Long-Term Assessment of Wound Healing in Damaged Residual Trees Under Continuous Cover Forestry in the Hyrcanian Broad-Leaved Forests
Abstract
1. Introduction
2. Materials and Methods
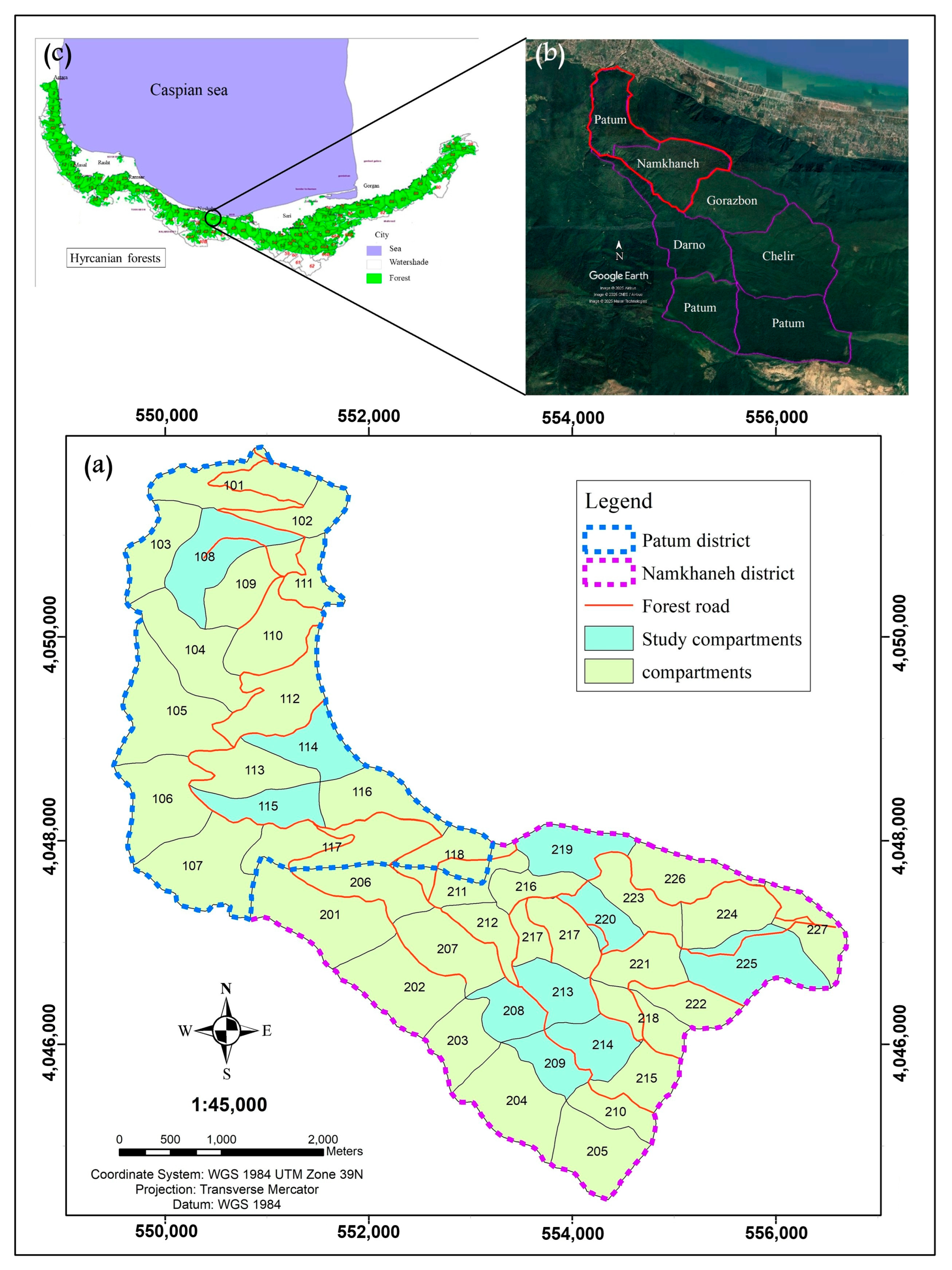
2.1. Study Area
2.2. Research Method
- (a)
- Species type: Oriental beech (Fagus orientalis Lipsky), Hornbeam (Carpinus betulus L.), Velvet maple (Acer velutinum Boiss.), Chestnut-leaved oak (Quercus castaneifolia C.A.M.), Caucasian alder (Alnus subcordata C.A.M.), and Date-plum (Diospyrus lotus L.)
- (b)
- Wound location: with code one (root collar), code two (up to 1 m height), code three (1–2 m height), and code four (height more than 2 m).
- (c)
- Wound severity: with code one (superficial; damage to bark), code two (deep; damage to cambium), and code three (very deep; deep gouging).
- (d)
- Wound area (cm2).
- (e)
- Distance from the skid trail: the distance from the center of the skid trail of each damaged tree was recorded, up to 5 m on both sides.
- (f)
- Traffic intensity: with code one (low intensity), code two (medium intensity), and code three (high intensity).
2.3. Data Analysis
3. Results
3.1. Relationship Between Species and Healing Ratio
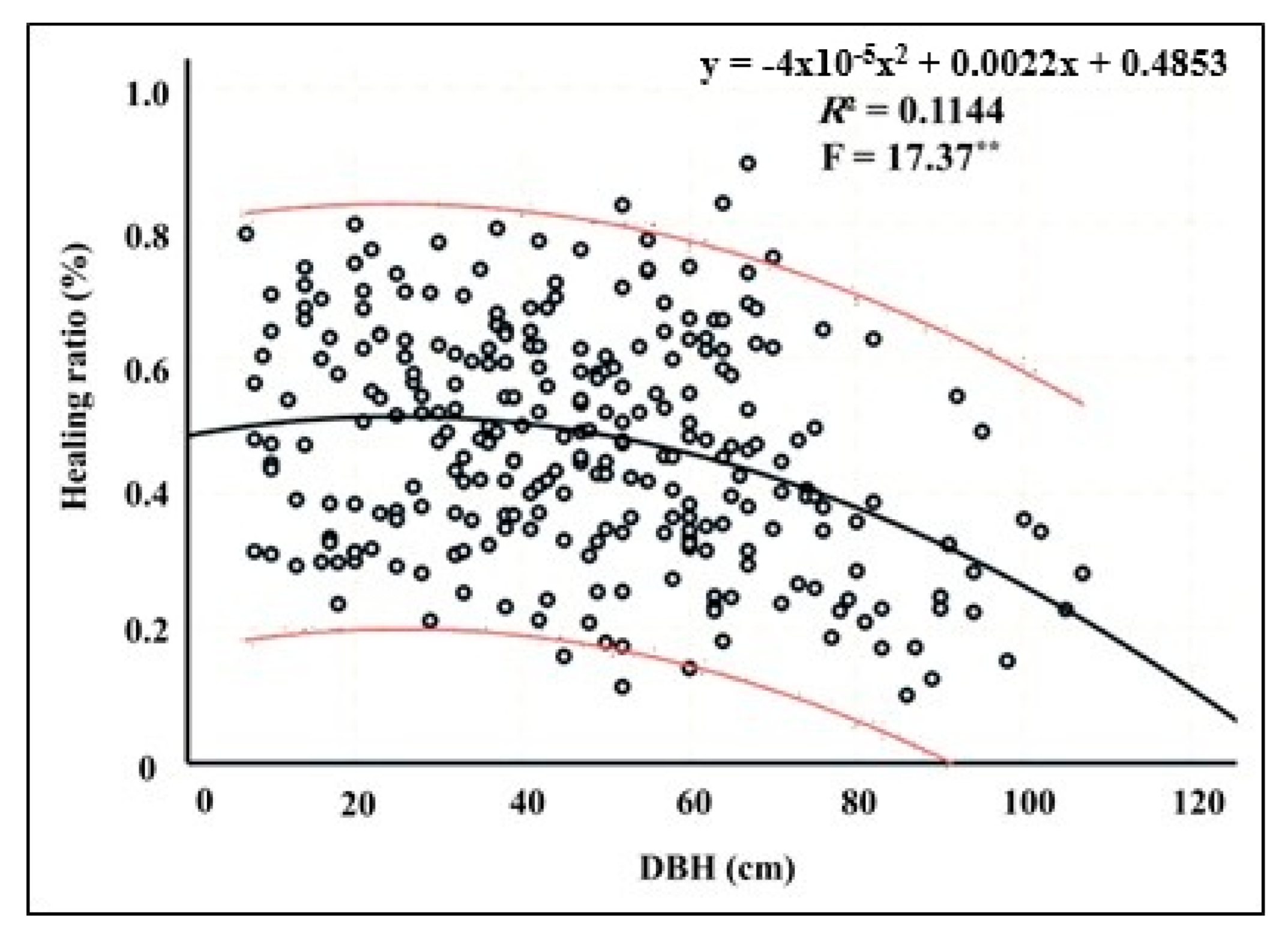
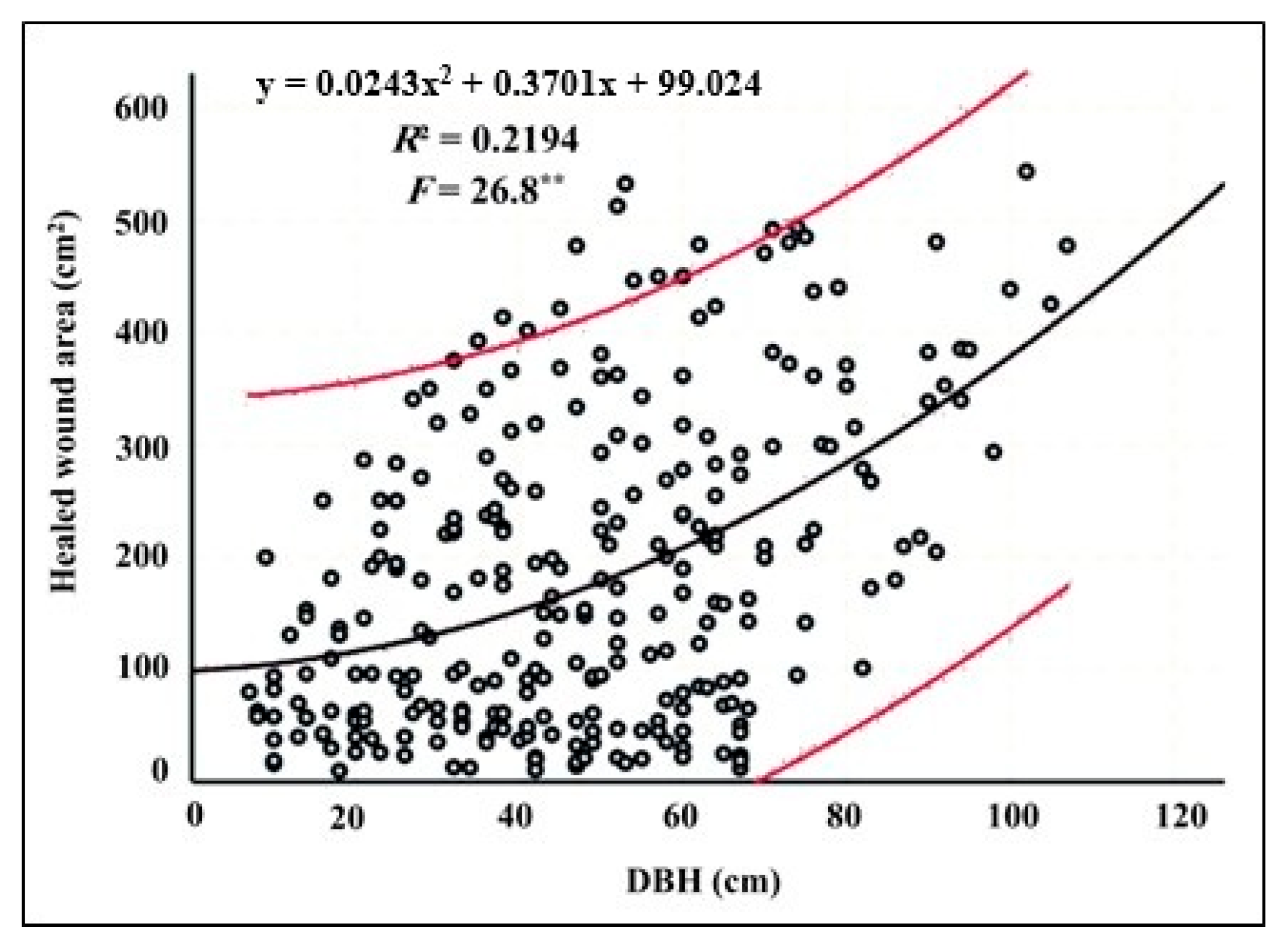
3.2. Relationship Between Tree Diameter and Healing
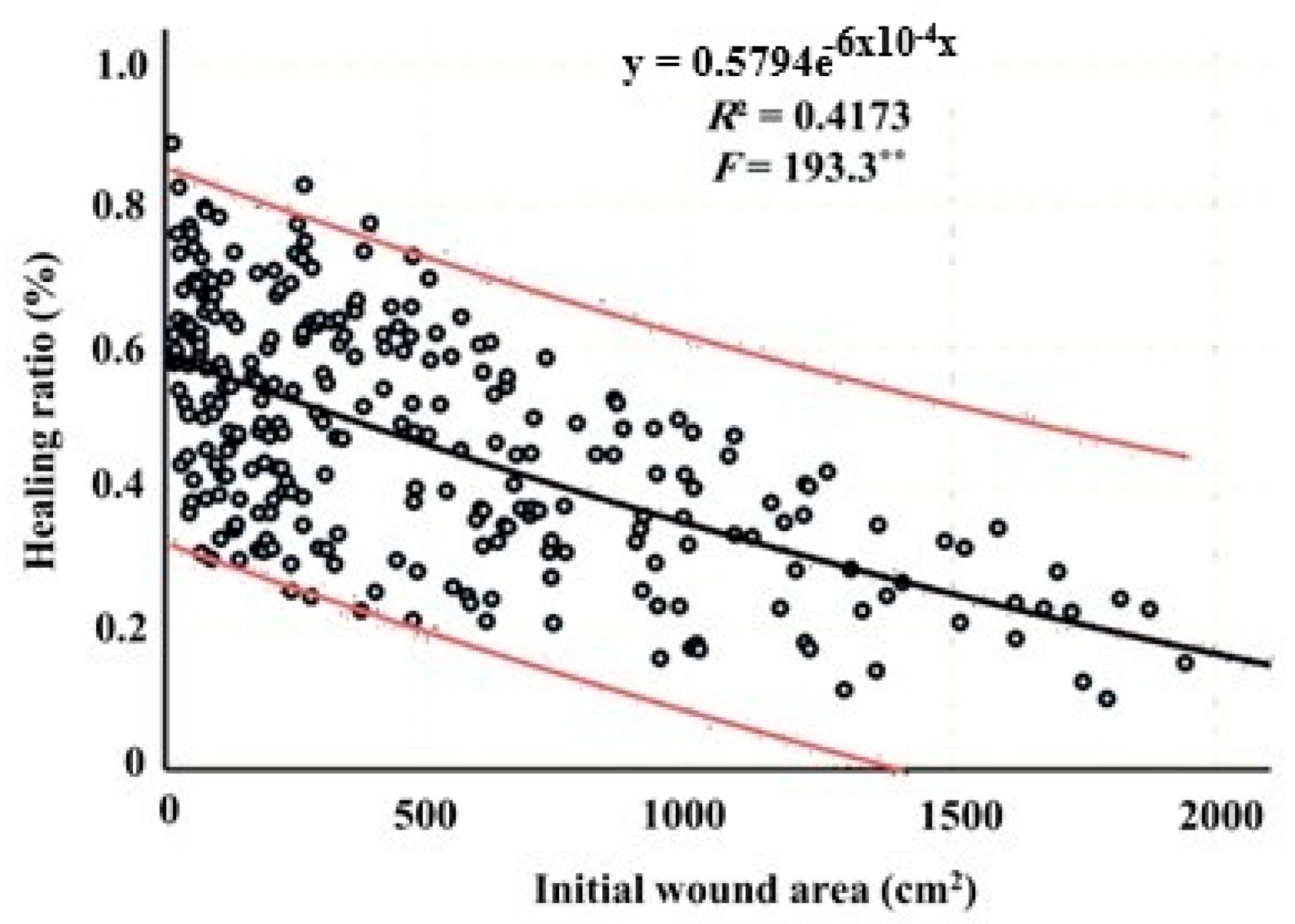
3.3. Relationship Between Initial Wound Area and Healing Ratio
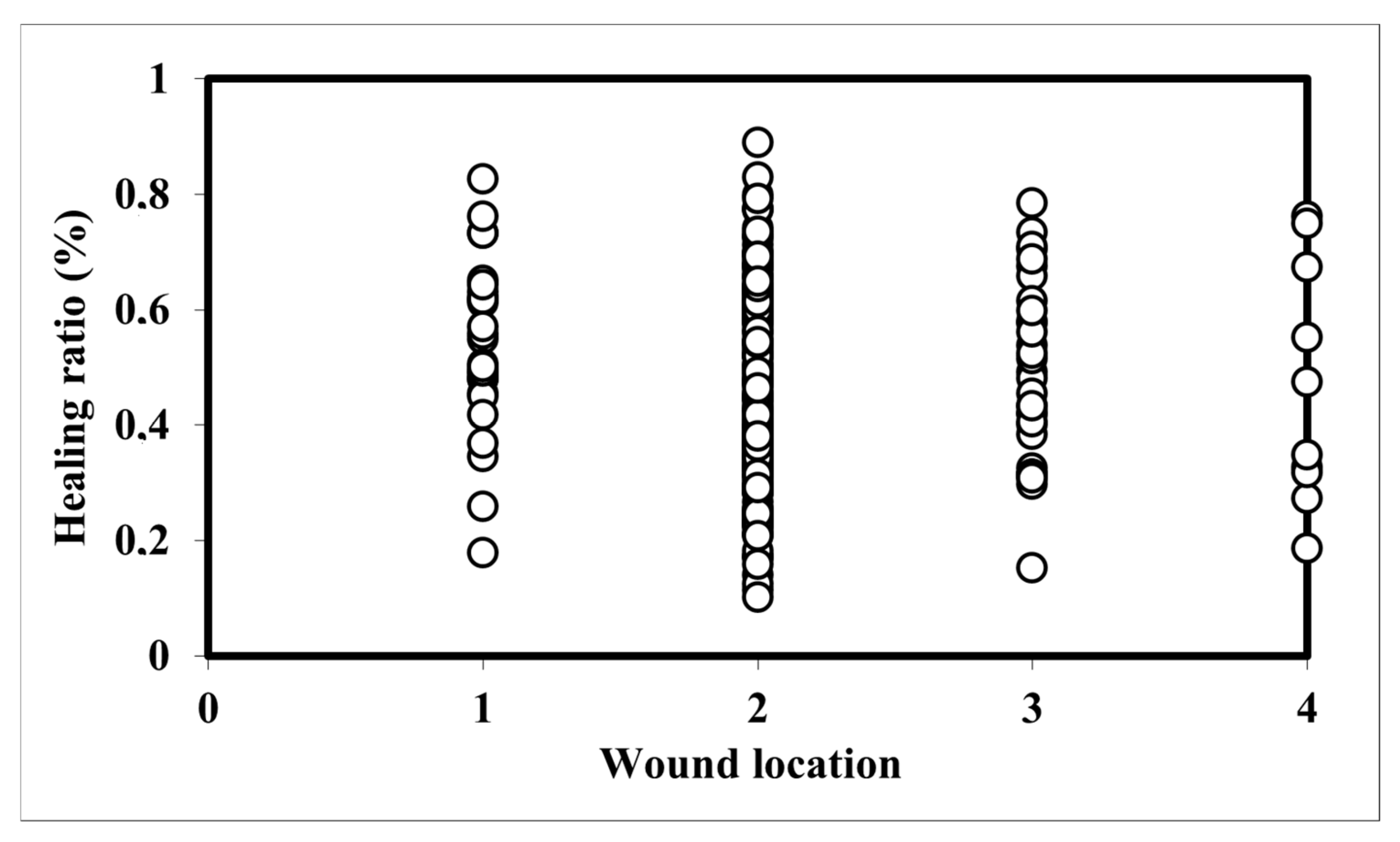
3.4. Relationship Between Wound Location and Healing Ratio
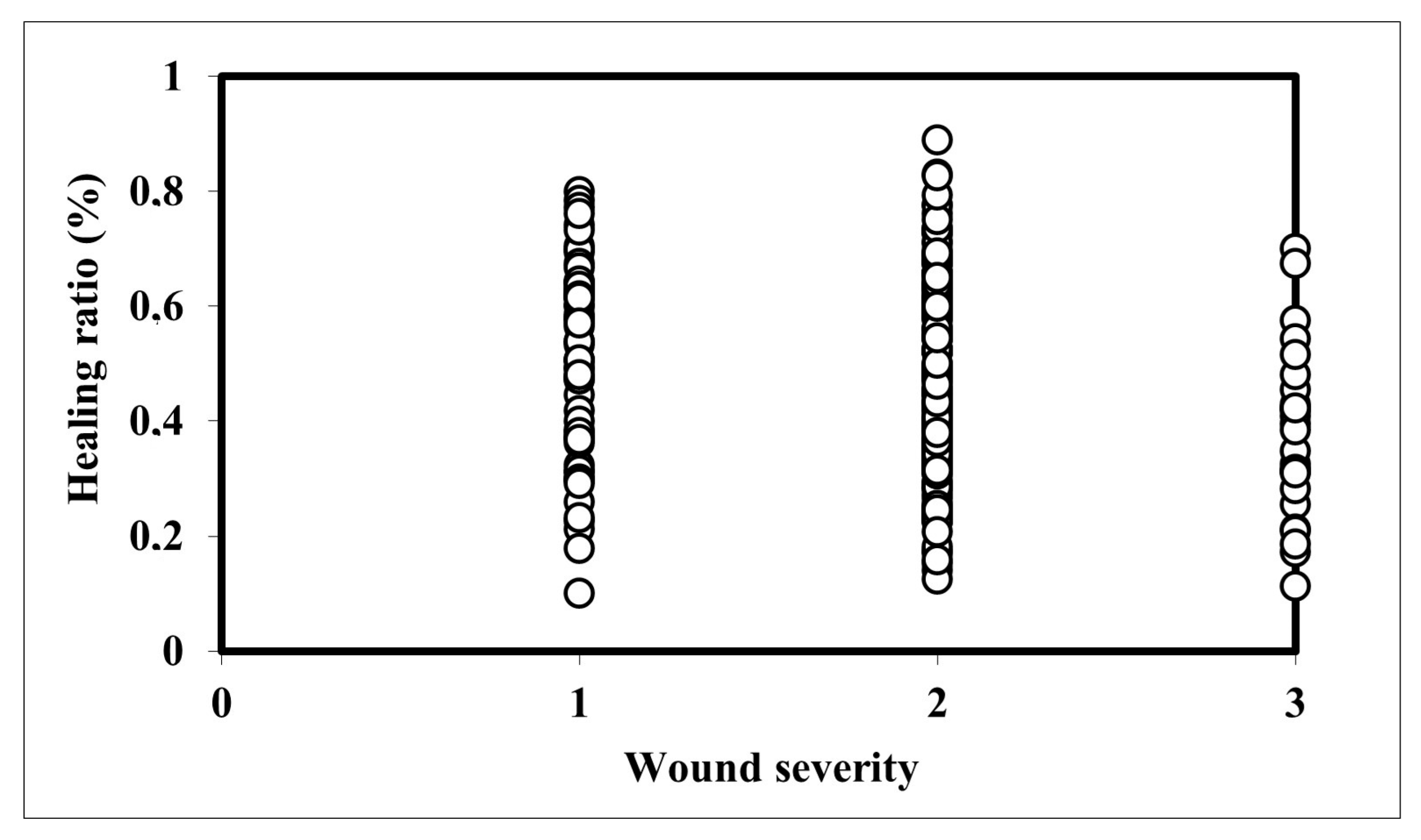
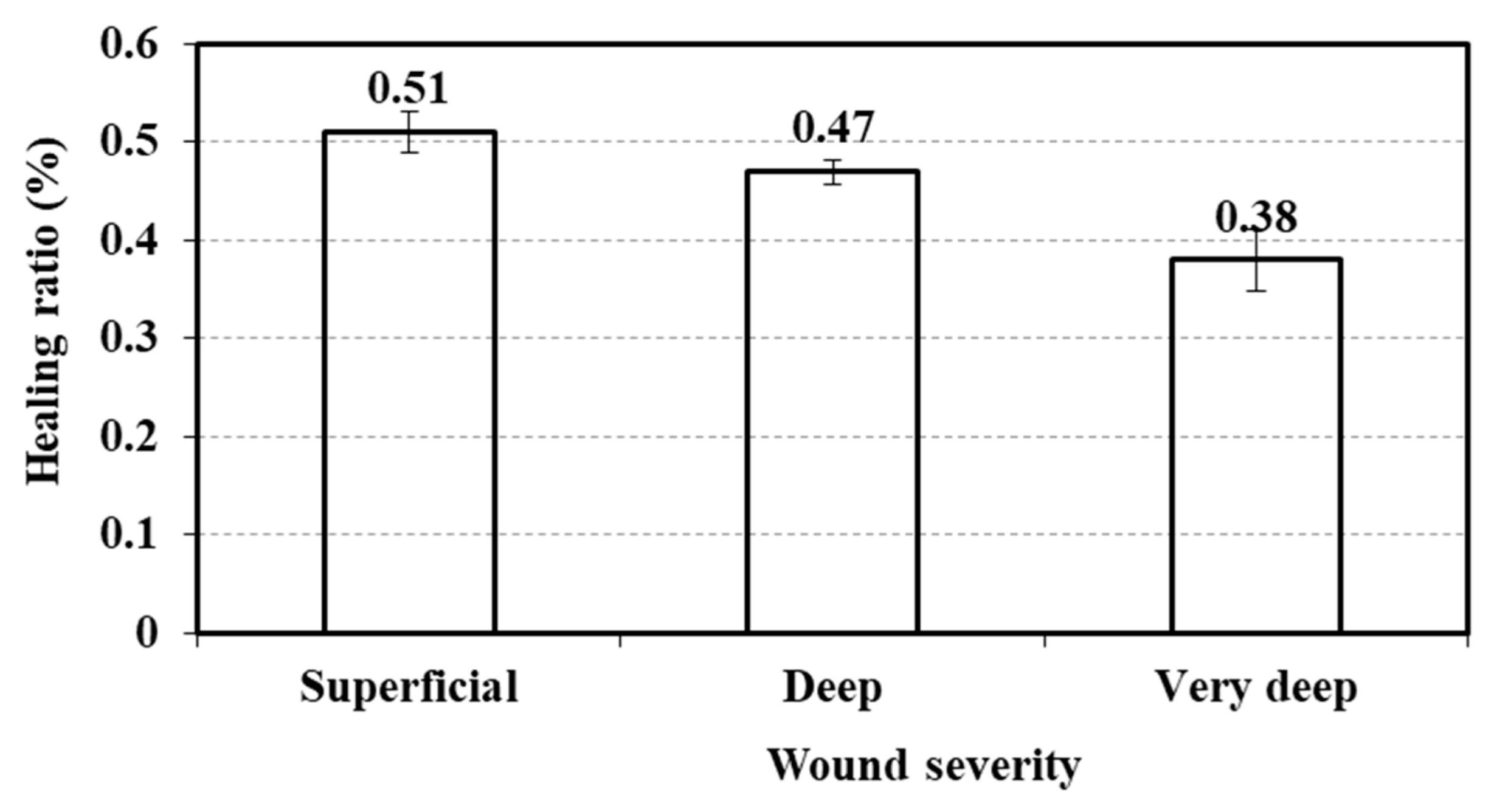
3.5. Relationship Between Wound Severity and Healing Ratio
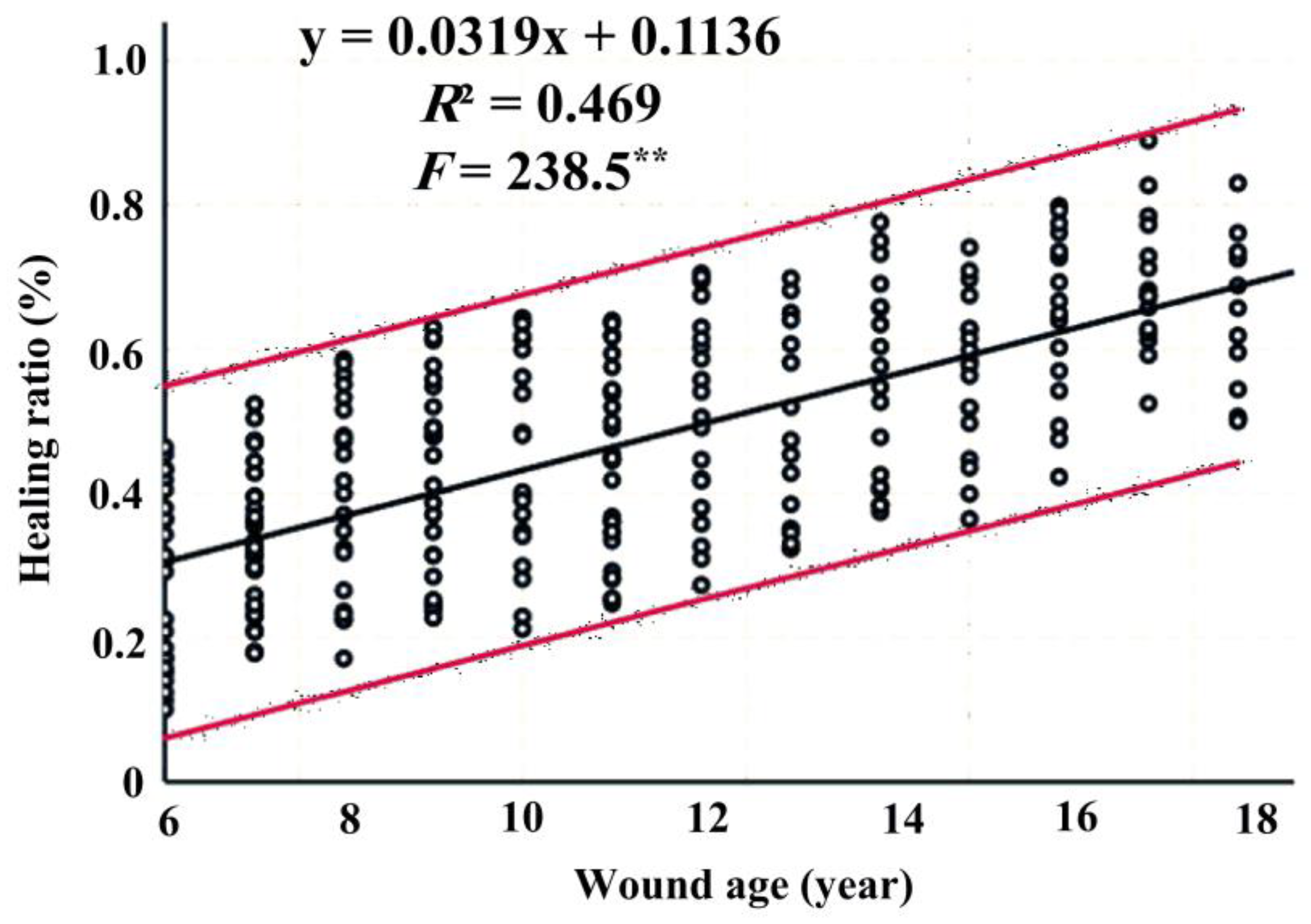
3.6. Relationship Between Wound Age and Healing Ratio
3.7. Relationship Between Traffic Intensity and Healing
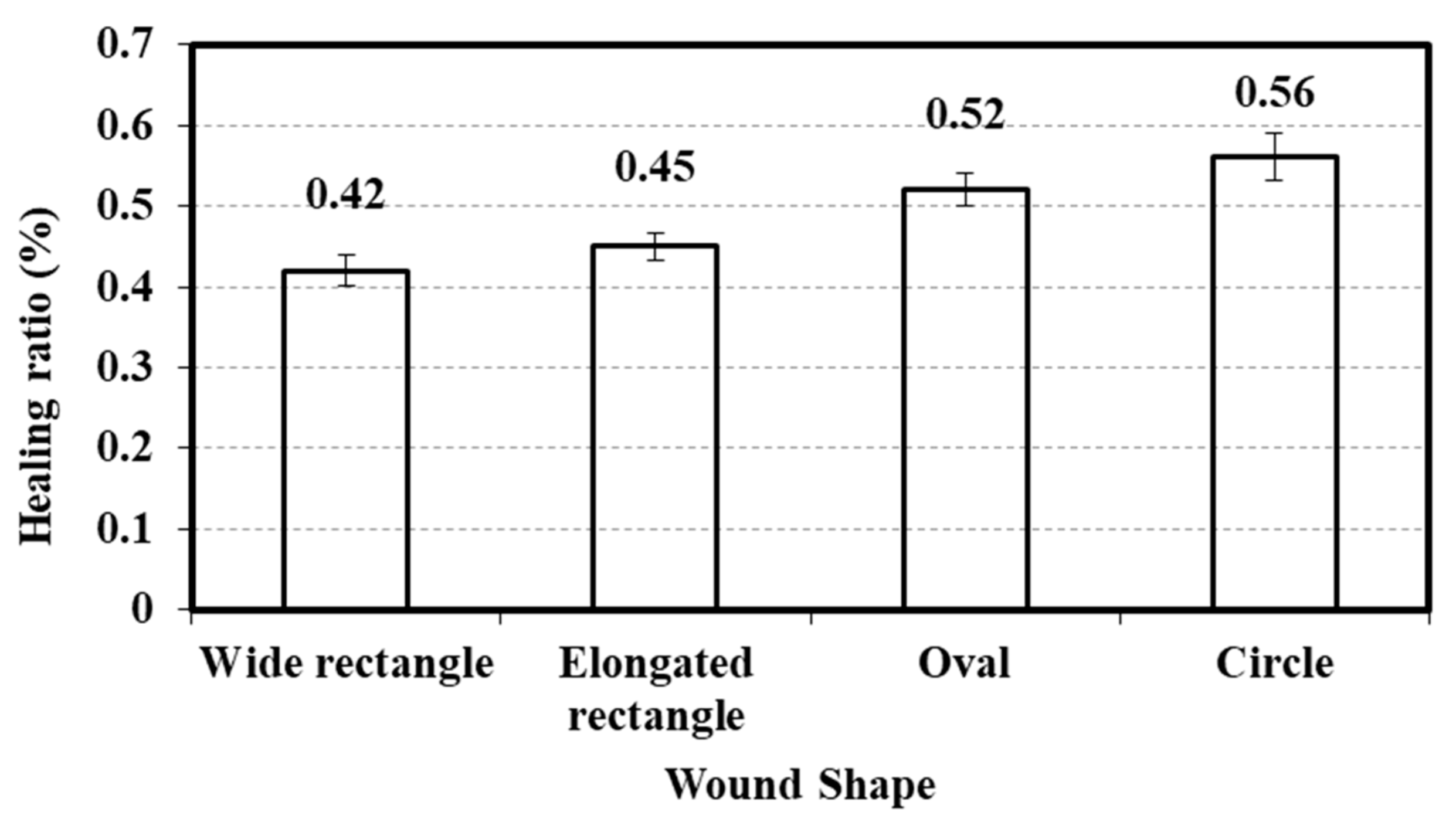
3.8. Relationship Between Wound Shape and Healing Ratio
4. Discussion
Management Recommendations and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jourgholami, M.; Rizvandi, V.; Majnounian, B. Evaluating the extent, patterns, size and distribution of tree scars following skidding operation (Case study: Kheyrud forest). Iran. J. For. 2012, 4, 187–196. [Google Scholar]
- Grünberg, J.; Ghaffariyan, M.R.; Jourgholami, M.; Labelle, E.R.; Kaakkurivaara, N.; Robert, R.C.G.; Kühmaier, M. Criteria for assessing the sustainability of logging operations—A systematic review. Curr. For. Rep. 2023, 9, 350–369. [Google Scholar] [CrossRef]
