Experimental Investigation of Biodiesel Fuels Obtained by Enriching the Content of Vegetable and Waste Oils with Nanoparticles and Modeling of Data Obtained from the Produced Fuel Samples Using Artificial Intelligence
Abstract
1. Introduction
2. Materials and Methods
2.1. Reaction Parameters
2.2. Fuel Sample Production
2.3. Chemical Analysis of Fuel Samples
2.4. Nanoparticle Addition to Fuel Samples
2.4.1. Mn2O3 Particle Technical Specifications
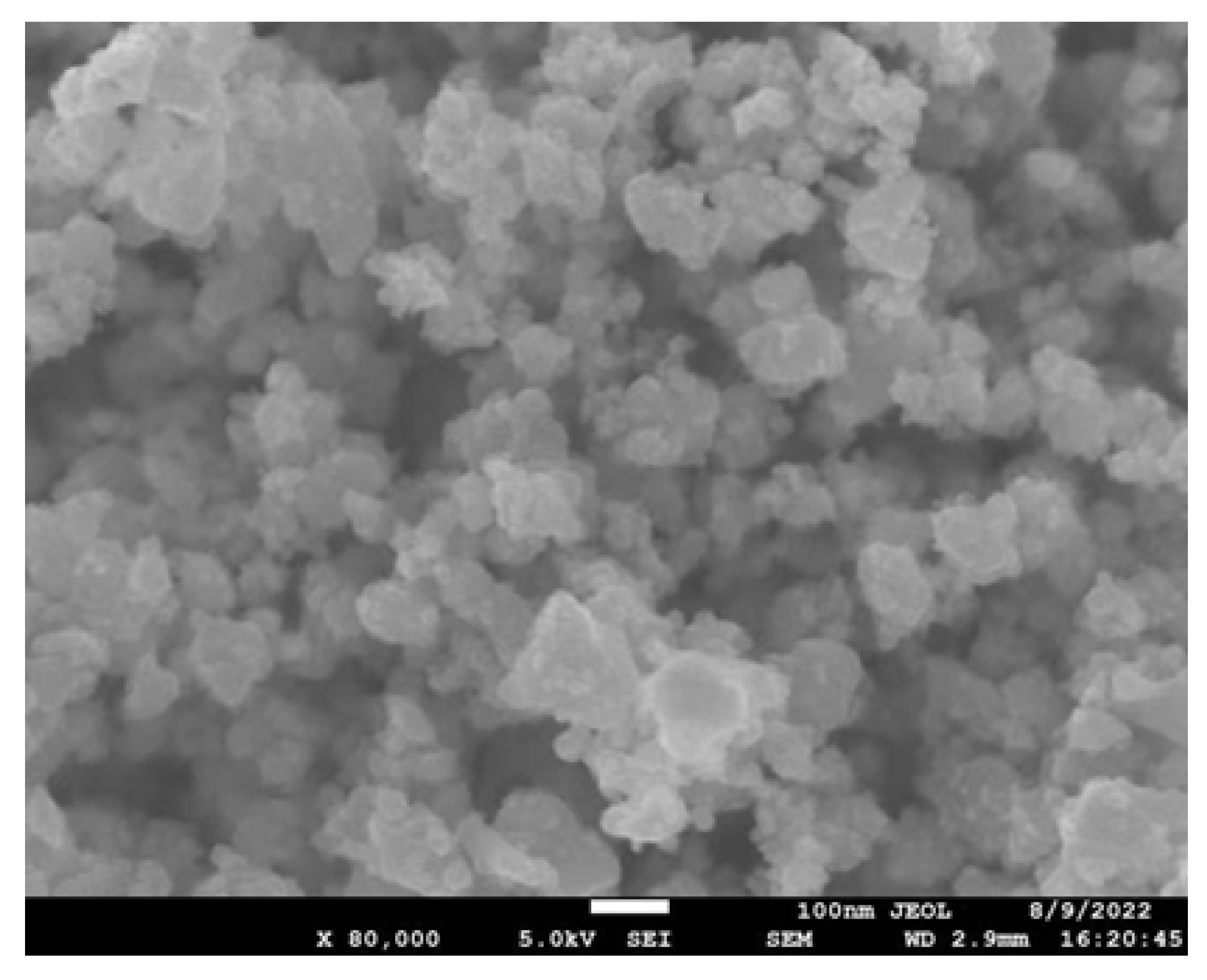
2.4.2. Mn2O3 Nanoparticle SEM Image
2.4.3. TEM Image of Mn2O3
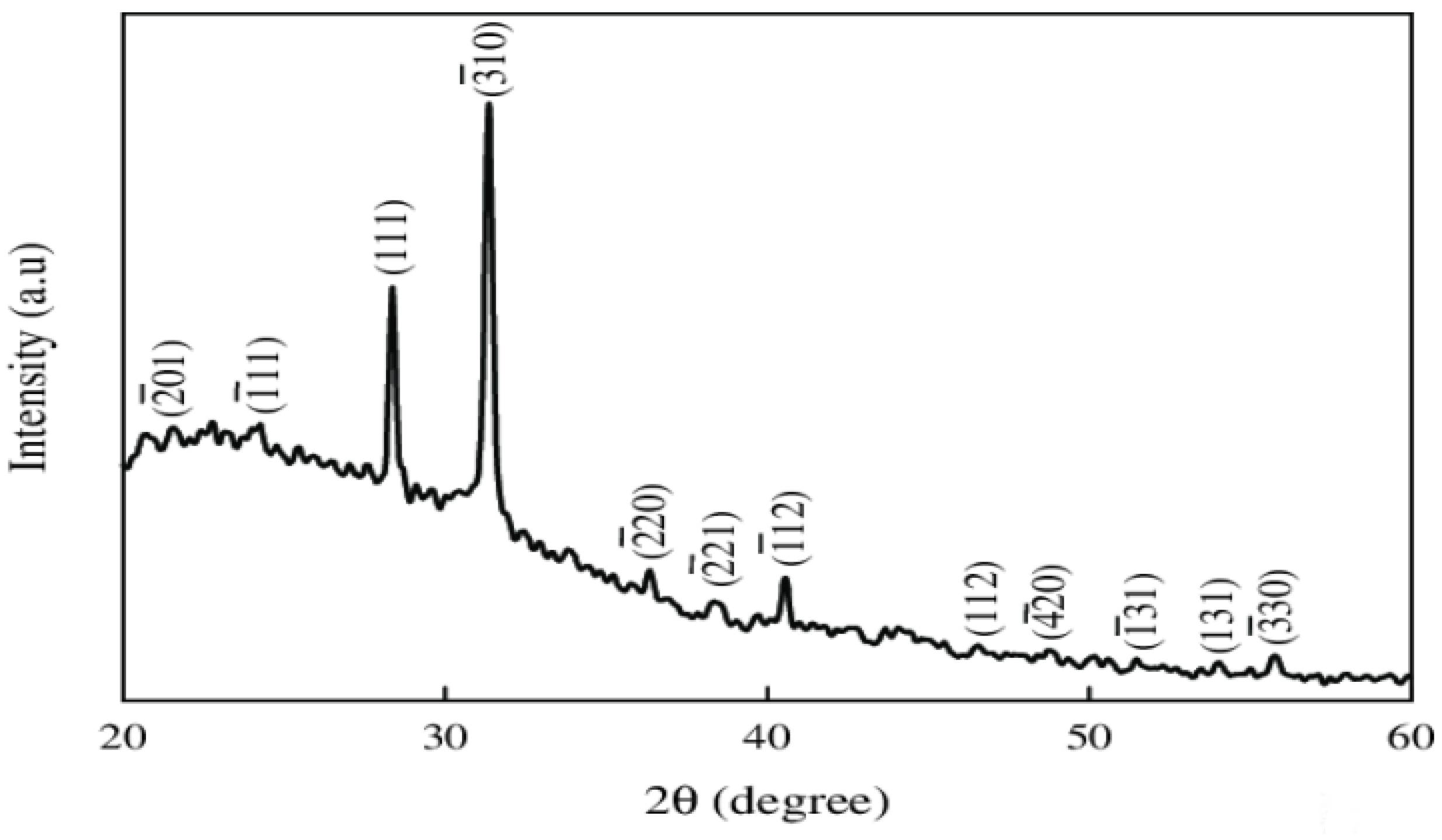
2.4.4. X-Ray Diffraction (XRD) Analysis of Mn2O3 Nanoparticles
2.5. Biodiesel Blend with Mn2O3 Nanoparticles
2.6. Experiment Setup
2.6.1. Calculation Method
Specific Fuel Consumption
- B: Hourly fuel consumption (kg/h);
- Δt: Time during which 500 mL of fuel is consumed;
- ρy: Fuel density (kg/L);
- be: Specific fuel consumption (kg/kWh);
- Pe: Engine power (kW).
Thermal Efficiency
- B: Hourly fuel consumption (kg/h);
- Ne: Effective motor power (kW);
- ηt: Thermal efficiency;
