Botanical Evaluation of the Two-Year-Old Flower Strip with Analysis of the Local Carabidae Population: Case Study
Abstract
1. Introduction
2. Materials and Methods
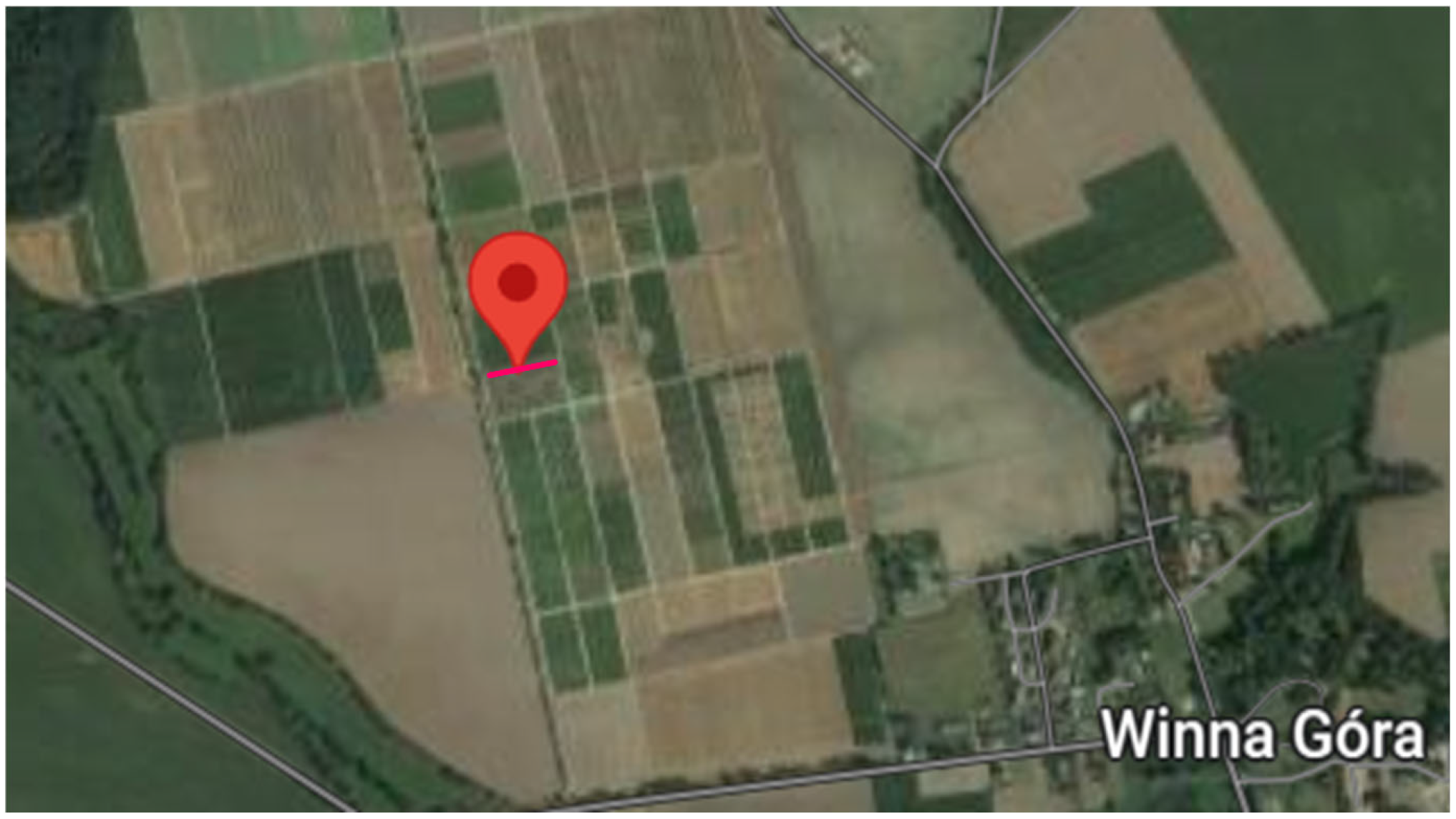
2.1. Study Area
2.2. Botanical Studies
2.3. Survey of Carabidae
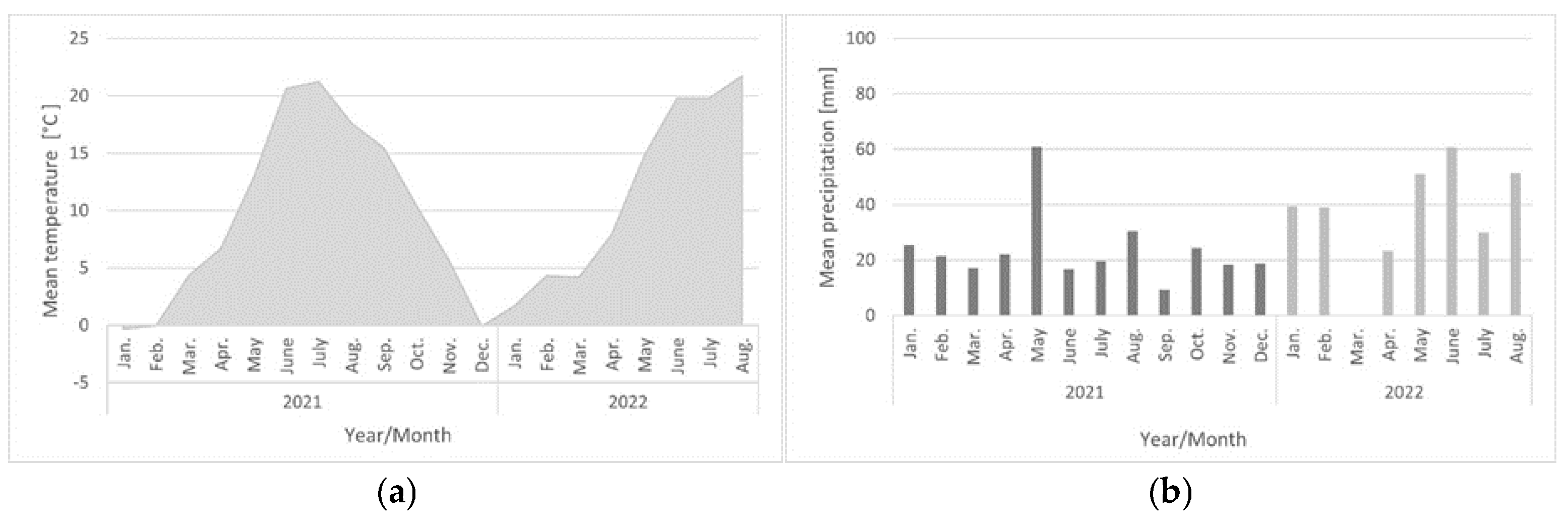
2.4. Location and Weather Conditions
2.5. Statistical Analysis
3. Results
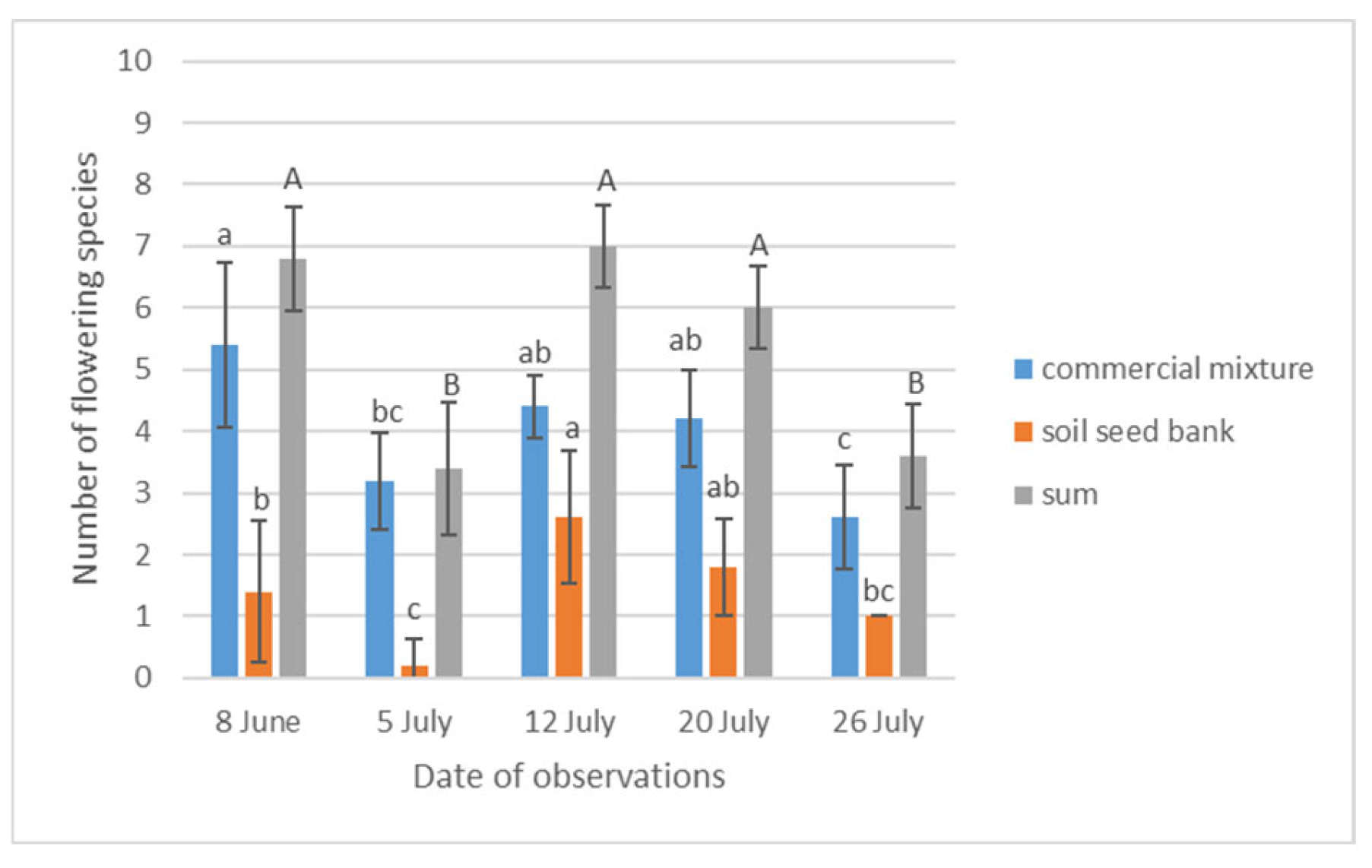
3.1. Botanical Studies
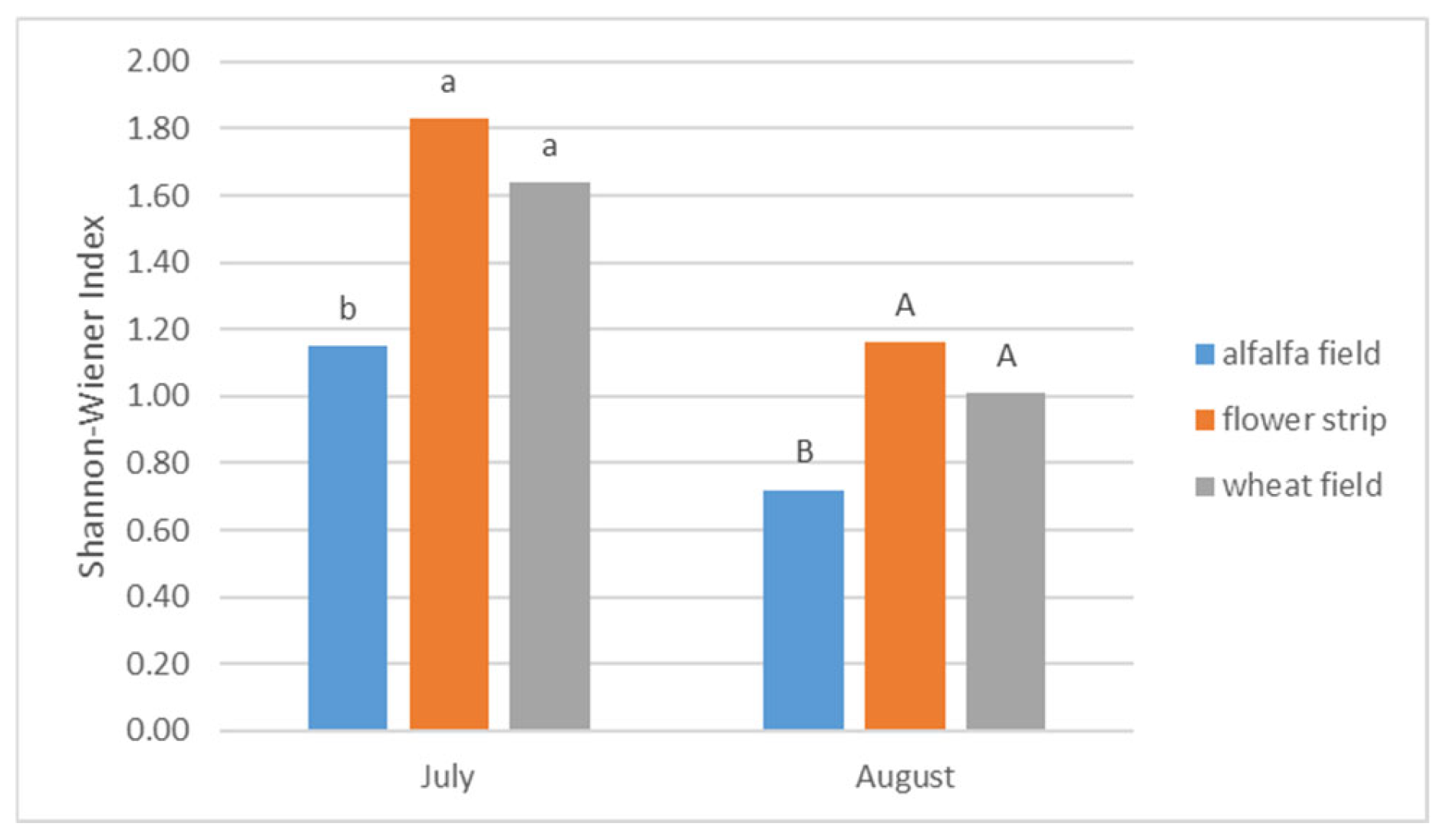
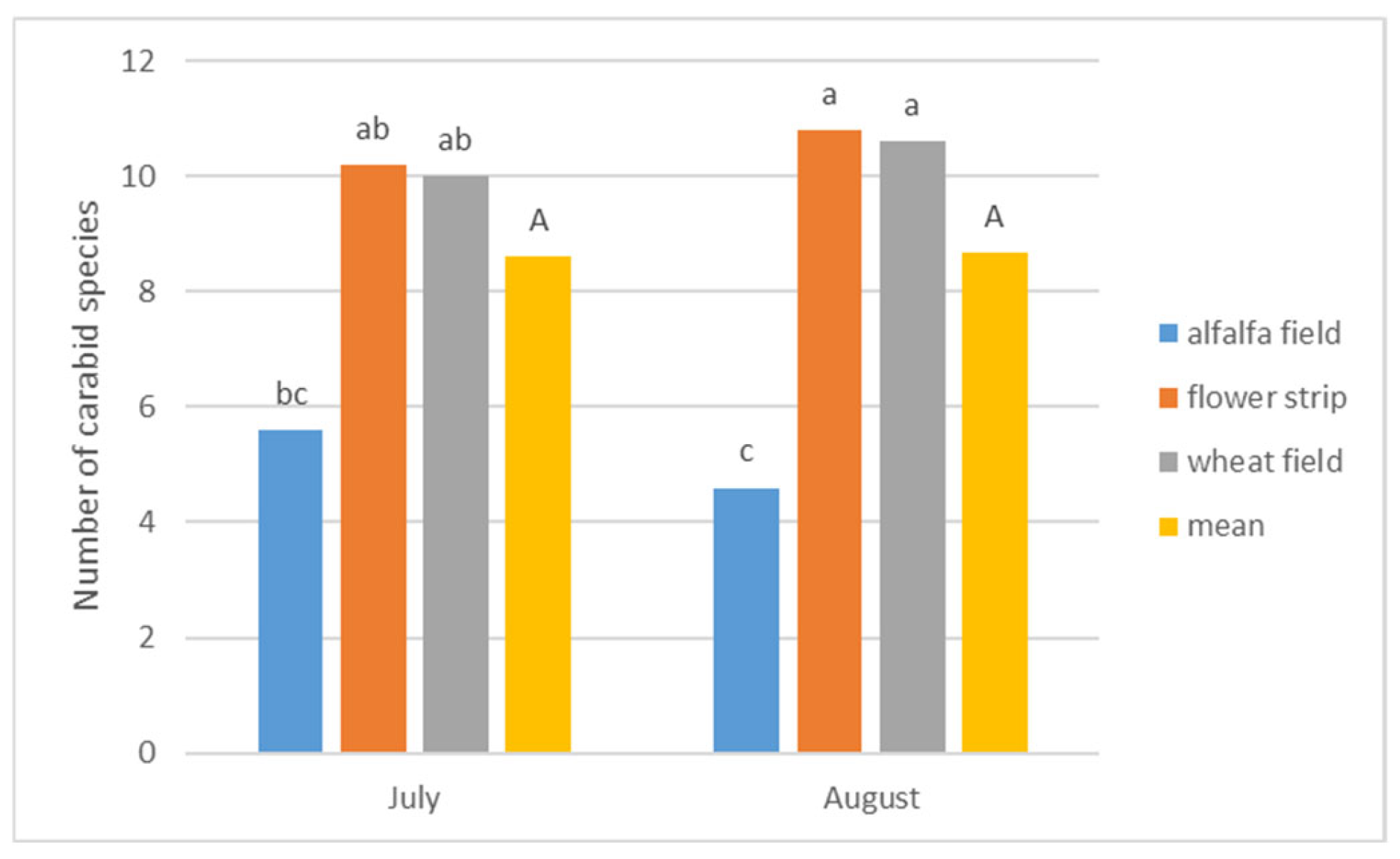
3.2. Survey of Carabidae
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matthews, A. Greening agricultural payments in the EU’s Common Agricultural Policy. Bio-Based Appl. Econ. 2013, 2, 1–114. [Google Scholar]
- Prandecki, K.; Wrzaszcz, W. Uwarunkowania środowiskowo-klimatyczne rozwoju rolnictwa w. In Środowiskowo-Klimatyczne U Warunkowania Rozwoju Rolnictwa i Obszarów Wiejskich w Polsce w Latach 2004–2030; Wrzaszcz, W., Wigier, M., Eds.; Instytut Ekonomiki Rolnictwa i Gospodarki Żywnościowej, Państwowy Instytut Badawczy: Warszawa, Poland, 2024; pp. 24–25. [Google Scholar]
- Kowalska, J.; Antkowiak, M.; Sienkiewicz, P. Flower strips and their ecological multifunctionality in agricultural fields. Agriculture 2022, 12, 1470. [Google Scholar] [CrossRef]
