Abstract
Transport and vehicle traffic are closely connected with particulate matter (PM) pollution, inducing various fractions into the atmosphere, some of them forming significant deposits on the surface of the car. They are washed away during carwash-inducing slurries collecting the PM deposits, which are characteristic of a large area. Crystalline PM matter was investigated by XRD coupled with polarized optical microscopy (POM). Organic matters were investigated by Fourier-Transform Infrared spectrometry (FTIR) and gas chromatography, GC-MS. Their microstructure and elemental composition were investigated by SEM-EDX. The crystalline features contain mainly quartz, calcite, and clay (muscovite and kaolinite) particles having traces of goethite and lepidocrocite. Slurry particle size distribution was established by sieving on the following meshes: 63 µm, 125 µm, 250 µm, 500 µm, 1000 µm, 2000 µm, and 4000 µm. Coarse fractions of 250–4000 μm are dominated by quartz and calcite particles. The quartz and calcite amount decreases with particle size, while the muscovite and kaolinite amount increases in the finest fractions of 0–125 μm. Organic matter was evidenced, firstly, by FTIR spectroscopy, revealing mostly CH2; C=O, and NH4 bonds that are more intense for the fine particulate fractions. The organic deposits form mainly amorphous crusts associated with micro- and nano-plastic particles related to the phthalates and traces of the washing detergents. Atomic Force Microscopy revealed their size range between 60 and 90 nm and evidenced nanoparticles within samples. The nanofractions adhere to the bigger particles in humid environments, assuring their immobilization to reduce their hazardous potential. Carwash slurry blending with fertile soil ensures proper grass seed germination and growth at mixtures of up to 60% slurry, allowing its sustainable reconversion as soil for landfill and dump rehabilitation, preventing the PM emission hazard. Blended compositions containing more than 60% slurry have noxious effects on the grass seeds, inhibiting their germination.
1. Introduction
The particulate matters (PMs) are major pollutants related to the natural and anthropogenic processes intermingling. Soil degradation and erosion associated with air currents is one of the common natural sources of particulate matters [1,2]. The anthropogenic sources of PMs are related to human activities such as industry [3], construction building [4], and mining [5]. Fuel consumption relating to transport activities is well known for fine and dangerous PM emissions, such as soot and ultrafine organic matters [6,7]. All these particulate pollutants are emitted into the atmosphere where they are mixed and subsequently fall under gravitation, affecting widespread areas.
Literature data show that all ranges of atmospheric PMs are found in street dust [8,9]. Furthermore, these PMs fell over the vehicles in traffic being covered with PM dust on the top sides of the chassis. The lower side of the car chassis collects large deposits of street dust formed during the particulate matter re-suspension [9,10] and street mud crusts when the cars circulate in humid weather. Finally, all of the PM deposits collected by a car are removed during a carwash and subsequently collected into the carwash facility decantation tank. The collected slurry is collected in a dumpsite where it is kept wet to prevent PM mobilization and atmospheric re-suspension. Thus, it represents the most relevant statistical homogenization of the PM emissions in a targeted area, especially when the carwash facility serves a transport company.
Fine and ultrafine particulate matter, such as PM2.5 and PM1, induced into the atmosphere due to the various mentioned sources are very dangerous for living beings, as clearly shown in the literature. Lung cancer is facilitated by prolonged exposure to carbonaceous particles [11,12], while mineral fractions are prone to cause asthma [13] and silicosis [14,15]. The conjugate effect of mineral and carbonaceous particulate matters proves to be more harmful to the lungs because the mineral fractions initiate silicosis, like lesions on the pulmonary alveoli, which facilitates the disruption of ultrafine carbonaceous particles into the lesions and allows them to be carried in the blood flow to the whole tissues in the body. The particle penetration into the blood flow through the wounded lung alveoli is facilitated by very small diameters, such as submicrons and nanoparticles. Previous studies already pointed out the presence of nanoparticles within the street dust [8]. The nanoparticles’ penetration ability through the living body tissue is sustained by the literature data [16,17].
The health risk related to carwash slurry strongly depends on its PM composition, mainly on the presence of ultrafine particles contaminated with organic matter. On the other hand, the dumping sites require permanent maintenance (e.g., to keep the slurry wet, preventing the fine fractions from spreading into the atmosphere), even after the carwash facility closure. In such cases, the dumped sites must be neutralized and ecologized on the company expenses; otherwise, they represent a major environmental risk. Both the health risk level and neutralization procedures strongly depend on the slurry composition regarding its particle distribution. In such cases, the particle size is one of the most important aspects to be carried out in addition to its chemical composition. The ultrafine fractions are more harmful than the bigger ones due to their penetration ability into the living tissues.
Literature data reveal that moving vehicles (cars, buses, and trucks) interact with the street and road particulate matters, like those previously discussed. They fell down onto the vehicle’s surface, forming a dust pellicle [18,19]. They act as vectors collecting the specific pollutants covering the circulated area. Thus, carwash slurry is the most representative sample for the complex characterization of these pollutant factors, which depend on the circulating area characteristics. This should be investigated, revealing the danger of the summarized particulate matter re-suspension into the atmosphere. This is very possible unless the dumped slurry is not properly controlled to keep its optimal moisture that prevents the finest fraction from spreading. If it is left to dry, many pollutant particles will be lifted into the air by the wind and affect the adjacent areas and living beings around the contaminated site. Carwash slurry landfill requires proper wetting, even after facility closure, implying significant additional costs.
On the other hand, industrial landfills have an increased hazardous potential than carwash slurry [20], implying more maintenance costs. We wonder if the carwash slurry can be converted to a coverage admixture suitable for the neutralization of industrial landfills. Such an approach ensures its sustainable reconversion by reducing its landfill size and subsequent maintenance costs.
The aim of the present article is to investigate carwash slurry collected from a dumpsite of a professional facility that serves both heavy traffic (e.g., buses and trucks) and personal cars. The physicochemical composition related to the particle size is investigated through complex analysis regarding the mineral and organic phase’s distribution. Such an approach allows for the identification of a proper neutralization method for each identified PM fraction. The subsequent aim of the present research is the proper conditioning of the slurry to obtain a viable admixture to cover other more hazardous landfills.
2. Materials and Methods
2.1. Sample Collection
The carwash slurry was collected from the dumpsite of a large washing facility that cleans cars, buses, and lorries covering a large area represented by the Romanian Euro Region 6 NV (Transylvania, Romania). The facility name and operator are kept anonymous due to economic reasons, and the present research is oriented only to the scientific aspects regarding the investigated samples. The slurry samples were collected with a shovel from three different points of the dump (N1, N2, and N3) into new recipients with a volume of 10 L and transported into the laboratory, where they were naturally dried at 22 °C until particles easily separate one from another. The dried slurry samples were stored in new glass vials. The mean representative sample was prepared by mixing equal quantities of slurry from N1, N2, and N3 collection points into a mechanical homogenizer for 30 min. This average representative sample of each particle class was used for further physicochemical investigations.
2.2. Particle Size Measurement
Particle size distribution was determined by sieving using a Retsch AS 200 (Retsch, Haan, Germany) sieving system with the following meshes: 63 µm, 125 µm, 250 µm, 500 µm, 1000 µm, 2000 µm, and 4000 µm. A total of 100 g of dry slurry was put on the bigger mesh sieve and further vibrated for 30 min at an amplitude of 60 mm. The residue from each sieve was collected and weighed with a Shimadzu AUX 120 (Shimadzu, Kyoto, Japan) analytical balance and stored in a labeled glass vial. The particle size distribution values were processed with Origin 2019 software (Microcal Company, Northampton, MA, USA) and statistically analyzed using the ANOVA test followed by Tukey’s post hoc test at a significance level of 0.05.
2.3. Physicochemical Investigations
The mineral phase identification was effectuated by X-ray diffraction (XRD) and was operated with a Bragg–Brentano diffractometer, Bruker D8 Advance (Bruker Co., Karlsruhe, Germany), using copper radiation Cu Kα1 (λ = 1.54056 Å). The diffraction patterns were registered between 10 and 80 2θ° with a speed of 1°/min. The diffraction peaks were identified using Match 1.0 software produced by Crystal Impact Company, Bonn, Germany equipped with Powder Diffraction Files database PDF 3.0.
The XRD results were correlated with mineralogical optical microscopy (MOM), which was effectuated on a Laboval 2 optical microscope (Carl Zeiss, Oberkochen, Germany) using the cross-polarized light method. The MOM images were digitally acquired using a Kodak camera 10 Mpx (Kodak, Rochester, NY, USA).
The PM microstructure was investigated by scanning electron microscopy (SEM) using a Hitachi SU8230 SEM (Hitachi Co., Tokyo, Japan) operated in high-vacuum mode at an acceleration voltage of 30 kv. SEM images were correlated with the sample elemental analysis effectuated with the energy-dispersive spectroscopy (EDS) X-Max 1160 EDS (Oxford Instruments, Abingdon, UK).
