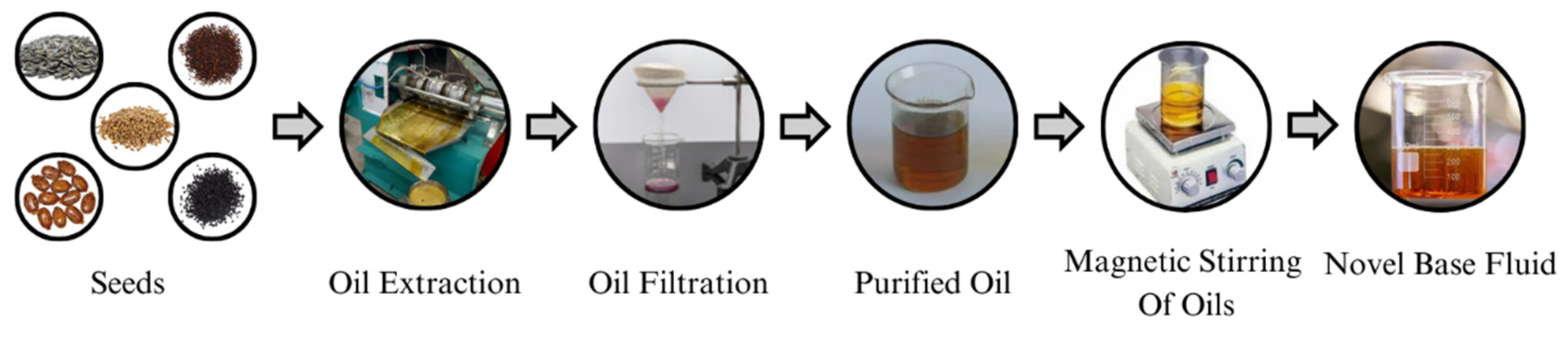
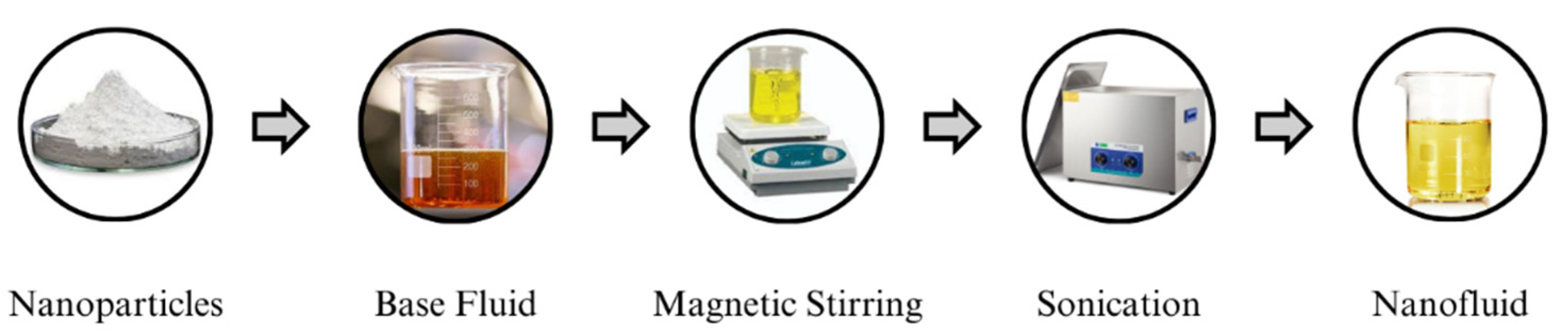
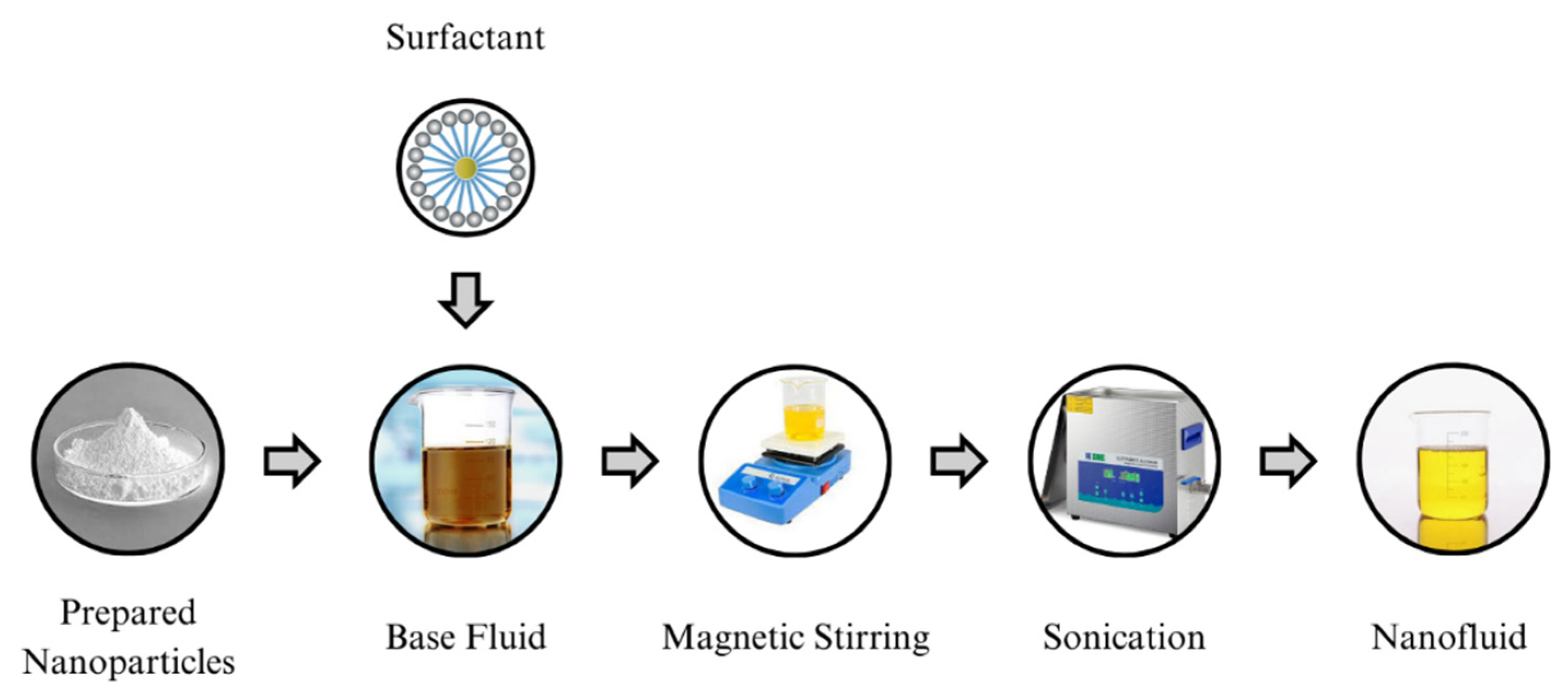
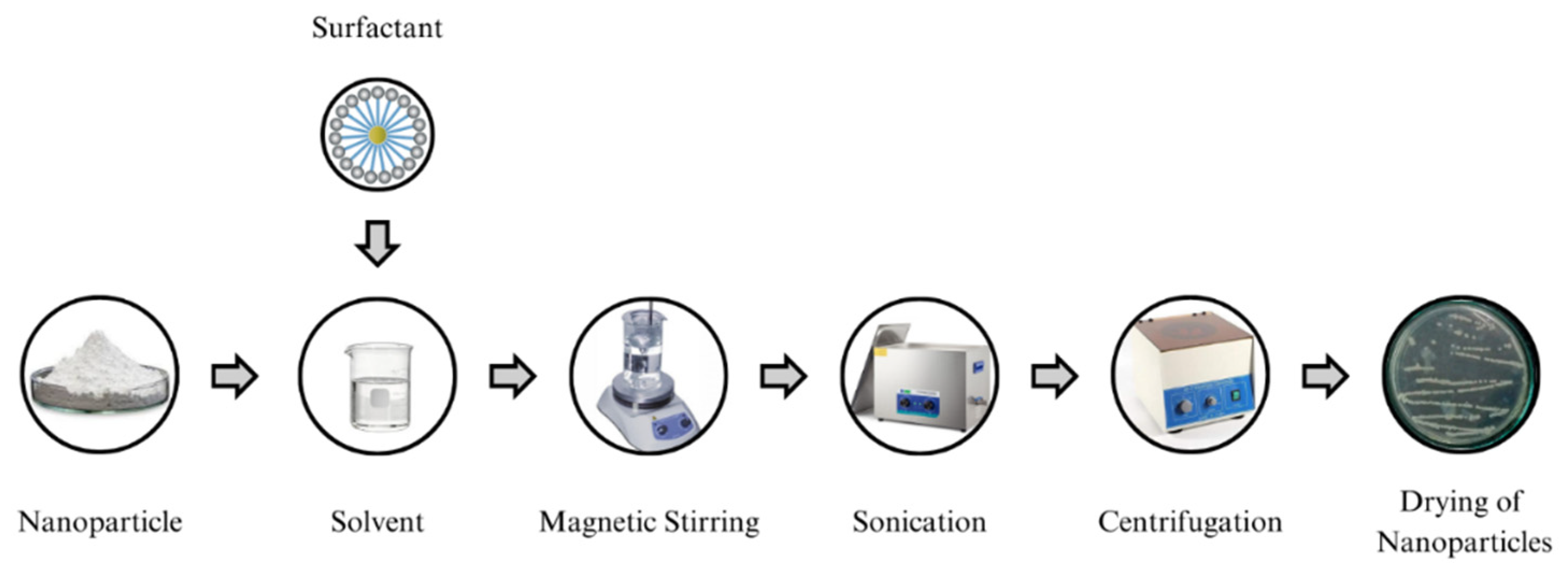
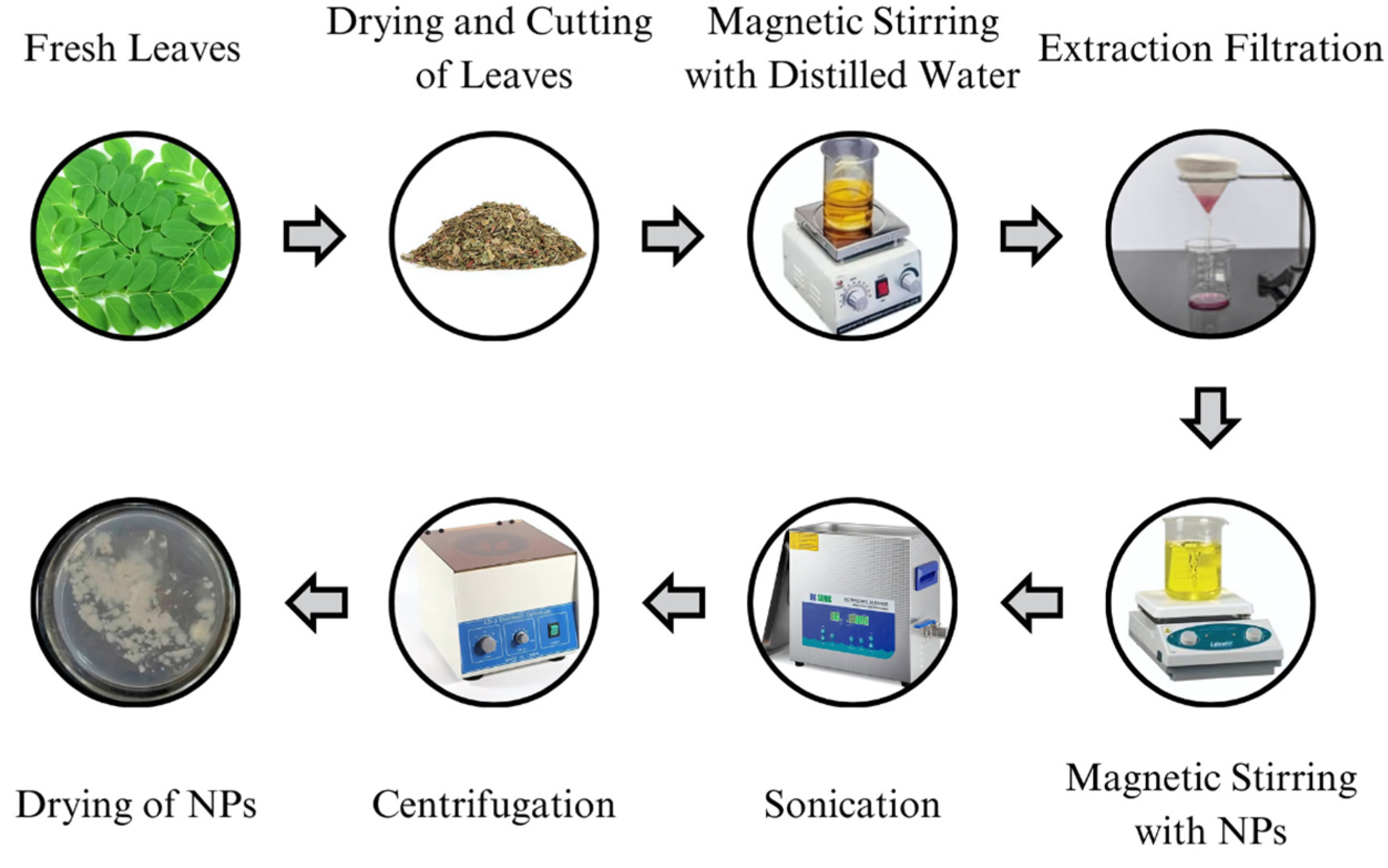
1. Introduction
The incessant and escalating demand for electrical power poses an enduring challenge for utilities, engineers, and researchers alike. This challenge necessitates continuous advancements in the realm of electrical apparatuses, particularly for the electrical, thermal, and chemical properties of fluid insulation systems. A compelling imperative emerges to engineer a new generation of fluid insulation systems characterized by heightened dielectric strength, augmented thermal conductivity, and consequential reductions in volume and capital expenditures. These advancements are not only pivotal for enhancing the efficiency and reliability of long-term operations [
1] but are also mandated to align with the contemporary imperative of environmental friendliness [
2,
3]. In essence, the evolution of fluid insulation systems stands as a critical frontier, harmonizing technological innovation with sustainability considerations to meet the evolving demands of the electrical power landscape.
Fluid insulation systems form the bedrock of electrical apparatuses, with key constituents comprising mineral [
4], natural ester (NE) [
5], and synthetic ester (SE) [
6] insulating oils (IO). These oils find pervasive applications in diverse electrical devices, including transformers, switchgear, and circuit breakers [
7]. Transformer oil (TO), particularly mineral oil (MO), has been a stalwart of electrical engineering, especially high-voltage engineering, for numerous decades [
8]. Its enduring popularity is attributed to its commendable insulating and thermal properties, coupled with remarkable oxidation stability, which render it indispensable for dual roles in insulation and cooling within electrical apparatuses, i.e., transformers [