- Westbrook, A.; Morris, S.; Stup, R.; Xia, R.; Coffey, R.; DiTommaso, A. Annual Flower strips increase biodiversity even if planting is delayed. Ann. Appl. Biol. 2024, 185, 81–90. [Google Scholar] [CrossRef]
- Tschumi, M.; Albrecht, M.; Collatz, J.; Dubsky, V.; Entling, M.H.; Najar-Rodriguez, A.J.; Jacot, K. Tailored Flower strips promote natural enemy biodiversity and pest control in potato crops. J. Appl. Ecol. 2016, 53, 1169–1176. [Google Scholar] [CrossRef]
- Amy, C.; Noël, G.; Hatt, S.; Uyttenbroeck, R.; Van de Meutter, F.; Genoud, D.; Francis, F. Flower strips in wheat intercropping system: Effect on pollinator abundance and diversity in Belgium. Insects 2018, 9, 114. [Google Scholar] [CrossRef]
- Buhk, C.; Oppermann, R.; Schanowski, A.; Bleil, R.; Ludemann, J.; Maus, C. Flower strip networks offer promising long term effects on pollinator species richness in intensively cultivated agricultural areas. BMC Ecol. 2018, 18, 55. [Google Scholar]
- Szitár, K.; Deák, B.; Halassy, M.; Steffen, C.; Batáry, P. Combination of organic farming and Flower strips in agricultural landscapes—A feasible method to maximise functional diversity of plant traits related to pollination. Glob. Ecol. Conserv. 2022, 38, e02229. [Google Scholar]
- Tomalak, M. Chrząszcze—Biegaczowate. In Organizmy Pożyteczne w Środowisku Rolniczym; Sosnowska, D., Ed.; Instytut Ochrony Roślin–Państwowy Instytut Badawczy: Poznań, Poland, 2008; pp. 50–53. [Google Scholar]
