Optimizing Nitrogen Management in Acidic Tea Orchard Soils: The Role of Biochar-Based Fertilizers in Reducing Losses and Enhancing Sequestration
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Collection and Preparation of Biochar-Based Fertilizer
2.2. Experimental Design
2.3. Analytical Methods
2.4. Statistical Analysis
3. Results
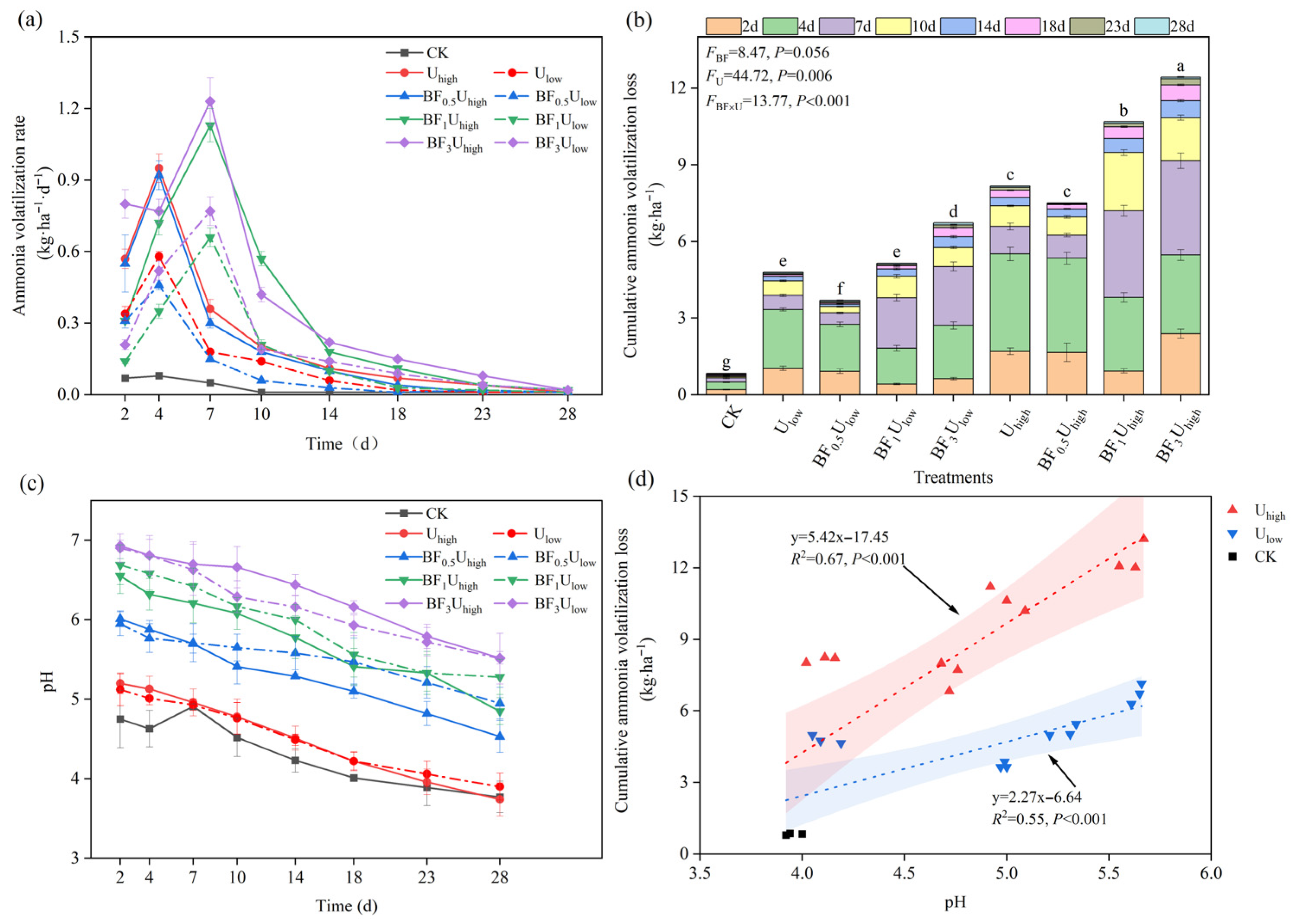
3.1. Dynamic Changes and Cumulative Amount of Ammonia Volatilization in Tea Orchard Soils
3.1.1. Dynamic Changes and Cumulative Losses of Ammonia Volatilization
3.1.2. Soil pH Dynamics and Its Relationship with Cumulative Ammonia Volatilization
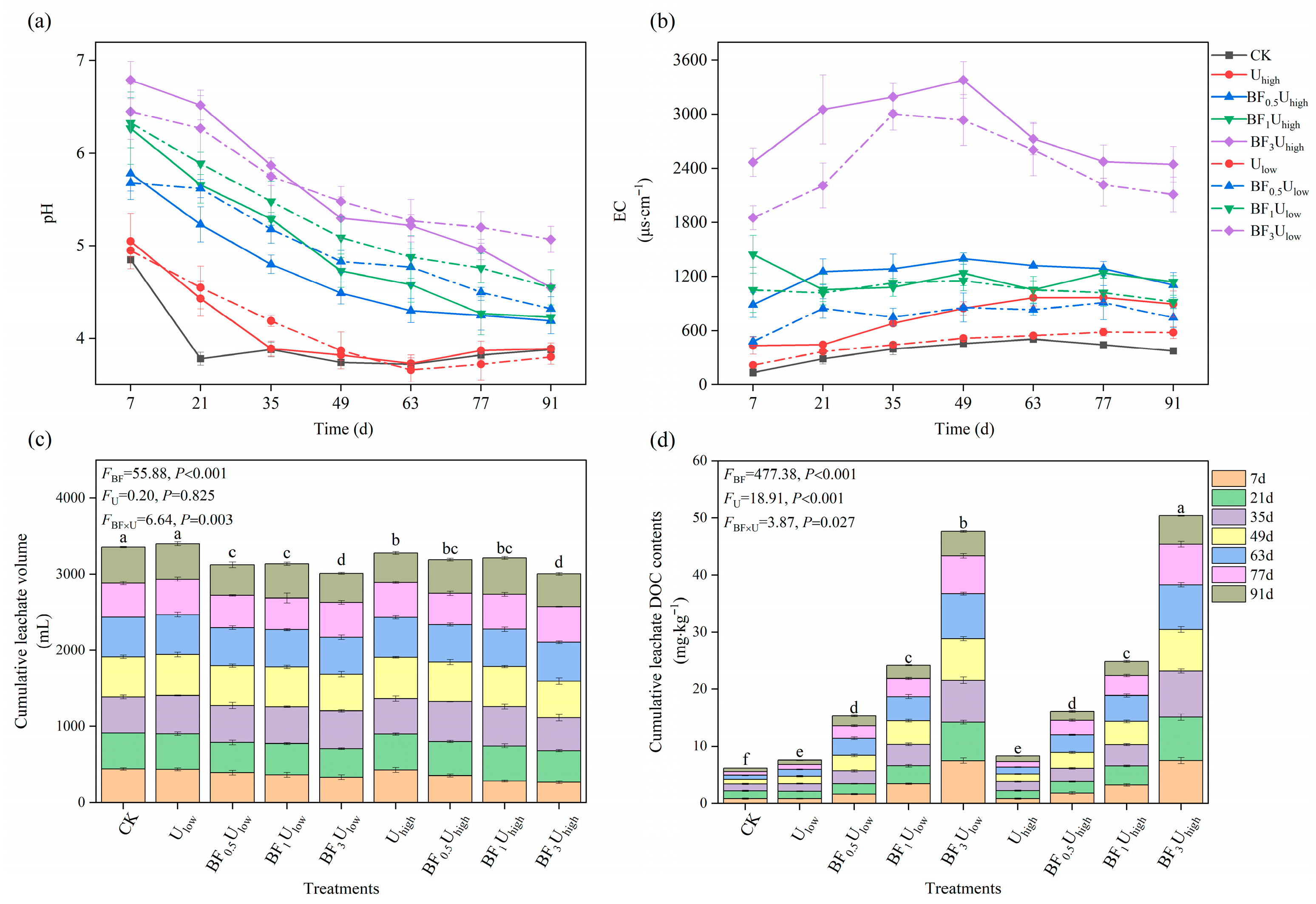
3.2. Dynamic Characteristics and Accumulation of Nitrogen Leaching in Tea Orchard Soils
3.2.1. pH and Electrical Conductivity of Leachate
3.2.2. Leachate Volume and DOC Content
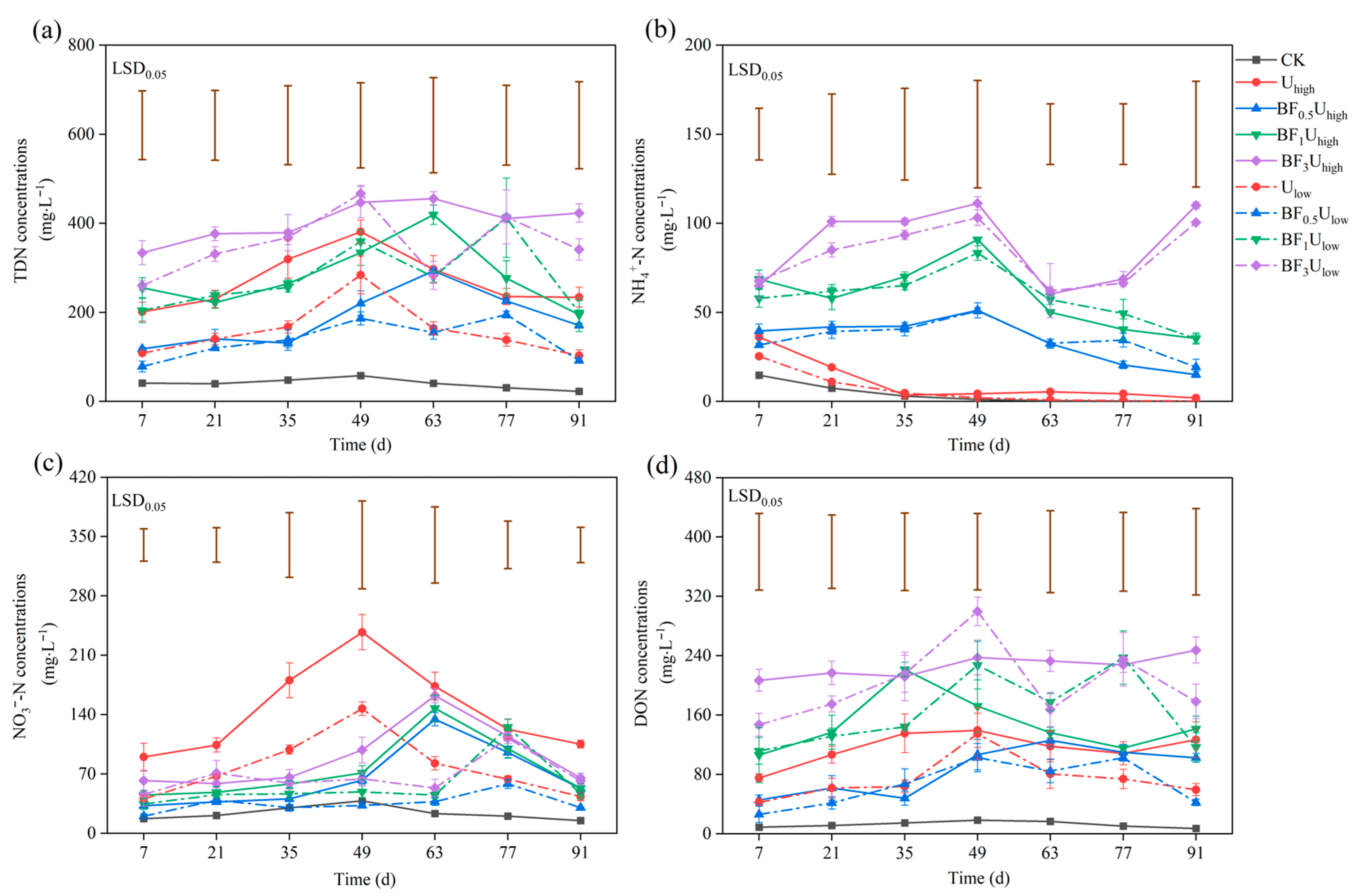
3.2.3. Nitrogen Leaching Characteristics
3.2.4. Cumulative Nitrogen Leaching Losses
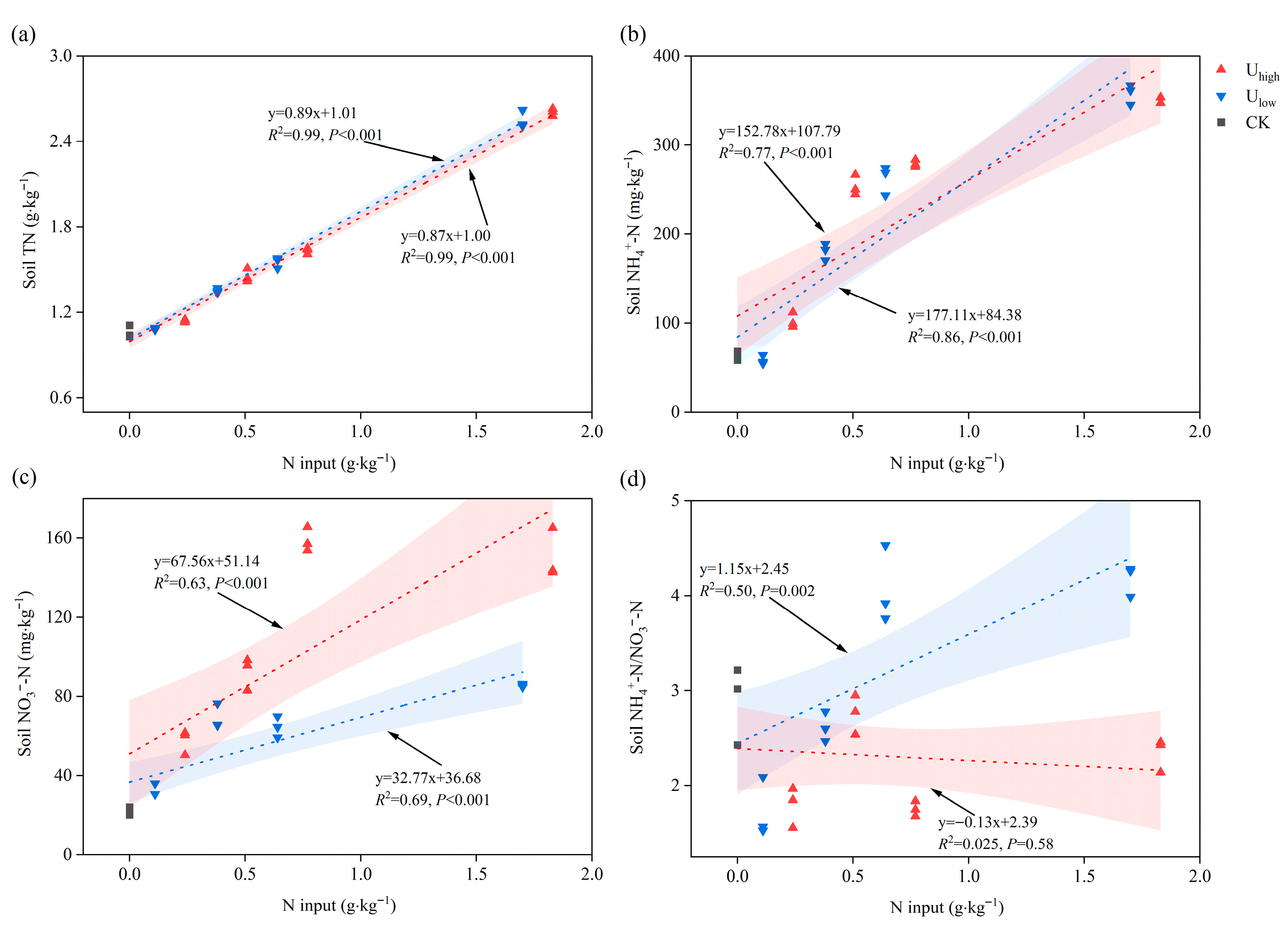
3.3. Soil Nitrogen Forms and Chemical Properties in the Tea Orchard
3.3.1. Effects of Fertilization on Soil Nitrogen Forms
3.3.2. Impact of Fertilization on Soil Chemical Properties
3.4. Analysis of Factors Influencing Nitrogen Migration and Transformation in Tea Orchard Soils
3.4.1. Effects of Fertilization on Soil Nitrogen Accumulation and Loss
3.4.2. Correlation and Random Forest Analysis of Nitrogen Drivers
3.4.3. Path Modeling of Nitrogen Transformation Mechanisms
3.4.4. Schematic Diagram of Nitrogen Migration and Transformation in Acidified Tea Orchard Soils
4. Discussion
4.1. Effect of Biochar-Based Fertilizer on Ammonia Volatilization in Tea Orchard Soils
4.2. Effects of Biochar-Based Fertilizer on Nitrogen Leaching in Tea Orchard Soils
4.3. Effects of Biochar-Based Fertilizer on Soil Nitrogen and Physicochemical Properties in Tea Orchard Soils
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| N | Nitrogen |
| BF | Biochar-based fertilizer |
| U | Urea |
| NH3 | Ammonia |
| TDN | Total dissolved nitrogen |
| TN | Total nitrogen |
| SON | Soil organic nitrogen |
| NH4+-N | Ammonium nitrogen |
| NO3−-N | Nitrate nitrogen |
| UE | Urease activity |
| SOC | Soil organic carbon |
| EC | Electrical conductivity |
| ENH3,tot | Cumulative NH3 volatilization loss |
| TV | Total leachate volume |
| DOC | Dissolved organic carbon |
| DON | Dissolved organic nitrogen |
| PLS-PM | Partial least squares path modeling |
References
- Yan, P.; Shen, C.; Fan, L.; Li, X.; Zhang, L.; Zhang, L.; Han, W. Tea planting affects soil acidification and nitrogen and phosphorus distribution in soil. Agric. Ecosyst. Environ. 2018, 254, 20–25. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Fu, X.; Liu, X.; Shen, J.; Wang, Y.; Wu, J. Three-dimensional spatial variability in soil microorganisms of nitrification and denitrification at a row-transect scale in a tea field. Soil Biol. Biochem. 2016, 103, 452–463. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, N.; Ma, Q.; Zhou, J.; Sun, T.; Zhang, X.; Wu, L. Applying Nutrient Expert system for rational fertilisation to tea (Camellia sinensis) reduces environmental risks and increases economic benefits. J. Clean. Prod. 2021, 305, 127197. [Google Scholar] [CrossRef]
