Vegetation Management Changes Community Assembly Rules in Mediterranean Urban Ecosystems—A Mechanistic Case Study
Abstract
1. Introduction
2. Materials and Methods
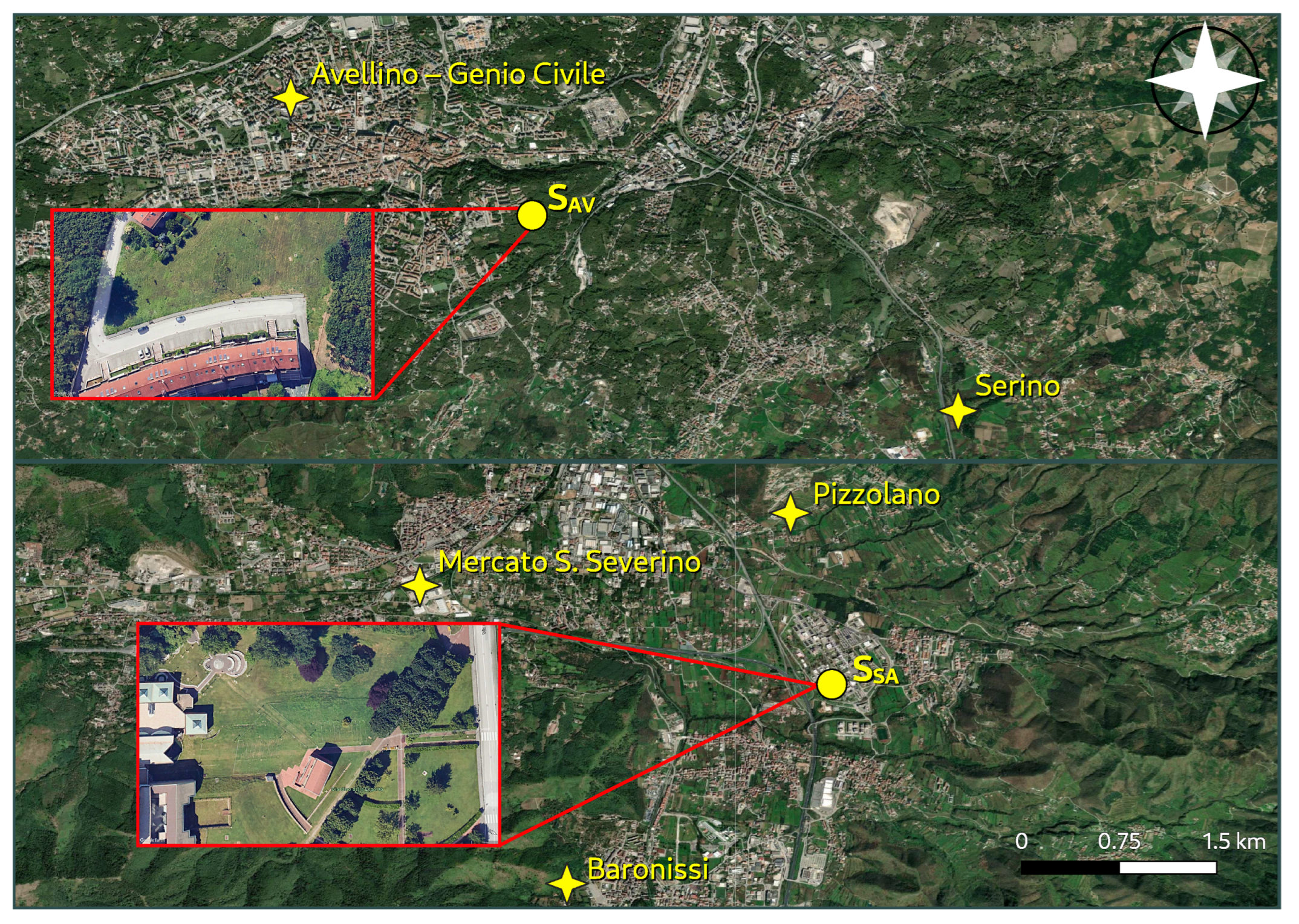
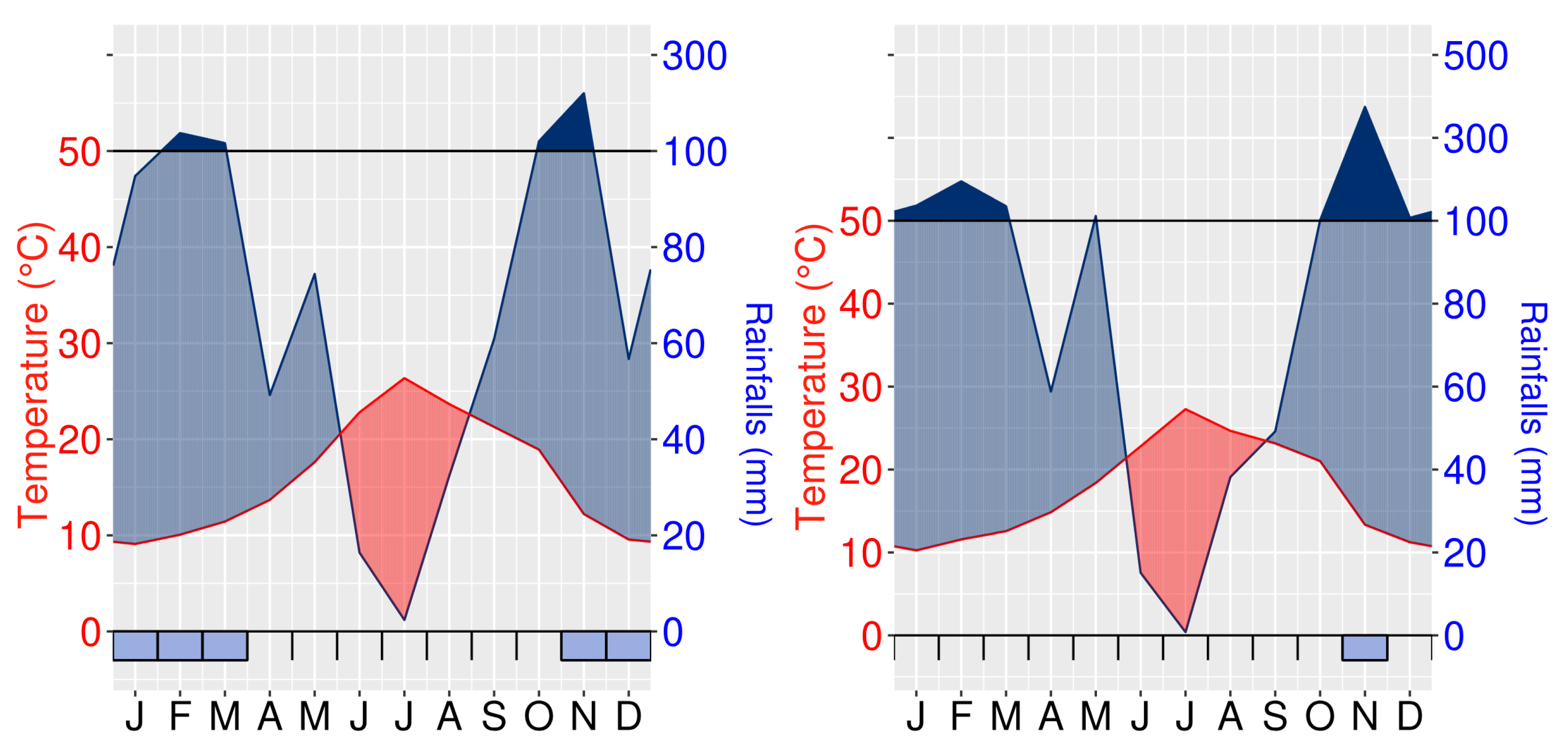
2.1. Study Areas
2.2. Phytosociological Surveys
2.3. Data Analysis
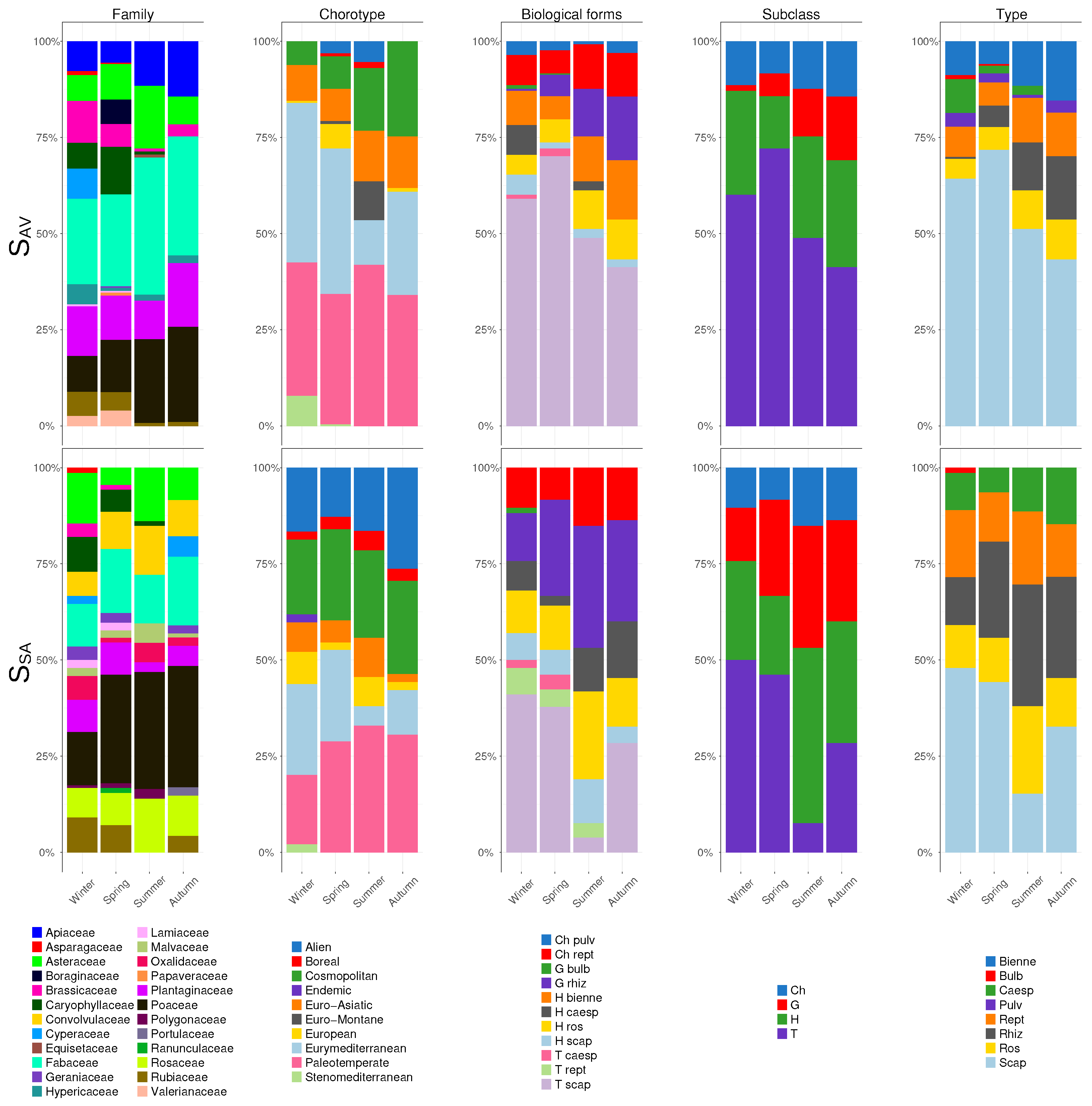
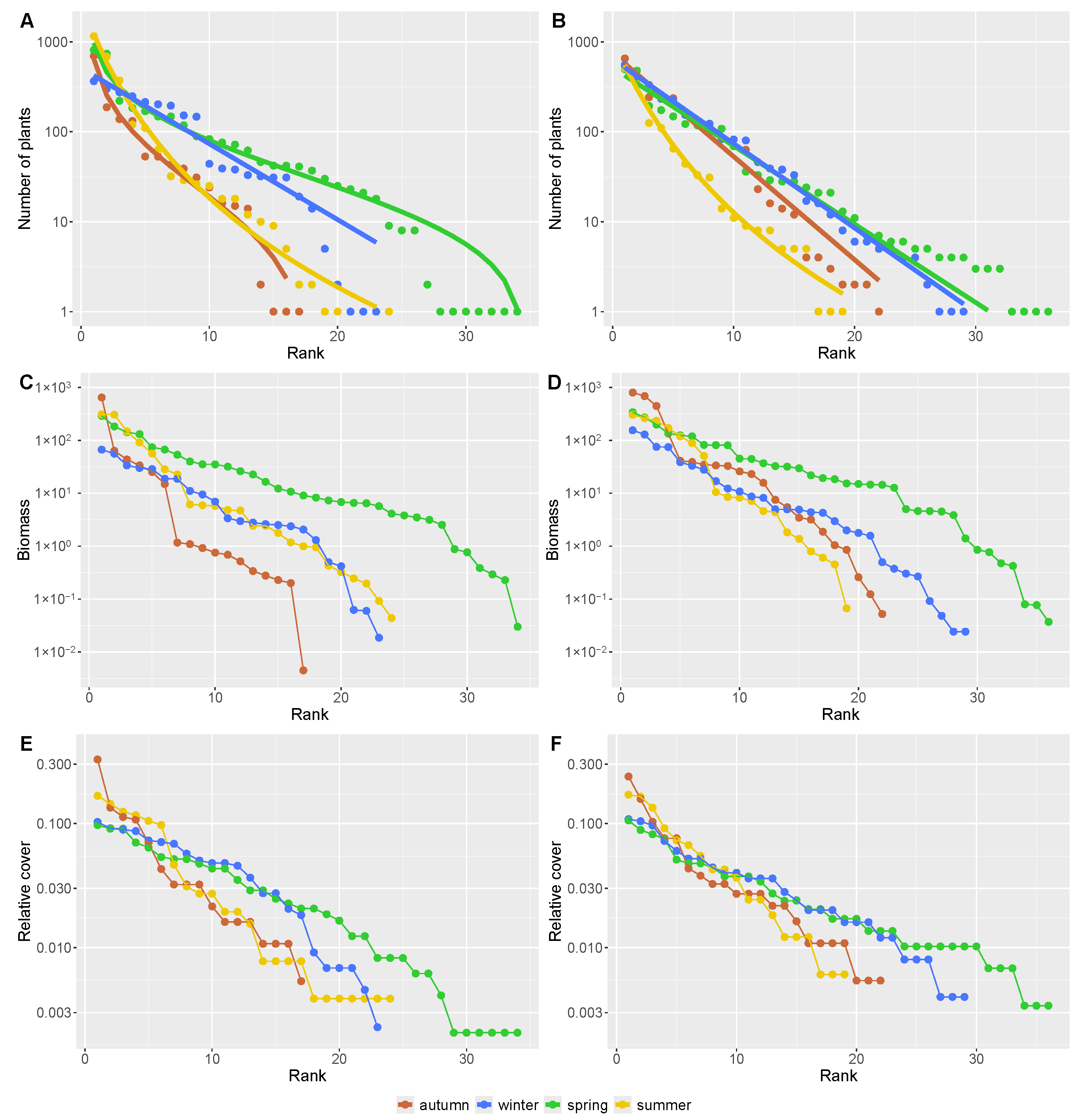
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SAV | Study area in the municipality of Avellino |
| SSA | Study area in the municipality of Fisciano |
| P/A | Presence-Absence |
| NMDS | Non-metric multidimensional scaling |
| PERMDISP | Analysis of multivariate homogeneity of group dispersions |
| PERMANOVA | Permutational multivariate analysis of variance |
| RAD | Rank abundance distribution |
| AIC | Akaike’s information criterion |
References
- Ouyang, X.; Luo, X. Models for assessing urban ecosystem services: Status and outlooks. Sustainability 2022, 14, 4725. [Google Scholar] [CrossRef]
- Teixeira, C.; Fernandes, C. Novel ecosystems: A review of the concept in non-urban and urban contexts. Landsc. Ecol. 2020, 35, 23–29. [Google Scholar] [CrossRef]
- Maibritt, P. Devising urban biodiversity habitat provision goals: Ecosystem services analysis. Forests 2019, 10, 391. [Google Scholar] [CrossRef]
- Etter, A.; McAlpine, C.; Seabrook, L.; Wilson, K. Incorporating temporality and biophysical vulnerability to quantify the human spatial footprint on ecosystems. Biol. Conserv. 2011, 144, 1585–1594. [Google Scholar] [CrossRef]
- Dai, X.; Zheng, H.; Yang, Y.; Meng, N.; Yang, Q.; Zhu, J.; Yan, D.; Li, Z.; Li, R. A new method to quantify the impacts of human activity on soil conservation service. J. Environ. Manag. 2024, 368, 122257. [Google Scholar] [CrossRef]
- Shi, Y.; Yuan, X.; Tang, Y.; Li, Y.; Wang, Q.; Ma, Q.; Zuo, J.; Liu, H. Localized regional life cycle model research for the impacts of carbon dioxide on human health and ecosystem. Sustain. Prod. Consum. 2022, 29, 36–45. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, Q.; He, C.; Wu, J. Impacts of urban expansion on ecosystem services in the Beijing-Tianjin-Hebei urban agglomeration, China: A scenario analysis based on the Shared Socioeconomic Pathways. Resour. Conserv. Recycl. 2017, 125, 115–130. [Google Scholar] [CrossRef]
- Napoletano, M.; Bellino, A.; Baldantoni, D. Depletion and recovery of soil organic matter: Ecological, economic and social implications. Ecol. Civiliz. 2025, 2, 10002. [Google Scholar] [CrossRef]
- Becher, J.; Englisch, C.; Griebler, C.; Bayer, P. Groundwater fauna downtown—Drivers, impacts and implications for subsurface ecosystems in urban areas. J. Contam. Hydrol. 2022, 248, 104021. [Google Scholar] [CrossRef] [PubMed]