- Hu: Lower heating value of fuel (kJ/kg).
2.6.2. Error Analysis
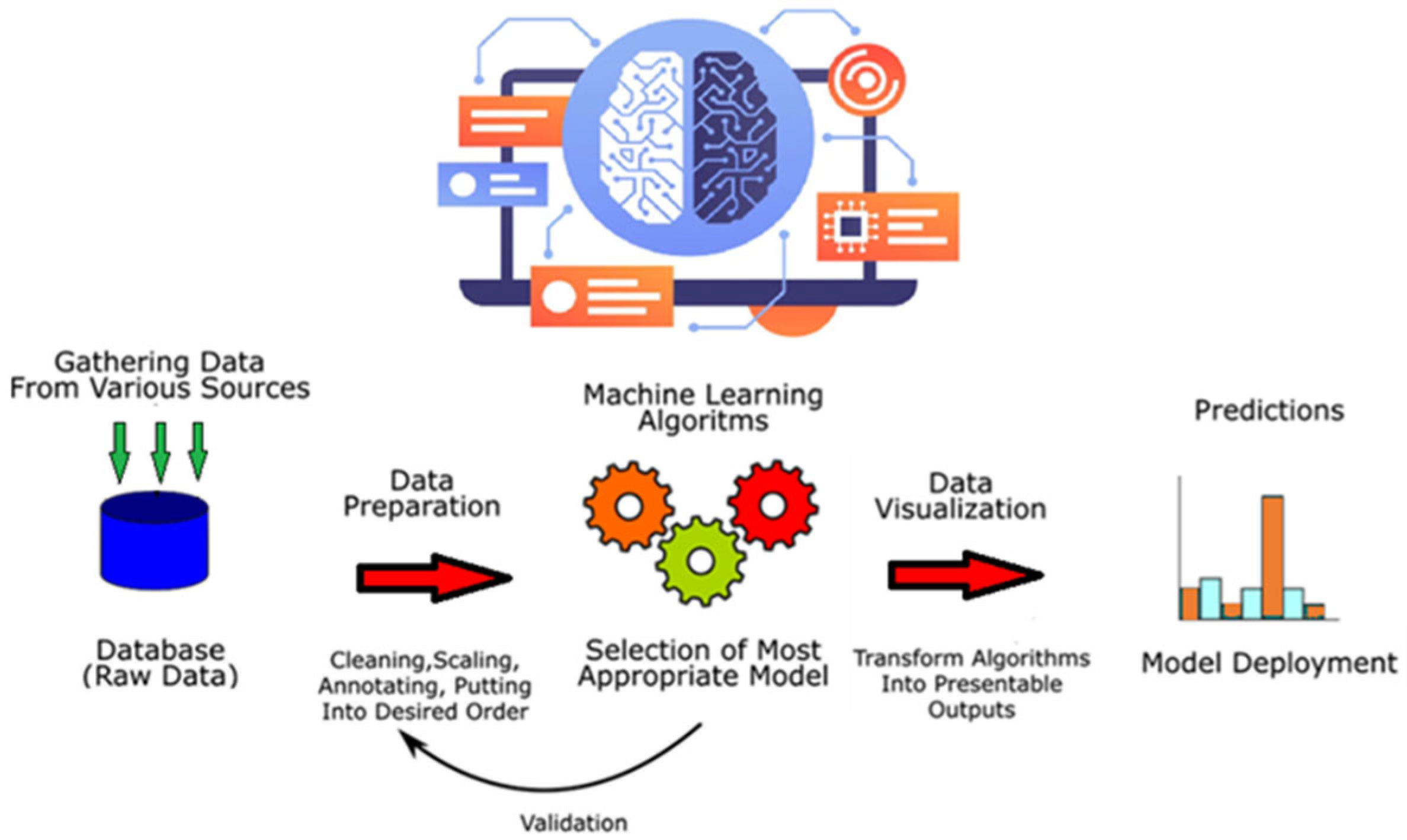
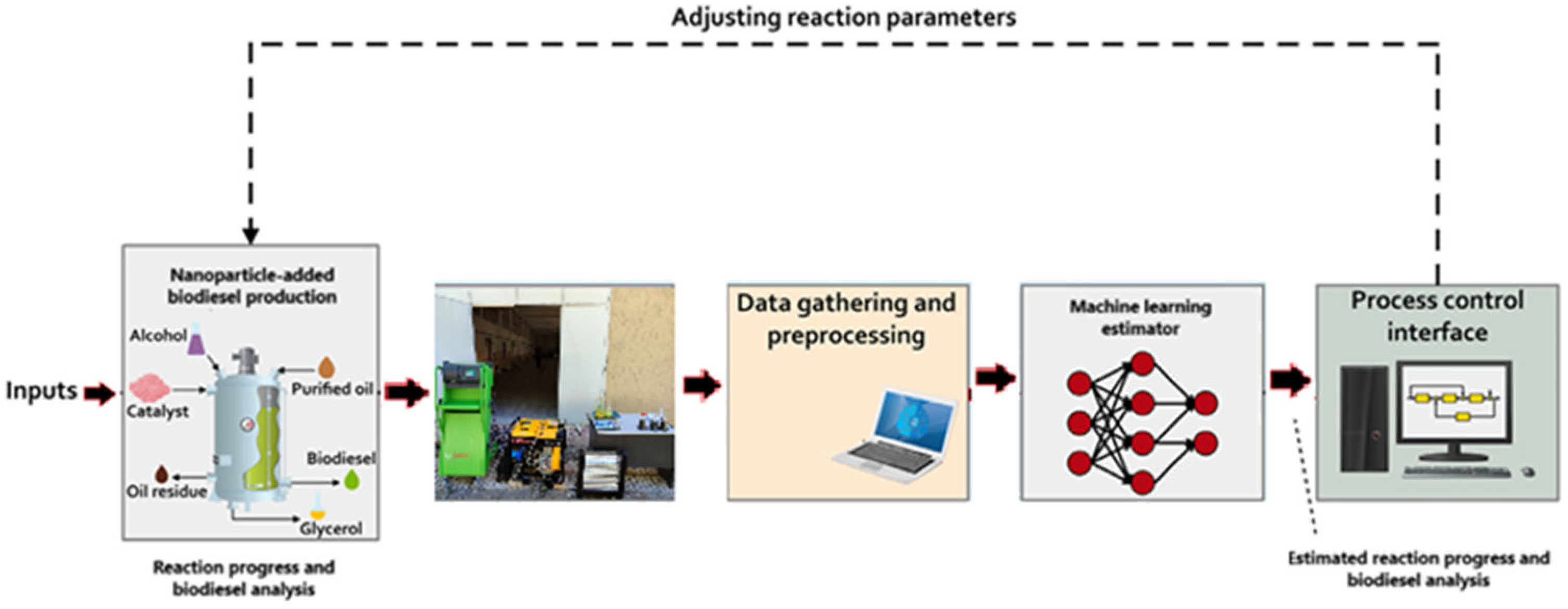
2.7. Machine Learning System (MLS)
2.8. Optimization and Artificial Intelligence Modeling
3. Results
3.1. Investigation of Specific Fuel Consumption Values
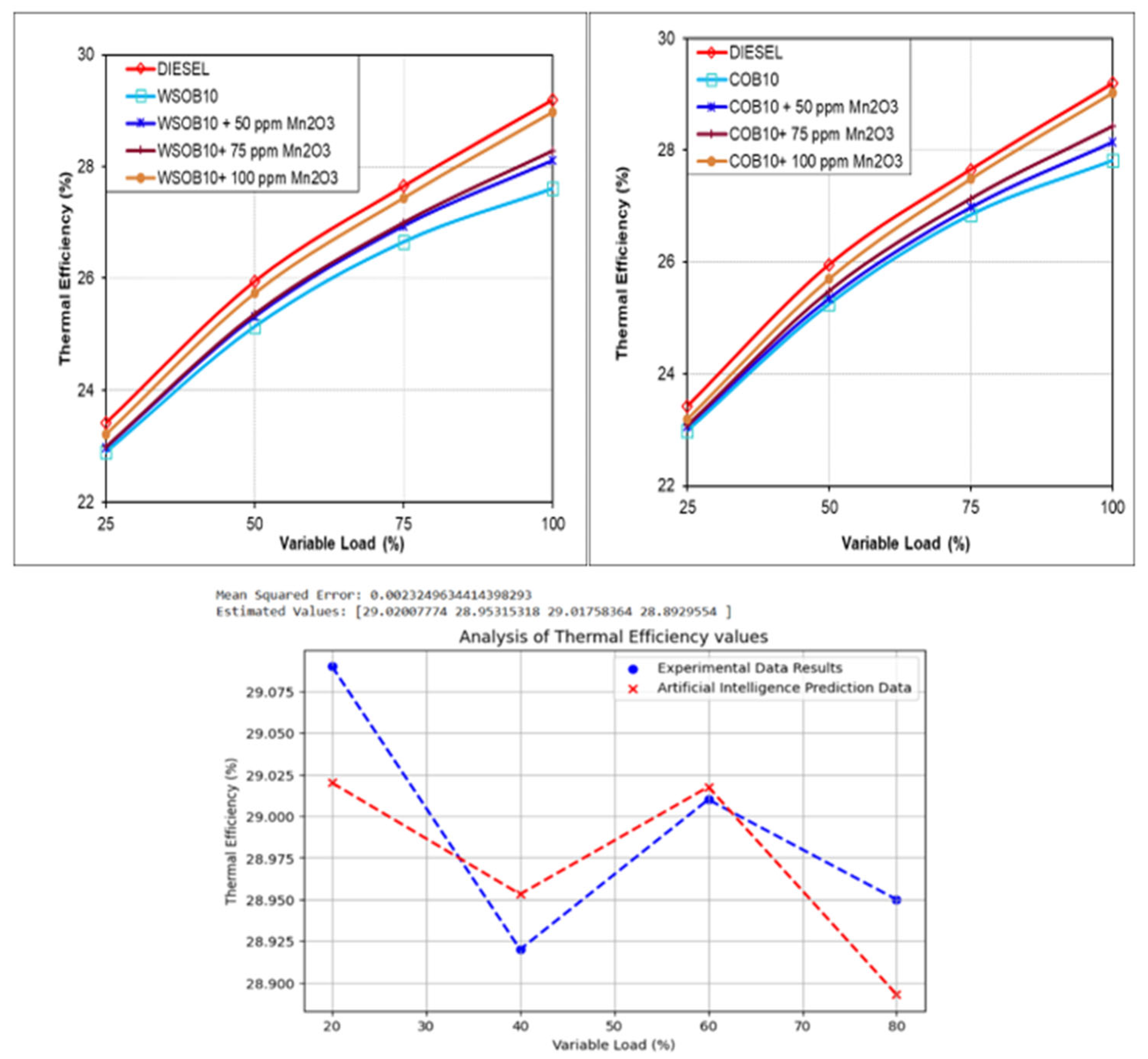
3.2. Analysis of Thermal Efficiency Parameters
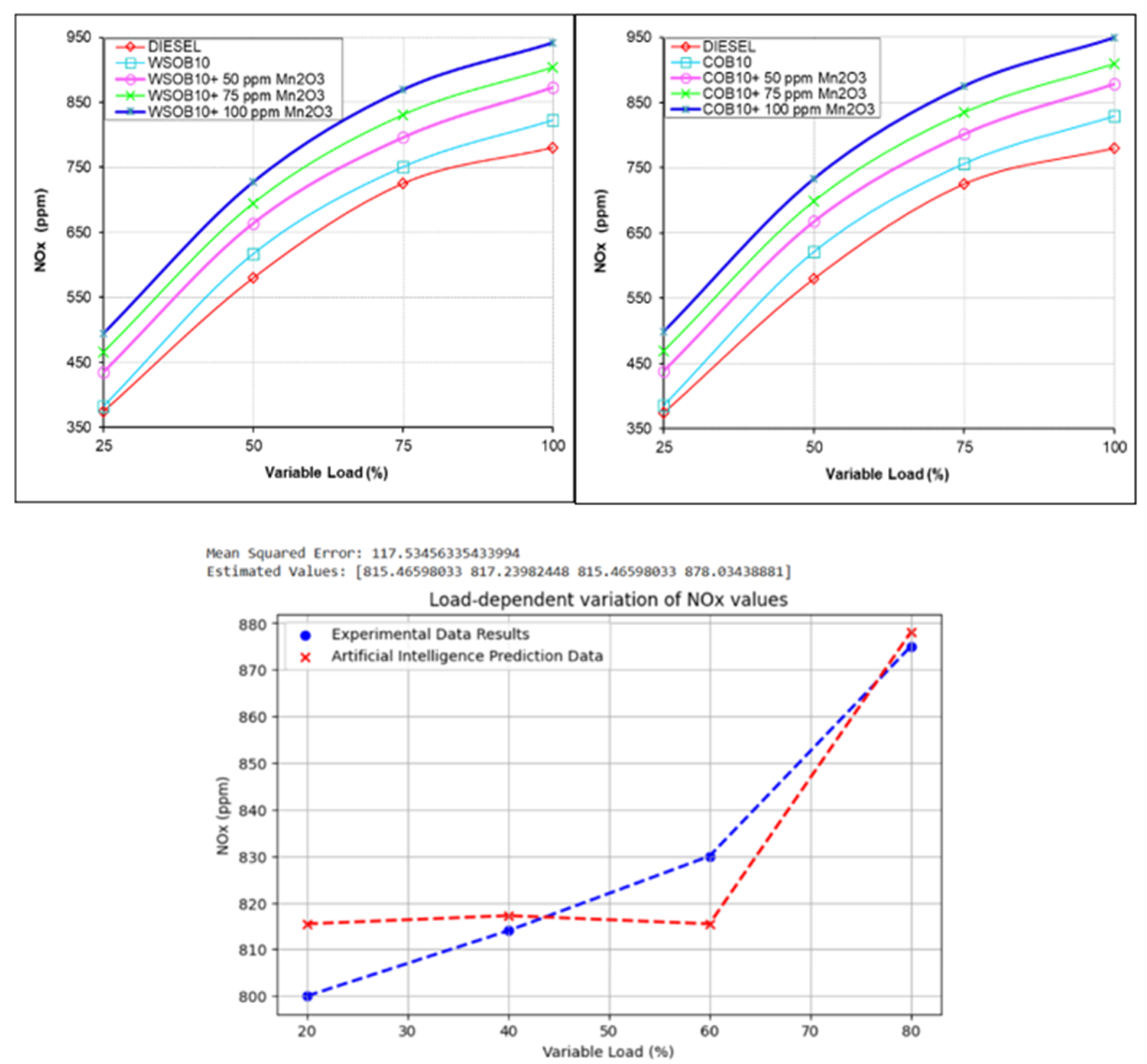
3.3. Investigation of NOx Emission Values
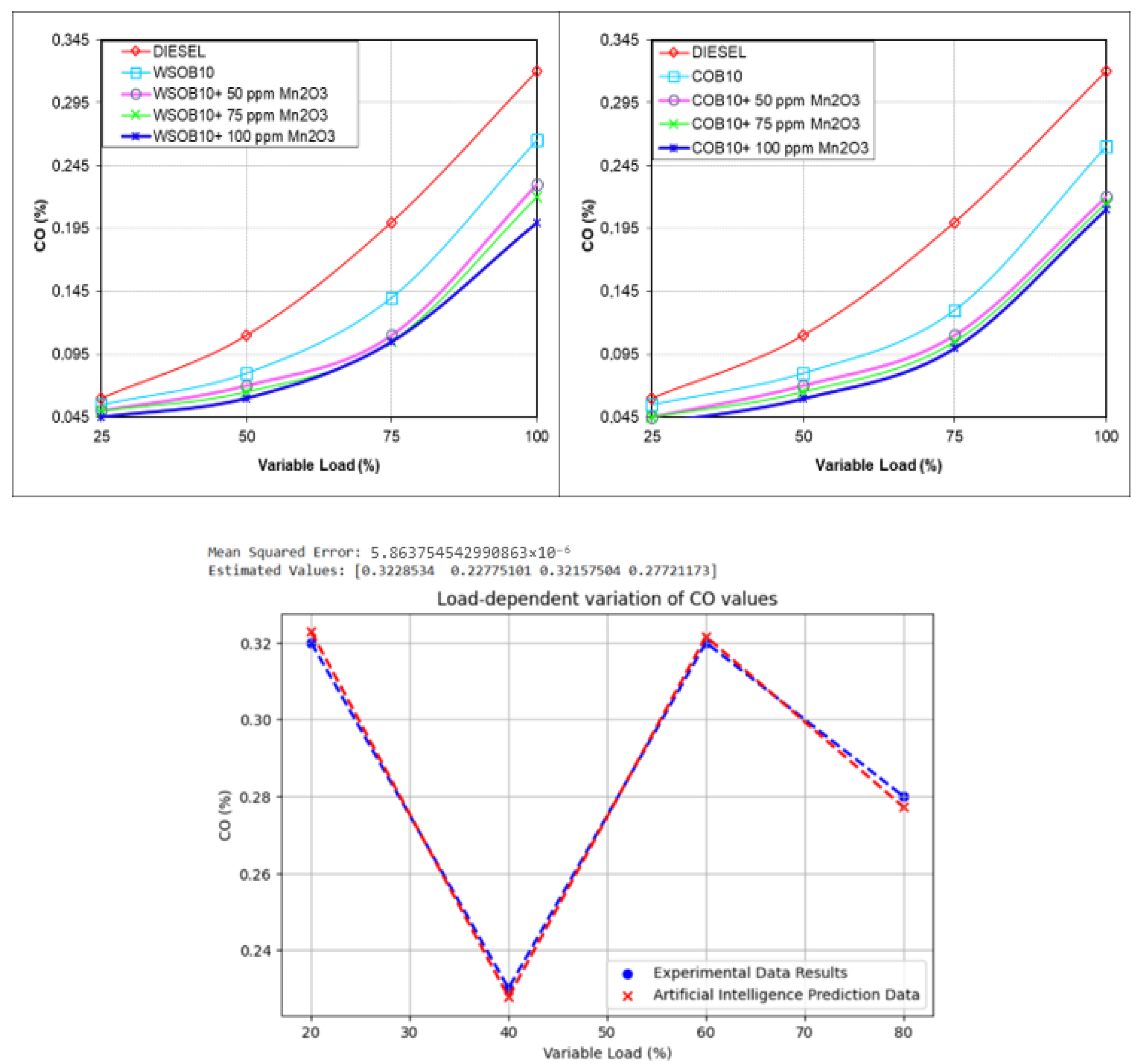
3.4. Investigation of CO Emission Values
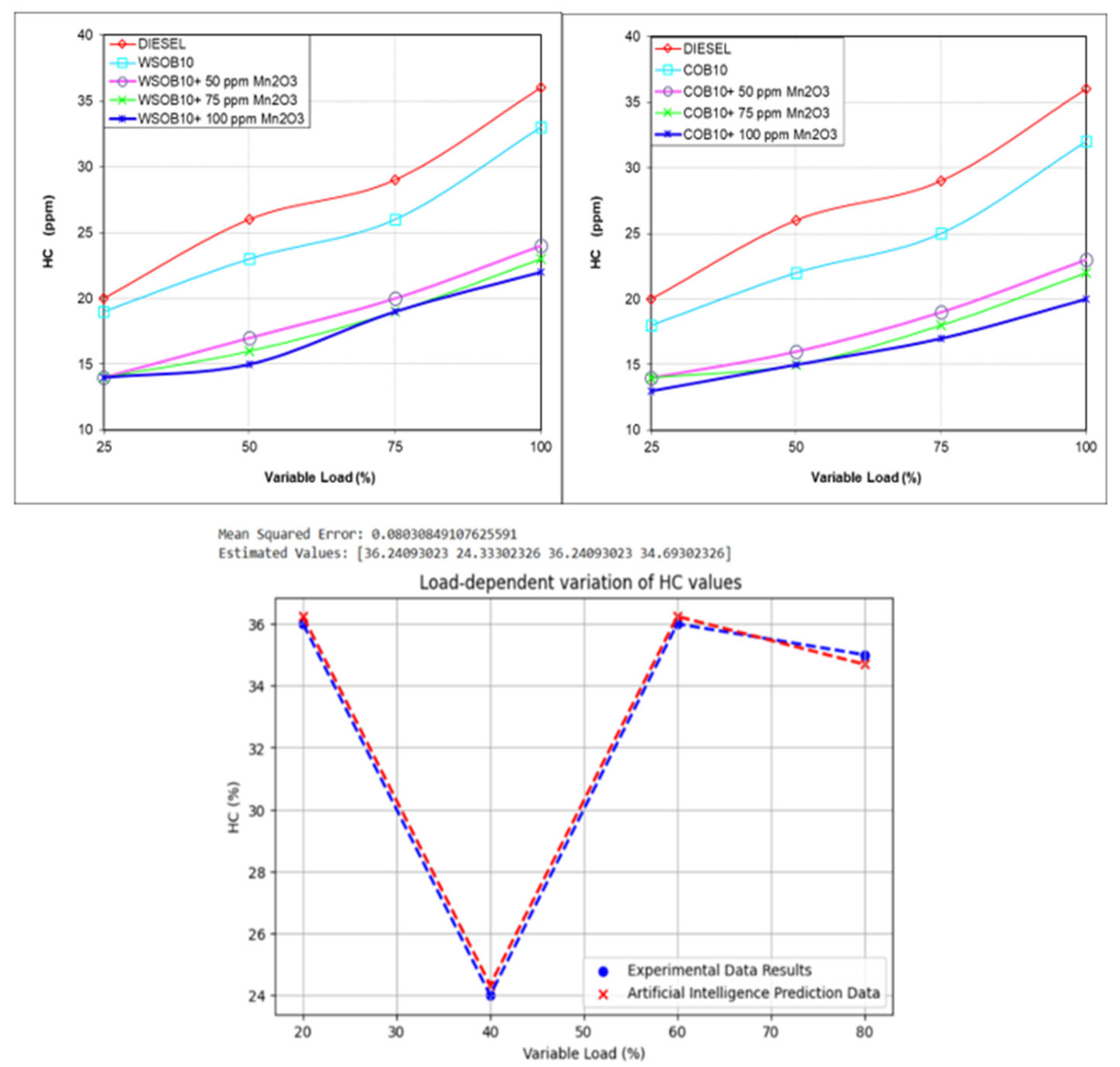
3.5. Investigation of HC Emission Values
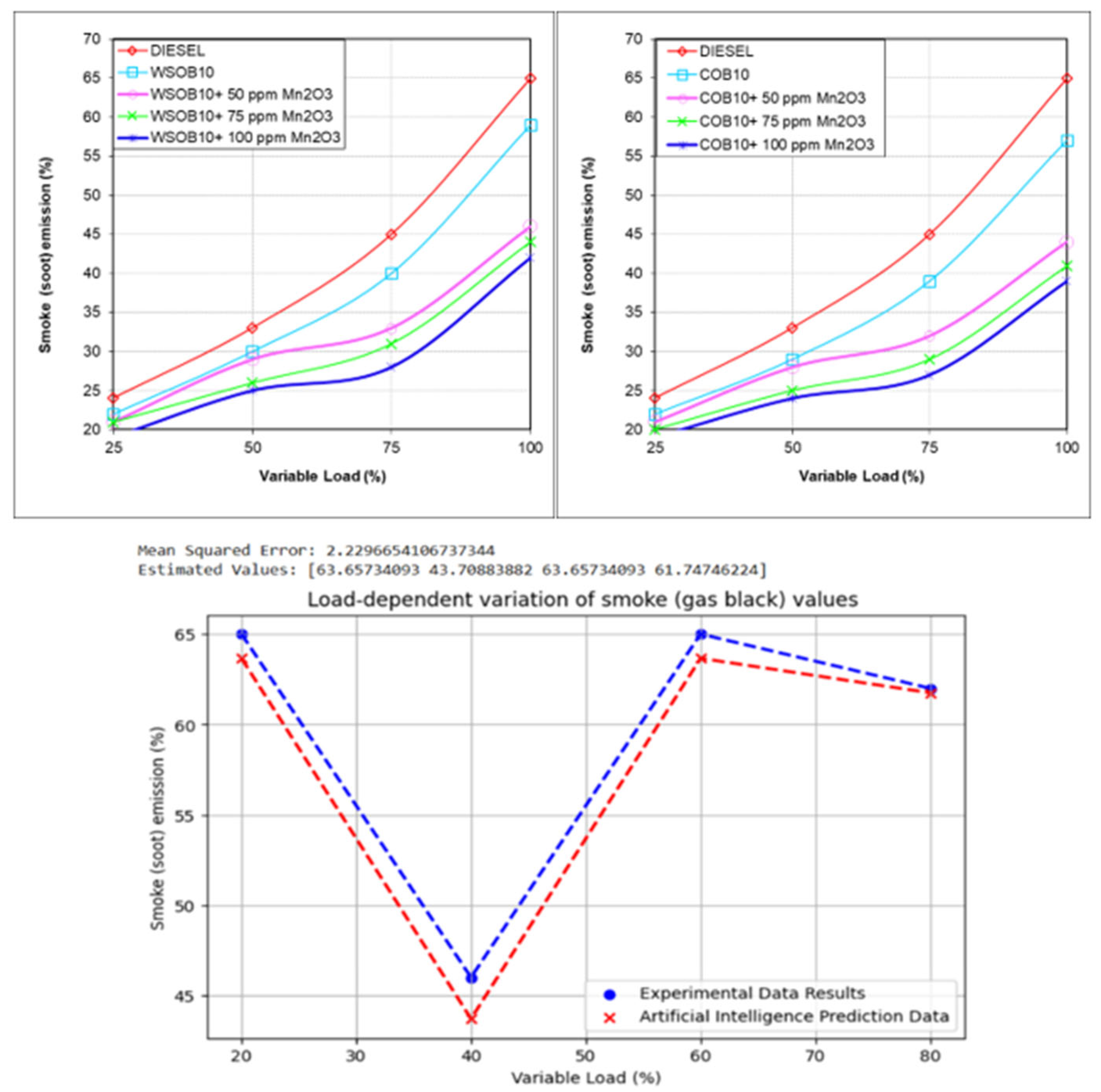
3.6. Investigation of Smoke Emission Values
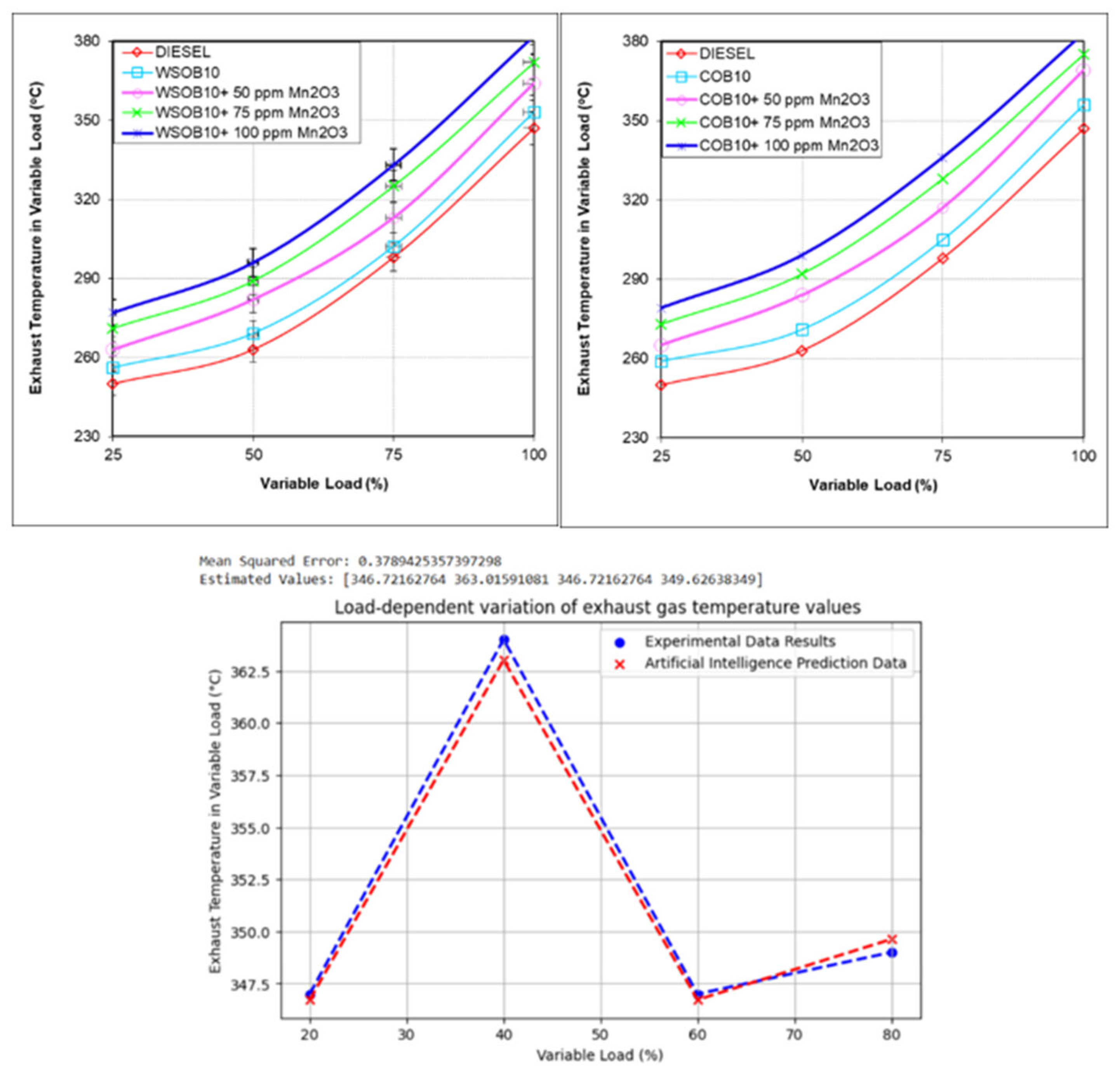
3.7. Investigation of Exhaust Gas Temperature Emission Values
4. Conclusions and Discussion
- Mn2O3 nanoparticle additives significantly improved the combustion efficiency and reduced harmful emissions in biodiesel–diesel blends;
