Screening Microalgae for Producing Biofuel Precursors from Industrial Off-Gases
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgal Species
2.2. Microalgae Cultivation
2.3. Monitoring and Analytical Measurements
3. Results
3.1. Microalgae Growth
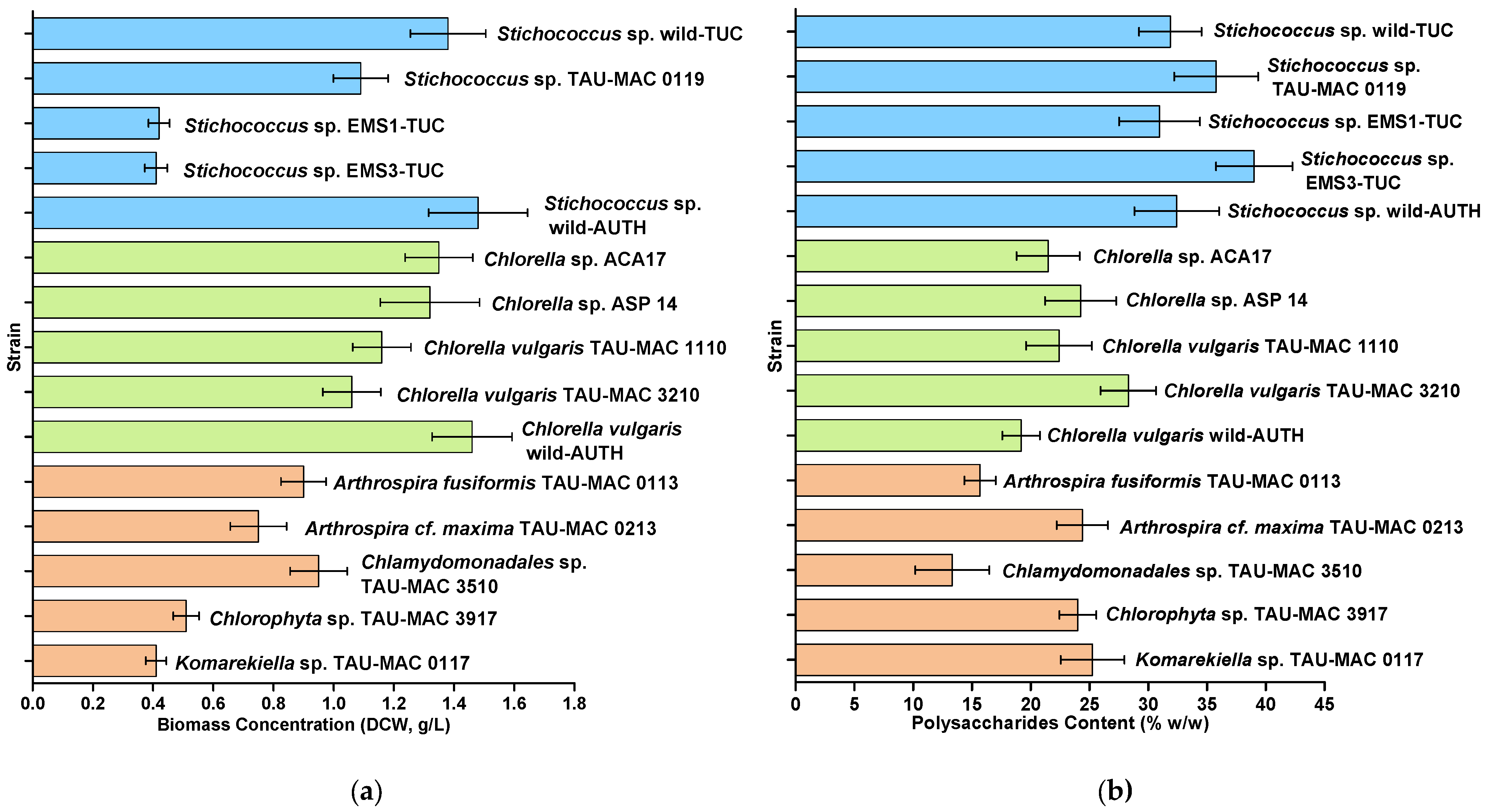
3.2. Biomass Production
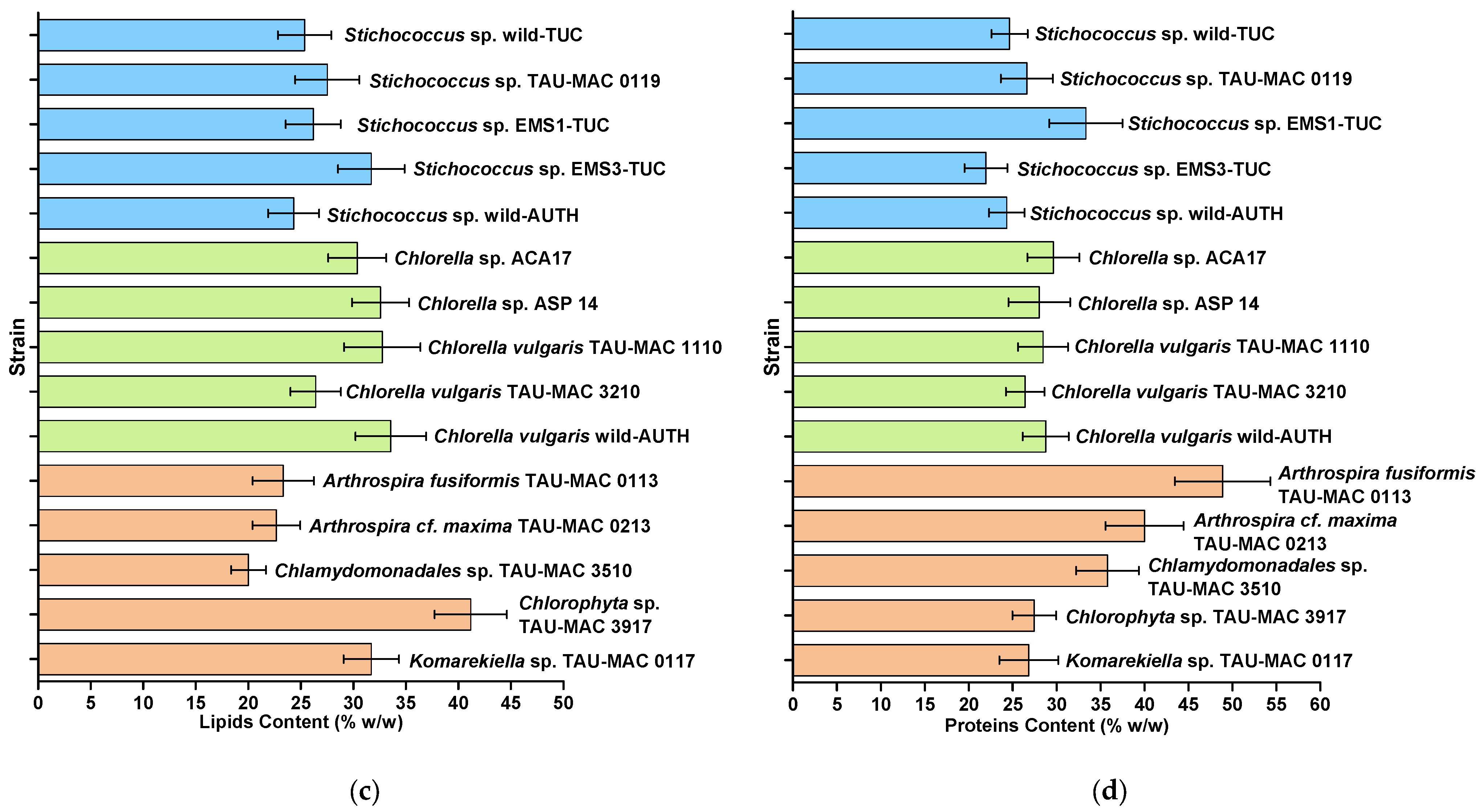
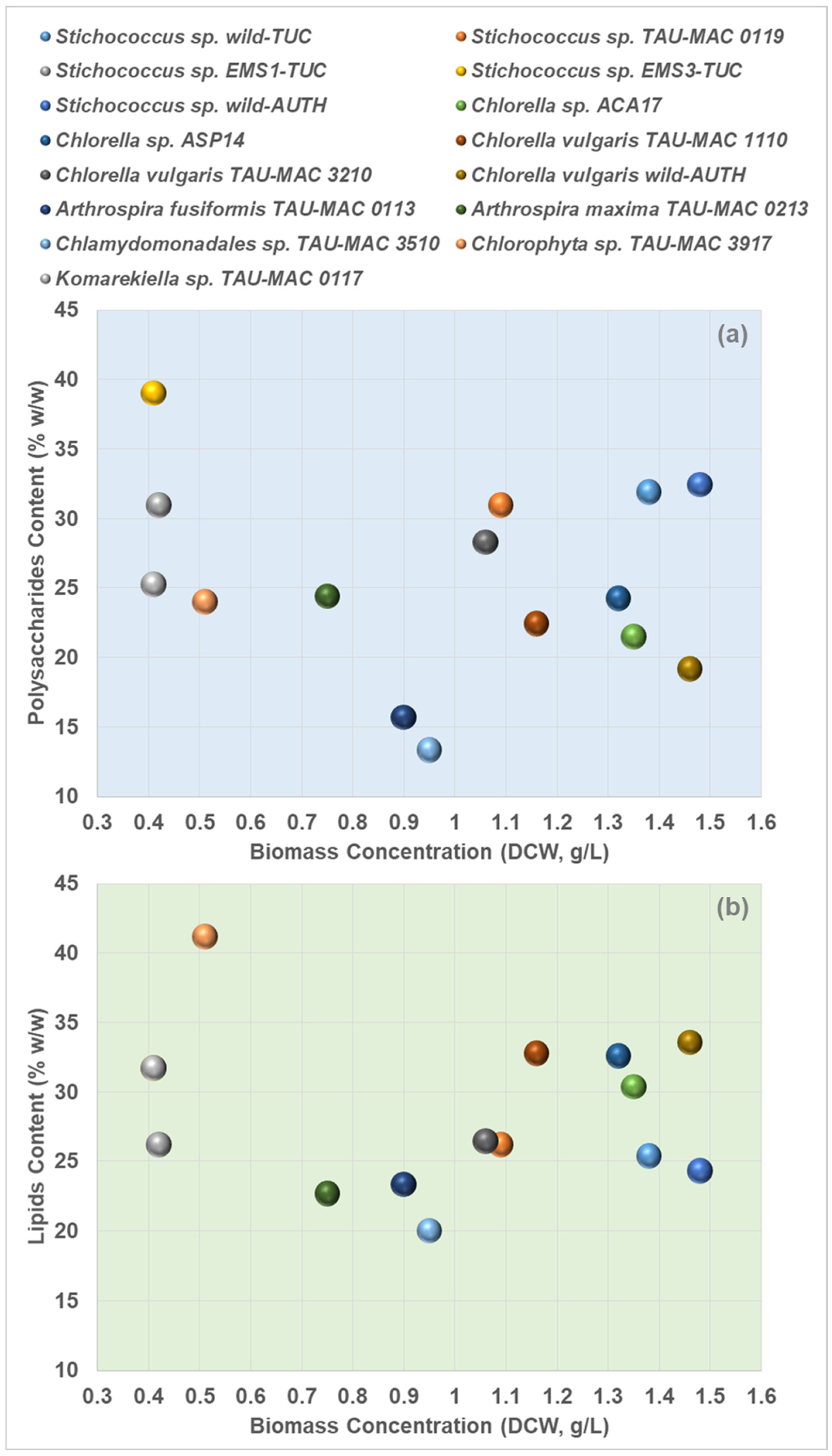
3.3. Biochemical Products Accumulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Filonchyk, M.; Peterson, M.P.; Zhang, L.; Hurynovich, V.; He, Y. Greenhouse gases emissions and global climate change: Examining the influence of CO2, CH4, and N2O. Sci. Total Environ. 2024, 935, 173359. [Google Scholar] [CrossRef] [PubMed]
- Minarta, R.; Ko, J. What are the stimulants on transportation carbon dioxide emissions? A nation-level analysis. Energy 2024, 296, 131179. [Google Scholar] [CrossRef]
- European Environmental Agency (EEA). Greenhouse Gas Emissions from Transport in Europe. Available online: https://www.eea.europa.eu/en/analysis/indicators/greenhouse-gas-emissions-from-transport?activeAccordion (accessed on 3 February 2025).
