Functional Trait-Based Responses of the Moroccan Menara Cultivar to Deficit Irrigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Design
2.2. Leaf Traits Sampling and Measurements
2.3. Biochemical Traits Determination
2.3.1. Total Soluble Sugar (SUC) Content
2.3.2. Proline Content (PRO)
2.3.3. Glycine Betaine Content (GLY)
2.3.4. Total Leaf Nitrogen Content (LNC)
2.3.5. Total Leaf Phosphorus Content (LPC)
2.3.6. Total Leaf Carbon Content (LCC)
2.3.7. Total Polyphenol Content (TPC)
2.3.8. Total Flavonoid Content (TFC)
2.4. Statistical Analyses
3. Results
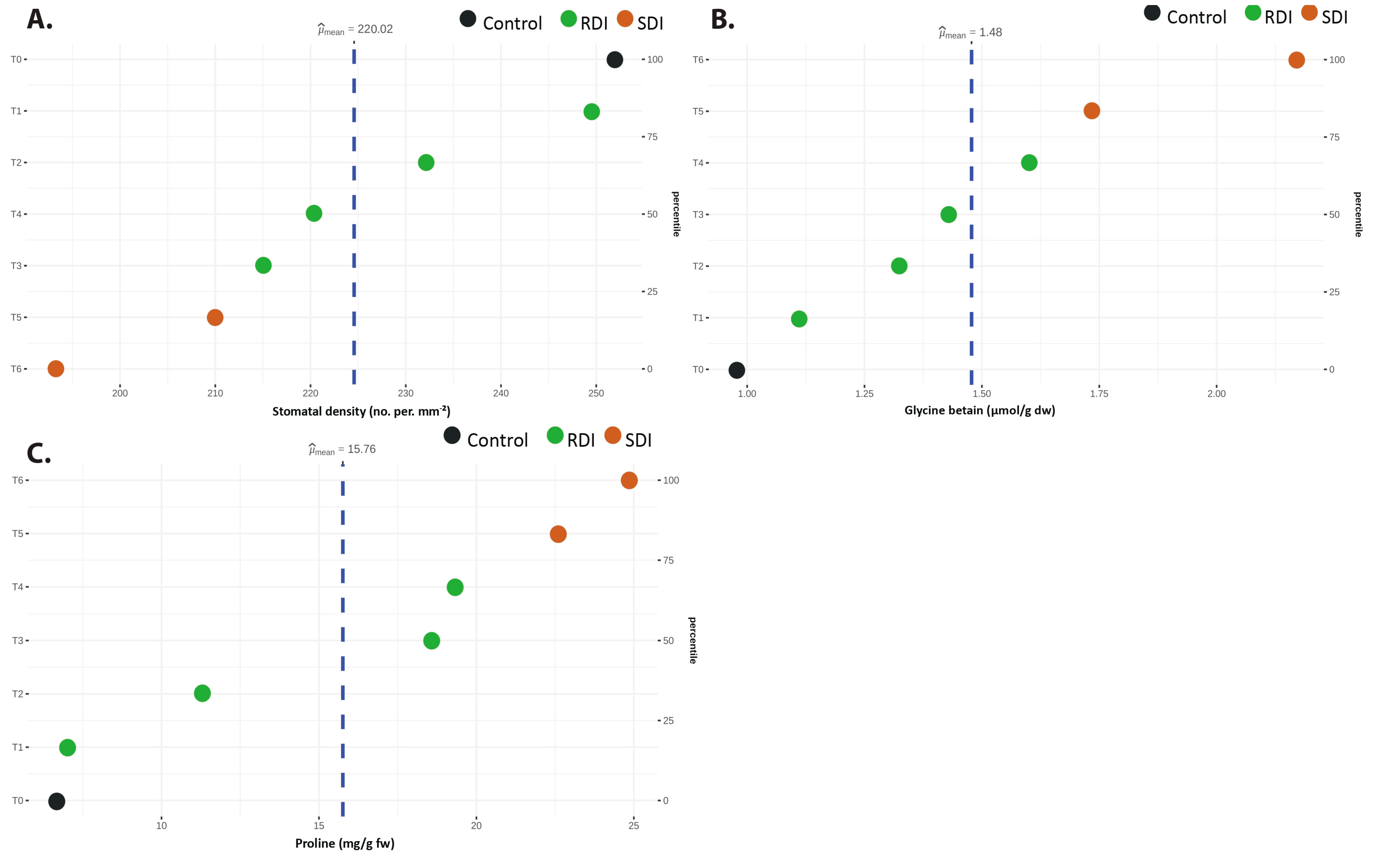
3.1. Trait Variation Across Deficit Irrigation Treatments
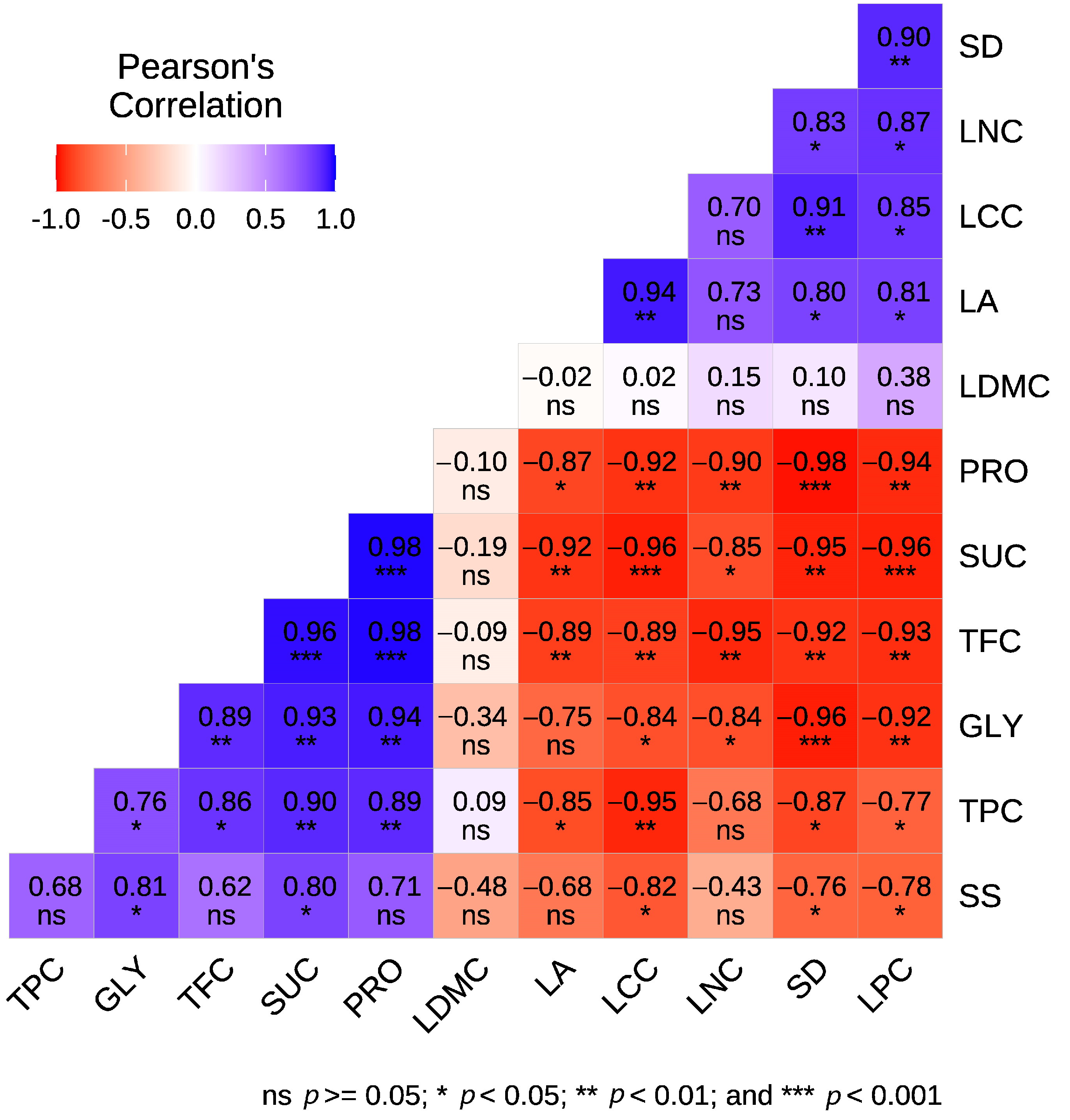
3.2. Correlations Among the Studied Traits
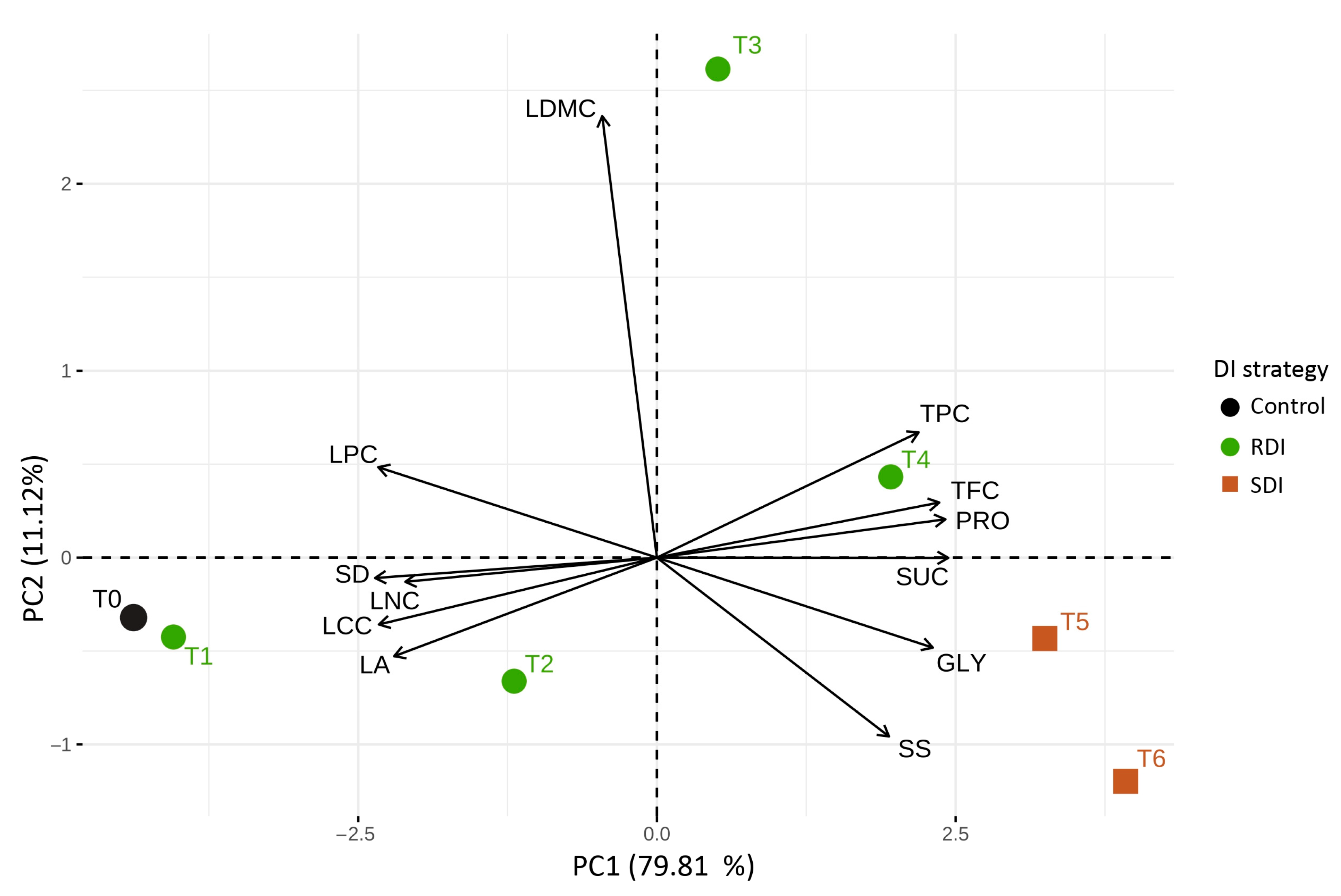
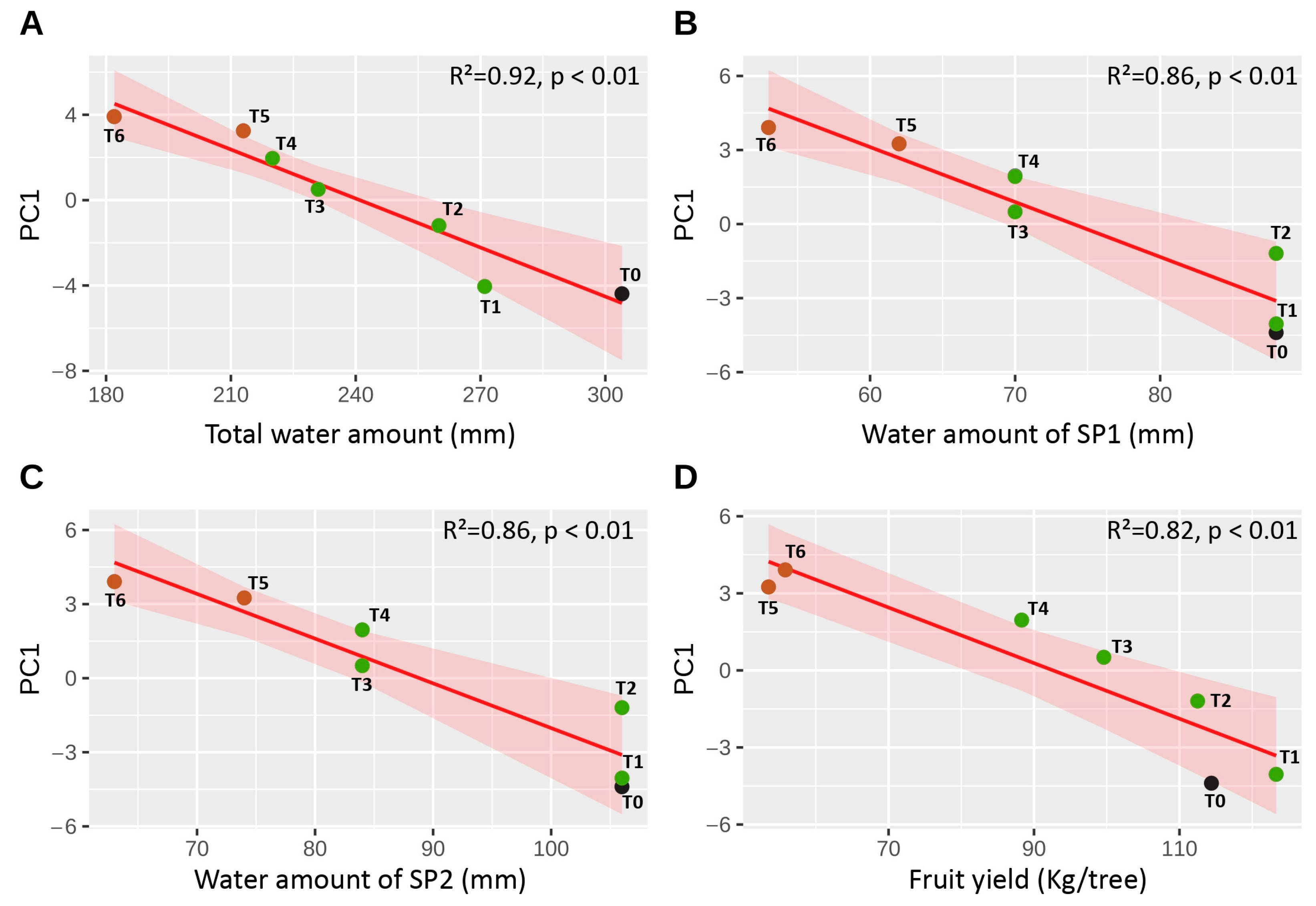
3.3. Structure and Covariation of Functional Traits Under Deficit Irrigation
4. Discussion
4.1. Variability of Menara Functional Responses Under Deficit Irrigation
4.2. Trade-Offs Underlying Functional Strategies of Menara Under Deficit Irrigation
4.3. Agronomic Implications of Deficit Irrigation
4.4. Innovations and Perspectives for Sustainable Irrigation of Olive Orchards Under Mediterranean Climatic Conditions
5. Conclusions
- The Menara cultivar exhibited significant morphological and biochemical plasticity under deficit irrigation, particularly through reductions in leaf area and adjustments in stomatal density, reflecting plastic responses to limited water availability.
- Accumulation of osmolytes (e.g., proline, soluble sugars) and enhanced synthesis of phenolic compounds contributed to maintaining cellular homeostasis and protecting leaf tissues under water stress.
- Moderate deficit irrigation maintained a favorable leaf nutrient status, suggesting efficient resource allocation and metabolic regulation that support stress tolerance without compromising essential physiological functions.
- Severe water deficit treatments (lowest ETc fractions) caused marked declines in performance, indicating critical thresholds beyond which Menara’s compensatory mechanisms are no longer effective.
