1. Introduction
The application of energetic materials in the mining sector represents the most time-efficient and cost-effective method for extracting hard rock. Despite its advantages, the underlying mechanisms of explosive-induced rock fragmentation are associated with a range of adverse impacts. The interplay of shock waves and the rapid expansion of large volumes of detonation gasses effectively fractures the rock [
1]. Yet, this process significantly contributes to environmental disturbances in the vicinity and impacts the natural ecosystem [
2,
3,
4].
A characteristic feature of energetic materials that are applied in the mining industry is the generation of substantial quantities of gaseous byproducts during detonation, predominantly carbon monoxide, carbon dioxide, and nitrogen oxides. The specific chemical composition of each explosive type results in varying emissions of these gasses [
5,
6]. These byproducts pose risks to worker health due to their toxicity [
7,
8,
9] and are recognized as environmental pollutants [
10,
11,
12,
13]. The risks to workers’ health are particularly pronounced in underground blasting operations, where blast fumes infiltrate the ventilation systems [
14,
15,
16], and in deep open-pit mines, where the natural dispersal of these fumes may be hindered [
17]. Blasting emissions are regarded as air pollutants in both surface and underground mining, with the environmental impact being more significant in surface operations due to the large-scale detonation of energetic materials in single events, releasing concentrated gaseous byproducts directly into the atmosphere.
The most commonly employed energetic materials in modern mining are ammonium nitrate fuel oil (ANFO), emulsion, and dynamite [
18,
19,
20]. The choice of energetic material for surface mining is generally informed by factors such as detonation velocity, thermal energy, density, impedance, and brisance [
21,
22]; the mechanical and geological properties of the rock (e.g., Protodyakonov coefficient, tensile strength, density, impedance, and fracture patterns) [
23,
24,
25]; parameters dependent on both the rock mass and explosives (e.g., powder factor) [
26]; mining conditions (e.g., water table depth, drilling configurations, and desired fragmentation) [
27]; and economic considerations [
28]. However, the production of gaseous detonation byproducts is seldom factored into the selection process. Furthermore, existing regulatory standards impose limits on permissible gas emissions (fumes) exclusively for underground mining [
29]. Consequently, fumes from blasting in open-pit mines are often overlooked, limiting the environmental sustainability of such operations.
Research into blasting emissions in open-pit mining is relatively sparse, likely due to the lower direct exposure of workers to these fumes. Existing studies predominantly focus on dust generation, dispersion, and mitigation [
30,
31,
32,
33]. Nonetheless, investigations into the emissions of carbon and nitrogen oxides from blasting operations have yielded insights into their measurement and reduction. For example, Oluwoye et al. [
12] quantified nitrogen oxide emissions from ammonium nitrate (V) (AN) based explosives. Zvyagintseva et al. [
34] explored the formation of dust and gaseous pollutants, including carbon monoxide and nitrogen oxides, during blasting in the Mikhailovsky GOK pit. Hosseini and Pourmirzaee employed integrated Monte Carlo simulations and artificial neural networks to predict dust dispersion during bench blasting [
30]. McCray [
35] utilized small unmanned aerial systems to measure nitrogen oxide emissions in large-scale blasting events, while Bui et al. [
36] investigated the potential of unmanned aerial vehicles for topographic mapping and air quality monitoring, including CO and NOx levels, in quarry environments.
Technological innovations for monitoring and mitigating gaseous emissions have also been explored. Dinchev and Gorbounov [
37] proposed the deployment of microelectromechanical sensors to detect carbon monoxide and nitrogen dioxide in open-pit atmospheres. Abdollahisharif et al. [
10] advocated for a biocompatible blasting approach incorporating calcium hydroxide to mitigate NOx and CO emissions. Similarly, Tverda et al. [
38] evaluated borehole stemming using zeolite-based sorption technologies for gas neutralization and dust suppression. Additional studies, such as those by Yi et al. [
39] and Solixov et al. [
40], examined innovative approaches to reduce toxic gas emissions during ANFO-based blasting. Konorev and Nesterenko [
41] discussed atmospheric normalization strategies in open-pit mines, emphasizing the removal of toxic gasses generated by blasting. Moreover, Biessikirski et al. [
29] investigated the composition and toxicity of blasting fumes, assessing their implications for industrial mining applications [
42].
