Synergistic Removal of Typical Heavy Metal and Organic Contaminants via FeS2/α-FeOOH/C from Electronic Industry Wastewater: Insights for Selective Degradation and Promotion
Abstract
1. Introduction
2. Materials and Methods
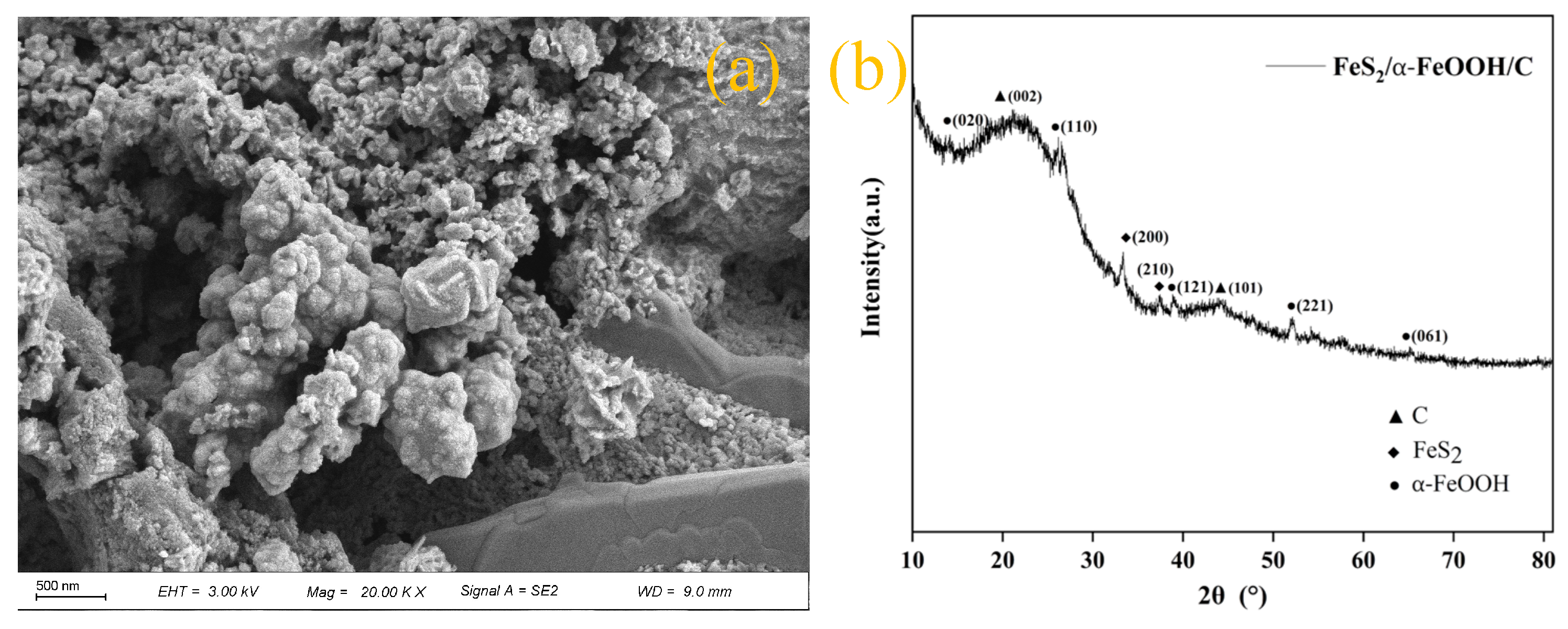
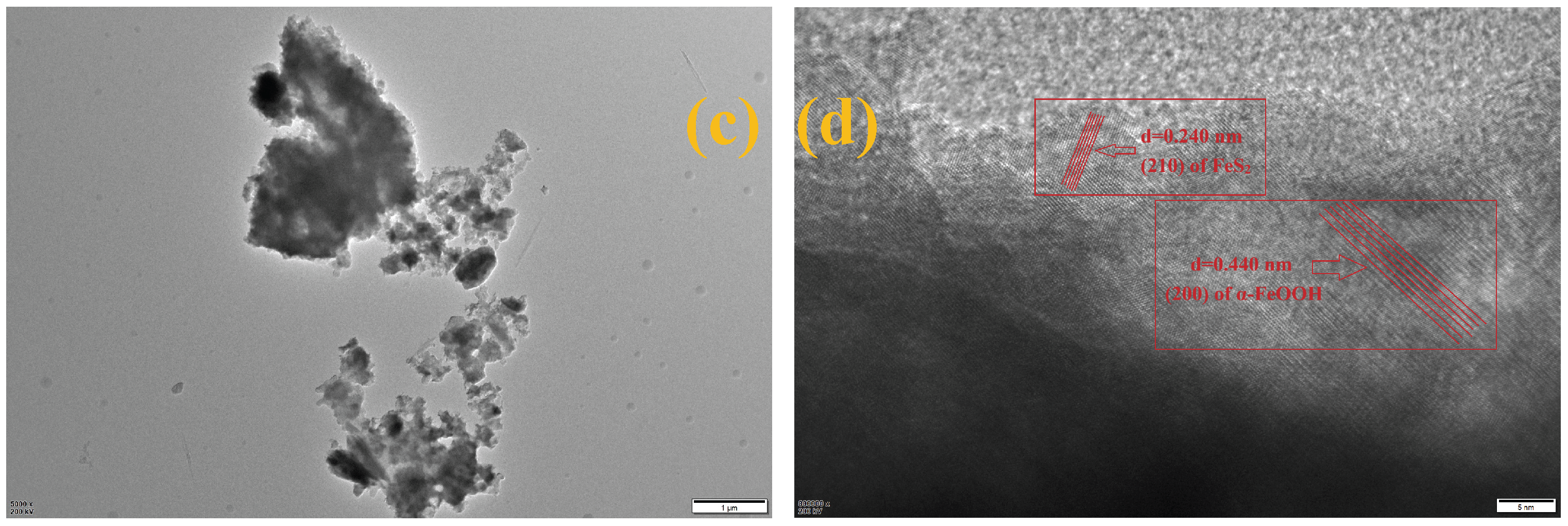
2.1. Fabrication of FeS2/α-FeOOH/C Composite and Reagents
2.2. Batch Experiments
2.3. Testing Method and Theoretical Calculation
3. Results and Discussion
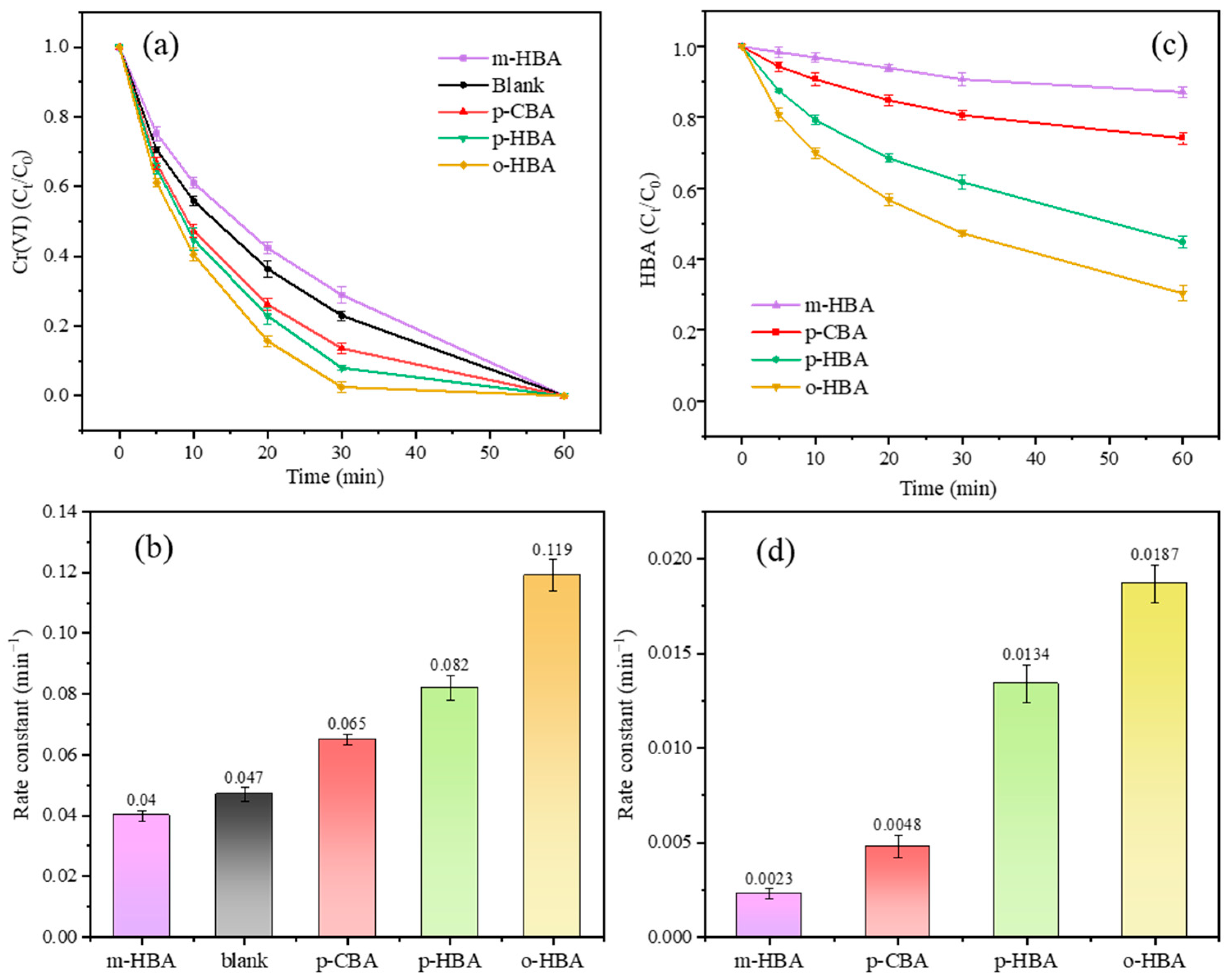
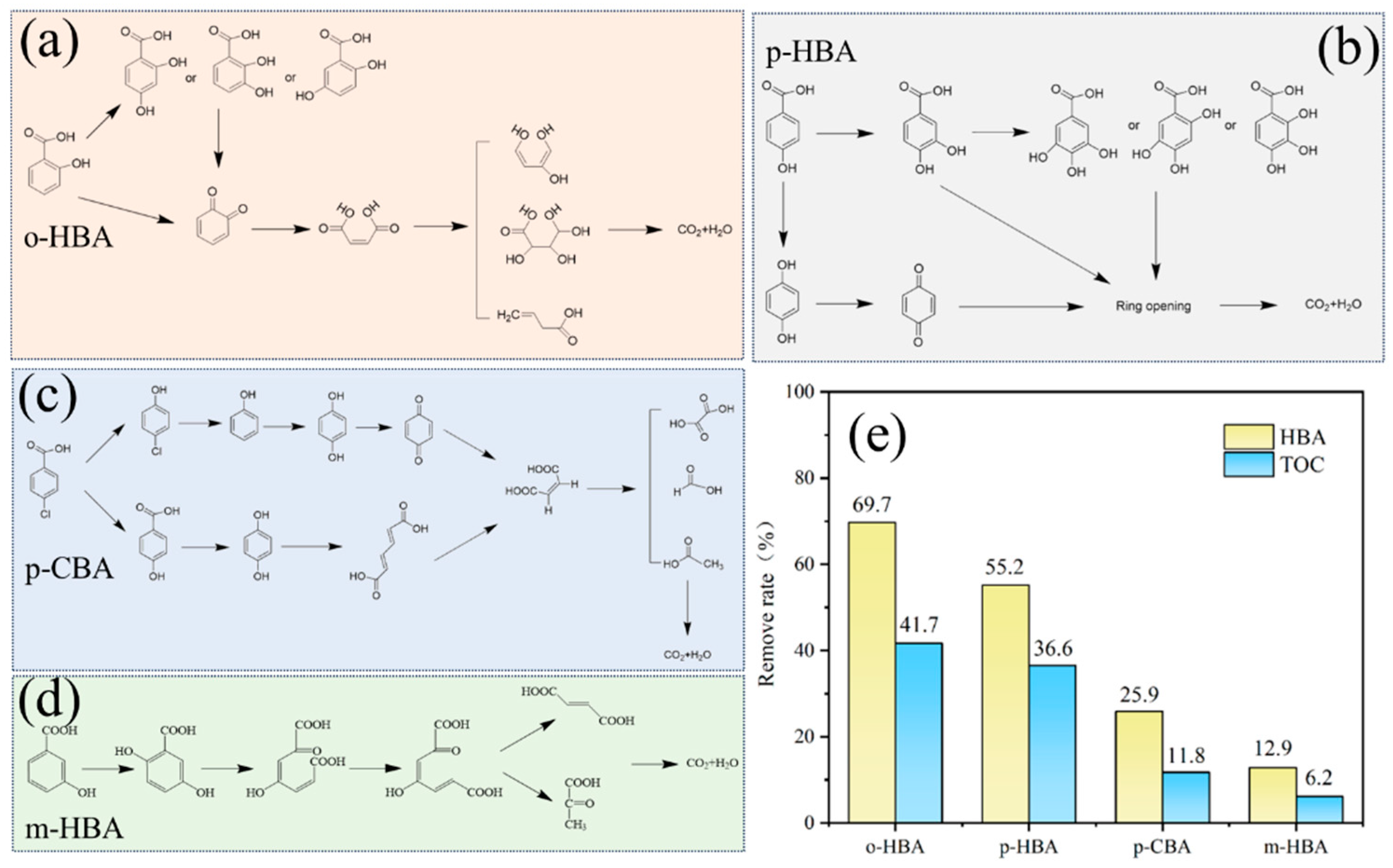
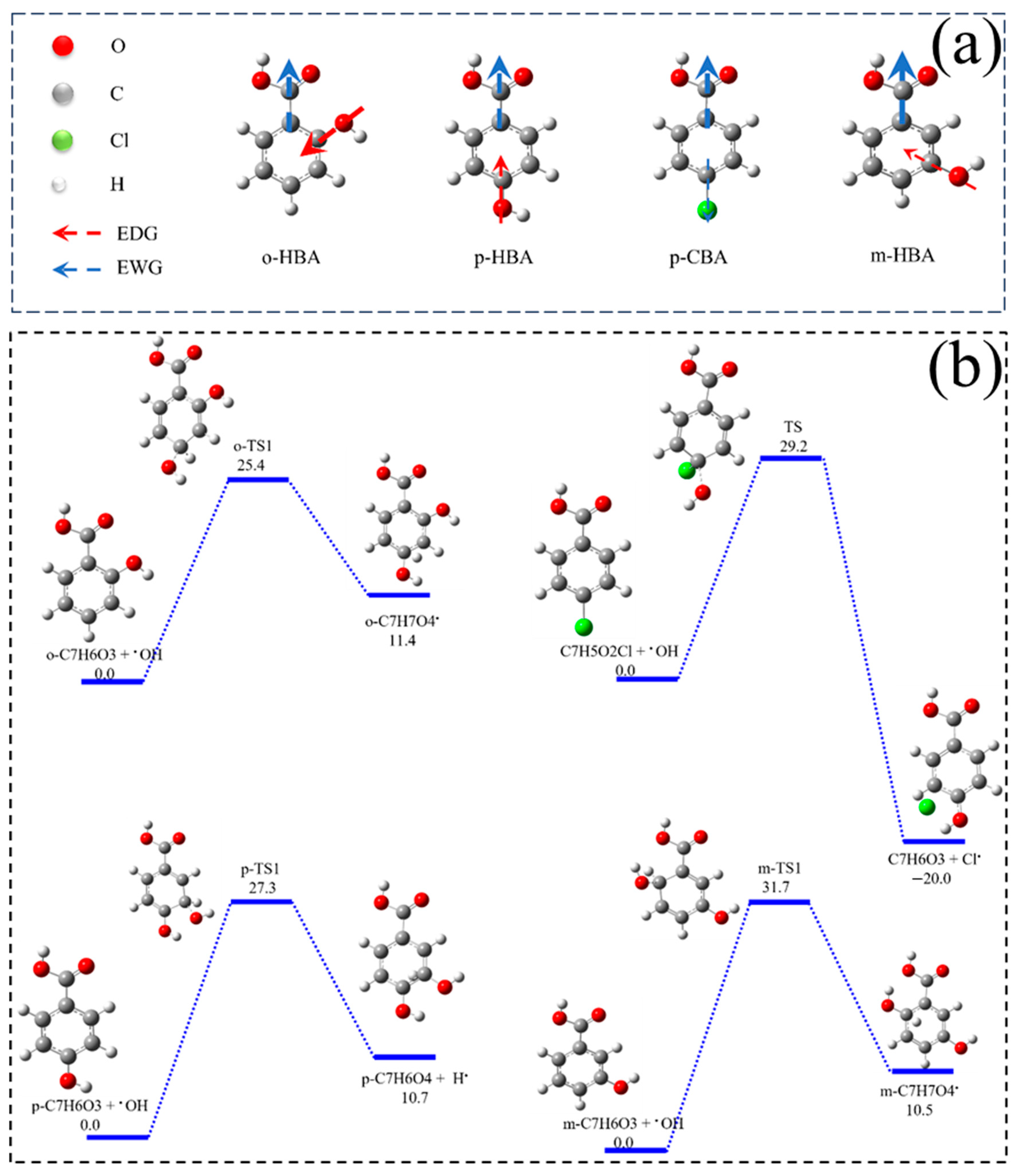
3.1. Influence of Benzoic Acids with Different Substituent Groups on Cr(VI) Reduction
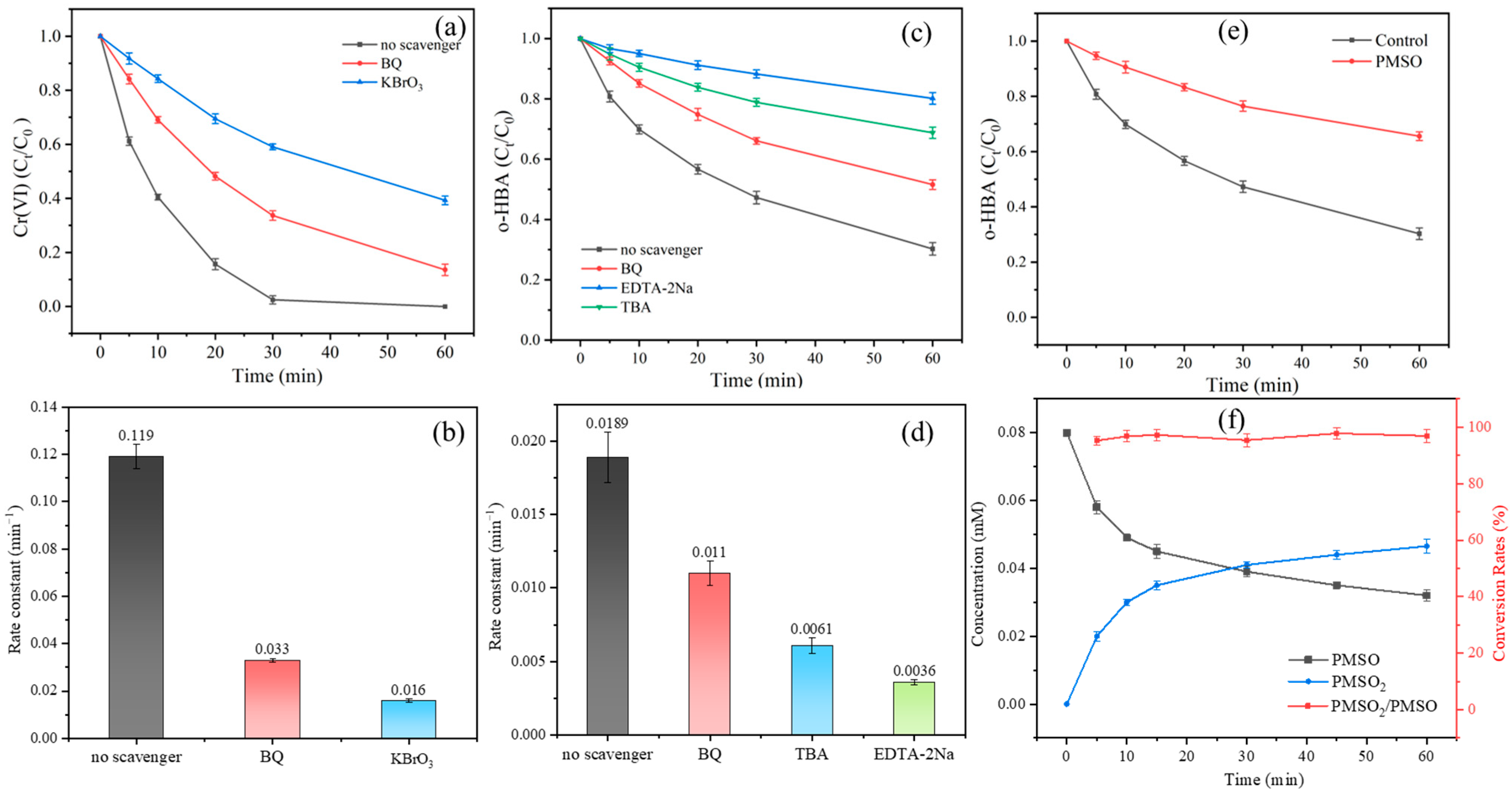
3.2. Identification of Main Reductive and Oxidative Reactive Species
3.3. Proposed Mechanism and Connection Between Degradation of Benzoic Acids and Their Effect on Cr(VI) Reduction
3.3.1. Proposed Degradation Paths for Different Benzoic Acids
3.3.2. Proposed Mechanisms for the Effect of Benzoic Acids on Cr(VI) Removal
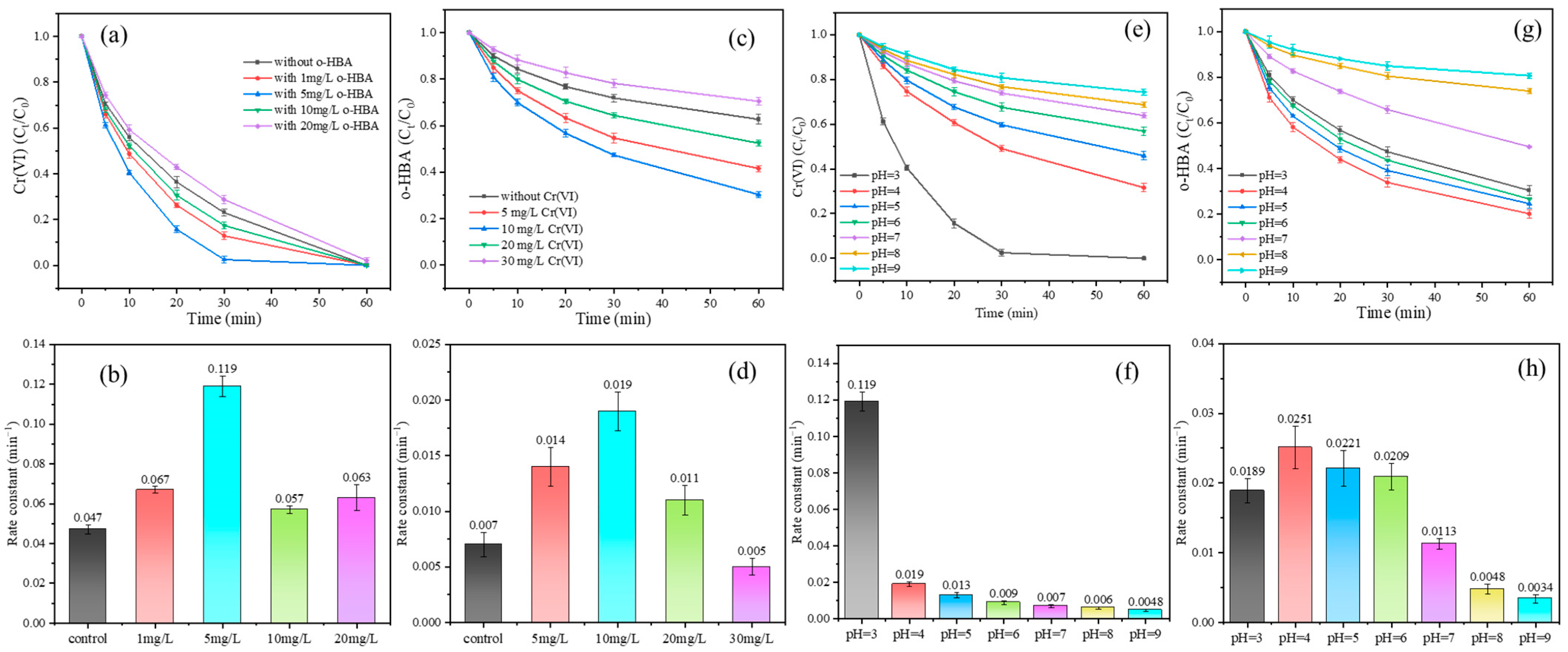
3.4. Influence of Typical Operation Conditions on Cr(VI) Reduction and Benzoic Acid Degradation
3.4.1. Initial Concentration
3.4.2. Initial pH
3.5. Comprehensive Evaluation for Real Electronic Industry Wastewater Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| p-HBA | p-hydroxybenzoic acid |
| o-HBA | o-hydroxybenzoic acid |
| m-HBA | m-hydroxybenzoic acid |
| p-CBA | parachlorobenzoic-acid |
References
- Tao, X.; Hu, X.; Wen, Z.; Ming, Y.a.; Li, J.; Liu, Y.; Chen, R. Highly efficient Cr(VI) removal from industrial electroplating wastewater over Bi2S3 nanostructures prepared by dual sulfur-precursors: Insights on the promotion effect of sulfate ions. J. Hazard. Mater. 2022, 424, 127423. [Google Scholar] [CrossRef]
