Valorization of Pumpkin Seed Flour in Biscuit Production: Nutritional Enhancement and Sensory Acceptability
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Materials
2.2. Production of Pumpkin Seed Flour
2.3. Formulation of Biscuits by Utilizing Pumpkin Seed Flour
2.4. Sensory Properties of Biscuits
2.5. Determination of Proximate Compositions of the Pumpkin Seed Flour and the Formulated Biscuits
2.6. Microbiological Analysis
2.6.1. Yeast and Mold
2.6.2. Total Plate Count
2.6.3. Detection of Escherichia coli
2.6.4. Detection of Salmonella spp.
2.7. Statistical Analysis
3. Results and Discussion
3.1. Proximate Analysis
3.1.1. Moisture Content
3.1.2. Ash Content
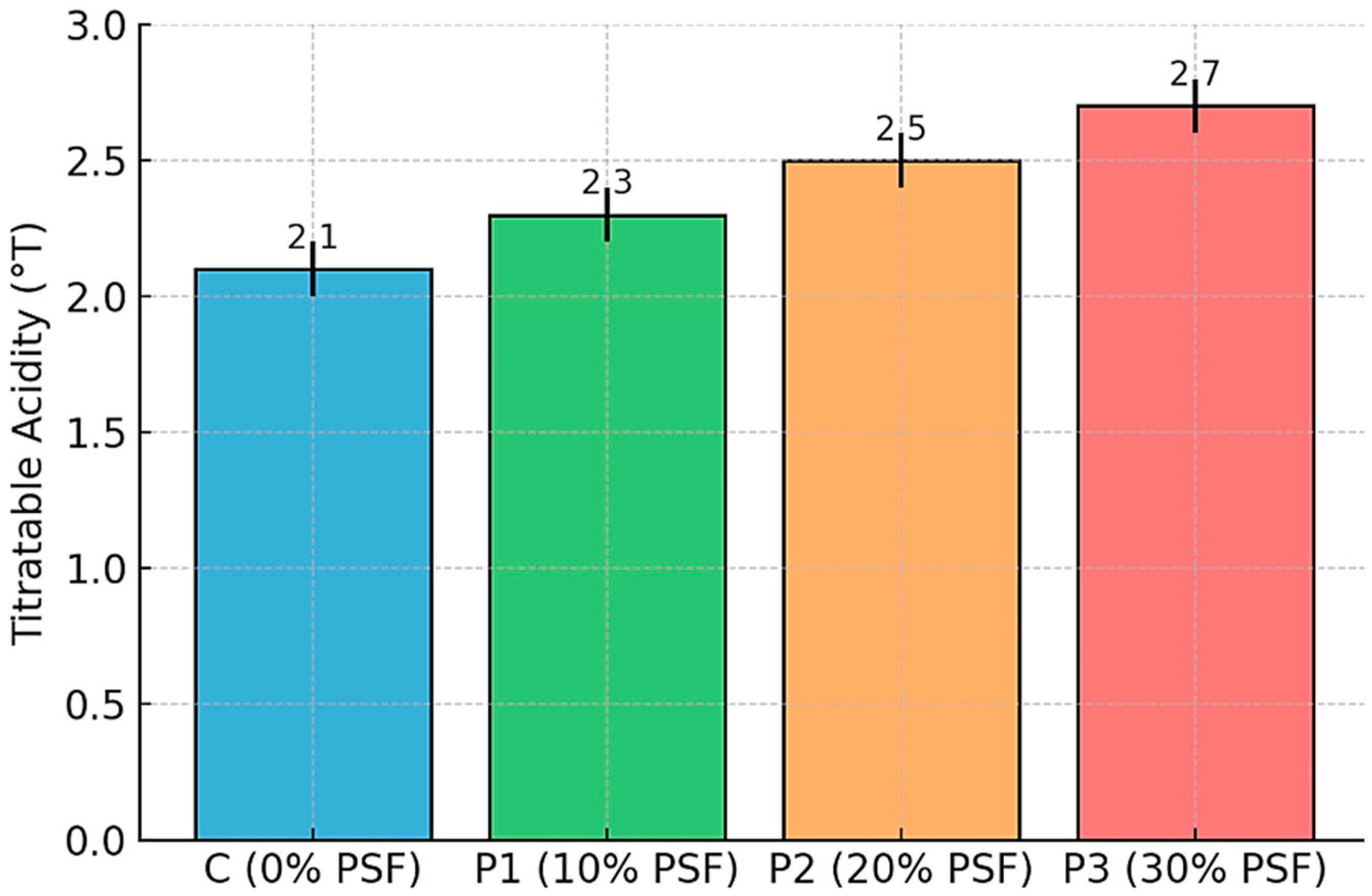
3.1.3. Titratable Acidity
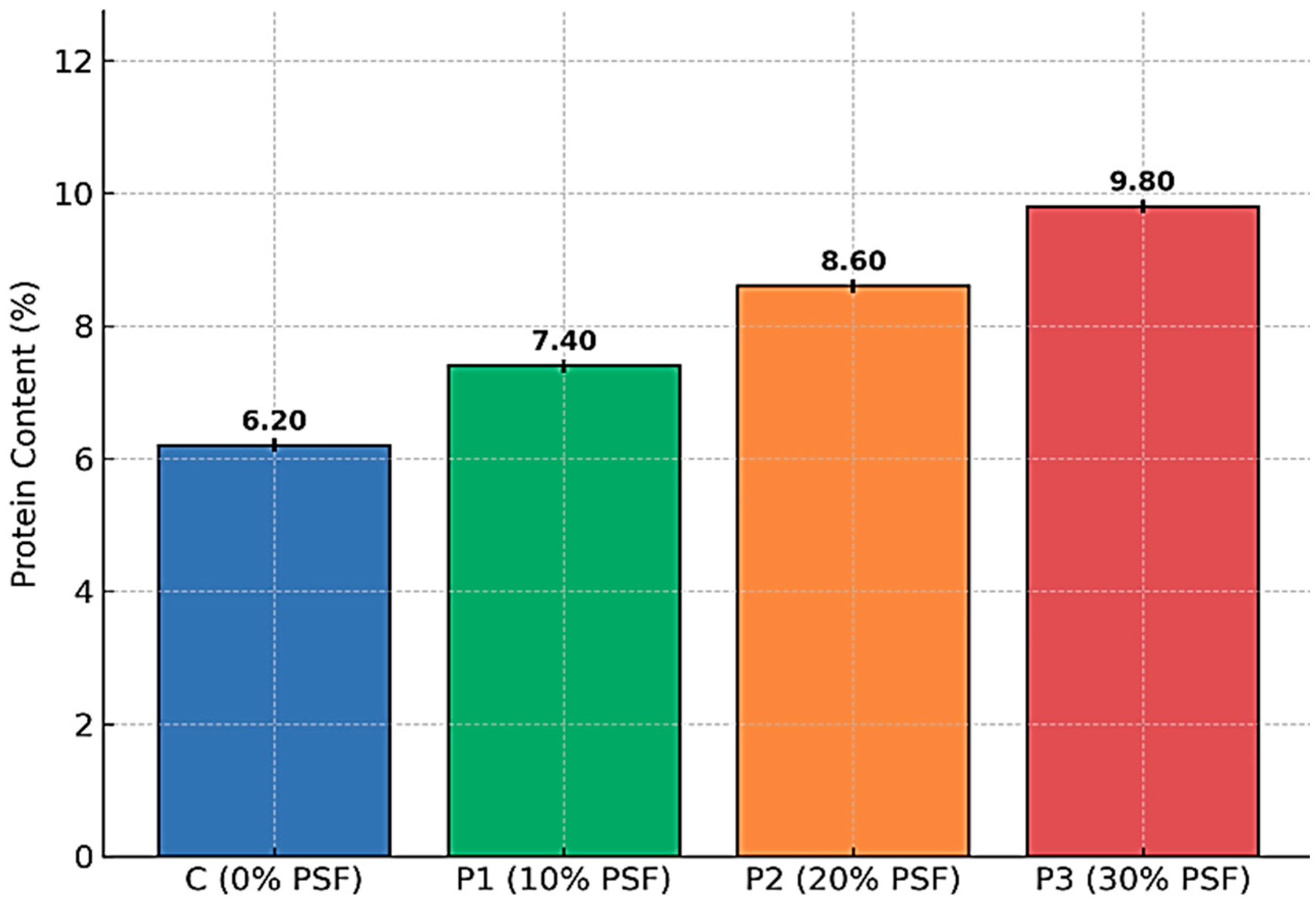
3.1.4. Protein Content
3.1.5. Lipid Content
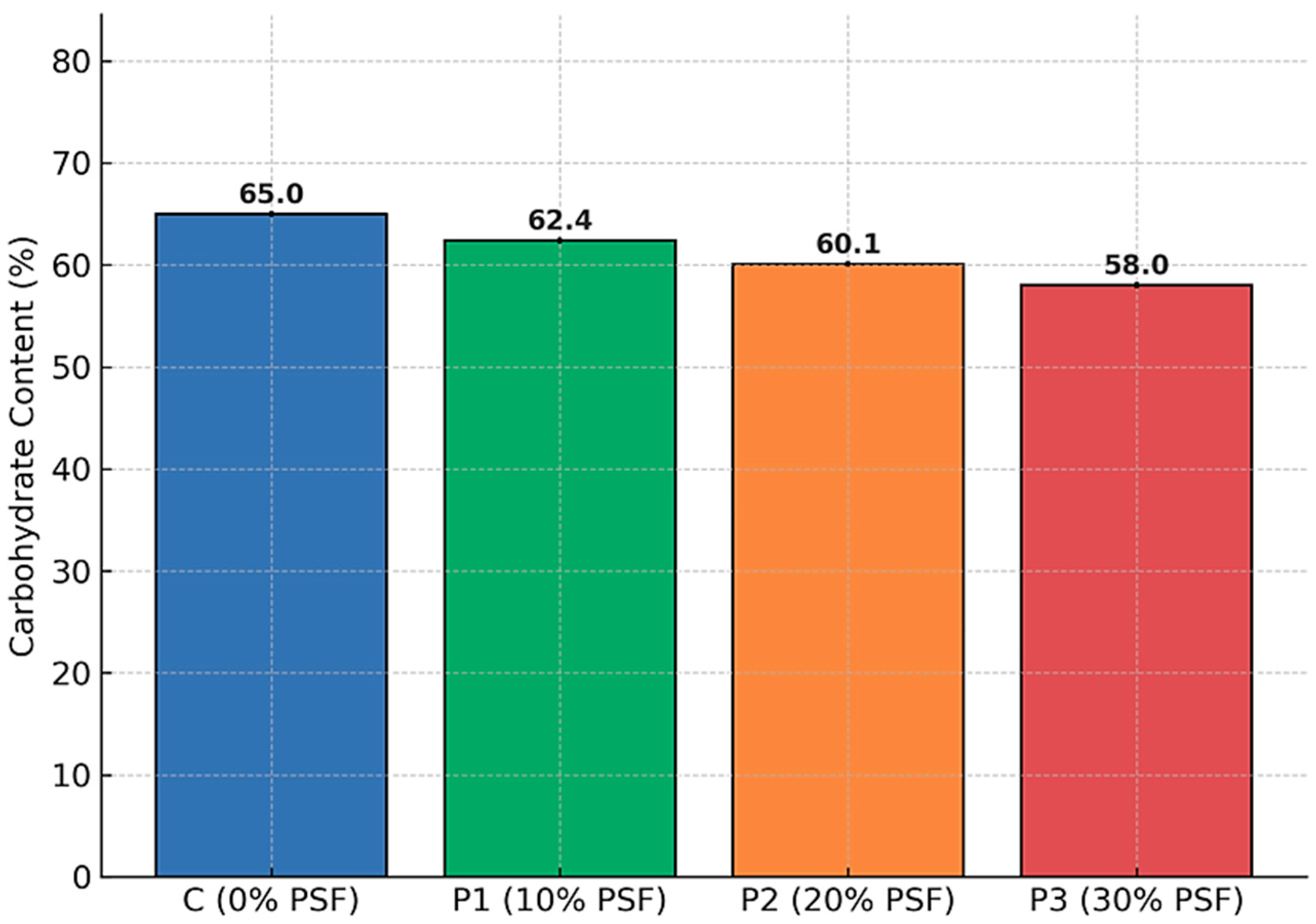
3.1.6. Carbohydrate Content
3.1.7. Crude Fiber Content of Biscuits Enriched with Pumpkin Seed Flour
3.2. Microbiological Analysis of Biscuits Enriched with Pumpkin Seed Flour
3.3. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| PSF | Pumpkin seed flour |
