Environmental Factors Influence Lichen Colonization and the Biodeterioration of Brick Carvings on Roof Ridges of Historic Buildings in Luoyang, China
Abstract
1. Introduction
2. Materials and Methods
2.1. The Research Area and Its Natural Environment
2.2. Visual Evaluation and Sample Collection
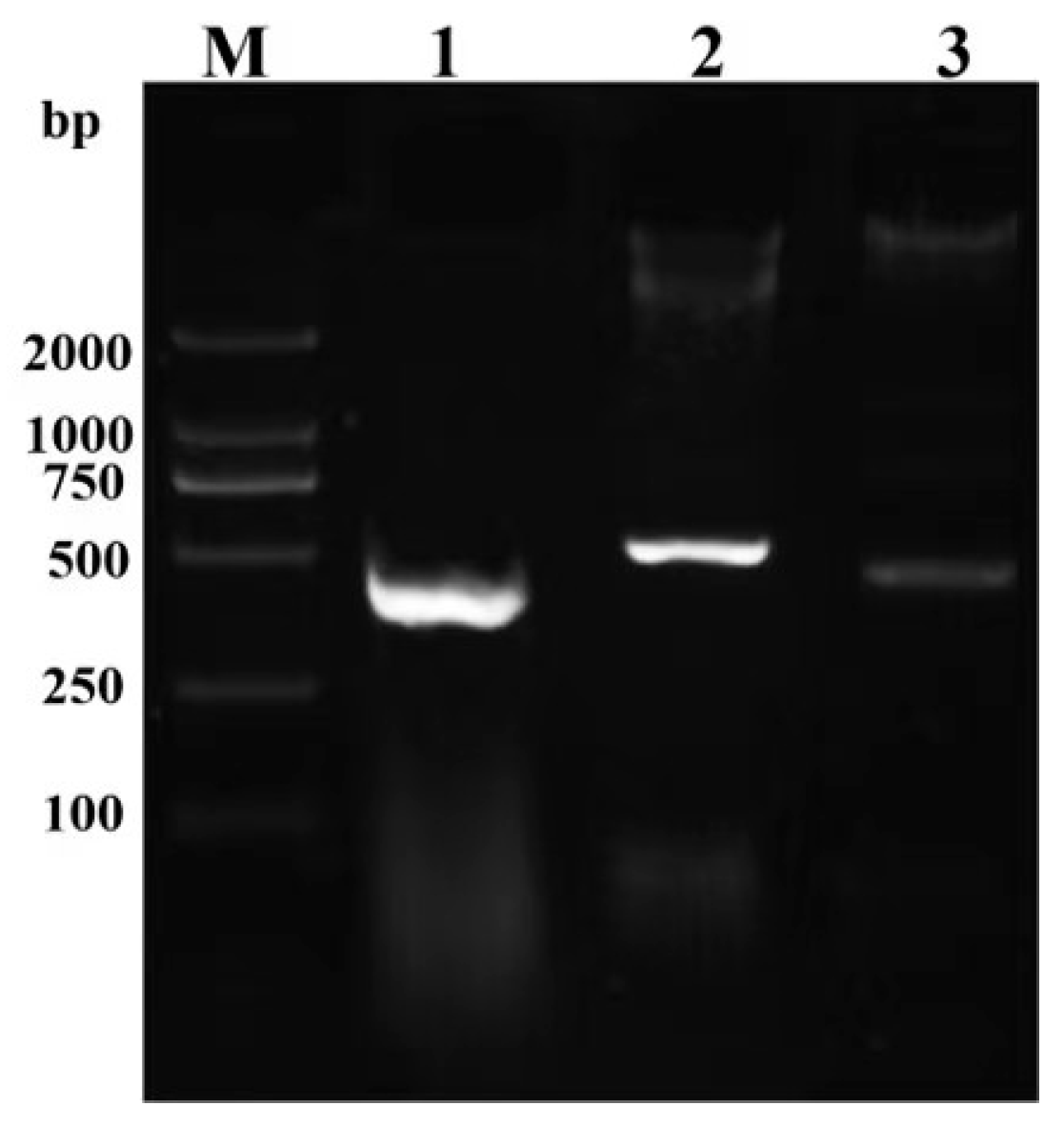
2.3. DNA Extraction and Polymerase Chain Reaction (PCR)
2.4. Nucleotide Sequence Analysis
3. Results
3.1. Visual Assessment of Lichen-Induced Biodegradation
3.2. Agarose Gel Electrophoresis Analysis of PCR Products from Lichen Samples
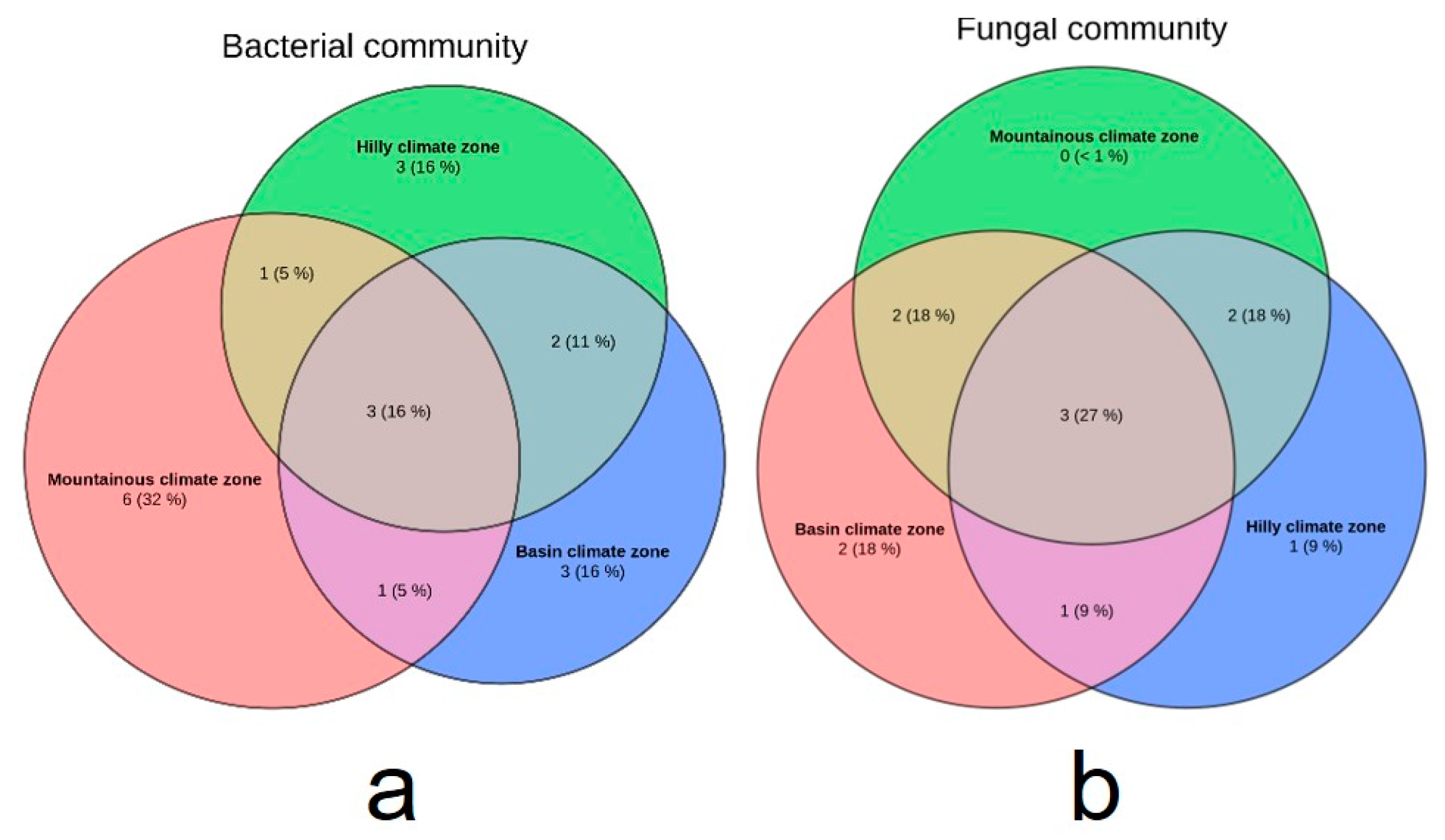
3.3. Analysis of Microbial Diversity in Lichen Samples
3.4. Microbial Communities of Lichens
4. Discussion
4.1. Impacts of Microclimatic Conditions and Environmental Factors on Lichen Microbial Communities
4.2. Effects of Physical Properties and Morphology of Brick Carvings on Colonization by Lichen Microorganisms
4.3. Microecology of Roof Lichens and Biodegradation of Brick Carvings
4.4. Implications for Sustainable Heritage Conservation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zayats, I.; Murgul, V. The institutional architecture—Art or building? Procedia Eng. 2016, 165, 1460–1467. [Google Scholar] [CrossRef]
- Sterflinger, K.; Piñar, G. Microbial deterioration of cultural heritage and works of art—Tilting at windmills? Appl. Microbiol. Biotechnol. 2013, 97, 9637–9646. [Google Scholar] [CrossRef]
- De Belie, N. Microorganisms versus stony materials: A love-hate relationship. Mater. Struct. 2010, 43, 1191–1202. [Google Scholar] [CrossRef]
- Beata, G. The use of -omics tools for assessing biodeterioration of cultural heritage: A review. J. Cult. Herit. 2020, 45, 351–361. [Google Scholar] [CrossRef]
- Gadd, G.M. Geomicrobiology of the built environment. Nat. Microbiol. 2017, 2, 16275. [Google Scholar] [CrossRef]
- Kakakhel, M.A.; Wu, F.; Gu, J.-D.; Feng, H.; Shah, K.; Wang, W. Controlling biodeterioration of cultural heritage objects with biocides: A review. Int. Biodeterior. Biodegrad. 2019, 143, 104721. [Google Scholar] [CrossRef]
- Wiktor, V.; De Leo, F.; Urzi, C.; Guyonnet, R.; Grosseau, P.; Garcia-Diaz, E. Accelerated laboratory test to study fungal biodeterioration of cementitious matrix. Int. Biodeter. Biodegr. 2009, 63, 1061–1065. [Google Scholar] [CrossRef]