This study aims to assess the emissions of carbon and nitrogen oxides from typical explosives employed in open-pit mining, with a focus on the influence of chemical composition on atmospheric pollution. The analysis is conducted using a gypsum mine case study, comparing the environmental impact of various energetic materials under identical mining conditions to propose strategies for reducing the release of toxic oxides. This approach holds significant importance when considering the total annual mass of energetic materials undergoing decomposition and possible fume emission from their detonation. According to the most recent data, in 2022, approximately 23.61 million tons of energetic materials were utilized in Polish open-pit mining. This total included 9.24 million tons of ANFO, 14.17 million tons of emulsion explosives, and 0.13 million tons of dynamite as a possible source of fumes [
43]. By identifying the significant influence of chemical composition and the priming method, the study offers pathways for reducing emissions as well as highlights the importance of optimized formulations and operational strategies, particularly in large-scale blasting operations.
3. Results and Discussion
Table 2 provides a comparative analysis of the fume emissions associated with various types of energetic materials. Emulsion energetic materials demonstrated the lowest emissions of CO
2 and CO, approximately 114.8 and 4.1 dm
3·kg
−1, respectively, when compared to other tested materials. Correspondingly, the total CO
x emissions were the lowest, accompanied by NOx emissions of approximately 0.55 dm
3·kg
−1. In contrast, research dynamite and ANFO exhibited significantly higher total COx emissions, measuring 157.0 dm
3·kg
−1 and 160.9 dm
3·kg
−1, respectively. Their NO
x emissions were also elevated, with dynamite emitting approximately 1.39 dm
3·kg
−1, and ANFO producing 13.1 dm
3·kg
−1. The observed discrepancies in fume emissions, among the energetic materials, are primarily attributed to variations in their chemical compositions. While energetic materials are typically designed to achieve a zero oxygen balance, aimed at optimizing energy output and minimizing fumes, the specific chemical constituents and their proportions significantly influence the resultant fumes volume. ANFO is conventionally formulated by blending AN with FO in a ratio of 94:6 (wt.). This composition, characterized by a high AN content compared to other tested types of energetic materials, leads to the highest NOx emissions (approximately 13.1 dm
3·kg
−1). Elevated CO levels (16.4 dm
3·kg
−1) may arise from oxygen deficiency or inadequate homogenization during the blending process. On the other hand, emulsions are typically formulated by blending AN, FO, water, and an emulsifier in a ratio of 78.5:4.8:15.0:1.7 (wt.%). These materials exhibit the lowest CO and CO
2 emissions due to the optimized composition and efficient decomposition mechanisms, as governed by decomposition rules such as Springall Roberts or Kistiakowski-Wilson [
46]. Dynamite is a complex blend of AN, nitrocellulose, nitroglycerin, nitroglycol, fuels, and modifiers in proportions such as 71.29:0.7:13.2:8.8:7.0:0.01 (wt.%). Its CO
2 emissions (151.3 dm
3·kg
−1) are attributed to the high carbon content from its components. From all tested samples, TNT is characterized by the highest CO
x emissions (approximately 931.1 dm
3·kg
−1), primarily as CO
2 and CO, due to its oxygen-deficient composition and a negative oxygen balance (approximately −74.0%). This factor can have a significant impact on the total fume emission, either from one blasting series or from all blasted series in one year.
Given the total ANFO mass (89,711.54 kg) and the densities of individual materials 0.82 kg·dm
−3 for ANFO, 1.50 kg·dm
−3 for dynamite, and 1.11 kg·dm
−3 for emulsions the total mass of each explosive type was calculated, as presented in
Table 3. Emulsion density was set up at 40 min of gasification at 20 °C, which is in line with Kramarczyk et al. findings [
47].