References
- Hussain, A.; Kausar, T.; Aslam, J.; Akhtar, M.; Arshad, M.U.; Ahmad, N. Development of nutritional biscuits for children, rich in Fe and Zn, by incorporation of pumpkin (Cucurbita maxima) seed powder. Pure Appl. Biol. 2023, 12, 392–403. [Google Scholar] [CrossRef]
- Noor, F.; Rahman, S.; Musa, R. Effect of germinated pumpkin seed flour on the nutritional and sensory properties of biscuits. Int. Food Res. J. 2024, 31, 1032–1041. [Google Scholar]
- Aljumaily, H.; Abbas, S.; Hussein, R. Development of functional cookies from wheat–pumpkin seed-based composite flour. Heliyon 2024, 10, e26013. [Google Scholar] [CrossRef]
- Esrafil, M.; Akter, S.; Alam, M.J.; Alim, M.A.; Reza, M.S.A.; Zubair, M.A.; Joy, P.R.; Jahan, M.; Khatun, M. Development and quality evaluation of novel biscuits by utilizing fruit and vegetable seed powders including pumpkin seed powder. Food Res. 2024, 8, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Adelerin, R.O.; Awolu, O.O.; Nwaogu, M.U.; Ifesan, B.O.T.; Walker, L. Pumpkin-based cookies formulated from optimized pumpkin flour blends: Nutritional and antidiabetic potentials. Food Humanit. 2024, 2, 100215. [Google Scholar] [CrossRef]