- Kędzior, R. Struktura Zgrupowań Biegaczowatych (Coleoptera; Carabidae) na Terenach Zalewowych Rzek Górskich. Ph.D. Thesis, Uniwersytet Jagielloński, Kraków, Poland, 2010; p. 206. [Google Scholar]
- El-Danasoury, H.; Cerecedo, C.; Córdoba, M.; Iglesias-Piñeiro, J. Predation by the carabid beetle Harpalus rufipes on the pest slug Deroceras reticulatum in the laboratory. Ann. Appl. Biol. 2017, 170, 251–262. [Google Scholar] [CrossRef]
- Lundgren, J.G. Ground beetle (Coleoptera: Carabidae) ecology: Their function and diversity in natural and agricultural habitats. Am. Entomol. 2005, 51, 218. [Google Scholar]
- Teofilova, T. The ground beetles (Coleoptera: Carabidae) and their role as bio-agents. For. Sci. 2020, 125–142. [Google Scholar]
- Langraf, V.; Petrovičová, K.; David, S.; Krumpálová, Z.; Schlarmannová, J.; Hreusová, N.; Klimentová, V.; Kuricová, P.; Urban, M.; Šatarová, H. Changes in the dispersion of epigeic groups of animals in different types of agricultural crops. J. Cent. Eur. Agric. 2021, 22, 798–806. [Google Scholar] [CrossRef]
- Egorov, L.V.; Aleksanov, V.V.; Alekseev, S.K.; Ruchin, A.B.; Artaev, O.N.; Esin, M.N.; Lukiyanov, S.V.; Lobachev, E.A.; Semishin, G.B. Dataset: Biodiversity of ground beetles (Coleoptera, Carabidae) of the Republic of Mordovia (Russia). Data 2023, 8, 161. [Google Scholar] [CrossRef]
