Effect of Silver Nanoparticles and Vermicompost on the Control of Aphelenchoides fragariae and Meloidogyne hapla in Jerusalem Artichoke (Helianthus tuberosus L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
- Control—no amendments;
- (Ag+)—silver nanoparticle solution (dose 120 mg per 1 L soil);
- (Ve)—20 L of vermicompost (with 150–200 specimens of E. fetida: 30% adult and 70% larvae).
2.2. Vermicompost Preparation
2.3. Silver Nanoparticle Application
2.4. Soil Analysis
2.5. Physiological Parameters of Plants
2.6. Nematode Extraction
2.7. Microorganisms in Soil and Dehydrogenase Activity
3. Results
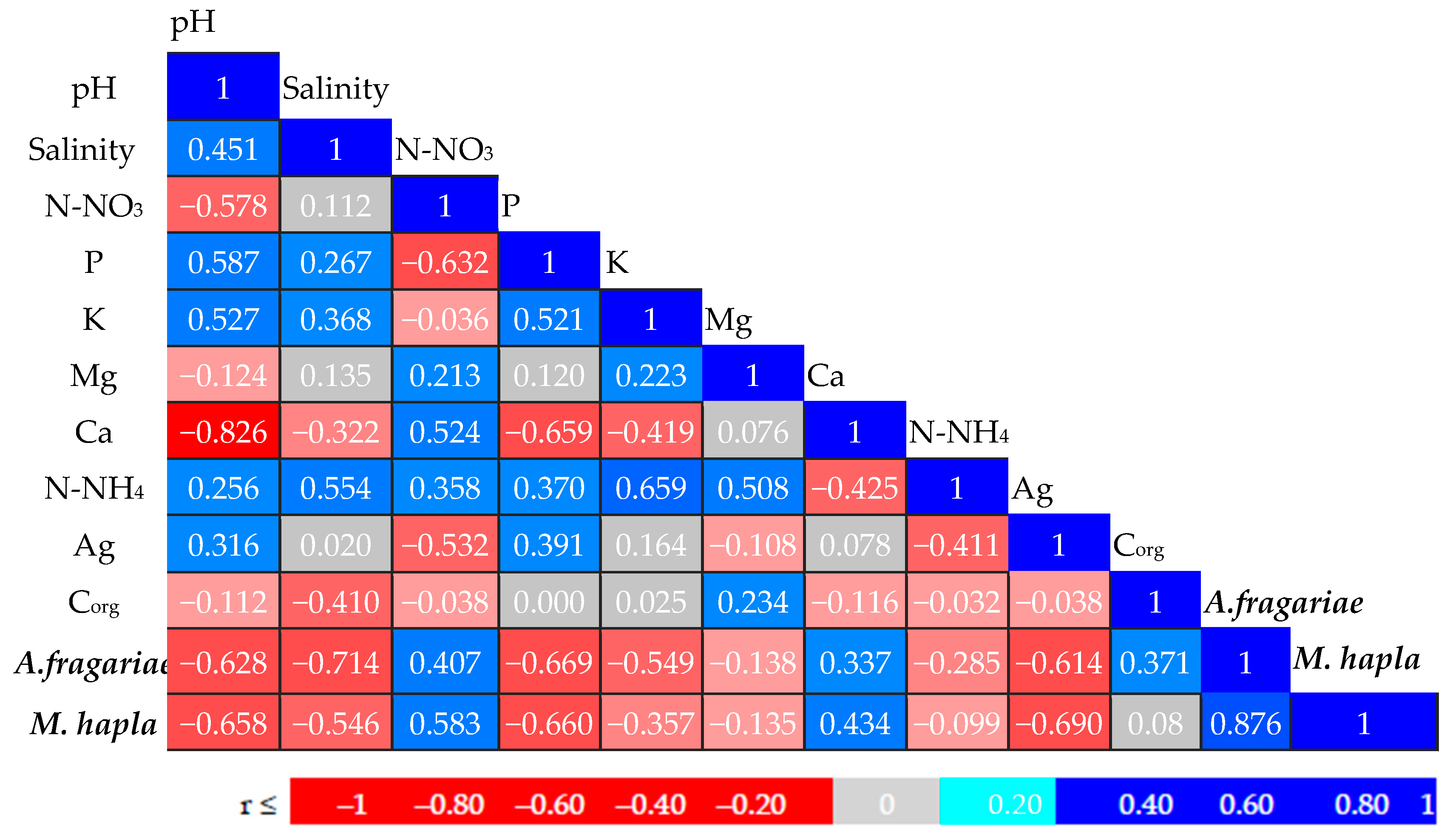
3.1. Soil Properties
3.2. Biometric Parameters of Plants