- The optimal fuel blend (COB10+ 100 ppm Mn2O3) achieved a 3.25% increase in thermal efficiency and 2.08% decrease in specific fuel consumption;
- Substantial reductions were observed in CO (37.50%), HC (38.8%), and smoke (33.84%) emissions compared to diesel fuel;
- Artificial intelligence modeling using the linear regression method accurately predicted emission parameters, with a mean squared error of 5.86 × 10−6 for CO;
- Mn2O3-doped biodiesel fuels produced from waste vegetable oils provide an economical and eco-friendly alternative without requiring engine modifications;
- Future research should focus on long-term durability tests, NOx mitigation strategies, and techno-economic analyses to enhance practical applicability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, V.; Singh, K.P. Machine learning-driven predictive modeling of biodiesel characteristics with heterogeneous catalysts: A review. Mach. Learn. 2023, 55, 4301–4315. [Google Scholar]
- Khan, O.; Ali, V.; Parvez, M.; Alhodaib, A.; Yahya, Z.; Yadav, A.K.; Ağbulut, Ü. Exploring the performance of biodiesel-hydrogen blends with diverse nanoparticles in diesel engine: A hybrid machine learning K-means clustering approach with weighted performance metrics. Int. J. Hydrogen Energy 2024, 78, 547–563. [Google Scholar] [CrossRef]
- Tiwari, C.; Dwivedi, G.; Verma, T.N. Experimental study of diesel engine performance and emissions from microalgae fuel blends with SiO2 nanoadditives: RSM and Taguchi optimization. Environ. Sci. Pollut. Res. 2025, 32, 19596–19617. [Google Scholar] [CrossRef]
- Rajak, U.; Panchal, M.; Dasore, A.; Verma, T.N.; Chaurasiya, P.K. Predict the characteristics of the DI engine with various injection timings by Glycine max oil biofuel using artificial neural networks. Environ. Sci. Pollut. Res. 2025, 32, 19546–19561. [Google Scholar] [CrossRef] [PubMed]
- Ardebili, S.M.S.; Kocakulak, T.; Aytav, E.; Calam, A. Investigation of the effect of JP-8 fuel and biodiesel fuel mixture on engine performance and emissions by experimental and statistical methods. Energy 2022, 254, 124155. [Google Scholar] [CrossRef]
