Abstract
Depression is becoming more common in the face of modern life’s obstacles. Antidepressants are a fast-expanding pharmaceutical category. Antidepressant residues in water must be closely monitored and kept at levels that do not endanger human health, just like those of other psychotropic medications. Additionally, research has shown that these pollutants severely hinder aquatic life’s ability to migrate, reproduce, and interact with one another when they enter natural ecosystems. Antidepressants released into the natural environment can therefore be expected to have an impact on exposed fish and other aquatic species. There is a lot of information available about how exposure affects fish, but much of it is for exposure levels higher than those seen in their natural habitats. Antidepressants can bioaccumulate in fish tissues, and some behavioral effects have been documented for exposures that are relevant to the environment. As a result, antidepressant residue removal methods must be incorporated into contemporary wastewater treatment plant technology. In addition to covering a wide range of suggested treatment options and their ecotoxicological consequences on non-target organisms, this study discusses recent efforts to accomplish this goal. First, a thorough analysis of the harmful impacts on non-target people is provided. This work describes a variety of adsorptive methods that can make use of modern materials like molecularly imprinted polymers or ion-exchange resins or can rely on well-known and efficient adsorbents like silicates or activated carbon. Although extractive methods are also taken into consideration, they are now impractical due to the lack of reasonably priced and ecologically suitable solvents. Lastly, sophisticated oxidation methods are discussed, such as electrochemical alternatives, UV and gamma radiation, and ozone therapy. Notably, some of these techniques could totally mineralize antidepressant toxicants, either alone or in combination. Lastly, the topic of biological treatment with microorganisms is covered. This method can be very specific, but it usually prevents full mineralization.
1. Introduction
Because of the rising incidence of anxiety and depression, which are among the most diagnosed psychiatric diseases, an increasing number of people are dealing with mental health issues. Antidepressants are the primary technique to treat psychiatric problems, and they are regularly one of the most often prescribed medications worldwide. Specifically, antidepressant prescriptions in England climbed considerably, going from 36 million to 70.9 million annually between 2008 and 2018 [1]. Exacerbating the situation, continuous societal uncertainty is expected to contribute to an increase in mental health problems. In terms of medication, selective serotonin reuptake inhibitors, tricyclic antidepressants, and serotonin-norepinephrine reuptake inhibitors remain the most widely prescribed antidepressant classes worldwide. Additional types of pharmaceuticals used to treat these problems include partial serotonin reuptake inhibitors, dopamine-norepinephrine reuptake inhibitors, and newer multimodal-action medications [2,3]. To improve a patient’s mood, all classes use manipulation of the brain’s adrenergic, serotonergic, and dopaminergic systems, although their main mechanisms of action vary slightly. The antidepressants used vary significantly by region. For instance, in the United States in 2018, venlafaxine (48.363 kg), sertraline (57.575 kg), and duloxetine (37.863 kg) were the three most prescribed medications by weight, whilst in England, the top three were sertraline (7.408 kg), amitriptyline (10.149 kg), and venlafaxine (4.402 kg) [1]. Nonetheless, sertraline was the most prescribed antidepressant in both the US and the UK. For a review of antidepressant prescription and use rates in the US, Brazil, Sweden, and Australia, see [1].
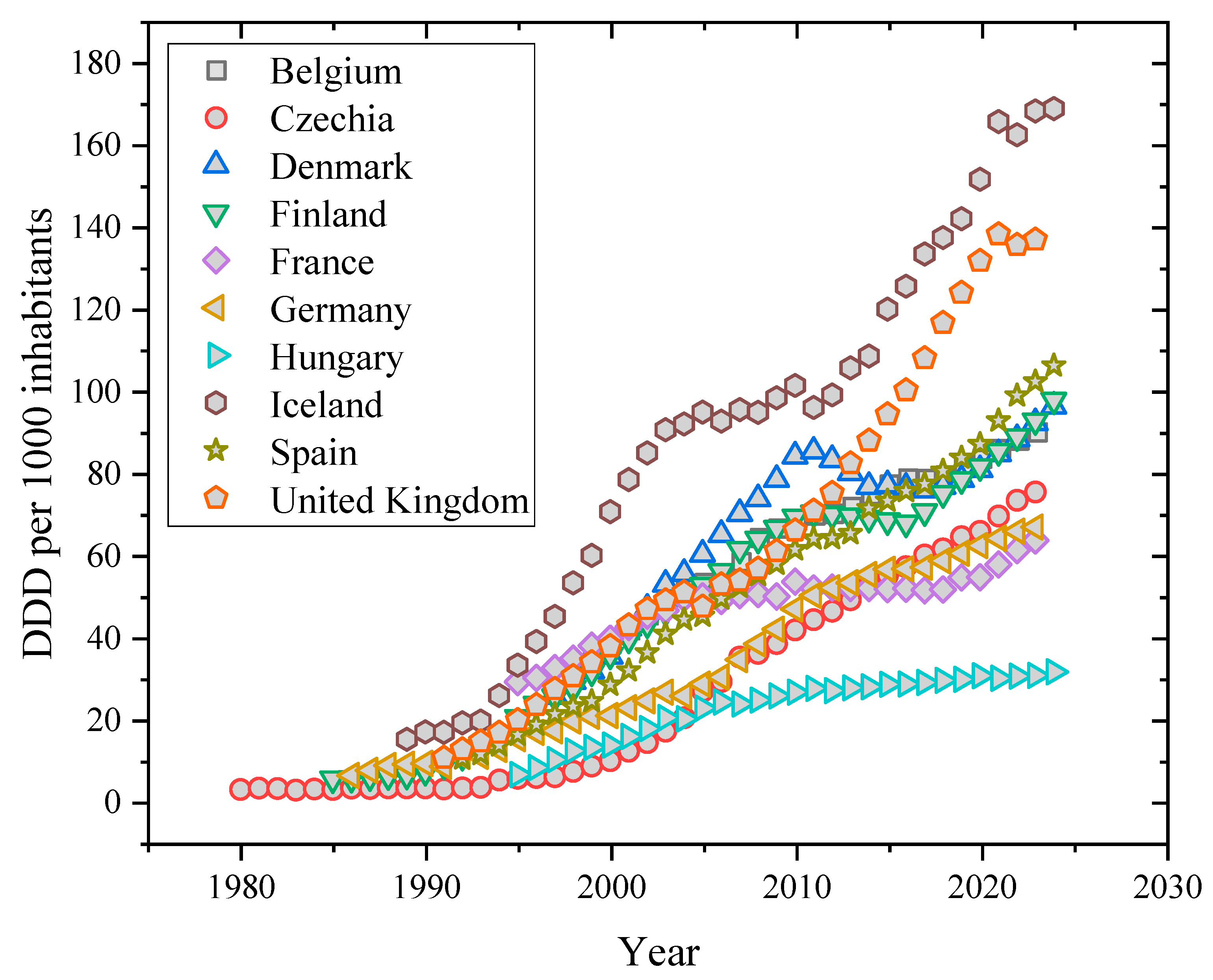
Due to the growing demands, difficulties, and pressures that modern people face in their daily and professional lives, a particular class of pharmaceutical products has been prescribed and used more frequently in European nations as well as other developed nations like Canada, Australia, New Zealand, South Korea, and Israel during the past 20 years [4]. Antidepressants are representative of this class. The following graph (Figure 1) illustrates how, based on the most recent data published by the Organization for Economic Cooperation and Development (OECD), the defined daily dosage (DDD) per 1.000 inhabitants has been gradually rising in several EU and EA nations in recent years. The definition of the daily dosage in this graph is “the assumed average maintenance dose per day for a drug used for its primary indication in adults.” The prescription and consumption of antidepressants have increased in all European societies, which is consistent with their yearly per capita healthcare expenditures, despite the fact that nations like Iceland, Portugal, Sweden, and the United Kingdom seem to be the biggest consumers of these medications [5]. A survey released in 2015 found that roughly 7.2 percent of adults throughout the 27 European Union member nations used antidepressants frequently, with rates varying greatly—from a peak of 15.7 percent seen in Portugal to a low of 2.7 percent in Greece [5]. Notably, these figures have increased since this first research and are anticipated to continue growing, mostly due to the impact of global COVID-19 restrictions, which have aggravated depressed symptoms through increased isolation and fewer social ties [6,7].
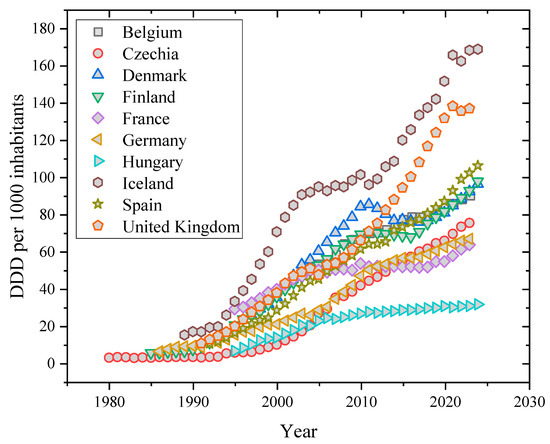
Figure 1.
Historical evolution of antidepressant consumption according to OECD information.
Notably, these figures have increased since the first study and are anticipated to continue growing, mostly due to the impact of global COVID-19 restrictions, which have aggravated depressive symptoms through increased isolation and fewer social ties [8,9]. Since then, several investigations have documented the presence of several antidepressants in surface waters across the globe at the ng L−1 level. There has been a correlation between high prescription and consumption rates and rising levels of drug and metabolite contamination in municipal waters or wastewater from hospitals or pharmaceutical companies [10,11]. Antidepressants account for just around 4% of all pharmaceuticals discovered in the environment, but their quantities in wastewater are unexpectedly high. Antidepressant contamination in raw wastewater treatment plant outflows has been measured from the limit of detection up to roughly 3000 ng L−1, whereas final treated effluents commonly contain amounts ranging from tens to hundreds of ng L−1 [12,13]. Considering how harmful these chemicals are to people and aquatic life, these are concerning levels [11]. While the molecular targets for antidepressants share considerable similarities between fish and mammals (exhibiting excellent structural conservation), measured doses of these medicines are usually lower than those expected to cause adverse effects in fish species. [14]. There is little information on long-term exposure to some of these medications, and they are known to bioaccumulate in fish [15,16]. Furthermore, accumulating data suggests the potential for antidepressant pollution to harm wild fish populations. Recent studies have revealed numerous behavioral and physiological abnormalities in fish exposed to doses that resemble those found in genuine aquatic habitats. Additionally, fish will be exposed to antidepressant mixes with comparable mechanisms of action, thus the possibility of combined effects is reasonable [17,18].
These elements have all strengthened the case for environmental remediation research on these medications. Secondary stage, activated sludge, and membrane bioreactors are examples of biological treatments that can degrade some antidepressants (like venlafaxine and desvenlafaxine) moderately well, frequently >70% in specialized systems. However, they struggle with more resistant compounds like carbamazepine and many selective serotonin reuptake inhibitors, usually achieving a removal efficiency of 50% or less [19]. Adsorption with metal oxide nanoparticles (such as magnetite red mud bioadsorbents), biochar, activated carbon, and membrane filtration techniques like reverse osmosis or nanofiltration are examples of physical methods. In lab tests, adsorption can eliminate 80% to 97% of some antidepressants, but reverse osmosis/nanofiltration can reject >95%, contingent on membrane pressure and circumstances [20,21]. Ozonation, ultraviolet/H2O2, Fenton, photo-Fenton, electro-Fenton, or photocatalysis are examples of advanced oxidation processes. These produce strong hydroxyl radicals that mineralize drugs into CO2 and other safe byproducts, frequently eliminating them with >95% efficiency and significantly lowering any remaining toxicity [22]. In recent years, the cocatalytic (or modified) Fenton system, as a branch of Advanced Oxidative Processes (AOP), has been increasingly used to remove refractory organic pollutants from wastewater [23]. This type of system combines the classic Fenton reagent (Fe2+ + H2O2) with additional catalysts or auxiliary substrates (such as high-iron industrial waste, doped nanoparticles, heterogeneous catalysts, carrier surfaces, etc.) with the aim of increasing the efficiency of oxidative radical generation, expanding the useful pH range, reducing reagent consumption, or minimizing the formation of unwanted byproducts. Recent studies demonstrate that iron-base hybrid catalysts can efficiently remove persistent dyes, antibiotics, or other resistant organic contaminants, achieving high removal rates (sometimes 90–100%) in relatively short times, especially when combined with irradiation (UV or ultrasound) or coupled processes. An integrated multi-barrier treatment is the most successful strategy. This usually consists of membrane filtration or biological secondary treatment, which is followed by tertiary polishing using sophisticated oxidative techniques and/or activated carbon adsorption. When it comes to eliminating antidepressants and lessening ecotoxicological effects, these combinations work best.
This study critically examines the available research on potential environmental concerns related to antidepressants by synthesizing published data on exposure levels and documented effects on both people and fish. Furthermore, the article investigates improvements in technologies aimed at removing these toxins before concluding by highlighting important data gaps and proposing additional research to increase our general understanding of the hazards posed by antidepressants in aquatic environments.
2. Environmental Occurrence and Pathways of Antidepressants
The biological activity of pharmaceuticals at very low concentrations and their engineered chemical stability, which increases persistence in the environment, have made them pollutants of increasing environmental concern [24]. It is just astounding how much information is already available regarding how pharmaceuticals affect the environment. A recent study found that over 4.000 different pharmacologically active substances, including human and animal prescription pharmaceuticals and over-the-counter drugs, are being given worldwide [25]. In 2020, an estimated 4.5 trillion doses of medications was taken globally [25]. The trend is likely to continue, based on the following factors: climate change may worsen existing diseases (both communicable and noncommunicable); economies are rising, particularly in emerging economies, raising expectations and capacity to treat chronic diseases and aging; aquaculture and livestock operations are intensifying to fulfill demand; and new medications are being developed [25].
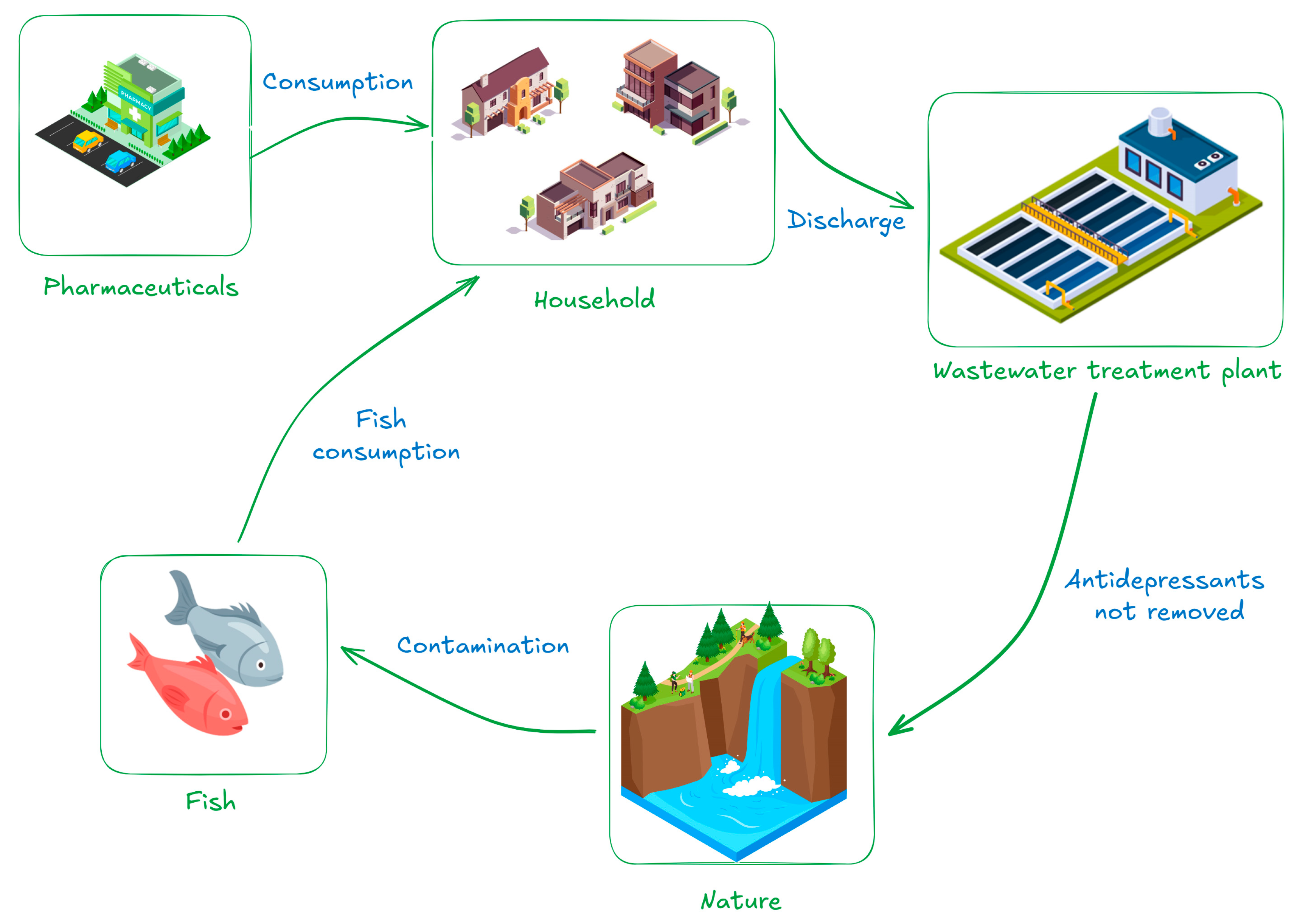
An illustration (Figure 2) of the antidepressant environmental cycle is shown below. Following manufacture and distribution, the public consumes these medications, which are then eliminated and end up in wastewater treatment facilities. A large percentage of these pollutants return to the environment and end up in rivers, lakes, and other bodies of water since traditional treatment methods frequently fall short of fully eliminating them. Aquatic creatures, including fish, are exposed in this situation and may accumulate these compounds in their tissues, which could have ecotoxicological effects and jeopardize biodiversity.
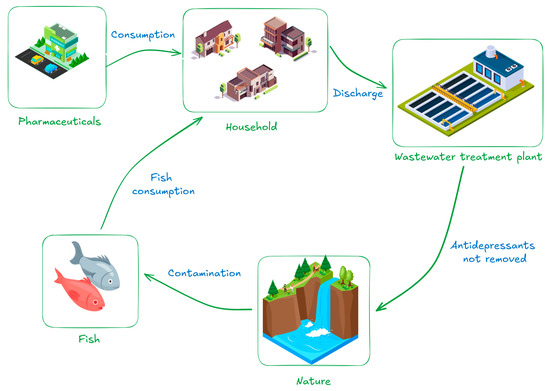
Figure 2.
Pathway of antidepressants from consumption to bioaccumulation.
Over the last 25 years, advances in mass spectrometry analytical techniques have allowed researchers to analyze pharmaceutical residues in the environment, which was previously impossible. Initially, infrequent reports of active medicinal substances in the environment, primarily resulting from factory wastes, received little attention [26,27,28]. However, the finding of estrogens in sewage effluents that cause fish feminization in the late 1990s radically transformed ecotoxicologists’ focus, which had previously been mostly on sewage and river effluents. Despite the pharmaceutical industry’s claims that financial reasons would prevent significant breaches, recent research has proven that these assumptions were incorrect [29]. The finding of diclofenac levels in cow carcasses serves as the first evidence tying the huge drop in vulture populations across India and Pakistan to exposure through a previously unknown mechanism [30]. A subsequent series of studies revealed that pharmaceutical companies headquartered in Patancheru, India, were discharging extremely high amounts of pharmaceuticals into the environment [31,32,33,34].
This area is a major hub to produce bulk pharmaceuticals worldwide due to its dense industrial concentration. A treatment plant that handled wastewater from about 90 production facilities had higher levels of several medications in its effluent than in the blood of patients who were taking the drug. High doses of various broad-spectrum antibiotics increase the chance of resistance development. Ciprofloxacin, the most often used medication, has been identified at concentrations as high as 31,000 µg L−1, exceeding many bacteria’s tolerance limits by over 1000 times [31]. Estimates suggested that 44 kg of ciprofloxacin were released per day, equivalent to Sweden’s entire five-day pharmaceutical intake enough to treat a city of 44,000 people. As a result, these accidents caused previously inconceivable amounts of contamination in surfaces, drinking water, and soil [34], river sediments [33], and irrigated soils [35]. Because of the extensive media coverage of this study, scientists and the general public became more interested in this exposure channel [29,36].
In a variety of environmental compartments, such as surface water, wastewater, groundwater, biota, air, soil, and even tap water, almost 700 distinct drug residues have been discovered. There are many books that look at how psychiatric drugs are used in the workplace [37]. Carbamazepine’s widespread use has even led to its suggestion as a marker for water bodies impacted by wastewater [38]. Additionally, once in the environment, the metabolites or original pharmaceuticals undergo complex metabolic processes and undergo changes due to a variety of species and physicochemical phenomena, including photodegradation and adsorption to solids. For instance, research on the photodegradation of psychiatric medications revealed that this leads to “transformation compounds” [39]. Due to their ability to stay unaltered in the environment for decades, certain compounds are especially persistent. Oxazepam is a common example in this instance, having remained unaltered at the bottom of Swedish lakes for more than 30 years [40].
The availability of antidepressants in the environment is influenced by factors such as patient dosage, how the body processes and eliminates the medicine, and the effectiveness of wastewater treatment plants in eliminating active drug molecules [41]. The majority of studies document that antidepressants are most frequently prescribed in developed countries, although patient use varies by socioeconomic status [42,43]. They are usually found in wastewater and effluents from surface water treatment plants at concentrations ranging from ng L−1 to low μg L−1. Table 1 and Table 2 provide a summary of the levels of different antidepressants in the environment and the dosages of these drugs consumed by nation. In the UK, the highest levels of amitriptyline, the most commonly used tricyclic antidepressant, have been found in river water and wastewater at 0.243 μg L−1, respectively [44] and 0.0716 μg L−1 [45]. Nonetheless, certain effluents or waterways have been found to have local hotspots of elevated antidepressant concentrations. Wastewater treatment facilities in India, such as those in India, discharged citalopram, a medicine that inhibits serotonin reabsorption, into the environment. Measurements revealed the substance at levels ranging from 76 to 430 micrograms per liter in both the effluent of these plants and the downstream river flows [34]. This area was not included in the analysis due to its high concentration of drug factories. Venlafaxine, the serotonin-norepinephrine reuptake inhibitor most commonly administered, was measured at 1.31 μg L−1 [46] and 2.19 μg L−1 [47]. In the United States, antidepressant metabolites have been found in surface water samples at higher amounts than the parent chemical in effluents from and downstream of treatment plants. For those who have biological activity, the existence of metabolites is especially crucial. O-desmethylvenlafaxine, for instance, is a metabolite of venlafaxine, and in Germany, it was found to be up to six times more abundant than the parent substance [48].

Table 1.
Data on antidepressant prescription numbers and their potential annual usage across countries with high antidepressant consumption.

Table 2.
Antidepressant presence in aquatic environments at diverse surface water sample locations in different nations worldwide.
