Application of a Multi-Component Conditioner as a Sustainable Management Practice for Enhancing Soil Properties and Hordeum vulgare L. Growth and Yield
Abstract
1. Introduction
2. Materials and Methods
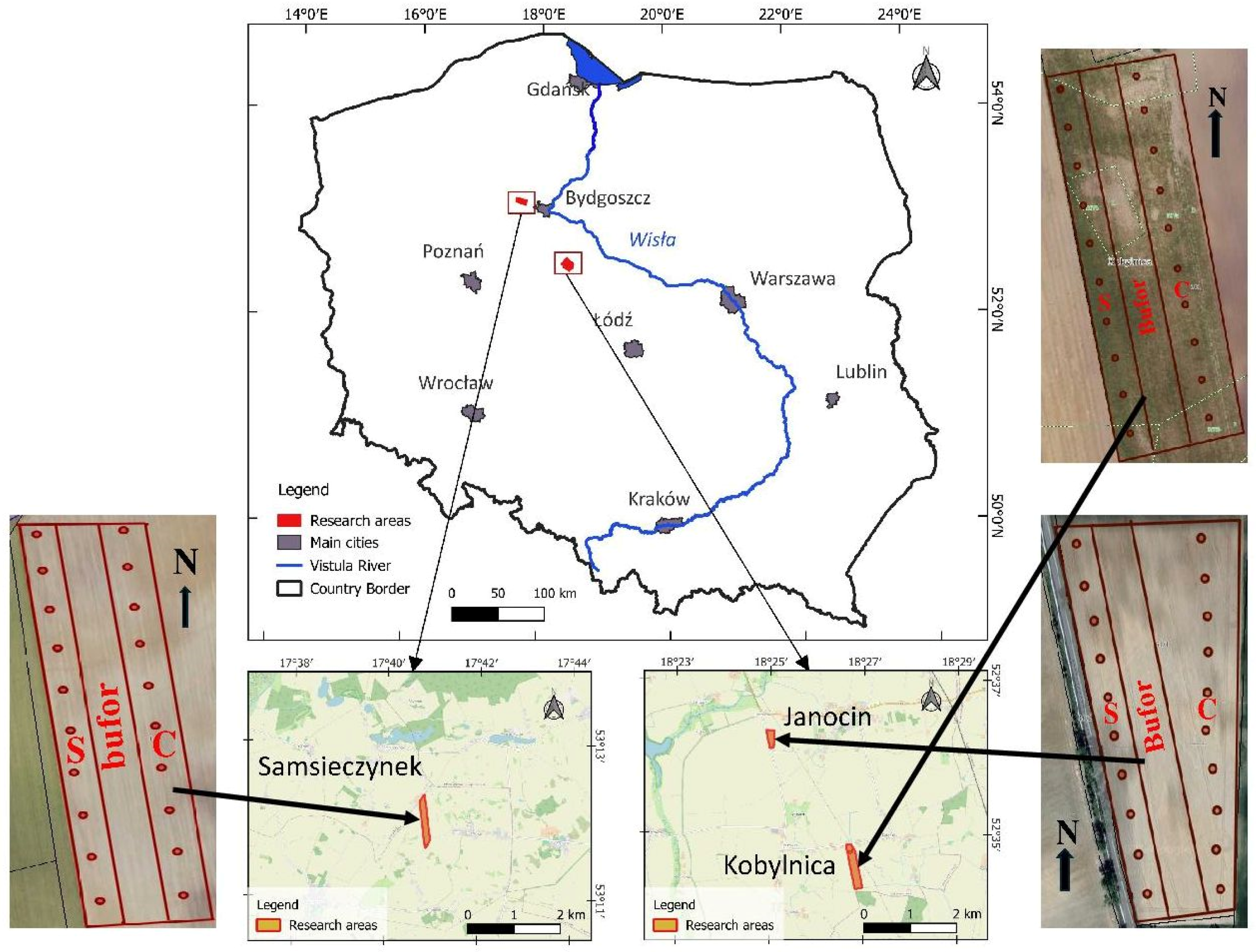
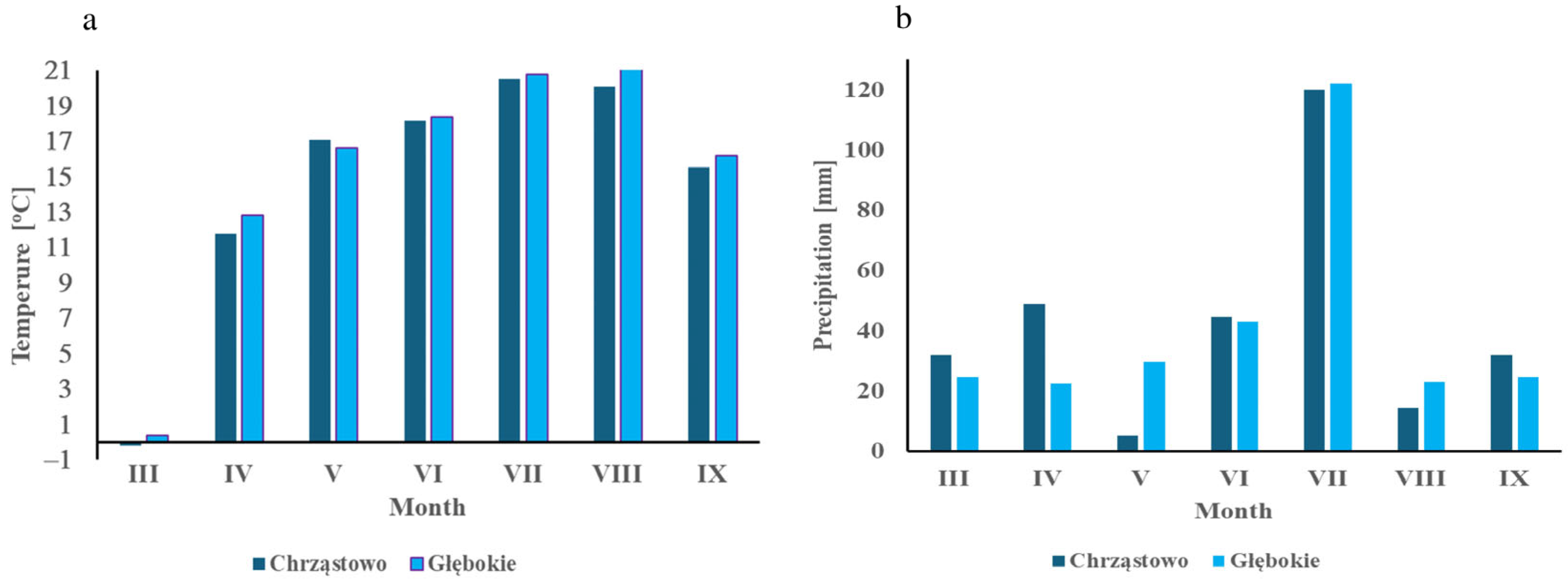
2.1. Description of the Experimental Fields and Soil Sampling
2.2. Determination of Soil Physicochemical Properties
2.3. Soil Enzymatic and Microbial Biomass Content
2.4. Plant Characteristics
2.5. Statistical Calculations
3. Results
3.1. Soil Chemical, Physical, and Water-Related Properties
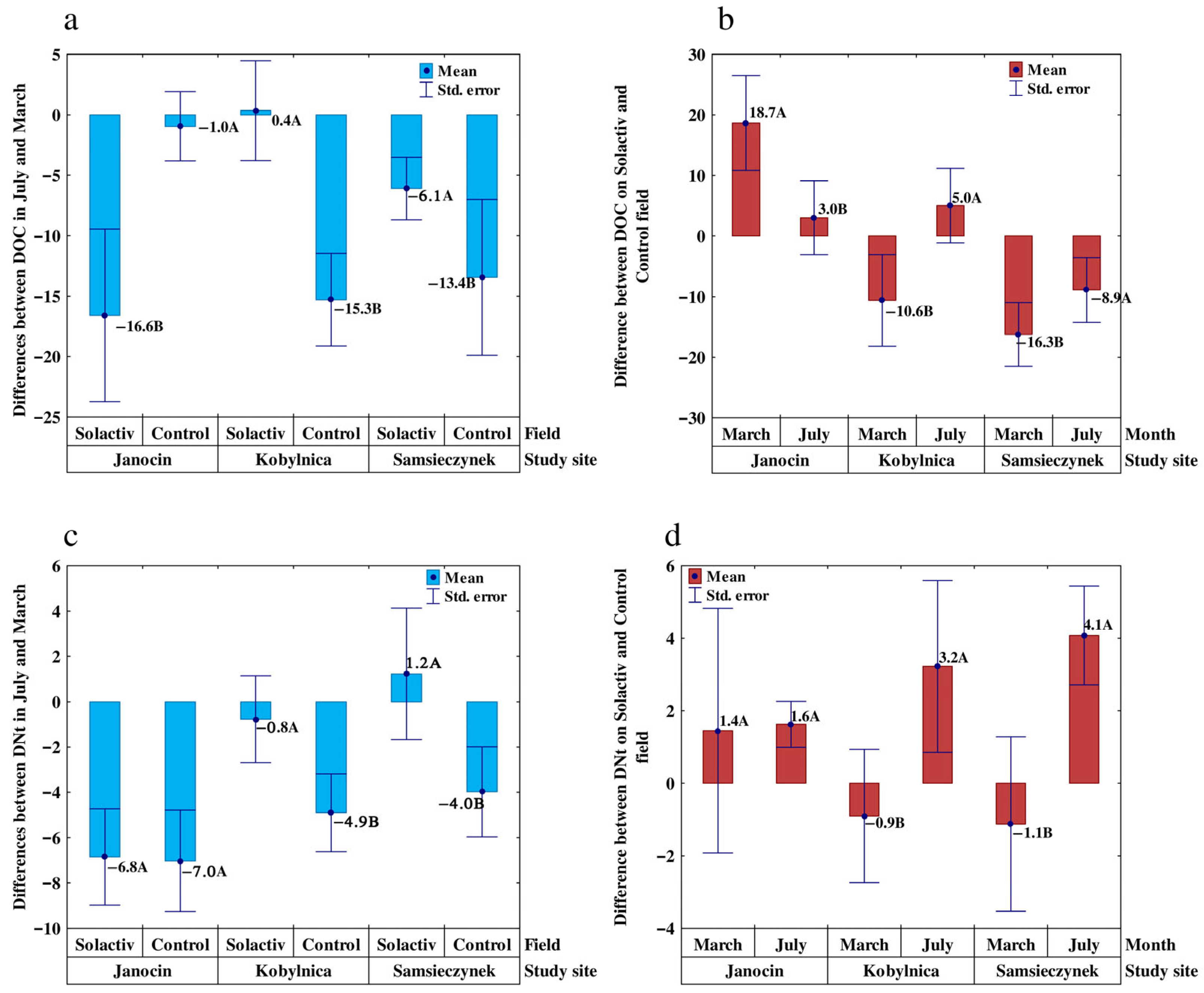
3.2. Carbon and Nitrogen: Dissolved Forms and Microbial Biomass
3.3. Soil Enzymatic and Microbial Properties
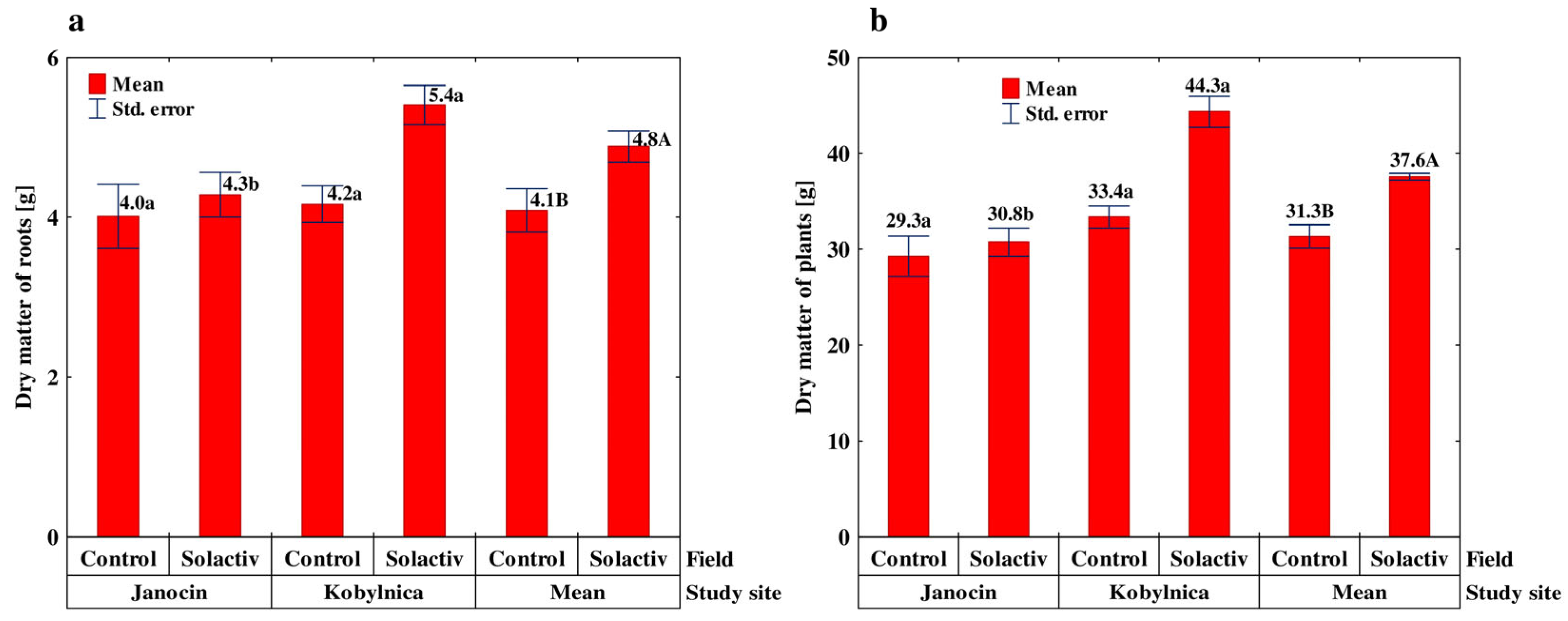
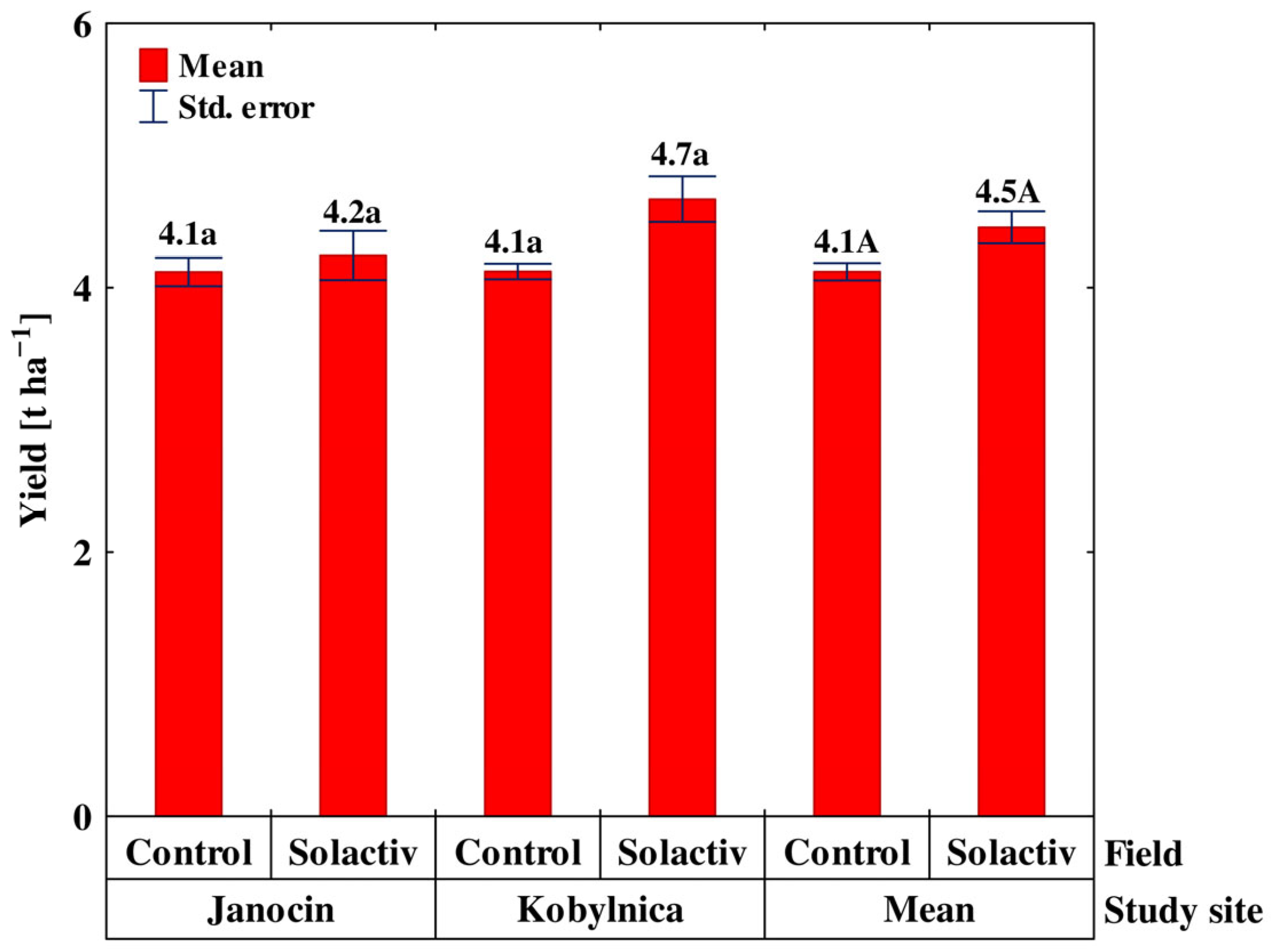
3.4. Properties Relating to the Growth and Yield of Spring Barley
3.5. Principal Component Analysis
4. Discussion
4.1. Selected Physicochemical Properties
4.2. Soil Enzymatic and Microbial Properties
4.3. Plant Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xing, Y.; Wang, X.; Mustafa, A. Exploring the link between soil health and crop productivity. Ecotoxicol. Environ. Saf. 2025, 289, 117703. [Google Scholar] [CrossRef]
- Furtak, K.; Wolińska, A. The impact of extreme weather events as a consequence of climate change on the soil moisture and on the quality of the soil environment and agriculture—A review. Catena 2023, 231, 107378. [Google Scholar] [CrossRef]
- Oishy, M.N.; Shemonty, N.A.; Fatema, S.I.; Mahbub, S.; Min, E.L.; Raisa, M.B.H.; Anik, A.H. Unravelling the effects of climate change on the soil-plant-atmosphere interactions: A critical review. Soil Environ. Health 2025, 3, 100130. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; McKenna, B.A.; Lombi, E. Soil and the intensification of agriculture for global food security. Environ. Int. 2019, 132, 105078. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, G.; Li, Q.; Zhou, Z.; Feng, Y.; Luo, Y.; Tan, H.; Tian, X. Soil moisture determines the consistency of organic matter decomposition in field and lab test patterns. Catena 2024, 246, 108485. [Google Scholar] [CrossRef]
- Krasowicz, S.; Matyka, M.; Madej, A. The rational use of Polish soils as a challenge for science, advice, and agricultural practice State Research Institute in Puławy. Acta Sci. Pol. Oecon. 2023, 22, 17–25. [Google Scholar] [CrossRef]
