Optimizing Physical Factors for the Ammonium Removal from Wastewater Using Bio-Electrochemical Systems
Abstract
1. Introduction
2. Materials and Methods
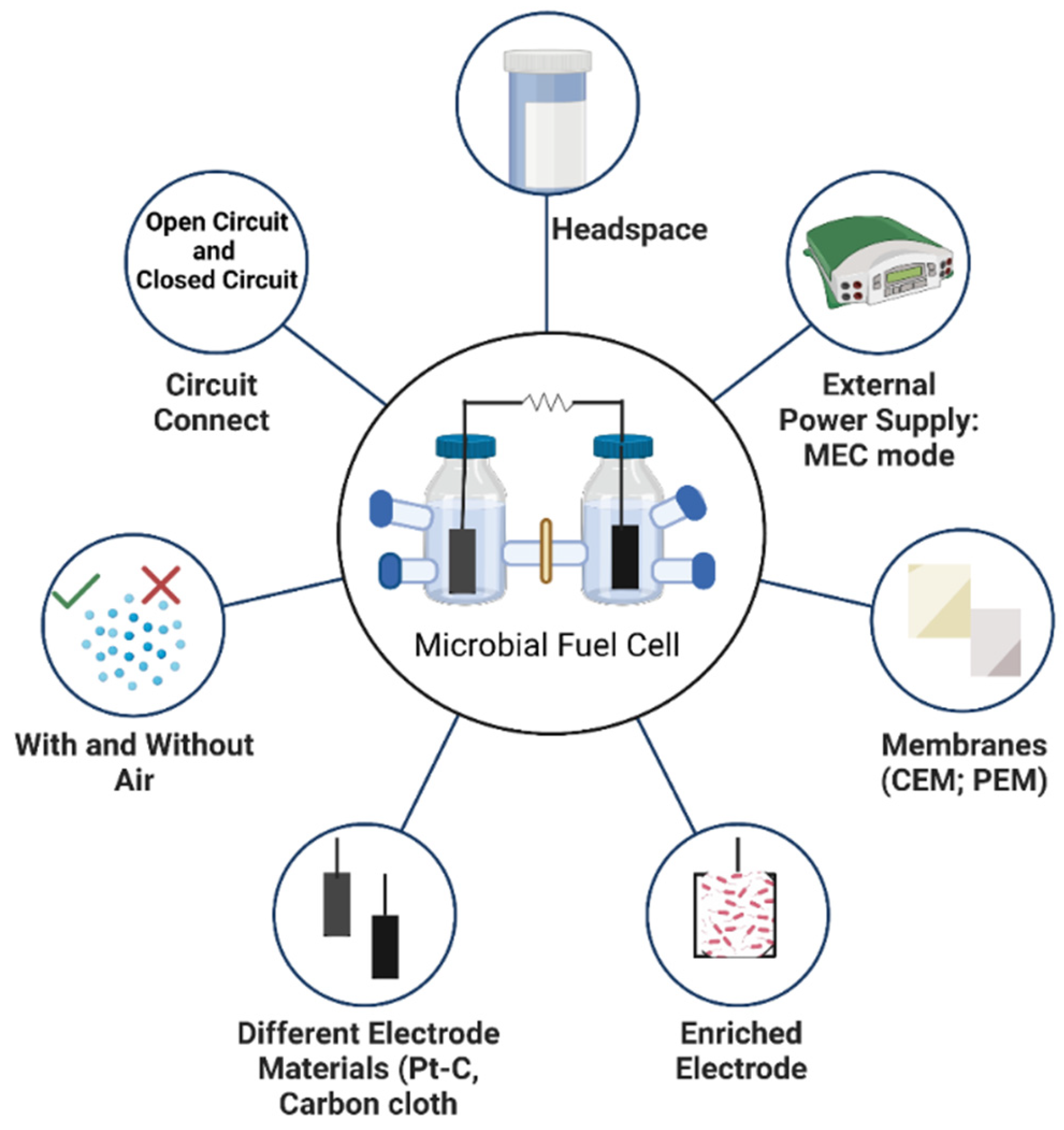
2.1. MFC Configuration and Operation
2.1.1. Effect of Different Membrane Type in Ammonium Diffusion
2.1.2. Effect of Circuit Connection and Different Electrode Material in Ammonium Removal
2.1.3. Effect of Presence of Air in Ammonium Removal
2.1.4. Effect of Headspace in Ammonium Removal
2.1.5. Effect of Applied Voltage in Ammonium Removal
2.2. Analyses and Calculations
2.3. Statistical Analysis
3. Results and Discussion
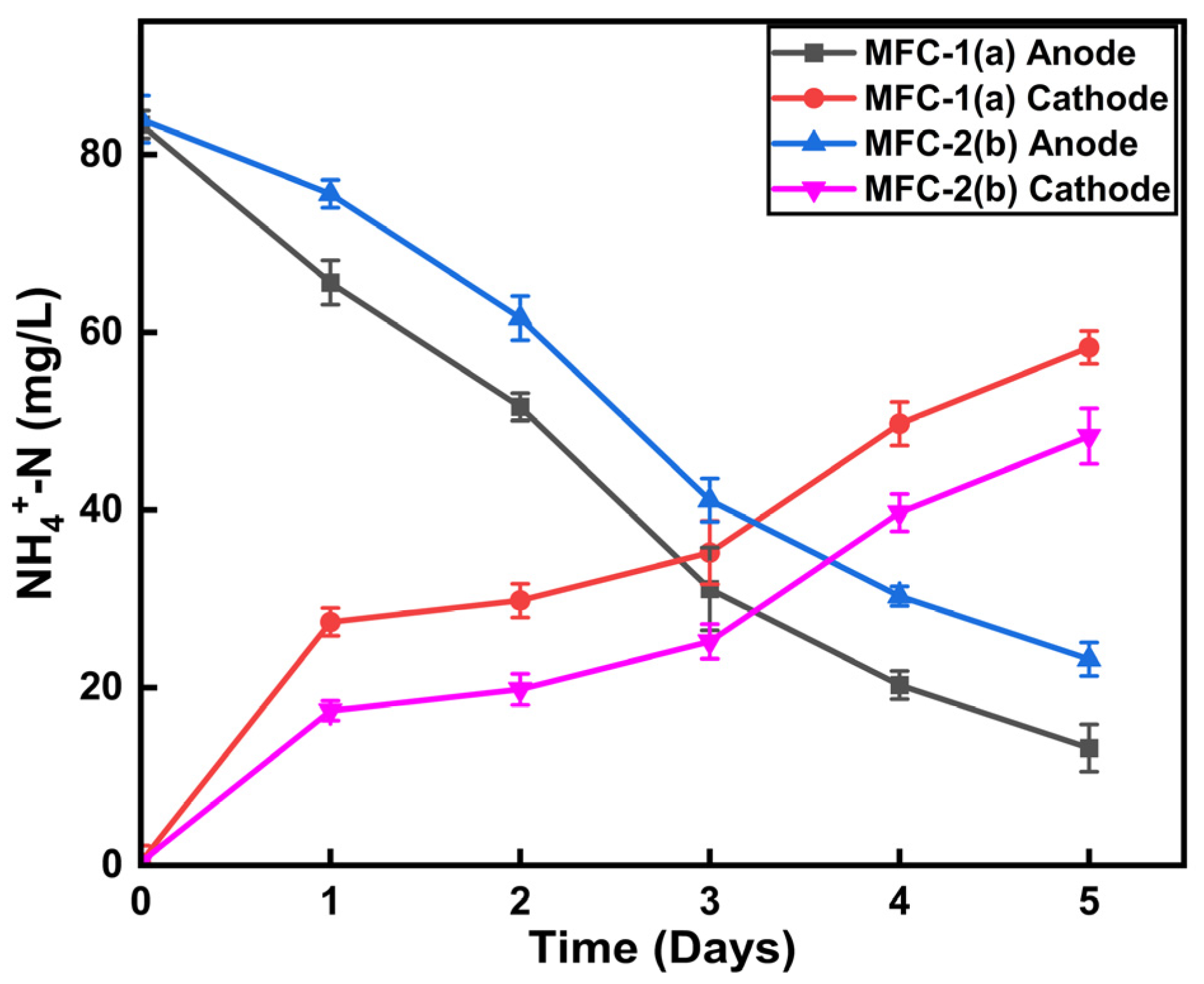
3.1. Ammonium Diffusion in Two-Chambered MFCs
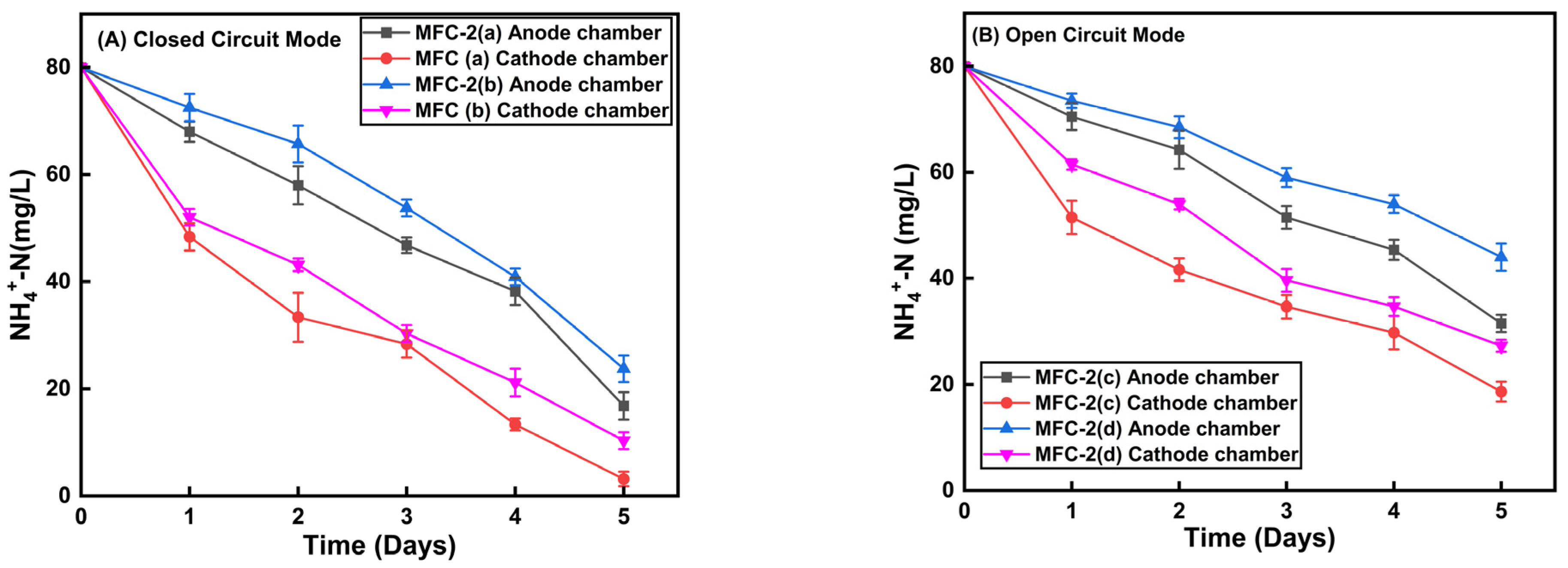
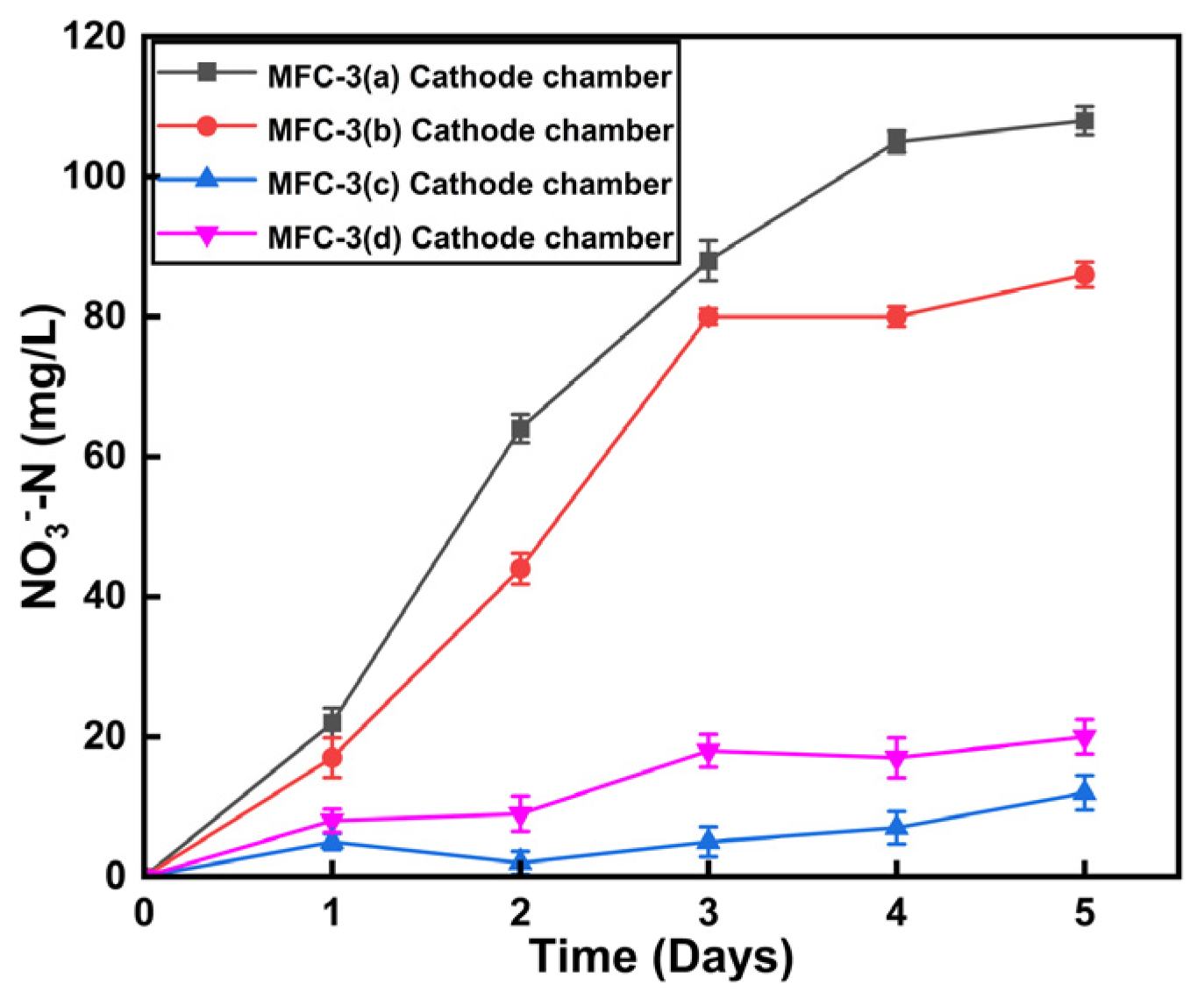
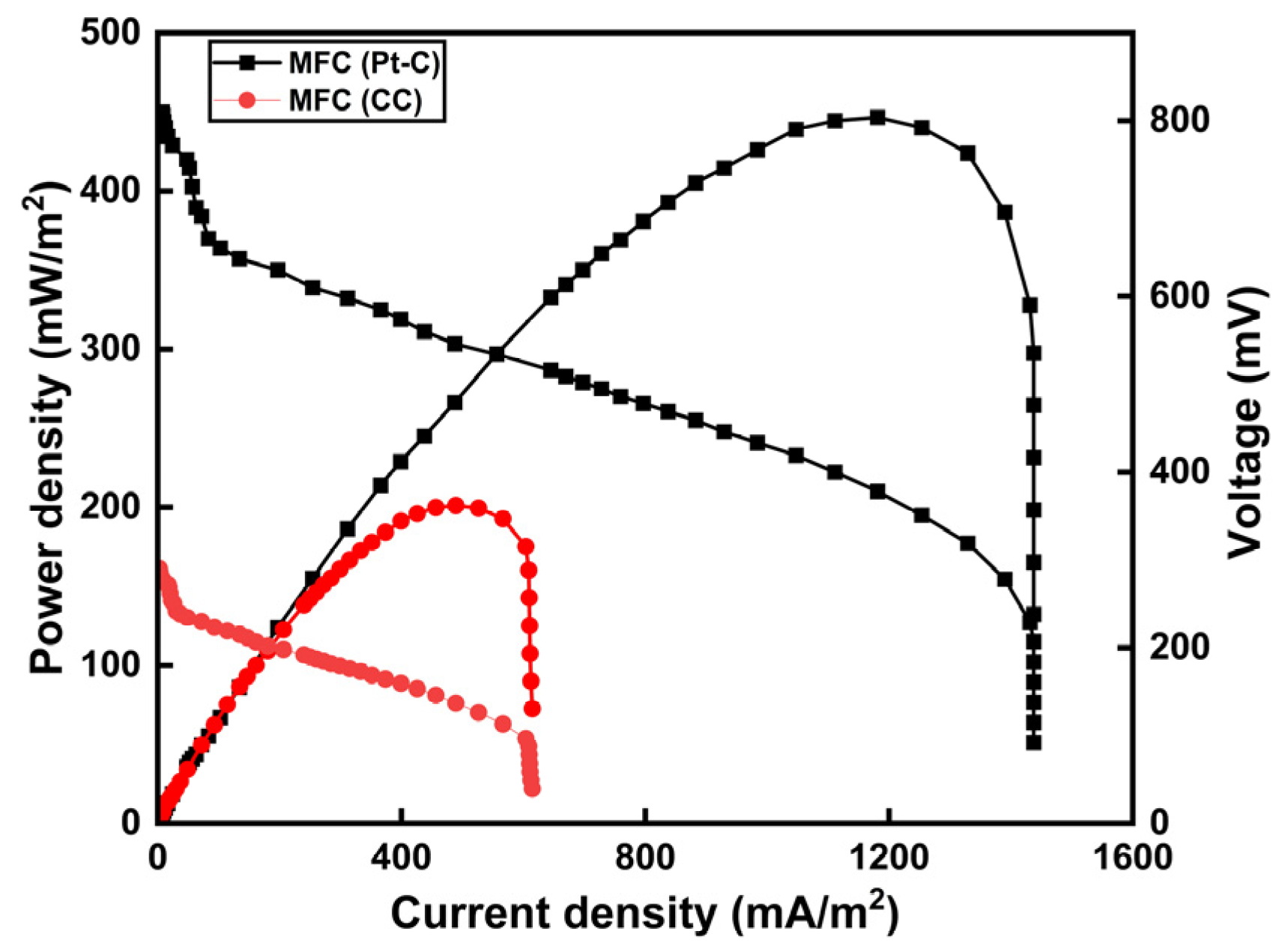
3.2. Effect of Circuit Connection and Electrode Materials
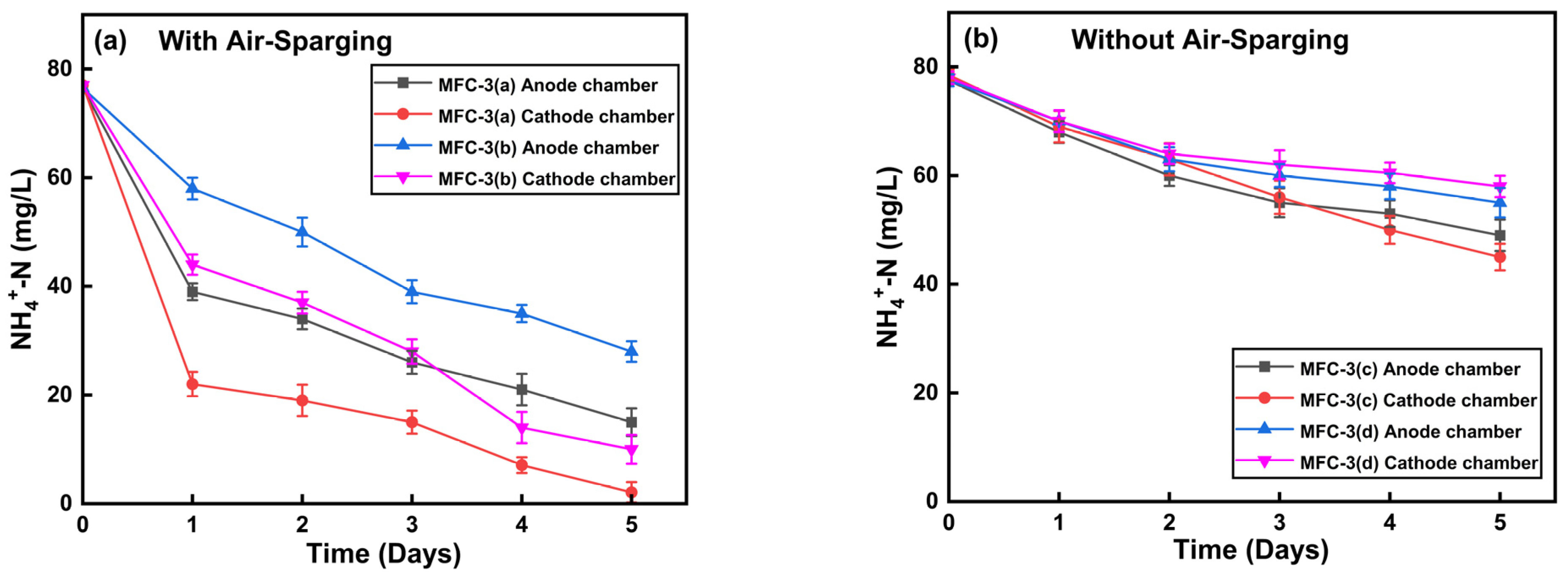
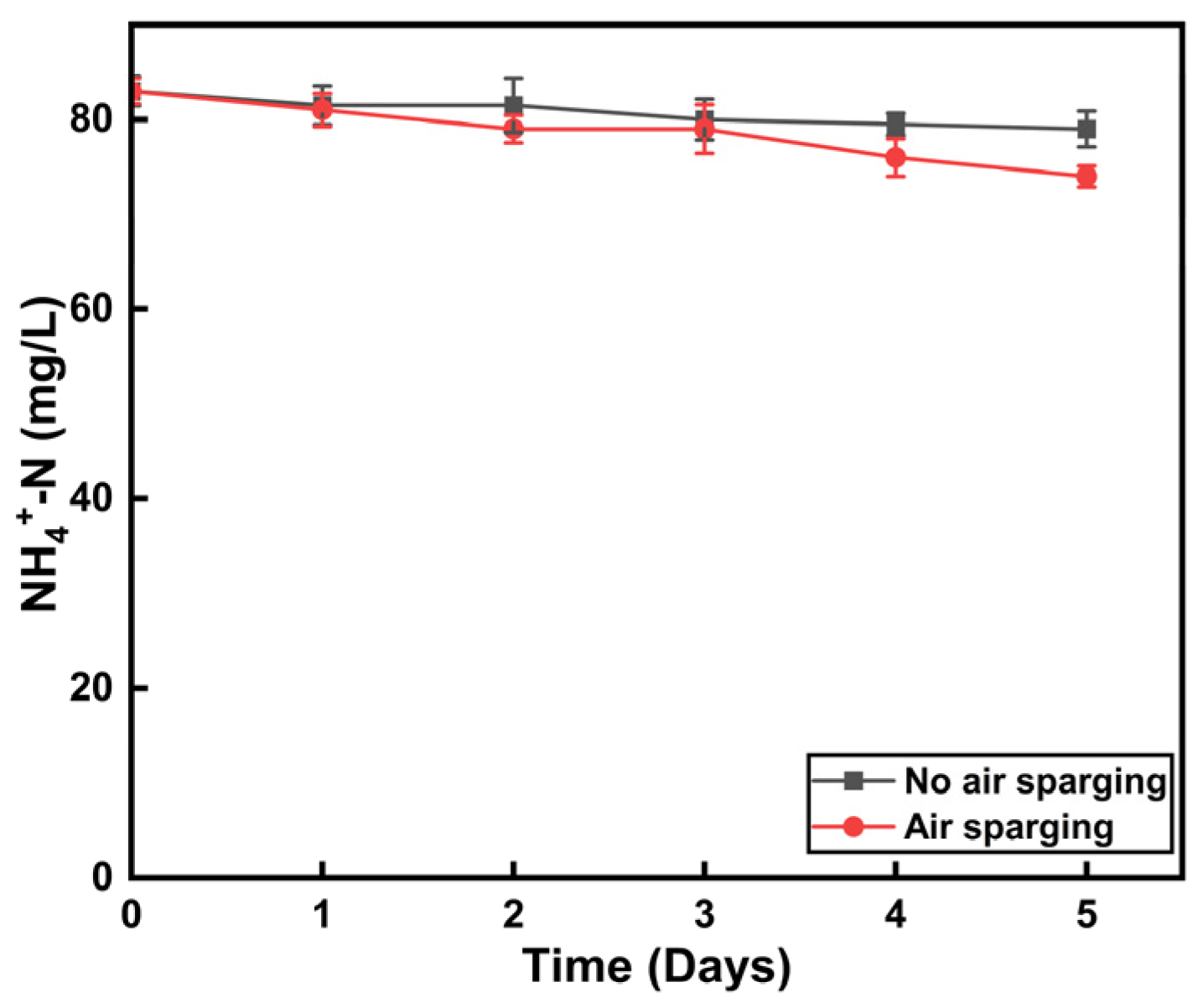
3.3. Effect of Dissolved Oxygen and Headspace
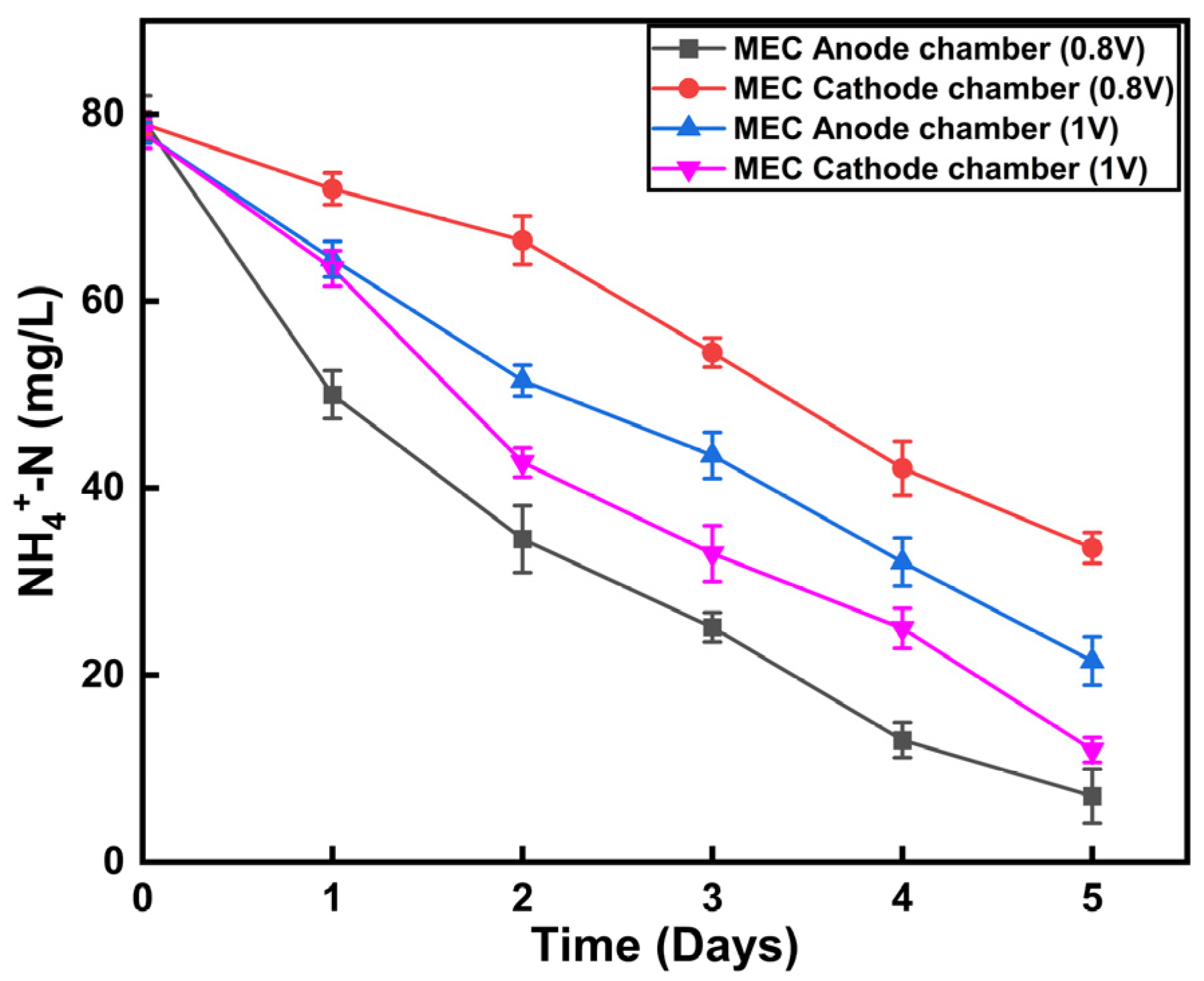
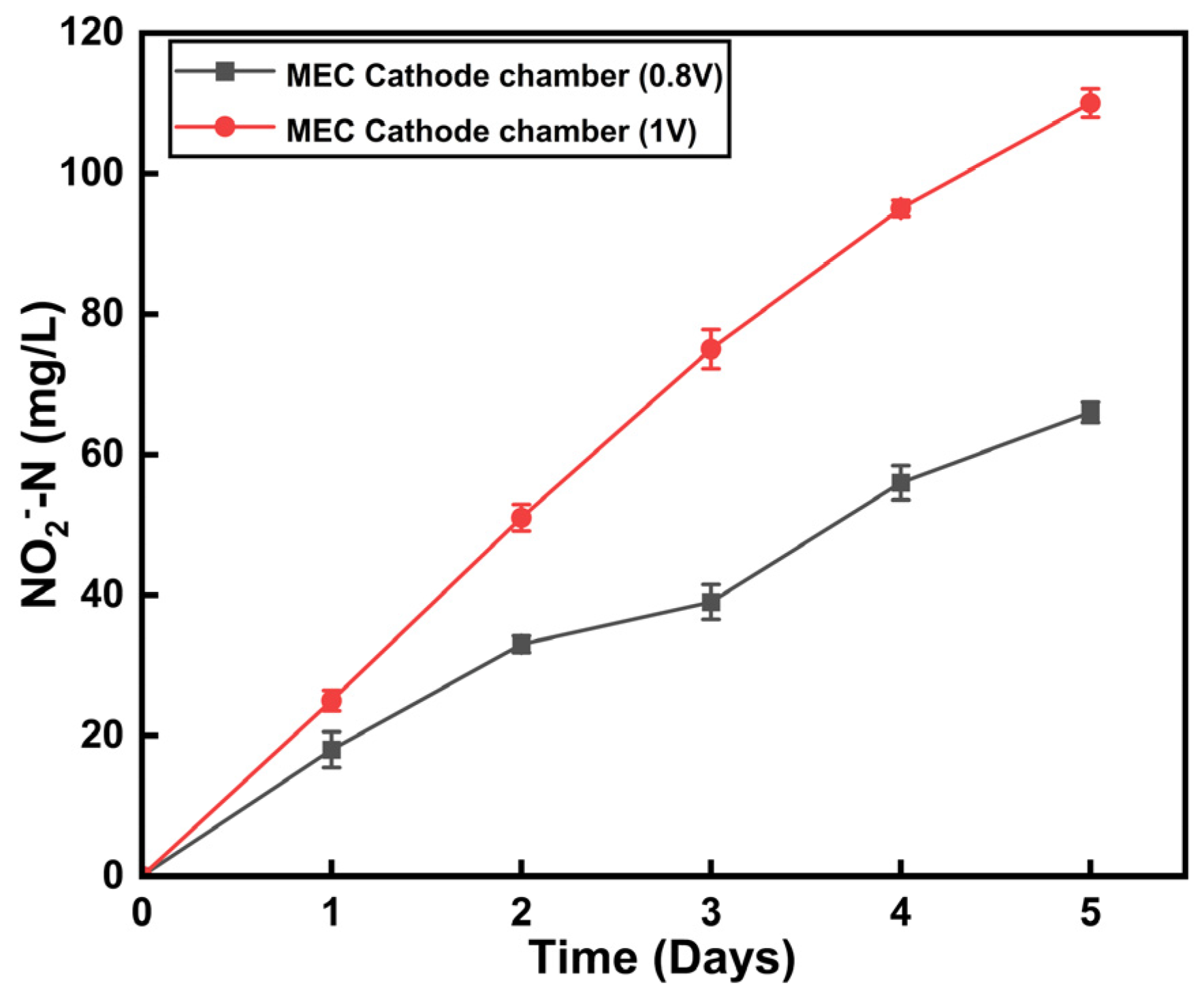
3.4. Effect of Ammonium Removal at a Different Applied Potential
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuntke, P.; Sleutels, T.H.J.A.; Rodríguez Arredondo, M.; Georg, S.; Barbosa, S.G.; ter Heijne, A.; Hamelers, H.V.M.; Buisman, C.J.N. (Bio)Electrochemical Ammonia Recovery: Progress and Perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 3865–3878. [Google Scholar] [CrossRef]
