Evolution Mechanisms of Gas-Solid Products in Multi-Source Sludge Pyrolysis: Synergistic Regulation by Temperature and Time Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
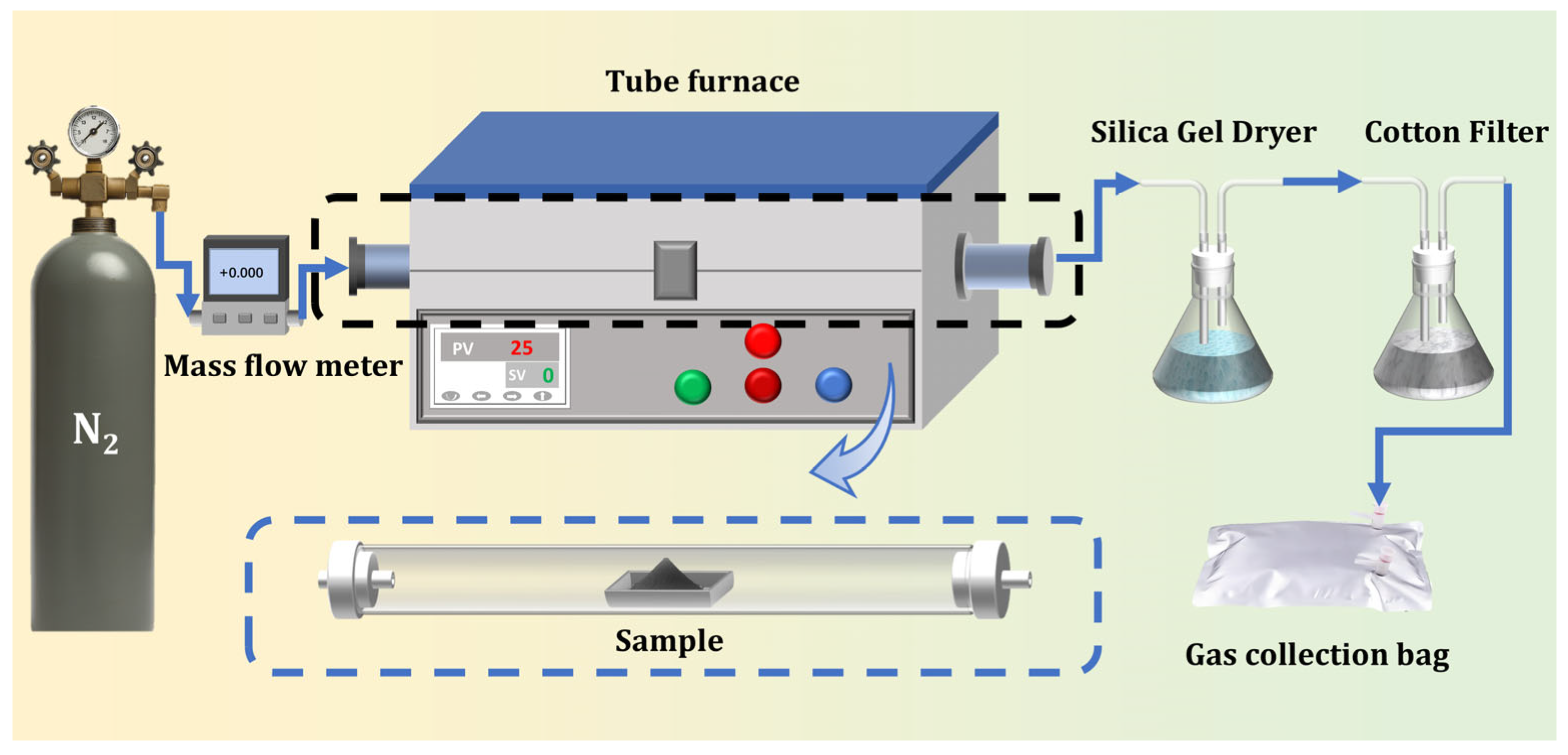
2.2. Experimental Setup and Operational Procedures
2.3. Statistical Analysis
3. Results
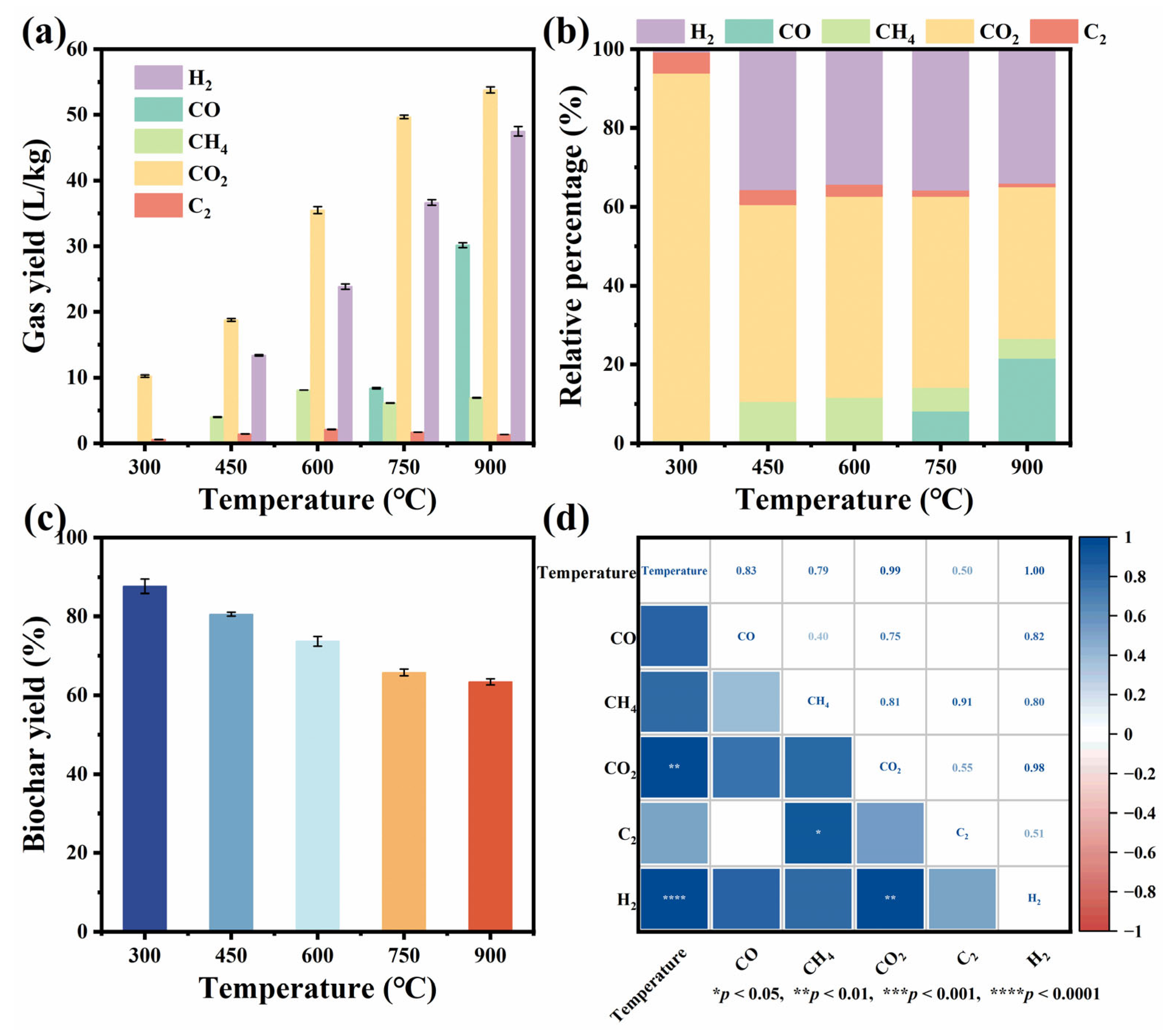
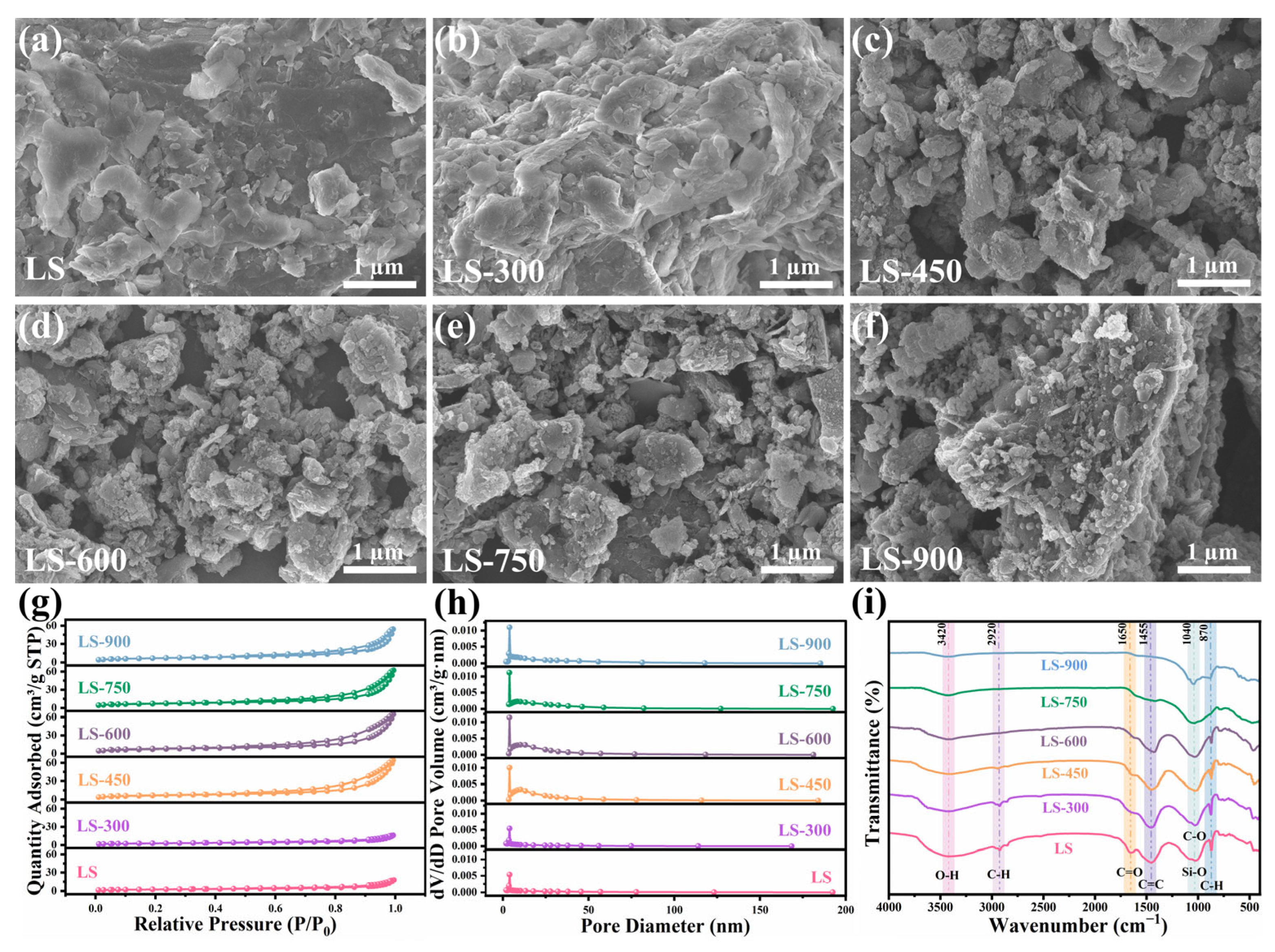
3.1. Effects of Pyrolysis Temperature on Product Distribution, Pyrolysis Gas Composition, and Biochar Properties
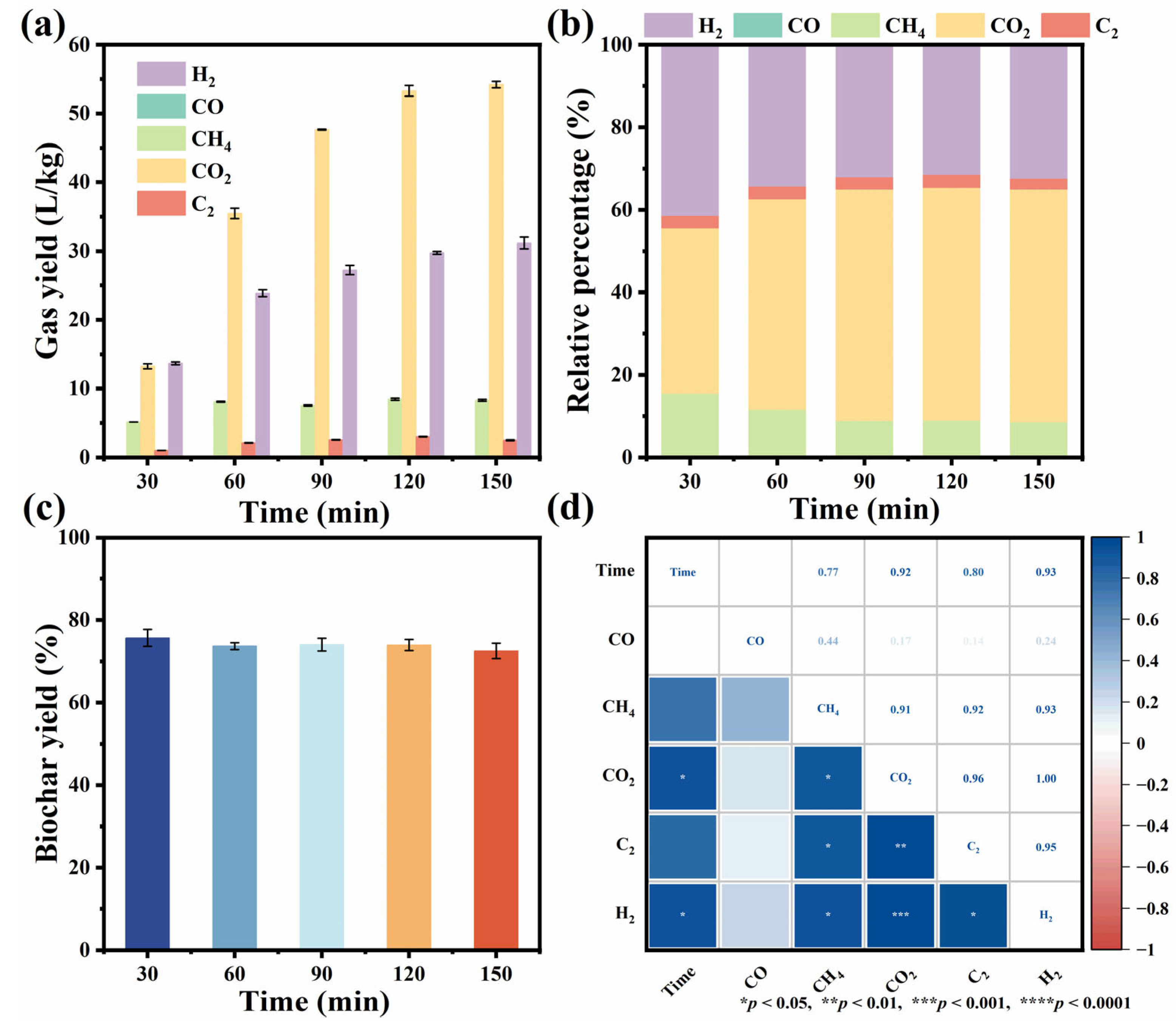
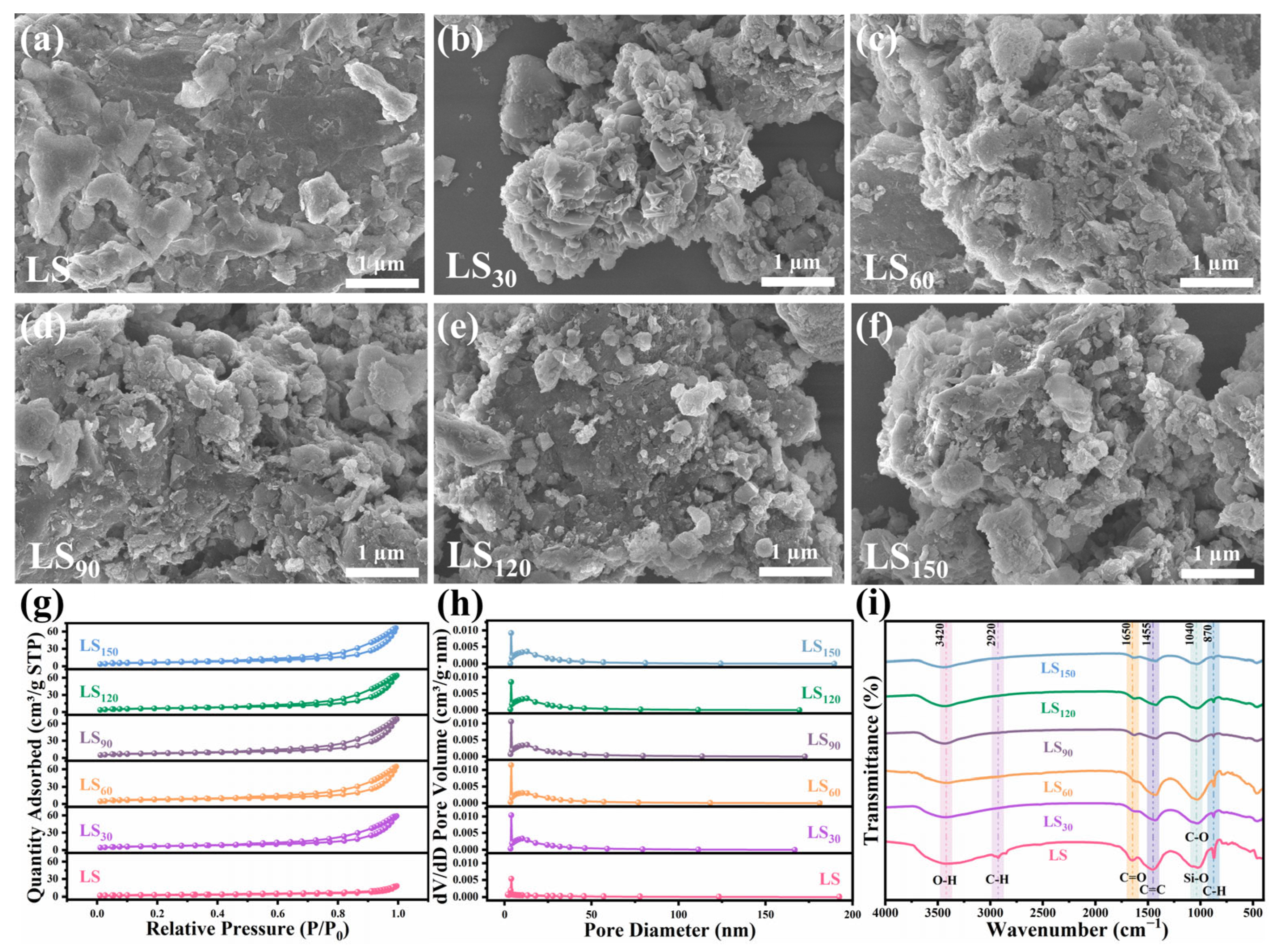
3.2. Effects of Residence Time on Product Distribution, Pyrolysis Gas Composition, and Biochar Properties
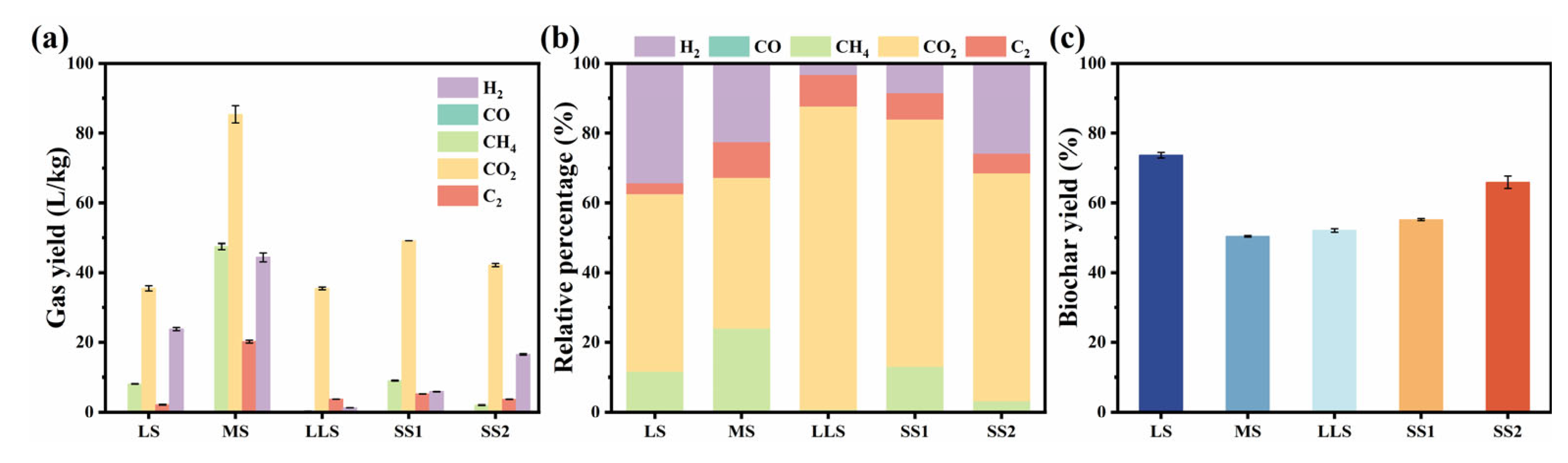
3.3. Effects of Sludge Type on Product Distribution, Pyrolysis Gas Composition, and Biochar Properties
3.4. Perspectives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, B.; Liu, Y.; Guan, Y.; Feng, Y. Characteristic of the production of hydrogen-rich combustible gas by pyrolysis of high-ash sewage sludge. J. Clean. Prod. 2022, 334, 130224. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, Z.; Yang, J.; Chen, G.; Yao, J.; Tu, C.; Zhao, X.; Qiu, Z.; Wu, Z. Insights into conditioning of landfill sludge by FeCl3 and lime. Water Res. 2019, 160, 167–177. [Google Scholar] [CrossRef]
- Zhou, A.; Wang, X.; Yu, S.; Deng, S.; Tan, H.; Mikulčić, H. Process design and optimization on self-sustaining pyrolysis and carbonization of municipal sewage sludge. Waste Manag. 2023, 159, 125–133. [Google Scholar] [CrossRef]
- Han, L.; Li, J.; Qu, C.; Shao, Z.; Yu, T.; Yang, B. Recent Progress in Sludge Co-Pyrolysis Technology. Sustainability 2022, 14, 7574. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, J.; Wang, M.; Naidu, R.; Liu, Y.; Man, Y.B.; Liang, X.; Wong, M.H.; Christie, P.; Zhang, Y.; et al. Co-pyrolysis of sewage sludge and rice husk/bamboo sawdust for biochar with high aromaticity and low metal mobility. Environ. Res. 2020, 191, 110034. [Google Scholar] [CrossRef]
