Addressing Challenges for Eco-Friendly and Sustainable Wastewater Treatment Solutions Using Extremophile Microorganisms
Abstract
1. Introduction
2. Methodology
2.1. Literature Search and Data Collection
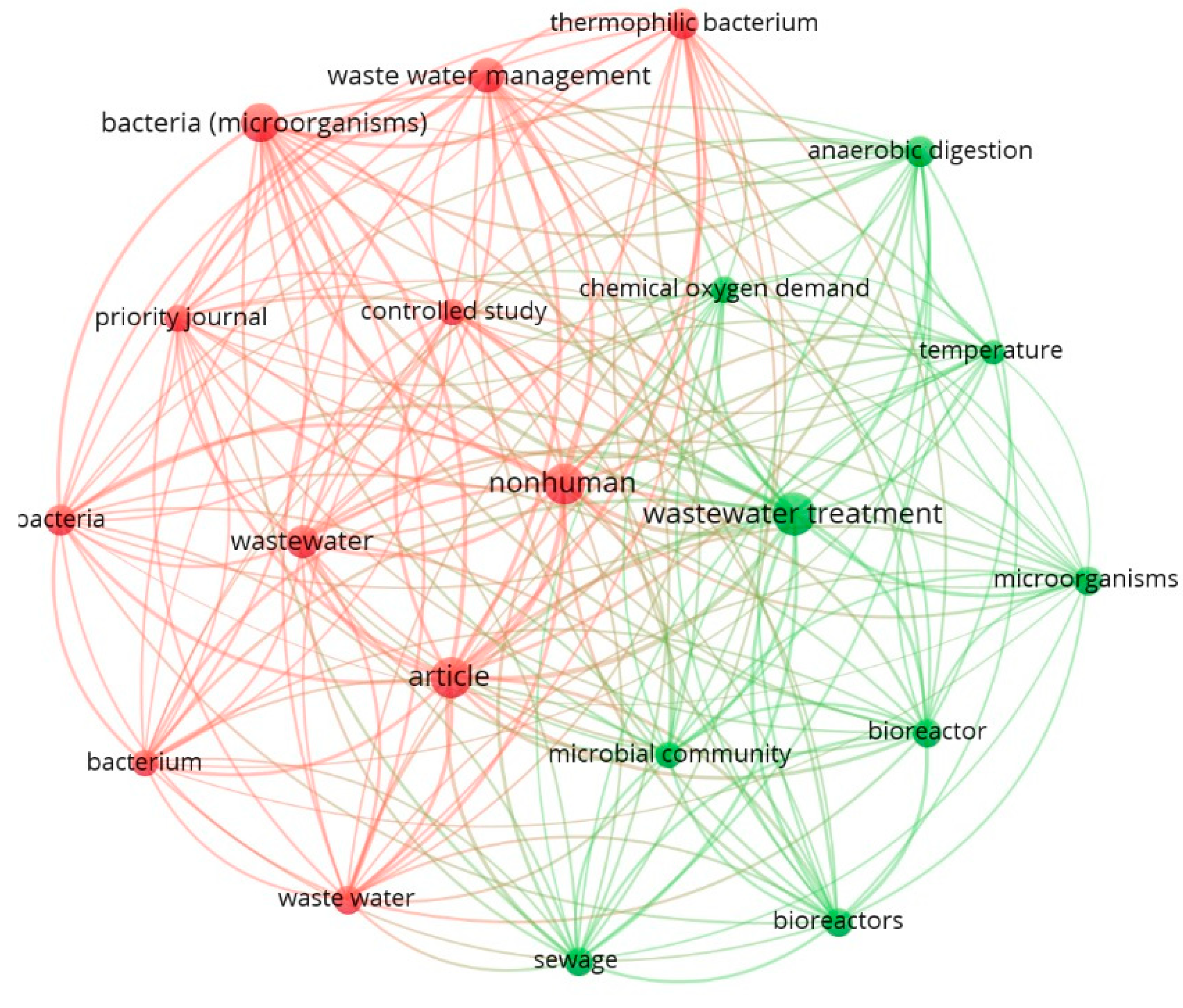
2.2. Data Analysis and Visualization
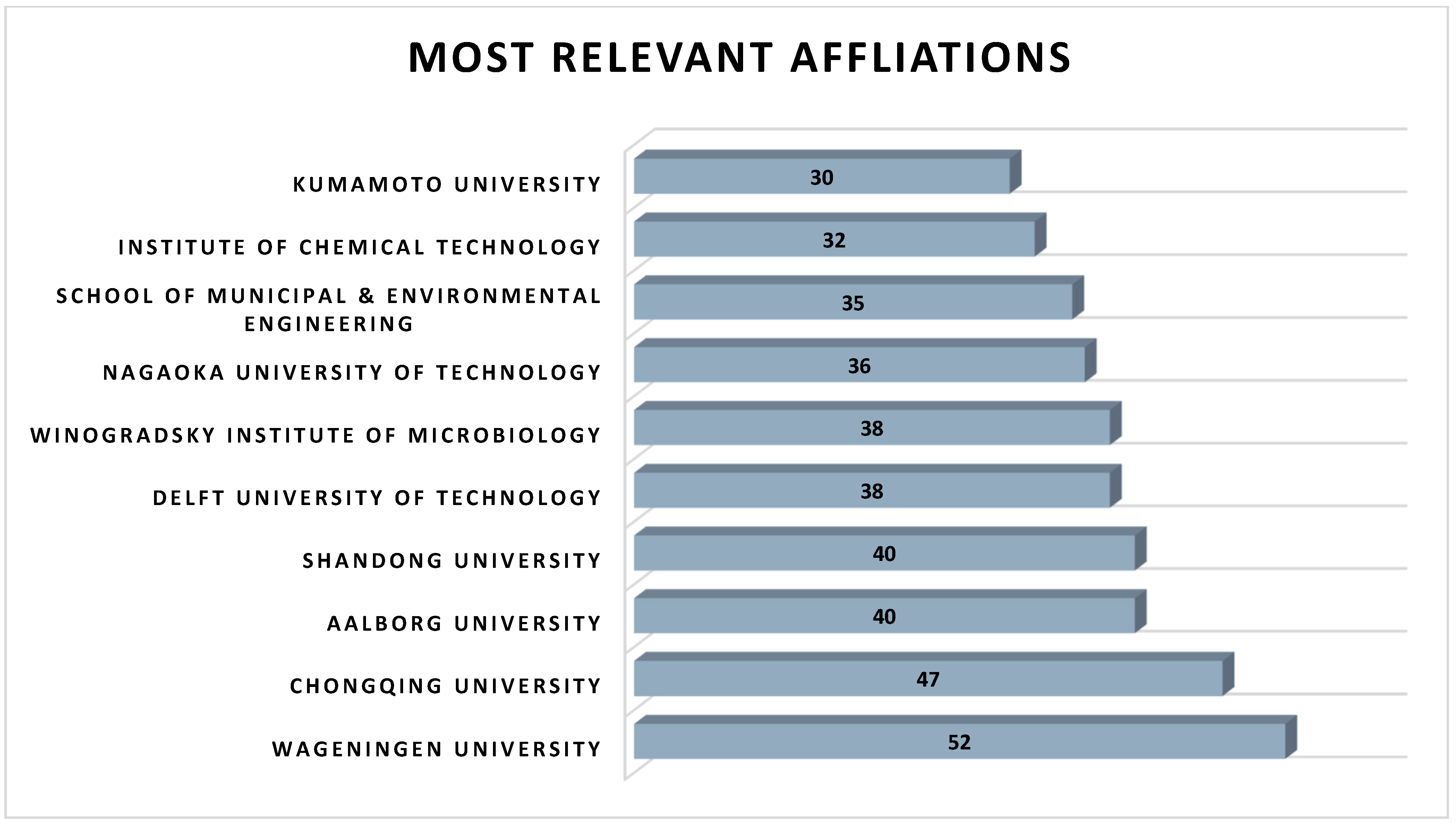
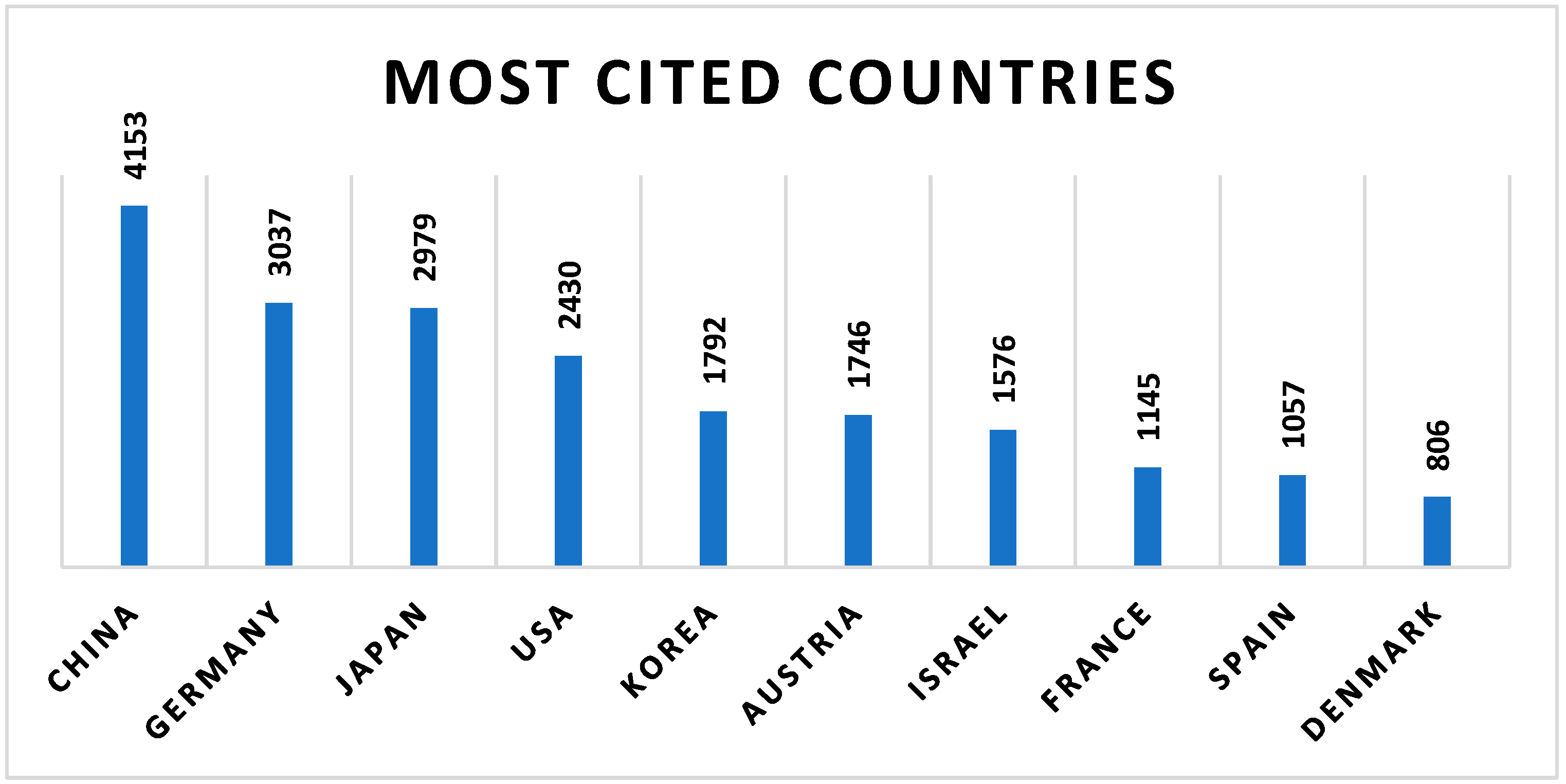
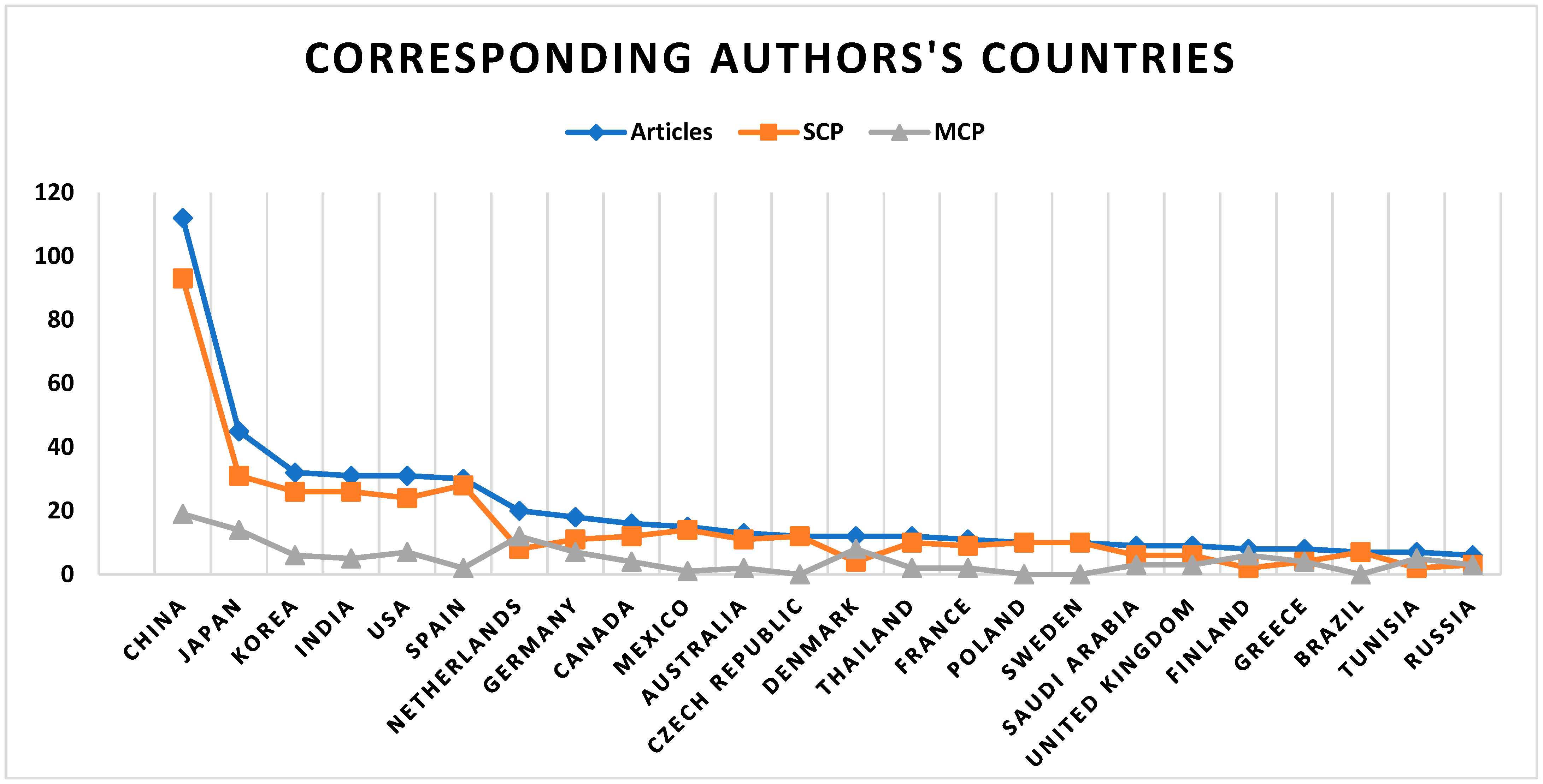
2.3. Geographic and Institutional Contribution to Research
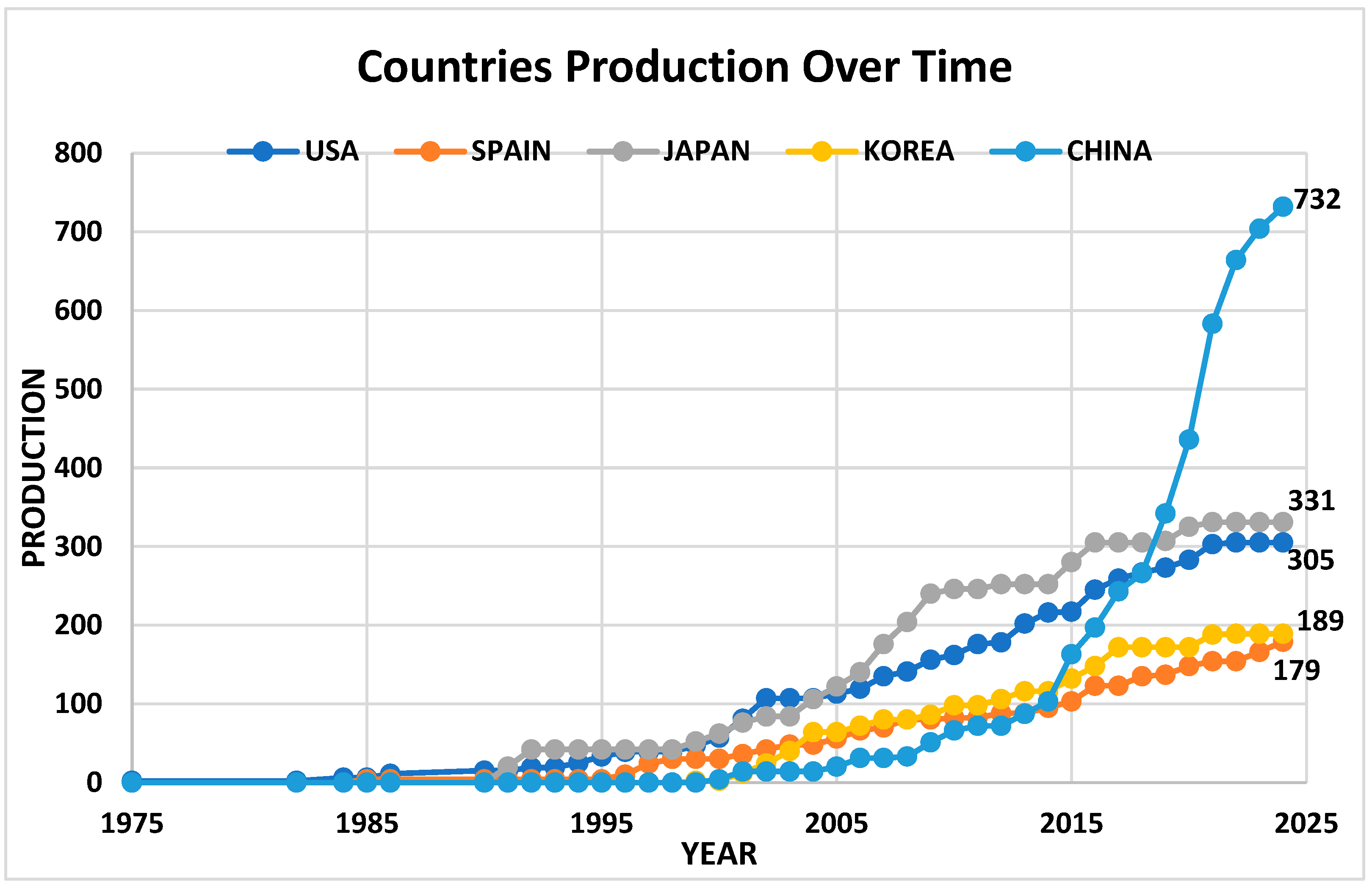
2.4. Analysis of Publication Trends in the Time
3. Extremophilic Microorganisms in Wastewater Treatment
3.1. Acidophilic Microorganisms
3.1.1. Acidophilic Microorganisms for Organic Wastewater
3.1.2. Acidophilic Microorganisms for Inorganic Wastewater
| Domain | Acidophiles | Inorganic Pollutants | Removal % | Duration | Pollutant Initial Concentration | References |
|---|---|---|---|---|---|---|
| Heavy Metals | ||||||
| Bacteria | Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans, cidithiobacillus ferrooxidans, Acidomicrobium ferrooxidans, Ferroplasma, Alicyclobacillus, Acidiphilium spp., Sulfobacillus spp., Acidocella spp. | Iron Reduction | 85–90% | 10–15 days | 500 mg/L | [82,83,84] |
| Desulfovibrio, Desulfomicrobium, Desulfobulbus, Desulfosarcina, Desulfobacter, Desulfotomaculum, Desulfosporosinus, Thermodesulfobacterium, Thermodesulfovibrio, Desulfovibrio desulfuricans, Desulfomicrobium baculatum | Sulfate Reduction | 70–80% | 12–20 days | 300 mg/L | [85,86,87] | |
| Desulfurella | Sulfur Reduction | 75% | 14 days | 200 mg/L | [88,89] | |
| Acidithiobacillus spp., Leptospirillum ferrooxidans, Acidiphilium cryptum | Symbiotic Role | N/A | N/A | N/A | [90,91,92] | |
| Algae | Anabaena, Cladophora, Oscillatoria, Phaeodactylum, Scenedesmus, Spirulina sp. | Bio-sorption and Bioaccumulation | 60–85% | 7–14 days | 100 mg/L | [92,93,94] |
| Radioactive Pollutants | ||||||
| Bacteria | A. ferrooxidans, Cupriavidus metallidurans | Bio-sorption | 80–90% | 10 days | 50 mg/L | [95,96] |
| Sulfolobus metallicu, Acidithiobacillus sp., Sulfolobus metallicus | Bioaccumulation | 85% | 12 days | 70 mg/L | [97,98,99] | |
3.1.3. Potential Impacts of Acidophiles
3.2. Alkaliphile Microorganisms in Wastewater Treatment
3.2.1. Alkaliphiles for Organic Wastewater
3.2.2. Alkaliphiles for Inorganic Pollutant Wastewater Treatment
3.3. Halophilic Microorganisms in Wastewater Treatment
3.3.1. Halophilic Organic Pollutant Wastewater Treatment
3.3.2. Halophilic Inorganic Pollutant Wastewater Treatment
| Domain | Halophiles | Pollutants | Mechanism | Removal % | References |
|---|---|---|---|---|---|
| Organic Pollutants | |||||
| Bacteria | Halomonas spp. | Phenol and other aromatic hydrocarbons | Biodegradation | 85% | [157] |
| Bacillus marmarensis | Ethanol fermentation | Fermentation | 90% ethanol yield | [158] | |
| Halophilic strains from oil-contaminated environments | Complete phenol degradation | Biodegradation | 95% | [159] | |
| Mixed halophilic cultures | Phenyl-phenol, bisphenol A (in anaerobic MBR systems) | Biodegradation in MBR systems | 80–85% | [160] | |
| Halophilic bacteria from marine mud | Phenol degradation (more than 80% removal in 48 h) | Biodegradation | 80% | [161] | |
| Bacillus spp. | Organic matter, hydrocarbons | Biodegradation | 85% | [162] | |
| Ochrobactrum, Marinobacter, Bacillus | Fish market wastewater (COD, TSS removal, energy production in MFC) | Biodegradation and energy production | 90% | [154] | |
| Lysinibacillus fusiformis and Providencia stuartii | Proteins, lipids, mucopolysaccharides from tannery wastewater | Biodegradation | 88% | [163] | |
| Archaea | Haloarchaea | Carbon, nitrogen, phosphorus, sulfur, and heavy metal metabolism in hypersaline wastewater | Metabolism and remediation | 75–90% | [164] |
| Inorganic Pollutants | |||||
| Bacteria | Ectothiorhodospira magna, Ectothiorhodospira shaposhnikovii | Zinc, lead, copper (via sulfide oxidation to sulfur) | Sulfide oxidation to sulfur | 85% | [165] |
| Thiomonas spp. | Arsenic (As (III) to As (V) conversion) | Oxidation | 90% | [166] | |
| Thioalkalivibrio spp. | Sulfur (H2S removal from biogas in the thiopaq process) | Oxidation | 88% | [155] | |
| Halophilic bacteria | Heavy metals, arsenic | Bioaccumulation and biosorption | 80% | [167] | |
| Ochrobactrum, Marinobacter, Rhodococcus, Bacillus | Seafood and pharmaceutical wastewater treatment with energy production | Biodegradation and energy generation | 85% | [168] | |
| Exiguobacterium mexicanum | Nitrogen removal from saline wastewater via heterotrophic nitrification and aerobic denitrification | Nitrification and denitrification | 78% | [169] | |
3.4. Thermophilic Microorganisms in the Treatment of Wastewater
3.4.1. Thermophilic Organic Pollutant Wastewater Treatment
| Domain | Thermophiles | Pollutants | Removal % | References |
|---|---|---|---|---|
| Organic pollutants | ||||
| Bacteria | Chloroflexus sp. | Lignin and macromolecules | 75–80% | [176] |
| Meiothermus sp. | Lignin and macromolecules | 80% | [177] | |
| Roseiflexus sp. | Lignin and macromolecules | 85% | [174] | |
| Thermophilic methanogens | Proteins, lipids, carbohydrates | 70–85% CH4 yield | [178] | |
| Thermoanaerobacterium sp. | Sewage sludge, industrial effluent (TAD process) | 75–88% | [179] | |
| Clostridium thermocellum | Organic matter, cellulose, complex biopolymers | 80–85% | [180] | |
| Thermoanaerobacterium thermosaccharolyticum | Carbohydrates, volatile fatty acids | 78% | [181] | |
| Caldicellulosiruptor saccharolyticus | Polysaccharides, organic matter | 85% | [182] | |
| Archaea | Thermophilic Anaerobic Digesters | Sewage sludge, high-concentration industrial waste | 80% | [183] |
| Methanothermobacter marburgensis | Sewage sludge, high-organic waste | 85% CH4 yield | [130] | |
| Methanosaeta sp., Methanosarcina sp. | Industrial wastewater (organic pollutants) | 75–85% CH4 yield | [184] | |
| Methanothermobacter thermautotrophicus | Sewage sludge, organic waste | 80% CH4 yield | [130] | |
| Inorganic pollutants | ||||
| Bacteria | Sulfur-oxidizing thermophiles (Thermothrix sp. (sulfur-oxidizing, Thermothrix thiopara) | Sulfides and sulfur-containing compounds | 85–90% 80–85% | [185] |
| Iron-oxidizing thermophiles | Iron (ferrous to ferric) | 80–85% | [186,187] | |
| Archaea | Acidianus brierleyiSulfolobus acidocaldarius | Sulfides | 90% | [188] |
3.4.2. Thermophilic Inorganic Pollutant Wastewater Treatment
3.5. Extremophilic Microalgae
3.5.1. Organic Pollutant Removal
3.5.2. Heavy Metal Removal
3.5.3. Nutrient Removal
3.5.4. Integration with Bacterial Systems
| Pollutant Type | Pollutants | Extremophilic Microalgae | Mechanism | Removal % | References |
|---|---|---|---|---|---|
| Organic Pollutants | Organic matter | Galdieria sulphuraria | Biodegradation of organic load | 85–90% | [32] |
| Pharmaceuticals | Scenedesmus sp. | Adsorption and biodegradation | 75–85% | [199] | |
| Textile dyes | Dunaliella salina | Decolorization and degradation | 80–88% | [200] | |
| Industrial effluents | Nannochloropsis gaditana | Reduction of organic and toxic load | 78–85% | [201] | |
| Organic pollutants (general) | Spirulina platensis | Biodegradation | 80% | [201] | |
| Inorganic Pollutants | Nutrient Removal (Nitrogen, phosphorus) | Scenedesmus sp., Nannochloropsis | Assimilation and nutrient uptake | 75–90% | [202] |
| Nutrients (Nitrates, phosphates) | Galdieria sulphuraria | Nutrient assimilation | 85% | [191] | |
| Heavy Metals (Aluminum (Al), Nickel (Ni), Copper (Cu), Vanadium, Uranium, Titanium | acidophila, Galdieria sulphuraria | Biosorption and bioaccumulation | 70–85% | [203] | |
| Sulfates | Microalgae in rotating biofilm reactors | Sulfate reduction | 80–90% | [27] | |
| Emerging Contaminants | Pharmaceuticals and personal care products (PPCPs) | Microalgae consortia | Biodegradation and biosorption | 75–85% | [204,205,206] |
4. Conclusions
5. Future Prospect