- Patel, D.; Rao, M.; Kumar, A. Valorization of agro-industrial by-products in bakery systems: A circular bioeconomy perspective. Sustainability 2024, 16, 985. [Google Scholar] [CrossRef]
- Kadan, R.S.; Bryant, R.J.; Miller, J.A. Effects of milling on functional properties of rice flour. J. Food Sci. 2008, 73, E151–E154. [Google Scholar] [CrossRef]
- ISO 4121:2003; Sensory Analysis: Guidelines for the Use of Quantitative Response Scales. International Organization for Standardization (ISO): Geneva, Switzerland, 2003.
- AOAC. Official Methods of Analysis of AOAC International, 22nd ed.; AOAC International: Rockville, MD, USA, 2023. [Google Scholar]
- AOAC. Official Method 925.10: Moisture in Flour. In Official Methods of Analysis of AOAC International, 22nd ed.; AOAC International: Rockville, MD, USA, 2023. [Google Scholar]
- AOAC. Official Method 923.03: Moisture in Nuts and Nut Products. In Official Methods of Analysis of AOAC International, 22nd ed.; AOAC International: Rockville, MD, USA, 2023. [Google Scholar]
- AOAC. Official Method 2001.11: Moisture in Foods. In Official Methods of Analysis of AOAC International, 22nd ed.; AOAC International: Rockville, MD, USA, 2023. [Google Scholar]
- AOAC. Official Method 985.29: Total Dietary Fiber in Foods—Enzymatic–Gravimetric Method. In Official Methods of Analysis of AOAC International, 22nd ed.; AOAC International: Rockville, MD, USA, 2023. [Google Scholar]
- ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique. International Organization for Standardization: Geneva, Switzerland, 2013.
- ISO 21527-2:2008; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 2: Colony Count Technique in Products with Water Activity Less than or Equal to 0.95. International Organization for Standardization: Geneva, Switzerland, 2008.
- ISO 16649-2:2001; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli—Part 2: Colony-Count Technique at 44 °C Using 5-Bromo-4-Chloro-3-Indolyl Beta-D-Glucuronide. International Organization for Standardization: Geneva, Switzerland, 2001.
- ISO 6579-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. International Organization for Standardization: Geneva, Switzerland, 2017.