- Korkanç, M.; Savran, A. Impact of the Surface Roughness of Stones Used in Historical Buildings on Biodeterioration. Constr. Build. Mater. 2015, 80, 279–294. [Google Scholar] [CrossRef]
- Grube, M.; Cardinale, M.; De Castro, J.V.; Müller, H.; Berg, G. Species-specific structural and functional diversity of bacterial communities in lichen symbioses. ISME J. 2009, 3, 1105–1115. [Google Scholar] [CrossRef]
- Margrét, A.S.; Vilhelmsson, O. Selective isolation of potentially phosphate-mobilizing, biosurfactant-producing and biodegradative bacteria associated with a sub-Arctic, terricolous lichen, Peltigera membranacea. FEMS Microbiol. Ecol. 2016, 92, 90. [Google Scholar]
- Lisci, M.; Monte, M.; Pacini, E. Lichens and Higher Plants on Stone: A Review. Int. Biodeterior. Biodegrad. 2003, 51, 1–17. [Google Scholar] [CrossRef]
- Garcia-Rowe, J.; Saiz-Jimenez, C. Lichens and Bryophytes as Agents of Deterioration of Building Materials in Spanish Cathedrals. Int. Biodeterior. 1991, 28, 151–163. [Google Scholar] [CrossRef]
- Adamo, P. Weathering of Rocks and Neogenesis of Minerals Associated with Lichen Activity. Appl. Clay Sci. 2000, 16, 229–256. [Google Scholar] [CrossRef]
- Hayek, M.; Salgues, M.; Souche, J.; Cunge, E.; Giraudel, C.; Paireau, O. Influence of the Intrinsic Characteristics of Cementitious Materials on Biofouling in the Marine Environment. Sustainability 2021, 13, 2625. [Google Scholar] [CrossRef]
- Stanaszek-Tomal, E. Environmental Factors Causing the Development of Microorganisms on the Surfaces of National Cultural Monuments Made of Mineral Building Materials-Review. Coatings 2020, 10, 1203. [Google Scholar] [CrossRef]
- Giannantonio, D.J.; Kurth, J.C.; Kurtis, K.E.; Sobecky, P.A. Effects of concrete properties and nutrients on fungal colonization and fouling. Int. Biodeter. Biodegr. 2009, 63, 252–259. [Google Scholar] [CrossRef]
- Motti, R.; Bonanomi, G.; Stinca, A. Biodeteriogens at a Southern Italian Heritage Site: Analysis and Management of Vascular Flora on the Walls of Villa Rufolo. Int. Biodeterior. Biodegrad. 2021, 162, 105252. [Google Scholar] [CrossRef]
