Material Design and Operation Strategy of the Electro-Fenton System for the Treatment of High Pollutant Load Wastewater
Abstract
1. Introduction
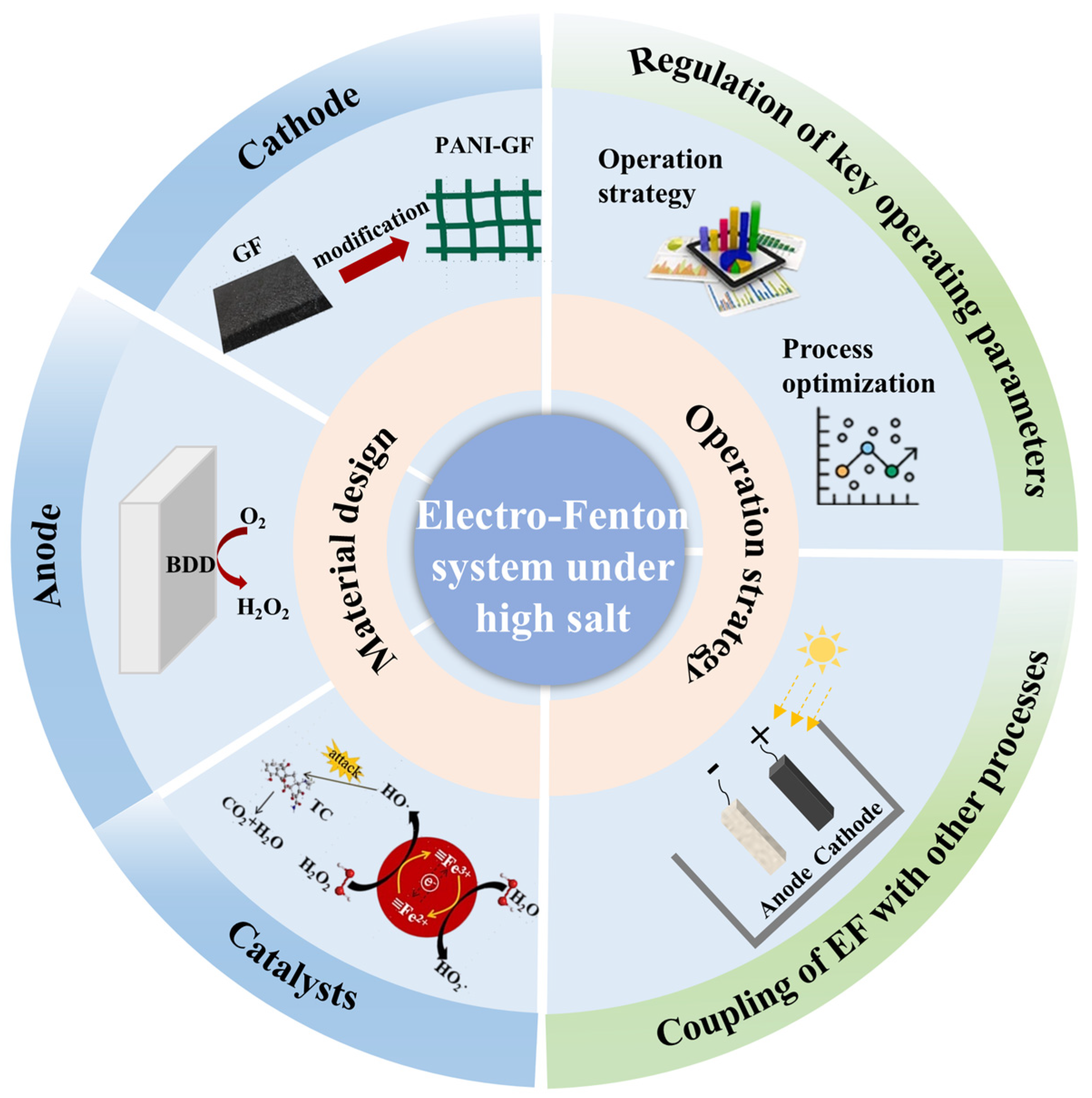
2. Material Design for Electro-Fenton Systems
2.1. Cathode Materials
2.2. Anode Materials
2.3. Catalysts
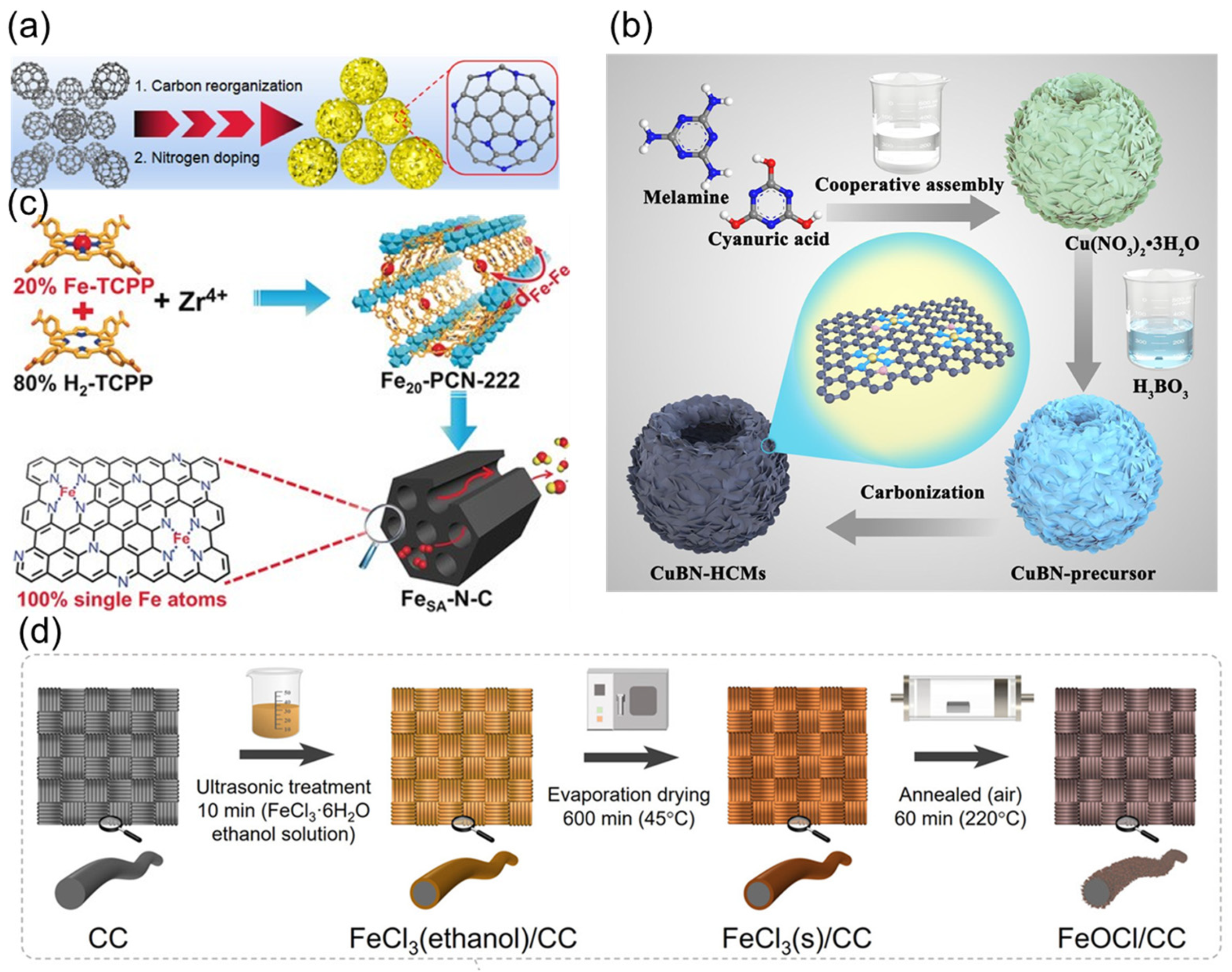
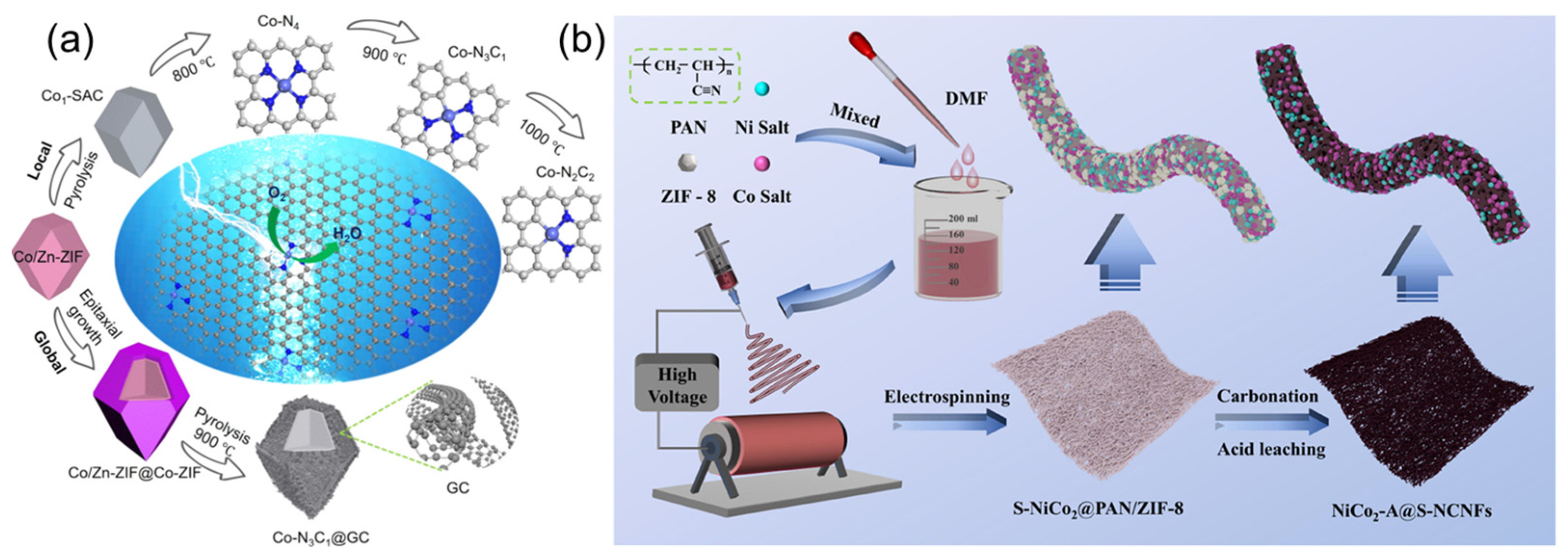
2.3.1. Heterogeneous Iron-Based Catalysts
2.3.2. Other Heterogeneous Catalysts
3. Operation Strategy and Process Optimization for Electro-Fenton Systems
3.1. Regulation of Key Operating Parameters
3.1.1. pH
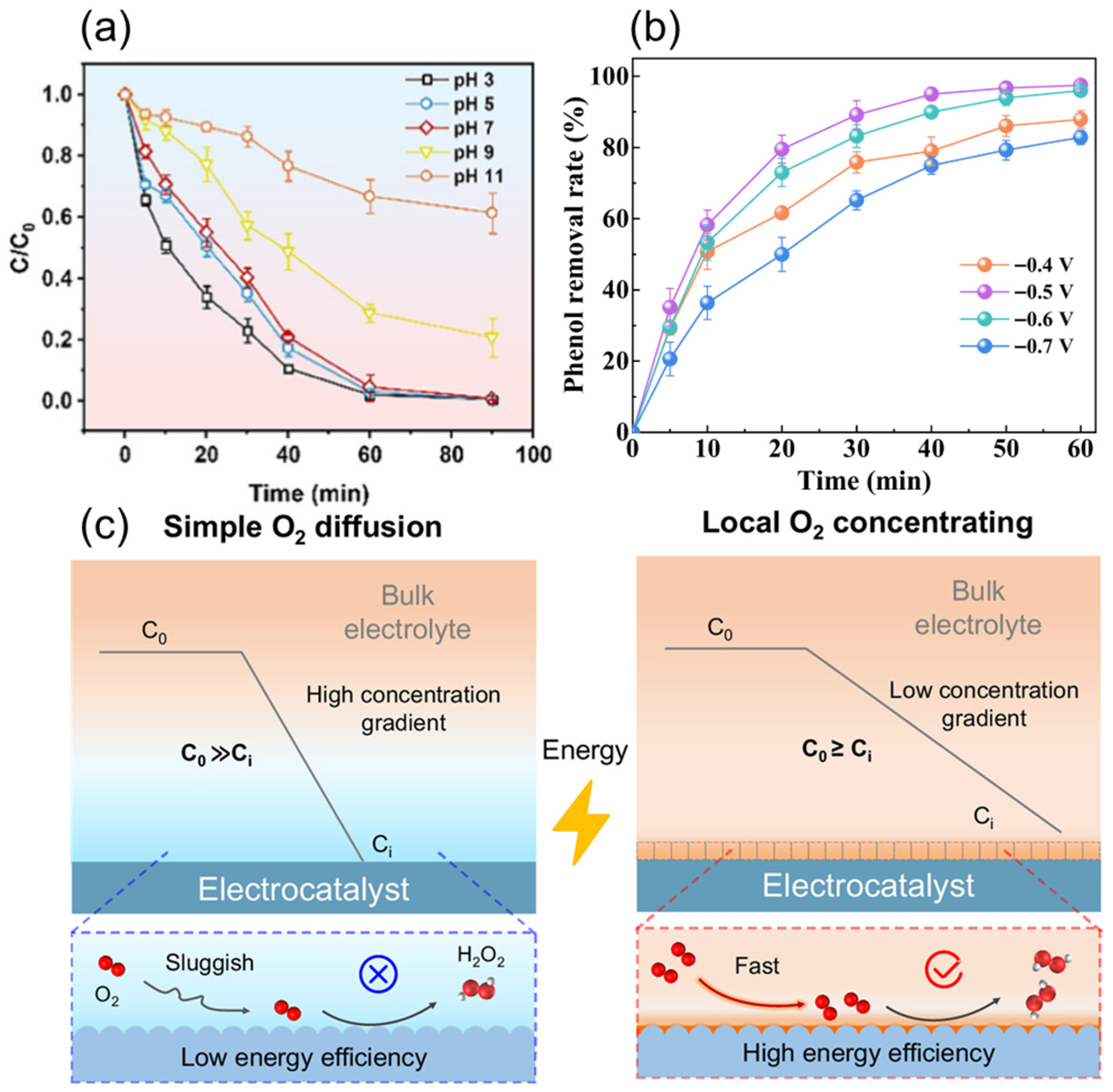
3.1.2. Current Density and Voltage
3.1.3. Aeration Rate
3.1.4. Catalyst Dosage
3.2. Coupling of Electro-Fenton with Other Processes