3. Modes of Action of Antidepressants
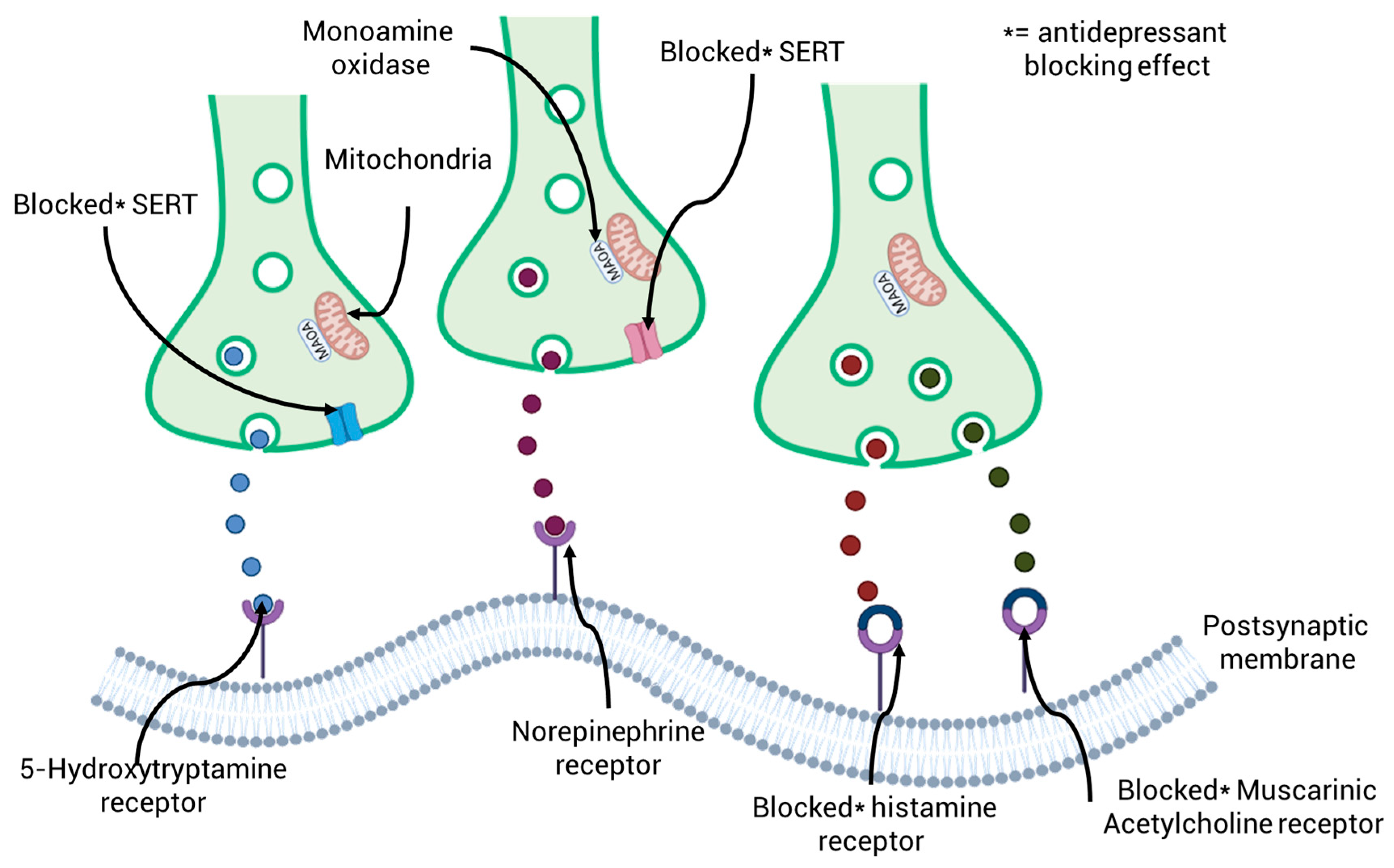
Tricyclic antidepressants, which were first made available in the 1980s, represent a significant class of drugs used to treat depression. However, due to better safety profiles and efficacy offered by newer medications, their usage has declined globally. The way these older antidepressants work is by blocking the uptake of norepinephrine and serotonin (5-hydroxytryptamine) at their respective transporters, which are in the brain’s presynaptic neurons and throughout the peripheral nervous system. By increasing these neurotransmitters locally, this dual action eventually reduces their release into the gaps between nerve cells [73]. The secondary pharmacology of both also varies, sometimes involving action on muscarinic acetylcholine and histamine receptors [74]. Figure 3 provides a schematic illustration of how the main kinds of antidepressants work. Amitriptyline, which is still used in some nations to treat serious depression, was once the most often prescribed positively ionized tricyclic antidepressant. N-demethylation in the patient converts amitriptyline to nortriptyline, which is also prescribed to treat depression-related conditions and has a similar mode of action to the parent drug [75]. By blocking the sertraline receptor, selective serotonin reuptake inhibitors raise serotonin levels in the synaptic cleft by preventing serotonin reuptake in presynaptic terminals (Figure 3). Anatomical Therapeutic Chemistry and serotonin and norepinephrine reuptake inhibitors block the norepinephrine transporter, which stops norepinephrine reuptake in addition to serotonin reuptake. Additionally, via postsynaptically antagonistically binding to histamine and muscarinic acetylcholine receptors, positively ionized tricyclic antidepressants demonstrate secondary pharmacological effects.
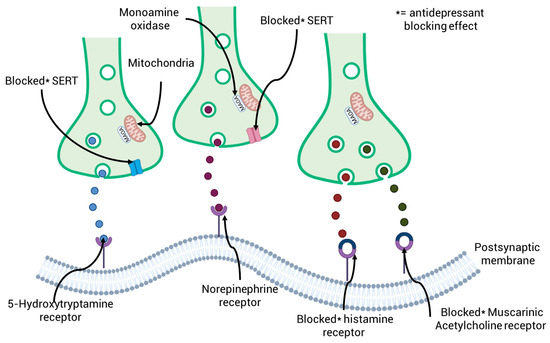
Figure 3.
A schematic diagram illustrating the primary therapeutic modes of action of the three primary groups of antidepressants: selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors, and anatomical therapeutic chemicals.
Due to their improved safety profiles, selective serotonin reuptake inhibitors have largely supplanted tricyclic antidepressants and are currently the most prescribed class of antidepressants [1]. Sertraline inhibition is how selective serotonin reuptake medications function, specifically preventing serotonin from being reabsorbed in presynaptic neurons [76] additionally, the potency level and secondary pharmacology profile of sertraline vary amongst the drug’s different molecules, as is the case with Anatomical Therapeutic Chemistry [77]. Fluoxetine, the first selective serotonin reuptake inhibitor to be commercially available, has a strong affinity for sertraline but almost no affinity for other receptors or neurotransmitters [78]. Both the S (+) and R (−) enantiomers of fluoxetine, which are sold as a racemic combination, prevent serotonin reuptake. However, the potency of norfluoxetine, the primary metabolite of fluoxetine, varies between its two enantiomers. Since S-norfluoxetine is 14 times more powerful than fluoxetine, it is thought to be the main cause of fluoxetine’s ability to inhibit serotonin reuptake [79]. Newer selective serotonin reuptake inhibitors, such sertraline and citalopram, have gained popularity recently due to advancements in pharmacokinetics and efficacy, despite fluoxetine being the first to be given widely [80]. Realizing that combination therapy was much more effective in treating depression, the third major class of antidepressants, known as serotonin-norepinephrine reuptake inhibitors, were created to target both sertraline and the norepinephrine transporter [81]. Venlafaxine, which was manufactured as a combination of S (+) and R (−) enantiomers, was the first serotonin-norepinephrine reuptake inhibitor to be sold commercially. The S enantiomer primarily inhibits sertraline, whereas the R enantiomer inhibits both sertraline and the norepinephrine transporter. Consequently, serotonin uptake inhibition is roughly three times higher than norepinephrine’s [82]. O-desmethylvenlafaxine, a pharmacologically active main metabolite of venlafaxine, blocks norepinephrine and serotonin reuptake just as effectively as the parent drug.
A neurotransmitter that is frequently present in vertebrates’ central nervous systems, serotonin regulates a number of fundamental processes in mammals, such as arousal, muscular control, sleep, social behavior, nutrition, memory, and learning [83]. The main source of serotonergic neurons in the mammalian central nervous system is the anterior and posterior raphe nuclei, which are made up of nine different structures that span several different parts of the brain [84]. It is interesting to note that, despite their different numbers, teleost fish only have six, the architecture of the serotonergic system in bony fish and teleosts is very similar to that found in mammals. Numerous regions of the teleost brain, including the spinal cord, vagal lobes, posterior tubercle/hypothalamus, teleost retina, and pretectum, have been found to have serotonin-positive cells [85]. Although the exact makeup and purpose of these cells are still not fully understood, a recent study looked at the chemical neuroanatomy of the zebrafish (Danio rerio) serotonergic system. [86]. Additionally, scientists have discovered two human sertraline isoforms in zebrafish, slc6a4b and slc6a4a, which exhibit 75% and 66–69% amino acid sequence identity with other mammalian serotonin transporters, respectively [1]. The zebrafish brain exhibits widespread expression of Slc6a4a, particularly in the raphe and pretectum, while the expression of Slc6a4b is limited to the paraventricular organ, hypothalamus, and medulla oblongata [87]. Three primary kinds of serotonin receptors 5-HT2, 5-HT1, and 5-HT7 have been found in all fish species. Three 5-HT1 subtypes have been identified in zebrafish: HT1ab, HT1aa, and HT1bd; ref. [87] divides five-HT2 into two further subgroups, five-HT2C and five-HT2A [88]. The human HTR1A protein is 76% and 69% similar with the two zebrafish-encoded proteins, htr1ab and htr1aa, respectively [87]. In total, zebrafish have orthologs that are equivalent to 66% of human serotonin drug targets [14]. Teleosts, like mammals, rely heavily on serotonin for appetite and feeding [89,90], in motor activity [91] and social behavior, including aggression. Through a variety of mechanisms, including promoting gonadotropin secretion and directly influencing oocyte maturation in Japanese medaka (Oryzias latipes), serotonin also influences fish reproductive processes [92,93].
The mammalian noradrenergic system plays a significant role in a variety of processes, encompassing areas such as memory formation [94], aggressive behavior [95] the modulation of pain [96], and the regulation of mood and anxiety [51]. The slc6a2 gene, which codes for the norepinephrine transporter, a major target of antidepressant drugs, is an essential part of this system. Zebrafish also have a comparable form of this gene [52]. Most noradrenergic projections throughout the mammalian body originate from the locus coeruleus, an area of the brain that contains about 45,000 neurons in humans [65,70]. Even teleost fish share this circuit is high degree of conservation with other vertebrate lineages. But there have only been reports of roughly 14 neurons in zebrafish overall [52]. The three primary adrenergic receptor groups found in teleosts are ß, α2, and α1, which are the same as those found in humans [54,67,68,69]. There are nine recognized subtypes of these in mammals, while zebrafish have five different α2 receptor genes, despite the lack of evidence in other species [54,66]. Of these, three (α2B, α2A, and α2C) are orthologs of the human receptors, while the other two are duplicate subtypes of α2Db, α2, and α2Da that do not have human orthologs. Together, these paralogs’ protein sequences show 80–87% closeness to those of mammalian genes [54,69]. Additionally, it is known that humans only have three ß adrenergic receptors, whereas zebrafish have five. Additionally, zebrafish have two orthologs for ß3 and ß2, and one for ß1 [67]. Norepinephrine helps control blood pressure in teleosts [97], in pigmentation regulation [67], and in the control of many behaviors, such as the degree of hostility. For instance, aqueous exposure to norepinephrine causes the gills of the Siamese fighting fish (Betta splendens) to dilate, indicating that this monoamine is involved in controlling aggression levels [60].
Many of these drugs also alter dopamine levels in the central nervous system, albeit this is not thought to be the main way that most antidepressants work. The pretectum, olfactory bulb, locus ceruleus, and retina of zebrafish larvae’s brains express the dopamine transporter (slc6a3, DAT) [63]. The dopaminergic system in mammals, like those of other monoamines, is involved in regulating a number of adaptive processes, such as reward [58], memory [94], motor control [98], attention and cognition [99]. Numerous behavioral consequences in teleosts have been linked to the dopaminergic system, including the decrease in actions that resemble enhanced boldness [100] and anxiety [72], as well as modify locomotion [50,71] and associative learning outcomes for Labroides dimidiatus, the cleaner wrasse [101] and zebrafish [102]. Although the receptor orthologs of humans, zebrafish, and other teleost species are broadly similar, their distribution varies [53,103,104]. Fish do not exhibit the D5 receptor subtype found in humans.
Bioavailability and Bioaccumulation in Aquatic Organisms
The physiochemistry of the water, including pH and the compound’s lipophilicity, are among the elements that influence how drug residues are partitioned into biological compartments after they are in the aquatic environment [105], as well as how much medication is digested and eliminated by organisms exposed to the environment [106]. Ionizable organic chemicals, which are present in antidepressants, might change their speciation depending on the pH of the water, which can impact their bioavailability [107,108]. For instance, it has been demonstrated that the bioconcentration of fluoxetine in Japanese medaka rises with increasing pH, making the drug more hazardous at higher pH values [105]. The bioconcentration factor, also known as the observable bioconcentration factor (or the ratio of internal to external concentrations), is a measure of bioaccumulation that varies between species and among body regions within a single fish. To demonstrate this, rainbow trout (Oncorhynchus mykiss) exposed to an antidepressant-containing wastewater treatment plant effluent for 13 days accumulated more citalopram in their liver (observed bioconcentration factor = 47) than in their brain (observed bioconcentration factor = 9) [56]. However, the reverse pattern was shown after 90 days of exposure to brook trout (Salvelinus fontinalis) for three months [109]. Similarly, citalopram was only found in plasma in a study involving the round gudgeon (Neogobius melanostomus), whereas venlafaxine and bupropion were found to bioconcentrate in plasma, brain, liver, gonads, and muscle [61]. Naturally, the possibility of biological effects in fish is primarily determined by the compound’s potency and is not solely determined by bioavailability or bioaccumulation. Consequently, even though venlafaxine is less bioavailable in fish than many other antidepressants [57,110,111,112], when male mosquitofish (Gambusia holbrooki) were exposed to the same doses of sertraline, fluoxetine, and venlafaxine, only venlafaxine changed their patterns of diurnal activity [113].
An additional method for forecasting the potential for hazardous compounds to bioaccumulate in non-target organisms is the use of mathematical models. Assuming evolutionary conservation of drug targets, the fish plasma model predicts the likelihood of an effect by comparing the internal concentrations of target organisms (humans) and non-target organisms (fish) [59,114]. Researchers demonstrate that after a chronic 28-day water exposure, the Fish Plasma Model may be used to accurately predict plasma concentrations of the antidepressant fluoxetine in fathead fish (Pimephales promelas) [115]. But for behavioral anxiety-related endpoints, the lowest impact concentrations were just marginally higher than human therapeutic levels [115]. But when it comes to ionizable medications, the Fish Plasma Model is less reliable in this respect [32].
4. Ecotoxicological Effects on Aquatic Biota
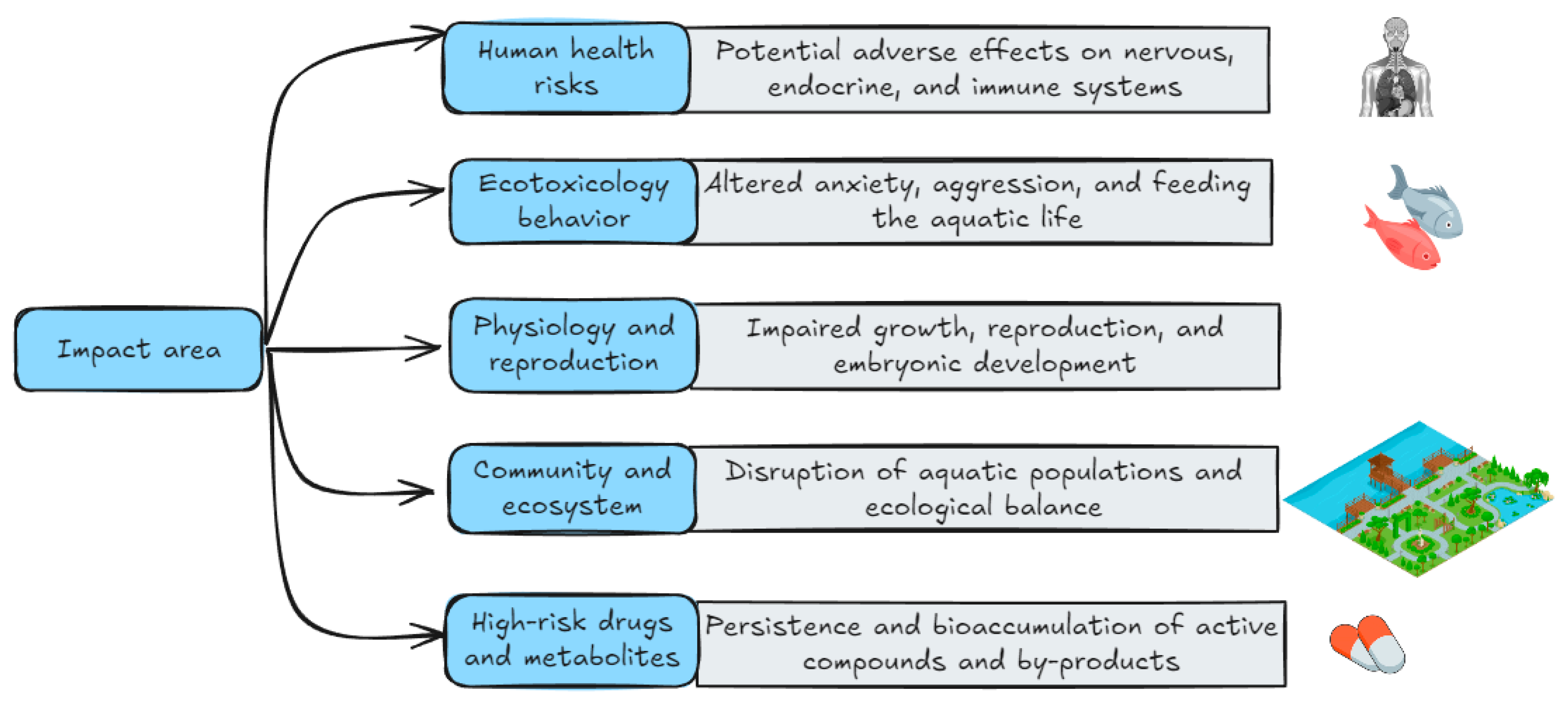
Despite limited research into the health impacts of pharmaceutical contamination, certain adverse effects have been noted, as depicted in Figure 4. However, a World Health Organization (WHO) assessment suggests that current drug levels detected in drinking water are unlikely to pose a significant risk [116]. A follow-up study conducted in China confirmed these findings [1]. However, the presence of pharmaceuticals in the environment can cause problems for particularly vulnerable patient populations, such as those with allergies [117]. Despite a lack of definitive research on the long-term health effects of multiple pollutants, significant uncertainties persist. Individuals are primarily exposed to consuming foods such as vegetables, fruits, meat, seafood, fish, and dairy products, as well as drinking water. Notably, the increasing prevalence of antibiotic-resistant bacteria is a critical global public health concern. We believe that the “One Health” concept, which emphasizes the interconnectedness of human, animal, and environmental well-being, is critical for addressing this issue. In general, the consumption of antidepressants during pregnancy can cause premature birth, low birth weight/fetal growth retardation/growth restriction/“small for gestational age” (SGA), problems in the neonatal period/adaptation to birth, respiratory disorders, jaundice, hypoglycemia and difficulties in regulating temperature also reported, in addition to persistent pulmonary hypertension in the newborn (PPHN) [118,119,120,121].
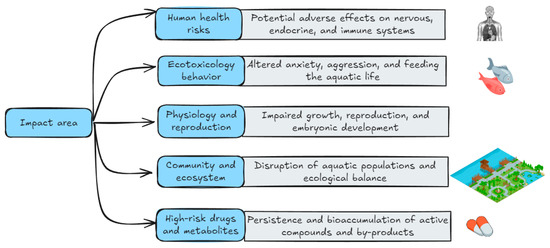
Figure 4.
Areas of impact of aquatic antidepressants: human health, ecotoxicological behavior, physiology and reproduction, communities and ecosystems, and high-risk compounds/metabolites.
Drugs can have harmful environmental effects that extend well beyond their effects on growth, reproduction, or death. For instance, by affecting an organism’s fitness and behavior, psychoactive chemicals might change population dynamics [71,72,98,100]. Both the therapeutic targets and the physiological systems that drugs can impact are not specific to humans. These signaling pathways and phylogenetically highly conserved traits are shared by many living things [50]. For instance, fish are used in a number of behavioral tests for experimental medications meant for human use, such as testing for stress, anxiety, and fear [102]. Numerous neurotransmitters and signaling pathways are similar in fish and humans. Indeed, biogenic monoamines like dopamine, serotonin, norepinephrine, and others are found in both invertebrates and vertebrates, including fish, insects, amphibians, and echinoderms [101,103]. According to evolutionary theory, some substances are so old that they are present in non-animals. For instance, plants contain serotonin [53] and acetylcholine in fungi and bacteria [104]. It has been demonstrated that fluoxetine affects cricket behavior differently [1]. Additionally, the antipsychotic clozapine makes fish constipated [41], by changing the microbial food chain, serotonin modifies nitrification processes [43] as well as plants that produce benzodiazepines that can affect your gabaergic system [42].
Given that different chemicals can use the same molecular pathways and are frequently found together in wastewater and surrounding waters, fish in aquatic environments are almost certainly exposed to contaminated mixtures. Antidepressants such as venlafaxine, nortriptyline, amitriptyline, fluoxetine, dosulepin, and norfluoxetine have been found at concentrations as low as nanograms per liter in UK waters [45]. Combining these concentrations yields a maximum of 207.9 ng L−1 in river water and 727.7 ng L−1 in effluent, putting exposure levels within the range previously shown to affect fish physiology and behavior [122,123,124,125]. Studies evaluating developmental retardation, death rates, and antioxidant enzyme activity over a 30-day period in common carp using nortriptyline, amitriptyline, and clomipramine indicate that exposure to antidepressant combinations may have cumulative effects [17]. Treatment with a combination of paroxetine, sertraline, mianserin, and fluoxetine resulted in a reduction in the survival and growth of hepatocytes within zebrafish larvae, as demonstrated by [18]. Due to the limited research currently available on this specific interaction, further investigation is needed. Moreover, it is possible that other non-antidepressant drugs or chemical contaminants present in surface water could influence the impact of these antidepressants.
4.1. Neurochemical and Molecular Effects
One important measure of the biological effect of an antidepressant is changes in monoamine levels in the central nervous system; this phenomenon has been seen in species different than the intended target. Studies have demonstrated that fish exposed to antidepressants like amitriptyline may have changed neurotransmitter levels. For example, studies involving zebrafish revealed that amitriptyline exposure led to a greater proportion of 5-hydroxyindoleacetic acid to serotonin, suggesting an elevated rate of serotonin neurotransmission within the synapses of the brain [55]. Amitriptyline’s pharmacological profile is consistent with the fact that no effect on norepinephrine production was seen [126]. Given that effects differ depending on the location of the brain, most published investigations used high-performance liquid chromatography to measure monoamine contents in whole-brain samples. This method is quite rudimentary. To demonstrate this, rainbow trout given 0.2 and 1 μg L−1 of venlafaxine showed increased dopamine, serotonin, and norepinephrine receptor levels, particularly in the midbrain [122]. Therefore, a crucial first step in assessing the potential impact of antidepressant therapy on fish is to use techniques that can discriminate between different levels of monoamines in various brain areas. Studies have demonstrated that antidepressants can impact on the genes that produce neurotransmitter transporters and receptors in fish, albeit the outcomes have been mixed. In particular, it has been noted that zebrafish exposed to fluoxetine exhibit decreased expression of genes including htr1ab, htr1aa, htr1b, htr5a, and htr2a [127]. This medication appears to affect the dopaminergic and adrenergic systems in addition to the serotonergic system in zebrafish. According to more recent studies on the effects of fluoxetine on zebrafish larvae and embryos, concentrations that mirrored those seen in the environment caused the expression of the serotonin transporters slc6a4b and slc6a4a to rise [128].