- Jha, S.; Singh, R.; Pandey, B.K.; Tiwari, A.K.; Shukla, S.; Dikshit, A.; Bhardwaj, A.K. Recent aspects of algal biomass for sustainable fuel production: A review. Discov. Sustain. 2024, 5, 300. [Google Scholar] [CrossRef]
- Rodrigues, A.A. “Fit for 55”: The EU plan for a green transition. In Sustainable Finances and the Law; Saraiva, R., Pardal, P.A., Eds.; Springer: Dordrecht, The Netherlands, 2024; pp. 333–344. [Google Scholar]
- Ahmad, A.; Ashraf, S.S. Harnessing microalgae: Innovations for achieving UN Sustainable Development Goals and climate resilience. J. Water Process Eng. 2024, 68, 106506. [Google Scholar] [CrossRef]
- Penloglou, G.; Pavlou, A.; Kiparissides, C. Recent advancements in photobioreactors for microalgae cultivation: A brief overview. Processes 2024, 12, 1104. [Google Scholar] [CrossRef]
- Wang, M.; Ye, X.; Bi, H.; Shen, Z. Microalgae biofuels: Illuminating the path to a sustainable future amidst challenges and opportunities. Biotechnol. Biofuels Bioprod. 2024, 17, 10. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, S.; Chen, J.P. Carbon capture and utilization by algae with high concentration CO2 or bicarbonate as a carbon source. Sci. Total Environ. 2024, 918, 170325. [Google Scholar] [CrossRef]
- Almomani, F.; Abdelbar, A.; Ghanimeh, S. A review of the recent advancement of bioconversion of carbon dioxide to added value products: A state of the art. Sustainability 2023, 15, 10438. [Google Scholar] [CrossRef]
- Yu, B.S.; Pyo, S.; Lee, J.; Han, K. Microalgae: A multifaceted catalyst for sustainable solutions in renewable energy, food security, and environmental management. Microb. Cell Fact. 2024, 23, 308. [Google Scholar] [CrossRef]
- Babu, S.S.; Gondi, R.; Vincent, G.S.; JohnSamuel, G.C.; Jeyakumar, R.B. Microalgae biomass and lipids as feedstock for biofuels: Sustainable biotechnology strategies. Sustainability 2022, 14, 15070. [Google Scholar] [CrossRef]
- Penloglou, G.; Chatzidoukas, C.; Kiparissides, C. A microalgae-based biorefinery plant for the production of valuable biochemicals: Design and economics. Comput. Aided Chem. Eng. 2016, 38, 1731–1736. [Google Scholar] [CrossRef]
- Piyatilleke, S.; Thevarajah, B.; Nimarshana, P.H.V.; Ariyadasa, T.U. Microalgal biofuels: Challenges and prospects in the framework of circular bioeconomy. Energy Nexus 2025, 17, 100338. [Google Scholar] [CrossRef]
- Onyeaka, H.; Miri, T.; Obileke, K.; Hart, A.; Anumudu, C.; Al-Sharify, Z.T. Minimizing carbon footprint via microalgae as a biological capture. Carbon Capture Sci. Technol. 2021, 1, 100007. [Google Scholar] [CrossRef]
- Al-Hammadi, M.; Güngörmüşler, M. New insights into Chlorella vulgaris applications. Biotechnol. Bioeng. 2024, 121, 1486–1502. [Google Scholar] [CrossRef]
