Importance of Traditional Vanilla Cultivation in the Conservation of Plant Diversity in Tropical Forests in Northern Veracruz, Mexico
Abstract
1. Introduction
2. Materials and Methods
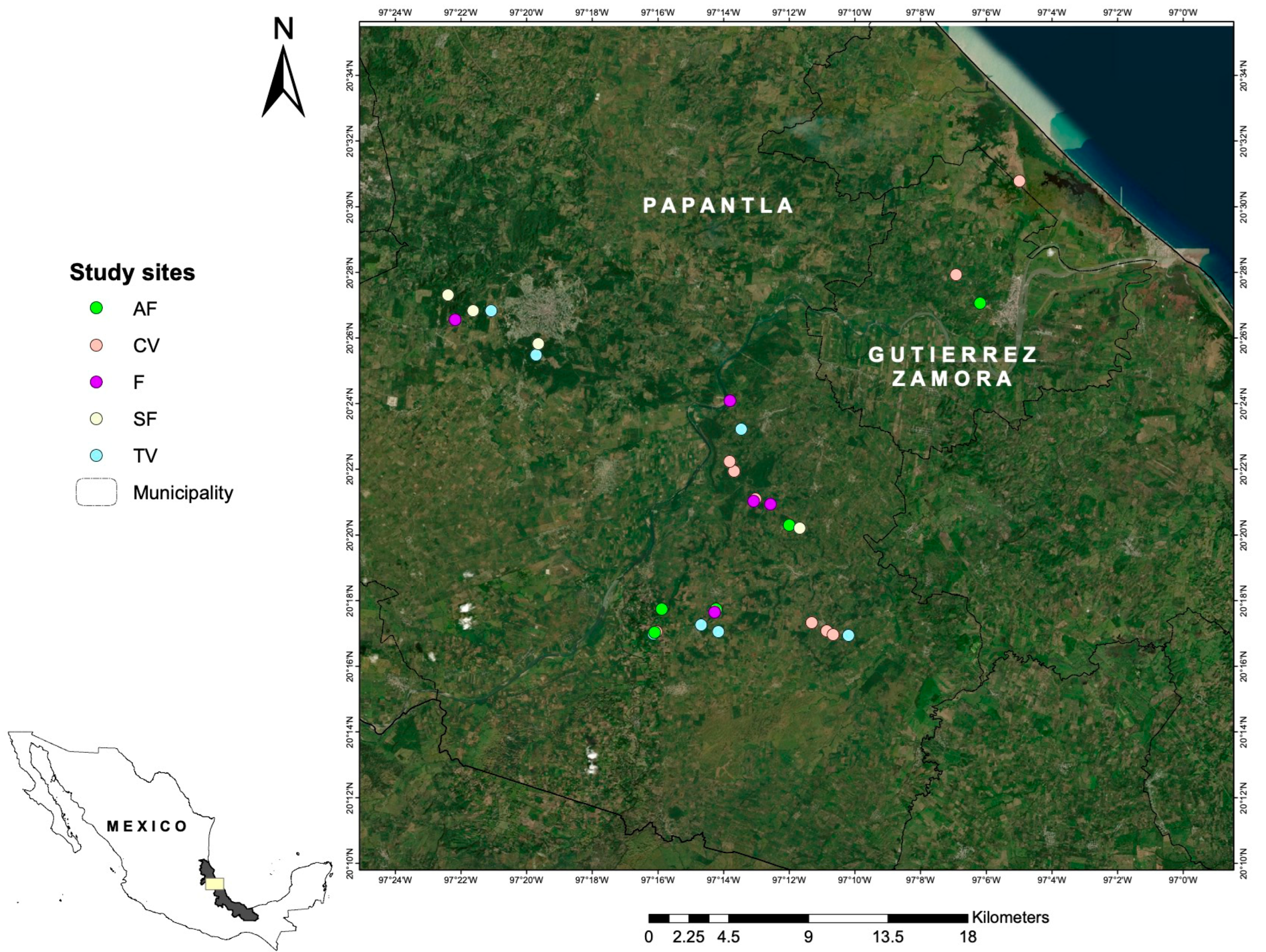
2.1. Study Area
2.2. Characterization of Vegetation in Elements of the Totonac Biocultural Landscape
2.3. Statistical Analysis
3. Results
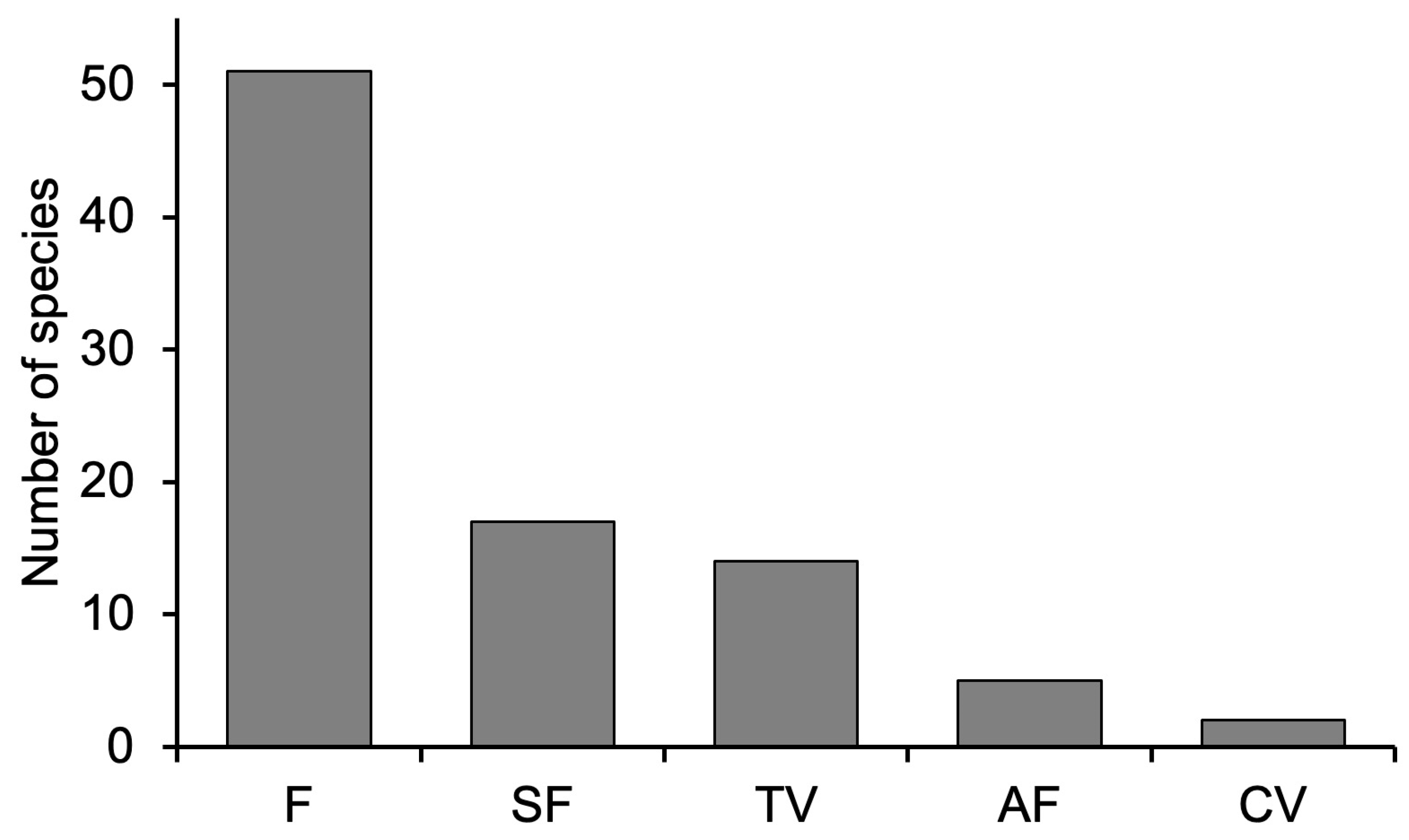
3.1. Richness and Diversity of Woody Plants
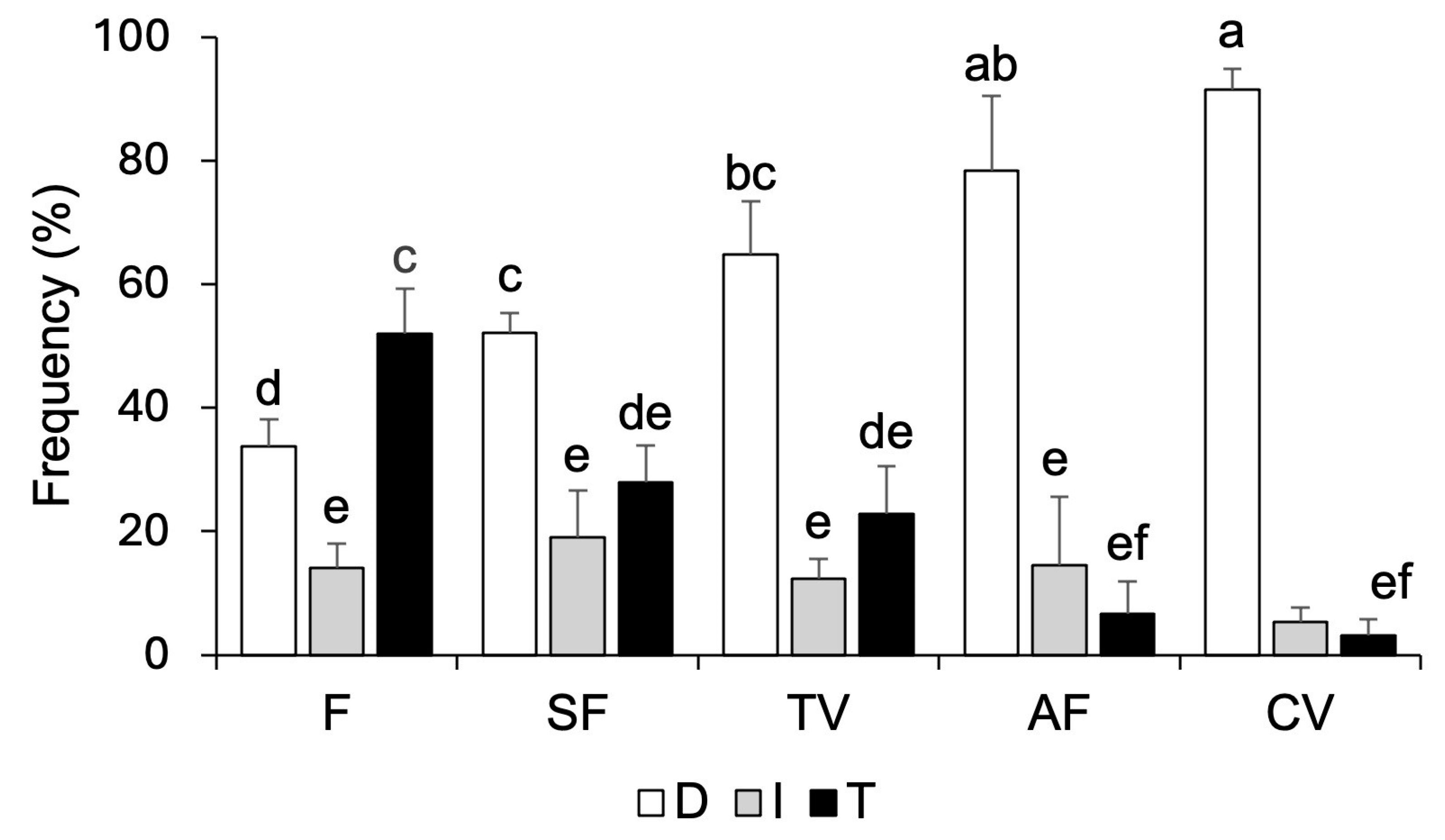
3.2. Structural Characteristics of Vegetation Fragments and Vanilla Cultivation Systems
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| F | Forest (medium semi-deciduous) |
| S | Secondary forest |
| TV | Traditional vanilla cultivation |
| AF | Vanilla agroforestry system |
| CV | Citrus–vanilla system |
| DBH | Diameter at breast height |
| NMDS | Non-metric multidimensional scaling |
| SIMPROF | Similarity profile analysis |
| SIMPER | Similarity percentage analysis |
Appendix A
| Family | Species | F | SF | TV | AF | CV |
|---|---|---|---|---|---|---|
| Acanthaceae | Odontonema callistachyum (Schltdl. & Cham.) Kuntze | X | ||||
| Annonaceae | Annona scleroderma Saff. | X | ||||
| Cymbopetalum baillonii R.E.Fr. | X | |||||
| Desmopsis trunciflora (Schltdl. &Cham.) G.E.Schatz ex Maas, E.A.Mennega & Westra | X | X | ||||
| Desmopsis uxpanapensis G.E.Schatz | X | |||||
| Mosannona depressa (Baill.) Chatrou | X | |||||
| Apocynaceae | Cascabela ovata (Cav.) Lippold | X | ||||
| Cascabela thevetioides (Humb.& Bonpl.) Lippold | X | X | ||||
| Tabernaemontana alba Mill. | X | X | X | X | ||
| Araliaceae | Dendropanax arboreus (L.) Decne. & Planch. | X | X | X | ||
| Arecaceae | Attalea butyracea (Mutis ex L.f.) Wess:Boer | X | X | |||
| Chamaedorea microspadix Burret | X | |||||
| Chamaedorea oblongata Mart. | X | |||||
| Asparagaceae | Yucca gigantea Lem. | X | ||||
| Asteraceae | Critonia morifolia (Mill.) R.M.King & H.Rob. | X | X | |||
| Flaveria trinervia (Spreng.) C.Mohr | X | X | X | X | ||
| Bignoniaceae | Parmentiera aculeata (Kunth) Seem. | X | X | X | ||
| Tabebuia rosea (Bertl.) DC. | X | X | X | |||
| Boraginaceae | Ehretia tinifolia L. | X | ||||
| Burseraceae | Bursera simaruba Sarg. | X | X | X | X | X |
| Protium copal (Schltdl. & Cham.) engl. | X | X | X | |||
| Cannabaceae | Aphananthe monoica (Hemsl.)J.-F.Leroy | X | ||||
| Celtis iguanaea (Jacq.) Sarg. | X | |||||
| Capparaceae | Morisonia quiriguensis (Standl.) Christenh. & Byng | X | ||||
| Caricaceae | Carica papaya L. | X | X | X | ||
| Celastraceae | Evonymopsis mexicanus Benth. | X | ||||
| Crossopetalum parviflorum (Hemsl.) Lundell | X | |||||
| Wimmeria concolor Schltdl. & Cham. | X | X | X | |||
| Wimmeria obtusifolia Standl. | X | X | X | |||
| Chrysobalanaceae | Couepia polyandra (Kunth) Rose | X | ||||
| Cleomaceae | Cleome uniglandulosa Cav. | X | ||||
| Euphorbiaceae | Acalypha macrostachya Jacq. | X | X | |||
| Adelia barbinervis Cham.& Schltdl. | X | X | X | |||