- Moderate deficit irrigation can optimize water use efficiency while preserving functional integrity, offering a viable strategy for sustainable olive cultivation under Mediterranean drought conditions.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AT | Adjusted Temperature |
| CV | Coefficient of Variation |
| cv. | Cultivar |
| DI | Deficit irrigation |
| ET0 | Daily Reference Evapotranspiration |
| ETc | Crop Water Evapotranspiration |
| FY | Fruit Yield |
| GLY | Glycine betaine content |
| INRA | National Institute of Agricultural Research |
| Kc | Crop coefficient |
| Kr | Reduction coefficient |
| LA | Leaf Area |
| LCC | Total Leaf Carbon Content |
| LDM | Leaf Dry Matter |
| LDMC | Leaf Dry Matter Content |
| LFM | Leaf Fresh Matter |
| LNC | Total Leaf Nitrogen Content |
| LPC | Total Leaf Phosphorus Content |
| NP | Non-Sensitive Period |
| PCA | Principal Component Analysis |
| PRO | Proline Content |
| RDI | Regulated Deficit Irrigation |
| SD | Stomatal Density |
| SDI | Sustained Deficit Irrigation |
| SL | Stomatal Length |
| SP | Sensitive Period |
| SS | Stomatal Size |
| SUC | Total Soluble Sugar Content |
| SW | Stomatal Width |
| TFC | Total Flavonoid Content |
| TPC | Total Polyphenol Content |
References
- Berdugo, M.; Delgado-Baquerizo, M.; Soliveres, S.; Hernández-Clemente, R.; Zhao, Y.; Gaitán, J.J.; Gross, N.; Saiz, H.; Maire, V.; Lehmann, A.; et al. Global Ecosystem Thresholds Driven by Aridity. Science 2020, 367, 787–790. [Google Scholar] [CrossRef]
- Xu, C.; McDowell, N.G.; Fisher, R.A.; Wei, L.; Sevanto, S.; Christoffersen, B.O.; Weng, E.; Middleton, R.S. Increasing Impacts of Extreme Droughts on Vegetation Productivity under Climate Change. Nat. Clim. Change 2019, 9, 948–953. [Google Scholar] [CrossRef]
- Ault, T.R. On the Essentials of Drought in a Changing Climate. Science 2020, 368, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Cramer, W.; Guiot, J.; Fader, M.; Garrabou, J.; Gattuso, J.-P.; Iglesias, A.; Lange, M.A.; Lionello, P.; Llasat, M.C.; Paz, S.; et al. Climate Change and Interconnected Risks to Sustainable Development in the Mediterranean. Nat. Clim. Change 2018, 8, 972–980. [Google Scholar] [CrossRef]
- Lionello, P.; Scarascia, L. The Relation between Climate Change in the Mediterranean Region and Global Warming. Reg. Environ. Change 2018, 18, 1481–1493. [Google Scholar] [CrossRef]
- Avolio, E.; Orlandi, F.; Bellecci, C.; Fornaciari, M.; Federico, S. Assessment of the Impact of Climate Change on the Olive Flowering in Calabria (Southern Italy). Theor. Appl. Climatol. 2012, 107, 531–540. [Google Scholar] [CrossRef]
- Picornell, A.; Abreu, I.; Ribeiro, H. Trends and Future Projections of Olea Flowering in the Western Mediterranean: The Example of the Alentejo Region (Portugal). Agric. For. Meteorol. 2023, 339, 109559. [Google Scholar] [CrossRef]
- Pokhrel, Y.; Felfelani, F.; Satoh, Y.; Boulange, J.; Burek, P.; Gädeke, A.; Gerten, D.; Gosling, S.N.; Grillakis, M.; Gudmundsson, L. Global Terrestrial Water Storage and Drought Severity under Climate Change. Nat. Clim. Change 2021, 11, 226–233. [Google Scholar] [CrossRef]
- Lembaid, I.; Moussadek, R.; Mrabet, R.; Bouhaouss, A. Modeling Soil Organic Carbon Changes under Alternative Climatic Scenarios and Soil Properties Using DNDC Model at a Semi-Arid Mediterranean Environment. Climate 2022, 10, 23. [Google Scholar] [CrossRef]
- Azenzem, R.; Koussa, T.; Alfeddy, M.N.; Kassout, J. Impacts and Mitigation Strategies of Abiotic and Biotic Stresses in Olive Orchards in the Era of Climate Change. In Water-Soil-Plant-Animal Nexus in the Era of Climate Change; IGI Global: Hershey, PA, USA, 2024; pp. 137–172. [Google Scholar]
- Caselli, A.; Petacchi, R. Climate Change and Major Pests of Mediterranean Olive Orchards: Are We Ready to Face the Global Heating? Insects 2021, 12, 802. [Google Scholar] [CrossRef]
- Ortiz-Bobea, A.; Ault, T.R.; Carrillo, C.M.; Chambers, R.G.; Lobell, D.B. Anthropogenic Climate Change Has Slowed Global Agricultural Productivity Growth. Nat. Clim. Change 2021, 11, 306–312. [Google Scholar] [CrossRef]
- Fraga, H.; Pinto, J.G.; Viola, F.; Santos, J.A. Climate Change Projections for Olive Yields in the Mediterranean Basin. Int. J. Climatol. 2020, 40, 769–781. [Google Scholar] [CrossRef]
- Kassout, J.; Terral, J.-F.; Azenzem, R.; El Ouahrani, A.; Sahli, A.; Houssni, M.; Chakkour, S.; Kadaoui, K.; Ater, M. Olea europaea and Stressful Conditions. In Medicinal Plant Responses to Stressful Conditions; CRC Press: Boca Raton, FL, USA, 2023; pp. 243–272. [Google Scholar]
- Kaniewski, D.; Marriner, N.; Terral, J.-F.; Besnard, G.; Tsitsou, L.; Topsakal, J.; Morhange, C.; Otto, T.; Luce, F.; Cheddadi, R. Olive Production in the 21st Century Will Be Threatened by Water Stress and Declining Solar Activity. Commun. Earth Environ. 2025, 6, 268. [Google Scholar] [CrossRef]
- Kaniewski, D.; Van Campo, E.; Boiy, T.; Terral, J.-F.; Khadari, B.; Besnard, G. Primary Domestication and Early Uses of the Emblematic Olive Tree: Palaeobotanical, Historical and Molecular Evidence from the Middle East. Biol. Rev. 2012, 87, 885–899. [Google Scholar] [CrossRef]
- FAO. Agricultural Production Statistics 2000–2022. In FAOSTAT Analytical Briefs; FAO: Rome, Italy, 2023. [Google Scholar]
- Besnard, G.; Terral, J.-F.; Cornille, A. On the Origins and Domestication of the Olive: A Review and Perspectives. Ann. Bot. 2018, 121, 385–403. [Google Scholar] [CrossRef] [PubMed]
- International Olive Oil Council. COI Statistics; International Olive Oil Council: Madrid, Spain, 2024. [Google Scholar]
- Terral, J.-F.; Creusot, P.; Limier, B.; Bombeau, A.; Bernazeau, B.; Lochon-Menseau, S.; Ater, M.; Barbara, H.; Pinatel, C.; Paradis, L.; et al. The Potential of Sap Conduction in the Olive Tree Is Linked to Aridity Conditions of the Main Cultivation Area of Varieties and Allow to Uncover Their Sensitivity to Ongoing Climate Change. Sci. Hortic. 2025, 339, 113856. [Google Scholar] [CrossRef]
- Abdallah, M.B.; Methenni, K.; Nouairi, I.; Zarrouk, M.; Youssef, N.B. Drought Priming Improves Subsequent More Severe Drought in a Drought-Sensitive Cultivar of Olive Cv. Chétoui. Sci. Hortic. 2017, 221, 43–52. [Google Scholar] [CrossRef]
- Ben Abdallah, M.; Methenni, K.; Taamalli, W.; Ben Youssef, N. Post-Stress Recovery from Drought and Salinity in Olive Plants Is an Active Process Associated to Physiological and Metabolic Changes. Acta Physiol. Plant. 2024, 46, 120. [Google Scholar] [CrossRef]
- Clavijo-Herrera, J.; Thetford, M.; Williamson, J.; Mulvaney, M.J.; Rossi, L.; Sarkhosh, A. Adaptation and Early Establishment of Olive Trees (Olea europaea L.) under the Humid Subtropical Climate of the Southeastern United States. HortScience 2025, 60, 1379–1388. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.-T.; Meijón, M.; Ferreira, H.; Pinto, G.; Moutinho-Pereira, J.; Correia, C. Salicylic Acid Modulates Olive Tree Physiological and Growth Responses to Drought and Rewatering Events in a Dose Dependent Manner. J. Plant Physiol. 2018, 230, 21–32. [Google Scholar] [CrossRef]
- Alderotti, F.; Piccolo, E.L.; Brunetti, C.; Stefano, G.; Ugolini, T.; Cecchi, L.; Beccaluva, M.; Renna, L.; Detti, C.; Ferrini, F. Cultivar-Specific Responses of Young Olive Trees to Water Deficit: Impacts on Physiology, Leaf Anatomy, and Fruit Quality in ’Arbequina’,’Leccio Del Corno’ and ‘Maurino’. Plant Physiol. Biochem. 2025, 229, 110331. [Google Scholar] [CrossRef] [PubMed]
