Decoding the Sustainability Code: Enzyme Thermodynamic and Kinetic Parameters Reveal the Efficacy of Straw, Biochar, and Nanocarbon in Black Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Materials
2.3. Experiment Preparation and Design
2.4. Soil Collection and Chemical Analysis
2.5. Enzyme Activity Assay
2.6. Calculations and Statistical Analyses
3. Results
3.1. Soil Physicochemical Properties
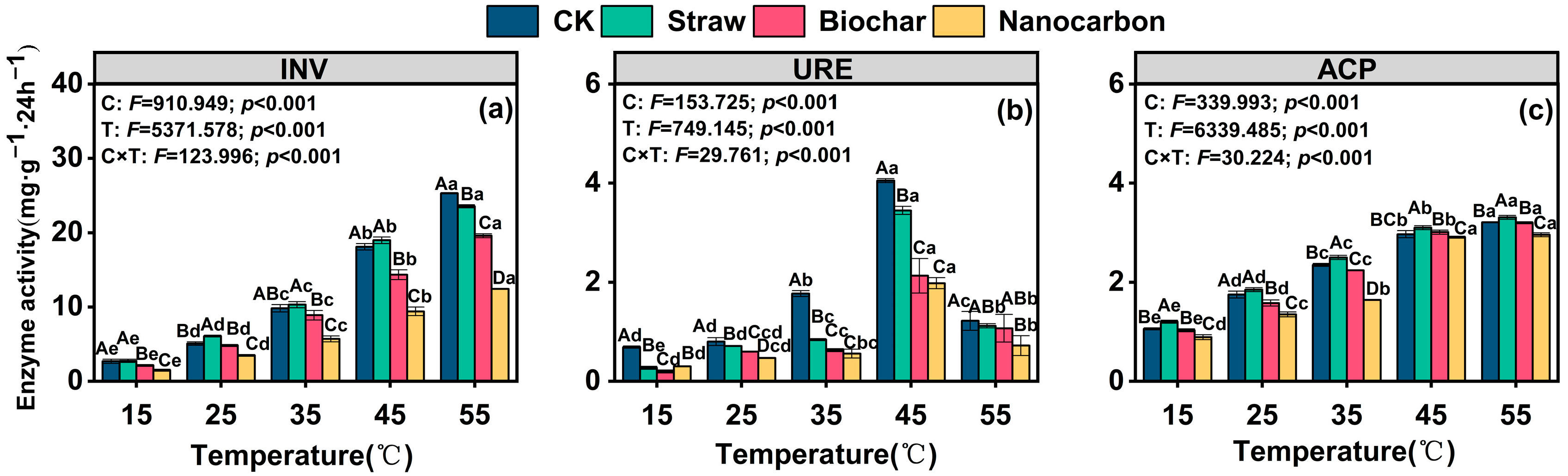
3.2. Soil Enzyme Activities
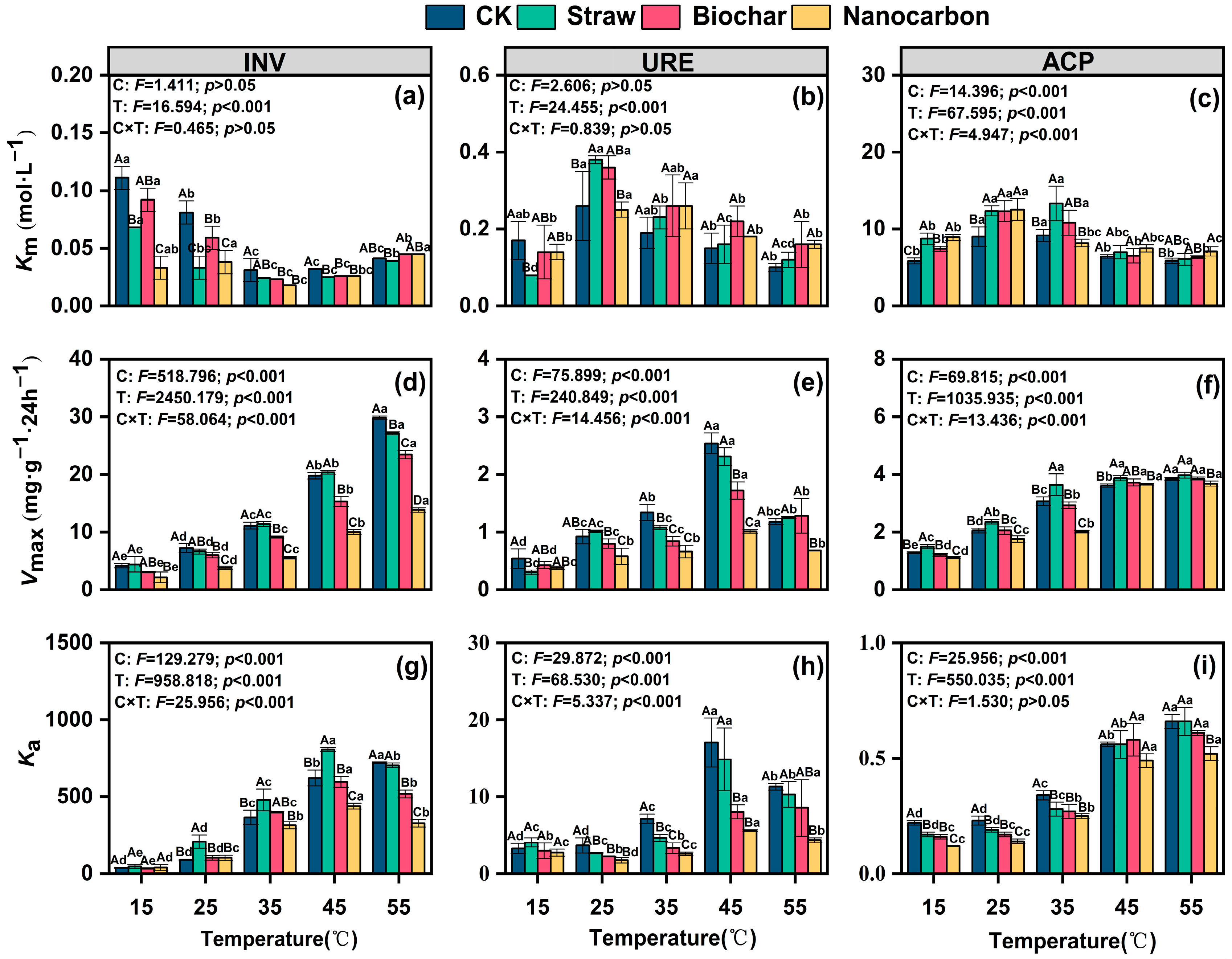
