.7 ± 1.3 | 0.140 ± 0.017 |
| MI—Extruded | 29.9 ± 0.9 | 0.814 ± 0.015 |
| UT—Extruded | 23.8 ± 0.1 | 1.01 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Appiah, H.; Bar-Ziv, E.; Klinger, J.L.; McDonald, A.G. Exploring New Applications of Municipal Solid Waste. Sustainability 2025, 17, 3719. https://doi.org/10.3390/su17083719
Appiah H, Bar-Ziv E, Klinger JL, McDonald AG. Exploring New Applications of Municipal Solid Waste. Sustainability. 2025; 17(8):3719. https://doi.org/10.3390/su17083719
Chicago/Turabian StyleAppiah, Harrison, Ezra Bar-Ziv, Jordan L. Klinger, and Armando G. McDonald. 2025. "Exploring New Applications of Municipal Solid Waste" Sustainability 17, no. 8: 3719. https://doi.org/10.3390/su17083719
APA StyleAppiah, H., Bar-Ziv, E., Klinger, J. L., & McDonald, A. G. (2025). Exploring New Applications of Municipal Solid Waste. Sustainability, 17(8), 3719. https://doi.org/10.3390/su17083719







