Abstract
Silver and gold nanoparticles (NPs) have gained considerable attention in recent years due to their wide-ranging applications in medicine, agriculture, industry, and other fields where they may interact with the environment. Green synthesis of NPs supports sustainability by reducing chemical waste and energy use while improving their biocompatibility through plant phytochemicals. Accordingly, it is important to assess the effects of metal NPs on microorganisms, which play vital roles in ecosystems and biogeochemical cycles. This study aimed to investigate microbial growth dynamics in the presence of green-synthesized silver and gold NPs (using an aqueous extract of Mentha × piperita leaves) and to evaluate potential mechanisms of their interaction. Microorganisms were cultivated in 96-well microtiter plates, and growth curves were analyzed alongside bacterial enumeration on Petri plates. Silver NPs affected the growth of Brevundimonas vesicularis USM1, Pseudarthrobacter oxydans USM2, and Pseudomonas putida USM4, although these strains exhibited partial resistance. In contrast, gold NPs did not inhibit the growth of the tested strains. The ability of Brevundimonas vesicularis USM1 to precipitate metal NPs highlights its potential for sustainable bioremediation applications. The findings contribute to a better understanding of the environmental impact and sustainability aspects of silver and gold NPs in microbial systems.
1. Introduction
Nanomaterials have been rapidly integrated into science, medicine, agriculture, and various other fields in recent years [1]. Among them, metal nanoparticles (NPs) have attracted particular attention, as metals in nanoscale form exhibit unique morphology, crystal structure, agglomeration behavior, surface charge, and physicochemical properties that enable a broad range of applications [2,3]. Metal NPs are employed to improve or modify the mechanical and functional properties of materials used in electronics, energy technologies, and related industries [4]. In agriculture, they are applied to enhance crop yields and plant resistance [3]. In medicine, metal NPs are valued for their antimicrobial activity and for reinforcing the mechanical performance of biomedical materials, such as dental restorative and prosthetic composites [5]. Moreover, they are considered promising agents for cancer diagnosis and therapy [6].
Owing to the growing interest in NPs, there is a need to develop advanced synthesis protocols that allow precise control over morphology, stability, and reproducibility. Modern nanoscience offers a variety of NP synthesis methods, including physical approaches—such as ball milling, laser ablation, vapor condensation, electric arc discharge, and sputtering—and chemical approaches, such as sol–gel synthesis, hydrothermal synthesis, and chemical reduction [7]. Physical methods have the advantage of avoiding the use of chemicals and solvents and enabling precise control over certain synthesis conditions. However, they are often associated with high energy consumption, require expensive equipment, and generally provide limited control over particle morphology. In contrast, chemical methods offer better control over the size, shape, and chemical composition of the resulting NPs, and they are typically cost-effective and scalable for mass production. Their main drawbacks include the use of potentially toxic reducing and capping agents, the generation of harmful byproducts, and challenges in achieving uniformity and long-term stability of the synthesized nanoparticles [8].
The aforementioned challenges can be mitigated by using intentionally non-toxic chemicals derived from biological sources. This strategy is referred to as green synthesis or biological synthesis, depending on the origin of the reagents. Green synthesis methods are time-, energy-, and cost-efficient and environmentally friendly. An additional advantage is the utilization of renewable and readily available resources, whose diversity is limited only by the variety of biological matter, such as plants, algae, or microorganisms [9,10]. For medicinal applications of nanoparticles, biocompatibility represents another significant benefit of this approach [11,12]. However, the use of biologically derived reagents introduces certain challenges, including complex chemical composition, regional and seasonal variability, and limited understanding of the mechanisms underlying NP formation [13].
Silver nanoparticles (AgNPs) are well known for their broad-spectrum antimicrobial activity and promising anticancer properties [14]. Even at low concentrations, such as 3.125 mg·L−1 (0.028 mM), AgNPs have been shown to inhibit the growth of microorganisms, including Escherichia coli and Staphylococcus aureus [15].
They exhibit antibacterial activity against E. coli [16], Pseudomonas aeruginosa [17], S. aureus, and Bacillus subtilis [18], Vibrio cholera, Salmonella typhus [19], and antifungal activity against Trichophyton mentagrophytes, Candida albicans [16], Candida krusei, Candida tropicalis, and Candida guilliermondii [20]. AgNPs have found applications in cosmetology, water treatment, agriculture, the textile industry [16], and food packaging [21]. Their functional properties can be tuned by controlling size and shape; however, their environmental instability can limit practical applications.
The properties of gold nanoparticles (AuNPs) depend strongly on their size, shape, and surface characteristics. They are stable and harmless compounds with minimal chemical activity, which makes them particularly well-suited for biomedical uses, including the transport of drugs and genetic material [14]. AuNPs possess high surface affinity, enabling facile conjugation with a variety of biomolecules to enhance targeted delivery [22]. Synthesis methods for AuNPs prioritize precise control over size, shape, solubility, and stability, which are critical determinants of their performance and applicability across different industries [14].
The increasing demand for metal NPs and their expanding range of applications have raised concerns about their potential release into the environment. While the benefits of metal NPs are well documented, their adverse effects on living organisms and ecosystems remain insufficiently understood. AgNPs, for instance, have been reported to exhibit genotoxicity and cytotoxicity [16], inducing oxidative stress, lipid and protein oxidation, and DNA damage [21]. The use of AgNPs is becoming increasingly widespread in various fields of science, including medicine, modern analytics, material sciences, and healthcare, largely due to their proven antimicrobial activity [19]. However, the impact of AgNPs on ecosystems is not well understood. The microbiome of natural ecosystems, in particular, is among the first to be affected by AgNPs. Therefore, studying microorganisms isolated from natural ecosystems is essential to understanding the resilience of microbiomes and ecosystems as a whole. Although AuNPs are generally considered chemically inert and less reactive, studies indicate that they can still interact with cellular components and influence cell physiology [23]. For example, AuNPs have been shown not to inhibit E. coli or S. aureus under certain conditions [15,24]. AuNPs were shown to inhibit S. aureus at 197 μg·mL−1 (0.985 mM). The antimicrobial activity of AuNPs depends on their size, concentration, surface modification, and purity. While results on size effects are inconsistent, antibacterial activity is consistently concentration-dependent and strongly enhanced by cationic or amine-containing coatings. Proper purification is essential, as residual ions or reagents can cause false-positive antibacterial results. Although gold is generally considered inert toward living organisms and AuNPs are often regarded as non-toxic to microorganisms, several studies have reported microbial inhibition at high AuNP concentrations. In addition to concentration, factors such as ligands, particle size, and other physicochemical properties also play a crucial role in determining their biological activity [24]. Moreover, their effects on a broad spectrum of microorganisms remain largely unexplored. With the increasing use of AuNPs across various applications, their release into the environment is also rising; therefore, their impact on environmental microorganisms is no less important than that of AgNPs.
Microorganisms play a critical role in ecosystems and are among the first to encounter metal NPs in natural environments. Understanding how these nanoparticles interact with microbial cells is therefore essential for assessing ecological risks. Although numerous studies have examined the inhibitory effects of metal ions and NPs on microbial growth, less is known about the mechanisms by which microorganisms interact with metal nanoparticles—specifically, whether they can transform NPs into insoluble or soluble forms, or modify their chemical or physical properties.
In this context, the present work aims to investigate the dynamics of microbial growth in the presence of AgNPs and AuNPs. In addition, it seeks to explore potential pathways of interaction between microorganisms and these metal NPs, including their capacity for precipitation, transformation, or detoxification. By addressing these questions, the study contributes to a better understanding of NP–microbe interactions, which is essential for evaluating the environmental impact and safe use of metal NPs.
Many existing studies have focused on the effects of metal NPs on pathogenic microorganisms [19,20,24], whereas environmental microbial populations have received considerably less attention [21]. Further research is required to elucidate both the high biomedical relevance of metal NPs and their potential applications in disease therapy [17,18,19]. In contrast, the environmental dimension—encompassing the release of metal NPs into natural ecosystems, their influence on microbial community structure, and their broader ecological interactions—remains insufficiently investigated [21].