- Qin, M.; Hynes, E.A.; Abu-Reesh, I.M.; He, Z. Ammonium Removal from Synthetic Wastewater Promoted by Current Generation and Water Flux in an Osmotic Microbial Fuel Cell. J. Clean. Prod. 2017, 149, 856–862. [Google Scholar] [CrossRef]
- Al-Breiki, M.; Bicer, Y. Comparative Life Cycle Assessment of Sustainable Energy Carriers Including Production, Storage, Overseas Transport and Utilization. J. Clean. Prod. 2021, 279, 123481. [Google Scholar] [CrossRef]
- Kitano, M.; Inoue, Y.; Yamazaki, Y.; Hayashi, F.; Kanbara, S.; Matsuishi, S.; Yokoyama, T.; Kim, S.-W.; Hara, M.; Hosono, H. Ammonia Synthesis Using a Stable Electride as an Electron Donor and Reversible Hydrogen Store. Nat. Chem. 2012, 4, 934–940. [Google Scholar] [CrossRef]
- Kim, T.; An, J.; Lee, H.; Jang, J.K.; Chang, I.S. pH-Dependent Ammonia Removal Pathways in Microbial Fuel Cell System. Bioresour. Technol. 2016, 215, 290–295. [Google Scholar] [CrossRef]
- Farghali, M.; Chen, Z.; Osman, A.I.; Ali, I.M.; Hassan, D.; Ihara, I.; Rooney, D.W.; Yap, P.-S. Strategies for Ammonia Recovery from Wastewater: A Review. Environ. Chem. Lett. 2024, 22, 2699–2751. [Google Scholar] [CrossRef]
- Sartori, D.; Macchia, S.; Gaion, A. Did You Consider Ammonium? A Possible Confounding Factor in Evaluating the Toxicity of Marine Sediments. Mar. Pollut. Bull. 2024, 199, 116021. [Google Scholar] [CrossRef]