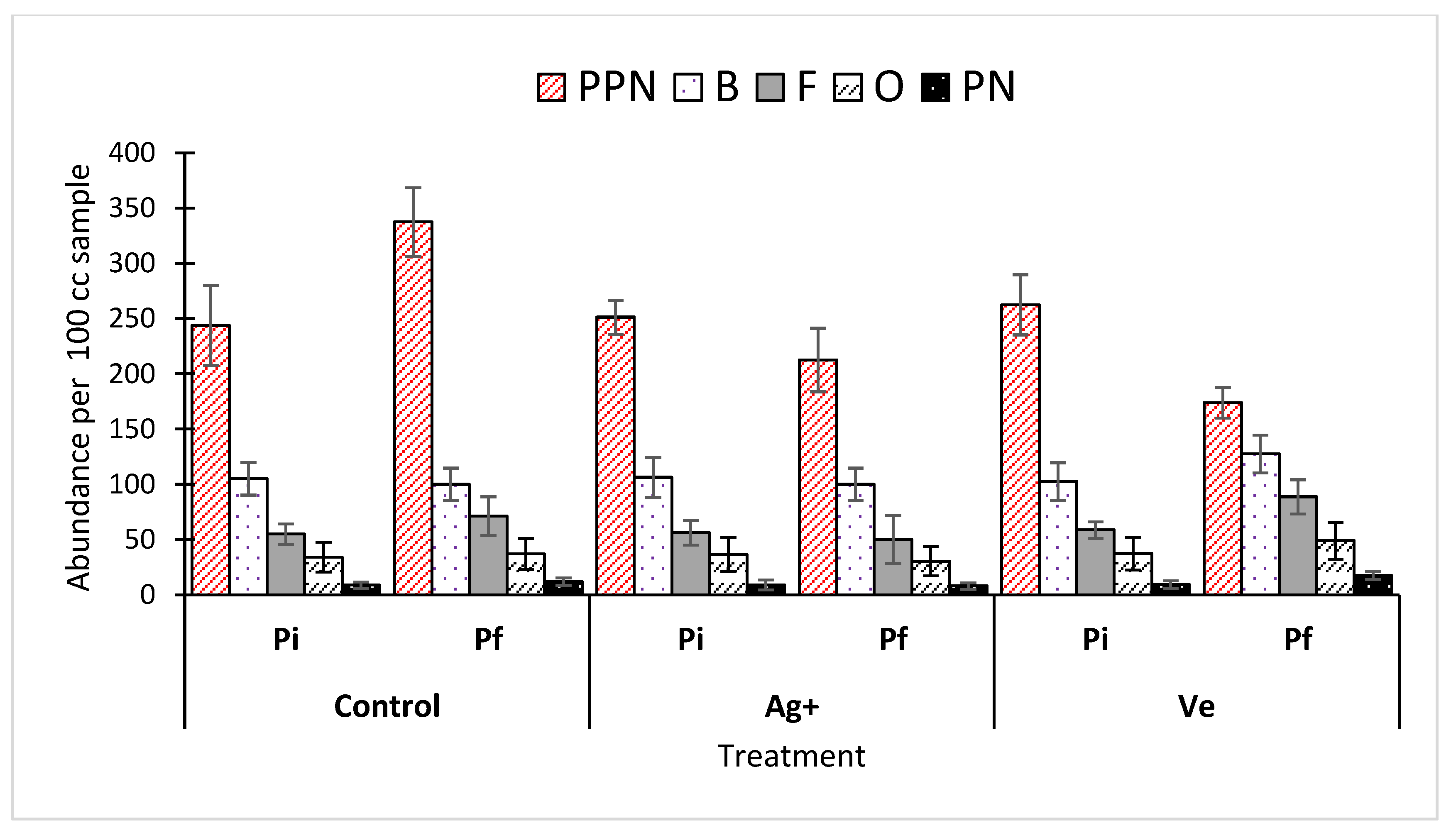
3.3. Nematode Population
3.4. Evaluations of the Microorganisms and Dehydrogenase Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matías, J.; Encinar, J.M.; González, J.; González, J.F. Optimisation of ethanol fermentation of Jerusalem artichoke tuber juice using simple technology for a decentralised and sustainable ethanol production. Energy Sustain. Dev. 2015, 25, 34–39. [Google Scholar] [CrossRef]
- Piskier, T. A method of estimation of the caloric value of the biomass. Part I. Biomass energy potential. J. Mech. Energy Eng. 2017, 1, 189–194. Available online: https://bibliotekanauki.pl/articles/95285 (accessed on 28 November 2024).