- Fasolino, I.; Cicalese, F.; Bellino, A.; Grimaldi, M.; del Caz-Enjuto, M.R.; Baldantoni, D. The ecological efficiency of green materials in sustainable urban planning—A model for its measurement. Sustainability 2023, 15, 16038. [Google Scholar] [CrossRef]
- Chen, X.; Di, Q.; Liang, C. The mechanism and path of pollution reduction and carbon reduction affecting high quality economic development—Taking the Yangtze River Delta urban agglomeration as an example. Appl. Energy 2024, 376, 124340. [Google Scholar] [CrossRef]
- White, P.; Pickett, S. Chapter 1—Natural Disturbance and Patch Dynamics: An Introduction. In The Ecology of Natural Disturbance and Patch Dynamics; Pickett, S.T.A., White, P.S., Eds.; Academic Press: San Diego, CA, USA, 1985; pp. 3–13. [Google Scholar] [CrossRef]
- Gámez, M.; López, I.; Shamandy, A. Open- and closed-loop equilibrium control of trophic chains. Ecol. Model 2010, 221, 1839–1846. [Google Scholar] [CrossRef]
- Newman, E. Disturbance Ecology in the Anthropocene. Front. Ecol. Evol. 2019, 7, 147. [Google Scholar] [CrossRef]
- Boldt, J.; Hazen, E.; Hunsicker, M.; Fu, C.; Perry, R.; Shan, X. Quantifying ecosystem responses to environmental and human pressures in the marine ecosystem off the west coast of Vancouver Island. Ecol. Indic. 2021, 132, 108232. [Google Scholar] [CrossRef]
- Portugal, A.; Carvalho, F.; de Macedo Carneiro, P.; Rossi, S.; de Oliveira Soares, M. Increased anthropogenic pressure decreases species richness in tropical intertidal reefs. Mar. Environ. Res. 2016, 120, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.; So, K.; Shum, B.; Hau, B. Optimal mowing regime in enhancing biodiversity in seasonal floodplains along engineered channels. Sustainability 2022, 14, 4002. [Google Scholar] [CrossRef]
- Toledo-Garibaldi, M.; Gallardo-Hernández, C.; Ulian, T.; Toledo-Aceves, T. Urban forests support natural regeneration of cloud forest trees and shrubs, albeit with limited occurrence of late-successional species. For. Ecol. Manag. 2023, 546, 121327. [Google Scholar] [CrossRef]
- Baldi, V.; Bellino, A.; Baldantoni, D. Small-scale land use effects on plant communities in Mediterranean urban ecosystems. Ecol. Indic. 2025, 170, 113051. [Google Scholar] [CrossRef]
- Milano, V.; Cortet, J.; Baldantoni, D.; Bellino, A.; Dubs, F.; Nahmani, J.; Strumia, S.; Maisto, G. Collembolan biodiversity in Mediterranean urban parks: Impact of history, urbanization, management and soil characteristics. Appl. Soil Ecol. 2017, 119, 428–437. [Google Scholar] [CrossRef]
- Milano, V.; Maisto, G.; Baldantoni, D.; Bellino, A.; Bernard, C.; Croce, A.; Dubs, F.; Strumia, S.; Cortet, J. The effect of urban park landscapes on soil Collembola diversity: A Mediterranean case study. Landsc. Urban Plan. 2018, 180, 135–147. [Google Scholar] [CrossRef]