- Porras-Rivera, G.; Górski, K.; Colin, N. Behavioral Biomarkers in Fishes: A Non-Lethal Approach to Assess the Effects of Chemical Pollution on Freshwater Ecosystems. Environ. Res. 2024, 260, 119607. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, Y.; Wang, S.; Tang, Y.; Tian, L.; Cai, W.; Wei, Z.; Wu, Z.; Zhu, Y.; Guo, Q. Photocatalytic Removal of Ammonia Nitrogen from Water: Investigations and Challenges for Enhanced Activity. Environ. Sci. Pollut. Res. 2024, 31, 41824–41843. [Google Scholar] [CrossRef]
- De Vries, W.; Posch, M.; Simpson, D.; de Leeuw, F.A.A.M.; van Grinsven, H.J.M.; Schulte-Uebbing, L.F.; Sutton, M.A.; Ros, G.H. Trends and Geographic Variation in Adverse Impacts of Nitrogen Use in Europe on Human Health, Climate, and Ecosystems: A Review. Earth-Sci. Rev. 2024, 253, 104789. [Google Scholar] [CrossRef]
- Xiang, S.; Liu, Y.; Zhang, G.; Ruan, R.; Wang, Y.; Wu, X.; Zheng, H.; Zhang, Q.; Cao, L. New Progress of Ammonia Recovery during Ammonia Nitrogen Removal from Various Wastewaters. World J. Microbiol. Biotechnol. 2020, 36, 144. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Shaoan, C.; Oh, S.E.; Logan, B.E. Power Generation Using Different Cation, Anion, and Ultrafiltration Membranes in Microbial Fuel Cells. Environ. Sci. Technol. 2007, 41, 1004–1009. [Google Scholar] [CrossRef]
- Park, Y.; Yu, J.; Nguyen, V.K.; Park, S.; Kim, J.; Lee, T. Understanding Complete Ammonium Removal Mechanism in Single-Chamber Microbial Fuel Cells Based on Microbial Ecology. Sci. Total Environ. 2021, 764, 144231. [Google Scholar] [CrossRef] [PubMed]