- Epie, K.E.; Santanen, A.; Mäkelä, P.S.A.; Stoddard, F.L. Fertilizer and intercropped legumes as nitrogen source for Jerusalem artichoke (Helianthus tuberosus L.) tops for bioenergy. Agric. Food Sci. 2018, 27, 199–205. [Google Scholar] [CrossRef]
- Rossini, F.; Provenzano, M.E.; Kuzmanović, L.; Ruggeri, R. Jerusalem artichoke (Helianthus tuberosus L.): A versatile and sustainable crop for renewable energy production in Europe. Agronomy 2019, 9, 528. [Google Scholar] [CrossRef]
- Jankowski, K.J.; Dubis, B.; Kozak, M. Sewage sludge and the energy balance of Jerusalem artichoke production. A six-year field experiment in Poland. Energy 2021, 276, 121545. [Google Scholar] [CrossRef]
- Bogucka, B.; Jankowski, K.J. The effect of harvest strategy on the energy potential of Jerusalem artichoke. Ind. Crops Prod. 2022, 177, 114473. [Google Scholar] [CrossRef]
- Jankowski, K.J.; Dubis, B. Jerusalem artichoke: Nitrogen Fertilization Strategy and Energy Balance in the Production Technology of Aerial Biomass. Energies 2024, 17, 5202. [Google Scholar] [CrossRef]
- Cieślik, E.; Gębusia, A.; Florkiewicz, A.; Mickowska, B. The content of protein and of amino acids in Jerusalem artichoke tubers (Helianthus tuberosus L.) of red variety Rote Zonenkugel. Acta Sci. Pol. Technol. Aliment. 2011, 10, 433–441. Available online: https://pubmed.ncbi.nlm.nih.gov/22230925/ (accessed on 28 November 2024).
- Sawicka, B.; Skiba, D.; Pszczółkowski, P.; Aslan, I.; Sharifi-Rad, J.; Krochmal-Marczak, B. Jerusalem artichoke (Helianthus tuberosus L.) as a medicinal plant and its natural products. Cell Mol. Biol. 2020, 66, 160–177. Available online: https://pubmed.ncbi.nlm.nih.gov/32583794/ (accessed on 28 November 2024). [CrossRef]
- Manokhina, A.A.; Dorokhov, A.S.; Kobozeva, T.P.; Fomina, T.N.; Starovoitov, V.I. Jerusalem artichoke as a Strategic Crop for Solving Food Problems. Agronomy 2022, 12, 465. [Google Scholar] [CrossRef]
- Cornescu, G.M.; Panaite, T.D.; Soica, C.; Cismileanu, A.; Matache, C.C. Jerusalem artichoke (Helianthus tuberosus L.) as a Promising Dietary Feed Ingredient for Monogastric Farm Animals. Appl. Sci. 2023, 13, 12748. [Google Scholar] [CrossRef]
- Breton, C.; Kiru, S.D.; Bervillé, A.; Anushkevich, N.Y. Breeding of Jerusalem artichoke with the desired traits for different directions of use: Retrospective, approaches, and prospects. Agric. Biol. 2017, 52, 940–951. [Google Scholar] [CrossRef][Green Version]
- Long, X.H.; Shao, H.B.; Liu, L.; Liu, L.P.; Liu, Z.P. Jerusalem artichoke: A sustainable biomass feedstock for biorefinery. Renew. Sustain. Energy Rev. 2016, 54, 1382–1388. [Google Scholar] [CrossRef]
- Manokhina, A.A.; Dorokhov, A.S.; Kobozeva, T.P.; Fomina, T.N.; Starovoitova, O.A. Varietal Characteristics of Jerusalem artichoke as a High Nutritional Value Crop for Herbivorous Animal Husbandry. Appl. Sci. 2022, 12, 4507. [Google Scholar] [CrossRef]
- Antonkiewicz, J.; Kołodziej, B.; Bielińska, E.J.; Witkowicz, R.; Tabor, S. Using Jerusalem artichoke to Extract Heavy Metals from Municipal Sewage Sludge Amended Soil. Pol. J. Environ. Stud. 2018, 27, 513–527. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Sood, P. Role of Microbial Enriched Vermicompost in Plant-Parasitic Nematode Management. In Nematodes-Recent Advances, Management and New Perspectives; Chapter 3; IntechOpen: London, UK, 2022; Available online: https://www.intechopen.com/chapters/77343 (accessed on 28 November 2024). [CrossRef]
- Gimenez, E.; Salinas, M.; Manzano-Agugliaro, F. Worldwide Research on Plant Defense against Biotic Stresses as Improvement for Sustainable Agriculture. Sustainability 2018, 10, 391. [Google Scholar] [CrossRef]