The PM ultrafine fractions were evidenced by Atomic Force Microscopy (AFM) effectuated with a Jeol JSPM 4210 Scanning Probe Microscope (Jeol Co., Tokyo, Japan). The ultrafine PM fractions were dispersed in deionized water and transferred onto the glass slide substrate via vertical adsorption followed by natural drying. The dry samples were scanned at a rate of 1 to 3 Hz in tapping mode using NSC 15 hard cantilevers produced by MikroMasch Company (Sofia, Bulgaria) having a resonant frequency of 325 kHz and a force constant of 40 N/m. The obtained images were analyzed with Jeol Win SPM 2.0 software.
The chemical bonds involved in PM samples were evidenced using Fourier-Transform Infrared spectroscopy (FTIR), which was effectuated with a JASCO 610 FTIR spectrophotometer (Jasco Co., Tokyo, Japan) using the KBr pellet method in the wave number domain 4000–400 cm−1. The spectra resolution is 4 cm−1, and the obtained curves are the average of 100 scans.
The organic volatile compounds associated with the slurry PM were determined by Gas Chromatography and Mass Spectrometry (GC-MS). An Agilent GC-MS Gas Chromatograph-7890A/5975/2008 (Agilent Technologies, Inc. Europe, Waldbronn, Germany) was used for analysis; GC-MS analyses were performed in scan mode on a DB-5MS (30 m × 0.25 mm × 0.25 µm) capillary column (Agilent 19091S-433M, Agilent Technologies, Inc., Europe, Waldbronn, Germany) with high-purity He carrier gas at a flow rate of 1 mL/min. The samples (0.5 g) were diluted in a 2 mL solvent (dichloromethane:hexane = 1:3) mixture for 30 min; then, they were ultrasonicated for 6 min and then centrifuged at 4400 rpm for 15 min. The volatile fraction was filtered and concentrated, and 1 μL was injected in GC. The temperature program included an initial temperature of 40 °C with a ramp rate of 5 °C/min up to 300 °C, an injection volume of 1 µL, a 100:1 split ratio, MS 70 eV, and a mass range u.a.m. 40–500. Data acquisition and processing were performed using MSD ChemStation software version 2.0. The NIST L14 library was used for identification/confirmation of the structure components. In addition, a C8-C20 standard alkane (Alkane Standard Solution C8-C20, Sigma Aldrich, St. Louis, MO, USA) was used for the calculation of the linear retention index (LRI) and matching the experimental values with those reported in the literature for similar chromatographic columns in the same condition. The qualitative analysis was based on the percentage area of each peak of the sample compounds.
2.4. Coverage Admixture Preparation
The vegetation-growing potential of the mineral fractions within the carwash slurry is worth special attention. Therefore, we perform a preliminary screening of the vegetal proliferation within the slurry samples. Fertile soil (FS), the finest slurry fraction (<63 µm), and integral soil were considered, along with their blended compositions, FS 50 wt.% + slurry < 63 µm 50 wt.% and FS 50 wt.% + integral slurry 50 wt.%, which were properly mixed into a homogeneous ground. The control sample contains only grass seeds with a small water quantity. The same quantity of seeds was planted in all tested soils, which were properly wetted. Their germination and subsequent growth were followed.
3. Results
3.1. Particle Size Distribution
The mean representative sample of carwash slurry, which includes equal parts collected from N1, N2, and N3 points, was subjected to the sieving analysis. Each sieve refuse was weighted, and the corresponding percent was established and plotted in Figure 1.
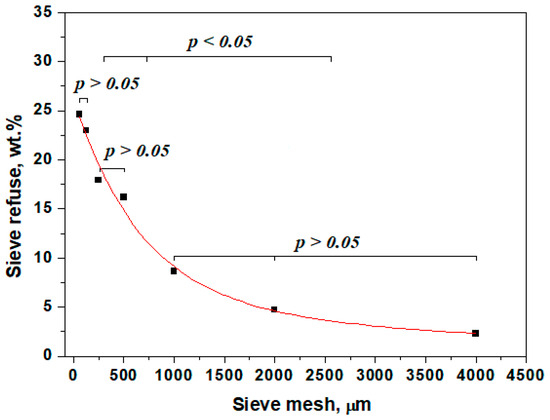
Figure 1.
Sieving analysis results.
Figure 1 reveals that the dominant fraction consists of the sieve pass 0–63 μm, followed closely by the fraction 63–125 μm. Statistical analysis reveals that those two fractions form a relevant statistical group (p > 0.05), representing about 46.75% of the slurry amount. The following fractions, 125–250 µm and 250–500 µm, have moderate particle amounts; the values are close to each other, forming another statistically relevant group (p > 0.05) that summarizes about 34.06% of the total amount of the slurry. Finally, the coarser fractions (500–1000 μm, 1000–2000 μm, and 2000–4000 μm) have fewer amounts of slurry particles, forming the last relevant statistical group (p > 0.05), which contains only 19.19% of the total amount. Moreover, these relevant groups were compared to each other, resulting in relevant statistical differences (p < 0.05). Consequently, the particle size distribution indicates that the investigated carwash slurry is dominated by the finest fractions and presents a scarcity in coarse fractions.
3.2. Mineral Components Assessment
The bigger fractions (e.g., 2–4 mm and <4 mm) are less representative due to their low amount. They contain conglomerates of the other smaller fractions, mainly quartz and calcite, which are associated with the other minerals and vegetal fragments. There are some silica particles with boulder shapes and rounded edges having about a 4 mm diameter that corresponds to quartz. Our previous study regarding these fractions reveals that calcite partial dissolution embeds the iron hydroxides from the car’s chassis rust, forming bigger particles of ferrous conglomerate [21].
The weight amount increases in the 1000–2000 μm fractions, which contain well-individualized particles that allow observing their characteristics under cross-polarized light, which became microstructural and mineralogical etalons for the present investigation, as shown in Figure 2.
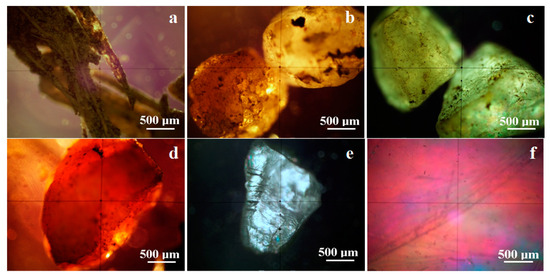
Figure 2.
Mineralogical optical microscopy of the particles observed in the particle class 1000–2000 µm: (a) vegetal fragments, (b) calcite, (c) quartz, (d) iron hydroxide (goethite and lepidocrocite), (e) kaolinite, and (f) muscovite.
Vegetal particles are predominantly elongated with a length of 1000–2000 µm and a diameter ranging from 50 to 150 µm, consisting of the lignified branch remains of the herbaceous plants and floral calyx having a strong mixture of cellulose and lignin that appear dark brown in the cross-polarized light due to their low crystallinity, as shown in Figure 2a. Calcite particles, as shown in Figure 2b, are predominantly rounded and exhibit a yellow shade that turns brown at minimum illumination and yellow-white at maximum enlightens. Several dark spots occur on the calcite particle surface, the upper right corner in Figure 2b, belonging to amorphous organic deposits. Quartz particles have predominantly polyhedral boulders with rounded edges, as seen in Figure 2c. They exhibit a green-gray shade under cross-polarized light; the color nuance depends on the particle’s position regarding the microscope’s optical axis ranging from deep gray at the minimum and pale green at the maximum illumination. Iron hydroxide particles (goethite and lepidocrocite) have scale shapes and are colored in orange-red nuance with a brownish-red hue at the light extinction, as shown in Figure 2d. Phyllosilicates are represented by kaolinite and muscovite and have tabular particles formed by successive sheets of silica tetrahedral. Thus, the margins of the tabular particles are uneven and eroded due to their partial fragmentation. The cross-polarized light observation reveals blue-white nuances for kaolinite and pink nuances for muscovite, as shown in Figure 2e,f. These observations are very useful for particulate matter identification under MOM microscopy, allowing their proper correlation with the XRD information.
The vegetal and mineral particles are very well mixed together in the 1000–2000 μm fraction, such as the example given in Figure 3a, where a bunch of vegetal remains embed a quartz particle. Vegetal fragments completely disappear in the fraction 500–1000 μm, which appear to be dominated by quartz and calcite particles, as shown in Figure 3b.