- Ochal, P.; Jadczyszyn, T.; Jurga, B.; Kopiński, J.; Matyka, M.; Madej, A.; Rutkowska, A.; Smreczek, B.; Łysiak, M. Environmental aspects of soil acidity in Poland. In Studies and Reports of Institute of Soil Science and Plant Cultivation—State Research Institute; Report Prepared as a Part of the Task 2.2; Institute of Soil Science and Plant Cultivation–State Research Institute: Puławy, Poland, 2017; p. 47. [Google Scholar]
- Shinde, R.; Sarkar, P.; Thombare, N.; Naik, S. Soil conservation: Today’s need for sustainable development. Agric. Food E-Newsl. 2019, 1, 175–183. [Google Scholar]
- Thakur, P.; Wadhwa, H.; Shubham; Kaushal, S. Soil conditioners: Refinement of soil health for better tomorrow. Curr. J. Appl. Sci. Technol. 2023, 42, 1–9. [Google Scholar] [CrossRef]
- Triphati, S.; Tiwari, T.; Sachan, R. Soil Conditioners: Substances that Enhance Physical Properties of Soil. In Current Research and Review in Soil Science; Bright Sky Publications: Delhi, India, 2023; Volume 3, pp. 19–29. [Google Scholar]
- Rakkammal, K.; Maharajan, T.; Ceasar, S.A.; Ramesh, M. Biostimulants and their role in improving plant growth under drought and salinity. Cereal Res. Commun. 2023, 51, 61–74. [Google Scholar] [CrossRef]
- Cataldo, E.; Fucile, M.; Manzi, D.; Masini, C.M.; Doni, S.; Mattii, G.B. Sustainable soil Managment: Effects of clinoptilolite and organic compost soil application on eco-physiology, quercitin, and hydroxylated, methoxylated anthocyanins on Vitis vinifera. Plants 2023, 12, 708. [Google Scholar] [CrossRef]
- Kukowska, S.; Szewczuk-Karpisz, K. Managment of the soil environment using biochar and zeolite in various combinations: Impact on soil condition and economical aspects. J. Soils Sediments 2025, 25, 77–102. [Google Scholar] [CrossRef]
- Galamini, G.; Ferretti, G.; Rosinger, C.; Huber, S.; Diaz-Pines, E.; Faccini, B.; Keiblinger, K.M. Potential for agricultural recycling of struvite and zeolites to improve soil microbial physiology and mitigate CO2 emission. Geoderma 2025, 453, 117149. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, A.P.; Raha, P.; Rakshit, A.; Singh, C.M.; Kishor, P. Potassium humate: A potential soil conditioner and plant growth promoter. Int. J. Agric. Environ. Biotechnol. 2013, 6, 441–446. [Google Scholar] [CrossRef]
- Kołodziej, B.; Sugier, D.; Bielińska, E. The effect of leonardite application and various plantation modalities on yielding and quality of roseroot (Rhodiola rosea L.) and soil enzymatic activity. J. Geochem. Explor. 2013, 129, 64–69. [Google Scholar] [CrossRef]
- Moreno, J.L.; Ondoño, S.; Torres, I.; Bastida, F. Compost, leonardite, and zeolite impacts on soil microbial community under barley crops. J. Soil Sci. Plant Nutr. 2017, 17, 214–230. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease Managment. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef]
