Aristotelia chilensis Leaf Powder as a Sustainable Alternative to Synthetic Antioxidants in Fresh Sausages: Advancing Toward More Natural and Ecological Meat Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Sausages with Maqui Leaves Powders
2.3. Proximal Composition Analysis
2.4. pH and Color
2.5. Fatty Acid Profile
2.6. Volatile Compound Profile
2.7. Sensory Evaluation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Changes in Proximal Composition, pH and Color
3.2. Volatile Compound Profile
3.3. Fatty Acid Profile
3.4. Organoleptic Analysis
3.5. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MLP | Maqui Leaf Powder |
| CON | Control Treatment |
| SA | Sodium Erythorbate Treatment |
| VOCs | Volatile Organic Compounds |
| PCA | Principal component analysis |
| SFA | Saturated Fatty Acids |
| MUFA | Monounsaturated Fatty Acids |
| PUFA | Polyunsaturated Fatty Acids |
References
- Halagarda, M.; Wójciak, K.M. Health and safety aspects of traditional European meat products. A review. Meat Sci. 2022, 184, 108623. [Google Scholar] [CrossRef] [PubMed]
- Luong, N.D.M.; Coroller, L.; Zagorec, M.; Membré, J.M.; Guillou, S. Spoilage of chilled fresh meat products during storage: A quantitative analysis of literature data. Microorganisms 2020, 8, 1198. [Google Scholar] [CrossRef] [PubMed]
- Bolívar-Monsalve, J.; Ramírez-Toro, C.; Bolívar, G.; Ceballos-González, C. Mechanisms of action of novel ingredients used in edible films to preserve microbial quality and oxidative stability in sausages—A review. Trends Food Sci. Technol. 2019, 89, 100–109. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Ferysiuk, K.; Wójciak, K.M. Reduction of nitrite in meat products through the application of various plant-based ingredients. Antioxidants 2020, 9, 711. [Google Scholar] [CrossRef]
- European Commission. Decisión 2008/448/CE, de 23 de Mayo de 2008, Relativa a Las Disposiciones Nacionales Notificadas por Dinamarca Sobre la Adición de Nitritos a Determinados Productos Cárnicos; Diario Oficial de la Unión Europea, L 156, de 14 de junio de 2008; European Commission: Brussels, Belgium, 2008; p. 41. [Google Scholar]
- U.S. Food and Drug Administration (FDA). Guidelines for the Validation of Chemical Methods in Food, Feed, Cosmetics, and Veterinary Products; FDA: Silver Spring, MD, USA, 2019. Available online: https://www.fda.gov/media/121751/download (accessed on 31 August 2025).
- Wang, W.; Kannan, K. Quantitative identification of and exposure to synthetic phenolic antioxidants, including butylated hydroxytoluene, in urine. Environ. Int. 2019, 128, 24–29. [Google Scholar] [CrossRef]
- Wang, W.; Xiong, P.; Zhang, H.; Zhu, Q.; Liao, C.; Jiang, G. Analysis, occurrence, toxicity and environmental health risks of synthetic phenolic antioxidants: A review. Environ. Res. 2021, 201, 111531. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Seol, K.H.; Seong, P.N.; Park, B.Y.; Kim, H.W. Effects of procyanidin on meat quality and shelf-life for preserving pork patties during chilled storage. Korean J. Food Sci. Anim. Resour. 2015, 35, 564–571. [Google Scholar] [CrossRef]
- Tur, J.A.; Bibiloni, M.M. Cap. Functional Foods. In Encyclopedia of Food and Health; Academic Press: Cambridge, MA, USA, 2016; pp. 157–161. [Google Scholar]
- Liu, X.; Huang, B. Changes in fatty acid composition and saturation in leaves and roots of creeping bentgrass exposed to high soil temperature. J. Am. Soc. Hortic. Sci. 2004, 129, 795–801. [Google Scholar] [CrossRef]
- Devatkal, S.K.; Thorat, P.R.; Manjunatha, M.; Anurag, R.K. Comparative antioxidant effect of aqueous extracts of curry leaves, fenugreek leaves and butylated hydroxytoluene in raw chicken patties. J. Food Sci. Technol. 2012, 49, 781–785. [Google Scholar] [CrossRef]
- Hur, S.J.; Jin, S.K.; Park, J.H.; Jung, S.W.; Lyu, H.J. Effect of modified atmosphere packaging and vacuum packaging on quality characteristics of low-grade beef during cold storage. Asian-Australas. J. Anim. Sci. 2013, 26, 1781–1789. [Google Scholar] [CrossRef]
- Alirezalu, A.; Ahmadi, N.; Salehi, P.; Sonboli, A.; Alirezalu, K.; Mousavi Khaneghah, A.; Barba, F.J.; Munekata, P.E.S.; Lorenzo, J.M. Physicochemical Characterization, Antioxidant Activity, and Phenolic Compounds of Hawthorn (Crataegus spp.) Fruits Species for Potential Use in Food Applications. Foods 2020, 9, 436. [Google Scholar] [CrossRef] [PubMed]
- Burri, S.C.M.; Granheimer, K.; Rémy, M.; Ekholm, A.; Håkansson, Å.; Rumpunen, K.; Tornberg, E. Lipid oxidation inhibition capacity of 11 plant materials and extracts evaluated in highly oxidized cooked meatballs. Foods 2019, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Borella, T.G.; Peccin, M.M.; Mazon, J.M.; Roman, S.S.; Cansian, R.L.; Soares, M.B.A. Effect of rosemary (Rosmarinus officinalis) antioxidant in industrial processing of frozen-mixed hamburger during shelf life. J. Food Process. Preserv. 2019, 43, e14092. [Google Scholar] [CrossRef]
- Velázquez, L.; Quiñones, J.; Inostroza, K.; Sepúlveda, G.; Díaz, R.; Scheuermann, E.; Domínguez, R.; Lorenzo, J.M.; Velásquez, C.; Sepúlveda, N. Maqui (Aristotelia chilensis (Mol.) Stuntz): A natural antioxidant to improve quality of meat patties. Antioxidants 2022, 11, 1405. [Google Scholar] [CrossRef]
- Rubilar, M.; Jara, C.; Poo, Y.; Acevedo, F.; Gutiérrez, C.; Sineiro, J.; Shene, C. Extracts of Maqui (Aristotelia chilensis) and Murta (Ugni molinae Turcz.): Sources of antioxidant compounds and α-glucosidase/α-amylase inhibitors. J. Agric. Food Chem. 2011, 59, 1630–1637. [Google Scholar] [CrossRef]
- Velázquez, L.; Quiñones, J.; Díaz, R.; Pateiro, M.; Lorenzo, J.M.; Sepúlveda, N. Natural antioxidants from endemic leaves in the elaboration of processed meat products: Current Status. Antioxidants 2021, 10, 1396. [Google Scholar] [CrossRef]
- AOCS Official Procedure Am 5-04; Rapid Determination of Oil/Fat Utilizing High Temperature Solvent Extraction. AOCS: Champaign, IL, USA, 2004.
- ISO 937:1978; Meat and Meat Products—Determination of Nitrogen Content. ISO: Geneva, Switzerland, 1969; pp. 1–7.
- ISO 936:1998; Meat and Meat Products—Determination of Ash Content. ISO: Geneva, Switzerland, 1998.
- ISO 1442:1997; Meat and Meat Products—Determination of Moisture Content. ISO: Geneva, Switzerland, 1997.