In this study, microorganisms isolated from natural ecosystems were employed [25]. These strains possess genetically determined protective mechanisms that confer resistance to the toxic effects of heavy metals in their ionic forms [26]. However, metals in nanoparticulate form exhibit distinct physicochemical properties compared with their ionic counterparts and are often highly toxic to non-resistant microorganisms. The response of metal-resistant strains to NPs, however, remains poorly understood. Investigating their resistance to metal NPs and elucidating the underlying mechanisms of interaction are essential to determine whether resistance to ionic metal species also translates into tolerance toward nanoscale forms.
2. Materials and Methods
2.1. Phytosynthesis of Silver and Gold Nanoparticles
2.1.1. Preparation and Characterization of Peppermint Extract
Leaves of Mentha × piperita L. cv. ‘Kristinka’ were collected in July 2024 from plants cultivated in the School field of the University of Prešov (Prešov, Slovakia). The plantation was established in 2022 by the vegetative propagation of plants of the ‘Kristinka’ cultivar. The extract was prepared by maceration of air-dried leaves in double-distilled water (DDW) following a previously published procedure [27]. Briefly, 20 g of dried leaves were boiled with 200 mL of DDW in a round-bottom flask equipped with a reflux condenser for 30 min. Plant material was subsequently removed by filtration using a Buchner funnel and KA 1-M filter paper (Papírna Pernštejn s.r.o., Perštejn, Czech Republic). To eliminate small particles that could affect nanoparticle formation, the extract was centrifuged at 1000 rpm for 30 min using a Centronic BL-II microprocessor-controlled centrifuge (J.P. Selecta, Barcelona, Spain). The final extract volume was adjusted to 200 mL with DDW. Dry matter content was determined in five replicates using a Shimadzu MOC-120H moisture balance (Shimadzu, Kyoto, Japan). Aliquots of 1.5 mL were stored in Eppendorf tubes at −18 °C until further use.
2.1.2. Quantification of Bioactive Compounds
Total phenolic content was measured using the Folin–Ciocalteu method and expressed as mg gallic acid equivalent per g of extract (mg GAE/g) [28] (standard curve: y = 73.6907x − 0.0003, r2 = 0.998). Total flavonoids were quantified by a modified aluminum chloride colorimetric assay and expressed as mg rutin equivalent per g of extract (mg RUE/g) [29] (standard curve: y = 10.28087x − 0.00623, r2 = 0.997). Carbohydrate content was determined using the phenol–sulfuric acid method [29,30] (standard curve: y = 1.5218x − 0.023275, r2 = 0.992). All results are reported as mg per g of extract’s dry matter.
2.1.3. HPLC Analysis
A gradient reversed-phase high-performance liquid chromatography (HPLC) system (Dionex UltiMate 3000 Quaternary Analytical System with diode array detector; Germering, Germany) equipped with a Dionex Acclaim 120 C18 column (5 μm, 250 × 4.6 mm) maintained at 25 °C was used for extract characterization. Samples were filtered through 0.45 μm nylon membrane syringe filters (Whatman Puradisc 13, Cytiva, Buckinghamshire, UK) prior to injection. The mobile phases consisted of water with 0.1% formic acid (A) and acetonitrile with 0.1% formic acid (B). The flow rate was 1.0 mL min−1, and the gradient program was as follows: 0 min, 5% B; 0–5 min, 5–15% B; 5–50 min, 15–28% B; 50–57 min, 28–40% B; 57–60 min, 40–100% B; 60–63 min, 100% B; 63–65 min, 100–5% B; 65–70 min, 5% B. Detection wavelengths were set at 280, 320, and 350 nm.
Peaks were identified based on retention times and UV–Vis spectra (200–500 nm) and compared with commercially available standards: caffeic acid (3,4-dihydroxycinnamic acid; Acros Organics, Geel, Belgium), rosmarinic acid (Sigma-Aldrich, St. Louis, MI, USA), eriocitrin (eriodictyol-7-O-rutinoside; Extrasynthese, Genay, France), isorhoifolin (apigenin-7-O-rutinoside; Extrasynthese, Genay, France), and hesperidin (hesperetin-7-O-rutinoside; Extrasynthese, Genay, France). Quantification of individual phenolics was performed using the external standard calibration method and expressed as μg per g of extract’s dry matter.
The glycosides of luteolin and apigenin were quantified using the calibration lines of luteolin (λ = 350 nm; y = 95.528x − 0.8747, r2 = 0.999) and apigenin (λ = 320 nm; y = 94.886x + 0.573, r2 > 0.999), respectively. Hesperidin was quantified at λ = 280 nm using its standard calibration line (y = 150.13x + 0.2838, r2 > 0.999). Eriocitrin and narirutin were measured at λ = 280 nm using the eriocitrin standard, with narirutin expressed as eriocitrin equivalents. Caffeic acid and rosmarinic acid were evaluated at λ = 320 nm using the calibration lines of the corresponding standards. Derivatives of caffeic acid and 3-(3,4-dihydroxyphenyl)-lactic acid were quantified using the caffeic acid standard and expressed in caffeic acid equivalents. All measurements were performed in triplicate.
2.1.4. Preparation and Characterization of Nanoparticles
AgNPs and AuNPs were synthesized at ambient laboratory temperature (20–23 °C) by directly mixing diluted peppermint extract with 1 mM aqueous solutions of AgNO3 (analytical grade, Mikrochem, Penzinok, Slovakia) or HAuCl4 (analytical grade, Merk, Bratislava, Slovakia) under continuous stirring. Based on the dry matter content, the initial extract was diluted with DDW to a concentration of 0.5 mg·mL−1. Equal volumes of the 0.5 mg·mL−1 peppermint extract and 1 mM metal salt solutions were then combined to yield final starting concentrations of 0.5 mM Ag+ or Au3+.
The optical properties of the prepared nanocolloids were characterized by UV–Vis spectroscopy using a Shimadzu UV-1800 spectrophotometer (Shimadzu Corporation, Kyoto, Japan) over the wavelength range of 350–1100 nm, employing 1 cm disposable semi-micro cuvettes (Brand, Wertheim, Germany). The morphology of the nanoparticles was examined by transmission electron microscopy (TEM) using a JEOL JEM-2100F microscope (JEOL Ltd., Yamagata, Japan) operated at an accelerating voltage of 200 kV. Selected-area electron diffraction (SAED) patterns were recorded with a post-column GIF TRIDIEM energy filter (Energy resolution = 0.7 eV, CCD image filter: 2 k × 2 k) attached to the microscope.
Attenuated total reflectance Fourier transform infrared (ATR–FTIR) spectra of the air-dry extract, AuNPs, and AgNPs deposited on Parafilm® M films were collected in the spectral range of 700–4000 cm−1, averaging 64 scans at a resolution of 2 cm−1. Measurements were carried out on a Shimadzu Prestige 21 spectrometer (Shimadzu Corporation, Kyoto, Japan) equipped with a PIKE single-reflection ATR accessory and a ZnSe crystal (PIKE Technologies, Madison, WI, USA).
The hydrodynamic particle size distribution of the nanocolloids was determined by dynamic light scattering (DLS) using a Zetasizer Nano ZS instrument (Malvern Instruments, Malvern, UK).
2.2. Bacterial Strains
To assess the effect of metal nanoparticles on microbial growth, the following bacterial strains were used: Brevundimonas vesicularis USM1 (GenBank accession no. JABTYI000000000), Pseudarthrobacter oxydans USM2 (GenBank accession no. JABTYH000000000), and Pseudomonas putida USM4 (GenBank accession no. JABTYF000000000) [25]. These strains have previously been shown to resist various toxic metal ions (Co2+, Cu2+, etc.) at concentrations ranging from 100 to 2500 ppm [26]. Since metals in NP form exhibit distinct physical and chemical properties compared to their ionic forms, this study aimed to determine whether these metal-resistant strains could grow in the presence of AgNPs and AuNPs.
These bacterial strains were selected because their established resistance to toxic metal ions makes them suitable model organisms for investigating potential differences in microbial responses to metals in nanoparticulate versus ionic forms. This approach enables the identification of nanoparticle-specific effects that extend beyond the general mechanisms of metal-ion resistance.