4.2. Impacts on Development, Growth, and Reproduction
Fish exposed to antidepressants showed changed neurotransmitter levels in their brains, which would predict subsequent behavioral and physiological impacts [129]. Numerous physiological and behavioral effects in fish exposed to antidepressants have been documented, which lends credence to this. Growth inhibition accelerated embryonic hatching, and effects on bone development are examples of developmental and morphological effects. For instance, it has been demonstrated that zebrafish eggs exposed to amitriptyline display dose-dependent rapid hatching at a low impact concentration of 0.01 μg L−1, which has been detected in certain surface waters [123]. Studies show that several positively ionized tricyclic antidepressants and selective serotonin reuptake inhibitors can adversely impact growth in a range of fish species and environmental circumstances. Serotonin-mediated appetite suppression, for instance, caused the brackish water fish, the croaker (Argrosomus regius), to lose weight and lengthen their bodies after being exposed to 3 μg L−1 of fluoxetine for 15 days [130]. Additionally, amitriptyline and fluoxetine treatment at low concentrations (0.1 μg L−1) inhibited embryonic–larval growth in zebrafish by downregulating genes associated with the early growth response (egr4 and egr1) and cell growth and differentiation (dusp5) [131]. Sertraline and citalopram also had an impact on zebrafish bone formation, but only at far higher concentrations than are normally present in the environment [132].
There is a lot more publicly available information about how antidepressants affect fish reproductive processes and associated behaviors than there is about mammalian reproduction [133,134]. For example, zebrafish exposed to 32 μg L−1 of fluoxetine for seven days produced a reduced number of eggs [135], and venlafaxine at a dosage of 10 μg L−1 for six weeks had the same effect [136]. For fathead minnows, fluoxetine at a dosage of 100 μg L−1 for four weeks also changed male reproductive behavior, including hostility toward the mate and nest maintenance [137]. Pre-copulatory mating behaviors have also been observed to be impacted by antidepressants. Male eastern mosquitofish, for instance, spent noticeably more time with females than controls after being exposed to fluoxetine at doses of 31 to 374 ng L−1 for 35 days [138]. Similarly, males exposed to fluoxetine for 30 days at 478.5 ng L−1 made more attempts to mate with females than control males when a competitor was present [139]. Additionally, these investigators noted that eastern mosquitofish exposed to fluoxetine for 30 days had higher sperm counts (29.51 and 379.5 ng L−1) [139]. All of these findings point to an improvement in male reproductive success on an individual basis.
4.3. Behavioral Change
Because food is frequently scarce and sporadic in natural settings, any change in feeding behavior can have a big impact on survival and the success of reproduction. In addition to being well-known for influencing feeding and appetite in mammals, antidepressants have also been shown to have an impact on fish consumption. S-fluoxetine was found to be 3.3 times more effective than R-fluoxetine in suppressing appetite in fathead minnows, with Lowest Observed Effect Concentrations of 51 and 170 μg L−1, respectively. The racemic mixture of S- and R-fluoxetine were found to have intermediate potency, with Lowest Observed Effect Concentrations of 106 μg L−1 [140]. Their relative potencies in mammals are supported by these results [141]. Further evidence of fluoxetine’s appetite-suppressing effects comes from the goldfish’s brain’s reported decrease in Neuropeptide Y, a crucial appetite-stimulating neurotransmitter, following repeated injections [142]. Interestingly, certain studies indicate antidepressants can stimulate appetite in fish. For example, exposure to citalopram at a concentration of 1.5 μg L−1 induced increased feeding behavior in three-spined sticklebacks (Gasterosteus aculeatus) [143].
The effect of antidepressants on fish anxiety has been well studied because they are commonly used to treat anxiety and depression. To quantify anxiety-related reactions, researchers frequently use behavioral measures like diving, wall-hugging (thigmotaxis), and dark-light preferring (scototaxis). The novel tank diving test is a particularly well-established and commonly employed method for evaluating anxiety in fish [144,145]. By keeping an eye on a number of crucial variables, such as how long the fish stays in the tank, how quickly it rises to the water’s surface, and how frequently it switches between the top and bottom of the tank, the novel tank diving test evaluates how a fish reacts to a new environment after being exposed to drugs [144]. The aforementioned anxiolytic effects were detected using this assay in a range of antidepressant drugs, species, and experimental schedules [146,147,148]. Likewise, thigmotaxis has been employed as a behavioral proxy for anxiety [149]. It makes sense that fish treated to antidepressants would become more daring and inquisitive given their anxiolytic impact, and this has been shown with several antidepressant drugs. For instance, Japanese medaka given 100 μg L−1 of fluoxetine for 10 days become more interested in their surroundings [146]. When evaluating scototaxis, the amount of time spent in light and dark zones as well as the quantity of entries are used to gauge a person’s preference for a dark compartment [150]. Like many other species, adult zebrafish naturally prefer the dark compartment, and spending more time in the light compartment is linked to becoming more daring [150]. After receiving daily injections of 10 mg kg−1 fluoxetine, researchers found that adult zebrafish spent more time in the light compartment during a two-week period than controls [151]. Increased boldness can enhance an individual’s susceptibility to predation in an ecological setting, and there is evidence that this may be reflected in the pituitary–hypothalamic–inter-renal axis. For instance, compared to untreated controls, adult zebrafish exposed to 0.01 mg L−1 of nortriptyline or fluoxetine for a week and then subjected to erratic chronic stressors demonstrated significant decreases in whole-body cortisol levels [152]. Conversely, it has been demonstrated that zebrafish exposed to a very high dose of fluoxetine (5000 μg L−1) for 120 min have an increase in cortisol levels throughout their trunk [153].
Research has also shown anxiogenic effects in fish after exposure to antidepressants, despite some contradictory findings. For instance, wild guppies (Poecilia reticulata) exposed to fluoxetine for 28 days at a dose of only 16 ng L−1 showed longer freezing times and a higher percentage of time sheltered beneath vegetative cover than control animals, and Japanese medaka larvae exposed to citalopram, sertraline, or fluoxetine for 72 h spent more time at the edges of a thigmotactic test arena [154,155]. It is unclear if these effects result from variations in the functional homology of the target between species or from the compound’s primary or secondary pharmacology. A person’s ability to forage, evade predators, and find a mate can all be negatively impacted by anxiogenic or anxiolytic traits.
A possible effect on social behaviors, such as aggression and shoaling in fish, is one of the wider repercussions of changed animal boldness. In fact, juvenile croakers exposed to venlafaxine at 160 μg kg−1 through feed or 20 μg L−1 through water for 28 days showed a decreased latency to shoaling [147], and crucian carp (Carassius carassius) subjected to sertraline for a week at concentrations ranging from 4.3 to 116 μg L−1 [156]. Conversely, research has shown antidepressants can also alter fish behavior in ways that appear beneficial. Exposure to citalopram caused male zebrafish to exhibit reduced transitions away from their schools [157], and it was also found that antidepressant exposure lowered fish aggression, as demonstrated in rainbow trout [158]; blue-headed wrasse (Thalassoma bifasciatum) [159]; round goby [160]; Arabian kingfish (Aphanius dispar) [161]; zebrafish [162], and three-spined stickleback [163]. On the other hand, some research has also linked exposure to antidepressants to higher levels of violence. For instance, in dominant male Gulf toadfish (Opsanus beta), fluoxetine was injected intraperitoneally at doses of 10 or 25 μg g−1 [164], and three-spined stickleback that were given citalopram at a dose of 1.5 μg L−1 through water for a month, then washed out with drug-free water for 100 days [143], lastly, both displayed heightened hostility. In nature, a shift in male aggression might make it harder to find mates if a more powerful rival takes over a territory, or it can make it more likely that someone would get hurt if rivals fight in situations where one would typically play the role of a subordinate [165].
4.4. Transgenerational and Epigenetic Effects
The fact that some pollutants can have an impact on future generations is becoming more widely acknowledged, and studies on the effects of antidepressants on fish have recently taken notice of this. Epigenomic alterations in the germline that are passed down to next generations may be a part of transgenerational inheritance. Since the F1 embryo and the germline of the F2 generation may have been accidentally exposed, any environmental phenotypic alterations in the offspring of exposed adults must be evident in at least the F3 generation in order to be considered transgenerational inheritance processes [166]. Recently, a number of zebrafish studies have been published that examine the transgenerational inheritance of different phenotypes after fluoxetine exposure [167,168]. After being exposed to 54 or 0.54 μg L−1 of fluoxetine for 0–6 days, the cortisol levels of succeeding generations showed persistent changes. Changes in kidney gene expression linked to cortisol production were found in analyses of these generations, F0 and their progeny (F1–F3) [167]. It was discovered that fluoxetine caused changes in certain steroidogenic pathways in both F3 and F0 larvae, including the downregulation of a gene linked to cortisol activation [168]. Notably, ancestral exposure can still affect the biological reactions of subsequent generations, especially if they are re-exposed, even if antidepressants are stopped. Venlafaxine exposure caused long-lasting changes in adult females’ cortisol levels, as seen with F4 generation larvae, underscoring the possibility of transgenerational effects [168]. Men did not exhibit this, indicating that women were more susceptible to the effects of venlafaxine due to prior exposure to fluoxetine. There may be higher health hazards following antidepressant exposure than previously thought due to the possibility of transgenerational inheritance effects, in which later generations may become more sensitive to the effects of the medications. Some of the studies discussed in this section are summarized in Table 3, along with their primary toxic effects on fish.

Table 3.
Compiled from peer-reviewed studies documenting the toxic effects of major antidepressants on fish species.
4.5. Synthesis: Environmental Relevance and Future Research Directions
Therefore, some evidence from the literature reports that typical environmental concentrations of antidepressants in domestic wastewater, surface water, etc., are often in the order of ng/L (or a few µg/L in the most contaminated cases). Several studies report measured concentrations in water ranging from a few ng/L to µg/L. Studies using “environmentally relevant” concentrations (i.e., ng/L or low µg/L) show subtle or specific effects, often on sensitive endpoints such as behavior, gene expression, larval development, and bioaccumulation of the drug or its metabolites. Studies with higher concentrations (µg/L) often show clearer, even severe, effects: changes in development, reproduction, behavior, histology, etc. However, these concentrations ≥ µg/L frequently exceed those typically found in aquatic environments, although this is not impossible (e.g., effluents from treatment plants or point-of-care discharges). The discrepancy (effects often detected at high concentrations vs. low environmental concentrations) has several implications. Endpoint sensitivity, where many effects at low environmental concentrations are subtle, involves sensitive endpoints (e.g., behavior, gene expression, bioaccumulation). These effects are more difficult to detect, require large sample sizes, replicates, good standardization, and are often overlooked or considered less “burdensome” in terms of regulatory risk. Dose/concentration extrapolation: effects observed at µg/L may or may not scale to ng/L. There is the possibility of threshold effects below which there is no detectable effect, or of nonlinear dose–response curves, or of the existence of “pendency effects” (small cumulative effects or effects across multiple generations). Some mechanisms (e.g., transport saturation, metabolism, antioxidant defense, etc.) may function nonlinearly: at low doses, organisms can compensate or repair damage; at higher doses, once these mechanisms have been overcome, clear effects are observed.
Bioaccumulation and persistence/interaction: even at low water concentrations, antidepressants can accumulate in tissues, include active metabolites, or be transformed, causing long-term effects or transfer between generations. Furthermore, continuous or semi-continuous exposure in environments with constant input can result in relatively higher internal concentrations (plasma, organs) than would be expected from aquatic concentrations alone. Mixtures and synergistic effects: in nature, organisms are exposed to mixtures of different drugs (and other pollutants), which may act through similar mechanisms (via serotonin, dopamine, etc.), or may interact (synergies, additivity, or even antagonisms). Mixture effects can manifest effects at concentrations where each drug alone shows no effect. Many studies still test isolated compounds or a few compounds in combination. Regarding exposure time and life stages, chronic effects or effects on sensitive stages (larvae, embryos, development, early reproduction) tend to reveal impacts when exposure is prolonged, even at low doses. Many laboratory studies are acute or subchronic, but do not capture exposure over a lifetime or generations. Finally, real-world environmental conditions (temperature variations, pH, predation, competition, food, stress, the presence of other pollutants) can increase or decrease sensitivity to antidepressants. For example, a stressed or food-deficient fish may respond differently to a very low concentration than in a devised experiment.
Based on what is known so far regarding the estimation and likely effects at realistic levels, for sudden or severe effects (mortality, severe deformities, significant reproductive impairment), with many of the classic endpoints, the currently measured environmental levels (ng/L-a few µg/L) generally appear insufficient to produce these effects in many organisms, although there are cases where these levels are already close to the threshold for some more sensitive endpoints. For more subtle effects, such as behavioral, neuromodulatory, gene expression, foraging behavior, or slightly altered reproduction, real environmental exposures may possibly cause detectable effects, especially if the exposure is chronic and in vulnerable organisms or populations. For mixtures, lifetime (or even multigenerational) exposures, early life stages, and organisms in more fragile ecosystems, there is a reasonable probability that even low concentrations (ng/L) are ecologically relevant.
To reduce uncertainty and improve risk assessment for real-world environments, some research directions are particularly important. Chronic and multigenerational studies using low concentrations, close to those that actually occur (ng/L), cover the entire lifespan of fish or other organisms. This allows for the detection of cumulative effects, developmental effects, delayed reproduction, or impacts on overall fitness. Complex mixtures: Often, only one or two isolated compounds are tested; mixtures of antidepressants, as well as combinations with other pollutants, need to be studied to identify potential interactions (synergistic, additive, or antagonistic). Similar mechanisms (e.g., affecting serotonin) often suggest that compounds act by adding smaller effects that together cause an impact. Internal concentration measurements (e.g., plasma, tissues, organs, fetal or embryonic development) can relate aquatic exposure to physiological and behavioral effects. Models such as the Fish Plasma Model are helpful, but require more empirical data. Standardization of endpoints and methodologies: Differences between species, age, and test conditions (temperature, diet, density, water quality) create significant variability. Studies should be designed to compare across species/taxonomies, including response dates under varying conditions. More realistic ecosystems/field experiments/mecosystems, mesocosms, and verification of effects on wild populations (monitoring) help confirm whether the effects observed in the laboratory manifest in the environment. Constant exposures, variations in multiple stresses, etc., are important. Studies have focused on sensitive and sublethal endpoints with behavior (foraging, predation risk, sociability), development, gene expression, histology, bioaccumulation, reproductive fitness, larval mortality, etc., rather than just survival or growth. Finally, ecological and population risk assessment studies not only individual effects but also whether these effects translate into population impacts, community changes, ecological interactions, and ecosystem balance.
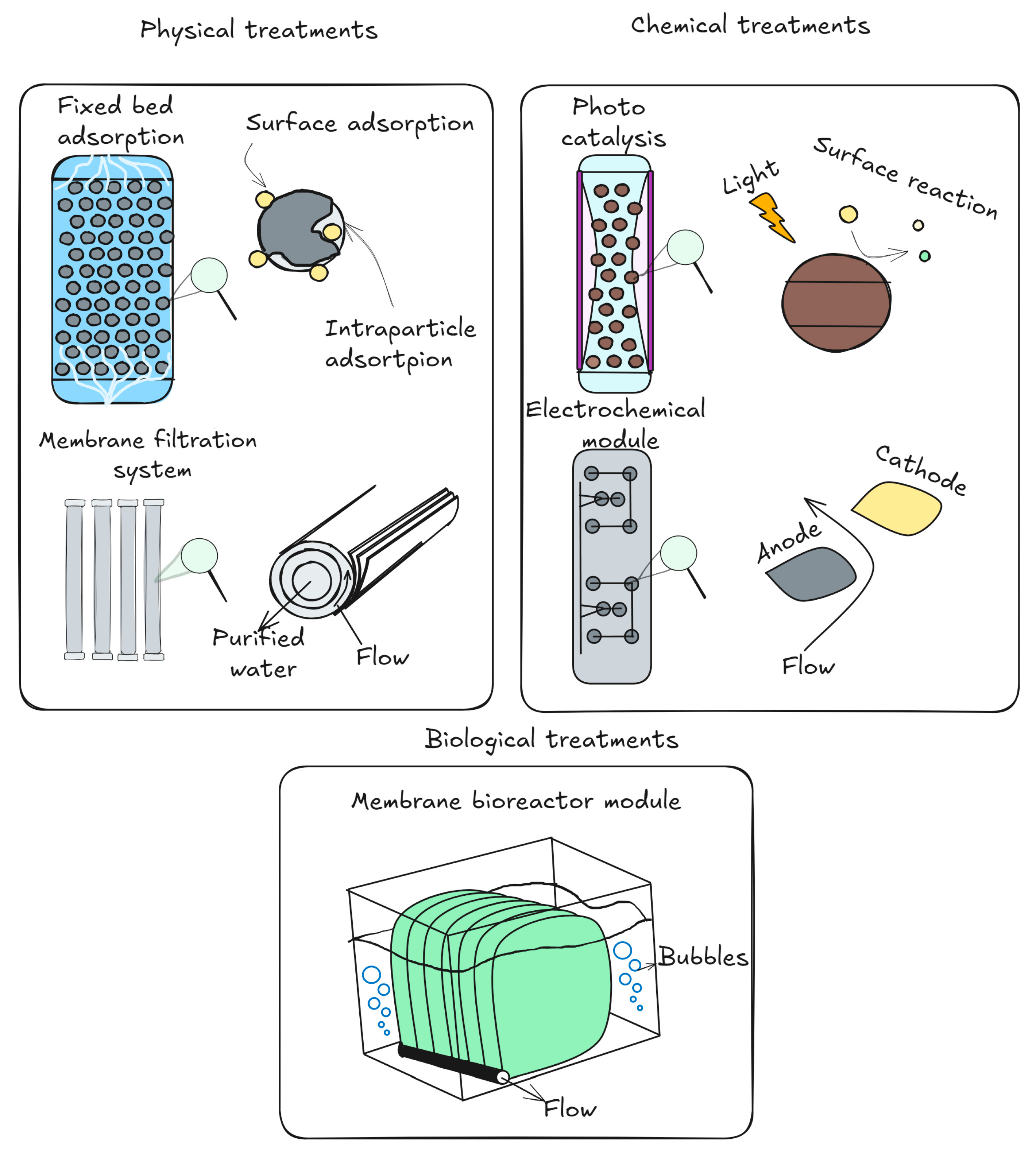
5. An Overview of Methods for Treating Water to Remove Antidepressant Residues
The methods now used to treat water to get rid of leftover antidepressant drugs are much the same as those used to address other kinds of contaminants of emerging concern. Options for treating drinking water and wastewater to eliminate contaminants of emerging concern include coagulation and sorption processes [169], physical–chemical separation [170,171], ozone-based advanced oxidation processes [172,173], including gamma radiation [174], ultraviolet treatment [175,176,177], enzymatic and biological treatment [178,179], ultrasonic degradation [180], nanofiltration and ultrafiltration through membranes [180], reverse osmosis using special membranes [181], and electrochemical oxidation [182]. When it comes to pharmaceutical and personal care goods, each of these approaches offers advantages and disadvantages (Table 4).

Table 4.
The primary chemical, biological, and physical methods for cleaning up antidepressants in water, as well as the benefits and drawbacks of each.
Pharmaceuticals and personal care items have typically been difficult to remove from water using conventional techniques like flocculation and coagulation. Advanced oxidation techniques can effectively eliminate these compounds, but they also produce potentially dangerous oxidation byproducts that might be much more poisonous than the original medications or cosmetics. Therefore, it is critical to evaluate both the risks to the environment posed by the original compounds and the risks posed by these newly produced intermediates [185,186]. Because of its ease of use and design, resistance to harmful compounds, and lack of undesired byproducts, adsorption is a particularly beneficial wastewater treatment method [187]. Adsorption is a particularly beneficial wastewater treatment method since it is easy to use and customize, resistant to harmful compounds, and produces no unwanted byproducts [188].
Even though used activated carbon can be recycled for future use, regeneration procedures come with extra expenses and hazards. Carbon loss may result from these processes, and after several cycles, the recovered material frequently shows a lower adsorption capacity than unused activated carbon [189]. This has led to efforts by a number of scientists and engineers to create inexpensive substitute adsorbents that can overcome the financial drawbacks of activated carbon and take its place in adsorption pollution management [187]. Regeneration procedures add expenses and hazards even though discarded activated carbon can be recycled for further use. These processes may result in carbon loss, and the recovered material frequently shows a lower adsorption capacity than utilized activated carbon after several cycles [190].
The trickling filter performed the worst when compared to other biological treatment techniques, such as waste stabilization ponds, biofilters, activated sludge, and biofilters. Waste stabilization ponds and biofilters showed removal efficiencies that were on par with or better than activated sludge [191]. Sometimes membrane filtration and ultrasound were added to biological treatment for optimal results [192]. To increase their efficacy against pharmaceutical pollutants, electro-oxidation and anodic oxidation methods in wastewater treatment rely on reactive oxygen species, such as H2O2 and HO•, or, especially in waters with high levels of chloride, chlorine species, such as ClO−, HClO, Cl2, and ClO2− [174]. So far, research into removing antidepressant drug residues from wastewater and contaminated waters has identified four distinct treatment approaches: extractive, adsorptive, biological degradation, and oxidative processes, such as ozone or advanced oxidative techniques (Figure 5).
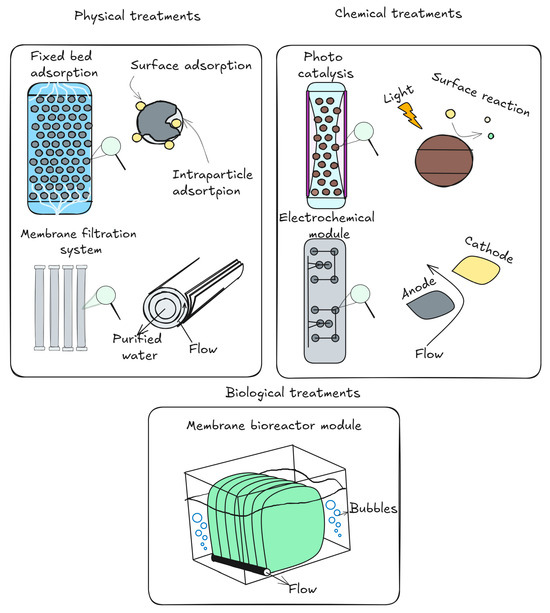
Figure 5.
Techniques for cleaning wastewater to remove antidepressant residues.
5.1. Adsorptive Methods
Antidepressants have been extracted from aqueous solutions thus far using a variety of adsorbents, such as silicates, activated carbon, titanium dioxide nanoparticles, cation exchange resins, and molecularly imprinted polymers [193,194,195]. The adsorption efficiencies of six distinct cation exchange resins toward tricyclic antidepressants were investigated by researchers [194]. The three that had carboxyl functionalities were Dowex MAC-3, Amberlite IRC-86, and Amberlite IRP-64, whereas the three that contained sulfone functional groups were Dowex 50WX4-200, Amberlite IR-120, and Dowex HCR W2. Using amitriptyline as a model pharmaceutical contaminant and one-point verification tests, they demonstrated that Dowex 50WX4-200 exhibited the maximum amitriptyline adsorption. It has been demonstrated that aqueous pH significantly affects adsorption efficiency; more specifically, above 7, Anatomical Therapeutic Chemistry, which has pKa values of approximately 9–10, tends to partially deprotonate and become insoluble in water. However, there was no discernible change in adsorption capacity between pH values 3 and 6.5; for this reason, a pH of 6.5, which is close to neutral, was selected for additional experiments. Adsorption isotherm models indicated that the highest resin capacities for the tested tricyclic antidepressants clomipramine, amitriptyline, imipramine, and desipramine ranged from 2.53 to 3.76 mmol g−1. Notably, desipramine removal from actual wastewater samples was less effective (56.66%) when the water was filtered, compared to unfiltered water (77.99%). This difference was attributed to an additional adsorptive contribution from the activated sludge present in the unfiltered wastewater [194].
