Valorization of Bioactive Compounds Extracted from Brewer’s Spent Grain (BSG) for Sustainable Food Waste Recycling
Abstract
1. Introduction
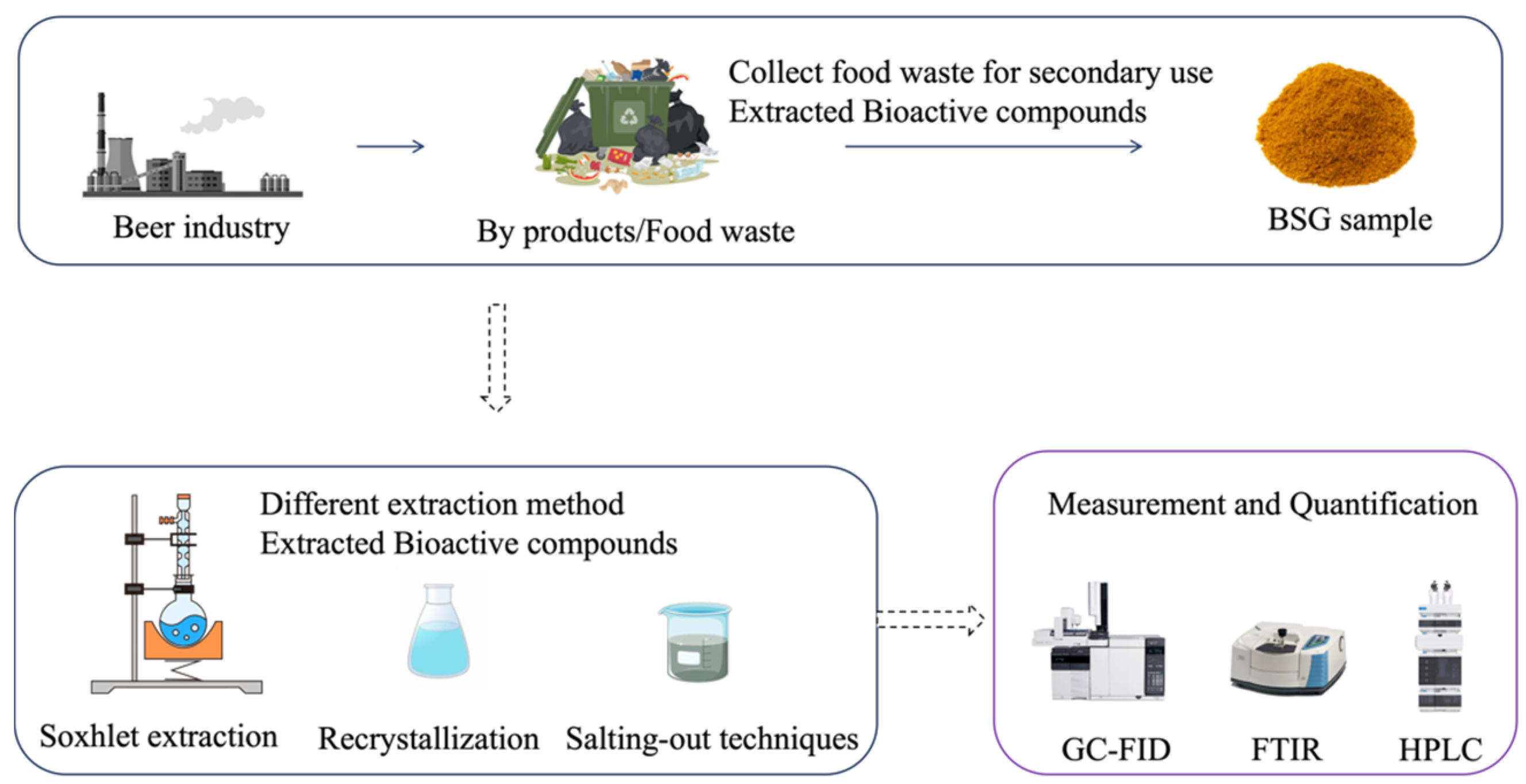
2. Materials and Method
2.1. Material and Chemicals
2.2. Extraction Method
2.2.1. Soxhlet Extraction
2.2.2. Recrystallization Extraction
2.2.3. Salting-Out Techniques
2.3. Measurement and Quantification
2.3.1. High-Performance Liquid Chromatography
Determination of Hemicellulose, Lignin, and Cellulose
2.3.2. Gas Chromatography-A Flame Ionization Detector
2.3.3. Fourier-Transform Infrared Spectroscopy
2.3.4. Fiber, Phenolic, and Antioxidant Quantitative Test
- B = Blank Correction
- R = Residue Weight
- P = Protein Weight
- A = Ash weight
- SW = Sample Weight
3. Results and Discussion
3.1. Measuring and Quantifying Fibers
3.2. Quantification of Phenolic Compounds and Antioxidants
4. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chattaraj, S.; Mitra, D.; Ganguly, A.; Thatoi, H.; Mohapatra, P.K.D. A critical review on the biotechnological potential of Brewers’ waste: Challenges and future alternatives. Curr. Res. Microb. Sci. 2024, 6, 100228. [Google Scholar] [CrossRef] [PubMed]
- Arellano, L.I.T. Travail de Fin d’études: Exploring the Supply Chain for Brewery’s By-Product Recycling: A Study on a Potential Alternative for a Brewery’s By-Product Valorization. 2023. Available online: https://matheo.uliege.be/handle/2268.2/17802 (accessed on 6 January 2025).