- Solaiman, A.Z.M.; Rashid, M.H.; Ferdous, T.; Rahman, M.S. Microbiological Quality Assessment of Selected Bakery Products from Local Markets in Dhaka, Bangladesh. J. Food Saf. Hyg. 2020, 6, 16–22. [Google Scholar]
- Nethathe, B.B.; Mashau, M.E.; Akinola, S.A. Microbial Safety and Nutritional Evaluation of Biscuits Enriched with Indigenous Plant Flours. Foods 2023, 12, 1452. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No. 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Off. J. Eur. Union 2005, 338, 1–26. [Google Scholar]
- Adebayo, S.F.; Azeez, L.A.; Idowu, M.A. Proximate composition and functional properties of defatted pumpkin (Cucurbita pepo) seed flour. Heliyon 2020, 6, e05219. [Google Scholar] [CrossRef]
- El-Adawy, T.A.; Taha, K.M. Characteristics and composition of different seed oils and flours. Food Chem. 2001, 74, 47–54. [Google Scholar] [CrossRef]
- Jarosz, M.; Żywica, R.; Waszkiewicz-Robak, B.; Kukułowicz, A.; Gustaw, W.; Przybylski, W. Effect of Pumpkin Seed Flour Supplementation on the Physical and Sensory Properties of Wheat Bread. Appl. Sci. 2019, 9, 2960. [Google Scholar] [CrossRef]
- Glew, R.H.; Glew, R.S.; Chuang, L.T.; Huang, Y.S.; Millson, M.; Constans, D.; VanderJagt, D.J. Amino Acid, Mineral and Fatty Acid Content of Pumpkin Seeds (Cucurbita spp.) and Cyperus Esculentus Nuts in the Republic of Niger. Plant Foods Hum. Nutr. 2006, 61, 49–54. [Google Scholar] [CrossRef]
- Bhat, M.A.; Wani, I.A.; Hamdani, A.M.; Gani, A.; Masoodi, F.A. Physicochemical, Microstructural and Antioxidant Properties of Cookies Incorporated with WaterChestnut Flour. LWT Food Sci. Technol. 2017, 75, 123–130. [Google Scholar] [CrossRef]
- Emmanuel-Ikpeme, C.A.; Eneji, C.A.; Essiet, U.A. Proximate, Mineral and Antinutrient Composition of Pumpkin (Cucurbita pepo L.) Seeds Flour. Int. J. Nutr. Metab. 2012, 4, 101–106. [Google Scholar]
- Nawirska-Olszańska, A.; Kita, A.; Biesiada, A.; Sokół-Łętowska, A.; Kucharska, A.Z. Characteristics of Antioxidant Activity and Composition of Pumpkin Seed Oils in 12 Cultivars. Food Chem. 2013, 139, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Rezig, L.; Chouaibi, M.; Meddeb, W.; Hamdi, S. Chemical Composition and Profile Characterisation of Pumpkin (Cucurbita maxima) Seed Oil. Ind. Crops Prod. 2012, 37, 82–87. [Google Scholar] [CrossRef]
- Lestari, R.A.; Kusnadi, J.; Febrianto, N.A. Pumpkin Seed Flour as a Partial Substitute for Wheat Flour in Biscuit Making: Effect on Nutritional and Sensory Properties. IOP Conf. Ser. Earth Environ. Sci. 2020, 475, 012051. [Google Scholar] [CrossRef]
- Gillingham, L.G.; Harris- Janz, S.; Jones, P.J.H. Dietary Monounsaturated Fatty Acids Are Protective against Metabolic Syndrome and Cardiovascular Disease Risk Factors. Lipids 2011, 46, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Rana, N.; Rafiq, S.; Rasool, A.; Ahmad, S.; Nazir, A.; Arshad, M.; Zahoor, T.; Raza, H.; Imran, M. Enhancing Nutritional Value and Quality of Cookies through Pumpkin Seed Powder. PLoS ONE 2024, 19, e0307506. [Google Scholar] [CrossRef]
- Bose, S.; Yadav, R.; Rai, A.; Yadav, B.S. Physical and Rheological Studies of Biscuits Developed with Different Replacement Levels of Pumpkin Peel, Flesh, and Seed Powders. J. Food Qual. 2023, 2023, 4362094. [Google Scholar] [CrossRef]
- Chauhan, A.; Saxena, D.C.; Singh, S. Nutritional Evaluation of Developed Value-Added Biscuits Incorporating Germinated Pumpkin Seed Flour. Prepr. Res. 2021, 9, 2802–2806. Available online: https://www.researchgate.net/publication/357887843 (accessed on 5 September 2025).
- Fernández-Peláez, J.; Guerra, P.; Gallego, C.; Gómez, M. Physical Properties of Flours Obtained from Wasted Bread Crusts and Crumbs. Foods 2021, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Đurović, V.; Radovanović, M.; Mandić, L.; Knežević, D.; Zornić, V.; Đukić, D. Chemical and Microbial Evaluation of Biscuits Made from Wheat Flour Substituted with Wheat Sprouts. Food Sci. Technol. Int. 2021, 27, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Mishra, A.; Mishra, H.N. Fuzzy Analysis of Sensory Attributes of Multigrain Biscuits and Correlation with Instrumental Parameters. J. Food Sci. Technol. 2018, 55, 438–448. [Google Scholar] [CrossRef]
- Ghendov-Mosanu, A.; Netreba, N.; Balan, G.; Cojocari, D.; Boestean, O.; Bulgaru, V.; Gurev, A.; Popescu, L.; Deseatnicova, O.; Resitca, V.; et al. Effect of Bioactive Compounds from Pumpkin Powder on the Quality and Textural Properties of Shortbread Cookies. Foods 2023, 12, 3907. [Google Scholar] [CrossRef]
- Silva, D.; Nunes, P.; Melo, J.; Quintas, C. Microbial Quality of Edible Seeds Commercially Available in Southern Portugal. AIMS Microbiol. 2022, 8, 199–215. [Google Scholar] [CrossRef]
- Ramashia, S.E.; Ntsanwisi, M.D.; Onipe, O.O.; Mashau, M.E.; Olamiti, G. Nutritional, Functional, and Microbial Quality of Wheat Biscuits Enriched with Malted Pearl Millet and Orange Peel Flours. Food Sci. Nutr. 2024, 12, e4562. [Google Scholar] [CrossRef]
- ISO 21527-1:2008; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 1: Colony Count Technique in Products with Water Activity Greater Than 0.95. International Organization for Standardization: Geneva, Switzerland, 2008.