- Herrera, L.K.; Arroyave, C.; Guiamet, P.; de Saravia, S.G.; Videla, H. Biodeterioration of Peridotite and Other Constructional Materials in a Building of the Colombian Cultural Heritage. Int. Biodeterior. Biodegrad. 2004, 54, 135–141. [Google Scholar] [CrossRef]
- Warscheid, T.; Braams, J. Biodeterioration of Stone: A Review. Int. Biodeterior. Biodegrad. 2000, 46, 343–368. [Google Scholar] [CrossRef]
- Miller, A.Z.; Sanmartín, P.; Pereira-Pardo, L.; Dionísio, A.; Saiz-Jimenez, C.; Macedo, M.F.; Prieto, B. Bioreceptivity of Building Stones: A Review. Sci. Total Environ. 2012, 426, 1–12. [Google Scholar] [CrossRef]
- Cámara, B.; Álvarez de Buergo, M.; Fort, R.; Souza-Egipsy, V.; Pérez-Ortega, S.; de los Ríos, A.; Wierzchos, J.; Ascaso, C. Anthropic effect on the lichen colonization in building stones from cultural heritage. Period. Di Mineralogia 2015, 84, 539–552. [Google Scholar]
- Luoyang Local Chronicles Compilation Committee. Annals of Luoyang City—Annals of Natural Environment; Zhongzhou Ancient Books Publishing House: Zhengzhou, China, 2000; pp. 169–182. [Google Scholar]
- Gardes, M.; Brun, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Heuer, H.; Krsek, M.; Baker, P.; Smalla, K.; Wellington, E.M. Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gelelectrophoretic separation in denaturing gradients. Appl. Environ. Microbiol. 1997, 63, 3233–3241. [Google Scholar] [CrossRef]
- Konkol, N.R.; McNamara, C.J.; Hellman, E.; Mitchell, R. Early detection of fungal biomass on library materials. J. Cult. Herit. 2012, 13, 115–119. [Google Scholar] [CrossRef]
- Ortega-Calvo, J.J.; Arino, X.; Hernandez-Marine, M.; Saiz-Jimenez, C. Factors affecting the weathering and colonization of monuments by phototrophic microorganisms. Sci. Total Environ. 1995, 167, 329–341. [Google Scholar] [CrossRef]
- Crispim, C.; Gaylarde, C. Cyanobacteria and biodeterioration of cultural heritage: A review. Microb. Ecol. 2005, 49, 1–9. [Google Scholar] [CrossRef]
- Hoang, C.P.; Kinney, K.A.; Corsi, R.L.; Szaniszlo, P.J. Resistance of green building materials to fungal growth. Int. Biodeterior. Biodegrad. 2010, 64, 104–113. [Google Scholar] [CrossRef]