- Bellino, A.; Baldantoni, D.; Milano, V.; Santorufo, L.; Cortet, J.; Maisto, G. Spatial patterns and scales of Collembola taxonomic and functional diversity in urban parks. Sustainability 2021, 13, 13029. [Google Scholar] [CrossRef]
- Ratajová, A. Study on the dynamics of grass microgametophytes from urban vegetation. Environ. Sci. Pollut. Res. 2014, 21, 6218–6220. [Google Scholar] [CrossRef]
- Jakobsson, S.; Bernes, C.; Bullock, J.; Verheyen, K.; Lindborg, R. How does roadside vegetation management affect the diversity of vascular plants and invertebrates? A systematic review. Environ. Evid. 2018, 7, 17. [Google Scholar] [CrossRef]
- Krajter Ostoić, S.; Salbitano, F.; Borelli, S.; Verlič, A. Urban forest research in the Mediterranean: A systematic review. Urban For. Urban Green. 2018, 31, 185–196. [Google Scholar] [CrossRef]
- Aurelle, D.; Thomas, S.; Albert, C.; Bally, M.; Bondeau, A.; Boudouresque, C.; Cahill, A.; Carlotti, F.; Chenuil, A.; Cramer, W.; et al. Biodiversity, climate change, and adaptation in the Mediterranean. Ecosphere 2022, 13, e3915. [Google Scholar] [CrossRef]
- Bellino, A.; Bellino, L.; Baldantoni, D.; Saracino, A. Evolution, ecology and systematics of Soldanella (Primulaceae) in the southern Apennines (Italy). BMC Evol. Biol. 2015, 15, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Buira, A.; Fernández-Mazuecos, M.; Aedo, C.; Molina-Venegas, R. The contribution of the edaphic factor as a driver of recent plant diversification in a Mediterranean biodiversity hotspot. J. Ecol. 2021, 109, 987–999. [Google Scholar] [CrossRef]
- Braz Pires, M.; Kougioumoutzis, K.; Norder, S.; Dimopoulos, P.; Strid, A.; Panitsa, M. The future of plant diversity within a Mediterranean endemism centre: Modelling the synergistic effects of climate and land-use change in Peloponnese, Greece. Sci. Total. Environ. 2024, 947, 174622. [Google Scholar] [CrossRef] [PubMed]
- Knapp, S.; Kühn, I.; Mosbrugger, V.; Klotz, S. Do protected areas in urban and rural landscapes differ in species diversity? Divers. Distrib. 2008, 14, 622–630. [Google Scholar] [CrossRef]
- Aronson, M.F.J.; La Sorte, F.A.; Nilon, C.H.; Katti, M.; Goddard, M.A.; Lepczyk, C.A.; Warren, P.S.; Williams, N.S.G.; Cilliers, S.; Clarkson, B.; et al. A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc. R. Soc. B 2014, 281, 20133330. [Google Scholar] [CrossRef]
- Williams, N.S.G.; Schwartz, M.W.; Vesk, P.A.; McCarthy, M.A.; Hahs, A.K.; Clemants, S.E.; Corlett, R.T.; Duncan, R.P.; Norton, B.A.; Thompson, K.; et al. A conceptual framework for predicting the effects of urban environments on floras. J. Ecol. 2009, 97, 4–9. [Google Scholar] [CrossRef]