- Dhanda, A.; Thulluru, L.P.; Mishra, S.; Chowdhury, S.; Dubey, B.K.; Ghangrekar, M.M. Integrated Fuel Cell System for Sustainable Wastewater Treatment, Ammonia Recovery, and Power Production. Environ. Res. 2024, 262, 119821. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Ni, B.J.; Zhang, X. Microbial Fuel Cell for Nutrient Recovery and Electricity Generation from Municipal Wastewater under Different Ammonium Concentrations. Bioresour. Technol. 2019, 292, 121992. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, M.; Luo, S.; He, Z.; Qiao, R. Understanding Ammonium Transport in Bioelectrochemical Systems towards Its Recovery. Sci. Rep. 2016, 6, 22547. [Google Scholar] [CrossRef]
- Rodríguez Arredondo, M.; Kuntke, P.; ter Heijne, A.; Buisman, C.J.N. The Concept of Load Ratio Applied to Bioelectrochemical Systems for Ammonia Recovery. J. Chem. Technol. Biotechnol. 2019, 94, 5992. [Google Scholar] [CrossRef]
- Baños, L. CRC Handbook of Chemistry and Physics Editor-in-Chief; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Agre, P. The Aquaporin Water Channels. Proc. Am. Thorac. Soc. 2006, 3, 5–13. [Google Scholar] [CrossRef]
- Lim, D.-W.; Sadakiyo, M.; Kitagawa, H. Proton Transfer in Hydrogen-Bonded Degenerate Systems of Water and Ammonia in Metal–Organic Frameworks. Chem. Sci. 2019, 10, 16–33. [Google Scholar] [CrossRef]