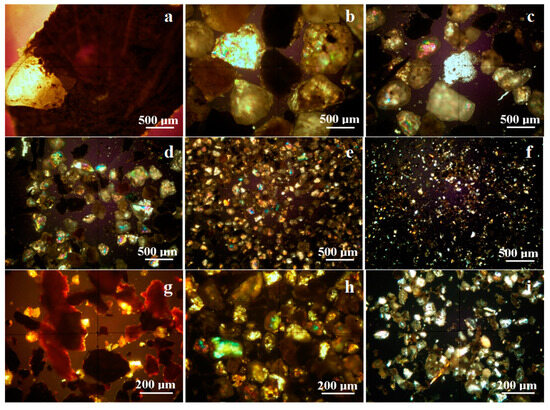
Figure 3.
Mineralogical optical microscopy of the particles observed in successive particle classes: (a) 1000–2000 μm, (b) 500–1000 µm, (c) 250–500 µm, (d) 125–250 µm, (e) 63–125 µm, and (f) 0–63 µm. (g–i) are microstructural details.
It is noteworthy that some of the quartz particles are coated with a compact crust having a dark shade. The dark nuance is given by the amorphous organic deposits, which become significant. The following fraction, 250–500 µm, evidences the appearance of larger tabular particles of muscovite and kaolinite in the center of the observation field in Figure 3d (the pink particle is muscovite and the blue-white particle is kaolinite).
There also occurs quartz particles coated with amorphous organic matter having a dark hue. The fraction 125–250 µm has a strong PM resemblance with the previous one in the matter of mineral and amorphous composition, but the particle size is slightly smaller. A local novelty is the appearance of some iron hydroxide particles well individualized among the other ones, which are colored reddish brown.
A major microstructural change is observed for the finest fractions, 0–63 μm and 63–125 μm, as observed in Figure 3e,f. The particle size is significantly reduced and facilitates the proliferation of fine muscovite and kaolinite particles despite quartz and calcite, which become a minority. There are small dark stains of organic dirt over the mineral particles, but there are also some black particles, indicating their amorphous and organic nature. These fine particles are rather related to the micro-plastic dispersions that contaminate the road areas, and the literature mentioned similar morphologies related to plastic contamination [22,23].
MOM observations are confirmed by the XRD results, as shown in Figure 4. The initial average representative sample (containing equal quantities of slurry from each collecting point) has a mean distribution of all size fractions.
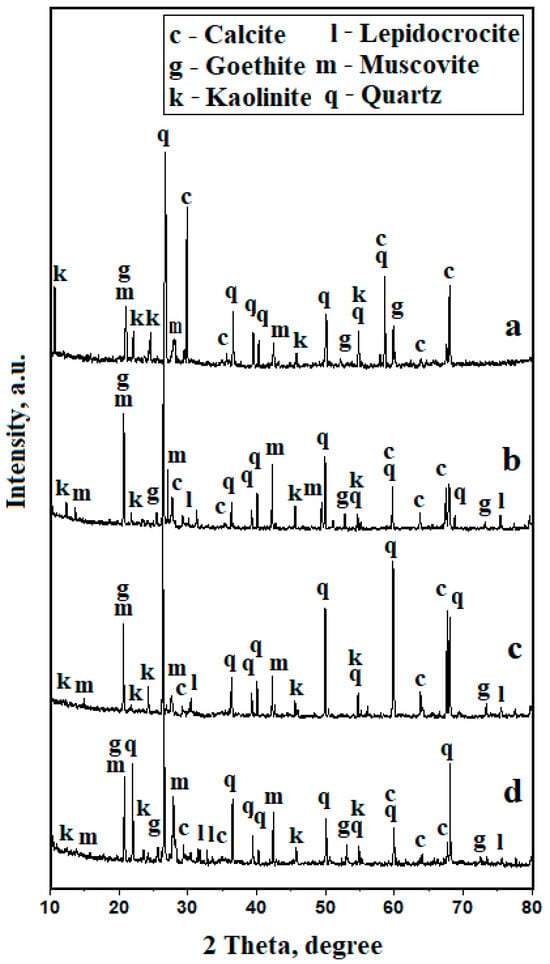
Figure 4.
XRD patterns for PM samples: (a) initial sample before sieving, (b) 125–250 µm, (c) 63–125 µm, and (d) 0–63 µm.
It reveals an XRD pattern having well-developed peaks, which corresponds to a high crystallinity, as shown in Figure 4a. Quartz and calcite present very intense and narrow peaks due to the presence of well-crystallized bigger particles. On the other hand, the peaks for muscovite and kaolinite are broadened and less intense due to the prevalence of fine particles. This fact is caused by the intense fragmentation of the phyllosilicate particles in the presence of quartz, which acts as a milling body [9,24].
The mean amount of each mineral can be determined based on the relative intensities of the most relevant diffraction peaks using the Reference Intensity Ratio (RIR) method [25]. Assuming the presence of two crystalline phases in a mixture, the following equation describes their relative ratio (1):
where Ia and Ib are the intensities of the most representative peaks of both phases a and b; xa and xb are their mass percentage; and fa and fb are the corundum factors of the involved phases.
Similar equations can be written for the other identified crystalline compounds. For example, the equation for another compound (c) looks in the following manner (2), with Ic and fc representing the intensity of the most relevant peak and the corundum factor of the other compound (c).
Considering that
the mean amount of each crystalline phase can be calculated. The equation system can be extended for each identified crystalline compound. The corundum factors are known from the XRD database, and the identification software reads the relative intensities. The obtained results are presented in Table 1.

Table 1.
Mineral weight content of the particle fractions determined from XRD patterns.
The initial sample has a significant amount of iron hydroxide crystallized as goethite of about 10 wt.%. It is very interesting that lepidocrocite presence is not detected in the initial sample, as shown in Table 1. This is due to the prevalence of the more stable form of iron hydroxide, which is goethite, which masks low amounts of lepidocrocite on the diffracting X-rays.
The XRD patterns of the separated particle class reveal diffraction peaks for the same minerals, but their intensities vary according to the particle distributions, as shown in Figure 4b–d. The quartz and calcite peaks decrease progressively with the refinement of the particle size, while the peaks of muscovite and kaolinite progressively increase. The particle dynamics during the deposit formations on the cars play a key role in the slurry formation. The bigger particles, like quartz and calcite, are abundantly found in the street dust mixed with the finest fraction that forms dense mud in humid weather and forms thick deposits on the lower side of the car chassis [26]. The mud interacts with chassis rust, forming complex particles containing street dust minerals embedded into a matrix of iron hydroxides [21,27]. The washing process removes the mud deposits along with some parts of the rust scale. This explains the presence of bigger rust conglomerate particles in the fraction 2000–4000 μm. The bigger quartz and calcite particles are immediately mobilized from the mud deposits and are capable of sedimentation from aqueous dispersion. The finest fractions of kaolinite and muscovite are more floatable and last longer in the water dispersion.
The iron hydroxide dynamics relate to the fragmentation of the rust. MOM and XRD investigation prove that the iron hydroxide amounts progressively increase with particle size refinement. It is very interesting that a coarse fraction of 125–250 μm contains about the same amount of goethite and lepidocrocite organized in compact micro-boulders, as evidenced in the microstructural detail in Figure 3g. The goethite amount progressively increases with particle size decreasing, while the lepidocrocite amount decreases. Larsen and Dieke show that goethite is a more stable form of the iron hydroxides and thus resists better in the fine particles, while lepidocrocite is a more degraded form and is prone to the hydration process and thus facilitates conglomerate formations [28].
The fine mineral fractions within PM10 and PM2.5 from the atmosphere form large and thin deposits over the top of the car’s bodies and on the windows [29,30]. These contain both mineral and organic components that are removed during the washing process and gather the finest fractions within the removed mud in the aqueous dispersion. The particle distribution of the slurry, as shown in Figure 1, clearly shows that the finest fractions 0–63 μm overwhelm the other bigger fractions, and, thus, these are the most dangerous. Both muscovite and kaolinite fine fractions have tabular fine particles with sharp edges that could act as microknives at the lung’s alveoli level, generating microscopic injuries of the tissue and allowing the organic matter to penetrate into the blood flow [13,14,15]. The diffraction peaks belonging to muscovite and kaolinite in Figure 4c,d are less intense and broadened due to the prevalence of the finest fractions. Therefore, we apply the Scherrer formula [31] on the most representative peaks, showing an average particle size of 60 nm for muscovite and 55 nm for kaolinite. This means that most of the finest fractions are nanostructured and subsequently present a greater health risk. MOM microscopy, as shown in Figure 3h,i, clearly shows some diffuse hallow around micron fractions, indicating the presence of nanoparticles. Their morphology requires more advanced microscopic techniques, such as scanning electron microscopy (SEM) and Atomic Force Microscopy (AFM), to be properly evidenced. The organic matter deposits observed as coating crusts deposited on the coarse fractions and, respectively, the individualized black particles observed in the finest fractions are difficult to assess by XRD. However, their presence has a limited influence on the XRD pattern baseline in Figure 4c,d, partly affecting their allure as amorphous matter. Their presence requires an elemental tracking of the carbon coalescence deposits.