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aljerf, L. Data of thematic analysis of farmer’s use behavior of recycled industrial wastewater. Data Brief. 2018, 21, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Bijekar, S.; Padariya, H.D.; Yadav, V.K.; Gacem, A.; Hasan, M.A.; Awwad, N.S.; Yadav, K.K.; Islam, S.; Park, S.; Jeon, B.-H. The state of the art and emerging trends in the wastewater treatment in developing nations. Water 2022, 14, 2537. [Google Scholar] [CrossRef]
- Dolan, F.; Lamontagne, J.; Link, R.; Hejazi, M.; Reed, P.; Edmonds, J. Evaluating the economic impact of water scarcity in a changing world. Nat. Commun. 2021, 12, 1915. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, H.; Gosling, S.N.; Kummu, M.; Flörke, M.; Pfister, S.; Hanasaki, N.; Wada, Y.; Zhang, X.; Zheng, C. Water scarcity assessments in the past, present, and future. Earth’s Future 2017, 5, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, S.; Priya, A.; Kumar, P.S.; Hoang, T.K.; Sekar, K.; Chong, K.Y.; Khoo, K.S.; Ng, H.S.; Show, P.L. A critical and recent developments on adsorption technique for removal of heavy metals from wastewater-A review. Chemosphere 2022, 303, 135146. [Google Scholar] [CrossRef]
- Wang, F.; Wang, B.; Duan, L.; Zhang, Y.; Zhou, Y.; Sui, Q.; Xu, D.; Qu, H.; Yu, G. Occurrence and distribution of microplastics in domestic, industrial, agricultural and aquacultural wastewater sources: A case study in Changzhou, China. Water Res. 2020, 182, 115956. [Google Scholar] [CrossRef] [PubMed]
- Bennish, M.L.; Ahmed, S. Shigellosis. In Hunter’s Tropical Medicine and Emerging Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2020; pp. 492–499. [Google Scholar]
- Çimen, A. Removal of chromium from wastewater by reverse osmosis. Russ. J. Phys. Chem. A 2015, 89, 1238–1243. [Google Scholar] [CrossRef]
- Petrinic, I.; Korenak, J.; Povodnik, D.; Hélix-Nielsen, C. A feasibility study of ultrafiltration/reverse osmosis (UF/RO)-based wastewater treatment and reuse in the metal finishing industry. J. Clean. Prod. 2015, 101, 292–300. [Google Scholar] [CrossRef]
- Gibson, J.; Drake, J.; Karney, B. UV disinfection of wastewater and combined sewer overflows. Ultrav. Light. Hum. Health Dis. Environ. 2017, 996, 267–275. [Google Scholar]
- Zhang, C.; Xu, L.; Wang, X.; Zhuang, K.; Liu, Q. Effects of ultraviolet disinfection on antibiotic-resistant Escherichia coli from wastewater: Inactivation, antibiotic resistance profiles and antibiotic resistance genes. J. Appl. Microbiol. 2017, 123, 295–306. [Google Scholar] [CrossRef]
- Kumar, V.; Othman, N.; Asharuddin, S. Applications of natural coagulants to treat wastewater—A review. Proc. MATEC Web Conf. 2017, 103, 06016. [Google Scholar] [CrossRef]
- Wenten, I.G. Reverse osmosis applications: Prospect and challenges. Desalination 2016, 391, 112–125. [Google Scholar] [CrossRef]
- Colla, V.; Branca, T.A.; Rosito, F.; Lucca, C.; Vivas, B.P.; Delmiro, V.M. Sustainable reverse osmosis application for wastewater treatment in the steel industry. J. Clean. Prod. 2016, 130, 103–115. [Google Scholar] [CrossRef]
- Shak, K.P.Y.; Wu, T.Y. Optimized use of alum together with unmodified Cassia obtusifolia seed gum as a coagulant aid in treatment of palm oil mill effluent under natural pH of wastewater. Ind. Crops Prod. 2015, 76, 1169–1178. [Google Scholar] [CrossRef]
- Jagaba, A.H.; Abubakar, S.; Lawal, I.M.; Latiff, A.A.A.; Umaru, I. Wastewater treatment using alum, the combinations of alum-ferric chloride, alum-chitosan, alum-zeolite and alum-moringa oleifera as adsorbent and coagulant. Int. J. Eng. Manag. 2018, 2, 67–75. [Google Scholar] [CrossRef]
- Masschelein, W.J.; Rice, R.G. Ultraviolet Light in Water and Wastewater Sanitation; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- He, H.; Chen, Y.; Li, X.; Cheng, Y.; Yang, C.; Zeng, G. Influence of salinity on microorganisms in activated sludge processes: A review. Int. Biodeterior. Biodegrad. 2017, 119, 520–527. [Google Scholar] [CrossRef]
- Orhon, D. Evolution of the activated sludge process: The first 50 years. J. Chem. Technol. Biotechnol. 2015, 90, 608–640. [Google Scholar] [CrossRef]
- Estrada-Arriaga, E.B.; Cortés-Muñoz, J.E.; González-Herrera, A.; Calderón-Mólgora, C.G.; de Lourdes Rivera-Huerta, M.; Ramírez-Camperos, E.; Montellano-Palacios, L.; Gelover-Santiago, S.L.; Pérez-Castrejón, S.; Cardoso-Vigueros, L. Assessment of full-scale biological nutrient removal systems upgraded with physico-chemical processes for the removal of emerging pollutants present in wastewaters from Mexico. Sci. Total Environ. 2016, 571, 1172–1182. [Google Scholar] [CrossRef]
- Huang, X.; Dong, W.; Wang, H.; Jiang, S. Biological nutrient removal and molecular biological characteristics in an anaerobic-multistage anaerobic/oxic (A-MAO) process to treat municipal wastewater. Bioresour. Technol. 2017, 241, 969–978. [Google Scholar] [CrossRef]
- Nancharaiah, Y.; Mohan, S.V.; Lens, P. Recent advances in nutrient removal and recovery in biological and bioelectrochemical systems. Bioresour. Technol. 2016, 215, 173–185. [Google Scholar] [CrossRef]
- Jegatheesan, V.; Pramanik, B.K.; Chen, J.; Navaratna, D.; Chang, C.-Y.; Shu, L. Treatment of textile wastewater with membrane bioreactor: A critical review. Bioresour. Technol. 2016, 204, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Liu, X.; Wang, D.; Wu, Y.; Wang, Q.; Liu, Y.; Li, X.; An, H.; Zhao, J.; Chen, F. Free ammonia-based pretreatment enhances phosphorus release and recovery from waste activated sludge. Chemosphere 2018, 213, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hu, H.; Liu, J.; Luo, J.; Qian, G.; Wang, A. pH dependent phosphorus release from waste activated sludge: Contributions of phosphorus speciation. Chem. Eng. J. 2015, 267, 260–265. [Google Scholar] [CrossRef]
- Pang, N.; Bergeron, A.D.; Gu, X.; Fu, X.; Dong, T.; Yao, Y.; Chen, S. Recycling of nutrients from dairy wastewater by extremophilic microalgae with high ammonia tolerance. Environ. Sci. Technol. 2020, 54, 15366–15375. [Google Scholar] [CrossRef]
- di Cicco, M.R.; Iovinella, M.; Palmieri, M.; Lubritto, C.; Ciniglia, C. Extremophilic microalgae Galdieria gen. for urban wastewater treatment: Current state, the case of “POWER” system, and future prospects. Plants 2021, 10, 2343. [Google Scholar] [CrossRef]
- Baati, H.; Siala, M.; Azri, C.; Ammar, E.; Dunlap, C.; Trigui, M. Resistance of a Halobacterium salinarum isolate from a solar saltern to cadmium, lead, nickel, zinc, and copper. Antonie Leeuwenhoek 2020, 113, 1699–1711. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.K.; Singh, A.K. Exploitation of potential extremophiles for bioremediation of xenobiotics compounds: A biotechnological approach. Curr. Genom. 2020, 21, 161–167. [Google Scholar] [CrossRef]
- Kaushik, S.; Alatawi, A.; Djiwanti, S.R.; Pande, A.; Skotti, E.; Soni, V. Potential of extremophiles for bioremediation. Microb. Rejuvenation Polluted Environ. 2021, 1, 293–328. [Google Scholar]
- di Cicco, M.R.; Palmieri, M.; Altieri, S.; Ciniglia, C.; Lubritto, C. Cultivation of the acidophilic microalgae galdieria phlegrea with wastewater: Process yields. Int. J. Environ. Res. Public Health 2021, 18, 2291. [Google Scholar] [CrossRef]
- Sadeghi, M.; Kor, Y.; Keramat, S.; Mehrbakhsh, Z. Improving the efficiency of saline wastewater treatment plant through adaptation of halophilic microorganisms. Desalin Water Treat. 2019, 157, 62–68. [Google Scholar] [CrossRef]
- Gerrity, S.; Kennelly, C.; Clifford, E.; Collins, G. Hydrogen sulfide oxidation in novel horizontal-flow biofilm reactors dominated by an Acidithiobacillus and a Thiobacillus species. Environ. Technol. 2016, 37, 2252–2264. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Zhou, M.; Chiu, T.H.; Sun, X.; Keller, J.; Bond, P.L. Wastewater-enhanced microbial corrosion of concrete sewers. Environ. Sci. Technol. 2016, 50, 8084–8092. [Google Scholar] [CrossRef]