- Gambaro, A.; Giménez, A.; Varela, P. Consumer sensory evaluation of bakery products. In Bakery Products Science and Technology, 2nd ed.; Zhou, W., Hui, Y.H., De Leyn, I., Pagani, M.A., Rosell, C.M., Selman, J.D., Therdthai, N., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2014; pp. 513–532. [Google Scholar]
- Giarnetti, M.; Paradiso, V.M.; Caponio, F.; Summo, C.; Pasqualone, A. Fat replacement in shortbread cookies using an emulsion filled gel based on inulin and extra virgin olive oil. LWT Food Sci. Technol. 2015, 63, 339–345. [Google Scholar] [CrossRef]
- Pareyt, B.; Delcour, J.A. The role of wheat flour constituents, sugar, and fat in low moisture cereal-based products: A review on sugar-snap cookies. Crit. Rev. Food Sci. Nutr. 2008, 48, 824–839. [Google Scholar] [CrossRef] [PubMed]






| Ingredients (g) | Control | P1 (10%) | P2 (20%) | P3 (30%) |
|---|---|---|---|---|
| Butter | 90 | 90 | 90 | 90 |
| Rice flour | 150 | 135 | 120 | 105 |
| Powdered sugar | 50 | 50 | 50 | 50 |
| Pumpkin seed flour | - | 15 | 30 | 45 |
| Egg yolk | 25 | 25 | 25 | 25 |
| Baking powder | 3 | 3 | 3 | 3 |
| Parameters | Pumpkin Seed Flour |
|---|---|
| Moisture content | 7.50 ± 0.10 |
| Crude protein | 39.35 ± 0.02 |
| Total fat | 7.26 ± 0.36 |
| Total ash | 3.65 ± 0.09 |
| Crude fiber | 11.29 ± 1.58 |
| Total carbohydrate | 7.60 ± 0.66 |
| Sample | Total Aerobic Mesophilic Bacteria (log CFU/g) | Yeasts and Molds (log CFU/g) | E. coli (log CFU/g) | Salmonella spp. (log CFU/g) |
|---|---|---|---|---|
| Control | 2.08 | 1.75 | Not detected | Absent |
| P1 (10%) | 2.15 | 1.79 | Not detected | Absent |
| P2 (20%) | 2.20 | 1.83 | Not detected | Absent |
| P3 (30%) | 2.26 | 1.87 | Not detected | Absent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungureanu, C.-V.; Morozova, I.; Horincar, G.; Răducanu, D. Valorization of Pumpkin Seed Flour in Biscuit Production: Nutritional Enhancement and Sensory Acceptability. Sustainability 2025, 17, 10103. https://doi.org/10.3390/su172210103
Ungureanu C-V, Morozova I, Horincar G, Răducanu D. Valorization of Pumpkin Seed Flour in Biscuit Production: Nutritional Enhancement and Sensory Acceptability. Sustainability. 2025; 17(22):10103. https://doi.org/10.3390/su172210103
Chicago/Turabian StyleUngureanu, Claudia-Veronica, Iana Morozova, Georgiana Horincar, and Dumitra Răducanu. 2025. "Valorization of Pumpkin Seed Flour in Biscuit Production: Nutritional Enhancement and Sensory Acceptability" Sustainability 17, no. 22: 10103. https://doi.org/10.3390/su172210103
APA StyleUngureanu, C.-V., Morozova, I., Horincar, G., & Răducanu, D. (2025). Valorization of Pumpkin Seed Flour in Biscuit Production: Nutritional Enhancement and Sensory Acceptability. Sustainability, 17(22), 10103. https://doi.org/10.3390/su172210103