- Marja, R.; Kleijn, D.; Tscharntke, T.; Klein, A.M.; Frank, T.; Batáry, P. Effectiveness of agri-environmental management on pollinators is moderated more by ecological contrast than by landscape structure or land-use intensity. Ecol. Lett. 2019, 22, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Favarin, S.; Sommaggio, D.; Fantinato, E.; Masiero, M.; Bufa, G. Ecological intensifcation: Multifunctional fower strips suport benefcial arthropods in an organic apple orchard. Plant Ecol. 2024, 225, 499–509. [Google Scholar] [CrossRef]
- Gurr, G.M.; Wratten, S.D.; Landis, D.A.; You, M. Habitat management to suppress pest populations: Progress and prospects. Annu. Rev. Entomol. 2017, 62, 91–109. [Google Scholar] [CrossRef]
- Kujawa, K.; Bernacki, Z.; Arczyńska-Chudy, E.; Janku, K.; Karg, J.; Kowalska, J.; Oleszczuk, M.; Sienkiewicz, P.; Sobczyk, D.; Weyssenhoff, D. Kwietne pasy: Rzadko stosowane w Polsce narzędzie wzmacniania integrowanej ochrony roślin uprawnych oraz zwiększania różnorodności biologicznej na terenach rolniczych. J. Prog. Plant Prot. 2018, 58, 115–128. [Google Scholar]
- Scheper, J.; Holzschuh, A.; Kuussaari, M.; Potts, S.G.; Rundlöf, M.; Smith, H.G.; Kleijn, D. Environmental factors driving the effectiveness of European agri-environmental measures in mitigating pollinator loss—A meta-analysis. Ecol. Lett. 2013, 16, 912–920. [Google Scholar] [CrossRef]
- Available online: www.imgw.pl (accessed on 7 March 2025).