- Liu, A.; Chen, W.; Zheng, L.; Song, L. Identification of high-lipid producers for biodiesel production from forty-three green algal isolates in China. Prog. Nat. Sci. Mater. Int. 2011, 21, 269–276. [Google Scholar] [CrossRef]
- Karapatsia, A.; Penloglou, G.; Chatzidoukas, C.; Kiparissides, C. An experimental investigation of Stichococcus sp. cultivation conditions for optimal co-production of carbohydrates, proteins, and lipids following a biorefinery concept. Biomass Bioenerg. 2016, 89, 123–132. [Google Scholar] [CrossRef]
- Penloglou, G.; Chatzidoukas, C.; Kiparissides, C. Scale-up and intensification of a microalgae cultivation process for the production of high-added value biochemicals. Mater. Today Proc. 2018, 5, 27463–27471. [Google Scholar] [CrossRef]
- Chunzhuk, E.A.; Grigorenko, A.V.; Chernova, N.I.; Kiseleva, S.V.; Ryndin, K.G.; Popel, O.S.; Malaniy, S.Y.; Slavkina, O.V.; Neves, F.F.; Leng, L.; et al. Direct study of CO2 capture efficiency during microalgae Arthrospira platensis cultivation at high CO2 concentrations. Energies 2023, 16, 822. [Google Scholar] [CrossRef]
- Podgórska-Kryszczuk, I. Spirulina—An invaluable source of macro- and micronutrients with broad biological activity and application potential. Molecules 2024, 29, 5387. [Google Scholar] [CrossRef]
- Morales-Sánchez, D.; Schulze, P.S.C.; Kiron, V.; Wijffels, R.H. Production of carbohydrates, lipids, and polyunsaturated fatty acids (PUFA) by the polar marine microalga Chlamydomonas malina RCC2488. Algal Res. 2020, 50, 102016. [Google Scholar] [CrossRef]
- Peralta, E.; Jerez, C.G.; Figueroa, F.L. Centrate grown Chlorella fusca (Chlorophyta): Potential for biomass production and centrate bioremediation. Algal Res. 2019, 39, 101458. [Google Scholar] [CrossRef]
- Banerjee, A.; Guria, C.; Maiti, S.K.; Banerjee, C.; Shukla, P. Carbon bio-fixation, effect of physicochemical factors and carbon supply strategies by Nannochloropsis sp. using flue gas and fertilizer. Biomass Bioenerg. 2019, 125, 95–104. [Google Scholar] [CrossRef]
- Kisielewska, M.; Zieliński, M.; Dębowski, M.; Kazimierowicz, J.; Romanowska-Duda, Z.; Dudek, M. Effectiveness of Scenedesmus sp. biomass growth and nutrient removal from the liquid phase of digestates. Energies 2020, 13, 1432. [Google Scholar] [CrossRef]
- Wong, J.F.; Hong, H.J.; Foo, S.C.; Yap, M.K.K.; Tan, J.W. A review on current and future advancements for commercialized microalgae species. Food Sci. Hum. Wellness 2022, 11, 1156–1170. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Xu, G.; Li, F.; Li, X. A review on biodiesel production from microalgae: Influencing parameters and recent advanced technologies. Front. Microbiol. 2022, 13, 970028. [Google Scholar] [CrossRef] [PubMed]