The removal of positively ionized tricyclic antidepressants based on the adsorption approach utilizing various adsorbent types is summarized in Table 5 [196,197,198]. Researchers found that the adsorbent reached adsorption equilibrium in 15 min and that the absorption of amitriptyline by palygorskite clay was 0.17 mmol g−1 [198]. Calcium montmorillonite, whose absorption was 1.05 mmol g−1 and greater than that of palygorskite clay, was also used by researchers to extract amitriptyline [196]. The removal efficiencies of activated carbon in various media, such as electrolyte solution and polyethylene glycol, were investigated for the removal of imipramine [4]. Additionally, a prior study demonstrated that the carbon sphere’s adsorption capabilities for removing imipramine and clomipramine were 0.36 mmol/g and 0.09 mmol/g, respectively [197]. Although high-performance cation-exchange resins are widely and frequently used for adsorptive removal applications, there are not many reports on their use in the adsorption of positively ionized tricyclic antidepressants. Prior drug removal research examined the use of an ion-exchange resin with a strong-base anion-exchange polymer to remove diclofenac from human urine [199]. Diclofenac was removed in excess of 90% of cases. Investigating the adsorption performance of ion-exchange resins for the removal of positively ionized tricyclic antidepressants is therefore clearly important when evaluating their adsorption capacity for drug-like compounds. Therefore, the goals of this study are to (1) determine the best high-performance cation-exchange resin for the removal of positively ionized tricyclic antidepressants among several high-performance cation-exchange resins; (2) compare the removal efficiencies of positively ionized tricyclic antidepressants using the selected high-performance cation-exchange resin in three different aqueous matrices: aerobic wastewater, distilled water, and filtered wastewater; and (3) examine the adsorption performance of the chosen high-performance cation-exchange resin in distilled water using kinetic, pH, and hash limits. Additionally, the adsorption of positively ionized tricyclic antidepressants was examined in relation to the impacts of competing cations and activated sludge found in actual wastewater.

Table 5.
Adsorbent uptakes for the removal of positively ionized tricyclic antidepressants that have been previously reported are compared.
The maximal adsorption capacity of activated carbon using imipramine was 372 mg g−1 at a pH of 6.8 and 610 mg g−1 at a pH of 8.5, according to the Langmuir adsorption model [4]. The deprotonated form of imipramine showed higher adsorption onto the carbonaceous substrate than the protonated form, in contrast to cation exchange resins. Additionally, a different study looked into the extraction of amitriptyline and nortriptyline from water using TiO2 nanoparticles, activated carbon, and diosmectite, a naturally occurring silicate [193]. The most efficient substance was found to be activated carbon, which had adsorption capacities of 1.87 and 3.75 mmol g−1 for amitriptyline and demethylated nortriptyline, respectively. With capacities of 1.82 and 3.3 mmol/g, diosmectite likewise shown encouraging performance; however, TiO2 nanoparticles demonstrated noticeably lower adsorption rates of 0.79 and 2.97 mmol g−1. A combination of electrostatic interactions between positively charged molecules and the negatively charged adsorbent surfaces, as well as π-π stacking interactions between the aromatic rings of the antidepressants and the carbon surface, were responsible for the observed adsorption [193]. Although activated carbon has a somewhat high production cost, there are a number of cost-effective regeneration solutions, such as biological regeneration [200], chemical [201] and electrochemistry [189].
A new development in adsorbent materials for environmental cleanup are molecularly imprinted polymers, sometimes known as synthetic receptors or artificial antibodies. These polymers have extremely selective recognition sites on their surface because they are made by imprinting a particular template during polymerization [195]. Using methacrylic acid as a monomer, either by itself or copolymerized with 2-hydroxymethyl methacrylate or methyl methacrylate, researchers have created these molecularly imprinted polymers [195]. Because the polymeric materials had a much smaller specific surface area (BET Superficial Area = 193.8 m2/g) than highly porous activated carbon (BET Superficial Area = 1400 m2/g), the adsorptive capacity was lower than that of activated carbon, for example, 72.6 mg g−1 for sertraline. However, the primary benefit of these molecularly imprinted polymers was their higher affinities and selectivities for their target contaminants. Using molecularly imprinted polymers, selective serotonin reuptake inhibitor antidepressants including citalopram, paroxetine, sertraline, and fluoxetine were extracted from water, leaving behind interfering chemicals like the structurally related medications bupropion and bupivacaine. In addition, the polymers needed to be regenerated more frequently than activated carbon, which is better for operating costs. Adsorptive techniques, such as regeneration and reuse technologies, continue to be a very viable option for eliminating antidepressant residues from contaminated water, even in the face of potentially high initial manufacturing costs.
5.2. Extractive Methods
Because liquid–liquid extraction techniques employ ecologically unfriendly solvents that increase wastewater contamination, they are primarily regarded as historically significant. In order to evaluate the possible environmental impact, researchers looked into how well sertraline could be extracted using halogenated and non-halogenated organic solvents, such as dichloromethane, chloroform, and ethyl acetate [202]. Although ethyl acetate produced the best extraction results, particularly when mixed with 2% ethanol, its intrinsic negative effects make it inappropriate for wastewater treatment [203]. Research has investigated more environmentally friendly solvent options and used water-in-oil microemulsions made with different carboxylic esters and the nonionic surfactant Brij 30 to successfully extract imipramine, amitriptyline, clomipramine hydrochloride, and sertraline from contaminated water. The extraction efficiencies ranged from 67.2 to 99.5% [204]. The best-performing microemulsions were propyl acetate-based ones, which maintained extraction yields between 88 and 99.3% even at high concentrations of antidepressant contaminants (up to 100 mg L−1). The water-in-oil microemulsion system showed great promise for antidepressant extraction, achieving yields of up to nearly 100% with varying wastewater contaminant loads, despite persistent issues with the cost of the nonionic surfactant and the use of organic ester solvents, even in smaller amounts.
Ionic liquids have also been suggested as environmentally friendly extraction solvents for antidepressants due to their well-known benefits of high thermal and chemical stability, minimal volatility, non-corrosive nature, and superior solvation capacity [205,206]. Amitriptyline was effectively extracted from pharmaceutical waste using aqueous two-phase systems that included phosphate buffers and tetrabutylphosphonium or tetrabutylammonium salts [206]. The extraction yields were notably increased by higher pH levels (6.6 to 9.6), reaching values of 94 percent to 100 percent. There was little change in this efficiency when the pH was raised to 13. Ionic liquids are a potential approach, but their expensive cost now prevents them from being widely used. Because traditional organic solvents are inherently harmful and more environmentally friendly alternatives are expensive, current extraction methods for removing antidepressant residues from wastewater are now inappropriate.
5.3. Advanced Oxidation Processes
When antidepressants or their derivatives contaminate water, ultraviolet irradiation whether UVA, UVB, or UVC by itself has been shown to be an effective method of decontamination [39]. Current research on the photodegradation of tricyclic antidepressants has shown that ultraviolet-C treatment has been more successful such as Amitriptyline and clomipramine hydrochloride [207] or trimipramine [208] in water with efficiencies more than 90%, whereas desipramine in water with a combination of UVA and UVB caused a relatively long half-life of 36 h [209] even after 114 days of exposure, UV-A radiation alone could only break down less than 30–40% of Venlafaxine and Doxepin [210]. The latter’s performance was only markedly enhanced when combined with a titanium dioxide photocatalyst or with H2O2 and Fe2+, a process known as photo-Fenton, which resulted in the degradation of 75% of imipramine hydrochloride in 4 h and full degradation in 24 h [211]. UV therapy is typically used in conjunction with another kind of sophisticated oxidation procedure for maximum efficacy.
Ozone has demonstrated remarkable efficacy in breaking down antidepressant residues found in wastewater. Using O3 + TiO2, O3, O3 + H2O2 + TiO2, and O3 + H2O2 (Peroxone process), researchers studied the oxidative disposal of fluoxetine from an aqueous environment with and without concurrent UV irradiation [212]. Depending on the effectiveness, fluoxetine may photomineralize partially or completely to create inorganic ions including NO3−, NO2−, F−, and CO2. Fluoxetine did not deplete either basic or acidic pH under dark circumstances. Because adsorption on the titanium dioxide surface was a prerequisite for degradation and because the positively charged oxide catalyst surface repelled cationic antidepressant species in an acidic environment, titanium dioxide-based methods were only effective in alkaline media. In acidic media, fluoxetine was likewise not degraded by ultraviolet-A irradiation (360 nm); however, following alkalization, the concentration of fluoxetine dropped by about 15% after 60 min of illumination. When a tiny amount of hydrogen peroxide was added to ultraviolet-A and O3 treatment, the degree of photodegradation doubled. In just one hour, 50% of the material was mineralized using the heterogeneous photoassisted ultraviolet-A + TiO2 + O3 process. The hybrid ultraviolet-A + H2O2 + O3 + TiO2 configuration obtained 97% mineralization, indicating that it is the optimum combination for wastewater treatment against such resistant drug contaminants. The inclusion of hydrogen peroxide greatly boosted photomineralization [212].
Ionizing radiation, also known as gamma (γ) radiation, is an alternative to ultraviolet radiation. Experts examined how γ rays degraded citalopram and sertraline in surface waters, especially when common radical scavengers such humic acid, CO32−, and NO3− were present [213]. Radiation dosages ranging from zero to 500 Gy were used in the experiments, which used a 60Co source. At a dosage of 1 mg/L, both antidepressants were totally broken down by a 100 Gy dose. At a concentration of 10 mg/L, 400 Gy of citalopram and 200 Gy of sertraline were needed to achieve full mineralization. When compared to ultraviolet light, this radiation dose was significantly lower, corresponding to only 11 min of irradiation at a rate of 2.15 kGy. Additionally, at a concentration of 1 mg/L and an absorbed dose of 100 Gy, the breakdown of sertraline was not significantly impacted by the addition of 10 mg/L of nitrate, carbonate, and humic acid—substances frequently found in wastewater. Nitrates had no effect on the breakdown of citalopram at the same concentration level, while carbonates and humic acid did. Degradation efficiency for a 50 Gy absorbed dosage was 70% when CO32− was present and 50% when humic acid was present. Carbonates maintained a moderate inhibition of citalopram degradation up to 200 Gy, where 80% efficiency was attained. Remarkably, with a dose of just 150 Gy, 100% citalopram breakdown was accomplished with humic acid addition, outperforming the lack of additional radical scavengers [213].
An effective technique for eliminating antidepressant impurities from water is electrooxidation, also known as anodic oxidation. Depending on whether chloride ions are present in the surrounding aquatic environment, this action can take place with or without active chlorine [174]. The physiosorbed OH radical is the most powerful of the reactive oxygen species produced when water is discharged onto the anode. Ozone (O3) and hydrogen peroxide are two more important oxidants that are created (H2O2). SnO2, PbO2, and boron-doped diamond are examples of “non-active” electrodes because they mainly produce physiosorbed OH radicals that have little interaction with the anode surface and cause organic oxidation and full mineralization very quickly. On the other hand, a more potent oxidation process is produced by electrodes like IrO2, Pt, or RuO2, which produce highly bonded and chemisorbed “active oxygen” or metal superoxide species. Usually, boron-doped diamond is the most efficient of these substances [174]. This study examined the breakdown of 130 mg L−1 of amitriptyline using three distinct electrochemical advanced oxidative methods. An electro-Fenton process, simple anodic oxidation, and a photo-electro-Fenton process, respectively, produced active OH radicals [214]. Researchers used fixed-current anodic oxidation (100 mA cm−1) to analyze total organic carbon and assess the performance of electrodes, such as RuO, Pt, and boron-doped diamond. After 360 min, the boron-doped diamond showed the highest efficiency, reaching 76% mineralization. Because of the chloride ions from the amitriptyline hydrochloride salt, RuO and Pt produced far poorer results, with mineralization rates of 27% and 21%, respectively. Chlorine is the predominant oxidant at pH levels below 3.3, but hypochlorite (ClO−) takes over as the main oxidant at higher pHs [214]. With applied currents of 33.3, 66.7, 100, and 150 mA/cm2, Melin et al. [214] also investigated the impact of current density using a boron-doped diamond anode, obtaining mineralization degrees of 50, 64, 76, and 83% in 360 min. As H2O2 is produced by coupling the OH radical during simple anodic oxidation, adding 0.5 mmol/L of Fe2+ catalyst to the medium generated conditions for an electro-Fenton reaction. In fact, the electro-Fenton method, which used the boron-doped diamond anode, generated 78% quicker mineralization in 240 min of electrolysis at 100 mA/cm2. The formation of iron hydroxide species limits the production of OH radicals in the mass, which lowers the electro-Fenton process’s efficiency. It is interesting to note that doubling the catalyst concentration did not enhance mineralization [214]. However, the combination of boron-doped diamond, bulk-homogeneous OH, and highly reactive physisorbed OH (360 nm) resulted in the greatest mineralization performance for amitriptyline in the photoelectro-Fenton process. The Fe2+ concentration was maintained at 0.5 mmol/L for 360 min of treatment, resulting in a current density of 100 mA/cm2 and 95% mineralization. The study’s authors also used gas chromatography-mass spectrometry analysis in combination with high-performance liquid chromatography to identify some of the intermediates that were produced from amitriptyline throughout its mineralization process. Polyaromatic hydrocarbons and their oxidized derivatives, including 5-dibenzosuberenone and dibenzosuberone, were among them, along with carboxylic acids such as malic, succinic, formic, and oxalic acids [214].
5.3.1. Pharmaceutical Photodegradation Pathways
Pharmaceuticals are compounds or blends that are used to cure or prevent human illnesses, but they also constitute a substantial class of pollutants that enter the environment as diffuse and point micropollutants. Since pharmaceutical pollutants are only partially removed by traditional treatment methods, municipal and hospital wastewater treatment plants are widely acknowledged as the main sources of pharmaceutical pollution discharge. Pharmaceutical concentrations in aquatic environments range from ng L−1 to µg L−1, and even at low levels, these substances can negatively impact human health and the environment, including causing aquatic life extinction and biodiversity loss [215,216,217,218]. Pharmaceutical compounds can now be effectively removed and degraded from wastewater and prevented from leaking into surface and groundwater thanks to the development of innovative technologies like advanced oxidative processes. As a tertiary treatment for wastewater treatment plant effluents, advanced oxidative processes have been suggested. Since advanced oxidative procedures involve several pathways for generating reactive oxygen species, mainly •OH, they are thought to be viable methods for treating contaminated wastewater, including non-biodegradable organic pollutants. Heterogeneous photocatalysis, which depends on the effective creation of reactive oxygen species in aqueous media, can completely remove a variety of pharmaceutical pollutants [219,220].
To improve removal efficiency, new methods for handling sewage effluents that are high in pharmaceuticals have been developed. Ultraviolet irradiation alone [221,222], advanced oxidative processes [223], as an effective oxidizing agent, hydroxyl is created when hydrogen peroxide and UV light are combined, [224,225] and enhance the efficiency of hydroxyl radical formation by combining hydrogen peroxide with Fe2+ ions, a process called photo-Fenton [226,227,228,229,230] are a few instances of various therapeutic approaches. More recently, heterogeneous photocatalysts like suspended TiO2 have been used to create hydroxyl radicals. The efficiency of titanium dioxide as a photocatalyst is determined by its physicochemical properties as a semiconductor [231].
5.3.2. Photocatalytic Nanomaterials for Drug Removal
Using advanced oxidation techniques for drug degradation is another novel way to eliminate pharmaceutical pollutants from water. These oxidation processes include ultraviolet/hydrogen peroxide oxidation, photocatalysis, photo-Fenton oxidation, and ozonation. Additional state-of-the-art techniques for drug breakdown include sonolysis and non-thermal plasma. The oxidizing agents generated by non-thermal plasma, such as OH, H2O2, and ozone, are used in the plasma technique [232,233,234,235], nowadays, photocatalytic drug degradation is the most widely used method [236]. Photocatalysts based on nanospheres are simple to make and can efficiently degrade pollutants. Here, TiO2, ZnO, and CeO2 nanoparticles are some of the most widely utilized and effective nanophotocatalysts [237]. When light is absorbed during photocatalytic degradation, excited valence band electrons shift to the conduction band. The valence band therefore forms holes that cause oxidation reactions on the surfaces of the photocatalyst, producing hydroxyls. By dividing the charge between the two bands, the electrons in the conduction band are in charge of performing reduction operations on the surface.
A narrow band gap, low charge recombination, advantageous band edge placement, many surface-active sites, effective charge separation, and robust charge transfer are all necessary for a photocatalyst to work well. The electrical and optical characteristics of a nanoparticle are greatly influenced by a high volume-to-surface ratio and a high concentration of surface atoms at the nanoscale. As nanoparticles shrink, surface atoms become more chemically active and less connected to the bulk material, resulting in high surface energy and enhanced catalytic activity. These changes are intrinsically linked to the material’s electrical structure and contribute to a quantum size effect, impacting its optical properties [238]. One important property that reflects a nanomaterial’s conductance and photoactivity is its bandgap. Zinc oxide nanoparticles, which have a smaller bandgap of 3.2 eV, show a high rate of photoexcitation recombination and photocorrosion, whereas titanium dioxide nanoparticles, which have a bandgap energy of 3.4 eV, are the most researched photocatalysts. CeO2 has a bandgap energy of 3.1 eV, which means that to absorb visible light, the bandgap must be lowered. This issue can be resolved by doping and combining noble metals with nanoparticles. CuO/ZnO and TiO2/ZnO nanocomposites work in concert to boost photoactivity [239].
Over the past ten years, a large variety of photocatalysts have been created. Of them, graphitic carbon nitride has a number of advantages that make it appropriate for a range of photocatalytic uses [240,241]. A smooth band gap of 2.7 eV, strong chemical stability, a straightforward preparation process, minimal toxicity, and a good responsiveness to visible light up to 460 nm are some of these aspects. Nevertheless, hospital wastewater has not been the subject of many published scientific investigations. Graphitic carbon nitride-spiked wastewater effluent is real and was extracted from a big wastewater treatment facility in Queensland, Australia [242]. The photocatalytic degradation of carbamazepine under simulated sun irradiation was investigated in this work, and it was discovered that within 15 min, 100% of the carbamazepine was destroyed. In a different study, graphitic carbon nitride was used to assess how carbamazepine photodegraded in response to UV radiation [243]. In a wastewater treatment facility and in actual hospital wastewater, specialists examined the removal efficiency of amoxicillin using a graphitic carbon nitride-based composite photocatalytic material known as Ag/TiO2/MgC3N4 [244]. Following one hour of the photocatalytic procedure, an amoxicillin clearance of 49 and 71%, respectively, was noted in both sample types [240].
Psychiatric drugs are widely distributed in habitats such as drinking water, lakes, rivers, and hospital effluents because they do not completely vanish when transported by patients’ urine and feces to wastewater treatment plants. Because present wastewater treatment equipment is not built to remove pharmaceuticals, these medications and their metabolites can be detected in almost any water source. As a result, concentrations in the environment are high enough to possibly affect aquatic life. Additionally, antipsychotics, which can gently change animal behavior and population dynamics, may bioaccumulate because of web feeding. Antipsychotic medication pollution’s environmental impact has been mainly overlooked [245].
5.4. Biological and Advanced Oxidation Processes for Antidepressant Removal
Microorganisms are used in the biological treatment of wastewater that contains residues of antidepressant drugs. These organisms usually provide the enzymes needed to break down hazardous and structurally complex pharmaceutical compounds into less harmful, simpler products. The two primary biological transformations of pharmaceutical waste in wastewater treatment plants are co-metabolism, in which drugs are broken down by enzymes released by microbial communities, and single-substrate degradation, in which the target compound is the only source of carbon and energy for the microbe [246]. Drug biotransformation methods can also be categorized as either anaerobic or aerobic based on their actual use and the oxygen availability to microorganisms. The solubility of pharmaceutical pollutants in wastewater is the primary determinant of their biodegradation efficacy. Drug compounds with low solubility, such hydrophobic ones, will remain in the sewage sludge longer, giving microorganisms more time to break them down, either via catabolic enzymes or as a source of carbon alone. Conversely, highly soluble drug micropollutants may evade the biodegradation process and depart the wastewater treatment facility after a brief period of residence [246].
Sertraline, the most often prescribed antidepressant, was the subject of a biotransformation study by researchers employing two distinct methodologies: batch sorption and biodegradation in the first case, and continuous flow wastewater treatment in the second [195]. About 90% of the sertraline was removed through sorption in lab tests using activated sludge. The availability of simpler carbon sources for microbial consumption had a significant impact on biodegradation, which had a little significance. Although sertraline ketone, norsertraline, and hydroxyl-sertraline were among the chemicals created by biodegradation, full breakdown into inorganic components was not achieved (as illustrated in Figure 6). The greatest removal rate of 94% was attained by the continuous-flow bioreactor. Sertraline removal efficiency varied from 77 to 81% when applied to wastewater samples obtained from a psychiatric hospital and two wastewater treatment facilities. Eight structurally complicated sertraline transformation products were found in trace levels in the final wastewater streams even after treatment [195].
Figure 6.
Techniques for cleaning wastewater to remove antidepressant residues.
The use of Pleurotus ostreatus to treat wastewater containing 2.5 mg L−1 of paroxetine, sertraline, fluoxetine, clomipramide, citalopram, amitriptyline, and venlafaxine was examined in a different study. The fungus was autoclaved at 120 °C for 15 min to deactivate its enzymes to isolate the effects of fungal biodegradation. After 96 h of fungal contact, clearance rates of more than 90% for paroxetine, sertraline, and amitriptyline were noted when enzymatic breakdown and adsorption were combined. Enzymatic degradation alone, however, produced lower clearance rates; fluoxetine was removed at about 85%, while venlafaxine and citalopram were removed at only 22% and 50%, respectively. Remarkably, citalopram and venlafaxine exhibited almost no enzymatic degradation, but amitriptyline and clonazepam showed over 80% degradation, and sertraline, paroxetine, and fluoxetine showed rates of 48%, 50%, and 24% degradation, respectively [247]. Based on these findings, the authors of the study hypothesized that the structural resemblance between the antidepressants and the monomeric components of lignin—which are the natural targets of fungal enzymes—was related to the efficiency of fungal degradation. The antidepressants with the highest rates of degradation were those with lignin-like structures, including fluoxetine, amitriptyline, sertraline, and clonazepam. The breakdown of fluoxetine was influenced by its three fluorine atoms, which reduced its aromaticity. Similarly, citalopram’s resistance may have stemmed from its distinct structure. Venlafaxine, containing only a single aromatic ring, was largely unaffected because it was not readily recognized by the fungal enzymes. Overall, the findings indicated that microorganisms exhibit varying capabilities when targeting specific types of environmental drug pollutants, as summarized in Table 6 [247].

Table 6.
Removal efficiencies of various antidepressant residues from wastewater by currently available treatment methods.