2.3. Growth Assay in the Presence of Nanoparticles
Microbial growth in the presence of metal nanoparticles was monitored via optical density measurements in peptone broth (PB; BioMaxima S.A., Lublin, Poland). The experimental setup included control cultures without nanoparticles and cultures with 0.125 or 0.25 mM of AgNPs or AuNPs. Pre-cultivated bacterial strains (24 h) were adjusted to optical density at 600 nm (OD600), OD600 ≈ 1.0 in a sterile 0.85% NaCl solution, and used as inoculum (10 µL per well). Each well contained 100 µL of nutrient medium, and the 96-well microtiter plates (TPP—Techno Plastic Products AG, Trasadingen, Switzerland) were incubated at 25 °C for 24 h. OD600 was measured hourly using a SPECTROstar Nano microplate reader (BMG LABTECH, Ortenberg, Germany), and growth curves were analyzed using MARS data analysis software 4.01 R2 (BMG LABTECH, Ortenberg, Germany).
2.4. Cell Viability Assay Before and After the Growth in the Presence of Metal Nanoparticles
Cell viability was evaluated based on the number of colony-forming units (CFUs) obtained on peptone agar plates. For this purpose, 10-fold serial dilutions of microbial suspensions were prepared, and 50 µL of each dilution was plated on peptone agar (PA; BioMaxima S.A., Lublin, Poland). CFUs were counted before cultivation in the 96-well plates and after 24 h of growth. All experiments were performed in triplicate.
2.5. Data Analysis
All experiments were conducted in triplicate. Statistical analysis was performed using MARS data analysis software 4.01 R2 (BMG LABTECH, Ortenberg, Germany). Differences between datasets (grouped by metal treatment and control) were assessed using one-way ANOVA followed by multiple comparisons with the Tukey–Kramer test. A significance level of p < 0.05 was applied.
3. Results
3.1. Properties of Phytosynthesized Silver and Gold Nanoparticles
The increasing demand for metallic NPs across diverse fields has driven the development of efficient and sustainable synthesis methods. Different applications impose distinct requirements on metal NPs: for instance, size and shape are critical for optical and catalytic properties, while biocompatibility and low toxicity are essential for biomedical uses. Among the various synthesis strategies, including physical, chemical, bottom-up, and top-down approaches, green synthesis has attracted particular attention. This approach emphasizes the use of non-toxic reagents and the avoidance of harmful byproducts. A subset of green synthesis known as phytosynthesis employs plant extracts as both reducing and capping agents, offering a biologically inspired route to NP formation.
Despite the advantages of phytosynthesis – such as cost- and time-efficiency, and the wide availability and diversity of phytochemicals –several challenges remain [31]. Reproducibility is often limited by the complex chemical composition of plant extracts, which varies with harvest location, season, and environmental conditions, and by the incomplete understanding of the mechanisms of NP formation in phytochemical environments.
To better understand the potential of peppermint extract for NP synthesis, the concentrations of key bioactive compounds were quantified. Aqueous peppermint leaf extract prepared according to [27] contained 5.141 mg·mL−1 of carbohydrates, 1.142 mg·mL−1 of total phenolics (expressed as gallic acid equivalents), and 12.769 mg·mL−1 of total flavonoids (expressed as rutin equivalents).
The phenolic composition of Mentha × piperita extract was further characterized using HPLC-DAD. Based on chromatographic and spectroscopic data, comparison with commercially available standards, and literature reports [32], fourteen phenolic compounds were identified and quantified (Figure 1, Table 1). The major compounds detected, frequently reported in peppermint, included caffeic acid, eriocitrin, luteolin-7-O-rutinoside, isorhoifolin, hesperidin, and rosmarinic acid. Among these, eriocitrin and rosmarinic acid were quantitatively dominant, while the relative abundance of luteolin-7-O-rutinoside and hesperidin varied depending on plant origin and extraction method [33,34].
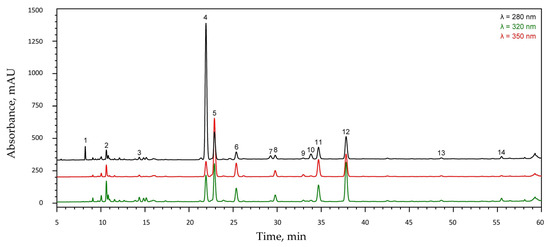
Figure 1.
HPLC chromatogram of the aqueous extract of Mentha × piperita cv. ‘Kristinka’ leaves. Peak numbers correspond to the compounds listed in Table 1.

Table 1.
Phenolic compounds in the Mentha × piperita cv. ‘Kristinka’ leaf extract (numbering of peaks related to Figure 1).
Flavonoids constituted the majority (67%) of the total phenolic compounds in the analyzed extract. Eriocitrin was the most abundant component, accounting for 43% of the total metabolite content, followed by luteolin-7-O-rutinoside (20%), rosmarinic acid (11%), and two unidentified salvianolic acids (peaks 6 and 11, approximately 8% each).
The FTIR spectrum of the peppermint extract (Figure S1) exhibits a broad absorption band in the 3300–3400 cm−1 region, corresponding to the O–H stretching vibrations of hydroxyl groups, primarily originating from water and phenolic compounds. Weak bands observed between 2700 and 2900 cm−1 are assigned to the C–H stretching vibrations of aromatic and aliphatic groups. A strong absorption band at 1591 cm−1 is attributed to the C=C stretching vibrations in phenyl rings and pyranone cycles, while the intense band at 1404 cm−1 arises from the bending vibrations of C–O–H groups. The prominent bands at 1262 cm−1 and 1074 cm−1 correspond to the C–O stretching vibrations of aryl–alkyl ether linkages commonly present in polyphenolic compounds. A medium-intensity band at 817 cm−1 is associated with the C=C bending vibrations of aromatic rings. In the spectra of extract-mediated AuNPs and AgNPs (Figure S1), these characteristic peaks undergo noticeable shifts and intensity changes, particularly in the O–H and C=O regions, suggesting the involvement of hydroxyl and carbonyl groups in the reduction of metal ions. The persistence of C–O-related bands indicates the presence of organic residues adsorbed on the nanoparticle surfaces, confirming the role of phytochemicals as both reducing and capping agents. These spectral modifications demonstrate that biomolecules within the peppermint extract facilitate the bioreduction of Au3+ and Ag+ ions while stabilizing the resulting colloids through surface functionalization.
The obtained nanocolloid solutions exhibited a pink color for AuNPs and a brown color for AgNPs. These colors arise from surface plasmon resonance (SPR) absorption, typically expected at 400–430 nm for AgNPs and 520–580 nm for AuNPs. However, the UV–Vis spectra of the prepared samples show notable deviations (Figure 2). In particular, the AuNP spectrum displays two broad absorption maxima at 560 and 790 nm, which can be attributed to the coexistence of spherical and irregularly shaped NPs, as reported previously [27] and confirmed by TEM images (Figure 3a). The synthesized AuNPs are polydisperse, comprising pseudospherical nanoparticles of 10–40 nm and some larger crystalline shapes (triangular/hexagonal facets) (Figure 3a).
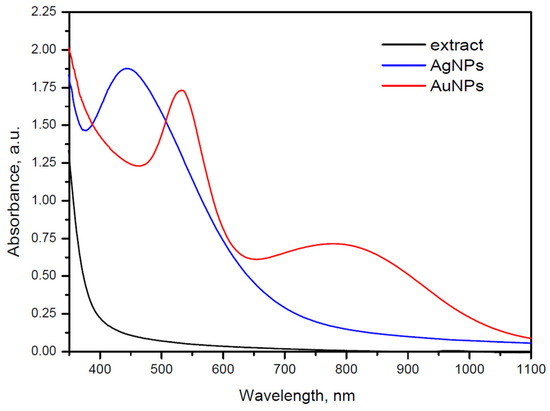
Figure 2.
UV–Vis spectra of peppermint extract (black) and peppermint extract-mediated AgNPs (blue) and AuNPs (red).