The fume emissions presented in
Table 4 and
Figure 1 were assessed under two scenarios. The first scenario reflects actual conditions, involving the blasting of 2059 boreholes with approximately 89.71 tons of ANFO over the course of one year. The second scenario is based on the calculated equivalent mass of energetic materials (emulsion and dynamite) to ANFO, as detailed in
Table 3.
For ANFO and emulsion, the volume of fumes emitted includes contributions from the primary charge (2059 kg of TNT). In contrast, for blasting operations using dynamite, initiation is conducted exclusively with a detonator. Consequently, no additional initiation charge is employed. As a result, the fume volumes emitted from dynamite decomposition, as provided in
Table 4, are calculated solely based on the mass of dynamite used.
Table 4 and
Figure 1 and
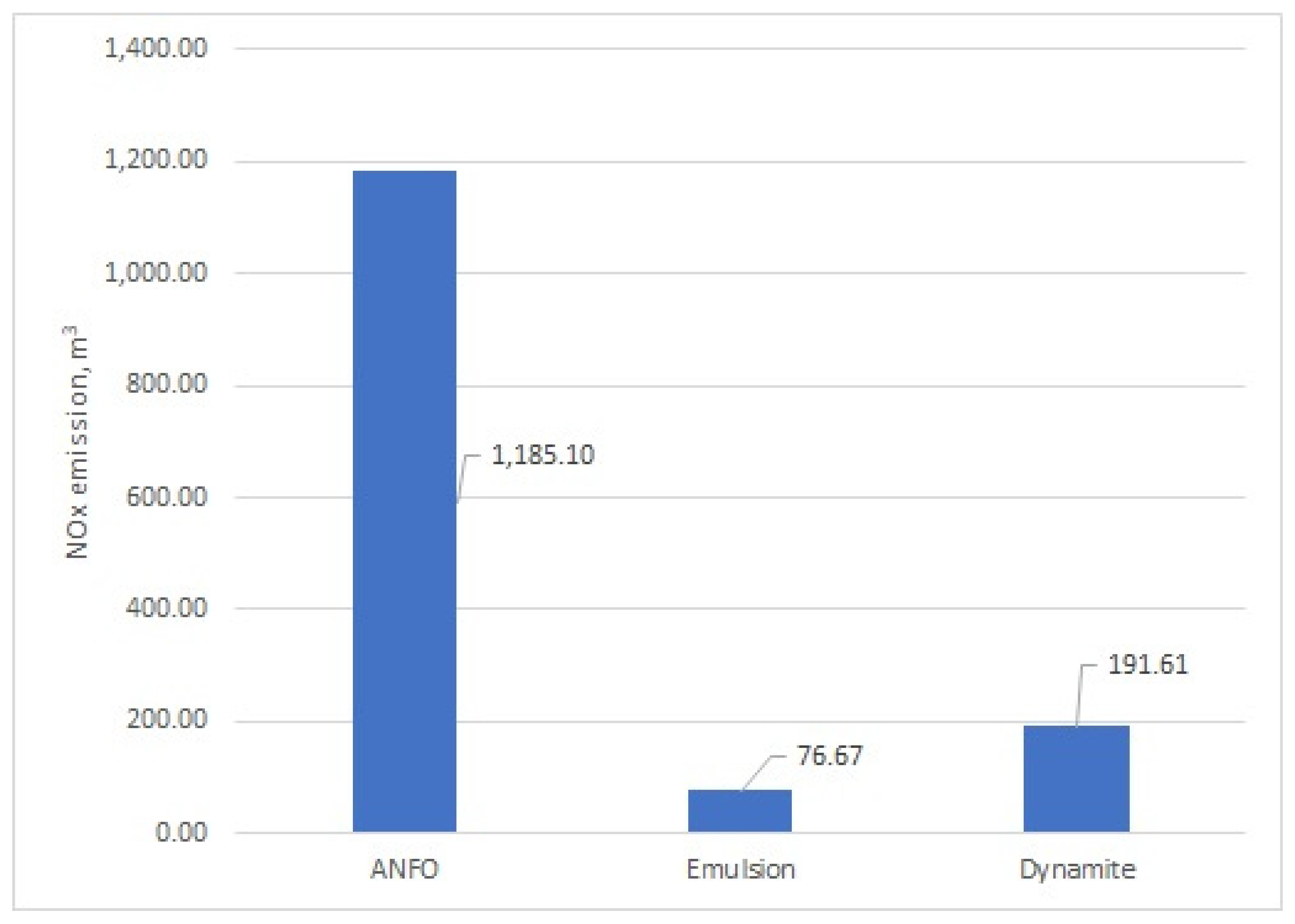
Figure 2 demonstrate the scale effect of the energetic material mass on overall fume emissions from the blasting process in an open-pit mine. The results indicate that the lowest emissions of CO
x (16,356.21 m
3) and NO
x (76.67 m
3), totaling approximately 16,432.88 m
3, were achieved using emulsion-type energetic materials. In contrast, the highest emissions of CO
x and NO
x were observed for dynamite, with a total of approximately 21,834.07 m
3. Notably, this dynamite emission level was calculated, without the additional use of a TNT booster. Blasting operations using ANFO resulted in an intermediate level of emissions, totaling approximately 17,536.83 m
3, comprising 14,856.77 m
3 tons of CO
2, 1494.95 m
3 of CO, and 1185.10 m
3 of NO
x These results confirm the influence of the chemical composition of energetic materials and scale effect on fume emissions.