- Shao, S.; Li, Y.; Li, Z.; Ma, X.; Zhu, Y.; Luo, Y.; Cai, P.; Jia, X.; Rensing, C.; Li, Q. Impact of tea tree cultivation on soil microbiota, soil organic matter, and nitrogen cycling in mountainous plantations. Agronomy 2024, 14, 638. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; Li, Y.; Zhang, Y.; Huang, X.; Yang, Y.; Zhu, H.; Xiong, H.; Jiang, T. Influence of nitrogen fertilizer application on soil acidification characteristics of tea plantations in karst areas of Southwest China. Agriculture 2023, 13, 849. [Google Scholar] [CrossRef]
- Ruan, J.; Ma, L.; Shi, Y. Aluminium in tea plantations: Mobility in soils and plants, and the influence of nitrogen fertilization. Environ. Geochem. Health 2006, 28, 519–528. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.; Yang, B.; Qian, C.; Wang, Y.; Chen, T.; Han, X.; Yang, L.; Xue, L. Nitrogen utilization and loss of the tea plantation system on sloped farmland: A short-term response to substitution with organic fertilizer. Agronomy 2024, 14, 392. [Google Scholar] [CrossRef]
- Li, S.; Li, H.; Yang, C.; Wang, Y.; Xue, H.; Niu, Y. Rates of soil acidification in tea plantations and possible causes. Agric. Ecosyst. Environ. 2016, 233, 60–66. [Google Scholar] [CrossRef]
- Yang, X.; Ni, K.; Shi, Y.; Yi, X.; Zhang, Q.; Fang, L.; Ma, L.; Ruan, J. Effects of long-term nitrogen application on soil acidification and solution chemistry of a tea plantation in China. Agric. Ecosyst. Environ. 2018, 252, 74–82. [Google Scholar] [CrossRef]
- Rauber, L.P.; Andrade, A.P.; Friederichs, A.; Mafra, Á.L.; Baretta, D.; Rosa, M.G.D.; Mafra, M.S.H.; Correa, J.C. Soil physical indicators of management systems in traditional agricultural areas under manure application. Sci. Agric. 2018, 75, 354–359. [Google Scholar] [CrossRef]
- Baffaut, C.; Ghidey, F.; Lerch, R.N.; Kitchen, N.R.; Sudduth, K.A.; Sadler, E.J. Long-term simulated runoff and water quality from grain cropping systems on restrictive layer soils. Agric. Water Manag. 2019, 213, 36–48. [Google Scholar] [CrossRef]
- Zhu, T.; Zhang, J.; Meng, T.; Zhang, Y.; Yang, J.; Müller, C.; Cai, Z. Tea plantation destroys soil retention of NO3− and increases N2O emissions in subtropical China. Soil Biol. Biochem. 2014, 73, 106–114. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; Da Silva, E.B.; De Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Castejón-del Pino, R.; Sánchez-Monedero, M.A.; Sánchez-García, M.; Cayuela, M.L. Fertilization strategies to reduce yield-scaled N2O emissions based on the use of biochar and biochar-based fertilizers. Nutr. Cycl. Agroecosyst. 2024, 129, 491–501. [Google Scholar] [CrossRef]
- Melo, L.C.A.; Lehmann, J.; Carneiro, J.S.D.S.; Camps-Arbestain, M. Biochar-based fertilizer effects on crop productivity: A meta-analysis. Plant Soil 2022, 472, 45–58. [Google Scholar] [CrossRef]
- Ding, Z.; Huang, R.; Li, X.; Fan, Q.; Hu, L.; Liu, S. Effects of biochar on soil organic carbon mineralization in citrus orchards. Sustainability 2024, 16, 9967. [Google Scholar] [CrossRef]