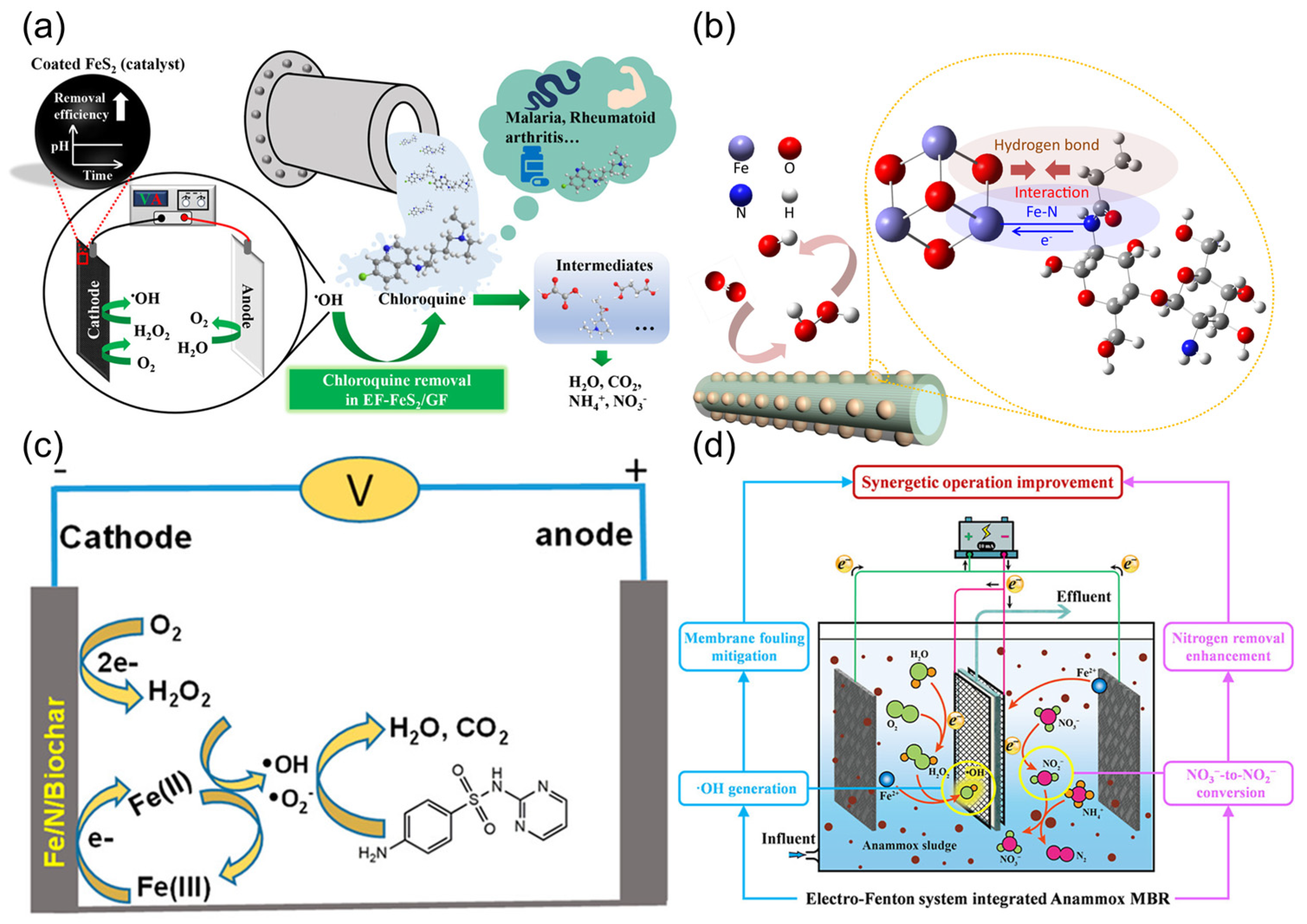
3.2.1. Electro-Fenton Coupled with Photocatalysis/Ultrasound
3.2.2. Electro-Fenton Coupled with Biological Treatment
3.2.3. Electro-Fenton Coupled with Membrane Technology
4. Challenges and Perspectives
4.1. Limitations of Existing Treatment Facilities and Opportunities
4.2. Material and Process Challenges
4.3. Prospective Solutions
- (1)
- Developing Intelligent Hybrid Materials: Design composite cathodes, anodes, and catalysts that integrate the strengths of multiple material classes while mitigating their weaknesses. For instance, constructing core–shell structures where conductive carbon matrices stably encapsulate highly active iron sites can simultaneously address activity, stability, and conductivity issues.