9]. The persistent exploration and investigation of liquid insulation have yielded a spectrum of alternatives to traditional mineral insulating oils (MIO). The historical trajectory reveals the evolution of transformer oils, with the initial iteration being the paraffinic mineral oil developed in 1890, which was subsequently supplanted by its naphthenic counterpart in 1925. A notable milestone in this progression was the advent of polychlorinated biphenyls (PCB) in 1930; however, these were phased out in 1978 owing to their recognized hazardous nature. The subsequent era witnessed the introduction of silicone oils in the early 1970s, although their application is presently limited to specialized use cases [
2]. This historical panorama illustrates the dynamic nature of liquid insulation research, which is marked by a continual quest for innovative solutions and an evolving landscape of alternatives driven by a pivotal objective to identify an insulating oil that is not only more cost-effective but also environmentally friendly, while concurrently demonstrating superior properties to meet the diverse demands of electrical applications.
A significant proportion of transformer insulation failure, approximately 30%, has been attributed to inadequate dielectric insulation [
10], while an additional 32% is ascribed to issues related to overheating and thermal stresses [
11]. This underscores the critical role of effective dielectric insulation and thermal management in mitigating the risk of insulation failure in transformers. Considering crucial parameters, such as insulation quality, load conditions, operating temperatures, and design intricacies, the anticipated lifespan of a transformer is estimated to range between 32 and 55 years [
12]. It is noteworthy, however, that in the absence of diligent monitoring of the transformer’s insulation system, the life expectancy of the device may be markedly diminished to less than 20 years [
13].