3.3. Micro- and Nanostructures and Their Elemental Composition
The unseparated initial sample has a heterogeneous microstructure, as observed in Figure 5a. The observation field is dominated by larger fractions having a boulder-like appearance.
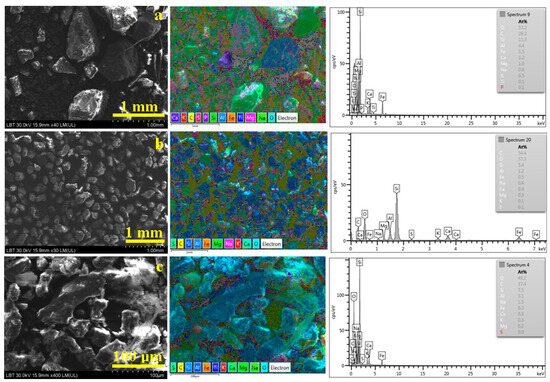
Figure 5.
SEM images of the large microstructural aspect with the corresponding elemental map and EDS spectrum for (a) initial sample before sieving, (b) 125–250 µm fraction, and (c) 0–63 µm fraction.
Quartz has the chemical formula SiO2, and its particles have sharp edges intersected under at least one sharp angle, as evidenced in the adjacent elemental map as green particles because of their Si and O content, a fact that is in good agreement with XRD observations. Calcite has a chemical formula of CaCO3, evidencing rounded boulder particles with a deep-blue aspect in the elemental map because of their Ca amount. Light blue is related to the aluminum presence within muscovite KAl2(AlSi3O10)(F,OH)2 and kaolinite Al2Si2O5(OH)4, which appear in very small spots, indicating their prevalence in the finest fractions. Several vegetal remains are observed on the right side in Figure 5a, especially a small sinuous straw, which appears yellow in the elemental map due to the high carbon amount. Carbon and oxygen are the dominant elements in the initial samples and are related to a wide range of compounds such as vegetal fragments, calcite, and organic pollutants crusts. Silicon and aluminum are the following elements predominating in the sample and are related to the quartz and aluminosilicates, which also explains the presence of potassium, sodium, and magnesium. A moderate amount of Fe is related to the iron hydroxides α-FeOOH (goethite) and γ-FeOOH (lepidocrocite) identified by XRD.
Particle fractions clearly evidence the segregation, and the quartz dominates fractions of 125–250 µm. The calcite mixture is dark blue, and there are green particles on the elemental map. Several pale blue particles are observed, indicating the presence of a few large muscovite and kaolinite particles. It is very interesting that some yellow spots occur on the particle surface dealing with local higher carbon concentrations related to the organic pollutant crusts. The relatively low aluminum amount confirms the low amount of aluminosilicates in this fraction.
The finest fraction, 0–64 µm, has an increased level of aluminum compared to the coarser fractions due to the prevalence of muscovite and kaolinite having tabular platelet particles, as shown in Figure 5c, which appears pale blue in the adjacent elemental map, dominating the observation field. A few sharp-edged quartz slivers and predominantly rounded calcite particles are observed in Figure 5c in addition to the muscovite and kaolinite platelets. SEM investigation at this point proves the presence of the PM1 category as an important constituent of the finest particle class. Its increased hazardous behavior is discussed in the literature [10,15]. The finest details require a high magnification observation of the SEM microscopy. Figure 6 focuses on individual quartz particle origination in the initial sample, as shown in Figure 6a, and in the finest fraction, 0–63 µm, in Figure 6b.
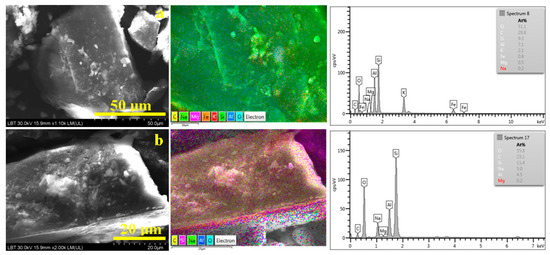
Figure 6.
SEM microstructural details with the corresponding elemental map and EDS spectrum for (a) initial sample before sieving and (b) 0–63 µm fraction.
The boulder-like aspect of these particles with edges intersected under sharp angles reveals typical quartz morphology. Their surface is coated with very fine fractions belonging predominantly to muscovite and kaolinite and are embedded into a continuous crust of organic matter dominated by a yellow color specific to carbon. This confirms the MOM observation that organic crusts occur. The unexpected thing is the evidence of small spots around 200–500 nm colored in intense yellow in the adjacent elemental maps, proving the presence of well-individualized submicron organic particles. This fact correlates with the black particles observed in MOM for the finest fractions and can be better observed in AFM images, as shown in Figure 7.
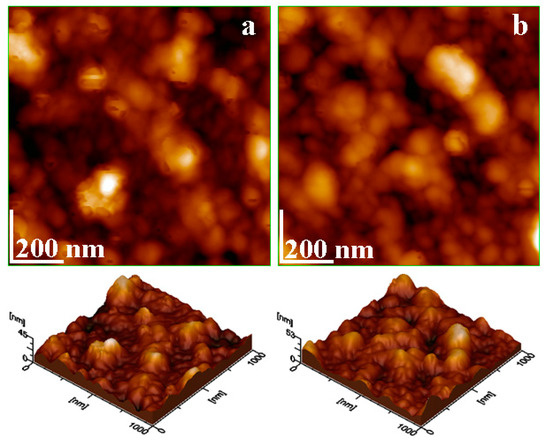
Figure 7.
Nanostructural details evidenced by AFM topographical images for (a) initial sample before sieving and (b) 0–63 µm fraction.
Dai uses AFM microscopy to evidence the micro-plastic components of ultrafine particulate matter, especially those deposited on larger particles, such as silica [22]. Therefore, a representative amount (e.g., 5 g) of the initial slurry and the 0–63 µm fraction were subjected to washing with 20 mL of ultrapure water under magnetically stirring for 30 min. The coating dirt containing ultrastructural particles was detached from the mineral particles, generating a dirty water suspension. Afterwards, the Berzelius vials were left to rest for 10 min, allowing the mineral fraction sedimentation to go to the bottom. The dirty dispersions were transferred to the other new Berzelius vials, where the dirty dispersion was transferred onto glass slides via vertical adsorption for 10 min. The adsorbed thin films were naturally dried and subjected to AFM investigation. The obtained images are presented in Figure 7.
The nanoparticles are well immobilized onto the glass substrate through a thin pellicle of organic matter, which seems to be partly dissolved into the water and solidified during the sample drying. It might be related to the car washing detergent remnant traces.
The AFM topographic image in Figure 7a reveals a well-adsorbed thin film of the nanoparticles, resulting from the initial sample. These are well individualized and present small tabular aspects. Most of the observed nanoparticles have a size of about 50 nm, which corresponds to the kaolinite, while fewer ones have about 60 nm, corresponding to muscovite, which is in good agreement with the mineralogical composition of the sample. Some irregular submicron particles are observed in Figure 7a with sizes ranging from 150 to 200 nm. Their shape and size correspond to the micro-plastics reported in the literature [12,22]. On the other hand, the nanoparticles resulting from the ultrafine fractions, as shown in Figure 7b, contain predominantly muscovite particles and have about 60 nm and less kaolinite, about 45 nm, which is in good agreement with the Scherrer formula results. The submicron plastic irregular clusters are also observed in Figure 7b. Their shape corresponds to the literature data and represents important clues regarding organic contamination spreading beyond the finest mineral fractions. They further require specific investigation for their assessment.
3.4. Organic Components Assessment
The resulting FTIR spectra, as shown in Figure 8, have a complex allure, corresponding to the chemical composition of the investigated fractions. The FTIR spectrum for the sample before sieving summarizes a median value of the absorption bands identified for the different particle classes.
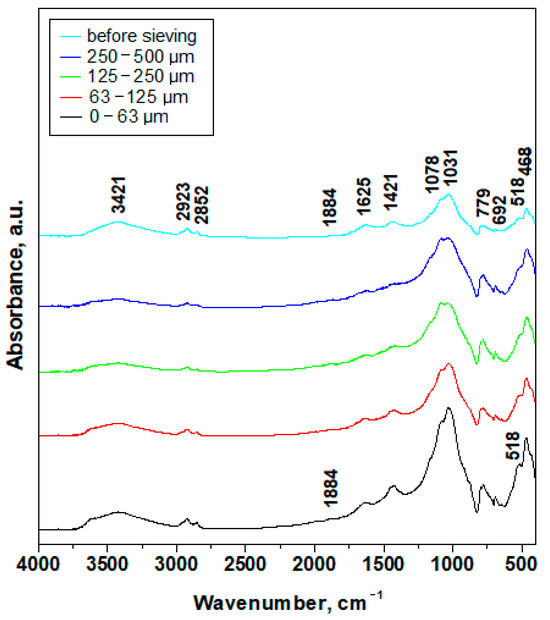
Figure 8.