- (2)
- Optimizing Reactor Design and System Integration: Increase electrode surface area, improve flow dynamics, and enhance oxygen transfer to improve the efficiency of oxidant generation. More critically and efficiently integrate EF as an intensification unit—coupling it with anaerobic (pre-treatment) or aerobic (post-treatment) biological processes to create synergistic treatment trains that balance efficiency and operational costs.
- (3)
- Implementing Intelligent Process Control: Develop machine learning and AI-driven systems to establish dynamic models correlating operational parameters (current density, aeration intensity, pH adjustment, H2O2 dosage) with treatment efficiency. Through real-time data analysis and predictive control, the system can automatically adjust parameters to adapt to influent quality variations, minimizing energy and chemical consumption while ensuring effluent compliance.
- (4)
- Conducting Life-Cycle and Techno-Economic Assessments: Embed Life Cycle Assessment (LCA) and Techno-Economic Analysis (TEA) into the EF technology development and evaluation framework. This ensures its role as a reliable and sustainable solution for high-pollutant-load wastewater treatment and clarifies its competitiveness and directions for improvement in practical applications.
5. Conclusions
- (1)
- Designing Robust Materials for High-Load Environments: Developing low-cost, highly durable electrodes and poisoning-resistant catalysts suitable for continuous operation in high-strength, complex wastewaters, with a focus on antifouling properties and self-regeneration capabilities.
- (2)
- Optimizing Reactor Configuration to Overcome Mass Transfer Limitations: Developing scalable, efficient reactor configurations (e.g., three-dimensional electrodes, fluidized-bed reactors) and hybrid EF systems (e.g., EF-membrane filtration) specifically designed to enhance mass transfer and degradation efficiency under high pollutant concentrations.
- (3)
- Implementing Intelligent, Real-Time Process Control: Implementing machine learning and AI-driven systems to dynamically adjust key operational parameters (e.g., current density, pH, aeration) in real-time response to fluctuations in complex wastewater composition, ensuring robust treatment performance and energy efficiency.
- (4)
- Advancing System Sustainability and Practical Application Assessment: Conducting comprehensive assessments of the environmental footprint (e.g., carbon footprint) and techno-economic feasibility of EF systems treating high-strength wastewaters and developing sustainable materials like biomass/waste-derived catalysts to ensure environmental friendliness and scalability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| •OH | Hydroxyl radicals |
| GF | Graphite felt |
| AC | Activated carbon |
| GO | Graphene oxide |
| AOPs | Advanced oxidation technology |
| MFC | Microbial fuel cells |
| BDD | Boron-doped diamond |
| MOFs | Metal–organic frameworks |
| BEF | Bio-electro-Fenton |
| ORR | Oxygen reduction reaction |
| COD | Chemical oxygen demand |
| SACs | Single-atom catalysts |
| DSAs | Dimensionally stable anodes |
| TOC | Total organic carbon |
| EF | Electro-Fenton |
| ZIFs | zeolitic imidazolate frameworks |
References
- Li, J.; Shi, W.; Jiang, C.; Bai, L.; Wang, T.; Yu, J.; Ruan, W. Evaluation of potassium as promoter on anaerobic digestion of saline organic wastewater. Bioresour. Technol. 2018, 266, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yao, H.; Li, X.; Deng, S.; Zhao, S.; Zhang, W. Enhanced Degradation of Sulfamethoxazole (SMX) in Toilet Wastewater by Photo-Fenton Reactive Membrane Filtration. Nanomaterials 2020, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liu, H.; Wang, M.; Xie, Y.; Ding, J.; Wang, Y. Research progress in new treatment technology for high-salinity wastewater. Mod. Chem. Ind 2022, 42, 68–71. [Google Scholar]
- Al-Amshawee, S.; Yunus, M.Y.B.M.; Azoddein, A.A.M.; Hassell, D.G.; Dakhil, I.H.; Hasan, H.A. Electrodialysis desalination for water and wastewater: A review. Chem. Eng. J. 2020, 380, 122231. [Google Scholar] [CrossRef]
- Maharaja, P.; Boopathy, R.; Anushree, V.; Mahesh, M.; Swarnalatha, S.; Ravindran, B.; Chang, S.W.; Sekaran, G. Bio removal of proteins, lipids and mucopolysaccharides in tannery hyper saline wastewater using halophilic bacteria. J. Water Process Eng. 2020, 38, 101674. [Google Scholar] [CrossRef]
- Deng, S.; Wang, C.; Ngo, H.H.; Guo, W.; You, N.; Tang, H.; Yu, H.; Tang, L.; Han, J. Comparative review on microbial electrochemical technologies for resource recovery from wastewater towards circular economy and carbon neutrality. Bioresour. Technol. 2023, 376, 128906. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Das, I.; Ghangrekar, M.M.; Blaney, L. Advanced oxidation processes: Performance, advantages, and scale-up of emerging technologies. J. Environ. Manag. 2022, 316, 115295. [Google Scholar] [CrossRef]
- Krishnan, R.Y.; Manikandan, S.; Subbaiya, R.; Biruntha, M.; Govarthanan, M.; Karmegam, N. Removal of emerging micropollutants originating from pharmaceuticals and personal care products (PPCPs) in water and wastewater by advanced oxidation processes: A review. Environ. Technol. Innov. 2021, 23, 101757. [Google Scholar] [CrossRef]
- Mukherjee, J.; Lodh, B.K.; Sharma, R.; Mahata, N.; Shah, M.P.; Mandal, S.; Ghanta, S.; Bhunia, B. Advanced oxidation process for the treatment of industrial wastewater: A review on strategies, mechanisms, bottlenecks and prospects. Chemosphere 2023, 345, 140473. [Google Scholar] [CrossRef]
- Diya, W.; Bing, C.; Jisi, Z.; Ganning, Z. Atrazine manufacturing wastewater treatment by photocatalytic ozonization with activated carbon supported ferric iron. Chin. J. Environ. Eng. 2020, 14, 349–358. [Google Scholar]
- Saravanan, A.; Deivayanai, V.C.; Kumar, P.S.; Rangasamy, G.; Hemavathy, R.V.; Harshana, T.; Gayathri, N.; Alagumalai, K. A detailed review on advanced oxidation process in treatment of wastewater: Mechanism, challenges and future outlook. Chemosphere 2022, 308, 136524. [Google Scholar] [CrossRef]
- You, N.; Deng, S.-H.; He, H.; Hu, J. Ferromanganese oxide-functionalized TiO2 for rapid catalytic ozonation of PPCPs through a coordinated oxidation process with adjusted composition and strengthened generation of reactive oxygen species. Water Res. 2024, 258, 121813. [Google Scholar] [CrossRef]
- Zhang, Z.; Yin, X. Kinetics on degradation of COD_(cr) in mustard comprehensive wastewater by electro-Fenton process. J. Chongqing Univ. 2013, 36, 115–120. [Google Scholar]
- Deng, S.; Wang, Q.; Cai, Q.; Ong, S.L.; Hu, J. Efficient bio-refractory industrial wastewater treatment with mitigated membrane fouling in a membrane bioreactor strengthened by the micro-scale ZVI@GAC galvanic-cells-initiated radical generation and coagulation processes. Water Res. 2022, 209, 117943. [Google Scholar] [CrossRef]
- Oturan, M.A.; and Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Song, W.; Chen, C.; Ma, W.; Zhao, J.; Tang, Y. Fenton Degradation of Organic Compounds Promoted by Dyes under Visible Irradiation. Environ. Sci. Technol. 2005, 39, 5810–5815. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Li, D.; Deng, S.; Liu, Y.; Ma, C.; Zhang, C. Combination with catalyzed Fe(0)-carbon microelectrolysis and activated carbon adsorption for advanced reclaimed water treatment: Simultaneous nitrate and biorefractory organics removal. Environ. Sci. Pollut. Res. 2019, 26, 5693–5703. [Google Scholar] [CrossRef]
- Zhang, P.; Meng, X.; Luo, X.; Wang, J.; Feng, J.; Ma, Y.; Li, K.; Liu, Y.; Wang, P.; Yin, C.; et al. In-situ interconnected 1D/2D/3D architectures of CNT/MoS2/Fe3O4 membranes for increasing degradation efficiency of penetrative electro-Fenton under low energy consumption. J. Environ. Chem. Eng. 2024, 12, 113735. [Google Scholar] [CrossRef]