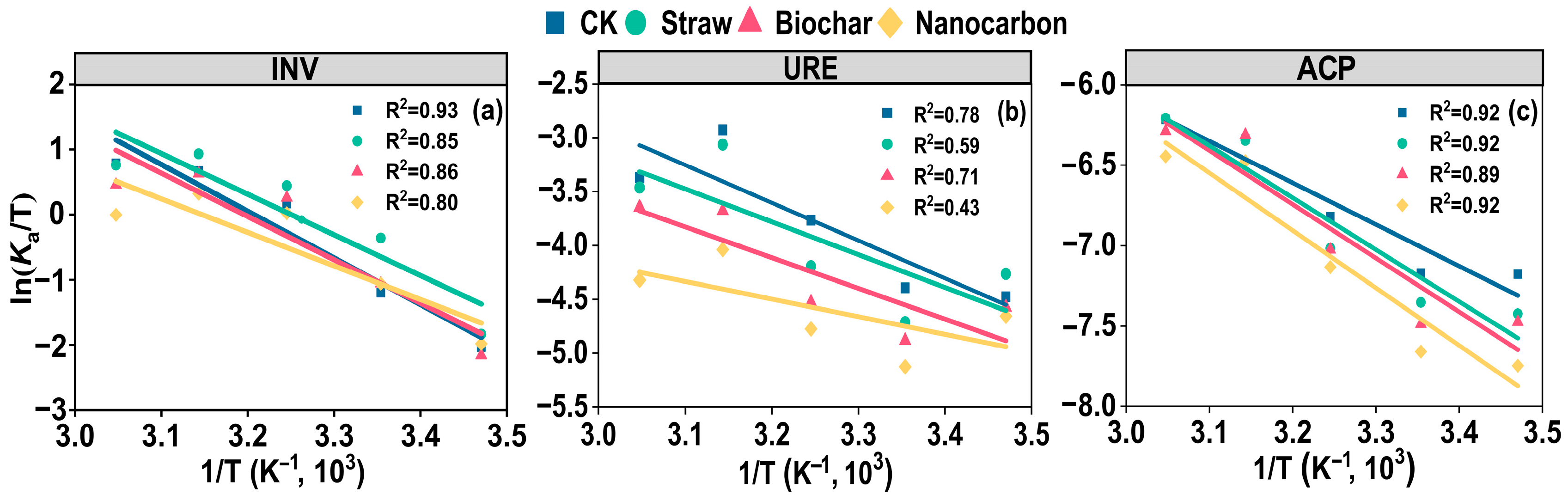
3.3. Soil Enzyme Kinetic Parameters
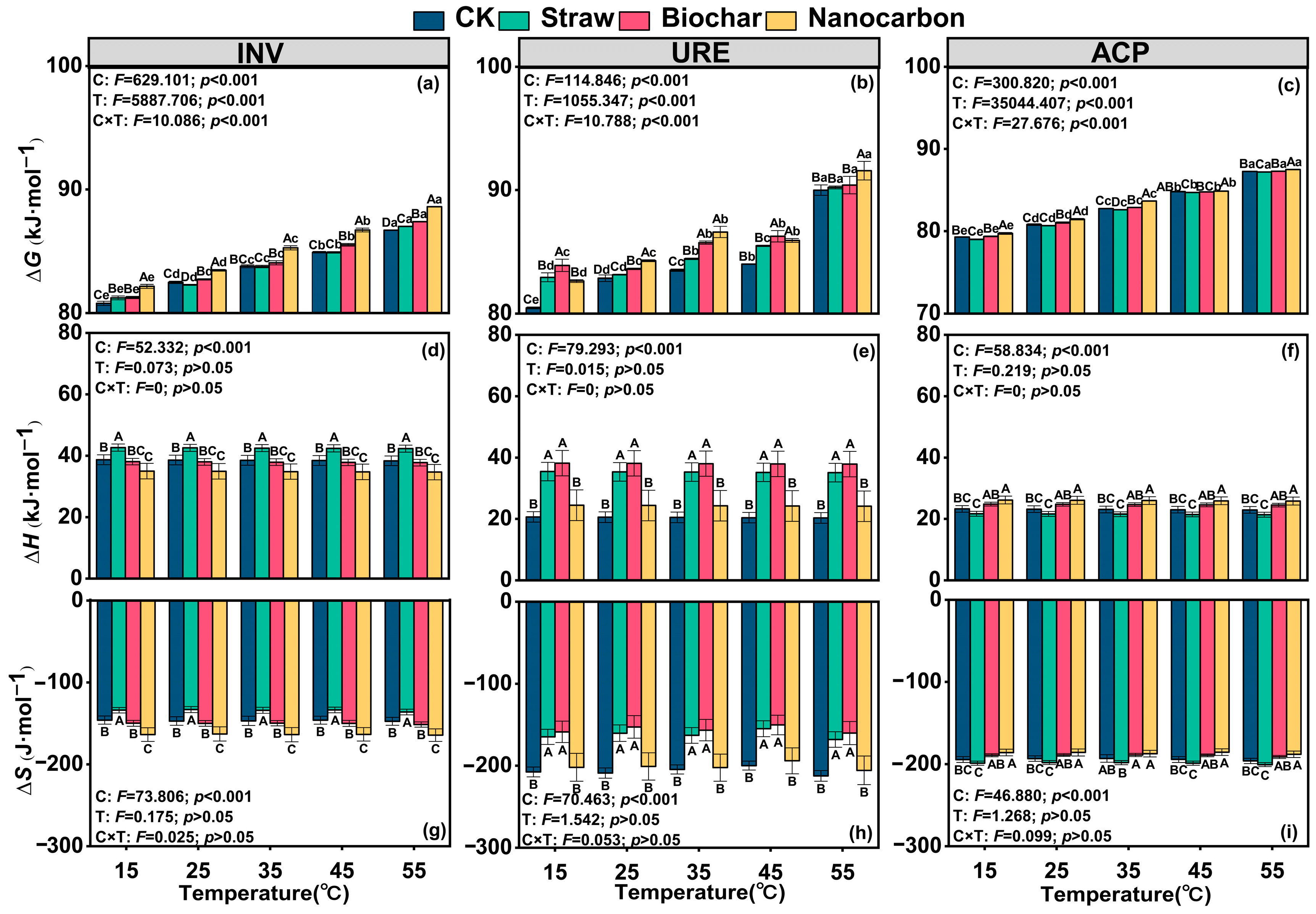
3.4. Soil Enzyme Thermodynamic Parameters
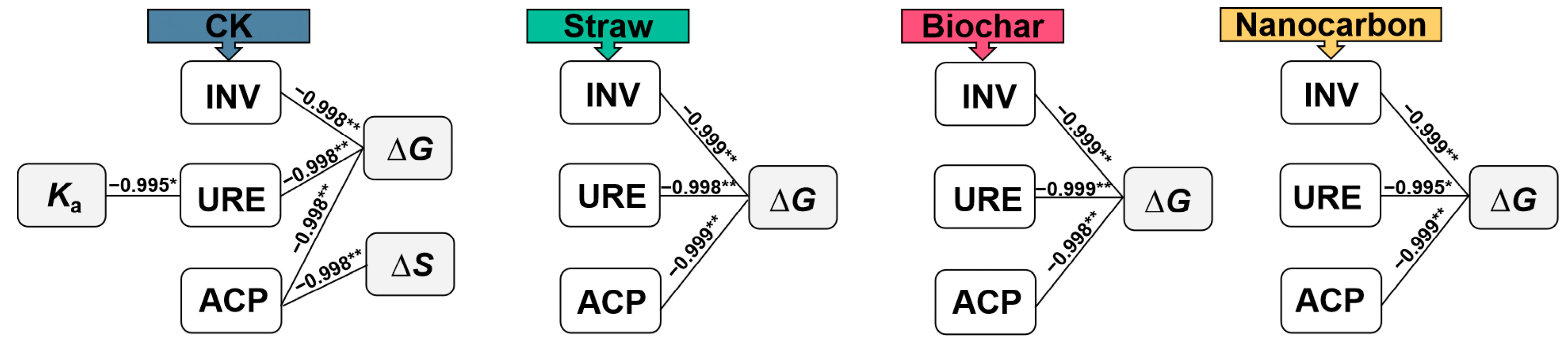
3.5. Correlation Analysis Between Hydrolase Activity and Catalytic Characteristics
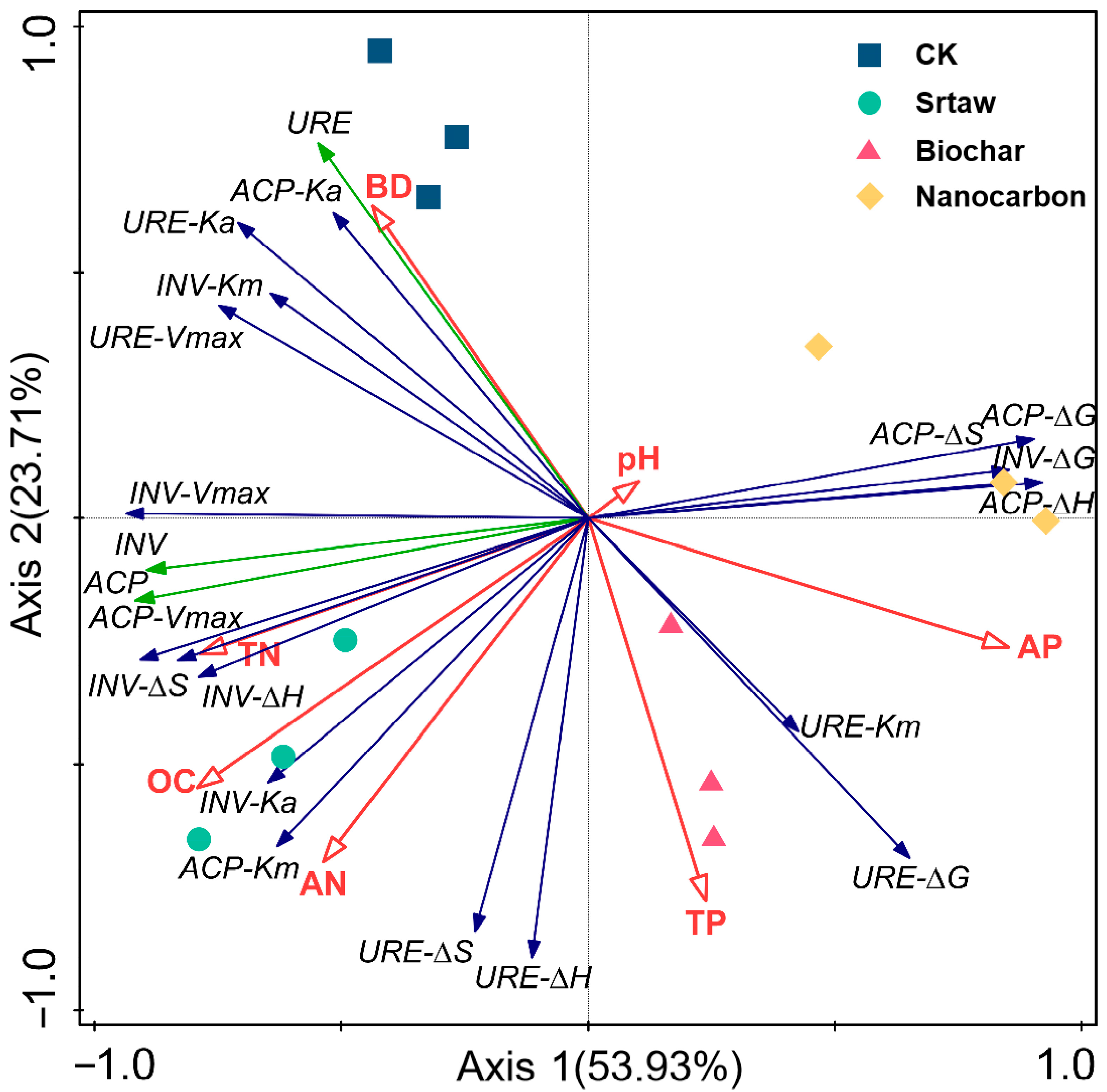
3.6. Redundancy Analysis
4. Discussion
4.1. Response of Hydrolase Activities to Straw, Biochar, and Nanocarbon in Black Soil
4.2. Response of Hydrolase Kinetic and Thermodynamic Characteristics to Straw, Biochar, and Nanocarbon in Black Soil
4.3. Factors Influencing Changes in Soil Hydrolase Activity
5. Conclusions
- (1)
- Although all carbon inputs did not alter the temperature response trends of INV, URE, and ACP, their effects on enzyme activities were distinct. Straw exerted different influences on the enzymes (no significant change in INV, a decrease in URE, and an increase in ACP), whereas both biochar and nanocarbon consistently inhibited all three enzymes, with nanocarbon exhibiting a stronger inhibitory effect.