- Pavlou, A.; Penloglou, G.; Kiparissides, C. Evaluation of tolerant to CO2 excess microalgae for the production of multiple biochemicals in a 3G biorefinery. Sustainability 2023, 15, 3889. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Kochert, G. Carbohydrate determination by the phenol-sulfuric acid method. Chemistry 1978, 2, 99274484. [Google Scholar]
- Chen, Y.; Vaidyanathan, S. Simultaneous assay of pigments, carbohydrates, proteins and lipids in microalgae. Anal. Chim. Acta 2013, 776, 31–40. [Google Scholar] [CrossRef]
- Bhola, V.; Swalaha, F.; Ranjith Kumar, R.; Singh, M.; Bux, F. Overview of the potential of microalgae for CO2 sequestration. Int. J. Environ. Sci. Technol. 2014, 11, 2103–2118. [Google Scholar] [CrossRef]
- Patel, A.K.; Vadrale, A.P.; Singhania, R.R.; Michaud, P.; Pandey, A.; Chen, S.J.; Chen, C.W.; Dong, C.D. Algal polysaccharides: Current status and future prospects. Phytochem. Rev. 2023, 22, 1167–1196. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Z.; Xiao, Y.; Yuan, M.; Zhou, C.; Liu, G.; Fang, J.; Yang, B. Biochemical and morphological changes triggered by nitrogen stress in the oleaginous microalga Chlorella vulgaris. Microorganisms 2022, 10, 566. [Google Scholar] [CrossRef]
- Aslam, A.; Thomas-Hall, S.R.; Mughal, T.A.; Schenk, P.M. Selection and adaptation of microalgae to growth in 100% unfiltered coal-fired flue gas. Bioresour. Technol. 2017, 233, 271–283. [Google Scholar] [CrossRef]
- Hossain, N.; Mahlia, T.M.I. Progress in physicochemical parameters of microalgae cultivation for biofuel production. Crit. Rev. Biotechnol. 2019, 39, 835–859. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, G.; Gargano, I.; Andreozzi, R.; Marotta, R.; Marzocchella, A.; Pinto, G.; Pollio, A. Effects of CO2 and pH on Stichococcus bacillaris in laboratory-scale photobioreactors. Chem. Eng. Trans. 2012, 27, 61–66. [Google Scholar] [CrossRef]
- Greque de Morais, M.; Vieira Costa, J.A. Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor. J. Biotechnol. 2007, 129, 439–445. [Google Scholar] [CrossRef]
- Zanolla, V.; Biondi, N.; Niccolai, A.; Abiusi, F.; Adessi, A.; Rodolfi, L.; Tredici, M.R. Protein, phycocyanin, and polysaccharide production by Arthrospira platensis grown with LED light in annular photobioreactors. J. Appl. Phycol. 2022, 34, 1189–1199. [Google Scholar] [CrossRef]
- Costa, E.; Ribeiro, M.; Filipe-Ribeiro, L.; Cosme, F.; Nunes, F.M. Protein extraction from Arthrospira platensis for use in food processing. Med. Sci. Forum 2024, 23, 8. [Google Scholar] [CrossRef]
- Chiu, S.Y.; Kao, C.Y.; Chen, C.H.; Kuan, T.C.; Ong, S.C.; Lin, C.S. Reduction of CO2 by a high-density culture of Chlorella sp. in a semicontinuous photobioreactor. Bioresour. Technol. 2008, 99, 3389–3396. [Google Scholar] [CrossRef]