- Olvera-Vargas, H.; Fernández González, Q.; Guillén-Garcés, R.A.; Rincón, M.E. Reverse-engineered Electro-Fenton for the selective synthesis of oxalic or oxamic acid through the degradation of acetaminophen: A novel green electrocatalytic refinery approach. Water Res. 2025, 272, 122914. [Google Scholar] [CrossRef]
- Tesnim, D.; Díez, A.M.; Amor Hédi, B.; Sanroman, M.Á.; Pazos, M. Sustainable removal of antipyrine from wastewater via an Eco-Friendly heterogeneous Electro-Fenton-like process employing Zero-Valent iron nanoparticles loaded activated carbon. Chem. Eng. J. 2024, 493, 152494. [Google Scholar] [CrossRef]
- Liu, X.; You, S.; Ma, F.; Zhou, H. Characterization of electrode fouling during electrochemical oxidation of phenolic pollutant. Front. Environ. Sci. Eng. 2021, 15, 53. [Google Scholar] [CrossRef]
- Afolabi, O.A.; Adekalu, K.O.; Okunade, D.A. Electro-Fenton treatment process for brewery wastewater: Effects of oxidant concentration and reaction time on BOD and COD removal efficiency. J. Eng. Appl. Sci. 2022, 69, 42. [Google Scholar] [CrossRef]
- Brillas, E.; Garcia-Segura, S. Benchmarking recent advances and innovative technology approaches of Fenton, photo-Fenton, electro-Fenton, and related processes: A review on the relevance of phenol as model molecule. Sep. Purif. Technol. 2020, 237, 116337. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, M.; Xiao, F.; Postole, G.; Zhao, H.; Zhao, G. Recent advances and trends of heterogeneous electro-Fenton process for wastewater treatment-review. Chin. Chem. Lett. 2022, 33, 653–662. [Google Scholar] [CrossRef]
- Nair, K.M.; Kumaravel, V.; Pillai, S.C. Carbonaceous cathode materials for electro-Fenton technology: Mechanism, kinetics, recent advances, opportunities and challenges. Chemosphere 2021, 269, 129325. [Google Scholar] [CrossRef]
- Khataee, A.; Hasanzadeh, A. Modified cathodes with carbon-based nanomaterials for electro-Fenton process. In Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2018; Volume 61, pp. 111–143. [Google Scholar]
- Shen, R.; Chen, W.; Peng, Q.; Lu, S.; Zheng, L.; Cao, X.; Wang, Y.; Zhu, W.; Zhang, J.; Zhuang, Z.; et al. High-Concentration Single Atomic Pt Sites on Hollow CuSx for Selective O2 Reduction to H2O2 in Acid Solution. Chem 2019, 5, 2099–2110. [Google Scholar] [CrossRef]
- Jiang, Y.; Ni, P.; Chen, C.; Lu, Y.; Yang, P.; Kong, B.; Fisher, A.; Wang, X. Selective Electrochemical H2O2 Production through Two-Electron Oxygen Electrochemistry. Adv. Energy Mater. 2018, 8, 1801909. [Google Scholar] [CrossRef]
- Govindaraj, D.; Nidheesh, P.V. Importance of Graphene in the Electro-Fenton Process. ACS Omega 2020, 5, 4725–4732. [Google Scholar] [CrossRef]
- Jiao, Y.; Ma, L.; Tian, Y.; Zhou, M. A flow-through electro-Fenton process using modified activated carbon fiber cathode for orange II removal. Chemosphere 2020, 252, 126483. [Google Scholar] [CrossRef]
- Wang, D.; Hu, J.; Liu, B.; Hou, H.; Yang, J.; Li, Y.; Zhu, Y.; Liang, S.; Xiao, K. Degradation of refractory organics in dual-cathode electro-Fenton using air-cathode for H2O2 electrogeneration and microbial fuel cell cathode for Fe2+ regeneration. J. Hazard. Mater. 2021, 412, 125269. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Le, T.X.H.; Bechelany, M.; Esposito, G.; Hullebusch, E.D.v.; Oturan, M.A.; Cretin, M. A hierarchical CoFe-layered double hydroxide modified carbon-felt cathode for heterogeneous electro-Fenton process. J. Mater. Chem. 2017, 5, 3655–3666. [Google Scholar] [CrossRef]
- Plakas, K.V.; Karabelas, A.J. Electro-Fenton Applications in the Water Industry. In Electro-Fenton Process: New Trends and Scale-Up; Zhou, M., Oturan, M.A., Sirés, I., Eds.; Springer: Singapore, 2018; pp. 343–378. [Google Scholar]
- Ren, W.; Tang, D.; Huang, M.; Sun, J.; Lv, K. Remarkable improved electro-Fenton efficiency by electric-field-induced catalysis of CeO2. J. Hazard. Mater. 2018, 350, 88–97. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, W.; Guo, K.; Xiong, M.; Zhang, J.; Lu, X. A Pentagonal Defect-Rich Metal-Free Carbon Electrocatalyst for Boosting Acidic O2 Reduction to H2O2 Production. J. Am. Chem. Soc. 2023, 145, 11589–11598. [Google Scholar] [CrossRef]
- Zhu, G.; Fan, X.; Yu, Y.; Liu, Y.; Quan, X. Regulating the Electronic Structure of Cu Single-Atom Catalysts toward Enhanced Electro-Fenton Degradation of Organic Contaminants via 1O2 and OH. Environ. Sci. Technol. 2024, 58, 19545–19554. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Han, M.; Shi, Y.; Guo, W.; Wu, Y.; Zhang, T.; Han, X.; Du, C.; Yu, C.; Feng, J.; et al. Construction of integrated oxygen-rich carbon-based metal-free cathode to simultaneous boost wastewater treatment performance and energy recovery in bio-electro-Fenton system. Chem. Eng. J. 2024, 487, 150532. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, J.; Zhang, K.; Zhang, Z.; Du, C.; Liu, Y.; Li, B.; Wu, Y.; Guo, W.; Dong, S.; et al. In-situ degradation of organic pollutants by bioelectrical-Fenton reaction with a metal-free polyaniline-derived nitrogen-doped carbon nanofibre electrode. J. Alloys Compd. 2022, 901, 163710. [Google Scholar] [CrossRef]
- Cui, L.; Zhao, X.; Xie, H.; Zhang, Z. Overcoming the Activity–Stability Trade-Off in Heterogeneous Electro-Fenton Catalysis: Encapsulating Carbon Cloth-Supported Iron Oxychloride within Graphitic Layers. ACS Catal. 2022, 12, 13334–13348. [Google Scholar] [CrossRef]
- Wang, K.; Cao, P.; Qin, X.; Chen, S.; Yu, H.; Quan, X. Electro-Fenton process with scalable modified carbonaceous electrode material processed by direct calcination for refractory organic pollutant degradation. Chem. Eng. J. 2023, 470, 144104. [Google Scholar] [CrossRef]
- Liu, M.; Feng, Z.; Luan, X.; Chu, W.; Zhao, H.; Zhao, G. Accelerated Fe2+ Regeneration in an Effective Electro-Fenton Process by Boosting Internal Electron Transfer to a Nitrogen-Conjugated Fe(III) Complex. Environ. Sci. Technol. 2021, 55, 6042–6051. [Google Scholar] [CrossRef]
- Liu, J.; Jia, J.; Yu, H.; Zhang, J.; Li, J.; Ge, H.; Zhao, Y. Graphite felt modified by nanoporous carbon as a novel cathode material for the EF process. New J. Chem. 2022, 46, 12696–12702. [Google Scholar] [CrossRef]
- Jiao, L.; Wan, G.; Zhang, R.; Zhou, H.; Yu, S.-H.; Jiang, H.-L. From Metal–Organic Frameworks to Single-Atom Fe Implanted N-doped Porous Carbons: Efficient Oxygen Reduction in Both Alkaline and Acidic Media. Angew. Chem. Int. Ed. 2018, 57, 8525–8529. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Cerrón-Calle, G.A.; Brillas, E.; Santos, M.C.; Garcia-Segura, S. Optimization of fipronil removal via electro-Fenton using a carbon cloth air-diffusion electrode. Sep. Purif. Technol. 2025, 367, 132955. [Google Scholar] [CrossRef]
- Zhang, P.; Meng, X.; Luo, X.; Wang, J.; Feng, J.; Ma, Y.; Li, K.; Liu, Y.; Ji, L. Enhanced heterogeneous Electro-Fenton catalysis using CNT/MoS2/FeCo-LDH cathode membranes for efficient phenol removal from wastewater. Int. J. Electrochem. Sci. 2024, 19, 100633. [Google Scholar] [CrossRef]
- Jia, Y.; Li, H.; Zhao, H.; Zhang, G.; Zhang, Z.; Zhang, X.; Zhou, W. A new strategy for improving the energy efficiency of electro-Fenton: Using N-doped activated carbon cathode with strong Fe(III) adsorption capacity to promote Fe(II) regeneration. J. Env. Manag. 2024, 357, 120823. [Google Scholar] [CrossRef]
- Huong Le, T.X.; Alemán, B.; Vilatela, J.J.; Bechelany, M.; Cretin, M. Enhanced Electro-Fenton Mineralization of Acid Orange 7 Using a Carbon Nanotube Fiber-Based Cathode. Front. Mater. 2018, 5, 9. [Google Scholar] [CrossRef]
- Muzenda, C.; Arotiba, O.A. Improved Magnetite Nanoparticle Immobilization on a Carbon Felt Cathode in the Heterogeneous Electro-Fenton Degradation of Aspirin in Wastewater. ACS Omega 2022, 7, 19261–19269. [Google Scholar] [CrossRef]