- Yang, Z.H.; Zhang, X.M.; Jiang, Z.; Li, Q.; Huang, P.C.; Zheng, C.J.; Liao, Q.; Yang, W.C. Reductive materials for remediation of hexavalent chromium contaminated soil—A review. Sci. Total Environ. 2021, 773, 145654. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Min, X.; Peng, T.; Zhao, F.; Ke, Y.; Wang, Y.; Jiang, G.; Xu, Q.; Wang, J. Mechanochemically Activated Microsized Zero-Valent Iron/Pyrite Composite for Effective Hexavalent Chromium Sequestration in Aqueous Solution. J. Chem. Eng. Data 2020, 65, 1936–1945. [Google Scholar] [CrossRef]
- Velazquez-Peña, S.; Barrera-Díaz, C.; Linares-Hernández, I.; Bilyeu, B.; Martínez-Delgadillo, S.A. An Effective Electrochemical Cr(VI) Removal Contained in Electroplating Industry Wastewater and the Chemical Characterization of the Sludge Produced. Ind. Eng. Chem. Res. 2012, 51, 5905–5910. [Google Scholar] [CrossRef]
- Ma, C.; Liu, M.; Yang, Z.; Zheng, Q.; Mei, J.; Yang, S. Highly efficient Cr (VI) removal from electroplating wastewater by regenerable copper sulfides: Mechanism and magical induction effect for Cr resource recovery. Environ. Res. 2023, 236, 116799. [Google Scholar] [CrossRef]
- Bae, S.; Sihn, Y.; Kyung, D.; Yoon, S.; Eom, T.; Kaplan, U.; Kim, H.; Schäfer, T.; Han, S.; Lee, W. Molecular Identification of Cr(VI) Removal Mechanism on Vivianite Surface. Environ. Sci. Technol. 2018, 52, 10647–10656. [Google Scholar] [CrossRef]
- Zhang, F.; Xi, L.; Zhao, M.; Du, Y.; Ma, L.; Chen, S.; Ye, H.; Du, D.; Zhang, T.C. Efficient removal of Cr(VI) from aqueous solutions by polypyrrole/natural pyrite composites. J. Mol. Liq. 2022, 365, 120041. [Google Scholar] [CrossRef]
- Lyu, H.; Tang, J.; Huang, Y.; Gai, L.; Zeng, E.Y.; Liber, K.; Gong, Y. Removal of hexavalent chromium from aqueous solutions by a novel biochar supported nanoscale iron sulfide composite. Chem. Eng. J. 2017, 322, 516–524. [Google Scholar] [CrossRef]
- Yu, J.; Chi, Y.; Tang, Z.; Wang, Y.; Wang, T.; Zhang, Z.; Gu, P.; Ren, X.; Xu, X.; Yang, K. Efficient photo-assisted reduction of Cr(VI) via activated carbon mediated Z-scheme FeS2/α-FeOOH/C heterojunction: Focusing on molecular oxygen fate and synergetic reduction mechanism. J. Water Process Eng. 2025, 69, 106661. [Google Scholar] [CrossRef]
