Biochar-Enhanced Nitrogen Removal in SBBR Under PFOA Stress: The Role of Quorum Sensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Reactor Operation and Synthetic Wastewater Composition
2.2. Analytical Methods
2.2.1. Wastewater Quality Analysis
2.2.2. Extraction and Determination of AHLs
2.2.3. Morphology and Spectroscopy Analysis of Biochar and Metabolic Analysis
2.2.4. Batch Experiments
2.2.5. Metagenomic Analysis
3. Results and Discussions
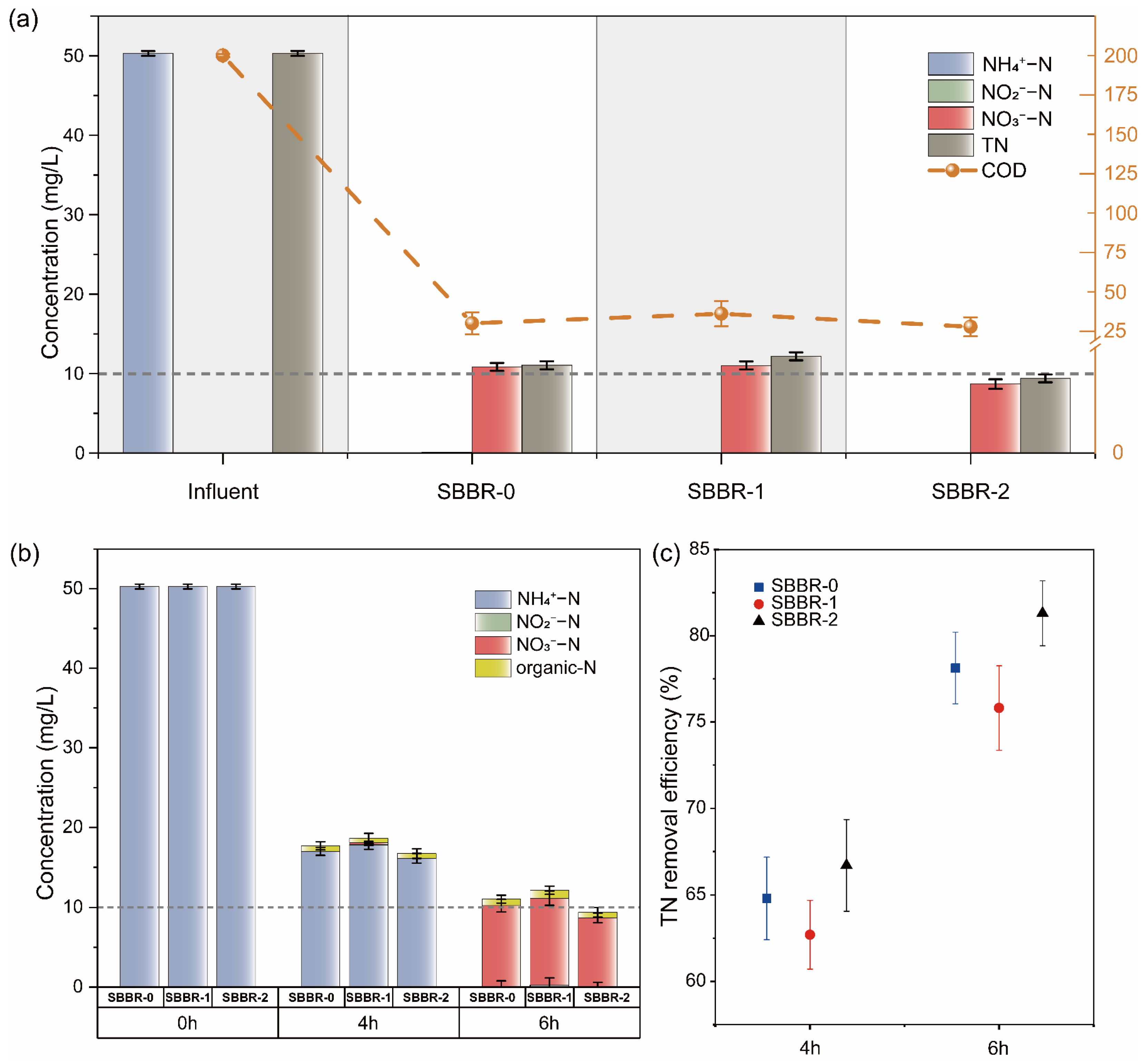
3.1. Profiles of Three SBBRs
3.2. Characterization of Biochar
3.3. Metabolic Analysis
3.4. Microbial Community Structure Analysis
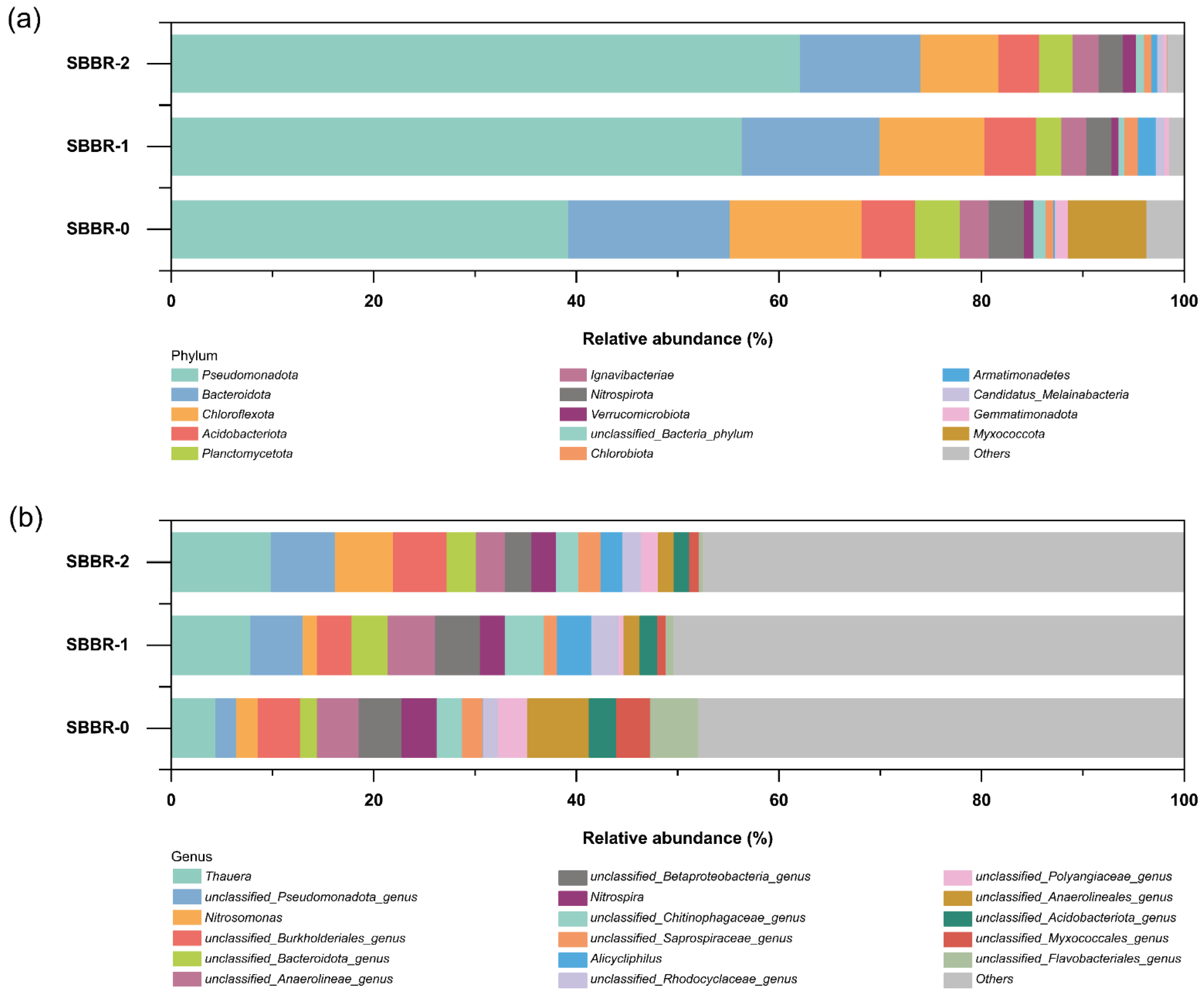
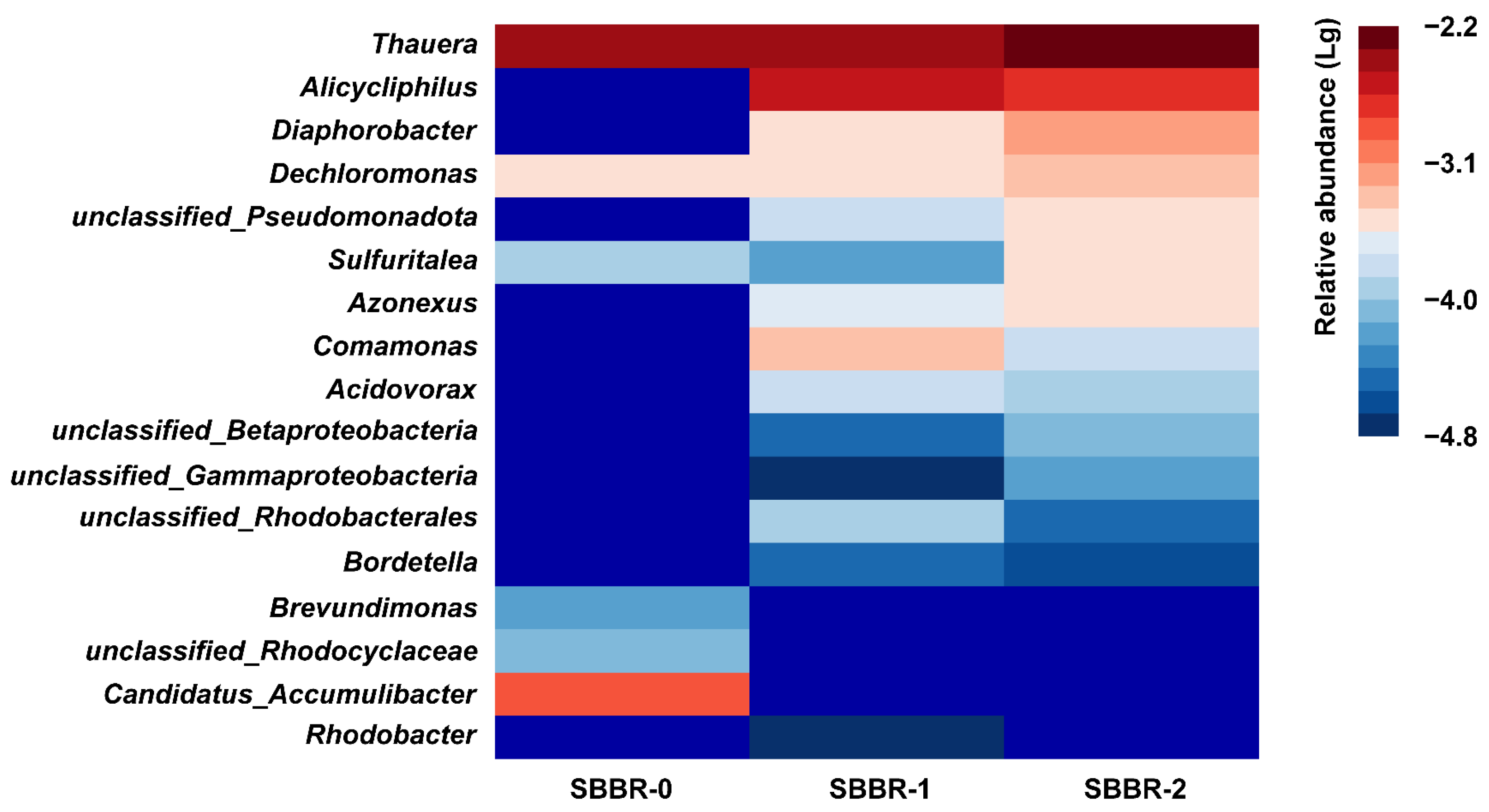
3.4.1. Community Composition Analysis
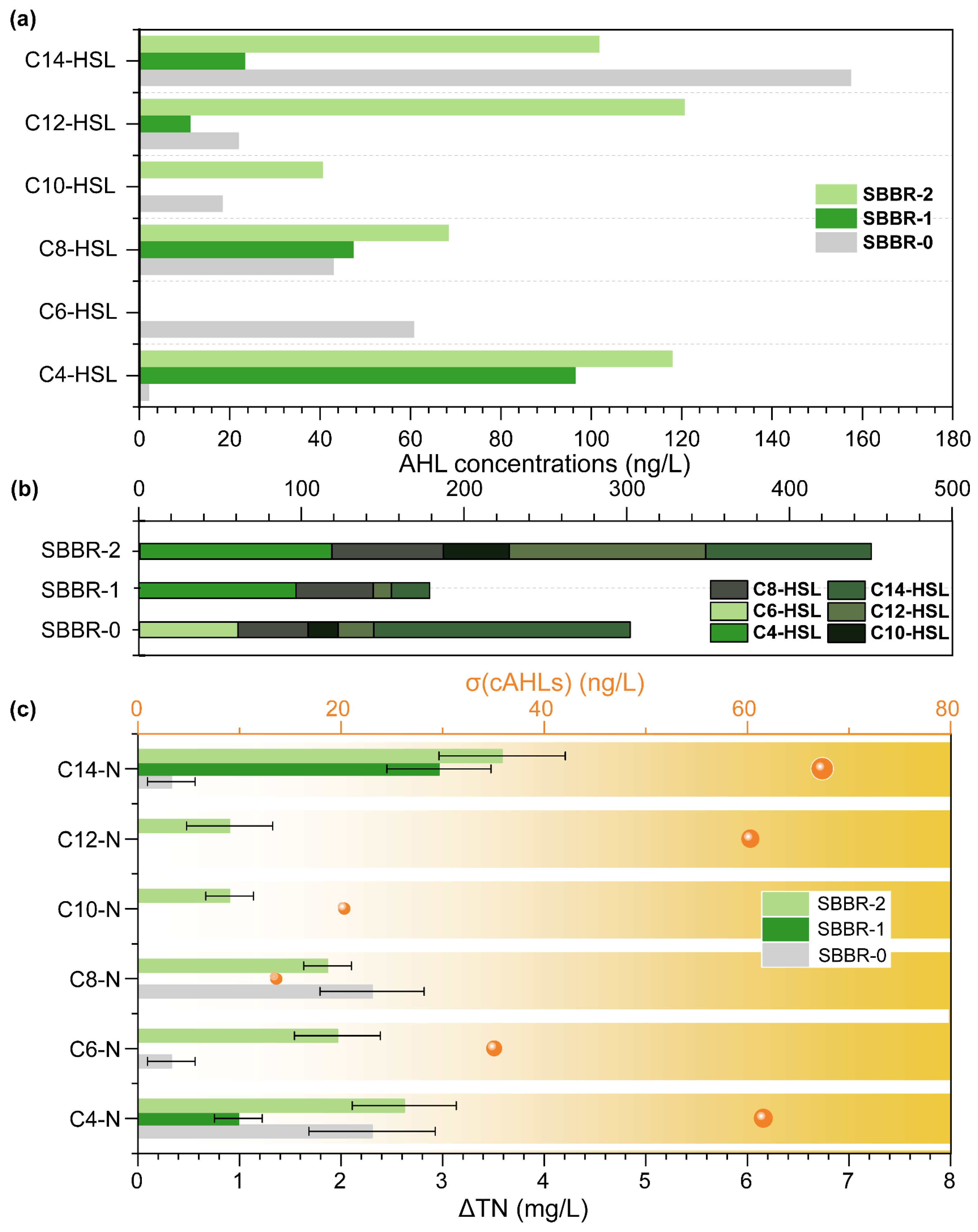
3.4.2. Quorum Sensing Analysis
3.5. Metagenomics Analysis
3.5.1. Quorum Sensing Genes
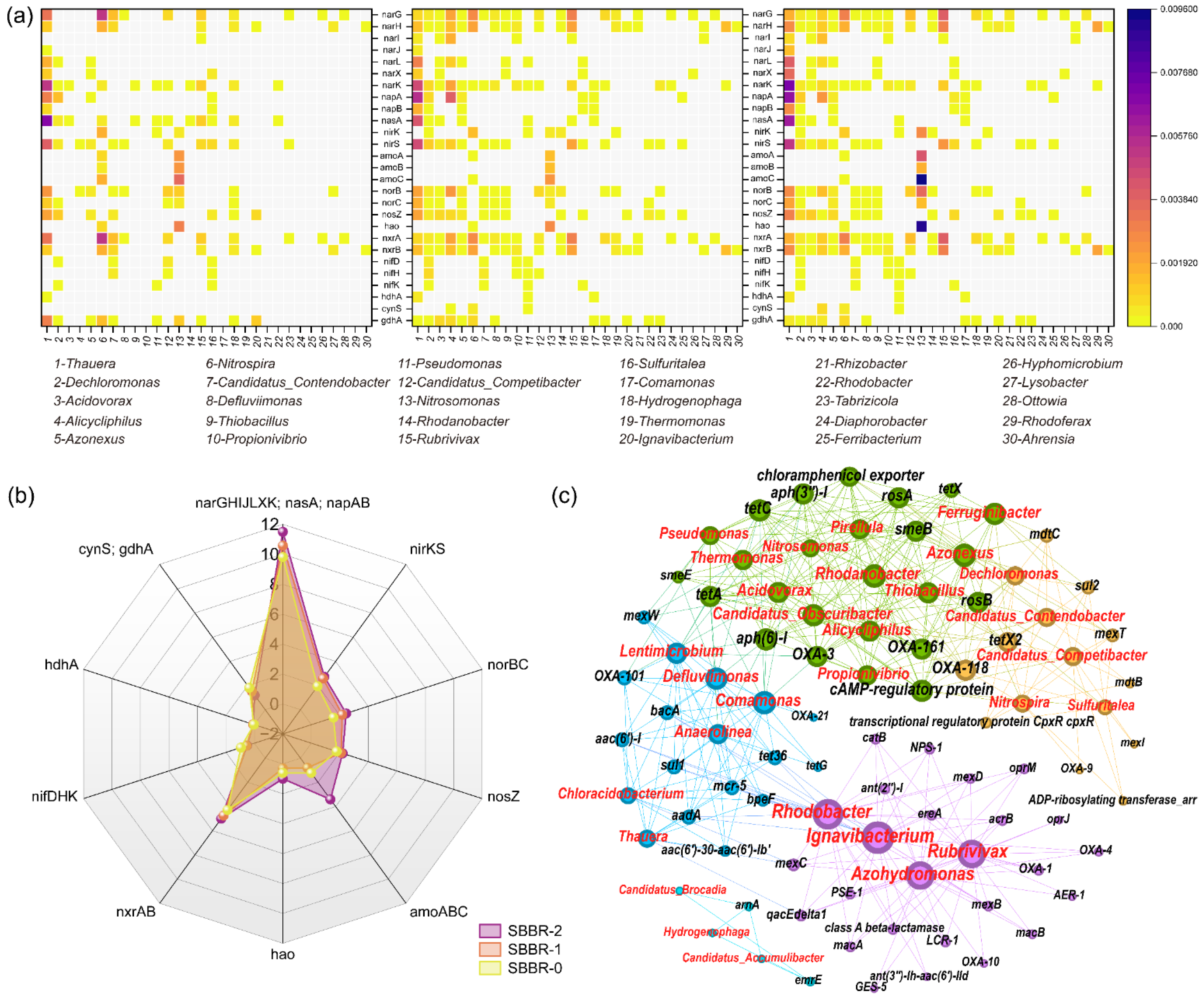
3.5.2. Nitrogen Cycle Genes
3.5.3. ARG Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, C.; Huang, X.; Chen, H.; Godfray, H.C.J.; Wright, J.S.; Hall, J.W.; Gong, P.; Ni, S.; Qiao, S.; Huang, G.; et al. Managing nitrogen to restore water quality in China. Nature 2019, 567, 516–520. [Google Scholar] [CrossRef]
- Rahimi, S.; Modin, O.; Mijakovic, I. Technologies for biological removal and recovery of nitrogen from wastewater. Biotechnol. Adv. 2020, 43, 107570. [Google Scholar] [CrossRef]
- Garfi, M.; Flores, L.; Ferrer, I. Life Cycle Assessment of wastewater treatment systems for small communities: Activated sludge, constructed wetlands and high rate algal ponds. J. Clean. Prod. 2017, 161, 211–219. [Google Scholar] [CrossRef]
- Liu, T.; Hu, S.; Guo, J. Enhancing mainstream nitrogen removal by employing nitrate/nitrite-dependent anaerobic methane oxidation processes. Crit. Rev. Biotechnol. 2019, 39, 732–745. [Google Scholar] [CrossRef]
- Cieslik, B.M.; Namiesnik, J.; Konieczka, P. Review of sewage sludge management: Standards, regulations and analytical methods. J. Clean. Prod. 2015, 90, 1–15. [Google Scholar] [CrossRef]
- Gallego-Schmid, A.; Tarpani, R.R.Z. Life cycle assessment of wastewater treatment in developing countries: A review. Water Res. 2019, 153, 63–79. [Google Scholar] [CrossRef]
- Loos, R.; Carvalho, R.; Antonio, D.C.; Cornero, S.; Locoro, G.; Tavazzi, S.; Paracchini, B.; Ghiani, M.; Lettieri, T.; Blaha, L.; et al. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. 2013, 47, 6475–6487. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Duan, J.; Tian, S.; Ji, H.; Zhu, Y.; Wei, Z.; Zhao, D. Short-chain per- and polyfluoroalkyl substances in aquatic systems: Occurrence, impacts and treatment. Chem. Eng. J. 2020, 380, 122506. [Google Scholar] [CrossRef]
- Xiao, J.; Huang, J.; Wang, Y.; Qian, X.; Cao, M. Evaluation of the ecological impacts of short- and long-chain perfluoroalkyl acids on constructed wetland systems: Perfluorobutyric acid and perfluorooctanoic acid. J. Hazard. Mater. 2022, 435, 128863. [Google Scholar] [CrossRef]