| Alchornea chiapasana Miranda | X | |||||
| Alchornea latifolia Sw. | X | |||||
| Balakata luzonica (S.Vidal) Esser | X | X | X | X | ||
| Bernardia dodecandra (Sessé ex Cav.) Govaerts | X | |||||
| Cnidoscolus multilobus (Pax) I.M.Johnst. | X | X | X | X | ||
| Croton draco Schltdl. | X | X | X | |||
| Croton soliman Cham. & Schltdl. | X | |||||
| Jatropha curcas L. | X | X | ||||
| Fabaceae | Pseudalbizzia tomentosa (Micheli) E.J.M.Koenen & Duno | X | ||||
| Pseudalbizzia tomentosa var. purpusii (Britton & Rose) E.J.M.Koenen & Duno | X | |||||
| Bauhinia divaricata L. | X | X | ||||
| Bauhinia ungulata L. | X | X | X | |||
| Cajanus cajan (L.) Millsp. | X | |||||
| Calliandra houstoniana var. anomala (Kunth) Barneby | X | |||||
| Calliandra trinervia var. arborea (Standl.) Barneby | X | |||||
| Cathormion umbellatum subsp. moniliforme (DC.) Brummitt | X | |||||
| Cojoba arborea (L.) Britton & Rose | X | |||||
| Diphysa carthagenensis Jacq. | X | |||||
| Diphysa microphylla Rydb. | X | |||||
| Diospyros nigra (J.F.Gmel.) Perr. | X | |||||
| Ehretia tinifolia L. | X | |||||
| Erythrina americana Mill. | X | X | ||||
| Erythrina folkersii Krukoff & Moldenke | X | |||||
| Erythrina macrophylla DC. | X | |||||
| Gliricidia maculata (Kunth) Walp. | X | X | X | |||
| Gliricidia sepium (Jacq.) Kunth | X | X | X | X | ||
| Inga acrocephala Steud. | X | |||||
| Inga punctata Willd. | X | |||||
| Inga sapindoides Willd. | X | |||||
| Leucaena leucocephala (Lam.) de Wit | X | X | X | X | ||
| Lonchocarpus eriocarinalis Micheli | X | |||||
| Lysiloma divaricatum (Jacq.) J.F:Macbr. | X | X | ||||
| Piscidia piscipula (L.) Sarg. | X | X | X | |||
| Pithecellobium dulce (Roxb.) Benth. | X | |||||
| Pithecellobium lanceolatum (Humb. & Bonpl. Ex Willd.) Benth. | X | X | X | |||
| Pterocarpus acapulcensis Rose | X | |||||
| Schnella glabra (Jacq.) Dugand | X | X | ||||
| Senna papillosa (Britton & Rose) H.S.Irwin & Barneby | X | X | X | |||
| Vachellia cornigera (L.) Seigler & Ebinger | X | X | X | |||
| Icacinaceae | Mappia racemosa Jacq. | X | ||||
| Lauraceae | Damburneya salicifolia (Kunth) Trofimov & Rohwer | X | X | X | X | |
| Licaria capitata (Cham. & Schltdl.) Kosterm. | X | |||||
| Nectandra hihua (Ruiz 6 Pav.) Rohmer | X | |||||
| Nectandra rubriflora (Mez) C.K.Allen | X | X | ||||
| Nectandra villosa Nees & Mart. | X | |||||
| Ocotea puberula (Rich.) Nees | X | X | ||||
| Persea americana Mill. | X | X | X | |||
| Persea cinerascens S.F.Blake | X | |||||
| Persea longipes (Schltdl.) Meisn. | X | X | ||||
| Malpighiaceae | Bunchosia biocellata Schltdl. | X | ||||
| Bunchosia lindeniana A.Juss. | X | X | X | X | X | |
| Byrsonima crassifolia Kunth | X | X | ||||
| Malpighia glabra L. | X | |||||
| Malvaceae | Carpodiptera cubensis Griseb. | X | X | |||
| Guazuma ulmifolia Lam. | X | X | X | X | ||
| Heliocarpus appendiculatus Turcz. | X | X | X | |||
| Heliocarpus donnellsmithii Rose | X | |||||