- Ahmed, M.; Ullah, H.; Piromsri, K.; Tiisarum, R.; Chaum, S.; Datta, A. Effect of an Ascophyllum nodosum seaweed extract application dose and method on growth, fruit yield, quality, and water productivity of tomato under water-deficit stress. S. Afr. J. Bot. 2022, 151, 95–107. [Google Scholar] [CrossRef]
- Liu, Q.; Cui, H.; Yang, W.; Wang, F.; Liao, H.; Zhu, Q.; Qin, S.; Lu, P. Soil conditioner improves soil properties, regulates microbial communities, and increases yield and quality of Uncaria rhynchophylla. Sci. Rep. 2024, 14, 13398. [Google Scholar] [CrossRef]
- Dueñas, J.F.; Kunze, E.; Li, H.; Riling, M.C. Soil conditioner mixtures as an agricultural Managment alternative to mitigate drought impacts: A proof of concept. Nat. Hazards Earth Syst. Sci. 2025, 25, 1377–1386. [Google Scholar] [CrossRef]
- Goulding, K.W.T. Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manag. 2016, 32, 3. [Google Scholar] [CrossRef]
- Ouni, Y.; Ghnaya, T.; Montemurro, F.; Abdelly, C.; Lakhdar, A. The role of humic substances in mitigating the harmful effects of soil salinity and improve plant productivity. Int. J. Plant Prod. 2014, 8, 353–374. [Google Scholar]
- Wu, Q.; Xia, D.; Chen, G.; Sun, T.; Song, Y. Effects of zeolite on drought resistance and water—nitrogen use efficiency in paddy rice. J. Irrig. Drain. Eng. 2019, 145, 04019024. [Google Scholar] [CrossRef]
- Piotrowska, A.; Długosz, J.; Zamorski, R.; Bogdanowicz, P. Changes in some biological and chemical properties of an arable soil treated with the microbial biofertilizer UGmax. Pol. J. Environ. Stud. 2012, 21, 455–463. [Google Scholar]
- Mayer, J.; Scheid, S.; Widmer, F.; Fließbach, A.; Oberholzer, H.R. How effective are “Effective micro-organisms (EM)”? Results from a field study in temperate climate. Appl. Soil Ecol. 2010, 46, 230–239. [Google Scholar] [CrossRef]
- Ravali, C.; Rao, K.J.; Anjaiah, T.; Suresh, K. Effect of zeolite on soil physical and physico-chemical properties. Int. Ref. Peer Rev. Index. Q. J. Sci. Agric. Eng. 2020, 10, 776–781. [Google Scholar]
- Mondal, M.; Biswas, B.; Garai, S.; Sarkar, S.; Banerjee, H.; Brahmachari, K.; Bandyopadhyay, P.K.; Maitra, S.; Brestic, M.; Skalicky, M.; et al. Zeolites enhance soil health, crop productivity and environmental safety. Agronomy 2021, 11, 448. [Google Scholar] [CrossRef]
- Ferretti, G.; Rosinger, C.; Diaz-Pines, E.; Faccini, B.; Coltorti, M.; Keiblinger, K.M. Soil quality increases with long-term chabazite-zeolite tuff amendments in arable and perennial cropping systems. J. Environ. Manag. 2024, 354, 120303. [Google Scholar] [CrossRef]