- Aragaw, T. Functions of various bacteria for specific pollutants degradation and their application in wastewater treatment: A review. Int. J. Environ. Sci. Technol. 2021, 18, 2063–2076. [Google Scholar] [CrossRef]
- Borkar, S. Alkaliphilic bacteria: Diversity, physiology and industrial applications. In Bioprospects of Coastal Eubacteria: Ecosystems of Goa; Springer: Berlin/Heidelberg, Germany, 2015; pp. 59–83. [Google Scholar]
- Abhay, P.; Rawat, P.; Singh, D. Isolation of alkaliphilic bacterium Citricoccus alkalitolerans CSB1: An efficient biosorbent for bioremediation of tannery waste water. Cell. Mol. Biol. 2016, 62, 135. [Google Scholar]
- Ratnasari, A.; Zaidi, N.S.; Syafiuddin, A.; Boopathy, R.; Kueh, A.B.H.; Amalia, R.; Prasetyo, D.D. Prospective biodegradation of organic and nitrogenous pollutants from palm oil mill effluent by acidophilic bacteria and archaea. Bioresour. Technol. Rep. 2021, 15, 100809. [Google Scholar] [CrossRef]
- Asadi, P.; Rad, H.A.; Qaderi, F. Comparison of Chlorella vulgaris and Chlorella sorokiniana pa. 91 in post treatment of dairy wastewater treatment plant effluents. Environ. Sci. Pollut. Res. 2019, 26, 29473–29489. [Google Scholar] [CrossRef]
- Delanka-Pedige, H.M.; Munasinghe-Arachchige, S.P.; Cornelius, J.; Henkanatte-Gedera, S.M.; Tchinda, D.; Zhang, Y.; Nirmalakhandan, N. Pathogen reduction in an algal-based wastewater treatment system employing Galdieria sulphuraria. Algal Res. 2019, 39, 101423. [Google Scholar] [CrossRef]
- Zhou, W.; Li, Y.; Gao, Y.; Zhao, H. Nutrients removal and recovery from saline wastewater by Spirulina platensis. Bioresour. Technol. 2017, 245, 10–17. [Google Scholar] [CrossRef]
- Li, J.; Zheng, X.; Liu, K.; Sun, S.; Li, X. Effect of tetracycline on the growth and nutrient removal capacity of Chlamydomonas reinhardtii in simulated effluent from wastewater treatment plants. Bioresour. Technol. 2016, 218, 1163–1169. [Google Scholar] [CrossRef]
- Liu, Y.; Yildiz, I. The effect of salinity concentration on algal biomass production and nutrient removal from municipal wastewater by Dunaliella salina. Int. J. Energy Res. 2018, 42, 2997–3006. [Google Scholar] [CrossRef]
- Pandey, H.; Singh, D.; Dhiman, V.K.; Dhiman, V.K.; Pandey, D. Extremophiles and their application in bioremediation. In Physiology, Genomics, and Biotechnological Applications of Extremophiles; IGI Global: Hershey, PA, USA, 2022; pp. 188–206. [Google Scholar]
- Quatrini, R.; Johnson, D.B. Microbiomes in extremely acidic environments: Functionalities and interactions that allow survival and growth of prokaryotes at low pH. Curr. Opin. Microbiol. 2018, 43, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, X.; Liang, Y.; Fan, F.; Zhang, X.; Yin, H. Metabolic diversity and adaptive mechanisms of iron-and/or sulfur-oxidizing autotrophic acidophiles in extremely acidic environments. Environ. Microbiol. Rep. 2016, 8, 738–751. [Google Scholar] [CrossRef] [PubMed]
- López-Archilla, A.I.; Marín, I.; Amils, R. Microbial community composition and ecology of an acidic aquatic environment: The Tinto River, Spain. Microb. Ecol. 2001, 41, 20–35. [Google Scholar] [CrossRef]
- Hedrich, S.; Schippers, A. Distribution of acidophilic microorganisms in natural and man-made acidic environments. Curr. Issues Mol. Biol. 2021, 40, 25–48. [Google Scholar] [CrossRef] [PubMed]
- Dopson, M.; González-Rosales, C.; Holmes, D.S.; Mykytczuk, N. Eurypsychrophilic acidophiles: From (meta)genomes to low-temperature biotechnologies. Front. Microbiol. 2023, 14, 1149903. [Google Scholar] [CrossRef]
- Sharma, M.; Agarwal, S.; Agarwal Malik, R.; Kumar, G.; Pal, D.B.; Mandal, M.; Sarkar, A.; Bantun, F.; Haque, S.; Singh, P.; et al. Recent advances in microbial engineering approaches for wastewater treatment: A review. Bioengineered 2023, 14, 2184518. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.R.; Desai, C.; van Hullebusch, E.D.; Madamwar, D. Editorial: Advanced Bioremediation Technologies and Processes for the Treatment of Synthetic Organic Compounds. Front. Bioeng. Biotechnol. 2021, 9, 721319. [Google Scholar] [CrossRef]
- Chen, G.; Bai, R.; Zhang, Y.; Zhao, B.; Xiao, Y. Application of metagenomics to biological wastewater treat-ment. Sci. Total Environ. 2022, 807, 150737. [Google Scholar] [CrossRef]
- Anabtawi, H.M.; Lee, W.H.; Al-Anazi, A.; Mohamed, M.M.; Aly Hassan, A. Advancements in Biological Strategies for Controlling Harmful Algal Blooms (HABs). Water 2024, 16, 224. [Google Scholar] [CrossRef]
- Gilbride, K.A.; Lee, D.Y.; Beaudette, L.A. Molecular techniques in wastewater: Understanding microbial communities, detecting pathogens, and real-time process control. J. Microbiol. Methods 2006, 66, 1–20. [Google Scholar] [CrossRef]
- Chen, L.-x.; Huang, L.-n.; Méndez-García, C.; Kuang, J.-l.; Hua, Z.-s.; Liu, J.; Shu, W.-s. Microbial communities, processes and functions in acid mine drainage ecosystems. Curr. Opin. Biotechnol. 2016, 38, 150–158. [Google Scholar] [CrossRef]
- Luís, A.T.; Córdoba, F.; Antunes, C.; Loayza-Muro, R.; Grande, J.A.; Silva, B.; Diaz-Curiel, J.; Ferreira da Silva, E. Extremely acidic eukaryotic (Micro) organisms: Life in acid mine drainage polluted environments—Mini-review. Int. J. Environ. Res. Public Health 2021, 19, 376. [Google Scholar] [CrossRef] [PubMed]
- Mesa, V.; Gallego, J.L.; González-Gil, R.; Lauga, B.; Sánchez, J.; Méndez-García, C.; Peláez, A.I. Bacterial, archaeal, and eukaryotic diversity across distinct microhabitats in an acid mine drainage. Front. Microbiol. 2017, 8, 281856. [Google Scholar] [CrossRef] [PubMed]
- Teng, W.; Liu, G.; Luo, H.; Zhang, R.; Xiang, Y. Simultaneous sulfate and zinc removal from acid wastewater using an acidophilic and autotrophic biocathode. J. Hazard. Mater. 2016, 304, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, K.; Kolmert, K.; Johnson, D.; Williams, P. A novel metabolic phenotype among acidophilic bacteria: Aromatic degradation and the potential use of these organisms for the treatment of wastewater containing organic and inorganic pollutants. In Process Metallurgy; Elsevier: Amsterdam, The Netherlands, 1999; Volume 9, pp. 719–728. [Google Scholar]
- Navarro, C.A.; von Bernath, D.; Jerez, C.A. Heavy metal resistance strategies of acidophilic bacteria and their acquisition: Importance for biomining and bioremediation. Biol. Res. 2013, 46, 363–371. [Google Scholar] [CrossRef]
- Arulazhagan, P.; Al-Shekri, K.; Huda, Q.; Godon, J.-J.; Basahi, J.M.; Jeyakumar, D. Biodegradation of polycyclic aromatic hydrocarbons by an acidophilic Stenotrophomonas maltophilia strain AJH1 isolated from a mineral mining site in Saudi Arabia. Extremophiles 2017, 21, 163–174. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Park, J.-H.; Seo, K.-H. Presence of Stenotrophomonas maltophilia exhibiting high genetic similarity to clinical isolates in final effluents of pig farm wastewater treatment plants. Int. J. Hyg. Environ. Health 2018, 221, 300–307. [Google Scholar] [CrossRef]
- Jarboui, R.; Baati, H.; Fetoui, F.; Gargouri, A.; Gharsallah, N.; Ammar, E. Yeast performance in wastewater treatment: Case study of Rhodotorula mucilaginosa. Environ. Technol. 2012, 33, 951–960. [Google Scholar] [CrossRef]
- Su, X.; Zhou, M.; Hu, P.; Xiao, Y.; Wang, Z.; Mei, R.; Hashmi, M.Z.; Lin, H.; Chen, J.; Sun, F. Whole-genome sequencing of an acidophilic Rhodotorula sp. ZM1 and its phenol-degrading capability under acidic conditions. Chemosphere 2019, 232, 76–86. [Google Scholar] [CrossRef]
- Comte, A.; Christen, P.; Davidson, S.; Pophillat, M.; Lorquin, J.; Auria, R.; Simon, G.; Casalot, L. Biochemical, transcriptional and translational evidences of the phenol-meta-degradation pathway by the hyperthermophilic Sulfolobus solfataricus 98/2. PLoS ONE 2013, 8, e82397. [Google Scholar] [CrossRef]
- Pandolfo, E.; Barra Caracciolo, A.; Rolando, L. Recent advances in bacterial degradation of hydrocarbons. Water 2023, 15, 375. [Google Scholar] [CrossRef]