- LaPaix, R.; Freedman, B. Vegetation structure and composition in urban habitats in Halifax, Nova Scotia, Canada. Environ. Manag. 2020, 65, 842–855. [Google Scholar] [CrossRef]
- Li, X.; Fan, S. Seasonal variation in plant functional traits and its implications for ecosystem functioning in a subtropical urban forest. Urban For. Urban Green. 2020, 55, 126824. [Google Scholar] [CrossRef]
- Yang, J.; Luo, X.; Peng, F.; Liu, H.; Sun, S. Temporal dynamics of plant functional traits in response to urbanization. Ecol. Indic. 2021, 129, 107976. [Google Scholar] [CrossRef]
- Lara, W.; Bravo, F.; Maguire, D. Modeling patterns between drought and tree biomass growth from dendrochronological data: A multilevel approach. Agric. For. Meteorol. 2013, 178–179, 140–151. [Google Scholar] [CrossRef]
- Baldantoni, D.; Bellino, A.; Morra, L.; Alfani, A. Compost amendment enhances natural revegetation of a Mediterranean degraded agricultural soil. Environ. Manag. 2015, 56, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Wildi, O. Data Analysis in Vegetation Ecology, 2nd ed.; Wiley: Oxford, UK, 2013; p. 330. [Google Scholar]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia, 2nd ed.; Edagricole: Milan, Italy, 2017. [Google Scholar]
- Pignatti, S. Indicator Values of the Vascular Plants of the Flora of Italy; Università degli Studi di Camerino: Macerata, Italy, 2005; p. 97. [Google Scholar]
- Di Biase, L.; Pace, L.; Mantoni, C.; Fattorini, S. Variations in plant richness, biogeographical composition, and life forms along an elevational gradient in a Mediterranean mountain. Plants 2021, 10, 2090. [Google Scholar] [CrossRef]
- Villéger, S.; Mason, N.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef]
- Whittaker, R. Dominance and diversity in land plant communities: Numerical relations of species express the importance of competition in community function and evolution. Science 1965, 147, 250–260. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Pizarro, M.; Hernangómez, D.; Fernández-Avilés, G. climaemet: Climate AEMET Tools; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar] [CrossRef]
- Grenié, M.; Gruson, H. Fundiversity: Easy Computation of Functional Diversity Indices, R Package Version 1.1.1; R Foundation for Statistical Computing: Vienna, Austria, 2023.
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Kassambara, A. ggpubr: ’ggplot2’ Based Publication Ready Plots, R Package Version 0.6.0; R Foundation for Statistical Computing: Vienna, Austria, 2023.
- Wilke, C.; Wiernik, B. ggtext: Improved Text Rendering Support for ’ggplot2’, R Package Version 0.1.2; R Foundation for Statistical Computing: Vienna, Austria, 2022.