- Bhattarai, B.; Neupane, J.; Dhakal, S.P.; Nepal, J.; Gnyawali, B.; Timalsina, R.; Poudel, A. Effect of biochar from different origin on physio-chemical properties of soil and yield of Garden Pea (Pisum sativum L.) at Paklihawa, Rupandehi. Nepal. World J. Agric. Res. 2015, 3, 129–138. [Google Scholar]
- Ergolu, N.; Emekci, M.; Athanassiou, C.G. Applications of natural zeolites on agriculture and food production. J. Sci. Food Agric. 2017, 97, 3487–3499. [Google Scholar] [CrossRef]
- Sangeetha, C.; Baskar, P. Zeolite and its potential uses in agriculture: A critical review. Agric. Rev. 2016, 37, 101–108. [Google Scholar] [CrossRef]
- Demirer, T. Effect of leonardite application on leaf nutrient content and fruit chemical parameters of cherry (Prunus avium L.). J. Plant Nutr. 2019, 42, 2532–2538. [Google Scholar] [CrossRef]
- Dinçsoy, M.; Sönmez, F. The effect of potassium and humic acid applications on yield and nutrient contents of wheat (Triticum aestivum L. var. Delfii) with same soil properties). J. Plant Nutr. 2019, 42, 2757–2772. [Google Scholar] [CrossRef]
- Alharbi, K.; Rashwan, E.; Hafez, E.; El-Dein Omara, A.; Mohamed, H.H.; Alshaal, T. Potassium Humate and Plant Growth-Promoting Microbes Jointly Mitigate Water Deficit Stress in Soybean Cultivated in Salt-Affected Soil. Plants 2022, 11, 3016. [Google Scholar] [CrossRef]
- Margal, P.B.; Thakare, R.S.; Kamble, B.M.; Patil, V.S.; Patil, K.B.; Titirmare, N.S. Effect of seaweed extracts on crop growth and soil: A review. J. Exp. Agric. Int. 2023, 45, 9–19. [Google Scholar] [CrossRef]
- Khan, W.; Palanisamy, R.; Critchley, A.T.; Smith, D.L.; Papadopoulos, Y.; Prithiviraj, B. Ascophyllum nodosum extract and its organic fractions stimulate Rhizobium root nodulation and growth of Medicago sativa (Alfalfa). Commun. Soil Sci. Plant Anal. 2013, 44, 900–908. [Google Scholar] [CrossRef]
- Banjare, L.; Banwasi, R.; Jataw, G.K.; Shrivastav, L.K. Effect of seaweed extract on yield and nutrient uptake of rice in a vertisol. Pharma Innov. J. 2022, 11, 2193–2198. [Google Scholar]
- Repke, R.A.; Ribeiro Silva, D.M.; Camilo dos Santos, J.C.; de Almeida Silva, M. Increased soybean tolerance to high-temperature through biostimulant based on Ascophyllum nodosum (L.) seaweed extract. J. Appl. Phycol. 2022, 34, 3205–3218. [Google Scholar] [CrossRef]
- Frioni, T.; Vander-Weide, J.; Palliotti, A.; Tombesi, S.; Poni, S.; Sabbatini, P. Foliar vs. soil application of Ascophyllum nodosum extracts to improve grapevine water stress tolerance. Sci. Hortic. 2021, 277, 109807. [Google Scholar] [CrossRef]