- Hyvärinen, A.; Meklin, T.; Vepsäläinen, A. Fungi and actinobacteria in moisture-damaged building materials—Concentrations and diversity. Int. Biodeterior. Biodegrad. 2002, 49, 27–37. [Google Scholar] [CrossRef]
- Sawadogo, A.Y.F.; Roux, S.; Lecomte, A. Bioreceptivity of Portland and calcium Sulphoaluminate cements in urban sewerage networks. Constr. Build. Mater. 2021, 293, 123425. [Google Scholar] [CrossRef]
- Ma, S.C.; Wu, Y.W.; Bao, P. Experimental study on the properties of modern blue clay brick for Kaifeng People’s Conference Hall. Sci. Rep. 2021, 11, 20631. [Google Scholar] [CrossRef]
- Tran, T.H.; Govin, A.; Guyonnet, R.; Grosseau, P.; Lors, C.; Garcia-Diaz, E.; Damidot, D.; Devès, O.; Ruot, B. Influence of the intrinsic characteristics of mortars on biofouling by Klebsormidium flaccidum. Int. Biodeterior. Biodegrad. 2012, 70, 31–39. [Google Scholar] [CrossRef]
- D’Orazio, M.; Cursio, G.; Graziani, L.; Aquilanti, L.; Osimani, A.; Clementi, F.; Yéprémian, C.; Lariccia, V.; Amoroso, S. Effects of water absorption and surface roughness on the bioreceptivity of ETICS compared to clay bricks. Build. Environ. 2014, 77, 20–28. [Google Scholar] [CrossRef]
- Barberoussea, H.; Ruota, B.; Yepremian, C.; Boulon, G. An assessment of façade coatings against colonization by aerial algae and cyanobacteria. Build. Environ. 2007, 42, 2555–2561. [Google Scholar] [CrossRef]
- Petrozzi, S.; Dunn, I.J.; Heinzle, E.; Kut, O.M. Carrier influence in anaerobic biofilm fluidized beds for treating vapor condensate from the sulfite cellulose process. Can. J. Chem. Eng. 1991, 69, 527–553. [Google Scholar] [CrossRef]
- Gutarowska, B. Metabolic activity of moulds as a factor of building materials biodegradation. Pol. J. Microbiol. 2010, 59, 119–124. [Google Scholar] [CrossRef]
- Kaarakainen, P.; Rintala, H.; Vepsalainen, A.; Hyvarinene, A.; Nevalainen, A.; Meklin, T. Microbial content of house dust samples determined with qPCR. Sci. Total Environ. 2009, 407, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Mascaro, M.E.; Pellegrino, G.; de Rose, I.; Palermo, A.M. Contribution to the Knowledge of Biodeteriogenic Flora on Three Historical Calabrian (Southern Italy) Churches. Open J. Ecol. 2021, 11, 287–300. [Google Scholar] [CrossRef]