- Majeed, M.; Ali Jaaf, S.; Li, Y.; Günal, E.; Ali El Enshasy, H.; Salmen, S.H.; Sürücü, A. The impact of corncob biochar and poultry litter on pepper (Capsicum annuum L.) growth and chemical properties of a silty-clay soil. Saudi J. Biol. Sci. 2022, 29, 2998–3005. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, X.; Singh, B.P.; Mandal, S.; Guo, J.; Che, L.; Wang, H. Effects of contrasting biochars on the leaching of inorganic nitrogen from soil. J. Soils Sediments 2020, 20, 3017–3026. [Google Scholar] [CrossRef]
- Chen, P.; Liu, Y.; Mo, C.; Jiang, Z.; Yang, J.; Lin, J. Microbial mechanism of biochar addition on nitrogen leaching and retention in tea soils from different plantation ages. Sci. Total Environ. 2021, 757, 143817. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, J.; Zhang, Y.; Li, S.; Chen, X.; Sha, Z. A meta-analysis study on the use of biochar to simultaneously mitigate emissions of reactive nitrogen gases (N2O and NO) from soils. Sustainability 2023, 15, 2384. [Google Scholar] [CrossRef]
- He, T.; Yuan, J.; Xiang, J.; Lin, Y.; Luo, J.; Lindsey, S.; Liao, X.; Liu, D.; Ding, W. Combined biochar and double inhibitor application offsets NH3 and N2O emissions and mitigates N leaching in paddy fields. Environ. Pollut. 2022, 292, 118344. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, B.; Zhang, Y.; Hu, T.; Lin, Z.; Liu, G.; Wang, X.; Ma, J.; Wang, H.; Jin, H.; et al. Biochar application as a tool to decrease soil nitrogen losses (NH3 volatilization, N2O emissions, and N leaching) from croplands: Options and mitigation strength in a global perspective. Glob. Change Biol. 2019, 25, 2077–2093. [Google Scholar] [CrossRef]
- Zhou, M.; Ying, S.; Chen, J.; Jiang, P.; Teng, Y. Effects of biochar-based fertilizer on nitrogen use efficiency and nitrogen losses via leaching and ammonia volatilization from an open vegetable field. Environ. Sci. Pollut. Res. 2021, 28, 65188–65199. [Google Scholar] [CrossRef]
- Jin, Q.; Zhang, Y.; Wang, Q.; Li, M.; Sun, H.; Liu, N.; Zhang, L.; Zhang, Y.; Liu, Z. Effects of potassium fulvic acid and potassium humate on microbial biodiversity in bulk soil and rhizosphere soil of panax ginseng. Microbiol. Res. 2022, 254, 126914. [Google Scholar] [CrossRef]
- Kanabar, P.; Wu, Y.; Nandwani, D. Enhancing sustainable cultivation of organic bell pepper through fulvic acid (FA) application: Impact on phytochemicals and antioxidant capacity under open-field conditions. Sustainability 2024, 16, 6745. [Google Scholar] [CrossRef]
- Abdelrasheed, K.G.; Mazrou, Y.; Omara, A.E.-D.; Osman, H.S.; Nehela, Y.; Hafez, E.M.; Rady, A.M.S.; El-Moneim, D.A.; Alowaiesh, B.F.; Gowayed, S.M. Soil amendment using biochar and application of K-humate enhance the growth, productivity, and nutritional value of onion (Allium cepa L.) under deficit irrigation conditions. Plants 2021, 10, 2598. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Chen, Q.; Guo, X.; Li, H.; Chen, X.; Men, K.; Liu, X.; Shang, X.; Gao, Y.; Zhang, L.; et al. Effect of potassium fulvate on continuous tobacco cropping soils and crop growth. Front. Plant Sci. 2024, 15, 1457793. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Rahim, H.U.; Mian, I.A.; Ali, W. Synergistic Biochar–nitrogen application enhances soil fertility and compensates for nutrient deficiency, improving wheat production in calcareous soil. Sustainability 2025, 17, 2321. [Google Scholar] [CrossRef]
