The Influence of Nitrogen and Phosphorus on Adsorption, Dissolution and Carbon Flux of Limestone Under Different Soil Layer Depths
Abstract
1. Introduction
2. Materials and Methods
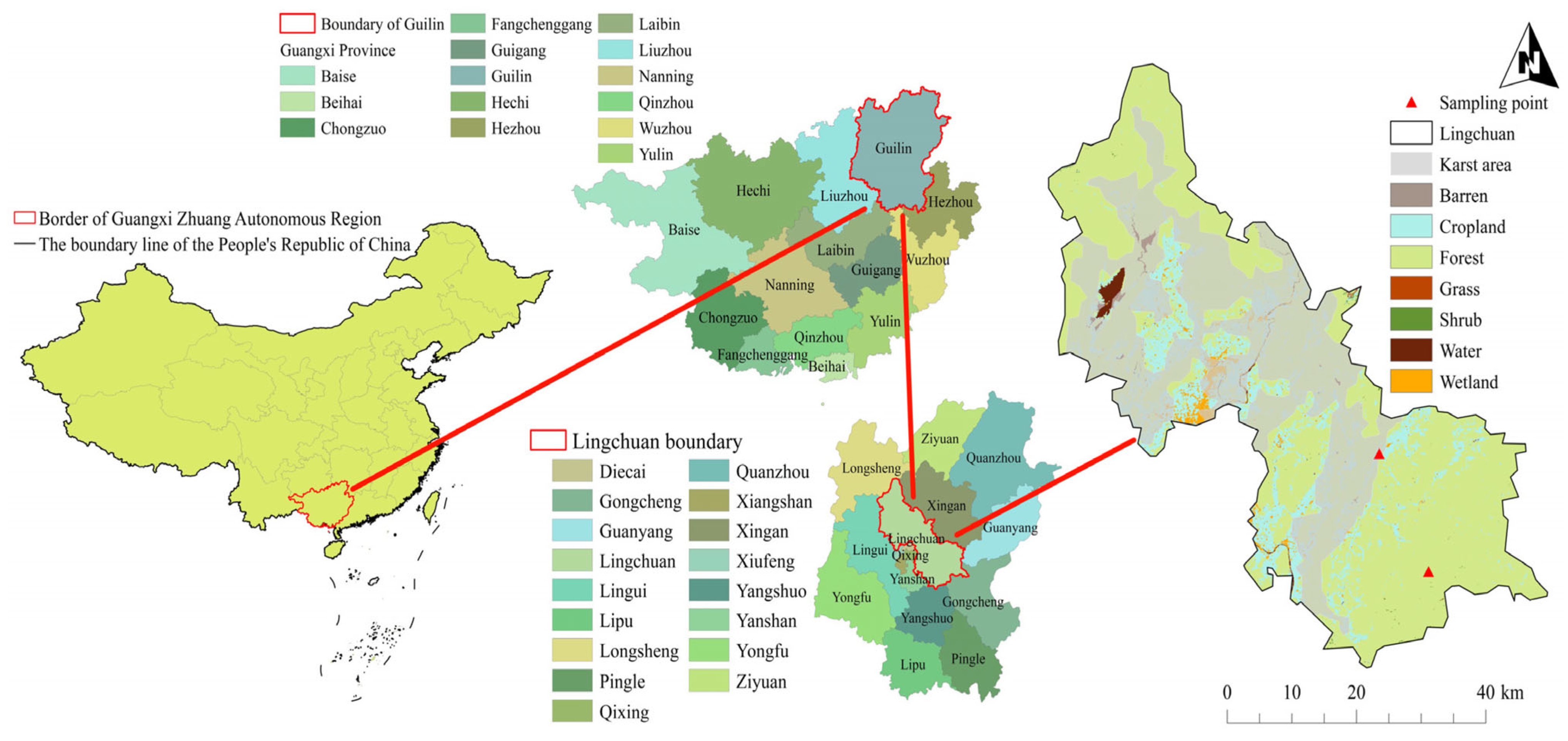
2.1. Study Area
2.2. Materials and Preparations
2.3. Characterization and Analysis Methods of Limestone
2.4. Experimental Setup and Methods
3. Results
3.1. Hydrochemical Processes in Soil–Limestone Systems
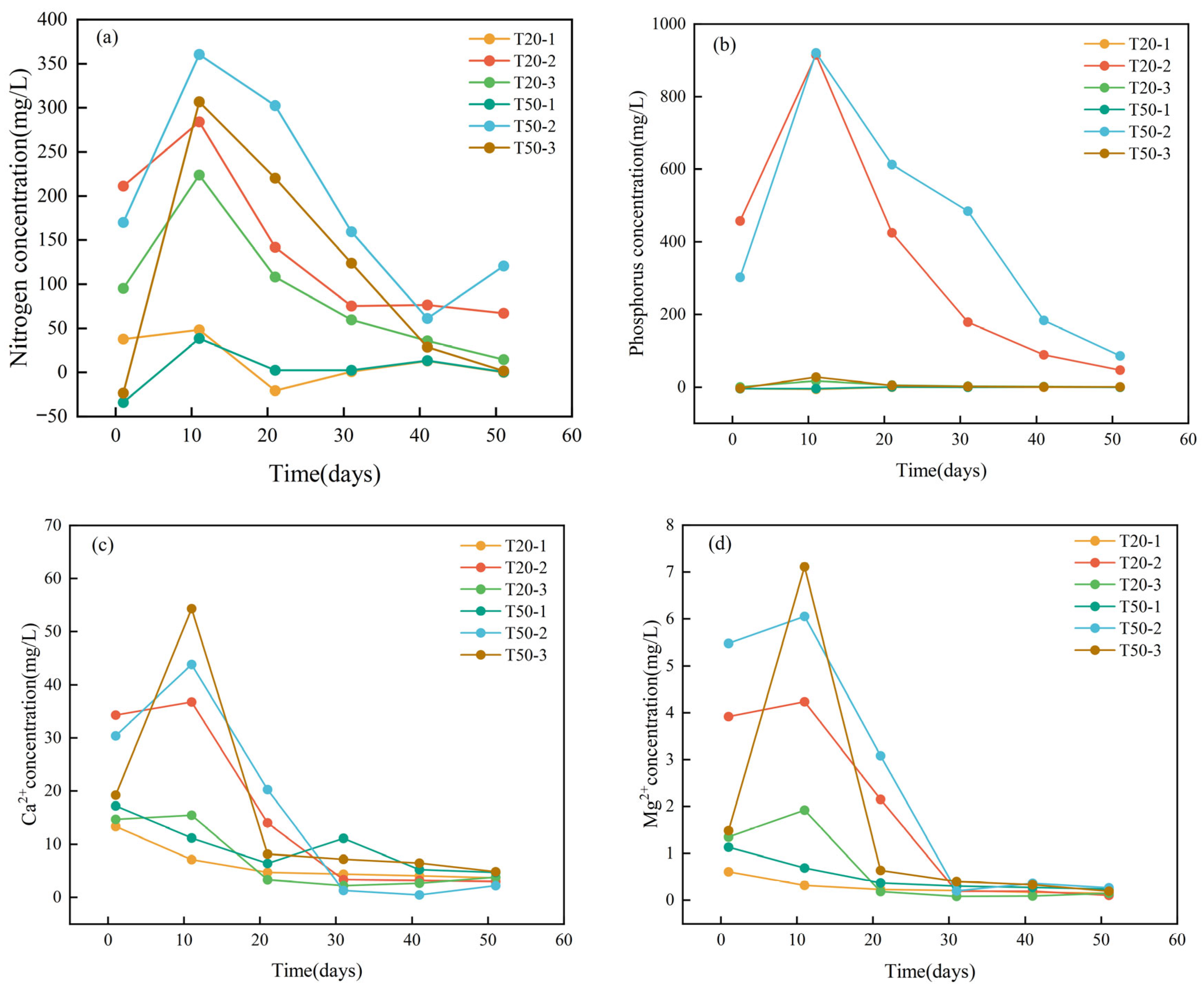
3.1.1. Analysis of the Influence of Nitrogen and Phosphorus Concentrations on the Concentrations of Ca2+ and Mg2+
3.1.2. Analysis of the Influence of Nitrogen and Phosphorus Concentrations on Water Chemical Parameters, Such as Inorganic Carbon
3.1.3. Analysis of the Dissolution Rate of Limestone
3.2. Morphological Characteristics of Limestone
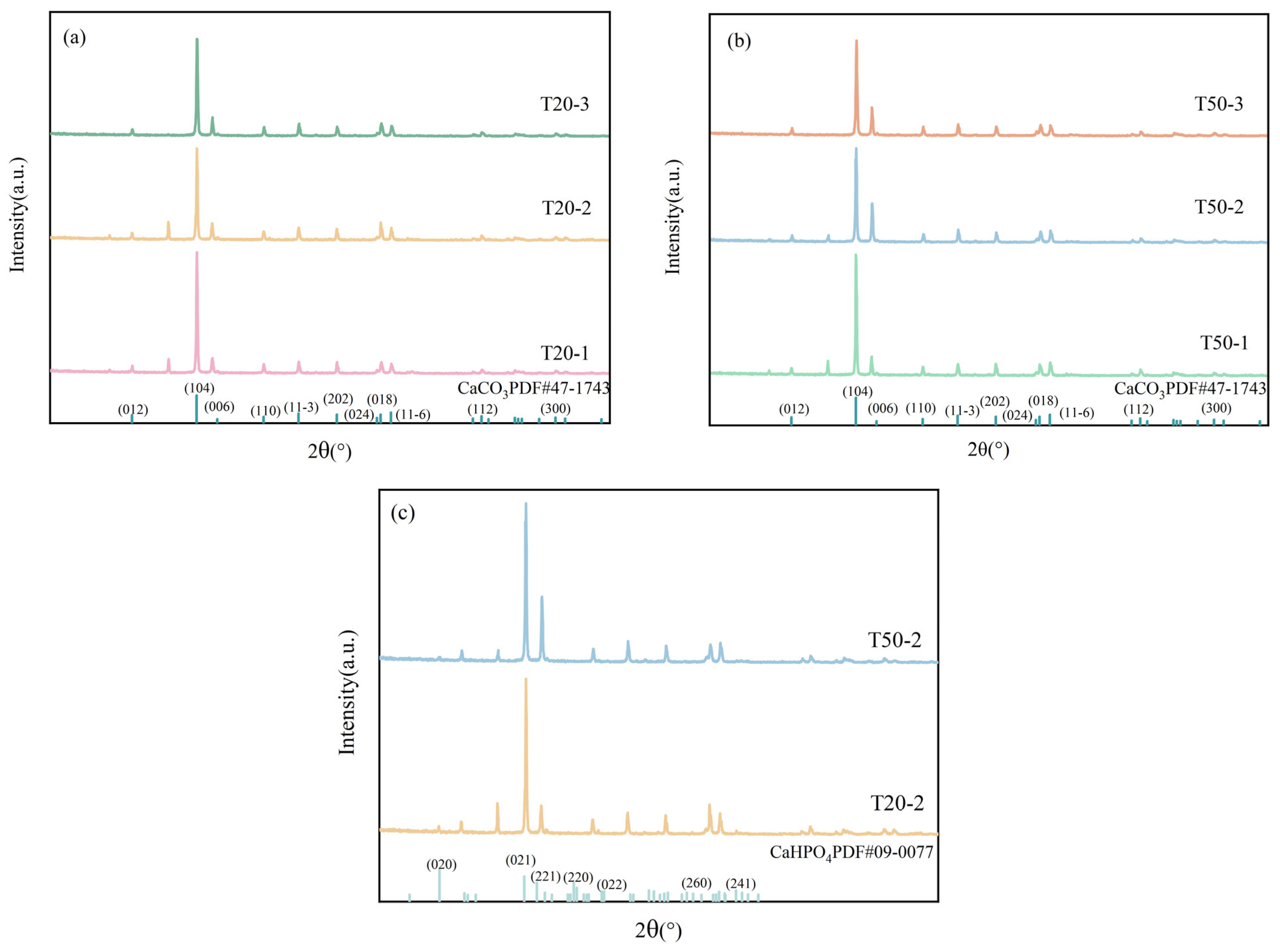
3.2.1. XRD Analysis of Limestone
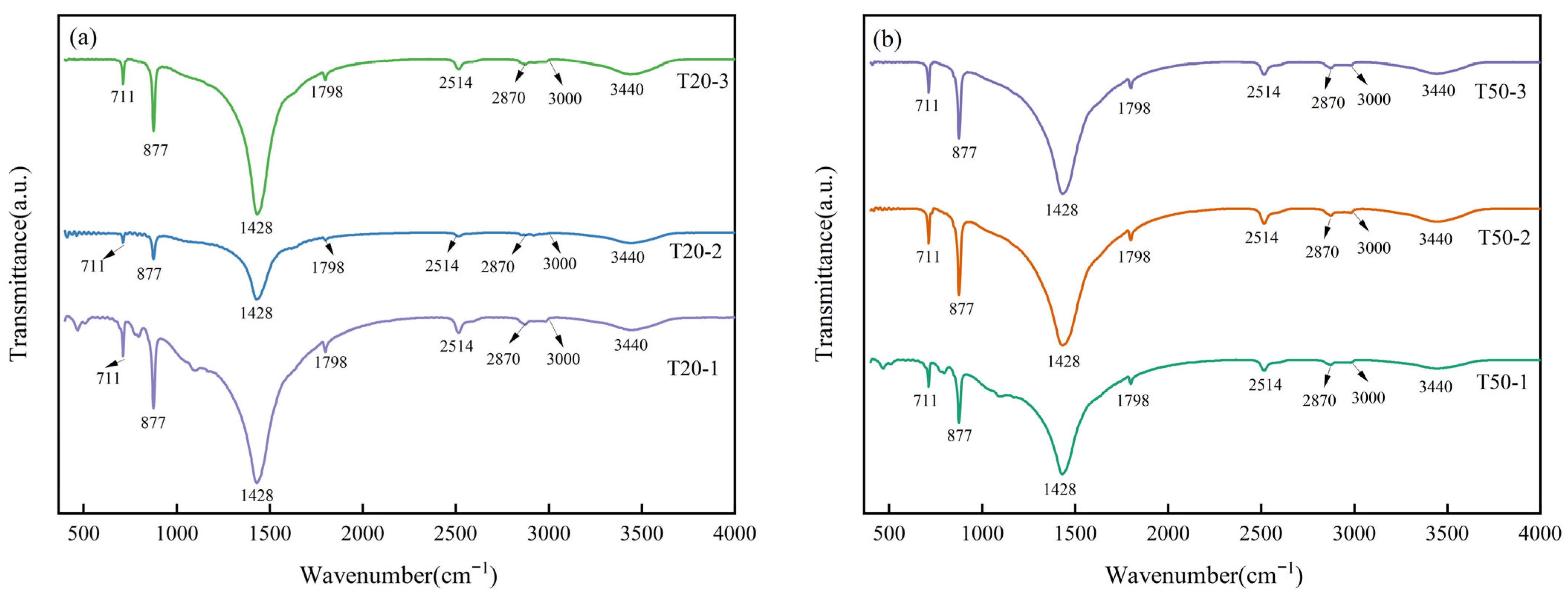
3.2.2. FT-IR Analysis of Limestone
3.2.3. XPS Analysis of Limestone
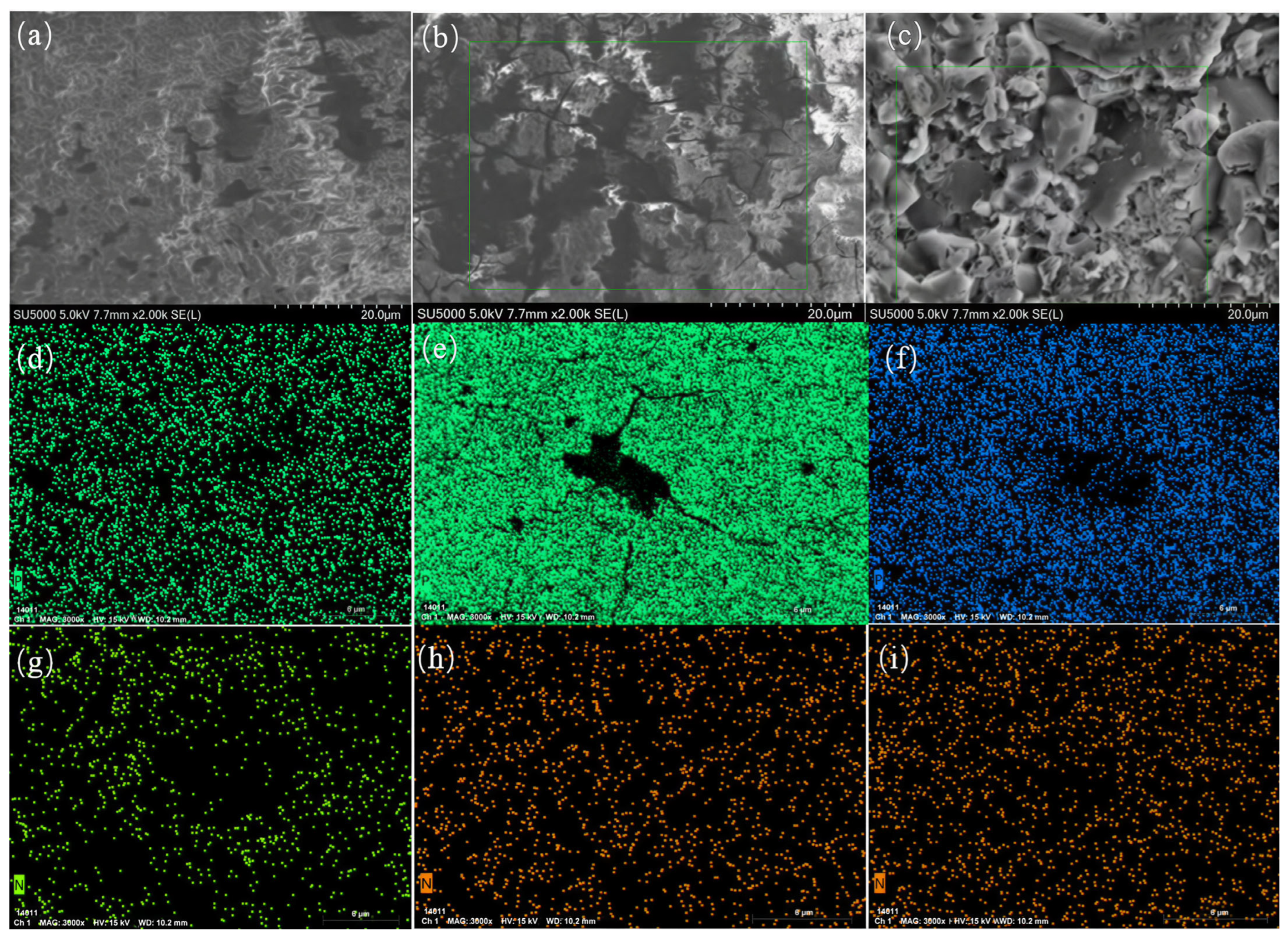
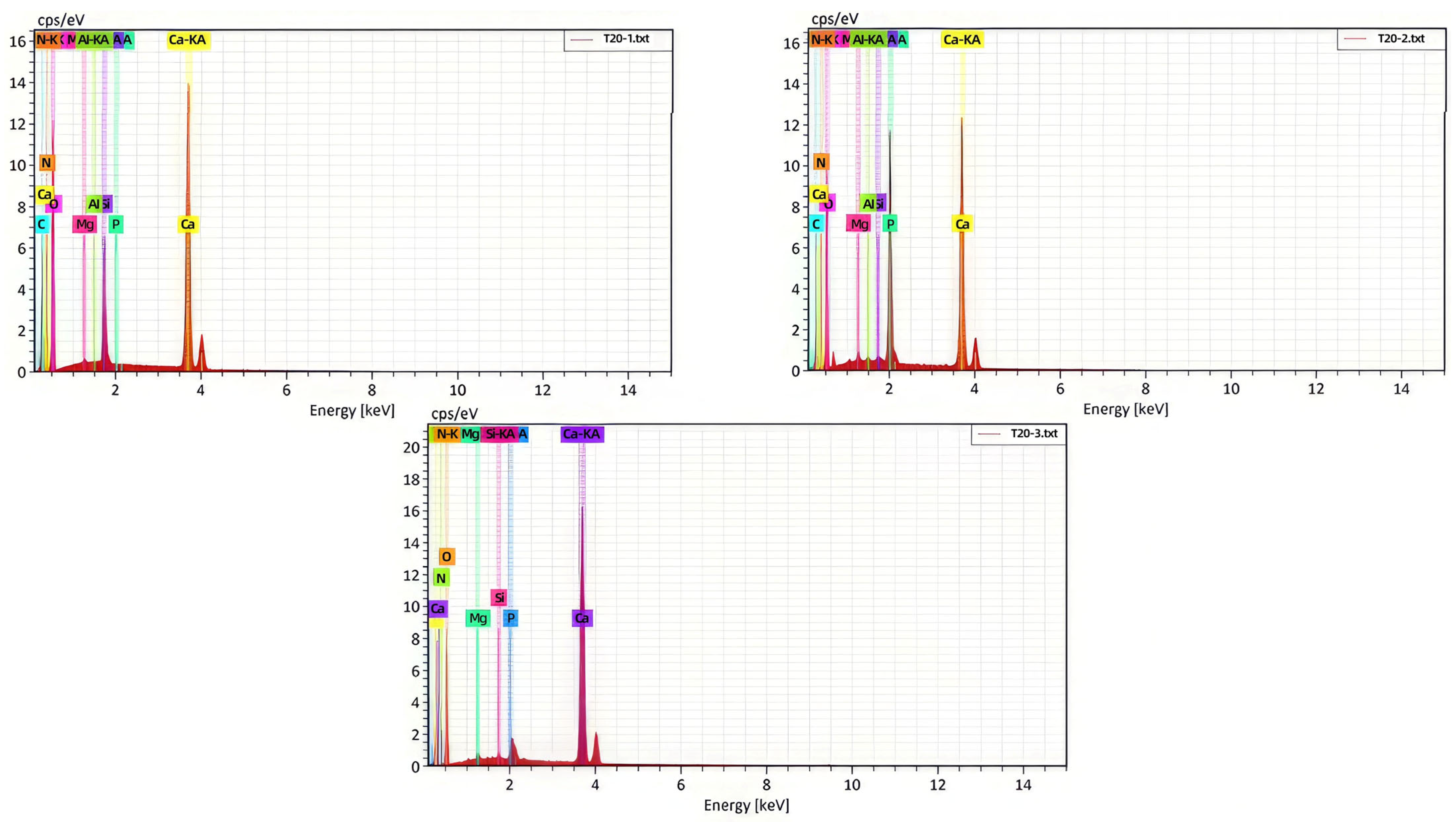
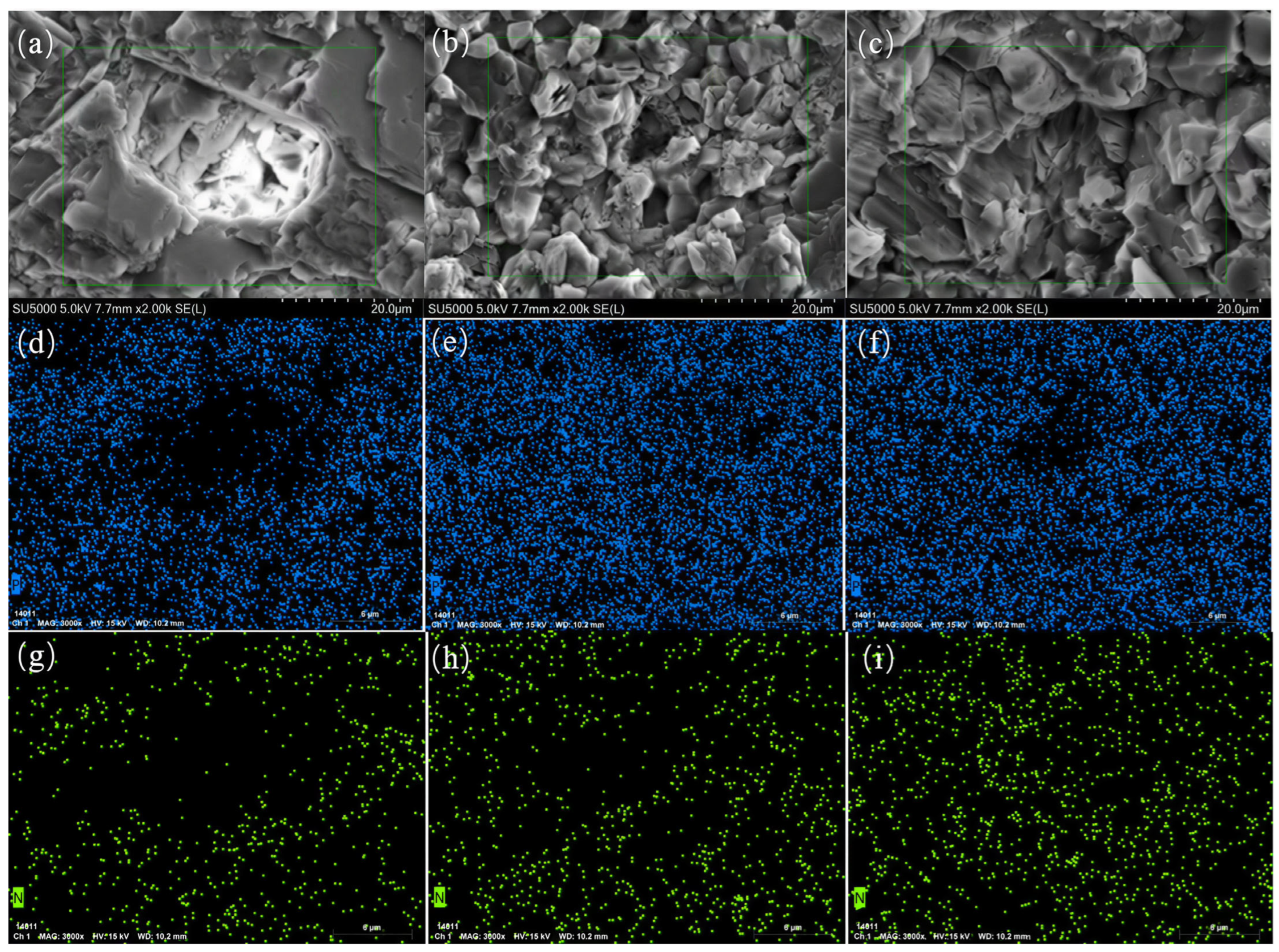
3.2.4. Characterization (SEM/EDS) Analysis of Limestone
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Y.; Luo, Z.; Luo, W.; Ma, T.; Wang, Y. Multiple Isotope Geochemistry and Hydrochemical Monitoring of Karst Water in a Rapidly Urbanized Region. J. Contam. Hydrol. 2018, 218, 44–58. [Google Scholar] [CrossRef]
- Ma, L.; Si, H.; Li, M.; Li, C.; Zhu, D.; Mao, Z.; Yan, Y.; Jiang, K.; Yu, P. Influence of Land Use Types on Soil Properties and Soil Quality in Karst Regions of Southwest China. Agronomy 2024, 14, 882. [Google Scholar] [CrossRef]
- Peng, J.; Jiang, H.; Liu, Q.; Green, S.M.; Quine, T.A.; Liu, H.; Qiu, S.; Liu, Y.; Meersmans, J. Human Activity vs. Climate Change: Distinguishing Dominant Drivers on LAI Dynamics in Karst Region of Southwest China. Sci. Total Environ. 2021, 769, 144297. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, T.Y.; Tu, W.; Pu, L. Analysis on the Changes of Fertilization Intensity and Efficiency in China’s Grain Production from 1980 to 2019. J. Sci. Food Agric. 2023, 103, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Jiang, M.; Su, R.; Luo, Y.; Jiang, Y.; Hu, R. Fertilization Intensities at the Buffer Zones of Ponds Regulate Nitrogen and Phosphorus Pollution in an Agricultural Watershed. Water Res. 2024, 250, 121033. [Google Scholar] [CrossRef] [PubMed]