- Skolotneva, E.; Tsygurina, K.; Mareev, S.; Melnikova, E.; Pismenskaya, N.; Nikonenko, V. High Diffusion Permeability of Anion-Exchange Membranes for Ammonium Chloride: Experiment and Modeling. Int. J. Mol. Sci. 2022, 23, 5782. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Mohamad Ibrahim, M.N.; Rafatullah, M.; Chua, Y.S.; Ahmad, A.; Umar, K. Recent Advances in Anodes for Microbial Fuel Cells: An Overview. Materials 2020, 13, 2078. [Google Scholar] [CrossRef] [PubMed]
- Anjum, A.; Ali Mazari, S.; Hashmi, Z.; Sattar Jatoi, A.; Abro, R. A Review of Role of Cathodes in the Performance of Microbial Fuel Cells. J. Electroanal. Chem. 2021, 899, 115673. [Google Scholar] [CrossRef]
- Zhao, L.; Deng, J.; Hou, H.; Li, J.; Yang, Y. Investigation of PAH and Oil Degradation along with Electricity Generation in Soil Using an Enhanced Plant-Microbial Fuel Cell. J. Clean. Prod. 2019, 221, 678–683. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Wen, Q. Microbial Fuel Cells: Enhancement with a Polyaniline/Carbon Felt Capacitive Bioanode and Reduction of Cr(VI) Using the Intermittent Operation. Environ. Chem. Lett. 2018, 16, 319–326. [Google Scholar] [CrossRef]
- Antolini, E. Photo-Assisted Methanol Oxidation on Pt-TiO2 Catalysts for Direct Methanol Fuel Cells: A Short Review. Appl. Catal. B Environ. 2018, 237, 491–503. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Mohamad Ibrahim, M.N. Role of Nanomaterials in the Treatment of Wastewater: A Review. Water 2020, 12, 495. [Google Scholar] [CrossRef]