- Sohrabi, H.; Jourgholami, M.; Tavankar, F.; Venanzi, R.; Picchio, R. Post-Harvest Evaluation of Soil Physical Properties and Natural Regeneration Growth in Steep-Slope Terrains. Forests 2019, 10, 1034. [Google Scholar] [CrossRef]
- Acuna, M.; Sessions, J.; Zamora, R.; Boston, K.; Brown, M.; Ghaffariyan, M.R. Methods to Manage and Optimize Forest Biomass Supply Chains: A Review. Curr. For. Rep. 2019, 5, 124–141. [Google Scholar] [CrossRef]
- Anderson, N.; Mitchell, D. Forest Operations and Woody Biomass Logistics to Improve Efficiency, Value, and Sustainability. BioEnergy Res. 2016, 9, 518–533. [Google Scholar] [CrossRef]
- Akay, A.E.; Yilmaz, M.; Tongue, F. Impact of Mechanized Harvesting Machines on Forest Ecosystem: Residual Stand Damage. J. Appl. Sci. 2006, 6, 2414–2419. [Google Scholar] [CrossRef]
- Zhou, W.; Sun, X.; Li, S.; Qu, B.; Zhang, J. How Organic Mulching Influences the Soil Bacterial Community Structure and Function in Urban Forests. Microorganisms 2024, 12, 520. [Google Scholar] [CrossRef] [PubMed]
- Kiumarsi, F.; Jourgholami, M.; Jafari, M.; Lo Monaco, A.; Venanzi, R.; Picchio, R. Restoring soil properties in the Hyrcanian forests from machine induced compaction: Reforestation of N2-fixing black alder (Alnus glutinosa (L.) Gaertn.). Land Degrad. Dev. 2024, 35, 2084–2096. [Google Scholar] [CrossRef]
- Hanna, D.E.L.; Harper, M.; Giroux-Bougard, X.; Richardson, J.S.; Rytwinski, T.; Bachhuber, A.; Hudgins, E.J.; Karimi, S.; Schuster, R.; Binley, A.D.; et al. An evidence map of research assessing the effects of timber harvesting on water quality, biotic and biodiversity indicators in running waters. For. Ecol. Manag. 2025, 580, 122425. [Google Scholar] [CrossRef]
- Labelle, E.R.; Hansson, L.; Högbom, L.; Jourgholami, M.; Laschi, A. Strategies to mitigate the effects of soil physical disturbances caused by forest machinery: A comprehensive review. Curr. For. Rep. 2022, 8, 20–37. [Google Scholar] [CrossRef]
- DeArmond, D.; Ferraz, J.B.S.; de Oliveira, L.R.; Lima, A.J.N.; de Souza Falcão, N.P.; Higuchi, N. Soil compaction in skid trails still affects topsoil recovery 28 years after logging in Central Amazonia. Geoderma 2023, 434, 116473. [Google Scholar] [CrossRef]
- Britto, P.C.; Jaeger, D.; Hoffmann, S.; Robert, R.C.G.; Vibrans, A.C.; Fantini, A.C. Impact Assessment of Timber Harvesting Operations for Enhancing Sustainable Management in a Secondary Atlantic Forest. Sustainability 2019, 11, 6272. [Google Scholar] [CrossRef]
- Latterini, F.; Dyderski, M.K.; Horodecki, P.; Picchio, R.; Venanzi, R.; Lapin, K.; Jagodziński, A.M. The effects of forest operations and silvicultural treatments on litter decomposition rate: A meta-analysis. Curr. For. Rep. 2023, 9, 276–290. [Google Scholar] [CrossRef]