- Rehman, S.U.; De Castro, F.; Aprile, A.; Benedetti, M.; Fanizzi, F.P. Vermicompost: Enhancing Plant Growth and Combating Abiotic and Biotic Stress. Agronomy 2023, 13, 1134. [Google Scholar] [CrossRef]
- Yatoo, A.M.; Ali, M.N.; Baba, Z.A.; Hassan, B. Sustainable management of diseases and pests in crops by vermicompost and vermicompost tea. A review. Agron. Sustain. Dev. 2021, 41, 7. [Google Scholar] [CrossRef]
- Chatterjee, R.; Debnath, A.; Mishra, S. Vermicompost and Soil Health. In Soil Health; Giri, B., Varma, A., Eds.; Soil Biology; Springer: Cham, Switzerland, 2020; Volume 59, pp. 69–88. [Google Scholar] [CrossRef]
- Gudeta, K.; Bhagat, A.; Julka, J.M.; Sinha, R.; Verma, R.; Kumar, A.; Kumari, S.; Ameen, F.; Bhat, S.A.; Amarowicz, R.; et al. Vermicompost and Its Derivatives against Phytopathogenic Fungi in the Soil: A Review. Horticulturae 2022, 8, 311. [Google Scholar] [CrossRef]
- Ntalli, N.; Adamski, Z.; Doula, M.; Monokrousos, N. Nematicidal Amendments and Soil Remediation. Plants 2020, 9, 429. [Google Scholar] [CrossRef]
- Silva, L.P.; Silveira, A.P.; Bonatto, C.C.; Reis, I.G.; Milreu, P.V. Silver Nanoparticles as Antimicrobial Agents: Past, Present, and Future. In Nanostructures for Antimicrobial Therapy; Nanostructures in Therapeutic Medicine Series; Elsevier: Amsterdam, The Netherlands, 2017; pp. 577–596. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.I.; Qari, H.A.; Umar, K.; Mohamad Ibrahim, M.N. Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications: A Review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanoparticle Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Dobosz, R.; Gawlak, M.; Karnkowski, W. Instrukcja Rozpoznawania Gatunków z Rodzaju Meloidogyne; Instytut Ochrony Roślin. Państwowy Instytut Badawczy: Poznan, Poland, 2008; ISBN 9788389867360. Available online: https://www.ior.poznan.pl/1004,2014?nobreakup#plik_2125 (accessed on 28 November 2024).
- Nowosielski, O. Rules for Fertilizer Recommendations in Gardening; PWRiL: Warsaw, Poland, 1988; Volume 310, Available online: https://www.scirp.org/reference/referencespapers?referenceid=2468331 (accessed on 28 November 2024).
- Boss, C.H.; Fredeen, K.J. Concepts, Instrumentation, and Techniques in Inductively Coupled Plasma Optical Emission Spectrometry, 3rd ed.; Perkin-Elmer: Shelton, CT, USA, 2004; Available online: https://www.scirp.org/reference/referencespapers?referenceid=2215107 (accessed on 28 November 2024).
- Available online: https://www.dijkstra.net/media/catalog/category/Dijkstra_Vereenigde_Gerhardt_Dumatherm.pdf (accessed on 28 November 2024).
- Zapałowska, A.; Skwiercz, A.T. Populations of parasitic nematodes colonizing Jerusalem artichoke (Helianthus tuberosus L.). Acta Soc. Bot. Pol 2018, 87, 3578. [Google Scholar] [CrossRef]
- Seinhorst, J.W. A rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematology 1959, 4, 67–69. [Google Scholar] [CrossRef]
- Brzeski, M.W. Nematodes of Tylenchina in Poland and Temperate Europe; Museum and Institute of Zoology, Polish Academy of Sciences: Warsaw, Poland, 1998. [Google Scholar]
- Andrássy, I. Free-living nematodes of Hungary. In Nematoda Errantia; Vol. II; Pedozoologica Hungarica, Volume 4; Hungarian Natural History Museum, Systematic Zoology Research Group of the Hungarian Academy of Sciences: Budapest, Hungary, 2007. [Google Scholar]
- Sinclair, J.B.; Dhingra, O.D. Basic Plant Pathology Methods, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar] [CrossRef]
- Gould, W.D.; Hagedorn, C.; Bardinelli, T.R.; Zablotowicz, R.M. New selective media for enumeration and recovery of fluorescent pseudomonads from various habitats. Appl. Environ. Microbiol. 1985, 49, 28–32. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC238339/ (accessed on 28 November 2024).
