Study on the Sustainable Degradation of Sulfur Hexafluoride by Thermal Plasma for Greenhouse Gas Abatement
Abstract
1. Introduction
2. Experimental Setup and Evaluation Method
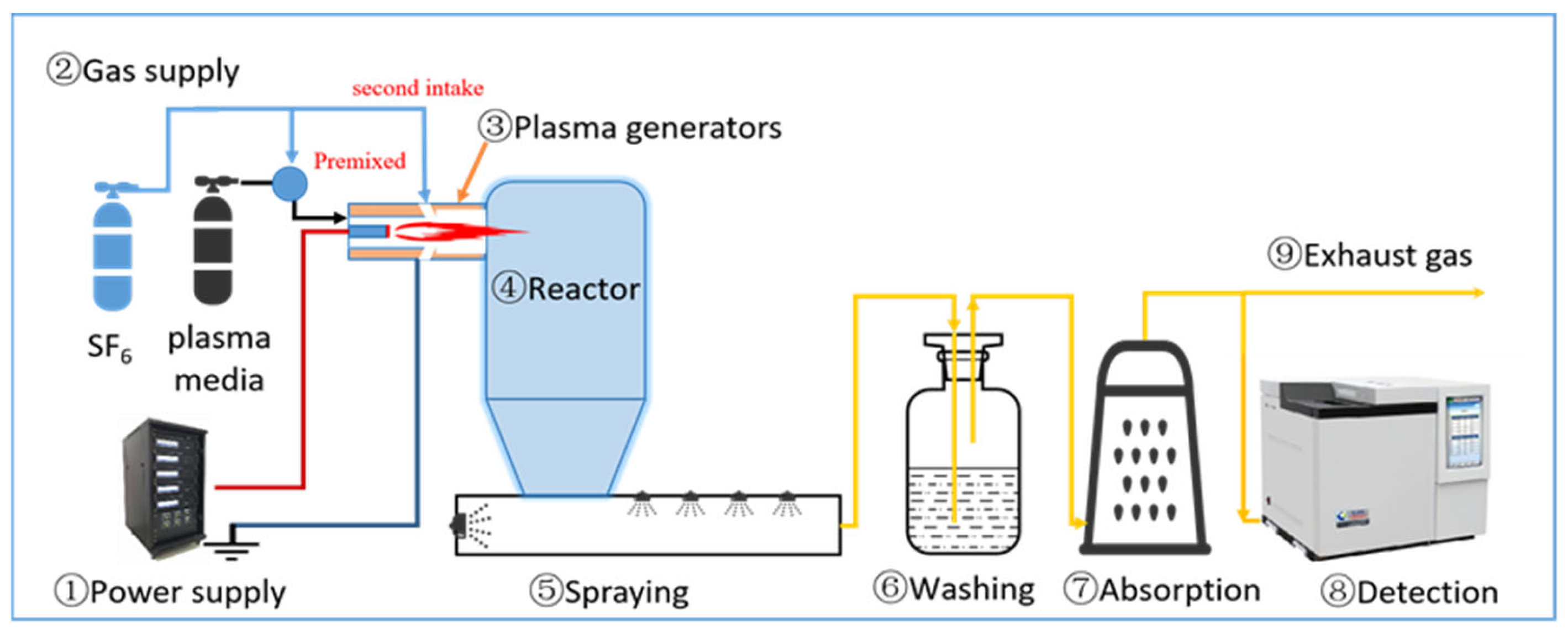
2.1. Experimental Setup
2.2. Evaluation Indexes
2.3. Analysis of Gas Degradation Products
3. Results and Discussion
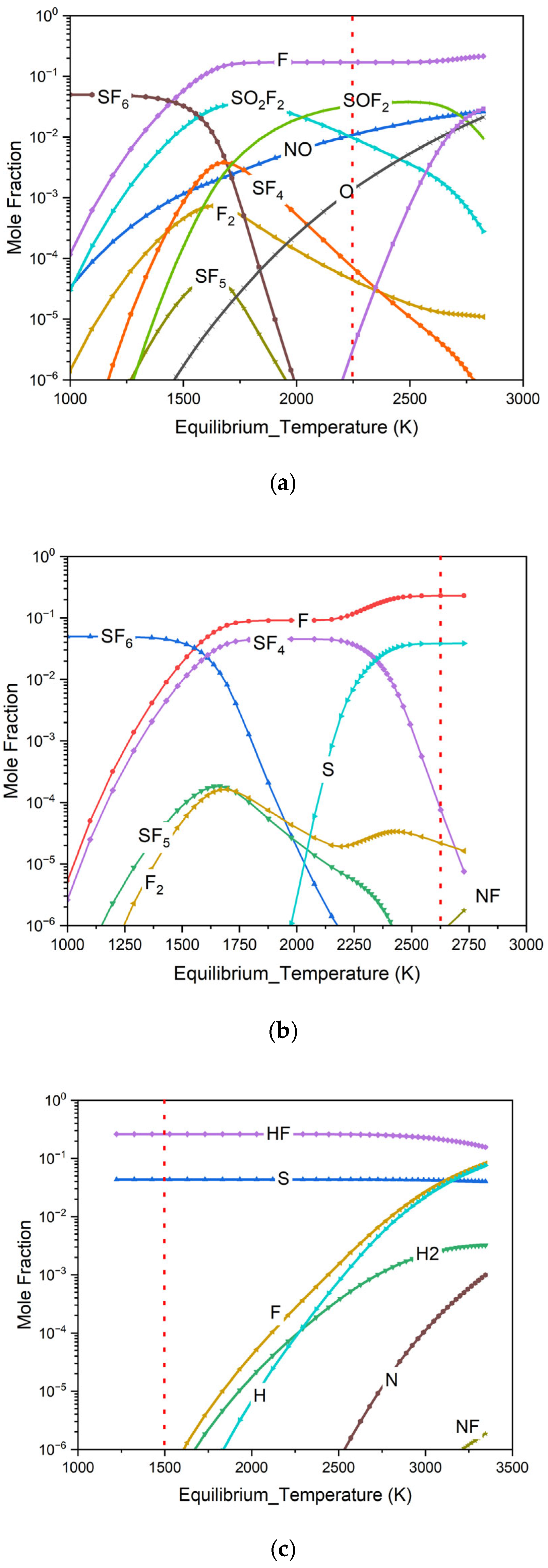