- Folch, J.; Lees, M.; Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Cutillas, L.; Lorenzo, M.; Munekata, P.E.S.; Purrin, L.; Teixeira, A.; Pateiro, M. Volatile Organic Compound Profile. In Advances in Food Research; Academic Press: Cambridge, MA, USA, 2022; pp. 133–140. [Google Scholar]
- NCh-ISO6658:2016; Análisis Sensorial de Alimentos—Metodología—Guía General. INN: Santiago, Chile, 2016; pp. 1–27.
- Hayes, J.E.; Stepanyan, V.; Allen, P.; O’Grady, M.N.; Kerry, J.P. Evaluation of the effects of selected plant-derived nutraceuticals on the quality and shelf-life stability of raw and cooked pork sausages. LWT-Food Sci. Technol. 2011, 44, 164–172. [Google Scholar] [CrossRef]
- Carballo, D.E.; Andrés, S.; Giraldez, F.J.; Khanjari, A.; Caro, I.; Llamazares, D.; Operta, S.; Mateo, J. The effects of storage and hop extract on aroma and flavor compounds in Balkan-style sausages packed under a CO2-containing anaerobic atmosphere. Heliyon 2020, 6, e05251. [Google Scholar] [CrossRef]
- Mottram, D.S. Flavour formation in meat and meat products: A review. Food Chem. 1998, 62, 415–424. [Google Scholar] [CrossRef]
- Casaburi, A.; Piombino, P.; Nychas, G.J.; Villani, F.; Ercolini, D. Bacterial populations and the Volatilome associated with meat spoilage. Food Microbiol. 2015, 45, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Purriños, L.; Pérez-Santaescolástica, C.; Pateiro, M.; Barba, F.J.; Tomasevic, I.; Bastianello Campagnol, P.C.; Lorenzo, J.M. Characterization of volatile compounds of dry-cured meat products using HS-SPME-GC/MS technique. Food Anal. Methods 2019, 12, 1263–1284. [Google Scholar] [CrossRef]
- Luong, N.D.M. Application of a path-modelling approach for deciphering causality relationships between microbiota, volatile organic compounds and off-odor profiles during meat spoilage. Int. J. Food Microbiol. 2021, 348, 109208. [Google Scholar] [CrossRef] [PubMed]
- Retamales, H.A.; Scherson, R.; Scharaschkin, T. Foliar micromorphology and anatomy of Ugni molinae Turcz. (Myrtaceae), with reference to schizogenous secretory cavities. Rev. Chil. Hist. Nat. 2014, 87, 27. [Google Scholar] [CrossRef]
- Cespedes, C.L.; Pavon, N.; Dominguez, M.; Alarcon, J.; Balbontin, C.; Kubo, I.; El-Hafidi, M.; Avila, J.G. The Chilean superfruit black-berry Aristotelia chilensis (Elaeocarpaceae), maqui as mediator in inflammation-associated disorders. Food Chem. Toxicol. 2017, 108, 438–450. [Google Scholar] [CrossRef]
- Cittadini, A.; Domínguez, R.; Pateiro, M.; Sarriés, M.V.; Lorenzo, J.M. Fatty acid composition and volatile profile of longissimus thoracis et lumborum muscle from Burguete and Jaca Navarra foals fattened with different finishing diets. Foods 2021, 10, 2914. [Google Scholar] [CrossRef]
- Holm, E.S.; Adamsen, A.P.S.; Feilberg, A.; Schäfer, A.; Løkke, M.M.; Petersen, M.A. Quality changes during storage of cooked and sliced meat products measured with PTR-MS and HS-GC-MS. Meat Sci. 2013, 95, 302–310. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Y.; Woldemariam, K.Y.; Zhong, S.; Yu, Q.; Wang, J. Antioxidant effect of yeast on lipid oxidation in salami sausage. Front. Microbiol. 2023, 13, 1113848. [Google Scholar] [CrossRef]
- Stanborough, T.; Fegan, N.; Powell, S.M.; Singh, T.; Tamplin, M.; Chandry, P.S. Genomic and metabolic characterization of spoilage-associated Pseudomonas species. Int. J. Food Microbiol. 2018, 268, 61–72. [Google Scholar] [CrossRef]