- Janzen, H.H. Carbon Cycling in Earth Systems—A Soil Science Perspective. Agric. Ecosyst. Environ. 2004, 104, 399–417. [Google Scholar] [CrossRef]
- Ozlu, E.; Kumar, S. Response of Soil Organic Carbon, pH, Electrical Conductivity, and Water Stable Aggregates to Long-term Annual Manure and Inorganic Fertilizer. Soil Sci. Soc. Am. J. 2018, 82, 1243–1251. [Google Scholar] [CrossRef]
- Yu, H.; Ling, N.; Wang, T.; Zhu, C.; Wang, Y.; Wang, S.; Gao, Q. Responses of Soil Biological Traits and Bacterial Communities to Nitrogen Fertilization Mediate Maize Yields across Three Soil Types. Soil Tillage Res. 2019, 185, 61–69. [Google Scholar] [CrossRef]
- Chen, S.; Wei, X.; Cai, Y.; Li, H.; Li, L.; Pu, J. Spatiotemporal Evolution of Rocky Desertification and Soil Erosion in Karst Area of Chongqing and Its Driving Factors. Catena 2024, 242, 108108. [Google Scholar] [CrossRef]
- Huang, Q.; Qin, X.; Liu, P.; Zhang, L.; Su, C. Impact of Sulfuric and Nitric Acids on Carbonate Dissolution, and the Associated Deficit of CO 2 Uptake in the Upper–Middle Reaches of the Wujiang River, China. J. Contam. Hydrol. 2017, 203, 18–27. [Google Scholar] [CrossRef]
- Chen, L.; Tan, L.; Zhao, M.; Sinha, A.; Wang, T.; Gao, Y. Karst Carbon Sink Processes and Effects: A Review. Quat. Int. 2023, 652, 63–73. [Google Scholar] [CrossRef]
- Wang, S.; Jin, Z.; Li, X.; Zhu, H.; Fang, F.; Luo, T.; Li, J. Characterization of Microbial Carbon Metabolism in Karst Soils from Citrus Orchards and Analysis of Its Environmental Drivers. Microorganisms 2025, 13, 267. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.; Xin, S.; Huang, F.; Cao, J.; Xiao, J. Characteristics of Dissolution Changes in Carbonate Rocks and Their Influencing Factors in the Maocun Basin, Guilin, China. Water 2023, 15, 3285. [Google Scholar] [CrossRef]
- Pain, A.J.; Martin, J.B.; Young, C.R. Sources and Sinks of CO2 and CH4 in Siliciclastic Subterranean Estuaries. Limnol. Oceanogr. 2019, 64, 1500–1514. [Google Scholar] [CrossRef]