- Naz, S.; Din Muhammad, H.M.; Ramzan, M.; Sadiq, B.; Ahmad, R.; Ali, S.; Alsahli, A.A.; Altal, A. Seaweed application enhanced the growth and yield of pea (Pisum sativum L.) by altering physiological indices. J. Soil Sci. Plant Nutr. 2023, 23, 6183–6195. [Google Scholar] [CrossRef]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal biostimulants and biofertilisers in crop productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]
- Siwik-Ziomek, A.; Szczepanek, M. Soil extracellular enzyme activities and uptake of N by oilseed rape depending on fertilization and seaweed biostimulant application. Agronomy 2019, 9, 480. [Google Scholar] [CrossRef]
- Villa e Vila, V.; Rezende, R.; Marques, P.A.A.; Wenneck, G.S.; Crepaldi de Faria Nocchi, R.; de Souza Terassi, D.; Barion Alves Andrean, A.F.; Matumoto-Pintro, P.T. Seaweed extract of Ascophyllum nodosum applied in tomato crop as a biostimulant for improving growth, yield and soil fertility in subtropical condition. J. Appl. Phycol. 2023, 35, 2531–2541. [Google Scholar] [CrossRef]
- Długosz, J.; Piotrowska-Długosz, A.; Kotwica, K.; Przybyszewska, E. Application of multi-component Conditioner with clinoptilolite and Ascophyllum nodosum extract for improving soil properties and Zea mays L. growth and yield. Agronomy 2020, 10, 2005. [Google Scholar] [CrossRef]
- Őztürk, H.S.; Türkmen, C.; Erdogan, E.; Baskan, O.; Dengiz, O.; Parlak, M. Effects of a soil conditioner on some physical and biological features of soils: Results from a greenhouse study. Biores. Technol. 2005, 96, 1950–1954. [Google Scholar] [CrossRef]
- Ebrahimi, E.; Asadi, G.; von Fragstein und Niemsdorff, P. A field study on the effect of organic soil conditioners with different placements on dry matter and yield of tomato (Lycopersicon esculentum L.). Int. J. Recycl. Org. Waste Agric. 2019, 8, 59–66. [Google Scholar] [CrossRef]
- Jeřábková, J.; Salaš, P.; Burgová, J. The Influence of the Application of Soil Conditioners on the Temperature and Moisture of the Soil Environment. Inż. Mineral. 2024, 1, 1–7. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World reference base for soil resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Gorbulewski, K.; Fronczak, J.; Leszczyńska, M. Specific surface area-basic parameter of reactive material’s characteristics. Sci. Rev. Eng. Environ. Sci. 2008, 17, 122–130. (In Polish) [Google Scholar]
- Soil Survey Staff. 2014 Soil Survey Staff, Keys to Soil Taxonomy, 11th ed.; USDA Natural Resources Conservation Service: Washington, DC, USA, 2010.