- Coloretti, F.; Tabanelli, G.; Chiavari, C.; Lanciotti, R.; Grazia, L.; Gardini, F.; Montanari, C. Effect of wine addition on microbiological characteristics, volatile molecule profiles and biogenic amine contents in fermented sausages. Meat Sci. 2014, 96, 1395–1402. [Google Scholar] [CrossRef]
- Xiao, Z.; Lu, J.R. Strategies for enhancing fermentative production of acetoin: A review. Biotechnol. Adv. 2014, 32, 492–503. [Google Scholar] [CrossRef]
- Xiao, Z.; Ma, C.; Xu, P.; Lu, J.R. Acetoin catabolism and acetylbutanediol formation by Bacillus pumilus in a chemically defined medium. PLoS ONE 2009, 4, e5627. [Google Scholar] [CrossRef]
- Haukeli, A.D.; Lee, S. Formation and removal of acetoin during yeast fermentation. J. Inst. Brew. 1975, 81, 58–64. [Google Scholar] [CrossRef]
- Møller, J.K.S.; Hinrichsen, L.L.; Andersen, H.J. Formation of amino acid (L-leucine, L-phenylalanine) derived volatile flavour compounds by Moraxella phenylpyruvica and Staphylococcus xylosus in cured meat model systems. Int. J. Food Microbiol. 1998, 42, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Ferrocino, I. Antimicrobial packaging to retard the growth of spoilage bacteria and to reduce the release of volatile metabolites in meat stored under vacuum at 1 °C. J. Food Prot. 2013, 76, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Agregán, R.; Lorenzo, J.M. Role of commercial starter cultures on microbiological, physicochemical characteristics, volatile compounds and sensory properties of dry-cured foal sausage. Asian Pac. J. Trop. Dis. 2016, 6, 396–403. [Google Scholar] [CrossRef]
- Yang, C.; Qi, Y.; Zheng, J.; Fan, X.; Liang, P.; Song, C. Efficacy of various preservatives on extending shelf life of vacuum-packaged raw pork during 4 °C storage. J. Food Prot. 2018, 81, 636–645. [Google Scholar] [CrossRef]
- Wettasinghe, M.; Vasanthan, T.; Temelli, F.; Swallow, K. Volatile flavour composition of cooked by-product blends of chicken, beef and pork: A quantitative GC–MS investigation. Food Res. Int. 2001, 34, 149–158. [Google Scholar] [CrossRef]
- Vega, M.S.; Vega, J. Detectable odor thresholds of selected lipid oxidation compounds at various temperatures in a gelatin model system. J. Food Lipids 1994, 1, 229–245. [Google Scholar] [CrossRef]
- Ahamed, Z.; Seo, J.; Eom, J.; Yang, H. Optimization of volatile compound extraction on cooked meat using HS-SPME-GC-MS, and evaluation of diagnosis to meat species using volatile compound by multivariate data analysis. LWT 2023, 188, 115374. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Rahman, A.; Kang, S.C. Chemical composition and inhibitory parameters of essential oil and extracts of Nandina domestica Thunb. to control food-borne pathogenic and spoilage bacteria. Int. J. Food Microbiol. 2008, 125, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Shireman, R. Essential Fatty Acids. Br. Med. J. Sci. 2003, 289, 2169–2176. [Google Scholar]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods—A review. J. Food Sci. Technol. 2014, 51, 2289–2303. [Google Scholar] [CrossRef]
- Boruzi, A.I.; Nour, V. Walnut (Juglans regia L.) leaf powder as a natural antioxidant in cooked pork patties. CYTA-J. Food 2019, 17, 431–438. [Google Scholar]
- Kim, J.Y.; Yi, B.R.; Lee, C.; Gim, S.Y.; Kim, M.J.; Lee, J.H. Effects of pH on the rates of lipid oxidation in oil–water system. Appl. Biol. Chem. 2016, 59, 157–161. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, W.; Richards, M.P. Attributes of lipid oxidation due to bovine myoglobin, hemoglobin and hemolysate. Food Chem. 2017, 234, 230–235. [Google Scholar] [CrossRef]