- Caneva, G.; Nugari, M.P.; Salvadori, O. Plant Biology for Cultural Heritage: Biodeterioration and Conservation; Getty Publications: Los Angeles, CA, USA, 2008. [Google Scholar]
- De los Ríos, A.; Ascaso, C. Contributions of in situ microscopy to the current understanding of stone biodeterioration. Int. Microbiol. 2005, 8, 181–188. [Google Scholar]
- Bock, E.; Ahlers, B.; Meyer, C. Biogene Korrosion von Beton und Natursteinen durch Salpetersäure bildende Bakterien. Bauphysik 1989, 4, 141–144. [Google Scholar]
- Frankeová, D.; Bauerová, P.; Náhunková, P.; Slížková, Z.; Polák, M. Characterization and damage of the historic brick masonry of the Zákupy castle stables. J. Phys. Conf. Ser. 2024, 2792, 012015. [Google Scholar] [CrossRef]
- Liu, J.B.; Zhang, Z.J. Characteristics and weathering mechanisms of the traditional Chinese blue brick from the ancient city of Ping Yao. R. Soc. Open Sci. 2020, 7, 200058. [Google Scholar] [CrossRef] [PubMed]
- Prieto, B.; Vázquez-Nion, D.; Fuentes, E.; Durán-Román, A.G. Response of subaerial biofilms growing on stone-built cultural heritage to changing water regime and CO2 conditions. Int. Biodeter. Biodegr. 2020, 148, 104882. [Google Scholar] [CrossRef]
- Filomena, D.L.; Marchetta, A.; Urzì, C. Black Fungi on Stone-Built Heritage: Current Knowledge and Future Outlook. Appl. Sci. 2022, 12, 3969. [Google Scholar] [CrossRef]
- Geoffrey, M.G.; Dyer, T.D. Bioprotection of the built environment and cultural heritage. Microb. Biotechnol. 2017, 10, 1152–1156. [Google Scholar]
- Gaylarde, C.C.; Rodrıguez, C.H.; Navarro, N.Y.; Ortega, M.B. Microbial biofilms on the sandstone monuments of the Angkor Wat Complex, Cambodia. Curr. Microbiol. 2012, 64, 85–92. [Google Scholar] [CrossRef]
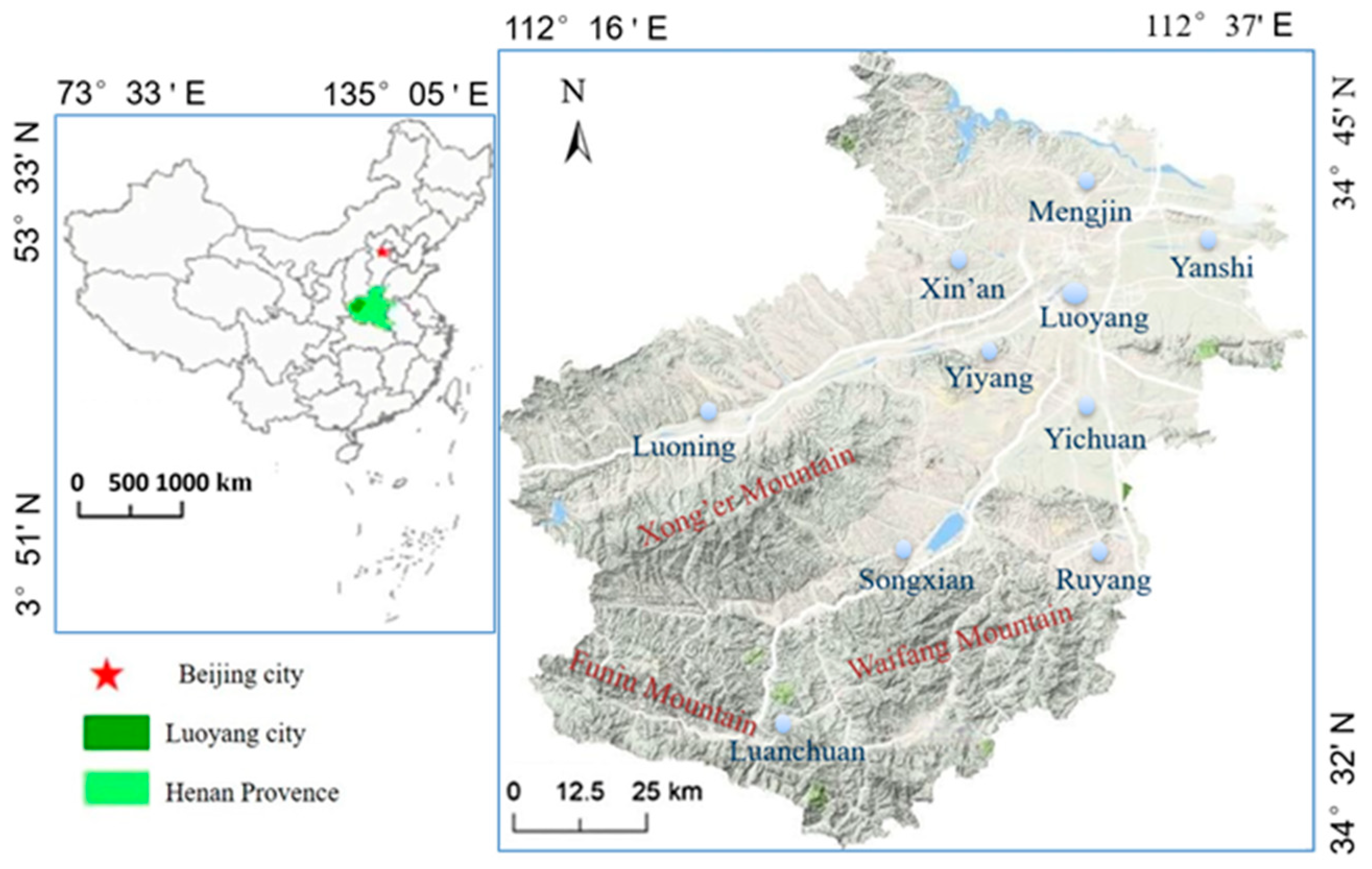


| Location of Historic Buildings | Historic Buildings Name | Construction Time (AD) | Sample No. |
|---|---|---|---|
| Luoyang urban area | Zhang’s residence | 1781 | LYU |
| Yichuan County | Wen’s residence | 1873 | YC |
| Guano | YC-G | ||
| Carving material | YC-C | ||
| Yi River beach | YC-R | ||
| Yanshi County | Guo’s residence | 1873 | YS |
| Mengjin County | Wei’s residence | 1804 | MJ |