- Zhao, M.; Ji, D. Investigations on the pyrolysis characteristics of sludge and peanut shell: Thermal decomposition behaviors, kinetic, products. Biomass Convers. Biorefinery 2025, 15, 9071–9082. [Google Scholar] [CrossRef]
- Kujawska, J.; Wojtaś, E.; Charmas, B. Biochar Derived from Sewage Sludge: The Impact of Pyrolysis Temperature on Chemical Properties and Agronomic Potential. Sustainability 2024, 16, 8225. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Wang, M.; Xia, Y.; Lan, G.; Yan, B.; Yu, Y.; Xiong, X.; Zou, J.; Zhu, Y. Study on the effect of cyclic catalytic pyrolysis on sludge pyrolysis products. J. Environ. Chem. Eng. 2024, 12, 111647. [Google Scholar] [CrossRef]
- Song, Y.; Hu, J.; Liu, J.; Evrendilek, F.; Buyukada, M. CO2-assisted co-pyrolysis of textile dyeing sludge and hyperaccumulator biomass: Dynamic and comparative analyses of evolved gases, bio-oils, biochars, and reaction mechanisms. J. Hazard. Mater. 2020, 400, 123190. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, R.; Zhao, B.; Fan, G.; Li, H.; Xu, X.; Zhang, M. Preparation of porous biochars by the co-pyrolysis of municipal sewage sludge and hazelnut shells and the mechanism of the nano-zinc oxide composite and Cu(II) adsorption kinetics. Sustainability 2020, 12, 8668. [Google Scholar] [CrossRef]
- Li, J.; Zheng, F.; Li, Q.; Farooq, M.Z.; Lin, F.; Yuan, D.; Yan, B.; Song, Y.; Chen, G. Effects of inherent minerals on oily sludge pyrolysis: Kinetics, products, and secondary pollutants. Chem. Eng. J. 2022, 431, 133218. [Google Scholar] [CrossRef]