- Karlović, A.; Jurić, A.; Ćorić, N.; Habschied, K.; Krstanović, V.; Mastanjević, K. By-products in the malting and brewing industries—Re-usage possibilities. Fermentation 2020, 6, 82. [Google Scholar] [CrossRef]
- Zeko-Pivač, A.; Tišma, M.; Žnidaršič-Plazl, P.; Kulisic, B.; Sakellaris, G.; Hao, J.; Planinić, M. The potential of brewer’s spent grain in the circular bioeconomy: State of the art and future perspectives. Front. Bioeng. Biotechnol. 2022, 10, 870744. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, A. Circular Economy: Best Practices and Future Perspectives for the Beer Brewing Industry; Louvain School of Management, Université Catholique de Louvain: Ottignies-Louvain-la-Neuve, Belgium, 2020; p. 24674. Available online: https://dial.uclouvain.be/downloader/downloader.php?pid=thesis%3A24674&datastream=PDF_01 (accessed on 29 November 2024).
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ spent grain: A review with an emphasis on food and health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Connolly, A. Generation and Characterisation of Novel Health Enhancing Ingredients from Brewers’ Spent Grain (BSG). Ph.D. Thesis, University of Limerick, Limerick, Ireland, 2013. [Google Scholar]
- Olagunju, A.I.; Omoba, O.S. High fibres functional products. In Functional Cereals and Cereal Foods: Properties, Functionality and Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 379–400. [Google Scholar]
- Radoš, K.; Čukelj Mustač, N.; Varga, K.; Drakula, S.; Voučko, B.; Ćurić, D.; Novotni, D. Development of high-fibre and low-FODMAP crackers. Foods 2022, 11, 2577. [Google Scholar] [CrossRef]
- Naibaho, J.; Korzeniowska, M.; Wojdyło, A.; Ayunda, H.M.; Foste, M.; Yang, B. Techno-functional properties of protein from protease-treated brewers’ spent grain (BSG) and investigation of antioxidant activity of extracted proteins and BSG residues. J. Cereal Sci. 2022, 107, 103524. [Google Scholar] [CrossRef]
- McCarthy, A.L.; O’Callaghan, Y.C.; Piggott, C.O.; FitzGerald, R.J.; O’Brien, N.M. Brewers’ spent grain; bioactivity of phenolic component, its role in animal nutrition and potential for incorporation in functional foods: A review. Proc. Nutr. Soc. 2013, 72, 117–125. [Google Scholar] [CrossRef]
- Choudhary, P.; Devi, T.B.; Tushir, S.; Kasana, R.C.; Popatrao, D.S.; K, N. Mango seed kernel: A bountiful source of nutritional and bioactive compounds. Food Bioprocess Technol. 2023, 16, 289–312. [Google Scholar] [CrossRef]
- Das, G.; Nath, R.; Das Talukdar, A.; Ağagündüz, D.; Yilmaz, B.; Capasso, R.; Shin, H.-S.; Patra, J.K. Major bioactive compounds from java plum seeds: An investigation of its extraction procedures and clinical effects. Plants 2023, 12, 1214. [Google Scholar] [CrossRef]
- Kovácik, A.; Kopecná, M.; Hrdinová, I.; Opálka, L.; Bettex, M.B.; Vávrová, K. Time-Dependent Differences in the Effects of Oleic Acid and Oleyl Alcohol on the Human Skin Barrier. Mol. Pharm. 2023, 20, 6237–6245. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism (s) of action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- He, X.; Wan, F.; Su, W.; Xie, W. Research progress on skin aging and active ingredients. Molecules 2023, 28, 5556. [Google Scholar] [CrossRef]
- Sun, J. The Effect Of Ellagic Acid On Anti-Aging. MedScien 2024, 1, 1–7. [Google Scholar] [CrossRef]
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the antioxidant and anti-inflammatory activities of selected plant compounds and their metal ions complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef]