- Zhao, R.; Zhou, Z.; Zhao, X.; Jing, G. Enhanced Cr(VI) removal from simulated electroplating rinse wastewater by amino-functionalized vermiculite-supported nanoscale zero-valent iron. Chemosphere 2019, 218, 458–467. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.-y.; Yang, X.; Wu, Y.; Huang, X.-f.; He, E.-k.; Qiu, R.-l.; Wang, S. Enhanced removal of Cr(VI) in the Fe(III)/natural polyphenols system: Role of the in situ generated Fe(II). J. Hazard. Mater. 2019, 377, 321–329. [Google Scholar] [CrossRef]
- Tian, H.; Liu, P.; Sun, H.; Huang, X. Efficient one-step removal and immobilization of recalcitrant Cr(III) and Cr(VI) fractions in real electroplating wastewater using electrolysis with O2 aeration. J. Water Process Eng. 2024, 65, 105783. [Google Scholar] [CrossRef]
- Aimaiti, G.; Ma, Y.; Shi, Y.; Wang, X.; Wang, S.; Wang, Z.; Li, Y.; Li, J.; Qi, X.; Chen, X. Nb2O5/red phosphorus S-scheme heterojunction photocatalyst for removal of organic contaminant and Cr(VI): Electrochemical performance and mechanism. Mater. Sci. Semicond. Process. 2023, 160, 107421. [Google Scholar] [CrossRef]
- Barbhuiya, M.H.; Dhar, S.S. MoS2-gC3N4 encapsulated in nitrogen-doped graphene oxide: An efficient visible light photocatalyst for removal of Cr (VI) from aqueous solution and mineralization of organic contaminant. Mater. Sci. Eng. B 2025, 318, 118286. [Google Scholar] [CrossRef]
- Vinu, R.; Madras, G. Kinetics of Simultaneous Photocatalytic Degradation of Phenolic Compounds and Reduction of Metal Ions with Nano-TiO2. Environ. Sci. Technol. 2008, 42, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Deng, B.; Li, F.; Huang, M.; Sun, Y.; Li, J.; Dong, F. Efficient photocatalytic toluene degradation over heterojunction of GQDs@BiOCl ultrathin nanosheets with selective benzoic acid activation. J. Hazard. Mater. 2021, 420, 126577. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Chi, Y.; Yang, Y.; Lou, Z.; Wang, T.; Wang, D.; Miao, H.; Xu, X. Synergistic effect of novel pyrite/N-doped reduced graphene oxide composite with heterojunction structure for enhanced photo-assisted reduction of Cr(VI) in oxic water: Specific role of molecular oxygen. Sci. Total Environ. 2024, 907, 168123. [Google Scholar] [CrossRef]