- Imperiale, V.; Caruso, T.; Ioppolo, A.; Carella, A.; Massenti, R.; Marra, F.P. Water Stress Effect on Hydraulic Architecture, Biomass Partitioning, and Gas Exchange of Four Different Olive Cultivars. Front. Plant Sci. 2025, 16, 1630454. [Google Scholar] [CrossRef] [PubMed]
- Ahmadipour, S.; Arji, I.; Ebadi, A.; Abdossi, V. Physiological and Biochemical Responses of Some Olive Cultivars (Olea europaea L.) to Water Stress. Cell. Mol. Biol. 2018, 64, 20–29. [Google Scholar] [CrossRef]
- Martín-Vertedor, A.I.; Rodríguez, J.M.P.; Losada, H.P.; Castiel, E.F. Interactive Responses to Water Deficits and Crop Load in Olive (Olea europaea L., Cv. Morisca) I.–Growth and Water Relations. Agric. Water Manag. 2011, 98, 941–949. [Google Scholar] [CrossRef]
- Mougiou, N.; Baalbaki, B.; Doupis, G.; Kavroulakis, N.; Poulios, S.; Vlachonasios, K.E.; Koubouris, G.C. The Effect of Low Temperature on Physiological, Biochemical and Flowering Functions of Olive Tree in Relation to Genotype. Sustainability 2020, 12, 10065. [Google Scholar] [CrossRef]
- Razouk, R.; Hssaini, L.; Alghoum, M.; Adiba, A.; Hamdani, A. Phenotyping Olive Cultivars for Drought Tolerance Using Leaf Macro-Characteristics. Horticulturae 2022, 8, 939. [Google Scholar] [CrossRef]
- Siakou, M.; Bruggeman, A.; Eliades, M.; Djuma, H.; Kyriacou, M.C.; Moriana, A. Phenology, Morphology and Physiology Responses of Deficit Irrigated ‘Koroneiki’Olive Trees as Affected by Environmental Conditions and Alternate Bearing. Agronomy 2022, 12, 879. [Google Scholar] [CrossRef]
- Marchioni, I.; Rodolfi, M.; Massa, D.; Cacini, S.; Ughini, V.; Bartolini, G.; Fabbri, A.; Petruccelli, R.; Ganino, T. Comparative Effects of Drought Stress on Three Olive Cultivars Focusing on Older Leaves. Sci. Hortic. 2024, 332, 113234. [Google Scholar] [CrossRef]
- Paoletti, A.; Cinosi, N.; Lodolini, E.M.; Famiani, F.; Rosati, A. Effects of Root Constriction on Vegetative Growth and Yield Efficiency in Young Trees of a Low-and a High-Vigor Olive Cultivar. Trees 2023, 37, 1179–1187. [Google Scholar] [CrossRef]
- Kaniewski, D.; Marriner, N.; Morhange, C.; Khater, C.; Terral, J.-F.; Besnard, G.; Otto, T.; Luce, F.; Couillebault, Q.; Tsitsou, L. Climate Change Threatens Olive Oil Production in the Levant. Nat. Plants 2023, 9, 219–227. [Google Scholar] [CrossRef]
- Orlandi, F.; Rojo, J.; Picornell, A.; Oteros, J.; Pérez-Badia, R.; Fornaciari, M. Impact of Climate Change on Olive Crop Production in Italy. Atmosphere 2020, 11, 595. [Google Scholar] [CrossRef]
- Rodrigo-Comino, J.; Salvia, R.; Quaranta, G.; Cudlín, P.; Salvati, L.; Gimenez-Morera, A. Climate Aridity and the Geographical Shift of Olive Trees in a Mediterranean Northern Region. Climate 2021, 9, 64. [Google Scholar] [CrossRef]
- Abou-Saaid, O.; El Yaacoubi, A.; Moukhli, A.; El Bakkali, A.; Oulbi, S.; Delalande, M.; Farrera, I.; Kelner, J.-J.; Lochon-Menseau, S.; El Modafar, C. Statistical Approach to Assess Chill and Heat Requirements of Olive Tree Based on Flowering Date and Temperatures Data: Towards Selection of Adapted Cultivars to Global Warming. Agronomy 2022, 12, 2975. [Google Scholar] [CrossRef]
- Ibba, K.; Kassout, J.; Boselli, V.; Er-Raki, S.; Oulbi, S.; Mansouri, L.E.; Bouizgaren, A.; Sikaoui, L.; Hadria, R. Assessing the Impact of Deficit Irrigation Strategies on Agronomic and Productive Parameters of Menara Olive Cultivar: Implications for Operational Water Management. Front. Environ. Sci. 2023, 11, 1100552. [Google Scholar] [CrossRef]
- Martins, S.; Pereira, S.; Dinis, L.-T.; Brito, C. Enhancing Olive Cultivation Resilience: Sustainable Long-Term and Short-Term Adaptation Strategies to Alleviate Climate Change Impacts. Horticulturae 2024, 10, 1066. [Google Scholar] [CrossRef]
- Kassout, J.; Souali, H.; Zahiri, A.; El Hilali, H.; Zaher, H.; Boselli, V.A.; Hadria, R.; Oulbi, S. Exploring Functional Trait Dynamics and Responses in New Olive Crossbreeds: Implications for Climate Resilience Strategies. Ecologies 2025, 6, 66. [Google Scholar] [CrossRef]
- Kassout, J.; Terral, J.-F.; Boselli, V.; Souali, H.; Ater, M. Roots of Domestication: Unveiling the Dynamics of Domestication through Trait-Based Analysis of Olive Trees in Northern Morocco. Philos. Trans. R. Soc. B: Biol. Sci. 2025, 380, 20240201. [Google Scholar] [CrossRef]
- Kassout, J.; Kadaoui, K.; Hmimsa, Y.; El Fatehi, S.; Chakkour, S.; Houssni, M.; Oulbi, S.; Azenzem, R.; Sahli, A.; Ater, M. The Missing Component: Intraspecific Traits Variation Enhances Wild Fruit Tree Tolerance to Climate Change. In Water-Soil-Plant-Animal Nexus in the Era of Climate Change; IGI Global: Hershey, PA, USA, 2024; pp. 194–210. [Google Scholar]
- Kassout, J.; Ater, M.; Ivorra, S.; Barbara, H.; Limier, B.; Ros, J.; Girard, V.; Paradis, L.; Terral, J.-F. Resisting Aridification: Adaptation of Sap Conduction Performance in Moroccan Wild Olive Subspecies Distributed over an Aridity Gradient. Front. Plant Sci. 2021, 12, 663721. [Google Scholar] [CrossRef] [PubMed]
- Kassout, J.; Terral, J.-F.; Hodgson, J.G.; Ater, M. Trait-Based Plant Ecology a Flawed Tool in Climate Studies? The Leaf Traits of Wild Olive That Pattern with Climate Are Not Those Routinely Measured. PLoS ONE 2019, 14, e0219908. [Google Scholar] [CrossRef] [PubMed]
- Terral, J.-F.; Badal, E.; Heinz, C.; Roiron, P.; Thiebault, S.; Figueiral, I. A Hydraulic Conductivity Model Points to Post-neogene Survival of the Mediterranean Olive. Ecology 2004, 85, 3158–3165. [Google Scholar] [CrossRef]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Colin Prentice, I. The Global Spectrum of Plant Form and Function. Nature 2016, 529, 167–171. [Google Scholar]
- Pavanetto, N.; Carmona, C.P.; Laanisto, L.; Niinemets, Ü.; Puglielli, G. Trait Dimensions of Abiotic Stress Tolerance in Woody Plants of the Northern Hemisphere. Glob. Ecol. Biogeogr. 2024, 33, 272–285. [Google Scholar] [CrossRef]
- Reich, P.B.; Wright, I.J.; Cavender-Bares, J.; Craine, J.M.; Oleksyn, J.; Westoby, M.; Walters, M.B. The Evolution of Plant Functional Variation: Traits, Spectra, and Strategies. Int. J. Plant Sci. 2003, 164, S143–S164. [Google Scholar] [CrossRef]
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the Concept of Trait Be Functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Díaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Law, E.A. Global Climatic Drivers of Leaf Size. Science 2017, 357, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Haworth, M.; Marino, G.; Materassi, A.; Raschi, A.; Scutt, C.P.; Centritto, M. The Functional Significance of the Stomatal Size to Density Relationship: Interaction with Atmospheric [CO2] and Role in Plant Physiological Behaviour. Sci. Total Environ. 2023, 863, 160908. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Gargallo-Garriga, A.; Urban, O.; Klem, K.; Walker, T.W.; Holub, P.; Janssens, I.A.; Peñuelas, J. Ecometabolomics for a Better Understanding of Plant Responses and Acclimation to Abiotic Factors Linked to Global Change. Metabolites 2020, 10, 239. [Google Scholar] [CrossRef]
- Wang, S.; Alseekh, S.; Fernie, A.R.; Luo, J. The Structure and Function of Major Plant Metabolite Modifications. Mol. Plant 2019, 12, 899–919. [Google Scholar] [CrossRef]
- Michaletz, S.T.; Weiser, M.D.; Zhou, J.; Kaspari, M.; Helliker, B.R.; Enquist, B.J. Plant Thermoregulation: Energetics, Trait–Environment Interactions, and Carbon Economics. Trends Ecol. Evol. 2015, 30, 714–724. [Google Scholar] [CrossRef]