- Sood, N.; Lal, B. Isolation of a novel yeast strain Candida digboiensis TERI ASN6 capable of degrading petroleum hydrocarbons in acidic conditions. J. Environ. Manag. 2009, 90, 1728–1736. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.; Zanaroli, G.; Wang, Z.; Xu, P.; Tang, H. Enhancing bioremediation potential of Pseudomonas putida by developing its acid stress tolerance with glutamate decarboxylase dependent system and global regulator of extreme radiation resistance. Front. Microbiol. 2019, 10, 2033. [Google Scholar] [CrossRef]
- Grant, C.; Woodley, J.M.; Baganz, F. Whole-cell bio-oxidation of n-dodecane using the alkane hydroxylase system of P. putida GPo1 expressed in E. coli. Enzym. Microb. Technol. 2011, 48, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Singh, S. Microbial degradation of n-hexadecane in mineral salt medium as mediated by degradative enzymes. Bioresour. Technol. 2012, 111, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Bogan, B.W.; Sullivan, W.R.; Kayser, K.J.; Derr, K.; Aldrich, H.C.; Paterek, J.R. Alkanindiges illinoisensis gen. nov., sp. nov., an obligately hydrocarbonoclastic, aerobic squalane-degrading bacterium isolated from oilfield soils. Int. J. Syst. Evol. Microbiol. 2003, 53, 1389–1395. [Google Scholar] [CrossRef]
- Dore, S.; Clancy, Q.; Rylee, S.; Kulpa, C. Naphthalene-utilizing and mercury-resistant bacteria isolated from an acidic environment. Appl. Microbiol. Biotechnol. 2003, 63, 194–199. [Google Scholar] [CrossRef]
- Shahi, A.; Aydin, S.; Ince, B.; Ince, O. Reconstruction of bacterial community structure and variation for enhanced petroleum hydrocarbons degradation through biostimulation of oil contaminated soil. Chem. Eng. J. 2016, 306, 60–66. [Google Scholar] [CrossRef]
- Uyttebroek, M.; Vermeir, S.; Wattiau, P.; Ryngaert, A.; Springael, D. Characterization of cultures enriched from acidic polycyclic aromatic hydrocarbon-contaminated soil for growth on pyrene at low pH. Appl. Environ. Microbiol. 2007, 73, 3159–3164. [Google Scholar] [CrossRef]
- Estévez, E.; Veiga, M.C.; Kennes, C. Biofiltration of waste gases with the fungi Exophiala oligosperma and Paecilomyces variotii. Appl. Microbiol. Biotechnol. 2005, 67, 563–568. [Google Scholar] [CrossRef]
- Cox, H.; Faber, B.; Van Heiningen, W.; Radhoe, H.; Doddema, H.; Harder, W. Styrene metabolism in Exophiala jeanselmei and involvement of a cytochrome P-450-dependent styrene monooxygenase. Appl. Environ. Microbiol. 1996, 62, 1471–1474. [Google Scholar] [CrossRef]
- Estévez, E.; Veiga, M.C.; Kennes, C. Biodegradation of toluene by the new fungal isolates Paecilomyces variotii and Exophiala oligosperma. J. Ind. Microbiol. Biotechnol. 2005, 32, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Bayramoğlu, G.; Çelik, G.; Arica, M.Y. Biosorption of Reactive Blue 4 dye by native and treated fungus Phanerocheate chrysosporium: Batch and continuous flow system studies. J. Hazard. Mater. 2006, 137, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Moe, W.; Kinney, K. Biodegradation of volatile organic compounds by five fungal species. Appl. Microbiol. Biotechnol. 2002, 58, 684–689. [Google Scholar]
- Johnson, D. Selective solid media for isolating and enumerating acidophilic bacteria. J. Microbiol. Methods 1995, 23, 205–218. [Google Scholar] [CrossRef]
- Beolchini, F.; Dell’Anno, A.; De Propris, L.; Ubaldini, S.; Cerrone, F.; Danovaro, R. Auto-and heterotrophic acidophilic bacteria enhance the bioremediation efficiency of sediments contaminated by heavy metals. Chemosphere 2009, 74, 1321–1326. [Google Scholar] [CrossRef]
- Pandey, S.; Fosso-Kankeu, E.; Redelinghuys, J.; Kim, J.; Kang, M. Implication of biofilms in the sustainability of acid mine drainage and metal dispersion near coal tailings. Sci. Total Environ. 2021, 788, 147851. [Google Scholar] [CrossRef]
- El Kik, O.; Lesage, G.; Zaviska, F.; Sauvêtre, A.; Heran, M.; Lestremau, F. Synergistic approach for enhanced wastewater treatment: Harnessing the potential of bioelectrochemical systems in integration with anaerobic membrane bioreactors. J. Environ. Chem. Eng. 2024, 12, 113162. [Google Scholar] [CrossRef]
- Malik, L.; Hedrich, S. Ferric iron reduction in extreme acidophiles. Front. Microbiol. 2022, 12, 818414. [Google Scholar] [CrossRef]
- Coupland, K.; Johnson, D.B. Evidence that the potential for dissimilatory ferric iron reduction is widespread among acidophilic heterotrophic bacteria. FEMS Microbiol. Lett. 2008, 279, 30–35. [Google Scholar] [CrossRef]
- Ghauri, M.A.; Okibe, N.; Johnson, D.B. Attachment of acidophilic bacteria to solid surfaces: The significance of species and strain variations. Hydrometallurgy 2007, 85, 72–80. [Google Scholar] [CrossRef]
- Sánchez-Andrea, I.; Stams, A.J.; Hedrich, S.; Ňancucheo, I.; Johnson, D.B. Desulfosporosinus acididurans sp. nov.: An acidophilic sulfate-reducing bacterium isolated from acidic sediments. Extremophiles 2015, 19, 39–47. [Google Scholar] [CrossRef]
- Sonne-Hansen, J.; Ahring, B.K. Thermodesulfobacterium hveragerdense sp. nov., and Thermodesulfovibrio islandicus sp. nov., two thermophilic sulfate reducing bacteria isolated from a Icelandic hot spring. Syst. Appl. Microbiol. 1999, 22, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Devereux, R.; Delaney, M.; Widdel, F.; Stahl, D.A. Natural relationships among sulfate-reducing eubacteria. J. Bacteriol. 1989, 171, 6689–6695. [Google Scholar] [CrossRef] [PubMed]
- Florentino, A.P.; Brienza, C.; Stams, A.J.; Sanchez-Andrea, I. Desulfurella amilsii sp. nov., a novel acidotolerant sulfur-respiring bacterium isolated from acidic river sediments. Int. J. Syst. Evol. Microbiol. 2016, 66, 1249–1253. [Google Scholar] [CrossRef]
- Bonch-Osmolovskaya, E.; Sokolova, T.; Kostrikina, N.; Zavarzin, G. Desulfurella acetivorans gen. nov. and sp. nov.—A new thermophilic sulfur-reducing eubacterium. Arch. Microbiol. 1990, 153, 151–155. [Google Scholar] [CrossRef]
- Johnson, D.B.; Rolfe, S.; Hallberg, K.B.; Iversen, E. Isolation and phylogenetic characterization of acidophilic microorganisms indigenous to acidic drainage waters at an abandoned Norwegian copper mine. Environ. Microbiol. 2001, 3, 630–637. [Google Scholar] [CrossRef]
- Gurung, A.; Chakraborty, R. The role of Acidithiobacillus ferrooxidans in alleviating the inhibitory effect of thiosulfate on the growth of acidophilic Acidiphilium species isolated from acid mine drainage samples from Garubathan, India. Can. J. Microbiol. 2009, 55, 1040–1048. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, H.; Zeng, W.; Liang, Y.; Liu, Y.; Baba, N.; Qiu, G.; Shen, L.; Fu, X.; Liu, X. The effect of the introduction of exogenous strain Acidithiobacillus thiooxidans A01 on functional gene expression, structure and function of indigenous consortium during pyrite bioleaching. Bioresour. Technol. 2011, 102, 8092–8098. [Google Scholar] [CrossRef]
- Singh, S.K.; Rahman, A.; Dixit, K.; Nath, A.; Sundaram, S. Evaluation of promising algal strains for sustainable exploitation coupled with CO2 fixation. Environ. Technol. 2016, 37, 613–622. [Google Scholar] [CrossRef]
- Dirbaz, M.; Roosta, A. Adsorption, kinetic and thermodynamic studies for the biosorption of cadmium onto microalgae Parachlorella sp. J. Environ. Chem. Eng. 2018, 6, 2302–2309. [Google Scholar] [CrossRef]
- Bütof, L.; Wiesemann, N.; Herzberg, M.; Altzschner, M.; Holleitner, A.; Reith, F.; Nies, D. Synergistic gold–copper detoxification at the core of gold biomineralisation in Cupriavidus metallidurans. Metallomics 2018, 10, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Desaunay, A.; Martins, J.M. Comparison of chemical washing and physical cell-disruption approaches to assess the surface adsorption and internalization of cadmium by Cupriavidus metallidurans CH34. J. Hazard. Mater. 2014, 273, 231–238. [Google Scholar] [CrossRef]