- Pikovskaya, R.I. Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Microbiology 1948, 17, 362–370. [Google Scholar]
- Martin, P. Use of acid, rose Bengal and streptomycin in the plate method for estimating soil fungi. Soil Sci. 1950, 69, 215–232. [Google Scholar] [CrossRef]
- Brzezińska, M.; Włodarczyk, T. Enzymy wewnątrzkomórkowych przemian redoks (oksydoreduktazy). Acta Agrophysica Rozpr. I Monogr. 2005, 3, 11–26. Available online: http://www.old.acta-agrophysica.org/artykuly/acta_agrophysica/ActaAgr_120_2005_0_3_11.pdf (accessed on 28 November 2024).
- Casida, L.; Johnson, J.; Klein, D. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Abdellatif, K.; Hamouda, R.; El-Ansary, M. Green Nanoparticles Engineering on Root-knot Nematode Infecting Eggplant plants and Their Effect on Plant DNA Modification. Iran. J. Biotechnol. 2016, 14, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Skwiercz, A.; Stefanovska, T.; Zhukov, O.; Zapałowska, A.; Masłoń, A. Effect of Silver Nanoparticles and Vermicompost on the Control of Longidorus elongatus (De Man, 1876) in Miscanthus × Giganteus and Its Growth and Development. Sustainability 2024, 16, 8093. [Google Scholar] [CrossRef]
- Khan, Z.; Son, S.H.; Moon, H.S.; Kim, S.G.; Shin, H.D.; Jeon, Y.H.; Kim, Y.H. Description of a Foliar Nematode, Aphelenchoides fragariae (Nematoda: Aphelenchida) with Additional Characteristics from Korea. J. Asia-Pac. Entomol. 2007, 10, 313–315. [Google Scholar] [CrossRef]
- Topalović, O.; Geisen, S. Nematodes as suppressors and facilitators of plant performance. New Phytol 2023, 238, 2305–2312. [Google Scholar] [CrossRef]
- Lazcano, C.; Domínguez, J. The use of vermicompost in sustainable agriculture: Impact on plant growth and soil fertility. In Soil Nutrients; Miransari, M., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2011; Volume 10, pp. 1–23. ISBN 978-1-61324-785-3. Available online: https://jdguez.webs.uvigo.es/wp-content/uploads/2012/01/the-use-of-vermicompost.pdf (accessed on 28 November 2024).
- Jayaswal, K.; Christian, J.; Singh, N.K.; Padhiyar, H.; Yadav, M.; Sanghvi, G. Effect of vermicompost on soil quality parameters for different land use patterns. In ASMI-2023 IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2023; Volume 1280, p. 012054. [Google Scholar] [CrossRef]
- Oyege, I.; Balaji Bhaskar, M.S. Effects of vermicompost on soil and plant health and promoting sustainable agriculture. Soil Syst. 2023, 7, 101. [Google Scholar] [CrossRef]
- Domínguez, J.; Aira, M.; Crandall, K.A.; Pérez-Losada, M. Earthworms drastically change fungal and bacterial communities during vermicomposting of sewage sludge. Sci. Rep. 2021, 11, 15556. [Google Scholar] [CrossRef]
- Collange, B.; Navarrete, M.; Peyre, G.; Mateille, T.; Tchamitchian, M. Root-knot nematode (Meloidogyne) management in vegetable crop production: The challenge of an agronomic system analysis. Crop Prot. 2011, 30, 1251–1262. [Google Scholar] [CrossRef]
- Oka, Y. Mechanisms of nematode suppression by organic soil amendments—A review. Appl. Soil Ecol. 2010, 44, 101–115. [Google Scholar] [CrossRef]