While mineral oil (MO)-based transformer insulating oil (TIO) remains in widespread use due to its inherent benefits, it is imperative to acknowledge the significant environmental consequences associated with its usage. Notably, mineral oil exhibits pronounced toxic effects when spilled into soil and waterways, featuring low biodegradability with detrimental environmental implications. A meager proportion of released oil undergoes self-degradation in the environment, exacerbating its persistence [
2]. The ramifications of such spills are profound, with a mere kilogram of oil leakage from a transformer rendering a staggering 5 million liters of water unfit for consumption [
14]. Mineral oil’s low flash- and fire points pose additional hazards, with the combustion byproducts, particularly polycyclic aromatic hydrocarbons, proving to be toxic and posing an acute threat to the environment in the case of a fire incident [
15]. The act of burning mineral oil further releases toxic gases into the environment, accompanied by the emission of heavy, dark smoke [
14]. The remediation of oil spills incurs substantial costs, with major environmental utilities expending millions of dollars annually to minimize the ecological consequences. Furthermore, mineral oil exhibits inadequate moisture tolerance and suboptimal performance at elevated temperatures, contributing to its environmental challenges. As a non-renewable fossil resource, there is a looming concern that the availability of mineral oil may be depleted in the next several decades [
16]. These multifaceted environmental considerations underscore the urgency for exploring alternative, more sustainable insulating mediums for transformers.
Given the aforementioned constraints associated with mineral oil (MO), a concerted emphasis is warranted toward exploring viable alternatives that offer heightened sustainability, environmental compatibility, and equivalent insulating and cooling properties [
17,
18]. Numerous endeavors have been undertaken to develop alternatives to mineral oil, leveraging readily available crops, such as corn oil [
19], coconut oil, neem oil [
20], sunflower oil, soybean oil [
21], rapeseed oil, and cottonseed oil [
22]. These alternatives, categorized as biodegradable oils [
23], exhibit commendable characteristics, manifesting as environmentally benign options with elevated flashpoints and fire points. However, it is essential to note that these alternatives may exhibit lower oxidation stability [
24], signaling the need for a judicious balance between ecological considerations and the technical requirements of transformer insulation systems. The quest for an optimal replacement for mineral oil is emblematic of the ongoing pursuit of sustainable, environmentally friendly alternatives in the domain of dielectric fluids.
This research work endeavors to advance the development of a cost-effective, environmentally friendly, and highly dielectric vegetable oil nanofluid (NF) that is specifically tailored for enhancing the insulation properties of green transformers. Many researchers have used a single oil as a base fluid, which has posed challenges in terms of viscosity, acidity, density, interfacial tension, etc. [
10,
25]. This research is focused on crafting a base fluid comprising a mixture of three vegetable oils to gain the inherent properties of each oil. The innovation of this study lies in the formulation of a unique base fluid (BF), comprising a distinctive blend of three vegetable oils in a carefully calibrated ratio. The selection of these oils and their proportionality are pivotal aspects; hence, the novelty of the research lies in its contribution to the distinctive character of the proposed nanofluid. Additionally, the incorporation of SiO
2 nanoparticles—a judicious choice detailed in the Materials Section—further enriches the nanofluid composition. Two paramount dielectric properties, namely dielectric constant/relative permittivity (
εr) and the dielectric dissipation factor (DDF)/tan
δ, are critically examined in this research, given their significance in preventing insulation failure in transformer insulating oil (IO). Comparative analyses with traditional mineral oil (MO) for both dielectric properties underscore the superior performance of the proposed nanofluid. Furthermore, the study delves into the comparison of different methods for nanofluid preparation, introducing a novel green synthesis method aligned with eco-friendly initiatives. The results from this green synthesis method are systematically contrasted with traditional approaches, culminating in the discerning conclusion that the green synthesis method outperforms its counterparts. This research thus contributes to the advancement of sustainable and high-performance liquid insulation solutions based on vegetable oil nanofluids, positioning itself at the intersection of scientific innovation and environmental responsibility in the field of electrical engineering and, more precisely, in high-voltage engineering.
3. Dielectric Properties
3.1. Dielectric Constant/Relative Permittivity (εr)
The concept of relative permittivity, also known as the dielectric constant, plays a pivotal role in the field of electrical insulation. Relative permittivity is a dimensionless quantity that characterizes the ability of a material to permit the transmission of electric flux under the influence of an electric field, relative to a vacuum. The background of relative permittivity lies in its profound impact on the behavior of dielectric materials when subjected to electric fields. The dielectric constant serves as a crucial parameter in determining the extent to which a material can store electrical energy and reduce the intensity of the electric field within an insulating medium. Understanding and evaluating the relative permittivity is paramount in designing effective insulating systems. A higher dielectric constant implies greater resistance to electric stress, making materials with high relative permittivity desirable for applications demanding efficient electrical insulation.
In practical terms, the dielectric constant assumes significance in insulation systems, contributing to the prevention of electrical breakdown or insulation failure and enhancing the overall reliability of the system. Liquid insulation materials with high relative permittivity are advantageous due to their ability to store and distribute electrical energy, reducing the risk of voltage stress and improving the insulation efficiency of components. This property is particularly beneficial in the design of transformers and other high-voltage devices where reliable insulation is essential. Moreover, in the context of liquid insulation, the dielectric constant of liquids becomes a critical parameter for assessing their suitability in high-voltage applications, i.e., transformers. Liquids with high relative permittivity are preferred for use in liquid insulation systems, as they contribute to increased dielectric strength and enhanced insulation performance, minimizing the risk of insulation failure. The meticulous consideration of relative permittivity in the context of insulation underscores its pivotal role in ensuring the safety and efficiency of the insulation of the system.
This section introduces an innovative methodology for elucidating the permittivity characteristics of the proposed nanofluid, which is specifically tailored for its application in transformers and other high-voltage equipment according to the International Electrotechnical Commission (IEC) 60247 standard [
39]. The primary focus is on advancing our understanding of the electrical properties of natural ester-based vegetable oil nanofluids to assess their suitability as insulating materials in high-voltage environments. Moreover, this investigation incorporates a comparative analysis, contrasting the electrical properties of the nanofluids with those of the traditional mineral oil-based transformer oil, a widely utilized dielectric fluid in the electrical industry. The juxtaposition with traditional mineral oil verifies the viability and superiority of the recommended nanofluid in the context of electrical insulation and dielectric performance, thereby contributing to the body of knowledge in electrical engineering and, more precisely, in high-voltage engineering. This comprehensive examination aims to provide valuable insights into the potential advancements and advantages offered by nanofluid-based insulation solutions in high-voltage applications.
The theoretical underpinning of this experiment is rooted in the electrostatics of parallel plate capacitors. The capacitance (
C) of a capacitor can be calculated by using Equation (2):
where
C is the capacitance,
ε is the absolute permittivity of the material between the plates,
A is the area of the capacitor plates, and
d is the separation between the plates. Rearranging Equation (2) yields the absolute permittivity:
The elucidation of the dielectric constant or relative permittivity (
εr) of a liquid is underpinned by a fundamental expression derived from the ratio of absolute permittivity (
ε) to the permittivity of free space (
εo). Equation (3) forms the cornerstone for quantifying the relative permittivity of a medium based on measured capacitance, plate area, and separation.
The utility of this expression is paramount in the forthcoming investigation, as it will be employed to ascertain the relative permittivity of the proposed nanofluids. The same methodology will be applied for the determination of the dielectric constant of mineral oil, establishing a foundation for the subsequent comparative analysis. The significance of these equations lies in their role as an analytical framework, allowing a quantitative assessment of the electrical properties of distinct dielectric materials.