- Auguie, B. gridExtra: Miscellaneous Functions for “Grid” Graphics, R Package Version 2.3; R Foundation for Statistical Computing: Vienna, Austria, 2017.
- Wickham, H.; Vaughan, D.; Girlich, M. tidyr: Tidy Messy Data, R Package Version 1.3.1; R Foundation for Statistical Computing: Vienna, Austria, 2024.
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Henry, M.; Stevens, H.; et al. vegan: Community Ecology Package, R Package Version 2.6-4; R Foundation for Statistical Computing: Vienna, Austria, 2022.
- Ellenberg, H.; Mueller-Dombois, D. A key to Raunkiær plant life forms with revised subdivisions. Ber. Geobot. Inst. Eth Stift RüBel 1965, 37, 56–73. [Google Scholar] [CrossRef]
- Narcizo, A.; Braga, J.; Sartori, R. Impact of exotic tree species on the natural regeneration of an urban restinga forest. Trees 2023, 37, 1643–1655. [Google Scholar] [CrossRef]
- Lubarda, B.; Rat, M.; Petronić, S.; Marić, N.; Maksimović, T.; Anačkov, G. Urban floristic diversity in Bosnia and Herzegovina—The reflection of nature. Urban Ecosyst. 2024, 27, 1067–1083. [Google Scholar] [CrossRef]
- Hayasaka, D.; Akasaka, M.; Miyauchi, D.; Box, E.O.; Uchida, T. Qualitative variation in roadside weed vegetation along an urban–rural road gradient. Flora 2012, 207, 126–132. [Google Scholar] [CrossRef]
- Fornal-Pieniak, B.; Stangierska-Mazurkiewicz, D.; Kamionowski, F.; Widera, K.; Żarska, B.; Latocha, P. Preferences of adults for synanthropic flora in the sustainable development of Polish cities’ green areas. Sustainability 2024, 16, 3610. [Google Scholar] [CrossRef]
- Moszkowicz, L.; Krzeptowska-Moszkowicz, I.; Porada, K.; Zieliński, M. The potential impact of changes in soil and climate conditions on development of the herb layer vegetation of public parks in Krakow (Southern Poland). Sustainability 2024, 16, 451. [Google Scholar] [CrossRef]
- Pianta, M.; Calbi, M.; Dagnino, D.; Turcato, C.; Roccotiello, E. Peri-urban Mediterranean plant communities are shaped by chronic anthropogenic disturbances. Urban For. Urban Green. 2024, 95, 128333. [Google Scholar] [CrossRef]
- Dylewski, L.; Banaszak-Cibicka, W.; Maćkowiak, L.; Dyderski, M. How do urbanization and alien species affect the plant taxonomic, functional, and phylogenetic diversity in different types of urban green areas? Environ. Sci. Pollut. Res. 2023, 30, 92390–92403. [Google Scholar] [CrossRef]
- He, R.; Li, L.; Wang, G.; Cao, L.; Xiong, G.; Yang, F. Plant diversity value of informal green spaces in tropical coastal urban areas: An empirical study of species, functional, and phylogenetic diversity. Sci. Total Environ. 2024, 955, 176741. [Google Scholar] [CrossRef]
- Zheng, B.; Su, L.; Hui, N.; Jumpponen, A.; Kotze, D.J.; Lu, C.; Pouyat, R.; Szlavecz, K.; Wardle, D.A.; Yesilonis, I.; et al. Urbanisation shapes microbial community composition and functional attributes more so than vegetation type in urban greenspaces across climatic zones. Soil. Biol. Biochem. 2024, 191, 109352. [Google Scholar] [CrossRef]
- Hassan, M.; Mohamed, H. Allelopathic interference of the exotic naturalized Paspalum dilatatum Poir. threatens diversity of native plants in urban gardens. Flora 2020, 266, 151593. [Google Scholar] [CrossRef]
- Du, M.; Fan, J.; Liu, M.; Niu, X.; Wang, S. Mowing and phosphorus affect plant diversity and soil carbon and nitrogen storage under nitrogen enrichment in the semi-arid alpine steppe. CATENA 2022, 217, 106458. [Google Scholar] [CrossRef]
- Benvenuti, S. Weed dynamics in the Mediterranean urban ecosystem: Ecology, biodiversity and management. Weed Res. 2004, 44, 341–354. [Google Scholar] [CrossRef]
- Cornelissen, J.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.; Reich, P.; Ter Steege, H.; Morgan, H.; Van Der Heijden, M.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Zeeman, B.; McDonnell, M.; Kendal, D.; Morgan, J. Biotic homogenization in an increasingly urbanized temperate grassland ecosystem. J. Veg. Sci. 2017, 28, 550–561. [Google Scholar] [CrossRef]
- Kelemen, A.; Török, P.; Valkó, O.; Deák, B.; Miglécz, T.; Tóth, K.; Ölvedi, T.; Tóthmérés, B. Sustaining recovered grasslands is not likely without proper management: Vegetation changes after cessation of mowing. Biodivers. Conserv. 2014, 23, 741–751. [Google Scholar] [CrossRef]
- Loreau, M.; de Mazancourt, C. Biodiversity and ecosystem stability: A synthesis of underlying mechanisms. Ecol. Lett. 2013, 16, 106–115. [Google Scholar] [CrossRef]
- Fukami, T. Historical contingency in community assembly: Integrating niches, species pools, and priority effects. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 1–23. [Google Scholar] [CrossRef]
- Shen, M.; Tang, Y.; Chen, J.; Yang, X.; Wang, C.; Cui, X.; Yang, Y.; Han, L.; Li, L.; Du, J.; et al. Earlier-season vegetation has greater temperature sensitivity of spring phenology in northern hemisphere. PLoS ONE 2014, 9, 88178. [Google Scholar] [CrossRef]