FTIR spectra of the investigated slurry fractions.
The mineral bonds within slurry PM range from 400 to 1100 cm−1. Thus, calcite presence is evidenced by the asymmetric and symmetric rotation of CO32− at 884 and 692 cm−1 followed by the asymmetric stretch at 1421 cm−1 [32]. Quartz and aluminosilicate (muscovite and kaolinite) particles evidence a strong absorption band of the planar stretch of the Si-O bond at 1031cm−1 followed by Si-O-Si deformation at 468 cm−1 and an asymmetric stretch of Si-O within quartz at 779 cm−1 [33,34]. Iron hydroxides are characterized by the Fe-O bond absorption band at 518 cm−1 [35].
Water presence is related to the hydration within iron hydroxide pointed out by the absorption band at 1625 cm−1, which is characteristic of O-H deformation [35]. The physically adsorbed water presence is proved by the absorption bands of H-O-H stretching at 3421 cm−1 and H-O-H rotation at 1625 cm−1 [36]. The water molecules penetrate the muscovite and kaolinite silica tetrahedrons sheets, influencing their interplanar distance [37].
The absorption bands related to quartz and calcite are stronger for the coarse fractions of 125–250 μm and 250–500 μm due to their prevalence as bigger particles.
The intensity of 779 cm−1 related to quartz particles significantly decreases in intensity for the finest fractions 0–63 μm and 63–125 μm and the band at 1031 cm−1 become more intense and narrower due to the prevalence of muscovite and kaolinite particles. This fact is in good agreement with the XRD observations.
The absorption bands for organic matter occur from 1100 to 3500 cm−1. One of the most intense absorption bands occurs at 1078 cm−1 induced by C-C stretch [38]. It appears as a shoulder band partly merged with the more intense vibrations related to silicate bonds [39,40]. The symmetric stretch of C=O saturated bond appears at 1884 cm−1. The absorption band at 2923 cm−1 belongs to the asymmetric stretch of CH2 and the symmetric stretch occurs at 2852 cm−1. The absorption band at 1421 cm−1 [36] evidences the NH4− groups. The evidenced organic bounds correspond to the phthalate’s structures [35], which are related to the presence of micro-plastic particles. The carwash detergents also contain organic compounds having the evidenced chemical bonds in their structure [41,42]. Its absorption bands progressively increase their intensity in the finest fractions 0–63 μm and 63–125 μm.
GC-MS analysis was employed to search the specific volatile compounds related to organic matter to establish the nature of identified nanoparticles and crust deposits. The obtained GC-MS spectra are presented in Figure 9.
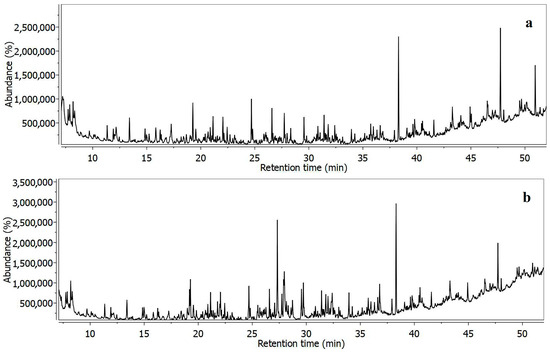
Figure 9.
GC—MS spectra for (a) the initial sample before sieving and (b) 0–63 µm fraction.
The identified compounds and their percent amount are centralized in Table 2. There were 75 volatile compounds for both the initial un-sieved sample and the finest fraction of 0–63 μm identified. There was a wide range of organic compound classes identified such as alcohols, phthalates, alkanes, cycloalkanes, alkenes, esters, halogens, etc.

Table 2.
Centralization of the organic compounds identified by GC—MS.
Phthalates such as dibutylphtalate, diisobutylphtalate, bis(2-ethylhexyl)phthalate, and bis(2-ethylhexyl)isophtalate are generated from the nano-plastic particles present in the investigated samples. These are most likely collected via the street dust and transported along with the mineral fractions.
Both investigated samples contain a significant amount of toluene, tetradecane, ethane1,1,2,2-tetrachloro (which are used as solvents for car engine mechanism cleaning), and various alkanes, such as docosane, which are involved in waxes for mechanisms; tricosane 2-methyl, which are used for dyes and organic paints preparations; and hexadecane,2,6,10,14-tetramethyl, belonging to petroleum products. On the other hand, 1,3-propanediol,ethyl,hexadecyl ether is used as a lubricant. These compounds relate to the professional activities of the carwash station, which include mechanical repairs and car paint refreshing.
Some of the volatile compounds are related to the traces of washing detergents, such as tetracosane, which acts as a non-polar surfactant, 2-bromotetradecane, which also acts as a surfactant, eicosane, which has an antifungal effect, and myristoylchloride, which involves organic synthesis. For example, octanal, 2-(phenylmethylene) and butyl angelate, 3-methyl have a floral scent, and they are commonly used in the perfume industry and might be related to the floral aroma of carwash detergents.
There are some other compounds, such as heneicosane, which is used as pheromone; oxyrane, which is used for anticancer drugs synthesis; oxirane,3-ethyl-2,2-dimethyl, which is also used in the pharmaceutical industry; and pentanoic acid and 2-propenyl ester, which are known for medical applications. Their presence is very difficult to explain related to their application and most likely occurs from the industrial transport cars, which might be used for the pharmaceutical industry.
3.5. Coverage Admixture Viability
The initial state (0 days) reveals an advanced moistening of the seed surface and the wetted ground surface without any sign of vegetation. The control sample has a significant number of green grass blades after 1 day, while the soil samples have no modification, as shown in Figure 10.
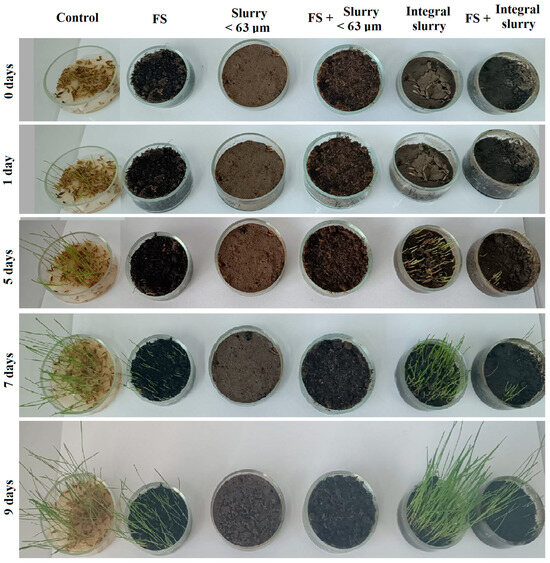
Figure 10.
Grass seed evolution in different soil compositions.
The first green grass blades appear in the integral slurry sample after 5 days and have a dense and uniform growth, while FS 50 wt.% + integral slurry 50 wt.% blend evidence only three weak blades in addition to the dish wall. The control sample has well-developed grass blade growth uniformly after 5 days. Soils containing slurry fine fractions do not evidence any sign of vegetal proliferation.
The control sample has the maximum development of the grass blades 7 days after seeding, followed by the integral slurry sample, which has dense and uniform grass blades having half of the control sample’s length. Fewer blades were noticed on the blended composition FS 50 wt.% + integral slurry 50 wt.%, which doubles their length from 5 to 7 days after seeding. Scarce grass blades appear uniformly on the fertile soil surface, while the soils containing slurry fine fractions are still empty of any vegetation.
Grass blades become weaker in the control sample, bowing their tip because of the complete consumption of the nutrients contained in the seeds after 9 days. Integral slurry develops dense and high grass blades, revealing the best growth after 9 days. Fertile soil has good results after 9 days and is comparable with the integral slurry after 7 days. The grass blades within blended composition FS 50 wt.% + integral slurry 50 wt.% grow well but are only a few. No sign of vegetation is noticed for the fine slurry fractions at 9 days after seeding.
4. Discussion
The correlation between the obtained experimental results reveals that the finest fraction of 0–63 μm dominates the dumped carwash slurry. It is very abundant in small micro-sized muscovite and kaolinite tabular particles belonging to PM2.5 and PM1 categories, a fact that is in good agreement with XRD and SEM—EDX observations. FTIR spectroscopy reveals the specific absorption bands for organic matter, which are closely connected with the mineral fractions. GC-MS spectroscopy precisely identifies the organic contaminant nature, such as petroleum residues and pesticides, substances that have an increased level of toxicity, making the situation worse and very hazardous for the health of living beings.