- Puga, A.P.; Queiroz, M.C.D.A.; Ligo, M.A.V.; Carvalho, C.S.; Pires, A.M.M.; Marcatto, J.D.O.S.; Andrade, C.A.D. Nitrogen availability and ammonia volatilization in biochar-based fertilizers. Arch. Agron. Soil Sci. 2020, 66, 992–1004. [Google Scholar] [CrossRef]
- Liu, L.; Fang, W.; Yuan, M.; Li, X.; Wang, X.; Dai, Y. Metolachlor-adsorption on the walnut shell biochar modified by the fulvic acid and citric acid in water. J. Environ. Chem. Eng. 2021, 9, 106238. [Google Scholar] [CrossRef]
- Yang, W.; Li, C.; Wang, S.; Zhou, B.; Mao, Y.; Rensing, C.; Xing, S. Influence of biochar and biochar-based fertilizer on yield, quality of tea and microbial community in an acid tea orchard soil. Appl. Soil Ecol. 2021, 166, 104005. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, X.; Zhao, Y.; Zhang, C.; Jin, Z.; Shan, S.; Ping, L. Effects of biochar application on enzyme activities in tea garden soil. Front. Bioeng. Biotechnol. 2021, 9, 728530. [Google Scholar] [CrossRef]
- Puga, A.P.; Grutzmacher, P.; Cerri, C.E.P.; Ribeirinho, V.S.; Andrade, C.A. Biochar-based nitrogen fertilizers: Greenhouse gas emissions, use efficiency, and maize yield in tropical soils. Sci. Total Environ. 2020, 704, 135375. [Google Scholar] [CrossRef] [PubMed]
- Plaimart, J.; Acharya, K.; Mrozik, W.; Davenport, R.J.; Vinitnantharat, S.; Werner, D. Coconut Husk Biochar amendment enhances nutrient retention by suppressing nitrification in agricultural soil following anaerobic digestate application. Environ. Pollut. 2021, 268, 115684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, J.; Ren, H.; Chen, H. Characterization of forest soil acidification in Wenzhou Daluoshan and Zhejiang Wuyanling National Nature Reserve. Sustainability 2024, 16, 7051. [Google Scholar] [CrossRef]
- Zhang, X.; Han, G. The effect of warming and nitrogen addition on soil aggregate enzyme activities in a desert steppe. Sustainability 2025, 17, 6031. [Google Scholar] [CrossRef]
- Gwenzi, W.; Nyambishi, T.J.; Chaukura, N.; Mapope, N. Synthesis and nutrient release patterns of a biochar-based N–P–K slow-release fertilizer. Int. J. Environ. Sci. Technol. 2018, 15, 405–414. [Google Scholar] [CrossRef]
- Soares, J.R.; Cantarella, H.; Menegale, M.L.d.C. Ammonia volatilization losses from surface-applied urea with urease and nitrification inhibitors. Soil Biol. Biochem. 2012, 52, 82–89. [Google Scholar] [CrossRef]
- Zhenghu, D.; Honglang, X. Effects of soil properties on ammonia volatilization. Soil Sci. Plant Nutr. 2000, 46, 845–852. [Google Scholar] [CrossRef]
- Jindo, K.; Audette, Y.; Higashikawa, F.S.; Silva, C.A.; Akashi, K.; Mastrolonardo, G.; Sánchez-Monedero, M.A.; Mondini, C. Role of biochar in promoting circular economy in the agriculture sector. Part 1: A review of the biochar roles in soil N, P and K cycles. Chem. Biol. Technol. Agric. 2020, 7, 15. [Google Scholar] [CrossRef]