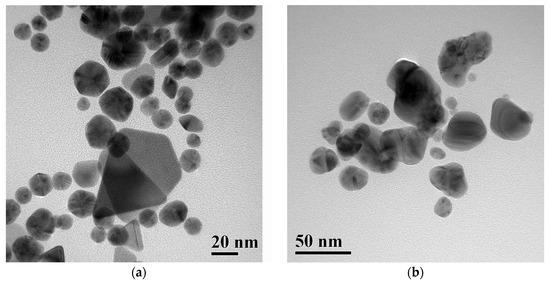
Figure 3.
Transmission electron microscopy (TEM) images of peppermint extract-mediated AuNPs (a) and AgNPs (b).
The hydrodynamic size distribution obtained from DLS (Figure S2a) exhibited a primary peak centered around 25–40 nm and minor contributions from larger aggregates (200–800 nm). These results are consistent with the TEM observations showing predominantly quasi-spherical AuNPs with core diameters of 5–15 nm and occasional larger faceted particles. The slight shift toward larger values in DLS is expected because the hydrodynamic diameter includes the organic stabilizing layer and any solvation effects, and because the intensity-weight distribution emphasizes larger particles. Thus, the DLS and TEM data collectively indicate the formation of small, moderately polydisperse gold nanoparticles with limited aggregation in colloidal suspension.
The UV–Vis spectrum of AgNPs (Figure 2) exhibits a single SPR maximum at 450 nm. This band is relatively broad compared to the SPR maximum of AuNPs, indicating a higher degree of polydispersity. TEM images of AgNPs (Figure 3b) confirm that the broadening of the SPR band results from light absorption by polydisperse, pseudospherical metal NPs deviating from the ideal spherical shape. The particle size predominantly falls within the range of 30–50 nm (Figure 3b).
Most AgNPs are in the 20–40 nm range, with several larger faceted particles exceeding 50 nm, confirming the polydisperse and partially aggregated morphology. The main DLS peak (~20 nm) agrees well with the TEM-measured core size (10–25 nm), confirming the presence of small, stable AgNPs (see Figure 3b and Figure S2b). The higher-size modes (>100 nm) arise from agglomeration in suspension or transient cluster formation, common in plant-extract-mediated AgNPs. Overall, the TEM and DLS data are in good agreement, differing only by the expected hydrodynamic broadening.
In the SAED patterns of both AuNPs and AgNPs, distinct bright diffraction spots superimposed on concentric rings are observed, indicating the coexistence of crystalline domains with preferred orientations and polycrystalline regions. These features confirm the nanocrystalline nature of the Au and Ag particles, characterized by randomly oriented crystallites. The diffraction rings can be indexed to the (111), (200), (220), and (311) planes of the face-centered cubic (FCC) structure, consistent with the standard reference data (JCPDS No. 04-0784 for Au and No. 04-0783 for Ag).
3.2. The Effect of Silver Nanoparticles on the Growth Patterns of Microorganisms
The analysis of the growth curves revealed that AgNPs inhibited the growth of the studied strains to varying degrees. The effect of AgNPs on Brevundimonas vesicularis USM1 remained ambiguous, as the growth curves showed similar increases in optical density under all conditions (Figure 4a). However, in PB medium without metal NPs, the optical density began to decrease after 12 h of cultivation. The persistently high OD600 values observed in the presence of 0.125 mM and 0.25 mM AgNPs may indicate the metal NP precipitation rather than biomass accumulation. This suggests that the strain is capable of decomposing the organic ligands stabilizing the metal NPs, thereby promoting their aggregation in solution.
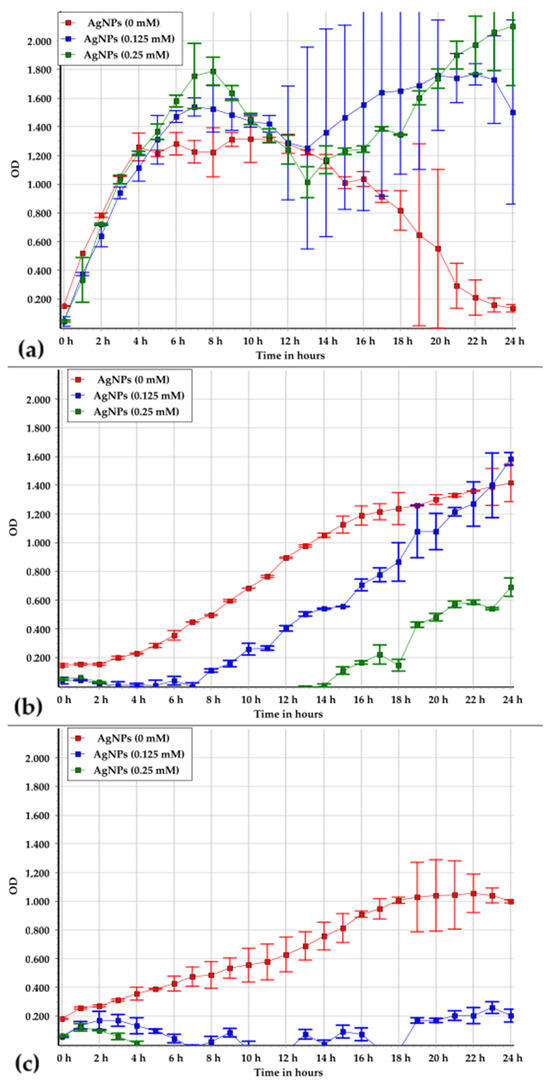
Figure 4.
Growth patterns of Brevundimonas vesicularis USM1 (a), Pseudarthrobacter oxydans USM2 (b), and Pseudomonas putida USM4 (c) in PB medium at AgNP concentrations of 0, 0.125, and 0.25 mM.
Pseudarthrobacter oxydans USM2 was shown to be sensitive to AgNPs (Figure 4b). After 24 h of cultivation, biomass accumulation was similar in PB medium without nanoparticles and in the presence of 0.125 mM AgNPs; however, the lag phase in PB was 2.3-fold shorter. At 0.25 mM AgNPs, biomass growth decreased by approximately 50%, and the lag phase was shortened by 4.7-fold, indicating a pronounced stress response.
Pseudomonas putida USM4 exhibited the highest sensitivity to AgNPs (Figure 4c). In the presence of silver, an increase in optical density was observed only during the first two hours of cultivation, after which growth ceased entirely.
Statistical analysis confirmed the inhibitory effect of AgNPs on the studied strains (Figure 5). Multiple comparison analysis of growth dynamics revealed that B. vesicularis USM1 exhibited a significant difference between growth without metal NPs and with 0.25 mM AgNPs (Figure 5a), whereas no significant differences were found between the 0 mM, 0.125 mM, and 0.25 mM conditions.
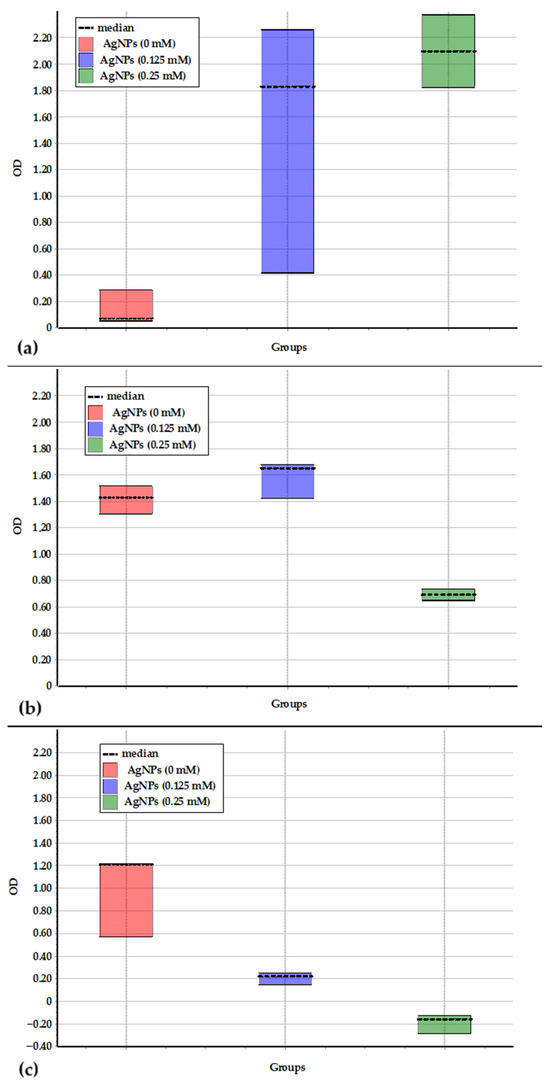
Figure 5.