- Jiang, Z.; Lian, Y.; Qin, X. Carbon Cycle in the Epikarst Systems and Its Ecological Effects in South China. Environ. Earth Sci. 2013, 68, 151–158. [Google Scholar] [CrossRef]
- Li, J.; Xie, H.; Li, J.; Yang, G.; Xie, Y.; Wang, J.; Zhou, C.; Zou, S. Influences of Anthropogenic Acids on Carbonate Weathering and CO2 Sink in an Agricultural Karst Wetland (South China). Ecol. Indic. 2023, 150, 110192. [Google Scholar] [CrossRef]
- Xie, Y.; Hao, Y.; Li, J.; Guo, Y.; Xiao, Q.; Huang, F. Influence of Anthropogenic Sulfuric Acid on Different Lithological Carbonate Weathering and the Related Carbon Sink Budget: Examples from Southwest China. Water 2023, 15, 2933. [Google Scholar] [CrossRef]
- Yu, S.; Du, W.; Sun, P.; He, S.; Kuo, Y.; Yuan, Y.; Huang, J. Study on the Hydrochemistry Character and Carbon Sink in the Middle and Upper Reaches of the Xijiang River Basin, China. Environ. Earth Sci. 2015, 74, 997–1005. [Google Scholar] [CrossRef]
- Raymond, P.A.; Oh, N.-H.; Turner, R.E.; Broussard, W. Anthropogenically Enhanced Fluxes of Water and Carbon from the Mississippi River. Nature 2008, 451, 449–452. [Google Scholar] [CrossRef]
- Semhi, K.; Amiotte Suchet, P.; Clauer, N.; Probst, J.-L. Impact of Nitrogen Fertilizers on the Natural Weathering-Erosion Processes and Fluvial Transport in the Garonne Basin. Appl. Geochem. 2000, 15, 865–878. [Google Scholar] [CrossRef]
- Luo, R.; Fan, J.; Wang, W.; Luo, J.; Kuzyakov, Y.; He, J.-S.; Chu, H.; Ding, W. Nitrogen and Phosphorus Enrichment Accelerates Soil Organic Carbon Loss in Alpine Grassland on the Qinghai-Tibetan Plateau. Sci. Total Environ. 2019, 650, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, M.; Sun, X.; Li, L.; Guo, X.; Huang, L.; Xiao, J.; Xu, D.; Liu, D. High Concentration Phosphate Removal by Calcite and Its Subsequent Utilization for Tetracycline Removal. J. Water Process Eng. 2020, 37, 101412. [Google Scholar] [CrossRef]
- Yan, Z.; Chen, S.; Li, J.; Alva, A.; Chen, Q. Manure and Nitrogen Application Enhances Soil Phosphorus Mobility in Calcareous Soil in Greenhouses. J. Environ. Manag. 2016, 181, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Tang, J.; Xu, J.; Kou, T.; Huang, H. Accelerated Phosphorus Accumulation and Acidification of Soils under Plastic Greenhouse Condition in Four Representative Organic Vegetable Cultivation Sites. Sci. Hortic. 2015, 195, 67–73. [Google Scholar] [CrossRef]