| Xin’an County | Whang’s residence | 1877 | XA |
| Yiyang County | Su’s residence | 1831 | YY |
| Luoning County | Cheng’s residence | 1861 | LN |
| Ruyang County | Wang’s residence | 1862 | RY |
| Song County | Chai’s residence | 1824 | SC |
| Luanchuan County | Li’s residence | 1817 | LC |
| Mountain rock | LC-M |
| Population | Primers | Sequence (5′-3′) | Reference Strains |
|---|---|---|---|
| Bacteria | 338F | ACTCCTACG GGAGGCAGCAG | Bacillus halmapalus (MW893675.1) |
| 806R | GGACTACCAGGGTATCTAAC | ||
| Fungi | ITS5 | GAAGTAAAAGTCGTAACAAGG | Aspergillus aureoterreus (OL772679.1) |
| ITS1F | CTTGGTCATTTAGAGGAAGTAA | ||
| Cyanobacteria | 309F | GGGGAATTTTCCGCAATGGG | Nostocaceae cyanobacterium (OP502075.1) |
| 732R | TACTGGGGTATCTAATCCCATT |
| Microclimatic Zone | Sample No. | Microbial Type | |
|---|---|---|---|
| Bacteria | Fungi | ||
| Basin climate zone | LYU | Actinobacteria; Bacillus wiedmannii | Cladosporium halotolerans; Aspergillus costaricensis |
| YC | Cyanobacteria; Bacillus licheniformis; Chroococcidiopsis lichenoides; Micromonosporaceae; Actinomycetospora flava | Cladosporium tenuissimum; Septoriella hibernica; Aspergillus terreus; Metarhizium marquandii | |
| YC-G | Bacillus licheniformis; Bacillus cereus | Aspergillus costaricensis | |
| YC-C | Actinobacteria; Bacillus licheniformis | Metarhizium marquandii; Aspergillus terreus | |
| YC-R | Cyanobacteria; Chroococcidiopsis lichenoides; Bacillus licheniformis; Scytonema stuposum; Actinobacteria | Penicillium atramentosum; Metarhizium marquandii; Aspergillus sydowii; Aspergillus terreus | |
| Hilly climate zone | YS | Cyanobacteria; Bacillus cereus; Actinomycetospora flava | Aspergillus terreus; Metarhizium marquandii |
| MJ | Cyanobacteria; Bacillus cereus; Actinomycetospora flava; Chroococcidiopsis | Aspergillus costaricensis; Metarhizium marquandii; Aspergillus terreus | |
| XA | Cyanobacteria; Bacillus rhizoplanae; Chroococcidiopsis lichenoides | Aspergillus versicolor; Aspergillus sydowii; Cladosporium sp.; | |
| YY | Cyanobacteria; Bacillus cereus; Micromonosporaceae; Scytonema crispum; Chroococcidiopsis; Actinobacteria | Chaetophorales; Cladosporium sp.; Aspergillus sydowii; Aspergillus terreus | |