- Huang, D.; Xu, R.; Sun, X.; Li, Y.; Xiao, E.; Xu, Z.; Wang, Q.; Gao, P.; Yang, Z.; Lin, H.; et al. Effects of perfluorooctanoic acid (PFOA) on activated sludge microbial community under aerobic and anaerobic conditions. Environ. Sci. Pollut. Res. 2022, 29, 63379–63392. [Google Scholar] [CrossRef]
- Ilieva, Z.; Hania, P.; Suehring, R.; Gilbride, K.; Hamza, R. Impact of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) on secondary sludge microorganisms: Removal, potential toxicity, and their implications on existing wastewater treatment regulations in Canada. Environ. Sci.-Proc. Imp. 2023, 25, 1604–1614. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zou, M.; Zhou, Y.; Zeng, L.; Yang, X. Monitoring the nitrous oxide emissions and biological nutrient removal from wastewater treatment: Impact of per fl uorooctanoic acid. J. Hazard. Mater. 2021, 402, 123469. [Google Scholar] [CrossRef]
- Cao, L.; Su, C.; Wu, J.; Wei, L.; Zhou, Y.; Tang, L.; Wang, Q.; Xian, Y. Impact of perfluorooctanoic acid on treatment wastewater by a tandem AnSBR-ASBR system: Performance, microbial community and metabolism pathway. Process Saf. Environ. Prot. 2022, 164, 373–383. [Google Scholar] [CrossRef]
- Liu, X.; Chen, L.; Yu, L.; Hua, Z.; Zhang, Y.; Ma, Y.; Lu, Y.; Dong, Y.; Wang, Y.; Zhang, Z.; et al. Removing nutrients from wastewater by constructed wetlands under perfluoroalkyl acids stress. Environ. Res. 2022, 212, 113334. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, N.; Yang, G.; Xu, Q.; Wang, D.; Tang, L.; Xia, J.; Li, P.; Li, X. Environmentally relevant level of perfluorooctanoic acid affect the formation of aerobic granular sludge. J. Environ. Manag. 2023, 336, 117659. [Google Scholar] [CrossRef]
- Chen, C.; Fang, Y.; Zhou, D. Selective pressure of PFOA on microbial community: Enrichment of denitrifiers harboring ARGs and the transfer of ferric-electrons. Water Res. 2023, 233, 119813. [Google Scholar] [CrossRef]
- Chen, C.; Fang, Y.; Wang, Y.; Zhang, C.; Zhou, D. Using CaCO3 armor to alleviate PFOA-induced stress on microorganisms in porous aquatic environments. Chem. Eng. J. 2024, 495, 153733. [Google Scholar] [CrossRef]
- Wen, X.; Gong, B.; Zhou, J.; He, Q.; Qing, X. Efficient simultaneous partial nitrification, anammox and denitrification (SNAD) system equipped with a real-time dissolved oxygen (DO) intelligent control system and microbial community shifts of different substrate concentrations. Water Res. 2017, 119, 201–211. [Google Scholar] [CrossRef]
- Gao, B.S.; Yang, X.; Dasi, E.A.; Lam, T.; Arias, M.E.; Ergas, S.J. Enhanced landfill leachate treatment in sequencing batch biofilm reactors (SBBRs) amended with zeolite and biochar. J. Chem. Technol. Biotechnol. 2022, 97, 759–770. [Google Scholar] [CrossRef]
- Wei, J.; Huang, X.; Wang, H.; Wang, F.; Liu, X.; Yan, Y.; Qu, Y. Insight into biofilm formation of wastewater treatment processes: Nitrogen removal performance and biological mechanisms. Sci. Total Environ. 2023, 903, 166550. [Google Scholar] [CrossRef]