- Liu, Y.; Ran, C.; Siddiqui, A.R.; Chtaeva, P.; Siyal, A.A.; Song, Y.; Dai, J.; Deng, Z.; Fu, J.; Ao, W.; et al. Pyrolysis of sewage sludge in a benchtop fluidized bed reactor: Characteristics of condensates and non-condensable gases. Renew. Energy 2020, 160, 707–720. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, Y.; Wang, Y.; Pan, X.; Shi, W.; Huang, Y.; Liao, Y.; Yang, Y.; Zuo, X. The effect of cyclic catalytic pyrolysis system on the co-pyrolysis products of sewage sludge and chicken manure, focusing on the yield and quality of syngas. Energy 2025, 314, 134182. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, B.; Liu, H.; Zhao, Y.; Li, L. Effects of pyrolysis temperature on biochar’s characteristics and speciation and environmental risks of heavy metals in sewage sludge biochars. Environ. Technol. Innov. 2022, 26, 102288. [Google Scholar] [CrossRef]
- Chen, F.; Ding, L.; Zhu, Y.; Ren, G.; Man, Y.; Hong, K.; Lang, L.; Ström, H.; Xiong, Q. Comprehensive kinetic modeling and product distribution for pyrolysis of pulp and paper mill sludge. Sci. Total Environ. 2024, 924, 171665. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Xiao, Y.; Zhao, X.; Li, D.; Ma, Z.; Li, W.; Gong, T.; Zhang, T.; Huang, N.; Xi, B. Transformation pathways of the carbon-containing group compounds during municipal sludge pyrolysis treatment. Waste Manag. 2024, 178, 26–34. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Fan, D.; Zhu, P.; Liu, Y.; Chao, L.; Zhou, C.; Yao, B.; Zhang, Y.; Dai, J. In situ catalytic pyrolysis of oily sludge using coal gangue char to produce naphthalene-rich oil and hydrogen-rich gas. Chem. Eng. J. 2025, 508, 160953. [Google Scholar] [CrossRef]
- Tang, B.; Fu, P.; Guo, Y.; Wang, Z.; Zhang, J.; Lin, X. Effect of temperature on product properties and synergies during the co-pyrolysis of paper sludge and corn stover. J. Environ. Chem. Eng. 2024, 12, 111817. [Google Scholar] [CrossRef]