- Gironés-Vilaplana, A.; Calín-Sánchez, Á.; Moreno, D.A.; Carbonell-Barrachina, Á.A.; García-Viguera, C. Novel maqui liquor using traditional pacharán processing. Food Chem. 2015, 173, 1228–1235. [Google Scholar] [CrossRef]
- Skwarek, P.; Lorenzo, J.M.; Purriños, L.; Karwowska, M. Development of volatile compounds in raw fermented sausages with reduced nitrogen compounds—The effect of tomato pomace addition. Molecules 2024, 29, 5826. [Google Scholar] [CrossRef]
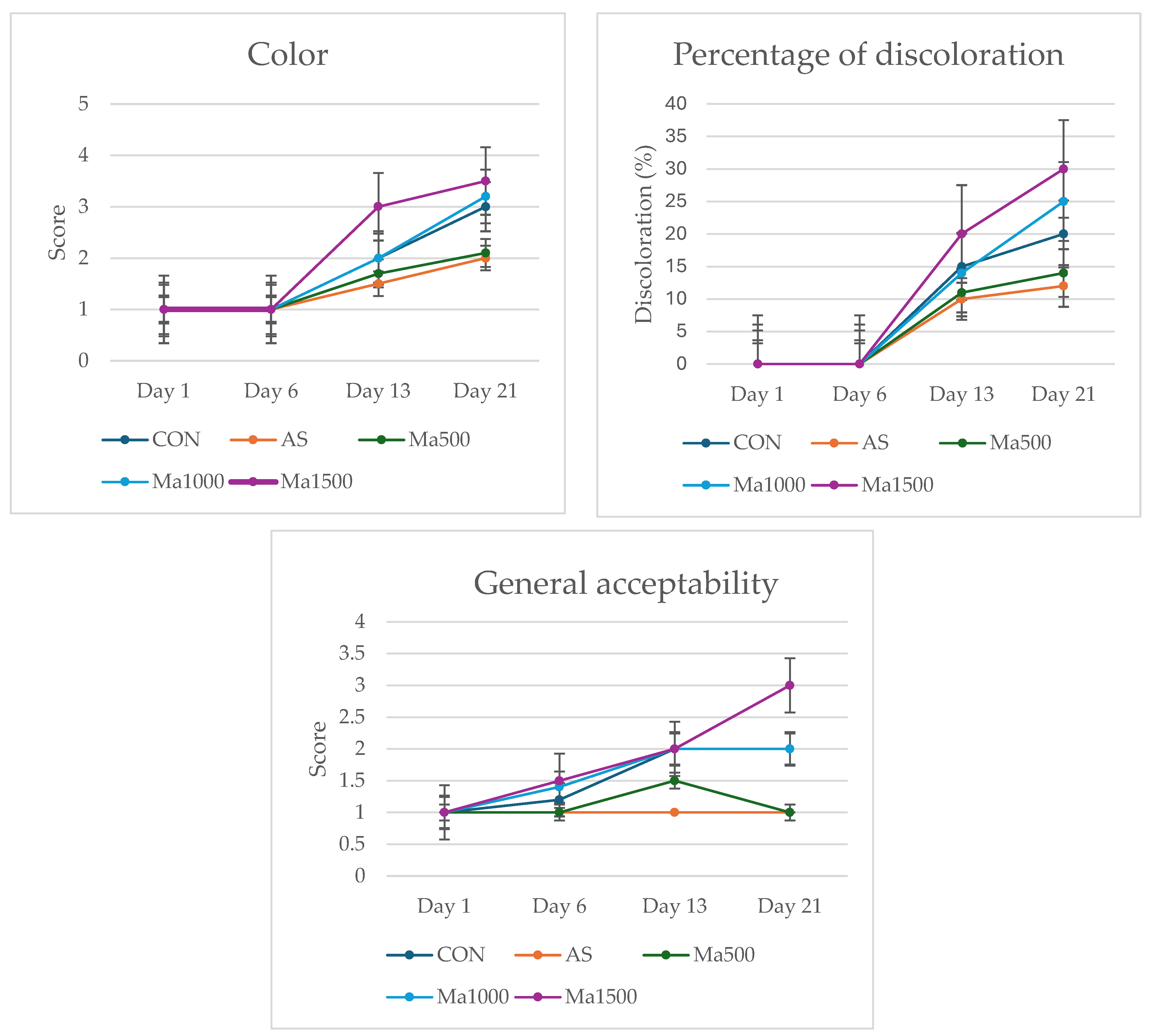
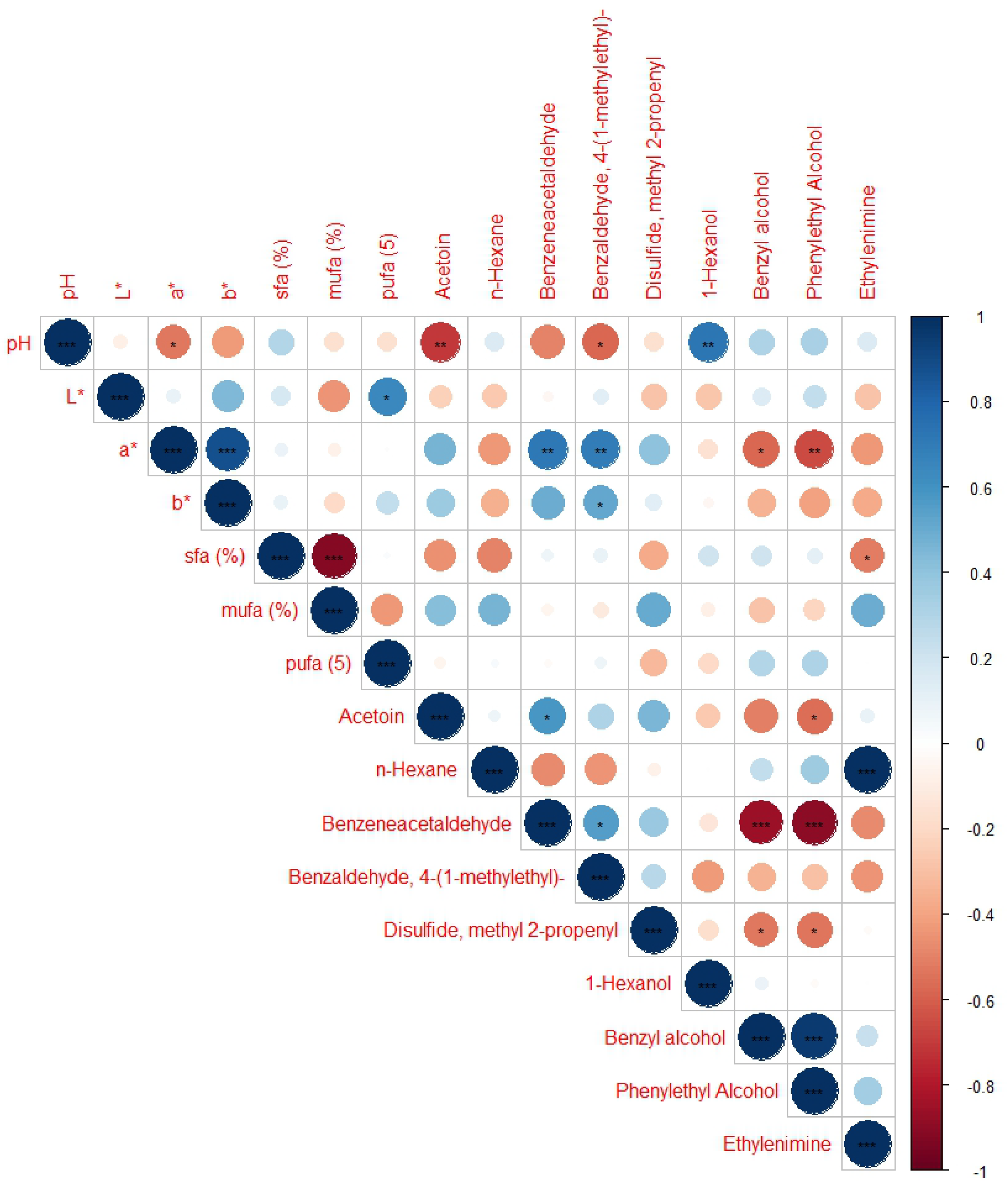
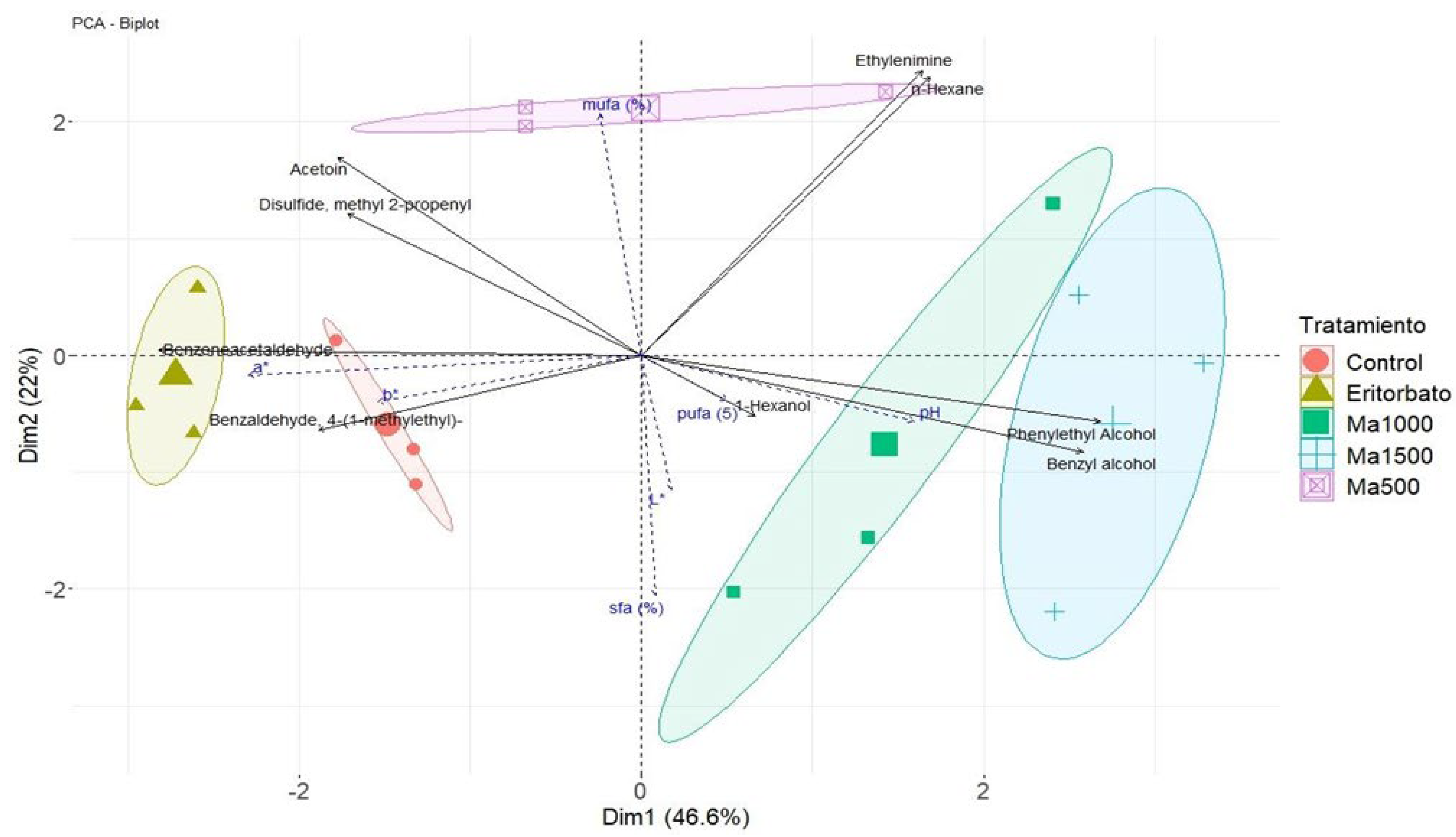
| Treatment | Moisture (%) | Fat (%) | Protein (%) | Ash (%) |
|---|---|---|---|---|
| CON | 58.76 ± 0.94 * | 20.42 ± 1.42 | 16.45 ± 0.40 | 1.98 ± 0.06 |
| SA | 59.20 ± 0.23 | 20.91 ± 0.72 | 15.98 ± 0.67 | 1.90 ± 0.08 |
| Ma500 | 58.38 ± 0.73 | 20.14 ± 1.27 | 16.45 ± 0.24 | 1.94 ± 0.09 |
| Ma1000 | 58.67 ± 0.69 | 20.64 ± 0.22 | 16.08 ± 0.34 | 1.94 ± 0.05 |
| Ma1500 | 59.71 ± 0.38 | 19.72 ± 0.35 | 15.99 ± 0.40 | 1.97 ± 0.02 |
| Sig | n.s. | n.s. | n.s. | n.s. |
| Storage Time at 2 °C | ||||||
|---|---|---|---|---|---|---|
| Parameter | Treatment | Day 1 | Day 6 | Day 13 | Day 21 | p-Value |
| pH | CON † | 5.82 ± 0.01 1 | 5.40 ± 0.02 2 | 5.52 ± 0.07 2 | 5.53 ± 0.06 2 | 0.000 |
| SA | 5.86 ± 0.08 1 | 5.39 ± 0.05 2 | 5.46 ± 0.05 2 | 5.44 ± 0.03 2 | 0.000 | |
| Ma500 | 5.76 ± 0.05 1 | 5.38 ± 0.04 2 | 5.58 ± 0.07 2 | 5.51 ± 0.05 2 | 0.000 | |
| Ma1000 | 5.78 ± 0.09 1 | 5.37 ± 0.06 2 | 5.44 ± 0.08 2 | 5.53 ± 0.03 2 | 0.000 | |
| Ma1500 | 5.79 ± 0.08 1 | 5.36 ± 0.03 2 | 5.49 ± 0.05 2 | 5.52 ± 0.03 2 | 0.000 | |
| p-value | n.s. | n.s. | n.s. | n.s. | ||
| Color | ||||||
| a* | CON | 26.16 ± 1.99 | 26.95 ± 2.19 | 27.13 ± 2.3 | 25.35 ± 0.60 | n.s. |
| SA | 26.23 ± 2.00 | 28.51 ± 0.21 | 29.09 ± 0.27 a | 27.45 ± 1.18 a | n.s. | |
| Ma500 | 26.16 ± 2.06 | 27.08 ± 0.74 | 26.50 ± 0.50 | 24.77 ± 0.57 | n.s. | |
| Ma1000 | 26.40 ± 1.95 | 26.29 ± 1.57 | 26.81 ± 0.69 | 24.82 ± 1.07 | n.s. | |
| Ma1500 | 25.25 ± 1.23 | 25.48 ± 0.38 | 24.49 ± 0.83 b | 23.54 ± 1.43 b | n.s. | |
| p-value | n.s. | n.s. | 0.010 | 0.011 | ||
| b* | CON | 32.64 ± 3.10 1 | 34.25 ± 2.17 | 32.96 ± 4.08 | 31.75 ± 1.63 | n.s. |
| SA | 35.16 ± 3.18 | 35.64 ± 2.3 1 | 37.29 ± 0.75 a2 | 34.33 ± 1.75 | 0.41 | |
| Ma500 | 33.49 ± 3.2 1 | 34.68 ± 1.971 | 34.08 ± 1.92 | 30.83 ± 2.20 | n.s. | |
| Ma1000 | 36.53 ± 0.58 1 | 33.32 ± 2.12 | 33.68 ± 1.45 | 31.81 ± 1.92 2 | n.s. | |
| Ma1500 | 34.09 ± 1.04 | 34.47 ± 1.42 | 30.92 ± 1.55 b | 30.60 ± 2.20 b | 0.020 | |
| p-value | n.s. | n.s. | 0.045 | n.s. | ||
| L* | CON | 46.71 ± 1.52 | 50.80 ± 2.00 | 49.05 ± 3.71 | 45.08 ± 3.49 | n.s. |
| SA | 51.04 ± 3.78 | 48.57 ± 3.59 | 51.73 ± 1.08 | 46.89 ± 1.67 | n.s. | |
| Ma500 | 47.51 ± 1.65 | 46.99 ± 3.28 | 48.57 ± 0.72 | 44.49 ± 3.05 | n.s. | |
| Ma1000 | 51.00 ± 3.10 | 47.69 ± 1.86 | 47.83 ± 3.54 | 46.89 ± 0.81 | n.s. | |
| Ma1500 | 48.66 ± 0.09 | 48.56 ± 2.01 | 46.70 ± 1.48 | 46.95 ± 0.65 | n.s. | |
| p-value | n.s. | n.s. | n.s. | n.s. | ||
| Day 1 | Day 21 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VOCs (%) | RT | Match Factor (%) | m/z | CON | AS | Ma500 | Ma1000 | Ma1500 | CON | AS | Ma500 | Ma1000 | Ma1500 |
| n- hexane | 4.88 | 98.94 | 56.0 | 0.84 | 0.82 | 0.42 1 | 0.15 a1 | 0.90 1 | 0.60 | 0.68 | 4.79 a2 | 2.56 b2 | 2.88 b2 |
| Trichloromethane | 7.37 | 98.28 | 83.0 | 0.13 | 0.16 | 0.07 1 | 0.03 1 | 0.17 1 | 0.08 | 0.11 | 1.12 a2 | 0.72 a2 | 0.70 a2 |
| Octane | 16.98 | 96.56 | 85.0 | 0.27 | 0.21 | 0.09 | 0.08 | 0.17 | 0.29 | 0.12 | 0.09 | 0.11 | 0.08 |