- Nothaaß, D.; Huth, A. Community recomposition caused by species extinction in the colonization-competition trade-off model for vegetation. Ecol. Model 2025, 499, 110906. [Google Scholar] [CrossRef]
- Bellino, A.; Mangano, M.; Baldantoni, D.; Russell, B.; Mannino, A.; Mazzola, A.; Vizzini, S.; Sarà, G. Seasonal patterns of biodiversity in Mediterranean coastal lagoons. Divers. Distrib. 2019, 25, 1512–1526. [Google Scholar] [CrossRef]
- Wilson, J.; Gitay, H.; Steel, J.; King, W. Relative abundance distributions in plant communities: Effects of species richness and of spatial scale. J. Veg. Sci. 1998, 9, 213–220. [Google Scholar] [CrossRef]
- Chiarucci, A.; Wilson, J.; Anderson, B.; De Dominicis, V. Cover versus biomass as an estimate of species abundance: Does it make a difference to the conclusions? J. Veg. Sci. 1999, 10, 35–42. [Google Scholar] [CrossRef]
- Steel, J.; Wilson, J.; Anderson, B.; Lodge, R.; Tangney, R. Are bryophyte communities different from higher-plant communities? Abundance relations. Oikos 2004, 104, 479–486. [Google Scholar] [CrossRef]
- Spatharis, S.; Tsirtsis, G. Zipf–Mandelbrot model behavior in marine eutrophication: Two way fitting on field and simulated phytoplankton assemblages. Hydrobiologia 2013, 714, 191–199. [Google Scholar] [CrossRef]
- Knapp, S.; Dinsmore, L.; Fissore, C.; Hobbie, S.E.; Jakobsdottir, I.; Kattge, J.; King, J.Y.; Klotz, S.; McFadden, J.P.; Cavender-Bares, J. Phylogenetic and functional characteristics of household yard floras and their changes along an urbanization gradient. Ecology 2012, 93, S83–S98. [Google Scholar] [CrossRef]
- Knapp, S.; Li, L.; Ladouceur, E.; Wang, J.; Yuan, C.; Wolters, V.; Hackländer, K.; Haddaway, N.R. A role for artificial night-time lighting in plant community dynamics in urban ecosystems. Front. Ecol. Environ. 2021, 19, 150–156. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]


| Species | Family | Form | Chorotype | L | T | C | U | R | N | S | Season | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | Anchusella cretica (Mill.) Bigazzi, E. Nardi & Selvi | Boraginaceae | T scap | Stenomediterranean | 7 | 8 | 6 | 3 | 5 | 6 | 0 | Spr |
| II | Arabidopsis thaliana (L.) Heynh. | Brassicaceae | T scap | Paleotemperate | 6 | 5 | 4 | 5 | 4 | 0 | Spr, Win | |
| III | Artemisia vulgaris L. | Asteraceae | H scap | Boreal | 9 | 7 | 8 | 4 | 5 | 0 | Spr | |
| IV | Avena barbata Pott ex Link | Poaceae | T scap | Eurymediterranean | 8 | 8 | 5 | 3 | 7 | 2 | 0 | Spr, Sum. Win |
| V | Briza media L. | Poaceae | H caesp | Euro-Asiatic | 6 | 4 | 2 | 0 | Sum | |||
| VI | Calepina irregularis (Asso) Thell. | Brassicaceae | T scap | Eurymediterranean | 8 | 8 | 4 | 3 | 5 | 3 | 0 | Spr |
| VII | Capsella bursa-pastoris (L.) Medik. | Brassicaceae | H bienne | Cosmopolitan | 7 | 5 | 5 | 5 | 4 | 0 | Spr, Win | |
| VIII | Cardamine hirsuta L. | Brassicaceae | T scap | Cosmopolitan | 7 | 8 | 5 | 3 | 5 | 4 | 0 | Win |
| IX | Carex distachya Desf. | Cyperaceae | H caesp | Stenomediterranean | 6 | 6 | 4 | 2 | 4 | 5 | 0 | Win |
| X | Cerastium glomeratum Thuill. | Caryophyllaceae | T scap | Eurymediterranean | 7 | 5 | 5 | 5 | 5 | 0 | Spr, Win | |
| XI | Cota tinctoria (L.) J. Gay | Asteraceae | H bienne | European | 8 | 6 | 5 | 2 | 6 | 4 | 0 | Aut, Win |
| XII | Crepis neglecta L. | Asteraceae | T scap | Euro-Montane | 7 | 6 | 3 | 4 | 6 | 3 | 0 | Sum |
| XIII | Crepis sancta L. | Asteraceae | T scap | Eurymediterranean | 11 | 9 | 6 | 2 | 2 | 0 | Aut, Spr, Win | |
| XIV | Cynodon dactylon L. | Poaceae | G rhiz | Cosmopolitan | 8 | 8 | 5 | 4 | 4 | 0 | Aut, Spr, Sum, Win | |
| XV | Daucus carota L. subsp. carota | Apiaceae | H bienne | Paleotemperate | 8 | 6 | 5 | 4 | 5 | 4 | 0 | Aut, Spr, Sum, Win |
| XVI | Digitaria sanguinalis (L.) Scop. | Poaceae | T scap | Cosmopolitan | 7 | 7 | 5 | 3 | 6 | 4 | 0 | Aut |
| XVII | Equisetum telmateja Ehrh. | Equisetaceae | G rhiz | Boreal | 5 | 7 | 4 | 8 | 8 | 5 | 0 | Sum |
| XVIII | Erigeron sumatrensis Retz. | Asteraceae | T scap | Alien | 8 | 8 | 5 | 3 | 7 | 0 | Spr, Sum | |
| XIX | Erodium cicutarium (L.) L’Hér | Geraniaceae | T scap | Cosmopolitan | 8 | 7 | 5 | 3 | 5 | 3 | 0 | Spr |
| XX | Hypericum perforatum L. | Hypericaceae | H scap | Paleotemperate | 7 | 8 | 6 | 0 | Aut, Spr, Sum, Win | |||
| XXI | Hypochaeris radicata L. | Asteraceae | H ros | European | 9 | 8 | 4 | 2 | 1 | 0 | Spr | |
| XXII | Lamium purpureum L. | Lamiaceae | T scap | Euro-Asiatic | 7 | 7 | 5 | 4 | 5 | 5 | 0 | Spr, Win |
| XXIII | Lolium perenne L. | Poaceae | H caesp | Boreal | 8 | 5 | 4 | 5 | 7 | 0 | Sum | |
| XXIV | Medicago arabica (L.) Huds. | Fabaceae | T scap | Eurymediterranean | 9 | 9 | 5 | 2 | 2 | 0 | Aut, Spr, Sum, Win | |
| XXV | Medicago lupulina L. | Fabaceae | T scap | Paleotemperate | 7 | 5 | 4 | 8 | 7 | 0 | Spr, Sum | |