- Guo, Y.; Li, C.; Gong, Z.; Guo, Y.; Wang, X.; Gao, B.; Qin, W.; Wang, G. Photocatalytic decontamination of tetracycline and Cr(VI) by a novel α-FeOOH/FeS2 photocatalyst: One-pot hydrothermal synthesis and Z-scheme reaction mechanism insight. J. Hazard. Mater. 2020, 397, 122580. [Google Scholar] [CrossRef]
- Aziz, F.F.A.; Jalil, A.A.; Hassan, N.S.; Hitam, C.N.C.; Rahman, A.F.A.; Fauzi, A.A. Enhanced visible-light driven multi-photoredox Cr(VI) and p-cresol by Si and Zr interplay in fibrous silica-zirconia. J. Hazard. Mater. 2021, 401, 123277. [Google Scholar] [CrossRef]
- Ding, L.; Zhou, Z.; Liu, C.; Yang, Y.; Li, X.; Ren, J.; Chang, H. Ultrafiltration membrane fouling mitigation mechanism induced by algal and natural organic matters: Dependence of photocatalysis pre-oxidation degree and membrane properties. Water Res. 2025, 286, 124199. [Google Scholar] [CrossRef]
- Suleman, S.; Guan, X.; Zhang, Y.; Waseem, A.; Metin, O.; Meng, Z.; Jiang, H.-L. Regulating the generation of reactive oxygen species for photocatalytic oxidation by metalloporphyrinic covalent organic frameworks. Chem. Eng. J. 2023, 476, 146623. [Google Scholar] [CrossRef]
- Liu, L.; Guo, D.; Ning, Z.; Liu, C.; Qiu, G. Solar irradiation induced oxidation and adsorption of arsenite on natural pyrite. Water Res. 2021, 203, 117545. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Browne, W.R. Photochemistry of iron complexes. Coord. Chem. Rev. 2018, 374, 15–35. [Google Scholar] [CrossRef]
- Guo, J.; Lei, M.; Yan, L.; Huang, J.; Liu, C.; Ye, L.; Li, B.; Xu, X.; Li, Y. Sustainable Water Decontamination: Advanced High-Valent Iron Active Species-Driven Peroxymonosulfate Activation for Global Challenges. CleanMat 2025, 2, 88–103. [Google Scholar] [CrossRef]