- Tsioras, P.A.; Liamas, D.K. Residual tree damage along skidding trails in beech stands in Greece. J. For. Res. 2015, 26, 523–531. [Google Scholar] [CrossRef]
- Bettinger, P.; Kellogg, L.D. Residual stand damage from cut-to-length thinning of second growth timber in the Cascade Range of western Oregon. For. Prod. J. 1993, 43, 59–64. [Google Scholar]
- Sidle, R.C.; Laurent, T.H. Site damage from mechanized thinning in southeast Alaska. North. J. Appl. For. 1986, 3, 94–97. [Google Scholar] [CrossRef]
- Froehlich, H.A. The influence of different thinning systems on damage to soil and trees. For. Commun. Bull. 1976, 55, 102–105. [Google Scholar]
- Kovbasa, N.P. Rasprostranenije i Razvitije Ranevoj Gnili v Elovyh Nasazdenijah Belarusi. Ph.D. Thesis, Belarusskij NII Zaschity Rastenij, Belarusian State University Priluki-Minsk, Minsk, Belarus, 1996; 18p. (In Russian with English Abstract). [Google Scholar]
- Han, H.S. Damage to Young Douglas-Fir Stand from Commercial Thinning with Various Timber Harvesting Systems and Silvicultural Prescriptions: Characteristics, Sampling Strategy for Assessment and Future Volume Loss. Ph.D. Thesis, Department of Forest Engineering, Faculty of Natural Resources, Oregon State University, Corvallis, OR, USA, 1998; p. 141. [Google Scholar]
- Froese, K.; Han, H.S. Residual Stand Damage from Cut-to-Length Thinning of a Mixed Conifer Stand in Northern Idaho. West. J. Appl. For. 2006, 21, 142–148. [Google Scholar]
- Tavankar, F.; Nikooy, M.; Lo Monaco, A.; Latterini, F.; Venanzi, R.; Picchio, R. Short-Term Recovery of Residual Tree Damage during Successive Thinning Operations. Forests 2020, 11, 731. [Google Scholar] [CrossRef]
- Aho, P.E.; Fiddler, G.; Srago, M. Logging Damage in Thinned, Young Growth True Fir Stands in California and Recommendations for Prevention; Northeastern Forest Experiment Station: Irvine, CA, USA, 1983; 7p. [Google Scholar] [CrossRef]
- Kamperidou, V.; Aidinidis, E.; Barboutis, I. Impact of Structural Defects on the Surface Quality of Hardwood Species Sliced Veneers. Appl. Sci. 2020, 10, 6265. [Google Scholar] [CrossRef]
- Youngblood, A. Damaged to residual trees and advanced regeneration from skyline and forwarding yarding in mixed-conifer stands of northeastern Oregon. West. J. App. For. 2000, 15, 101–107. [Google Scholar] [CrossRef]
- Ostrofsky, W.D.; Seymour, R.S.; Lemin, R.C. Damage to northern hardwoods from thinning using whole-tree harvesting technology. Can. J. For. Res. 1986, 16, 1238–1244. [Google Scholar] [CrossRef]