- (2)
- The changes in hydrolase activity following carbon addition were regulated through the coupling of kinetic and thermodynamic processes.
- (3)
- Thermodynamic properties, particularly ∆G, were identified as the key limiting factors driving enzyme activity changes, whereas soil OC and TP acted as important physicochemical regulators.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| INV | Invertase |
| URE | Urease |
| ACP | Phosphatase |
| BD | Bulk density |
| OC | Organic carbon |
| TN | Total nitrogen |
| AN | Available nitrogen |
| TP | Total phosphorus |
| AP | Available phosphorus |
References
- Smerald, A.; Kraus, D.; Rahimi, J.; Fuchs, K.; Kiese, R.; Butterbach, B.K.; Scheer, C. A redistribution of nitrogen fertiliser across global croplands can help achieve food security within environmental boundaries. Commun. Earth Environ. 2023, 4, 315. [Google Scholar] [CrossRef]
- Zhao, Q.; Hao, X.Y.; Ali, I.; Iqbal, A.; Ullah, S.; Huang, M.; Kong, F.Y.; Li, T.Y.; Xuan, Y.; Li, F.Q.; et al. Characterization and grouping of all primary branches at various positions on a rice panicle based on grain growth dynamics. Agronomy 2020, 10, 223. [Google Scholar] [CrossRef]
- Shen, W.Z.; He, J.; Li, S.S.; Zhuang, Y.H.; Wang, H.Y.; Liu, H.B.; Zhang, L.; Kappler, A. Opportunity and shift of nitrogen use in China. Geogr. Sustain. 2024, 5, 33–40. [Google Scholar] [CrossRef]
- Li, Q.S.; Wang, D.Q.; Du, C.Y.; Li, X.D.; Zhang, G.C.; Wang, S.K.; Guan, E.S.; Wang, S.Q. Effects of exogenous carbon sources on nitrogen transformation and N2O emissions in tobacco-planting soil. Chin. Tob. Sci. 2020, 41, 13–19. [Google Scholar] [CrossRef]
- Jin, Z.Q.; Shah, T.; Zhang, L.; Liu, H.Y.; Peng, S.B.; Nie, L.X. Effect of straw returning on soil organic carbon in rice–wheat rotation system: A review. Food Energy Secur. 2020, 9, e200. [Google Scholar] [CrossRef]
- Rivka, B.F.; David, A.L.; Timothy, B.P. Impact of six lignocellulosic biochars on C and N dynamics of two contrasting soils. GCB Bioenergy 2017, 9, 1279–1291. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, P.; Nie, T.Z.; Zhang, Z.X.; Li, T.C.; Dai, C.L.; Jiang, L.L.; Wu, Y.; Sun, Z.Y.; Yin, S. Long-term straw incorporation under controlled irrigation improves soil quality of paddy field and rice yield in northeast China. Plants 2024, 13, 1357. [Google Scholar] [CrossRef]
- Arshad, U.; Altaf, M.T.; Liaqat, W.; Ali, M.; Shah, M.N.; Jabran, M.; Ali, M.A. Biochar: Black gold for sustainable agriculture and fortification against plant pathogens—A review. J. Crop Health 2023, 76, 385–396. [Google Scholar] [CrossRef]
- Silvana, A.; Omar, G.P. Bamboo-based biochar: A still too little-studied black gold and its current applications. J. Xenobiotics 2024, 14, 416–451. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Skjemstad, J.O.; Czimczik, C.I.; Glaser, B.; Prentice, K.M.; Gelinas, Y.; Thomas, A.J.K. Comparative analysis of black carbon in soils. Glob. Biogeochem. Cycles 2001, 15, 163–167. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Ahmed, I.O.; Mohamed, F.; Ahmed, K.R. Life cycle assessment of biochar as a green sorbent for soil remediation. Curr. Opin. Green Sustain. Chem. 2024, 46, 100882. [Google Scholar] [CrossRef]
- Wang, D.J.; Zhang, W.; Hao, X.Z.; Zhou, D.M. Transport of biochar particles in saturated granular media: Effects of pyrolysis temperature and particle size. Environ. Sci. Technol. 2013, 47, 821–828. [Google Scholar] [CrossRef]
- Spokas, K.A.; Novak, J.M.; Masiello, C.A. Physical disintegration of biochar: An overlooked process. Environ. Sci. Technol. Lett. 2014, 1, 326–332. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, H.Y.; Wu, W.H.; Shang, H.P.; Zhang, D.; Zhan, X.H.; Xing, B.S. Role of nano-biochar in attenuating the allelopathic effect from Imperata cylindrica on rice seedlings. Environ. Sci. Nano 2019, 7, 116–126. [Google Scholar] [CrossRef]