| Malvaviscus arboreus Dill. ex Cav. | X | X | X | |||
| Pachira aquatica Aubl. | X | |||||
| Robinsonella mirandae Gómez Pompa | X | X | ||||
| Meliaceae | Cedrela odorata L. | X | X | X | X | X |
| Swietenia humilis Zucc. | X | X | ||||
| Trichilia havanensis Jacq. | X | X | X | X | X | |
| Trichilia hirta L. | X | X | ||||
| Trichilia minutiflora Standl. | X | |||||
| Trichilia moschata Sw. | X | |||||
| Trichilia septentrionalis C.DC. | X | |||||
| Moraceae | Brosimum alicastrum Sw. | X | X | X | ||
| Ficus aurea Nutt. | X | |||||
| Ficus benjamina L. | X | |||||
| Ficus maxima Mill. | X | X | X | |||
| Ficus obtusifolia Kunth | X | X | X | |||
| Ficus yoponensis Desv. | X | |||||
| Trophis mexicana (Liemb.) Bureau | X | X | ||||
| Trophis racemosa Urb. | X | X | X | |||
| Myrtaceae | Calycorectes mexicanus O.Berg | X | ||||
| Eugenia capuli Schltdl. | X | X | X | X | X | |
| Myrcia bartlettii (Standl.) A.R.Lourenço & Sánchez-Cháv. | X | X | ||||
| Myrcia chytraculia (L.) A.R.Lourenço & E.Lucas | X | |||||
| Myrcia chytraculia var. pauciflora (O.Berg) G.P.Burton & E. Lucas | X | |||||
| Myrcia schlechtendaliana (O.Berg) A.R.Lourenço & Sánchez-Cháv. | X | |||||
| Pimenta dioica (L.) Merr. | X | X | X | X | ||
| Psidium guajava L. | X | X | ||||
| Nyctaginaceae | Pisonia aculeata L. | X | ||||
| Pentaphylaceae | Ternstroemia tepezapote Schltdl. & Cham. | X | X | |||
| Picramniaceae | Picramnia antidesma Sw. | X | X | |||
| Picramnia teapensis Tul. | X | |||||
| Piperaceae | Piper aduncum L. | X | ||||
| Piper amalago L. | X | |||||
| Piper aequale Vahl | X | X | ||||
| Piper hispidum sw. | X | X | ||||
| Piper sanctum Miq. | X | |||||
| Polygonaceae | Coccoloba barbadensis Jacq. | X | ||||
| Coccoloba hirtella Lundell | X | |||||
| Coccoloba hondurensis Lundell | X | |||||
| Coccoloba montana Standl. | X | X | ||||
| Primulaceae | Ardisia compressa Kunth | X | ||||
| Ardisia tuerckheimii Donn.Sm. | X | |||||
| Bonellia macrocarpa subsp. pungens (A.Gray) B.Stahl & Källersjö | X | |||||
| Myrsine coriacea (Sw.) R.Br. Ex Roem. & Schult. | X | |||||
| Parathesis revoluta Kunth | X | |||||
| Rosaceae | Prunus brachybotrya Zucc. | X | X | |||
| Prunus hainanensis (G.A.Fu & Y.S.Lin) H.Yu, N.H.Xia & H.G.Ye | X | |||||
| Rubiaceae | Chione venosa var. mexicana (Standl.) David W.Taylor | X | X | |||
| Chomelia spinosa Jacq. | X | X | ||||
| Exostema caribaeum (Jaq.)Roem & Schult. | X | |||||
| Hamelia patens Jacq. | X | X | X | X | ||
| Palicourea padifolia (Roem. & Schult.) C.M.Taylor & Lorence | X | |||||
| Palicourea pseudinundata (Wernham) Delprete & J.H.Kirbr. | X | |||||
| Palicourea sousae (Lorence & Dwyer) Lorence | X | X | X | |||
| Posoqueria latifolia (Rudge) Roem. & Schult. | X | |||||
| Psychotria costivenia Griseb. | X | |||||
| Psychotria erythrocarpa Schltdl. | X | |||||
| Psychotria nervosa Sw. | X | X | X | |||
| Psychotria papantlensis (Oerst.) Hemsl. | X | |||||
| Psychotria sarapiquensis Standl. | X | |||||