- Domżał, H.; Słowińska-Jurkiewicz, A.; Turski, R. Przewodnik do Ćwiczeń z Gleboznawstwa z Elementami Geologii i Mechaniki Gleb, 1st ed.; Wydawnictwo Akademii Rolniczej: Lublin, Poland, 1976; pp. 12–16. (In Polish) [Google Scholar]
- Gonet, S.S.; Dębska, B.; Pakula, J. The Content of Dissolved Organic Carbon in Soils and Organic Fertilisers, 1st ed.; PTSH: Wrocław, Poland, 2002. (In Polish) [Google Scholar]
- PN-R-04020:1994; Chemical-Agricultural Analysis of Soil—Determination of Available Magnesium Content. PKN Press: Warsaw, Poland, 2018.
- Egner, H.; Riehm, H.; Domingo, W.R. Studies concerning the chemical analysis of soils as background for soil nutrient assessment. II. Chemical extracting methods to determinate the phosphorous and potassium content of soil. Kungl. Lantbr. Ann. 1960, 26, 199–215. (In German) [Google Scholar]
- PN-ISO 11260.2018; Soil Quality—Determination of Effective Cationic Exchange Capacity and Base Saturation with Barium Chloride. PKN Press: Warsaw, Poland, 2018.
- Van Reeuwijk, L.P. Procedures for Soil Analysis, 6th ed.; ISRIC Technical Paper 9; International Soil Reference and Information Centre, Food and Agriculture Organization of the United Nations: Wageningen, The Netherlands, 2002; p. 14. [Google Scholar]
- Jaremko, D.; Kalembasa, D. A comparison of methods for the determination of cation exchange capacity of soils. Ecol. Chem. Eng. S. 2014, 3, 487–498. [Google Scholar]
- PN-ISO 11465.1999; Soil quality—Determination of Soil Dry Matter and Soil Water Content Expressed as Dry Matter of Soil—Gravimetric Method. PKN Press: Warsaw, Poland, 1999.
- Walczak, R.; Ostrowski, J.; Witkowska-Walczak, B.; Sławiński, C. Spatial characteristics of water conductivity in the surface level of Polish arable soils. Int. Agrophys. 2002, 16, 239–247. [Google Scholar]
- Thalmann, A. Zur Methodik der Bestimmung der Dehydrodgenaseaktivitat im Boden mittels Triphenyltetrazolium-chlorid (TTC). Landwirtsch. Forsch. 1968, 21, 249–258. [Google Scholar]
- Adam, G.; Duncan, H. Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar] [CrossRef]
- Schinner, F.; von Mersi, W. Xylanase-, CM-cellulase- and invertase activity in soil: An improved method. Soil Biol. Biochem. 1990, 22, 511–515. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinsen, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen—Total. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeny, D.R., Eds.; SSSA: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Joergensen, R.G.; Mueller, T. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the kENvalue. Soil Biol. Biochem. 1996, 28, 33–37. [Google Scholar] [CrossRef]
- PN-88/R-04013.1994; Chemical-Agricultural Analysis of Plants. Determination of Air-Dry and Dry Mass. Polish Committee: Warsaw, Poland, 1996.
- Wilding, L.P. Spatial variability: Its documentation, accommodation, and implication to soil surveys. In Soil Spatial Variability; Nielsen, D.R., Bouma, J., Eds.; PUDOC: Wageningen, The Netherlands, 1985; pp. 166–194. [Google Scholar]
- PN-R-04023.1996; Agrochemical Soil Analysis—Determination of Available Phosphorus in Mineral Soils. Polish Committee: Warsaw, Poland, 1996.
- Sherrod, L.A.; Peterson, G.A.; Westfall, D.G.; Ahuja, L.R. Cropping intensity enhances soil organic carbon and nitrogen in a no-till agroecosystem. Soil Sci. Soc. Am. 2003, 67, 1533–1543. [Google Scholar] [CrossRef]
- Morante-Carballo, F.; Montalván-Burbano, N.; Carrión-Mero, P.; Espinoza-Santos, N. Cation exchange of natural zeolites: Worldwide research. Sustainability 2021, 13, 7751. [Google Scholar] [CrossRef]
- De Campos Bernardi, A.C.; Polidoro, J.C.; de Melo Monte, M.B.; Pereira, E.I.; Ribeiro, C.; Ramesh, K. Enhancing nutrient use efficiency using zeolites minerals—A review. Adv. Chem. Eng. Sci. 2016, 6, 295–304. [Google Scholar] [CrossRef]
- Józefaciuk, G.; Szatniak-Kloc, A.; Ambrozewicz-Nita, A. The surface area of zeolite-amended soils exceeds the sum of the inherent surface areas of soil and zeolite. Eur. J. Soil Sci. 2018, 69, 787–790. [Google Scholar] [CrossRef]
- Tatlier, M.; Munz, G.; Henninger, S.K. Relation of water adsorption capacities of zeolites with their structural properties. Microporous Mesoporous Mat. 2018, 264, 70–75. [Google Scholar] [CrossRef]
- Palanivell, P.; Ahmed, O.H.; Susilawati, K.; Majid, N.M.A. Mitigating ammonia volatilization urea in waterlogged condition using clinoptilolite zeolite. Int. J. Agric. Biol. 2015, 17, 149–155. [Google Scholar]
- Abdel-Hassan, A.N.; Abdullah Radi, A.M. Effect of zeolite on some physical properties of wheat plant growth (Triticum aestivum L.). Plant Arch. 2017, 18, 2641–2648. [Google Scholar]
- Githinji, L.J.M.; Dane, J.H.; Walker, R.H. Physical and hydraulic properties of inorganic amendments and modelling their e ects on water movement in sand-based root zones. Irrig. Sci. 2011, 29, 65–77. [Google Scholar] [CrossRef]