- Wang, T.; Yang, Y.; Tang, Z.; Zhao, P.; Yang, K.; Xu, X. Iron/nitrogen/sulfur co-doped cyanobacteria derived carbon composites for enhanced hydroxychloroquine degradation via primary nonradical pathway in peroxymonosulfate-based fenton-like system. J. Clean. Prod. 2025, 486, 144530. [Google Scholar] [CrossRef]
- Li, H.; Shan, C.; Pan, B. Fe(III)-Doped g-C3N4 Mediated Peroxymonosulfate Activation for Selective Degradation of Phenolic Compounds via High-Valent Iron-Oxo Species. Environ. Sci. Technol. 2018, 52, 2197–2205. [Google Scholar] [CrossRef]
- Avenier, F.; Herrero, C.; Leibl, W.; Desbois, A.; Guillot, R.; Mahy, J.-P.; Aukauloo, A. Photoassisted Generation of a Dinuclear Iron(III) Peroxo Species and Oxygen-Atom Transfer. Angew. Chem. Int. Ed. 2013, 52, 3634–3637. [Google Scholar] [CrossRef]
- Chandra, B.; Singh, K.K.; Gupta, S.S. Selective photocatalytic hydroxylation and epoxidation reactions by an iron complex using water as the oxygen source. Chem. Sci. 2017, 8, 7545–7551. [Google Scholar] [CrossRef]
- Miller, C.J.; Chang, Y.; Wegeberg, C.; McKenzie, C.J.; Waite, T.D. Kinetic Analysis of H2O2 Activation by an Iron(III) Complex in Water Reveals a Nonhomolytic Generation Pathway to an Iron(IV)oxo Complex. ACS Catal. 2021, 11, 787–799. [Google Scholar] [CrossRef]
- Yang, Y.; Chi, Y.; Yang, K.; Zhang, Z.; Gu, P.; Ren, X.; Wang, X.; Miao, H.; Xu, X. Iron/nitrogen co-doped biochar derived from salvaged cyanobacterial for efficient peroxymonosulfate activation and ofloxacin degradation: Synergistic effect of Fe/N in non-radical path. J. Colloid Interface Sci. 2023, 652, 350–361. [Google Scholar] [CrossRef]
- Bai, X.; Du, Y.; Hu, X.; He, Y.; He, C.; Liu, E.; Fan, J. Synergy removal of Cr (VI) and organic pollutants over RP-MoS2/rGO photocatalyst. Appl. Catal. B Environ. 2018, 239, 204–213. [Google Scholar] [CrossRef]
- Lu, W.; Wang, C.; Bai, Y.; Xie, C.; Zhang, Z.; Song, W.; Wang, J. A novel covalent organic framework for efficient photocatalytic reduction of Cr(vi) and synergistic removal of organic pollutants under visible light irradiation. Environ. Sci. Nano 2024, 11, 229–240. [Google Scholar] [CrossRef]