- Xin, R.J.; Nam, H.N.; Phung, Q.M.; Tang, J.; Ma, S.C.; Markus, J.; Dai, Y.C.; Alowasheeir, A.; Khaorapapong, N.; Wang, J.; et al. Trimodal Hierarchical Porous Carbon Nanoplates with Edge Curvature for Faster Mass Transfer and Enhanced Oxygen Reduction. ACS Nano 2025, 19, 11648–11663. [Google Scholar] [CrossRef] [PubMed]
- Santhi, M.B.; Abhishek, H.; Akhil, R.; Mousumi, B.; Binoy, K.S. Hierarchical porous carbon derived from petroleum coke via one-step chemical activation for the fabrication of a supercapacitor and real time clock application. RSC Adv. 2024, 14, 21411–21424. [Google Scholar] [CrossRef]
- Wang, Y.P.; Liu, Y.; Zhang, H.Y.; Duan, X.G.; Ma, J.; Sun, H.Q.; Tian, W.J.; Wang, S.B. Carbonaceous materials in structural dimensions for advanced oxidation processes. Chem. Soc. Rev. 2025, 54, 2436–2482. [Google Scholar] [CrossRef]
- Kumar, D.; Abraham, J.E.; Varghese, M.; George, J.; Balachandran, M.; Cherusseri, J. Nanocarbon assisted green hydrogen production: Development and recent trends. Int. J. Hydrogen Energy 2024, 50, 118–141. [Google Scholar] [CrossRef]
- Sainju, U.M.; Liptzin, D.; Dangi, S.M. Enzyme activities as soil health indicators in relation to soil characteristics and crop production. Agrosyst. Geosci. Environ. 2022, 5, e20297. [Google Scholar] [CrossRef]
- Li, J.; Wu, J.Z.; Yu, J.Y.; Wang, K.B.; Li, J.P.; Cui, Y.X.; Shangguan, Z.P.; Deng, L. Soil enzyme activity and stoichiometry in response to precipitation changes in terrestrial ecosystems. Soil Biol. Biochem. 2024, 191, 109321. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Wang, Y.S. Soil enzyme activities with greenhouse subsurface irrigation. Pedosphere 2006, 16, 512–518. [Google Scholar] [CrossRef]
- Jia, R.; Zhou, J.; Yang, L.; Blagodatskaya, E.; Jones, D.L.; Razavi, B.S.; Yang, Y.D.; Kuzyakov, Y.; Zeng, Z.H.; Zang, H.D. Trade-off between soil enzyme activities and hotspots area depends on long-term fertilization: In situ field zymography. Sci. Total Environ. 2024, 954, 176386. [Google Scholar] [CrossRef]
- Marx, M.C.; Kandeler, E.; Wood, M.; Wermbter, N.; Jarvis, S.C. Exploring the enzymatic landscape: Distribution and kinetics of hydrolytic enzymes in soil particle-size fractions. Soil Biol. Biochem. 2004, 37, 35–48. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Wang, X.; Sun, Y.X.; Wu, J.H.; Deng, T.; Yuan, M.H.; Duan, W.H.; Zhao, Y.F. Hydrolases control soil carbon sequestration in alpine grasslands in the Tibetan plateau. Sustainability 2024, 16, 3508. [Google Scholar] [CrossRef]
- Guan, S.Y. Soil Enzymes and Their Research Methods, 1st ed.; Chinese Agricultural Science and Technology Press: Beijing, China, 1986; pp. 19–21. [Google Scholar]
- Bai, M.X.; Situ, G.M.; Li, S.H.; Wu, Q.F.; Liang, C.F.; Qin, H.; Chen, J.H. Effects of combined application of biochar with organic amendments on enzyme activity and microbial metabolic function of carbon sources in infertile red soil. Chin. J. Appl. Ecol. 2022, 33, 1283–1290. [Google Scholar] [CrossRef]
- Shaon, K.D.; Goutam, K.G.; Ravikant, A.; Burhan, U.C.; Vinay, K.M.; Manik, C.K.; Aniruddha, R.; Tilak, M.; Achal, L.; Dhakre, D.S. Organic nutrient sources and biochar technology on microbial biomass carbon and soil enzyme activity in maize-black gram cropping system. Biomass Convers. Biorefin. 2023, 13, 9277–9287. [Google Scholar] [CrossRef]
- Wu, H.; Sun, W.; Zhu, F.; Jiang, Y.F.; Huang, S.W.; Goloran, J.; Xue, S.G. Straw addition increases enzyme activities and microbial carbon metabolism activities in bauxite residue. J. Environ. Sci. 2024, 135, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tang, Z.W.; Weng, Y.; Cai, H.M.; Wu, Y.; Zheng, B.Q.; Li, J.C. Effects of 15-year straw incorporation on soil carbon composition and microbial community under wheat-maize rotation system in the Huang-Huai-Hai Plain. BMC Plant Biol. 2025, 25, 522. [Google Scholar] [CrossRef] [PubMed]
