From Prescription to Pollution: Assessing the Ecological Impact and Treatment Technologies for Antidepressant Contaminants
Abstract
1. Introduction
2. Environmental Occurrence and Pathways of Antidepressants
3. Modes of Action of Antidepressants
Bioavailability and Bioaccumulation in Aquatic Organisms
4. Ecotoxicological Effects on Aquatic Biota
4.1. Neurochemical and Molecular Effects
4.2. Impacts on Development, Growth, and Reproduction
4.3. Behavioral Change
4.4. Transgenerational and Epigenetic Effects
4.5. Synthesis: Environmental Relevance and Future Research Directions
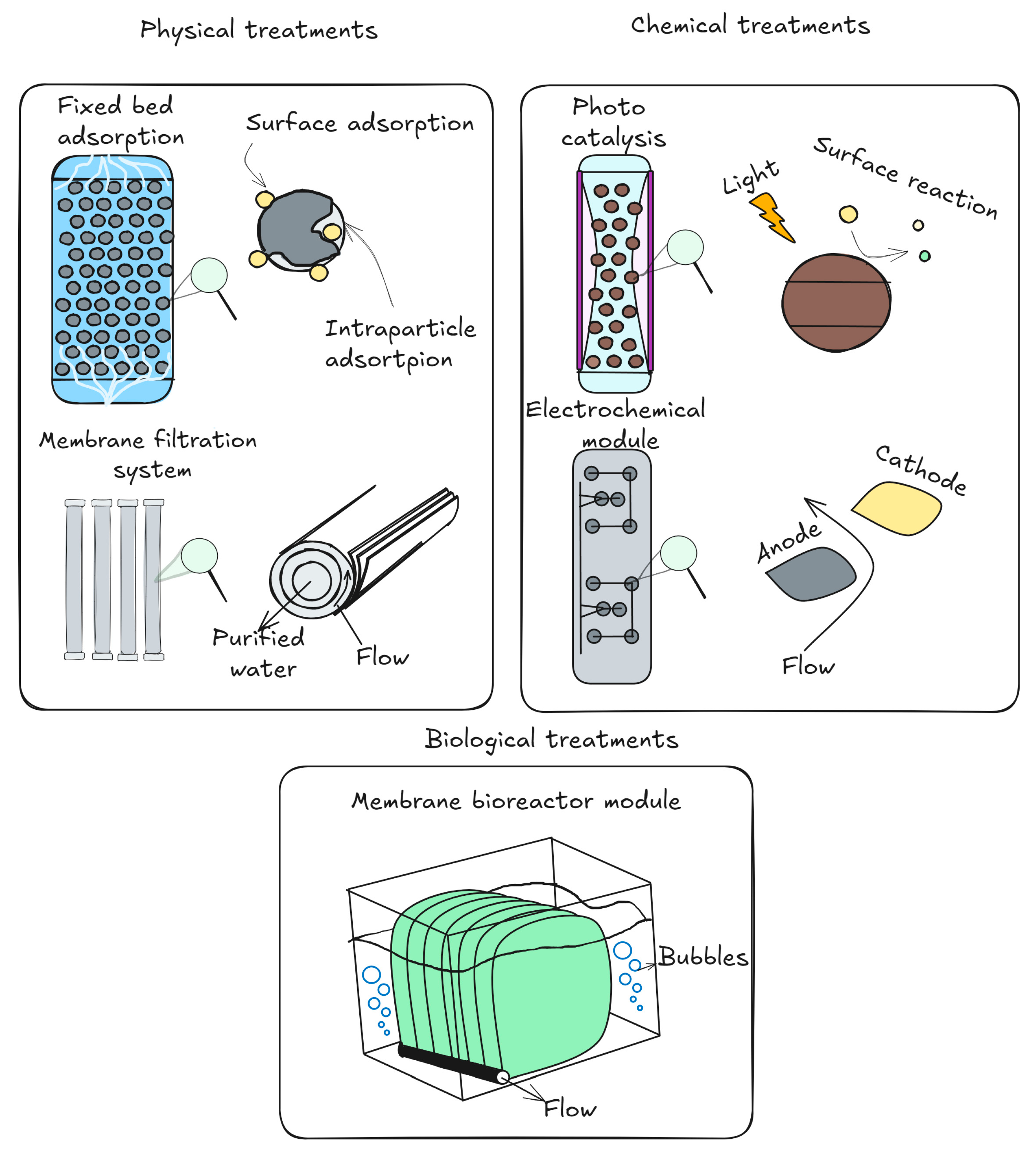
5. An Overview of Methods for Treating Water to Remove Antidepressant Residues
5.1. Adsorptive Methods
5.2. Extractive Methods
5.3. Advanced Oxidation Processes
5.3.1. Pharmaceutical Photodegradation Pathways
5.3.2. Photocatalytic Nanomaterials for Drug Removal
5.4. Biological and Advanced Oxidation Processes for Antidepressant Removal
5.5. Comparative Analysis of Different Technologies
6. Gaps and Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OECD | Organization for Economic Cooperation and Development |
| DDD | defined daily dosage |
| ASP | activated sludge |
| MBR | membrane bioreactor |
| MBBR | moving-bed biofilm reactor |
| UF | ultrafiltration |
| NF | nanofiltration |
| RO | reverse osmosis |
| AOPs | advanced oxidation processes |
| UV | ultraviolet |
| GAC | granular activated carbon |
References
- Gould, S.L.; Winter, M.J.; Norton, W.H.J.; Tyler, C.R. The Potential for Adverse Effects in Fish Exposed to Antidepressants in the Aquatic Environment. Environ. Sci. Technol. 2021, 55, 16299–16312. [Google Scholar] [CrossRef]
- Fava, M.; Rush, A.J.; Thase, M.E.; Clayton, A.; Stahl, S.M.; Pradko, J.F.; Johnston, J.A. 15 Years of Clinical Experience with Bupropion HCl: From Bupropion to Bupropion SR to Bupropion XL. Prim. Care Companion J. Clin. Psychiatry 2005, 7, 106–113. [Google Scholar] [CrossRef]
- Sanchez, C.; Asin, K.E.; Artigas, F. Vortioxetine, a Novel Antidepressant with Multimodal Activity: Review of Preclinical and Clinical Data. Pharmacol. Ther. 2015, 145, 43–57. [Google Scholar] [CrossRef]
- Racovita, R.C.; Ciuca, M.D. Wastewater Treatment Approaches for the Removal of Antidepressant Residues. In Wastewater Treatment and Sludge Management Systems; Taşeli, B.K., Jacob-Lopes, E., Deprá, M.C., Zepka, L.Q., Eds.; IntechOpen: Rijeka, Croatia, 2024. [Google Scholar]
- Lewer, D.; O’Reilly, C.; Mojtabai, R.; Evans-Lacko, S. Antidepressant Use in 27 European Countries: Associations with Sociodemographic, Cultural and Economic Factors. Br. J. Psychiatry 2015, 207, 221–226. [Google Scholar] [CrossRef]
- Benke, C.; Autenrieth, L.K.; Asselmann, E.; Pané-Farré, C.A. Lockdown, Quarantine Measures, and Social Distancing: Associations with Depression, Anxiety and Distress at the Beginning of the COVID-19 Pandemic among Adults from Germany. Psychiatry Res. 2020, 293, 113462. [Google Scholar] [CrossRef] [PubMed]
- Thoresen, S.; Blix, I.; Wentzel-Larsen, T.; Birkeland, M.S. Trusting Others During a Pandemic: Investigating Potential Changes in Generalized Trust and Its Relationship With Pandemic-Related Experiences and Worry. Front. Psychol. 2021, 12, 698519. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999−2000: A National Reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- DiPippa, A.D.; Mou, X. Meta-Analysis of the Distribution of Pharmaceuticals and Personal Care Products in Natural Streams of United States and Its Correlations with Anthropogenic Factors. ACS EST Water 2024, 4, 427–435. [Google Scholar] [CrossRef]
- Castillo-Zacarías, C.; Barocio, M.E.; Hidalgo-Vázquez, E.; Sosa-Hernández, J.E.; Parra-Arroyo, L.; López-Pacheco, I.Y.; Barceló, D.; Iqbal, H.N.M.; Parra-Saldívar, R. Antidepressant Drugs as Emerging Contaminants: Occurrence in Urban and Non-Urban Waters and Analytical Methods for Their Detection. Sci. Total Environ. 2021, 757, 143722. [Google Scholar] [CrossRef]
- Melchor-Martínez, E.M.; Jiménez-Rodríguez, M.G.; Martínez-Ruiz, M.; Peña-Benavides, S.A.; Iqbal, H.M.N.; Parra-Saldívar, R.; Sosa-Hernández, J.E. Antidepressants Surveillance in Wastewater: Overview Extraction and Detection. Case Stud. Chem. Environ. Eng. 2021, 3, 100074. [Google Scholar] [CrossRef]
- Santos, L.H.M.L.M.; Araújo, A.N.; Fachini, A.; Pena, A.; Delerue-Matos, C.; Montenegro, M.C.B.S.M. Ecotoxicological Aspects Related to the Presence of Pharmaceuticals in the Aquatic Environment. J. Hazard. Mater. 2010, 175, 45–95. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, E. Pharmaceuticals in the Environment: Expected and Unexpected Effects on Aquatic Fauna. Ann. N. Y. Acad. Sci. 2015, 1340, 20–28. [Google Scholar] [CrossRef]
- Gunnarsson, L.; Jauhiainen, A.; Kristiansson, E.; Nerman, O.; Larsson, D.G.J. Evolutionary Conservation of Human Drug Targets in Organisms Used for Environmental Risk Assessments. Environ. Sci. Technol. 2008, 42, 5807–5813. [Google Scholar] [CrossRef]
- Tanoue, R.; Nomiyama, K.; Nakamura, H.; Kim, J.W.; Isobe, T.; Shinohara, R.; Kunisue, T.; Tanabe, S. Uptake and Tissue Distribution of Pharmaceuticals and Personal Care Products in Wild Fish from Treated-Wastewater-Impacted Streams. Environ. Sci. Technol. 2015, 49, 11649–11658. [Google Scholar] [CrossRef]
- Huerta, B.; Rodriguez-Mozaz, S.; Lazorchak, J.; Barcelo, D.; Batt, A.; Wathen, J.; Stahl, L. Presence of Pharmaceuticals in Fish Collected from Urban Rivers in the U.S. EPA 2008–2009 National Rivers and Streams Assessment. Sci. Total Environ. 2018, 634, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Sehonova, P.; Plhalova, L.; Blahova, J.; Doubkova, V.; Marsalek, P.; Prokes, M.; Tichy, F.; Skladana, M.; Fiorino, E.; Mikula, P.; et al. Effects of Selected Tricyclic Antidepressants on Early-Life Stages of Common Carp (Cyprinus carpio). Chemosphere 2017, 185, 1072–1080. [Google Scholar] [CrossRef]
- Nowakowska, K.; Giebułtowicz, J.; Kamaszewski, M.; Adamski, A.; Szudrowicz, H.; Ostaszewska, T.; Solarska-Dzięciołowska, U.; Nałęcz-Jawecki, G.; Wroczyński, P.; Drobniewska, A. Acute Exposure of Zebrafish (Danio rerio) Larvae to Environmental Concentrations of Selected Antidepressants: Bioaccumulation, Physiological and Histological Changes. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 229, 108670. [Google Scholar] [CrossRef] [PubMed]
- Mahtab, M.S.; Farooqi, I.H. An Overview of Occurrence and Removal of Pharmaceuticals from Sewage/Wastewater. In Sewage; Zhang, T., Ed.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Georgin, J.; Franco, D.S.P.; Meili, L.; Dehmani, Y.; dos Reis, G.S.; Lima, E.C. Main Advances and Future Prospects in the Remediation of the Antibiotic Amoxicillin with a Focus on Adsorption Technology: A Critical Review. J. Water Process Eng. 2023, 56, 104407. [Google Scholar] [CrossRef]
- Georgin, J.; Franco, D.S.P.; Ramos, C.G.; Piccilli, D.G.A.; Lima, E.C.; Sher, F. A Review of the Antibiotic Ofloxacin: Current Status of Ecotoxicology and Scientific Advances in Its Removal from Aqueous Systems by Adsorption Technology. Chem. Eng. Res. Des. 2023, 193, 99–120. [Google Scholar] [CrossRef]
- Lama, G.; Meijide, J.; Sanromán, A.; Pazos, M. Heterogeneous Advanced Oxidation Processes: Current Approaches for Wastewater Treatment. Catalysts 2022, 12, 344. [Google Scholar] [CrossRef]
- Xiao, C.; Hu, Y.; Li, Q.; Liu, J.; Li, X.; Shi, Y.; Chen, Y.; Cheng, J. Carbon-Doped Defect MoS2 Co-Catalytic Fe3+/Peroxymonosulfate Process for Efficient Sulfadiazine Degradation: Accelerating Fe3+/Fe2+ Cycle and 1O2 Dominated Oxidation. Sci. Total Environ. 2023, 858, 159587. [Google Scholar] [CrossRef]
- Daughton, C.G. Pharmaceuticals and the Environment (PiE): Evolution and Impact of the Published Literature Revealed by Bibliometric Analysis. Sci. Total Environ. 2016, 562, 391–426. [Google Scholar] [CrossRef]
- Argaluza, J.; Domingo-Echaburu, S.; Orive, G.; Medrano, J.; Hernandez, R.; Lertxundi, U. Environmental Pollution with Psychiatric Drugs. World J. Psychiatry 2021, 11, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Reddersen, K.; Heberer, T.; Dünnbier, U. Identification and Significance of Phenazone Drugs and Their Metabolites in Ground- and Drinking Water. Chemosphere 2002, 49, 539–544. [Google Scholar] [CrossRef]
- Zühlke, S.; Dünnbier, U.; Heberer, T. Detection and Identification of Phenazone-Type Drugs and Their Microbial Metabolites in Ground and Drinking Water Applying Solid-Phase Extraction and Gas Chromatography with Mass Spectrometric Detection. J. Chromatogr. A 2004, 1050, 201–209. [Google Scholar] [CrossRef]
- Lee, W.Y.; Arnold, C.R. Chronic Toxicity of Ocean-Dumped Pharmaceutical Wastes to the Marine Amphipod Amphithoe Valida. Mar. Pollut. Bull. 1983, 14, 150–153. [Google Scholar] [CrossRef]
- Larsson, D.G.J.J. Pollution from Drug Manufacturing: Review and Perspectives. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130571. [Google Scholar] [CrossRef]
- Oaks, J.L.; Gilbert, M.; Virani, M.Z.; Watson, R.T.; Meteyer, C.U.; Rideout, B.A.; Shivaprasad, H.L.; Ahmed, S.; Iqbal Chaudhry, M.J.; Arshad, M.; et al. Diclofenac Residues as the Cause of Vulture Population Decline in Pakistan. Nature 2004, 427, 630–633. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; de Pedro, C.; Paxeus, N. Effluent from Drug Manufactures Contains Extremely High Levels of Pharmaceuticals. J. Hazard. Mater. 2007, 148, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.N.; Paxéus, N.; Förlin, L.; Larsson, D.G.J. Variations in Bioconcentration of Human Pharmaceuticals from Sewage Effluents into Fish Blood Plasma. Environ. Toxicol. Pharmacol. 2007, 24, 267–274. [Google Scholar] [CrossRef]
- Kristiansson, E.; Fick, J.; Janzon, A.; Grabic, R.; Rutgersson, C.; Weijdegård, B.; Söderström, H.; Joakim Larsson, D.G. Pyrosequencing of Antibiotic-Contaminated River Sediments Reveals High Levels of Resistance and Gene Transfer Elements. PLoS ONE 2011, 6, e17038. [Google Scholar] [CrossRef]
- Fick, J.; Söderström, H.; Lindberg, R.H.; Phan, C.; Tysklind, M.; Larsson, D.G.J. Contamination of Surface, Ground, and Drinking Water from Pharmaceutical Production. Environ. Toxicol. Chem. 2009, 28, 2522–2527. [Google Scholar] [CrossRef]
- Rutgersson, C.; Fick, J.; Marathe, N.; Kristiansson, E.; Janzon, A.; Angelin, M.; Johansson, A.; Shouche, Y.; Flach, C.F.; Larsson, D.G.J. Fluoroquinolones and Qnr Genes in Sediment, Water, Soil, and Human Fecal Flora in an Environment Polluted by Manufacturing Discharges. Environ. Sci. Technol. 2014, 48, 7825–7832. [Google Scholar] [CrossRef] [PubMed]
- Lubick, N. India’s Drug Problem: Chemists Show How Waste-Water Contamination Affects Ecosystem. Nature 2009, 457, 640–642. [Google Scholar] [CrossRef]
- Silva, B.; Costa, F.; Neves, I.C.; Tavares, T. Psychiatric Pharmaceuticals as Emerging Contaminants in Wastewater; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-319-20492-5. [Google Scholar]