- Tan, C.; Xu, H.; Cui, D.; Zuo, J.; Li, J.; Ji, Y.; Qiu, S.; Yao, L.; Chen, Y.; Liu, Y. Effects of tourmaline on nitrogen removal performance and biofilm structures in the sequencing batch biofilm reactor. J. Environ. Sci. 2018, 67, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Xiang, Y.; Liu, S.; Shao, Z.; Liu, Y.; Ma, H.; He, Q.; Chai, H. Insights into simultaneous nitrogen and phosphorus removal in biofilm: The overlooked comammox Nitrospira and the positive role of glycogen-accumulating organisms. Sci. Total Environ. 2023, 887, 164130. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.; Eldyasti, A. Ammonia-Oxidizing Bacteria (AOB): Opportunities and applications—A review. Rev. Environ. Sci. Biotechnol. 2018, 17, 285–321. [Google Scholar] [CrossRef]
- Wang, J.; Gong, B.; Huang, W.; Wang, Y.; Zhou, J. Bacterial community structure in simultaneous nitrification, denitrification and organic matter removal process treating saline mustard tuber wastewater as revealed by 16S rRNA sequencing. Bioresour. Technol. 2017, 228, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.T.; Chen, J.; Yuan, S.C.; Sang, W.J.; Ban, Y.H.; Zhang, S.Y. Impact of aeration frequency on performance of mixotrophic sequencing batch biofilm reactor (SBBR) treating real domestic wastewater: Removal efficiency, pathways, and mechanisms. J. Clean. Prod. 2023, 385, 135747. [Google Scholar] [CrossRef]
- Srivastava, A.; Ambekar, R.S.; Gupta, B.; Tiwary, C.S.; Gupta, A.K. Schwarzite-based 3D-printed carriers for enhanced performance of sequencing batch biofilm reactor (SBBR) for wastewater treatment. J. Environ. Chem. Eng. 2024, 12, 111794. [Google Scholar] [CrossRef]
- Jagaba, A.H.; Kutty, S.R.M.; Noor, A.; Birniwa, A.H.; Affam, A.C.; Lawal, I.M.; Kankia, M.U.; Kilaco, A.U. A systematic literature review of biocarriers: Central elements for biofilm formation, organic and nutrients removal in sequencing batch biofilm reactor. J. Water Process Eng. 2021, 42, 102178. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, X.; Zhang, X.; Ren, J.; Li, H.; Wang, Z.; Guo, W.; Ngo, H.H. Recent advances and trends of carbon-based biocarriers for performance enhancement of anaerobic membrane bioreactor system. J. Water Process Eng. 2024, 59, 104949. [Google Scholar] [CrossRef]
- Coleman, B.S.L.; Easton, Z.M.; Bock, E.M. Biochar fails to enhance nutrient removal in woodchip bioreactor columns following saturation. J. Environ. Manag. 2019, 232, 490–498. [Google Scholar] [CrossRef]
- Zheng, Z.; Ali, A.; Su, J.; Fan, Y.; Zhang, S. Layered double hydroxide modified biochar combined with sodium alginate: A powerful biomaterial for enhancing bioreactor performance to remove nitrate. Bioresour. Technol. 2021, 323, 124630. [Google Scholar] [CrossRef]