- Krishnasamy, V.; Otte, J.; Silbergeld, E. Antimicrobial Use in Chinese Swine and Broiler Poultry Production. Antimicrob. Resist. Infect. Control 2015, 4, 17. [Google Scholar] [CrossRef]
- Gao, M.; Li, B.; Yu, A.; Liang, F.; Yang, L.; Sun, Y. The Effect of Aeration Rate on Forced-Aeration Composting of Chicken Manure and Sawdust. Bioresour. Technol. 2010, 101, 1899–1903. [Google Scholar] [CrossRef]
- Qian, X.; Gu, J.; Sun, W.; Wang, X.-J.; Su, J.-Q.; Stedfeld, R. Diversity, Abundance, and Persistence of Antibiotic Resistance Genes in Various Types of Animal Manure Following Industrial Composting. J. Hazard. Mater. 2018, 344, 716–722. [Google Scholar] [CrossRef]
- Chen, K.; Li, J.; Lin, L.; Qin, W.; Gao, Y.; Hu, E.; Jiang, J. Occurrence, Fate and Control Strategies of Heavy Metals and Antibiotics in Livestock Manure Compost Land Application: A Review. Sci. Total Environ. 2024, 957, 177381. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, B.; Wang, Y.; Xiao, J.; Wang, X. Composting Process and Odor Emission Varied in Windrow and Trough Composting System under Different Air Humidity Conditions. Bioresour. Technol. 2020, 297, 122482. [Google Scholar] [CrossRef]
- DZ/T 0295-2016; Specification of Land Quality Geochemical Assessment. Ministry of Land and Resources of the People’s Republic of China. Geological Publishing House: Beijing, China, 2016.
- Li, L.; Wang, W.; Jiang, Z.; Luo, A. Phosphate in Aqueous Solution Adsorbs on Limestone Surfaces and Promotes Dissolution. Water 2023, 15, 3230. [Google Scholar] [CrossRef]
- Gong, Y.; Chen, X.; Wu, W. Application of Fourier Transform Infrared (FTIR) Spectroscopy in Sample Preparation: Material Characterization and Mechanism Investigation. Adv. Sample Prep. 2024, 11, 100122. [Google Scholar] [CrossRef]
- Powell, C.J.; Jablonski, A. Progress in Quantitative Surface Analysis by X-Ray Photoelectron Spectroscopy: Current Status and Perspectives. J. Electron Spectrosc. Relat. Phenom. 2010, 178–179, 331–346. [Google Scholar] [CrossRef]
- Ural, N. The Significance of Scanning Electron Microscopy (SEM) Analysis on the Microstructure of Improved Clay: An Overview. Open Geosci. 2021, 13, 197–218. [Google Scholar] [CrossRef]