- Hai, F.I.; Yang, S.; Asif, M.B.; Sencadas, V.; Shawkat, S.; Sanderson-Smith, M.; Gorman, J.; Xu, Z.Q.; Yamamoto, K. Carbamazepine as a Possible Anthropogenic Marker in Water: Occurrences, Toxicological Effects, Regulations and Removal by Wastewater Treatment Technologies. Water 2018, 10, 107. [Google Scholar] [CrossRef]
- Trawiński, J.; Skibiński, R. Studies on Photodegradation Process of Psychotropic Drugs: A Review. Environ. Sci. Pollut. Res. 2017, 24, 1152–1199. [Google Scholar] [CrossRef]
- Brodin, T.; Piovano, S.; Fick, J.; Klaminder, J.; Heynen, M.; Jonsson, M. Ecological Effects of Pharmaceuticals in Aquatic Systems—Impacts through Behavioural Alterations. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130580. [Google Scholar] [CrossRef] [PubMed]
- Onesios, K.M.; Yu, J.T.; Bouwer, E.J. Biodegradation and Removal of Pharmaceuticals and Personal Care Products in Treatment Systems: A Review. Biodegradation 2009, 20, 441–466. [Google Scholar] [CrossRef]
- Silva, L.J.G.; Lino, C.M.; Meisel, L.M.; Pena, A. Selective Serotonin Re-Uptake Inhibitors (SSRIs) in the Aquatic Environment: An Ecopharmacovigilance Approach. Sci. Total Environ. 2012, 437, 185–195. [Google Scholar] [CrossRef]
- Mole, R.A.; Brooks, B.W. Global Scanning of Selective Serotonin Reuptake Inhibitors: Occurrence, Wastewater Treatment and Hazards in Aquatic Systems. Environ. Pollut. 2019, 250, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.R.; Kasprzyk-Hordern, B. Spatial and Temporal Occurrence of Pharmaceuticals and Illicit Drugs in the Aqueous Environment and during Wastewater Treatment: New Developments. Sci. Total Environ. 2013, 454–455, 442–456. [Google Scholar] [CrossRef]
- Baker, D.R.; Kasprzyk-Hordern, B. Multi-Residue Analysis of Drugs of Abuse in Wastewater and Surface Water by Solid-Phase Extraction and Liquid Chromatography-Positive Electrospray Ionisation Tandem Mass Spectrometry. J. Chromatogr. A 2011, 1218, 1620–1631. [Google Scholar] [CrossRef]
- Schultz, M.M.; Furlong, E.T. Trace Analysis of Antidepressant Pharmaceuticals and Their Select Degradates in Aquatic Matrixes by LC/ESI/MS/MS. Anal. Chem. 2008, 80, 1756–1762. [Google Scholar] [CrossRef]
- Metcalfe, C.D.; Chu, S.; Judt, C.; Li, H.; Oakes, K.D.; Servos, M.R.; Andrews, D.M. Antidepressants and Their Metabolites in Municipal Wastewater, and Downstream Exposure in an Urban Watershed. Environ. Toxicol. Chem. 2010, 29, 79–89. [Google Scholar] [CrossRef]
- Rúa-Gómez, P.C.; Püttmann, W. Impact of Wastewater Treatment Plant Discharge of Lidocaine, Tramadol, Venlafaxine and Their Metabolites on the Quality of Surface Waters and Groundwater. J. Environ. Monit. 2012, 14, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Zmarlicka, M.; Ehret, M.J. Vilazodone: A Novel Antidepressant. Am. J. Health Pharm. 2012, 69, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.; Nowicki, M.; Muraleetharan, A.; Gerlai, R. Differential Effects of Dopamine D1 and D2/3 Receptor Antagonism on Motor Responses. Psychopharmacology 2015, 232, 795–806. [Google Scholar] [CrossRef]
- Brunello, N.; Blier, P.; Judd, L.L.; Mendlewicz, J.; Nelson, C.J.; Souery, D.; Zohar, J.; Racagni, G. Noradrenaline in Mood and Anxiety Disorders: Basic and Clinical Studies. Int. Clin. Psychopharmacol. 2003, 18, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Farrar, M.J.; Kolkman, K.E.; Fetcho, J.R. Features of the Structure, Development, and Activity of the Zebrafish Noradrenergic System Explored in New CRISPR Transgenic Lines. J. Comp. Neurol. 2018, 526, 2493–2508. [Google Scholar] [CrossRef]
- Li, P.; Shah, S.; Huang, L.; Carr, A.L.; Gao, Y.; Thisse, C.; Thisse, B.; Li, L. Cloning and Spatial and Temporal Expression of the Zebrafish Dopamine D1 Receptor. Dev. Dyn. 2007, 236, 1339–1346. [Google Scholar] [CrossRef]
- Ruuskanen, J.O.; Xhaard, H.; Marjamäki, A.; Salaneck, E.; Salminen, T.; Yan, Y.L.; Postlethwait, J.H.; Johnson, M.S.; Larhammar, D.; Scheinin, M. Identification of Duplicated Fourth A2-Adrenergic Receptor Subtype by Cloning and Mapping of Five Receptor Genes in Zebrafish. Mol. Biol. Evol. 2004, 21, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Demin, K.A.; Kolesnikova, T.O.; Khatsko, S.L.; Meshalkina, D.A.; Efimova, E.V.; Morzherin, Y.Y.; Kalueff, A.V. Acute Effects of Amitriptyline on Adult Zebrafish: Potential Relevance to Antidepressant Drug Screening and Modeling Human Toxidromes. Neurotoxicol. Teratol. 2017, 62, 27–33. [Google Scholar] [CrossRef]
- Grabicova, K.; Lindberg, R.H.; Östman, M.; Grabic, R.; Randak, T.; Joakim Larsson, D.G.; Fick, J. Tissue-Specific Bioconcentration of Antidepressants in Fish Exposed to Effluent from a Municipal Sewage Treatment Plant. Sci. Total Environ. 2014, 488–489, 46–50. [Google Scholar] [CrossRef]
- Koba, O.; Grabicova, K.; Cerveny, D.; Turek, J.; Kolarova, J.; Randak, T.; Zlabek, V.; Grabic, R. Transport of Pharmaceuticals and Their Metabolites between Water and Sediments as a Further Potential Exposure for Aquatic Organisms. J. Hazard. Mater. 2018, 342, 401–407. [Google Scholar] [CrossRef]
- Schultz, W. Behavioral Dopamine Signals. Trends Neurosci. 2007, 30, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Huggett, D.B.; Cook, J.C.; Ericson, J.F.; Williams, R.T. A Theoretical Model for Utilizing Mammalian Pharmacology and Safety Data to Prioritize Potential Impacts of Human Pharmaceuticals to Fish. Hum. Ecol. Risk Assess. 2003, 9, 1789–1799. [Google Scholar] [CrossRef]
- Marrone, R.L.; Pray, S.L.; Bridges, C.C. Norepinephrine Elicitation of Aggressive Display Responses in Betta Splendens. Psychon. Sci. 1966, 5, 207–208. [Google Scholar] [CrossRef]
- McCallum, E.S.; Krutzelmann, E.; Brodin, T.; Fick, J.; Sundelin, A.; Balshine, S. Exposure to Wastewater Effluent Affects Fish Behaviour and Tissue-Specific Uptake of Pharmaceuticals. Sci. Total Environ. 2017, 605–606, 578–588. [Google Scholar] [CrossRef]
- Brun, G.L.; Bernier, M.; Losier, R.; Doe, K.; Jackman, P.; Lee, H.B. Pharmaceutically Active Compounds in Atlantic Canadian Sewage Treatment Plant Effluents and Receiving Waters, and Potential for Environmental Effects as Measured by Acute and Chronic Aquatic Toxicity. Environ. Toxicol. Chem. 2006, 25, 2163–2176. [Google Scholar] [CrossRef]
- Holzschuh, J.; Ryu, S.; Aberger, F.; Driever, W. Dopamine Transporter Expression Distinguishes Dopaminergic Neurons from Other Catecholaminergic Neurons in the Developing Zebrafish Embryo. Mech. Dev. 2001, 101, 237–243. [Google Scholar] [CrossRef]
- Cunha, V.; Rodrigues, P.; Santos, M.M.; Moradas-Ferreira, P.; Ferreira, M. Fluoxetine Modulates the Transcription of Genes Involved in Serotonin, Dopamine and Adrenergic Signalling in Zebrafish Embryos. Chemosphere 2018, 191, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.G.; Törk, I.; Hornung, J.P.; Halasz, P. The Human Locus Coeruleus Complex: An Immunohistochemical and Three Dimensional Reconstruction Study. Exp. Brain Res. 1989, 77, 257–270. [Google Scholar] [CrossRef]
- Ruffolo, R.R.; Hieble, J.P. α-Adrenoceptors. Pharmacol. Ther. 1994, 61, 1–64. [Google Scholar] [CrossRef]
- Wang, Z.; Nishimura, Y.; Shimada, Y.; Umemoto, N.; Hirano, M.; Zang, L.; Oka, T.; Sakamoto, C.; Kuroyanagi, J.; Tanaka, T. Zebrafish β-Adrenergic Receptor MRNA Expression and Control of Pigmentation. Gene 2009, 446, 18–27. [Google Scholar] [CrossRef]
- Zikopoulos, B.; Dermon, C.R. Comparative Anatomy of A2 and β Adrenoceptors in the Adult and Developing Brain of the Marine Teleost the Red Porgy (Pagrus Pagrus, Sparidae): [3H]Clonidine and [3H]Dihydroalprenolol Quantitative Autoradiography and Receptor Subtypes Immunohistochemistry. J. Comp. Neurol. 2005, 489, 217–240. [Google Scholar] [CrossRef] [PubMed]
- Ruuskanen, J.O.; Peitsaro, N.; Kaslin, J.V.M.; Panula, P.; Scheinin, M. Expression and Function of A2-Adrenoceptors in Zebrafish: Drug Effects, MRNA and Receptor Distributions. J. Neurochem. 2005, 94, 1559–1569. [Google Scholar] [CrossRef]
- Sharma, Y.; Xu, T.; Graf, W.M.; Fobbs, A.; Sherwood, C.C.; Hof, P.R.; Allman, J.M.; Manaye, K.F. Comparative Anatomy of the Locus Coeruleus in Humans and Nonhuman Primates. J. Comp. Neurol. 2010, 518, 963–971. [Google Scholar] [CrossRef]
- Irons, T.D.; Kelly, P.E.; Hunter, D.L.; MacPhail, R.C.; Padilla, S. Acute Administration of Dopaminergic Drugs Has Differential Effects on Locomotion in Larval Zebrafish. Pharmacol. Biochem. Behav. 2013, 103, 792–813. [Google Scholar] [CrossRef]
- Kacprzak, V.; Patel, N.A.; Riley, E.; Yu, L.; Yeh, J.R.J.; Zhdanova, I.V. Dopaminergic Control of Anxiety in Young and Aged Zebrafish. Pharmacol. Biochem. Behav. 2017, 157, 1–8. [Google Scholar] [CrossRef]
- Gillman, P.K. Tricyclic Antidepressant Pharmacology and Therapeutic Drug Interactions Updated. Br. J. Pharmacol. 2007, 151, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Rehavi, M.; Maayani, S.; Sokolovsky, M. Tricyclic Antidepressants as Antimuscarinic Drugs: In Vivo and in Vitro Studies. Biochem. Pharmacol. 1977, 26, 1559–1567. [Google Scholar] [CrossRef]
- Wong, D.T.; Bymaster, F.P.; Engleman, E.A. Prozac (Fluoxetine, Lilly 110140), the First Selective Serotonin Uptake Inhibitor and an Antidepressant Drug: Twenty Years since Its First Publication. Life Sci. 1995, 57, 411–441. [Google Scholar] [CrossRef]
- McDonald, M.D. An AOP Analysis of Selective Serotonin Reuptake Inhibitors (SSRIs) for Fish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 197, 19–31. [Google Scholar] [CrossRef]
- Walker, F.R. A Critical Review of the Mechanism of Action for the Selective Serotonin Reuptake Inhibitors: Do These Drugs Possess Anti-Inflammatory Properties and How Relevant Is This in the Treatment of Depression? Neuropharmacology 2013, 67, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.T.; Bymaster, F.P.; Reid, L.R.; Threlkeld, P.G. Fluoxetine and Two Other Serotonin Uptake Inhibitors without Affinity for Neuronal Receptors. Biochem. Pharmacol. 1983, 32, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.; Bymaster, F.P.; Reid, L.R.; Mayle, D.A.; Krushinski, J.H.; Robertson, D.W. Norfluoxetine Enantiomers as Inhibitors of Serotonin Uptake in Rat Brain. Neuropsychopharmacology 1993, 8, 337–344. [Google Scholar] [CrossRef]
- Marken, P.A.; Stuart Munro, J. Selecting a Selective Serotonin Reuptake Inhibitor: Clinically Important Distinguishing Features. Prim. Care Companion J. Clin. Psychiatry 2000, 2, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Seth, R.; Jennings, A.L.; Bindman, J.; Phillips, J.; Bergmann, K. Combination Treatment with Noradrenalin and Serotonin Reuptake Inhibitors in Resistant Depression. Br. J. Psychiatry 1992, 161, 562–565. [Google Scholar] [CrossRef]
- Horst, W.D.; Preskorn, S.H. Mechanisms of Action and Clinical Characteristics of Three Atypical Antidepressants: Venlafaxine, Nefazodone, Bupropion. J. Affect. Disord. 1998, 51, 237–254. [Google Scholar] [CrossRef]