- Rao, S.S.; Paparao, J.; Raju, M.V.J.; Kumar, S. Effect of nanoparticle-doped biofuel in a dual-fuel diesel engine with oxy-hydrogen gas. Int. J. Hydrogen Energy 2024, 70, 146–158. [Google Scholar]
- Ramalingam, S.; Munuswamy, D.B.; Devarajan, Y. Combustion enhancement and emission reduction in RCCI engine using green synthesized CuO nanoparticles with Cymbopogon martinii methyl ester and phytol blends. Ind. Crops Prod. 2024, 218, 118969. [Google Scholar] [CrossRef]
- Akalın, M. Hydrothermal liquefaction of microalgae with metal halides for bio-crude production. J. Fac. Eng. Archit. Gazi Univ. 2019, 34, 845–853. [Google Scholar]
- Uyar, M.; Aydın, H. Production of low sulfur diesel-like fuel from crude oil wastes by pyrolytic distillation and its usage in a diesel engine. Energy 2022, 244, 122683. [Google Scholar] [CrossRef]
- Sathya, A.B.; Thirunavukkarasu, A.; Nithya, R.; Nandan, A.; Sakthishobana, K.; Kola, A.K.; Deepanraj, B. Microalgal biofuel production: Potential challenges and prospective research. Fuel 2023, 332, 126199. [Google Scholar] [CrossRef]