- Liang, H.; Chen, Q.; Liang, B.; Hu, K. Modeling the effects of long-term reduced N application on soil N losses and yield in a greenhouse tomato production system. Agric. Syst. 2020, 185, 102951. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Liu, B.; Amonette, J.E.; Lin, Z.; Liu, G.; Ambus, P.; Xie, Z. How does biochar influence soil N cycle? A meta-analysis. Plant Soil 2018, 426, 211–225. [Google Scholar] [CrossRef]
- Xu, R.; Zhao, A.; Yuan, J.; Jiang, J. pH buffering capacity of acid soils from tropical and subtropical regions of China as influenced by incorporation of crop straw biochars. J. Soils Sediments 2012, 12, 494–502. [Google Scholar] [CrossRef]
- Clough, T.J.; Condron, L.M. Biochar and the nitrogen cycle: Introduction. J. Environ. Qual. 2010, 39, 1218–1223. [Google Scholar] [CrossRef]
- Quilliam, R.S.; Glanville, H.C.; Wade, S.C.; Jones, D.L. Life in the ‘charosphere’—Does biochar in agricultural soil provide a significant habitat for microorganisms? Soil Biol. Biochem. 2013, 65, 287–293. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zimmerman, A.R. Organic carbon and nutrient release from a range of laboratory-produced biochars and biochar–soil mixtures. Geoderma 2013, 193–194, 122–130. [Google Scholar] [CrossRef]
- Taghizadeh-Toosi, A.; Clough, T.J.; Sherlock, R.R.; Condron, L.M. Biochar adsorbed ammonia is bioavailable. Plant Soil 2012, 350, 57–69. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, J.; Yao, R.; Wang, X.; Xie, W.; Zhu, W.; Liu, X.; Cao, Y.; Tao, J. Interactive effects of soil amendments (biochar and gypsum) and salinity on ammonia volatilization in coastal saline soil. Catena 2020, 190, 104527. [Google Scholar] [CrossRef]
- Jílková, V.; Angst, G. Biochar and compost amendments to a coarse-textured temperate agricultural soil lead to nutrient leaching. Appl. Soil Ecol. 2022, 173, 104393. [Google Scholar] [CrossRef]
- Huang, C.; Sun, X.; Wang, L.; Storer, P.; Siddique, K.H.M.; Solaiman, Z.M. Nutrients leaching from tillage soil amended with wheat straw biochar influenced by fertilizer type. Agriculture 2021, 11, 1132. [Google Scholar] [CrossRef]
- Ma, Y.; Xie, W.; Yao, R.; Feng, Y.; Wang, X.; Xie, H.; Feng, Y.; Yang, J. Biochar and hydrochar application influence soil ammonia volatilization and the dissolved organic matter in salt-affected soils. Sci. Total Environ. 2024, 926, 171845. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.Z.; Robertson, G.P.; Basso, B.; Hamilton, S.K. Leaching losses of dissolved organic carbon and nitrogen from agricultural soils in the upper US Midwest. Sci. Total Environ. 2020, 734, 139379. [Google Scholar] [CrossRef]
- Zhang, P.; Yue, F.-J.; Wang, X.-D.; Chen, S.-N. Dynamic transformation and leaching processes of nitrogen in a karst agricultural soil under simulated rainfall conditions. J. Contam. Hydrol. 2025, 269, 104494. [Google Scholar] [CrossRef]
- Shi, W.; Ju, Y.; Bian, R.; Li, L.; Joseph, S.; Mitchell, D.R.G.; Munroe, P.; Taherymoosavi, S.; Pan, G. Biochar bound urea boosts plant growth and reduces nitrogen leaching. Sci. Total Environ. 2020, 701, 134424. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cheng, J.; Lee, X.; Chen, Y.; Gao, W.; Pan, W.; Tang, Y. Effects of biochar-based fertilizers on nutrient leaching in a tobacco-planting soil. Acta Geochim. 2019, 38, 1–7. [Google Scholar] [CrossRef]