3.1. Chemical Equilibrium Calculation and Analysis Under Different Plasma Working Gas Atmospheres
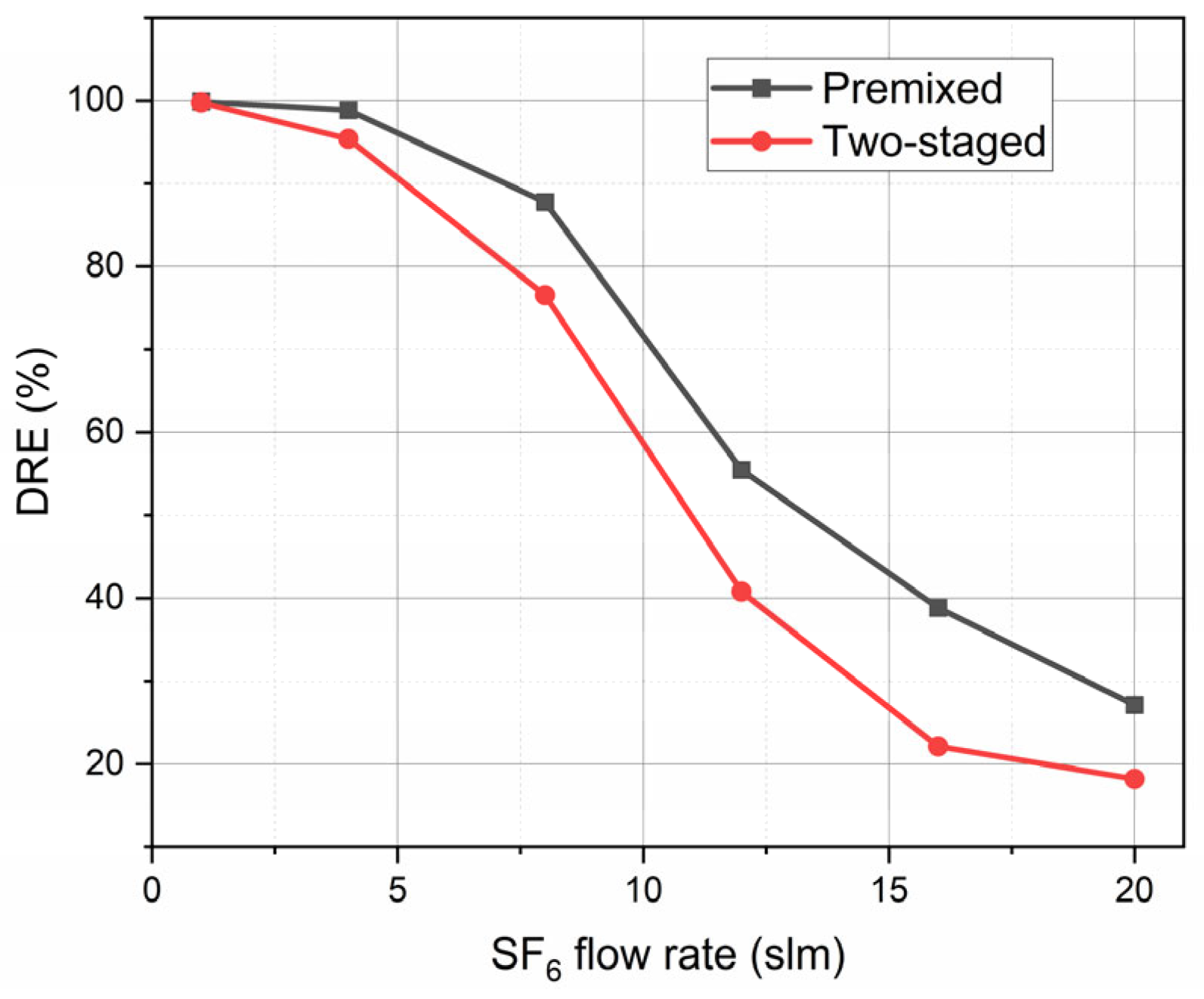
3.2. Influence of Mixing Methods on the Degradation Effect of Thermal Plasma
3.3. Influence of Discharge Current on the Degradation Effect of Thermal Plasma
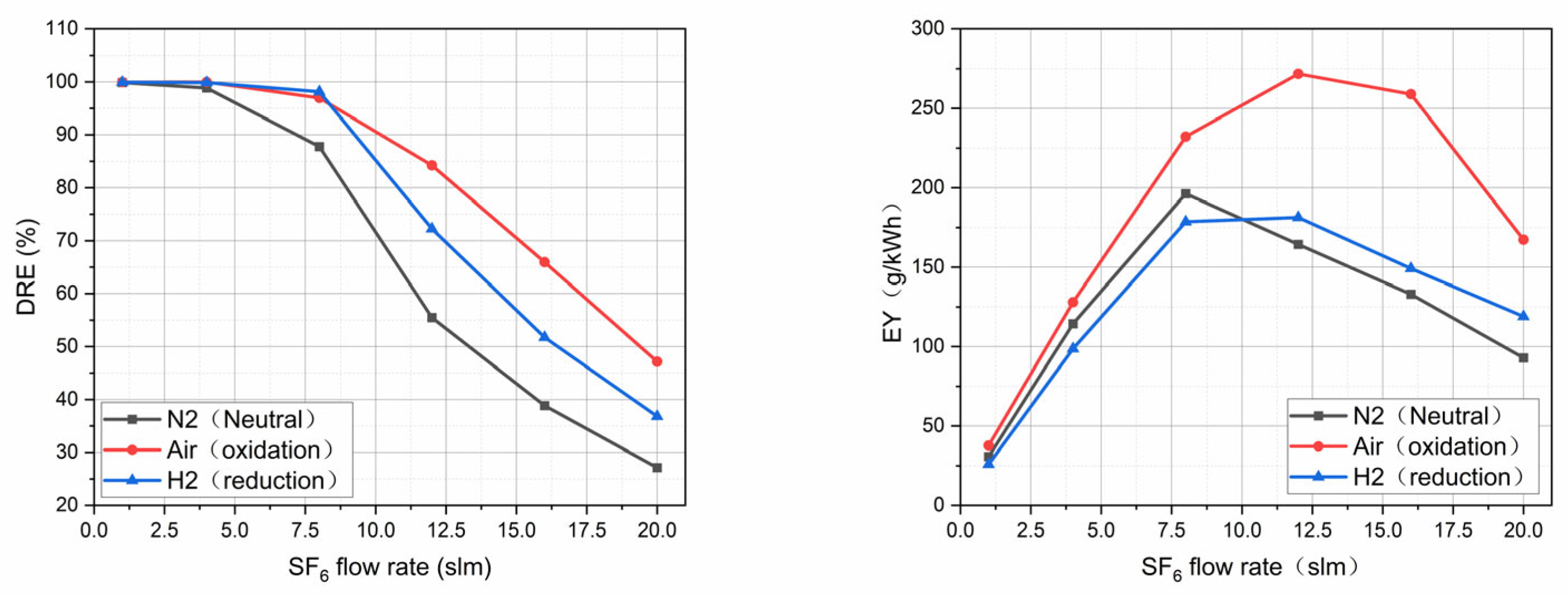
3.4. Influence of Different Atmospheres on the Degradation Effect of Thermal Plasma
4. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, Z.; Hao, P.; Yan, L.; Li, L.; Wang, Y.; Zhou, C.; Zhang, X. Review on Harmless Abatement of SF6 Waste Gas. Proc. CSEE 2023, 43, 7720–7736. [Google Scholar]
- Global Monitoring Laboratory. Earth System Research Laboratories. Available online: https://gml.noaa.gov/hats/combined/SF6.html (accessed on 15 August 2025).