Furthermore,
Table 2 highlights that the use of a 1 kg TNT booster per borehole contributes 1893.46 m
3 of CO
2, 23.68 m
3 of CO, and 9.88 m
3 of NO
x. This contribution represents 14.6% and 13.6% of the total CO
2 emissions, 1.6% and 4.8% of CO emissions, and 0.8% and 14.8% of NO
x emissions for ANFO and emulsion, respectively.
A shift in priming from TNT to dynamite, which is occasionally implemented at blast sites, would reduce fume emissions. In the evaluated case study, the use of 1 kg of dynamite per borehole would generate 311.53 m
3 of CO
2, 11.74 m
3 of CO, and 2.86 m
3 of NO
x (
Table 2). This represents reductions of approximately 6-fold for CO
2, 2-fold for CO, and 3.5-fold for NO
x compared to emissions from the equivalent mass of TNT. In open-pit mining operations, where tens to hundreds of thousands of primers are utilized, a change in priming materials has the potential to significantly reduce overall fume emissions.
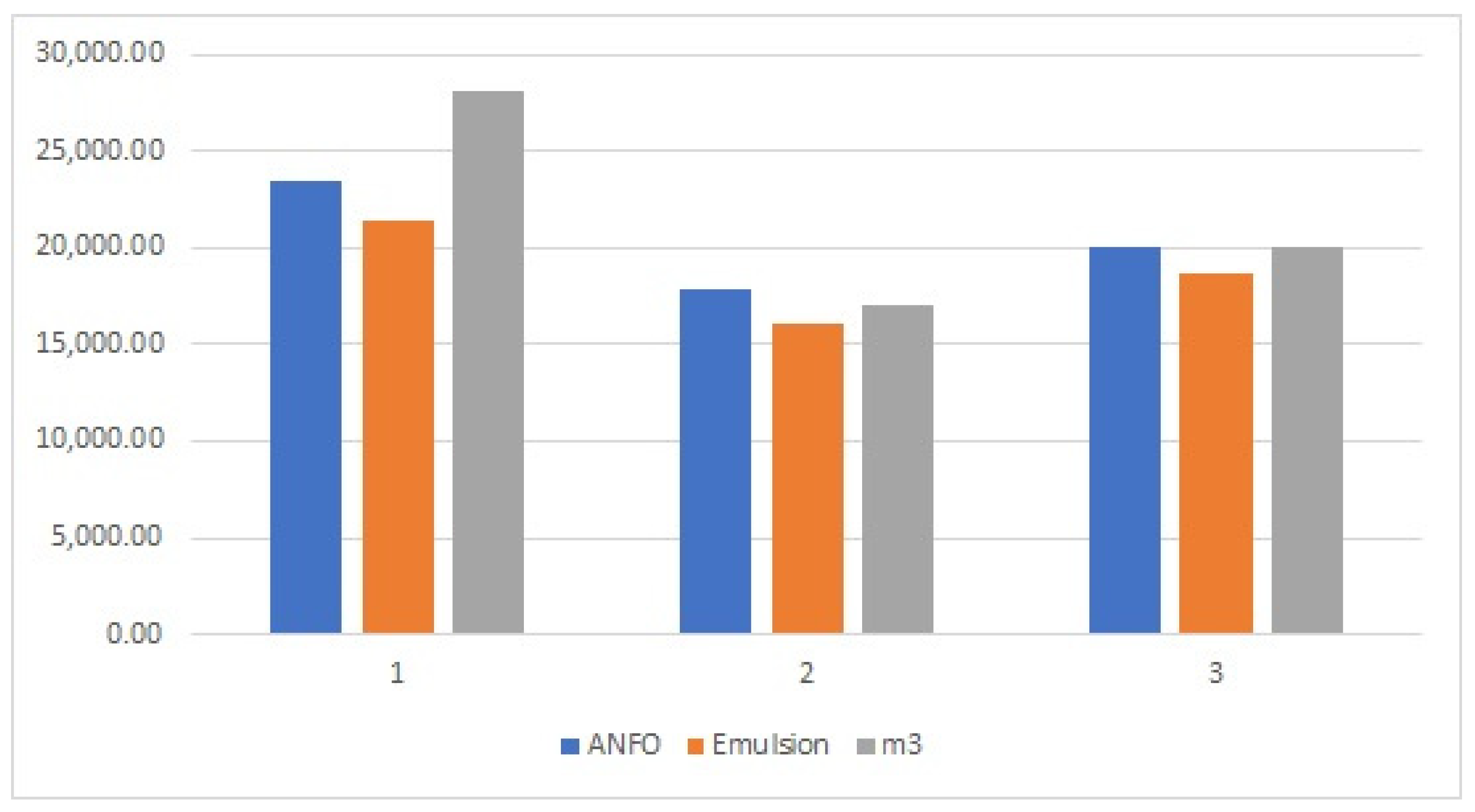
Based on the 5 years of blasting data (
Table 5), the 3-year prediction model of the energetic material consumption and fumes, presented in
Figure 3 and
Table A1 (
Appendix A), was established by the Prophet algorithm.
Based on the input data presented in
Table 5, a three-year prediction model for energetic material consumption was developed, as shown in
Figure 3. The analysis indicates that the annual consumption of ANFO fluctuates between approximately 77 and 120 tons. Over time, the prediction uncertainty increases, which can be attributed to the limited dataset obtained from the case study of the open-pit mine.
As discussed in