Difference between data sets obtained during the growth of Brevundimonas vesicularis USM1 (a), Pseudarthrobacter oxydans USM2 (b), and Pseudomonas putida USM4 (c) in PB medium at AgNPs concentrations of 0, 0.125, and 0.25 mM.
For P. oxydans USM2, significant differences were observed between the growth without metal NPs and with 0.25 mM AgNPs, as well as between 0.125 mM and 0.25 mM AgNPs (Figure 5b). No significant difference was detected between growth without metal NPs and with 0.125 mM AgNPs, confirming the strain’s resistance to 0.125 mM but sensitivity to 0.25 mM AgNPs.
P. putida USM4 showed statistically significant differences between the growth data in PB medium without metal NPs and in the presence of both 0.125 mM and 0.25 mM AgNPs (Figure 5c), confirming its high sensitivity to AgNPs as an extreme environmental factor.
Thus, the obtained experimental data showed that Brevundimonas vesicularis USM1 can induce the precipitation of AgNPs; however, its resistance to them remains uncertain. Pseudarthrobacter oxydans USM2 demonstrated resistance to 0.125 mM AgNPs, although its lag phase was longer compared to growth without metal NPs. In contrast, growth was inhibited at 0.25 mM AgNPs. Pseudomonas putida USM4 was found to be sensitive to all tested concentrations of AgNPs.
3.3. The Growth of Microorganisms in the Presence of Gold Nanoparticles
The analysis of the growth dynamics of the studied strains in the presence of AuNPs revealed distinct patterns. Brevundimonas vesicularis USM1 was shown to induce the precipitation of gold; therefore, the observed effects of metal NPs on its growth remain uncertain (Figure 6a). Statistical analysis indicated a significant difference between the growth data obtained in the absence and presence of AuNPs (Figure 7a). However, this difference is likely attributable to metal NP precipitation rather than genuine metal resistance.
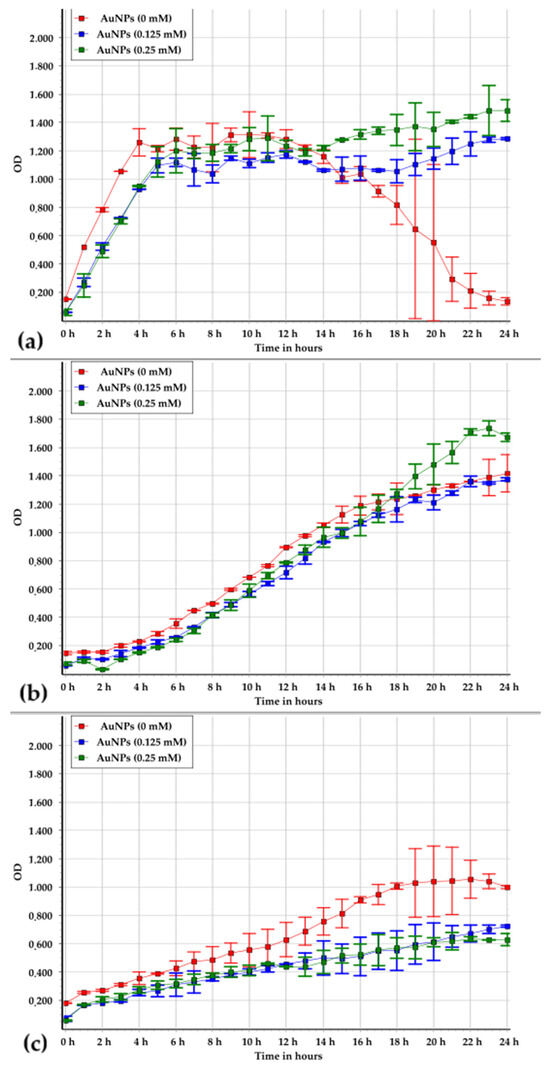
Figure 6.
Growth patterns of Brevundimonas vesicularis USM1 (a), Pseudarthrobacter oxydans USM2 (b), and Pseudomonas putida USM4 (c) in PB medium at Au NP concentrations of 0, 0.125, and 0.25 mM.
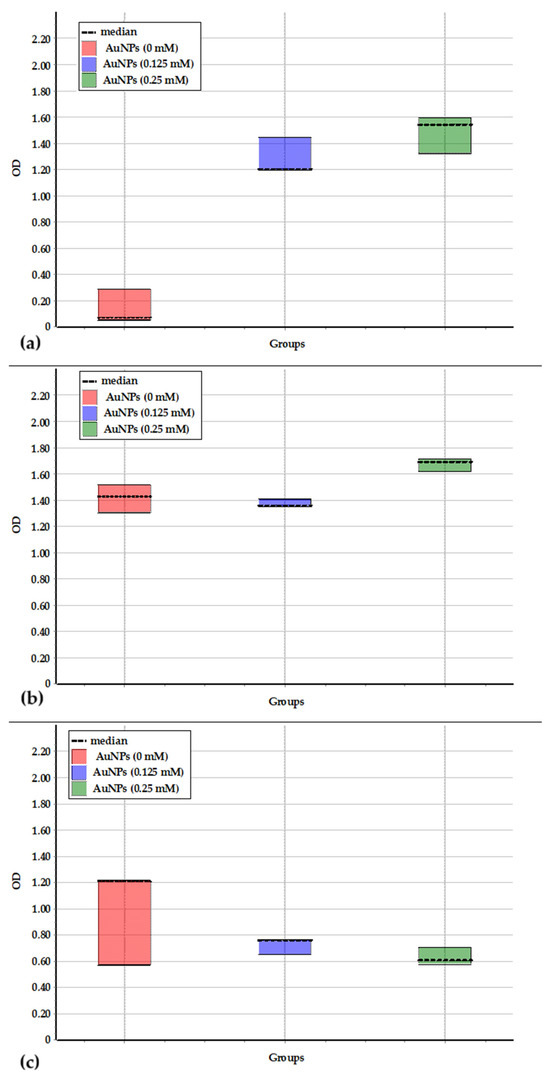
Figure 7.
Difference between data sets obtained during the growth of Brevundimonas vesicularis USM1 (a), Pseudarthrobacter oxydans USM2 (b), and Pseudomonas putida USM4 (c) in PB medium containing AuNPs at concentrations of 0, 0.125, and 0.25 mM.
Pseudarthrobacter oxydans USM2 exhibited tolerance to AuNPs at both 0.125 mM and 0.25 mM, as indicated by similar biomass growth dynamics under these conditions. Statistical analysis confirmed no significant difference between the data sets for growth in PB medium without NPs and at 0.125 mM AuNPs (Figure 7b). The slight deviation observed at 0.25 mM may be associated with partial NP precipitation, which could have contributed to the apparent increase in optical density.
In the case of Pseudomonas putida USM4, the increase in optical density during cultivation in the presence of AuNPs was lower than that observed in PB medium alone (Figure 6c). Nevertheless, statistical analysis revealed no significant difference between the growth data with and without AuNPs (Figure 7c).
Overall, the growth curve analysis demonstrated that the presence of AuNPs did not exert a significant inhibitory effect on any of the studied microorganisms.
3.4. The Number of Viable Microorganisms in the Presence of Metal Nanoparticles
The results demonstrated whether the increase in optical density of the medium was due to microbial growth or to the precipitation of metal NPs (Figure 8). The initial microbial counts in the medium before cultivation ranged from n × 106 to n × 107 CFU·mL−1. After 24 h of cultivation in 96-well microtiter plates, followed by plating on PA, no growth of Brevundimonas vesicularis USM1 was observed. In contrast, Pseudarthrobacter oxydans USM2 reached from n × 109 to n × 1010 CFU·mL−1, while Pseudomonas putida USM4 reached n × 107 CFU·mL−1.