- Bacqué-cazenave, J.; Bharatiya, R.; Barrière, G.; Delbecque, J.P.; Bouguiyoud, N.; Di Giovanni, G.; Cattaert, D.; De Deurwaerdère, P. Serotonin in Animal Cognition and Behavior. Int. J. Mol. Sci. 2020, 21, 1649. [Google Scholar] [CrossRef]
- Cordes, S.P. Molecular Genetics of the Early Development of Hindbrain Serotonergic Neurons. Clin. Genet. 2005, 68, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, P.; Lillesaar, C. Probing the Diversity of Serotonin Neurons. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2382–2394. [Google Scholar] [CrossRef]
- Herculano, A.M.; Maximino, C. Serotonergic Modulation of Zebrafish Behavior: Towards a Paradox. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 55, 50–66. [Google Scholar] [CrossRef]
- Norton, W.H.J.; Folchert, A.; Bally-Cuif, L. Comparative Analysis of Serotonin Receptor (HTR1A/HTR1B Families) and Transporter (Slc6a4a/b) Gene Expression in the Zebrafish Brain. J. Comp. Neurol. 2008, 511, 521–542. [Google Scholar] [CrossRef]
- Schneider, H.; Fritzky, L.; Williams, J.; Heumann, C.; Yochum, M.; Pattar, K.; Noppert, G.; Mock, V.; Hawley, E. Cloning and Expression of a Zebrafish 5-HT 2C Receptor Gene. Gene 2012, 502, 108–117. [Google Scholar] [CrossRef]
- De Pedro, N.; Pinillos, M.L.; Valenciano, A.I.; Alonso-Bedate, M.; Delgado, M.J. Inhibitory Effect of Serotonin on Feeding Behavior in Goldfish: Involvement of CRF. Peptides 1998, 19, 505–511. [Google Scholar] [CrossRef]
- Pérez-Maceira, J.J.; Otero-Rodiño, C.; Mancebo, M.J.; Soengas, J.L.; Aldegunde, M. Food Intake Inhibition in Rainbow Trout Induced by Activation of Serotonin 5-HT2C Receptors Is Associated with Increases in POMC, CART and CRF MRNA Abundance in Hypothalamus. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2016, 186, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.L.; Fornal, C.A. Serotonin and Motor Activity. Curr. Opin. Neurobiol. 1997, 7, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Iwamatsu, T.; Toya, Y.; Sakai, N.; Terada, Y.; Nagata, R.; Nagahama, Y. Effect of 5-Hydroxytryptamine on Steroidogenesis and Oocyte Maturation in Pre-ovulatory Follicles of the Medaka Oryzias latipes Medaka/Steroidogenesis/5-hydroxytryptamine/Foillicle/Oocyte Maturation. Dev. Growth Differ. 1993, 35, 625–630. [Google Scholar] [CrossRef]
- Khan, I.A.; Thomas, P. Stimulatory Effects of Serotonin on Maturational Gonadotropin Release in the Atlantic Croaker, Micropogonias Undulatus. Gen. Comp. Endocrinol. 1992, 88, 388–396. [Google Scholar] [CrossRef]
- Hauser, T.U.; Eldar, E.; Purg, N.; Moutoussis, M.; Dolan, R.J. Distinct Roles of Dopamine and Noradrenaline in Incidental Memory. J. Neurosci. 2019, 39, 7715–7721. [Google Scholar] [CrossRef] [PubMed]
- Haller, J.; Makara, G.B.; Kruk, M.R. Catecholaminergic Involvement in the Control of Aggression: Hormones, the Peripheral Sympathetic, and Central Noradrenergic Systems. Neurosci. Biobehav. Rev. 1997, 22, 85–97. [Google Scholar] [CrossRef]
- Pertovaara, A. Noradrenergic Pain Modulation. Prog. Neurobiol. 2006, 80, 53–83. [Google Scholar] [CrossRef] [PubMed]
- Bernier, N.J.; McKendry, J.E.; Perry, S.F. Blood Pressure Regulation during Hypotension in Two Teleost Species: Differential Involvement of the Renin–Angiotensin and Adrenergic Systems. J. Exp. Biol. 1999, 202, 1677–1690. [Google Scholar] [CrossRef]
- Sharples, S.A.; Koblinger, K.; Humphreys, J.M.; Whelan, P.J. Dopamine: A Parallel Pathway for the Modulation of Spinal Locomotor Networks. Front. Neural Circuits 2014, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Nieoullon, A. Dopamine and the Regulation of Cognition and Attention. Prog. Neurobiol. 2002, 67, 53–83. [Google Scholar] [CrossRef]
- Thörnqvist, P.O.; McCarrick, S.; Ericsson, M.; Roman, E.; Winberg, S. Bold Zebrafish (Danio rerio) Express Higher Levels of Delta Opioid and Dopamine D2 Receptors in the Brain Compared to Shy Fish. Behav. Brain Res. 2019, 359, 927–934. [Google Scholar] [CrossRef]
- Messias, J.P.M.; Santos, T.P.; Pinto, M.; Soares, M.C. Stimulation of Dopamine D1 Receptor Improves Learning Capacity in Cooperating Cleaner Fish. Proc. R. Soc. B Biol. Sci. 2016, 283, 20152272. [Google Scholar] [CrossRef]
- Naderi, M.; Jamwal, A.; Chivers, D.P.; Niyogi, S. Modulatory Effects of Dopamine Receptors on Associative Learning Performance in Zebrafish (Danio rerio). Behav. Brain Res. 2016, 303, 109–119. [Google Scholar] [CrossRef]
- Boehmier, W.; Obrecht-Pflumio, S.; Canfield, V.; Thisse, C.; Thisse, B.; Levenson, R. Evolution and Expression of D2 and D3 Dopamine Receptor Genes in Zebrafish. Dev. Dyn. 2004, 230, 481–493. [Google Scholar] [CrossRef]
- Boehmler, W.; Carr, T.; Thisse, C.; Thisse, B.; Canfield, V.A.; Levenson, R. D4 Dopamine Receptor Genes of Zebrafish and Effects of the Antipsychotic Clozapine on Larval Swimming Behaviour. Genes Brain Behav. 2007, 6, 155–166. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yamamoto, H.; Sekizawa, J.; Kondo, T.; Hirai, N.; Tatarazako, N. The Effects of PH on Fluoxetine in Japanese Medaka (Oryzias latipes): Acute Toxicity in Fish Larvae and Bioaccumulation in Juvenile Fish. Chemosphere 2008, 70, 865–873. [Google Scholar] [CrossRef]
- Arnnok, P.; Singh, R.R.; Burakham, R.; Pérez-Fuentetaja, A.; Aga, D.S. Selective Uptake and Bioaccumulation of Antidepressants in Fish from Effluent-Impacted Niagara River. Environ. Sci. Technol. 2017, 51, 10652–10662. [Google Scholar] [CrossRef]
- Bittner, L.; Klüver, N.; Henneberger, L.; Mühlenbrink, M.; Zarfl, C.; Escher, B.I. Combined Ion-Trapping and Mass Balance Models to Describe the PH-Dependent Uptake and Toxicity of Acidic and Basic Pharmaceuticals in Zebrafish Embryos (Danio rerio). Environ. Sci. Technol. 2019, 53, 7877–7886. [Google Scholar] [CrossRef]
- Escher, B.I.; Abagyan, R.; Embry, M.; Klüver, N.; Redman, A.D.; Zarfl, C.; Parkerton, T.F. Recommendations for Improving Methods and Models for Aquatic Hazard Assessment of Ionizable Organic Chemicals. Environ. Toxicol. Chem. 2020, 39, 269–286. [Google Scholar] [CrossRef]
- Lajeunesse, A.; Gagnon, C.; Gagné, F.; Louis, S.; Čejka, P.; Sauvé, S. Distribution of Antidepressants and Their Metabolites in Brook Trout Exposed to Municipal Wastewaters before and after Ozone Treatment—Evidence of Biological Effects. Chemosphere 2011, 83, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Grabicova, K.; Grabic, R.; Fedorova, G.; Fick, J.; Cerveny, D.; Kolarova, J.; Turek, J.; Zlabek, V.; Randak, T. Bioaccumulation of Psychoactive Pharmaceuticals in Fish in an Effluent Dominated Stream. Water Res. 2017, 124, 654–662. [Google Scholar] [CrossRef]
- David, A.; Lange, A.; Tyler, C.R.; Hill, E.M. Concentrating Mixtures of Neuroactive Pharmaceuticals and Altered Neurotransmitter Levels in the Brain of Fish Exposed to a Wastewater Effluent. Sci. Total Environ. 2018, 621, 782–790. [Google Scholar] [CrossRef]
- Grabicová, K.; Grabic, R.; Fedorova, G.; Vojs Staňová, A.; Bláha, M.; Randák, T.; Brooks, B.W.; Žlábek, V. Water Reuse and Aquaculture: Pharmaceutical Bioaccumulation by Fish during Tertiary Treatment in a Wastewater Stabilization Pond. Environ. Pollut. 2020, 267, 115593. [Google Scholar] [CrossRef] [PubMed]
- Melvin, S.D. Effect of Antidepressants on Circadian Rhythms in Fish: Insights and Implications Regarding the Design of Behavioural Toxicity Tests. Aquat. Toxicol. 2017, 182, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimmons, P.N.; Fernandez, J.D.; Hoffman, A.D.; Butterworth, B.C.; Nichols, J.W. Branchial Elimination of Superhydrophobic Organic Compounds by Rainbow Trout (Oncorhynchus mykiss). Aquat. Toxicol. 2001, 55, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Margiotta-Casaluci, L.; Owen, S.F.; Cumming, R.I.; De Polo, A.; Winter, M.J.; Panter, G.H.; Rand-Weaver, M.; Sumpter, J.P. Quantitative Cross-Species Extrapolation between Humans and Fish: The Case of the Anti-Depressant Fluoxetine. PLoS ONE 2014, 9, e110467. [Google Scholar] [CrossRef]
- World Health Organization. WHO Pharmaceuticals in Drinking Water; WHO Press: World Health Organization: Geneva, Switzerland, 2012; ISBN 978 9241502085. [Google Scholar]
- Wang, F.; Xiang, L.; Sze-Yin Leung, K.; Elsner, M.; Zhang, Y.; Guo, Y.; Pan, B.; Sun, H.; An, T.; Ying, G.; et al. Emerging Contaminants: A One Health Perspective. Innovation 2024, 5, 100612. [Google Scholar] [CrossRef] [PubMed]
- Marchocki, Z.; Russell, N.E.; Donoghue, K.O. Selective Serotonin Reuptake Inhibitors and Pregnancy: A Review of Maternal, Fetal and Neonatal Risks and Benefits. Obstet. Med. 2013, 6, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Toh, S.; Mitchell, A.A.; Louik, C.; Werler, M.M.; Chambers, C.D.; Hernández-Díaz, S. Antidepressant Use during Pregnancy and the Risk of Preterm Delivery and Fetal Growth Restriction. J. Clin. Psychopharmacol. 2009, 29, 555–560. [Google Scholar] [CrossRef]
- El Marroun, H.; Jaddoe, V.W.V.; Hudziak, J.J.; Roza, S.J.; Steegers, E.A.P.; Hofman, A.; Verhulst, F.C.; White, T.J.H.; Stricker, B.H.C.; Tiemeier, H. Maternal Use of Selective Serotonin Reuptake Inhibitors, Fetal Growth, and Risk of Adverse Birth Outcomes. Arch. Gen. Psychiatry 2012, 69, 706–714. [Google Scholar] [CrossRef]
- Rommel, A.-S.; Momen, N.C.; Molenaar, N.M.; Agerbo, E.; Bergink, V.; Munk-Olsen, T.; Liu, X. Antidepressant Use during Pregnancy and Risk of Adverse Neonatal Outcomes: A Comprehensive Investigation of Previously Identified Associations. Acta Psychiatr. Scand. 2022, 145, 544–556. [Google Scholar] [CrossRef]
- Melnyk-Lamont, N.; Best, C.; Gesto, M.; Vijayan, M.M. The Antidepressant Venlafaxine Disrupts Brain Monoamine Levels and Neuroendocrine Responses to Stress in Rainbow Trout. Environ. Sci. Technol. 2014, 48, 13434–13442. [Google Scholar] [CrossRef]
- Yang, M.; Qiu, W.; Chen, J.; Zhan, J.; Pan, C.; Lei, X.; Wu, M. Growth Inhibition and Coordinated Physiological Regulation of Zebrafish (Danio rerio) Embryos upon Sublethal Exposure to Antidepressant Amitriptyline. Aquat. Toxicol. 2014, 151, 68–76. [Google Scholar] [CrossRef]
- Pelli, M.; Connaughton, V.P. Chronic Exposure to Environmentally-Relevant Concentrations of Fluoxetine (Prozac) Decreases Survival, Increases Abnormal Behaviors, and Delays Predator Escape Responses in Guppies. Chemosphere 2015, 139, 202–209. [Google Scholar] [CrossRef]
- Martin, J.M.; Saaristo, M.; Bertram, M.G.; Lewis, P.J.; Coggan, T.L.; Clarke, B.O.; Wong, B.B.M. The Psychoactive Pollutant Fluoxetine Compromises Antipredator Behaviour in Fish. Environ. Pollut. 2017, 222, 592–599. [Google Scholar] [CrossRef]
- Fuxe, K.; Ungerstedt, U. Histochemical studies on the effect of (+)-amphetamine, drugs of the imipramine group and tryptamine on central catecholamine and 5-hydroxytryptamine neurons after intraventricular injection of catecholamines and 5-hydroxytryptamine. Eur. J. Pharmacol. 1968, 4, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Liu, L.; Zhong, Z.; Wang, H.; Lin, S.; Shang, J. Risk of Prenatal Depression and Stress Treatment: Alteration on Serotonin System of Offspring through Exposure to Fluoxetine. Sci. Rep. 2016, 6, 33822. [Google Scholar] [CrossRef] [PubMed]