- Ugya, A.Y.; Meguellati, K. Microalgae biomass modeling and optimization for sustainable biotechnology—A concise review. J. Ecol. Eng. 2022, 23, 309–318. [Google Scholar] [CrossRef]
- Guccione, A.; Biondi, N.; Sampietro, G.; Rodolfi, L.; Bassi, N.; Tredici, M.R. Chlorella for protein and biofuels: From strain selection to outdoor cultivation in a Green Wall Panel photobioreactor. Biotechnol. Biofuels 2014, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Tounsi, L.; Hentati, F.; Ben Hlima, H.; Barkallah, M.; Smaoui, S.; Fendri, I.; Michaud, P.; Abdelkafi, S. Microalgae as feedstock for bioactive polysaccharides. Int. J. Biol. Macromol. 2022, 221, 1238–1250. [Google Scholar] [CrossRef] [PubMed]
- Mohler, D.; Wilson, M.H.; Kesner, S.; Schambach, J.Y.; Vaughn, D.; Frazar, M.; Stewart, J.; Groppo, J.; Pace, R.; Crocker, M. Beneficial re-use of industrial CO2 emissions using microalgae: Demonstration assessment and biomass characterization. Bioresour. Technol. 2024, 15, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Iglina, T.; Iglin, P.; Pashchenko, D. Industrial CO2 capture by algae: A review and recent advances. Sustainability 2022, 14, 3801. [Google Scholar] [CrossRef]
- Yang, Y.; Ge, S.; Pan, Y.; Qian, W.; Wang, S.; Zhang, J.; Zhuang, L.-L. Screening of microalgae species and evaluation of algal-lipid stimulation strategies for biodiesel production. Sci. Total Environ. 2022, 789, 159281. [Google Scholar] [CrossRef]
- Comley, J.G.; Scott, J.A.; Laamanen, C.A. Utilizing CO2 in industrial off-gas for microalgae cultivation: Considerations and solutions. Crit. Rev. Biotechnol. 2024, 44, 910–923. [Google Scholar] [CrossRef]
- Razzak, S.A.; Bahar, K.; Islam, K.M.O.; Haniffa, A.K.; Faruque, M.O.; Hossain, S.M.Z.; Hossain, M.M. Microalgae cultivation in photobioreactors: Sustainable solutions for a greener future. Green Chem. Eng. 2024, 5, 418–439. [Google Scholar] [CrossRef]
- Figueroa-Torres, G.M.; Pittman, J.K.; Theodoropoulos, C. Optimisation of microalgal cultivation via nutrient-enhanced strategies: The biorefinery paradigm. Biotechnol. Biofuels 2021, 14, 64. [Google Scholar] [CrossRef]

| Number (#), Microalgal Species, and Reference Code | ||
|---|---|---|
| #1 Stichococcus sp. wild-TUC | #6 Chlorella sp. ACA17 | #11 Arthrospira fusiformis TAU-MAC 0113 |
| #2 Stichococcus sp. TAU-MAC 0119 | #7 Chlorella sp. ASP14 | #12 Arthrospira maxima TAU-MAC 0213 |
| #3 Stichococcus sp. EMS1-TUC | #8 Chlorella vulgaris TAU-MAC 1110 | #13 Chlamydomonadales sp. TAU-MAC 3510 |
| #4 Stichococcus sp. EMS3-TUC | #9 Chlorella vulgaris TAU-MAC 3210 | #14 Chlorophyta sp. TAU-MAC 3917 |
| #5 Stichococcus sp. wild-AUTH | #10 Chlorella vulgaris wild-AUTH | #15 Komarekiella sp. TAU-MAC 0117 |
| Major Components | Concentration (g/L) | Traces and Vitamins | Concentration (g/L) |