| Randia armata (Sw.) DC. | X | X | ||||
| Randia laetevirens Standl. | X | |||||
| Rutaceae | Amyris monofoliaris P.E.Sánchez | X | ||||
| Citrus aurantiaca Swingle | X | |||||
| Citrus × aurantium L. | X | X | X | X | X | |
| Citrus limon (L.) Osbeck | X | |||||
| Esenbeckia pentaphylla Griseb. | X | |||||
| Murraya paniculata (L.) Jack | X | |||||
| Salicaceae | Casearia corymbosa Kunth | X | ||||
| Casearia laetioides (A.Rich.) Warb. | X | X | X | X | ||
| Pleuranthodendron lindenii (Turcz.) Sleumer | X | X | X | X | ||
| Xylosma pubescens Griseb. | X | X | ||||
| Sapindaceae | Cupania glabra Sw. | X | X | X | X | |
| Litchi chinensis Sonn. | X | |||||
| Matayba oppositifolia Britton | X | X | ||||
| Sapindus saponaria L. | X | X | ||||
| Talisia macrophylla Radlk. | X | |||||
| Sapotaceae | Chrysophyllum mexicanum Brandegee ex Standl. | X | X | |||
| Manilkara chicle (Pitter) Gilly | X | X | X | |||
| Manilkara sapota Van Royen | X | X | X | |||
| Sideroxylon obtusifolium (Roem. & Schult.) T.D.Penn. | X | X | ||||
| Sideroxylon obtusifolium subsp. buxifolium (Roem. & Schult.) T.D.Penn. | X | |||||
| Sideroxylon persimile (Hemsl.) T.D.Penn. | X | |||||
| Solanaceae | Cestrum racemosum Ruiz & Pav. | X | X | X | ||
| Solanum umbellatum Mill. | X | X | ||||
| Urticaceae | Cecropia obtusifolia Bertol. | X | ||||
| Myriocarpa longipes Liebm. | X | X | ||||
| Urera simplex Wedd. | X | |||||
| Verbenaceae | Lippia myriocephala Schltdl. & Cham. | X | X | |||
| Violaceae | Rinorea guatemalensis Bartlett | X |
References
- Díaz, S.; Fargione, J.; Iii, F.S.C.; Tilman, D. Biodiversity Loss Threatens Human Well-Being. PLoS Biol. 2006, 4, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Dent, D.H.; DeWalt, S.J.; Denslow, J.S. Secondary forests of central Panama increase in similarity to old-growth forest over time in shade tolerance but not species composition. J. Veg. Sci. 2013, 24, 530–542. [Google Scholar] [CrossRef]
- van Breugel, M.; Hall, J.S.; Craven, D.; Bailon, M.; Hernandez, A.; Abbene, M.; van Breugel, P. Succession of ephemeral secondary forests and their limited role for the conservation of floristic diversity in a human-modified tropical landscape. PLoS ONE 2013, 8, e82433. [Google Scholar] [CrossRef]
- Bhagwat, S.A.; Willis, K.J.; Birks, H.J.B.; Whittaker, R.J. Agroforestry: A refuge for tropical biodiversity? Trends Ecol. Evol. 2008, 23, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Malhi, Y.; Gardner, T.A.; Goldsmith, G.R.; Silman, M.R.; Zelazowski, P. Tropical Forest in the Antropocene. Annu. Rev. Env. Resour. 2014, 39, 125–159. [Google Scholar] [CrossRef]
- Aide, T.M. Biodiversity (Wilson & Peters, 1988) revisited: How has tropical conservation science changed in the last 35 years? Biotropica 2023, 55, 729–736. [Google Scholar]