- Barcelos, M.C.; Ramos, C.L.; Kuddus, M.; Rodriguez-Couto, S.; Srivastava, N.; Ramteke, P.W.; Mishra, P.K.; Molina, G. Enzymatic potential for the valorization of agro-industrial by-products. Biotechnol. Lett. 2020, 42, 1799–1827. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhou, T.; Wang, Y.; Cao, X.; Wu, S.; Zhao, M.; Wang, H.; Xu, M.; Zheng, B.; Zheng, J. Pretreatment of wheat straw leads to structural changes and improved enzymatic hydrolysis. Sci. Rep. 2018, 8, 1321. [Google Scholar] [CrossRef]
- Merali, Z.; Collins, S.R.; Elliston, A.; Wilson, D.R.; Käsper, A.; Waldron, K.W. Characterization of cell wall components of wheat bran following hydrothermal pretreatment and fractionation. Biotechnol. Biofuels 2015, 8, 23. [Google Scholar] [CrossRef]
- Kristensen, J.B.; Thygesen, L.G.; Felby, C.; Jørgensen, H.; Elder, T. Cell-wall structural changes in wheat straw pretreated for bioethanol production. Biotechnol. Biofuels 2008, 1, 5. [Google Scholar] [CrossRef]
- Chen, X.; Guo, Y.; Song, T.; Dou, Y.; Zhang, J.; Zhang, X.; Dou, H. Investigation on the stability of low-density lipoproteins modified by phospholipase A2 using asymmetrical flow field-flow fractionation. J. Food Meas. Charact. 2021, 15, 3350–3356. [Google Scholar] [CrossRef]
- Fărcaș, A.C.; Socaci, S.A.; Nemeș, S.A.; Pop, O.L.; Coldea, T.E.; Fogarasi, M.; Biriș-Dorhoi, E.S. An update regarding the bioactive compound of cereal by-products: Health benefits and potential applications. Nutrients 2022, 14, 3470. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Birsan, R. Enrichment of Brewer’s Spent Grain Polyphenols and Assessment of Their Role in Inhibition of Cholinesterases, Amylase and Glucosidase. Ph.D. Thesis, University of East Anglia, Norwich, UK, 2022. [Google Scholar]
- Klepacka, J.; Fornal, Ł. Ferulic acid and its position among the phenolic compounds of wheat. Crit. Rev. Food Sci. Nutr. 2006, 46, 639–647. [Google Scholar] [CrossRef]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; Foletto-Felipe, M.d.P.; Abrahão, J. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Daud, N.M.; Putra, N.R.; Jamaludin, R.; Norodin, N.S.M.; Sarkawi, N.S.; Hamzah, M.H.S.; Nasir, H.M.; Zaidel, D.N.A.; Yunus, M.A.C.; Salleh, L.M. Valorisation of plant seed as natural bioactive compounds by various extraction methods: A review. Trends Food Sci. Technol. 2022, 119, 201–214. [Google Scholar] [CrossRef]
- Agapay, R.C.; Ju, Y.H.; Tran-Nguyen, P.L.; Ismadji, S.; Angkawijaya, A.E.; Go, A.W. Process evaluation of solvent-free lipase-catalyzed esterification schemes in the synthesis of structured triglycerides from oleic and palmitic acids. Asia-Pac. J. Chem. Eng. 2021, 16, e2606. [Google Scholar] [CrossRef]
- Liu, J.; Wang, F.F.; Jiang, Z.M.; Liu, E.H. Identification of antidiabetic components in Uncariae Rammulus Cum Uncis based on phytochemical isolation and spectrum–effect relationship analysis. Phytochem. Anal. 2022, 33, 659–669. [Google Scholar] [CrossRef]
- Chen, P.-C.; Dlamini, B.S.; Chen, C.-R.; Kuo, Y.-H.; Shih, W.-L.; Lin, Y.-S.; Lee, C.-H.; Chang, C.-I. Structure related α-glucosidase inhibitory activity and molecular docking analyses of phenolic compounds from Paeonia suffruticosa. Med. Chem. Res. 2022, 31, 293–306. [Google Scholar] [CrossRef]
- Burgess, R.R. Protein precipitation techniques. Methods Enzymol. 2009, 463, 331–342. [Google Scholar] [CrossRef]
- Duong-Ly, K.C.; Gabelli, S.B. Salting out of proteins using ammonium sulfate precipitation. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 541, pp. 85–94. [Google Scholar]
- de Oliveira, D.N.; Claro, P.I.; de Freitas, R.R.; Martins, M.A.; Souza, T.M.; Silva, B.M.; Mendes, L.M.; Bufalino, L. Enhancement of the Amazonian açaí waste fibers through variations of alkali pretreatment parameters. Chem. Biodivers. 2019, 16, e1900275. [Google Scholar] [CrossRef]