- Zhao, X.; Yan, X.; Wang, S.; Xing, G.; Zhou, Y. Effects of the addition of rice-straw-based biochar on leaching and retention of fertilizer N in highly fertilized cropland soils. Soil Sci. Plant Nutr. 2013, 59, 771–782. [Google Scholar] [CrossRef]
- Tao, W.-Q.; Wu, Q.-Q.; Zhang, J.; Chang, T.-T.; Liu, X.-N. Effects of applying organic amendments on soil aggregate structure and tomato yield in facility agriculture. Plants 2024, 13, 3064. [Google Scholar] [CrossRef]
- Xin, W.; Zhang, J.; Yu, Y.; Tian, Y.; Li, H.; Chen, X.; Li, W.; Liu, Y.; Lu, T.; He, B.; et al. Root microbiota of tea plants regulate nitrogen homeostasis and theanine synthesis to influence tea quality. Curr. Biol. 2024, 34, 868–880.e6. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Z.; Deng, X.; Herbert, S.; Xing, B. Impacts of adding biochar on nitrogen retention and bioavailability in agricultural soil. Geoderma 2013, 206, 32–39. [Google Scholar] [CrossRef]
- Lehman, R.M.; Acosta-Martinez, V.; Buyer, J.S.; Cambardella, C.A.; Collins, H.P.; Ducey, T.F.; Halvorson, J.J.; Jin, V.L.; Johnson, J.M.F.; Kremer, R.J.; et al. Soil biology for resilient, healthy soil. J. Soil Water Conserv. 2015, 70, 12A–18A. [Google Scholar] [CrossRef]
- García-Jaramillo, M.; Meyer, K.M.; Phillips, C.L.; Acosta-Martínez, V.; Osborne, J.; Levin, A.D.; Trippe, K.M. Biochar addition to vineyard soils: Effects on soil functions, grape yield and wine quality. Biochar 2021, 3, 565–577. [Google Scholar] [CrossRef]
- Sun, X.; Yang, X.; Hu, Z.; Liu, F.; Xie, Z.; Li, S.; Wang, G.; Li, M.; Sun, Z.; Bol, R. Biochar effects on soil nitrogen retention, leaching and yield of perennial citron daylily under three irrigation regimes. Agric. Water Manag. 2024, 296, 108788. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Wei, Q.; Gou, J. Biochar enhances the retention capacity of nitrogen fertilizer and affects the diversity of nitrifying functional microbial communities in karst soil of Southwest China. Ecotoxicol. Environ. Saf. 2021, 226, 112819. [Google Scholar] [CrossRef]
- Sun, H.; Lu, H.; Chu, L.; Shao, H.; Shi, W. Biochar applied with appropriate rates can reduce N leaching, keep N retention and not increase NH3 volatilization in a coastal saline soil. Sci. Total Environ. 2017, 575, 820–825. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Whitman, T.; Singh, B.P.; Zimmerman, A.R. Priming effects in biochar-amended soils: Implications of biochar-soil organic matter interactions for carbon storage. In Biochar for Environmental Management, 2nd ed.; Lehmann, J., Joseph, S., Eds.; Routledge: London, UK, 2015; pp. 487–520. [Google Scholar]
- Javeed, H.M.R.; Ali, M.; Ahmed, I.; Wang, X.; Al-Ashkar, I.; Qamar, R.; Ibrahim, A.; Habib-Ur-Rahman, M.; Ditta, A.; El Sabagh, A. Biochar enriched with buffalo slurry improved soil nitrogen and carbon dynamics, nutrient uptake and growth attributes of wheat by reducing leaching losses of nutrients. Land 2021, 10, 1392. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef]