- Xu, C.; Sun, L.; Tong, S.; Quyang, J.; Gu, X. Cellulase immobilization on zeolitic imidazolate frameworks for boosting cellulose hydrolysis at high solids loading. Ind. Crops Prod. 2023, 206, 117693. [Google Scholar] [CrossRef]
- Sun, L.; Xu, C.; Tong, S.; Gu, X. Enhancing cellulose hydrolysis via cellulase immobilization on zeolitic imidazolate frameworks using physical adsorption. Bioprocess Biosyst. Eng. 2024, 47, 1071–1080. [Google Scholar] [CrossRef]
- Jampana, S.R.; Jia, L.; Ramarao, B.V.; Kumar, D. Experimental investigation of the adsorption and desorption of cellulase enzymes on zeolite-β for enzyme recycling applications. Bioprocess Biosyst. Eng. 2021, 44, 495–505. [Google Scholar] [CrossRef]
- Wolny-Koładka, K.; Jarosz, R.; Marcińska-Mazur, L.; Mierzwa-Hersztek, M. Effect of mineral and organic additions on soil microbial composition. Int. Agrophys. 2022, 63, 131–138. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Doni, S.; Gispert, M.; Peruzzi, E.; Macci, C.; Mattii, G.B.; Manzi, D.; Masini, C.M.; Grazia, M. Impact of natural zeolite on chemical and biochemical properties of vineyard soils. Soil Use Manag. 2020, 37, 1–11. [Google Scholar] [CrossRef]
- Shivakumara, M.N.; Rangaish, K.M.; Subbarayappa, C.T.; Chamegowda, T.C.; Thimmegowda, M.N.; Ramaiah, M. Effect of zeolite and fertilizer application on soil microbial biomass and enzyme activity in finger millet. Int. J. Curr. Microbiol. App. Sci. 2019, 8, 1939–1957. [Google Scholar] [CrossRef]
- Nabti, E.; Jha, B.; Hartmann, A. Impact of seaweeds on agricultural crop production as biofertilizer. Int. J. Environ. Sci. Technol. 2016, 14, 1119–1134. [Google Scholar] [CrossRef]
- Wang, M.; Chen, L.; Li, Y.; Chen, L.; Liu, Z.; Wang, X.; Qin, S. Responses of soil microbial communities to a short-term application of seaweed fertilizer revealed by deep amplicon sequencing. Appl. Soil Ecol. 2018, 125, 288–296. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Huang, Z.; Su, G.; Li, X.; Sun, Z.; Qin, Y. Impact of short-term application of seaweed fertilizer on bacterial diversity and community structure, soil nitrogen contents, and plant growth in maize rhizosphere soil. Folia Microbiol. 2020, 65, 591–603. [Google Scholar] [CrossRef]
- Xavier James, V.C.; Pushpa Thiraviam, A.G.; Al-Dosary, M.A.; Hatamleh, A.A.; Bukhari, N.A.; Arokiyaraj, S.; Kalaiyarasi, M. Evaluation of nutrient composition and biostimulant properties of seaweeds for improving soil microbial population and tomato plant growth. BioResources 2025, 20, 1431–1451. [Google Scholar] [CrossRef]
- Hernández, I.Z.; Zamopra-Natera, J.F.; Garcia, P.M.; Ramirez, E.; Trujillo, N. Biological activity in soils treated with green manures of Lupinus spp. (Leguminosae) using the hydrolysis of fluorescein diacetate method (FDA) in Jalisco, Mexico. Hortic. Int. J. 2020, 4, 203–206. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Tarkalson, D.D.; Lehrsch, G.A. Zeolite soil application method a ects inorganic nitrogen, moisture, and corn growth. Soil Sci. 2011, 176, 136–142. [Google Scholar] [CrossRef]
- Szatanik-Kloc, A.; Szerement, J.; Adamczuk, A.; Józefaciuk, G. Effect of low zeolite doses on plants and soil physicochemical properties. Materials 2021, 14, 2617. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.Z.A.; Mahmoud, A.W.M. The combined effect of bentonite and natural zeolite on sandy soil properties and productivity of some crops. Topclass J. Agric. Res. 2013, 1, 22–28. [Google Scholar]
- Alam, M.Z.; Braun, G.; Norrie, J.; Hodges, D.M. Ascophyllum extract application can promote plant growth and root yield in carrot associated with increased rootzone soil microbial activity. Can. J. Plant Sci. 2013, 94, 337–348. [Google Scholar] [CrossRef]
- Alam, M.Z.; Braun, G.; Norrie, J.; Hodges, D.M. Effect of Ascophyllum extract application on plant growth, fruit yield and soil microbial communities of strawberry. Can. J. Plant Sci. 2013, 93, 23–36. [Google Scholar] [CrossRef]
- Lötze, E.; Homan, E.W. Nutrient composition and content of various biological active compounds of three South African-based commercial seaweed biostimulants. J. Appl. Phycol. 2016, 28, 1379–1386. [Google Scholar] [CrossRef]
- Hartz, T.K. Humic substances generally ineffective in improving vegetable crop nutrient uptake or productivity. Hortic. Sci. 2010, 45, 906–910. [Google Scholar] [CrossRef]
- Little, K.R.; Rose, M.T.; Jackson, W.R.; Cavagnaro, T.R.; Patti, A.F. Do lignite-derived organic amendments improve early-stage pasture growth and key soil biological and physicochemical properties? Crop Pasture Sci. 2014, 65, 899–910. [Google Scholar] [CrossRef]
- Akimbekov, N.; Qiao, X.; Digel, I.; Abdieva, G.; Ualieva, P.; Zhubanova, A. The effect of leonardite-derived amendments on soil microbiome structure and potato yield. Agriculture 2020, 10, 147. [Google Scholar] [CrossRef]