- Neely, D. Tree wounds and wound closure. Arboric. Urban For. AUF 1979, 5, 135–140. [Google Scholar] [CrossRef]
- Stobbe, H.; Schmitt, U.; Eckstein, D.; Dujesiefken, D. Developmental Stages and Fine Structure of Surface Callus Formed after Debarking of Living Lime Trees (Tilia sp.). Ann. Bot. 2002, 89, 773–782. [Google Scholar] [CrossRef]
- Neely, D. Healing of wounds on trees. J. Am. Soc. Hortic. Sci. 1970, 95, 536–540. [Google Scholar] [CrossRef]
- Vasiliauskas, R. Wound Healing Rate and Its Influence on Spread of Decay in Spruce; Lithuanian Forest Research Institute: Girionys, Lithuania, 1994; Volume 34, pp. 207–212. [Google Scholar]
- Vasiliauskas, R.; Stenlid, J. Discoloration following bark stripping wounds on Fraxinus excelsior. Eur. J. Plant Pathol. 1998, 28, 383–390. [Google Scholar] [CrossRef]
- Staines, B.W.; Welch, D. Habitat selection and impact of red (Cervus elaphus L.) and roe (Capreolus capreolus L.) deer in a Sitka spruce plantation. Proc. R. Soc. Edinb. 1984, 82, 303–319. [Google Scholar] [CrossRef]
- Welch, D.; Scott, D.; Staines, B.W. Bark stripping damage by red deer in a Sitka spruce forest in Western Scotland. III. Trends in wound condition. Forestry 1997, 70, 11–120. [Google Scholar]
- Hosius, D. Bark stripping consequences on beech. Allg. Forst-Und Jagdztg. 1967, 22, 484–487. [Google Scholar]
- Smith, H.C.; Miller, G.W.; Schuler, T.M. Closure of Logging Wounds after 10 Years, USDA Forest Service; Northeastern Forest Experiment Station: Irvine, CA, USA, 1994; p. 6. [Google Scholar]
- Naghdi, R. Investigation and Comparison of Two Harvesting Systems: Tree Length and Cut-to-Length Method in Order to Optimize Road Network Planning in Neka, Iran. Ph.D. Thesis, Tarbiat Modares University, Tehran, Iran, 2005; p. 177. (In Persian). [Google Scholar]
- Nikooy, M. Production Optimization and Reduction Impact on Forest by Preparing Harvest Planning in Nav, Iran. Ph.D. Thesis, Tehran University, Tehran, Iran, 2007; p. 165. (In Persian). [Google Scholar]
- Ezzati, S.; Najafi, A. Long-term impact evaluation of ground-based skidding on residual damaged trees in the Hyrcanian forest, Iran. Int. J. For. Res. 2010, 2010, 183735. [Google Scholar] [CrossRef]
- Tavankar, F.; Bonyad, A. Long-term Effects of Logging Damages on Quality of Residual Trees in the Asalem Nav Forest. J. Environ. Stud. 2014, 40, 39–50. [Google Scholar]
- Naghdi, R.; Alizadeh, N.; Nikooy, M.; Dalir, P.; Tavankar, F.; Mirzaei, M. Cable-Skidder Logging Wounds on Tree Species in the Small-Scale Forestry. Small-Scale For. 2025, 24, 115–132. [Google Scholar] [CrossRef]