- Zhao, S.; Baik, O.-D.; Choi, Y.J.; Kim, S.-M. Pretreatments for the efficient extraction of bioactive compounds from plant-based biomaterials. Crit. Rev. Food Sci. Nutr. 2014, 54, 1283–1297. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Akyüz, A.; Tekin, İ.; Aksoy, Z.; Ersus, S. Plant Protein Resources, Novel Extraction and Precipitation Methods: A Review. J. Food Process Eng. 2024, 47, e14758. [Google Scholar] [CrossRef]
- Xie, S.; Li, Z.; Zhang, W. Techno-Economic Analysis of Upgrading Corn Stover-Based Acetone, n-Butanol, and Ethanol to Higher Ketones and Alcohols: Fuels or Fine Chemicals? ACS Sustain. Chem. Eng. 2023, 11, 3474–3485. [Google Scholar] [CrossRef]
- Mradu, G.; Saumyakanti, S.; Sohini, M.; Arup, M. HPLC profiles of standard phenolic compounds present in medicinal plants. Int. J. Pharmacogn. Phytochem. Res. 2012, 4, 162–167. [Google Scholar]
- Dubuis, A.; Le Masle, A.; Chahen, L.; Destandau, E.; Charon, N. Centrifugal partition chromatography as a fractionation tool for the analysis of lignocellulosic biomass products by liquid chromatography coupled to mass spectrometry. J. Chromatogr. A 2019, 1597, 159–166. [Google Scholar] [CrossRef]
- Reza, M.T.; Rottler, E.; Herklotz, L.; Wirth, B. Hydrothermal carbonization (HTC) of wheat straw: Influence of feedwater pH prepared by acetic acid and potassium hydroxide. Bioresour. Technol. 2015, 182, 336–344. [Google Scholar] [CrossRef]
- Reymond, C.; Dubuis, A.; Le Masle, A.; Colas, C.; Chahen, L.; Destandau, E.; Charon, N. Characterization of liquid–liquid extraction fractions from lignocellulosic biomass by high performance liquid chromatography hyphenated to tandem high-resolution mass spectrometry. J. Chromatogr. A 2020, 1610, 460569. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Liu, O. Development and validation of a GC–FID method for quantitative analysis of oleic acid and related fatty acids. J. Pharm. Anal. 2015, 5, 223–230. [Google Scholar] [CrossRef]
- McCleary, B.V.; McLoughlin, C. Determination of Insoluble, Soluble, and Total Dietary Fiber in Foods Using a Rapid Integrated Procedure of Enzymatic-Gravimetric-Liquid Chromatography: First Action 2022.01. J. AOAC Int. 2023, 106, 127–145. [Google Scholar] [CrossRef]
- Bird, D.; Ho, S. Nutritive values of whole-animal diets for captive birds of prey. J. Raptor Res. 2024, 10, 2. [Google Scholar]
- Myrvold, B.O. Salting-out and salting-in experiments with lignosulfonates (LSs). Holzforschung 2013, 67, 549–557. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Wang, Y.; Liu, Z.; Ni, Y. Effects of extraction methods on the structural characteristics and functional properties of dietary fiber extracted from kiwifruit (Actinidia deliciosa). Food Hydrocoll. 2021, 110, 106162. [Google Scholar] [CrossRef]
- Gupta, M.; Abu-Ghannam, N.; Gallaghar, E. Barley for Brewing: Characteristic Changes during Malting, Brewing and Applications of Its By-Products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Ikram, S.; Huang, L.; Zhang, H.; Wang, J.; Yin, M. Composition and nutrient value proposition of brewers spent grain. J. Food Sci. 2017, 82, 2232–2242. [Google Scholar] [CrossRef]
- Naibaho, J.; Korzeniowska, M. Brewers’ spent grain in food systems: Processing and final products quality as a function of fiber modification treatment. J. Food Sci. 2021, 86, 1532–1551. [Google Scholar] [CrossRef]
- Garside, P.; Wyeth, P. Identification of cellulosic fibres by FTIR spectroscopy-thread and single fibre analysis by attenuated total reflectance. Stud. Conserv. 2003, 48, 269–275. [Google Scholar] [CrossRef]
- Collins, S.R.; Wellner, N.; Martinez Bordonado, I.; Harper, A.L.; Miller, C.N.; Bancroft, I.; Waldron, K.W. Variation in the chemical composition of wheat straw: The role of tissue ratio and composition. Biotechnol. Biofuels 2014, 7, 121. [Google Scholar] [CrossRef]