3.1.1. Experimental Verification
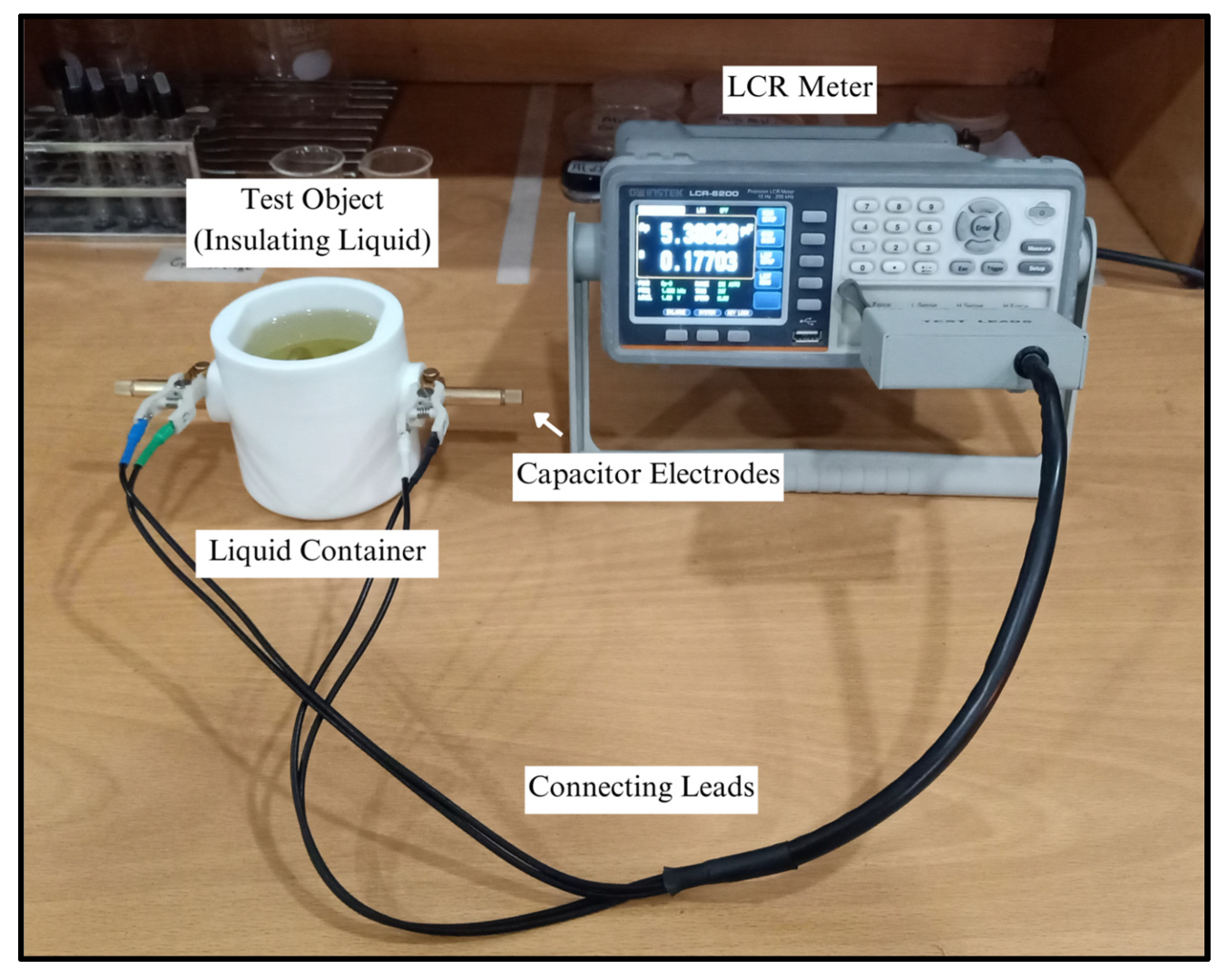
The experimental arrangement encompasses a precisely engineered parallel plate capacitor submerged in the targeted liquid, i.e., the proposed nanofluids and conventional mineral oil in this case. The primary constituents include a parallel plate capacitor, consisting of two conductive plates with a precisely defined surface area (
A) and separation distance (
d). The LCR meter is a sophisticated instrument proficient in quantifying capacitance (
C), inductance (
L), and resistance (
R). The liquid container is a specialized receptacle housing the liquid under investigation.
Figure 6 shows the experimental setup utilized for evaluating the relative permittivity (
εr) of the dielectric liquid.
The meticulous immersion of the parallel plate capacitor in the liquid ensures complete submersion and establishes optimal conditions for the experiment. The LCR meter is intricately connected to the electrodes of the capacitor via connecting leads for real-time measurement of capacitance. The design of the liquid container assumes pivotal significance to minimize external influences on the capacitor, i.e., environmental effects on the liquid housing, with precise consideration of its dimensions factored into the permittivity calculation. This rigorous setup guarantees accurate and controlled conditions, forming the foundation for the subsequent determination of the relative permittivity of the liquid through detailed capacitance measurements.
The GW INSTEK (LCR-6200) precision LCR meter sourced from GW INSTEK, Taipei, Taiwan, stands as a sophisticated instrument that is integral to this experimental framework, particularly in its capacity for measuring impedance parameters. Within the confines of this study, emphasis is placed on its capacitance measurement capabilities, which play a pivotal role in the determination of relative permittivity.
The GW INSTEK (LCR-6200) is equipped with four specialized sensors, ‘L-force, L-sense, H-force, and H-sense’, incorporating a two-wire configuration. This design ensures a comprehensive and nuanced measurement approach, allowing precise analysis of impedance characteristics in the experimental setup.
Utilizing an alternating current (AC) signal, the LCR meter, when connected to the parallel plate capacitor, applies a voltage across its terminals, inducing a current. The phase difference between the applied voltage and the ensuing current serves as the foundational parameter for capacitance determination. The LCR meter’s capacity to precisely discern this phase relationship enables accurate calculation of capacitance, a key element in the broader assessment of relative permittivity.
The integrity of the experimental process lies in the impeccable condition of the parallel plate capacitor, necessitating a meticulous cleaning regimen to eliminate potential contaminants. The liquid container, a crucial component of the setup, is meticulously established, and the targeted liquid is introduced with precision. The immersion of the parallel plate capacitor into the liquid is conducted with precision, precluding the introduction of air bubbles or extraneous perturbations that may compromise measurement accuracy. To ensure minimal interference, the parallel plate capacitor is intricately connected to the LCR meter. The configuration of the LCR meter for capacitance measurement is executed with the utmost attention to detail; this is a step of paramount importance in guaranteeing accurate and reliable results.
The relative permittivity (
εr) measurement in this article was conducted according to International Electrotechnical Commission (IEC) 60247 standards. This standard deals with measuring the relative permittivity (
εr) of insulating liquids. After connecting and configuring the LCR meter, capacitance can be measured, and paying heed to Equation (4) reveals that
εr is a ratio of the capacitances, as shown in Equation (5).
where
Cl is the capacitance of the insulating liquid measured by the LCR meter, and
Co is the capacitance of free space measured in the same fashion.
3.1.2. Results and Discussion
Table 4 presents the measured results for the diverse array of test samples investigated in this study. By employing Equation (5), the computed relative permittivity (
εr) values are documented in
Table 4. Studies show that natural ester-based nanofluids have higher values of
εr in contrast to mineral oil [
40], and similar results were found in our research.