- Aquilina, L.; Poszwa, A.; Walter, C.; Vergnaud, V.; Pierson-Wickmann, A.-C.; Ruiz, L. Long-Term Effects of High Nitrogen Loads on Cation and Carbon Riverine Export in Agricultural Catchments. Environ. Sci. Technol. 2012, 46, 9447–9455. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Huang, F.; Yang, H.; Yu, S. Role of Anthropogenic Sulfuric and Nitric Acids in Carbonate Weathering and Associated Carbon Sink Budget in a Karst Catchment (Guohua), Southwestern China. J. Hydrol. 2021, 599, 126287. [Google Scholar] [CrossRef]
- Sawyer, C.N.; McCarty, P.L.; Parkin, G.F. Chemistry for Environmental Engineering and Science; McGraw–Hill: New York, NY, USA, 2003. [Google Scholar]
- Teir, S.; Eloneva, S.; Fogelholm, C.-J.; Zevenhoven, R. Stability of Calcium Carbonate and Magnesium Carbonate in Rainwater and Nitric Acid Solutions. Energy Convers. Manag. 2006, 47, 3059–3068. [Google Scholar] [CrossRef]
- Ruiz-Agudo, E.; Putnis, C.V.; Jiménez-López, C.; Rodriguez-Navarro, C. An Atomic Force Microscopy Study of Calcite Dissolution in Saline Solutions: The Role of Magnesium Ions. Geochim. Cosmochim. Acta 2009, 73, 3201–3217. [Google Scholar] [CrossRef]
- Ren, C.; Li, Y.; Zhou, Q.; Li, W. Phosphate Uptake by Calcite: Constraints of Concentration and pH on the Formation of Calcium Phosphate Precipitates. Chem. Geol. 2021, 579, 120365. [Google Scholar] [CrossRef]
- Torit, J.; Phihusut, D. Phosphorus Removal from Wastewater Using Eggshell Ash. Environ. Sci. Pollut. Res. 2019, 26, 34101–34109. [Google Scholar] [CrossRef]
- Ruffolo, S.A.; Comite, V.; La Russa, M.F.; Belfiore, C.M.; Barca, D.; Bonazza, A.; Crisci, G.M.; Pezzino, A.; Sabbioni, C. An Analysis of the Black Crusts from the Seville Cathedral: A Challenge to Deepen the Understanding of the Relationships among Microstructure, Microchemical Features and Pollution Sources. Sci. Total Environ. 2015, 502, 157–166. [Google Scholar] [CrossRef]
- Samanta, A.; Chanda, D.K.; Das, P.S.; Ghosh, J.; Mukhopadhyay, A.K.; Dey, A. Synthesis of Nano Calcium Hydroxide in Aqueous Medium. J. Am. Ceram. Soc. 2016, 99, 787–795. [Google Scholar] [CrossRef]
- Sdiri, A.; Higashi, T. Simultaneous Removal of Heavy Metals from Aqueous Solution by Natural Limestones. Appl. Water Sci. 2013, 3, 29–39. [Google Scholar] [CrossRef]