- Sennaoui, A.; Alahiane, S.; Sakr, F.; Tamimi, M.; Ait Addi, E.H.; Hamdani, M.; Assabbane, A. Comparative degradation of benzoic acid and its hydroxylated derivatives by electro-Fenton technology using BDD/carbon-felt cells. J. Environ. Chem. Eng. 2019, 7, 103033. [Google Scholar] [CrossRef]
- Criquet, J.; Leitner, N.K.V. Reaction pathway of the degradation of the p-hydroxybenzoic acid by sulfate radical generated by ionizing radiations. Radiat. Phys. Chem. 2015, 106, 307–314. [Google Scholar] [CrossRef]
- Cong, J.; Wen, G.; Huang, T.; Deng, L.; Ma, J. Study on enhanced ozonation degradation of para-chlorobenzoic acid by peroxymonosulfate in aqueous solution. Chem. Eng. J. 2015, 264, 399–403. [Google Scholar] [CrossRef]
- Bielicka-Daszkiewicz, K.; Krawczyk, P.; Nowicka, K. Examination of benzoquinone electrooxidation pathway as crucial step of phenol degradation process. Electrochim. Acta 2012, 80, 22–26. [Google Scholar] [CrossRef]
- Ran, J.; Zhai, B.; Zhao, J.; Li, S.; Duan, H.; Chen, Y.; Yin, S.; Zhang, L.; Li, Z. Oxidation of 4-hydroxybenzoic acid in strongly alkaline and high-salt solutions via ultrasonic-assisted ozone: Helping radioactive waste disposal and environmental safety. Desalination 2025, 593, 118252. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Tang, S.; Tian, J.; Wang, Y.; Yang, Y. Thermodynamics, kinetics and structure-activity relationship of hydroxyanthraquinones scavenging free radicals. Food Biosci. 2023, 53, 102705. [Google Scholar] [CrossRef]
- Yang, J.; Qu, D.; Wang, J.; Wu, Y.; Bu, L.; Huang, Y.; Zhou, S. UV-induced degradation of contaminants of emerging concern in the presence of monobromoamine: Role of N-Br bond and degradation mechanisms. J. Environ. Chem. Eng. 2023, 11, 109646. [Google Scholar] [CrossRef]