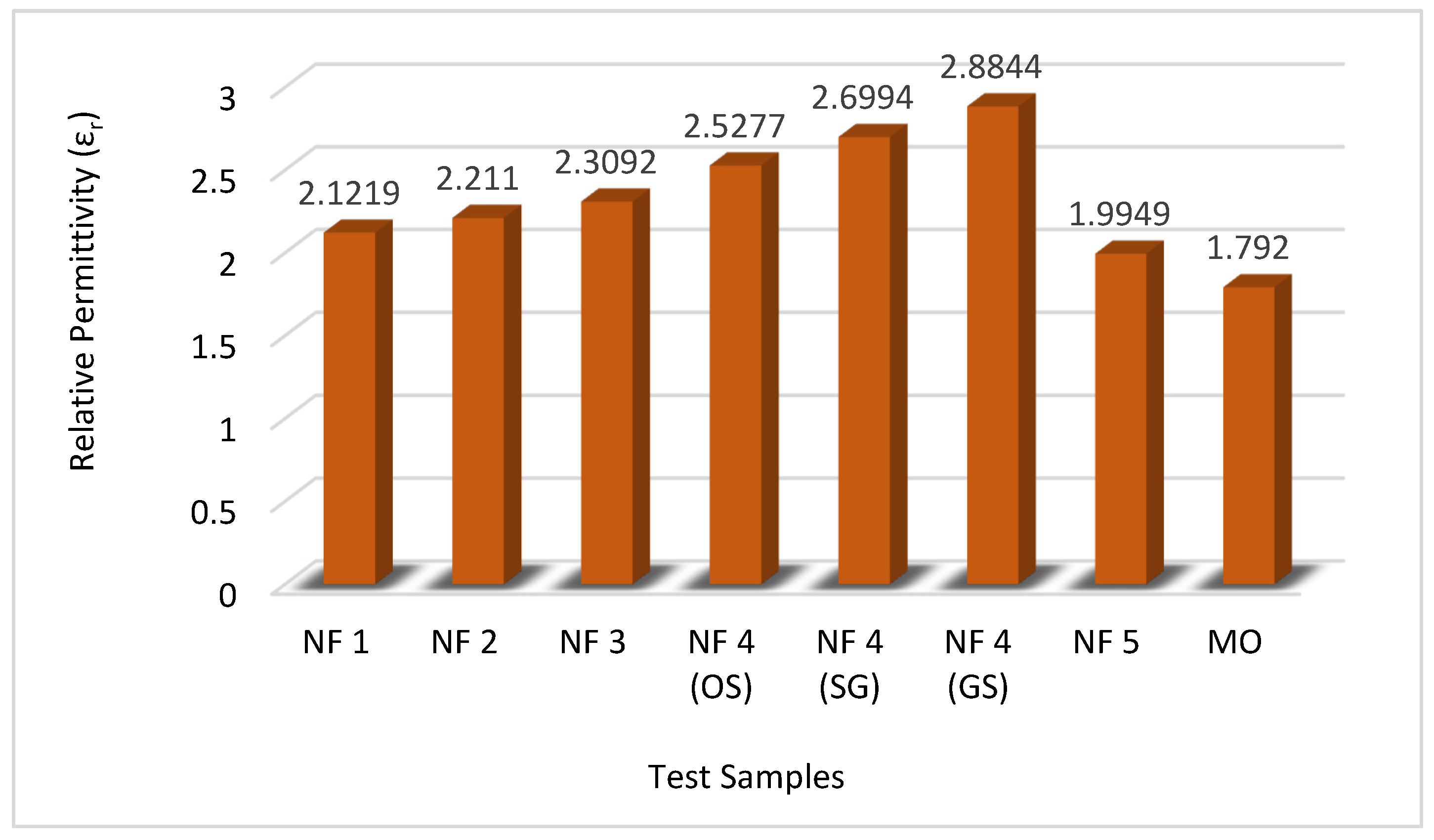
The outcomes of our investigation reveal that nanofluids based on natural esters exhibit considerable promise as viable substitutes for conventional mineral oil. Notably, the measured capacitances and corresponding relative permittivity (
εr) values surpass those associated with mineral oil. Moreover, discernible differences in the outcomes of natural ester-based nanofluids are evident, with the method of preparation playing a vital role. Of particular significance is the observation that the proposed nanofluid synthesized through environmentally friendly, green methods outperforms other counterparts. The proposed nanofluid, synthesized via the green synthesis approach, exhibited a significantly higher relative permittivity (
εr) of 2.8844 compared to the conventionally employed mineral oil, which possesses an
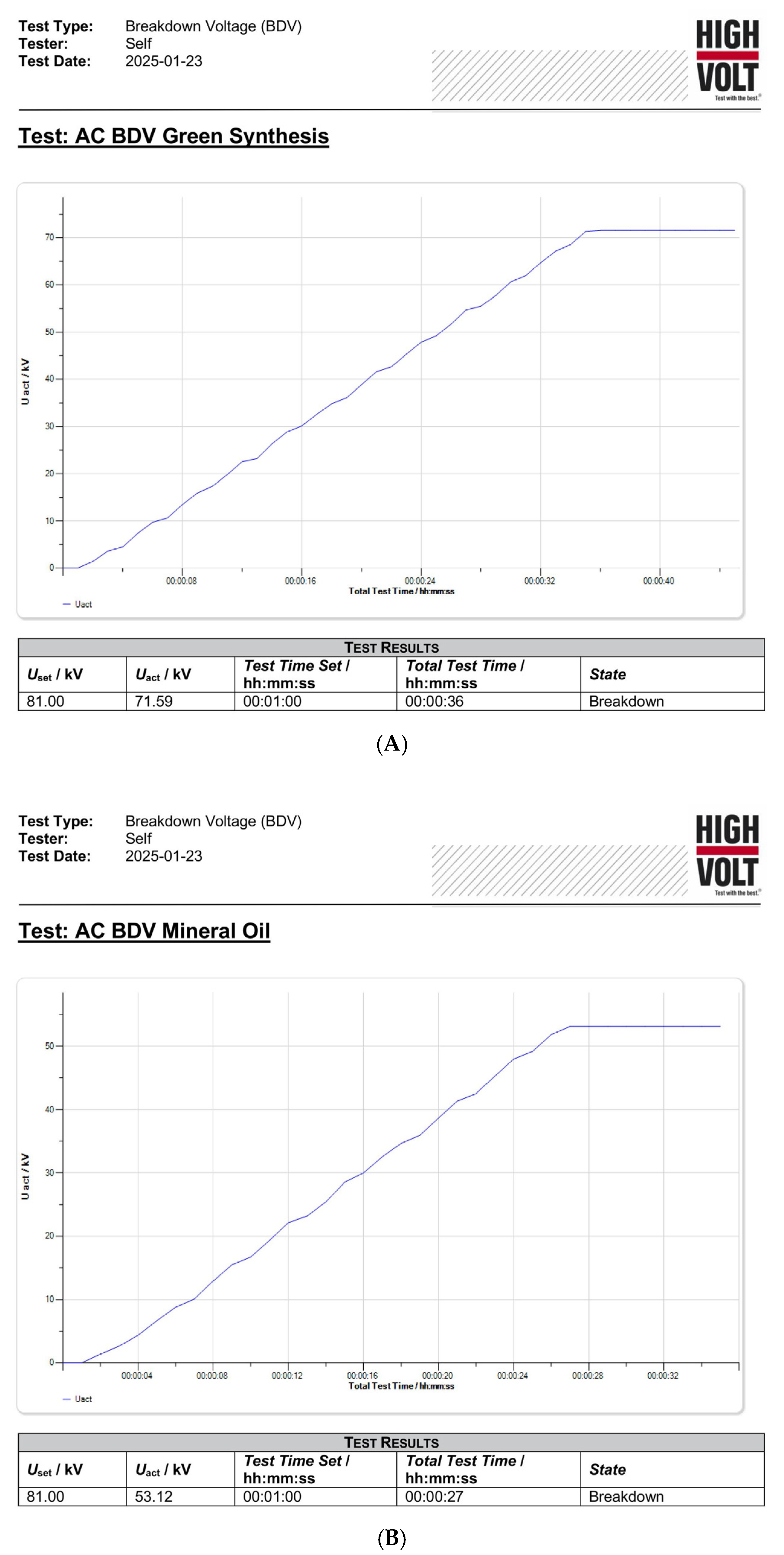
εr of 1.7920. The breakdown voltage (BDV) test was performed on test samples to verify the enhancement of the dielectric properties of the proposed sample. The results of the BDV test are presented in
Table 4, showing its potential to be used as the replacement for MO. The pictorial evidence of the DBV results is shown in
Figure 7. A visual representation of relative permittivity (
εr) is elucidated in
Figure 8. This superior performance underscores the potential of such nanofluids as exceptional insulating liquids for high-voltage equipment. The pronounced advantages exhibited by the green-synthesized nanofluids advocate for their strategic consideration in enhancing the performance and reliability of high-voltage systems.
A graphical illustration depicting the comparative analysis of relative permittivity (
εr) across all test samples of nanofluids is presented in
Figure 9.
3.2. Dielectric Dissipation Factor (Tan δ)
The dielectric dissipation factor (DDF), often denoted as tan δ, is a crucial parameter in the field of electrical insulation, serving as a measure of energy loss in insulating materials. This dimensionless quantity reflects the ratio of the resistive component to the reactive component in a dielectric material when subjected to an alternating electric field. The DDF holds significant importance in the realm of electrical insulation design as it provides insights into the efficiency of insulating materials. High tan δ values indicate greater energy dissipation in the form of heat, suggesting potential inefficiencies in the insulation system. Understanding the dielectric dissipation factor (DDF) is essential for assessing the overall performance and reliability of insulation, especially in applications where minimizing energy loss is critical, such as in high-voltage equipment like transformers.
The dielectric dissipation factor (DDF), tan δ, is a measure of the energy dissipated as heat in an insulating material when subjected to an alternating electric field. In insulation systems, the DDF is a key parameter used to evaluate the efficiency and quality of the insulation. A lower tan δ signifies reduced energy loss and enhanced insulation performance. This parameter is particularly valuable in liquid insulation, where the dielectric dissipation factor (DDF) can influence the overall effectiveness of the insulation system. In the context of liquid insulation for high-voltage applications like transformers, maintaining a low tan δ is crucial for minimizing heat generation and optimizing the dielectric performance of the insulating fluid. Careful consideration of tan δ is paramount in ensuring the reliability and safety of electrical insulation systems, especially in situations where efficient energy transfer and heat dissipation are critical factors for the system’s operational integrity.