- Fadhil, A.B. Evaluation of apricot (Prunus armeniaca L.) seed kernel as a potential feedstock for the production of liquid bio-fuels and activated carbons. Energy Convers. Manag. 2017, 133, 307–317. [Google Scholar] [CrossRef]
- Aydın, B.; Çelik, M. Investigation of the effect of cerium oxide (CeO2) nanoparticle added biodiesel-diesel (B20) blend on performance and emissions. Afyon Kocatepe Univ. J. Sci. Eng. Sci. 2022, 22, 689–702. [Google Scholar]
- Cinar, G.; Akyuz, O. Investigation of the effect of Cu2O and Al2O3 nanoparticles on engine performance and emissions. Eur. J. Sci. Technol. 2022, 53–61. [Google Scholar] [CrossRef]
- Hazar, H.; Uyar, M. Experimental investigation of isopropyl alcohol (IPA)/diesel blends in a diesel engine for improved exhaust emissions. Int. J. Automot. Eng. Technol. 2015, 4, 1–6. [Google Scholar] [CrossRef]
- Khan, O.; Parvez, M.; Alhodaib, A.; Yahya, Z.; Ahamad, T.; Yadav, A.K.; Shukla, A.K. Development and selection of lignocellulose biomass and nano-additive combination for co-pyrolysis operation in power generation using hybrid prediction and machine learning model: A K-means cluster approach. Sustain. Energy Technol. Assess. 2024, 72, 104061. [Google Scholar] [CrossRef]
- Ghanbari, M.; Najafi, G.; Ghobadian, B.; Yusaf, T.; Carlucci, A.P.; Kiani, M.K.D. Performance and emission characteristics of a CI engine using nanoparticle additives in biodiesel-diesel blends and modeling with GP approach. Fuel 2017, 202, 699–716. [Google Scholar] [CrossRef]
- Singh, B.; Srivastava, A.K.; Prakash, O. Effect on combustion performance and emission behavior of diesel engine fueled with hybrid biodiesel produced from a novel ternary oil mixture incorporated with nano additive. Energy Sources Part A Recover. Util. Environ. Eff. 2024, 46, 6566–6585. [Google Scholar] [CrossRef]
- Hazar, H.; Uyar, M.; Aydın, H.; Sap, E. Usage of methyl ester produced from waste grape and Mn additive as alternative diesel fuel. Sci. Bull. Petru Maior Univ. Targu Mures 2017, 14, 5–11. [Google Scholar] [CrossRef]
- ISO/TR 18196:2016; Nanotechnologies—Terminology and Definitions for Nano-Objects. International Organization for Standardization: Geneva, Switzerland, 2016.