| Cyclopentane | 18.38 | 88.85 | 70.0 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | 0.11 | 0.03 | 0.07 | 0.07 | 0.07 |
| Ethylbenzene | 21.43 | 98.02 | 91.0 | 0.63 | 0.44 | 0.51 | 0.37 | 0.46 | 0.52 | 0.41 | 0.40 | 0.39 | 0.41 |
| Benzene, 1,3-dimethyl- | 21.84 | 99.50 | 91.0 | 2.36 | 1.70 | 1.97 | 1.46 | 1.82 | 1.93 | 1.58 | 1.49 | 1.50 | 1.58 |
| o-Xylene | 23.08 | 97.74 | 91.0 | 0.53 | 0.39 | 0.46 | 0.34 | 0.43 | 0.49 | 0.39 | 0.38 | 0.39 | 0.40 |
| Bicyclo [3,1,0] hex-2-ene, 2-methyl-5-(1-methylethyl)- | 23.83 | 97.36 | 91.0 | 0.27 | 0.27 | 0.21 | 0.21 | 0.28 | 0.29 | 0.29 | 0.26 | 0.25 | 0.26 |
| (1R)-2,6,6-Trimethylbicyclo[3,1,1]hept-2-ene | 24.17 | 98.40 | 93.0 | 1.52 | 1.28 | 1.22 | 1.40 | 1.55 | 1.62 | 1.47 | 1.49 | 1.43 | 1.5 |
| Bicyclo [3,1,1] heptane, 6,6-dimethyl-2-methylene-, (1S)- | 26.26 | 98.00 | 77.0 | 2.39 | 2.20 | 1.90 | 1.94 | 2.40 | 2.18 | 2.14 | 1.97 | 1.92 | 1.94 |
| Heptane, 2,2,4,6,6-pentamethyl- | 26.39 | 96.42 | 56.0 | 0.21 | 0.20 | 0.21 | 0.22 | 0.23 | 0.23 | 0.25 | 0.25 | 0.22 | 0.21 |
| Nonane, 3,7-dimethyl- | 30.33 | 88.93 | 71.0 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.08 | 0.15 | 0.25 | 0.26 | 0.23 |
| Dodecane | 33.79 | 95.56 | 71.0 | 0.02 | 0.05 | 0.02 | 0.02 | 0.03 | 0.36 | 0.64 | 1.01 | 0.96 | 0.84 |
| Decane, 2,9-dimethyl- | 33.80 | 87.53 | 57.0 | 0.03 1 | 0.07 1 | 0.02 1 | 0.02 1 | 0.04 1 | 0.53 2 | 0.93 2 | 1.47 2 | 1.39 2 | 1.20 2 |
| Butane, 2,2-dimethyl- | 36.98 | 94.31 | 71.0 | 0.01 1 | 0.03 1 | 0.01 1 | 0.00 1 | 0.01 1 | 0.36 a2 | 0.55 2 | 0.79 2 | 0.70 2 | 0.73 2 |
| Octane, 2,7-dimethyl- | 36.99 | 90.92 | 57.0 | 0.01 1 | 0.051 | 0.01 1 | 0.01 1 | 0.01 1 | 0.49 2 | 0.75 2 | 1.07 2 | 0.94 | 0.99 2 |
| HDROCARBONS | 8.22 | 6.821 | 6.6 | 6.06 | 7.39 | 8.1 | 7.47 | 7.66 1 | 8.44 1 | 8.25 1,2 | |||
| 1-Butanol, 3-methyl- | 16.61 | 99.36 | 70.0 | 1.18 1 | 2.07 1 | 2.59 1 | 3.32 1 | 3.88 1 | 0.24 2 | 0.19 2 | 0.44 2 | 0.94 2 | 2.01 2 |
| 1-Butanol, 2-methyl- | 16.79 | 95.94 | 56.0 | 0.07 | 0.15 | 0.16 | 0.23 | 0.28 | 0.04 | 0.03 | 0.07 | 0.16 | 0.41 |
| Silanediol, dimethyl- | 18.32 | 98.93 | 45.0 | 0.55 | 0.22 | 0.42 | 0.50 | 0.02 | 0.21 | 0.07 | 0.06 | 0.06 | 0.37 |
| 1-Pentanol | 18.37 | 95.43 | 55.0 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.14 | 0.04 | 0.09 | 0.09 | 0.09 |
| 2,3-Butanediol | 21.30 | 95.58 | 45.0 | 0.43 1 | 0.43 | 0.66 | 0.49 | 0.22 | 1.88 2 | 0.68 | 0.51 | 0.67 | 0.44 |
| 1-Hexanol | 23.24 | 97.43 | 56.0 | 0.05 | 0.06 | 0.06 | 0.07 | 0.11 | 0.82 | 0.24 | 0.48 | 0.56 | 0.48 |
| 2-Ethyl-1-hexanol | 29.61 | 96.75 | 57.0 | 0.09 | 0.05 | 0.06 | 0.03 | 0.06 | 0.06 | 0.07 | 0.07 | 0.09 | 0.05 |
| 1-Hexanol, 2-ethyl- | 29.61 | 95.82 | 83.0 | 0.03 | 0.01 | 0.02 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 |
| Benzyl alcohol | 31.12 | 95.59 | 79.0 | 0.13 | 0.16 | 0.16 | 0.33 | 0.74 1 | 0.29 | 0.30 | 0.61 | 0.99 | 1.61 2 |
| Cyclohexanol, 2,6-dimethyl- | 33.17 | 86.89 | 71.0 | 0.07 | 0.08 | 0.06 | 0.08 | 0.10 | 0.06 | 0.06 | 0.07 | 0.07 | 0.07 |
| Phenylethyl Alcohol | 33.77 | 99.11 | 91.0 | 0.11 1 | 0.19 1 | 0.36 1 | 0.71 1 | 0.97 1 | 3.38 2 | 4.94 2 | 8.46 a2 | 13.36 b2 | 17.77 c2 |
| 3-Cyclohexen-1-ol, 4-methyl-1-(1-methylethyl)-, (R)- | 34.86 | 90.65 | 111.0 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.04 | 0.04 | 0.04 | 0.05 | 0.05 |
| p-Menth-2-en-7-ol, trans- | 38.12 | 87.98 | 93.0 | 0.02 | 0.03 | 0.03 | 0.03 | 0.04 | 0.05 | 0.05 | 0.03 | 0.05 | 0.05 |
| p-Cymen-7-ol | 39.34 | 98.38 | 135.0 | 0.23 | 0.27 | 0.35 | 0.59 | 1.29 | 0.55 | 0.58 | 0.60 | 0.90 | 1.27 |