- Tavankar, F.; Ezzati, S.; Latterini, F.; Lo Monaco, A.; Venanzi, R.; Picchio, R. Assessment of Wound Recovery and Radial Growth 10 Years after Forest Operations in Hardwood Stands. Forests 2022, 13, 1393. [Google Scholar] [CrossRef]
- Ezzati, S.; Malek, I.; Tavankar, F.; Parsakhoo, A. The role of management practices and trail layouts on postharvest healing of residual trees in mountain broadleaves forests. Eur. J. For. Res. 2025, 144, 621–633. [Google Scholar] [CrossRef]
- Han, H.S.; Kellogg, L.D. A comparison of sampling methods for measuring residual stand damage from commercial thinning. J. For. Eng. 2000, 11, 8–17. [Google Scholar]
- Cassens, D.L. Factors determining the suitability of trees and logs for the face veneer industry. In Proceedings of the 14th Central Hardwood Forest Conference, Wooster, OH, USA, 16–19 March 2004; pp. 130–139. [Google Scholar]
- Romero, C.; Bolker, B.M.; Edwards, C.E. Stem responses to damage: The evolutionary ecology of Quercus species in contrasting fire regimes. New Phytol. 2009, 182, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W.A.; Flake, S.W.; Rossatto, D.R.; De Antonio, A.C.; Durigan, G.; Abreu, R.C.R. Stem wound healing is dependent upon bark and trunk growth rates in Brazilian savanna. Trees 2024, 38, 197–204. [Google Scholar] [CrossRef]
- Vasiliauskas, R. Damage to trees due to forestry operations and its pathological significance in temperate forests: A literature review. Forestry 2001, 74, 319–336. [Google Scholar] [CrossRef]
- Vacek, Z.; Cukor, J.; Linda, R.; Vacek, S.; Šimůnek, V.; Brichta, J.; Gallo, J.; Prokůpková, A. Bark stripping, the crucial factor affecting stem rot development and timber production of Norway spruce forests in Central Europe. For. Ecol. Manag. 2020, 474, 118360. [Google Scholar] [CrossRef]
- Knoke, T.; Stang, S.; Remler, N.; Seifert, T. Ranking the importance of quality variables for the price of high quality beech timber (Fagus sylvatica L.). Ann. For. Sci. 2006, 63, 399–413. [Google Scholar] [CrossRef]
- Fillip, G.M.; Parks, C.A.; Wickman, B.E.; Mitchell, R.G. Tree wound dynamics in thinned and unthinned stands of grand fir, ponderosa pine, and lodgepole pine in eastern Oregon. Northwest Sci. 1995, 69, 276–283. [Google Scholar]
- Hecht, U.; Kohnle, U.; Nill, M.; Metzler, B. Bark wounds caused by felling are more susceptible to discoloration and decay than wounds caused by extraction in European beech. Ann. For. Sci. 2015, 72, 731–740. [Google Scholar] [CrossRef]