- Peng, W.; Wang, L.; Ohkoshi, M.; Zhang, M. Separation of hemicelluloses from Eucalyptus species: Investigating the residue after alkaline treatment. Cellul. Chem. Technol. 2015, 49, 756–764. [Google Scholar]
- Agwuncha, S.C.; Owonubi, S.; Fapojuwo, D.P.; Abdulkarim, A.; Okonkwo, T.P.; Makhatha, E.M. Evaluation of mercerization treatment conditions on extracted cellulose from shea nut shell using FTIR and thermogravimetric analysis. Mater. Today Proc. 2021, 38, 958–963. [Google Scholar] [CrossRef]
- Martins, J.R.; Abe, M.M.; Brienzo, M. Chemical Modification Strategies for Developing Functionalized Hemicellulose: Advanced Applications of Modified Hemicellulose. In Hemicellulose Biorefinery: A Sustainable Solution for Value Addition to Bio-Based Products and Bioenergy; Springer: Berlin/Heidelberg, Germany, 2022; pp. 171–205. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, H.; Yang, B.; Weng, Y. Hemicellulose-based film: Potential green films for food packaging. Polymers 2020, 12, 1775. [Google Scholar] [CrossRef]
- Hu, L.; Fang, X.; Du, M.; Luo, F.; Guo, S. Hemicellulose-based polymers processing and application. Am. J. Plant Sci. 2020, 11, 2066–2079. [Google Scholar] [CrossRef]
- Kavkler, K.; Šmit, Ž.; Jezeršek, D.; Eichert, D.; Demšar, A. Investigation of biodeteriorated historical textiles by conventional and synchrotron radiation FTIR spectroscopy. Polym. Degrad. Stab. 2011, 96, 1081–1086. [Google Scholar] [CrossRef]
- Badii, K.; Church, J.S.; Golkarnarenji, G.; Naebe, M.; Khayyam, H. Chemical structure based prediction of PAN and oxidized PAN fiber density through a non-linear mathematical model. Polym. Degrad. Stab. 2016, 131, 53–61. [Google Scholar] [CrossRef]
- Galanakis, C.M. Separation of functional macromolecules and micromolecules: From ultrafiltration to the border of nanofiltration. Trends Food Sci. Technol. 2015, 42, 44–63. [Google Scholar] [CrossRef]
- Gallagher, W. FTIR analysis of protein structure. Course Man. Chem 2009, 455, 1–8. [Google Scholar]
- Sadat, A.; Joye, I.J. Peak fitting applied to fourier transform infrared and raman spectroscopic analysis of proteins. Appl. Sci. 2020, 10, 5918. [Google Scholar] [CrossRef]
- Ricci, A.; Olejar, K.J.; Parpinello, G.P.; Kilmartin, P.A.; Versari, A. Application of Fourier transform infrared (FTIR) spectroscopy in the characterization of tannins. Appl. Spectrosc. Rev. 2015, 50, 407–442. [Google Scholar] [CrossRef]
- Asriza, R.; Humaira, D.; Ryaldi, G. Characterization of cellulose acetate functional groups synthesized from corn husk (Zea mays). Proc. IOP Conf. Ser. Earth Environ. Sci. 2021, 926, 012060. [Google Scholar] [CrossRef]
- Alamri, H.; Low, I.M. Mechanical properties and water absorption behaviour of recycled cellulose fibre reinforced epoxy composites. Polym. Test. 2012, 31, 620–628. [Google Scholar] [CrossRef]
- Barbosa, B.M.; Vaz, S., Jr.; Colodette, J.L.; De Aguiar, A.R.; Cabral, C.P.T.; de Freitas Homem de Faria, B. Structural and chemical characterization of lignin and hemicellulose isolated from corn fibers toward agroindustrial residue valorization. Cellulose 2022, 29, 8117–8132. [Google Scholar] [CrossRef]
- Reyes-Rivera, J.; Terrazas, T. Lignin Analysis by HPLC and FTIR: Spectra Deconvolution and S/G Ratio Determination. In Xylem: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2023; pp. 149–169. [Google Scholar] [CrossRef]