- Yao, B.; Li, X.; Zhou, C.; Lv, F.; Zhang, C.; Wang, L.; Yu, M.; Yuan, Y.; Zhang, Y.; Jin, Y.; et al. Co-pyrolysis of dyeing sludge and pine sawdust in a fluidized bed: Characterization and analysis of pyrolytic products and investigation of synergetic effects. Waste Manag. 2023, 167, 122–134. [Google Scholar] [CrossRef]
- Wang, J.X.; Liu, P.; Lai, F.Y.; Huang, H.J. Pyrolysis of different sewage sludge feedstocks for biochar products: Characterization and application. J. Cent. South Univ. 2020, 27, 3302–3319. [Google Scholar] [CrossRef]
- Vali, N.; Zabihi, S.; Shamim, S.; Mohsenzadeh, A.; Pettersson, A. Slow-pyrolysis of municipal sewage sludge: Biochar characteristics and advanced thermodynamics. Biomass Convers. Biorefinery 2025, 15, 21045–21065. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Y.; Li, J.; Xue, Y.; Liu, J.; Mei, M.; Hou, H.; Chen, S. Co-pyrolysis behavior of sewage sludge and rice husk by TG-MS and residue analysis. J. Clean. Prod. 2020, 250, 119557. [Google Scholar] [CrossRef]
- Liu, Y.; Ran, C.; Siyal, A.A.; Song, Y.; Jiang, Z.; Dai, J.; Chtaeva, P.; Fu, J.; Ao, W.; Deng, Z.; et al. Comparative study for fluidized bed pyrolysis of textile dyeing sludge and municipal sewage sludge. J. Hazard. Mater. 2020, 396, 122619. [Google Scholar] [CrossRef]
- Xing, X.; Zhao, H.; Zhou, L.; Wang, Y.; Chen, H.; Gao, Y.; Wang, Y.; Zhu, Y. Pyrolysis kinetics, thermodynamics of PTA sludge and product characterization of cyclic in-situ catalytic pyrolysis by using recycled char as a catalyst. Energy 2022, 251, 123821. [Google Scholar] [CrossRef]