- Walkowska, A.; Półrolniczak, M.; Kolendowicz, L. Dynamika zmian sumy i struktury opadów w Wielkopolsce w latach 1981–2020. Badania Fizjogr. R. XIII–Ser. A–Geogr. Fiz. 2022, a73, 207–226. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeont. Electr. 2001, 4, 1. [Google Scholar]
- Czyżewska-Suchoń, N. Pasy kwietne—Rolnictwo przyjazne środowisku. Kuj.-Pomor. Ośrodek Doradz. Rol. W Minikowie KODR Minikowo 2021, 23, 39–40. [Google Scholar]
- Schmied, H.; Getrost, L.; Hamm, A.; Dünzkofer, T. The flower strip dilemma (FSD): An overlooked challenge in nature conservation and a possible first step towards a solution by combining different aged Flower strips. Agric. Ecosyst. Environ. 2023, 347, 108375. [Google Scholar] [CrossRef]
- Pankanin-Franczyk, M.; Bilewicz-Pawińska, T. Drapieżne owady (Chrysopidae, Coccinellidae, Nabidae, Anthocoridae, Syrphidae) w śródpolnych zbiorowiskach trawiastych. Wiad. Entomol. 2000, 19, 29–36. [Google Scholar]
- Antkowiak, M.; Kowalska, J.; Trzciński, P. Flower strips as an ecological tool to Strengthen the environmental balance of fields: Case study of a national park zone in western Poland. Sustainability 2024, 16, 1251. [Google Scholar] [CrossRef]
- Kowalska, J.; Antkowiak, M.; Tymoszuk, A. Effect of plant seed mixture on overwintering and floristic attractiveness of the flower strip in Western Poland. Agriculture 2023, 13, 467. [Google Scholar] [CrossRef]
- Ouvrard, P.; Transon, J.; Jacquemart, A.L. Flower-strip agri-environment schemes provide diverse and valuable summer flower resources for pollinating insects. Biodivers. Conserv. 2018, 27, 2193–2216. [Google Scholar]
- Holland, J.M.; Bianchi, F.J.; Entling, M.H.; Moonen, A.-C.; Smith, B.M.; Jeanneret, P. Structure function and management of semi-natural habitats for conservation biological control: A review of European studies. Pest. Manag. Sci. 2016, 72, 1638–1651. [Google Scholar]
- Bilski, Z.; Kajdan-Zysnarska, I. Uprawa roślin bobowatych drobnonasiennych. In Centrum Doradztwa Ekologicznego w Brwinowie; Oddział w Poznaniu: Poznań, Poland, 2019. [Google Scholar]
- Dobrzański, A. Zwalczanie chabra. Warzywa I Owoce Miękkie 2019, 10, 50–51. [Google Scholar]
- Ślósarz, J.; Kostuch, J. Wykorzystanie wybranych roślin miododajnych do poprawy bazy pożytkowej pszczoły miodnej. In Małopolski Ośrodek Doradztwa Rolniczego w Karniowicach; Małopolski Ośrodek Doradztwa Rolniczego w Karniowicach: Karniowice, Poland, 2019. [Google Scholar]
- Marczewska-Kolasa, K.; Sekutowski, T.; Bortniak, M. Determination of allelopathy Centaurea cyanus seeds by using bioassays. Prog. Plant Prot. 2012, 52, 733–736. [Google Scholar]
- Reddy, E.R.S.; Adi, B.S.; Dabolkar, J.; Adi, G.B. Chrysanthemum leucanthemum: A review. Int. J. Hom. Sci. 2019, 3, A:20–A:22. [Google Scholar]
- Babicki, J. Jastrun Zwyczajny = Złocień Właściwy = Jastrun Wczesny (Leucanthemum vulgare (Vail.) Lam. = Chrysanthemum leucanthemum L.)–Monography: Plant Pharmacology and Properties. 2019. Available online: https://www.researchgate.net/publication/360845106_Jastrun_zwyczajny_Zlocien_wlasciwy_jastrun_wczesny_Leucanthemum_vulgare_Vail_Lam_Chrysanthemum_leucanthemum_L_-_Monography_Plant_Pharmacology_and_Properties?channel=doi&linkId=628e38848d19206823da4fe2&showFulltext=true (accessed on 7 March 2025).