- Ottah, V.E.; Ezugwu, A.L.; Ezike, T.C.; Chilaka, F.C. Comparative analysis of alkaline-extracted hemicelluloses from Beech, African rose and Agba woods using FTIR and HPLC. Heliyon 2022, 8, e09714. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Li, H.; Saravanamurugan, S. Removal of lignin and silica from rice straw for enhanced accessibility of holocellulose for the production of high-value chemicals. Bioresour. Technol. 2022, 361, 127661. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Mohanty, K. Optimization of lignin extraction from bamboo by ultrasound-assisted organosolv pretreatment. Bioresour. Technol. 2023, 376, 128884. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Xu, B.; Wang, X.; Lang, J.; Zhang, H. Chemical and structural elucidation of lignin and cellulose isolated using DES from bagasse based on alkaline and hydrothermal pretreatment. Polymers 2022, 14, 2756. [Google Scholar] [CrossRef]
- Cho, E.J.; Trinh, L.T.P.; Song, Y.; Lee, Y.G.; Bae, H.-J. Bioconversion of biomass waste into high value chemicals. Bioresour. Technol. 2020, 298, 122386. [Google Scholar] [CrossRef]
- Ali, A.; Riaz, S.; Sameen, A.; Naumovski, N.; Iqbal, M.W.; Rehman, A.; Mehany, T.; Zeng, X.-A.; Manzoor, M.F. The disposition of bioactive compounds from fruit waste, their extraction, and analysis using novel technologies: A review. Processes 2022, 10, 2014. [Google Scholar] [CrossRef]
- Liu, Y.; Sui, X.; Zhao, X.; Wang, S.; Yang, Q. Antioxidative Activity Evaluation of High Purity and Micronized Tartary Buckwheat Flavonoids Prepared by Antisolvent Recrystallization. Foods 2022, 11, 1346. [Google Scholar] [CrossRef]
- Chezanoglou, E.; Goula, A.M. Properties and Stability of Encapsulated Pomegranate Peel Extract Prepared by Co-Crystallization. Appl. Sci. 2023, 13, 8680. [Google Scholar] [CrossRef]
- Iadecola, R.; Ciccoritti, R.; Ceccantoni, B.; Bellincontro, A.; Amoriello, T. Optimization of phenolic compound extraction from brewers’ spent grain using ultrasound technologies coupled with response surface methodology. Sustainability 2022, 14, 3309. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Z.; Li, W.; Tang, Y.; Meng, H.; Wang, S. Separation and Purification of Taxanes from Crude Taxus cuspidata Extract by Antisolvent Recrystallization Method. Separations 2022, 9, 304. [Google Scholar] [CrossRef]
- Salleh, W.M.N.H.W.; Shakri, N.M.; Jauri, M.H.; Nafiah, M.A. Alkaloids and Flavonoids from Polyalthia cauliflora. Chem. Nat. Compd. 2022, 58, 986–988. [Google Scholar] [CrossRef]
- Al Juhaimi, F.; Özcan, M.M.; Ghafoor, K.; Babiker, E.E.; Hussain, S. Comparison of cold-pressing and soxhlet extraction systems for bioactive compounds, antioxidant properties, polyphenols, fatty acids and tocopherols in eight nut oils. J. Food Sci. Technol. 2018, 55, 3163–3173. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.A.; Shoukat, A.; Khalid, W.; Ejaz, A.; Itrat, N.; Majeed, I.; Koraqi, H.; Imran, M.; Nisa, M.U.; Nazir, A. A narrative review on various oil extraction methods, encapsulation processes, fatty acid profiles, oxidative stability, and medicinal properties of black seed (Nigella sativa). Foods 2022, 11, 2826. [Google Scholar] [CrossRef] [PubMed]
- Shunmugiah Veluchamy, R.; Mary, R.; Beegum Puthiya P, S.; Pandiselvam, R.; Padmanabhan, S.; Sathyan, N.; Shil, S.; Niral, V.; Musuvadi Ramarathinam, M.; Lokesha, A.N. Physicochemical characterization and fatty acid profiles of testa oils from various coconut (Cocos nucifera L.) genotypes. J. Sci. Food Agric. 2023, 103, 370–379. [Google Scholar] [CrossRef]
- Boyadzhieva, S.; Coelho, J.A.; Errico, M.; Reynel-Avilla, H.E.; Yankov, D.S.; Bonilla-Petriciolet, A.; Stateva, R.P. Assessment of Gnaphalium viscosum (Kunth) valorization prospects: Sustainable recovery of antioxidants by different techniques. Antioxidants 2022, 11, 2495. [Google Scholar] [CrossRef]
- Chetrariu, A.; Ursachi, V.F.; Dabija, A. Evaluation of the fatty acids and amino acids profiles in spent grain from brewing and malt whisky. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2022, 23, 167–177. [Google Scholar]