- Vanitha, N.; Jeyalakshmi, R. Structural Study of the Effect of Nano Additives on the Thermal Properties of Metakaolin Phosphate Geopolymer by MASNMR, XPS and SEM Analysis. Inorg. Chem. Commun. 2023, 153, 110758. [Google Scholar] [CrossRef]
- Jia, Z.; Zeng, W.; Xu, H.; Li, S.; Peng, Y. Adsorption Removal and Reuse of Phosphate from Wastewater Using a Novel Adsorbent of Lanthanum-Modified Platanus Biochar. Process Saf. Environ. Prot. 2020, 140, 221–232. [Google Scholar] [CrossRef]
- Baltrusaitis, J.; Grassian, V.H. Atomic Force Microscopy and X-Ray Photoelectron Spectroscopy Study of NO2 Reactions on CaCO3 (101̅4) Surfaces in Humid Environments. J. Phys. Chem. A 2012, 116, 9001–9009. [Google Scholar] [CrossRef]
- Lin, F.; Wang, Z.; Ma, Q.; Yang, Y.; Whiddon, R.; Zhu, Y.; Cen, K. Catalytic Deep Oxidation of NO by Ozone over MnO x Loaded Spherical Alumina Catalyst. Appl. Catal. B Environ. 2016, 198, 100–111. [Google Scholar] [CrossRef]
- Cáceres, R.; Malińska, K.; Marfà, O. Nitrification within Composting: A Review. Waste Manag. 2018, 72, 119–137. [Google Scholar] [CrossRef]
- Kong, J.; Zhou, Z.; Xie, R.; Chen, Z.; Li, R.; Li, L.; Cao, W. Anthropogenic Exogenous Nitric and Sulfuric Acids in Karst Plateau Reservoirs and Their Impact on Carbon Sinks. J. Hydrol. 2025, 660, 133394. [Google Scholar] [CrossRef]
- Yin, H.; Kong, M.; Fan, C. Batch Investigations on P Immobilization from Wastewaters and Sediment Using Natural Calcium Rich Sepiolite as a Reactive Material. Water Res. 2013, 47, 4247–4258. [Google Scholar] [CrossRef]
- Kõiv, M.; Liira, M.; Mander, Ü.; Mõtlep, R.; Vohla, C.; Kirsimäe, K. Phosphorus Removal Using Ca-Rich Hydrated Oil Shale Ash as Filter Material–the Effect of Different Phosphorus Loadings and Wastewater Compositions. Water Res. 2010, 44, 5232–5239. [Google Scholar] [CrossRef]
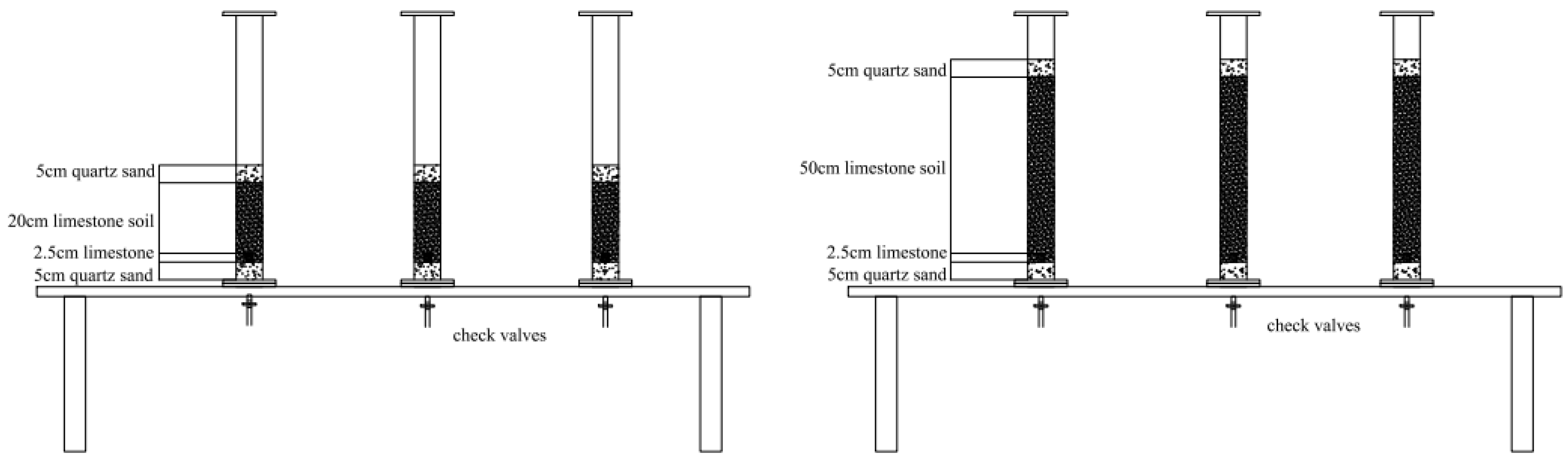






| 20 cm Column Experimental Group | Control Soil Column (T20–1) | 970 g calcareous soil + 13.3666 g limestone |
| Compound Fertilizer Soil Column (T20–2) | 970 g calcareous soil + 50 g compound fertilizer + 13.3974 g limestone | |