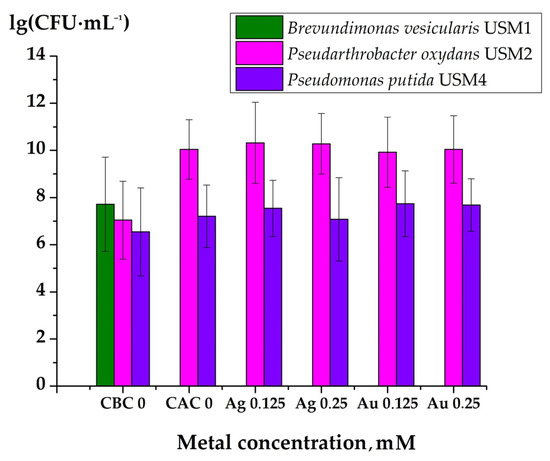
Figure 8.
Number of microorganisms before the cultivation in 96-well microtiter plates and after 24 h of growth: CBC 0—control sample without metal NPs before cultivation; CAC 0—control sample without metal NPs after cultivation; Ag 0.125, Ag 0.25, Au 0.125, and Au 0.25 represent samples containing AgNPs or AuNPs at concentrations ranging from 0.125 mM to 0.25 mM, respectively.
Comparative analysis of the growth curves and the number of CFU per milliliter of culture medium revealed that Brevundimonas vesicularis USM1 may be affected by AgNPs and AuNPs, although it was capable of precipitating these compounds. Since the optical density of the medium without metal NPs increased similarly to that with metal NPs, the increase was attributed to microbial growth rather than NP precipitation. However, in the absence of metal NPs, the growth curve showed that the stationary phase occurred between 4 and 12 h, followed by cell lysis and a decrease in optical density. This was confirmed by plating the microbial suspensions on PA before and after cultivation: the initial number of microorganisms was 5.2 × 107 CFU·mL−1, while no viable cells were detected after 24 h. Thus, B. vesicularis USM1 demonstrated the ability to precipitate AgNPs and AuNPs, but their effects on the cells require further investigation.
Analysis of the Pseudarthrobacter oxydans USM2 growth curves and CFU counts supported these findings. This strain was shown to be sensitive to AgNPs, whereas AuNPs had no observable inhibitory effect. After 24 h of cultivation, the number of microbial cells increased both in the presence and absence of metal NPs.
Plating results also confirmed the effect of AgNPs on the biomass increase of Pseudomonas putida USM4 and the absence of growth inhibition in the presence of AuNPs.
4. Discussion
The toxicity of NP colloidal solutions depends not only on the intrinsic properties of the NPs but also on the characteristics of the capping agents that stabilize the nanocolloidal system, residual reducing agents used during NP synthesis, and the solvent employed. In the present study, aqueous peppermint (Mentha × piperita) leaf extract was used for the green synthesis of AgNPs and AuNPs. The extract concentration in the obtained NP solutions was 250 mg·L−1. For microbiological experiments, these solutions were diluted two- and fourfold with growth medium to achieve nanometal concentrations of 0.25 and 0.125 mM, respectively. According to literature data, peppermint extracts exhibit moderate antimicrobial activity against microorganisms such as Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa, with minimum inhibitory concentrations (MICs) of 400, 500, and 600 mg·L−1, respectively [35]. A related study involving S. aureus, Salmonella typhimurium, P. aeruginosa, and E. coli reported similar MIC values (~400 mg·L−1) for peppermint stem and leaf extracts [36]. Thus, aqueous peppermint extracts demonstrate relatively weak antimicrobial activity [37]. Considering the extract concentrations used in this study, their effect on the growth of the tested microorganisms was deemed negligible.
It is well established that AgNPs exert strong inhibitory effects on microorganisms. Among them, Pseudomonas putida is one of the most frequently studied bacterial species due to its notable resistance to silver. Although the growth of P. putida is generally inhibited by AgNPs [38], the degree of resistance varies depending on the strain. For instance, the minimal inhibitory concentration of AgNPs for P. putida mt-2 was reported to be 620 mg·L−1 (5.58 mM) [39], whereas another study demonstrated strong antimicrobial activity at concentrations ranging from 90 mg·L−1 (0.81 mM) to 170 (1.53 mM) mg·L−1 [40]. P. aeruginosa was shown to be inhibited by AgNPs at 75 μg·mL−1 (0.675 mM) [19]. In the present work, biomass accumulation of Pseudomonas putida USM4 was inhibited; however, the cells remained viable, as confirmed by subsequent growth on PA plates. Information regarding the resistance of Pseudarthrobacter oxydans and Brevundimonas vesicularis to silver nanoparticles is scarce in the literature. The current study thus provides new insight into the resistance levels of these lesser-studied microorganisms, opening avenues for further investigation. The Pseudarthrobacter oxydans USM2 strain exhibited considerable resistance to AgNPs at 0.125 mM, while biomass accumulation was inhibited at 0.25 mM without causing complete cell death, as confirmed by regrowth on PA following cultivation in the presence of AgNPs. The case of Brevundimonas vesicularis USM1 was more complex. Its growth curve showed an initial increase in optical density during the first 24 h, followed by cell lysis and a subsequent decrease in optical density even in the absence of metal. In the presence of AgNPs, silver precipitation was observed, suggesting an interaction involving organic ligand decomposition and silver destabilization. No regrowth on PA was detected after exposure, either with or without AgNPs, indicating that the cells could interact with and transform silver before lysis. This behavior is particularly noteworthy, as it suggests a potential for Brevundimonas vesicularis USM1 to participate in biogenic silver precipitation and detoxification, which may be useful in environmental biotechnological applications such as NP recovery and wastewater purification.
The resistance of the studied microorganisms to AuNPs was confirmed by experimental data. Brevundimonas vesicularis USM1 exhibited growth dynamics similar to those observed in the presence of AgNPs and induced visible precipitation of AuNPs, likely due to the decomposition of organic ligands and subsequent destabilization of the colloid. The strains Pseudarthrobacter oxydans USM2 and Pseudomonas putida USM4 demonstrated comparable growth behavior, showing no significant inhibition upon exposure to AuNPs.
These findings are in good agreement with literature reports indicating that AuNPs generally exert minimal antimicrobial activity [41]. Previous studies have shown that Pseudomonas putida strains display no measurable response to AuNPs [42], while Pseudomonas aeruginosa also exhibits resistance to their presence [41]. However, no published data were found regarding the effects of AuNPs on Brevundimonas vesicularis or Pseudarthrobacter oxydans. Thus, the present results help to fill an existing gap in the understanding of microbial interactions with gold nanoparticles, particularly for these lesser-studied bacterial strains.
The increasing release of engineered metal NPs into the atmosphere, aquatic environments, and soils raises serious ecological concerns. As non-degradable and persistent materials, NPs can accumulate in environmental compartments, interact with living organisms, and potentially disrupt ecosystem balance. Although numerous studies have demonstrated the toxicity of metal-based NPs toward aquatic and soil microorganisms, a comprehensive understanding of their environmental fate, transformation pathways, and long-term effects in terrestrial ecosystems remains limited. This knowledge gap underscores the need for expanded research and improved regulatory frameworks to ensure the safe use and disposal of nanomaterials. Nanometals are introduced into soils through agricultural applications, atmospheric deposition, and wastewater discharge, where they exhibit low mobility and tend to accumulate. Once in the soil matrix, NPs interact with organic and inorganic constituents, influencing soil aggregation, water retention, and microbial activity. Such interactions can lead to the generation of reactive oxygen species (ROS), disruption of microbial membranes, enzyme inactivation, and DNA damage, ultimately reducing microbial biomass, diversity, and ecological stability. The severity of these effects depends on NP type, concentration, surface chemistry, and soil physicochemical conditions [43]. Similarly, in aquatic systems, metal NPs can be highly toxic to microorganisms that play critical roles in nutrient cycling and the marine food web. While many bacterial species possess adaptive defense mechanisms, excessive ROS production induced by NP exposure can compromise membrane integrity, alter permeability, and lead to cell death [44].