- Sui, M.; Li, Y.; Jiang, Y.; Wang, L.; Zhang, W.; Sathishkumar, K.; Zakaria, H. Sediment-based biochar facilitates highly efficient nitrate removal: Physicochemical properties, biological responses and potential mechanism. Chem. Eng. J. 2021, 405, 126645. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Q.; Xue, H.; Wang, T.; Fan, Y.; Zhang, Z.; Wang, H.; Wang, Y. The feasibility and mechanism of redox-active biochar for promoting anammox performance. Sci. Total Environ. 2022, 814, 152813. [Google Scholar] [CrossRef]
- Waters, C.M.; Bassler, B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef]
- Davies, D.G.; Parsek, M.R.; Pearson, J.P.; Iglewski, B.H.; Costerton, J.W.; Greenberg, E.P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 1998, 280, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.T.; Bassler, B.L. Bacterial Quorum Sensing: Its Role in Virulence and Possibilities for Its Control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef]
- Williams, P.; Camara, M.; Hardman, A.; Swift, S.; Milton, D.; Hope, V.J.; Winzer, K.; Middleton, B.; Pritchard, D.I.; Bycroft, B.W. Quorum sensing and the population-dependent control of virulence. Philos. Trans. R. Soc. B 2000, 355, 667–680. [Google Scholar] [CrossRef]
- Valentini, M.; Filloux, A. Biofilms and Cyclic di-GMP (c-di-GMP) Signaling: Lessons from Pseudomonas aeruginosa and Other Bacteria. J. Biol. Chem. 2016, 291, 12547–12555. [Google Scholar] [CrossRef]
- Toyofuku, M.; Nomura, N.; Fujii, T.; Takaya, N.; Maseda, H.; Sawada, I.; Nakajima, T.; Uchiyama, H. Quorum sensing regulates denitrification in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2007, 189, 4969–4972. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, X.; Zhao, Y.; Wang, J.; Ding, L.; Wang, Y.; Ren, H. Biofilm Reactor Performance and Shifts of Microbial Community during the Start-Up and Operation Phase of MBBRs at 8 °C: Effect of Exogenous Quorum Sensing Bacteria. ACS EST Water 2023, 3, 804–816. [Google Scholar] [CrossRef]
- Han, H.; Li, J.; Zhang, J.; Peng, Y.; Li, Y.; Zhang, K.; Zhang, Y.; Wei, P.; Luo, R. Enhancing the treatment performance of partial denitrification/Anammox process at high nitrogen load: Effects of immobilized strain HFQ8C/N on the sludge characteristics. Bioresour. Technol. 2021, 341, 125870. [Google Scholar] [CrossRef]
- Tripathi, S.; Chandra, R.; Purchase, D.; Bilal, M.; Mythili, R.; Yadav, S. Quorum sensing—A promising tool for degradation of industrial waste containing persistent organic pollutants. Environ. Pollut. 2022, 292, 118342. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, B.; Zhang, N.; Zhang, H.; Ma, Y.; Song, Y.; Zhang, H. Regulating performance of CANON process via adding external quorum sensing signal molecules in membrane bioreactor. Bioresour. Technol. 2023, 369, 128465. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; He, J.; Liu, J.; Yu, H.; Zhang, J. Biofilm activity and sludge characteristics affected by exogenous N-acyl homoserine lactones in biofilm reactors. Bioresour. Technol. 2016, 211, 339–347. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Huang, R.X.; Jiang, L.G.; Lu, Z.C.; Wu, G.L.; Lei, J.X.; Liao, S.F.; Liu, G.Q.; Li, B.W.; Wang, J.M. Enhancing nitrogen removal and reducing aeration energy for wastewater treatment with intermittent Modified Ludzack-Ettinger process: A field demonstration. J. Water Process Eng. 2021, 43, 102303. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Y.; Zhang, H.; Feng, D.; Bu, H.; Li, L.; Lu, S. A critical review of characteristics of domestic wastewater and key treatment techniques in Chinese villages. Sci. Total Environ. 2024, 927, 172155. [Google Scholar] [CrossRef] [PubMed]
- Majone, M.; Aulenta, F.; Dionisi, D.; D’Addario, E.N.; Sbardellati, R.; Bolzonella, D.; Beccari, M. High-rate anaerobic treatment of Fischer-Tropsch wastewater in a packed-bed biofilm reactor. Water Res. 2010, 44, 2745–2752. [Google Scholar] [CrossRef]
- Hua, Z.-l.; Gao, C.; Zhang, J.-y.; Li, X.-q. Perfluoroalkyl acids in the aquatic environment of a fluorine industry-impacted region: Spatiotemporal distribution, partition behavior, source, and risk assessment. Sci. Total Environ. 2023, 857, 159452. [Google Scholar] [CrossRef]
- Liu, S.; Jin, B.; Arp, H.P.H.; Chen, W.; Liu, Y.; Zhang, G. The Fate and Transport of Chlorinated Polyfluorinated Ether Sulfonates and Other PFAS through Industrial Wastewater Treatment Facilities in China. Environ. Sci. Technol. 2022, 56, 3002–3010. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Huang, S.; Zhang, H.; Ng, T.C.A.; Xu, B.; Shi, X.; Ng, H.Y. Analysis of N-Acy-L-homoserine lactones (AHLs) in wastewater treatment systems using SPE-LLE with LC-MS/MS. Water Res. 2020, 177, 115756. [Google Scholar] [CrossRef]
- Sun, Y.; He, K.; Yin, Q.; Echigo, S.; Wu, G.; Guan, Y. Determination of quorum-sensing signal substances in water and solid phases of activated sludge systems using liquid chromatography-mass spectrometry. J. Environ. Sci. 2018, 69, 85–94. [Google Scholar] [CrossRef]
- Wang, J.; Ding, L.; Li, K.; Schmieder, W.; Geng, J.; Xu, K.; Zhang, Y.; Ren, H. Development of an extraction method and LC-MS analysis for N-acylated-L-homoserine lactones (AHLs) in wastewater treatment biofilms. J. Chromatogr. B 2017, 1041, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence excitation—Emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Wu, Y.-H.; Luo, L.-W.; Wang, Q.; Tong, X.; Bai, Y.; Ni, X.-Y.; Wang, H.-B.; Chen, G.-Q.; Nozomu, I.; et al. Metagenomics analysis of the key functional genes related to biofouling aggravation of reverse osmosis membranes after chlorine disinfection. J. Hazard. Mater. 2021, 410, 124602. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Jiang, X.-T.; Chai, B.; Li, L.; Yang, Y.; Cole, J.R.; Tiedje, J.M.; Zhang, T. ARGs-OAP v2.0 with an expanded SARG database and Hidden Markov Models for enhancement characterization and quantification of antibiotic resistance genes in environmental metagenomes. Bioinformatics 2018, 34, 2263–2270. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, J.; Chen, H.; Bond, P.L.; Yuan, Z. Metagenomic analysis reveals wastewater treatment plants as hotspots of antibiotic resistance genes and mobile genetic elements. Water Res. 2017, 123, 468–478. [Google Scholar] [CrossRef]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef]