- Tong, S.; Zhang, S.; Yin, H.; Wang, J.; Chen, M. Study on co-hydrothermal treatment combined with pyrolysis of rice straw/sewage sludge: Biochar properties and heavy metals behavior. J. Anal. Appl. Pyrolysis 2021, 155, 105074. [Google Scholar] [CrossRef]
- Yu, D.; Li, Z.; Li, J.; He, J.; Li, B.; Wang, Y. Enhancement of H2 and light oil production and CO2 emission mitigation during co-pyrolysis of oily sludge and incineration fly ash. J. Hazard. Mater. 2024, 462, 132618. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, L.; Hu, Q.; Hu, Z. Influence of pyrolysis temperature on speciation, leaching and environmental risk assessment of heavy metals in biochar and bio-oil from pyrolysis of wet sewage sludge. Biomass Convers. Biorefinery 2025, 15, 19363–19376. [Google Scholar] [CrossRef]
- Raj, A.; Yadav, A.; Arya, S.; Sirohi, R.; Kumar, S.; Rawat, A.P.; Thakur, R.S.; Patel, D.K.; Bahadur, L.; Pandey, A. Preparation, characterization and agri applications of biochar produced by pyrolysis of sewage sludge at different temperatures. Sci. Total Environ. 2021, 795, 148722. [Google Scholar] [CrossRef]
- Yu, F.; Lv, H.; Fan, L.; Chen, L.; Hu, Y.; Wang, X.; Guo, Q.; Cui, X.; Zhou, N.; Jiao, L. Co-pyrolysis of sewage sludge and poplar sawdust under controlled low-oxygen conditions: Biochar properties and heavy metals behavior. J. Anal. Appl. Pyrolysis 2023, 169, 105868. [Google Scholar] [CrossRef]
- Han, Y.; Guo, M.; Xu, Z.; Fu, T.; Li, F.; Huang, X.; Liu, N.; Chen, Z.; Wang, T. Effect of steel slag acting as dewatering agent on the subsequent pyrolysis properties of sewage sludge. J. Clean. Prod. 2022, 376, 134249. [Google Scholar] [CrossRef]
- Qu, J.; Wang, D.; Deng, Z.; Yu, H.; Dai, J.; Bi, X. Biochar Prepared by Microwave-Assisted Co-Pyrolysis of Sewage Sludge and Cotton Stalk: A Potential Soil Conditioner. Sustainability 2023, 15, 7265. [Google Scholar] [CrossRef]