- Zhang, X.; Wang, Y.; Tian, S.; Chen, F.; Hu, J. Influence of O2 on the Thermal Catalytic Degradation of SF6 Waste Gas by CePO4. High Volt. Eng. 2022, 48, 2152–2158. [Google Scholar]
- Nanjo, Y.; Ohyama, R. An experimental study on vacuum-ultraviolet photochemical reaction to non-thermal plasma oxidized SF/sub 6/ gases. In Proceedings of the CEIDP ‘05. 2005 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Nashville, TN, USA, 16–19 October 2005. [Google Scholar]
- Shih, M.; Lee, W.J.; Tsai, C.H.; Tsai, P.J.; Chen, C.Y. Decomposition of SF6 in an RF Plasma Environment. J. Air Waste Manag. Assoc. 2002, 52, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.V. Decomposition of SF6–R134a effluents by RF plasma. Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrom Detect. Assoc. Equip. 2012, 661, S245–S248. [Google Scholar] [CrossRef]
- Marilena, R.; Shahid, H. Microwave plasma removal of sulphur hexafluoride. J. Hazard. Mater. 2009, 164, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cho, C.; Shin, D.; Hong, Y.; Shin, Y. Abatement of fluorinated compounds using a 2.45GHz microwave plasma torch with a reverse vortex plasma reactor. J. Hazard. Mater. 2015, 294, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zhang, X.; Yuan, T.; Xing, P.; Luo, Y.; Tang, J. Plasma-assisted abatement of SF6 in a dielectric barrier discharge reactor: Investigation of the effect of packing materials. J. Phys. Appl. Phys. 2019, 53, 025205. [Google Scholar] [CrossRef]
- Zhuang, Q.; Clements, B.; McFarlan, A.; Fasoyinu, Y. Decomposition of the most potent greenhouse gas (GHG) sulphur hexafluoride (SF6) using a dielectric barrier discharge (DBD) plasma. Can. J. Chem. Eng. 2013, 92, 32–35. [Google Scholar] [CrossRef]
- Sun, H.; Long, H.; Wu, Y.; Guo, Y.; Rong, M. Simulation and experimental study on the degradation of the greenhouse gas SF6 by thermal plasma. Environ. Res. 2022, 216, 114411. [Google Scholar] [CrossRef]
- Yu, D.; Hao, H.; Yun, G.; Yi, W.; Yun, Z.; Yang, L. Comparison of the Effect of H2 and O2 on SF6 Degradation by Hot Plasma. In Proceedings of the 2024 7th International Conference on Electric Power Equipment-Switching Technology (ICEPE-ST), Xiamen, China, 10–13 November 2024. [Google Scholar]
- Choi, S.-S.; Park, D.-W.; Watanabe, T. Thermal plasma decomposition of fluorinated greenhouse gases. Nucl. Eng. Technol. 2012, 44, 21–32. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Zhao, Y.; Hu, H.; Ma, F.; Cao, J.; Lin, T.; Li, J.; Chen, X. Study on the Sustainable Degradation of Sulfur Hexafluoride by Thermal Plasma for Greenhouse Gas Abatement. Sustainability 2025, 17, 10030. https://doi.org/10.3390/su172210030
Zhu S, Zhao Y, Hu H, Ma F, Cao J, Lin T, Li J, Chen X. Study on the Sustainable Degradation of Sulfur Hexafluoride by Thermal Plasma for Greenhouse Gas Abatement. Sustainability. 2025; 17(22):10030. https://doi.org/10.3390/su172210030
Chicago/Turabian StyleZhu, Shan, Yue Zhao, Haoxin Hu, Fengxiang Ma, Jun Cao, Tao Lin, Jiachen Li, and Xianhui Chen. 2025. "Study on the Sustainable Degradation of Sulfur Hexafluoride by Thermal Plasma for Greenhouse Gas Abatement" Sustainability 17, no. 22: 10030. https://doi.org/10.3390/su172210030
APA StyleZhu, S., Zhao, Y., Hu, H., Ma, F., Cao, J., Lin, T., Li, J., & Chen, X. (2025). Study on the Sustainable Degradation of Sulfur Hexafluoride by Thermal Plasma for Greenhouse Gas Abatement. Sustainability, 17(22), 10030. https://doi.org/10.3390/su172210030