- Itoh, T.; Miura, T.; Sakai, H.D.; Kato, S.; Ohkuma, M.; Takashina, T. Sulfuracidifex tepidarius gen. nov., sp. nov. and transfer of Sulfolobus metallicus Huber and Stetter 1992 to the genus Sulfuracidifex as Sulfuracidifex metallicus comb. nov. Int. J. Syst. Evol. Microbiol. 2020, 70, 1837–1842. [Google Scholar] [CrossRef]
- Barrows, J.M.; Goley, E.D. Synchronized swarmers and sticky stalks: Caulobacter crescentus as a model for bacterial cell biology. J. Bacteriol. 2023, 205, e00384-22. [Google Scholar] [CrossRef]
- Hu, P.; Brodie, E.L.; Suzuki, Y.; McAdams, H.H.; Andersen, G.L. Whole-genome transcriptional analysis of heavy metal stresses in Caulobacter crescentus. J. Bacteriol. 2005, 187, 8437–8449. [Google Scholar] [CrossRef] [PubMed]
- Zouch, H.; Cabrol, L.; Chifflet, S.; Tedetti, M.; Karray, F.; Zaghden, H.; Sayadi, S.; Quéméneur, M. Effect of Acidic Industrial Effluent Release on Microbial Diversity and Trace Metal Dynamics During Resuspension of Coastal Sediment. Front. Microbiol. 2018, 9, 3103. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Khan, A.H.; Pinê Américo-Pinheiro, J.H.; Ahmad, K. Innovative and Hybrid Technologies for Wastewater Treatment and Recycling; Routledge: London, UK, 2024. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, Y.; Yang, J.; Chen, P.; Sun, Y.; Wang, M.; Ma, Y. A bioflocculant from Corynebacterium glutamicum and its application in acid mine wastewater treatment. Front. Bioeng. Biotechnol. 2023, 11, 1136473. [Google Scholar] [CrossRef]
- Li, J.; Yun, Y.-q.; Xing, L.; Song, L. Novel bioflocculant produced by salt-tolerant, alkaliphilic strain Oceanobacillus polygoni HG6 and its application in tannery wastewater treatment. Biosci. Biotechnol. Biochem. 2017, 81, 1018–1025. [Google Scholar] [CrossRef]
- Gong, W.-X.; Wang, S.-G.; Sun, X.-F.; Liu, X.-W.; Yue, Q.-Y.; Gao, B.-Y. Bioflocculant production by culture of Serratia ficaria and its application in wastewater treatment. Bioresour. Technol. 2008, 99, 4668–4674. [Google Scholar] [CrossRef]
- Picos-Corrales, L.A.; Sarmiento-Sánchez, J.I.; Ruelas-Leyva, J.P.; Crini, G.; Hermosillo-Ochoa, E.; Gutierrez-Montes, J.A. Environment-friendly approach toward the treatment of raw agricultural wastewater and river water via flocculation using chitosan and bean straw flour as bioflocculants. ACS Omega 2020, 5, 3943–3951. [Google Scholar] [CrossRef] [PubMed]
- Maliehe, T.S.; Basson, A.K.; Dlamini, N.G. Removal of pollutants in mine wastewater by a non-cytotoxic polymeric bioflocculant from Alcaligenes faecalis HCB2. Int. J. Environ. Res. Public Health 2019, 16, 4001. [Google Scholar] [CrossRef] [PubMed]
- Qiao, N.; Gao, M.; Zhang, X.; Du, Y.; Fan, X.; Wang, L.; Liu, N.; Yu, D. Trichosporon fermentans biomass flocculation from soybean oil refinery wastewater using bioflocculant produced from Paecilomyces sp. M2-1. Appl. Microbiol. Biotechnol. 2019, 103, 2821–2831. [Google Scholar] [CrossRef] [PubMed]
- Mamo, G.; Mattiasson, B. Alkaliphilic microorganisms in biotechnology. In Biotechnology of Extremophiles: Advances and Challenges; Springer: Berlin/Heidelberg, Germany, 2016; pp. 243–272. [Google Scholar]
- Bhattacharya, A.; Goyal, N.; Gupta, A. Degradation of azo dye methyl red by alkaliphilic, halotolerant Nesterenkonia lacusekhoensis EMLA3: Application in alkaline and salt-rich dyeing effluent treatment. Extremophiles 2017, 21, 479–490. [Google Scholar] [CrossRef]
- Medić, A.; Lješević, M.; Inui, H.; Beškoski, V.; Kojić, I.; Stojanović, K.; Karadžić, I. Efficient biodegradation of petroleum n-alkanes and polycyclic aromatic hydrocarbons by polyextremophilic Pseudomonas aeruginosa san ai with multidegradative capacity. RSC Adv. 2020, 10, 14060–14070. [Google Scholar] [CrossRef]
- Nourbakhsh, M.N.; Kiliçarslan, S.; Ilhan, S.; Ozdag, H. Biosorption of Cr6+, Pb2+ and Cu2+ ions in industrial waste water on Bacillus sp. Chem. Eng. J. 2002, 85, 351–355. [Google Scholar] [CrossRef]
- Horikoshi, K. Alkaliphiles: Some applications of their products for biotechnology. Microbiol. Mol. Biol. Rev. 1999, 63, 735–750. [Google Scholar] [CrossRef]
- Ke, Q.; Zhang, Y.; Wu, X.; Su, X.; Wang, Y.; Lin, H.; Mei, R.; Zhang, Y.; Hashmi, M.Z.; Chen, C. Sustainable biodegradation of phenol by immobilized Bacillus sp. SAS19 with porous carbonaceous gels as carriers. J. Environ. Manag. 2018, 222, 185–189. [Google Scholar] [CrossRef]
- Qi, Y.B.; Wang, C.Y.; Lv, C.Y.; Lun, Z.M.; Zheng, C.G. Removal Capacities of Polycyclic Aromatic Hydrocarbons (PAHs) by a Newly Isolated Strain from Oilfield Produced Water. Int. J. Environ. Res. Public Health 2017, 14, 215. [Google Scholar] [CrossRef]
- McGenity, T.J.; Whitby, C.; Fahy, A. Alkaliphilic Hydrocarbon Degraders. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1931–1937. [Google Scholar] [CrossRef]
- Kumari, S.; Regar, R.K.; Manickam, N. Improved polycyclic aromatic hydrocarbon degradation in a crude oil by individual and a consortium of bacteria. Bioresour. Technol. 2018, 254, 174–179. [Google Scholar] [CrossRef]
- Arulazhagan, P.; Vasudevan, N. Biodegradation of polycyclic aromatic hydrocarbons by a halotolerant bacterial strain Ochrobactrum sp. VA1. Mar. Pollut. Bull. 2011, 62, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Gou, Y.; Zhao, Q.; Yang, S.; Wang, H.; Qiao, P.; Song, Y.; Cheng, Y.; Li, P. Removal of polycyclic aromatic hydrocarbons (PAHs) and the response of indigenous bacteria in highly contaminated aged soil after persulfate oxidation. Ecotoxicol. Environ. Saf. 2020, 190, 110092. [Google Scholar] [CrossRef]
- Fernández-López, M.G.; Batista-García, R.A.; Aréchiga-Carvajal, E.T. Alkaliphilic/Alkali-Tolerant Fungi: Molecular, Biochemical, and Biotechnological Aspects. J. Fungi 2023, 9, 652. [Google Scholar] [CrossRef] [PubMed]
- Plate, L.; Marletta, M.A. Nitric oxide-sensing H-NOX proteins govern bacterial communal behavior. Trends Biochem. Sci. 2013, 38, 566–575. [Google Scholar] [CrossRef]
- Yu, F.-B.; Li, X.-D.; Ali, S.W.; Shan, S.-D.; Luo, L.-P.; Guan, L.-B. Further characterization of o-nitrobenzaldehyde degrading bacterium Pseudomonas sp: ONBA-17 and deduction on its metabolic pathway. Braz. J. Microbiol. 2014, 45, 1303–1308. [Google Scholar] [CrossRef]
- Mangaiyarkarasi, M.M.; Vincent, S.; Janarthanan, S.; Rao, T.S.; Tata, B. Bioreduction of Cr (VI) by alkaliphilic Bacillus subtilis and interaction of the membrane groups. Saudi J. Biol. Sci. 2011, 18, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Oie, C.S.; Albaugh, C.E.; Peyton, B.M. Benzoate and salicylate degradation by Halomonas campisalis, an alkaliphilic and moderately halophilic microorganism. Water Res. 2007, 41, 1235–1242. [Google Scholar] [CrossRef]
- Amin, S.; Rastogi, R.P.; Chaubey, M.G.; Jain, K.; Divecha, J.; Desai, C.; Madamwar, D. Degradation and toxicity analysis of a reactive textile diazo dye-Direct Red 81 by newly isolated Bacillus sp. DMS2. Front. Microbiol. 2020, 11, 576680. [Google Scholar] [CrossRef]
- Manara, A.; DalCorso, G.; Baliardini, C.; Farinati, S.; Cecconi, D.; Furini, A. Pseudomonas putida response to cadmium: Changes in membrane and cytosolic proteomes. J. Proteome Res. 2012, 11, 4169–4179. [Google Scholar] [CrossRef]
- Sorokin, D.Y.; Tourova, T.P.; Lysenko, A.M.; Mityushina, L.L.; Kuenen, J.G. Thioalkalivibrio thiocyanoxidans sp. nov. and Thioalkalivibrio paradoxus sp. nov., novel alkaliphilic, obligately autotrophic, sulfur-oxidizing bacteria capable of growth on thiocyanate, from soda lakes. Int. J. Syst. Evol. Microbiol. 2002, 52, 657–664. [Google Scholar] [CrossRef]
- Hao, X.; Mu, T.; Sharshar, M.M.; Yang, M.; Zhong, W.; Jia, Y.; Chen, Z.; Yang, G.; Xing, J. Revealing sulfate role in empowering the sulfur-oxidizing capacity of Thioalkalivibrio versutus D301 for an enhanced desulfurization process. Bioresour. Technol. 2021, 337, 125367. [Google Scholar] [CrossRef]