- Haouas, D.; Halima-Kamel, B.M.; Hamouda, B.M.H. Insecticidal activity of flower and leaf extracts from Chrysanthemum species against Tribolium confusum. Tunis. J. Plant Prot. 2008, 3, 87–93. [Google Scholar]
- Moradi, L.A.; Shafaghat, A. GC/MS analyzes of essential oil from Trifolium pretense. In Proceedings of the 5th International Conference on Recent Innovations, Chemistry and Chemical Engineering, Teheran, Iran, 15 September 2023; pp. 1–4. [Google Scholar]
- Ditner, N.; Balmer, O.; Beck, J.; Blick, T.; Nagel, P.; Luka, H. Effects of experimentally planting non-crop flowers into cabbage fields on the abundance and diversity of predators. Biodivers. Conserv. 2013, 22, 1049–1061. [Google Scholar]
- Twardowski, J.P.; Hurej, M.; Jaworska, T. An effect of strip-management on carabid beetles (Col., Carabidae) in sugar beet crop. J. Plant Prot. 2006, 46, 61–71. [Google Scholar]
- Kataev, B.M. Ground-Beetles of the Genus Harpalus Latr. (Coleoptera, Carabidae) of the World: Systematics, Zoogeography, Phylogeny. Ph.D. Thesis, Russian Academy of Sciences, Saint Petersburg, Russia, 2009; pp. 1–23. [Google Scholar]
- Luff, M. The biology of the ground beetle Harpalus rufipes in a strawberry field in Northumberland. Ann. Appl. Biol. 1980, 94, 153–164. [Google Scholar]
- Triquet, C.; Roume, A.; Wezel, A.; Tolon, V.; Ferrer, A. In-field cover crop strips support carabid communities and shape the ecological trait repartition in maize fields. Agric. For. Entomol. 2022, 25, 152–163. [Google Scholar] [CrossRef]
- Kosewska, A.; Skalski, T.; Nietupski, M. Effect of conventional and non-inversion tillage systems on the abundance and some life history traits of carabid beetles (Coleoptera: Carabidae) in winter triticale fields. Eur. J. Entomol. 2014, 111, 669–676. [Google Scholar] [CrossRef]
- Skłodowski, J. Zgrupowania biegaczowatych jako zooindykatory różnych sposobów przygotowania gleby na zrębach oraz ich zagospodarowania. Sylwan 2010, 154, 625–638. [Google Scholar]
- Devi, N. Sustainable agriculture. J. Adv. Sch. Res. Allied Educ. 2022, 19, 106–111. [Google Scholar]



| No. | Species | Family | Month of Flowering | Life Cycle |
|---|---|---|---|---|
| Commercial Mixture | ||||
| 1 | Calendula officinalis L. | Asteraceae | June–September | annual |
| 2 | Centaurea cyanus L. | July–September | annual | |
| 3 | Chrysanthemum leucanthemum L. | June–September | perennial | |
| 4 | Coreopsis tinctoria Nutt. | July–October | annual | |
| 5 | Cosmos bipinatus Cav. | July–October | annual | |
| 6 | Echinacea purpurea (L.) Moench | June–September | perennial | |
| 7 | Guizotia abyssinica (L. f.) Cass | August–September | annual | |
| 8 | Borago officinalis L. | Boraginaceae | June–August | annual |
| 9 | Echium creticum L. | July–September | annual | |
| 10 | Echium plantagineum L. | June–October | annual | |
| 11 | Echium vulgare L. | June–October | biennial | |
| 12 | Phacelia tanacetifolia Benth. | May–June | annual | |
| 13 | Gypsophila elegans M.Bieb. | Caryophyllaceae | June–August | perennial |
| 14 | Lotus corniculatus L. | Fabaceae | May–August | perennial |
| 15 | Lupinus angustifolius L. | June–September | annual | |
| 16 | Melilotus albus Medik. | June–October | biennial | |
| 17 | Melilotus officinalis (L.) Pallas | July–October | biennial | |
| 18 | Trifolium incarnatum L. | May–September | annual | |
| 19 | Trifolium pratense L. | May–September | perennial | |
| 20 | Vicia sativa L. | May–August | annual | |
| 21 | Dracocephalum moldavicum L. | Lamiaceae | July–August | annual |
| 22 | Melissa officinalis L. | June–September | perennial | |
| 23 | Origanum majorana L. | June–November | annual | |
| 24 | Salvia officinalis L. | May–June | perennial | |
| 25 | Linum usitatissimum L. | Linaceae | June–August | annual |
| 26 | Mirabilis jalapa L. | Nyctaginaceae | June–September | annual |
| 27 | Oenothera paradoxa Hudziok | Onagraceae | July–October | biennial |
| 28 | Papaver rhoeas L. | Papaveraceae | May–August | annual |
| 29 | Fagopyrum acutatum (Lehm.) Mansf. ex K.Hammer | Polygonaceae | July | annual |
| 30 | Nigella sativa L. | Ranunculaceae | May–September | annual |
| No. | Species | Family | Month of Flowering | Life Cycle | |||
|---|---|---|---|---|---|---|---|
| Soil Seed Bank | |||||||
| 1 | Chenopodium album L. | Amaranthaceae | June–October | annual | |||
| 2 | Anthemis arvensis L. | Asteraceae | May–October | annual/perennial | |||
| 3 | Artemisia vulgaris L. | July–September | perennial | ||||
| 4 | Matricaria chamomilla L. | May–August | annual | ||||
| 5 | Tripleurospermum maritimum (L.) W.D.J. Koch | June–September | annual | ||||
| 6 | Myosotis arvensis (L.) Hill | Boraginaceae | May–August | annual | |||
| 7 | Capsella bursa-pastoris | Brassicaceae | May–October | annual | |||
| 8 | Raphanus sativus L. var. oleiformis Pers. | May–October | annual | ||||