This section undertakes a comprehensive comparative analysis between natural ester-based vegetable oil nanofluids and conventionally employed mineral oil. The methodological approach involves the utilization of a dissipation factor measuring bridge, sourced from Presco AG, to conduct tan δ tests according to the IEC 60247 standard. This rigorous testing protocol is designed to facilitate an in-depth examination of the tan δ values across various test objects. The primary emphasis of this analysis is on discerning the dielectric properties of the liquids, with a specific focus on identifying a suitable dielectric liquid for insulation purposes in high-voltage equipment, such as transformers with low dissipation. Through tan δ tests, we aim to meticulously assess and compare the dissipation factor behaviors exhibited by natural ester-based nanofluids and traditionally used mineral oil. This investigation is motivated by the imperative to ascertain which liquid demonstrates superior dielectric characteristics, thereby qualifying it for optimal use in electrical insulation systems. The overarching goal is to contribute valuable insights to the scholarly discourse on dielectric materials, particularly within the domain of high-voltage applications. This section aligns with the broader research objective of enhancing our understanding of dielectric behavior in liquid insulation by a material that is cost-effective, environmentally friendly, and exhibits superior dielectric properties, thereby informing the selection and design of improved insulation solutions for high-voltage equipment.
The dielectric dissipation factor (DDF)/tan
δ is a significant parameter in the field of electrical insulation, i.e., the ratio of the resistive component to the reactive component in a dielectric material subjected to an alternating electric field. Mathematically, tan
δ is expressed as the tangent of the phase angle (δ) between the voltage and current waveforms in a capacitive circuit. This can be formulated as follows:
In this equation, the imaginary part of capacitance is indicative of the energy stored and released as reactive power, while the real part of capacitance represents the energy dissipated as resistive power. The dielectric dissipation factor (DDF) is particularly relevant in the context of insulation materials, where a low tan
δ value signifies efficient energy transfer and minimal heat generation. The dielectric dissipation factor can also be related to the quality factor (
Q) of the material, which describes the efficiency of energy storage and release. For a parallel RLC circuit, the relationship between tan
δ and
Q is given by:
This expression underscores the reciprocal relationship between the dielectric dissipation factor (DDF) and the quality factor (QF), further emphasizing the significance of tan δ in characterizing the performance of dielectric materials. Understanding the mathematical foundations of tan δ is crucial for assessing the suitability of materials in insulation systems, as it provides insights into the efficiency of energy transfer, heat dissipation, and overall dielectric behavior in high-voltage applications.
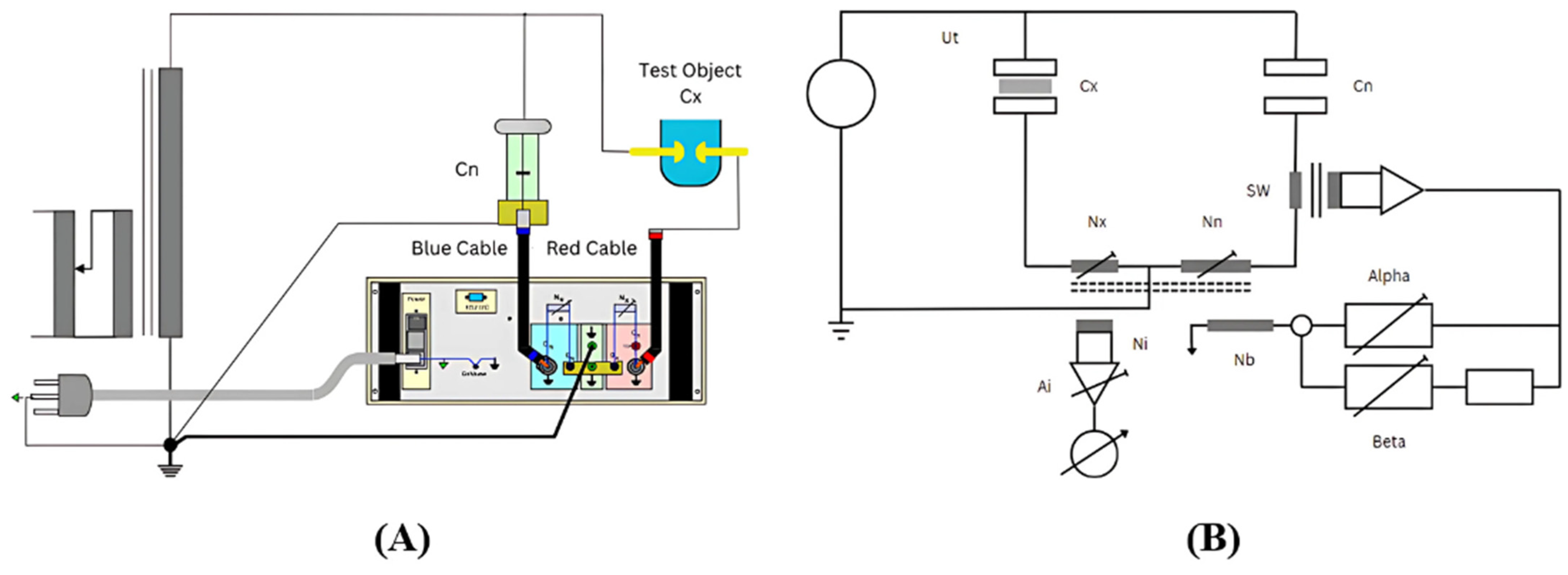
3.2.1. Experimental Verification
The experimental setup using a dissipation factor measuring bridge, sourced from Presco AG is shown in
Figure 10A. The connections have to be made according to the circuit diagram in
Figure 10A. Establishing a robust grounding connection between the laboratory’s ground and the ground terminal of the instrument is imperative. Given that the instrument conforms to safety class I of IEC 359, the mains cable incorporates a dedicated ground conductor, necessitating its connection to the local mains ground. The low-voltage output from the standard capacitor should be linked to the
CN input of the instrument using the cable marked in blue. It is noteworthy that many standard capacitors feature a metallic guard ring in their low-voltage sections, which should be appropriately connected to the ground. This connection is ideally established directly to a central ground terminal to effectively divert discharge currents in the event of a breakdown, thereby safeguarding the instrument from damage. The test object, exemplified by the test object in
Figure 10A, must be linked to the
CX input using the cable marked in red. In instances where the input current (
IX) exceeds 5 A (
IX = U
test ×
ω ×
CX), it is advisable to substitute the provided coaxial cable with a stranded wire, dimensioned at 5 A/mm
2, for the X-connection. The final step involves configuring the high-voltage supply according to the schematic in
Figure 10A.
The capacitance and dissipation factor measuring bridge, specifically the type TG-3MOD manufactured by Presco AG, stands as a sophisticated instrument designed for precise measurement in electrical testing. This state-of-the-art bridge is engineered to determine the dissipation factor, denoted as tan δ, which is a crucial parameter in evaluating the quality and efficiency of dielectric materials. Presco AG’s TG-3MOD demonstrates advanced capabilities in conducting tan δ tests, which is our primary focus in this test, providing researchers and engineers with a reliable tool for characterizing the electrical properties of insulation materials. Renowned for its accuracy and versatility, this measuring bridge plays a pivotal role in enhancing our understanding of dielectric behavior and contributes to advancements in electrical insulation technology.
The measurement apparatus performs a comparative analysis of the current in the unknown capacitor (test object CX) with that of the standardized capacitor CN. Termed the “Current Comparator”, this device operates on magnetic principles, utilizing a high-permeability iron core configured in a ring shape with multiple windings. The establishment of a zero sum in the ampere windings results in a corresponding nullification of the magnetic flux within the core. The proportional relationship between the voltage on the “Ni” winding and the magnetic flux is crucial, as a decrease in flux to zero yields a corresponding voltage reduction to zero on the Ni winding. Amplification of the minimal voltage on the Ni winding is executed by the “Ai” amplifier, subsequently detected by the microprocessor, and visually represented as a bar graph on the main screen.