| XXVI | Minuartia hybrida (Vill.) Shischk | Caryophyllaceae | T scap | Paleotemperate | 7 | 7 | 5 | 3 | 6 | 2 | 0 | Spr |
| XXVII | Myosotis ramosissima Rochel subsp. ramosissima | Boraginaceae | T scap | European | 9 | 8 | 5 | 2 | 4 | 3 | 0 | Spr |
| XXVIII | Ornithogalum umbellatum L. | Asparagaceae | G bulb | Eurymediterranean | 5 | 6 | 5 | 5 | 7 | 5 | 0 | Spr, Win |
| XXIX | Ornithopus compressus L. | Fabaceae | T scap | Eurymediterranean | 11 | 9 | 5 | 2 | 2 | 1 | 0 | Aut, Spr, Sum, Win |
| XXX | Papaver rhoeas L. | Papaveraceae | T scap | Euro-Montane | 6 | 6 | 5 | 5 | 7 | 0 | Spr | |
| XXXI | Petrorhagia prolifera (L.) P.W. Ball & Heywood | Caryophyllaceae | T scap | Eurymediterranean | 8 | 5 | 5 | 2 | 2 | 0 | Sum | |
| XXXII | Picris hieraciodes L. | Asteraceae | H scap | Euro-Asiatic | 8 | 5 | 4 | 8 | 4 | 0 | Sum | |
| XXXIII | Plantago lanceolata L. | Plantaginacee | H ros | Euro-Asiatic | 6 | 7 | 5 | 0 | Aut, Spr, Sum, Win | |||
| XXXIV | Poa annua L. | Poaceae | T caesp | Cosmopolitan | 7 | 5 | 6 | 8 | 0 | Spr, Win | ||
| XXXV | Raphanus raphanistrum L. | Brassicaceae | T scap | Eurymediterranean | 11 | 5 | 5 | 4 | 5 | 0 | Aut, Sum | |
| XXXVI | Senecio vulgaris L. | Asteraceae | T scap | Eurymediterranean | 7 | 5 | 8 | 0 | Spr | |||
| XXXVII | Setaria pumila (Poir.) Roem & Schult. | Poaceae | T scap | Cosmopolitan | 7 | 7 | 5 | 4 | 5 | 6 | 0 | Aut, Sum |
| XXXVIII | Sherardia arvensis L. | Rubiaceae | T scap | Eurymediterranean | 8 | 6 | 5 | 5 | 8 | 5 | 0 | Aut, Spr, Sum, Win |
| XXXIX | Silene gallica L. | Caryophyllaceae | T scap | Eurymediterranean | 8 | 9 | 5 | 3 | 2 | 1 | 0 | Spr |
| XL | Sorghum halepense (L.) Pers. | Poaceae | G rhiz | Cosmopolitan | 8 | 8 | 7 | 8 | 8 | 0 | Aut, Sum | |
| XLI | Trifolium arvense | Fabaceae | T scap | Paleotemperate | 8 | 5 | 5 | 2 | 2 | 1 | 0 | Spr |
| XLII | Trifolium campestre Schreb. | Fabaceae | T scap | Paleotemperate | 8 | 5 | 5 | 4 | 3 | 0 | Spr, Sum | |
| XLIII | Trifolium pratense L. | Fabaceae | Ch pulv | Euro-Asiatic | 7 | 4 | 0 | Aut, Spr, Sum, Win | ||||
| XLIV | Trifolium repens L. | Fabaceae | Ch rept | Paleotemperate | 8 | 7 | 0 | Aut, Spr, Sum, Win | ||||
| XLV | Valerianella carinata Loisel. | Valerianaceae | T scap | Eurymediterranean | 7 | 8 | 5 | 4 | 8 | 0 | Spr, Win | |
| XLVI | Veronica arvensis L. | Plantaginacee | T scap | Paleotemperate | 5 | 5 | 5 | 5 | 6 | 0 | Aut, Spr, Win | |
| XLVII | Vicia sativa L. | Fabaceae | T scap | Eurymediterranean | 5 | 5 | 6 | 0 | Aut, Spr, Sum, Win |
| Species | Family | Form | Chorotype | L | T | C | U | R | N | S | Season | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | Artemisia vulgaris L. | Asteraceae | H scap | Boreal | 9 | 7 | 8 | 4 | 5 | 0 | Aut, Spr, Sum, Win | |
| II | Avena barbata Pott ex Link | Poaceae | T scap | Eurymediterranean | 8 | 8 | 5 | 3 | 7 | 2 | 0 | Spr |
| III | Bromus hordeaceus L. | Poaceae | T scap | Cosmopolitan | 7 | 6 | 5 | 0 | Spr | |||
| IV | Cardamine hirsuta L. | Brassicaceae | T scap | Cosmopolitan | 7 | 8 | 5 | 3 | 5 | 4 | 0 | Spr, Win |
| V | Carex distachya Desf. | Cyperaceae | H caesp | Stenomediterranean | 6 | 6 | 4 | 2 | 4 | 5 | 0 | Win |
| VI | Catapodium rigidum (L.) C.E. Hubb. | Poaceae | T scap | Eurymediterranean | 8 | 8 | 5 | 2 | 5 | 4 | 0 | Spr |
| VII | Cerastium glomeratum Thuill. | Caryophillaceae | T scap | Eurymediterranean | 7 | 5 | 5 | 5 | 5 | 0 | Spr, Win | |
| VIII | Convolvolus arvensis L. | Convolvulaceae | G rhiz | Paleotemperate | 7 | 7 | 5 | 4 | 5 | 5 | 0 | Aut, Spr, Sum |
| IX | Crepis bursifolia L. | Asteraceae | H scap | Endemic | 9 | 6 | 4 | 3 | 8 | 2 | 0 | Win |
| X | Crepis neglecta L. | Asteraceae | T scap | European | 7 | 6 | 3 | 4 | 6 | 3 | 0 | Win |
| XI | Cynodon dactylon L. | Poaceae | G rhiz | Cosmopolitan | 8 | 8 | 5 | 4 | 4 | 0 | Aut, Spr, Sum, Win | |
| XII | Cyperus rotundus L. | Cyperaceae | G rhiz | Cosmopolitan | 8 | 10 | 5 | 6 | 8 | 5 | 0 | Aut |
| XIII | Dactylis glomerata L. | Poaceae | H caesp | Paleotemperate | 7 | 6 | 5 | 4 | 5 | 6 | 0 | Spr, Sum, Win |
| XIV | Dichondra micrantha Urb. | Convolvulaceae | G rhiz | Alien | 5 | 8 | 5 | 6 | 3 | 2 | 0 | Aut, Spr, Sum, Win |
| XV | Digitaria sanguinalis (L.) Scop. | Poaceae | T scap | Cosmopolitan | 7 | 7 | 5 | 3 | 6 | 4 | 0 | Aut |
| XVI | Erigeron canadiensis L. | Asteraceae | T scap | Alien | 8 | 8 | 5 | 3 | 7 | 0 | Aut | |
| XVII | Festuca ciliata Gouan | Poaceae | T caesp | Eurymediterranean | 8 | 9 | 5 | 2 | 4 | 2 | 0 | Spr |
| XVIII | Geranium rotundifolium L. | Geraniaceae | T scap | Paleotemperate | 7 | 8 | 5 | 3 | 6 | 3 | 0 | Aut, Spr, Win |
| XIX | Hypochaeris radicata L. | Asteraceae | H ros | European | 9 | 8 | 4 | 2 | 1 | 0 | Aut, Spr, Sum, Win | |
| XX | Lamium purpureum L. | Lamiaceae | T scap | Euro-Asiatic | 7 | 7 | 5 | 4 | 5 | 5 | 0 | Spr, Win |