- Imhoff, J.F.; Kyndt, J.A.; Meyer, T.E. Genomic Comparison, Phylogeny and Taxonomic Reevaluation of the Ectothiorhodospiraceae and Description of Halorhodospiraceae fam. nov. and Halochlorospira gen. nov. Microorganisms 2022, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Vainshtein, M.; Gogotova, G.; Heinritz, N.-J. Removal of H2S by the purple sulphur bacterium Ectothiorhodospira shaposhnikovii. World J. Microbiol. Biotechnol. 1994, 10, 110–111. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; He, Z.; Hu, Y.; Hu, L.; Zhong, H. Both cell envelope and cytoplasm were the locations for chromium (VI) reduction by Bacillus sp. M6. Bioresour. Technol. 2019, 273, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Narihiro, T.; Terada, T.; Kikuchi, K.; Iguchi, A.; Ikeda, M.; Yamauchi, T.; Shiraishi, K.; Kamagata, Y.; Nakamura, K.; Sekiguchi, Y. Comparative analysis of bacterial and archaeal communities in methanogenic sludge granules from upflow anaerobic sludge blanket reactors treating various food-processing, high-strength organic wastewaters. Microbes Environ. 2009, 24, 88–96. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, Z.; Wang, Q.; Zhu, C.; Wu, Z. An anaerobic dynamic membrane bioreactor (AnDMBR) for landfill leachate treatment: Performance and microbial community identification. Bioresour. Technol. 2014, 161, 29–39. [Google Scholar] [CrossRef]
- Lee, J.; Mahandra, H.; Hein, G.A.; Ramsay, J.; Ghahreman, A. Toward sustainable solution for biooxidation of waste and refractory materials using neutrophilic and alkaliphilic microorganisms—A review. ACS Appl. Bio Mater. 2021, 4, 2274–2292. [Google Scholar] [CrossRef]
- Sharshar, M.M.; Samak, N.A.; Hao, X.; Mu, T.; Zhong, W.; Yang, M.; Peh, S.; Ambreen, S.; Xing, J. Enhanced growth-driven stepwise inducible expression system development in haloalkaliphilic desulfurizing Thioalkalivibrio versutus. Bioresour. Technol. 2019, 288, 121486. [Google Scholar] [CrossRef]
- Šoštarić, T.D.; Petrović, M.S.; Pastor, F.T.; Lončarević, D.R.; Petrović, J.T.; Milojković, J.V.; Stojanović, M.D. Study of heavy metals biosorption on native and alkali-treated apricot shells and its application in wastewater treatment. J. Mol. Liq. 2018, 259, 340–349. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Chen, M. Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater. Bioresour. Technol. 2016, 214, 836–851. [Google Scholar] [CrossRef]
- Mallick, I.; Bhattacharyya, C.; Mukherji, S.; Dey, D.; Sarkar, S.C.; Mukhopadhyay, U.K.; Ghosh, A. Effective rhizoinoculation and biofilm formation by arsenic immobilizing halophilic plant growth promoting bacteria (PGPB) isolated from mangrove rhizosphere: A step towards arsenic rhizoremediation. Sci. Total Environ. 2018, 610, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Preiss, L.; Hicks, D.B.; Suzuki, S.; Meier, T.; Krulwich, T.A. Alkaliphilic Bacteria with Impact on Industrial Applications, Concepts of Early Life Forms, and Bioenergetics of ATP Synthesis. Front. Bioeng. Biotechnol. 2015, 3, 75. [Google Scholar] [CrossRef]
- Kanekar, P.P.; Nilegaonkar, S.S.; Sarnaik, S.S.; Kelkar, A.S. Optimization of protease activity of alkaliphilic bacteria isolated from an alkaline lake in India. Bioresour. Technol. 2002, 85, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Carvajal, L.C.; Sanz-Martín, J.L.; Barragán-Huerta, B.E. Biodegradation of organic pollutants in saline wastewater by halophilic microorganisms: A review. Environ. Sci. Pollut. Res. 2014, 21, 9578–9588. [Google Scholar] [CrossRef]
- Gunjal, A.B.; Badodekar, N.P. Halophiles. In Physiology, Genomics, and Biotechnological Applications of Extremophiles; IGI Global: Hershey, PA, USA, 2022; pp. 13–34. [Google Scholar]
- Saum, S.H.; Pfeiffer, F.; Palm, P.; Rampp, M.; Schuster, S.C.; Müller, V.; Oesterhelt, D. Chloride and organic osmolytes: A hybrid strategy to cope with elevated salinities by the moderately halophilic, chloride-dependent bacterium Halobacillus halophilus. Environ. Microbiol. 2013, 15, 1619–1633. [Google Scholar] [CrossRef]
- García, M.T.; Mellado, E.; Ostos, J.C.; Ventosa, A. Halomonas organivorans sp. nov., a moderate halophile able to degrade aromatic compounds. Int. J. Syst. Evol. Microbiol. 2004, 54, 1723–1728. [Google Scholar] [CrossRef] [PubMed]
- Mainka, T.; Weirathmüller, D.; Herwig, C.; Pflügl, S. Potential applications of halophilic microorganisms for biological treatment of industrial process brines contaminated with aromatics. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab015. [Google Scholar] [CrossRef]
- Zhuang, X.; Han, Z.; Bai, Z.; Zhuang, G.; Shim, H. Progress in decontamination by halophilic microorganisms in saline wastewater and soil. Environ. Pollut. 2010, 158, 1119–1126. [Google Scholar] [CrossRef]
- Edbeib, M.F.; Wahab, R.A.; Huyop, F. Halophiles: Biology, adaptation, and their role in decontamination of hypersaline environments. World J. Microbiol. Biotechnol. 2016, 32, 1–23. [Google Scholar] [CrossRef]
- Aljerf, L. High-efficiency extraction of bromocresol purple dye and heavy metals as chromium from industrial effluent by adsorption onto a modified surface of zeolite: Kinetics and equilibrium study. J. Environ. Manag. 2018, 225, 120–132. [Google Scholar] [CrossRef]
- Xiao, Y.; Roberts, D.J. A review of anaerobic treatment of saline wastewater. Environ. Technol. 2010, 31, 1025–1043. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, O.; Moletta, R. Treatment of organic pollution in industrial saline wastewater: A literature review. Water Res. 2006, 40, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Pugazhendi, A.; Jamal, M.T.; Al-Mur, B.A.; Jeyakumar, R.B. Bioaugmentation of electrogenic halophiles in the treatment of pharmaceutical industrial wastewater and energy production in microbial fuel cell under saline condition. Chemosphere 2022, 288, 132515. [Google Scholar] [CrossRef]
- Shi, K.; Zhou, W.; Zhao, H.; Zhang, Y. Performance of halophilic marine bacteria inocula on nutrient removal from hypersaline wastewater in an intermittently aerated biological filter. Bioresour. Technol. 2012, 113, 280–287. [Google Scholar] [CrossRef]
- Huang, J.-L.; Wang, H.-H.; Alam, F.; Cui, Y.-W. Granulation of halophilic sludge inoculated with estuarine sediments for saline wastewater treatment. Sci. Total Environ. 2019, 682, 532–540. [Google Scholar] [CrossRef]
- Torbaghan, M.E.; Torghabeh, G.H.K. Biological removal of iron and sulfate from synthetic wastewater of cotton delinting factory by using halophilic sulfate-reducing bacteria. Heliyon 2019, 5, e02948. [Google Scholar] [CrossRef]
- Jamal, M.T.; Pugazhendi, A. Treatment of fish market wastewater and energy production using halophiles in air cathode microbial fuel cell. J. Environ. Manag. 2021, 292, 112752. [Google Scholar] [CrossRef]
- Sorokin, D.Y.; Van Den Bosch, P.; Abbas, B.; Janssen, A.; Muyzer, G. Microbiological analysis of the population of extremely haloalkaliphilic sulfur-oxidizing bacteria dominating in lab-scale sulfide-removing bioreactors. Appl. Microbiol. Biotechnol. 2008, 80, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Sharshar, M.M.; Samak, N.A.; Ambreen, S.; Hao, X.; Mu, T.; Maarouf, M.; Zheng, C.; Gao, Y.; Liu, Z.; Jia, Y. Improving confirmed nanometric sulfur bioproduction using engineered Thioalkalivibrio versutus. Bioresour. Technol. 2020, 317, 124018. [Google Scholar] [CrossRef]
- García, M.T.; Ventosa, A.; Mellado, E. Catabolic versatility of aromatic compound-degrading halophilic bacteria. FEMS Microbiol. Ecol. 2005, 54, 97–109. [Google Scholar] [CrossRef]
- Wernick, D.G.; Pontrelli, S.P.; Pollock, A.W.; Liao, J.C. Sustainable biorefining in wastewater by engineered extreme alkaliphile Bacillus marmarensis. Sci. Rep. 2016, 6, 20224. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Wang, W.; Tian, S.; Jiang, P.; Qi, Q.; Li, F.; Li, H.; Wang, Q.; Li, H. Potential biodegradation of phenanthrene by isolated halotolerant bacterial strains from petroleum oil polluted soil in Yellow River Delta. Sci. Total Environ. 2019, 664, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Phan, H.V.; Hai, F.I.; Price, W.E.; Guo, W.; Ngo, H.H.; Yamamoto, K.; Nghiem, L.D. Effects of salinity build-up on the performance and bacterial community structure of a membrane bioreactor. Bioresour. Technol. 2016, 200, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Wang, D.; Zou, Y.; Tian, J.; Tian, Y.; Liao, X. Glycine betaine enhances biodegradation of phenol in high saline environments by the halophilic strain Oceanobacillus sp. PT-20. RSC Adv. 2019, 9, 29205–29216. [Google Scholar] [CrossRef] [PubMed]