In light of these findings, the establishment of comprehensive risk assessment frameworks and centralized databases capturing the physicochemical properties, environmental behavior, and toxicity profiles of nanomaterials is essential. Continuous monitoring and regulation of NP emissions into environmental media, coupled with strategies to stabilize reactive surfaces and minimize ROS generation, may effectively mitigate ecological risks. Furthermore, systematic classification of nanomaterials by size, morphology, and reactivity, together with focused studies on bioaccumulation and mechanistic toxicity, will be critical for guiding safer nanotechnology practices and informed regulatory decisions [45].
The results obtained in this study also demonstrate that certain microorganisms can contribute to metal detoxification by converting soluble metal ions into insoluble, biologically inert forms. In particular, the strain Brevundimonas vesicularis USM1 was found to promote the precipitation of dissolved metal species, indicating its potential for biotransformation and immobilization of toxic metals. These findings provide a promising foundation for the development of environmentally sustainable biotechnological strategies aimed at removing metals from biogeochemical cycles and mitigating NP-related pollution.
5. Conclusions
AgNPs generally inhibit microbial growth, although the degree of resistance varies among strains. The Pseudomonas putida USM4 strain showed reduced biomass accumulation in the presence of AgNPs; however, the cells remained viable, as confirmed by subsequent growth on Petri agar plates. Similarly, Pseudarthrobacter oxydans USM2 exhibited resistance to AgNPs at 0.125 mM, while biomass accumulation was inhibited at 0.25 mM without complete cell death. In Brevundimonas vesicularis USM1, initial growth was followed by cell lysis, and the strain demonstrated the ability to interact with and precipitate silver, suggesting potential applications in environmental biotechnology. In contrast, AuNPs did not significantly inhibit Pseudomonas putida USM4 or Pseudarthrobacter oxydans USM2. Brevundimonas vesicularis USM1 also precipitated gold, likely through organic ligand decomposition, which aligns with the generally minimal impact of AuNPs on microorganisms. These findings provide a foundation for further research into the mechanisms of interaction between microorganisms and metal nanoparticles.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su172210232/s1, Figure S1: FTIR spectra of peppermint extract and extract mediated AuNPs and AgNPs; Figure S2: DLS measurements for peppermint extract mediated gold (a) and silver (b) nanoparticles; Figure S3: Selected area electron diffraction (SAED) patterns of gold (left) and silver (right) NPs.
Author Contributions
Conceptualization, O.T. and R.M.; methodology, O.T., V.H. and R.M.; software, V.H., I.B. and R.M.; validation, I.B., H.M. and J.M.-F.; formal analysis, R.M.; investigation, V.H., I.B., R.M., R.S., A.E., V.V.L., L.M.G., H.M., J.M.-F., E.M. and O.T.; resources, E.M. and R.M.; data curation, R.M.; writing—original draft preparation, V.H. and R.M.; writing—review and editing, V.H., I.B., R.M., R.S., A.E., V.V.L., L.M.G., H.M., J.M.-F., E.M. and O.T.; visualization, V.H. and R.M.; supervision, R.M., O.T. and E.M.; project administration, O.T. and R.M.; funding acquisition, O.T. and R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Polish National Agency for Academic Exchange (NAWA) under the NAWA Joint Research Projects programme between the Republic of Poland and Slovakia, grant number BPN/BSK/2023/1/00027/U/00001 and the Slovak Research and Development Agency, grant number APVV SK-PL-23-0032.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mazari, S.A.; Ali, E.; Abro, R.; Khan, F.S.A.; Ahmed, I.; Ahmed, M.; Nizamuddin, S.; Siddiqui, T.H.; Hossain, N.; Mubarak, N.M.; et al. Nanomaterials: Applications, Waste-Handling, Environmental Toxicities, and Future Challenges—A Review. J. Environ. Chem. Eng. 2021, 9, 105028. [Google Scholar] [CrossRef]
- He, L.-B.; Zhang, L.; Tang, L.-P.; Sun, J.; Zhang, Q.-B.; Sun, L.-T. Novel Behaviors/Properties of Nanometals Induced by Surface Effects. Mater. Today Nano 2018, 1, 8–21. [Google Scholar] [CrossRef]
- Kolbert, Z.; Szőllősi, R.; Rónavári, A.; Molnár, Á. Nanoforms of Essential Metals: From Hormetic Phytoeffects to Agricultural Potential. J. Exp. Bot. 2022, 73, 1825–1840. [Google Scholar] [CrossRef]
- Wu, Q.; Miao, W.; Zhang, Y.; Gao, H.; Hui, D. Mechanical Properties of Nanomaterials: A Review. Nanotechnol. Rev. 2020, 9, 259–273. [Google Scholar] [CrossRef]
- Agnihotri, R.; Gaur, S.; Albin, S. Nanometals in Dentistry: Applications and Toxicological Implications—A Systematic Review. Biol. Trace Elem. Res. 2020, 197, 70–88. [Google Scholar] [CrossRef] [PubMed]
- Azooz, E.A.; Abduladheem, N.A.; El Abbadi, N.K. An Overview of the Green Synthesis of Coinage Metallic Nanoparticles: Protocols, Challenges, and Cancer Treatments. Nano LIFE 2025, 15, 2430011. [Google Scholar] [CrossRef]
- Al-Harbi, N.; Abd-Elrahman, N.K. Physical Methods for Preparation of Nanomaterials, Their Characterization and Applications: A Review. J. Umm Al-Qura Univ. Appl. Sci. 2025, 11, 356–377. [Google Scholar] [CrossRef]
- Srinivasan, L.V.; Rana, S.S. A Critical Review of Various Synthesis Methods of Nanoparticles and Their Applications in Biomedical, Regenerative Medicine, Food Packaging, and Environment. Discov. Appl. Sci. 2024, 6, 371. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef]
- Edo, G.I.; Mafe, A.N.; Ali, A.B.M.; Akpoghelie, P.O.; Yousif, E.; Isoje, E.F.; Igbuku, U.A.; Zainulabdeen, K.; Owheruo, J.O.; Essaghah, A.E.A.; et al. Eco-Friendly Nanoparticle Phytosynthesis via Plant Extracts: Mechanistic Insights, Recent Advances, and Multifaceted Uses. Nano TransMed 2025, 4, 100080. [Google Scholar] [CrossRef]
- Hossain, A.; Manik, M.H.; Rakib, S.; Mahmud, N.; Khan, S.; Ahsan, Z.; Islam, M.S.; Hossain, N.; Akter, M.A. Green Nanotechnology for Implantable Biosensors: Biocompatibility and Functional Integration in Medical Applications. Biosens. Bioelectron. X 2025, 27, 100678. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, C.; Zhang, X.; Liu, Y.; Li, Y.; Li, D.; Xi, Q. Plant-Synthesized Gold Nanotheranostics for Photothermal Treatment and Imaging of Breast Cancer: Harnessing Flavonoids for Dual-Functionality. Ind. Crops Prod. 2025, 235, 121718. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green Synthesis of Nanoparticles: Current Developments and Limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Prado-López, S.; González-Ballesteros, N.; Carmen Rodríguez-Argüelles, M. Nanometals in Cancer Diagnosis and Therapy. In Biomaterials in Clinical Practice: Advances in Clinical Research and Medical Devices; Zivic, F., Affatato, S., Trajanovic, M., Schnabelrauch, M., Grujovic, N., Choy, K.L., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 407–428. ISBN 978-3-319-68025-5. [Google Scholar]