- Jaffar, M.T.; Zhen, S.A.; Han, J.L.; Zhang, J.G.; Dar, A.; Mushtaq, Z.; Hussain, Q.; Zahir, A.; Siddique, K.H.M. Orange peel biochar: An effective amendment to improve the maize resilience by regulating the soil enzymatic activities, nutrient uptake, and ionic homeostasis under salinity stress. Ind. Crops Prod. 2024, 222, 120081. [Google Scholar] [CrossRef]
- Jaffar, M.T.; Chang, W.Q.; Zhang, J.G.; Mukhtar, A.; Mushtaq, Z.; Ahmed, M.; Zahir, A.; Siddique, K.H.M. Sugarcane bagasse biochar boosts maize growth and yield in salt-affected soil by improving soil enzymatic activities. J. Environ. Manag. 2024, 363, 121418. [Google Scholar] [CrossRef]
- Rahmanian, M.; Khadem, A. The effects of biochar on soil extra and intracellular enzymes activity. Biomass Convers. Biorefin. 2023, 14, 21993–22005. [Google Scholar] [CrossRef]
- Chen, S.; Dong, C.W.; Gao, Y.; Li, Y.J.; Shi, Y. Effects of nanocarbon and nano-calcium carbonate on soil enzyme activities and soil microbial community in wheat (Triticum aestivum L.) rhizosphere soil. J. Plant Nutr. Soil Sci. 2023, 187, 639–652. [Google Scholar] [CrossRef]
- Das, P.; Barker, C.; Park, Y.; Perreault, F.; Westerhoff, P.; Penton, C.R. Impact of graphite nano amendments on soil enzyme activities, functional genes and microbiome composition in a soil-plant system. Soil Biol. Biochem. 2025, 203, 109714. [Google Scholar] [CrossRef]
- Zhu, M.E. Soil Enzyme Kinetics and Thermodynamics, 1st ed.; Science Press: Beijing, China, 2011; pp. 1–41. [Google Scholar]
- Stone, M.M.; Plante, A.F. Changes in phosphatase kinetics with soil depth across a variable tropical landscape. Soil Biol. Biochem. 2014, 71, 61–67. [Google Scholar] [CrossRef]
- Allison, S.D.; Romero-Olivares, A.L.; Lu, Y.; Taylor, J.W.; Treseder, K.K. Temperature sensitivities of extracellular enzyme Vmax and Km across thermal environments. Glob. Change Biol. 2018, 24, 2884–2897. [Google Scholar] [CrossRef]
- Guo, Y.X.; Zhao, J.Y.; Li, J.W.; Dong, Y.L.; Yue, Z.H.; Yin, Y.; Li, W.; Ren, Q.N.; Wu, X.Y. Straw, biochar, and nanocarbon altered the enzymatic reaction kinetics and thermodynamic process of catalase in the black soil under continuous warming. Arch. Agron. Soil Sci. 2023, 69, 3637–3650. [Google Scholar] [CrossRef]
- Chintala, R.; Owen, R.K.; Schumacher, T.E.; Spokas, K.A.; McDonald, L.M.; Kumar, S.; Clay, D.E.; Malo, D.D.; Bleakley, B. Denitrification kinetics in biomass- and biochar-amended soils of different landscape positions. Environ. Sci. Pollut. Res. Int. 2015, 22, 5152–5163. [Google Scholar] [CrossRef]
- Yan, Q.T.; Tian, H.X.; Huang, Y.; Mu, X.L.; Tang, G.M.; Ma, H.G.; Megharaj, M.; Xu, W.L.; He, W.X. Recycled wheat straw biochar enhances nutrient-poor soil: Enzymatic kinetics of carbon, nitrogen, and phosphorus cycling. J. Environ. Manag. 2025, 380, 124950. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.D. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2008; pp. 30–34, 42–58, 74–81. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Michaelis constants of soil enzymes. Soil Biol. Biochem. 1971, 3, 317–323. [Google Scholar] [CrossRef]
- Shaaban, M.; Wu, Y.P.; Delgado, A.; Kuzyakov, Y.; Peng, Q.A.; Lin, S.; Hu, R.G. Enzyme activities and organic matter mineralization in response to application of gypsum, manure and rice straw in saline and sodic soils. Environ. Res. 2023, 224, 115393. [Google Scholar] [CrossRef]
- Xu, H.D.; Sun, J.N.; Zhao, Z.Q.; Gao, Y.; Tian, L.J.; Wei, X.M. Long-term straw return promotes soil phosphorus cycling by enhancing soil microbial functional genes responsible for phosphorus mobilization in the rice rhizosphere. Agric. Ecosyst. Environ. 2025, 381, 109422. [Google Scholar] [CrossRef]
- Xie, X.F.; Xu, Z.Q.; Tian, Z.Y.; Bu, X.G.; Xu, F.; Liang, J.; Pu, L.J. Effects of Supplementation of Different Amendments on Soil Heavy Metals and Enzyme Activities in Coastal Saline Land. Huanjing Kexue 2023, 44, 5649–5656. [Google Scholar] [CrossRef]