- Thoden, T.C.; Korthals, G.W.; Termorshuizen, A.J. Organic amendments and their influences on plant-parasitic and free-living nematodes: A promising method for nematode management? Nematology 2011, 13, 133–153. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, M.; Jiang, L.; Chen, X.; Griffiths, B.; Li, H.; Hu, F. Vermicompost increases defense against root-knot nematode (Meloidogyne incognita) in tomato plants. Appl. Soil Ecol. 2016, 105, 177–186. [Google Scholar] [CrossRef]
- Kaur, J.; Kaur, G. Dehydrogenase activity as a biological indicator of soil health. Chem. Sci. Rev. Lett. 2021, 10, 326–329. [Google Scholar] [CrossRef]
- Maková, J.; Javoreková, S.; Elbl, J.; Medo, J.; Hricáková, N.; Kovacik, P. Impact of vermicompost on biological indicators of the quality of soil under maize in a greenhouse experiment. J. Elem. 2019, 24, 319–330. [Google Scholar] [CrossRef]
- Szczech, M.M. Suppressiveness of vermicompost against Fusarium wilt of tomato. J. Phytopathol. 1999, 147, 155–161. [Google Scholar] [CrossRef]
- Iftikhar, A.; Farooq, R.; Akhtar, M.; Khalid, H.; Hussain, N.; Ali, Q.; Malook, S.U.; Ali, D. Ecological and sustainable implications of phosphorous-solubilizing microorganisms in soil. Discov. Appl. Sci. 2024, 6, 33. [Google Scholar] [CrossRef]
- Shouche, S.; Tiwari, D. Role of earthworms and actinomycetes in ecofriendly degradation of floral waste via vermicomposting and antimicrobial potential of actinomycetes. Biol. Forum Int. J. 2023, 15, 632–636. Available online: https://www.researchtrend.net/bfij/pdf/Role-of-Earthworms-and-Actinomycetes-in-Ecofriendly-Degradation-of-Floral-Waste-via-Vermicomposting-and-Antimicrobial-Potential-of-Actinomycetes-Deepika-Tiwari-98.pdf (accessed on 28 November 2024).
- Crisan, C.M.; Mocan, T.; Manolea, M.; Lasca, L.I.; Tăbăran, F.A.; Mocan, L. Review on silver nanoparticles as a novel class of antibacterial solutions. Appl. Sci. 2021, 11, 1120. [Google Scholar] [CrossRef]
- Mittal, D.; Kumar, A.; Balasubramaniam, B.; Thakur, R.; Siwal, S.S.; Saini, R.V.; Gupta, R.K.; Saini, A.K. Synthesis of biogenic silver nanoparticles using plant growth-promoting bacteria: Potential use as biocontrol agent against phytopathogens. Biomater. Polym. Horiz. 2022, 1, 22–31. [Google Scholar] [CrossRef]
- Mossa, M.I.; El-Sharkawy, E.E.S.; El-Sharkawy, A.A. Antifungal activity of silver nanoparticles green biosynthesis from the extract of Zygophyllum album (L.f.) on Fusarium wilt. J. Plant Prot. Res. 2023, 63, 340–349. [Google Scholar] [CrossRef]
- Li, L.; Pan, H.; Deng, L.; Qian, G.; Wang, Z.; Li, W.; Zhong, C. The antifungal activity and mechanism of silver nanoparticles against four pathogens causing kiwifruit post-harvest rot. Front. Microbiol. 2022, 13, 988633. [Google Scholar] [CrossRef]
- Thammachote, N.; Sripong, K.; Uthairatanakij, A.; Laohakunjit, N.; Limmatvapirat, S.; Ma, G.; Zhang, L.; Kato, M.; Jitareerat, P. Influence of silver nanoparticles on postharvest disease, pericarp hardening, and quality of mangosteen. Postharvest Biol. Technol. 2024, 204, 112470. [Google Scholar] [CrossRef]
- Kim, D.Y.; Patel, S.K.S.; Rasool, K.; Lone, N.; Bhatia, S.K.; Seth, C.S.; Ghodake, G.S. Bioinspired silver nanoparticle-based nanocomposites for effective control of plant pathogens: A review. Sci. Total Environ. 2024, 908, 168318. [Google Scholar] [CrossRef] [PubMed]