| Chicken Manure Fertilizer Soil Column (T20–3) | 970 g calcareous soil + 50 g chicken manure fertilizer + 14.0047 g limestone | |
| 50 cm Column Experimental Group | Control Soil Column (T50–1) | 2430 g calcareous soil + 13.6705 g limestone |
| Compound Fertilizer Soil Column (T50–2) | 2430 g calcareous soil + 50 g compound fertilizer + 13.9890 g limestone | |
| Chicken Manure Fertilizer Soil Column (T50–3) | 2430 g calcareous soil + 50 g chicken manure fertilizer + 13.7280 g limestone |
| T20–1 | T20–2 | T20–3 | T50–1 | T50–2 | T50–3 | |
|---|---|---|---|---|---|---|
| Weight before the experiment (g) | 13.3666 | 13.3974 | 14.0047 | 13.6705 | 13.9890 | 13.7280 |
| Weight after the experiment (g) | 13.3599 | 13.3818 | 13.9982 | 13.6631 | 13.9723 | 13.7211 |
| Erosion amount (g) | 0.0067 | 0.0156 | 0.0065 | 0.0074 | 0.0167 | 0.0069 |
| Dissolution rate (mg·cm−2.d−1) | 0.0069 | 0.0160 | 0.0066 | 0.0076 | 0.0171 | 0.0071 |
| Project Name | ω(B)% |
|---|---|
| SiO2 | 2.10 |
| Al2O3 | 0.06 |
| Fe2O3 | 0.015 |
| CaO | 54.38 |
| MgO | 0.64 |
| K2O | 0.25 |
| Na2O | 0.21 |
| MnO | 0.004 |
| P2O5 | 0.015 |
| Element | Line Type | Apparent Concentration | wt.% | wt.% Sigma |
|---|---|---|---|---|
| O | K–line system | 65,464 | 28.79 | 5.77 |
| C | K–line system | 24,292 | 10.68 | 1.76 |
| Ca | K–line system | 112,478 | 49.47 | 0.77 |
| Mg | K–line system | 24,972 | 10.98 | 0.21 |
| P | K–line system | 72 | 0.03 | 0 |
| N | K–line system | 96 | 0.05 | 0.13 |
| Total quantity: | 100.00 | |||
| Element | Line Type | Apparent Concentration | wt.% | wt.% Sigma |
|---|---|---|---|---|
| O | K–line system | 51,552 | 15.82 | 4.76 |
| C | K–line system | 10,372 | 3.18 | 1.21 |
| Ca | K–line system | 156,528 | 48.04 | 0.96 |
| Mg | K–line system | 2900 | 0.89 | 0.05 |
| P | K–line system | 103,837 | 31.87 | 0.47 |
| N | K–line system | 606 | 0.2 | 0.36 |
| Total quantity: | 100.00 | |||
| Element | Line Type | Apparent Concentration | wt.% | wt.% Sigma |
|---|---|---|---|---|
| O | K–line system | 18,264 | 12.34 | 4.21 |
| C | K–line system | 7845 | 5.30 | 1.01 |
| Ca | K–line system | 118,688 | 80.21 | 1.24 |
| Mg | K–line system | 396 | 0.27 | 0.04 |
| P | K–line system | 2539 | 1.72 | 0.05 |
| N | K–line system | 241 | 0.16 | 0.41 |
| Total quantity: | 100.00 | |||