- Parolini, M.; Ghilardi, A.; De Felice, B.; Del Giacco, L. Environmental Concentration of Fluoxetine Disturbs Larvae Behavior and Increases the Defense Response at Molecular Level in Zebrafish (Danio rerio). Environ. Sci. Pollut. Res. 2019, 26, 34943–34952. [Google Scholar] [CrossRef]
- Sehonova, P.; Svobodova, Z.; Dolezelova, P.; Vosmerova, P.; Faggio, C. Effects of Waterborne Antidepressants on Non-Target Animals Living in the Aquatic Environment: A Review. Sci. Total Environ. 2018, 631–632, 789–794. [Google Scholar] [CrossRef]
- Duarte, I.A.; Reis-Santos, P.; Novais, S.C.; Rato, L.D.; Lemos, M.F.L.; Freitas, A.; Pouca, A.S.V.; Barbosa, J.; Cabral, H.N.; Fonseca, V.F. Depressed, Hypertense and Sore: Long-Term Effects of Fluoxetine, Propranolol and Diclofenac Exposure in a Top Predator Fish. Sci. Total Environ. 2020, 712, 136564. [Google Scholar] [CrossRef]
- Wu, M.; Liu, S.; Hu, L.; Qu, H.; Pan, C.; Lei, P.; Shen, Y.; Yang, M. Global Transcriptomic Analysis of Zebrafish in Response to Embryonic Exposure to Three Antidepressants, Amitriptyline, Fluoxetine and Mianserin. Aquat. Toxicol. 2017, 192, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Fraher, D.; Hodge, J.M.; Collier, F.M.; McMillan, J.S.; Kennedy, R.L.; Ellis, M.; Nicholson, G.C.; Walder, K.; Dodd, S.; Berk, M.; et al. Citalopram and Sertraline Exposure Compromises Embryonic Bone Development. Mol. Psychiatry 2016, 21, 656–664. [Google Scholar] [CrossRef]
- Bataineh, H.N.; Daradka, T. Effects of Long-Term Use of Fluoxetin on Fertility Parameters in Adults Male Rats. Neuroendocrinol. Lett. 2007, 28, 321–325. [Google Scholar]
- Tanrikut, C.; Feldman, A.S.; Altemus, M.; Paduch, D.A.; Schlegel, P.N. Adverse Effect of Paroxetine on Sperm. Fertil. Steril. 2010, 94, 1021–1026. [Google Scholar] [CrossRef]
- Lister, A.; Regan, C.; Van Zwol, J.; Van Der Kraak, G. Inhibition of Egg Production in Zebrafish by Fluoxetine and Municipal Effluents: A Mechanistic Evaluation. Aquat. Toxicol. 2009, 95, 320–329. [Google Scholar] [CrossRef]
- Galus, M.; Kirischian, N.; Higgins, S.; Purdy, J.; Chow, J.; Rangaranjan, S.; Li, H.; Metcalfe, C.; Wilson, J.Y. Chronic, Low Concentration Exposure to Pharmaceuticals Impacts Multiple Organ Systems in Zebrafish. Aquat. Toxicol. 2013, 132–133, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, J.; Klaper, R. Environmental Concentrations of the Selective Serotonin Reuptake Inhibitor Fluoxetine Impact Specific Behaviors Involved in Reproduction, Feeding and Predator Avoidance in the Fish Pimephales Promelas (Fathead minnow). Aquat. Toxicol. 2014, 151, 77–83. [Google Scholar] [CrossRef]
- Martin, J.M.; Bertram, M.G.; Saaristo, M.; Ecker, T.E.; Hannington, S.L.; Tanner, J.L.; Michelangeli, M.; O’Bryan, M.K.; Wong, B.B.M. Impact of the Widespread Pharmaceutical Pollutant Fluoxetine on Behaviour and Sperm Traits in a Freshwater Fish. Sci. Total Environ. 2019, 650, 1771–1778. [Google Scholar] [CrossRef]
- Bertram, M.G.; Ecker, T.E.; Wong, B.B.M.; O’Bryan, M.K.; Baumgartner, J.B.; Martin, J.M.; Saaristo, M. The Antidepressant Fluoxetine Alters Mechanisms of Pre- and Post-Copulatory Sexual Selection in the Eastern Mosquitofish (Gambusia holbrooki). Environ. Pollut. 2018, 238, 238–247. [Google Scholar] [CrossRef]
- Stanley, J.K.; Ramirez, A.J.; Chambliss, C.K.; Brooks, B.W. Enantiospecific Sublethal Effects of the Antidepressant Fluoxetine to a Model Aquatic Vertebrate and Invertebrate. Chemosphere 2007, 69, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.T.; Reid, L.R.; Threlkeld, P.G. Suppression of Food Intake in Rats by Fluoxetine: Comparison of Enantiomers and Effects of Serotonin Antagonists. Pharmacol. Biochem. Behav. 1988, 31, 475–479. [Google Scholar] [CrossRef]
- Mennigen, J.A.; Harris, E.A.; Chang, J.P.; Moon, T.W.; Trudeau, V.L. Fluoxetine Affects Weight Gain and Expression of Feeding Peptides in the Female Goldfish Brain. Regul. Pept. 2009, 155, 99–104. [Google Scholar] [CrossRef]
- Kellner, M.; Porseryd, T.; Porsch-Hällström, I.; Borg, B.; Roufidou, C.; Olsén, K.H. Developmental Exposure to the SSRI Citalopram Causes Long-Lasting Behavioural Effects in the Three-Spined Stickleback (Gasterosteus aculeatus). Ecotoxicology 2018, 27, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding Behavioral and Physiological Phenotypes of Stress and Anxiety in Zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef]
- Maximino, C.; de Brito, T.M.; da Silva Batista, A.W.; Herculano, A.M.; Morato, S.; Gouveia, A. Measuring Anxiety in Zebrafish: A Critical Review. Behav. Brain Res. 2010, 214, 157–171. [Google Scholar] [CrossRef]
- Ansai, S.; Hosokawa, H.; Maegawa, S.; Kinoshita, M. Chronic Fluoxetine Treatment Induces Anxiolytic Responses and Altered Social Behaviors in Medaka, Oryzias latipes. Behav. Brain Res. 2016, 303, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Maulvault, A.L.; Santos, L.H.M.L.M.; Paula, J.R.; Camacho, C.; Pissarra, V.; Fogaça, F.; Barbosa, V.; Alves, R.; Ferreira, P.P.; Barceló, D.; et al. Differential Behavioural Responses to Venlafaxine Exposure Route, Warming and Acidification in Juvenile Fish (Argyrosomus regius). Sci. Total Environ. 2018, 634, 1136–1147. [Google Scholar] [CrossRef]
- Giacomini, A.C.V.V.; Piassetta, A.S.; Genario, R.; Bonan, C.D.; Piato, A.; Barcellos, L.J.G.; de Abreu, M.S. Tryptophan Alleviates Neuroendocrine and Behavioral Responses to Stress in Zebrafish. Behav. Brain Res. 2020, 378, 112264. [Google Scholar] [CrossRef]
- Treit, D.; Fundytus, M. Thigmotaxis as a Test for Anxiolytic Activity in Rats. Pharmacol. Biochem. Behav. 1988, 31, 959–962. [Google Scholar] [CrossRef]
- Maximino, C.; Marques De Brito, T.; De Mattos Dias, C.A.G.; Gouveia, A.; Morato, S. Scototaxis as Anxiety-like Behavior in Fish. Nat. Protoc. 2010, 5, 221–228. [Google Scholar] [CrossRef]
- Maximino, C.; da Silva, A.W.B.; Gouveia, A.; Herculano, A.M. Pharmacological Analysis of Zebrafish (Danio rerio) Scototaxis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Marcon, M.; Herrmann, A.P.; Mocelin, R.; Rambo, C.L.; Koakoski, G.; Abreu, M.S.; Conterato, G.M.M.; Kist, L.W.; Bogo, M.R.; Zanatta, L.; et al. Prevention of Unpredictable Chronic Stress-Related Phenomena in Zebrafish Exposed to Bromazepam, Fluoxetine and Nortriptyline. Psychopharmacology 2016, 233, 3815–3824. [Google Scholar] [CrossRef]
- Theodoridi, A.; Tsalafouta, A.; Pavlidis, M. Acute Exposure to Fluoxetine Alters Aggressive Behavior of Zebrafish and Expression of Genes Involved in Serotonergic System Regulation. Front. Neurosci. 2017, 11, 223. [Google Scholar] [CrossRef] [PubMed]
- Chiffre, A.; Clérandeau, C.; Dwoinikoff, C.; Le Bihanic, F.; Budzinski, H.; Geret, F.; Cachot, J. Psychotropic Drugs in Mixture Alter Swimming Behaviour of Japanese Medaka (Oryzias latipes) Larvae above Environmental Concentrations. Environ. Sci. Pollut. Res. 2016, 23, 4964–4977. [Google Scholar] [CrossRef]
- Saaristo, M.; McLennan, A.; Johnstone, C.P.; Clarke, B.O.; Wong, B.B.M. Impacts of the Antidepressant Fluoxetine on the Anti-Predator Behaviours of Wild Guppies (Poecilia reticulata). Aquat. Toxicol. 2017, 183, 38–45. [Google Scholar] [CrossRef]
- Xie, Z.; Lu, G.; Li, S.; Nie, Y.; Ma, B.; Liu, J. Behavioral and Biochemical Responses in Freshwater Fish Carassius Auratus Exposed to Sertraline. Chemosphere 2015, 135, 146–155. [Google Scholar] [CrossRef]
- Porseryd, T.; Kellner, M.; Reyhanian Caspillo, N.; Volkova, K.; Elabbas, L.; Ullah, S.; Olsén, H.; Dinnétz, P.; Porsch Hällström, I. Combinatory Effects of Low Concentrations of 17A-Etinylestradiol and Citalopram on Non-Reproductive Behavior in Adult Zebrafish (Danio rerio). Aquat. Toxicol. 2017, 193, 9–17. [Google Scholar] [CrossRef]
- Lepage, O.; Larson, E.T.; Mayer, I.; Winberg, S. Serotonin, but Not Melatonin, Plays a Role in Shaping Dominant-Subordinate Relationships and Aggression in Rainbow Trout. Horm. Behav. 2005, 48, 233–242. [Google Scholar] [CrossRef]
- Perreault, H.A.N.; Semsar, K.; Godwin, J. Fluoxetine Treatment Decreases Territorial Aggression in a Coral Reef Fish. Physiol. Behav. 2003, 79, 719–724. [Google Scholar] [CrossRef]
- McCallum, E.S.; Bose, A.P.H.; Warriner, T.R.; Balshine, S. An Evaluation of Behavioural Endpoints: The Pharmaceutical Pollutant Fluoxetine Decreases Aggression across Multiple Contexts in Round Goby (Neogobius melanostomus). Chemosphere 2017, 175, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.J. Effects of Fluoxetine on the Swimming and Behavioural Responses of the Arabian Killifish. Ecotoxicology 2013, 22, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Norton, W.H.J.; Stumpenhorst, K.; Faus-Kessler, T.; Folchert, A.; Rohner, N.; Harris, M.P.; Callebert, J.; Bally-Cuif, L. Modulation of Fgfr1a Signaling in Zebrafish Reveals a Genetic Basis for the Aggression-Boldness Syndrome. J. Neurosci. 2011, 31, 13796–13807. [Google Scholar] [CrossRef] [PubMed]
- Kellner, M.; Olsén, K.H. Divergent Response to the SSRI Citalopram in Male and Female Three-Spine Sticklebacks (Gasterosteus aculeatus). Arch. Environ. Contam. Toxicol. 2020, 79, 478–487. [Google Scholar] [CrossRef]
- McDonald, M.D.; Gonzalez, A.; Sloman, K.A. Higher Levels of Aggression Are Observed in Socially Dominant Toadfish Treated with the Selective Serotonin Reuptake Inhibitor, Fluoxetine. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 107–112. [Google Scholar] [CrossRef]
- Greaney, N.E.; Mannion, K.L.; Dzieweczynski, T.L. Signaling on Prozac: Altered Audience Effects on Male-Male Interactions after Fluoxetine Exposure in Siamese Fighting Fish. Behav. Ecol. Sociobiol. 2015, 69, 1925–1932. [Google Scholar] [CrossRef]
- Jirtle, R.L.; Skinner, M.K. Environmental Epigenomics and Disease Susceptibility. Nat. Rev. Genet. 2007, 8, 253–262. [Google Scholar] [CrossRef]
- Vera-Chang, M.N.; St-Jacques, A.D.; Gagné, R.; Martyniuk, C.J.; Yauk, C.L.; Moon, T.W.; Trudeau, V.L. Transgenerational Hypocortisolism and Behavioral Disruption Are Induced by the Antidepressant Fluoxetine in Male Zebrafish Danio rerio. Proc. Natl. Acad. Sci. USA. 2018, 115, E12435–E12442. [Google Scholar] [CrossRef]
- Vera-Chang, M.N.; Moon, T.W.; Trudeau, V.L. Cortisol Disruption and Transgenerational Alteration in the Expression of Stress-Related Genes in Zebrafish Larvae Following Fluoxetine Exposure. Toxicol. Appl. Pharmacol. 2019, 382, 114742. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Abdel-Shafy, H.I.; Mansour, M.S.M. Removal of Pharmaceutical Compounds from Urine via Chemical Coagulation by Green Synthesized ZnO-Nanoparticles Followed by Microfiltration for Safe Reuse. Arab. J. Chem. 2019, 12, 4074–4083. [Google Scholar] [CrossRef]