- Mandree, P.; Masika, W.; Naicker, J.; Moonsamy, G.; Ramchuran, S.; Lalloo, R. Bioremediation of polycyclic aromatic hydrocarbons from industry contaminated soil using indigenous Bacillus spp. Processes 2021, 9, 1606. [Google Scholar] [CrossRef]
- Maharaja, P.; Boopathy, R.; Anushree, V.; Mahesh, M.; Swarnalatha, S.; Ravindran, B.; Chang, S.W.; Sekaran, G. Bio removal of proteins, lipids and mucopolysaccharides in tannery hyper saline wastewater using halophilic bacteria. J. Water Process Eng. 2020, 38, 101674. [Google Scholar] [CrossRef]
- Sorokin, D.Y.; Messina, E.; Smedile, F.; La Cono, V.; Hallsworth, J.E.; Yakimov, M.M. Carbohydrate-dependent sulfur respiration in halo (alkali) philic archaea. Environ. Microbiol. 2021, 23, 3789–3808. [Google Scholar] [CrossRef]
- Bryantseva, I.; Tourova, T.; Kovaleva, O.; Kostrikina, N.; Gorlenko, V. Ectothiorhodospira magna sp. nov., a new large alkaliphilic purple sulfur bacterium. Microbiology 2010, 79, 780–790. [Google Scholar] [CrossRef]
- Freel, K.C.; Krueger, M.C.; Farasin, J.; Brochier-Armanet, C.; Barbe, V.; Andres, J.; Cholley, P.-E.; Dillies, M.-A.; Jagla, B.; Koechler, S. Adaptation in toxic environments: Arsenic genomic islands in the bacterial genus Thiomonas. PLoS ONE 2015, 10, e0139011. [Google Scholar] [CrossRef]
- Sher, S.; Hussain, S.Z.; Rehman, A. Phenotypic and genomic analysis of multiple heavy metal–resistant Micrococcus luteus strain AS2 isolated from industrial waste water and its potential use in arsenic bioremediation. Appl. Microbiol. Biotechnol. 2020, 104, 2243–2254. [Google Scholar] [CrossRef]
- Pugazhendi, A.; Al-Mutairi, A.E.; Jamal, M.T.; Jeyakumar, R.B.; Palanisamy, K. Treatment of seafood industrial wastewater coupled with electricity production using air cathode microbial fuel cell under saline condition. Int. J. Energy Res. 2020, 44, 12535–12545. [Google Scholar] [CrossRef]
- Cui, Y.; Cui, Y.-W.; Huang, J.-L. A novel halophilic Exiguobacterium mexicanum strain removes nitrogen from saline wastewater via heterotrophic nitrification and aerobic denitrification. Bioresour. Technol. 2021, 333, 125189. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.A.; Tawabini, B.; Nazal, M.; AlThaqfi, J.; Khalil, A. Efficiency of thermophilic bacteria in wastewater treatment. Arab. J. Sci. Eng. 2021, 46, 123–128. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Bertanza, G.; Abbà, A.; Torretta, V.; Katsoyiannis, I.A. Wastewater treatment by means of thermophilic aerobic membrane reactors: Respirometric tests and numerical models for the determination of stoichiometric/kinetic parameters. Environ. Technol. 2019, 40, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Atalah, J.; Cáceres-Moreno, P.; Espina, G.; Blamey, J.M. Thermophiles and the applications of their enzymes as new biocatalysts. Bioresour. Technol. 2019, 280, 478–488. [Google Scholar] [CrossRef]
- Fontanier, V.; Farines, V.; Albet, J.; Baig, S.; Molinier, J. Study of catalyzed ozonation for advanced treatment of pulp and paper mill effluents. Water Res. 2006, 40, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Lin, Z.; Wang, Y.; Zhao, P.; Kuang, F.; Zhou, J. Coupling of thermophilic biofilm-based systems and ozonation for enhanced organics removal from high-temperature pulping wastewater: Performance, microbial communities, and pollutant transformations. Sci. Total Environ. 2020, 714, 136802. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Campoy, R.A.; Álvarez-Gallego, C.; García, L.R. Enhancement in hydrogen production by thermophilic anaerobic co-digestion of organic fraction of municipal solid waste and sewage sludge–optimization of treatment conditions. Bioresour. Technol. 2014, 164, 408–415. [Google Scholar] [CrossRef]
- Chauhan, P.S. Role of various bacterial enzymes in complete depolymerization of lignin: A review. Biocatal. Agric. Biotechnol. 2020, 23, 101498. [Google Scholar] [CrossRef]
- Behbahani, M.; Mohabatkar, H.; Nosrati, M. Analysis and comparison of lignin peroxidases between fungi and bacteria using three different modes of Chou’s general pseudo amino acid composition. J. Theor. Biol. 2016, 411, 1–5. [Google Scholar] [CrossRef]
- Sasaki, K.; Morita, M.; Sasaki, D.; Nagaoka, J.; Matsumoto, N.; Ohmura, N.; Shinozaki, H. Syntrophic degradation of proteinaceous materials by the thermophilic strains Coprothermobacter proteolyticus and Methanothermobacter thermautotrophicus. J. Biosci. Bioeng. 2011, 112, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, M.; Braguglia, C.; Gianico, A.; Mininni, G.; Nakamura, K.; Rossetti, S. Thermophilic anaerobic digestion of thermal pretreated sludge: Role of microbial community structure and correlation with process performances. Water Res. 2015, 68, 498–509. [Google Scholar] [CrossRef]
- Zverlov, V.V.; Schwarz, W.H. Bacterial Cellulose Hydrolysis in Anaerobic Environmental Subsystems—Clostridium thermocellum and Clostridium stercorarium, Thermophilic Plant-fiber Degraders. Ann. N. Y. Acad. Sci. 2008, 1125, 298–307. [Google Scholar] [CrossRef]
- Sanchez-Ledesma, L.M.; Ramírez-Malule, H.; Rodríguez-Victoria, J.A. Volatile fatty acids production by acidogenic fermentation of wastewater: A bibliometric analysis. Sustainability 2023, 15, 2370. [Google Scholar] [CrossRef]
- Blumer-Schuette, S.E.; Ozdemir, I.; Mistry, D.; Lucas, S.; Lapidus, A.; Cheng, J.-F.; Goodwin, L.A.; Pitluck, S.; Land, M.L.; Hauser, L.J. Complete genome sequences for the anaerobic, extremely thermophilic plant biomass-degrading bacteria Caldicellulosiruptor hydrothermalis, Caldicellulosiruptor kristjanssonii, Caldicellulosiruptor kronotskyensis, Caldicellulosiruptor owensensis, and Caldicellulosiruptor lactoaceticus. J. Bacteriol. 2011, 193, 1483–1484. [Google Scholar] [PubMed]
- Jiang, C.; Peces, M.; Andersen, M.H.; Kucheryavskiy, S.; Nierychlo, M.; Yashiro, E.; Andersen, K.S.; Kirkegaard, R.H.; Hao, L.; Høgh, J. Characterizing the growing microorganisms at species level in 46 anaerobic digesters at Danish wastewater treatment plants: A six-year survey on microbial community structure and key drivers. Water Res. 2021, 193, 116871. [Google Scholar] [CrossRef]
- Vítězová, M.; Kohoutová, A.; Vítěz, T.; Hanišáková, N.; Kushkevych, I. Methanogenic microorganisms in industrial wastewater anaerobic treatment. Processes 2020, 8, 1546. [Google Scholar] [CrossRef]
- Brannan, D.K.; Caldwell, D.E. Thermothrix thiopara: Growth and metabolism of a newly isolated thermophile capable of oxidizing sulfur and sulfur compounds. Appl. Environ. Microbiol. 1980, 40, 211–216. [Google Scholar] [CrossRef]
- Afzal Ghauri, M.; Johnson, D.B. Physiological diversity amongst some moderately thermophilic iron-oxidising bacteria. FEMS Microbiol. Lett. 1991, 85, 327–333. [Google Scholar] [CrossRef]
- Etique, M.; Jorand, F.d.r.P.; Zegeye, A.; Grégoire, B.; Despas, C.; Ruby, C. Abiotic process for Fe (II) oxidation and green rust mineralization driven by a heterotrophic nitrate reducing bacteria (Klebsiella mobilis). Environ. Sci. Technol. 2014, 48, 3742–3751. [Google Scholar] [CrossRef]
- Zeldes, B.M.; Loder, A.J.; Counts, J.A.; Haque, M.; Widney, K.A.; Keller, L.M.; Albers, S.V.; Kelly, R.M. Determinants of sulphur chemolithoautotrophy in the extremely thermoacidophilic Sulfolobales. Environ. Microbiol. 2019, 21, 3696–3710. [Google Scholar] [CrossRef]