5.5. Comparative Analysis of Different Technologies
Adsorption refers to the physical (and sometimes chemical) capture of drug molecules on adsorbent surfaces. Common adsorbents include activated carbon (granular or powder), biochar, clays, zeolites, nanoparticles (e.g., magnetite, functionalized), etc. Removal does not occur through degradation of the molecule (although some transformation may occur in the adsorbent or by ancillary processes), but rather through entrapment, reducing its concentration in water. Certain psychiatric drugs have favorable hydrophobicity, ionic (or weak) behavior, high log Kow, etc., which favor adsorption. For example, in one study, magnetite-red-mud nanoparticles (RM-NPs) obtained high adsorption capacity (90.5 mg/g) for carbamazepine under certain conditions, and removal in real WWTP effluent was good [248]. Another example is polystyrene-coated magnetite nanoparticles which removed amitriptyline and sertraline from hospital wastewater [249]. Also, the removal of several psychiatric drugs in commercial activated charcoal (venlafaxine, paroxetine, oxazepam, etc.) has been shown to be efficient [250]. In terms of removal efficiency, very high values have been observed in many benchtop and laboratory experiments: for example, >80–90%, sometimes >95%, especially if contact times are sufficient, adsorbent dosage is high, and pH is favorable. However, these high efficiencies often occur at relatively high concentrations or in unitary/controlled systems rather than in real wastewater with many competing components. For drugs with weak affinity for the adsorbent (hydrophilic, strongly ionic, small molecules, etc.), the efficiency is significantly lower.
Limitations include the presence of dissolved organic matter (DOM), other micropollutants, colloids, salts, etc., which compete for adsorption sites, reducing capacity, and causing premature saturation (breakthrough) before the expected time in clean water. Activated carbon (or other adsorbents) eventually saturate. Regeneration (via heat, chemicals, etc.) or disposal is necessary. Regeneration consumes energy and chemicals, or can degrade the adsorbent. Disposal of drug-laden adsorbents is a concern (leachation, incineration, and high costs may occur). Although adsorption is often “cheaper” compared to some AOPs (especially on a small scale), the cost per volume for large-scale treatment, especially when high dosages or frequent regeneration are required, can be significant. Adsorption merely transfers the contaminant from the aqueous phase to the adsorbent. The drug remains intact (or nearly so); If the adsorbent is not adequately treated, or desorption occurs, the pollutant can return to the environment. Achieving high removal rates requires considerable contact time (and therefore reactor volume/retention time). In large plants or those with high flow rates, this requires large structures, high costs, and a larger footprint. Some psychiatric drugs have poor adsorption due to their high hydrophilicity, high ionic charge, or other molecular characteristics. Behaviors observed at the bench scale (batch) are not always repeated in continuous-flow systems. Convection, mass transfer, flow distribution, hydrodynamics, etc., significantly affect these processes.
AOPs are processes that generate highly reactive radicals (especially hydroxyl radicals •OH, sulfate radicals, etc.), ozone, UV radiation, Fenton, photo-Fenton, electrochemical oxidation, combinations (hybrid, UV/O3, UV/H2O2, photocatalysis, non-thermal plasma, etc.). The goal is to degrade parent pharmaceutical molecules, ideally mineralizing them into CO2, H2O, and harmless salts, or at least transform them into less hazardous products. For psychiatric drugs, the idea is to disrupt their complex structures (aromatic rings, heterocycles, etc.). In terms of adsorption efficiency, many laboratory or pilot studies report high removals (>90%) for psychiatric drugs under optimized conditions. For example, in a comparative study of 3 AOPs (photocatalysis, ozonation, pulsed corona discharge), all achieved >90% removal for selected pharmaceuticals [251]. Another example is the ozonation of antidepressants and carbamazepine which produced substantial removal, whereas conventional sewage treatments remove very little (19% on average) before ozonation [252]. Reviews confirm that the efficiency for removal of the parent compound is good, but complete mineralization is often much lower (TOC removal follows slowly), or some fractions remain [253]. One of the limitations involves the high energy consumption through UV lamps, ozone generation, electricity for electrochemical oxidation, etc. For example, achieving ~90% removal via photocatalysis may require tens of kWh/m3, while ozonation or other processes may demand much less, but it depends very much on the case [251]. Furthermore, partial oxidation can generate metabolites or intermediates that may be more toxic, more persistent, or more mobile than the original drug. Examples: ozonation of antidepressants can generate N-demethylated compounds, etc. Also, residues of oxidants or radicals may remain [253]. Natural water contains bicarbonate, naturally occurring organic matter, chloride, etc., which compete for radicals (the scavengers) and reduce the effective concentration of radicals needed to degrade pharmaceuticals. Many AOPs require an acidic pH (such as 2–4, in the case of Fenton/photo-Fenton), requiring chemical adjustments before and after treatment, which is practical but costly. They require oxidants (H2O2, ozone), catalysts (TiO2, etc.), the replacement of UV lamps, the maintenance of ozone generators or electrochemical systems, etc. To treat large volumes (effluent from a WWTP), reactors must ensure adequate exposure, efficient mixing, light penetration (in the case of photocatalysis), contact with oxidants, etc. Mass transfer, optical path, catalytic contact, etc., make it difficult to maintain the same performance as at laboratory scale. Often, the parent compound is removed, but some of the organic carbon remains, and intermediates persist. Residual toxicity tests can reveal risks stemming from the use of strong oxidants (ozone, hydrogen peroxide, radicals), gaseous emissions, the potential formation of regulated byproducts (chlorinated, brominated, etc.), and operations. And in many places, especially developing countries, energy, reagent, and capital costs are prohibitive; therefore, these methods are more viable as tertiary treatment or for specific streams (such as hospital water).
Biological treatment relies on microorganisms (bacteria, fungi, algae, mixed consortia) that metabolize (or co-metabolize) drug molecules. Adsorption by biomass (sludge) can also contribute; biodegradation includes partial transformation, co-metabolism pathways, etc. There are also specialized systems (biotransformative fungi, white-rot fungi, algae, constructed drainage ponds, artificial wetlands, etc.). For many psychiatric drugs, conventional activated sludge plants remove only partial fractions. In one study, in laboratory-scale hospital wastewater, the average removal of “psychotropic” drugs was 19 ± 26% (i.e., highly variable, with many compounds showing low removal) [254]. For sertraline, one study showed up to ~94% removal in pilot reactors, but much of this was due to adsorption rather than biodegradation, and transformation products were detected [195]. Microalgae-based systems such as high-rate algae ponds demonstrate moderate to high removal (40–60%, in many cases > 60%) for several drugs; however, drugs such as carbamazepine are often among the most difficult to remove [255]. MBRs can improve over conventional activated sludge, especially with long SRTs, but removal remains variable depending on the compound [255]. Many psychiatric drugs are designed to resist metabolism (in the human body), possess lipophilicity, and exhibit structural stability (aromatic rings, halogens, etc.), which makes them resistant to biological treatment. When biodegradation occurs, metabolites or byproducts may be generated whose ecological or toxicological effects are poorly understood. Sorption to sludge removes the drug from the water, but the drug remains bound to the sludge. If the sludge is applied to soil, incinerated, or otherwise treated, the sorbed compounds or their products may return to the environment. Sorption may also be reversible under certain conditions: SRT, temperature, presence of co-substrates (biodegradable carbon), microbial composition, etc. [255]. If not optimized, removal will be low. To allow for slow degradation, reactors need to be large or have long retention times, which increases space, construction, and operating costs. Psychiatric drugs are typically present in very low concentrations (ng-µg/L); low concentrations can limit biodegradation because microorganisms may not be induced to metabolize these molecules, or there may be competition/inhibition. Temperature, organic matter input load, pH, nutrient availability, etc., vary throughout the year or by location, affecting microbial activity and, therefore, removal. Some promising systems (specialized fungi, etc.) show high removal rates, but over long periods (days to weeks), limiting practical applicability unless the system is designed for long retentions or used as a polisher. Table 7 below provides a summary comparing these technologies across several key dimensions. This table is somewhat qualitative but based on reported data and studies. Qualitative values or ratings reflect typical or likely real-world performance/costs/limitations, not absolute best lab numbers (which may be optimistic).

Table 7.
Comparative analysis of AOPs, adsorption and biological treatment processes in the remediation of drugs for psychiatric use.
6. Gaps and Future Perspectives
According to laboratory research conducted on a variety of fish species, there is a greater chance of biological effects in fish populations in the wild since antidepressant targets in fish and mammals share a large amount of structural similarities. Recent research is starting to cast doubt on this assumption, possibly because of differences seen in behavioral outcomes, even though generally low concentrations of these drugs are found in aquatic environments. This is because, when compared to their effective concentrations in fish, this represents a currently low environmental risk [256,257]. Applying a systematic review approach, as outlined by [258], could strengthen assessments of differences between studies and their results. Importantly, relatively few published studies have examined the long-term impacts of antidepressant medications on fish, including their potential for bioaccumulation and transfer within the food chain, or the effects of exposure to complex environmental mixtures—all of which represent key areas requiring further research [259]. Antidepressant concentrations in the environment are expected to increase due to rising prescription rates, which calls for more research. The significance of looking at transgenerational and epigenetic effects in fish is further highlighted by recent studies in mammals. Importantly, there is still a great need for additional research on fish, especially studies that investigate the behavioral effects of antidepressant exposure, which are still mostly unexplored.
Strong measures are required to ascertain how behaviors are impacted and how these modifications affect an animal’s capacity to react to environmental changes, such as mating, predation, or social interactions, and to precisely evaluate the effects of antidepressant treatment. Since changes in an individual’s behavior, such as mate choice, can affect population structure and the resulting diversity in certain traits across generations, it is imperative to comprehend these behavioral changes. We can learn more about the neuronal circuits at play by using transgenic fish models and current techniques for evaluating how the brain reacts to chemical exposure [260,261,262]. Examining these methods may open up fruitful lines of inquiry into how antidepressants affect fish behavior, which in turn may affect fish survival and fitness in their natural habitats. Future research would benefit greatly from a meta-analysis of published studies on the repeatability, sensitivity, and reliability of behavioral measurements. Additionally, given that most of the previous research has been carried out in lab settings, future studies should focus on assessing the effects of antidepressants in more natural settings, since environmental influences may have a big impact on behavioral outcomes.
Pollution exposure, especially from some antidepressant medications, can change an organism’s behavior, which can then affect interspecies relationships and, ultimately, the health of ecological systems as a whole [40,263]. These drugs have been shown to reduce a fish’s capacity to consume food efficiently, which may have a domino effect on populations at all levels of the food chain, especially those at higher or lower trophic levels [264,265]. Despite the inherent challenges in carrying out such research, it is essential to obtain a more thorough understanding of how environmental exposure to antidepressants may alter the structure and function of ecological communities. Pharmaceuticals are used extensively to treat and prevent diseases. They include both single substances and combinations. Nevertheless, they are a significant class of contaminants that can enter the environment directly or indirectly. Municipal and hospital wastewater facilities are major contributors to the discharge of pharmaceutical pollutants because traditional wastewater treatment techniques usually only remove a small portion of them. In aquatic environments, pharmaceutical chemicals are commonly found at concentrations between parts per billion and parts per million. Like other pharmaceuticals, psychiatric drugs are frequently discovered in the environment, frequently coexisting in complex mixes, even at concentrations that do not immediately endanger human health or produce acute toxicity in animals.
Innovative solutions are needed to address the growing issue of organic compound-based environmental contamination, which is a result of growing populations and industrialization. While well-established methods such as adsorption, coagulation, and ultrafiltration are commonly used to remove organic contaminants from liquids and wastewater, new methods are required to chemically change many newly discovered man-made toxins. Since these compounds frequently have significant toxicity even at trace levels, special care should be paid for their transformation. A variety of methods are required to quickly eliminate natural contaminants. In addition to being sustainable and affordable, these solutions should ideally prevent the production of new pollutants. A possible method for removing pharmaceutical pollutants from wastewater and stopping their spread into surface and groundwater is to use technologies such as AOPs. These procedures, which are frequently applied as the last phase of wastewater treatment, use the production of reactive oxygen species, mainly •OH, to totally break down contaminants. Studies conducted in the last ten years have concentrated on creating a variety of photocatalysts for the degradation of such drugs, showing that the main reactive species at play are photogenerated holes and •OH. The important unanswered concerns and possible directions for further research on antidepressant treatment in aquatic environments are listed in Table 8.

Table 8.
Knowledge gaps and future perspectives in the remediation of antidepressants present in water effluents.
7. Conclusions
Given the growing prescription and consumption of antidepressants globally, and their subsequent release into the aquatic environment, this study reinforces the urgency of considering these drugs as emerging contaminants of significant ecotoxicological relevance. Scientific evidence indicates that even at environmental concentrations ranging from ng L−1 to μg L−1, these psychoactive compounds can trigger behavioral, reproductive, physiological, and biochemical alterations in aquatic organisms, especially fish, affecting their neuroendocrine and social functions due to the evolutionary conservation of serotonergic and noradrenergic pathways between fish and humans. Furthermore, the simultaneous presence of multiple antidepressants and their active metabolites potentiates synergistic risks and cumulative adverse effects, highlighting the importance of investigating the impacts of complex mixtures. This review also highlights the limitations of conventional wastewater treatment systems in completely removing these compounds, especially the most persistent ones, such as carbamazepine and fluoxetine. Technologies such as activated carbon adsorption, AOPs, membrane filtration, and biological treatments with specialized microorganisms have demonstrated variable efficacy, with integrated multi-barrier systems standing out as the most promising approaches. However, challenges associated with economic viability, the generation of toxic byproducts, and large-scale applicability remain. Therefore, effective mitigation of antidepressant contamination requires not only the optimization of remediation technologies, but also public policies aimed at managing drug consumption, encouraging rational prescribing, developing environmentally degradable compounds, and strengthening the “One Health” concept, which recognizes the interdependence between human, animal, and environmental health. Consolidating interdisciplinary research, coupled with systematic monitoring and appropriate regulation, will be essential to reduce the environmental impacts of these contaminants and ensure the long-term protection of aquatic biodiversity and public health.
Author Contributions
J.G., original draft, writing. C.G.R. writing and preparation, J.S.d.O., writing and review, Y.D. writing and review, N.E.M., review, L.M. writing and reviewing, M.d.A.C. writing and reviewing, D.S.P.F. reviewing, preparation, visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Any requested data will be given upon request.
Acknowledgments
During the preparation of this manuscript/study, the authors used Obsidian with Excalidraw, Biorender, Markinsketh for drawing images.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| OECD | Organization for Economic Cooperation and Development |
| DDD | defined daily dosage |
| ASP | activated sludge |
| MBR | membrane bioreactor |
| MBBR | moving-bed biofilm reactor |
| UF | ultrafiltration |
| NF | nanofiltration |
| RO | reverse osmosis |
| AOPs | advanced oxidation processes |
| UV | ultraviolet |
| GAC | granular activated carbon |
References
- Gould, S.L.; Winter, M.J.; Norton, W.H.J.; Tyler, C.R. The Potential for Adverse Effects in Fish Exposed to Antidepressants in the Aquatic Environment. Environ. Sci. Technol. 2021, 55, 16299–16312. [Google Scholar] [CrossRef]
- Fava, M.; Rush, A.J.; Thase, M.E.; Clayton, A.; Stahl, S.M.; Pradko, J.F.; Johnston, J.A. 15 Years of Clinical Experience with Bupropion HCl: From Bupropion to Bupropion SR to Bupropion XL. Prim. Care Companion J. Clin. Psychiatry 2005, 7, 106–113. [Google Scholar] [CrossRef]
- Sanchez, C.; Asin, K.E.; Artigas, F. Vortioxetine, a Novel Antidepressant with Multimodal Activity: Review of Preclinical and Clinical Data. Pharmacol. Ther. 2015, 145, 43–57. [Google Scholar] [CrossRef]
- Racovita, R.C.; Ciuca, M.D. Wastewater Treatment Approaches for the Removal of Antidepressant Residues. In Wastewater Treatment and Sludge Management Systems; Taşeli, B.K., Jacob-Lopes, E., Deprá, M.C., Zepka, L.Q., Eds.; IntechOpen: Rijeka, Croatia, 2024. [Google Scholar]
- Lewer, D.; O’Reilly, C.; Mojtabai, R.; Evans-Lacko, S. Antidepressant Use in 27 European Countries: Associations with Sociodemographic, Cultural and Economic Factors. Br. J. Psychiatry 2015, 207, 221–226. [Google Scholar] [CrossRef]
- Benke, C.; Autenrieth, L.K.; Asselmann, E.; Pané-Farré, C.A. Lockdown, Quarantine Measures, and Social Distancing: Associations with Depression, Anxiety and Distress at the Beginning of the COVID-19 Pandemic among Adults from Germany. Psychiatry Res. 2020, 293, 113462. [Google Scholar] [CrossRef] [PubMed]
- Thoresen, S.; Blix, I.; Wentzel-Larsen, T.; Birkeland, M.S. Trusting Others During a Pandemic: Investigating Potential Changes in Generalized Trust and Its Relationship With Pandemic-Related Experiences and Worry. Front. Psychol. 2021, 12, 698519. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999−2000: A National Reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- DiPippa, A.D.; Mou, X. Meta-Analysis of the Distribution of Pharmaceuticals and Personal Care Products in Natural Streams of United States and Its Correlations with Anthropogenic Factors. ACS EST Water 2024, 4, 427–435. [Google Scholar] [CrossRef]