- Petrie, B.; McAdam, E.J.; Hassard, F.; Stephenson, T.; Lester, J.N.; Cartmell, E. Diagnostic Investigation of Steroid Estrogen Removal by Activated Sludge at Varying Solids Retention Time. Chemosphere 2014, 113, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Georgin, J.; Meili, L.; Franco, D. A Review of the Application of Low-Cost Adsorbents as an Alternative Method for Biosorption of Contaminants Present in Water. Lat. Am. Dev. Energy. Eng. 2023, 4, 1–20. [Google Scholar] [CrossRef]
- Ibáñez, M.; Gracia-Lor, E.; Bijlsma, L.; Morales, E.; Pastor, L.; Hernández, F. Removal of Emerging Contaminants in Sewage Water Subjected to Advanced Oxidation with Ozone. J. Hazard. Mater. 2013, 260, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Rosal, R.; Rodríguez, A.; Perdigón-Melón, J.A.; Petre, A.; García-Calvo, E.; Gómez, M.J.; Agüera, A.; Fernández-Alba, A.R. Occurrence of Emerging Pollutants in Urban Wastewater and Their Removal through Biological Treatment Followed by Ozonation. Water Res. 2010, 44, 578–588. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as Emerging Contaminants and Their Removal from Water. A Review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef]
- Jung, Y.J.; Kim, W.G.; Yoon, Y.; Kang, J.W.; Hong, Y.M.; Kim, H.W. Removal of Amoxicillin by UV and UV/H2O2 Processes. Sci. Total Environ. 2012, 420, 160–167. [Google Scholar] [CrossRef]
- Keen, O.S.; Baik, S.; Linden, K.G.; Aga, D.S.; Love, N.G. Enhanced Biodegradation of Carbamazepine after UV/H2O 2 Advanced Oxidation. Environ. Sci. Technol. 2012, 46, 6222–6227. [Google Scholar] [CrossRef]
- Yonar, T.; Kestioglu, K.; Azbar, N. Treatability Studies on Domestic Wastewater Using UV/H2O2 Process. Appl. Catal. B Environ. 2006, 67, 223–228. [Google Scholar] [CrossRef]
- Matamoros, V.; Gutiérrez, R.; Ferrer, I.; García, J.; Bayona, J.M. Capability of Microalgae-Based Wastewater Treatment Systems to Remove Emerging Organic Contaminants: A Pilot-Scale Study. J. Hazard. Mater. 2015, 288, 34–42. [Google Scholar] [CrossRef]
- Taseli, B.K.; Gokcay, C.F. Biological Treatment of Paper Pulping Effluents by Using a Fungal Reactor. Water Sci. Technol. 1999, 40, 93–99. [Google Scholar] [CrossRef]
- Secondes, M.F.N.; Naddeo, V.; Belgiorno, V.; Ballesteros, F. Removal of Emerging Contaminants by Simultaneous Application of Membrane Ultrafiltration, Activated Carbon Adsorption, and Ultrasound Irradiation. J. Hazard. Mater. 2014, 264, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mozaz, S.; Ricart, M.; Köck-Schulmeyer, M.; Guasch, H.; Bonnineau, C.; Proia, L.; de Alda, M.L.; Sabater, S.; Barceló, D. Pharmaceuticals and Pesticides in Reclaimed Water: Efficiency Assessment of a Microfiltration-Reverse Osmosis (MF-RO) Pilot Plant. J. Hazard. Mater. 2015, 282, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.A.; Yonar, T.; Zakaria, K. The Electrochemical Generation of Ozone: A Review. Ozone Sci. Eng. 2013, 35, 149–167. [Google Scholar] [CrossRef]
- Hernández Martínez, S.A.; Melchor-Martínez, E.M.; González-González, R.B.; Sosa-Hernández, J.E.; Araújo, R.G.; Rodríguez-Hernández, J.A.; Barceló, D.; Parra-Saldívar, R.; Iqbal, H.M.N. Environmental Concerns and Bioaccumulation of Psychiatric Drugs in Water Bodies—Conventional versus Biocatalytic Systems of Mitigation. Environ. Res. 2023, 229, 115892. [Google Scholar] [CrossRef]
- Leyva-Díaz, J.C.; Batlles-Delafuente, A.; Molina-Moreno, V.; Molina, J.S.; Belmonte-Ureña, L.J. Removal of Pharmaceuticals from Wastewater: Analysis of the Past and Present Global Research Activities. Water 2021, 13, 2353. [Google Scholar] [CrossRef]
- Celiz, M.D.; Tso, J.; Aga, D.S.; Risks, E. Pharmaceutical Metabolites in the Environment: Analytical Challenges and Ecological Risks. Environ. Toxicol. Chem. 2009, 28, 2473–2484. [Google Scholar] [CrossRef]
- Escher, B.I.; Fenner, K. Recent Advances in Environmental Risk Assessment of Transformation Products. Environ. Sci. Technol. 2011, 45, 3835–3847. [Google Scholar] [CrossRef]
- Tong, D.S.; Zhou, C.H.; Lu, Y.; Yu, H.; Zhang, G.F.; Yu, W.H. Adsorption of Acid Red G Dye on Octadecyl Trimethylammonium Montmorillonite. Appl. Clay Sci. 2010, 50, 427–431. [Google Scholar] [CrossRef]
- Katsigiannis, A.; Noutsopoulos, C.; Mantziaras, J.; Gioldasi, M. Removal of Emerging Pollutants through Granular Activated Carbon. Chem. Eng. J. 2015, 280, 49–57. [Google Scholar] [CrossRef]
- Gazigil, L.; Er, E.; Yonar, T. Determination of the Optimum Conditions for Electrochemical Regeneration of Exhausted Activated Carbon. Diam. Relat. Mater. 2023, 133, 109741. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Utilization of Agro-Industrial and Municipal Waste Materials as Potential Adsorbents for Water Treatment-A Review. Chem. Eng. J. 2010, 157, 277–296. [Google Scholar] [CrossRef]
- Dagnino, S.; Gomez, E.; Picot, B.; Cavaillès, V.; Casellas, C.; Balaguer, P.; Fenet, H. Estrogenic and AhR Activities in Dissolved Phase and Suspended Solids from Wastewater Treatment Plants. Sci. Total Environ. 2010, 408, 2608–2615. [Google Scholar] [CrossRef] [PubMed]
- Alfonso-Muniozguren, P.; Serna-Galvis, E.A.; Bussemaker, M.; Torres-Palma, R.A.; Lee, J. A Review on Pharmaceuticals Removal from Waters by Single and Combined Biological, Membrane Filtration and Ultrasound Systems. Ultrason. Sonochem. 2021, 76, 105656. [Google Scholar] [CrossRef]
- Maršálek, R.; Švidrnoch, M. The Adsorption of Amitriptyline and Nortriptyline on Activated Carbon, Diosmectite and Titanium Dioxide. Environ. Chall. 2020, 1, 100005. [Google Scholar] [CrossRef]
- Choi, J.W.; Bediako, J.K.; Zhao, Y.; Lin, S.; Sarkar, A.K.; Han, M.; Song, M.H.; Cho, C.W.; Yun, Y.S. Adsorptive Removal of Cationic Tricyclic Antidepressants Using Cation-Exchange Resin. Environ. Sci. Pollut. Res. 2020, 27, 24760–24771. [Google Scholar] [CrossRef]
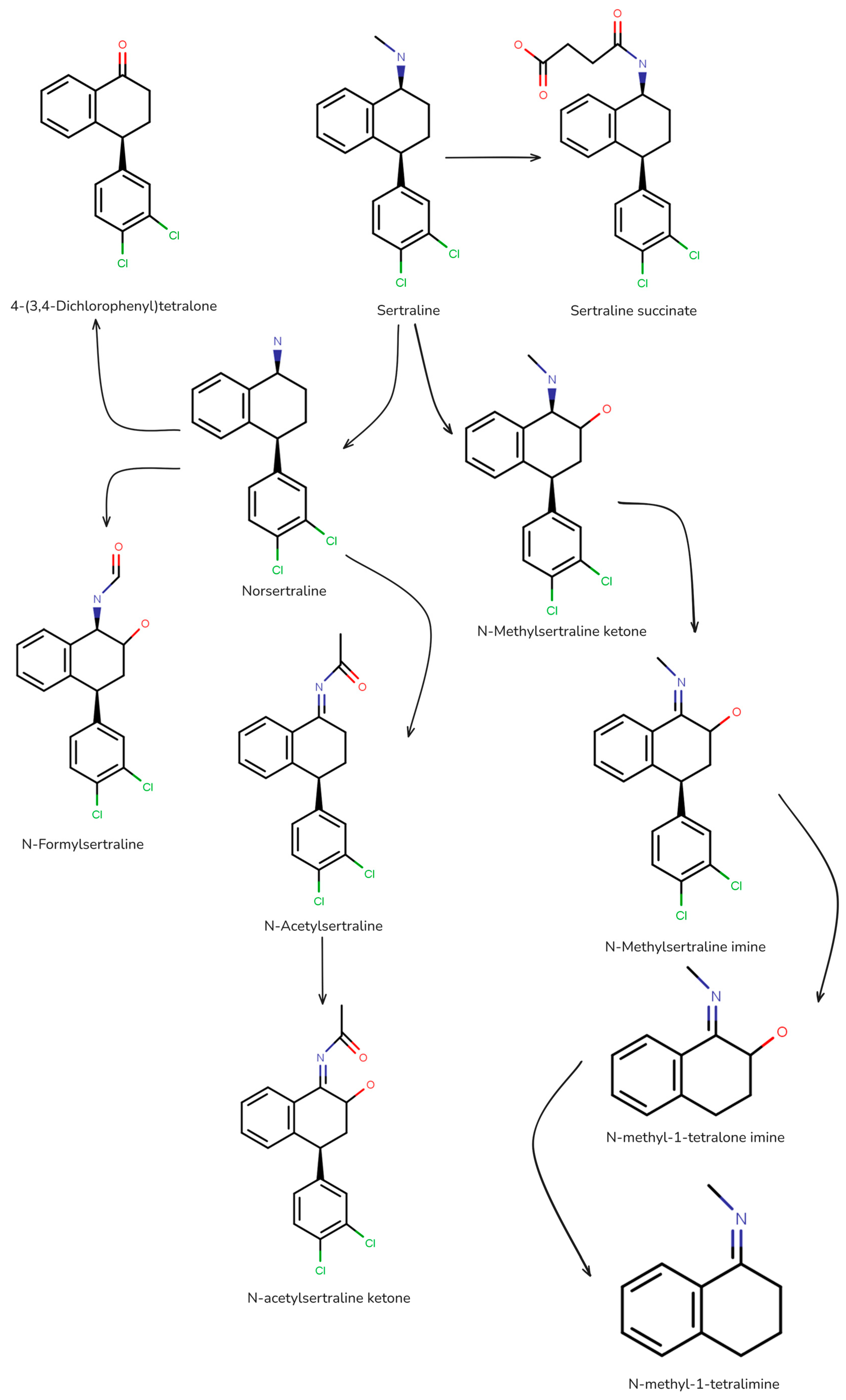
- Gornik, T.; Kovacic, A.; Heath, E.; Hollender, J.; Kosjek, T. Biotransformation Study of Antidepressant Sertraline and Its Removal during Biological Wastewater Treatment. Water Res. 2020, 181, 115864. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.H.; Jiang, W.T.; Li, Z.; Kuo, C.Y.; Jean, J.S.; Chen, W.R.; Lv, G. Mechanism of Amitriptyline Adsorption on Ca-Montmorillonite (SAz-2). J. Hazard. Mater. 2014, 277, 44–52. [Google Scholar] [CrossRef]
- Toyoguchi, T.; Ebihara, M.; Ojima, F.; Hosoya, J.; Nakagawa, Y. In Vitro Study of the Adsorption Characteristics of Drugs. Biol. Pharm. Bull. 2005, 28, 841–844. [Google Scholar] [CrossRef]
- Tsai, Y.L.; Chang, P.H.; Gao, Z.Y.; Xu, X.Y.; Chen, Y.H.; Wang, Z.H.; Chen, X.Y.; Yang, Z.Y.; Wang, T.H.; Jean, J.S.; et al. Amitriptyline Removal Using Palygorskite Clay. Chemosphere 2016, 155, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Landry, K.A.; Boyer, T.H. Diclofenac Removal in Urine Using Strong-Base Anion Exchange Polymer Resins. Water Res. 2013, 47, 6432–6444. [Google Scholar] [CrossRef]
- El Gamal, M.; Mousa, H.A.; El-Naas, M.H.; Zacharia, R.; Judd, S. Bio-Regeneration of Activated Carbon: A Comprehensive Review. Sep. Purif. Technol. 2018, 197, 345–359. [Google Scholar] [CrossRef]
- Larasati, A.; Fowler, G.D.; Graham, N.J.D. Insights into Chemical Regeneration of Activated Carbon for Water Treatment. J. Environ. Chem. Eng. 2021, 9, 105555. [Google Scholar] [CrossRef]
- Koçoğlu, E.S.; Bakırdere, S.; Keyf, S. A Novel Liquid-Liquid Extraction for the Determination of Sertraline in Tap Water and Waste Water at Trace Levels by GC-MS. Bull. Environ. Contam. Toxicol. 2017, 99, 354–359. [Google Scholar] [CrossRef]
- Ikeda, M. Public Health Problems of Organic Solvents. Toxicol. Lett. 1992, 64–65, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Racovita, R.C.; Ciuca, M.D.; Catana, D.; Comanescu, C.; Ciocirlan, O. Microemulsions of Nonionic Surfactant with Water and Various Homologous Esters: Preparation, Phase Transitions, Physical Property Measurements, and Application for Extraction of Tricyclic Antidepressant Drugs from Aqueous Media. Nanomaterials 2023, 13, 2311. [Google Scholar] [CrossRef]
- Ge, D.; Lee, H.K. Ionic Liquid Based Dispersive Liquid-Liquid Microextraction Coupled with Micro-Solid Phase Extraction of Antidepressant Drugs from Environmental Water Samples. J. Chromatogr. A 2013, 1317, 217–222. [Google Scholar] [CrossRef]
- Zawadzki, M.; e Silva, F.A.; Domańska, U.; Coutinho, J.A.P.; Ventura, S.P.M. Recovery of an Antidepressant from Pharmaceutical Wastes Using Ionic Liquid-Based Aqueous Biphasic Systems. Green Chem. 2016, 18, 3527–3536. [Google Scholar] [CrossRef]
- Nassar, R.; Trivella, A.; Mokh, S.; Al-Iskandarani, M.; Budzinski, H.; Mazellier, P. Photodegradation of Sulfamethazine, Sulfamethoxypiridazine, Amitriptyline, and Clomipramine Drugs in Aqueous Media. J. Photochem. Photobiol. A Chem. 2017, 336, 176–182. [Google Scholar] [CrossRef]
- Khaleel, N.D.H.; Mahmoud, W.M.M.; Olsson, O.; Kümmerer, K. Initial Fate Assessment of Teratogenic Drug Trimipramine and Its Photo-Transformation Products—Role of PH, Concentration and Temperature. Water Res. 2017, 108, 197–211. [Google Scholar] [CrossRef]
- Gros, M.; Williams, M.; Llorca, M.; Rodriguez-Mozaz, S.; Barceló, D.; Kookana, R.S. Photolysis of the Antidepressants Amisulpride and Desipramine in Wastewaters: Identification of Transformation Products Formed and Their Fate. Sci. Total Environ. 2015, 530–531, 434–444. [Google Scholar] [CrossRef]
- Maślanka, A.; Żmudzki, P.; Szlósarczyk, M.; Talik, P.; Hubicka, U. Photodegradation Assessment of Amisulpride, Doxepin, Haloperidol, Risperidone, Venlafaxine, and Zopiclone in Bulk Drug and in the Presence of Excipients. Monatshefte Fur Chem. 2020, 151, 483–493. [Google Scholar] [CrossRef]
- Calza, P.; Sakkas, V.A.; Villioti, A.; Massolino, C.; Boti, V.; Pelizzetti, E.; Albanis, T. Multivariate Experimental Design for the Photocatalytic Degradation of Imipramine. Determination of the Reaction Pathway and Identification of Intermediate Products. Appl. Catal. B Environ. 2008, 84, 379–388. [Google Scholar] [CrossRef]
- Méndez-Arriaga, F.; Otsu, T.; Oyama, T.; Gimenez, J.; Esplugas, S.; Hidaka, H.; Serpone, N. Photooxidation of the Antidepressant Drug Fluoxetine (Prozac®) in Aqueous Media by Hybrid Catalytic/Ozonation Processes. Water Res. 2011, 45, 2782–2794. [Google Scholar] [CrossRef] [PubMed]
- Bojanowska-Czajka, A.; Pyszynska, M.; Majkowska-Pilip, A.; Wawrowicz, K. Degradation of Selected Antidepressants Sertraline and Citalopram in Ultrapure Water and Surface Water Using Gamma Radiation. Processes 2022, 10, 63. [Google Scholar] [CrossRef]
- Melin, V.; Salgado, P.; Thiam, A.; Henríquez, A.; Mansilla, H.D.; Yáñez, J.; Salazar, C. Study of Degradation of Amitriptyline Antidepressant by Different Electrochemical Advanced Oxidation Processes. Chemosphere 2021, 274, 129683. [Google Scholar] [CrossRef]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Comprehensive Study of the Antidiabetic Drug Metformin and Its Transformation Product Guanylurea in Greek Wastewaters. Water Res. 2015, 70, 436–448. [Google Scholar] [CrossRef]