- Kalaimurugan, K.; Karthikeyan, S.; Periyasamy, M.; Mahendran, G. Emission analysis of CI engine with CeO2 nanoparticles added Perchloric oleoabundans biodiesel-diesel fuel blends. Mater. Today Proc. 2020, 33, 2877–2881. [Google Scholar] [CrossRef]
- Banapurmath, N.R.; Tewari, P.G.; Hosmath, R.S. Performance and emission characteristics of a DI compression ignition engine operated on Honge, Jatropha and Sesame oil methyl esters. Renew. Energy 2008, 33, 1982–1988. [Google Scholar] [CrossRef]
- Mathiyazhagan, M.; Ganapathi, A. Factors affecting biodiesel production. Res. Plant Biol. 2011, 1, 1–5. [Google Scholar]
- Lapuerta, M.; Armas, O.; Rodriguez-Fernandez, J. Effect of biodiesel fuels on engine emissions. Prog. Energy Combust. Sci. 2008, 34, 198–223. [Google Scholar] [CrossRef]
- Lapuerta, M.; Rodriguez-Fernandez, J.; Agudelo, J.R. Diesel particulate emissions from used cooking oil biodiesel. Bioresour. Technol. 2008, 99, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.S.; Kerihuel, A.; Belletre, J.; Tazerout, M. Experimental investigation on the use of preheated animal fat as fuel in a compression ignition engine. Renew. Energy 2005, 30, 1443–1456. [Google Scholar] [CrossRef]
- Gülüm, M.; Yesilyurt, M.K.; Bilgin, A. The modeling and analysis of transesterification reaction conditions in the selection of optimal biodiesel yield and viscosity. Environ. Sci. Pollut. Res. 2020, 27, 10351–10366. [Google Scholar] [CrossRef] [PubMed]











| Fuel Type | Density (g/cm3) | Viscosity (mm2/s) | Cetane Number (CN) | Lower Heating Value (kJ/kg) |
|---|---|---|---|---|
| Diesel Fuel | 0.8370 | 3.321 | 51.50 | 42,816 |
| %100 COB | 0.8650 | 3.612 | 54.15 | 40,202 |
| %100 WSOB | 0.8790 | 4.380 | 53.90 | 39,420 |
| COB10 | 0.8405 | 3.349 | 51.85 | 42,555 |
| COB10+ 50 ppm Mn2O3 | 0.8538 | 4.171 | 54.50 | 42,760 |
| COB10+ 75 ppm Mn2O3 | 0.8544 | 4.204 | 54.90 | 43,075 |
| COB10+ 100 ppm Mn2O3 | 0.8551 | 4.236 | 55.55 | 43,375 |
| WSOB10 | 0.8412 | 3.427 | 51.80 | 42,478 |
| WSOB10+ 50 ppm Mn2O3 | 0.8546 | 4.251 | 54.45 | 42,685 |
| WSOB10+ 75 ppm Mn2O3 | 0.8554 | 4.273 | 54.80 | 42,998 |
| WSOB10+ 100 ppm Mn2O3 | 0.8563 | 4.298 | 55.45 | 43,300 |
| Mn2O3 Information | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Name | Mn2O3 | |||||||||
| CAS Number | 1317-34-6 | |||||||||
| Number | NG04SO2501 | |||||||||
| Notation | Mn2O3 | |||||||||
| Purity Percentage | 99.5+% | |||||||||
| Elemental Analysis Certificate Information | ||||||||||
| K | Si | Ca | Co | Cu | Fe | Mg | Na | P | Sr | Zn |
| 17.3 µg/g | 21.5 µg/g | 88.6 µg/g | 0.02% | 28.9 µg/g | 0.02% | 108 µg/g | 0.16% | 0.03% | 1.53 µg/g | 33.4 µg/g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirpolat, A.B.; Uyar, M.M.; Çıtlak, A. Experimental Investigation of Biodiesel Fuels Obtained by Enriching the Content of Vegetable and Waste Oils with Nanoparticles and Modeling of Data Obtained from the Produced Fuel Samples Using Artificial Intelligence. Sustainability 2025, 17, 10689. https://doi.org/10.3390/su172310689
Demirpolat AB, Uyar MM, Çıtlak A. Experimental Investigation of Biodiesel Fuels Obtained by Enriching the Content of Vegetable and Waste Oils with Nanoparticles and Modeling of Data Obtained from the Produced Fuel Samples Using Artificial Intelligence. Sustainability. 2025; 17(23):10689. https://doi.org/10.3390/su172310689
Chicago/Turabian StyleDemirpolat, Ahmet Beyzade, Muhammed Mustafa Uyar, and Aydın Çıtlak. 2025. "Experimental Investigation of Biodiesel Fuels Obtained by Enriching the Content of Vegetable and Waste Oils with Nanoparticles and Modeling of Data Obtained from the Produced Fuel Samples Using Artificial Intelligence" Sustainability 17, no. 23: 10689. https://doi.org/10.3390/su172310689
APA StyleDemirpolat, A. B., Uyar, M. M., & Çıtlak, A. (2025). Experimental Investigation of Biodiesel Fuels Obtained by Enriching the Content of Vegetable and Waste Oils with Nanoparticles and Modeling of Data Obtained from the Produced Fuel Samples Using Artificial Intelligence. Sustainability, 17(23), 10689. https://doi.org/10.3390/su172310689