- Ma, J.; Li, J.; Wu, W.; Liu, J. Global Forest fragmentation change from 2000 to 2020. Nat. Commun. 2023, 14, 3752. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Peres, C.A.; Dent, D.; Sheil, D.; Lugo, A.E.; Lamb, D.; Stork, N.E.; Miller, S.E. The potential for species conservation in tropical secondary forests. Conserv. Biol. 2009, 23, 1406–1417. [Google Scholar] [CrossRef]
- Chazdon, R.L. Landscape restoration, natural regeneration, and the forest of the future. Ann. Mo. Bot. Gard. 2017, 102, 251–257. [Google Scholar] [CrossRef]
- Toledo, V.M.; Ortiz, B.; Medellín Morales, S. Biodiversity Island in a Sea of Pasturelands: Indigenous Resource management in the Humid Tropics of Mexico. Etnoecológica 1994, 2, 37–49. [Google Scholar]
- Quin, Y.; Xia, X.; Liu, F.; de Sa e Silva, F.; Shimabukuro, Y.; Arai, E.; Fearnside, P.M. Forest conservation in Indigenous territories and protected areas in the Brazilian Amazon. Nat. Sustain. 2023, 6, 295–305. [Google Scholar] [CrossRef]
- Finer, M.; Mamani, N. Protected Areas & Indigenous Territories Effective Against Deforestation Across Amazon. MAAP, 176. Available online: https://www.maapprogram.org/protected-indigenous-amazon/ (accessed on 24 December 2024).
- Moreno-Calles, A.I.; Galicia-Luna, V.J.; Casas, A.; Toledo, V.M.; Vallejo-Ramos, M.; Santos-Fita, D.; Camou-Guerrero, A. La etnoagroforesteria: El estudio de los sistemas agoforestales tradicioanles de México. Etnobiología 2014, 12, 1–16. [Google Scholar]
- Rendón-Sandoval, F.J.; Casas, A.; Moreno-Calles, A.I.; Torres-García, I.; García-Frapolli, E. Traditional Agroforestry Systems and Conservation of Native Plant Diversity of Seasonally Dry Tropical Forests. Sustainability 2020, 12, 4600. [Google Scholar] [CrossRef]
- Pérez-Silva, A.; Gunata, Z.; Lepoutre, J.P.; Odoux, E. New insight on the genesis and fate of odor- active compounds in vanilla beans (Vanilla planifolia G. Jackson) during traditional curing. Food Res. Int. 2011, 44, 2930–2937. [Google Scholar] [CrossRef]
- Lubinsky, P.; Bory, S.; Hernández-Hernández, J.; Kim, S.C.; Gómez-Pompa, A. Origins and Dispersal of Cultivated Vanilla (Vanilla planifolia Jacks. [Orchidaceae]). Econ. Bot. 2008, 62, 127–138. [Google Scholar] [CrossRef]
- Chenaut, V. Historia de los Pueblos Indígenas de México. Aquellos que Vuelan. Los Totonacos en el Siglo XIX; Centro de Investigaciones y Estudios Superiores en Antropologia Social, Instituto Nacional Indigenista: Mexico City, Mexico, 1995. [Google Scholar]
- Barrera-Bassols, N.; Medellín-Morales, S.; Ortíz-Espejel, B. The never-ending battle over corn: Facets and local provisioning; Case Study C: Exceptional abundance: Traditional resource management in the Totonac community of Plan de Hidalgo, Veracruz. In Economic Restructuring and Rural Subsistence in Mexico: Corn and the Crisis of the 1980’s; Transforming of Rural Mexico Serie Number 2; H. de Alcántara, C., Ed.; University of California-San Diego, Center for U.S.-Mexican Studies: San Diego, CA, USA, 1994; pp. 69–80. [Google Scholar]
- Valderrama Rouy, P. El Totonacapan, una región indígena en la vertiente del Golfo de México. Ollin 2016, 1, 31–39. [Google Scholar]