| Element | Line Type | Apparent Concentration | wt.% | wt.% Sigma |
|---|---|---|---|---|
| O | K–line system | 30,019 | 23.08 | 5.10 |
| C | K–line system | 7441 | 5.72 | 1.35 |
| Ca | K–line system | 58,492 | 44.98 | 0.73 |
| Mg | K–line system | 33,950 | 26.11 | 0.53 |
| P | K–line system | 4 | 0.01 | 0.01 |
| N | K–line system | 131 | 0.10 | 0.24 |
| Total quantity: | 100.00 | |||
| Element | Line Type | Apparent Concentration | wt.% | wt.% Sigma |
|---|---|---|---|---|
| O | K–line system | 24,022 | 15.17 | 4.18 |
| C | K–line system | 10,197 | 6.44 | 1.12 |
| Ca | K–line system | 119,208 | 75.26 | 1.10 |
| Mg | K–line system | 401 | 0.25 | 0.11 |
| P | K–line system | 4382 | 2.76 | 0.06 |
| N | K–line system | 183 | 0.12 | 0.24 |
| Total quantity: | 100.00 | |||
| Element | Line Type | Apparent Concentration | wt.% | wt.% Sigma |
|---|---|---|---|---|
| O | K–line system | 21,015 | 14.64 | 4.47 |
| C | K–line system | 5776 | 4.02 | 0.85 |
| Ca | K–line system | 110,454 | 76.94 | 1.15 |
| Mg | K–line system | 4691 | 3.27 | 0.12 |
| P | K–line system | 1406 | 0.98 | 0.04 |
| N | K–line system | 211 | 0.15 | 0.34 |
| Total quantity: | 100.00 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; He, H.; Li, J.; Wang, W.; Jiang, Z. The Influence of Nitrogen and Phosphorus on Adsorption, Dissolution and Carbon Flux of Limestone Under Different Soil Layer Depths. Sustainability 2025, 17, 11326. https://doi.org/10.3390/su172411326
Li L, He H, Li J, Wang W, Jiang Z. The Influence of Nitrogen and Phosphorus on Adsorption, Dissolution and Carbon Flux of Limestone Under Different Soil Layer Depths. Sustainability. 2025; 17(24):11326. https://doi.org/10.3390/su172411326
Chicago/Turabian StyleLi, Liang, Haiping He, Jiacai Li, Wenhai Wang, and Zhiwei Jiang. 2025. "The Influence of Nitrogen and Phosphorus on Adsorption, Dissolution and Carbon Flux of Limestone Under Different Soil Layer Depths" Sustainability 17, no. 24: 11326. https://doi.org/10.3390/su172411326
APA StyleLi, L., He, H., Li, J., Wang, W., & Jiang, Z. (2025). The Influence of Nitrogen and Phosphorus on Adsorption, Dissolution and Carbon Flux of Limestone Under Different Soil Layer Depths. Sustainability, 17(24), 11326. https://doi.org/10.3390/su172411326