- Rudke, A.R.; Heleno, S.A.; Fernandes, I.P.; Prieto, M.A.; Gonçalves, O.H.; Rodrigues, A.E.; Ferreira, I.C.F.R.; Barreiro, M.F. Microencapsulation of Ergosterol and Agaricus Bisporus L. Extracts by Complex Coacervation Using Whey Protein and Chitosan: Optimization Study Using Response Surface Methodology. LWT 2019, 103, 228–237. [Google Scholar] [CrossRef]
- Van Doorslaer, X.; Dewulf, J.; Van Langenhove, H.; Demeestere, K. Fluoroquinolone Antibiotics: An Emerging Class of Environmental Micropollutants. Sci. Total Environ. 2014, 500–501, 250–269. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Titanium Dioxide Photocatalysis for Pharmaceutical Wastewater Treatment. Environ. Chem. Lett. 2014, 12, 27–47. [Google Scholar] [CrossRef]
- Rekhate, C.V.; Srivastava, J.K. Recent Advances in Ozone-Based Advanced Oxidation Processes for Treatment of Wastewater- A Review. Chem. Eng. J. Adv. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Marotta, R.; Radovnikovic, A. Ozonation and H2O2/UV Treatment of Clofibric Acid in Water: A Kinetic Investigation. J. Hazard. Mater. 2003, 103, 233–246. [Google Scholar] [CrossRef]
- Liu, Q.-T.; Williams, H.E. Kinetics and Degradation Products for Direct Photolysis of β-Blockers in Water. Environ. Sci. Technol. 2007, 41, 803–810. [Google Scholar] [CrossRef]
- Li, Y.N.B.; Moore, D.E.; Tattam, B.N. Photodegradation of Amiloride in Aqueous Solution. Int. J. Pharm. 1999, 183, 109–116. [Google Scholar] [CrossRef]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of Residual Pharmaceuticals from Aqueous Systems by Advanced Oxidation Processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lu, S.; Qiu, Z.; Lin, K. Clofibric Acid Degradation in UV254/H2O2 Process: Effect of Temperature. J. Hazard. Mater. 2010, 176, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Rosario-Ortiz, F.L.; Wert, E.C.; Snyder, S.A. Evaluation of UV/H2O2 Treatment for the Oxidation of Pharmaceuticals in Wastewater. Water Res. 2010, 44, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Ravina, M.; Campanella, L.; Kiwi, J. Accelerated Mineralization of the Drug Diclofenac via Fenton Reactions in a Concentric Photo-Reactor. Water Res. 2002, 36, 3553–3560. [Google Scholar] [CrossRef]
- Pérez-Estrada, L.A.; Maldonado, M.I.; Gernjak, W.; Agüera, A.; Fernández-Alba, A.R.; Ballesteros, M.M.; Malato, S. Decomposition of Diclofenac by Solar Driven Photocatalysis at Pilot Plant Scale. Catal. Today 2005, 101, 219–226. [Google Scholar] [CrossRef]
- Goi, A.; Veressinina, Y.; Trapido, M. Degradation of Salicylic Acid by Fenton and Modified Fenton Treatment. Chem. Eng. J. 2008, 143, 1–9. [Google Scholar] [CrossRef]
- Trovó, A.G.; Melo, S.A.S.; Nogueira, R.F.P. Photodegradation of the Pharmaceuticals Amoxicillin, Bezafibrate and Paracetamol by the Photo-Fenton Process-Application to Sewage Treatment Plant Effluent. J. Photochem. Photobiol. A Chem. 2008, 198, 215–220. [Google Scholar] [CrossRef]
- Melo, S.A.S.; Trovó, A.G.; Bautitz, I.R.; Nogueira, R.F.P. Degradação de Fármacos Residuais Por Processos Oxidativos Avançados. Quim. Nova 2009, 32, 188–197. [Google Scholar] [CrossRef]
- Tong, A.Y.C.; Braund, R.; Warren, D.S.; Peake, B.M. TiO 2-Assisted Photodegradation of Pharmaceuticals—A Review. Cent. Eur. J. Chem. 2012, 10, 989–1027. [Google Scholar] [CrossRef]
- Magureanu, M.; Mandache, N.B.; Parvulescu, V.I. Degradation of Pharmaceutical Compounds in Water by Non-Thermal Plasma Treatment. Water Res. 2015, 81, 124–136. [Google Scholar] [CrossRef]
- Bruggeman, P.; Schram, D.; González, M.Á.; Rego, R.; Kong, M.G.; Leys, C. Characterization of a Direct Dc-Excited Discharge in Water by Optical Emission Spectroscopy. Plasma Sources Sci. Technol. 2009, 18, 25017. [Google Scholar] [CrossRef]
- Kanazawa, S.; Kawano, H.; Watanabe, S.; Furuki, T.; Akamine, S.; Ichiki, R.; Ohkubo, T.; Kocik, M.; Mizeraczyk, J. Observation of OH Radicals Produced by Pulsed Discharges on the Surface of a Liquid. Plasma Sources Sci. Technol. 2011, 20, 34010. [Google Scholar] [CrossRef]
- Bruggeman, P.; Schram, D.C. On OH Production in Water Containing Atmospheric Pressure Plasmas. Plasma Sources Sci. Technol. 2010, 19, 045025. [Google Scholar] [CrossRef]
- Papagiannis, I.; Koutsikou, G.; Frontistis, Z.; Konstantinou, I.; Avgouropoulos, G.; Mantzavinos, D.; Lianos, P. Photoelectrocatalytic vs. Photocatalytic Degradation of Organic Water Born Pollutants. Catalysts 2018, 8, 455. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent Progress in Metal-Doped TiO2, Non-Metal Doped/Codoped TiO2 and TiO2 Nanostructured Hybrids for Enhanced Photocatalysis. Int. J. Hydrogen Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green Synthesis of Nanoparticles: Current Developments and Limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Rapti, I.; Kourkouta, T.; Malisova, E.M.; Albanis, T.; Konstantinou, I. Photocatalytic Degradation of Inherent Pharmaceutical Concentration Levels in Real Hospital WWTP Effluents Using G-C3N4 Catalyst on CPC Pilot Scale Reactor. Molecules 2023, 28, 1170. [Google Scholar] [CrossRef] [PubMed]
- Bairamis, F.; Konstantinou, I. Wo3 Fibers/g-C3n4 z-Scheme Heterostructure Photocatalysts for Simultaneous Oxidation/Reduction of Phenol/Cr (VI) in Aquatic Media. Catalysts 2021, 11, 792. [Google Scholar] [CrossRef]
- Zhong, J.; Jiang, H.; Wang, Z.; Yu, Z.; Wang, L.; Mueller, J.F.; Guo, J. Efficient Photocatalytic Destruction of Recalcitrant Micropollutants Using Graphitic Carbon Nitride under Simulated Sunlight Irradiation. Environ. Sci. Ecotechnology 2021, 5, 100079. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.; Chafiq, L.; Dalhatou, S.; Bonnet, P.; Nasr, M.; Gaillard, N.; Dikdim, J.M.D.; Monier, G.; Assadie, A.A.; Zeghioud, H. G-C3N4/TiO2 S-Scheme Heterojunction Photocatalyst with Enhanced Photocatalytic Carbamazepine Degradation and Mineralization. J. Photochem. Photobiol. A Chem. 2022, 430, 113971. [Google Scholar] [CrossRef]
- Gao, B.; Wang, J.; Dou, M.; Xu, C.; Huang, X. Enhanced Photocatalytic Removal of Amoxicillin with Ag/TiO2/Mesoporous g-C3N4 under Visible Light: Property and Mechanistic Studies. Environ. Sci. Pollut. Res. 2020, 27, 7025–7039. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Tiwari, B.; Sellamuthu, B.; Ouarda, Y.; Drogui, P.; Tyagi, R.D.; Buelna, G. Review on Fate and Mechanism of Removal of Pharmaceutical Pollutants from Wastewater Using Biological Approach. Bioresour. Technol. 2017, 224, 1–12. [Google Scholar] [CrossRef]
- Kózka, B.; Nałęcz-Jawecki, G.; Turło, J.; Giebułtowicz, J. Application of Pleurotus Ostreatus to Efficient Removal of Selected Antidepressants and Immunosuppressant. J. Environ. Manage. 2020, 273, 111131. [Google Scholar] [CrossRef] [PubMed]
- Aydın, S.; Bedük, F.; Ulvi, A.; Aydın, M.E. Simple and Effective Removal of Psychiatric Pharmaceuticals from Wastewater Treatment Plant Effluents by Magnetite Red Mud Nanoparticles. Sci. Total Environ. 2021, 784, 147174. [Google Scholar] [CrossRef]
- Bozyiğit, G.D.; Zaman, B.T.; Özdemir, O.K.; Kılınç, Y.; Chormey, D.S.; Bakırdere, S.; Engin, G.O. Removal of Two Antidepressant Active Pharmaceutical Ingredients from Hospital Wastewater by Polystyrene-Coated Magnetite Nanoparticles-Assisted Batch Adsorption Process. Environ. Monit. Assess. 2023, 196, 77. [Google Scholar] [CrossRef]
- Calisto, V.; Ferreira, C.I.A.; Oliveira, J.A.B.P.; Otero, M.; Esteves, V.I. Adsorptive Removal of Pharmaceuticals from Water by Commercial and Waste-Based Carbons. J. Environ. Manag. 2015, 152, 83–90. [Google Scholar] [CrossRef]
- Skalska-Tuomi, K.; Kaijanen, L.; Monteagudo, J.M.; Mänttäri, M. Efficient Removal of Pharmaceuticals from Wastewater: Comparative Study of Three Advanced Oxidation Processes. J. Environ. Manag. 2025, 375, 124276. [Google Scholar] [CrossRef] [PubMed]
- Lajeunesse, A.; Blais, M.; Barbeau, B.; Sauvé, S.; Gagnon, C. Ozone Oxidation of Antidepressants in Wastewater: Treatment Evaluation and Characterization of New By-Products by LC-QToFMS. Chem. Cent. J. 2013, 7, 15. [Google Scholar] [CrossRef]
- Hama Aziz, K.H.; Mustafa, F.S.; Karim, M.A.H.; Hama, S. Pharmaceutical Pollution in the Aquatic Environment: Advanced Oxidation Processes as Efficient Treatment Approaches: A Review. Mater. Adv. 2025, 6, 3433–3454. [Google Scholar] [CrossRef]
- Azuma, T.; Otomo, K.; Kunitou, M.; Shimizu, M.; Hosomaru, K.; Mikata, S.; Mino, Y.; Hayashi, T. Performance and Efficiency of Removal of Pharmaceutical Compounds from Hospital Wastewater by Lab-Scale Biological Treatment System. Environ. Sci. Pollut. Res. Int. 2018, 25, 14647–14655. [Google Scholar] [CrossRef]
- Alobaidi, R.A.K.; Ulucan-Altuntas, K.; Mhemid, R.K.S.; Manav-Demir, N.; Cinar, O. Biodegradation of Emerging Pharmaceuticals from Domestic Wastewater by Membrane Bioreactor: The Effect of Solid Retention Time. Int. J. Environ. Res. Public Health 2021, 18, 3395. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Polverino, G.; Martin, J.M.; Bertram, M.G.; Wiles, S.C.; Palacios, M.M.; Bywater, C.L.; White, C.R.; Wong, B.B.M. Chronic Exposure to a Pervasive Pharmaceutical Pollutant Erodes Among-Individual Phenotypic Variation in a Fish. Environ. Pollut. 2020, 263, 114450. [Google Scholar] [CrossRef]
- Wiles, S.C.; Bertram, M.G.; Martin, J.M.; Tan, H.; Lehtonen, T.K.; Wong, B.B.M. Long-Term Pharmaceutical Contamination and Temperature Stress Disrupt Fish Behavior. Environ. Sci. Technol. 2020, 54, 8072–8082. [Google Scholar] [CrossRef] [PubMed]
- Moermond, C.T.A.; Kase, R.; Korkaric, M.; Ågerstrand, M. CRED: Criteria for Reporting and Evaluating Ecotoxicity Data. Environ. Toxicol. Chem. 2016, 35, 1297–1309. [Google Scholar] [CrossRef]
- Parrott, J.L.; Metcalfe, C.D. Assessing the Effects of the Antidepressant Venlafaxine to Fathead minnows Exposed to Environmentally Relevant Concentrations over a Full Life Cycle. Environ. Pollut. 2017, 229, 403–411. [Google Scholar] [CrossRef]
- Vladimirov, N.; Mu, Y.; Kawashima, T.; Bennett, D.V.; Yang, C.T.; Looger, L.L.; Keller, P.J.; Freeman, J.; Ahrens, M.B. Light-Sheet Functional Imaging in Fictively Behaving Zebrafish. Nat. Methods 2014, 11, 883–884. [Google Scholar] [CrossRef]
- Dunn, T.W.; Gebhardt, C.; Naumann, E.A.; Riegler, C.; Ahrens, M.B.; Engert, F.; Del Bene, F. Neural Circuits Underlying Visually Evoked Escapes in Larval Zebrafish. Neuron 2016, 89, 613–628. [Google Scholar] [CrossRef]
- Yoon, Y.-G.; Wang, Z.; Pak, N.; Park, D.; Dai, P.; Kang, J.S.; Suk, H.-J.; Symvoulidis, P.; Guner-Ataman, B.; Wang, K.; et al. Sparse Decomposition Light-Field Microscopy for High Speed Imaging of Neuronal Activity. Optica 2020, 7, 1457. [Google Scholar] [CrossRef]
- Arnold, K.E.; Brown, A.R.; Brown, A.R.; Ankley, G.T.; Sumpter, J.P. Medicating the Environment: Assessing Risks of Pharmaceuticals to Wildlife and Ecosystems. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130569. [Google Scholar] [CrossRef]