- Castillo-Zacarías, C.; Barocio, M.E.; Hidalgo-Vázquez, E.; Sosa-Hernández, J.E.; Parra-Arroyo, L.; López-Pacheco, I.Y.; Barceló, D.; Iqbal, H.N.M.; Parra-Saldívar, R. Antidepressant Drugs as Emerging Contaminants: Occurrence in Urban and Non-Urban Waters and Analytical Methods for Their Detection. Sci. Total Environ. 2021, 757, 143722. [Google Scholar] [CrossRef]
- Melchor-Martínez, E.M.; Jiménez-Rodríguez, M.G.; Martínez-Ruiz, M.; Peña-Benavides, S.A.; Iqbal, H.M.N.; Parra-Saldívar, R.; Sosa-Hernández, J.E. Antidepressants Surveillance in Wastewater: Overview Extraction and Detection. Case Stud. Chem. Environ. Eng. 2021, 3, 100074. [Google Scholar] [CrossRef]
- Santos, L.H.M.L.M.; Araújo, A.N.; Fachini, A.; Pena, A.; Delerue-Matos, C.; Montenegro, M.C.B.S.M. Ecotoxicological Aspects Related to the Presence of Pharmaceuticals in the Aquatic Environment. J. Hazard. Mater. 2010, 175, 45–95. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, E. Pharmaceuticals in the Environment: Expected and Unexpected Effects on Aquatic Fauna. Ann. N. Y. Acad. Sci. 2015, 1340, 20–28. [Google Scholar] [CrossRef]
- Gunnarsson, L.; Jauhiainen, A.; Kristiansson, E.; Nerman, O.; Larsson, D.G.J. Evolutionary Conservation of Human Drug Targets in Organisms Used for Environmental Risk Assessments. Environ. Sci. Technol. 2008, 42, 5807–5813. [Google Scholar] [CrossRef]
- Tanoue, R.; Nomiyama, K.; Nakamura, H.; Kim, J.W.; Isobe, T.; Shinohara, R.; Kunisue, T.; Tanabe, S. Uptake and Tissue Distribution of Pharmaceuticals and Personal Care Products in Wild Fish from Treated-Wastewater-Impacted Streams. Environ. Sci. Technol. 2015, 49, 11649–11658. [Google Scholar] [CrossRef]
- Huerta, B.; Rodriguez-Mozaz, S.; Lazorchak, J.; Barcelo, D.; Batt, A.; Wathen, J.; Stahl, L. Presence of Pharmaceuticals in Fish Collected from Urban Rivers in the U.S. EPA 2008–2009 National Rivers and Streams Assessment. Sci. Total Environ. 2018, 634, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Sehonova, P.; Plhalova, L.; Blahova, J.; Doubkova, V.; Marsalek, P.; Prokes, M.; Tichy, F.; Skladana, M.; Fiorino, E.; Mikula, P.; et al. Effects of Selected Tricyclic Antidepressants on Early-Life Stages of Common Carp (Cyprinus carpio). Chemosphere 2017, 185, 1072–1080. [Google Scholar] [CrossRef]
- Nowakowska, K.; Giebułtowicz, J.; Kamaszewski, M.; Adamski, A.; Szudrowicz, H.; Ostaszewska, T.; Solarska-Dzięciołowska, U.; Nałęcz-Jawecki, G.; Wroczyński, P.; Drobniewska, A. Acute Exposure of Zebrafish (Danio rerio) Larvae to Environmental Concentrations of Selected Antidepressants: Bioaccumulation, Physiological and Histological Changes. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 229, 108670. [Google Scholar] [CrossRef] [PubMed]
- Mahtab, M.S.; Farooqi, I.H. An Overview of Occurrence and Removal of Pharmaceuticals from Sewage/Wastewater. In Sewage; Zhang, T., Ed.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Georgin, J.; Franco, D.S.P.; Meili, L.; Dehmani, Y.; dos Reis, G.S.; Lima, E.C. Main Advances and Future Prospects in the Remediation of the Antibiotic Amoxicillin with a Focus on Adsorption Technology: A Critical Review. J. Water Process Eng. 2023, 56, 104407. [Google Scholar] [CrossRef]
- Georgin, J.; Franco, D.S.P.; Ramos, C.G.; Piccilli, D.G.A.; Lima, E.C.; Sher, F. A Review of the Antibiotic Ofloxacin: Current Status of Ecotoxicology and Scientific Advances in Its Removal from Aqueous Systems by Adsorption Technology. Chem. Eng. Res. Des. 2023, 193, 99–120. [Google Scholar] [CrossRef]
- Lama, G.; Meijide, J.; Sanromán, A.; Pazos, M. Heterogeneous Advanced Oxidation Processes: Current Approaches for Wastewater Treatment. Catalysts 2022, 12, 344. [Google Scholar] [CrossRef]
- Xiao, C.; Hu, Y.; Li, Q.; Liu, J.; Li, X.; Shi, Y.; Chen, Y.; Cheng, J. Carbon-Doped Defect MoS2 Co-Catalytic Fe3+/Peroxymonosulfate Process for Efficient Sulfadiazine Degradation: Accelerating Fe3+/Fe2+ Cycle and 1O2 Dominated Oxidation. Sci. Total Environ. 2023, 858, 159587. [Google Scholar] [CrossRef]
- Daughton, C.G. Pharmaceuticals and the Environment (PiE): Evolution and Impact of the Published Literature Revealed by Bibliometric Analysis. Sci. Total Environ. 2016, 562, 391–426. [Google Scholar] [CrossRef]
- Argaluza, J.; Domingo-Echaburu, S.; Orive, G.; Medrano, J.; Hernandez, R.; Lertxundi, U. Environmental Pollution with Psychiatric Drugs. World J. Psychiatry 2021, 11, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Reddersen, K.; Heberer, T.; Dünnbier, U. Identification and Significance of Phenazone Drugs and Their Metabolites in Ground- and Drinking Water. Chemosphere 2002, 49, 539–544. [Google Scholar] [CrossRef]
- Zühlke, S.; Dünnbier, U.; Heberer, T. Detection and Identification of Phenazone-Type Drugs and Their Microbial Metabolites in Ground and Drinking Water Applying Solid-Phase Extraction and Gas Chromatography with Mass Spectrometric Detection. J. Chromatogr. A 2004, 1050, 201–209. [Google Scholar] [CrossRef]
- Lee, W.Y.; Arnold, C.R. Chronic Toxicity of Ocean-Dumped Pharmaceutical Wastes to the Marine Amphipod Amphithoe Valida. Mar. Pollut. Bull. 1983, 14, 150–153. [Google Scholar] [CrossRef]
- Larsson, D.G.J.J. Pollution from Drug Manufacturing: Review and Perspectives. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130571. [Google Scholar] [CrossRef]
- Oaks, J.L.; Gilbert, M.; Virani, M.Z.; Watson, R.T.; Meteyer, C.U.; Rideout, B.A.; Shivaprasad, H.L.; Ahmed, S.; Iqbal Chaudhry, M.J.; Arshad, M.; et al. Diclofenac Residues as the Cause of Vulture Population Decline in Pakistan. Nature 2004, 427, 630–633. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; de Pedro, C.; Paxeus, N. Effluent from Drug Manufactures Contains Extremely High Levels of Pharmaceuticals. J. Hazard. Mater. 2007, 148, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.N.; Paxéus, N.; Förlin, L.; Larsson, D.G.J. Variations in Bioconcentration of Human Pharmaceuticals from Sewage Effluents into Fish Blood Plasma. Environ. Toxicol. Pharmacol. 2007, 24, 267–274. [Google Scholar] [CrossRef]
- Kristiansson, E.; Fick, J.; Janzon, A.; Grabic, R.; Rutgersson, C.; Weijdegård, B.; Söderström, H.; Joakim Larsson, D.G. Pyrosequencing of Antibiotic-Contaminated River Sediments Reveals High Levels of Resistance and Gene Transfer Elements. PLoS ONE 2011, 6, e17038. [Google Scholar] [CrossRef]
- Fick, J.; Söderström, H.; Lindberg, R.H.; Phan, C.; Tysklind, M.; Larsson, D.G.J. Contamination of Surface, Ground, and Drinking Water from Pharmaceutical Production. Environ. Toxicol. Chem. 2009, 28, 2522–2527. [Google Scholar] [CrossRef]
- Rutgersson, C.; Fick, J.; Marathe, N.; Kristiansson, E.; Janzon, A.; Angelin, M.; Johansson, A.; Shouche, Y.; Flach, C.F.; Larsson, D.G.J. Fluoroquinolones and Qnr Genes in Sediment, Water, Soil, and Human Fecal Flora in an Environment Polluted by Manufacturing Discharges. Environ. Sci. Technol. 2014, 48, 7825–7832. [Google Scholar] [CrossRef] [PubMed]
- Lubick, N. India’s Drug Problem: Chemists Show How Waste-Water Contamination Affects Ecosystem. Nature 2009, 457, 640–642. [Google Scholar] [CrossRef]
- Silva, B.; Costa, F.; Neves, I.C.; Tavares, T. Psychiatric Pharmaceuticals as Emerging Contaminants in Wastewater; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-319-20492-5. [Google Scholar]
- Hai, F.I.; Yang, S.; Asif, M.B.; Sencadas, V.; Shawkat, S.; Sanderson-Smith, M.; Gorman, J.; Xu, Z.Q.; Yamamoto, K. Carbamazepine as a Possible Anthropogenic Marker in Water: Occurrences, Toxicological Effects, Regulations and Removal by Wastewater Treatment Technologies. Water 2018, 10, 107. [Google Scholar] [CrossRef]
- Trawiński, J.; Skibiński, R. Studies on Photodegradation Process of Psychotropic Drugs: A Review. Environ. Sci. Pollut. Res. 2017, 24, 1152–1199. [Google Scholar] [CrossRef]
- Brodin, T.; Piovano, S.; Fick, J.; Klaminder, J.; Heynen, M.; Jonsson, M. Ecological Effects of Pharmaceuticals in Aquatic Systems—Impacts through Behavioural Alterations. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130580. [Google Scholar] [CrossRef] [PubMed]
- Onesios, K.M.; Yu, J.T.; Bouwer, E.J. Biodegradation and Removal of Pharmaceuticals and Personal Care Products in Treatment Systems: A Review. Biodegradation 2009, 20, 441–466. [Google Scholar] [CrossRef]
- Silva, L.J.G.; Lino, C.M.; Meisel, L.M.; Pena, A. Selective Serotonin Re-Uptake Inhibitors (SSRIs) in the Aquatic Environment: An Ecopharmacovigilance Approach. Sci. Total Environ. 2012, 437, 185–195. [Google Scholar] [CrossRef]
- Mole, R.A.; Brooks, B.W. Global Scanning of Selective Serotonin Reuptake Inhibitors: Occurrence, Wastewater Treatment and Hazards in Aquatic Systems. Environ. Pollut. 2019, 250, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.R.; Kasprzyk-Hordern, B. Spatial and Temporal Occurrence of Pharmaceuticals and Illicit Drugs in the Aqueous Environment and during Wastewater Treatment: New Developments. Sci. Total Environ. 2013, 454–455, 442–456. [Google Scholar] [CrossRef]
- Baker, D.R.; Kasprzyk-Hordern, B. Multi-Residue Analysis of Drugs of Abuse in Wastewater and Surface Water by Solid-Phase Extraction and Liquid Chromatography-Positive Electrospray Ionisation Tandem Mass Spectrometry. J. Chromatogr. A 2011, 1218, 1620–1631. [Google Scholar] [CrossRef]
- Schultz, M.M.; Furlong, E.T. Trace Analysis of Antidepressant Pharmaceuticals and Their Select Degradates in Aquatic Matrixes by LC/ESI/MS/MS. Anal. Chem. 2008, 80, 1756–1762. [Google Scholar] [CrossRef]
- Metcalfe, C.D.; Chu, S.; Judt, C.; Li, H.; Oakes, K.D.; Servos, M.R.; Andrews, D.M. Antidepressants and Their Metabolites in Municipal Wastewater, and Downstream Exposure in an Urban Watershed. Environ. Toxicol. Chem. 2010, 29, 79–89. [Google Scholar] [CrossRef]
- Rúa-Gómez, P.C.; Püttmann, W. Impact of Wastewater Treatment Plant Discharge of Lidocaine, Tramadol, Venlafaxine and Their Metabolites on the Quality of Surface Waters and Groundwater. J. Environ. Monit. 2012, 14, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Zmarlicka, M.; Ehret, M.J. Vilazodone: A Novel Antidepressant. Am. J. Health Pharm. 2012, 69, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.; Nowicki, M.; Muraleetharan, A.; Gerlai, R. Differential Effects of Dopamine D1 and D2/3 Receptor Antagonism on Motor Responses. Psychopharmacology 2015, 232, 795–806. [Google Scholar] [CrossRef]
- Brunello, N.; Blier, P.; Judd, L.L.; Mendlewicz, J.; Nelson, C.J.; Souery, D.; Zohar, J.; Racagni, G. Noradrenaline in Mood and Anxiety Disorders: Basic and Clinical Studies. Int. Clin. Psychopharmacol. 2003, 18, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Farrar, M.J.; Kolkman, K.E.; Fetcho, J.R. Features of the Structure, Development, and Activity of the Zebrafish Noradrenergic System Explored in New CRISPR Transgenic Lines. J. Comp. Neurol. 2018, 526, 2493–2508. [Google Scholar] [CrossRef]
- Li, P.; Shah, S.; Huang, L.; Carr, A.L.; Gao, Y.; Thisse, C.; Thisse, B.; Li, L. Cloning and Spatial and Temporal Expression of the Zebrafish Dopamine D1 Receptor. Dev. Dyn. 2007, 236, 1339–1346. [Google Scholar] [CrossRef]
- Ruuskanen, J.O.; Xhaard, H.; Marjamäki, A.; Salaneck, E.; Salminen, T.; Yan, Y.L.; Postlethwait, J.H.; Johnson, M.S.; Larhammar, D.; Scheinin, M. Identification of Duplicated Fourth A2-Adrenergic Receptor Subtype by Cloning and Mapping of Five Receptor Genes in Zebrafish. Mol. Biol. Evol. 2004, 21, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Demin, K.A.; Kolesnikova, T.O.; Khatsko, S.L.; Meshalkina, D.A.; Efimova, E.V.; Morzherin, Y.Y.; Kalueff, A.V. Acute Effects of Amitriptyline on Adult Zebrafish: Potential Relevance to Antidepressant Drug Screening and Modeling Human Toxidromes. Neurotoxicol. Teratol. 2017, 62, 27–33. [Google Scholar] [CrossRef]
- Grabicova, K.; Lindberg, R.H.; Östman, M.; Grabic, R.; Randak, T.; Joakim Larsson, D.G.; Fick, J. Tissue-Specific Bioconcentration of Antidepressants in Fish Exposed to Effluent from a Municipal Sewage Treatment Plant. Sci. Total Environ. 2014, 488–489, 46–50. [Google Scholar] [CrossRef]
- Koba, O.; Grabicova, K.; Cerveny, D.; Turek, J.; Kolarova, J.; Randak, T.; Zlabek, V.; Grabic, R. Transport of Pharmaceuticals and Their Metabolites between Water and Sediments as a Further Potential Exposure for Aquatic Organisms. J. Hazard. Mater. 2018, 342, 401–407. [Google Scholar] [CrossRef]
- Schultz, W. Behavioral Dopamine Signals. Trends Neurosci. 2007, 30, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Huggett, D.B.; Cook, J.C.; Ericson, J.F.; Williams, R.T. A Theoretical Model for Utilizing Mammalian Pharmacology and Safety Data to Prioritize Potential Impacts of Human Pharmaceuticals to Fish. Hum. Ecol. Risk Assess. 2003, 9, 1789–1799. [Google Scholar] [CrossRef]
- Marrone, R.L.; Pray, S.L.; Bridges, C.C. Norepinephrine Elicitation of Aggressive Display Responses in Betta Splendens. Psychon. Sci. 1966, 5, 207–208. [Google Scholar] [CrossRef]
- McCallum, E.S.; Krutzelmann, E.; Brodin, T.; Fick, J.; Sundelin, A.; Balshine, S. Exposure to Wastewater Effluent Affects Fish Behaviour and Tissue-Specific Uptake of Pharmaceuticals. Sci. Total Environ. 2017, 605–606, 578–588. [Google Scholar] [CrossRef]
- Brun, G.L.; Bernier, M.; Losier, R.; Doe, K.; Jackman, P.; Lee, H.B. Pharmaceutically Active Compounds in Atlantic Canadian Sewage Treatment Plant Effluents and Receiving Waters, and Potential for Environmental Effects as Measured by Acute and Chronic Aquatic Toxicity. Environ. Toxicol. Chem. 2006, 25, 2163–2176. [Google Scholar] [CrossRef]
- Holzschuh, J.; Ryu, S.; Aberger, F.; Driever, W. Dopamine Transporter Expression Distinguishes Dopaminergic Neurons from Other Catecholaminergic Neurons in the Developing Zebrafish Embryo. Mech. Dev. 2001, 101, 237–243. [Google Scholar] [CrossRef]
- Cunha, V.; Rodrigues, P.; Santos, M.M.; Moradas-Ferreira, P.; Ferreira, M. Fluoxetine Modulates the Transcription of Genes Involved in Serotonin, Dopamine and Adrenergic Signalling in Zebrafish Embryos. Chemosphere 2018, 191, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.G.; Törk, I.; Hornung, J.P.; Halasz, P. The Human Locus Coeruleus Complex: An Immunohistochemical and Three Dimensional Reconstruction Study. Exp. Brain Res. 1989, 77, 257–270. [Google Scholar] [CrossRef]
- Ruffolo, R.R.; Hieble, J.P. α-Adrenoceptors. Pharmacol. Ther. 1994, 61, 1–64. [Google Scholar] [CrossRef]
- Wang, Z.; Nishimura, Y.; Shimada, Y.; Umemoto, N.; Hirano, M.; Zang, L.; Oka, T.; Sakamoto, C.; Kuroyanagi, J.; Tanaka, T. Zebrafish β-Adrenergic Receptor MRNA Expression and Control of Pigmentation. Gene 2009, 446, 18–27. [Google Scholar] [CrossRef]
- Zikopoulos, B.; Dermon, C.R. Comparative Anatomy of A2 and β Adrenoceptors in the Adult and Developing Brain of the Marine Teleost the Red Porgy (Pagrus Pagrus, Sparidae): [3H]Clonidine and [3H]Dihydroalprenolol Quantitative Autoradiography and Receptor Subtypes Immunohistochemistry. J. Comp. Neurol. 2005, 489, 217–240. [Google Scholar] [CrossRef] [PubMed]
- Ruuskanen, J.O.; Peitsaro, N.; Kaslin, J.V.M.; Panula, P.; Scheinin, M. Expression and Function of A2-Adrenoceptors in Zebrafish: Drug Effects, MRNA and Receptor Distributions. J. Neurochem. 2005, 94, 1559–1569. [Google Scholar] [CrossRef]
- Sharma, Y.; Xu, T.; Graf, W.M.; Fobbs, A.; Sherwood, C.C.; Hof, P.R.; Allman, J.M.; Manaye, K.F. Comparative Anatomy of the Locus Coeruleus in Humans and Nonhuman Primates. J. Comp. Neurol. 2010, 518, 963–971. [Google Scholar] [CrossRef]
- Irons, T.D.; Kelly, P.E.; Hunter, D.L.; MacPhail, R.C.; Padilla, S. Acute Administration of Dopaminergic Drugs Has Differential Effects on Locomotion in Larval Zebrafish. Pharmacol. Biochem. Behav. 2013, 103, 792–813. [Google Scholar] [CrossRef]
- Kacprzak, V.; Patel, N.A.; Riley, E.; Yu, L.; Yeh, J.R.J.; Zhdanova, I.V. Dopaminergic Control of Anxiety in Young and Aged Zebrafish. Pharmacol. Biochem. Behav. 2017, 157, 1–8. [Google Scholar] [CrossRef]
- Gillman, P.K. Tricyclic Antidepressant Pharmacology and Therapeutic Drug Interactions Updated. Br. J. Pharmacol. 2007, 151, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Rehavi, M.; Maayani, S.; Sokolovsky, M. Tricyclic Antidepressants as Antimuscarinic Drugs: In Vivo and in Vitro Studies. Biochem. Pharmacol. 1977, 26, 1559–1567. [Google Scholar] [CrossRef]
- Wong, D.T.; Bymaster, F.P.; Engleman, E.A. Prozac (Fluoxetine, Lilly 110140), the First Selective Serotonin Uptake Inhibitor and an Antidepressant Drug: Twenty Years since Its First Publication. Life Sci. 1995, 57, 411–441. [Google Scholar] [CrossRef]
- McDonald, M.D. An AOP Analysis of Selective Serotonin Reuptake Inhibitors (SSRIs) for Fish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 197, 19–31. [Google Scholar] [CrossRef]
- Walker, F.R. A Critical Review of the Mechanism of Action for the Selective Serotonin Reuptake Inhibitors: Do These Drugs Possess Anti-Inflammatory Properties and How Relevant Is This in the Treatment of Depression? Neuropharmacology 2013, 67, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.T.; Bymaster, F.P.; Reid, L.R.; Threlkeld, P.G. Fluoxetine and Two Other Serotonin Uptake Inhibitors without Affinity for Neuronal Receptors. Biochem. Pharmacol. 1983, 32, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.; Bymaster, F.P.; Reid, L.R.; Mayle, D.A.; Krushinski, J.H.; Robertson, D.W. Norfluoxetine Enantiomers as Inhibitors of Serotonin Uptake in Rat Brain. Neuropsychopharmacology 1993, 8, 337–344. [Google Scholar] [CrossRef]
- Marken, P.A.; Stuart Munro, J. Selecting a Selective Serotonin Reuptake Inhibitor: Clinically Important Distinguishing Features. Prim. Care Companion J. Clin. Psychiatry 2000, 2, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Seth, R.; Jennings, A.L.; Bindman, J.; Phillips, J.; Bergmann, K. Combination Treatment with Noradrenalin and Serotonin Reuptake Inhibitors in Resistant Depression. Br. J. Psychiatry 1992, 161, 562–565. [Google Scholar] [CrossRef]
- Horst, W.D.; Preskorn, S.H. Mechanisms of Action and Clinical Characteristics of Three Atypical Antidepressants: Venlafaxine, Nefazodone, Bupropion. J. Affect. Disord. 1998, 51, 237–254. [Google Scholar] [CrossRef]
- Bacqué-cazenave, J.; Bharatiya, R.; Barrière, G.; Delbecque, J.P.; Bouguiyoud, N.; Di Giovanni, G.; Cattaert, D.; De Deurwaerdère, P. Serotonin in Animal Cognition and Behavior. Int. J. Mol. Sci. 2020, 21, 1649. [Google Scholar] [CrossRef]
- Cordes, S.P. Molecular Genetics of the Early Development of Hindbrain Serotonergic Neurons. Clin. Genet. 2005, 68, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, P.; Lillesaar, C. Probing the Diversity of Serotonin Neurons. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2382–2394. [Google Scholar] [CrossRef]
- Herculano, A.M.; Maximino, C. Serotonergic Modulation of Zebrafish Behavior: Towards a Paradox. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 55, 50–66. [Google Scholar] [CrossRef]
- Norton, W.H.J.; Folchert, A.; Bally-Cuif, L. Comparative Analysis of Serotonin Receptor (HTR1A/HTR1B Families) and Transporter (Slc6a4a/b) Gene Expression in the Zebrafish Brain. J. Comp. Neurol. 2008, 511, 521–542. [Google Scholar] [CrossRef]
- Schneider, H.; Fritzky, L.; Williams, J.; Heumann, C.; Yochum, M.; Pattar, K.; Noppert, G.; Mock, V.; Hawley, E. Cloning and Expression of a Zebrafish 5-HT 2C Receptor Gene. Gene 2012, 502, 108–117. [Google Scholar] [CrossRef]
- De Pedro, N.; Pinillos, M.L.; Valenciano, A.I.; Alonso-Bedate, M.; Delgado, M.J. Inhibitory Effect of Serotonin on Feeding Behavior in Goldfish: Involvement of CRF. Peptides 1998, 19, 505–511. [Google Scholar] [CrossRef]
- Pérez-Maceira, J.J.; Otero-Rodiño, C.; Mancebo, M.J.; Soengas, J.L.; Aldegunde, M. Food Intake Inhibition in Rainbow Trout Induced by Activation of Serotonin 5-HT2C Receptors Is Associated with Increases in POMC, CART and CRF MRNA Abundance in Hypothalamus. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2016, 186, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.L.; Fornal, C.A. Serotonin and Motor Activity. Curr. Opin. Neurobiol. 1997, 7, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Iwamatsu, T.; Toya, Y.; Sakai, N.; Terada, Y.; Nagata, R.; Nagahama, Y. Effect of 5-Hydroxytryptamine on Steroidogenesis and Oocyte Maturation in Pre-ovulatory Follicles of the Medaka Oryzias latipes Medaka/Steroidogenesis/5-hydroxytryptamine/Foillicle/Oocyte Maturation. Dev. Growth Differ. 1993, 35, 625–630. [Google Scholar] [CrossRef]
- Khan, I.A.; Thomas, P. Stimulatory Effects of Serotonin on Maturational Gonadotropin Release in the Atlantic Croaker, Micropogonias Undulatus. Gen. Comp. Endocrinol. 1992, 88, 388–396. [Google Scholar] [CrossRef]
- Hauser, T.U.; Eldar, E.; Purg, N.; Moutoussis, M.; Dolan, R.J. Distinct Roles of Dopamine and Noradrenaline in Incidental Memory. J. Neurosci. 2019, 39, 7715–7721. [Google Scholar] [CrossRef] [PubMed]