- Reisinger, T.W.; Pope, P.E. Impact of timber harvesting on tress in a central hardwood forest in Indiana. In Proceedings of the 8th Central Hardwood Forest Conference, University Park, PA, USA, 4–6 March 1991; Northeastern Forest Experiment Station: Radnor, PA, USA, 1991; pp. 82–91. [Google Scholar]
- Gallagher, P.W.; Sydnor, T.D. Variation in Wound Response among Cultivars of Red Maple. J. Am. Soc. Hortic. Sci. 1983, 108, 744–746. [Google Scholar] [CrossRef]
- Khan, A.L.; Asaf, S.; Numan, M.; AbdulKareem, N.; Imran, M.; Riethoven, J.J.M.; Kim, H.Y.; Al-Harrasi, A.; Schachtman, D.P.; Al-Rawahi, A.; et al. Transcriptomics of tapping and healing process in frankincense tree during resin production. Genomics 2021, 113, 4337–4351. [Google Scholar] [CrossRef]
- Wiedenbeck, J.; Smith, K.T. Hardwood Management, Tree Wound Response, and Wood Product Value. For. Chron. 2019, 94, 292–306. [Google Scholar]
- Kiser, J. Histochemical and geometric alterations of sapwood in coastal Douglas-fir following mechanical damage during commercial thinning. Silva Fenn. 2011, 45, 729–741. [Google Scholar] [CrossRef]
- Han, H.S.; Kellogg, L.D.; Filip, G.M.; Brown, T.D. Scar closure and future timber value losses from thinning damage in western Oregon. For. Prod. J. 2000, 50, 36–42. [Google Scholar]
- Karaszewski, Z.; Bembenek, M.; Mederski, P.S.; Szczepanska-Alvarez, A.; Byczkowski, R.; Kozlowska, A.; Michnowicz, K.; Przytula, W.; Giefing, D.F. Identifying beech round wood quality—Distribution of beech timber qualities and influencing defects. Drewno 2013, 56, 39–54. [Google Scholar] [CrossRef]
- Vasaitis, R.; Lygis, V.; Vasiliauskaite, I.; Vasiliauskas, A. Wound occlusion and decay in Picea abies stems. Eur. J. For. Res. 2012, 131, 1211–1216. [Google Scholar] [CrossRef]
- Clatterbuck, W.K. Logging damage to residual trees following commercial harvesting to different over story retention levels in a mature hardwood stand in Tennessee. In Proceedings of the 13th Biennial Southern Silvicultural Research Conference, Memphis, TN, USA, 28 February–4 March 2005. [Google Scholar]
- Shigo, A.L. How to assess the defect status of stand. North. J. Appl. For. 1984, 10, 41–49. [Google Scholar]
- Dimitri, L. Wound decay following tree injury in forestry: Establishment, significance and possibilities of its prevention. Forestry 1983, 102, 68–79. [Google Scholar]
- Suzuki, Y. Damage to residual stands from thinning with short-span tower yarders: Re-examination of wounds after five years. J. For. Res. 2000, 5, 201–204. [Google Scholar] [CrossRef]