Section 2.1, both mechanical excavation and blasting operations are employed in the studied open-pit mine. The consumption of energetic materials is heavily influenced by blasting restriction zones, particularly due to the proximity of housing structures, as well as by the overall area of the open-pit mine. Over time, the demand for energetic materials is expected to decrease as the availability of the deposit for excavation diminishes. However, potential factors such as the opening of new excavation levels or the acquisition of additional land may alter the demand for blasting operations and associated energetic materials.
Similar trends were observed for emulsion-energetic materials and dynamite, as their equivalent masses were calculated relative to ANFO, as shown in
Table 5. The predicted demand ranges for these materials were approximately 97–126 tons for emulsion and 107–126 tons for dynamite per year. For TNT boosters, the predicted demand was estimated to range between 1.6 and 2.6 tons per year.
Using the annual consumption data for each energetic material, presented in
Figure 3 and
Table A1 in
Appendix A, the corresponding total and individual fume emissions (respectively
Figure 4 and
Figure 5) were calculated and are summarized in
Table A2.
The overall predicted fume emissions, depending on the type of energetic material used, were estimated to range between approximately 16,000 and 28,000 m3. This value may increase if the frequency of blasting operations rises. As previously demonstrated, a potential reduction in fume emissions of approximately 9.13% for ANFO and 9.74% for emulsion can be achieved by altering the priming energetic material. This represents a notable reduction when considering annual emissions.