- Walker, T.W.; Alexander, J.M.; Allard, P.-M.; Baines, O.; Baldy, V.; Bardgett, R.D.; Capdevila, P.; Coley, P.D.; David, B.; Defossez, E. Functional Traits 2.0: The Power of the Metabolome for Ecology. J. Ecol. 2022, 110, 4–20. [Google Scholar]
- Walker, T.W.; Schrodt, F.; Allard, P.-M.; Defossez, E.; Jassey, V.E.; Schuman, M.C.; Alexander, J.M.; Baines, O.; Baldy, V.; Bardgett, R.D. Leaf Metabolic Traits Reveal Hidden Dimensions of Plant Form and Function. Sci. Adv. 2023, 9, eadi4029. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M. The Worldwide Leaf Economics Spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Yang, Y.; Gou, R.; Zhao, J.; Qi, N.; Wen, Z.; Kassout, J.; Li, R.; Lin, G.; Zheng, H. Variation in Carbon Isotope Composition of Plants across an Aridity Gradient on the Loess Plateau, China. Glob. Ecol. Conserv. 2022, 33, e01948. [Google Scholar] [CrossRef]
- Volaire, F. A Unified Framework of Plant Adaptive Strategies to Drought: Crossing Scales and Disciplines. Glob. Change Biol. 2018, 24, 2929–2938. [Google Scholar] [CrossRef]
- Mouillot, D.; Loiseau, N.; Grenié, M.; Algar, A.C.; Allegra, M.; Cadotte, M.W.; Casajus, N.; Denelle, P.; Guéguen, M.; Maire, A. The Dimensionality and Structure of Species Trait Spaces. Ecol. Lett. 2021, 24, 1988–2009. [Google Scholar] [CrossRef]
- Díaz, S. Plant Functional Traits and the Entangled Phenotype. Funct. Ecol. 2025, 39, 1144–1159. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.-T.; Moutinho-Pereira, J.; Correia, C.M. Drought Stress Effects and Olive Tree Acclimation under a Changing Climate. Plants 2019, 8, 232. [Google Scholar] [CrossRef]
- El Antari, A.; Sikaoui, L. Catalogues Des Variétés d’olivier Cultivées et Authochtones Du Maroc, 1st ed.; Institut National de la Recherche Agronomique: Paris, France, 2022. [Google Scholar]
- Khadari, B.; Charafi, J.; Moukhli, A.; Ater, M. Substantial Genetic Diversity in Cultivated Moroccan Olive despite a Single Major Cultivar: A Paradoxical Situation Evidenced by the Use of SSR Loci. Tree Genet. Genomes 2008, 4, 213–221. [Google Scholar] [CrossRef]
- Costa, J.M.; Ortuño, M.F.; Chaves, M.M. Deficit Irrigation as a Strategy to Save Water: Physiology and Potential Application to Horticulture. J. Integr. Plant Biol. 2007, 49, 1421–1434. [Google Scholar] [CrossRef]
- Parkash, V.; Singh, S. A Review on Potential Plant-Based Water Stress Indicators for Vegetable Crops. Sustainability 2020, 12, 3945. [Google Scholar] [CrossRef]
- Er-Raki, S.; Chehbouni, A.; Khabba, S.; Simonneaux, V.; Jarlan, L.; Ouldbba, A.; Rodriguez, J.C.; Allen, R. Assessment of Reference Evapotranspiration Methods in Semi-Arid Regions: Can Weather Forecast Data Be Used as Alternate of Ground Meteorological Parameters? J. Arid Environ. 2010, 74, 1587–1596. [Google Scholar] [CrossRef]
- Ater, M.; Barbara, H.; Kassout, J. Importance Des Variétés Locales, de l’oléastre et Des Pratiques Traditionnelles de l’oléiculture Dans La Région de Chefchaouen (Nord Du Maroc). In L’Oléiculture au Maroc de la Préhistoire à nos Jours: Pratiques, Diversité, Adaptation, Usages, Commerce et Politiques; CIHEAM: Montpellier, France, 2016; Volume 118, pp. 109–121. ISBN 978-2-85352-560-0. [Google Scholar]
- Sanz-Cortés, F.; MARTINEZ-CALVO, J.; Badenes, M.L.; Bleiholder, H.; Hack, H.; Llácer, G.; Meier, U. Phenological Growth Stages of Olive Trees (Olea europaea). Ann. Appl. Biol. 2002, 140, 151–157. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New Handbook for Standardised Measurement of Plant Functional Traits Worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Kassout, J.; Terral, J.-F.; Souali, H.; Ater, M. Environment-Dependent and Intraspecific Variations in Leaf and Size Traits of a Native Wild Olive (Olea europaea L.) along an Aridity Gradient in Morocco: A Functional Perspective. Plant Ecol. 2024, 225, 943–959. [Google Scholar] [CrossRef]
- Ryser, P.; Bernardi, J.; Merla, A. Determination of Leaf Fresh Mass after Storage between Moist Paper Towels: Constraints and Reliability of the Method. J. Exp. Bot. 2008, 59, 2461–2467. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Liu, C.; He, N.; Zhang, J.; Li, Y.; Wang, Q.; Sack, L.; Yu, G. Variation of Stomatal Traits from Cold Temperate to Tropical Forests and Association with Water Use Efficiency. Funct. Ecol. 2018, 32, 20–28. [Google Scholar] [CrossRef]
- Eris, A.; Gulen, H.; Barut, E.; Cansev, A. Annual Patterns of Total Soluble Sugars and Proteins Related to Coldhardiness in Olive (Olea europaea L.‘Gemlik’). J. Hortic. Sci. Biotechnol. 2007, 82, 597–604. [Google Scholar] [CrossRef]
- Van Handel, E. Determination of Fructose and Fructose-Yielding Carbohydrates with Cold Anthrone. Anal. Biochem. 1965, 11, 266–267. [Google Scholar] [CrossRef] [PubMed]
- Paquin, R.; Lechasseur, P. Observations Sur Une Méthode de Dosage de La Proline Libre Dans Les Extraits de Plantes. Can. J. Bot. 1979, 57, 1851–1854. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid Assay for Determination of Water Soluble Quaternary Ammonium Compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Barbano, D.M.; Clark, J.L.; Dunham, C.E.; Flemin, R.J. Kjeldahl Method for Determination of Total Nitrogen Content of Milk: Collaborative Study. J. Assoc. Off. Anal. Chem. 1990, 73, 849–859. [Google Scholar] [CrossRef]
- Oukaltouma, K.; El Moukhtari, A.; Lahrizi, Y.; Makoudi, B.; Mouradi, M.; Farissi, M.; Willems, A.; Qaddoury, A.; Bekkaoui, F.; Ghoulam, C. Physiological, Biochemical and Morphological Tolerance Mechanisms of Faba Bean (Vicia faba L.) to the Combined Stress of Water Deficit and Phosphorus Limitation. J. Soil Sci. Plant Nutr. 2022, 22, 1632–1646. [Google Scholar] [CrossRef]
- ISO 10694; Soil Quality. Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis). International Oraganization for Standarization: Geneva, Switzerland, 1995.
- De Vos, B.; Lettens, S.; Muys, B.; Deckers, J.A. Walkley–Black Analysis of Forest Soil Organic Carbon: Recovery, Limitations and Uncertainty. Soil Use Manag. 2007, 23, 221–229. [Google Scholar] [CrossRef]
- Albu, S.; Joyce, E.; Paniwnyk, L.; Lorimer, J.P.; Mason, T.J. Potential for the Use of Ultrasound in the Extraction of Antioxidants from Rosmarinus Officinalis for the Food and Pharmaceutical Industry. Ultrason. Sonochemistry 2004, 11, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Tunç, Y.; Yaman, M.; Keçe, Y.M.; Yilmaz, K.U.; Yildiz, E.; Güneş, A. Characterization of Olive (Olea europaea L.) Cultivars; Colour Properties, Biochemical Contents, Antioxidant Activity and Nutrient Contents. Genet. Resour. Crop Evol. 2025, 72, 529–541. [Google Scholar] [CrossRef]
- Ribarova, F.; Atanassova, M.; Marinova, D.; Ribarova, F.; Atanassova, M. Total Phenolics and Flavonoids in Bulgarian Fruits and Vegetables. JU Chem. Met. 2005, 40, 255–260. [Google Scholar]
- Ryu, C. dlookr: Tools for Data Diagnosis, Exploration, Transformation. Available online: https://CRAN.R-project.org/package=dlookr (accessed on 15 May 2025).
- Sjoberg, D.D.; Whiting, K.; Curry, M.; Lavery, J.A.; Larmarange, J. Reproducible Summary Tables with the Gtsummary Package. R J. 2021, 13, 570–580. [Google Scholar] [CrossRef]
- de Mendiburu, F, Agricolae: Statistical Procedures for Agricultural Research. Available online: https://cran.r-project.org/web/packages/agricolae (accessed on 15 May 2025).
- Olivoto, T.; Lúcio, A.D. Metan: An R Package for Multi-environment Trial Analysis. Methods Ecol. Evol. 2020, 11, 783–789. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses. Available online: https://cran.r-project.org/web/packages/factoextra (accessed on 15 May 2025).