- Benzie, J.W.; Hesterberg, G.; Ohman, J.H. Pathological effects of logging damages four years after selective cutting in old growth northern hardwoods. J. For. Res. 1963, 61, 786–792. [Google Scholar]
- Furutani, S.; Sakai, T.; Kawanabe, S. Observation for branch stub after pruning (Ill) Changes of branch stub from 2 to 4 years after pruning and knot analysis. Bull. Kyoto Univ. For. 1987, 59, 176–186. [Google Scholar]
- Bobilk, M. Damages to Residual Stand in Commercial Thinning. Master’s Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2008. [Google Scholar]
- Kizha, A.R.; Nahor, E.; Coogen, N.; Louis, L.T.; George, A.K. Residual Stand Damage under Different Harvesting Methods and Mitigation Strategies. Sustainability 2021, 13, 7641. [Google Scholar] [CrossRef]
- Johns, J.S.; Barreto, P.; Uhl, C. Logging damage during planned and unplanned logging operations in the eastern Amazon. For. Ecol. Manag. 1996, 89, 59–77. [Google Scholar] [CrossRef]
| Species | Parameter | Wound Area (cm2) | Healed Area (cm2) | Healing Ratio (%) |
|---|---|---|---|---|
| Oriental beech | Mean (±SE) | 380.1 ± 33.14 | 160.0 ± 11.29 | 50.65 ± 1.36 |
| Max | 1793.5 | 513.0 | 88.89 | |
| Min | 13.5 | 9.8 | 10.04 | |
| Hornbeam | Mean (±SE) | 589.89 ± 50.48 | 200.0 ± 13.98 | 43.03 ± 1.75 |
| Max | 1943.0 | 494.0 | 78.43 | |
| Min | 25.0 | 16.0 | 11.31 | |
| Velvet maple | Mean (±SE) | 454.85 ± 95.84 | 169.0 ± 28.48 | 47.36 ± 3.85 |
| Max | 1748.0 | 544.0 | 79.84 | |
| Min | 43.5 | 25.0 | 12.47 | |
| Chestnut-leaved oak | Mean (±SE) | 789.62 ± 197.26 | 247.0 ± 37.69 | 37.53 ± 5.96 |
| Max | 1619.0 | 383.0 | 53.79 | |
| Min | 243.5 | 131.0 | 17.02 | |
| Caucasian alder | Mean (±SE) | 971.5 ± 165.06 | 349.0 ± 47.36 | 40.23 ± 4.10 |
| Max | 1700.0 | 533.0 | 60.66 | |
| Min | 125.0 | 68.0 | 0.26 | |
| Date-plum | Mean (±SE) | 417.48 ± 125.86 | 171.0 ± 67.61 | 39.42 ± 10.05 |
| Max | 735.0 | 350.0 | 69.71 | |
| Min | 193.0 | 63.0 | 27.28 |
| Parameter | Species | N | Mean Rank | Test Statistics |
|---|---|---|---|---|
| Healing ratio (%) | Hornbeam | 94 | 119.41 | Chi-Square: 14.975 df: 5 Sig.: 0.01 |
| Oriental beech | 135 | 153.39 | ||
| Date-plum | 4 | 97.75 | ||
| Velvet maple | 24 | 137.96 | ||
| Chestnut-leaved oak | 6 | 94.5 | ||
| Caucasian alder | 9 | 103.0 | ||
| Total | 272 |
| Parameter | Wound Location | N | Mean Rank | Test Statistics |
|---|---|---|---|---|
| Healing ratio (%) | Root collar | 28 | 168.96 | Chi-Square: 5.89 df: 3 Sig.: 0.117 |
| ≤1 m stem height | 200 | 131.16 | ||
| 1–2 m stem height | 34 | 142.29 | ||
| >2 m stem height | 10 | 132.7 | ||
| Total | 272 |