- Cai, A.; Deng, J.; Ling, X.; Ye, C.; Sun, H.; Deng, Y.; Zhou, S.; Li, X. Degradation of bisphenol A by UV/persulfate process in the presence of bromide: Role of reactive bromine. Water Res. 2022, 215, 118288. [Google Scholar] [CrossRef]
- Liao, S.; Liang, K.; Shu, J.; Han, Y.; Chen, J.; Chen, S.; Luo, J.; Wang, S.; Chen, M.; Wei, H.; et al. Enhanced removal of Cr(VI) from aqueous solutions by an electric field and iron–manganese oxalate composites. J. Environ. Chem. Eng. 2025, 13, 115129. [Google Scholar] [CrossRef]
- Yin, X.; Liu, W.; Ni, J. Removal of coexisting Cr(VI) and 4-chlorophenol through reduction and Fenton reaction in a single system. Chem. Eng. J. 2014, 248, 89–97. [Google Scholar] [CrossRef]
- Sarma, J.; Mahiuddin, S. Comparative adsorption involving ortho- and para-hydroxybenzoic acids in mixed-adsorbate mode onto α-alumina surface: Effect of molecular structure. J. Environ. Chem. Eng. 2014, 2, 90–99. [Google Scholar] [CrossRef]
- Gan, R.; Ye, Y.; Zhan, Z.; Zhang, Q.; Deng, Y.; Liu, Y.; Li, H.; Wan, J.; Pei, X.; Li, Q.; et al. One-step strategy for efficient Cr(VI) removal via phytate modified zero-valent iron: Accelerated electron transfer and enhanced coordination effect. J. Hazard. Mater. 2024, 466, 133636. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, M.; Giannakas, A.; Konstantinou, I. Simultaneous Photocatalytic Reduction of Cr(VI) and Oxidation of Benzoic Acid in Aqueous N-F-Codoped TiO2 Suspensions: Optimization and Modeling Using the Response Surface Methodology. Int. J. Photoenergy 2012, 2012, 520123. [Google Scholar] [CrossRef]
- Xu, N.; Chen, J.; Hu, C.; Zhu, Z.; Wang, W.; Liu, B. Supported photocatalyst for Cr (VI) conversion and removal of organic pollutants. Environ. Sci. Pollut. Res. 2023, 30, 44130–44147. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Li, X.; Huang, Y.; Zhou, Z.; Wei, J. Z-scheme heterojunction and photothermal effect synergistic induced simultaneous removal and mechanism of Cr(VI) and RhB by GQDs/BiOBr/g-C3N4. J. Water Process Eng. 2024, 66, 105914. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Antonopoulou, M.; Daikopoulos, C.; Deligiannakis, Y.; Konstantinou, I. Characterization and catalytic performance of B-doped, B–N co-doped and B–N–F tri-doped TiO2 towards simultaneous Cr(VI) reduction and benzoic acid oxidation. Appl. Catal. B Environ. 2016, 184, 44–54. [Google Scholar] [CrossRef]