- Chai, Q.; Gan, Y.; Zhao, C.; Xu, H.-L.; Waskom, R.M.; Niu, Y.; Siddique, K.H. Regulated Deficit Irrigation for Crop Production under Drought Stress. A Review. Agron. Sustain. Dev. 2016, 36, 3. [Google Scholar] [CrossRef]
- Panda, R.K.; Behera, S.K.; Kashyap, P.S. Effective Management of Irrigation Water for Maize under Stressed Conditions. Agric. Water Manag. 2004, 66, 181–203. [Google Scholar] [CrossRef]
- Sadok, W.; Lopez, J.R.; Smith, K.P. Transpiration Increases under High-temperature Stress: Potential Mechanisms, Trade-offs and Prospects for Crop Resilience in a Warming World. Plant Cell Environ. 2021, 44, 2102–2116. [Google Scholar] [CrossRef]
- Royer, D.L.; McElwain, J.C.; Adams, J.M.; Wilf, P. Sensitivity of Leaf Size and Shape to Climate within Acer Rubrum and Quercus Kelloggii. New Phytol. 2008, 179, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Luza, A.L.; Barneche, D.R.; Cordeiro, C.A.; Dambros, C.S.; Ferreira, C.E.; Floeter, S.R.; Giglio, V.J.; Luiz, O.J.; Mendes, T.C.; Picolotto, V.A. Going across Taxa in Functional Ecology: Review and Perspectives of an Emerging Field. Funct. Ecol. 2023, 37, 3091–3110. [Google Scholar] [CrossRef]
- Westerband, A.C.; Funk, J.L.; Barton, K.E. Intraspecific Trait Variation in Plants: A Renewed Focus on Its Role in Ecological Processes. Ann. Bot. 2021, 127, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Kadaoui, K.; Kassout, J.; Boselli, V.A.; Chakkour, S.; Sahli, A.; Houssni, M.; Bouziane, H.; Ater, M. Leaf Trait Variability and CSR Strategy Shifts in Mediterranean Woody Species along an Edaphic Gradient. Ecologies 2024, 5, 455–469. [Google Scholar] [CrossRef]
- Wong, M.K.; Carmona, C.P. Including Intraspecific Trait Variability to Avoid Distortion of Functional Diversity and Ecological Inference: Lessons from Natural Assemblages. Methods Ecol. Evol. 2021, 12, 946–957. [Google Scholar] [CrossRef]
- Terral, J.-F.; Bédé, G.; Paradis, L.; Ivorra, S.; Ros, J.; Girard, V.; Ater, M.; Limier, B.; Kassout, J. Understanding How the Argan Tree Adapts Its Performance in a Heterogeneous Environment Using Wood Anatomical and Leaf Functional Traits. J. Arid Environ. 2025, 231, 105473. [Google Scholar] [CrossRef]
- Gratani, L. Plant Phenotypic Plasticity in Response to Environmental Factors. Adv. Bot. 2014, 2014, 208747. [Google Scholar] [CrossRef]
- Stotz, G.C.; Salgado-Luarte, C.; Escobedo, V.M.; Valladares, F.; Gianoli, E. Phenotypic Plasticity and the Leaf Economics Spectrum: Plasticity Is Positively Associated with Specific Leaf Area. Oikos 2022, 2022, e09342. [Google Scholar] [CrossRef]
- Rodríguez Romero, M.; Gallardo Soler, A.; Pulido, F.J. Geographical and Within-Population Variation of Constitutive Chemical Defences in a Mediterranean Oak (“Quercus Ilex”). For. Syst. 2020, 29, e011. [Google Scholar] [CrossRef]
- Steen, J.S.; Asplund, J.; Lie, M.H.; Nybakken, L. Environment Rather than Provenance Explains Levels of Foliar Phenolics in European Beech (Fagus sylvatica L.) Seedlings. Trees 2021, 35, 1555–1569. [Google Scholar] [CrossRef]
- Tian, D.; Yan, Z.; Schmid, B.; Kattge, J.; Fang, J.; Stocker, B.D. Environmental versus Phylogenetic Controls on Leaf Nitrogen and Phosphorous Concentrations in Vascular Plants. Nat. Commun. 2024, 15, 5346. [Google Scholar] [CrossRef]
- Gholami, R.; Zahedi, S.M. Identifying Superior Drought-Tolerant Olive Genotypes and Their Biochemical and Some Physiological Responses to Various Irrigation Levels. J. Plant Nutr. 2019, 42, 2057–2069. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Osnas, J.L.; Lichstein, J.W.; Reich, P.B.; Pacala, S.W. Global Leaf Trait Relationships: Mass, Area, and the Leaf Economics Spectrum. Science 2013, 340, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Sperry, J.S.; Hacke, U.G.; Oren, R.; Comstock, J.P. Water Deficits and Hydraulic Limits to Leaf Water Supply. Plant Cell Environ. 2002, 25, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Vialet-Chabrand, S. Speedy Stomata, Photosynthesis and Plant Water Use Efficiency. New Phytol. 2019, 221, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Baccari, S.; Elloumi, O.; Chaari-Rkhis, A.; Fenollosa, E.; Morales, M.; Drira, N.; Ben Abdallah, F.; Fki, L.; Munné-Bosch, S. Linking Leaf Water Potential, Photosynthesis and Chlorophyll Loss with Mechanisms of Photo-and Antioxidant Protection in Juvenile Olive Trees Subjected to Severe Drought. Front. Plant Sci. 2020, 11, 614144. [Google Scholar] [CrossRef]
- Medda, S.; Fadda, A.; Mulas, M. Influence of Climate Change on Metabolism and Biological Characteristics in Perennial Woody Fruit Crops in the Mediterranean Environment. Horticulturae 2022, 8, 273. [Google Scholar] [CrossRef]
- Domingues, T.F.; Meir, P.; Feldpausch, T.R.; Saiz, G.; Veenendaal, E.M.; Schrodt, F.; Bird, M.; Djagbletey, G.; Hien, F.; Compaore, H. Co-limitation of Photosynthetic Capacity by Nitrogen and Phosphorus in West Africa Woodlands. Plant Cell Environ. 2010, 33, 959–980. [Google Scholar] [CrossRef]
- Walker, A.P.; Beckerman, A.P.; Gu, L.; Kattge, J.; Cernusak, L.A.; Domingues, T.F.; Scales, J.C.; Wohlfahrt, G.; Wullschleger, S.D.; Woodward, F.I. The Relationship of Leaf Photosynthetic Traits–Vcmax and Jmax–to Leaf Nitrogen, Leaf Phosphorus, and Specific Leaf Area: A Meta-analysis and Modeling Study. Ecol. Evol. 2014, 4, 3218–3235. [Google Scholar] [CrossRef]
- Ordoñez, J.C.; Van Bodegom, P.M.; Witte, J.-P.M.; Wright, I.J.; Reich, P.B.; Aerts, R. A Global Study of Relationships between Leaf Traits, Climate and Soil Measures of Nutrient Fertility. Glob. Ecol. Biogeogr. 2009, 18, 137–149. [Google Scholar] [CrossRef]
- Reich, P.B. The World-Wide ‘Fast–Slow’ Plant Economics Spectrum: A Traits Manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Herms, D.A.; Mattson, W.J. The Dilemma of Plants: To Grow or Defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef]
- Monson, R.K.; Trowbridge, A.M.; Lindroth, R.L.; Lerdau, M.T. Coordinated Resource Allocation to Plant Growth–Defense Tradeoffs. New Phytol. 2022, 233, 1051–1066. [Google Scholar] [CrossRef]
- Massad, T.J.; Dyer, L.A.; Vega, C.G. Costs of Defense and a Test of the Carbon-Nutrient Balance and Growth-Differentiation Balance Hypotheses for Two Co-Occurring Classes of Plant Defense. PLoS ONE 2012, 7, e47554. [Google Scholar] [CrossRef]
- Ibba, K.; Er-Raki, S.; Bouizgaren, A.; Hadria, R. Effects of Regulated and Sustained Deficit Irrigation on Water Use, Physiology and Yield of ‘Menara’Olive Trees, in Morocco. Irrig. Sci. 2024, 42, 829–848. [Google Scholar] [CrossRef]