- Ortega, R.; Miralles, I.; Soria, R.; RodríguezBerbel, N.; Villafuerte, A.B.; Zema, D.A.; Borja, M.E.L. Short-term effects of post-fire soil mulching with wheat straw and wood chips on the enzymatic activities in a Mediterranean pine forest. Sci. Total Environ. 2022, 857, 159489. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Tian, H.X.; Wang, Z.Q.; Liu, C.Y.; Nurzhan, A.; Megharaj, M.; He, W.X. Potential effect of warming on soil microbial nutrient limitations as determined by enzymatic stoichiometry in the farmland from different climate zones. Sci. Total Environ. 2022, 802, 149657. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.L.; Zhang, Y.H.; Han, I.; Wang, P.; Mei, Q.W.; Huang, Y.J. Effects of different straw biochars on soil organic carbon, nitrogen, available phosphorus, and enzyme activity in paddy soil. Sci. Rep. 2020, 10, 8837. [Google Scholar] [CrossRef]
- Liu, L.; Cheng, M.; Yang, L.; Gu, X.Y.; Jin, J.Y.; Fu, M.J. Regulation of straw decomposition and its effect on soil function by the amount of returned straw in a cool zone rice crop system. Sci. Rep. 2023, 13, 15673. [Google Scholar] [CrossRef]
- Bekchanova, M.; Campion, L.; Bruns, S.; Kuppens, T.; Lehmann, J.; Jozefczak, M.; Cuypers, A.; Malina, R. Biochar improves the nutrient cycle in sandy-textured soils and increases crop yield: A systematic review. Environ. Evid. 2024, 13, 3. [Google Scholar] [CrossRef]
- Bhandari, G.; Gangola, S.; Dhasmana, A.; Rajput, V.; Gupta, S.; Malik, S.; Slama, P. Nano-biochar: Recent progress, challenges, and opportunities for sustainable environmental remediation. Front. Microbiol. 2023, 14, 1214870. [Google Scholar] [CrossRef]
- Song, Y.B.; Tan, J.W.; Jin, M.Y.; Liu, Z.; Zhu, J.F.; Mohamed, E.A.; Abdelhafeez, I.A.; Zhang, Y.L.; Shen, Z. Effect of pyrolysis temperature and heating rate on the physicochemical properties of alkali lignin-derived biochar: A comparative study of fast and slow pyrolysis. J. Anal. Appl. Pyrolysis 2025, 191, 107236. [Google Scholar] [CrossRef]
- Yan, J.F.; Xu, J.D.; Zhang, R.Y.; Zhou, P.; Yuan, Y.F.; Li, Y.M. Nanocarbon molecules—The fascination of synthetic chemistry. Prog. Chem. 2023, 35, 699–708. [Google Scholar] [CrossRef]
- Zeng, L.Q.; Zimmerman, A.R.; Huang, R.X. Adsorption of extracellular enzymes by biochar: Impacts of enzyme and biochar properties. Geoderma 2024, 451, 117082. [Google Scholar] [CrossRef]
- Raliya, R.; Saharan, V.; Dimkpa, C.; Biswas, P. Nanofertilizer for precision and sustainable agriculture: Current state and future perspectives. J. Agric. Food Chem. 2017, 66, 6487–6503. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Cao, Y.N.; Ma, C.X.; Yan, W.D. Nano-biochar as a potential amendment for metal(loid) remediation: Implications for soil quality improvement and stress alleviation. J. Environ. Manag. 2024, 351, 119658. [Google Scholar] [CrossRef]
- Malik, A.A.; Martiny, J.B.H.; Brodie, E.L.; Martiny, A.C.; Treseder, K.K.; Allison, S.D. Defining trait-based microbial strategies with consequences for soil carbon cycling under climate change. ISME J. 2020, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Weverka, J.R.; Moeller, H.V.; Schimel, J.P. Chemodiversity controls microbial assimilation of soil organic carbon: A theoretical model. Soil Biol. Biochem. 2023, 187, 109161. [Google Scholar] [CrossRef]
- Shang, W.H.; Razavi, B.S.; Yao, S.H.; Hao, C.K.; Kuzyakov, Y.; Blagodatskaya, E.; Tian, J. Contrasting mechanisms of nutrient mobilization in rhizosphere hotspots driven by straw and biochar amendment. Soil Biol. Biochem. 2023, 187, 109212. [Google Scholar] [CrossRef]
- Raiesi, F.; Khadem, A. Short-term effects of maize residue biochar on kinetic and thermodynamic parameters of soil β-glucosidase. Biochar 2019, 1, 213–227. [Google Scholar] [CrossRef]
- Khadem, A.; Besharati, H.; Khalaj, M.A. Biochar application changed arylsulfatase activity, kinetic and thermodynamic aspects. Eur. J. Soil Biol. 2019, 95, 103134. [Google Scholar] [CrossRef]