- Liou, J.S.C.; Szostek, B.; DeRito, C.M.; Madsen, E.L. Investigating the biodegradability of perfluorooctanoic acid. Chemosphere 2010, 80, 176–183. [Google Scholar] [CrossRef]
- Barker, D.J.; Stuckey, D.C. A review of soluble microbial products (SMP) in wastewater treatment systems. Water Res. 1999, 33, 3063–3082. [Google Scholar] [CrossRef]
- Farges, R.; Gharzouni, A.; Ravier, B.; Jeulin, P.; Rossignol, S. Insulating Foams and Dense Geopolymers from Biochar By-Products. J. Ceram. Sci. Technol. 2018, 9, 193–200. [Google Scholar]
- Cole, E.J.; Zandvakili, O.R.; Xing, B.; Hashemi, M.; Herbert, S.; Mashayekhi, H.H. Dataset on the effect of hardwood biochar on soil gravimetric moisture content and nitrate dynamics at different soil depths with FTIR analysis of fresh and aged biochar. Data Brief 2019, 25, 104073. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Wei, W.; Huang, Q.-S.; Wang, C.; Wang, Y.; Ni, B.-J. Insights into the microbial response of anaerobic granular sludge during long-term exposure to polyethylene terephthalate microplastics. Water Res. 2020, 179, 115898. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, S.; Liu, L.; Meng, F.; Wang, D.; Qiu, C. Effect of perfluorooctanoic acid on denitrifying phosphorus removal system under short-term stress. J. Environ. Sci. 2025, 154, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zheng, X.; Yang, S.; He, H.; Han, Z.; Li, W.; Lin, T.; Xu, H. Influence of perfluorooctanoic acid on alkaline anaerobic fermentation of waste activated sludge: Perspective from volatile fatty acids production and sludge reduction. J. Environ. Manag. 2024, 370, 122581. [Google Scholar] [CrossRef]
- Zhang, S.; Merino, N.; Wang, N.; Ruan, T.; Lu, X. Impact of 6:2 fluorotelomer alcohol aerobic biotransformation on a sediment microbial community. Sci. Total Environ. 2017, 575, 1361–1368. [Google Scholar] [CrossRef]
- Vackova, L.; Srb, M.; Stloukal, R.; Wanner, J. Comparison of denitrification at low temperature using encapsulated Paracoccus denitrificans, Pseudomonas fluorescens and mixed culture. Bioresour. Technol. 2011, 102, 4661–4666. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Bai, L.; Hou, C.; Zheng, C.; Li, W. Biodegradation of polypropylene microplastics by Bacillus pasteurii isolated from a gold mine tailing. Emerg. Contam. 2025, 11, 100397. [Google Scholar] [CrossRef]
- Bunbury, F.; Rivas, C.; Calatrava, V.; Malkovskiy, A.V.; Joubert, L.-M.; Parvate, A.D.; Evans, J.E.; Grossman, A.R.; Bhaya, D. Cyanobacteria and Chloroflexota cooperate to structure light-responsive biofilms. Proc. Natl. Acad. Sci. USA 2025, 122, e2423574122. [Google Scholar] [CrossRef]
- Dyksma, S.; Pester, M. Oxygen respiration and polysaccharide degradation by a sulfate-reducing acidobacterium. Nat. Commun. 2023, 14, 6337. [Google Scholar] [CrossRef]