| ALCOHOLS | 3.00 | 3.76 | 4.95 1 | 6.43 a1 | 7.78 b1 | 7.78 2 | 7.32 2 | 11.55 a2 | 18.56 b2 | 24.68 c2 | |||
| Butanal, 3-methyl- | 9.80 | 99.48 | 58.0 | 0.47 | 0.52 | 0.29 | 0.13 | 0.06 | 0.10 | 0.09 | 0.12 | 0.10 | 0.10 |
| Butanal, 2-methyl- | 10.41 | 97.57 | 57.0 | 0.19 | 0.25 | 0.14 | 0.07 | 0.02 | 0.02 | 0.03 | 0.07 | 0.09 | 0.09 |
| Hexanal | 19.17 | 98.42 | 56.0 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.68 1 | 0.08 | 0.17 2 | 0.13 2 | 0.07 |
| Benzeneacetaldehyde | 30.86 | 91.81 | 91.0 | 0.17 1 | 0.26 1 | 0.22 1 | 0.22 1 | 0.13 1 | 21.58 2 | 21.15 2 | 11.65 2 | 8.05 2 | 5.23 2 |
| Nonanal | 32.08 | 99.23 | 98.0 | 0.12 | 0.08 | 0.02 | 0.01 | 0.01 | 0.14 | 0.12 | 0.08 | 0.08 | 0.04 |
| 3-p-Menthen-7-al | 35.57 | 96.99 | 109.0 | 0.07 | 0.11 | 0.04 | 0.03 | 0.02 | 0.05 | 0.09 | 0.05 | 0.05 | 0.04 |
| Benzaldehyde, 4-(1-methylethyl) | 37.62 | 98.65 | 133.0 | 5.98 1 | 8.92 | 4.87 | 3.87 1 | 4.03 | 7.06 2 | 9.85 | 6.79 | 7.44 2 | 6.04 |
| 4-Isopropylcyclohexa-1,3-dienecarbaldehyde | 39.15 | 97.46 | 79.0 | 0.89 | 1.25 | 0.74 | 0.67 | 1.05 | 1.12 | 1.41 | 1.12 | 1.15 | 1.03 |
| ALDEHYDES | 7.91 a1 | 11.40 b1 | 6.33 ac1 | 5.00 ac1 | 5.35 ac1 | 30.75 a2 | 32.82 a2 | 20.03 b2 | 17.09 b2 | 12.65 c2 | |||
| 2,3-Butanedione | 6.36 | 96.95 | 86.0 | 0.10 | 0.25 | 0.43 | 0.31 | 1.08 1 | 0.07 | 0.18 | 0.09 | 0.03 | 0.03 2 |
| 2-Butanone | 6.60 | 92.04 | 72.0 | 0.00 1 | 0.00 1 | 0.00 1 | 0.02 1 | 0.08 1 | 0.44 2 | 0.19 2 | 0.49 2 | 0.58 2 | 0.53 2 |
| Acetoin | 15.78 | 96.15 | 45.0 | 31.64 a1 | 31.51 a1 | 40.35 b1 | 38.00 b1 | 19.31 c1 | 2.50 2 | 2.65 2 | 2.78 2 | 1.67 2 | 1.89 2 |
| Ethanone. 1-(1H-pyrrol-2-yl) | 32.35 | 97.86 | 94.0 | 0.07 | 0.10 | 0.08 | 0.09 | 0.11 | 0.13 | 0.14 | 0.14 | 0.14 | 0.13 |
| 6-Methyl-3,5-heptadiene-2-one | 32.97 | 87.13 | 109.0 | 0.07 | 0.09 | 0.07 | 0.07 | 0.09 | 0.12 | 0.13 | 0.11 | 0.12 | 0.09 |
| KETONES | 31.88 a1 | 31.95 b1 | 40.93 c1 | 38.49 c1 | 30.67 d1 | 3.25 a2 | 3.29 2 | 3.60 2 | 2.54 2 | 2.67 b | |||
| Thiirane, methyl- | 5.47 | 97.21 | 74.0 | 3.71 1 | 2.29 1 | 3.85 1 | 3.34 1 | 3.89 1 | 0.03 2 | 0.02 2 | 0.01 2 | 0.01 2 | 0.01 2 |
| Sulfide, allyl methyl | 11.58 | 98.21 | 88.0 | 1.02 | 0.78 | 0.64 | 0.66 | 0.85 | 0.74 | 0.62 | 0.46 | 0.54 | 0.45 |
| Disulfide, methyl 2-propenyl | 24.48 | 96.51 | 120.0 | 0.23 | 0.18 | 0.26 | 0.20 | 0.27 | 1.23 | 1.20 | 1.46 | 1.10 | 0.95 |
| Diallyl disulphide | 31.22 | 99.44 | 113.0 | 1.04 | 1.03 | 0.71 | 0.79 | 0.75 | 0.94 | 1.05 | 0.69 | 0.74 | 0.60 |
| SULFUR | 6.01 1 | 4.28 1 | 5.46 1 | 5.00 1 | 5.76 1 | 2.95 2 | 2.89 2 | 2.62 2 | 2.39 2 | 2.01 2 | |||
| Ethylenimine | 4.88 | 87.76 | 41.0 | 0.75 1 | 0.74 1 | 0.36 1 | 0.14 1 | 0.80 1 | 0.54 2 | 0.60 2 | 2.44 2 | 2.24 2 | 2.54 2 |
| 1H-Pyrrole, 3-methyl- | 15.98 | 94.29 | 81.0 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Propane. 2-nitro- | 24.17 | 92.77 | 41.0 | 0.14 | 0.10 | 0.08 | 0.07 | 0.06 | 0.14 | 0.10 | 0.11 | 0.10 | 0.11 |
| 3-Methylpyridazine | 30.65 | 88.55 | 94.0 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 | 0.03 |
| NITROGEN | 0.92 | 0.87 | 0.47 1 | 0.24 1 | 0.9 1 | 0.72 a2 | 0.74 a2 | 2.59 b2 | 2.39 b2 | 2.7 b2 | |||
| alpha-Pinene | 24.17 | 98.34 | 93.0 | 1.53 | 1.28 | 1.23 | 1.41 | 1.55 | 1.62 | 1.47 | 1.49 | 1.43 | 1.50 |
| beta-Pinene | 26.26 | 97.18 | 91.0 | 3.30 | 2.97 | 2.58 | 2.64 | 3.27 | 3.02 | 2.96 | 2.70 | 2.67 | 2.69 |
| beta-Myrcene | 26.66 | 97.81 | 93.0 | 0.96 | 0.97 | 0.81 | 0.93 | 1.16 | 1.28 | 1.43 | 1.14 | 1.19 | 1.25 |
| 3-Carene | 27.48 | 98.80 | 93.0 | 2.84 | 2.23 | 2.40 | 2.93 | 3.00 | 3.45 | 2.95 | 3.08 | 3.43 | 3.45 |