- Song, H.; Choi, O.; Pandey, A.; Kim, Y.G.; Joo, J.S.; Sang, B.-I. Simultaneous production of methane and acetate by thermophilic mixed culture from carbon dioxide in bioelectrochemical system. Bioresour. Technol. 2019, 281, 474–479. [Google Scholar] [CrossRef] [PubMed]
- De Vrieze, J.; Smet, D.; Klok, J.; Colsen, J.; Angenent, L.T.; Vlaeminck, S.E. Thermophilic sludge digestion improves energy balance and nutrient recovery potential in full-scale municipal wastewater treatment plants. Bioresour. Technol. 2016, 218, 1237–1245. [Google Scholar] [CrossRef]
- Wollmann, F.; Dietze, S.; Ackermann, J.U.; Bley, T.; Walther, T.; Steingroewer, J.; Krujatz, F. Microalgae wastewater treatment: Biological and technological approaches. Eng. Life Sci. 2019, 19, 860–871. [Google Scholar] [CrossRef]
- Ostroumov, S.; Shestakova, T.; Tropin, I. Biosorption of copper by biomass of extremophilic algae. Russ. J. Gen. Chem. 2015, 85, 2961–2964. [Google Scholar] [CrossRef]
- Shen, L.; Wang, J.; Li, Z.; Fan, L.; Chen, R.; Wu, X.; Li, J.; Zeng, W. A high-efficiency Fe2O3@ Microalgae composite for heavy metal removal from aqueous solution. J. Water Process Eng. 2020, 33, 101026. [Google Scholar] [CrossRef]
- De-Bashan, L.E.; Bashan, Y. Immobilized microalgae for removing pollutants: Review of practical aspects. Bioresour. Technol. 2010, 101, 1611–1627. [Google Scholar] [CrossRef]
- Leong, Y.K.; Huang, C.-Y.; Chang, J.-S. Pollution prevention and waste phycoremediation by algal-based wastewater treatment technologies: The applications of high-rate algal ponds (HRAPs) and algal turf scrubber (ATS). J. Environ. Manag. 2021, 296, 113193. [Google Scholar] [CrossRef]
- Maad, A.; Aljerf, L.; AlJerf, A.; Dayoob, C.; Sukkar, A.; Alajlani, M. A Cross-Sectional Study on Demographic Characteristics, Nutritional Knowledge, and Supplement Use Patterns. Int. J. Kinesiol. Sports Sci. 2024, 12, 21–30. [Google Scholar] [CrossRef]
- Al-Jabri, H.; Das, P.; Khan, S.; Thaher, M.; AbdulQuadir, M. Treatment of wastewaters by microalgae and the potential applications of the produced biomass—A review. Water 2020, 13, 27. [Google Scholar] [CrossRef]
- Cheng, F.; Mallick, K.; Gedara, S.M.H.; Jarvis, J.M.; Schaub, T.; Jena, U.; Nirmalakhandan, N.; Brewer, C.E. Hydrothermal liquefaction of Galdieria sulphuraria grown on municipal wastewater. Bioresour. Technol. 2019, 292, 121884. [Google Scholar] [CrossRef]
- Pleissner, D.; Händel, N. Algae Cultivation as Measure for the Sanitation of Organic Waste—A Case Study Based on the Alga Galdieria sulphuraria Grown on Food Waste Hydrolysate in a Continuous Flow Culture. Sustainability 2023, 15, 14313. [Google Scholar] [CrossRef]
- Guo, G.; Hao, J.; Tian, F.; Liu, C.; Ding, K.; Zhang, C.; Yang, F.; Xu, J. Decolorization of Metanil Yellow G by a halophilic alkalithermophilic bacterial consortium. Bioresour. Technol. 2020, 316, 123923. [Google Scholar] [CrossRef] [PubMed]
- Renuka, N.; Sood, A.; Prasanna, R.; Ahluwalia, A. Phycoremediation of wastewaters: A synergistic approach using microalgae for bioremediation and biomass generation. Int. J. Environ. Sci. Technol. 2015, 12, 1443–1460. [Google Scholar] [CrossRef]
- Qu, W.; Zhang, C.; Zhang, Y.; Ho, S.-H. Optimizing real swine wastewater treatment with maximum carbohydrate production by a newly isolated indigenous microalga Parachlorella kessleri QWY28. Bioresour. Technol. 2019, 289, 121702. [Google Scholar] [CrossRef] [PubMed]
- Alalawy, A.I.; Yang, Y.; Almutairi, F.M.; El Rabey, H.A.; Al-Duais, M.A.; Abomohra, A.; Salama, E.-S. Freshwater microalgae-based wastewater treatment under abiotic. AIMS Environ. Sci. 2023, 10, 504–515. [Google Scholar] [CrossRef]
- Hena, S.; Gutierrez, L.; Croué, J.-P. Removal of pharmaceutical and personal care products (PPCPs) from wastewater using microalgae: A review. J. Hazard. Mater. 2021, 403, 124041. [Google Scholar] [CrossRef]



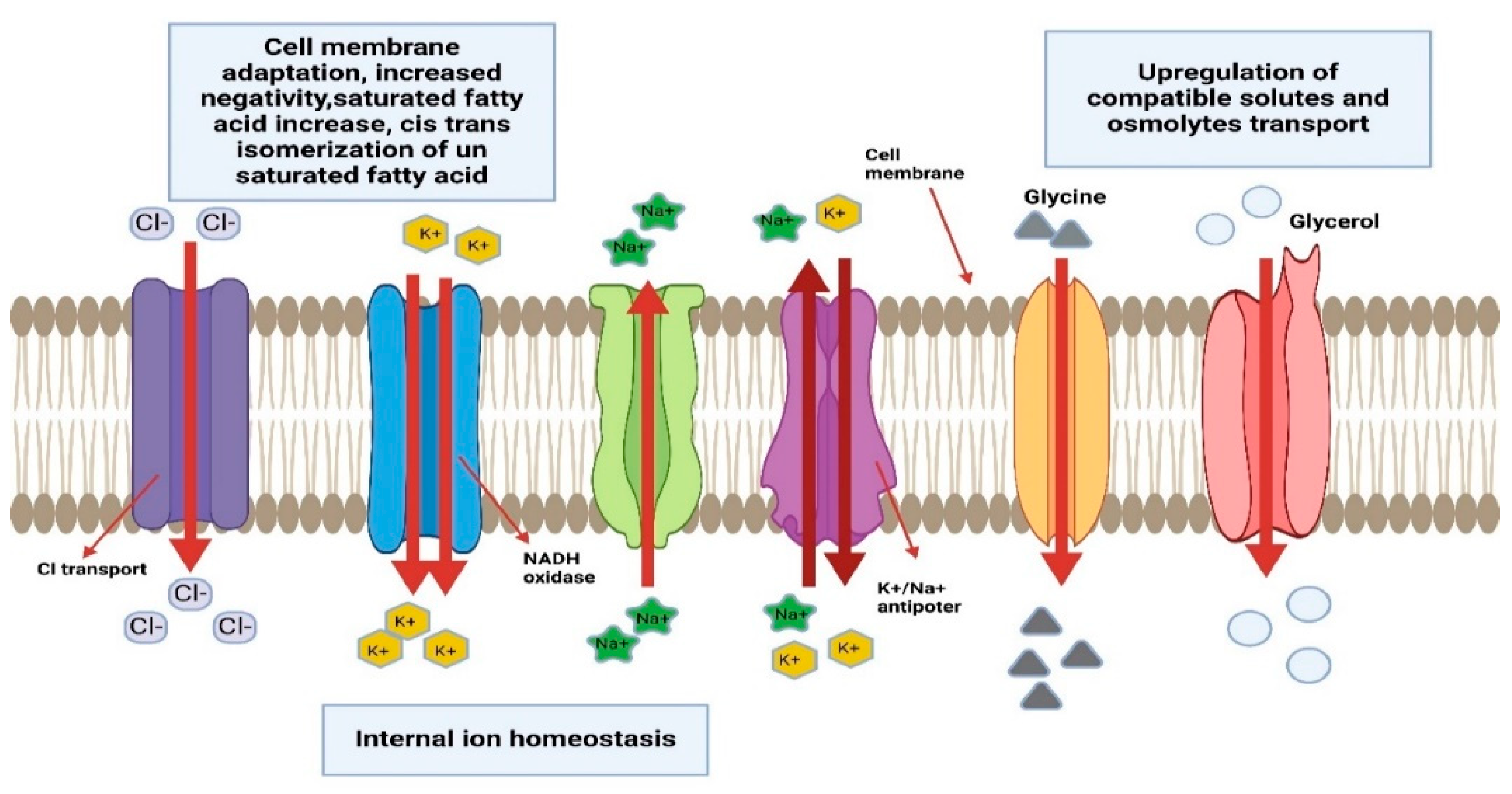
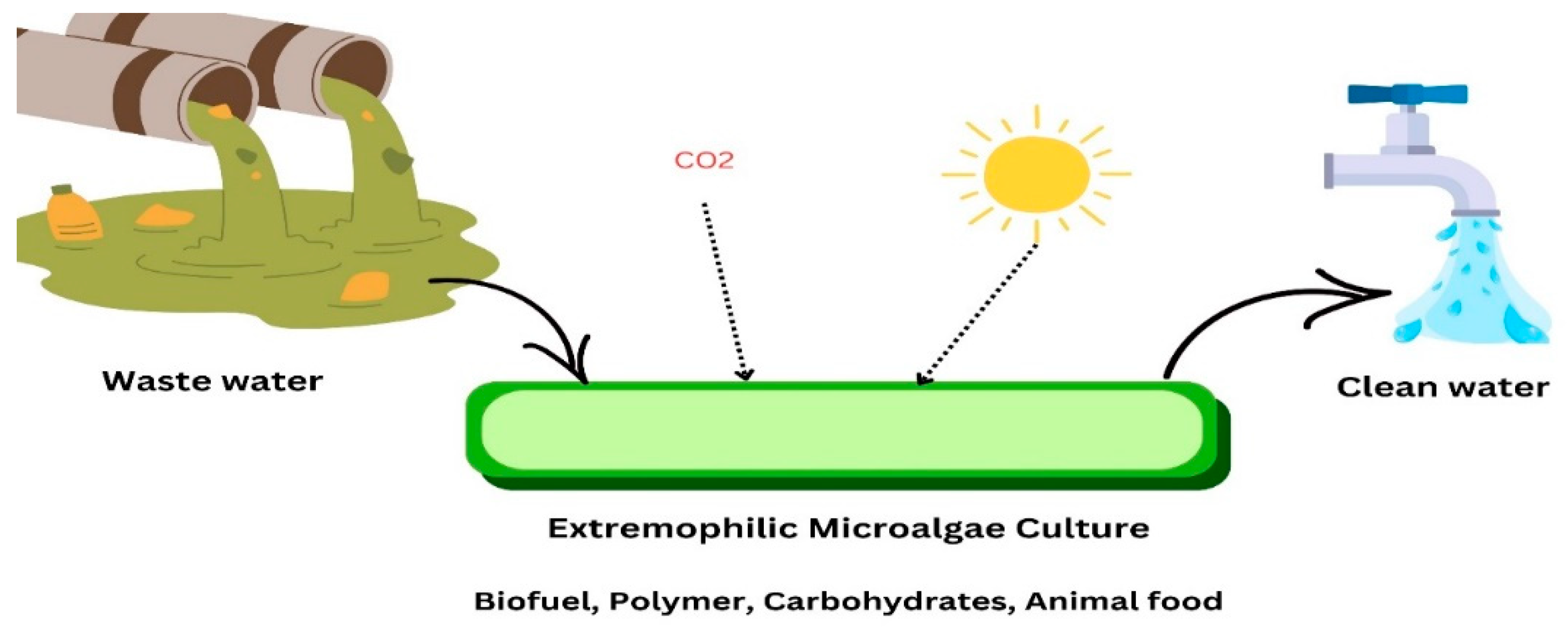
| Domain | Acidophiles | Organic Pollutants | Degradation % | Duration | References |
|---|---|---|---|---|---|
| Bacteria | Acidocella sp. WJB-3 | Aliphatic acid (dodecanoic acid) | 85% | 7 days | [67] |
| Pseudomonas putida S16(genetically modified) | Benzoate | 90% | 5 days | [66] | |
| Acidosphaera sp. C197 | Dodecane, Hexadecane | 75% | 10 days | [68] | |
| Acidocella sp. LGS-3 | Dodecane, hexadecane | 78% | 10 days | [69] | |
| Acidocella sp. IS10 | Naphthalene | 80% | 6 days | [70] | |
| Stenotrophomonas maltophilia strain AJH1, Acidiphilium | PAHs | 82% | 12 days | [59] | |
| Acidiphilium, Acidocella, Acidisphaera | Petroleum oil | 87% | 14 days | [71] | |
| Mycobacterium montefiorense | Phenanthrene, pyrene | 85% | 8 days | [72] | |
| Archaeon | S. solfataricus 98/2 | Phenol | 70% | 5 days | [63] |
| Yeast | Zymomonas mobilis (ZM1) | Phenol | 72% | 4 days | [62] |
| Candida digboiensis TERI ASN6 | Total petroleum hydrocarbons (TPHs) | 90% | 15 days | [65] | |
| Fungi | Exophiala oligosperma | Toluene | 80% | 6 days | [73] |
| Exophiala jeanselmei | Styrene | 85% | 7 days | [74] | |
| Paecilomyces variotii | Toluene | 83% | 6 days | [75] | |
| Phanerocheate chrysosporium | Toluene | 88% | 8 days | [76] | |
| Exophiala lecanii-corni | Toluene, ethylbenzene, benzene, styrene | 90% | 10 days | [77] |
| Domain | Alkaliphiles | Pollutants | Removal % | Duration | References |
|---|---|---|---|---|---|
| Organic Pollutants | |||||
| Bacteria | Citricoccus alkalitolerans CSB1 | Tannery effluents (COD, BOD, total chromium) | 80–85% | 10 days | [38] |
| Bacillus sp. | Phenol | 90% | 7 days | [112,113,114] | |
| Pseudomonas and Bacillus strains | PAHs (Polycyclic Aromatic Hydrocarbons) | 75–88% | 12–15 days | [115,116,117,118] | |
| Halanaerobium lacipiscis | NOx-N, NH3-N | 80% | 14 days | [119] | |
| Halomonas, Marinobacter | High salinity and heavy metals | 70–85% | 12 days | [36] | |
| Pseudomonas putida | o-nitro-benzaldehyde (ONBA) | 85% | 8 days | [120] | |
| Alkaliphilic Bacillus sp. | Chromium (Cr VI) | 90% | 6 days | [121] | |
| Halomonas campisalis | Benzoate and Salicylate | 88% | 10 days | [122] | |
| Bacillus sp. Bacillus circulans | Textile dyes | 80% | 12 days | [123] | |
| Archaea | Alkaliphilic Pseudomonas sp. | Industrial effluents | 75% | 14 days | [124] |
| Bacteria | Thioalkalivibrio spp. (Thioalkalivibrio halophilus, Thioalkalivibrio versutus) | Sulfides, polysulfides, thiosulfates, tetrathionates, thiocyanates | 80–85% | 15 days | [125,126] |
| Ectothiorhodospira magna, Ectothiorhodospira shaposhnikovii | Zinc, lead, copper (via sulfide oxidation to sulfur) | 90% | 7 days | [127,128] | |
| Bacillus spp. | Cr6+ (Chromium reduction to Cr3+) | 80% | [129] | ||
| Archaea | Methanobacteria and Methanomicrobia | Methanogenesis in high-strength organic wastewaters | 75% CH4 yield | 20 days | [130] |
| Methanosarcina | methanogenesis and ammonia oxidation | 18 days | 400 mg/L COD | [131] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anabtawi, H.M.; Ikhlaq, A.; Kumar, S.; Rafique, S.; Aly Hassan, A. Addressing Challenges for Eco-Friendly and Sustainable Wastewater Treatment Solutions Using Extremophile Microorganisms. Sustainability 2025, 17, 2339. https://doi.org/10.3390/su17062339
Anabtawi HM, Ikhlaq A, Kumar S, Rafique S, Aly Hassan A. Addressing Challenges for Eco-Friendly and Sustainable Wastewater Treatment Solutions Using Extremophile Microorganisms. Sustainability. 2025; 17(6):2339. https://doi.org/10.3390/su17062339
Chicago/Turabian StyleAnabtawi, Hassan Mohamad, Amir Ikhlaq, Sandeep Kumar, Safa Rafique, and Ashraf Aly Hassan. 2025. "Addressing Challenges for Eco-Friendly and Sustainable Wastewater Treatment Solutions Using Extremophile Microorganisms" Sustainability 17, no. 6: 2339. https://doi.org/10.3390/su17062339
APA StyleAnabtawi, H. M., Ikhlaq, A., Kumar, S., Rafique, S., & Aly Hassan, A. (2025). Addressing Challenges for Eco-Friendly and Sustainable Wastewater Treatment Solutions Using Extremophile Microorganisms. Sustainability, 17(6), 2339. https://doi.org/10.3390/su17062339