- Ruiz-Ruiz, J.C.; Aldana, G.d.C.E.; Cruz, A.I.C.; Segura-Campos, M.R. Antioxidant activity of polyphenols extracted from hop used in craft beer. In Biotechnological Progress and Beverage Consumption; Elsevier: Amsterdam, The Netherlands, 2020; pp. 283–310. [Google Scholar] [CrossRef]
- Nuchdang, S.; Phruetthinan, N.; Paleeleam, P.; Domrongpokkaphan, V.; Chuetor, S.; Chirathivat, P.; Phalakornkule, C. Soxhlet, microwave-assisted, and room temperature liquid extraction of oil and bioactive compounds from palm kernel cake using isopropanol as solvent. Ind. Crops Prod. 2022, 176, 114379. [Google Scholar] [CrossRef]
- Singh, N.; Sudha, M. Natural food flavours: A healthier alternative for bakery industry—A review. J. Food Sci. Technol. 2024, 61, 642–650. [Google Scholar] [CrossRef]
- Shemet, V.; Hulai, O. Food additives of natural origin: Short review. Commod. Bull. 2023, 1, 6–18. [Google Scholar] [CrossRef]
- Belwal, T.; Cravotto, C.; Ramola, S.; Thakur, M.; Chemat, F.; Cravotto, G. Bioactive compounds from cocoa husk: Extraction, analysis and applications in food production chain. Foods 2022, 11, 798. [Google Scholar] [CrossRef]
- Wang, M.; Lu, Y.; Yang, Y.; Yu, J.; Chen, Y.; Tu, F.; Hou, J.; Yang, Z.; Jiang, X. Source identification of vanillin in sesame oil by HPLC-MS/MS. Food Control 2023, 143, 109283. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, J.; Bayertai; Tang, S.; Zhou, X. Analysis of gallic acid and ellagic acid in leaves of Elaeagnus angustifolia L. from different habitats and times in Xinjiang by HPLC with cluster analysis. Acta Chromatogr. 2021, 33, 195–201. [Google Scholar] [CrossRef]
- Vilas-Franquesa, A.; Casertano, M.; Tresserra-Rimbau, A.; Vallverdú-Queralt, A.; Torres-León, C. Recent advances in bio-based extraction processes for the recovery of bound phenolics from agro-industrial by-products and their biological activity. Crit. Rev. Food Sci. Nutr. 2023, 64, 10643–10667. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Rojas, W.V.; Hernández, D.M.; Cano, M.P.; Reali, T.F. Extraction and Analytical Characterization of Phenolic Compounds from Brazil Nut (Bertholletia excelsa) Skin Industrial by-Product. Trends Sci. 2023, 20, 5457. [Google Scholar] [CrossRef]
- El-Shahir, A.A.; El-Wakil, D.A.; Abdel Latef, A.A.H.; Youssef, N.H. Bioactive Compounds and Antifungal Activity of Leaves and Fruits Methanolic Extracts of Ziziphus spina-christi L. Plants 2022, 11, 746. [Google Scholar] [CrossRef]
- Lopes, M.T.B.V. Development and Characterization of Functional Ingredients Through Valorization from Brewer’s Spent Grain. P.D. Thesis, University of Minho, Braga, Portugal, University of Aveiro, Aveiro, Portugal, 2022. [Google Scholar]
- Bonifacio-Lopes, T.; Vilas-Boas, A.; Machado, M.; Costa, E.M.; Silva, S.; Pereira, R.N.; Campos, D.; Teixeira, J.A.; Pintado, M. Exploring the bioactive potential of brewers spent grain ohmic extracts. Innov. Food Sci. Emerg. Technol. 2022, 76, 102943. [Google Scholar] [CrossRef]
- Minervini, F.; Comitini, F.; De Boni, A.; Fiorino, G.M.; Rodrigues, F.; Tlais, A.Z.A.; Carafa, I.; De Angelis, M. Sustainable and Health-Protecting Food Ingredients from Bioprocessed Food by-Products and Wastes. Sustainability 2022, 14, 15283. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.; Latha, L.Y. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef]
- Arora, N.K.; Mishra, I. Responsible consumption and production: A roadmap to sustainable development. Environ. Sustain. 2023, 6, 1–6. [Google Scholar] [CrossRef]
- Purnell, P.J. A comparison of different methods of identifying publications related to the United Nations Sustainable Development Goals: Case study of SDG 13—Climate Action. Quant. Sci. Stud. 2022, 3, 976–1002. [Google Scholar] [CrossRef]