| α-Terpinene | 27.88 | 93.12 | 121.0 | 0.09 | 0.10 | 0.08 | 0.09 | 0.12 | 0.12 | 0.15 | 0.11 | 0.13 | 0.13 |
| D-Limonene | 28.29 | 98.64 | 68.0 | 1.32 | 1.19 | 1.08 | 1.43 | 1.62 | 1.68 | 1.61 | 1.58 | 1.69 | 1.69 |
| o-Cymene | 28.49 | 98.47 | 119.0 | 20.12 a | 20.46 a | 16.94 b | 18.11 ab | 23.77 c | 22.14 | 20.17 | 19.92 | 20.84 | 19.35 |
| Eucalyptol | 28.77 | 97.47 | 154.0 | 0.03 | 0.04 | 0.03 | 0.03 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| γ-Terpinene | 29.42 | 99.11 | 91.0 | 8.36 | 8.48 | 6.98 | 7.40 | 10.03 a1 | 7.47 | 8.35 | 7.02 | 7.23 | 7.15 |
| α-Terpinolene | 30.54 | 93.79 | 136.0 | 0.03 | 0.04 | 0.03 | 0.03 | 0.04 | 0.05 | 0.06 | 0.05 | 0.06 | 0.06 |
| Terpinen-4-ol | 34.85 | 92.34 | 93.0 | 0.02 | 0.03 | 0.02 | 0.02 | 0.04 | 0.04 | 0.05 | 0.04 | 0.05 | 0.05 |
| Caryophyllene | 42.04 | 99.62 | 91.0 | 0.44 | 0.48 | 0.43 | 0.44 | 0.65 | 0.71 | 0.78 | 0.73 | 0.80 | 0.79 |
| Humulene | 42.92 | 94.42 | 93.0 | 0.04 | 0.04 | 0.04 | 0.04 | 0.05 | 0.08 | 0.09 | 0.08 | 0.09 | 0.09 |
| TERPENES | 39.08 1 | 38.30 1 | 32.65 1 | 35.49 1 | 45.35 1 | 41.69 2 | 40.12 2 | 37.97 2 | 39.65 2 | 38.23 2 | |||
| Ethyl Acetate | 6.79 | 88.20 | 43.0 | 0.05 | 0.01 | 0.06 | 0.04 | 0.05 | 0.48 | 0.42 | 0.46 | 0.64 | 0.64 |
| Acetic acid ethenyl ester | 9.80 | 86.04 | 43.0 | 0.46 | 0.44 | 0.25 | 0.11 | 0.05 | 0.08 | 0.08 | 0.09 | 0.09 | 0.08 |
| Methyl isovalerate | 17.30 | 90.51 | 74.0 | 0.00 | 0.01 | 0.01 | 0.02 | 0.05 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Butanoic acid, 2-methyl- ethyl ester | 21.04 | 91.41 | 102.0 | 0.00 | 0.00 | 0.01 | 0.02 | 0.04 | 0.03 | 0.03 | 0.03 | 0.04 | 0.05 |
| Butanoic acid, 3-methyl- ethyl ester | 21.26 | 95.47 | 85.0 | 0.02 | 0.00 | 0.01 | 0.03 | 0.07 | 0.03 | 0.03 | 0.04 | 0.05 | 0.06 |
| 1-Butanol, 3-methyl- acetate | 22.50 | 99.02 | 70.0 | 0.02 | 0.02 | 0.04 | 0.11 | 0.37 | 0.02 | 0.01 | 0.02 | 0.02 | 0.04 |
| 1,5-Dimethyl-1-vinyl-4-hexenyl butyrate | 32.00 | 87.89 | 93.0 | 0.04 | 0.05 | 0.04 | 0.04 | 0.07 | 0.07 | 0.08 | 0.07 | 0.08 | 0.07 |
| Linalyl acetate | 32.01 | 86.47 | 71.0 | 0.03 | 0.04 | 0.03 | 0.04 | 0.05 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 |
| Methyl salicylate | 35.74 | 96.09 | 120.0 | 0.02 | 0.02 | 0.02 | 0.02 | 0.04 | 0.06 | 0.07 | 0.06 | 0.07 | 0.06 |
| ESTER | 0.64 1 | 0.59 1 | 0.48 1 | 0.44 1 | 0.78 1 | 0.83 2 | 0.77 2 | 0.84 2 | 1.06 2 | 1.09 2 | |||
| Dimethyl ether | 2.92 | 92.89 | 46.0 | 0.08 | 0.07 | 0.09 | 0.13 1 | 0.08 | 0.91 | 0.71 | 0.85 | 1.53 2 | 0.97 |
| ETHER | 0.08 1 | 0.07 1 | 0.09 1 | 0.13 a1 | 0.08 1 | 0.91 2 | 0.71 2 | 0.85 2 | 1.53 2 | 0.97 2 | |||
| Fatty Acid (%) | CON | SA | Ma500 | Ma1000 | Ma1500 |
|---|---|---|---|---|---|
| C10:0 | 0.088 ± 0.02 | 0.082 ± 0.02 | 0.082 ± 0.08 | 0.096 ± 0.01 | 0.079 ± 0.01 |
| C12:0 | 0.149 ± 0.01 | 0.111 ± 0.03 | 0.123 ± 0.06 | 0.128 ± 0.01 | 0.080 ± 0.03 a |
| C14:0 | 1.28 ± 0.05 | 1.20 ± 0.06 a | 1.28 ± 0.36 | 1.22 ± 0.06 a | 1.27 ± 0.03 |
| C16:0 | 22.6 ± 0.145 | 22.6 ± 0.34 | 22.4 ± 0.77 | 22.6 ± 0.08 | 22.18 ± 0.20 |
| C16:1n-7 | 1.92 ± 0.07 | 1.92 ± 0.12 | 1.80 ± 0.08 | 1.91 ± 0.08 | 1.66 ± 0.12 a |
| C17:0 | 0.443 ± 0.05 | 0.373 ± 0.08 | 0.356 ± 0.87 | 0.482 ± 0.06 | 0.569 ± 0.11 |
| C17:1n-7 | 0.404 ± 0.08 a | 0.323 ± 0.06 b | 0.268± 0.23 c | 0.352 ± 0.08 ab | 0.460 ± 0.10 c |
| C18:0 | 13.0 ± 0.10 | 13.1 ± 0.12 | 13.2 ± 0.51 | 13.3 ± 0.08 | 13.1 ± 0.07 |
| C18:1n-9 | 39.1 ± 0.38 a | 39.9 ± 0.39 bc | 40.2 ± 0.20 c | 39.6 ± 0.51 b | 39.7 ± 0.33 bc |
| 9t,12t-C18:2 | 1.82 ± 0.12 a | 1.55 ± 0.15 b | 1.55 ± 0.07 b | 1.45 ± 0.01 c | 1.396 ± 0.08 c |