- Mariychuk, R.; Porubská, J.; Ostafin, M.; Čaplovičová, M.; Eliašová, A. Green Synthesis of Stable Nanocolloids of Monodisperse Silver and Gold Nanoparticles Using Natural Polyphenols from Fruits of Sambucus nigra L. Appl. Nanosci. 2020, 10, 4545–4558. [Google Scholar] [CrossRef]
- Choudhary, A.; Singh, S.; Ravichandiran, V. Toxicity, Preparation Methods and Applications of Silver Nanoparticles: An Update. Toxicol. Mech. Methods 2022, 32, 650–661. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Effect of Size, Shape and Surface Functionalization on the Antibacterial Activity of Silver Nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef]
- Kailasa, S.K.; Park, T.-J.; Rohit, J.V.; Koduru, J.R. Chapter 14—Antimicrobial Activity of Silver Nanoparticles. In Nanoparticles in Pharmacotherapy; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 461–484. ISBN 978-0-12-816504-1. [Google Scholar]
- Ribeiro, L.G.; Roque, G.S.C.; Conrado, R.; De Souza, A.O. Antifungal Activity of Mycogenic Silver Nanoparticles on Clinical Yeasts and Phytopathogens. Antibiotics 2023, 12, 91. [Google Scholar] [CrossRef]
- Nie, P.; Zhao, Y.; Xu, H. Synthesis, Applications, Toxicity and Toxicity Mechanisms of Silver Nanoparticles: A Review. Ecotoxicol. Environ. Saf. 2023, 253, 114636. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Y.; Wei, Z.; Wang, L. Toxicity of Gold Nanoparticles Complicated by the Co-Existence Multiscale Plastics. Front. Microbiol. 2024, 15, 1447046. [Google Scholar] [CrossRef]
- Jarrar, Q.; Al-Doaiss, A.; Jarrar, B.M.; Alshehri, M. On the Toxicity of Gold Nanoparticles: Histological, Histochemical and Ultrastructural Alterations. Toxicol. Ind. Health 2022, 38, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shareena Dasari, T.P.; Deng, H.; Yu, H. Antimicrobial Activity of Gold Nanoparticles and Ionic Gold. J. Environ. Sci. Health Part C 2015, 33, 286–327. [Google Scholar] [CrossRef]
- Hovorukha, V.; Bhattacharyya, A.; Iungin, O.; Tashyreva, H.; Romanovska, V.; Havryliuk, O.; Bielikova, O.; Blackwell, C.; Burks, B.; Cothern, C.; et al. Draft Genome Sequences of Six Strains Isolated from the Rhizosphere of Wheat Grown in Cadmium-Contaminated Soil. Microbiol. Resour. Announc. 2020, 9, e00676-20. [Google Scholar] [CrossRef]
- Hovorukha, V.; Moliszewska, E.; Havryliuk, O.; Bida, I.; Tashyrev, O. Metal Resistance of Microorganisms as a Crucial Factor for Their Homeostasis and Sustainable Environment. Sustainability 2024, 16, 9655. [Google Scholar] [CrossRef]
- Mariychuk, R.; Smolková, R.; Bartošová, V.; Eliašová, A.; Grishchenko, L.M.; Diyuk, V.E.; Lisnyak, V.V. The Regularities of the Mentha piperita L. Extract Mediated Synthesis of Gold Nanoparticles with a Response in the Infrared Range. Appl. Nanosci. 2022, 12, 1071–1083. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Acree, T.E.; Decker, E.A.; Penner, M.H.; Reid, D.S.; Schwartz, S.J.; Shoemaker, C.F.; Smith, D.M.; Sporns, P. Handbook of Food Analytical Chemistry, Volume 1: Water, Proteins, Enzymes, Lipids, and Carbohydrates; John Wiley & Sons: Hoboken, NJ, USA, 2005; ISBN 978-0-471-70909-1. [Google Scholar]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Liu, D.; Wong, P.T.S.; Dutka, B.J. Determination of Carbohydrate in Lake Sediment by a Modified Phenol-Sulfuric Acid Method. Water Res. 1973, 7, 741–746. [Google Scholar] [CrossRef]
- Ahmad, T.; Iqbal, J.; Bustam, M.A.; Irfan, M.; Anwaar Asghar, H.M. A Critical Review on Phytosynthesis of Gold Nanoparticles: Issues, Challenges and Future Perspectives. J. Clean. Prod. 2021, 309, 127460. [Google Scholar] [CrossRef]
- Bodalska, A.; Kowalczyk, A.; Włodarczyk, M.; Fecka, I. Analysis of Polyphenolic Composition of a Herbal Medicinal Product—Peppermint Tincture. Molecules 2020, 25, 69. [Google Scholar] [CrossRef]
- Areias, F.M.; Valentão, P.; Andrade, P.B.; Ferreres, F.; Seabra, R.M. Phenolic Fingerprint of Peppermint Leaves. Food Chem. 2001, 73, 307–311. [Google Scholar] [CrossRef]
- Hudz, N.; Kobylinska, L.; Pokajewicz, K.; Horčinová Sedláčková, V.; Fedin, R.; Voloshyn, M.; Myskiv, I.; Brindza, J.; Wieczorek, P.P.; Lipok, J. Mentha Piperita: Essential Oil and Extracts, Their Biological Activities, and Perspectives on the Development of New Medicinal and Cosmetic Products. Molecules 2023, 28, 7444. [Google Scholar] [CrossRef]
- Desai, R.; Anuradha, B.S.; Pindi, P.K. Determination of the Antibacterial and Anti-Biofilm Capability of Selected Leaf Extracts. Curr. Trends Biotechnol. Pharm. 2023, 17, 1340–1351. [Google Scholar] [CrossRef]
- Ghazwani, M.; Hakami, A.; Sani, S.S.; Sultana, S.; Sultana, T.; Bashir, W.; Rafique, A. Antibacterial Activity of Aqueous and Methanolic Extract of Mentha Piperita against Pervasive Bacteria Isolated from Urial the Ovis Vignei. Pak. Vet. J. 2023, 43, 103–108. Available online: https://www.pvj.com.pk/pdf-files/23-044.pdf (accessed on 12 November 2025).
- Noor, S. Screening of Some Commonly Used Plant Extracts for Their Effects on Some Gut Pathogens and Probiotics. J. Pure Appl. Microbiol. 2017, 11, 163–171. [Google Scholar] [CrossRef]
- Sudheer Khan, S.; Ghouse, S.S.; Chandran, P. Toxic Effect of Environmentally Relevant Concentration of Silver Nanoparticles on Environmentally Beneficial Bacterium Pseudomonas Putida. Bioprocess Biosyst. Eng. 2015, 38, 1243–1249. [Google Scholar] [CrossRef]
- Hachicho, N.; Hoffmann, P.; Ahlert, K.; Heipieper, H.J. Effect of Silver Nanoparticles and Silver Ions on Growth and Adaptive Response Mechanisms of Pseudomonas Putida Mt-2. FEMS Microbiol. Lett. 2014, 355, 71–77. [Google Scholar] [CrossRef]
- Sarkar, R.; Anil Kumar, C.; Kumbhakar, P.; Mandal, T. Aqueous Synthesis and Antibacterial Activity of Silver Nanoparticles against Pseudomonas Putida. Mater. Today Proc. 2019, 11, 686–694. [Google Scholar] [CrossRef]
- Yu, Q.; Li, J.; Zhang, Y.; Wang, Y.; Liu, L.; Li, M. Inhibition of Gold Nanoparticles (AuNPs) on Pathogenic Biofilm Formation and Invasion to Host Cells. Sci. Rep. 2016, 6, 26667. [Google Scholar] [CrossRef]
- Shukla, S.K.; Kumar, R.; Mishra, R.K.; Pandey, A.; Pathak, A.; Zaidi, M.G.H.; Srivastava, S.K.; Dikshit, A. Prediction and Validation of Gold Nanoparticles (GNPs) on Plant Growth Promoting Rhizobacteria (PGPR): A Step toward Development of Nano-Biofertilizers. Nanotechnol. Rev. 2015, 4, 439–448. [Google Scholar] [CrossRef]
- Xu, Z.; Long, X.; Jia, Y.; Zhao, D.; Pan, X. Occurrence, Transport, and Toxicity of Nanomaterials in Soil Ecosystems: A Review. Environ. Chem. Lett. 2022, 20, 3943–3969. [Google Scholar] [CrossRef]
- Singh, S.; Prasad, S.M.; Bashri, G. Fate and Toxicity of Nanoparticles in Aquatic Systems. Acta Geochim. 2023, 42, 63–76. [Google Scholar] [CrossRef]
- Alizadeh, M.; Qarachal, J.F.; Sheidaee, E. Understanding the Ecological Impacts of Nanoparticles: Risks, Monitoring, and Mitigation Strategies. Nanotechnol. Environ. Eng. 2025, 10, 6. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).