- Ruan, J.; Zhang, F.; Wong, M.H. Effect of nitrogen form and phosphorus source on the growth, nutrient uptake and rhizosphere soil property of Camellia sinensis L. Plant Soil 2000, 223, 65–73. [Google Scholar] [CrossRef]
- Ruan, J.; Gerendás, J.; Härdter, R.; Sattelmacher, B. Effect of nitrogen form and root-zone pH on growth and nitrogen uptake of tea (Camellia sinensis) plants. Ann. Bot. 2007, 99, 301–310. [Google Scholar] [CrossRef]
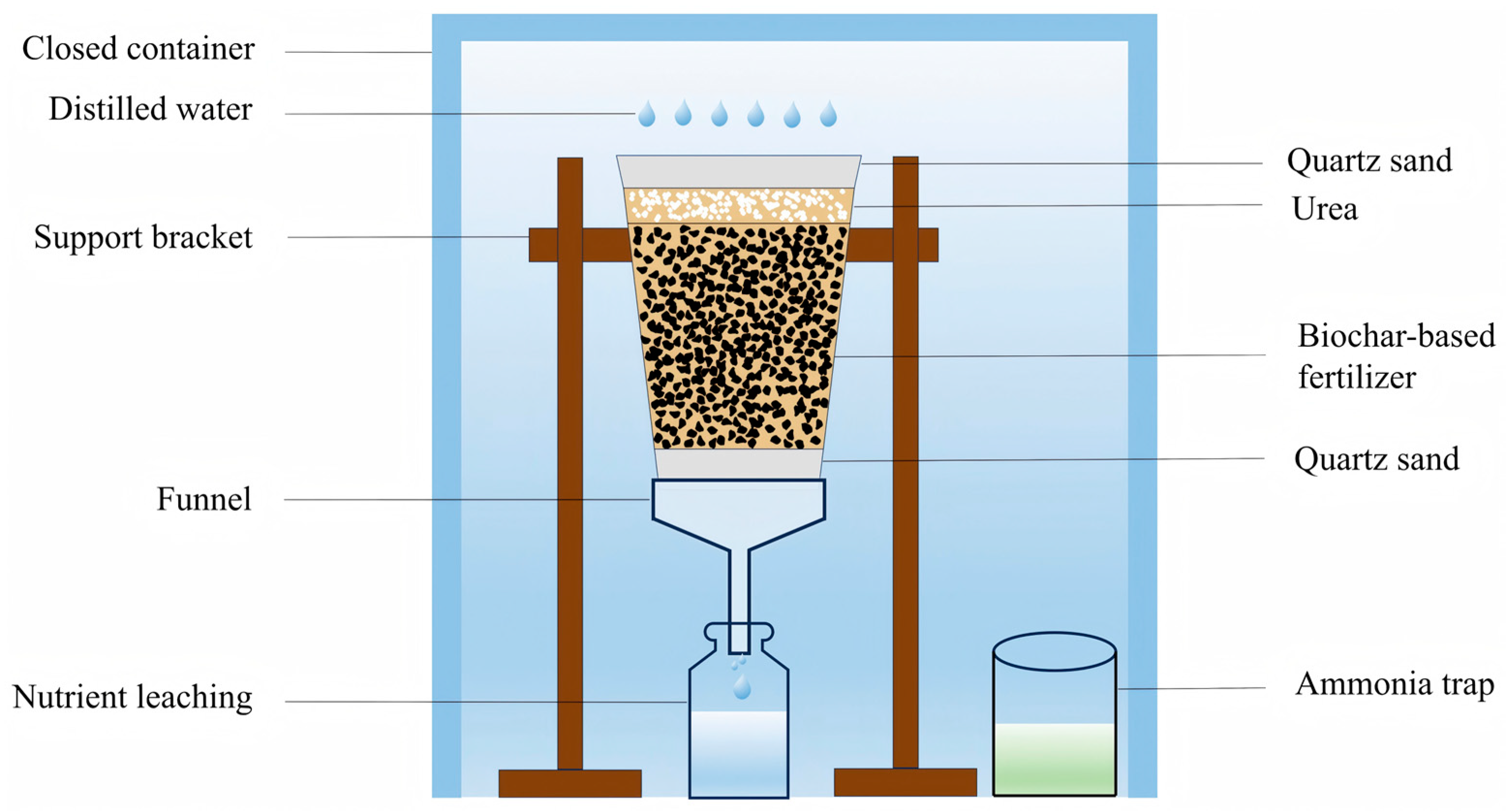



| Dose (t·ha−1) | CK | Uhigh | BF0.5Uhigh | BF1Uhigh | BF3Uhigh | Ulow | BF0.5Ulow | BF1Ulow | BF3Ulow |
|---|---|---|---|---|---|---|---|---|---|
| U | – | 1.50 | 1.50 | 1.50 | 1.50 | 0.72 | 0.72 | 0.72 | 0.72 |
| BF | – | – | 15.00 | 30.00 | 90.00 | – | 15.00 | 30.00 | 90.00 |
| Treatment | TDN (mg·kg−1) | NH4+-N (mg·kg−1) | NO3−-N (mg·kg−1) | DON (mg·kg−1) | NH4+-N/NO3−-N |
|---|---|---|---|---|---|
| CK | 23.49 ± 1.22 g | 2.13 ± 0.03 g | 13.99 ± 0.25 g | 7.37 ± 1.02 g | 0.15 ± 0.00 f |
| Ulow | 95.09 ± 3.78 e | 3.52 ± 0.16 f | 47.09 ± 0.93 b | 44.48 ± 3.99 ef | 0.07 ± 0.00 f |
| BF0.5Ulow | 76.27 ± 1.79 f | 19.59 ± 0.85 d | 19.27 ± 0.80 f | 37.42 ± 2.31 f | 1.02 ± 0.06 bc |
| BF1Ulow | 152.32 ± 1.66 c | 32.21 ± 1.23 c | 30.25 ± 1.60 e | 89.87 ± 1.06 c | 1.07 ± 0.09 b |
| BF3Ulow | 187.03 ± 11.30 b | 43.36 ± 2.57 b | 35.27 ± 2.03 d | 108.39 ± 9.91 b | 1.23 ± 0.03 a |
| Uhigh | 156.80 ± 5.94 c | 5.91 ± 0.25 e | 84.74 ± 3.87 a | 66.14 ± 2.12 d | 0.07 ± 0.00 f |
| BF0.5Uhigh | 104.17 ± 3.70 d | 19.53 ± 0.70 d | 36.67 ± 1.81 d | 47.97 ± 3.56 e | 0.53 ± 0.02 e |
| BF1Uhigh | 158.19 ± 5.59 c | 32.98 ± 0.11 c | 42.70 ± 0.79 c | 82.51 ± 6.28 c | 0.77 ± 0.02 d |
| BF3Uhigh | 213.66 ± 4.55 a | 46.36 ± 0.57 a | 48.74 ± 0.97 b | 118.56 ± 4.62 a | 0.95 ± 0.03 c |
| BF | * | ** | ns | * | * |
| U | * | ns | * | * | ns |
| BF × U | ** | ns | ** | ** | ** |
| Treatment | Soil TN (g·kg−1) | Soil NH4+-N (mg·kg−1) | Soil NO3−-N (mg·kg−1) | Soil NH4+-N/NO3−-N | SOC (g·kg−1) | EC (μs·cm−1) | UE (mg·g−1·24 h−1) | Soil C/N | pH |
|---|---|---|---|---|---|---|---|---|---|
| CK | 1.06 ± 0.05 g | 64.12 ± 5.00 f | 22.35 ± 1.97 e | 2.89 ± 0.41 b | 11.71 ± 0.64 d | 89.58 ± 5.83 g | 0.56 ± 0.04 e | 11.05 ± 0.22 a | 4.17 ± 0.03 d |
| Ulow | 1.08 ± 0.00 fg | 58.63 ± 4.88 f | 34.24 ± 3.09 d | 1.73 ± 0.31 c | 11.53 ± 0.11 d | 143.21 ± 9.71 fg | 0.95 ± 0.07 d | 10.63 ± 0.13 ab | 4.05 ± 0.02 e |
| BF0.5Ulow | 1.35 ± 0.02 e | 180.60 ± 9.24 d | 69.23 ± 6.30 c | 2.62 ± 0.16 b | 13.02 ± 0.34 c | 293.70 ± 16.48 e | 1.38 ± 0.12 c | 9.62 ± 0.13 c | 4.46 ± 0.04 bc |
| BF1Ulow | 1.55 ± 0.04 c | 262.07 ± 16.29 c | 64.65 ± 5.23 c | 4.07 ± 0.40 a | 14.55 ± 0.48 b | 465.40 ± 14.90 d | 1.60 ± 0.10 bc | 9.36 ± 0.35 c | 4.55 ± 0.05 b |
| BF3Ulow | 2.55 ± 0.06 a | 357.72 ± 11.26 a | 85.64 ± 0.85 b | 4.18 ± 0.16 a | 20.64 ± 1.06 a | 783.25 ± 64.97 b | 2.07 ± 0.15 a | 8.10 ± 0.50 d | 5.00 ± 0.05 a |
| Uhigh | 1.14 ± 0.01 f | 102.45 ± 8.45 e | 57.59 ± 6.14 c | 1.79 ± 0.21 c | 11.64 ± 0.58 d | 179.30 ± 15.91 f | 0.90 ± 0.07 d | 10.21 ± 0.49 b | 4.01 ± 0.03 e |
| BF0.5Uhigh | 1.46 ± 0.05 d | 253.82 ± 11.33 c | 92.45 ± 8.25 b | 2.76 ± 0.21 b | 13.05 ± 0.36 c | 352.30 ± 15.83 e | 1.44 ± 0.09 c | 8.97 ± 0.47 c | 4.36 ± 0.07 c |