For the balanced state, the following conditions are valid. The current
IX through the test object is
The current
IN through the standard capacitor is
The current
IN is converted through a current transformer and a current–voltage converter, yielding a voltage directly correlated to the magnitude of
IN. Subsequently, employing the analog–digital converter ADC (in phase) and ADtg (with a 90° phase lag), a fraction of the
IN current is channeled into the winding
Nb of the current comparator.
Figure 10B shows the principle of comparison.
Upon the meticulous adjustment of the
NX and
NN windings, in conjunction with precise calibration of the analog–digital converters A/DC and A/Dtgδ, the magnetic flux within the iron core is rendered inert. This state is often denoted as the balanced state; it signifies the system’s equilibrium and ensures optimal performance and accurate measurements. Mathematically, the condition for the balanced state is:
By utilizing the mathematical expression outlined in Equation (10), one can derive the values of
Cxp and
Gxp. In practical scenarios, the focus shifts from
Gxp to the computation of the dissipation factor, known as tangent delta (tan
δ). This parameter, tan
δ, is rigorously defined as the ratio of active power within the test object to its reactive power. In sinusoidal signal conditions, tan
δ precisely corresponds to the phase angle of the impedance vector, providing valuable insights into the degree of power dissipation concerning the reactive elements within the system.
From Equation (10), we obtain the following:
With the judicious selection of parameters k and Nb, Cxp can be determined with extraordinary precision. The fine-tuning capacity of the NN winding spans three logarithmic scales (10 × 100 + 10 × 10 + 10 × 1), and the 12-bit A/D converter contributes an incremental three digits. The cumulative resolution exceeds six decades, presenting an accuracy level below one part per million (ppm).
Initially, it is imperative to adhere to pre-conditioning methodologies, as per IEC 60247, while establishing the connections delineated in
Figure 10A. Subsequently, the configuration of the tan
δ measuring bridge becomes paramount. The pivotal element in this process is the balancing of the bridge, accomplished through utilizing a specialized algorithm. The bridge autonomously achieves equilibrium, with the measurement outcomes presented on the displays. The initial auto-ranging or first balancing operation requires approximately 6 s. Following this, the bridge perpetually self-balances, updating the measurement results at intervals of 0.6 s. The conclusive step involves the application of power to the test object and standard capacitor, thus completing the setup process.
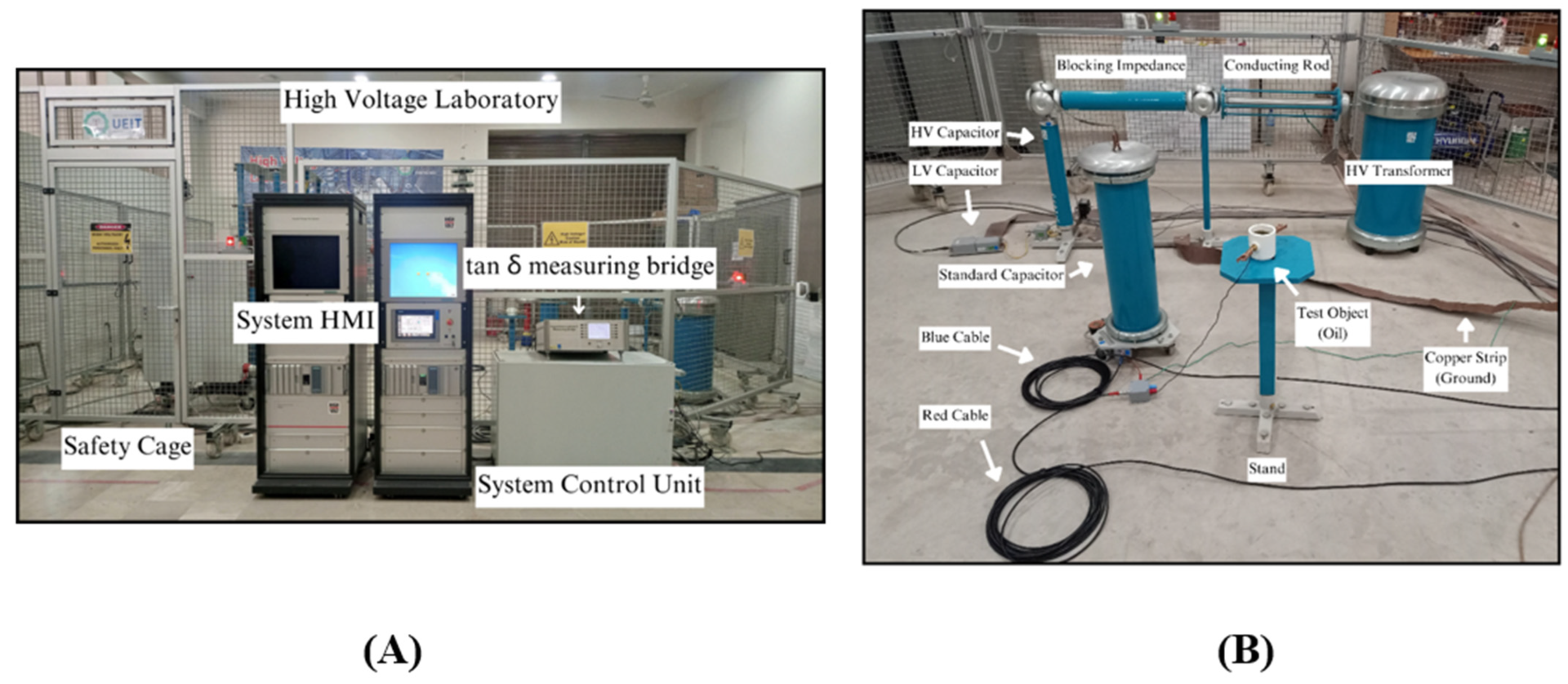
Figure 11 provides a comprehensive overview of the laboratory setting, offering a panoramic glimpse into the experimental environment under scrutiny.
Figure 11B provides a meticulous portrayal, offering a detailed examination of the schematic delineated in
Figure 10A. This finer-grained illustration facilitates a nuanced exploration of the components and intricacies embedded within the overarching experimental framework.
3.2.2. Results and Discussion
The tan
δ assessments of diverse nanofluids were conducted in accordance with the IEC 60247 standard (@ 2.2–2.5 kV and 50 Hz), a meticulous protocol specifically designed for the measurement of the dissipation factor (tan
δ) [
39].
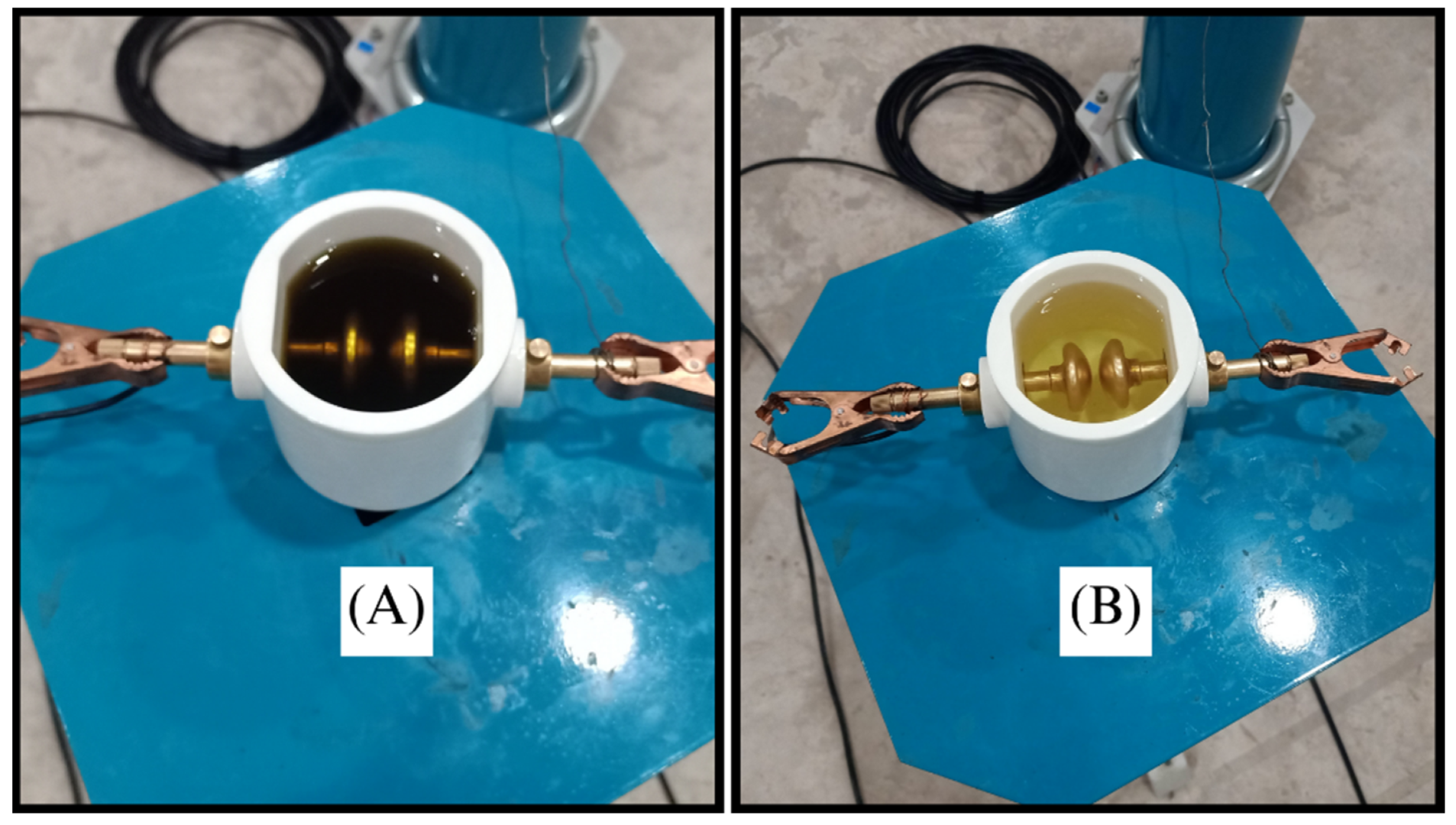
Figure 12 visually captures the test samples undergoing the tan
δ evaluations, offering a glimpse into the rigorous testing procedures implemented as per the established standards.