A transition to mechanical excavation as an alternative to blasting operations is not recommended. As demonstrated by Biessikirski et al., mechanical excavation is both less efficient and more energy-intensive compared to blasting methods. Additionally, comparable ore extraction using mechanical excavation generates over six times the fume emissions of blasting operations [
42]. A detailed breakdown of fume emissions from possible annual consumption of energetic material decomposition is provided in
Figure 5.
The detailed distribution of fume emissions, as presented in
Figure 5 and
Table A2 in
Appendix A, indicates that, particularly when considering the scale effect, emulsion bulk energetic materials emerge as the most suitable and environmentally friendly option. As previously noted, the composition of fume emissions is directly influenced by the chemical composition of the energetic material. Although emulsion energetic materials can produce slightly higher carbon dioxide emissions (averaging approximately 784 tons, as shown in
Table A2 in
Appendix A), the emissions of other fumes, specifically CO and NO
x, are significantly lower compared to ANFO. Moreover, Kramarczyk et al. demonstrated that an extended gasification period for emulsion energetic materials can effectively reduce their density, thereby decreasing material consumption and subsequently lowering fume emissions [
47]. Furthermore, it should be emphasized that, for both ANFO and emulsion materials, additional reductions in CO
2 emissions can be achieved by altering the type of primer used. For dynamites, the impact of coating on fume composition must also be considered. It is generally estimated that approximately 1–2% of the oxygen is allocated to the combustion of the coating material. Consequently, coated dynamite is likely to generate higher CO
x emissions than standard calculations suggest.
Based on the results illustrated in
Figure 5, it can be concluded that, unless economic factors (production cost of energetic material) or geological conditions (mechanical strength of the deposit, presence of cracks and fractures, presence of water in boreholes) necessitate otherwise, emulsion bulk explosives should be prioritized as the primary energetic material due to their favorable emission profile.
4. Conclusions
The analysis of fume emissions from various energetic materials used in open-pit mining operations demonstrates that the chemical composition and formulation of these materials significantly influence their environmental impact. Among the evaluated materials, emulsion bulk explosives emerged as the most environmentally favorable option, producing the lowest emissions of CO and NOx, despite slightly elevated CO2 emissions compared to ANFO.
ANFO exhibited the highest NOx emissions due to its high ammonium nitrate (V) content, while dynamite showed elevated COx emissions attributed to its complex composition, including nitroglycerin and nitroglycol. TNT boosters further contributed to the emissions of CO2, CO, and NOx, but substituting TNT with dynamite for priming significantly reduced these emissions, as demonstrated in the case study. The change in priming high-energy material from TNT to dynamite can reduce the overall fume emission by approximately 9–9.5% depending on the high-energy material in the borehole. Future research concerning the impact of primer on fumes should be made towards the characterization of the chemical decomposition pathways of primer materials during detonation, with an emphasis on the effect of the chemical composition of primers based on traditional and new more environmentally friendly compositions, including the occupational and hazard safety, which concern the aspect of the main charge detonation reliability for both cap sensitive and insensitive energetic materials.
The scale effect of the energetic material mass on fume emissions highlights the importance of selecting materials with optimized compositions for large-scale blasting operations. The three-year prediction model indicated fluctuations in the demand for energetic materials, with reductions expected as the availability of deposits diminishes. However, additional factors, such as the expansion of mining operations, could influence this trend.
Taking into account environmental impact, emulsion bulk energetic materials are recommended as the primary energetic material for open-pit mining operations due to their superior environmental performance and reduced fume emissions (lowest fume emission of approximately 16,432.88 m3 obtained from decomposition of 121.4 tons of energetic material). Furthermore, altering the type of primer used in conjunction with emulsion explosives can further minimize emissions, making them a sustainable choice for blasting operations.
Transitioning from blasting to mechanical excavation is not advisable, as it would result in significantly higher fume emissions and reduced efficiency.
The adoption of emulsion bulk explosives, coupled with a careful selection of primers, represents a strategic approach to minimizing the environmental impact of blasting operations in open-pit mining while maintaining operational efficiency.