There appears to be a risk of PM1 and PM2.5 fraction re-suspension into the atmosphere if the landfill deposit becomes dry. The air currents lift the finest mineral fraction contaminated with organic matter and spread it all over the adjacent area [18,19]. Its size is too small to be trapped in the upper respiratory system mucosa and get into the lungs. Rainfall interacts with these fine fractions, increasing the pluvial water turbidity, which is further collected in rivers and lakes. The association between the finest clay particles and plastic nanoparticles enhances their liquid suspension ability, preventing their sedimentation. Their shiny aspect reflects sunlight, which becomes unable to penetrate into the water’s deeper layer, hindering their natural disinfection through the ultraviolet beams within solar light. The bottom aquatic vegetation is also deprived of sunlight, which becomes unable to sustain the feeding process. This fact is known in the literature as water eutrophication [43], which is favored by organic matter, such as antibiotics and pesticides [44].
AFM microscopy reveals the nanostructured components within the carwash slurry’s fine fractions. The hazard increases once more if these fractions are inhaled due to the presence of nano-plastic associated with nanomuscovite and nanokaolinite covered with organic matter such as petroleum, grease residues, and pesticides. Sharp-edged clay particles act as microscopic knives, sectioning the lungs’ alveoli [15]. These nanopollutants can penetrate directly into the blood through the sectioned alveoli, delivering the toxic matter to whole organs, which is in good agreement with the literature observation [16,17]. The nano-plastic particles contain many phthalates, which have carcinogenic effects at high bio-accumulation doses [45,46]. Bouhamidi evidence the high ability of phthalates adsorption on the other organic carbon structure [47].
Paltinean and Rusca show that all smallest PM fractions below 1 μm are very sensitive to the humid environment, mitigating their re-suspension tendency [8,9], a fact that is confirmed by the other studies in the literature [48]. Thus, the carwash slurry dumpsite is relatively safe if it is maintained at the proper humidity. If the top layers of the dump become dry, the air currents will cause the finest fraction suspension into the atmosphere and put living beings in danger.
Since the carwash facility is closed and the landfill might be neglected, there must be some rehabilitation actions made to prevent the finest particle re-suspension into the atmosphere. Landfill coverage with fertile soil is the simplest method of preventing slurry erosion, and, subsequently, particulate matter emissions have the benefit of the development of a dense vegetation layer. Such action could be relatively difficult and costly.
We found in the carwash slurry many minerals containing Ca, Mg, and other ions, which could sustain a vegetative layer [49,50], but in fact, it strongly depends on the contamination of organic matter. The obtained results reveal that the finest fractions are the most contaminated with significant amounts of oil and grease spillage from vehicle engine maintenance along with other organic traces such as detergents, pigments, and pesticides. Such organic matter might affect vegetal seed germination, jeopardizing the further development of the green barrier. For instance, Klokk shows that low amounts of petroleum residues and grease matter delay the seeds’ germination, but their excess has a deeply negative effect [51]. Pesticides have a negative influence on targeted plants but also might affect the germination of other species [52]. Bigger fractions are less contaminated and contain significant amounts of calcite, bringing a Ca amount useful for plant growth, as previously mentioned in the literature [53].
The novelty of the present study is the aim of converting the carwash slurry into a proper admixture able to sustain a vegetative layer. The slurry usage as a raw material for admixture preparation ensures its landfill liquidation. The blending with fertile soil brings an extra binding PM of fine fractions through the naturally occurring humus compounds. Quartz particles bring stability in a similar manner to composite material filler. Calcite is a useful mineral, supporting the vegetation requirements for Ca, and the clay particles mediate the material’s humidity through their lamellar structure. All these mineral components of the carwash slurry fit well with the composition of fertile soil, enriching it with minerals. Such blending is a sustainable process that converts potentially dangerous waste into a material useful for the mitigation of other dangerous waste spreading. Several shortcomings might interfere because of the increased organic contamination of the finest fractions. Thus, the optimal blending composition must be found through a vegetal proliferation test.
The carwash slurry composition depends on the car’s circulation area characteristics, including soil structure and industrial activities. Both aspects generate particulate matter emissions, which are deposited on the surface of the car. Quartz, calcite, and clay particles are commonly found in variable proportions in soils all over the world. Therefore, the presented aspects are not limited to a strict area and can be adapted to the specificities of any area of interest.
The preliminary tests reveal that integral slurry sustains a proper development of the grass germination, and, subsequently, growth, ensuring optimal results after 9 days. Fertile soil has a slow retardation of two days in promoting grass germination because of the humidity distribution within humus bonds within the mineral particles. Healthy organic matter within fertile soil ensures a proper life cycle for the grass [54,55], which might be poor in the integral slurry without fertile additives. Thus, integral slurry blending with fertile soil should develop the best sustainable effect on the growing grass. Indeed, the germinated grass blades evolve in a proper manner and are situated between the precursor results. It certainly requires further investigation of the particle interaction with the grass seeds to establish the proper blending mode.
The obtained results clearly show that the finest fractions of the slurry are completely not suitable for vegetal life development, but the integral slurry is effective and proper for fast vegetal proliferation. However, the literature data indicate the necessity of fertile compounds support to ensure sustainable growth of the vegetal species lasting well in time [54,55]. Therefore, the integral slurry blending process requires further investigation to reveal the optimal dosage.
The optimal blended composition should be used as a coverage admixture for the neutralization of other more dangerous landfills such as slag deposits from the copper–nickel industry [56], copper slag [57], and silico–manganese slag [58]. These slag dumps might emit deadly aerosols into the atmosphere if they are not properly maintained. Their coverage with the properly blended integral carwash slurry would be the best cost-effective sustainable approach for hazard mitigation.
Literature data reveal that vegetal species (e.g., grass and bushes) growth on the coverage layer ensures complete protection against fine hazardous particle emission [53,59]. Bush roots fix the dump stability, and their progressive growth has a significant contribution to the global carbon footprint reduction by embedding CO2 into the lignin structure [60,61]. The mowed grass is not affordable for the production of hay because of pollutant matter bioaccumulation, but it is feasible for composting and enriching the fertile component of the covering layer.
5. Conclusions
Carwash slurry is dominated by the smallest particle fraction, 0–63 μm, in which fine and sharp particles of muscovite and kaolinite predominate, in addition to some quartz and calcite particles. Nanofractions of muscovite of about 60 nm and kaolinite of about 50 nm were identified along with nano-plastic clusters of 150–200 nm. These are contaminated with organic dirt spots and crusts formed by the car washing detergent remains along with traces of solvents and petroleum products related to car and lorry maintenance. The organic contamination of the ultrafine particles increases their potential hazard if they are re-suspended into the atmosphere. The mitigation strategy is maintaining the carwash slurry dump at the optimal humidity level. A permanent solution for the finest fraction re-suspension would be the growing of a green grass top layer achieved by landfill seeding or its sustainable conversion as cover admixture. Carwash slurry finest fractions are unable to promote seed germination because of the increased level of pollutants. The integral slurry can be converted into a sustainable covering material for the neutralization of the most hazardous dumps.
Author Contributions
Conceptualization, S.E.A. and I.P.; methodology, S.E.A. and I.P.; software, G.B. and I.C.; validation, S.E.A. and I.P.; formal analysis, M.R.F. and I.P.; investigation, S.E.A., L.B.T., G.B., M.R.F., I.C. and I.P.; resources, S.E.A.; data curation, I.P.; writing—original draft preparation, S.E.A. and I.P.; writing—review and editing, I.P.; visualization, L.B.T. and I.P.; supervision, S.E.A. and I.P.; project administration, S.E.A. and I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article.