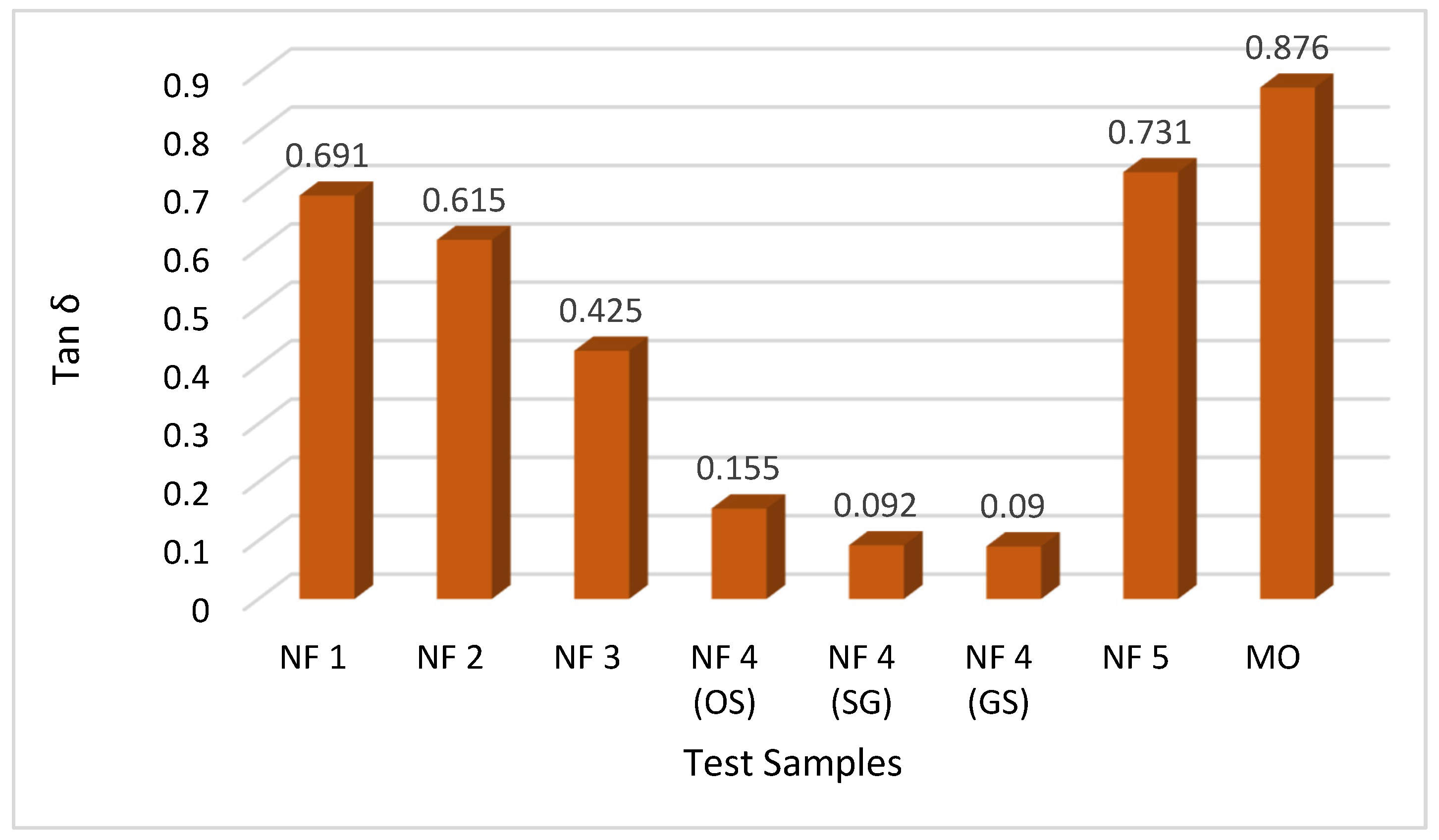
The ensuing results illuminate the superior performance of natural ester-based vegetable oil nanofluids in comparison to the conventionally utilized mineral oil, as visually demonstrated in
Figure 13. Evident from the outcomes is a notable dissimilarity between the dissipation factors, with mineral oil exhibiting a higher dissipation of 0.876 compared to its natural ester-based counterparts. Upon a closer examination of the results within the realm of natural ester-based nanofluids, a compelling revelation emerges. The proposed nanofluid synthesized through an environmentally friendly approach, namely green synthesis, manifests the lowest dissipation of 0.09 among all the other insulating liquids. The higher values for the other samples indicate the potential challenges associated with the homogeneous mixing of nanoparticles within the base fluid. This noteworthy distinction positions green synthesis-derived nanofluids as highly promising candidates for deployment as insulating liquids, suggesting a pioneering avenue for advancing insulation technologies in high-voltage applications.
A graphical illustration depicting the comparative analysis of tan
δ across all the test samples of the nanofluids is presented in
Figure 14.
4. Conclusions
This research work aimed to develop a cost-effective, environmentally sustainable, and technologically advanced dielectric fluid by harnessing the enriching properties of natural ester-based vegetable oils, thereby presenting a promising alternative for transformer insulation and cooling applications. The novelty lies in the formulation of a nanofluid comprising a carefully crafted blend of three distinct vegetable oils, i.e., castor, flaxseed, and blackseed as the base fluid, which was a distinctive aspect that distinguished this research. The incorporation of SiO2 nanoparticles into the fluid matrix was pursued owing to their myriad advantageous characteristics. Extensive experimentation was conducted to assess the superior attributes of the proposed nanofluid, with a focus on critical dielectric properties, including relative permittivity (εr) and the dielectric dissipation factor (tan δ). Comparative analyses were performed against conventional mineral oil, which served as the benchmark; the analyses revealed the pronounced advantages of the natural ester-based vegetable oil nanofluid. Notably, the novel formulation surpassed all other tested samples with a 38% enhancement in εr and a 90% decrease in tan δ compared to MO, underscoring its exceptional performance. Furthermore, the preparation methodologies were meticulously scrutinized, culminating in the identification of a superior technique, namely the green synthesis method, for fabricating the proposed nanofluid, which exhibited unparalleled properties. Through a comprehensive exposition of empirical evidence and cogent arguments, this study substantiates the proposition that natural ester-based vegetable oil nanofluids hold immense promise as a superior alternative; this is attributable to their inherent properties and the innovative methodologies employed in their synthesis. Given the promising results, future research should focus on long-term stability and aging studies under various operating conditions to further validate this novel nanofluid’s long-term performance and reliability in real-world transformer applications.