- Cao, L.; Liao, Y.; Su, C.; Tang, L.; Qi, Z.; Wei, L.; Wu, J.; Gao, S. Effects of PFOA on the physicochemical properties of anaerobic granular sludge: Performance evaluation, microbial community and metagenomic analysis. J. Environ. Manag. 2022, 313, 114936. [Google Scholar] [CrossRef]
- Wang, B.; Peng, Y.; Guo, Y.; Zhao, M.; Wang, S. Illumina MiSeq sequencing reveals the key microorganisms involved in partial nitritation followed by simultaneous sludge fermentation, denitrification and anammox process. Bioresour. Technol. 2016, 207, 118–125. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, X.; Sun, Z. Degradation mechanism of montmorillonite-enhanced antibiotic wastewater: Performance, antibiotic resistance genes, microbial communities, and functional metabolism. Bioresour. Technol. 2022, 352, 127098. [Google Scholar] [CrossRef]
- Chen, C.; Yin, G.; Hou, L.; Liu, M.; Jiang, Y.; Zheng, D.; Gao, D.; Liu, C.; Zheng, Y.; Han, P. Effects of sulfamethoxazole on coupling of nitrogen removal with nitrification in Yangtze Estuary sediments. Environ. Pollut. 2021, 271, 116382. [Google Scholar] [CrossRef]
- Liu, S.; Chen, D.; Wang, Z.; Zhang, M.; Zhu, M.; Yin, M.; Zhang, T.; Wang, X. Shifts of bacterial community and molecular ecological network in activated sludge system under ibuprofen stress. Chemosphere 2022, 295, 133888. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Chi, Y.; Wang, Y.; Shi, X.; Jin, X.; Jin, P. Diversified metabolism makes novel Thauera strain highly competitive in low carbon wastewater treatment. Water Res. 2021, 206, 117742. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Liu, W.-Z.; Li, Z.-L.; Zhang, S.-M.; Chen, F.; Yu, H.-R.; Shao, S.-L.; Nan, J.; Wang, A.-J. High recycling efficiency and elemental sulfur purity achieved in a biofilm formed membrane filtration reactor. Water Res. 2018, 130, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Fujisawa, S.; Nakai, S.; Hosomi, M. Biodegradation of natural and synthetic estrogens by nitrifying activated sludge and ammonia-oxidizing bacterium Nitrosomonas europaea. Water Res. 2004, 38, 2323–2330. [Google Scholar] [CrossRef]
- Perez-Pantoja, D.; Donoso, R.; Agullo, L.; Cordova, M.; Seeger, M.; Pieper, D.H.; Gonzalez, B. Genomic analysis of the potential for aromatic compounds biodegradation in Burkholderiales. Environ. Microbiol. 2012, 14, 1091–1117. [Google Scholar] [CrossRef]
- Tong, H.; Hu, M.; Li, F.; Chen, M.; Lv, Y. Burkholderiales participating in pentachlorophenol biodegradation in iron-reducing paddy soil as identified by stable isotope probing. Environ. Sci.-Proc. Imp. 2015, 17, 1282–1289. [Google Scholar] [CrossRef]
- Mattmann, M.E.; Blackwell, H.E. Small Molecules That Modulate Quorum Sensing and Control Virulence in Pseudomonas aeruginosa. J. Org. Chem. 2010, 75, 6737–6746. [Google Scholar] [CrossRef]