| 9 | Silene vulgaris (Salisb.) Sm. | Caryophyllaceae | May–September | perennial | |||
| 10 | Geranium pusillum L. | Geraniaceae | April–October | annual | |||
| 11 | Plantago lanceolata L. | Plantaginaceae | May–September | perennial | |||
| 12 | Rumex crispus L. | Polygonaceae | June–August | perennial | |||
| 13 | Viola tricolor L. | Violaceae | May–October | annual | |||
| No. | Species | Color of Inflorescences | Date of Inventory (2022) | ||||
| 8 June | 5 July | 12 July | 20 July | 26 July | |||
| Commercial Mixture | |||||||
| 1 | Calendula officinalis | orange | 0.48 | 0.00 | 0.00 | 0.86 | 2.13 |
| 2 | Centaurea cyanus | white/pink/blue/violet | 31.43 | 17.59 | 4.17 | 6.03 | 8.51 |
| 3 | Chrysanthemum leucanthemum | white/yellow | 15.71 | 1.85 | 2.98 | 2.59 | 0.00 |
| 4 | Coreopsis tinctoria | pink | 0.00 | 0.00 | 5.95 | 18.10 | 4.26 |
| 5 | Echium vulgare | violet | 1.43 | 3.70 | 1.19 | 1.72 | 8.51 |
| 6 | Gypsophila elegans | white | 9.05 | 0.00 | 0.00 | 0.00 | 0.00 |
| 7 | Melilotus albus | white | 0.00 | 0.00 | 2.38 | 3.45 | 6.38 |
| 8 | Melilotus officinalis | yellow | 0.95 | 1.85 | 1.19 | 0.00 | 0.00 |
| 9 | Trifolium incarnatum | dark pink | 8.10 | 0.00 | 0.00 | 0.00 | 0.00 |
| 10 | Trifolium pratense | pink | 9.52 | 67.59 | 41.07 | 56.90 | 38.30 |
| 11 | Papaver rhoeas | red | 0.00 | 0.00 | 1.19 | 0.86 | 0.00 |
| 12 | Fagopyrum acutatum | white/pink | 8.10 | 0.00 | 0.00 | 0.00 | 0.00 |
| Soil Seed Bank | |||||||
| 1 | Chenopodium album | green | 0.00 | 0.00 | 1.19 | 0.00 | 0.00 |
| 2 | Anthemis arvensis | white/yellow | 0.00 | 0.00 | 23.81 | 0.00 | 0.00 |
| 3 | Artemisia vulgaris | green/yellow | 0.00 | 0.00 | 0.00 | 4.31 | 4.26 |
| 4 | Matricaria chamomilla | white/yellow | 0.00 | 7.41 | 4.76 | 0.00 | 0.00 |
| 5 | Tripleurospermum maritimum | white/yellow | 0.00 | 0.00 | 0.00 | 0.00 | 25.53 |
| 6 | Myosotis arvensis | blue | 0.00 | 0.00 | 0.00 | 0.86 | 0.00 |
| 7 | Capsella bursa-pastoris | white | 8.10 | 0.00 | 0.00 | 0.00 | 0.00 |
| 8 | Raphanus sativus | white | 0.00 | 0.00 | 5.95 | 0.00 | 0.00 |
| 9 | Silene vulgaris | white/pink | 0.00 | 0.00 | 1.79 | 3.45 | 0.00 |
| 10 | Geranium pusillum | violet | 4.29 | 0.00 | 0.00 | 0.00 | 0.00 |
| 11 | Plantago lanceolata | green/white | 0.00 | 0.00 | 0.60 | 0.00 | 2.13 |
| 12 | Rumex crispus | green/white/pink | 0.00 | 0.00 | 1.79 | 0.86 | 0.00 |
| 13 | Viola tricolor | violet/white/yellow | 2.86 | 0.00 | 0.00 | 0.00 | 0.00 |
| Date of Inventory | (SD) | Me * (Min./Max.) | Mean Rank | Kruskal-Wallis Test (4, N = 25) |
|---|---|---|---|---|
| 8 June | 38.60 (±12.89) | 38.00 (25.00/60.00) | 19.30 a ** | H = 14.498 p =0.0059 |
| 5 July | 21.60 (±5.95) | 20.00 (14.00/29.00) | 12.00 ab | |
| 12 July | 33.60 (±8.31) | 35.00 (23.00/46.00) | 18.00 a | |
| 20 July | 23.20 (±10.19) | 25.00 (11.00/36.00) | 12.20 ab | |
| 26 July | 9.60 (±2.24) | 11.00 (6.00/12.00) | 3.50 b | |
| Test statistics | χ2 = 8.974 | df = 4 | p = 0.0617 | |
| Significant difference | 1–5 | 3–5 | ||
| Species | Site (Preferences to Habitats) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alfalfa Field | FS | Wheat Field | ||||||||||
| July | August | July | August | July | August | |||||||
| IS | [%] | IS | [%] | IS | [%] | IS | [%] | IS | [%] | IS | [%] | |
| Agonum muelleri (Herbst) | 0.00 | 0.00 | 0.00 | 0.00 | 2.00 | 1.49 | 0.00 | 0.00 | 3.00 | 1.69 | 0.00 | 0.00 |
| Amara aenea (De Geer) | 1.00 | 1.10 | 1.00 | 0.27 | 5.00 | 3.73 | 2.00 | 0.28 | 5.00 | 2.82 | 12.00 | 1.78 |
| Amara aulica (Panz.) | 0.00 | 0.00 | 0.00 | 0.00 | 2.00 | 1.49 | 17.00 | 2.34 | 4.00 | 2.26 | 0.00 | 0.00 |
| Amara bifrons (Gyll.) | 0.00 | 0.00 | 0.00 | 0.00 | 5.00 | 3.73 | 1.00 | 0.14 | 1.00 | 0.56 | 0.00 | 0.00 |
| Amara lunicollis (Schiödte) | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.75 | 1.00 | 0.14 | 0.00 | 0.00 | 1.00 | 0.15 |
| Amara plebeja (Gyll.) | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.75 | 1.00 | 0.14 | 1.00 | 0.56 | 0.00 | 0.00 |
| Amara similata (Dej.) | 0.00 | 0.00 | 0.00 | 0.00 | 3.00 | 2.24 | 5.00 | 0.69 | 6.00 | 3.39 | 2.00 | 0.30 |
| Anisodactylus binotatus (F.) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.14 | 0.00 | 0.00 | 0.00 | 0.00 |
| Bembidion lampros (Herbst) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.14 | 0.00 | 0.00 | 1.00 | 0.15 |
| Bembidion properans (Steph.) | 7.00 | 7.69 | 1.00 | 0.27 | 11.00 | 8.21 | 0.00 | 0.00 | 10.00 | 5.65 | 2.00 | 0.30 |
| Bembidion quadrimaculatum (L.) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.00 | 1.13 | 2.00 | 0.30 |
| Calathus ambiguus (Payk.) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.56 | 2.00 | 0.30 |
| Calathus erratus (Schalb.) | 0.00 | 0.00 | 10.00 | 2.67 | 6.00 | 4.48 | 15.00 | 2.07 | 2.00 | 1.13 | 13.00 | 1.93 |
| Calathus fuscipes (Goeze) | 2.00 | 2.20 | 40.00 | 10.67 | 7.00 | 5.22 | 36.00 | 4.97 | 3.00 | 1.69 | 32.00 | 4.74 |