- Zhou, W.; Rajic, L.; Chen, L.; Kou, K.; Ding, Y.; Meng, X.; Wang, Y.; Mulaw, B.; Gao, J.; Qin, Y.; et al. Activated carbon as effective cathode material in iron-free Electro-Fenton process: Integrated H2O2 electrogeneration, activation, and pollutants adsorption. Electrochim Acta 2019, 296, 317–326. [Google Scholar] [CrossRef]
- Lv, J.; Zhao, Q.; Wang, K.; Jiang, J.; Ding, J.; Wei, L. A critical review of approaches to enhance the performance of bio-electro-Fenton and photo-bio-electro-Fenton systems. J. Environ. Manag. 2024, 365, 121633. [Google Scholar] [CrossRef]
- Rai, D.; Sinha, S. Impact of different anode materials on electro-Fenton process and tannery wastewater treatment using sequential electro-Fenton and electrocoagulation. Chemosphere 2023, 336, 139225. [Google Scholar] [CrossRef]
- Kim, T.H.; Jeong, C.; Choi, J.-H.; Park, H.-s.; Lee, K.-W.; Lee, T.S. Fabrication of nanofibrous PbO2 electrode embedded with Pt for decomposition of organic chelating agents. Chemosphere 2023, 344, 140386. [Google Scholar] [CrossRef]
- He, H.; Zhou, Z. Electro-Fenton process for water and wastewater treatment. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2100–2131. [Google Scholar] [CrossRef]
- Rajoria, S.; Vashishtha, M.; Sangal, V.K. Oxidative degradation of electroplating wastewater by an electro-Fenton process using GO/TiO2NTs electrode. Environ. Eng. Res. 2024, 29, 230056. [Google Scholar] [CrossRef]
- Wang, J.; Xu, M.; Liang, X.; Zhang, Y.; Yang, D.; Pan, L.; Fang, W.; Zhu, C.; Wang, F. Development of a novel 2D Ni-MOF derived NiO@C nanosheet arrays modified Ti/TiO2NTs/PbO2 electrode for efficient electrochemical degradation of salicylic acid wastewater. Sep. Purif. Technol. 2021, 263, 118368. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, H.; Ji, Y.; Gao, Y.; Ni, S.; Huang, Y.; Han, W.; Wei, K. Electrocatalytic mechanism of titanium-based anodes and research progress of chemical saline wastewater treatment: A short review. Water Resour. Ind. 2024, 31, 100242. [Google Scholar] [CrossRef]
- Tao, N.C.; Luu, T.L. Different behaviours of biologically textile wastewater treatment using persulfate catalyzed electrochemical oxidation process on Ti/BDD and Ti/SnO2-Nb2O5 anodes. Environ. Eng. Res. 2023, 28, 220555. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Y.; Cao, D.; Xiao, K.; Guo, T.; Zhao, X. Enhanced photoelectrocatalytic degradation of 2,4-dichlorophenol by TiO2/Ru-IrO2 bifacial electrode. Chem. Eng. J. 2018, 343, 69–77. [Google Scholar] [CrossRef]
- Liu, M.; Liu, G.; Shao, P.; Tian, Y.; Zou, H. Electrochemical removal of Ni–EDTA mediated by chlorine and hydroxyl radicals on BDD anodes. ACS EST Water 2023, 3, 827–837. [Google Scholar] [CrossRef]
- Okur, M.C.; Akyol, A.; Nayir, T.Y.; Kara, S.; Ozturk, D.; Civas, A. Performance of Ti/RuO2-IrO2 electrodes and comparison with BDD electrodes in the treatment of textile wastewater by electro-oxidation process. Chem. Eng. Res. Des. 2022, 183, 398–410. [Google Scholar] [CrossRef]
- He, Y.; Zhao, D.; Lin, H.; Huang, H.; Li, H.; Guo, Z. Design of diamond anodes in electrochemical degradation of organic pollutants. Curr. Opin. Electrochem. 2022, 32, 100878. [Google Scholar] [CrossRef]
- Zhang, J.; Qiu, S.; Feng, H.; Hu, T.; Wu, Y.; Luo, T.; Tang, W.; Wang, D. Efficient degradation of tetracycline using core–shell Fe@Fe2O3-CeO2 composite as novel heterogeneous electro-Fenton catalyst. Chem. Eng. J. 2022, 428, 131403. [Google Scholar] [CrossRef]
- Li, J.; Li, D.; Cui, Y.; Xing, W.; Deng, S. Micro-electrolysis/retinervus luffae-based simultaneous autotrophic and heterotrophic denitrification for low C/N wastewater treatment. Environ. Sci. Pollut. Res. 2017, 24, 16651–16658. [Google Scholar] [CrossRef]
- Liu, H.-y.; Jiang, J.; Tang, L.; Liang, Y.; Xue, S.-G. Recent progress in electrocatalytic selectivity in heterogeneous electro-Fenton processes. J. Mater. Chem. A 2023, 11, 7387–7408. [Google Scholar] [CrossRef]
- Fayazi, M.; Ghanei-Motlagh, M. Electrochemical mineralization of methylene blue dye using electro-Fenton oxidation catalyzed by a novel sepiolite/pyrite nanocomposite. Int. J. Environ. Sci. Technol. 2020, 17, 4541–4548. [Google Scholar] [CrossRef]
- Huang, X.; Wei, J.; Jiang, X.; Nan, Z. FeS2/SiO2 mesoporous hollow spheres formation and catalytic properties in the Fenton reaction. Mater. Lett. 2020, 277, 128408. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, Y.; Chen, J.; Chen, J.; Yang, L.; Wei, W.; Ni, B.-J.; Chen, X. Efficient Chloroquine Removal by Electro-Fenton with FeS2-Modified Cathode: Performance, Influencing Factors, Pathway Contributions, and Degradation Mechanisms. ACS EST Water 2023, 3, 2786–2796. [Google Scholar] [CrossRef]
- Jiang, S.; Sun, B.; Han, Y.; Yang, C.; Zhou, T.; Xiao, K.; Gong, J. Low-toxicity natural pyrite on electro-Fenton catalytic reaction in a wide pH range. Sci. Total Environ. 2024, 950, 175295. [Google Scholar] [CrossRef]
- Liu, X.C.; Zhang, K.X.; Song, J.S.; Zhou, G.N.; Li, W.Q.; Ding, R.R.; Wang, J.; Zheng, X.; Wang, G.; Mu, Y. Tuning Fe3O4 for sustainable cathodic heterogeneous electro-Fenton catalysis by acetylated chitosan. Proc. Natl. Acad. Sci. USA 2023, 120, e2213480120. [Google Scholar] [CrossRef]
- Li, H.-C.; Ji, X.-Y.; Pan, X.-Q.; Liu, C.; Liu, W.-J. Ionothermal Carbonization of Biomass to Construct Fe, N-Doped Biochar with Prominent Activity and Recyclability as Cathodic Catalysts in Heterogeneous Electro-Fenton. ACS EST Eng. 2021, 1, 21–31. [Google Scholar] [CrossRef]
- Ni, L.; Wang, P.; Wang, Y. Sustainable Electro-Fenton Antifouling Strategy Simultaneously Improves Nitrogen-Removal Efficiency of Anammox in Membrane Bioreactors. Environ. Sci. Technol. 2025, 59, 17545–17557. [Google Scholar] [CrossRef]
- Görmez, F.; Görmez, Ö.; Gözmen, B.; Kalderis, D. Degradation of chloramphenicol and metronidazole by electro-Fenton process using graphene oxide-Fe3O4 as heterogeneous catalyst. J. Environ. Chem. Eng. 2019, 7, 102990. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, Y. Study on Synthesis and Catalytic Performance of Fe3O4@C Nanosphere. Hans J. Nanotechnol. 2023, 13, 1–6. [Google Scholar] [CrossRef]
- Cheng, M.; Lai, C.; Liu, Y.; Zeng, G.; Huang, D.; Zhang, C.; Qin, L.; Hu, L.; Zhou, C.; Xiong, W. Metal-organic frameworks for highly efficient heterogeneous Fenton-like catalysis. Coord. Chem. Rev. 2018, 368, 80–92. [Google Scholar] [CrossRef]
- Taha, A.A.; Huang, L.; Ramakrishna, S.; Liu, Y. MOF [NH2-MIL-101(Fe)] as a powerful and reusable Fenton-like catalyst. J. Water Process Eng. 2020, 33, 101004. [Google Scholar] [CrossRef]
- Najafzadeh, A.; Ayati, B. Improvement of electro-Fenton process by using heterogeneous Fe-MIL-88B nanocatalyst and simultaneous rotation of cathode and anode for dye removal. Sci. Rep. 2024, 14, 24038. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, Y.; He, D.; Pan, X. Application of iron-based materials in heterogeneous advanced oxidation processes for wastewater treatment: A review. Chem. Eng. J. 2021, 407, 127191. [Google Scholar] [CrossRef]
- Tian, Y.; Fu, W.; Wang, Q.; Tang, Y.; Zhou, M. High electron transfer rate and efficiency on Fe0 modified by sulfidation and pre-magnetization for carbamazepine degradation by heterogeneous electro-Fenton in wide pH ranges. Chem. Eng. J. 2022, 427, 131694. [Google Scholar] [CrossRef]
- Ye, Z.; Padilla, J.A.; Xuriguera, E.; Brillas, E.; Sirés, I. Magnetic MIL(Fe)-type MOF-derived N-doped nano-ZVI@C rods as heterogeneous catalyst for the electro-Fenton degradation of gemfibrozil in a complex aqueous matrix. Appl. Catal. B Environ. 2020, 266, 118604. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Xu, S.; Yang, Y.; Li, F. Heterogeneous electro-Fenton removal of polyacrylamide in aqueous solution over CoFe2O4 catalyst. Water Sci. Technol. 2024, 89, 3309–3324. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Cao, Z.; Tong, X.; Yang, P.; Li, S.; Chen, X.; Liu, D.; Huang, W. Investigation of CuCoFe-LDH as an efficient and stable catalyst for the degradation of acetaminophen in heterogeneous electro-Fenton system: Key operating parameters, mechanisms and pathways. J. Environ. Manag. 2023, 327, 116787. [Google Scholar] [CrossRef]