| Property | Sampling Month | Control | Solactiv | ||
|---|---|---|---|---|---|
| Mean ± SE | CV [%] | Mean ± SE | CV [%] | ||
| Corg [g kg−1] | * III | 8.69 (±0.48) | 19.2 | 8.99 (±0.60) | 23.2 |
| ** VII | 8.20 (±0.26) | 10.8 | 8.92 (±0.54) | 21.0 | |
| Ntot [g kg−1] | III | 1.07 (±0.03) | 11.0 | 1.12 (±0.05) | 15.4 |
| VII | 1.09 (±0.03) | 8.7 | 1.13 (±0.04) | 10.9 | |
| pH in 1M KCl | III | 6.34 (±0.15) | 12.0 | 6.24 (±0.19) | 15.2 |
| VII | 6.51 (±0.15) | 11.2 | 6.35 (±0.18) | 14.1 | |
| Hh [cmol kg−1] | III | 0.79 (±0.10) B | 63.0 | 1.16 (±0.18) A | 73.9 |
| VII | 0.84 (±0.11) B | 66.5 | 1.10 (±0.17) A | 75.0 | |
| CEC [cmol kg−1] | III | 9.43 (±0.61) | 31.5 | 10.3 (±0.65) | 31.2 |
| VII | 11.3 (±1.41) | 31.4 | 12.2 (±0.71) | 28.8 | |
| Basic saturation (BS) [%] | III | 89.4 (±1.8) | 9.6 | 86.0 (±2.2) | 12.7 |
| VII | 91.1 (±1.4) | 7.6 | 89.4 (±1.7) | 9.3 | |
| Mg-exchan [cmol kg−1] | III | 0.48 (±0.04) | 43.0 | 0.49 (±0.03) | 30.0 |
| VII | 0.49 (±0.04) | 42.5 | 0.48 (±0.03) | 33.6 | |
| Ca-exchan [cmol kg−1] | III | 7.58 (±0.64) | 41.2 | 7.97 (±0.76) | 46.8 |
| VII | 9.41 (±0.72) | 37.4 | 9.95 (±0.78) | 38.6 | |
| K-exchan [cmol kg−1] | III | 0.34 (±0.02) | 27.9 | 0.37 (±0.01) | 14.0 |
| VII | 0.33 (±0.03) | 36.7 | 0.38 (±0.01) | 15.5 | |
| Na-exchan [cmol kg−1] | III | 0.23 (±0.01) | 13.6 | 0.29 (±0.01) | 21.4 |
| VII | 0.21 (±0.02) | 44.8 | 0.25 (±0.02) | 31.3 | |
| P-avail [mg kg−1] | III | 63.4 (±1.3) | 10.2 | 67.1 (±2.8) | 20.3 |
| VII | 66.0 (±2.1) | 15.4 | 70.0 (±3.4) | 24.1 | |
| K-avail [mg kg−1] | III | 155.8 (±9.7) a | 30.5 | 158.1 (±7.1) a | 21.9 |
| VII | 138.6 (±7.7) b | 27.4 | 139.6 (±5.5) b | 19.2 | |
| Mg-avail [mg kg−1] | III | 53.1 (±3.0) | 27.3 | 56.9 (±2.7) | 23.5 |
| VII | 57.9 (±3.4) | 28.4 | 59.6 (±2.4) | 19.9 | |
| Property | Control | Solactiv | ||
|---|---|---|---|---|
| Mean ± SE | CV [%] | Mean ± SE | CV [%] | |
| Clay [%] | 5.0 (±0.3) A | 22.0 | 5.2 (±0.3) A | 21.3 |
| Silt [%] | 38.5 (±2.9) A | 26.3 | 39.9 (±2.4) A | 21.2 |
| Bulk density | 1.61 (±0.0) B | 1.1 | 1.57 (±0.01) A | 2.8 |
| Total porosity | 35.2 (±0.30) A | 3.6 | 36.2 (±0.29) A | 3.4 |
| Macropores | 7.22 (±0.34) B | 19.7 | 8.51 (±0.45) A | 22.3 |
| AWC | 17.2 (±0.25) B | 6.2 | 18.8 (±0.17) A | 3.8 |
| RAWC | 9.51 (±0.17) B | 7.7 | 11.01(±0.17) A | 6.4 |
| SAWC | 7.64 (±0.32) A | 18.0 | 7.66 (±0.29) A | 16.0 |
| Micropores | 10.8 (±0.26) A | 10.4 | 8.94 (±0.28) B | 13.4 |
| Property | Sampling Month | Control | Solactiv | ||
|---|---|---|---|---|---|
| Mean ± SE | CV [%] | Mean ± SE | CV [%] | ||
| DOC | * III | 101.1 (±4.0) Aa | 13.7 | 98.4 (±2.8) Aa | 9.8 |
| ** VII | 91.2 (±2.3) Ab | 8.7 | 91.0 (±2.8) Ab | 10.6 | |
| DNt | III | 23.6 (±0.9) Aa | 12.8 | 23.3 (±1.0) Aa | 15.5 |
| VII | 18.2 (±0.5) Bb | 18.2 | 21.1 (±1.0) Aa | 16.1 | |
| MBC | III | 118.3 (±7.9) Bb | 32.8 | 133.5 (±6.8) Aa | 24.8 |
| VII | 131.3 (±8.8) Aa | 32.8 | 135.9 (±5.5) Aa | 19.9 | |
| MBN | III | 19.7 (±1.33) Bb | 33.1 | 23.1 (±1.1) Aa | 22.5 |
| VII | 21.5 (±1.6) Aa | 35.5 | 23.1 (±1.0) Aa | 22.0 | |
| Property | Sampling Month | Control | Solactiv | ||
|---|---|---|---|---|---|
| Mean ± SE | CV [%] | Mean ± SE | CV [%] | ||
| FDAH | * III | 21.1 (±0.66) Ba | 15.3 | 24.4 (±0.89) Aa | 17.9 |
| ** VII | 19.4 (±0.59) Bb | 15.0 | 22.4 (±0.47) Aa | 10.2 | |
| DHA | III | 7.28 (±1.00) Ba | 67.2 | 9.28 (±1.05) Aa | 55.5 |
| VII | 8.08 (±1.01) Ba | 61.4 | 9.20 (±0.67) Aa | 35.6 | |
| CMC | III | 5.17 (±0.20) Bb | 18.8 | 6.19 (±0.17) Ab | 13.4 |
| VII | 8.73 (±0.19) Ba | 10.6 | 11.0 (±0.30) Aa | 13.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Długosz, J.; Kotwica, K.; Przybyszewska, E.; Piotrowska-Długosz, A. Application of a Multi-Component Conditioner as a Sustainable Management Practice for Enhancing Soil Properties and Hordeum vulgare L. Growth and Yield. Sustainability 2025, 17, 10169. https://doi.org/10.3390/su172210169
Długosz J, Kotwica K, Przybyszewska E, Piotrowska-Długosz A. Application of a Multi-Component Conditioner as a Sustainable Management Practice for Enhancing Soil Properties and Hordeum vulgare L. Growth and Yield. Sustainability. 2025; 17(22):10169. https://doi.org/10.3390/su172210169
Chicago/Turabian StyleDługosz, Jacek, Karol Kotwica, Ewelina Przybyszewska, and Anna Piotrowska-Długosz. 2025. "Application of a Multi-Component Conditioner as a Sustainable Management Practice for Enhancing Soil Properties and Hordeum vulgare L. Growth and Yield" Sustainability 17, no. 22: 10169. https://doi.org/10.3390/su172210169
APA StyleDługosz, J., Kotwica, K., Przybyszewska, E., & Piotrowska-Długosz, A. (2025). Application of a Multi-Component Conditioner as a Sustainable Management Practice for Enhancing Soil Properties and Hordeum vulgare L. Growth and Yield. Sustainability, 17(22), 10169. https://doi.org/10.3390/su172210169