- Chenaut, V. Los totonacas de Veracruz. Población, cultura y sociedad. In Atlas del Patrimonio Natural, Histórico y Cultural de Veracruz; Florescano, E., Ortíz-Escamilla, O., Eds.; Gobierno del Estado de Veracruz y Universidad Veracruzano: Veracruz, Mexico, 2010; Volume 2, pp. 45–66. [Google Scholar]
- Rodríguez-Luna, E.; Gómez-Pompa, A.; López Acosta, J.C.; Velázquez-Rosas, N.; Aguilar-Domínguez, Y.; Torres-Vázquez, S.M. Atlas de los Espacios Naturales Protegidos de Veracruz; Gobierno del Estado de Veracruz, Universidad Veracruzana: Veracruz, Mexico, 2011. [Google Scholar]
- Ellis, E.A.; Martínez-Bello, M. Vegetación y uso de suelo. In Atlas del Patrimonio Natural, Históriuco y Cultural del Estado de Veracruz; Comisión para la Conmemoración del Bicentenario de la Independencia Nacional y del Centenario de la Revolución Mexicana; Gobierno del Estado de Veracruz: Veracruz, Mexico, 2010; pp. 203–226. [Google Scholar]
- Instituto Nacional de Estadística y Geografía (INEGI). Compendio de Información Geográfica Municipal 2010; Veracruz Ignacio de la Llave: Papantla, Mexico, 2010. [Google Scholar]
- Castelan Culebro, S.F. Producción y Calidad de Frutos de Vanilla planifolia Jacks. ex Andrews, en un Gradiente Altitudinal. Master’s Thesis, Universidad Veracruzana, Veracruz, México, 2022. [Google Scholar]
- Mostacedo, B.; Fredericksen, T.S. Manual de Métodos Básicos de Muestreo y Análisis en Ecología Vegetal; BOLFOR: Santa Cruz, Bolivia, 2000. [Google Scholar]
- Hill, J.L.; Curran, P.J. Area, shape and isolation of tropical forest fragments: Effects on tree species diversity and implications for conservation. J. Biogeogr. 2003, 30, 1391–1403. [Google Scholar] [CrossRef]
- Martínez Ramos, M. Regeneraciín natural y diversidad de especies arbóreas en selvas húmedas. Bot. Sci. 1994, 54, 179–224. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Chao, A.; Ma, K.H.; Hsieh, T.C. iNEXT (iNterpolation and EXTrapolation) Online: Software for Interpolation and Extrapolation of Species Diversity. Program and User’s Guide. 2016. Available online: http://chao.stat.nthu.edu.tw/wordpress/software_download/inext-online/ (accessed on 24 March 2024).
- Clarke, K.R.; Somerfield, P.J.; Gorley, E.N. Testing of null hypotheses in exploratory community analyses: Similarity profiles and biota-environment linkage. J. Exp. Mar. Biol. Ecol. 2008, 366, 56–69. [Google Scholar] [CrossRef]
- Faith, D.P.; Minchin, P.R.; Belbin, L. Compositional dissimilarity as a robust measure of ecological distance. Vegetatio 1987, 69, 57–68. [Google Scholar] [CrossRef]
- Minchin, P.R. An evaluation of the relative robustness of techniques for ecological ordination. Vegetatio 1987, 69, 89–107. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Marjokorpi, A.; Ruokolainen, K. The role of traditional forest gardens in the conservation of tree species in West Kalimantan, Indonesia. Biodivers. Conserv. 2003, 12, 799–822. [Google Scholar] [CrossRef]