|---|---|---|---|
| NaNO3 | 0.75 | DiNa-EDTA | 0.0045 |
| K2HPO4·3H2O | 0.075 | FeCl3·6H2O | 0.000582 |
| KH2PO4 | 0.175 | MnCl2·4H2O | 0.000246 |
| NaCl | 0.025 | ZnCl2 | 0.00003 |
| MgSO4·7H2O | 0.075 | CoCl2·6H2O | 0.000012 |
| CaCl2·2H2O | 0.025 | Na2MoO4·2H2O | 0.000024 |
| Vitamin B1 | 0.00012 | ||
| Vitamin B12 | 0.0001 |
| Microalgal Species | Polysaccharides (g/L) | Lipids (g/L) | Proteins (g/L) | Total Macromolecules (g/L) |
|---|---|---|---|---|
| Stichococcus sp. wild-TUC | 0.44 ± 0.013 | 0.35 ± 0.039 | 0.34 ± 0.043 | 1.13 ± 0.095 |
| Stichococcus sp. TAU-MAC 0119 | 0.39 ± 0.016 | 0.30 ± 0.025 | 0.29 ± 0.036 | 0.98 ± 0.077 |
| Stichococcus sp. EMS1-TUC | 0.13 ± 0.020 | 0.11 ± 0.009 | 0.14 ± 0.016 | 0.38 ± 0.045 |
| Stichococcus sp. EMS3-TUC | 0.16 ± 0.032 | 0.13 ± 0.011 | 0.09 ± 0.009 | 0.38 ± 0.052 |
| Stichococcus sp. wild-AUTH | 0.48 ± 0.036 | 0.36 ± 0.040 | 0.36 ± 0.045 | 1.2 ± 0.121 |
| Chlorella sp. ACA17 | 0.29 ± 0.033 | 0.41 ± 0.034 | 0.40 ± 0.044 | 1.1 ± 0.111 |
| Chlorella sp. ASP14 | 0.32 ± 0.038 | 0.43 ± 0.039 | 0.37 ± 0.046 | 1.12 ± 0.123 |
| Chlorella vulgaris TAU-MAC 1110 | 0.26 ± 0.028 | 0.38 ± 0.032 | 0.33 ± 0.037 | 0.97 ± 0.097 |
| Chlorella vulgaris TAU-MAC 3210 | 0.30 ± 0.010 | 0.28 ± 0.026 | 0.28 ± 0.035 | 0.86 ± 0.071 |
| Chlorella vulgaris wild-AUTH | 0.28 ± 0.011 | 0.49 ± 0.061 | 0.42 ± 0.047 | 1.19 ± 0.119 |
| Arthrospira fusiformis TAU-MAC 0113 | 0.08 ± 0.037 | 0.21 ± 0.021 | 0.44 ± 0.040 | 0.73 ± 0.098 |
| Arthrospira maxima TAU-MAC 0213 | 0.10 ± 0.049 | 0.17 ± 0.022 | 0.30 ± 0.033 | 0.57 ± 0.104 |
| Chlamydomonadales sp. TAU-MAC 3510 | 0.12 ± 0.016 | 0.19 ± 0.019 | 0.34 ± 0.034 | 0.65 ± 0.069 |
| Chlorophyta sp. TAU-MAC 3917 | 0.18 ± 0.020 | 0.21 ± 0.023 | 0.14 ± 0.016 | 0.53 ± 0.059 |
| Komarekiella sp. TAU-MAC 0117 | 0.24 ± 0.053 | 0.13 ± 0.014 | 0.11 ± 0.009 | 0.48 ± 0.076 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penloglou, G.; Pavlou, A.; Kiparissides, C. Screening Microalgae for Producing Biofuel Precursors from Industrial Off-Gases. Sustainability 2025, 17, 2964. https://doi.org/10.3390/su17072964
Penloglou G, Pavlou A, Kiparissides C. Screening Microalgae for Producing Biofuel Precursors from Industrial Off-Gases. Sustainability. 2025; 17(7):2964. https://doi.org/10.3390/su17072964
Chicago/Turabian StylePenloglou, Giannis, Alexandros Pavlou, and Costas Kiparissides. 2025. "Screening Microalgae for Producing Biofuel Precursors from Industrial Off-Gases" Sustainability 17, no. 7: 2964. https://doi.org/10.3390/su17072964
APA StylePenloglou, G., Pavlou, A., & Kiparissides, C. (2025). Screening Microalgae for Producing Biofuel Precursors from Industrial Off-Gases. Sustainability, 17(7), 2964. https://doi.org/10.3390/su17072964