| XXI | Malva sylvestris L. | Malvaceae | H scap | Euro-Asiatic | 8 | 6 | 4 | 4 | 8 | 0 | Aut, Spr, Sum, Win | |
| XXII | Medicago arabica (L.) Huds. | Fabaceae | T scap | Eurymediterranean | 9 | 9 | 5 | 2 | 2 | 0 | Aut, Spr, Win | |
| XXIII | Medicago lupulina L. | Fabaceae | T scap | Paleotemperate | 7 | 5 | 4 | 8 | 7 | 0 | Aut, Spr, Sum | |
| XXIV | Medicago sativa L. | Fabaceae | H scap | Euro-Asiatic | 8 | 5 | 7 | 3 | 9 | 3 | 0 | Sum |
| XXV | Medicago tenoreana Ser. | Fabaceae | T scap | European | 11 | 9 | 6 | 2 | 2 | 0 | Win | |
| XXVI | Ornithogalum umbellatum L. | Asparagaceae | G bulb | Eurymediterranean | 5 | 6 | 5 | 5 | 7 | 5 | 0 | Win |
| XXVII | Oxalis corniculata L. | Oxalidaceae | Ch rept | Eurymediterranean | 7 | 7 | 0 | 4 | 6 | 0 | Aut, Spr, Sum, Win | |
| XXVIII | Paspalum dilatatum Poir. | Poaceae | H caesp | Alien | 8 | 10 | 8 | 8 | 0 | Aut, Sum, Win | ||
| XXIX | Plantago lanceolata L. | Plantaginaceae | H ros | Euro-Asiatic | 6 | 7 | 5 | 0 | Spr, Sum, Win | |||
| XXX | Poa annua L. | Poaceae | T caesp | Cosmopolitan | 7 | 5 | 6 | 8 | 0 | Spr, Win | ||
| XXXI | Polygonum aviculare L. | Polygonaceae | T rept | Cosmopolitan | 7 | 7 | 5 | 3 | 6 | 1 | 0 | Spr, Sum |
| XXXII | Portulaca oleracea L. | Portulacaceae | T scap | Cosmopolitan | 7 | 8 | 5 | 4 | 7 | 7 | 0 | Aut |
| XXXIII | Potentilla reptans L. | Rosaceae | H ros | Paleotemperate | 6 | 6 | 5 | 6 | 7 | 5 | 0 | Aut, Spr, Sum, Win |
| XXXIV | Prunella vulgaris L. | Lamiaceae | H scap | Boreal | 7 | 6 | 4 | 6 | 4 | 0 | Spr | |
| XXXV | Ranunculus arvensis L. | Ranunculaceae | T scap | Paleotemperate | 6 | 6 | 5 | 4 | 8 | 0 | Spr | |
| XXXVI | Raphanus raphanistrum L. | Brassicaceae | T scap | Eurymediterranean | 11 | 5 | 5 | 4 | 5 | 0 | Spr | |
| XXXVII | Rumex crispus L. | Polygonaceae | H scap | Cosmopolitan | 7 | 5 | 5 | 6 | 5 | 0 | Spr, Win | |
| XXXVIII | Senecio vulgaris L. | Asteraceae | T scap | Eurymediterranean | 7 | 5 | 8 | 0 | Win | |||
| XXXIX | Sherardia arvensis L. | Rubiaceae | T scap | Eurymediterranean | 8 | 6 | 5 | 5 | 8 | 5 | 0 | Aut, Spr, Win |
| XL | Silene dioica (L.) Clairv. | Caryophillaceae | H scap | Paleotemperate | 7 | 5 | 5 | 6 | 7 | 8 | 0 | Spr, Sum, Win |
| XLI | Sonchus oleraceus L. | Asteraceae | T scap | Euro-Asiatic | 7 | 5 | Aut, Spr, Sum, Win | |||||
| XLII | Sorghum halepense (L.) Pers. | Poaceae | G rhiz | Cosmopolitan | 8 | 8 | 7 | 8 | 8 | 0 | Aut, Spr, Sum | |
| XLIII | Stellaria media (L.) Vill. | Caryophillaceae | T rept | Cosmopolitan | 6 | 4 | 7 | 8 | 0 | Spr, Sum, Win | ||
| XLIV | Symphyotrichum squamatum (Spreng.) G.L. Nesom | Asteraceae | T scap | Alien | 8 | 8 | 5 | 4 | 7 | 7 | 0 | Aut |
| XLV | Trifolium campestre Schreb. | Fabaceae | T scap | Paleotemperate | 8 | 5 | 5 | 4 | 3 | 0 | Spr | |
| XLVI | Trifolium repens L. | Fabaceae | Ch rept | Paleotemperate | 8 | 7 | 0 | Aut, Spr, Sum, Win | ||||
| XLVII | Trifolium resupinatum L. | Fabaceae | T rept | Paleotemperate | 8 | 8 | 5 | 5 | 5 | 0 | Spr | |
| XLVIII | Veronica persica Poir. | Plantaginaceae | T scap | Alien | 8 | 7 | 5 | 5 | 5 | 6 | 0 | Aut, Win, Spr |
| XLIX | Vicia sativa L. | Fabaceae | T scap | Euro-Asiatic | 5 | 5 | 6 | 0 | Spr, Win |
| PERMANOVA | PERMDISP | ||||||
|---|---|---|---|---|---|---|---|
| Season | SAV | SSA | ϱ (%) | SAV | SSA | ϱ (%) | |
| Structural | P/A | 32.6 * | 7.43 * | −77.2 | 0.0559 | 0.0703 | 25.7 |
| Number | 16.3 * | 3.87 * | −76.2 | 0.291 | 0.409 | 40.6 | |
| Biomass | 19.5 * | 6.28 * | −67.8 | 0.194 | 0.369 | 90.0 | |
| Cover | 25.3 * | 4.99 * | −80.3 | 0.106 | 0.128 | 20.9 | |
| Functional | P/A | 13.3 * | 2.96 * | −77.7 | 1.28 | 2.06 | 60.9 |
| Number | 8.50 * | 1.90 | −77.6 | 1.28 | 1.88 | 46.9 | |
| Biomass | 8.72 * | 1.27 | −85.4 | 1.54 | 1.87 | 21.4 | |
| Cover | 8.81 * | 1.94 | −78.0 | 1.27 | 2.03 | 59.8 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldi, V.; Bellino, A.; Napoletano, M.; Baldantoni, D. Vegetation Management Changes Community Assembly Rules in Mediterranean Urban Ecosystems—A Mechanistic Case Study. Sustainability 2025, 17, 9516. https://doi.org/10.3390/su17219516
Baldi V, Bellino A, Napoletano M, Baldantoni D. Vegetation Management Changes Community Assembly Rules in Mediterranean Urban Ecosystems—A Mechanistic Case Study. Sustainability. 2025; 17(21):9516. https://doi.org/10.3390/su17219516
Chicago/Turabian StyleBaldi, Vincenzo, Alessandro Bellino, Mattia Napoletano, and Daniela Baldantoni. 2025. "Vegetation Management Changes Community Assembly Rules in Mediterranean Urban Ecosystems—A Mechanistic Case Study" Sustainability 17, no. 21: 9516. https://doi.org/10.3390/su17219516
APA StyleBaldi, V., Bellino, A., Napoletano, M., & Baldantoni, D. (2025). Vegetation Management Changes Community Assembly Rules in Mediterranean Urban Ecosystems—A Mechanistic Case Study. Sustainability, 17(21), 9516. https://doi.org/10.3390/su17219516