Acknowledgments
The authors acknowledge the XRD diffractometer maintenance, which was supported by the Ministry of Research, Innovation, and Digitization through Program 1—Development of the National Research and Development System, Subprogram 1.2—Institutional Performance-Funding Projects for Excellence in RDI, Contract No. 37PFE/30.12.2021.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AFM | Atomic Force Microscopy |
| ASPs | Atmosphere Suspended Particles |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| MOM | Mineralogical Optical Microscopy |
| SEM | Scanning Electron Microscopy |
| SD | Street Dust |
| TVOCs | Total Volatile Organic Compounds |
| PMs | Particulate Matters |
| XRD | X-ray Diffraction |
References
- Chang, X.; Sun, L.; Yu, X.; Liu, Z.; Jia, G.; Wang, Y.; Zhu, X. Windbreak efficiency in controlling wind erosion and particulate matter concentrations from farmlands. Agriculture. Ecosyst. Environ. 2021, 308, 107269. [Google Scholar] [CrossRef]
- Ramirez Haberkon, N.B.; Aparicio, V.C.; De Gerónimo, E.; Mendez, M.J. Multi residues of pesticides in the particulate matter (PM10) emitted by rural soils of the semiarid pampas, Argentina. A potential source of air pollution. Environ. Pollut. 2024, 360, 124617. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, G.; Falandysz, J.; Yang, L.; Zhao, C.; Chen, C.; Sun, Y.; Zheng, M.; Jiang, G. Atmospheric emissions of particulate matter-bound heavy metals from industrial sources. Sci. Total Environ. 2024, 947, 174467. [Google Scholar] [CrossRef]
- Fang, X.; Chang, R.; Zhang, Y.; Zuo, J.; Zou, Y.; Han, Y. Monitoring airborne particulate matter from building construction: A systematic review. J. Build. Eng. 2024, 86, 108708. [Google Scholar] [CrossRef]
- Zafra-Pérez, A.; Boente, C.; García-Díaz, M.; Gómez-Galán, J.A.; Sánchez de la Campa, A.; De la Rosa, D. Aerial monitoring of atmospheric particulate matter produced by open-pit mining using low-cost airborne sensors. Sci. Total Environ. 2023, 904, 166743. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.M.; Memon, L.A.; Selim, M.Y.E. Experimental study of particulate matter emission for a diesel engine fueled with nanoparticles and biofuel/diesel blends. Int. J. Thermofluids 2024, 23, 100738. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, L.; Chen, Z.; Meng, L.; Li, Z.; Fang, Y. Effect of ash-bridge deposition in asymmetric diesel particulate filter channels on the pressure drop and particulate matter trapping characteristics. Part. Sci. Technol. 2024, 42, 1395–1406. [Google Scholar] [CrossRef]
- Paltinean, A.G.; Petean, I.; Arghir, G.; Muntean, D.F.; Bobos, L.D.; Tomoaia-Cotisel, M. Atmospheric induced nanoparticles due to the urban street dust. Part. Sci. Technol. 2015, 34, 580–585. [Google Scholar] [CrossRef]
- Rusca, M.; Rusu, T.; Avram, S.E.; Prodan, D.; Paltinean, G.A.; Filip, M.R.; Ciotlaus, I.; Pascuta, P.; Rusu, T.A.; Petean, I. Physicochemical Assessment of the Road Vehicle Traffic Pollution Impact on the Urban Environment. Atmosphere 2023, 14, 862. [Google Scholar] [CrossRef]
- Wei, T.; Wijesiri, B.; Li, Y.; Goonetilleke, A. Particulate matter exchange between atmosphere and roads surfaces in urban areas. J. Environ. Sci. 2020, 98, 118–123. [Google Scholar] [CrossRef]
- Mcclellan, R.O. Lung cancer in rats from prolonged exposure to high concentrations of carbonaceous particles: Implications for human risk assessment. Part. Sci. Technol. 1996, 14, 89–122. [Google Scholar] [CrossRef]
- Xu, L.; Ma, W.; Huo, X.; Luo, J.; Li, R.; Zhu, X.; Kong, X.; Zhao, K.; Jin, Y.; Zhang, M.; et al. New insights into the function and mechanisms of piRNA PMLCPIR in promoting PM2.5-induced lung cancer. J. Adv. Res. 2024, in press. [Google Scholar] [CrossRef]
- Zhou, X.; Sampath, V.; Nadeau, K.C. Effect of air pollution on asthma. Ann. Allergy Asthma Immunol. 2024, 132, 426–432. [Google Scholar] [CrossRef]
- Seaton, A.; Legge, J.S.; Henderson, J.; Kerr, K.M. Accelerated silicosis in Scottish stonemasons. Lancet 1991, 337, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Penchala, A.; Patra, A.K.; Santra, S.; Dubey, R.; Mishra, N.; Nazneen, A.; Pradhan, D.S. Assessment of vertical transport of PM in a surface iron ore mine due to in-pit mining operations. Measurement 2025, 240, 115580. [Google Scholar] [CrossRef]
- Child, H.; Berry, C.C. A novel 3D model for the study of functionalised-nanoparticle penetration into human tissue. Drug Discov. Today 2010, 15, 1086–1087. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, W.; Wang, W.; Gu, Z.; Shen, Y. Particle-scale study on extracellular penetration of nanoparticles in tumor tissues. Mater. Today Phys. 2024, 44, 101428. [Google Scholar] [CrossRef]
- Kang, T.; Kim, H. An Experimental Study on the Component Analysis and Variation in Concentration of Tire and Road Wear Particles Collected from the Roadside. Sustainability 2023, 15, 12815. [Google Scholar] [CrossRef]
- Avram, S.E.; Tudoran, L.B.; Borodi, G.; Filip, M.R.; Petean, I. Urban Traffic’s Influence on Noise and Particulate Matter Pollution. Sustainability 2025, 17, 2077. [Google Scholar] [CrossRef]
- Devarangadi, M.; Shankar, U.M. Correlation studies on geotechnical properties of various industrial byproducts generated from thermal power plants, iron and steel industries as liners in a landfill—A detailed review. J. Clean. Prod. 2020, 261, 121207. [Google Scholar] [CrossRef]
- Avram, S.E.; Filip, M.R.; Barbu Tudoran, L.; Borodi, G.; Petean, I. Investigation of ferrous conglomerate particles found in carwash slurry and their environmental implications. Stud. UBB Chem. 2023, 68, 57–70. [Google Scholar] [CrossRef]
- Dai, H.; Li, H.; Qiu, W.; Deng, S.; Han, J.; Aminabhavi, T. Nondestructive analysis of plastic debris from micro to nano sizes: A state-of-the-art review on Raman spectroscopy-based techniques. TrAC Trends Anal. Chem. 2024, 176, 117750. [Google Scholar] [CrossRef]
- Eisen, A.; Pioro, E.P.; Goutman, S.A.; Kiernan, M.C. Nanoplastics and Neurodegeneration in ALS. Brain Sci. 2024, 14, 471. [Google Scholar] [CrossRef] [PubMed]
- Păltinean, G.A.; Petean, I.; Arghir, G.; Muntean, D.F.; Tomoaia-Cotişel, M. Silicates Fragmentation a Source of Atmosphere Dispersed Nano—Particulate Matter. Rev. Chim. 2016, 67, 1118–1123. [Google Scholar]
- Avram, S.E.; Birle, B.V.; Tudoran, L.B.; Borodi, G.; Petean, I. Investigation of Used Water Sediments from Ceramic Tile Fabrication. Water 2024, 16, 1027. [Google Scholar] [CrossRef]
- Amato, F.; Querol, X.; Johansson, C.; Nagl, C.; Alastuey, A. A review on the effectiveness of street sweeping, washing and dust suppressants as urban PM control methods. Sci. Total Environ. 2010, 408, 3070–3084. [Google Scholar] [CrossRef]
- Górka-Kostrubiec, B.; Świetlik, R.; Szumiata, T.; Dytłow, S.; Trojanowska, M. Integration of chemical fractionation, Mössbauer spectrometry, and magnetic methods for identification of Fe phases bonding heavy metals in street dust. J. Environ. Sci. 2023, 124, 875–891. [Google Scholar] [CrossRef]
- Larsen, O.; Postma, D. Kinetics of reductive bulk dissolution of lepidocrocite, ferrihydrite, and goethite. Geochim. Cosmochim. Acta 2001, 65, 1367–1379. [Google Scholar] [CrossRef]
- Karanasiou, A.; Moreno, T.; Amato, F.; Lumbreras, J.; Narros, A.; Borge, R.; Tobías, A.; Boldo, E.; Linares, C.; Pey, J.; et al. Road dust contribution to PM levels—Evaluation of the effectiveness of street washing activities by means of Positive Matrix Factorization. Atmos. Environ. 2011, 45, 2193–2201. [Google Scholar] [CrossRef]
- Kakavas, S.; Pandis, S.N. Effects of urban dust emissions on fine and coarse PM levels and composition. Atmos. Environ. 2021, 246, 118006. [Google Scholar] [CrossRef]