- Hending, D.; Andrianiaina, A.; Maxfield, P.; Rakotomalala, Z.; Cotton, S. Floral species richness, structural diversity and conservation value of vanilla agroecosystems in Madagascar. Afr. J. Ecol. 2019, 58, 100–111. [Google Scholar] [CrossRef]
- Noble, I.R.; Dirzo, R. Forest as Human-Dominated Ecosystems. Science 1997, 277, 522–525. [Google Scholar] [CrossRef]
- Velázquez-Rosas, N.; Silva-Rivera, E.; Ruiz-Guerra, B.; Armenta-Montero, S.; González, J.T. Traditional Ecological Knowledge as a tool for biocultural landscape restoration in northern Veracruz, Mexico. Ecol. Soc. 2018, 23, 6. [Google Scholar] [CrossRef]
- Velasco-Murguía, A.; Silva-Rivera, E.; Velázquez-Rosas, N. Socio-Ecological Changes in Traditional Vanilla Agro-Ecosystems and Its Key Role in Biocultural Landscape Restoration; Centro de Investigaciones Tropicales, Universidad Veracruzan, Xalapa: Vercruz, México, 2025; (manuscript in preparation; to be submitted). [Google Scholar]
- Arasa-Gisbert, R.; Arroyo-Rodríguez, V.; Ortiz-Díaz, J.J.; Martínez, E. Regeneración de plantas leñosas en frgemnetos de bsoque tropcial húmedo: Estructura de la comunidad y registros nuevos para Chiapas, Tabasco y México. Rev. Mex. Biodivers. 2021, 92, e923502. [Google Scholar]
- Clark, D.B.; Clark, D.A. Abundance, growth and mortality of very large trees in neotropical lowland rain forests. Forest Ecol. Manag. 1996, 80, 235–244. [Google Scholar] [CrossRef]
- Ramachandran Nair, P.K.; Nair, V.D.; Kumar, B.M.; Showalter, J.M. Carbon Sequestration. In Agroforestry Systems. Advances in Agronomy 108, 1st ed.; Sparks, D.L., Ed.; Academic Press: San Diego, CA, USA, 2010; pp. 237–307. [Google Scholar]
- Casanova-Lugo, F.; Ramírez-Avilés, L.; Parsons, D.; Caamal-Maldonado, A.; Piñeiro-Vázquez, A.T.; Díaz-Echeverria, V. Environmental services from tropical agroforestry systems. Rev. Chapingo Ser. Cie. 2015, 22, 269–284. [Google Scholar]
- Ruiz-Guerra, B.; Guevara, R.; Mariano, N.; Dirzo, R. Insect herbivory declines with forest fragmentation and covaries with plant regeneration mode: Evidence from a Mexican tropical rainforest. Oikos 2010, 119, 317–325. [Google Scholar] [CrossRef]
- Tabarelli, M.; Peres, C.A.; Melo, F.P.L. The ‘few winners and many losers’ paradigm revisited: Emerging prospects for tropical forest biodiversity. Biol. Conserv. 2012, 155, 136–140. [Google Scholar] [CrossRef]
- Leakey, R.B. The Role of Trees in Agroecology and Sustainable Agriculture in the Tropics. Annu. Rev. Phytopathol. 2014, 52, 113–133. [Google Scholar] [CrossRef] [PubMed]
- Armenta-Montero, S.; Menchaca-García, R.; Pérez Silva, A.; Velázquez-Rosas, N. Changes in the Potential Distribution of Vanilla planifolia Andrews under Different Climate Change Projections in Mexico. Sustainability 2022, 14, 2881. [Google Scholar] [CrossRef]
- Velázquez-Rosas, N.; Ruíz-Guerra, B.; Martínez-Mota, R.; Silva Rivera, E.; Vázquez Domínguez, G.; Cortés Galindo, R. ¿Los vainillales tradicionales pueden favorecer la conservación de los bosques tropicales? Desde El Herb. CICY 2024, 16, 57–61. [Google Scholar]