- Karuppannan, M.; Kim, Y.; Gok, S.; Lee, E.; Hwang, J.Y.; Jang, J.-H.; Cho, Y.-H.; Lim, T.; Sung, Y.-E.; Kwon, O.J. A Highly Durable Carbon-Nanofiber-Supported Pt–C Core–Shell Cathode Catalyst for Ultra-Low Pt Loading Proton Exchange Membrane Fuel Cells: Facile Carbon Encapsulation. Energy Environ. Sci. 2019, 12, 2820–2829. [Google Scholar] [CrossRef]
- León, M.I.; Castañeda, L.F.; Márquez, A.A.; Walsh, F.C.; Nava, J.L. Review—Carbon Cloth as a Versatile Electrode: Manufacture, Properties, Reaction Environment, and Applications. J. Electrochem. Soc. 2022, 169, 053503. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, H.; Zheng, P.; Zhang, J.; Cai, J.; Abbas, G. Influence and Mechanism of Dissolved Oxygen on the Performance of Ammonia-Oxidation Microbial Fuel Cell. Int. J. Hydrogen Energy 2013, 38, 10607–10615. [Google Scholar] [CrossRef]
- Kondaveeti, S.; Choi, D.-H.; Noori, M.T.; Min, B. Ammonia Removal by Simultaneous Nitrification and Denitrification in a Single Dual-Chamber Microbial Electrolysis Cell. Energies 2022, 15, 9171. [Google Scholar] [CrossRef]
- Koffi, N.J.; Okabe, S. Bioelectrochemical Anoxic Ammonium Nitrogen Removal by an MFC Driven Single Chamber Microbial Electrolysis Cell. Chemosphere 2021, 274, 129715. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cheng, J.; Wang, J.; Zhang, Z.; Xie, X.; Chu, P. Effects of Temporary External Voltage on the Performance and Community of Microbial Fuel Cells. Water Sci. Technol. 2020, 81, 1972–1982. [Google Scholar] [CrossRef] [PubMed]