- Mbuthia, L.W.; Acosta-Martínez, V.; DeBruyn, J.; Schaeffer, S.; Tyler, D.; Odoi, E.; Mpheshea, M.; Walker, F.; Eash, N. Long term tillage, cover crop, and fertilization effects on microbial community structure, activity: Implications for soil quality. Soil Biol. Biochem. 2015, 89, 24–34. [Google Scholar] [CrossRef]
- Qiu, L.P.; Wang, Y.Q.; Liu, J.; Zhang, X.C. The dynamic and thermodynamic characteristics of soil reactions catalyzed by soil enzymes under long-term fertilization in Loess Plateau. Plant Nutr. Fertil. Sci. 2007, 13, 1028–1034. [Google Scholar]
- Panettieri, M.; Sosa, D.L.L.; Domínguez, T.M.; Madejón, E. Long-term impacts of conservation tillage on Mediterranean agricultural soils: Shifts in microbial communities despite limited effects on chemical properties. Agric. Ecosyst. Environ. 2020, 304, 107144. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Wu, Y.; Chen, W.J.; Sun, W.; Wang, Z.H.; Liu, G.B.; Xue, S. Soil enzyme kinetics and thermodynamics in response to long-term vegetation succession. Sci. Total Environ. 2023, 882, 163542. [Google Scholar] [CrossRef] [PubMed]
- Misiak, M.; Goodall Copestake, W.P.; Sparks, T.H.; Worland, M.R.; Boddy, L.; Magan, N.; Convey, P.; Hopkins, D.W.; Newsham, K.K. Inhibitory effects of climate change on the growth and extracellular enzyme activities of a widespread Antarctic soil fungus. Glob. Change Biol. 2021, 27, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.X.; Yu, G.H.; Hu, S.J.; Liang, C.; Kappler, A.; Jorgenson, M.T.; Guo, L.D.; Guggenberger, G. Deciphering the intricate control of minerals on deep soil carbon stability and persistence in Alaskan permafrost. Glob. Change Biol. 2024, 30, e17552. [Google Scholar] [CrossRef]
- Mooshammer, M.; Hofhansl, F.; Frank, A.H.; Wanek, W.; Hämmerle, I.; Leitner, S.; Schnecker, J.; Wild, B.; Watzka, M.; Keiblinger, K.M.; et al. Decoupling of microbial carbon, nitrogen, and phosphorus cycling in response to extreme temperature events. Sci. Adv. 2017, 3, e1602781. [Google Scholar] [CrossRef]
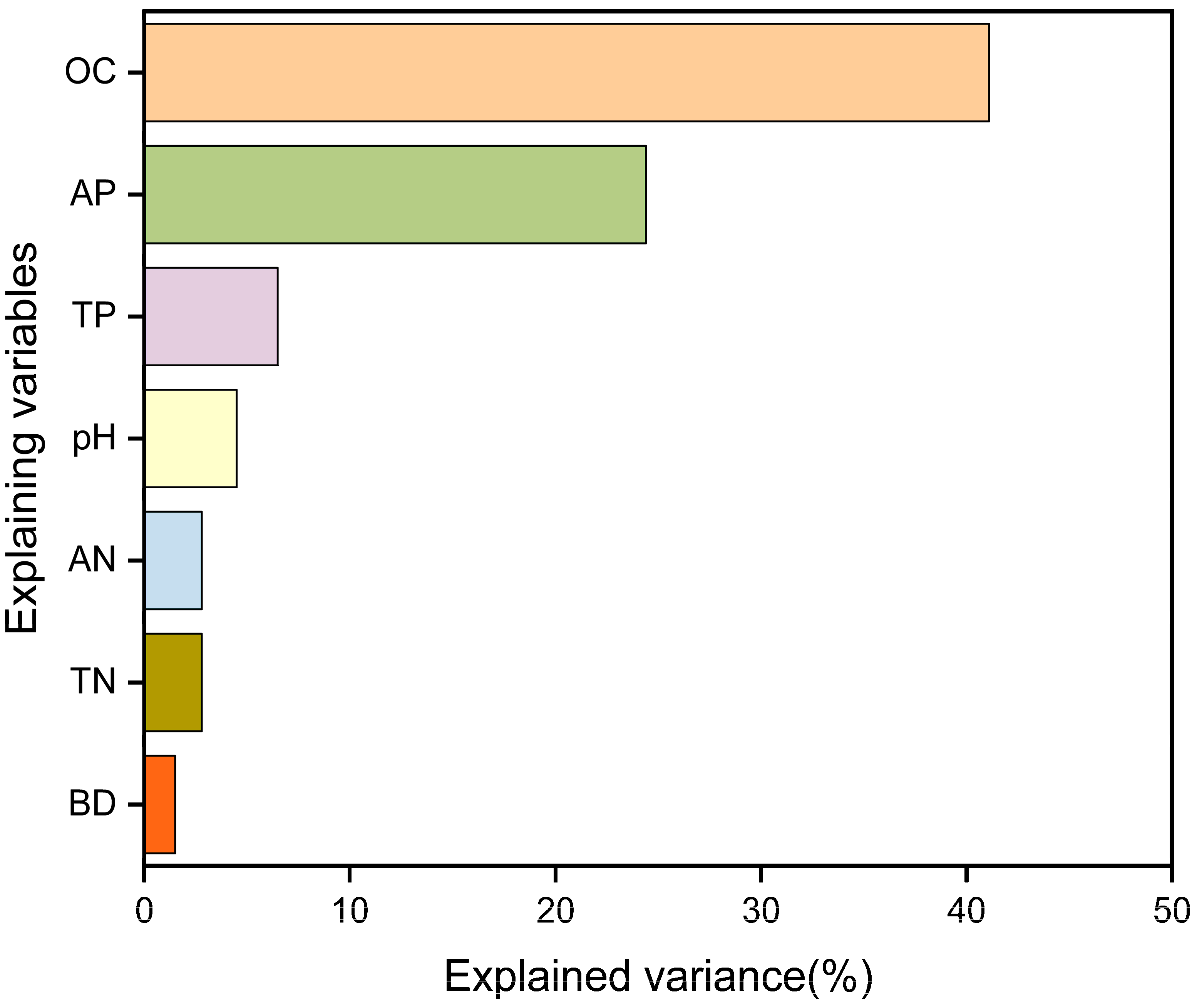
| Treatment | pH | Bulk Density (BD) (g·cm−3) | Organic Carbon (OC) (g·kg−1) | Total Nitrogen (TN) (g·kg−1) | Available Nitrogen (AN) (mg·kg−1) | Total Phosphorus (TP) (g·kg−1) | Available Phosphorus (AP) (mg·kg−1) |
|---|---|---|---|---|---|---|---|
| CK | 6.66 ± 0.02 a | 1.52 ± 0.01 a | 9.28 ± 0.10 b | 0.84 ± 0.03 ab | 66.22 ± 0.88 b | 0.92 ± 0.02 b | 74.72 ± 1.26 b |
| Straw | 6.65 ± 0.00 a | 1.47 ± 0.03 ab | 11.11 ± 0.10 a | 0.97 ± 0.06 a | 85.17 ± 6.15 a | 1.03 ± 0.06 a | 72.93 ± 3.79 b |
| Biochar | 6.69 ± 0.08 a | 1.45 ± 0.04 b | 9.88 ± 0.10 b | 0.76 ± 0.03 b | 83.71 ± 3.15 a | 1.02 ± 0.02 a | 100.71 ± 6.94 a |
| Nanocarbon | 6.66 ± 0.00 a | 1.46 ± 0.02 b | 8.31 ± 0.60 c | 0.73 ± 0.08 b | 61.83 ± 8.75 b | 1.01 ± 0.02 a | 98.49 ± 4.73 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Wu, X.; Wang, P.; Zhao, J.; Yue, Z.; Bai, X.; Li, J.; Yin, Y.; Huang, J. Decoding the Sustainability Code: Enzyme Thermodynamic and Kinetic Parameters Reveal the Efficacy of Straw, Biochar, and Nanocarbon in Black Soil. Sustainability 2025, 17, 10436. https://doi.org/10.3390/su172310436
Xu J, Wu X, Wang P, Zhao J, Yue Z, Bai X, Li J, Yin Y, Huang J. Decoding the Sustainability Code: Enzyme Thermodynamic and Kinetic Parameters Reveal the Efficacy of Straw, Biochar, and Nanocarbon in Black Soil. Sustainability. 2025; 17(23):10436. https://doi.org/10.3390/su172310436
Chicago/Turabian StyleXu, Jia, Xiangyu Wu, Pengwei Wang, Jingyi Zhao, Zhonghui Yue, Xin Bai, Jiawang Li, Yuan Yin, and Jianhao Huang. 2025. "Decoding the Sustainability Code: Enzyme Thermodynamic and Kinetic Parameters Reveal the Efficacy of Straw, Biochar, and Nanocarbon in Black Soil" Sustainability 17, no. 23: 10436. https://doi.org/10.3390/su172310436
APA StyleXu, J., Wu, X., Wang, P., Zhao, J., Yue, Z., Bai, X., Li, J., Yin, Y., & Huang, J. (2025). Decoding the Sustainability Code: Enzyme Thermodynamic and Kinetic Parameters Reveal the Efficacy of Straw, Biochar, and Nanocarbon in Black Soil. Sustainability, 17(23), 10436. https://doi.org/10.3390/su172310436