- Liu, D.; Dai, L.; Lin, X.; Chen, J.-F.; Zhang, J.; Feng, X.; Müllen, K.; Zhu, X.; Dai, S. Chemical Approaches to Carbon-Based Metal-Free Catalysts. Adv. Mater. 2019, 31, 1804863. [Google Scholar] [CrossRef]
- Shang, Y.; Xu, X.; Gao, B.; Wang, S.; Duan, X. Single-atom catalysis in advanced oxidation processes for environmental remediation. Chem. Soc. Rev. 2021, 50, 5281–5322. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.E. State-of-the-art developments in carbon-based metal nanocomposites as a catalyst: Photocatalysis. Nanoscale Adv. 2021, 3, 1887–1900. [Google Scholar] [CrossRef]
- Qin, X.; Zhao, K.; Quan, X.; Cao, P.; Chen, S.; Yu, H. Highly efficient metal-free electro-Fenton degradation of organic contaminants on a bifunctional catalyst. J. Hazard. Mater. 2021, 416, 125859. [Google Scholar] [CrossRef]
- Shang, Y.; Duan, X.; Wang, S.; Yue, Q.; Gao, B.; Xu, X. Carbon-based single atom catalyst: Synthesis, characterization, DFT calculations. Chin. Chem. Lett. 2022, 33, 663–673. [Google Scholar] [CrossRef]
- Hai, X.; Zhao, X.; Guo, N.; Yao, C.; Chen, C.; Liu, W.; Du, Y.; Yan, H.; Li, J.; Chen, Z.; et al. Engineering Local and Global Structures of Single Co Atoms for a Superior Oxygen Reduction Reaction. ACS Catal. 2020, 10, 5862–5870. [Google Scholar] [CrossRef]
- Wang, L.; Guo, S.; Yu, S.; Chen, F.; Zhou, Q.; Guo, M.; Zhang, C.; Li, C. NiCo Alloy Encapsulated in Sulfur and Nitrogen Codoped Carbon Nanofiber as Efficient Oxygen Reduction Electrocatalysts for Direct Methanol Fuel Cells. ACS Sustain. Chem. Eng. 2025, 13, 7740–7749. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Zhou, M.; Martínez-Huitle, C.A. Heterogeneous electro-Fenton and photoelectro-Fenton processes: A critical review of fundamental principles and application for water/wastewater treatment. Appl. Catal. B Environ. 2018, 235, 103–129. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Trellu, C.; Vargas, H.O.; Mousset, E.; Ganiyu, S.O.; Oturan, M.A. Electro-Fenton process in combination with other advanced oxidation processes: Challenges and opportunities. Curr. Opin. Electrochem. 2023, 37, 101171. [Google Scholar] [CrossRef]
- Heidari, Z.; Pelalak, R.; Zhou, M. A critical review on the recent progress in application of electro-Fenton process for decontamination of wastewater at near-neutral pH. Chem. Eng. J. 2023, 474, 145741. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, M. A critical review of the application of chelating agents to enable Fenton and Fenton-like reactions at high pH values. J. Hazard. Mater. 2019, 362, 436–450. [Google Scholar] [CrossRef]
- Rezaei, F.; Vione, D. Effect of pH on Zero Valent Iron Performance in Heterogeneous Fenton and Fenton-Like Processes: A Review. Molecules 2018, 23, 3127. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, H.; Liu, Y.; Ren, Y.; Zhou, P.; He, C.-S.; Xiong, Z.; Liu, W.; Dai, X.; Lai, B. Surface structure regulation of sulfidated zero-valent iron by H2O2 for efficient pH self-regulation and proton cycle to boost heterogeneous Fenton-like reaction for pollutant control. Appl. Catal. B Environ. Energy 2024, 345, 123667. [Google Scholar] [CrossRef]
- Zhu, S.; Qiang, J.; Hu, L.; Li, X.; Li, Y.; Chen, X.; Jia, Z.; Yang, Y. A Dual-Cathode System with a Naturally Aspirated Cathode and a Bimetallic Quasi-MOF Derived Electrode for Efficient Heterogeneous Fenton over a Wide pH Range. Adv. Funct. Mater. 2025, e02912. [Google Scholar] [CrossRef]
- Xu, S.-L.; Wang, W.; Song, Y.; Tang, R.; Hu, Z.-H.; Zhou, X.; Yu, H.-Q. Expanding the pH range of Fenton-like reactions for pollutant degradation: The impact of acidic microenvironments. Water Res. 2025, 270, 122851. [Google Scholar] [CrossRef]
- Cui, L.; Huang, H.; Ding, P.; Zhu, S.; Jing, W.; Gu, X. Cogeneration of H2O2 and OH via a novel Fe3O4/MWCNTs composite cathode in a dual-compartment electro-Fenton membrane reactor. Sep. Purif. Technol. 2020, 237, 116380. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Y.; Ma, H.; Zhou, M. Hydrothermal synthesis of FeS2 as a highly efficient heterogeneous electro-Fenton catalyst to degrade diclofenac via molecular oxygen effects for Fe(II)/Fe(III) cycle. Sep. Purif. Technol. 2020, 248, 117022. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, K.; Ma, X.; Sun, Z.; Hu, X. Degradation of diuron by heterogeneous electro-Fenton using modified magnetic activated carbon as the catalyst. RSC Adv. 2018, 8, 19971–19978. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Sun, Z. Cu-doped g-C3N4 catalyst with stable Cu0 and Cu+ for enhanced amoxicillin degradation by heterogeneous electro-Fenton process at neutral pH. Chemosphere 2021, 283, 131257. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Martínez-Huitle, C.A.; Nidheesh, P.V. An overview of chelate modified electro-Fenton processes. J. Environ. Chem. Eng. 2022, 10, 107183. [Google Scholar] [CrossRef]
- Varindani, A.; Singh, T.S.A.; Poornima, M.; and Nidheesh, P.V. Chelate-modified Electro-Fenton process for mixed industrial wastewater treatment. Environ. Technol. 2022, 43, 3497–3506. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, G.; Wang, Q.; Zhang, Y.; Zhou, M. Kinetic study of the degradation of rhodamine B using a flow-through UV/electro-Fenton process with the presence of ethylenediaminetetraacetic acid. Chemosphere 2020, 240, 124929. [Google Scholar] [CrossRef]
- Xu, B.; Lin, Z.; Li, F.; Tao, T.; Zhang, G.; Wang, Y. Local O2 concentrating boosts the electro-Fenton process for energy-efficient water remediation. Proc. Natl. Acad. Sci. USA 2024, 121, e2317702121. [Google Scholar] [CrossRef] [PubMed]
- Barhoumi, N.; Oturan, N.; Ammar, S.; Gadri, A.; Oturan, M.A.; Brillas, E. Enhanced degradation of the antibiotic tetracycline by heterogeneous electro-Fenton with pyrite catalysis. Environ. Chem. Lett. 2017, 15, 689–693. [Google Scholar] [CrossRef]
- Shokri, A.; Nasernejad, B.; Sanavi Fard, M. Challenges and Future Roadmaps in Heterogeneous Electro-Fenton Process for Wastewater Treatment. Water Air Soil Pollut. 2023, 234, 153. [Google Scholar] [CrossRef]
- Jiang, M.; Zhao, Y.; Guo, L.; Tong, L.; Gong, W.; Wang, J.; Zhang, H.; Tang, X.; Liang, H. Membrane fouling behaviors in membrane Fenton process without acid-base agents controlled by current density for nanofiltration concentrate treatment. J. Membr. Sci. 2025, 717, 123527. [Google Scholar] [CrossRef]
- Deng, F.; Li, S.; Cao, Y.; Fang, M.A.; Qu, J.; Chen, Z.; Qiu, S. A dual-cathode pulsed current electro-Fenton system: Improvement for H2O2 accumulation and Fe3+ reduction. J. Power Sources 2020, 466, 228342. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Brillas, E.; Santos, M.C.; Garcia-Segura, S. Electro-Fenton treatment of benzophenone-4 solutions: A sustainable approach for its removal using an air-diffusion cathode. Process Saf. Environ. Prot. 2025, 199, 107342. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J.; Song, C.; Shi, X.; Du, H. An Eco-friendly Iron Cathode Electro-Fenton System Coupled With a pH-Regulation Electrolysis Cell for p-nitrophenol Degradation. Front. Chem. 2022, 9, 837761. [Google Scholar] [CrossRef]
- Sun, H.; Yao, Y.; Wei, F.; Zhao, Q.; Liu, B.; Zhang, L. Process optimization and mechanism study of acid red G degradation by electro-Fenton-Feox process as an in situ generation of H2O2. Turk. J. Chem. 2021, 45, 5–16. [Google Scholar] [CrossRef]
- Marlina, E.; Purwanto, P. Electro-Fenton for Industrial Wastewater Treatment: A Review. E3S Web Conf. 2019, 125, 03003. [Google Scholar] [CrossRef]