- Chae, K.-J.; Choi, M.-J.; Kim, K.-Y.; Ajayi, F.F.; Chang, I.-S.; Kim, I.S. A Solar-Powered Microbial Electrolysis Cell with a Platinum Catalyst-Free Cathode To Produce Hydrogen. Environ. Sci. Technol. 2009, 43, 9525–9530. [Google Scholar] [CrossRef] [PubMed]
- Cerrillo, M.; Riau, V.; Bonmatí, A. Recent Advances in Bioelectrochemical Systems for Nitrogen and Phosphorus Recovery Using Membranes. Membranes 2023, 13, 186. [Google Scholar] [CrossRef]
| S. No. | Anode | Cathode | Membrane | Circuit Connection | Air Sparging | MEC Mode | Inoculum (Anode Chamber) | Inoculum (Cathode Chamber) |
|---|---|---|---|---|---|---|---|---|
| MFC 1(a) | X | X | CEM | X | X | X | X | X |
| MFC 1(b) | X | X | PEM | X | X | X | X | X |
| MFC 2(a) | CC | Pt-C | CEM | CCM | O | X | O | New carbon cloth |
| MFC 2(b) | CC | CC | CEM | CCM | O | X | O | New carbon cloth |
| MFC 2(c) | CC | Pt-C | CEM | OCM | O | X | O | New carbon cloth |
| MFC 2(d) | CC | CC | CEM | OCM | O | X | O | New carbon cloth |
| MFC 3(a) | CC | Pt-C | CEM | CCM | O | X | O | Enriched Cathode |
| MFC 3(b) | CC | CC | CEM | CCM | O | X | O | Enriched Cathode |
| MFC 3(c) | CC | Pt-C | CEM | CCM | X | X | O | Enriched Cathode |
| MFC 3(d) | CC | CC | CEM | CCM | X | X | O | Enriched Cathode |