| Mountainous climate zone | LN | Cyanobacteria; Bacillus licheniformis; Chroococcidiopsis lichenoides; Microcoleus chthonoplastes; Actinobacteria | Chaetophorales; Aspergillus terreus; Aspergillus costaricensi |
| RY | Microcoleus paludosus; Chroococcidiopsis lichenoides; Nostocaceae; Micromonosporaceae | Cladosporium halotolerans; Aspergillus sydowii; Penicillium atramentosum | |
| SC | Cyanobacteria; Bacillus licheniformis; Microcoleus paludosus; Chroococcidiopsis lichenoides; Nostoc edaphicum | Cladosporium halotolerans; Chaetophorales; Penicillium atramentosum; Aspergillus sydowii | |
| LC | Cyanobacteria; Bacillus licheniformis; Chroococcidiopsis lichenoides; Nostoc edaphicum; Scytonema crispum | Chaetophorales; Cladosporium sp.; Cladosporium halotolerans; Aspergillus costaricensis; Penicillium atramentosum | |
| LC-M | Cyanobacteria; Bacillus licheniformis; Chroococcidiopsis muralis; Micromonosporaceae; Nostoc edaphicum; Scytonema crispum; Microcoleus paludosus | Cladosporium halotolerans; Chaetophorales; Penicillium atramentosum; Aspergillus sydowii; Aspergillus costaricensis | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Ye, P.; Li, Y. Environmental Factors Influence Lichen Colonization and the Biodeterioration of Brick Carvings on Roof Ridges of Historic Buildings in Luoyang, China. Sustainability 2025, 17, 3721. https://doi.org/10.3390/su17083721
Li Z, Ye P, Li Y. Environmental Factors Influence Lichen Colonization and the Biodeterioration of Brick Carvings on Roof Ridges of Historic Buildings in Luoyang, China. Sustainability. 2025; 17(8):3721. https://doi.org/10.3390/su17083721
Chicago/Turabian StyleLi, Zijing, Ping Ye, and Yinju Li. 2025. "Environmental Factors Influence Lichen Colonization and the Biodeterioration of Brick Carvings on Roof Ridges of Historic Buildings in Luoyang, China" Sustainability 17, no. 8: 3721. https://doi.org/10.3390/su17083721
APA StyleLi, Z., Ye, P., & Li, Y. (2025). Environmental Factors Influence Lichen Colonization and the Biodeterioration of Brick Carvings on Roof Ridges of Historic Buildings in Luoyang, China. Sustainability, 17(8), 3721. https://doi.org/10.3390/su17083721