- Ni, Q.; Chen, Y.; Lu, L.; Liu, M. C4-HSL-mediated quorum sensing regulates nitrogen removal in activated sludge process at Low temperatures. Environ. Res. 2024, 244, 117928. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Meng, J.; Sun, K. Understanding of signaling molecule controlled anammox through regulating C/N ratio. Bioresour. Technol. 2020, 315, 123863. [Google Scholar] [CrossRef]
- Chiavola, A.; Di Marcantonio, C.; Boni, M.R.; Biagioli, S.; Frugis, A.; Cecchini, G. Experimental investigation on the perfluorooctanoic and perfluorooctane sulfonic acids fate and behaviour in the activated sludge reactor. Process Saf. Environ. Prot. 2020, 134, 406–415. [Google Scholar] [CrossRef]
- Whiteley, M.; Diggle, S.P.; Greenberg, E.P. Progress in and promise of bacterial quorum sensing research. Nature 2017, 551, 313–320. [Google Scholar] [CrossRef]
- Zhang, B.; Li, W.; Guo, Y.; Zhang, Z.; Shi, W.; Cui, F.; Lens, P.N.L.; Tay, J.H. A sustainable strategy for effective regulation of aerobic granulation: Augmentation of the signaling molecule content by cultivating AHL-producing strains. Water Res. 2020, 169, 115193. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef] [PubMed]
- She, Z.; Zhao, L.; Zhang, X.; Jin, C.; Guo, L.; Yang, S.; Zhao, Y.; Gao, M. Partial nitrification and denitrification in a sequencing batch reactor treating high-salinity wastewater. Chem. Eng. J. 2016, 288, 207–215. [Google Scholar] [CrossRef]
- Wang, M.; Lian, Y.; Wang, Y.; Zhu, L. The role and mechanism of quorum sensing on environmental antimicrobial resistance. Environ. Pollut. 2023, 322, 121238. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wen, X.; Li, J.; Niu, Q.; Tang, A.; Li, Q. Metagenomic insights into role of red mud in regulating fate of compost antibiotic resistance genes mediated by both direct and indirect ways. Environ. Pollut. 2023, 317, 120795. [Google Scholar] [CrossRef]
- Xiang, Y.; Jia, M.; Xu, R.; Xu, J.; He, L.; Peng, H.; Sun, W.; Wang, D.; Xiong, W.; Yang, Z. Carbamazepine facilitated horizontal transfer of antibiotic resistance genes by enhancing microbial communication and aggregation. Bioresour. Technol. 2024, 391, 129983. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Zhao, M.; He, X.; Li, H. Biochar-Enhanced Nitrogen Removal in SBBR Under PFOA Stress: The Role of Quorum Sensing. Sustainability 2025, 17, 3359. https://doi.org/10.3390/su17083359
Lu Z, Zhao M, He X, Li H. Biochar-Enhanced Nitrogen Removal in SBBR Under PFOA Stress: The Role of Quorum Sensing. Sustainability. 2025; 17(8):3359. https://doi.org/10.3390/su17083359
Chicago/Turabian StyleLu, Zhiqi, Mengzhe Zhao, Xianglong He, and Hongjing Li. 2025. "Biochar-Enhanced Nitrogen Removal in SBBR Under PFOA Stress: The Role of Quorum Sensing" Sustainability 17, no. 8: 3359. https://doi.org/10.3390/su17083359
APA StyleLu, Z., Zhao, M., He, X., & Li, H. (2025). Biochar-Enhanced Nitrogen Removal in SBBR Under PFOA Stress: The Role of Quorum Sensing. Sustainability, 17(8), 3359. https://doi.org/10.3390/su17083359