| MEC (0.8 V) | CC | Pt-C | CEM | MEC | O | O | O | O |
| MEC (1.0 V) | CC | Pt-C | CEM | MEC | O | O | O | O |
| MFC 4(a) | CC | Pt-C | CEM | CCM | O | X | X | X |
| MFC 4(b) | CC | Pt-C | CEM | CCM | O | X | X | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Gurung, A.; Mehdi, S.E.H.; Shahzad, S.; Hussain, F.; Kang, W.; Pandey, S.; Khan, A.H.A.; Oh, S.-E. Optimizing Physical Factors for the Ammonium Removal from Wastewater Using Bio-Electrochemical Systems. Sustainability 2025, 17, 2543. https://doi.org/10.3390/su17062543
Sharma A, Gurung A, Mehdi SEH, Shahzad S, Hussain F, Kang W, Pandey S, Khan AHA, Oh S-E. Optimizing Physical Factors for the Ammonium Removal from Wastewater Using Bio-Electrochemical Systems. Sustainability. 2025; 17(6):2543. https://doi.org/10.3390/su17062543
Chicago/Turabian StyleSharma, Aparna, Anup Gurung, Syed Ejaz Hussain Mehdi, Suleman Shahzad, Fida Hussain, Woochang Kang, Sandesh Pandey, Aqib Hassan Ali Khan, and Sang-Eun Oh. 2025. "Optimizing Physical Factors for the Ammonium Removal from Wastewater Using Bio-Electrochemical Systems" Sustainability 17, no. 6: 2543. https://doi.org/10.3390/su17062543
APA StyleSharma, A., Gurung, A., Mehdi, S. E. H., Shahzad, S., Hussain, F., Kang, W., Pandey, S., Khan, A. H. A., & Oh, S.-E. (2025). Optimizing Physical Factors for the Ammonium Removal from Wastewater Using Bio-Electrochemical Systems. Sustainability, 17(6), 2543. https://doi.org/10.3390/su17062543