- De Ita, A. Sembrando Envidia. In Comunidad y Autonomia frente a Sembrando Vida, 1st ed.; Vera-Herrera, R., Ed.; Centro de Estudios para el Cambio en el Campo Mexicano, CECCAM: Guerrero, Mexico, 2021; pp. 13–28. [Google Scholar]
- Mardero, S.; Schmook, B.; Calmé, S.; Casanova, G. Drifting Past Policy Coherence? Rhetoric and Realities of the Mexican Sembrando Vida Program’s Sustainability Goals. Land 2025, 14, 278. [Google Scholar] [CrossRef]


| Site | Richness | Number of Individuals | Estimate Sample Coverage | q0 | q1 | q2 |
|---|---|---|---|---|---|---|
| Forest | 115 | 2332 | 0.99 | 114.9 (9.4) | 45.4 (2.3) | 27.1 (1.7) |
| Secondary forest | 82 | 1004 | 0.96 | 81.9 (19.2) | 25.3 (2.6) | 15.2 (1.3) |
| Traditional vanilla cultivation | 78 | 1027 | 0.98 | 77.9 (9.5) | 28.7 (2.3) | 17.3 (1.6) |
| Agroforestry system | 4 | 711 | 0.97 | 39.9 (26.8) | 12. 0 (1.2) | 7.8 (0.7) |
| Citrus–vanilla | 21 | 287 | 0.97 | 20.9 (13.3) | 5.2 (1.0) | 3.1 (0.5) |
| Group | A | B | C | D | E |
|---|---|---|---|---|---|
| A | |||||
| B | 17.19 | ||||
| C | 11.5 | 18.54 | |||
| D | 7.04 | 6.54 | 2.04 | ||
| E | 10.9 | 17.39 | 6.47 | 27.2 | |
| F | 4.75 | 12.34 | 2.61 | 9.68 | 21.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velázquez-Rosas, N.; Sinaca Colin, S.; Vázquez-Domínguez, G.; Velasco-Murguía, A.; Silva Rivera, E.; Ruiz-Guerra, B.; Friedrich, F.L.; Cortés Galindo, R.; Armenta-Montero, S.; Martínez-Mota, R. Importance of Traditional Vanilla Cultivation in the Conservation of Plant Diversity in Tropical Forests in Northern Veracruz, Mexico. Sustainability 2025, 17, 2598. https://doi.org/10.3390/su17062598
Velázquez-Rosas N, Sinaca Colin S, Vázquez-Domínguez G, Velasco-Murguía A, Silva Rivera E, Ruiz-Guerra B, Friedrich FL, Cortés Galindo R, Armenta-Montero S, Martínez-Mota R. Importance of Traditional Vanilla Cultivation in the Conservation of Plant Diversity in Tropical Forests in Northern Veracruz, Mexico. Sustainability. 2025; 17(6):2598. https://doi.org/10.3390/su17062598
Chicago/Turabian StyleVelázquez-Rosas, Noé, Santiago Sinaca Colin, Guillermo Vázquez-Domínguez, Abril Velasco-Murguía, Evodia Silva Rivera, Betsabé Ruiz-Guerra, Fabio Levi Friedrich, Rosenda Cortés Galindo, Samaria Armenta-Montero, and Rodolfo Martínez-Mota. 2025. "Importance of Traditional Vanilla Cultivation in the Conservation of Plant Diversity in Tropical Forests in Northern Veracruz, Mexico" Sustainability 17, no. 6: 2598. https://doi.org/10.3390/su17062598
APA StyleVelázquez-Rosas, N., Sinaca Colin, S., Vázquez-Domínguez, G., Velasco-Murguía, A., Silva Rivera, E., Ruiz-Guerra, B., Friedrich, F. L., Cortés Galindo, R., Armenta-Montero, S., & Martínez-Mota, R. (2025). Importance of Traditional Vanilla Cultivation in the Conservation of Plant Diversity in Tropical Forests in Northern Veracruz, Mexico. Sustainability, 17(6), 2598. https://doi.org/10.3390/su17062598