- Valenti, T.W.; Perez-Hurtado, P.; Chambliss, C.K.; Brooks, B.W. Aquatic Toxicity of Sertraline to Pimephales Promelas at Environmentally Relevant Surface Water PH. Environ. Toxicol. Chem. 2009, 28, 2685–2694. [Google Scholar] [CrossRef] [PubMed]
- Bisesi, J.H.; Bridges, W.; Klaine, S.J. Reprint of: Effects of the Antidepressant Venlafaxine on Fish Brain Serotonin and Predation Behavior. Aquat. Toxicol. 2014, 151, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Ortúzar, M.; Esterhuizen, M.; Olicón-Hernández, D.R.; González-López, J.; Aranda, E. Pharmaceutical Pollution in Aquatic Environments: A Concise Review of Environmental Impacts and Bioremediation Systems. Front. Microbiol. 2022, 13, 869332. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, F.; Chouchene, K.; Roche, N.; Ksibi, M. Removal of Pharmaceuticals from Water by Adsorption and Advanced Oxidation Processes: State of the Art and Trends. Appl. Sci. 2021, 11, 6659. [Google Scholar] [CrossRef]
- Caban, M.; Stepnowski, P. How to Decrease Pharmaceuticals in the Environment? A Review. Environ. Chem. Lett. 2021, 19, 3115–3138. [Google Scholar] [CrossRef]
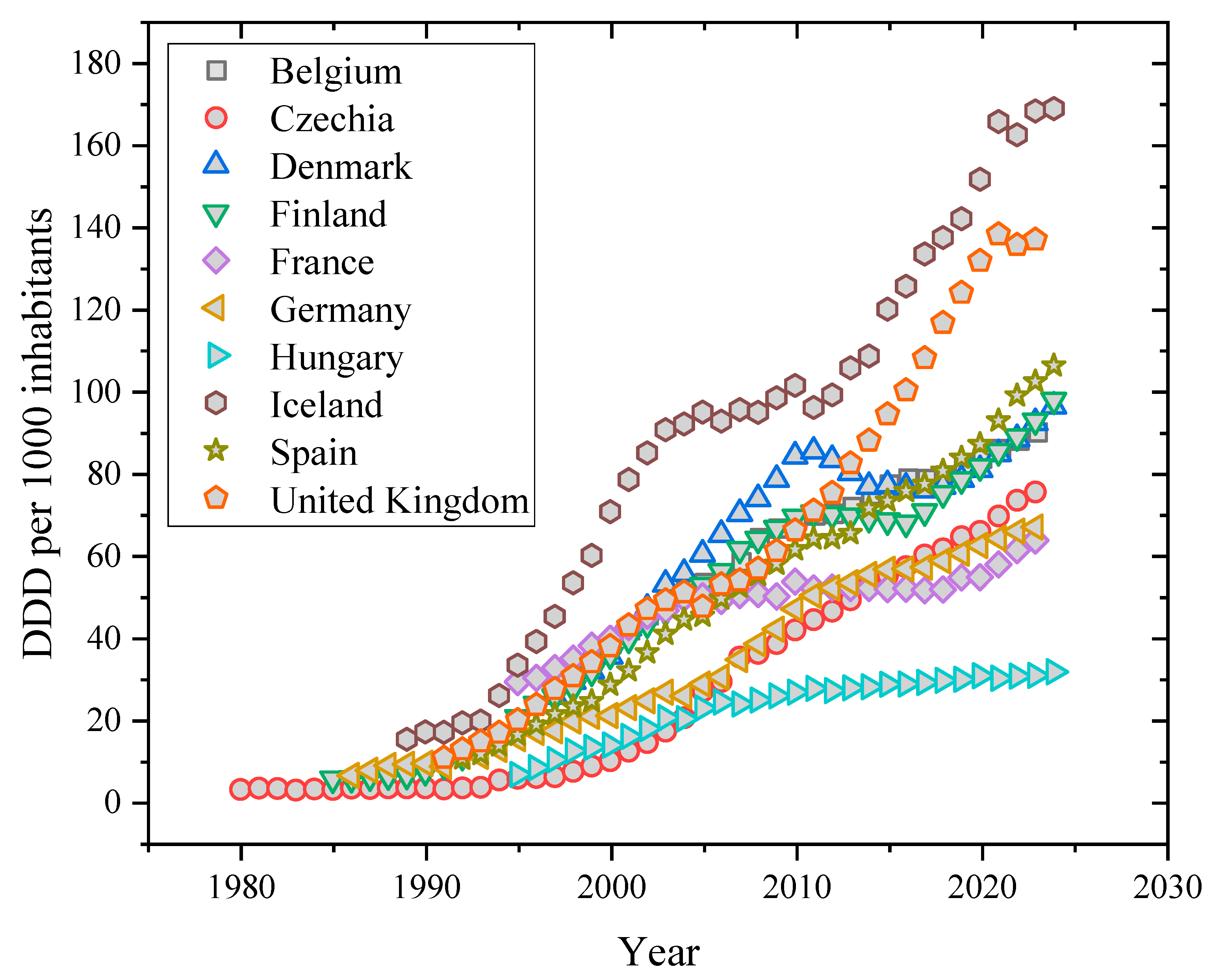
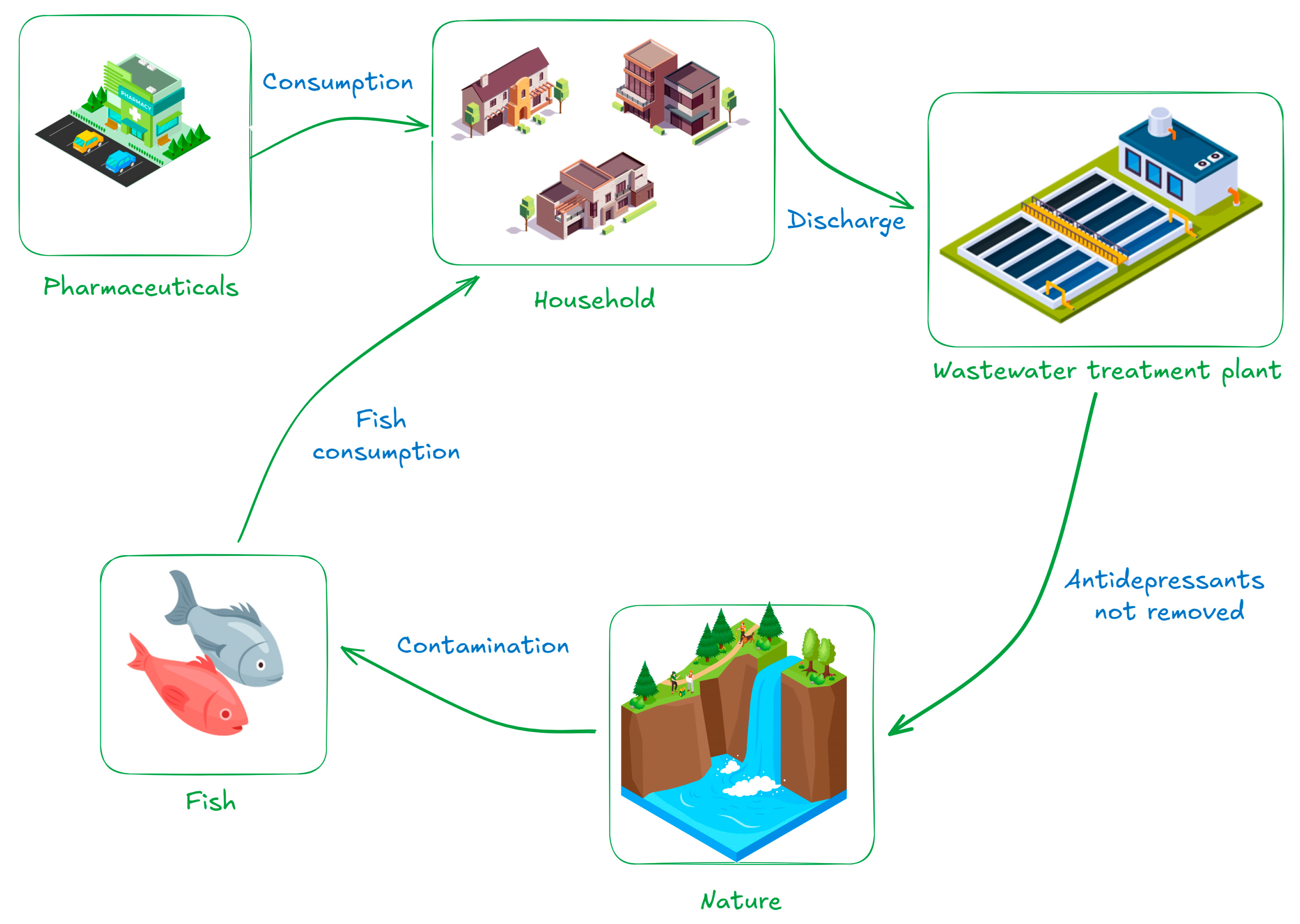
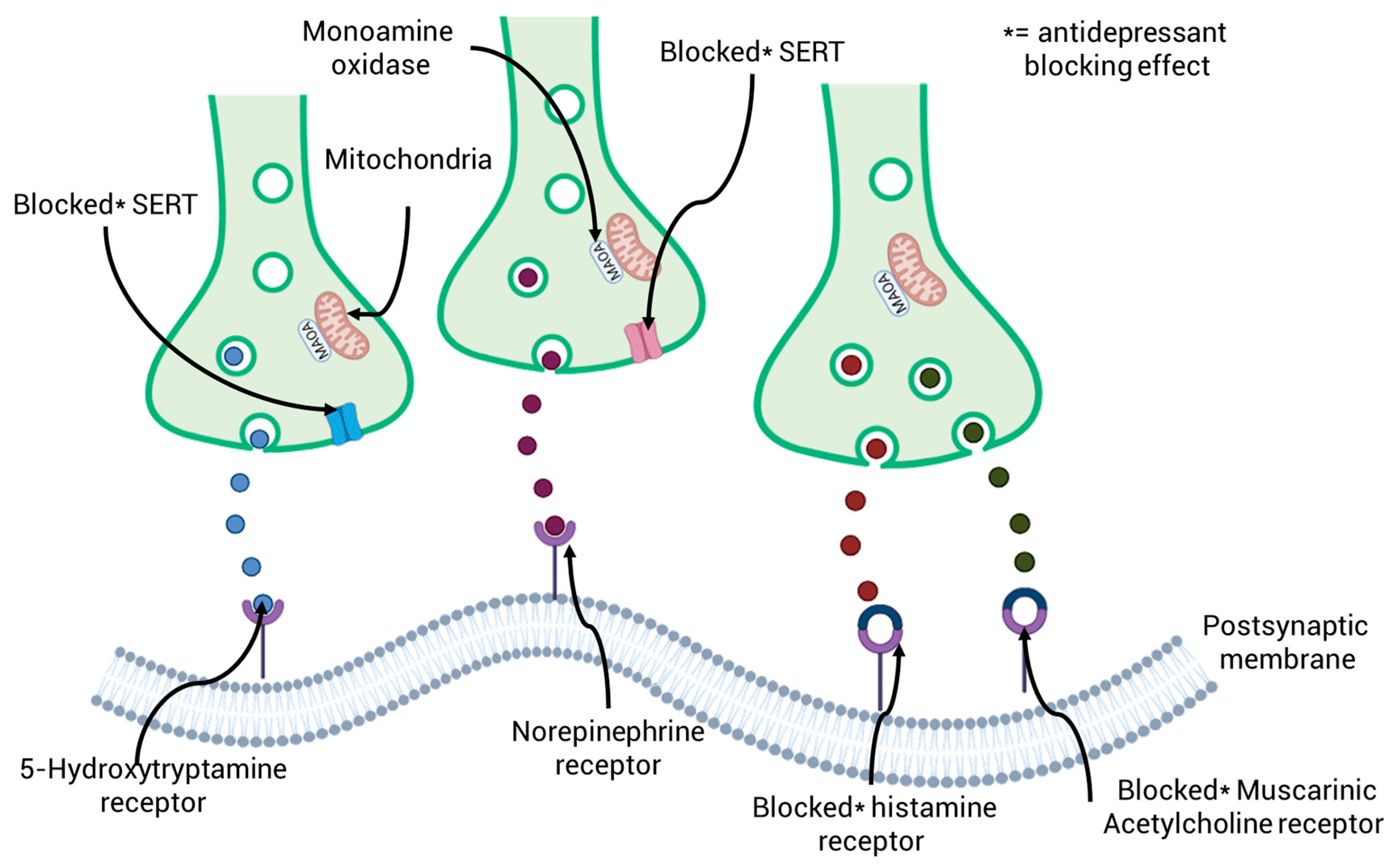
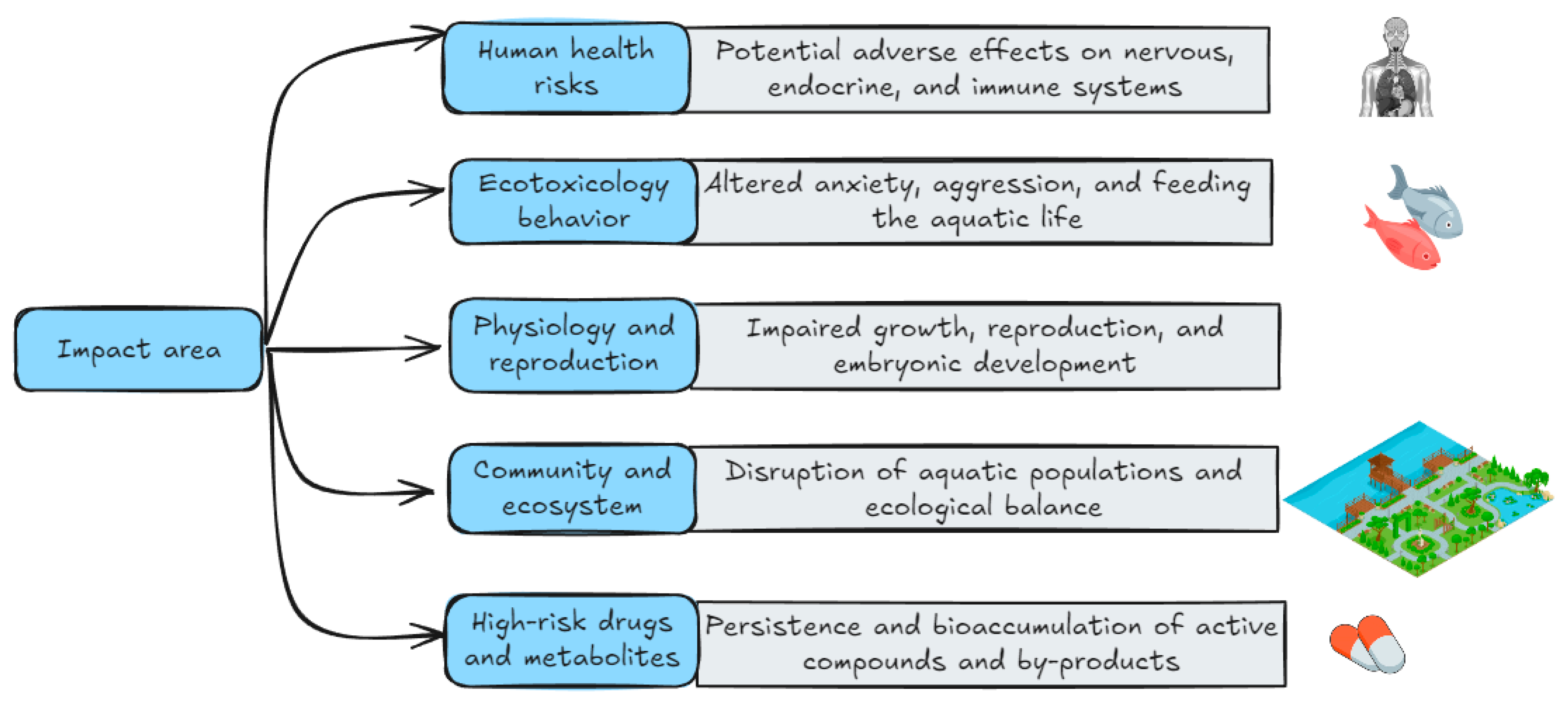
| Country | Antidepressant | Number of Prescriptions and DDD | Predicted Usage (g/year) | Reference |
|---|---|---|---|---|
| Sweden | Fluoxetine | 276,214 and 20 | 55.2 | [3] |
| Australia | Fluoxetine | 2,039,596 and 20 | 407.9 | [3] |
| Brazil | Fluoxetine | 8,054,380 and 20 | 1610.9 | [49] |
| England | Fluoxetine | 6,713,763 and 20 | 1342.8 | [2] |
| USA | Fluoxetine | 25,578,000 and 20 | 15,346.8 | [1] |
| Sweden | Amitriptyline | 419,437 and 75 | 314.6 | [3] |
| Australia | Amitriptyline | 2,366,938 and 75 | 1775.2 | [3] |
| Brazil | Amitriptyline | 8,265,629 and 75 | 6199.2 | [49] |
| England | Amitriptyline | 13,532,567 and 75 | 10,149.4 | [2] |
| USA | Amitriptyline | 10,265,000 and 75 | 23,096.3 | [1] |
| Sweden | Escitalopram | 603,580 and 10 | 60.4 | [3] |
| Australia | Escitalopram | 4,406,535 and 10 | 440.7 | [3] |
| Brazil | Escitalopram | 10,852,868 and 10 | 1085.3 | [49] |
| England | Escitalopram | 1,100,349 and 10 | 110.0 | [2] |
| USA | Escitalopram | 25,979,000 and 10 | 7793.7 | [1] |
| Sweden | Citalopram | 1,135,550 and 20 | 227.1 | [3] |
| Australia | Citalopram | 1,762,614 and 20 | 352.5 | [3] |
| Brazil | Citalopram | 5,051,155 and 20 | 1010.2 | [49] |
| England | Citalopram | 14,136,645 and 20 | 2827.3 | [2] |
| USA | Citalopram | 22,224,000 and 20 | 13,334.4 | [1] |
| Sweden | Duloxetine | 327,365 and 60 | 1055.8 | [2] |
| Brazil | Duloxetine | 4,460,523 and 60 | 37,863.0 | [1] |
| England | Duloxetine | 1,812,334 and 60 | 1087.4 | [2] |
| USA | Duloxetine | 21,035,000 and 60 | 37,863.0 | [1] |
| Brazil | Nortriptyline | 1,793,719 and 75 | 1345.3 | [49] |
| England | Nortriptyline | 609,775 and 75 | 457.3 | [2] |
| USA | Nortriptyline | 4,347,000 and 75 | 9780.8 | [1] |
| Australia | Desvenlafaxine | 2,266,218 and 50 | 1133.1 | [3] |
| England | Dosulepin | 703,879 and 150 | 1055.8 | [2] |
| England | Clomipramine | 282,652 and 100 | 282.7 | [2] |
| Sweden | Venlafaxine | 542,199 and 100 | 813.3 | [3] |
| Australia | Venlafaxine | 3,097,164 and 100 | 4645.7 | [3] |
| Brazil | Venlafaxine | 5,361,432 and 100 | 8042.1 | [49] |
| England | Venlafaxine | 4,401,567 and 100 | 4401.6 | [2] |
| USA | Venlafaxine | 16,121,000 and 100 | 48,363.0 | [1] |
| England | Imipramine | 150,526 and 100 | 150.5 | [2] |
| Brazil | Paroxetine | 5,320,964 and 20 | 1064.2 | [49] |
| England | Paroxetine | 1,351,979 and 20 | 270.4 | [2] |
| USA | Paroxetine | 11,229,000 and 20 | 6737.4 | [1] |
| Sweden | Sertraline | 1,340,154 and 50 | 670.1 | [3] |
| Australia | Sertraline | 4,255,453 and 50 | 2127.7 | [3] |
| Brazil | Sertraline | 9,636,908 and 50 | 4818.5 | [49] |
| England | Sertraline | 14,815,719 and 50 | 7407.9 | [2] |
| England | Lofepramine | 197,325 and 105 | 207.2 | [2] |
| USA | Sertraline | 38,383,000 and 50 | 57,574.5 | [1] |
| Country | Antidepressant | Wastewater Treatment Plant Effluent (ng L−1) | Surface Water (ng L−1) | Reference |
|---|---|---|---|---|
| Australia | Venlafaxine | - | <0.1–86.2 | [50] |
| Canada | Venlafaxine | - | 0.796–573 | [51] |
| China | Venlafaxine | - | <LOQ–3.03 | [52] |
| Czech Republic | Venlafaxine | - | 11–110 | [53] |
| EU-wide (17 countries) | Venlafaxine | 548 (max) | - | [54] |
| Finland | Venlafaxine | - | 1–15 | [1] |
| Germany | Venlafaxine | - | <LOQ–100 | [42] |
| Portugal | Venlafaxine | - | 13.68–25.80 | [55] |
| Spain | Venlafaxine | 582.7–999.5 | 4.1–177.7 | [56] |
| Sweden | Venlafaxine | - | <0.21–5.6 | [57] |
| UK | Venlafaxine | 21.4–285.1 | 0.9–85.4 | [58] |
| US | Venlafaxine | 188–690 | 73.3–359 | [59] |
| Brazil | Escitalopram | 25–1136 | nd–520 | [49] |
| Turkey | Escitalopram | - | <LOQ–3.08 | [60] |
| Norway | Fluoxetine | nd–1.3 | - | [47] |
| Portugal | Fluoxetine | 68.16–88.46 | [55] | |
| Spain | Fluoxetine | 22.70 (max) | <0.18–10.6 | [61] |
| Sweden | Fluoxetine | <LOQ–94 | <LOQ–32 | [62] |
| Turkey | Fluoxetine | - | <LOQ–2.36 | [60] |
| UK | Fluoxetine | 43.3 (max) | 13.5 (max) | [63] |
| US | Fluoxetine | 104–119 | 5.1–14.4 | [64] |
| Norway | Fluvoxamine | nd–0.8 | - | [47] |
| US | Fluvoxamine | nd–4.6 | nd–0.83 | [59] |
| China | Imipramine | nd–2.1, nd | - | [65] |
| Spain | Imipramine | 30–55 | - | [66] |
| Brazil | Nortriptyline | nd–786 | - | [49] |
| UK | Nortriptyline | 0.9–53.8 | 0.2–19 | [58] |
| Germany | Fluoxetine | - | <LOQ–3.8 | [42] |
| EU-wide (17 countries) | Fluoxetine | 21.5 (max) | - | [54] |
| China | Fluoxetine | - | <LOQ–0.24 | [52] |
| Canada | Fluoxetine | - | 0.76–25.1 | [51] |
| Brazil | Fluoxetine | 25–160 | 25–90 | [49] |
| Australia | Fluoxetine | - | <0.1–36 | [50] |
| Spain | Amitriptyline | 13–20 | nd–0.4 | [67] |
| Portugal | Amitriptyline | - | 1 to 2 | [68] |
| France | Amitriptyline | nd-6.0 | Nd | [69] |
| EU-wide (17 countries) | Amitriptyline | 14.6 (max) | - | [54] |
| Czech Republic | Amitriptyline | - | nd–3.3 | [70] |
| China | Amitriptyline | nd–4.8, nd–0.2 | - | [65] |
| China | Amitriptyline | - | 0.12–0.64 | [52] |
| Canada | Amitriptyline | - | 0.156–54.8 | [51] |
| Brazil | Amitriptyline | nd–80 | 157–196 | [49] |
| Wales | Amitriptyline | <2–357 | <0.5–9 | [71] |
| US | Amitriptyline | 88–119 | - | [72] |
| Drug | Fish Species/Life Stage | Exposure (ng/µg-L or µM) | Observed Effects |
|---|---|---|---|
| Fluoxetine | Fathead Minnow (Pimephales promelas) adult | 100 ng/L–100 µg/L | Changes in eating, predator avoidance, and reproductive behavior; long-term, four-week exposure at ambient levels |
| Fluoxetine | Zebrafish (Danio rerio) embryos | ≥0.88 µg/L (locomotor), ≥6 µg/L (AChE inhibition) | Even close to the environmental maximum, delayed hatching, loss of balance, decreased swimming, and neurotoxicity |
| Fluoxetine/Venlafaxine mixture | Hybrid striped bass | Low µg/L concentrations (additive format) | Slower prey capture, decreased brain serotonin, and a combination that is more effective at low dosages than individual medications |
| Fluoxetine | Cichlid (Cichlasoma dimerus)—adult | Injection: ~2 µg drug/g fish | Testicular foam cells, changed pituitary LH, and no increase in plasma vitellogenin indicate mild endocrine disturbance. |
| Fluoxetine (chronic, multigenerational) | Guppies (Poecilia reticulata) wild/lab over 5 years | Acclimated to ~30 ng/L and 300 ng/L | decreased sperm velocity and body condition; longer gonopodium; and less behavioral flexibility |
| Sertraline | Mosquitofish (Gambusia holbrooki) male & group behavior | Environmental µg/L levels typical | Reduced ability to escape predators; altered foraging and social hostility in group contexts rather than solo tests |
| Citalopram | Stickleback (Gasterosteus aculeatus) | 0.15–1.5 µg/L | decreased stress response, aggressiveness, and eating rate |
| Venlafaxine | Brown trout (Salmo trutta) larvae and juveniles | 10–1000 µg/L | less severe than citalopram at the same dosage; some oxidative stress and mild liver inflammation |
| Mixed SSRIs (fluoxetine, sertraline, venlafaxine) | Mosquitofish circadian behavior | 1, 10, 100 µg/L mixture | At 100 µg/L, venlafaxine alone interfered with rhythms; at 1 and 100 µg/L, the mixture changed diurnal activity, and the dose response was not monotonic |
| Process Category | Technology /Method | Advantages | Disadvantages/Limitations |
|---|---|---|---|
| Physical | Adsorption (such as metal–oxide nanoparticles, biochar, and activated carbon) | High elimination efficiency for many antidepressants (e.g., >80–97%); Flexible adsorbents, a straightforward design, and a comparatively low operating cost [183]; | Moves contaminants to an adsorbent, where they must be disposed of or renewed; performance is influenced by affinity, competing substances, and contact time [184]. |
| Physical | Membrane filtration (UF, NF, RO) | extremely high rejection rates, including trace-level elimination (up to >95%); produces reusable, high-quality permeate. | High energy and running expenses; problems with the disposal of concentrate (brine); membrane fouling and necessary maintenance [19] |
| Biological | Activated sludge (ASP), Membrane bioreactor (MBR), moving-bed biofilm reactor (MBBR) | Broadly used, reasonably priced, and compatible with current plants, MBR/MBBR achieves better removal because of its lengthy SRT and retained biomass. | Many antidepressants, such as carbamazepine and SSRIs, have low biodegradability (less than 55–75%); the effectiveness of removal is largely dependent on the compound’s characteristics and operating conditions [19] |
| Biological | Constructed wetlands/fungal mycoremediation | inexpensive, environmentally benign, and capable of breaking down resistant substances through the action of fungal enzymes or plant microbial synergy | Mostly at the lab or pilot scale, scalability is difficult; slow rates, retention duration, and the requirement for substrate modifications; Less shown performance in complex actual wastewater |
| Chemical (AOPs) | Ozonation, UV, UV/H2O2, Fenton, photo-Fenton, photocatalysis | Extremely high rates of drug breakdown and mineralization (>90%, frequently >95%); total conversion to inorganic end products is feasible; non-selective, broad-spectrum oxidation using OH radicals | High operating and capital costs (catalysts, chemicals, and energy); If partial oxidation occurs, potentially hazardous transformation byproducts may arise; post-biological polishing may be necessary. Radical scavengers (such bicarbonate ions) impair efficient operation. |