- Haller, J.; Makara, G.B.; Kruk, M.R. Catecholaminergic Involvement in the Control of Aggression: Hormones, the Peripheral Sympathetic, and Central Noradrenergic Systems. Neurosci. Biobehav. Rev. 1997, 22, 85–97. [Google Scholar] [CrossRef]
- Pertovaara, A. Noradrenergic Pain Modulation. Prog. Neurobiol. 2006, 80, 53–83. [Google Scholar] [CrossRef] [PubMed]
- Bernier, N.J.; McKendry, J.E.; Perry, S.F. Blood Pressure Regulation during Hypotension in Two Teleost Species: Differential Involvement of the Renin–Angiotensin and Adrenergic Systems. J. Exp. Biol. 1999, 202, 1677–1690. [Google Scholar] [CrossRef]
- Sharples, S.A.; Koblinger, K.; Humphreys, J.M.; Whelan, P.J. Dopamine: A Parallel Pathway for the Modulation of Spinal Locomotor Networks. Front. Neural Circuits 2014, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Nieoullon, A. Dopamine and the Regulation of Cognition and Attention. Prog. Neurobiol. 2002, 67, 53–83. [Google Scholar] [CrossRef]
- Thörnqvist, P.O.; McCarrick, S.; Ericsson, M.; Roman, E.; Winberg, S. Bold Zebrafish (Danio rerio) Express Higher Levels of Delta Opioid and Dopamine D2 Receptors in the Brain Compared to Shy Fish. Behav. Brain Res. 2019, 359, 927–934. [Google Scholar] [CrossRef]
- Messias, J.P.M.; Santos, T.P.; Pinto, M.; Soares, M.C. Stimulation of Dopamine D1 Receptor Improves Learning Capacity in Cooperating Cleaner Fish. Proc. R. Soc. B Biol. Sci. 2016, 283, 20152272. [Google Scholar] [CrossRef]
- Naderi, M.; Jamwal, A.; Chivers, D.P.; Niyogi, S. Modulatory Effects of Dopamine Receptors on Associative Learning Performance in Zebrafish (Danio rerio). Behav. Brain Res. 2016, 303, 109–119. [Google Scholar] [CrossRef]
- Boehmier, W.; Obrecht-Pflumio, S.; Canfield, V.; Thisse, C.; Thisse, B.; Levenson, R. Evolution and Expression of D2 and D3 Dopamine Receptor Genes in Zebrafish. Dev. Dyn. 2004, 230, 481–493. [Google Scholar] [CrossRef]
- Boehmler, W.; Carr, T.; Thisse, C.; Thisse, B.; Canfield, V.A.; Levenson, R. D4 Dopamine Receptor Genes of Zebrafish and Effects of the Antipsychotic Clozapine on Larval Swimming Behaviour. Genes Brain Behav. 2007, 6, 155–166. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yamamoto, H.; Sekizawa, J.; Kondo, T.; Hirai, N.; Tatarazako, N. The Effects of PH on Fluoxetine in Japanese Medaka (Oryzias latipes): Acute Toxicity in Fish Larvae and Bioaccumulation in Juvenile Fish. Chemosphere 2008, 70, 865–873. [Google Scholar] [CrossRef]
- Arnnok, P.; Singh, R.R.; Burakham, R.; Pérez-Fuentetaja, A.; Aga, D.S. Selective Uptake and Bioaccumulation of Antidepressants in Fish from Effluent-Impacted Niagara River. Environ. Sci. Technol. 2017, 51, 10652–10662. [Google Scholar] [CrossRef]
- Bittner, L.; Klüver, N.; Henneberger, L.; Mühlenbrink, M.; Zarfl, C.; Escher, B.I. Combined Ion-Trapping and Mass Balance Models to Describe the PH-Dependent Uptake and Toxicity of Acidic and Basic Pharmaceuticals in Zebrafish Embryos (Danio rerio). Environ. Sci. Technol. 2019, 53, 7877–7886. [Google Scholar] [CrossRef]
- Escher, B.I.; Abagyan, R.; Embry, M.; Klüver, N.; Redman, A.D.; Zarfl, C.; Parkerton, T.F. Recommendations for Improving Methods and Models for Aquatic Hazard Assessment of Ionizable Organic Chemicals. Environ. Toxicol. Chem. 2020, 39, 269–286. [Google Scholar] [CrossRef]
- Lajeunesse, A.; Gagnon, C.; Gagné, F.; Louis, S.; Čejka, P.; Sauvé, S. Distribution of Antidepressants and Their Metabolites in Brook Trout Exposed to Municipal Wastewaters before and after Ozone Treatment—Evidence of Biological Effects. Chemosphere 2011, 83, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Grabicova, K.; Grabic, R.; Fedorova, G.; Fick, J.; Cerveny, D.; Kolarova, J.; Turek, J.; Zlabek, V.; Randak, T. Bioaccumulation of Psychoactive Pharmaceuticals in Fish in an Effluent Dominated Stream. Water Res. 2017, 124, 654–662. [Google Scholar] [CrossRef]
- David, A.; Lange, A.; Tyler, C.R.; Hill, E.M. Concentrating Mixtures of Neuroactive Pharmaceuticals and Altered Neurotransmitter Levels in the Brain of Fish Exposed to a Wastewater Effluent. Sci. Total Environ. 2018, 621, 782–790. [Google Scholar] [CrossRef]
- Grabicová, K.; Grabic, R.; Fedorova, G.; Vojs Staňová, A.; Bláha, M.; Randák, T.; Brooks, B.W.; Žlábek, V. Water Reuse and Aquaculture: Pharmaceutical Bioaccumulation by Fish during Tertiary Treatment in a Wastewater Stabilization Pond. Environ. Pollut. 2020, 267, 115593. [Google Scholar] [CrossRef] [PubMed]
- Melvin, S.D. Effect of Antidepressants on Circadian Rhythms in Fish: Insights and Implications Regarding the Design of Behavioural Toxicity Tests. Aquat. Toxicol. 2017, 182, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimmons, P.N.; Fernandez, J.D.; Hoffman, A.D.; Butterworth, B.C.; Nichols, J.W. Branchial Elimination of Superhydrophobic Organic Compounds by Rainbow Trout (Oncorhynchus mykiss). Aquat. Toxicol. 2001, 55, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Margiotta-Casaluci, L.; Owen, S.F.; Cumming, R.I.; De Polo, A.; Winter, M.J.; Panter, G.H.; Rand-Weaver, M.; Sumpter, J.P. Quantitative Cross-Species Extrapolation between Humans and Fish: The Case of the Anti-Depressant Fluoxetine. PLoS ONE 2014, 9, e110467. [Google Scholar] [CrossRef]
- World Health Organization. WHO Pharmaceuticals in Drinking Water; WHO Press: World Health Organization: Geneva, Switzerland, 2012; ISBN 978 9241502085. [Google Scholar]
- Wang, F.; Xiang, L.; Sze-Yin Leung, K.; Elsner, M.; Zhang, Y.; Guo, Y.; Pan, B.; Sun, H.; An, T.; Ying, G.; et al. Emerging Contaminants: A One Health Perspective. Innovation 2024, 5, 100612. [Google Scholar] [CrossRef] [PubMed]
- Marchocki, Z.; Russell, N.E.; Donoghue, K.O. Selective Serotonin Reuptake Inhibitors and Pregnancy: A Review of Maternal, Fetal and Neonatal Risks and Benefits. Obstet. Med. 2013, 6, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Toh, S.; Mitchell, A.A.; Louik, C.; Werler, M.M.; Chambers, C.D.; Hernández-Díaz, S. Antidepressant Use during Pregnancy and the Risk of Preterm Delivery and Fetal Growth Restriction. J. Clin. Psychopharmacol. 2009, 29, 555–560. [Google Scholar] [CrossRef]
- El Marroun, H.; Jaddoe, V.W.V.; Hudziak, J.J.; Roza, S.J.; Steegers, E.A.P.; Hofman, A.; Verhulst, F.C.; White, T.J.H.; Stricker, B.H.C.; Tiemeier, H. Maternal Use of Selective Serotonin Reuptake Inhibitors, Fetal Growth, and Risk of Adverse Birth Outcomes. Arch. Gen. Psychiatry 2012, 69, 706–714. [Google Scholar] [CrossRef]
- Rommel, A.-S.; Momen, N.C.; Molenaar, N.M.; Agerbo, E.; Bergink, V.; Munk-Olsen, T.; Liu, X. Antidepressant Use during Pregnancy and Risk of Adverse Neonatal Outcomes: A Comprehensive Investigation of Previously Identified Associations. Acta Psychiatr. Scand. 2022, 145, 544–556. [Google Scholar] [CrossRef]
- Melnyk-Lamont, N.; Best, C.; Gesto, M.; Vijayan, M.M. The Antidepressant Venlafaxine Disrupts Brain Monoamine Levels and Neuroendocrine Responses to Stress in Rainbow Trout. Environ. Sci. Technol. 2014, 48, 13434–13442. [Google Scholar] [CrossRef]
- Yang, M.; Qiu, W.; Chen, J.; Zhan, J.; Pan, C.; Lei, X.; Wu, M. Growth Inhibition and Coordinated Physiological Regulation of Zebrafish (Danio rerio) Embryos upon Sublethal Exposure to Antidepressant Amitriptyline. Aquat. Toxicol. 2014, 151, 68–76. [Google Scholar] [CrossRef]
- Pelli, M.; Connaughton, V.P. Chronic Exposure to Environmentally-Relevant Concentrations of Fluoxetine (Prozac) Decreases Survival, Increases Abnormal Behaviors, and Delays Predator Escape Responses in Guppies. Chemosphere 2015, 139, 202–209. [Google Scholar] [CrossRef]
- Martin, J.M.; Saaristo, M.; Bertram, M.G.; Lewis, P.J.; Coggan, T.L.; Clarke, B.O.; Wong, B.B.M. The Psychoactive Pollutant Fluoxetine Compromises Antipredator Behaviour in Fish. Environ. Pollut. 2017, 222, 592–599. [Google Scholar] [CrossRef]
- Fuxe, K.; Ungerstedt, U. Histochemical studies on the effect of (+)-amphetamine, drugs of the imipramine group and tryptamine on central catecholamine and 5-hydroxytryptamine neurons after intraventricular injection of catecholamines and 5-hydroxytryptamine. Eur. J. Pharmacol. 1968, 4, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Liu, L.; Zhong, Z.; Wang, H.; Lin, S.; Shang, J. Risk of Prenatal Depression and Stress Treatment: Alteration on Serotonin System of Offspring through Exposure to Fluoxetine. Sci. Rep. 2016, 6, 33822. [Google Scholar] [CrossRef] [PubMed]
- Parolini, M.; Ghilardi, A.; De Felice, B.; Del Giacco, L. Environmental Concentration of Fluoxetine Disturbs Larvae Behavior and Increases the Defense Response at Molecular Level in Zebrafish (Danio rerio). Environ. Sci. Pollut. Res. 2019, 26, 34943–34952. [Google Scholar] [CrossRef]
- Sehonova, P.; Svobodova, Z.; Dolezelova, P.; Vosmerova, P.; Faggio, C. Effects of Waterborne Antidepressants on Non-Target Animals Living in the Aquatic Environment: A Review. Sci. Total Environ. 2018, 631–632, 789–794. [Google Scholar] [CrossRef]
- Duarte, I.A.; Reis-Santos, P.; Novais, S.C.; Rato, L.D.; Lemos, M.F.L.; Freitas, A.; Pouca, A.S.V.; Barbosa, J.; Cabral, H.N.; Fonseca, V.F. Depressed, Hypertense and Sore: Long-Term Effects of Fluoxetine, Propranolol and Diclofenac Exposure in a Top Predator Fish. Sci. Total Environ. 2020, 712, 136564. [Google Scholar] [CrossRef]
- Wu, M.; Liu, S.; Hu, L.; Qu, H.; Pan, C.; Lei, P.; Shen, Y.; Yang, M. Global Transcriptomic Analysis of Zebrafish in Response to Embryonic Exposure to Three Antidepressants, Amitriptyline, Fluoxetine and Mianserin. Aquat. Toxicol. 2017, 192, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Fraher, D.; Hodge, J.M.; Collier, F.M.; McMillan, J.S.; Kennedy, R.L.; Ellis, M.; Nicholson, G.C.; Walder, K.; Dodd, S.; Berk, M.; et al. Citalopram and Sertraline Exposure Compromises Embryonic Bone Development. Mol. Psychiatry 2016, 21, 656–664. [Google Scholar] [CrossRef]
- Bataineh, H.N.; Daradka, T. Effects of Long-Term Use of Fluoxetin on Fertility Parameters in Adults Male Rats. Neuroendocrinol. Lett. 2007, 28, 321–325. [Google Scholar]
- Tanrikut, C.; Feldman, A.S.; Altemus, M.; Paduch, D.A.; Schlegel, P.N. Adverse Effect of Paroxetine on Sperm. Fertil. Steril. 2010, 94, 1021–1026. [Google Scholar] [CrossRef]
- Lister, A.; Regan, C.; Van Zwol, J.; Van Der Kraak, G. Inhibition of Egg Production in Zebrafish by Fluoxetine and Municipal Effluents: A Mechanistic Evaluation. Aquat. Toxicol. 2009, 95, 320–329. [Google Scholar] [CrossRef]
- Galus, M.; Kirischian, N.; Higgins, S.; Purdy, J.; Chow, J.; Rangaranjan, S.; Li, H.; Metcalfe, C.; Wilson, J.Y. Chronic, Low Concentration Exposure to Pharmaceuticals Impacts Multiple Organ Systems in Zebrafish. Aquat. Toxicol. 2013, 132–133, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, J.; Klaper, R. Environmental Concentrations of the Selective Serotonin Reuptake Inhibitor Fluoxetine Impact Specific Behaviors Involved in Reproduction, Feeding and Predator Avoidance in the Fish Pimephales Promelas (Fathead minnow). Aquat. Toxicol. 2014, 151, 77–83. [Google Scholar] [CrossRef]
- Martin, J.M.; Bertram, M.G.; Saaristo, M.; Ecker, T.E.; Hannington, S.L.; Tanner, J.L.; Michelangeli, M.; O’Bryan, M.K.; Wong, B.B.M. Impact of the Widespread Pharmaceutical Pollutant Fluoxetine on Behaviour and Sperm Traits in a Freshwater Fish. Sci. Total Environ. 2019, 650, 1771–1778. [Google Scholar] [CrossRef]
- Bertram, M.G.; Ecker, T.E.; Wong, B.B.M.; O’Bryan, M.K.; Baumgartner, J.B.; Martin, J.M.; Saaristo, M. The Antidepressant Fluoxetine Alters Mechanisms of Pre- and Post-Copulatory Sexual Selection in the Eastern Mosquitofish (Gambusia holbrooki). Environ. Pollut. 2018, 238, 238–247. [Google Scholar] [CrossRef]
- Stanley, J.K.; Ramirez, A.J.; Chambliss, C.K.; Brooks, B.W. Enantiospecific Sublethal Effects of the Antidepressant Fluoxetine to a Model Aquatic Vertebrate and Invertebrate. Chemosphere 2007, 69, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.T.; Reid, L.R.; Threlkeld, P.G. Suppression of Food Intake in Rats by Fluoxetine: Comparison of Enantiomers and Effects of Serotonin Antagonists. Pharmacol. Biochem. Behav. 1988, 31, 475–479. [Google Scholar] [CrossRef]
- Mennigen, J.A.; Harris, E.A.; Chang, J.P.; Moon, T.W.; Trudeau, V.L. Fluoxetine Affects Weight Gain and Expression of Feeding Peptides in the Female Goldfish Brain. Regul. Pept. 2009, 155, 99–104. [Google Scholar] [CrossRef]
- Kellner, M.; Porseryd, T.; Porsch-Hällström, I.; Borg, B.; Roufidou, C.; Olsén, K.H. Developmental Exposure to the SSRI Citalopram Causes Long-Lasting Behavioural Effects in the Three-Spined Stickleback (Gasterosteus aculeatus). Ecotoxicology 2018, 27, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding Behavioral and Physiological Phenotypes of Stress and Anxiety in Zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef]
- Maximino, C.; de Brito, T.M.; da Silva Batista, A.W.; Herculano, A.M.; Morato, S.; Gouveia, A. Measuring Anxiety in Zebrafish: A Critical Review. Behav. Brain Res. 2010, 214, 157–171. [Google Scholar] [CrossRef]
- Ansai, S.; Hosokawa, H.; Maegawa, S.; Kinoshita, M. Chronic Fluoxetine Treatment Induces Anxiolytic Responses and Altered Social Behaviors in Medaka, Oryzias latipes. Behav. Brain Res. 2016, 303, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Maulvault, A.L.; Santos, L.H.M.L.M.; Paula, J.R.; Camacho, C.; Pissarra, V.; Fogaça, F.; Barbosa, V.; Alves, R.; Ferreira, P.P.; Barceló, D.; et al. Differential Behavioural Responses to Venlafaxine Exposure Route, Warming and Acidification in Juvenile Fish (Argyrosomus regius). Sci. Total Environ. 2018, 634, 1136–1147. [Google Scholar] [CrossRef]
- Giacomini, A.C.V.V.; Piassetta, A.S.; Genario, R.; Bonan, C.D.; Piato, A.; Barcellos, L.J.G.; de Abreu, M.S. Tryptophan Alleviates Neuroendocrine and Behavioral Responses to Stress in Zebrafish. Behav. Brain Res. 2020, 378, 112264. [Google Scholar] [CrossRef]
- Treit, D.; Fundytus, M. Thigmotaxis as a Test for Anxiolytic Activity in Rats. Pharmacol. Biochem. Behav. 1988, 31, 959–962. [Google Scholar] [CrossRef]
- Maximino, C.; Marques De Brito, T.; De Mattos Dias, C.A.G.; Gouveia, A.; Morato, S. Scototaxis as Anxiety-like Behavior in Fish. Nat. Protoc. 2010, 5, 221–228. [Google Scholar] [CrossRef]
- Maximino, C.; da Silva, A.W.B.; Gouveia, A.; Herculano, A.M. Pharmacological Analysis of Zebrafish (Danio rerio) Scototaxis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Marcon, M.; Herrmann, A.P.; Mocelin, R.; Rambo, C.L.; Koakoski, G.; Abreu, M.S.; Conterato, G.M.M.; Kist, L.W.; Bogo, M.R.; Zanatta, L.; et al. Prevention of Unpredictable Chronic Stress-Related Phenomena in Zebrafish Exposed to Bromazepam, Fluoxetine and Nortriptyline. Psychopharmacology 2016, 233, 3815–3824. [Google Scholar] [CrossRef]
- Theodoridi, A.; Tsalafouta, A.; Pavlidis, M. Acute Exposure to Fluoxetine Alters Aggressive Behavior of Zebrafish and Expression of Genes Involved in Serotonergic System Regulation. Front. Neurosci. 2017, 11, 223. [Google Scholar] [CrossRef] [PubMed]
- Chiffre, A.; Clérandeau, C.; Dwoinikoff, C.; Le Bihanic, F.; Budzinski, H.; Geret, F.; Cachot, J. Psychotropic Drugs in Mixture Alter Swimming Behaviour of Japanese Medaka (Oryzias latipes) Larvae above Environmental Concentrations. Environ. Sci. Pollut. Res. 2016, 23, 4964–4977. [Google Scholar] [CrossRef]
- Saaristo, M.; McLennan, A.; Johnstone, C.P.; Clarke, B.O.; Wong, B.B.M. Impacts of the Antidepressant Fluoxetine on the Anti-Predator Behaviours of Wild Guppies (Poecilia reticulata). Aquat. Toxicol. 2017, 183, 38–45. [Google Scholar] [CrossRef]
- Xie, Z.; Lu, G.; Li, S.; Nie, Y.; Ma, B.; Liu, J. Behavioral and Biochemical Responses in Freshwater Fish Carassius Auratus Exposed to Sertraline. Chemosphere 2015, 135, 146–155. [Google Scholar] [CrossRef]
- Porseryd, T.; Kellner, M.; Reyhanian Caspillo, N.; Volkova, K.; Elabbas, L.; Ullah, S.; Olsén, H.; Dinnétz, P.; Porsch Hällström, I. Combinatory Effects of Low Concentrations of 17A-Etinylestradiol and Citalopram on Non-Reproductive Behavior in Adult Zebrafish (Danio rerio). Aquat. Toxicol. 2017, 193, 9–17. [Google Scholar] [CrossRef]
- Lepage, O.; Larson, E.T.; Mayer, I.; Winberg, S. Serotonin, but Not Melatonin, Plays a Role in Shaping Dominant-Subordinate Relationships and Aggression in Rainbow Trout. Horm. Behav. 2005, 48, 233–242. [Google Scholar] [CrossRef]
- Perreault, H.A.N.; Semsar, K.; Godwin, J. Fluoxetine Treatment Decreases Territorial Aggression in a Coral Reef Fish. Physiol. Behav. 2003, 79, 719–724. [Google Scholar] [CrossRef]
- McCallum, E.S.; Bose, A.P.H.; Warriner, T.R.; Balshine, S. An Evaluation of Behavioural Endpoints: The Pharmaceutical Pollutant Fluoxetine Decreases Aggression across Multiple Contexts in Round Goby (Neogobius melanostomus). Chemosphere 2017, 175, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.J. Effects of Fluoxetine on the Swimming and Behavioural Responses of the Arabian Killifish. Ecotoxicology 2013, 22, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Norton, W.H.J.; Stumpenhorst, K.; Faus-Kessler, T.; Folchert, A.; Rohner, N.; Harris, M.P.; Callebert, J.; Bally-Cuif, L. Modulation of Fgfr1a Signaling in Zebrafish Reveals a Genetic Basis for the Aggression-Boldness Syndrome. J. Neurosci. 2011, 31, 13796–13807. [Google Scholar] [CrossRef] [PubMed]
- Kellner, M.; Olsén, K.H. Divergent Response to the SSRI Citalopram in Male and Female Three-Spine Sticklebacks (Gasterosteus aculeatus). Arch. Environ. Contam. Toxicol. 2020, 79, 478–487. [Google Scholar] [CrossRef]
- McDonald, M.D.; Gonzalez, A.; Sloman, K.A. Higher Levels of Aggression Are Observed in Socially Dominant Toadfish Treated with the Selective Serotonin Reuptake Inhibitor, Fluoxetine. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 107–112. [Google Scholar] [CrossRef]
- Greaney, N.E.; Mannion, K.L.; Dzieweczynski, T.L. Signaling on Prozac: Altered Audience Effects on Male-Male Interactions after Fluoxetine Exposure in Siamese Fighting Fish. Behav. Ecol. Sociobiol. 2015, 69, 1925–1932. [Google Scholar] [CrossRef]
- Jirtle, R.L.; Skinner, M.K. Environmental Epigenomics and Disease Susceptibility. Nat. Rev. Genet. 2007, 8, 253–262. [Google Scholar] [CrossRef]
- Vera-Chang, M.N.; St-Jacques, A.D.; Gagné, R.; Martyniuk, C.J.; Yauk, C.L.; Moon, T.W.; Trudeau, V.L. Transgenerational Hypocortisolism and Behavioral Disruption Are Induced by the Antidepressant Fluoxetine in Male Zebrafish Danio rerio. Proc. Natl. Acad. Sci. USA. 2018, 115, E12435–E12442. [Google Scholar] [CrossRef]
- Vera-Chang, M.N.; Moon, T.W.; Trudeau, V.L. Cortisol Disruption and Transgenerational Alteration in the Expression of Stress-Related Genes in Zebrafish Larvae Following Fluoxetine Exposure. Toxicol. Appl. Pharmacol. 2019, 382, 114742. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Abdel-Shafy, H.I.; Mansour, M.S.M. Removal of Pharmaceutical Compounds from Urine via Chemical Coagulation by Green Synthesized ZnO-Nanoparticles Followed by Microfiltration for Safe Reuse. Arab. J. Chem. 2019, 12, 4074–4083. [Google Scholar] [CrossRef]
- Petrie, B.; McAdam, E.J.; Hassard, F.; Stephenson, T.; Lester, J.N.; Cartmell, E. Diagnostic Investigation of Steroid Estrogen Removal by Activated Sludge at Varying Solids Retention Time. Chemosphere 2014, 113, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Georgin, J.; Meili, L.; Franco, D. A Review of the Application of Low-Cost Adsorbents as an Alternative Method for Biosorption of Contaminants Present in Water. Lat. Am. Dev. Energy. Eng. 2023, 4, 1–20. [Google Scholar] [CrossRef]
- Ibáñez, M.; Gracia-Lor, E.; Bijlsma, L.; Morales, E.; Pastor, L.; Hernández, F. Removal of Emerging Contaminants in Sewage Water Subjected to Advanced Oxidation with Ozone. J. Hazard. Mater. 2013, 260, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Rosal, R.; Rodríguez, A.; Perdigón-Melón, J.A.; Petre, A.; García-Calvo, E.; Gómez, M.J.; Agüera, A.; Fernández-Alba, A.R. Occurrence of Emerging Pollutants in Urban Wastewater and Their Removal through Biological Treatment Followed by Ozonation. Water Res. 2010, 44, 578–588. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as Emerging Contaminants and Their Removal from Water. A Review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef]
- Jung, Y.J.; Kim, W.G.; Yoon, Y.; Kang, J.W.; Hong, Y.M.; Kim, H.W. Removal of Amoxicillin by UV and UV/H2O2 Processes. Sci. Total Environ. 2012, 420, 160–167. [Google Scholar] [CrossRef]
- Keen, O.S.; Baik, S.; Linden, K.G.; Aga, D.S.; Love, N.G. Enhanced Biodegradation of Carbamazepine after UV/H2O 2 Advanced Oxidation. Environ. Sci. Technol. 2012, 46, 6222–6227. [Google Scholar] [CrossRef]
- Yonar, T.; Kestioglu, K.; Azbar, N. Treatability Studies on Domestic Wastewater Using UV/H2O2 Process. Appl. Catal. B Environ. 2006, 67, 223–228. [Google Scholar] [CrossRef]
- Matamoros, V.; Gutiérrez, R.; Ferrer, I.; García, J.; Bayona, J.M. Capability of Microalgae-Based Wastewater Treatment Systems to Remove Emerging Organic Contaminants: A Pilot-Scale Study. J. Hazard. Mater. 2015, 288, 34–42. [Google Scholar] [CrossRef]