- Xia, Y.; Shang, H.; Zhang, Q.; Zhou, Y.; Hu, X. Electrogeneration of hydrogen peroxide using phosphorus-doped carbon nanotubes gas diffusion electrodes and its application in electro-Fenton. J. Electroanal. Chem. 2019, 840, 400–408. [Google Scholar] [CrossRef]
- Chai, Y.; Qin, P.; Zhang, J.; Li, T.; Dai, Z.; Wu, Z. Simultaneous removal of Fe(II) and Mn(II) from acid mine wastewater by electro-Fenton process. Process Saf. Environ. Prot. 2020, 143, 76–90. [Google Scholar] [CrossRef]
- Cao, P.; Quan, X.; Zhao, K.; Chen, S.; Yu, H.; Su, Y. High-Efficiency Electrocatalysis of Molecular Oxygen toward Hydroxyl Radicals Enabled by an Atomically Dispersed Iron Catalyst. Environ. Sci. Technol. 2020, 54, 12662–12672. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Cao, P.; Quan, X.; Zhao, K.; Chen, S.; Yu, H.; Su, Y. Highly Efficient Hydroxyl Radicals Production Boosted by the Atomically Dispersed Fe and Co Sites for Heterogeneous Electro-Fenton Oxidation. Environ. Sci. Technol. 2023, 57, 2907–2917. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhou, M.; Ren, G.; Ma, L. A novel dual gas diffusion electrodes system for efficient hydrogen peroxide generation used in electro-Fenton. Chem. Eng. J. 2015, 263, 92–100. [Google Scholar] [CrossRef]
- Zhou, W.; Meng, X.; Gao, J.; Alshawabkeh, A.N. Hydrogen peroxide generation from O2 electroreduction for environmental remediation: A state-of-the-art review. Chemosphere 2019, 225, 588–607. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Angelidaki, I.; Zhang, Y. Bio-electro-Fenton processes for wastewater treatment: Advances and prospects. Chem. Eng. J. 2018, 354, 492–506. [Google Scholar] [CrossRef]
- Zhang, M.-h.; Dong, H.; Zhao, L.; Wang, D.-x.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef]
- Dominguez, C.M.; Oturan, N.; Romero, A.; Santos, A.; Oturan, M.A. Optimization of electro-Fenton process for effective degradation of organochlorine pesticide lindane. Catal. Today 2018, 313, 196–202. [Google Scholar] [CrossRef]
- Shokri, A.; Sanavi Fard, M. Using α-Fe2O3/SiO2 as a heterogeneous Fenton catalyst for the removal of chlorophenol in aqueous environment: Thermodynamic and kinetic studies. Int. J. Environ. Sci. Technol. 2023, 20, 383–396. [Google Scholar] [CrossRef]
- Yu, N.; Bai, J.; Cao, H.; Yao, H.; Shi, G.; Yuan, H.; Xu, Z.; Luo, F.; Li, M.; Si, R. Electrocatalysis coupled heterogeneous electro-Fenton like treatment of coal gasification wastewater using tourmaline as catalyst: Process parameters and response surface. Environ. Sci. Pollut. Res. 2024, 31, 20207–20221. [Google Scholar] [CrossRef]
- Roy, S.V.; Raychaudhuri, A.; Behera, M.; Neelancherry, R. Elimination of pharmaceuticals from wastewater using microbial fuel cell-based bio-electro-Fenton process. Environ. Sci. Pollut. Res. 2023, 1–13. [Google Scholar] [CrossRef]
- Mohammadi, M.; Davarnejad, R.; Sillanpää, M. A novel catalyst based on zero-valent iron nanoparticles for assisting electro-fenton process applied to a toxic wastewater. Results Eng. 2024, 24, 102938. [Google Scholar] [CrossRef]
- Li, P.; Yin, Z.; Chi, C.; Wang, Y.; Wang, Y.; Liu, H.; Lv, Y.; Jiang, N.; Wu, S. Treatment of membrane-concentrated landfill leachate by heterogeneous chemical and electrical Fenton processes with Iron-loaded granular activated carbon catalysts. J. Environ. Chem. Eng. 2024, 12, 112337. [Google Scholar] [CrossRef]
- Zhang, L.; Fu, W.; Huo, S.; Li, J.; Gao, M. Preparation of CuFeO_2@PVP for the photo-electro-Fenton degradation of ofloxacin. Chin. J. Environ. Eng. 2023, 17, 416–430. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Hussain, N.A.S.; Stafford, J.L.; Gamal El-Din, M. Electrocatalytic activation of peroxomonosulfate (PMS) under aerobic condition for the remediation of oil sands process water: Insight into one-pot synergistic coupling of PMS electro-activation and heterogeneous electro-Fenton processes. Chem. Eng. J. 2024, 480, 147737. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, P.; Zhang, X.; Zhang, Y.; Liu, Y. Three-dimensional electro-Fenton system with iron-carbon packing as a particle electrode for nitrobenzene wastewater treatment. Front. Environ. Sci. Eng. 2024, 18, 138. [Google Scholar] [CrossRef]
- Pirhadi, M.; Mohammadi, M.; Davarnejad, R. Simulation of pesticide wastewater treatment process using electro-Fenton method: In situ production of H2O2. Can. J. Chem. Eng. 2025. [Google Scholar] [CrossRef]
- Elumalai, G.; Sowmya, B.; Rajan, R.K.; Shanmugam, V. Experimental study of photo electro-Fenton method for the removal of Reactive Yellow 186: Influence of operational parameters. Environ. Prog. Sustain. Energy 2023, 42, e14061. [Google Scholar] [CrossRef]
- Xie, L.; Liya, W.; Shuyi, Z.; Wenting, C.; Jinyan, H.; Fanglin, L.; and Gan, Y. Photocatalysis coupled electro-Fenton process for treatment of acrylamide wastewater: An optimised study. Environ. Technol. 2025, 46, 2080–2091. [Google Scholar] [CrossRef] [PubMed]
- Brillas, E. A review on the photoelectro-Fenton process as efficient electrochemical advanced oxidation for wastewater remediation. Treatment with UV light, sunlight, and coupling with conventional and other photo-assisted advanced technologies. Chemosphere 2020, 250, 126198. [Google Scholar] [CrossRef]
- Mousset, E.; Huang Weiqi, V.; Foong Yang Kai, B.; Koh, J.S.; Tng, J.W.; Wang, Z.; Lefebvre, O. A new 3D-printed photoelectrocatalytic reactor combining the benefits of a transparent electrode and the Fenton reaction for advanced wastewater treatment. J. Mater. Chem. A 2017, 5, 24951–24964. [Google Scholar] [CrossRef]
- Jamal, M.; Khan Nabi, G.A.; Sun, H.; Ullah, K.; Khattak, O.A.; Kashif, M.; Khan, S.; Alam, M.; Hussain, S.; Ullah, M.; et al. Preparation of Manganese-Doped Bismuth Oxide for the Photocatalytic Degradation of Methylene Blue. Arch. Adv. Eng. Sci. 2024, 1–7. [Google Scholar] [CrossRef]
- Chen, J.; He, J.; Yin, Z.; Wang, T.; Liu, S.; Cao, S. One-pot synthesis of porous TiO2/BiOI adsorbent with high removal efficiency and excellent recyclability towards tetracyclines. Ceram. Int. 2023, 49, 22139–22148. [Google Scholar] [CrossRef]
- Jiad, M.M.; Abbar, A.H. Efficient wastewater treatment in petroleum refineries: Hybrid electro-fenton and photocatalysis (UV/ZnO) process. Chem. Eng. Res. Des. 2023, 200, 431–444. [Google Scholar] [CrossRef]
- Ghanbari, F.; Hassani, A.; Wacławek, S.; Wang, Z.; Matyszczak, G.; Lin, K.-Y.A.; Dolatabadi, M. Insights into paracetamol degradation in aqueous solutions by ultrasound-assisted heterogeneous electro-Fenton process: Key operating parameters, mineralization and toxicity assessment. Sep. Purif. Technol. 2021, 266, 118533. [Google Scholar] [CrossRef]
- Ahmadi, M.; Haghighifard, N.J.; Soltani, R.D.C.; Tobeishi, M.; Jorfi, S. Treatment of a saline petrochemical wastewater containing recalcitrant organics using Electro-Fenton process: Persulfate and ultrasonic intensification. Desalination Water Treat. 2019, 169, 241–250. [Google Scholar] [CrossRef]
- Ghjair, A.Y.; Abbar, A.H. Applications of advanced oxidation processes (Electro-Fenton and sono-electro-Fenton) for COD removal from hospital wastewater: Optimization using response surface methodology. Process Saf. Environ. Prot. 2023, 169, 481–492. [Google Scholar] [CrossRef]
- Nazari, R.; Rajić, L.; Xue, Y.; Zhou, W.; Alshawabkeh, A.N. Degradation of 4-Chlorophenol in Aqueous Solution by Sono-Electro-Fenton Process. Int. J. Electrochem. Sci. 2018, 13, 9214–9230. [Google Scholar] [CrossRef]
- Wang, W.; Lu, Y.; Luo, H.; Liu, G.; Zhang, R.; Jin, S. A microbial electro-fenton cell for removing carbamazepine in wastewater with electricity output. Water Res. 2018, 139, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Sathe, S.M.; Chakraborty, I.; Dubey, B.K.; Ghangrekar, M.M. Microbial fuel cell coupled Fenton oxidation for the cathodic degradation of emerging contaminants from wastewater: Applications and challenges. Environ. Res. 2022, 204, 112135. [Google Scholar] [CrossRef] [PubMed]