| Study | Extraction Method | Total Dietary Fiber (TDF) Extraction Rate | Structural Differences | Key Enhancements | Precipitation Method | Ref. |
|---|---|---|---|---|---|---|
| This Study (BSG) | Salting-out + Ultrasound-assisted alkaline treatment | 93.3% | BSG fiber structure was more loosely bound due to brewing pretreatment, which partially degraded cellulose and lignin | Ultrasound-assisted treatment induced cavitation effects, breaking cell walls and accelerating fiber release | 1:1 ethanol precipitation to isolate high-purity dietary fiber | |
| Wang et al. (Kiwi Fruit) | Alkaline treatment | 92.9% | Kiwi fruit fiber structure remained more intact as no prior processing was involved | No ultrasound treatment was applied | Not specified | [48] |
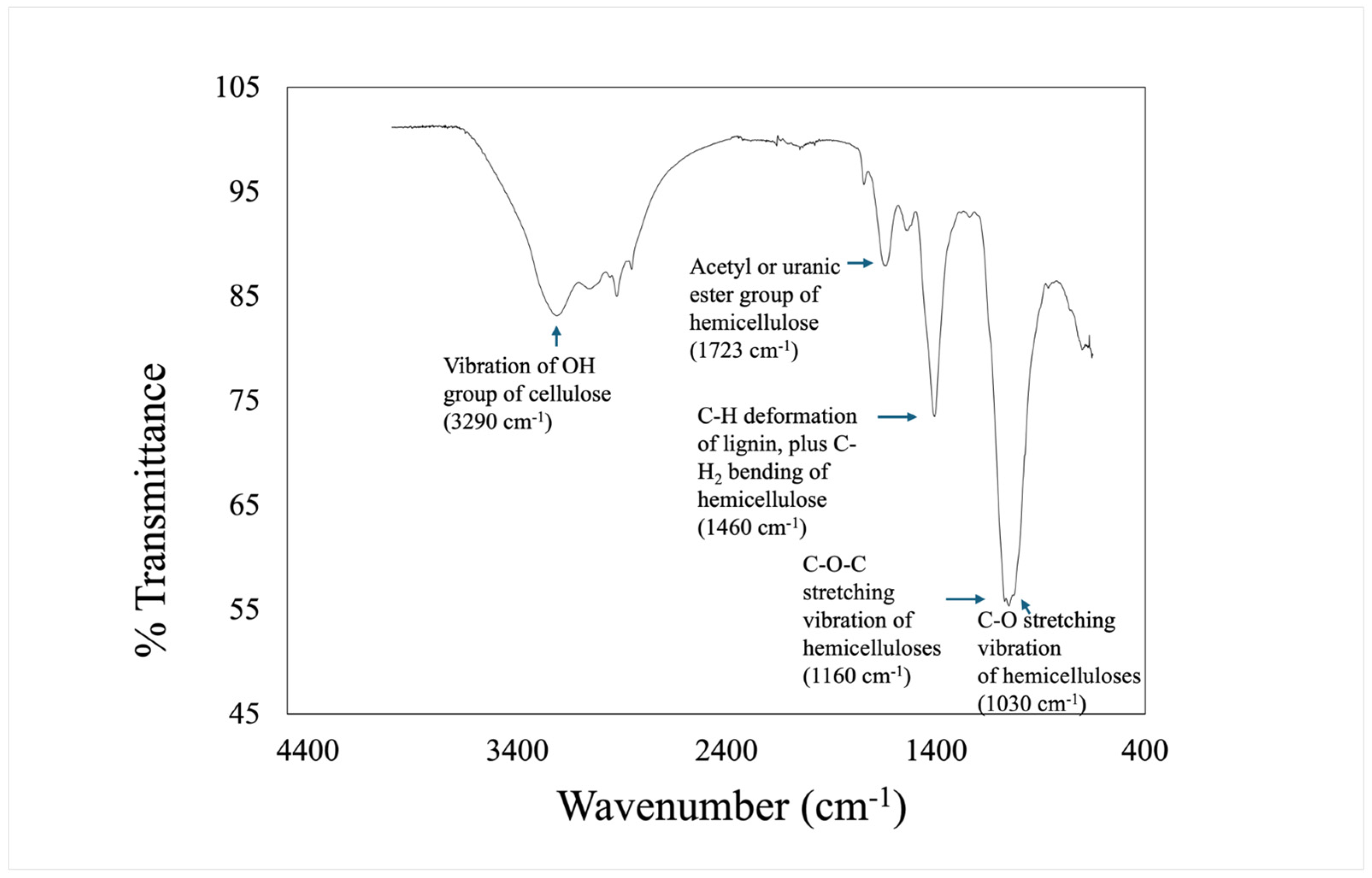
| Wavenumber (cm−1) | Assignment |
|---|---|
| 1030 | C-O stretching vibration of hemicelluloses |
| 1160 | C-O-C stretching vibration of hemicelluloses |
| 1380 | CH3 and CH deformation |
| 1460 | C-H deformation of lignin, and C-H2 bending of hemicellulose |
| 1540 | N-H bending vibration, amide II |
| 1657 | C=O, amide I |
| 1723 | Acetyl or uronic ester group of hemicellulose |
| 2700 | Bending OH overtone vibrations for tannins |
| 2853 | Asymmetric and symmetric CH2 and CH3 of epoxy resin |
| 3290 | Vibration of OH group of cellulose |
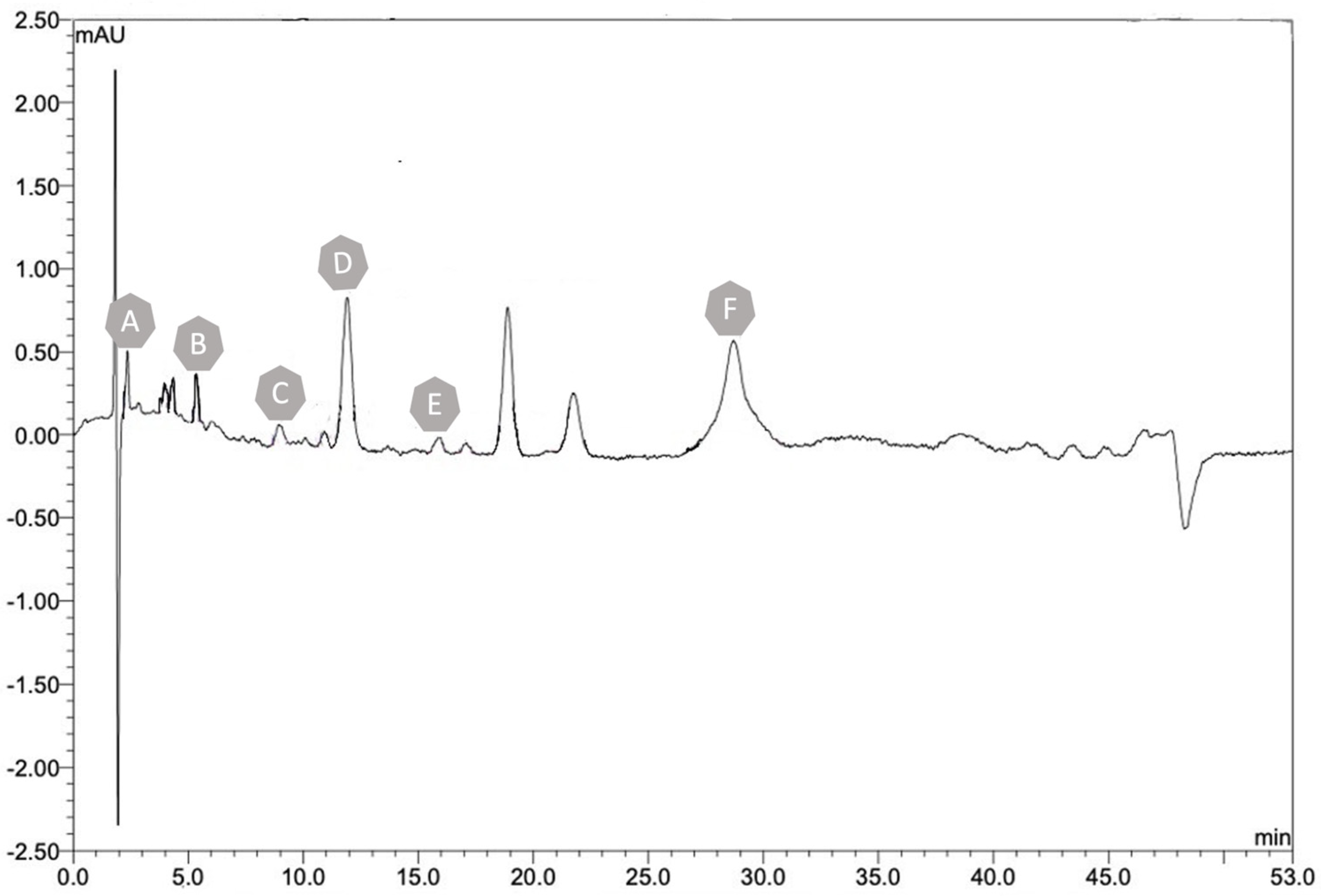
| Peak | Compound Name | Molecular Formula | Concentration (mg/L) | Extraction Rate |
|---|---|---|---|---|
| A | Hemicellulose | C5H8O4 | 8245.2 | 54.9% |
| B | Lignin | (C31H34O11)n | 10,435.4 | 69.5% |
| C | Cellulose | (C6H10O5)n | 13,245.4 | 88.3% |
| Study and Sample | Hemicellulose Extraction Rate (%) | Lignin Extraction Rate (%) | Cellulose Extraction Rate (%) | Extraction Method | Ref. |
|---|---|---|---|---|---|
| This study (BSG) | 54.9% | 69.5% | 88.3% | Alkaline Pretreatment (5% NaOH) + Ultrasonication (1000 Hz, 1 h) + Ethanol Precipitation | |
| Pal et al. (Rice Straw) | 60.0% | NH3 in Water-THF | [70] | ||
| A. Das et al., Optimized Ultrasound-Assisted (Bamboo) | 65.8% | Ultrasound-Assisted Extraction | [71] | ||
| N. Wang., Hydrothermal Pretreatment (Bagasse) | 32.6% | 81.2% | Hydrothermal Pretreatment | [72] |
| Peak | Compound Name | Molecular Formula | Concentration (mg/L) |
|---|---|---|---|
| A | Oleanolic acid | C30H48O3 | 0.243 |
| B | Oleic acid | C18H34O2 | 0.057 |
| C | Linoleic acid | C18H32O2 | 0.547 |
| D | Arachidic acid | C20H40O2 | 0.1737 |
| Peak | Compound Name | Concentration (mg/L) | Extraction Rate |
|---|---|---|---|
| A | Ascorbic acid | 1.5923 | |
| B | Gallic acid | 2.314 | |
| C | Catechol | 2.739 | |
| D | Ellagic acid | 162.88 | |
| E | Acetylsalicylic acid | 0.63 | |
| F | Vanillin | 590.1688 | 45.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, H.-Y.I.; Miri, T.; Onyeaka, H. Valorization of Bioactive Compounds Extracted from Brewer’s Spent Grain (BSG) for Sustainable Food Waste Recycling. Sustainability 2025, 17, 2477. https://doi.org/10.3390/su17062477
Chu H-YI, Miri T, Onyeaka H. Valorization of Bioactive Compounds Extracted from Brewer’s Spent Grain (BSG) for Sustainable Food Waste Recycling. Sustainability. 2025; 17(6):2477. https://doi.org/10.3390/su17062477
Chicago/Turabian StyleChu, Hao-Yu Ivory, Taghi Miri, and Helen Onyeaka. 2025. "Valorization of Bioactive Compounds Extracted from Brewer’s Spent Grain (BSG) for Sustainable Food Waste Recycling" Sustainability 17, no. 6: 2477. https://doi.org/10.3390/su17062477
APA StyleChu, H.-Y. I., Miri, T., & Onyeaka, H. (2025). Valorization of Bioactive Compounds Extracted from Brewer’s Spent Grain (BSG) for Sustainable Food Waste Recycling. Sustainability, 17(6), 2477. https://doi.org/10.3390/su17062477