- Taseli, B.K.; Gokcay, C.F. Biological Treatment of Paper Pulping Effluents by Using a Fungal Reactor. Water Sci. Technol. 1999, 40, 93–99. [Google Scholar] [CrossRef]
- Secondes, M.F.N.; Naddeo, V.; Belgiorno, V.; Ballesteros, F. Removal of Emerging Contaminants by Simultaneous Application of Membrane Ultrafiltration, Activated Carbon Adsorption, and Ultrasound Irradiation. J. Hazard. Mater. 2014, 264, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mozaz, S.; Ricart, M.; Köck-Schulmeyer, M.; Guasch, H.; Bonnineau, C.; Proia, L.; de Alda, M.L.; Sabater, S.; Barceló, D. Pharmaceuticals and Pesticides in Reclaimed Water: Efficiency Assessment of a Microfiltration-Reverse Osmosis (MF-RO) Pilot Plant. J. Hazard. Mater. 2015, 282, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.A.; Yonar, T.; Zakaria, K. The Electrochemical Generation of Ozone: A Review. Ozone Sci. Eng. 2013, 35, 149–167. [Google Scholar] [CrossRef]
- Hernández Martínez, S.A.; Melchor-Martínez, E.M.; González-González, R.B.; Sosa-Hernández, J.E.; Araújo, R.G.; Rodríguez-Hernández, J.A.; Barceló, D.; Parra-Saldívar, R.; Iqbal, H.M.N. Environmental Concerns and Bioaccumulation of Psychiatric Drugs in Water Bodies—Conventional versus Biocatalytic Systems of Mitigation. Environ. Res. 2023, 229, 115892. [Google Scholar] [CrossRef]
- Leyva-Díaz, J.C.; Batlles-Delafuente, A.; Molina-Moreno, V.; Molina, J.S.; Belmonte-Ureña, L.J. Removal of Pharmaceuticals from Wastewater: Analysis of the Past and Present Global Research Activities. Water 2021, 13, 2353. [Google Scholar] [CrossRef]
- Celiz, M.D.; Tso, J.; Aga, D.S.; Risks, E. Pharmaceutical Metabolites in the Environment: Analytical Challenges and Ecological Risks. Environ. Toxicol. Chem. 2009, 28, 2473–2484. [Google Scholar] [CrossRef]
- Escher, B.I.; Fenner, K. Recent Advances in Environmental Risk Assessment of Transformation Products. Environ. Sci. Technol. 2011, 45, 3835–3847. [Google Scholar] [CrossRef]
- Tong, D.S.; Zhou, C.H.; Lu, Y.; Yu, H.; Zhang, G.F.; Yu, W.H. Adsorption of Acid Red G Dye on Octadecyl Trimethylammonium Montmorillonite. Appl. Clay Sci. 2010, 50, 427–431. [Google Scholar] [CrossRef]
- Katsigiannis, A.; Noutsopoulos, C.; Mantziaras, J.; Gioldasi, M. Removal of Emerging Pollutants through Granular Activated Carbon. Chem. Eng. J. 2015, 280, 49–57. [Google Scholar] [CrossRef]
- Gazigil, L.; Er, E.; Yonar, T. Determination of the Optimum Conditions for Electrochemical Regeneration of Exhausted Activated Carbon. Diam. Relat. Mater. 2023, 133, 109741. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Utilization of Agro-Industrial and Municipal Waste Materials as Potential Adsorbents for Water Treatment-A Review. Chem. Eng. J. 2010, 157, 277–296. [Google Scholar] [CrossRef]
- Dagnino, S.; Gomez, E.; Picot, B.; Cavaillès, V.; Casellas, C.; Balaguer, P.; Fenet, H. Estrogenic and AhR Activities in Dissolved Phase and Suspended Solids from Wastewater Treatment Plants. Sci. Total Environ. 2010, 408, 2608–2615. [Google Scholar] [CrossRef] [PubMed]
- Alfonso-Muniozguren, P.; Serna-Galvis, E.A.; Bussemaker, M.; Torres-Palma, R.A.; Lee, J. A Review on Pharmaceuticals Removal from Waters by Single and Combined Biological, Membrane Filtration and Ultrasound Systems. Ultrason. Sonochem. 2021, 76, 105656. [Google Scholar] [CrossRef]
- Maršálek, R.; Švidrnoch, M. The Adsorption of Amitriptyline and Nortriptyline on Activated Carbon, Diosmectite and Titanium Dioxide. Environ. Chall. 2020, 1, 100005. [Google Scholar] [CrossRef]
- Choi, J.W.; Bediako, J.K.; Zhao, Y.; Lin, S.; Sarkar, A.K.; Han, M.; Song, M.H.; Cho, C.W.; Yun, Y.S. Adsorptive Removal of Cationic Tricyclic Antidepressants Using Cation-Exchange Resin. Environ. Sci. Pollut. Res. 2020, 27, 24760–24771. [Google Scholar] [CrossRef]
- Gornik, T.; Kovacic, A.; Heath, E.; Hollender, J.; Kosjek, T. Biotransformation Study of Antidepressant Sertraline and Its Removal during Biological Wastewater Treatment. Water Res. 2020, 181, 115864. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.H.; Jiang, W.T.; Li, Z.; Kuo, C.Y.; Jean, J.S.; Chen, W.R.; Lv, G. Mechanism of Amitriptyline Adsorption on Ca-Montmorillonite (SAz-2). J. Hazard. Mater. 2014, 277, 44–52. [Google Scholar] [CrossRef]
- Toyoguchi, T.; Ebihara, M.; Ojima, F.; Hosoya, J.; Nakagawa, Y. In Vitro Study of the Adsorption Characteristics of Drugs. Biol. Pharm. Bull. 2005, 28, 841–844. [Google Scholar] [CrossRef][Green Version]
- Tsai, Y.L.; Chang, P.H.; Gao, Z.Y.; Xu, X.Y.; Chen, Y.H.; Wang, Z.H.; Chen, X.Y.; Yang, Z.Y.; Wang, T.H.; Jean, J.S.; et al. Amitriptyline Removal Using Palygorskite Clay. Chemosphere 2016, 155, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Landry, K.A.; Boyer, T.H. Diclofenac Removal in Urine Using Strong-Base Anion Exchange Polymer Resins. Water Res. 2013, 47, 6432–6444. [Google Scholar] [CrossRef]
- El Gamal, M.; Mousa, H.A.; El-Naas, M.H.; Zacharia, R.; Judd, S. Bio-Regeneration of Activated Carbon: A Comprehensive Review. Sep. Purif. Technol. 2018, 197, 345–359. [Google Scholar] [CrossRef]
- Larasati, A.; Fowler, G.D.; Graham, N.J.D. Insights into Chemical Regeneration of Activated Carbon for Water Treatment. J. Environ. Chem. Eng. 2021, 9, 105555. [Google Scholar] [CrossRef]
- Koçoğlu, E.S.; Bakırdere, S.; Keyf, S. A Novel Liquid-Liquid Extraction for the Determination of Sertraline in Tap Water and Waste Water at Trace Levels by GC-MS. Bull. Environ. Contam. Toxicol. 2017, 99, 354–359. [Google Scholar] [CrossRef]
- Ikeda, M. Public Health Problems of Organic Solvents. Toxicol. Lett. 1992, 64–65, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Racovita, R.C.; Ciuca, M.D.; Catana, D.; Comanescu, C.; Ciocirlan, O. Microemulsions of Nonionic Surfactant with Water and Various Homologous Esters: Preparation, Phase Transitions, Physical Property Measurements, and Application for Extraction of Tricyclic Antidepressant Drugs from Aqueous Media. Nanomaterials 2023, 13, 2311. [Google Scholar] [CrossRef]
- Ge, D.; Lee, H.K. Ionic Liquid Based Dispersive Liquid-Liquid Microextraction Coupled with Micro-Solid Phase Extraction of Antidepressant Drugs from Environmental Water Samples. J. Chromatogr. A 2013, 1317, 217–222. [Google Scholar] [CrossRef]
- Zawadzki, M.; e Silva, F.A.; Domańska, U.; Coutinho, J.A.P.; Ventura, S.P.M. Recovery of an Antidepressant from Pharmaceutical Wastes Using Ionic Liquid-Based Aqueous Biphasic Systems. Green Chem. 2016, 18, 3527–3536. [Google Scholar] [CrossRef]
- Nassar, R.; Trivella, A.; Mokh, S.; Al-Iskandarani, M.; Budzinski, H.; Mazellier, P. Photodegradation of Sulfamethazine, Sulfamethoxypiridazine, Amitriptyline, and Clomipramine Drugs in Aqueous Media. J. Photochem. Photobiol. A Chem. 2017, 336, 176–182. [Google Scholar] [CrossRef]
- Khaleel, N.D.H.; Mahmoud, W.M.M.; Olsson, O.; Kümmerer, K. Initial Fate Assessment of Teratogenic Drug Trimipramine and Its Photo-Transformation Products—Role of PH, Concentration and Temperature. Water Res. 2017, 108, 197–211. [Google Scholar] [CrossRef]
- Gros, M.; Williams, M.; Llorca, M.; Rodriguez-Mozaz, S.; Barceló, D.; Kookana, R.S. Photolysis of the Antidepressants Amisulpride and Desipramine in Wastewaters: Identification of Transformation Products Formed and Their Fate. Sci. Total Environ. 2015, 530–531, 434–444. [Google Scholar] [CrossRef]
- Maślanka, A.; Żmudzki, P.; Szlósarczyk, M.; Talik, P.; Hubicka, U. Photodegradation Assessment of Amisulpride, Doxepin, Haloperidol, Risperidone, Venlafaxine, and Zopiclone in Bulk Drug and in the Presence of Excipients. Monatshefte Fur Chem. 2020, 151, 483–493. [Google Scholar] [CrossRef]
- Calza, P.; Sakkas, V.A.; Villioti, A.; Massolino, C.; Boti, V.; Pelizzetti, E.; Albanis, T. Multivariate Experimental Design for the Photocatalytic Degradation of Imipramine. Determination of the Reaction Pathway and Identification of Intermediate Products. Appl. Catal. B Environ. 2008, 84, 379–388. [Google Scholar] [CrossRef]
- Méndez-Arriaga, F.; Otsu, T.; Oyama, T.; Gimenez, J.; Esplugas, S.; Hidaka, H.; Serpone, N. Photooxidation of the Antidepressant Drug Fluoxetine (Prozac®) in Aqueous Media by Hybrid Catalytic/Ozonation Processes. Water Res. 2011, 45, 2782–2794. [Google Scholar] [CrossRef] [PubMed]
- Bojanowska-Czajka, A.; Pyszynska, M.; Majkowska-Pilip, A.; Wawrowicz, K. Degradation of Selected Antidepressants Sertraline and Citalopram in Ultrapure Water and Surface Water Using Gamma Radiation. Processes 2022, 10, 63. [Google Scholar] [CrossRef]
- Melin, V.; Salgado, P.; Thiam, A.; Henríquez, A.; Mansilla, H.D.; Yáñez, J.; Salazar, C. Study of Degradation of Amitriptyline Antidepressant by Different Electrochemical Advanced Oxidation Processes. Chemosphere 2021, 274, 129683. [Google Scholar] [CrossRef]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Comprehensive Study of the Antidiabetic Drug Metformin and Its Transformation Product Guanylurea in Greek Wastewaters. Water Res. 2015, 70, 436–448. [Google Scholar] [CrossRef]
- Rudke, A.R.; Heleno, S.A.; Fernandes, I.P.; Prieto, M.A.; Gonçalves, O.H.; Rodrigues, A.E.; Ferreira, I.C.F.R.; Barreiro, M.F. Microencapsulation of Ergosterol and Agaricus Bisporus L. Extracts by Complex Coacervation Using Whey Protein and Chitosan: Optimization Study Using Response Surface Methodology. LWT 2019, 103, 228–237. [Google Scholar] [CrossRef]
- Van Doorslaer, X.; Dewulf, J.; Van Langenhove, H.; Demeestere, K. Fluoroquinolone Antibiotics: An Emerging Class of Environmental Micropollutants. Sci. Total Environ. 2014, 500–501, 250–269. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Titanium Dioxide Photocatalysis for Pharmaceutical Wastewater Treatment. Environ. Chem. Lett. 2014, 12, 27–47. [Google Scholar] [CrossRef]
- Rekhate, C.V.; Srivastava, J.K. Recent Advances in Ozone-Based Advanced Oxidation Processes for Treatment of Wastewater- A Review. Chem. Eng. J. Adv. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Marotta, R.; Radovnikovic, A. Ozonation and H2O2/UV Treatment of Clofibric Acid in Water: A Kinetic Investigation. J. Hazard. Mater. 2003, 103, 233–246. [Google Scholar] [CrossRef]
- Liu, Q.-T.; Williams, H.E. Kinetics and Degradation Products for Direct Photolysis of β-Blockers in Water. Environ. Sci. Technol. 2007, 41, 803–810. [Google Scholar] [CrossRef]
- Li, Y.N.B.; Moore, D.E.; Tattam, B.N. Photodegradation of Amiloride in Aqueous Solution. Int. J. Pharm. 1999, 183, 109–116. [Google Scholar] [CrossRef]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of Residual Pharmaceuticals from Aqueous Systems by Advanced Oxidation Processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lu, S.; Qiu, Z.; Lin, K. Clofibric Acid Degradation in UV254/H2O2 Process: Effect of Temperature. J. Hazard. Mater. 2010, 176, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Rosario-Ortiz, F.L.; Wert, E.C.; Snyder, S.A. Evaluation of UV/H2O2 Treatment for the Oxidation of Pharmaceuticals in Wastewater. Water Res. 2010, 44, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Ravina, M.; Campanella, L.; Kiwi, J. Accelerated Mineralization of the Drug Diclofenac via Fenton Reactions in a Concentric Photo-Reactor. Water Res. 2002, 36, 3553–3560. [Google Scholar] [CrossRef]
- Pérez-Estrada, L.A.; Maldonado, M.I.; Gernjak, W.; Agüera, A.; Fernández-Alba, A.R.; Ballesteros, M.M.; Malato, S. Decomposition of Diclofenac by Solar Driven Photocatalysis at Pilot Plant Scale. Catal. Today 2005, 101, 219–226. [Google Scholar] [CrossRef]
- Goi, A.; Veressinina, Y.; Trapido, M. Degradation of Salicylic Acid by Fenton and Modified Fenton Treatment. Chem. Eng. J. 2008, 143, 1–9. [Google Scholar] [CrossRef]
- Trovó, A.G.; Melo, S.A.S.; Nogueira, R.F.P. Photodegradation of the Pharmaceuticals Amoxicillin, Bezafibrate and Paracetamol by the Photo-Fenton Process-Application to Sewage Treatment Plant Effluent. J. Photochem. Photobiol. A Chem. 2008, 198, 215–220. [Google Scholar] [CrossRef]
- Melo, S.A.S.; Trovó, A.G.; Bautitz, I.R.; Nogueira, R.F.P. Degradação de Fármacos Residuais Por Processos Oxidativos Avançados. Quim. Nova 2009, 32, 188–197. [Google Scholar] [CrossRef]
- Tong, A.Y.C.; Braund, R.; Warren, D.S.; Peake, B.M. TiO 2-Assisted Photodegradation of Pharmaceuticals—A Review. Cent. Eur. J. Chem. 2012, 10, 989–1027. [Google Scholar] [CrossRef]
- Magureanu, M.; Mandache, N.B.; Parvulescu, V.I. Degradation of Pharmaceutical Compounds in Water by Non-Thermal Plasma Treatment. Water Res. 2015, 81, 124–136. [Google Scholar] [CrossRef]
- Bruggeman, P.; Schram, D.; González, M.Á.; Rego, R.; Kong, M.G.; Leys, C. Characterization of a Direct Dc-Excited Discharge in Water by Optical Emission Spectroscopy. Plasma Sources Sci. Technol. 2009, 18, 25017. [Google Scholar] [CrossRef]
- Kanazawa, S.; Kawano, H.; Watanabe, S.; Furuki, T.; Akamine, S.; Ichiki, R.; Ohkubo, T.; Kocik, M.; Mizeraczyk, J. Observation of OH Radicals Produced by Pulsed Discharges on the Surface of a Liquid. Plasma Sources Sci. Technol. 2011, 20, 34010. [Google Scholar] [CrossRef]
- Bruggeman, P.; Schram, D.C. On OH Production in Water Containing Atmospheric Pressure Plasmas. Plasma Sources Sci. Technol. 2010, 19, 045025. [Google Scholar] [CrossRef]
- Papagiannis, I.; Koutsikou, G.; Frontistis, Z.; Konstantinou, I.; Avgouropoulos, G.; Mantzavinos, D.; Lianos, P. Photoelectrocatalytic vs. Photocatalytic Degradation of Organic Water Born Pollutants. Catalysts 2018, 8, 455. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent Progress in Metal-Doped TiO2, Non-Metal Doped/Codoped TiO2 and TiO2 Nanostructured Hybrids for Enhanced Photocatalysis. Int. J. Hydrogen Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green Synthesis of Nanoparticles: Current Developments and Limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Rapti, I.; Kourkouta, T.; Malisova, E.M.; Albanis, T.; Konstantinou, I. Photocatalytic Degradation of Inherent Pharmaceutical Concentration Levels in Real Hospital WWTP Effluents Using G-C3N4 Catalyst on CPC Pilot Scale Reactor. Molecules 2023, 28, 1170. [Google Scholar] [CrossRef] [PubMed]
- Bairamis, F.; Konstantinou, I. Wo3 Fibers/g-C3n4 z-Scheme Heterostructure Photocatalysts for Simultaneous Oxidation/Reduction of Phenol/Cr (VI) in Aquatic Media. Catalysts 2021, 11, 792. [Google Scholar] [CrossRef]
- Zhong, J.; Jiang, H.; Wang, Z.; Yu, Z.; Wang, L.; Mueller, J.F.; Guo, J. Efficient Photocatalytic Destruction of Recalcitrant Micropollutants Using Graphitic Carbon Nitride under Simulated Sunlight Irradiation. Environ. Sci. Ecotechnology 2021, 5, 100079. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.; Chafiq, L.; Dalhatou, S.; Bonnet, P.; Nasr, M.; Gaillard, N.; Dikdim, J.M.D.; Monier, G.; Assadie, A.A.; Zeghioud, H. G-C3N4/TiO2 S-Scheme Heterojunction Photocatalyst with Enhanced Photocatalytic Carbamazepine Degradation and Mineralization. J. Photochem. Photobiol. A Chem. 2022, 430, 113971. [Google Scholar] [CrossRef]
- Gao, B.; Wang, J.; Dou, M.; Xu, C.; Huang, X. Enhanced Photocatalytic Removal of Amoxicillin with Ag/TiO2/Mesoporous g-C3N4 under Visible Light: Property and Mechanistic Studies. Environ. Sci. Pollut. Res. 2020, 27, 7025–7039. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Tiwari, B.; Sellamuthu, B.; Ouarda, Y.; Drogui, P.; Tyagi, R.D.; Buelna, G. Review on Fate and Mechanism of Removal of Pharmaceutical Pollutants from Wastewater Using Biological Approach. Bioresour. Technol. 2017, 224, 1–12. [Google Scholar] [CrossRef]
- Kózka, B.; Nałęcz-Jawecki, G.; Turło, J.; Giebułtowicz, J. Application of Pleurotus Ostreatus to Efficient Removal of Selected Antidepressants and Immunosuppressant. J. Environ. Manage. 2020, 273, 111131. [Google Scholar] [CrossRef] [PubMed]
- Aydın, S.; Bedük, F.; Ulvi, A.; Aydın, M.E. Simple and Effective Removal of Psychiatric Pharmaceuticals from Wastewater Treatment Plant Effluents by Magnetite Red Mud Nanoparticles. Sci. Total Environ. 2021, 784, 147174. [Google Scholar] [CrossRef]
- Bozyiğit, G.D.; Zaman, B.T.; Özdemir, O.K.; Kılınç, Y.; Chormey, D.S.; Bakırdere, S.; Engin, G.O. Removal of Two Antidepressant Active Pharmaceutical Ingredients from Hospital Wastewater by Polystyrene-Coated Magnetite Nanoparticles-Assisted Batch Adsorption Process. Environ. Monit. Assess. 2023, 196, 77. [Google Scholar] [CrossRef]
- Calisto, V.; Ferreira, C.I.A.; Oliveira, J.A.B.P.; Otero, M.; Esteves, V.I. Adsorptive Removal of Pharmaceuticals from Water by Commercial and Waste-Based Carbons. J. Environ. Manag. 2015, 152, 83–90. [Google Scholar] [CrossRef]
- Skalska-Tuomi, K.; Kaijanen, L.; Monteagudo, J.M.; Mänttäri, M. Efficient Removal of Pharmaceuticals from Wastewater: Comparative Study of Three Advanced Oxidation Processes. J. Environ. Manag. 2025, 375, 124276. [Google Scholar] [CrossRef] [PubMed]
- Lajeunesse, A.; Blais, M.; Barbeau, B.; Sauvé, S.; Gagnon, C. Ozone Oxidation of Antidepressants in Wastewater: Treatment Evaluation and Characterization of New By-Products by LC-QToFMS. Chem. Cent. J. 2013, 7, 15. [Google Scholar] [CrossRef]
- Hama Aziz, K.H.; Mustafa, F.S.; Karim, M.A.H.; Hama, S. Pharmaceutical Pollution in the Aquatic Environment: Advanced Oxidation Processes as Efficient Treatment Approaches: A Review. Mater. Adv. 2025, 6, 3433–3454. [Google Scholar] [CrossRef]
- Azuma, T.; Otomo, K.; Kunitou, M.; Shimizu, M.; Hosomaru, K.; Mikata, S.; Mino, Y.; Hayashi, T. Performance and Efficiency of Removal of Pharmaceutical Compounds from Hospital Wastewater by Lab-Scale Biological Treatment System. Environ. Sci. Pollut. Res. Int. 2018, 25, 14647–14655. [Google Scholar] [CrossRef]
- Alobaidi, R.A.K.; Ulucan-Altuntas, K.; Mhemid, R.K.S.; Manav-Demir, N.; Cinar, O. Biodegradation of Emerging Pharmaceuticals from Domestic Wastewater by Membrane Bioreactor: The Effect of Solid Retention Time. Int. J. Environ. Res. Public Health 2021, 18, 3395. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Polverino, G.; Martin, J.M.; Bertram, M.G.; Wiles, S.C.; Palacios, M.M.; Bywater, C.L.; White, C.R.; Wong, B.B.M. Chronic Exposure to a Pervasive Pharmaceutical Pollutant Erodes Among-Individual Phenotypic Variation in a Fish. Environ. Pollut. 2020, 263, 114450. [Google Scholar] [CrossRef]
- Wiles, S.C.; Bertram, M.G.; Martin, J.M.; Tan, H.; Lehtonen, T.K.; Wong, B.B.M. Long-Term Pharmaceutical Contamination and Temperature Stress Disrupt Fish Behavior. Environ. Sci. Technol. 2020, 54, 8072–8082. [Google Scholar] [CrossRef] [PubMed]
- Moermond, C.T.A.; Kase, R.; Korkaric, M.; Ågerstrand, M. CRED: Criteria for Reporting and Evaluating Ecotoxicity Data. Environ. Toxicol. Chem. 2016, 35, 1297–1309. [Google Scholar] [CrossRef]
- Parrott, J.L.; Metcalfe, C.D. Assessing the Effects of the Antidepressant Venlafaxine to Fathead minnows Exposed to Environmentally Relevant Concentrations over a Full Life Cycle. Environ. Pollut. 2017, 229, 403–411. [Google Scholar] [CrossRef]
- Vladimirov, N.; Mu, Y.; Kawashima, T.; Bennett, D.V.; Yang, C.T.; Looger, L.L.; Keller, P.J.; Freeman, J.; Ahrens, M.B. Light-Sheet Functional Imaging in Fictively Behaving Zebrafish. Nat. Methods 2014, 11, 883–884. [Google Scholar] [CrossRef]
- Dunn, T.W.; Gebhardt, C.; Naumann, E.A.; Riegler, C.; Ahrens, M.B.; Engert, F.; Del Bene, F. Neural Circuits Underlying Visually Evoked Escapes in Larval Zebrafish. Neuron 2016, 89, 613–628. [Google Scholar] [CrossRef]
- Yoon, Y.-G.; Wang, Z.; Pak, N.; Park, D.; Dai, P.; Kang, J.S.; Suk, H.-J.; Symvoulidis, P.; Guner-Ataman, B.; Wang, K.; et al. Sparse Decomposition Light-Field Microscopy for High Speed Imaging of Neuronal Activity. Optica 2020, 7, 1457. [Google Scholar] [CrossRef]
- Arnold, K.E.; Brown, A.R.; Brown, A.R.; Ankley, G.T.; Sumpter, J.P. Medicating the Environment: Assessing Risks of Pharmaceuticals to Wildlife and Ecosystems. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130569. [Google Scholar] [CrossRef]
- Valenti, T.W.; Perez-Hurtado, P.; Chambliss, C.K.; Brooks, B.W. Aquatic Toxicity of Sertraline to Pimephales Promelas at Environmentally Relevant Surface Water PH. Environ. Toxicol. Chem. 2009, 28, 2685–2694. [Google Scholar] [CrossRef] [PubMed]
- Bisesi, J.H.; Bridges, W.; Klaine, S.J. Reprint of: Effects of the Antidepressant Venlafaxine on Fish Brain Serotonin and Predation Behavior. Aquat. Toxicol. 2014, 151, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Ortúzar, M.; Esterhuizen, M.; Olicón-Hernández, D.R.; González-López, J.; Aranda, E. Pharmaceutical Pollution in Aquatic Environments: A Concise Review of Environmental Impacts and Bioremediation Systems. Front. Microbiol. 2022, 13, 869332. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, F.; Chouchene, K.; Roche, N.; Ksibi, M. Removal of Pharmaceuticals from Water by Adsorption and Advanced Oxidation Processes: State of the Art and Trends. Appl. Sci. 2021, 11, 6659. [Google Scholar] [CrossRef]
- Caban, M.; Stepnowski, P. How to Decrease Pharmaceuticals in the Environment? A Review. Environ. Chem. Lett. 2021, 19, 3115–3138. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).