- Cha-um, S.; Samphumphuang, T.; Kirdmanee, C. Glycinebetaine Alleviates Water Deficit Stress in Indica Rice Using Proline Accumulation, Photosynthetic Efficiencies, Growth Performances and Yield Attributes. Aust. J. Crop Sci. 2013, 7, 213–218. [Google Scholar]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a Multifaceted Signalling Molecule in Plant Responses to Abiotic Stress: Understanding the Physiological Mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of Osmoprotectants in Improving Salinity and Drought Tolerance in Plants: A Review. Rev. Environ. Sci. Bio/Technol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Parri, S.; Romi, M.; Hoshika, Y.; Giovannelli, A.; Dias, M.C.; Piritore, F.C.; Cai, G.; Cantini, C. Morpho-Physiological Responses of Three Italian Olive Tree (Olea europaea L.) Cultivars to Drought Stress. Horticulturae 2023, 9, 830. [Google Scholar] [CrossRef]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble Sugars: Metabolism, Sensing and Abiotic Stress: A Complex Network in the Life of Plants. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef]
- Bacelar, E.A.; Moutinho-Pereira, J.M.; Gonçalves, B.C.; Lopes, J.I.; Correia, C.M. Physiological Responses of Different Olive Genotypes to Drought Conditions. Acta Physiol. Plant. 2009, 31, 611–621. [Google Scholar] [CrossRef]
- Boughalleb, F.; Mhamdi, M. Possible Involvement of Proline and the Antioxidant Defense Systems in the Drought Tolerance of Three Olive Cultivars Grown under Increasing Water Deficit Regimes. Agric. J. 2011, 6, 378–391. [Google Scholar] [CrossRef]
- Chehab, H.; Mechri, B.; Mariem, F.B.; Hammami, M.; Elhadj, S.B.; Braham, M. Effect of Different Irrigation Regimes on Carbohydrate Partitioning in Leaves and Wood of Two Table Olive Cultivars (Olea europaea L. Cv. Meski and Picholine). Agric. Water Manag. 2009, 96, 293–298. [Google Scholar] [CrossRef]
- Petrussa, E.; Braidot, E.; Zancani, M.; Peresson, C.; Bertolini, A.; Patui, S.; Vianello, A. Plant Flavonoids—Biosynthesis, Transport and Involvement in Stress Responses. Int. J. Mol. Sci. 2013, 14, 14950–14973. [Google Scholar] [CrossRef] [PubMed]
- Gershenzon, J. Changes in the Levels of Plant Secondary Metabolites under Water and Nutrient Stress. In Phytochemical Adaptations to Stress; Springer: Berlin/Heidelberg, Germany, 1984; pp. 273–320. [Google Scholar]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Caruso, G.; Gucci, R.; Urbani, S.; Esposto, S.; Taticchi, A.; Di Maio, I.; Selvaggini, R.; Servili, M. Effect of Different Irrigation Volumes during Fruit Development on Quality of Virgin Olive Oil of Cv. Frantoio. Agric. Water Manag. 2014, 134, 94–103. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.; Figueiredo, C.; Santos, C.; Silva, A.M. Phenolic and Lipophilic Metabolite Adjustments in Olea europaea (Olive) Trees during Drought Stress and Recovery. Phytochemistry 2021, 185, 112695. [Google Scholar] [CrossRef]
- Greven, M.; Neal, S.; Green, S.; Dichio, B.; Clothier, B. The Effects of Drought on the Water Use, Fruit Development and Oil Yield from Young Olive Trees. Agric. Water Manag. 2009, 96, 1525–1531. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Fabiani, R.; Urbani, S.; Taticchi, A.; Mariucci, F.; Selvaggini, R.; Montedoro, G.F. Phenolic Compounds in Olive Oil: Antioxidant, Health and Organoleptic Activities According to Their Chemical Structure. Inflammopharmacology 2009, 17, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Bykova, O.; Chuine, I.; Morin, X. Highlighting the Importance of Water Availability in Reproductive Processes to Understand Climate Change Impacts on Plant Biodiversity. Perspect. Plant Ecol. Evol. Syst. 2019, 37, 20–25. [Google Scholar] [CrossRef]
- Çakir, R. Effect of Water Stress at Different Development Stages on Vegetative and Reproductive Growth of Corn. Field Crops Res. 2004, 89, 1–16. [Google Scholar] [CrossRef]
- Pierantozzi, P.; Torres, M.; Tivani, M.; Contreras, C.; Gentili, L.; Parera, C.; Maestri, D. Spring Deficit Irrigation in Olive (Cv. Genovesa) Growing under Arid Continental Climate: Effects on Vegetative Growth and Productive Parameters. Agric. Water Manag. 2020, 238, 106212. [Google Scholar] [CrossRef]
- Jackson, R.D. Canopy Temperature and Crop Water Stress. In Advances in Irrigation; Elsevier: Amsterdam, The Netherlands, 1982; Volume 1, pp. 43–85. ISBN 0275-7915. [Google Scholar]
- Araújo, M.; Prada, J.; Mariz-Ponte, N.; Santos, C.; Pereira, J.A.; Pinto, D.C.; Silva, A.M.; Dias, M.C. Antioxidant Adjustments of Olive Trees (Olea europaea) under Field Stress Conditions. Plants 2021, 10, 684. [Google Scholar] [CrossRef]
- Lesk, C.; Anderson, W.; Rigden, A.; Coast, O.; Jägermeyr, J.; McDermid, S.; Davis, K.F.; Konar, M. Compound Heat and Moisture Extreme Impacts on Global Crop Yields under Climate Change. Nat. Rev. Earth Environ. 2022, 3, 872–889. [Google Scholar] [CrossRef]
- Garofalo, P.; Gaeta, L.; Vitti, C.; Giglio, L.; Leogrande, R. Optimizing Water Footprint, Productivity, and Sustainability in Southern Italian Olive Groves: The Role of Organic Fertilizers and Irrigation Management. Land 2025, 14, 318. [Google Scholar] [CrossRef]
- Fernández, J.E. Plant-Based Methods for Irrigation Scheduling of Woody Crops. Horticulturae 2017, 3, 35. [Google Scholar] [CrossRef]
- Lakhiar, I.A.; Yan, H.; Zhang, C.; Wang, G.; He, B.; Hao, B.; Han, Y.; Wang, B.; Bao, R.; Syed, T.N. A Review of Precision Irrigation Water-Saving Technology under Changing Climate for Enhancing Water Use Efficiency, Crop Yield, and Environmental Footprints. Agriculture 2024, 14, 1141. [Google Scholar] [CrossRef]
- Abou-Shady, A.; Siddique, M.S.; Yu, W. A Critical Review of Innovations and Perspectives for Providing Adequate Water for Sustainable Irrigation. Water 2023, 15, 3023. [Google Scholar] [CrossRef]
- Miller, T.; Mikiciuk, G.; Durlik, I.; Mikiciuk, M.; Łobodzińska, A.; Śnieg, M. The IoT and AI in Agriculture: The Time Is Now—A Systematic Review of Smart Sensing Technologies. Sensors 2025, 25, 3583. [Google Scholar] [CrossRef]
- Messina, G.; Modica, G. Twenty Years of Remote Sensing Applications Targeting Landscape Analysis and Environmental Issues in Olive Growing: A Review. Remote Sens. 2022, 14, 5430. [Google Scholar] [CrossRef]
- Bouslihim, Y.; Bouasria, A.; Jelloul, A.; Khiari, L.; Dahhani, S.; Mrabet, R.; Moussadek, R. Baseline High-Resolution Maps of Soil Nutrients in Morocco to Support Sustainable Agriculture. Sci. Data 2025, 12, 1389. [Google Scholar] [CrossRef]
- Christou, A.; Beretsou, V.G.; Iakovides, I.C.; Karaolia, P.; Michael, C.; Benmarhnia, T.; Chefetz, B.; Donner, E.; Gawlik, B.M.; Lee, Y. Sustainable Wastewater Reuse for Agriculture. Nat. Rev. Earth Environ. 2024, 5, 504–521. [Google Scholar] [CrossRef]
- Obijianya, C.C.; Yakamercan, E.; Karimi, M.; Veluru, S.; Simko, I.; Eshkabilov, S.; Simsek, H. Agricultural Irrigation Using Treated Wastewater: Challenges and Opportunities. Water 2025, 17, 2083. [Google Scholar] [CrossRef]
- Vanella, D.; Consoli, S.; Continella, A.; Chinnici, G.; Milani, M.; Cirelli, G.L.; D’Amico, M.; Maesano, G.; Gentile, A.; La Spada, P. Environmental and Agro-Economic Sustainability of Olive Orchards Irrigated with Reclaimed Water under Deficit Irrigation. Sustainability 2023, 15, 15101. [Google Scholar] [CrossRef]