- Bodo, K. Antimicrobial Peptides in Eisenia andrei Earthworms: Their Role in Immune Response, Regeneration Process and Interactions with Metal Nanoparticles. Ph.D. Thesis, University of Pecs, Pécs, Hungary, 2020. [Google Scholar]
- Bodo, K.; Hayashi, Y.; Gerencser, G.; Laszlo, Z.; Keri, A.; Galbacs, G.; Telek, E.; Meszaros, M.; Deli, M.A.; Kokhanyuk, B.; et al. Species-Specific Sensitivity of Eisenia Earthworms Towards Noble Metal Nanoparticles: A Multiparametric In Vitro Study. Environ. Sci: Nano 2020, 7, 3509–3525. [Google Scholar] [CrossRef]
- Baccaro, M. Quantification and Modelling of Accumulation Kinetics of Nanomaterials in Soil Organisms Under Environmentally Relevant Conditions. Chapter 3: Particle or Ion, what is the Influence of Dissolution on the Uptake of Metal Nanoparticles in Earthworm? Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2020; pp. 61–64. [Google Scholar] [CrossRef]
- Baccaro, M.; Montano, M.D.; Cui, X.; Mackevica, M.; Lynch, L.; von den Kammer, F.; Lodge, R.W.; Khlobystov, A.N.; van den Brink, N.W. Influence of dissolution on the uptake of bimetallic nanoparticles Au@Ag-NPs in soil organisms Eisenia fetida. Chemosphere 2022, 302, 134909. [Google Scholar] [CrossRef]
- Kokhanyuk, D.; Vantus, B.; Radnai, V.B.; Vamos, B.; Kajner, E.; Galbacs, G.; Telek, G.; Meszaros, E.; Deli, M.; Nemeth, M.A.; et al. Distinct Uptake Routes Participate in Silver Nanoparticle Engulfment by Earthworm and Human Immune Cells. Nanomaterials 2020, 12, 2818. [Google Scholar] [CrossRef]

| Location: Zduny, Poland (52°1484331′ N, 19°8112456′ E) Host Plant: Fragaria × ananassa | ||
|---|---|---|
| Character | (X1–X10) | Mean ± SD |
| Body length L | 598.4–835.0 | 752.0 ± 90.2 |
| Stylet length | 10.2–11.5 | 10.7 ± 0.4 |
| Tail length | 34.6–52.8 | 44.2± 6.3 |
| PUS% | 33.3–73.5 | 57.7 ± 0.1 |
| a | 48.2–58.6 | 53.1 ± 3.0 |
| b′ | 4.4–6.3 | 6.2 ± 0.6 |
| c | 12.6–19.3 | 17.2 ± 1.9 |
| c′ | 4.3–5.9 | 4.9 ± 0.4 |
| V/AT | 2.9–5.1 | 4.2 ± 0.7 |
| V% | 61.0–72.7 | 68.2 ± 3.4 |
| Location: Skierniewice, Poland (51°5717′5″ N, 20°9301″ E) Host Plant: Daucus carota L. | ||
|---|---|---|
| Character | (X1–X10) | Mean ± SD |
| Stylet length | 17–23 | 19.5 ± 2.06 |
| J2 body length | 305–517 | 446.4 ± 75.09 |
| J2 tail length | 30–69 | 50.5 ±12.36 |
| J2 hyaline length | 6–20 | 13.8 ±4.9 |
| Stylet knob shape | oval | |
| Parameter | Treatment (Mean ± SD) | ||
|---|---|---|---|
| Control | Ag+ | Ve | |
| pH | 6.71 ± 0.18 a | 6.77 ± 0.08 a | 6.82 ± 0.14 a |
| Salinity [NaClg·L−1] | 0.198 ± 0.03 ba | 0.278 ± 0.02 a | 0.253 ± 0.04 a |
| N-NO3 [mg·kg−1] | 26.66 ± 13.21 a | 25.22 ± 9.95 a | 30.33 ± 9.39 a |
| P [mg·kg−1] | 202.66 ± 38.17 b | 236.44 ± 9.73 a | 240.889 ± 19.52 a |
| K [mg·kg−1] | 41.33 ± 18.9 b | 59.77 ± 37.15 b | 113.66 ± 25.98 a |
| Mg [mg·kg−1] | 161 ± 12.01 a | 167.3± 11.82 a | 170.1 ± 14.33 a |
| Ca [mg·kg−1] | 2417.2 ± 144.58 a | 2362.4 ± 78.42 a | 2315 ± 76.11 a |
| N-NH4 [mg·kg−1] | 453.33 ± 91.92 b | 544.33 ± 68.2 b | 2126.66 ± 76.32 a |
| Ag [mg·kg−1] | 0 ± 0.00 b | 0.229 ± 0.08 a | 0 ± 0.00 b |
| Corg [%] | 3.84 ± 0.43 a | 3.77 ± 0.25 a | 3.93 ± 0.24 a |
| Parameter | Treatment (Mean ± SD) | ||