- Ali, L.; Palamanit, A.; Techato, K.; Ullah, A.; Chowdhury, M.S.; Phoungthong, K. Characteristics of Biochars Derived from the Pyrolysis and Co-Pyrolysis of Rubberwood Sawdust and Sewage Sludge for Further Applications. Sustainability 2022, 14, 3829. [Google Scholar] [CrossRef]
- Liu, Y.; Song, Y.; Zhang, T.; Jiang, Z.; Siyal, A.A.; Dai, J.; Fu, J.; Zhou, C.; Wang, L.; Li, X.; et al. Microwave-assisted pyrolysis of oily sludge from offshore oilfield for recovery of high-quality products. J. Hazard. Mater. 2021, 420, 126578. [Google Scholar] [CrossRef]
- Xue, Y.; Zhou, Y.; Liu, J.; Xiao, Y.; Wang, T. Comparative analysis for pyrolysis of sewage sludge in tube reactor heated by electromagnetic induction and electrical resistance furnace. Waste Manag. 2021, 120, 513–521. [Google Scholar] [CrossRef]
- Khodaparasti, M.S.; Khorasani, R.; Tavakoli, O.; Khodadadi, A.A. Optimal Co-pyrolysis of municipal sewage sludge and microalgae Chlorella Vulgaris: Products characterization, synergistic effects, mechanism, and reaction pathways. J. Clean. Prod. 2023, 390, 135991. [Google Scholar] [CrossRef]
- Wang, C.; Wang, W.; Lin, L.; Zhang, F.; Zhang, R.; Sun, J.; Song, Z.; Mao, Y.; Zhao, X. A stepwise microwave synergistic pyrolysis approach to produce sludge-based biochars: Feasibility study simulated by laboratory experiments. Fuel 2020, 272, 117628. [Google Scholar] [CrossRef]
- Liu, J.; Huang, S.; Chen, K.; Wang, T.; Mei, M.; Li, J. Preparation of biochar from food waste digestate: Pyrolysis behavior and product properties. Bioresour. Technol. 2020, 302, 122841. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, J. Adsorption of ciprofloxacin on co-pyrolyzed biochar from fish scale and pine needle. Chin. J. Anal. Chem. 2024, 52, 100350. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Y.; Cui, M.