- Dippong, T.; Deac, I.G.; Petean, I.; Levei, E.A.; Cadar, O. Evolution of morphology, structure and magnetic behavior of CdxZn1−xFe2O4@SiO2 nanocomposites with Cd2+ content and heat treatment. Opt. Mater. 2025, 162, 116936. [Google Scholar] [CrossRef]
- Lettieri, M.; Giannotta, M.T. Investigations by Ft-Ir Spectroscopy on Residues in Pottery Cosmetic Vases from Archaeological Sites in the Mediterranean Basin. Int. J. Exp. Spectrosc. Tech. 2017, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.M.; Qiao, X.C.; Yu, J.G. Influences of quartz and muscovite on the formation of mullite from kaolinite. Appl. Clay Sci. 2013, 80–81, 176–181. [Google Scholar] [CrossRef]
- Zheng, K.; Xie, X.; Gou, G.; Chen, X.; Huang, Y.; Gao, J. Comparative study on characteristics and microstructure of magnesium silicate hydrate utilizing quartz and silica fume as siliceous raw materials. Case Stud. Constr. Mater. 2023, 19, e02313. [Google Scholar] [CrossRef]
- Yang, Y.; Du, J.; Jing, C. Dynamic adsorption process of phthalate at goethite/aqueous interface: An ATR-FTIR study. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 504–509. [Google Scholar] [CrossRef]
- Varrica, D.; Tamburo, E.; Vultaggio, M.; Di Carlo, I. ATR–FTIR Spectral Analysis and Soluble Components of PM10 And PM2.5 Particulate Matter over the Urban Area of Palermo (Italy) during Normal Days and Saharan Events. Int. J. Environ. Res. Public Health 2019, 16, 2507. [Google Scholar] [CrossRef]
- Avram, S.E.; Barbu Tudoran, L.; Cuc, S.; Borodi, G.; Birle, B.V.; Petean, I. Microstructural Investigations Regarding Sustainable Recycling of Ceramic Slurry Collected from Industrial Waste Waters. Sustainability 2024, 16, 1123. [Google Scholar] [CrossRef]
- Malek, K.; Wood, B.; Bambery, K. FTIR imaging of tissues: Techniques and methods of analysis. In Optical Spectroscopy and Computational Methods in Biology and Medicine; Springer Science & Business Media: Berlin, Germany, 2014; pp. 419–437. ISBN 978-94-007-7831-3. [Google Scholar]
- Bora, J.; Deka, P.; Bhuyan, P.; Sarma, K.P.; Hoque, R.R. Morphology and mineralogy of ambient particulate matter over mid-Brahmaputra Valley: Application of SEM–EDX, XRD, and FTIR techniques. SN Appl. Sci. 2021, 3, 137. [Google Scholar] [CrossRef]
- Usman, F.; Zeb, B.; Alam, K.; Huang, Z.; Shah, A.; Ahmad, I.; Ullah, S. In-Depth Analysis of Physicochemical Properties of Particulate Matter (PM10, PM2.5 and PM1) and Its Characterization through FTIR, XRD and SEM–EDX Techniques in the Foothills of the Hindu Kush Region of Northern Pakistan. Atmosphere 2022, 13, 124. [Google Scholar] [CrossRef]
- Kazembeigi, F.; Bayad, S.; Yousefi Nasab, A.; Doraghi, M.; Parseh, I. Techno-environmental study on the consequences of carwash wastewater and its management methods. Heliyon 2023, 9, e19764. [Google Scholar] [CrossRef]
- Hoseinzadeh, E.; Gholifam, A.; Faramarzi, M. Treatment of real carwash wastewater using high-efficiency and energy-saving electrocoagulation technique. Sustain. Chem. Pharm. 2024, 41, 101688. [Google Scholar] [CrossRef]
- Amancio, R.C.H.; Pacheco, S.P.; Moura, K.A.F.; Valle, B.L.; Alves, J.T.C.; Melo, F.F.; Silva, V.J.G.; Botelho, L.S.; Rocha, R.T.; Pelegrine, D.R.; et al. Influence of Optically Active Substances on Light Attenuation in a Tropical Eutrophic Urban Reservoir. Limnogical Rev. 2025, 25, 7. [Google Scholar] [CrossRef]
- Li, S.; Murava, R.T.; Zhang, Q.; Zhou, T.; Omoregie, A.I.; Rajasekar, A.; Ouahbi, T. Linking Antibiotic Residues and Antibiotic Resistance Genes to Water Quality Parameters in Urban Reservoirs: A Seasonal Perspective. Environment 2025, 12, 96. [Google Scholar] [CrossRef]
- Meng, M.; Yang, Y.; Song, L.; Peng, J.; Li, S.; Gao, Z.; Bu, Y.; Gao, J. Association between urinary phthalates and phthalate metabolites and cancer risk: A systematic review and meta-analysis. Heliyon 2024, 10, e29684. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, Z.; Jin, Y.; Yang, M.; Zhang, Z.; Zhou, X.; Qiu, S.; Zou, X. Exploring the relationships between exposure levels of bisphenols and phthalates and prostate cancer occurrence. J. Hazard. Mater. 2024, 474, 134736. [Google Scholar] [CrossRef]
- Bouhamidi, Y.; Kaouah, F.; Nouri, L.; Boumaza, S.; Trari, M.; Bendjama, Z. Kinetic, thermodynamic, and isosteric heat of dibutyl and diethyl phthalate removal onto activated carbon from Albizzia julibrissin pods. Part. Sci. Technol. 2016, 36, 235–243. [Google Scholar] [CrossRef]
- Peng, Y.; Sui, Z.; Zhang, Y.; Wang, T.; Norris, P.; Pan, W.P. The effect of moisture on particulate matter measurements in an ultra-low emission power plant. Fuel 2019, 238, 430–439. [Google Scholar] [CrossRef]
- de Jesus, H.I.; Cassity-Duffey, K.; Dutta, B.; da Silva, A.L.B.R.; Coolong, T. Influence of Soil Type and Temperature on Nitrogen Mineralization from Organic Fertilizers. Nitrogen 2024, 5, 47–61. [Google Scholar] [CrossRef]
- Smart, K.E.; Singer, D.M. Surface Coal Mine Soils: Evidence for Chronosequence Development. Soil Syst. 2023, 7, 59. [Google Scholar] [CrossRef]
- Klokk, T. Effects of oil pollution on the germination and vegetative growth of five species of vascular plant. Oil Petrochem. Pollut. 1984, 2, 25–30. [Google Scholar] [CrossRef]
- Jing, C.; Wang, J.; Wu, Y.; Zhou, Y.; Zhu, H.; Zhang, Y.; Dong, S.; Li, X.; Zhao, J.; Cao, J.; et al. The Effect of Weed Control with Pre-Emergence Herbicides on the Yield Level of Mung Bean Yield. Plants 2025, 14, 275. [Google Scholar] [CrossRef]
- Braşovan, A.; Mandroc, V.; Câmpean, R.F.; Petean, I.; Codrea, V.; Arghir, G. Calcium and Magnesium Content in Brier (Rosa Canina L.) Fruits at The ”Campul lui Neag” Sterile Coal Dump. Analele Univ. Din Oradea Fasc. Biol. 2011, 18, 5–9. [Google Scholar]
- Cuo, M.; Xu, L.; Yuan, B.; Nie, Y.; Wei, J. Pastureland Soil Organic Carbon Storage Regulated by Pasture Species and Age Under Nitrogen and Water Addition in Northern China. Agronomy 2025, 15, 399. [Google Scholar] [CrossRef]
- Tripolskaja, L.; Kazlauskaite-Jadzevice, A.; Razukas, A.; Baksiene, E. Perennial Grasses on Stony Sandy Loam Arenosol: Summary of Results of Long-Term Experiment in Northern Europe Region (1995–2024). Plants 2025, 14, 166. [Google Scholar] [CrossRef]
- Kasikov, A.G.; Shchelokova, E.A.; Timoshchik, O.A.; Semushin, V.V. Deep Processing of Dump Slag from the Copper-Nickel Industry. Metals 2023, 13, 1265. [Google Scholar] [CrossRef]
- Gabasiane, T.S.; Danha, G.; Mamvura, T.A.; Mashifana, T.; Dzinomwa, G. Environmental and Socioeconomic Impact of Copper Slag—A Review. Crystals 2021, 11, 1504. [Google Scholar] [CrossRef]
- Buruiana, D.L.; Obreja, C.-D.; Herbei, E.E.; Ghisman, V. Re-Use of Silico-Manganese Slag. Sustainability 2021, 13, 11771. [Google Scholar] [CrossRef]
- Fernández-Caliani, J.C.; Álvarez-Lozano, J.; García-Navarro, E.; Fernández-Landero, S.; Cantero, C.; Giráldez, M.I. A Novel Technosol Formulation for Sustainable Landfill Top Covers Using Non-Hazardous Wastes. Appl. Sci. 2024, 14, 6166. [Google Scholar] [CrossRef]
- Cobos-Torres, J.-C.; Idrovo-Ortiz, L.-H.; Cobos-Mora, S.L.; Santillan, V. Renewable Energies and Biochar: A Green Alternative for Reducing Carbon Footprints Using Tree Species from the Southern Andean Region of Ecuador. Energies 2025, 18, 1027. [Google Scholar] [CrossRef]
- Atchadé, A.J.; Kanda, M.; Folega, F.; Diouf, A.A.; Agbahoungba, S.; Dourma, M.; Wala, K.; Akpagana, K. Urban Flora Structure and Carbon Storage Potential of Woody Trees in Different Land Use Units of Cotonou (West Africa). Urban Sci. 2023, 7, 106. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).