| BF1Uhigh | 1.63 ± 0.02 b | 279.00 ± 4.00 b | 158.96 ± 6.20 a | 1.76 ± 0.08 c | 14.65 ± 0.09 b | 568.85 ± 49.54 c | 1.69 ± 0.10 b | 8.96 ± 0.05 c | 4.57 ± 0.04 b |
| BF3Uhigh | 2.61 ± 0.03 a | 351.35 ± 3.51 a | 150.66 ± 12.77 a | 2.34 ± 0.18 b | 20.69 ± 0.90 a | 947.70 ± 54.34 a | 2.12 ± 0.18 a | 7.94 ± 0.33 d | 5.04 ± 0.1 a |
| BF | ** | ** | ns | ns | ** | ** | ** | ** | ** |
| U | * | ns | ns | ns | ns | ns | * | * | ns |
| BF × U | ns | ** | ** | ** | ns | * | ns | ns | ns |
| N Content | CK | Uhigh | Ulow | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Uhigh | BF0.5 | BF1 | BF3 | Ulow | BF0.5 | BF1 | BF3 | ||
| Fertilizer application (g·kg−1) | 0 | 0.24 | 0.51 | 0.77 | 1.83 | 0.11 | 0.38 | 0.64 | 1.70 |
| Mineral N residue (g·kg−1) | 1.06 g | 1.14 f | 1.46 d | 1.63 b | 2.61 a | 1.08 g | 1.35 e | 1.55 c | 2.55 a |
| Losses via leaching and NH3 volatilization (mg·kg−1) | 24.40 h | 165.80 c | 112.46 e | 169.96 c | 227.37 a | 100.37 f | 80.37 g | 158.00 d | 194.44 b |
| NH3 volatilization loss (mg·kg−1) | 0.91 g | 9.00 c | 8.28 c | 11.77 b | 13.71 a | 5.28 e | 4.10 f | 5.68 e | 7.41 d |
| Leaching loss of total N (mg·kg−1) | 23.49 g | 156.80 c | 104.17 d | 158.19 c | 213.66 a | 95.09 e | 76.27 f | 152.32 c | 187.03 b |
| Ratio * of Applied N (%) | Uhigh | Ulow | ||||||
|---|---|---|---|---|---|---|---|---|
| Uhigh | BF0.5 | BF1 | BF3 | Ulow | BF0.5 | BF1 | BF3 | |
| Minerals N residue | 33.37 b | 77.84 a | 74.68 a | 84.54 a | 18.73 bc | 77.46 a | 77.29 a | 87.67 a |
| Losses via leaching and NH3 volatilization | 58.91 b | 17.27 cd | 18.90 c | 11.09 e | 69.06 a | 14.73 d | 20.88 c | 10.00 e |
| NH3 volatilization loss | 3.37 b | 1.44 c | 1.41 c | 0.70 d | 3.97 a | 0.84 d | 0.74 d | 0.38 e |
| Leaching loss of total N | 55.55 b | 15.82 d | 17.49 cd | 10.39 e | 65.10 a | 13.89 d | 20.13 c | 9.62 e |
| Undefined # | 7.61 b | 4.89 d | 6.42 c | 4.37 d | 12.21 a | 7.82 b | 1.83 f | 2.33 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Zhang, Y.; Fang, Y.; Xia, X.; Tao, T.; Liao, J.; Wang, Y.; Su, Y. Optimizing Nitrogen Management in Acidic Tea Orchard Soils: The Role of Biochar-Based Fertilizers in Reducing Losses and Enhancing Sequestration. Sustainability 2025, 17, 9751. https://doi.org/10.3390/su17219751
Sun Y, Zhang Y, Fang Y, Xia X, Tao T, Liao J, Wang Y, Su Y. Optimizing Nitrogen Management in Acidic Tea Orchard Soils: The Role of Biochar-Based Fertilizers in Reducing Losses and Enhancing Sequestration. Sustainability. 2025; 17(21):9751. https://doi.org/10.3390/su17219751
Chicago/Turabian StyleSun, Yulong, Yongli Zhang, Yage Fang, Xianjiang Xia, Tao Tao, Jun Liao, Yejun Wang, and Youjian Su. 2025. "Optimizing Nitrogen Management in Acidic Tea Orchard Soils: The Role of Biochar-Based Fertilizers in Reducing Losses and Enhancing Sequestration" Sustainability 17, no. 21: 9751. https://doi.org/10.3390/su17219751
APA StyleSun, Y., Zhang, Y., Fang, Y., Xia, X., Tao, T., Liao, J., Wang, Y., & Su, Y. (2025). Optimizing Nitrogen Management in Acidic Tea Orchard Soils: The Role of Biochar-Based Fertilizers in Reducing Losses and Enhancing Sequestration. Sustainability, 17(21), 9751. https://doi.org/10.3390/su17219751