-h.; Liu, H.; Liu, H.; Zheng, Z.; Zheng, W.; Zhang, C.; Wen, D. Pyrolyzing pharmaceutical sludge to biochar as an efficient adsorbent for deep removal of fluoroquinolone antibiotics from pharmaceutical wastewater: Performance and mechanism. J. Hazard. Mater. 2022, 426, 127798. [Google Scholar] [CrossRef]
- Petrovič, A.; Vohl, S.; Cenčič Predikaka, T.; Bedoić, R.; Simonič, M.; Ban, I.; Čuček, L. Pyrolysis of solid digestate from sewage sludge and lignocellulosic biomass: Kinetic and thermodynamic analysis, characterization of biochar. Sustainability 2021, 13, 9642. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, N.; Guo, Q.; Luo, Z.; Xia, Z.; Hu, Y. Synergetic effects during co-pyrolysis of waste textiles and Ca/Fe-rich industrial sewage sludge: Reaction kinetics and product distribution. Waste Manag. 2024, 186, 141–151. [Google Scholar] [CrossRef]
- Peng, B.; Liu, Q.; Li, X.; Zhou, Z.; Wu, C.; Zhang, H. Co-pyrolysis of industrial sludge and rice straw: Synergistic effects of biomass on reaction characteristics, biochar properties and heavy metals solidification. Fuel Process. Technol. 2022, 230, 107211. [Google Scholar] [CrossRef]
- Balmuk, G.; Videgain, M.; Manyà, J.J.; Duman, G.; Yanik, J. Effects of pyrolysis temperature and pressure on agronomic properties of biochar. J. Anal. Appl. Pyrolysis 2023, 169, 105858. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, Y.; Yang, L.; Zhu, Y. High quality syngas produced from the co-pyrolysis of wet sewage sludge with sawdust. Int. J. Hydrog. Energy 2018, 43, 5463–5472. [Google Scholar] [CrossRef]
- Yu, H.; Liu, J.; Yang, H. Catalytic reforming of volatiles from pyrolysis of biomass components over a novel Ni/magnesium slag catalyst. J. Anal. Appl. Pyrolysis 2021, 159, 105316. [Google Scholar] [CrossRef]