| Parameter | Wound Severity | N | Mean Rank | Test Statistics |
|---|---|---|---|---|
| Healing ratio (%) | Superficial | 64 | 154.7 | Chi-Square: 11.502 df: 2 Sig.: 0.003 |
| Deep | 181 | 136.48 | ||
| Very deep | 27 | 93.48 | ||
| Total | 272 |
| Wound Age (Year) | N | Mean Wound Area (cm2) | Mean Healed Area (cm2) | Healing Ratio (%) |
|---|---|---|---|---|
| 6 | 26 | 787.01 | 166.31 | 28 |
| 7 | 32 | 551.53 | 161.8 | 33 |
| 8 | 22 | 602.41 | 195.13 | 40 |
| 9 | 25 | 644.83 | 220.21 | 42 |
| 10 | 21 | 454.69 | 177.76 | 45 |
| 11 | 29 | 621.59 | 253.04 | 44 |
| 12 | 20 | 363.63 | 167.94 | 50 |
| 13 | 16 | 620.5 | 264.34 | 50 |
| 14 | 18 | 353.96 | 187.59 | 56 |
| 15 | 18 | 324.26 | 174.17 | 57 |
| 16 | 18 | 259.65 | 149.77 | 65 |
| 17 | 16 | 153.56 | 103.13 | 69 |
| 18 | 11 | 158.96 | 104.86 | 65 |
| Total | 272 | 488.36 | 183.00 | 46.9 |
| Parameter | Traffic Intensity | N | Mean Rank | Test Statistics |
|---|---|---|---|---|
| Healing ratio % | Low | 60 | 129.4 | Chi-Square: 0.85 df: 2 Sig.: 0.65 |
| Medium | 127 | 140.6 | ||
| High | 85 | 135.39 | ||
| Total | 272 |
| Parameter | Wound Shape | N | Mean Rank | Test Statistics |
|---|---|---|---|---|
| Healing ratio (%) | Wide rectangle | 75 | 110.96 | Chi-Square: 24.92 |
| Elongated rectangle | 98 | 160.79 | df: 3 | |
| Oval | 68 | 178.35 | Sig.: 0.000 | |
| Circle | 31 | - | ||
| Total | 272 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nooryazdan, N.; Jourgholami, M.; Picchio, R.; Venanzi, R.; Lo Monaco, A. Long-Term Assessment of Wound Healing in Damaged Residual Trees Under Continuous Cover Forestry in the Hyrcanian Broad-Leaved Forests. Sustainability 2025, 17, 9319. https://doi.org/10.3390/su17209319
Nooryazdan N, Jourgholami M, Picchio R, Venanzi R, Lo Monaco A. Long-Term Assessment of Wound Healing in Damaged Residual Trees Under Continuous Cover Forestry in the Hyrcanian Broad-Leaved Forests. Sustainability. 2025; 17(20):9319. https://doi.org/10.3390/su17209319
Chicago/Turabian StyleNooryazdan, Niloufar, Meghdad Jourgholami, Rodolfo Picchio, Rachele Venanzi, and Angela Lo Monaco. 2025. "Long-Term Assessment of Wound Healing in Damaged Residual Trees Under Continuous Cover Forestry in the Hyrcanian Broad-Leaved Forests" Sustainability 17, no. 20: 9319. https://doi.org/10.3390/su17209319
APA StyleNooryazdan, N., Jourgholami, M., Picchio, R., Venanzi, R., & Lo Monaco, A. (2025). Long-Term Assessment of Wound Healing in Damaged Residual Trees Under Continuous Cover Forestry in the Hyrcanian Broad-Leaved Forests. Sustainability, 17(20), 9319. https://doi.org/10.3390/su17209319