| Calathus melanocephalus (L.) | 1.00 | 01.10 | 2.00 | 0.53 | 1.00 | 0.75 | 3.00 | 0.41 | 1.00 | 0.56 | 1.00 | 0.15 |
| Calathus rotundicollis (Dej.) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.14 | 0.00 | 0.00 | 0.00 | 0.00 |
| Clivina fossor (L.) | 1.00 | 1.10 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.15 |
| Dolichus halensis (Schaller) | 5.00 | 5.49 | 5.00 | 1.33 | 7.00 | 5.22 | 147.00 | 20.28 | 1.00 | 0.56 | 4.00 | 0.59 |
| Harpalus affinis (Schalb.) | 0.00 | 0.00 | 4.00 | 01.07 | 0.00 | 0.00 | 2.00 | 0.28 | 9.00 | 05.08 | 18.00 | 2.67 |
| Harpalus anxius (Duft.) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.00 | 0.44 |
| Harpalus froelichii (Sturm) | 2.00 | 2.20 | 3.00 | 0.80 | 3.00 | 2.24 | 4.00 | 0.55 | 0.00 | 0.00 | 1.00 | 0.15 |
| Harpalus griseus (Panz.) | 4.00 | 4.40 | 0.00 | 0.00 | 5.00 | 3.73 | 3.00 | 0.41 | 1.00 | 0.56 | 7.00 | 01.04 |
| Harpalus luteicornis (Duft.) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.56 | 0.00 | 0.00 |
| Harpalus distinguendus (Duft.) | 2.00 | 2.20 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.14 | 4.00 | 2.26 | 0.00 | 0.00 |
| Harpalus rufipes (De Geer) | 55.00 | 60.44 | 300.00 | 80.00 | 59.00 | 44.03 | 451.00 | 62.21 | 89.00 | 50.28 | 528.00 | 78.22 |
| Harpalus signaticornis (Duft.) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.00 | 1.69 | 19.00 | 2.81 |
| Harpalus smaragdinus (Duft.) | 2.00 | 2.20 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 6.00 | 3.39 | 7.00 | 01.04 |
| Harpalus tardus (Panz.) | 2.00 | 2.20 | 0.00 | 0.00 | 1.00 | 0.75 | 1.00 | 0.14 | 0.00 | 0.00 | 0.00 | 0.00 |
| Harpalus pumilus (Sturm) | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.75 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Harpalus xanthopus (Schaub.) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.15 |
| Anchomenus dorsalis (Pont.) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 11.00 | 1.52 | 0.00 | 0.00 | 0.00 | 0.00 |
| Loricera pilicorns (F.) | 2.00 | 2.20 | 0.00 | 0.00 | 2.00 | 1.49 | 1.00 | 0.14 | 0.00 | 0.00 | 0.00 | 0.00 |
| Microlestes minutulus (Goeze) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 7.00 | 3.95 | 0.00 | 0.00 |
| Poecilus cupreus (L.) | 0.00 | 0.00 | 1.00 | 0.27 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Poecilus lepidus (Leske) | 0.00 | 0.00 | 2.00 | 0.53 | 1.00 | 0.75 | 1.00 | 0.14 | 15.00 | 8.47 | 14.00 | 02.07 |
| Poecilus versicolor (Sturm) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.00 | 0.28 | 0.00 | 0.00 | 0.00 | 0.00 |
| Pterostichus melanarius (Ill.) | 3.00 | 3.30 | 6.00 | 1.60 | 5.00 | 3.73 | 16.00 | 2.21 | 1.00 | 0.56 | 3.00 | 0.44 |
| Syntomus foveatus (Geoff.) | 0.00 | 0.00 | 0.00 | 0.00 | 3.00 | 2.24 | 0.00 | 0.00 | 1.00 | 0.56 | 0.00 | 0.00 |
| Trechus quadristriatus (Schrank) | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.75 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Zabrus tenebrionides (Goeze) | 2.00 | 2.20 | 0.00 | 0.00 | 2.00 | 1.49 | 1.00 | 0.14 | 0.00 | 0.00 | 1.00 | 0.15 |
| Individuals Species (Σ) | 91.00 | 375.00 | 134.00 | 725.00 | 177.00 | 675.00 | ||||||
| Habitats | (SD) | Me * (Min./Max.) | Mean Rank | Kruskal-Wallis Test (2, N = 30) |
|---|---|---|---|---|
| Alfalfa Field | 46.60 (±63.34) | 22.00 (4.00/210.00) | 11.45 | H = 3.213 p = 0.2006 |
| Flower Strip | 85.90 (±74.45) | 50.50 (20.00/215.00) | 17.90 | |
| Wheat Field | 85.20 (±82.09) | 55.00 (4.00/249.00) | 17.15 | |
| Test Statistics | χ2 = 3.20 | df = 2 | p = 0.2019 | |
| Significant difference | not significant | |||
| Habitats | Month | |
|---|---|---|
| July | August | |
| Alfalfa Field | 18.20 b | 75.00 a |
| Flower Strip | 26.80 b | 145.00 a |
| Wheat Field | 35.40 b | 135.00 a |
| Mean | 26.80 B | 118.33 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalska, J.; Antkowiak, M.; Tymoszuk, A.; Matysiak, K.; Sienkiewicz, P. Botanical Evaluation of the Two-Year-Old Flower Strip with Analysis of the Local Carabidae Population: Case Study. Sustainability 2025, 17, 3223. https://doi.org/10.3390/su17073223
Kowalska J, Antkowiak M, Tymoszuk A, Matysiak K, Sienkiewicz P. Botanical Evaluation of the Two-Year-Old Flower Strip with Analysis of the Local Carabidae Population: Case Study. Sustainability. 2025; 17(7):3223. https://doi.org/10.3390/su17073223
Chicago/Turabian StyleKowalska, Jolanta, Małgorzata Antkowiak, Alicja Tymoszuk, Kinga Matysiak, and Paweł Sienkiewicz. 2025. "Botanical Evaluation of the Two-Year-Old Flower Strip with Analysis of the Local Carabidae Population: Case Study" Sustainability 17, no. 7: 3223. https://doi.org/10.3390/su17073223
APA StyleKowalska, J., Antkowiak, M., Tymoszuk, A., Matysiak, K., & Sienkiewicz, P. (2025). Botanical Evaluation of the Two-Year-Old Flower Strip with Analysis of the Local Carabidae Population: Case Study. Sustainability, 17(7), 3223. https://doi.org/10.3390/su17073223