- Rafaqat, S.; Ali, N.; Torres, C.; Rittmann, B. Recent progress in treatment of dyes wastewater using microbial-electro-Fenton technology. RSC Adv. 2022, 12, 17104–17137. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Martínez-Huitle, C.A.; Rodrigo, M.A. Renewable energies driven electrochemical wastewater/soil decontamination technologies: A critical review of fundamental concepts and applications. Appl. Catal. B Environ. 2020, 270, 118857. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Martinez-Huitle, C.A. The use of renewable energies driving electrochemical technologies for environmental applications. Curr. Opin. Electrochem. 2020, 22, 211–220. [Google Scholar] [CrossRef]
- Birjandi, N.; Younesi, H.; Ghoreyshi, A.A.; Rahimnejad, M. Enhanced medicinal herbs wastewater treatment in continuous flow bio-electro-Fenton operations along with power generation. Renew. Energy 2020, 155, 1079–1090. [Google Scholar] [CrossRef]
- Sathe, S.M.; Chakraborty, I.; Sankar Cheela, V.R.; Chowdhury, S.; Dubey, B.K.; Ghangrekar, M.M. A novel bio-electro-Fenton process for eliminating sodium dodecyl sulphate from wastewater using dual chamber microbial fuel cell. Bioresour. Technol. 2021, 341, 125850. [Google Scholar] [CrossRef]
- Ren, G.; Li, R.; Zhao, M.; Hou, Q.; Rao, T.; Zhou, M.; Ma, X. Membrane electrodes for electrochemical advanced oxidation processes: Preparation, self-cleaning mechanisms and prospects. Chem. Eng. J. 2023, 451, 138907. [Google Scholar] [CrossRef]
- Chen, M.; Ren, L.; Qi, K.; Li, Q.; Lai, M.; Li, Y.; Li, X.; Wang, Z. Enhanced removal of pharmaceuticals and personal care products from real municipal wastewater using an electrochemical membrane bioreactor. Bioresour. Technol. 2020, 311, 123579. [Google Scholar] [CrossRef]
- Liu, H.; Vecitis, C.D. Reactive Transport Mechanism for Organic Oxidation during Electrochemical Filtration: Mass-Transfer, Physical Adsorption, and Electron-Transfer. J. Phys. Chem. C 2012, 116, 374–383. [Google Scholar] [CrossRef]
- Yin, Z.; Zhu, J.; Wang, Z.; Liu, Y.; Yang, Z.; Yang, W. Novel Fe/N co-doping biochar based electro-Fenton catalytic membrane enabling enhanced tetracycline removal and self-cleaning performance. J. Clean. Prod. 2023, 402, 136731. [Google Scholar] [CrossRef]
- Guo, D.; Jiang, S.; Jin, L.; Huang, K.; Lu, P.; Liu, Y. CNT encapsulated MnOx for an enhanced flow-through electro-Fenton process: The involvement of Mn(iv). J. Mater. Chem. A 2022, 10, 15981–15989. [Google Scholar] [CrossRef]
| Cathode Material | Operating Conditions | Target Pollutant | Removal Efficiency | Reference |
|---|---|---|---|---|
| Carbon cloth gas-diffusion electrode | pH 3.0, 30 mA cm−2, 0.5 mM Fe2+ | Fipronil | 100% (20 mg L−1 fipronil in 60 min) | [45] |
| CNT/MoS2/FeCo-LDH membrane | pH 3–9, 1.5 mA cm−2 | Phenol | 98.8% (60 min) | [46] |
| N-doped AC | pH 3–5 | Phenol | 93.7% | [47] |
| Carbon nanotube fiber (CNTF) | Constant current | Acid Orange 7 | TOC removal 73.9% (2 h) | [48] |
| Magnetite nanoparticles on carbon felt | - | Aspirin | Enhanced aspirin degradation | [49] |
| AC/stainless steel mesh (ACSS) | Neutral pH, 100 mA | Reactive Blue 19 | H2O2: 8.9 mg L−1, RB19 removal: 61.5% (90 min) | [50] |
| Fe3+-loaded N-doped carbon nanotubes | - | Multiple organics | Accelerated Fe2+ regeneration, high degradation rate | [42] |
| Target Pollutant | Operating Conditions | Current Density | Removal Efficiency | Reference |
|---|---|---|---|---|
| Fipronil | pH 3.0, 0.50 mM Fe2+, 60 min | 30 mA cm−2 | ~100% removal (20 mg L−1) | [45] |
| Benzophenone-4 | pH 3.0, 0.75 mM Fe2+, 2–7 min | 20 mA cm−2 | 100% mineralization (1–40 mg L−1) | [111] |
| p-Nitrophenol | pH regulated via secondary cell | 2 mA cm−2 | Near-complete degradation | [112] |
| Acid Red G dye | pH 3.0, 80 min | 20 mA cm−2 | 94.05% removal (300 mg L−1) | [113] |
| Pollutants | Electrode Material | Operational Conditions | Catalyst Dosage | Removal Efficiency | Reference |
|---|---|---|---|---|---|
| Polyacrylamide | GF+ Ru-Ir/Ti electrodes | pH = 3, [Na2SO4] = 0.1 M, air flow rate = 1.0 L min−1, current density = 5 mA cm−2 | CoFe2O4, 0.3 g L−1 | 92.01% | [82] |
| Fipronil | BDD anode + carbon cloth air-diffusion cathode | pH = 3.0, current density = 30 mA cm−2 | Fe2+, 0.50 mM | 85% | [45] |
| Benzophenone-4 | BDD anode + air- diffusion cathode (PTFE-treated carbon cloth) | pH = 3.0, [Na2SO4] = 0.050 M, current density = 20 mA cm−2, [pollutant] = 40 mg L−1 | Fe2+, 0.75 mM | 88% | [111] |
| Coal gasification wastewater | IrO2-RuO2 electrode anode + carbon felt cathode | current density = 82.4 mA cm−2, electrode gap = 1 cm | Fe-based tourmaline, 7.57 g L−1 | 88.25% | [125] |
| Synthetic pharmaceutical wastewater | graphite plate anode + Fe@Fe2O3/GF cathode | pH = 3, air flow rate = 10 mL min−1 | iron, 18.56% | 89.9% | [126] |
| Pesticide wastewater | SS 316 anode + graphite cathode | time = 125 min, current intensity = 272 mA, [H2O2] = 8.102 × 10−3 M | zeolite Y-nZVI, 1.78 g | 83.69% | [127] |
| Membrane-concentrated landfill leachate | DSA + needle coke electrode cathode | pH = 6, applied voltage = 8 V, the reaction time = 4 h | granular AC loaded with iron oxides, 16.67 g L−1 | 95.7% | [128] |
| Antipyrine | BDD anode + carbon-felt cathode | current density = 10 mA cm−2, [Na2SO4] = 0.05 M, air flow rate = 1 L min−1, electrode gap = 2.5 cm | AC -NZVI, 1.4 g L−1 | 97% | [21] |
| Ofloxacin | Pt anode + carbon felt cathode. | [Na2SO4] = 0.05 M, [pollutant] = 10 mg L−1, current density = 4 mA cm−2 | CuFeO2@polyvinylpyrrolidone, (PVP), 0.4 g L−1 | 94.3% | [129] |
| Surrogate naphthenic acids | Ti/IrO2 DSA + GF cathode | pH = 8.6, air flow rate = 1 L min−1, current density = 6.25 mA cm−2 | Fe-modified biochar, 0.5 mg L−1 | 70% | [130] |
| Acid Blue 25 | graphite electrode (cathode and anode both made of graphite plates) | pH = 3, air flow rate = 4 L min−1, current density = 0.228 A, [pollutant] = 75 mg·L−1 | Fe-MIL-88B nanocatalyst, 0.3 g L−1 | 92.3% | [77] |
| Nitrobenzene | titanium-based ruthenium dioxide anode + titanium mesh cathode | iron: carbon = 3:1, current density = 30 mA cm−2, [H2O2] = 50 mM, cathodic aeration = 0.8 L min−1 | iron-carbon particle electrode, 100 g L−1 | 67.38% | [131] |
| Pesticide wastewater | graphite modified with industrial carbon black (anode) and iron plate (cathode) | current intensity = 253 mA, air flow rate = 1.56 L min−1, hydraulic retention time = 126 min | Fe2+, 0.63 g | 77.1% | [132] |
| Reactive Yellow 186 azo dye | Ti anode + stainless steel cathode | pH = 3, [pollutant]= 0.15 g L−1, current density = 0.1 mA cm−2, [H2O2] = 0.2 g L−1 | Fe, 0.015 g L−1 | 99% | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, H.; Ma, Q.; Zhang, X.; Wang, C.; You, N.; Deng, S. Material Design and Operation Strategy of the Electro-Fenton System for the Treatment of High Pollutant Load Wastewater. Sustainability 2025, 17, 10501. https://doi.org/10.3390/su172310501
Ding H, Ma Q, Zhang X, Wang C, You N, Deng S. Material Design and Operation Strategy of the Electro-Fenton System for the Treatment of High Pollutant Load Wastewater. Sustainability. 2025; 17(23):10501. https://doi.org/10.3390/su172310501
Chicago/Turabian StyleDing, Hong, Qiqi Ma, Xiaoke Zhang, Chaoqi Wang, Na You, and Shihai Deng. 2025. "Material Design and Operation Strategy of the Electro-Fenton System for the Treatment of High Pollutant Load Wastewater" Sustainability 17, no. 23: 10501. https://doi.org/10.3390/su172310501
APA StyleDing, H., Ma, Q., Zhang, X., Wang, C., You, N., & Deng, S. (2025). Material Design and Operation Strategy of the Electro-Fenton System for the Treatment of High Pollutant Load Wastewater. Sustainability, 17(23), 10501. https://doi.org/10.3390/su172310501