| C18:2n-6 | 15.4 ± 0.11 | 15.4 ± 0.45 | 15.2 ± 0.03 | 15.5 ± 0.09 | 15.4 ± 0.19 |
| C20:0 | 0.304 ± 0.01 a | 0.224 ± 0.06 | 0.2155 ± 0.06 | 0.224 ± 0.09 | 0.295 ± 0.04 a |
| C18:3n-3 | 0.800 ± 0.01 | 0.731 ± 0.07 a | 0.762 ± 0.06 a | 0.737 ± 0.07 a | 0.840 ± 0.07 |
| C20:1n-9 | 0.906 ± 0.03 | 0.870 ± 0.09 | 0.924 ± 0.043 | 0.806 ± 0.00 a | 0.913 ± 0.09 |
| C21:0 | 0.785 ± 0.08 | 0.717 ± 0.00 a | 0.764 ± 0.00 | 0.734 ± 0.00 a | 0.793 ± 0.00 |
| C20:4n-6 | 0.369 ± 0.01 | 0.383 ± 0.02 | 0.396 ± 0.02 a | 0.377 ± 0.01 | 0.380 ± 0.06 a |
| C20:5n-3 | 0.225 ± 0.04 a | 0.177 ± 0.09 b | 0.154 ± 0.06 b | 0.371 ± 0.04 c | 0.223 ± 0.07 a |
| C24:0 | 0.125 ± 0.01 a | 0.208 ± 0.05 | 0.186 ± 0.04 | 0.117 ± 0.02 a | 0.179 ± 0.00 |
| C22:6n-3 | 0.099 ± 0.01 | 0.072 ± 0.05 a | 0.061 ± 0.018 a | 0.127 ± 0.02 | 0.072 ± 0.07 |
| SFA | 38.9 ± 0.50 | 38.5 ± 0.84 | 38.5 ± 1.97 | 38.9 ± 0.55 | 38.5 ± 0.60 |
| MUFA | 44.1 ± 0.57 | 44.5 ± 0.67 | 44.8 ± 1.34 | 44.1 ± 0.66 | 44.2 ± 0.63 |
| PUFA | 18.7 ± 0.34 a | 18.3 ± 0.86 | 18.1 ± 0.26 | 18.3 ± 0.38 | 18.5 ± 0.57 a |
| n-3 | 1.12 ± 0.07 | 0.98 ± 0.21 | 0.98 ± 0.13 | 1.24 ± 0.1 | 1.14 ± 0.20 |
| n-6 | 17.2 ± 0.24 | 17.0 ± 0.62 | 16.8 ± 0.11 | 17.0 ± 0.20 | 17.0 ± 0.33 |
| n-9 | 40.0 ± 0.411 | 40.8 ± 0.481 | 41.2 ± 0.2 a | 40.4 ± 0.51 | 40.7 ± 0.41 |
| Trans | 1.82 ± 0.12 | 1.55 ± 0.15 a | 1.55 ± 0.07 a | 1.45 ± 0.10 c | 1.39 ± 0.08 c |
| PUFA/MUFA | 0.424 | 0.411 | 0.404 | 0.4315 | 0.420 |
| Treatment | Appearance | Color | Juiciness | Odor | Flavor | Texture | General Acceptability | |
|---|---|---|---|---|---|---|---|---|
| CON | 7.0 | 7.2 a | 7.0 | 6.6 a | 6.6 a | 6.4 a | 6.2 a | |
| AS | 7.6 | 7.8 b | 7.8 | 7.8 | 8.0 | 7.6 | 7.6 | |
| Ma500 | 7.8 | 7.8 b | 7.0 | 8.2 | 8.4 | 8.0 | 7.8 | |
| Ma1000 | 6.8 | 6.2 a | 8.0 | 7.4 | 7.6 | 8.0 | 7.6 | |
| Ma1500 | 7.2 | 6.4 c | 7.2 | 7.2 | 7.4 | 7.2 | 7.2 | |
| p-value | 0.100 | 0.028 | 0.084 | 0.045 | 0.050 | 0.041 | 0.037 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velázquez, L.; Quiñones, J.; Sepúlveda-Truan, G.; Díaz, R.; Pateiro, M.; Lorenzo, J.M.; Domínguez-Valencia, R.; Sepúlveda, N. Aristotelia chilensis Leaf Powder as a Sustainable Alternative to Synthetic Antioxidants in Fresh Sausages: Advancing Toward More Natural and Ecological Meat Production. Sustainability 2025, 17, 9624. https://doi.org/10.3390/su17219624
Velázquez L, Quiñones J, Sepúlveda-Truan G, Díaz R, Pateiro M, Lorenzo JM, Domínguez-Valencia R, Sepúlveda N. Aristotelia chilensis Leaf Powder as a Sustainable Alternative to Synthetic Antioxidants in Fresh Sausages: Advancing Toward More Natural and Ecological Meat Production. Sustainability. 2025; 17(21):9624. https://doi.org/10.3390/su17219624
Chicago/Turabian StyleVelázquez, Lidiana, John Quiñones, Gastón Sepúlveda-Truan, Rommy Díaz, Mirian Pateiro, José Manuel Lorenzo, Rubén Domínguez-Valencia, and Néstor Sepúlveda. 2025. "Aristotelia chilensis Leaf Powder as a Sustainable Alternative to Synthetic Antioxidants in Fresh Sausages: Advancing Toward More Natural and Ecological Meat Production" Sustainability 17, no. 21: 9624. https://doi.org/10.3390/su17219624
APA StyleVelázquez, L., Quiñones, J., Sepúlveda-Truan, G., Díaz, R., Pateiro, M., Lorenzo, J. M., Domínguez-Valencia, R., & Sepúlveda, N. (2025). Aristotelia chilensis Leaf Powder as a Sustainable Alternative to Synthetic Antioxidants in Fresh Sausages: Advancing Toward More Natural and Ecological Meat Production. Sustainability, 17(21), 9624. https://doi.org/10.3390/su17219624