| Quencher | ROS | FeS2/α-FeOOH/C/Cr(VI) + o-HBA/Light | ||
|---|---|---|---|---|
| kobs (min−1) | Contribution Rate (%) | |||
| Cr(VI) | control | -- | 0.1191 | -- |
| KBrO3 | e− | 0.0165 | 86 | |
| BQ | •O2− | 0.0332 | 65 | |
| o-HBA | control | -- | 0.0189 | -- |
| TBA | •OH | 0.0061 | 67 | |
| BQ | •O2− | 0.0112 | 40 | |
| EDTA-2Na | hv | 0.0036 | 80 | |
| PMSO | FeIV=O | 0.0069 | 61 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Zhang, W.; Liu, Q.; Chen, G.; Yan, M.; Chi, Y.; Yang, K. Synergistic Removal of Typical Heavy Metal and Organic Contaminants via FeS2/α-FeOOH/C from Electronic Industry Wastewater: Insights for Selective Degradation and Promotion. Sustainability 2025, 17, 9239. https://doi.org/10.3390/su17209239
Zhou J, Zhang W, Liu Q, Chen G, Yan M, Chi Y, Yang K. Synergistic Removal of Typical Heavy Metal and Organic Contaminants via FeS2/α-FeOOH/C from Electronic Industry Wastewater: Insights for Selective Degradation and Promotion. Sustainability. 2025; 17(20):9239. https://doi.org/10.3390/su17209239
Chicago/Turabian StyleZhou, Jinqun, Wei Zhang, Qinghui Liu, Gang Chen, Mengbo Yan, Yanxiao Chi, and Kunlun Yang. 2025. "Synergistic Removal of Typical Heavy Metal and Organic Contaminants via FeS2/α-FeOOH/C from Electronic Industry Wastewater: Insights for Selective Degradation and Promotion" Sustainability 17, no. 20: 9239. https://doi.org/10.3390/su17209239
APA StyleZhou, J., Zhang, W., Liu, Q., Chen, G., Yan, M., Chi, Y., & Yang, K. (2025). Synergistic Removal of Typical Heavy Metal and Organic Contaminants via FeS2/α-FeOOH/C from Electronic Industry Wastewater: Insights for Selective Degradation and Promotion. Sustainability, 17(20), 9239. https://doi.org/10.3390/su17209239