- Yu, D.; Li, Z.; Li, J.; Li, B.; Yu, H.; He, J.; Wang, Y. Role of municipal solid waste incineration fly ash components in co-pyrolysis of oily sludge: Pyrolysis products and catalytic mechanism. J. Hazard. Mater. 2024, 471, 134368. [Google Scholar] [CrossRef]
- Lin, J.; Sun, J.; Chen, Y.; Luo, J.; Cui, C.; Sun, S. Valorization of sludge using microwave pyrolysis for green bio-energy: Combined effects of key parameters on the directional optimization of high-quality syngas. Fuel 2022, 326, 125010. [Google Scholar] [CrossRef]
- Ghodke, P.K.; Sharma, A.K.; Pandey, J.K.; Chen, W.H.; Patel, A.; Ashokkumar, V. Pyrolysis of sewage sludge for sustainable biofuels and value-added biochar production. J. Environ. Manag. 2021, 298, 113450. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wu, S.; Xing, X.; Zhou, T.; Zhao, Y. Evolution Mechanisms of Gas-Solid Products in Multi-Source Sludge Pyrolysis: Synergistic Regulation by Temperature and Time Parameters. Sustainability 2025, 17, 10270. https://doi.org/10.3390/su172210270
Li X, Wu S, Xing X, Zhou T, Zhao Y. Evolution Mechanisms of Gas-Solid Products in Multi-Source Sludge Pyrolysis: Synergistic Regulation by Temperature and Time Parameters. Sustainability. 2025; 17(22):10270. https://doi.org/10.3390/su172210270
Chicago/Turabian StyleLi, Xiaoya, Shuya Wu, Xu Xing, Tao Zhou, and Youcai Zhao. 2025. "Evolution Mechanisms of Gas-Solid Products in Multi-Source Sludge Pyrolysis: Synergistic Regulation by Temperature and Time Parameters" Sustainability 17, no. 22: 10270. https://doi.org/10.3390/su172210270
APA StyleLi, X., Wu, S., Xing, X., Zhou, T., & Zhao, Y. (2025). Evolution Mechanisms of Gas-Solid Products in Multi-Source Sludge Pyrolysis: Synergistic Regulation by Temperature and Time Parameters. Sustainability, 17(22), 10270. https://doi.org/10.3390/su172210270