|---|---|---|---|
| Control | Ag+ | Ve | |
| Net photosynthetic rate [µmol [CO2·m−2s−1] | 12.36 ± 0.51 a | 13.52 ± 0.48 b | 13.69 ± 0.53 b |
| Transpiration rate [mmlo [H2O·m−2s−1] | 1.58 ± 0.28 a | 2.77 ± 0.36 b | 3.14 ± 0.41 c |
| PSII [Fv/Fm] | 0.83 ± 0.09 a | 0.82 ± 0.08 a | 0.82 ± 0.10 a |
| Chlorophyll concentration index (CCI) | 19.84 ± 0.64 a | 19.85 ± 0.66 a | 20.58 ± 0.71 b |
| Stem count [number] | 39 | 39 | 40 |
| Stem thickness [cm] | 3.06 ± 0.72 a | 3.67± 0.62 a | 3.85 ± 0.61 a |
| Stem dry yield [kg·m−2] | 2.22 ± 0.26 a | 2.57 ± 0.28 a | 2.49 ± 0.27 a |
| Tuber yield [kg/m−2] | 6.38 ± 0.31 a | 8.54 ± 0.35 b | 9.26 ± 0.36 c |
| Plant height [cm] | 238.00 ± 47.13 a | 259.00 ± 23.80 a | 247.00 ± 22.17 a |
| Pf | Treatment (Mean ± SD) | ||
|---|---|---|---|
| Control | Ag+ | Ve | |
| Aphelenchoides fragariae | 116.25 ± 11.08 a | 38.00 ± 8.48 b | 58.58 ± 12.79 b |
| Meloidogyne hapla | 133.75 ± 21.36 a | 33.5 ± 8.06 c | 76.75 ± 10.75 b |
| Microorganisms | Treatment (Mean ± SD) | ||
|---|---|---|---|
| Control | Ag+ | Ve | |
| Bacteria cfu × 107 | 5.23 ± 0.74 a | 4.17 ± 0.83 a | 4.51 ± 2.13 a |
| Actinomycetes cfu × 107 | 2.43 ± 1.23 a | 1.442 ± 0.42 a | 3.1075 ± 1.74 a |
| Fluorescent Pseudomonas cfu × 105 | 2.102 ± 2.02 b | 0.8725 ± 0.47 ab | 0.2075 ± 0.04 a |
| Phosphate-solubilized bacteria cfu × 105 | 5.525 ± 4.33 a | 4.575 ± 2.43 a | 7.65 ± 2.73 a |
| Fungi cfu × 104 | 2.36 ± 2.36 a | 1.205 ± 1.51 a | 2.025 ± 3.54 a |
| Dehydrogenase activity µmol TPF/g | 33.727 ± 3.53 b | 25.362 ± 1.51 a | 36.412 ± 1.45 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skwiercz, A.T.; Zapałowska, A.; Szczech, M.; Kowalska, B.; Kozacki, D.; Stefanovska, T.; Zhukov, O.; Sekrecka, M.; Wójcik, K.; Klamkowski, K. Effect of Silver Nanoparticles and Vermicompost on the Control of Aphelenchoides fragariae and Meloidogyne hapla in Jerusalem Artichoke (Helianthus tuberosus L.). Sustainability 2025, 17, 2997. https://doi.org/10.3390/su17072997
Skwiercz AT, Zapałowska A, Szczech M, Kowalska B, Kozacki D, Stefanovska T, Zhukov O, Sekrecka M, Wójcik K, Klamkowski K. Effect of Silver Nanoparticles and Vermicompost on the Control of Aphelenchoides fragariae and Meloidogyne hapla in Jerusalem Artichoke (Helianthus tuberosus L.). Sustainability. 2025; 17(7):2997. https://doi.org/10.3390/su17072997
Chicago/Turabian StyleSkwiercz, Andrzej Tomasz, Anita Zapałowska, Magdalena Szczech, Beata Kowalska, Dawid Kozacki, Tatyana Stefanovska, Olexander Zhukov, Małgorzata Sekrecka, Katarzyna Wójcik, and Krzysztof Klamkowski. 2025. "Effect of Silver Nanoparticles and Vermicompost on the Control of Aphelenchoides fragariae and Meloidogyne hapla in Jerusalem Artichoke (Helianthus tuberosus L.)" Sustainability 17, no. 7: 2997. https://doi.org/10.3390/su17072997
APA StyleSkwiercz, A. T., Zapałowska, A., Szczech, M., Kowalska, B., Kozacki, D., Stefanovska, T., Zhukov, O., Sekrecka, M., Wójcik, K., & Klamkowski, K. (2025). Effect of Silver Nanoparticles and Vermicompost on the Control of Aphelenchoides fragariae and Meloidogyne hapla in Jerusalem Artichoke (Helianthus tuberosus L.). Sustainability, 17(7), 2997. https://doi.org/10.3390/su17072997