| Strategy | Treatment | Phenological Growth Stages and Corresponding Dates | Water Irrigation Amount (mm) 1 | Fruit Yield (kg/Tree) 1 | |||
|---|---|---|---|---|---|---|---|
| Total Water | SP1 | NP | SP2 | ||||
| Control | T0 | Full season (all phases) | 304 | 88 (100%ETc) | 110 (100%ETc) | 106 (100%ETc) | 114.4 |
| Regulated Deficit Irrigation (RDI) | T1 | SP1: Full flowering to the beginning of pit hardening (5 April–1 June) (58) 2 NP: From pit hardening to the beginning of oil synthesis (2 June–3 August) (63) 2 SP2: From the beginning of oil synthesis to harvest (4 August–3 November) (92) 2 | 246 | 88 (100%ETc) | 77 (70%ETc) | 106 (100%ETc) | 123.3 |
| T2 | 235 | 88 (100%ETc) | 66 (60%ETc) | 106 (100%ETc) | 112.5 | ||
| T3 | 212 | 70 (80%ETc) | 77 (70%ETc) | 84 (80%ETc) | 99.6 | ||
| T4 | 201 | 70 (80%ETc) | 66 (60%ETc) | 84 (80%ETc) | 88.3 | ||
| Sustained Deficit Irrigation (SDI) | T5 | 196 | 62 (70%ETc) | 77 (70%ETc) | 74 (70%ETc) | 53.5 | |
| T6 | 168 | 53 (60%ETc) | 66 (60%ETc) | 63 (60%ETc) | 55.8 | ||
| Trait Group | Traits (Units) | Mean | SD 2 | Min | Max | CV% | ANOVA F |
|---|---|---|---|---|---|---|---|
| Structural allocation and ecophysiological | LDMC (mg g−1) | 561.20 | 48.81 | 200.38 | 929.07 | 8.69 | 42.984 *** |
| LA (cm2) | 4.15 | 0.90 | 2.42 | 7.74 | 21.80 | 38.743 *** | |
| SS (μm) | 80.49 | 22.70 | 20.09 | 144.52 | 28.21 | 142.68 *** | |
| SD 1 (no. per mm−2) | 220.02 | 39.74 | 100 | 346.66 | 18.06 | 440.119 *** | |
| Biochemical | LNC (mg/g dw) | 19.73 | 1.17 | 17.64 | 21.98 | 5.96 | 18.170 *** |
| LCC (mg/g dw) | 143.75 | 8.89 | 134 | 159.1 | 6.18 | 76.098 *** | |
| LPC (mg/g dw) | 1.23 | 0.31 | 0.77 | 1.64 | 25.25 | 59.568 *** | |
| TFC (mg CE/g dw) | 4.01 | 1.17 | 2.48 | 5.34 | 29.38 | 505.257 *** | |
| TPC (mg GAE/g dw) | 23.89 | 3.40 | 17.68 | 28.19 | 14.27 | 118.952 *** | |
| PRO (mg/g fw) | 15.76 | 7.05 | 6.51 | 25.65 | 44.74 | 373.262 *** | |
| SUC (mg/g fw) | 21.26 | 6.19 | 12.41 | 29.20 | 29.12 | 334.646 *** | |
| GLY (μmol/g dw) | 1.48 | 0.395 | 0.92 | 2.36 | 26.76 | 30.616 *** |
| Strategy | Treatment | LDMC | LA | SS | SD |
|---|---|---|---|---|---|
| Control | T0 | 562.70 ± 29.324 b | 4.881 ± 0.882 a | 70.129 ± 10.34 c | 251.867 ± 33.153 a |
| RDI | T1 1 | 555.434 ± 41.821 b | 4.878 ± 0.919 a | 70.457 ± 12.952 c | 249.466 ± 38.001 a |
| T2 | 555.882 ± 49.994 b | 4.327 ± 0.873 a.b | 85.774 ± 11.057 a | 232.177 ± 31.068 b | |
| T3 | 606.394 ± 27.173 a | 4.102 ± 0.914 b.c | 74.612 ± 11.850 b.c | 215.066 ± 36.603 b.c | |
| T4 | 563.155 ± 38.647 b | 3.646 ± 0.821 c | 80.385 ± 9.555 a.b.c | 220.355 ± 34.923 b.c | |
| SDI | T5 | 545.468 ± 62.075 b | 3.9263 ± 0.629 b.c | 84.108 ± 11.192 b.c | 209.955 ± 37.296 c.d |
| T6 | 540.893 ± 35.439 b | 4.051 ± 0.746 b.c | 87.622 ± 12.8250 a | 193.155 ± 35.464 d | |
| ANOVA F | 42.984 *** | 38.743 *** | 142.68 *** | 440.119 *** |
| Strategy | Treatment | LNC | LCC | LPC | TFC |
|---|---|---|---|---|---|
| Control | T0 | 21.140 ± 0.779 a | 157.367 ± 2.267 a | 1.624 ± 0.019 a | 2.574 ± 0.135 e |
| RDI | T1 | 20.902 ± 0.492 a,b | 156.400 ± 2.500 a | 1.554 ± 0.127 a,b | 2.669 ± 0.124 e |
| T2 | 20.665 ± 0.482 a,b | 142.433 ± 1.115 b | 1.381 ± 0.023 b,c | 2.937 ± 0.008 d | |
| T3 | 19.730 ± 0.140 b,c | 139.700 ± 0.200 b,c | 1.323 ± 0.068 c | 4.387 ± 0.116 c | |
| T4 | 18.890 ± 0.042 c,d | 137.933 ± 0.550 b,c | 1.064 ± 0.035 d | 4.976 ± 0.079 b | |
| SDI | T5 | 18.694 ± 0.424 c,d | 135.600 ± 1.558 c | 0.847 ± 0.098 e | 5.278 ± 0.032 a |
| T6 | 18.251 ± 0.542 d | 136.833 ± 2.850 c | 0.835 ± 0.060 e | 5.242 ± 0.094 a,b | |
| ANOVA F | 18.170 *** | 76.098 *** | 59.568 *** | 505.257 *** | |
| TPC | PRO | SUC | GLY | ||
| Control | T0 | 18.190 ± 0.545 f | 6.690 ± 0.171 e | 12.842 ± 0.137 e | 0.978 ± 0.044 e |
| RDI | T1 | 20.198 ± 0.258 e | 6.985 ± 0.734 e | 13.078 ± 0.804 e | 1.111 ± 0.017 d,e |
| T2 | 23.652 ± 0.532 d | 11.285 ± 0.885 d | 19.288 ± 0.934 d | 1.324 ± 0.018 c,d,e | |
| T3 | 26.729 ± 0.914 a,b | 18.578 ± 0.602 c | 21.948 ± 0.518 c | 1.429 ± 0.005 b,c,d | |
| T4 | 24.674 ± 0.716 c,d | 19.312 ± 0.321 c | 25.575 ± 0.103 b | 1.601 ± 0.052 b,c | |
| SDI | T5 | 28.129 ± 0.060 a | 22.605 ± 0.873 b | 27.668 ± 0.647 a | 1.735 ± 0.064 b |
| T6 | 25.678 ± 0.484 b,c | 24.852 ± 0.711 a | 28.476 ± 0.611 a | 2.169 ± 0.318 a | |
| ANOVA F | 118.952 *** | 373.262 *** | 334.646 *** | 30.616 *** |
| Variables | Regression Parameters | PC1 a | PC2 a |
|---|---|---|---|
| Total irrigation water amount | R2 | 0.922 | 0.067 |
| p-value | 0.002 | 0.550 | |
| Relationship | - | n.s. | |
| Estimates | −0.076 | 0.003 | |
| Standard error | 0.012 | 0.004 | |
| Water amount during SP1 b | R2 | 0.860 | 0.025 |
| p-value | 0.002 | 0.737 | |
| Relationship | - | n.s. | |
| Estimates | −0.222 | 0.007 | |
| Standard error | 0.038 | 0.021 | |
| Water amount during SP2 b | R2 | 0.860 | 0.026 |
| p-value | 0.002 | 0.730 | |
| Relationship | - | n.s. | |
| Estimates | −0.181 | 0.006 | |
| Standard error | 0.031 | 0.017 | |
| Water amount during NP b | R2 | 0.371 | 0.007 |
| p-value | 0.005 | 0.756 | |
| Relationship | - | n.s. | |
| Estimates | −0.135 | 0.003 | |
| Standard error | 0.028 | 0.011 | |
| Fruit yield | R2 | 0.824 | 0.039 |
| p-value | 0.001 | 0.482 | |
| Relationship | - | n.s. | |
| Estimates | −0.108 | 0.005 | |
| Standard error | 0.016 | 0.007 | |
| Adjusted temperature index (AT) | R2 | 0.852 | 0.048 |
| p-value | 0.002 | 0.653 | |
| Relationship | + | n.s. | |
| Estimates | 1.443 | −0.055 | |
| Standard error | 0.248 | 0.117 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souali, H.; Ibba, K.; Ahrouch, H.; Zahiri, A.; El Issaoui, K.; Rabi, B.; Choukrane, B.; Boselli, V.A.; Hadria, R.; Er-Raki, S.; et al. Functional Trait-Based Responses of the Moroccan Menara Cultivar to Deficit Irrigation. Sustainability 2025, 17, 10614. https://doi.org/10.3390/su172310614
Souali H, Ibba K, Ahrouch H, Zahiri A, El Issaoui K, Rabi B, Choukrane B, Boselli VA, Hadria R, Er-Raki S, et al. Functional Trait-Based Responses of the Moroccan Menara Cultivar to Deficit Irrigation. Sustainability. 2025; 17(23):10614. https://doi.org/10.3390/su172310614
Chicago/Turabian StyleSouali, Houda, Khaoula Ibba, Hamza Ahrouch, Asma Zahiri, Kaoutar El Issaoui, Bouchra Rabi, Basma Choukrane, Vladimiro Andrea Boselli, Rachid Hadria, Salah Er-Raki, and et al. 2025. "Functional Trait-Based Responses of the Moroccan Menara Cultivar to Deficit Irrigation" Sustainability 17, no. 23: 10614. https://doi.org/10.3390/su172310614
APA StyleSouali, H., Ibba, K., Ahrouch, H., Zahiri, A., El Issaoui, K., Rabi, B., Choukrane, B., Boselli, V. A., Hadria, R., Er-Raki, S., Oulbi, S., Hsissou, D., Ater, M., & Kassout, J. (2025). Functional Trait-Based Responses of the Moroccan Menara Cultivar to Deficit Irrigation. Sustainability, 17(23), 10614. https://doi.org/10.3390/su172310614