| Chemical (AOPs) | Electrochemical oxidation/electro-Fenton | Effectively breaking down resistant medications; can combine with adsorption (like GAC) to improve removal and lessen byproducts | It requires electricity and specific electrodes; it can be expensive. may result in oxidative byproducts such as chlorinated ones, contingent on the chemistry of the water. |
| Positively Ionized Tricyclic Antidepressants | Adsorbents | qm (mmol g−1) | pH | Reference |
|---|---|---|---|---|
| Desipramine | Dowex 50WX4-200 | 3.22 | 6.5 | [194] |
| Clomipramine | Dowex 50WX4-200 | 2.53 | 6.5 | [194] |
| Clomipramine | Carbon sphere | 0.09 | 6.8 | [197] |
| Imipramine | Dowex 50WX4-200 | 3.42 | 6.5 | [194] |
| Imipramine | Carbon sphere | 0.36 | 6.8 | [197] |
| Imipramine | Activated charcoal | 1.17 | 6.8 | [4] |
| Amitriptyline | Dowex 50WX4-200 | 3.76 | 6.5 | [194] |
| Amitriptyline | Ca-montmorillonite | 1.05 | 6–7 | [196] |
| Specific Conditions | Wastewater Treatment Methods | Efficiency Removal (%) | Antidepressant Removed | Reference |
|---|---|---|---|---|
| Enzymatic degradation + fungal adsorption | Biological degradation | 22–98 | sertraline, paroxetine, clomipramine, mianserin, fluoxetine, citalopram, venlafaxine | [247] |
| Activated sludge | Biological degradation | 77–81 | sertraline | [195] |
| Photo-electro-Fenton | Advanced oxidation processes | 95 | amitriptyline | [214] |
| Electro-Fenton | Advanced oxidation processes | 78 | amitriptyline | [214] |
| UVA irradiation | Advanced oxidation processes | 30–40 | doxepine, venlafaxine | [210] |
| Electro-oxidation using BDD anode | Advanced oxidation processes | 76 | amitriptyline | [214] |
| Gamma (γ) radiation | Advanced oxidation processes | 80–100 | sertraline, citalopram | [213] |
| UVB + UVA irradiation | Advanced oxidation processes | 50 | desipramine | [209] |
| UVA + TiO2 + O3 + H2O2 | Advanced oxidation processes | 97 | fluoxetine | [212] |
| H2O2 (PEROXONE) + O3 + UVA | Advanced oxidation processes | 70 | fluoxetine | [212] |
| O3 + UVA + TiO2 | Advanced oxidation processes | 50 | fluoxetine | [212] |
| UVC irradiation | Advanced oxidation processes | 88–100 | amitriptyline, clomipramine | [207] |
| UVC irradiation | Advanced oxidation processes | 92 | trimipramine | [208] |
| Water microemulsion +Brij 30 + Propyl acetate | Extraction | 88–100 | amitriptyline, doxepine, imipramine, clomipramine | [204] |
| Phosphate buffer + phosphonium salt/Tetrabutyl-ammonium | Extraction | 94–100 | amitriptyline | [206] |
| Ethyl acetate | Extraction | 86–95 | sertraline | [202] |
| Molecularly imprinted polymers | Adsorption | 70–100 | sertraline, fluoxetine, citalopram, paroxetine | [195] |
| Molecularly imprinted polymers | Adsorption | 55–90 | bupropion | [195] |
| Diosmectite | Adsorption | 16–31 | amitriptyline, nortriptyline | [4] |
| TiO2 nanoparticles | Adsorption | 7–22 | amitriptyline, nortriptyline | [4] |
| Activated carbon | Adsorption | 17–37 | amitriptyline, nortriptyline | [193] |
| Activated carbon | Adsorption | 37–61 | imipramine | [4] |
| Cation-exchange resins | Adsorption | 57–78 | amitriptyline, clomipramine, imipramine, desipramine | [194] |
| Metric | Adsorption | AOPs | Biological Treatment |
|---|---|---|---|
| Removal efficiency (for psychiatric drugs/trace compounds) | Moderate to high for compounds with good adsorption affinity; can reach >80–95% in favorable lab conditions; lower for hydrophilic or small, ionic molecules. | High for many compounds; often >90% parent compound removal under optimized lab or pilot conditions; mineralization often less complete. | Low to moderate for many psychiatric drugs; some compounds removed well, many recalcitrant ones remain; performance heavily dependent on type of compound and conditions. |
| Energy requirements | Low to moderate: mostly energy for pumping, mixing, possibly regeneration; no high intensity light/radical generation. | High for many types (ozone generation, UV lamps, electrochemical systems etc.); some hybrid or newer AOPs can reduce energy, but still significant. | Low: microbial metabolism, ambient temperature/light (if applicable) predominates; energy mainly for aeration, mixing, pumping. |
| Chemical/reagent costs | Adsorbent material; cost of regeneration or replacement; possible pre-treatment. | High: oxidants (e.g., H2O2, ozone), catalysts, UV lamps, electricity; steady supply; maintenance. | Moderate: nutrients, sometimes external carbon sources, but generally fewer specialized chemicals required. |
| Byproduct risk/toxicity | Low in terms of new chemicals, though removal of parent compound only; risk if desorption or leaching; disposal/regeneration must be considered. | Moderate to High: possible formation of oxidation byproducts, partial transformation products, chlorinated/brominated byproducts (depending on matrix), residual radicals/oxidants; require careful monitoring. | Variable: transformation products may be produced; microbial byproducts (sludge) may accumulate pharmaceuticals; risk in sludge disposal; generally lower risk of “new radical byproducts” but still possible. |
| Scalability/real-world feasibility | Good: can retrofit existing plants with adsorber units; adsorbents relatively well known; GAC, PAC have history; but scale increases cost, logistics of regeneration, footprint are challenges. | Challenging: scaling reactors, infrastructural modifications, ensuring consistent oxidant delivery, safety, handling, capital costs. More feasible as polishing/tertiary treatment rather than primary removal. | Strong: many WWTPs already use biological treatment; improvements (MBRs, longer SRTs, specialized microbes) are possible; nature-based systems (algae, constructed wetlands etc.) promising but require large land, longer times. |
| Operational complexity/monitoring/maintenance | Moderate: maintaining adsorbent performance, avoiding fouling, regular regeneration/disposal, monitoring breakthrough. | High: need to monitor oxidant dosages, byproduct formation, pH, light, etc.; safety issues; maintenance of UV lamps/ozone generators or catalysts; energy systems need overseeing. | Moderate: maintaining microbial health, community composition, handling fluctuations in influent; but generally lower technical sophistication than AOP for certain aspects. |
| Cost per m3 treated (for high removal polishing/trace compound removal) | Moderate to High depending on adsorbent cost/regeneration; on order of some USD or EUR per m3 for high-fidelity trace removal; relatively lower than AOP in many cases. | High: energy + chemical costs make cost per m3 for high removal large; only competitive in specific cases/high value water/regulatory pressure. | Lower: mainstream biological treatment is relatively economical; improvements (e.g., MBRs or longer retention times) add cost but often less than full-scale AOPs. |
| Category | Gaps/Challenges | Future Prospects/Opportunities |
|---|---|---|
| Analytical and Monitoring | Lack of standard methods and quality control for measuring antidepressants and metabolites in water; Poor data on chronic low-dose exposure, mixture interactions, behavioral/developmental endpoints | Development of prioritized sampling frameworks and validated analytical protocols targeting environmentally relevant compounds and mixtures |
| Biological Treatment | Conventional activated sludge systems show poor removal of many antidepressants, including SSRIs and carbamazepine; Limited understanding of how microbial community structure and redox conditions affect biodegradation | Optimization of redox dosing and specialized microbial consortia; bioaugmentation with fungi (e.g., Mucor, Trametes) in MBRs for enhanced degradation [266] |
| Physical Methods (Adsorption, Membranes) | Need for lower-cost, selective adsorbents compatible with real wastewater matrices; Disposal or regeneration of spent adsorbents and handling of concentrated reject streams | Advanced adsorbents (e.g., MOFs, modified biochars) designed for high selectivity and reusability; hybrid approaches (e.g., membrane adsorption AOP) to enhance removal efficiency [267] |
| Chemical/AOPs | High energy and chemical costs; formation of toxic byproducts from partial oxidation; Performance variability depending on water chemistry (e.g., radical scavengers like bicarbonate) | Development of visible-light photocatalysts (e.g., doped TiO2, g-C3N4), fluidized-bed Fenton reactors, sonophotocatalysis to improve efficiency and reduce energy use |
| Hybrid Systems | Few full-scale pilots integrating biological + AOP or adsorption systems; design integration and economics underexplored [267] | Pilot/full-scale implementation of hybrid configurations (e.g., biological + ozonation, adsorption + electrochemical oxidation) optimized for cost-effectiveness and performance [267] |
| Novel/Emerging Tech | Limited real-world application data for mycoremediation, nanoremediation, bioelectrochemical wetlands, permeable reactive barriers | Explore mycoreactors in WWTPs, bioelectrochemical wetlands (BES), nanoadsorbents and PRBs for decentralized or source-control remediation [266] |
| Risk and Regulation | Lack of strong regulatory frameworks addressing pharmaceutical discharge; few tools to assess exposure risk of mixtures [268]; No universal prioritization of antidepressants for monitoring or remediation | Policy development for hospital/mixed-source effluent pre-treatment, standardized risk assessment methods, and prioritization frameworks for tracking key antidepressants [268] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgin, J.; Schumacher de Oliveira, J.; Dehmani, Y.; El Messaoudi, N.; de Albuquerque Carvalho, M.; Meili, L.; Ramos, C.G.; Franco, D.S.P. From Prescription to Pollution: Assessing the Ecological Impact and Treatment Technologies for Antidepressant Contaminants. Sustainability 2025, 17, 9752. https://doi.org/10.3390/su17219752
Georgin J, Schumacher de Oliveira J, Dehmani Y, El Messaoudi N, de Albuquerque Carvalho M, Meili L, Ramos CG, Franco DSP. From Prescription to Pollution: Assessing the Ecological Impact and Treatment Technologies for Antidepressant Contaminants. Sustainability. 2025; 17(21):9752. https://doi.org/10.3390/su17219752
Chicago/Turabian StyleGeorgin, Jordana, Jivago Schumacher de Oliveira, Younes Dehmani, Noureddine El Messaoudi, Matheus de Albuquerque Carvalho, Lucas Meili, Claudete Gindri Ramos, and Dison S. P. Franco. 2025. "From Prescription to Pollution: Assessing the Ecological Impact and Treatment Technologies for Antidepressant Contaminants" Sustainability 17, no. 21: 9752. https://doi.org/10.3390/su17219752
APA StyleGeorgin, J., Schumacher de Oliveira, J., Dehmani, Y., El Messaoudi, N., de Albuquerque Carvalho, M., Meili, L., Ramos, C. G., & Franco, D. S. P. (2025). From Prescription to Pollution: Assessing the Ecological Impact and Treatment Technologies for Antidepressant Contaminants. Sustainability, 17(21), 9752. https://doi.org/10.3390/su17219752










