Sustainable Ozonation Using Natural Zeolite-Based Catalysts for Petrochemical Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
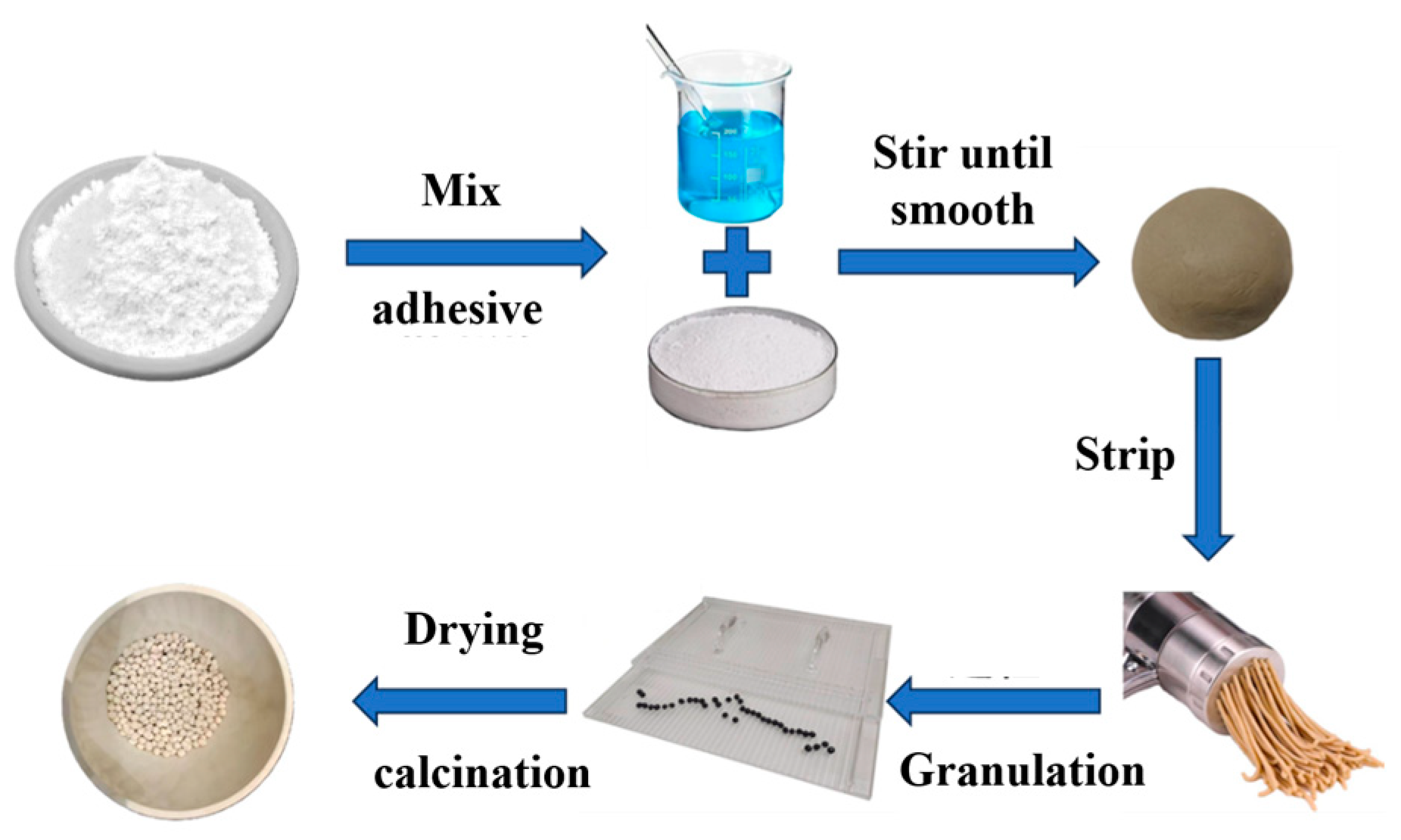
2.2. Experimental Methods
2.3. Characterization Methods
3. Results and Discussion
3.1. Optimization of Catalyst Preparation
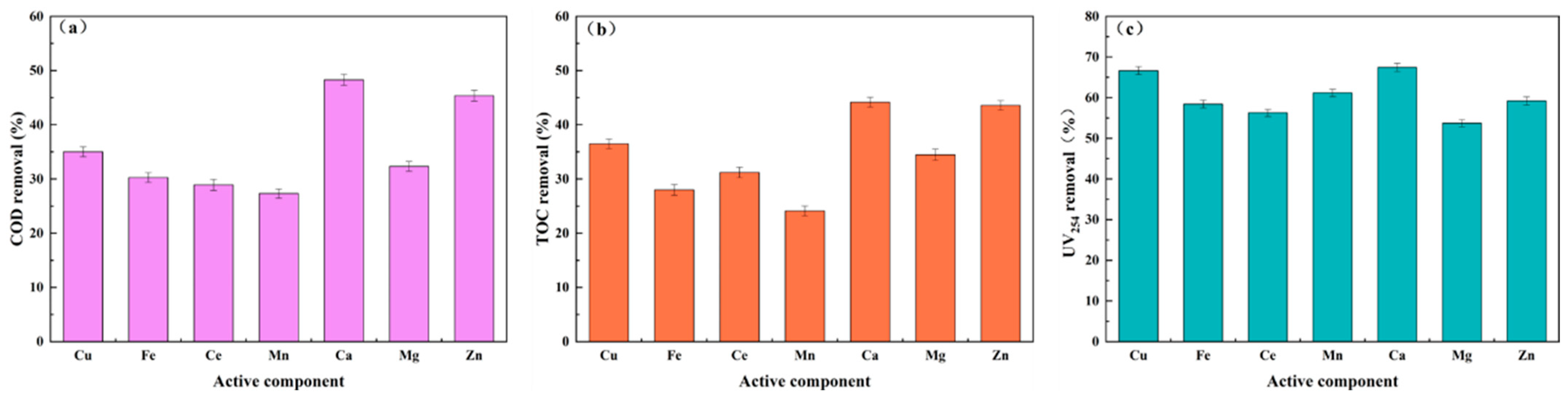
3.1.1. Single-Component Screening of Active Components
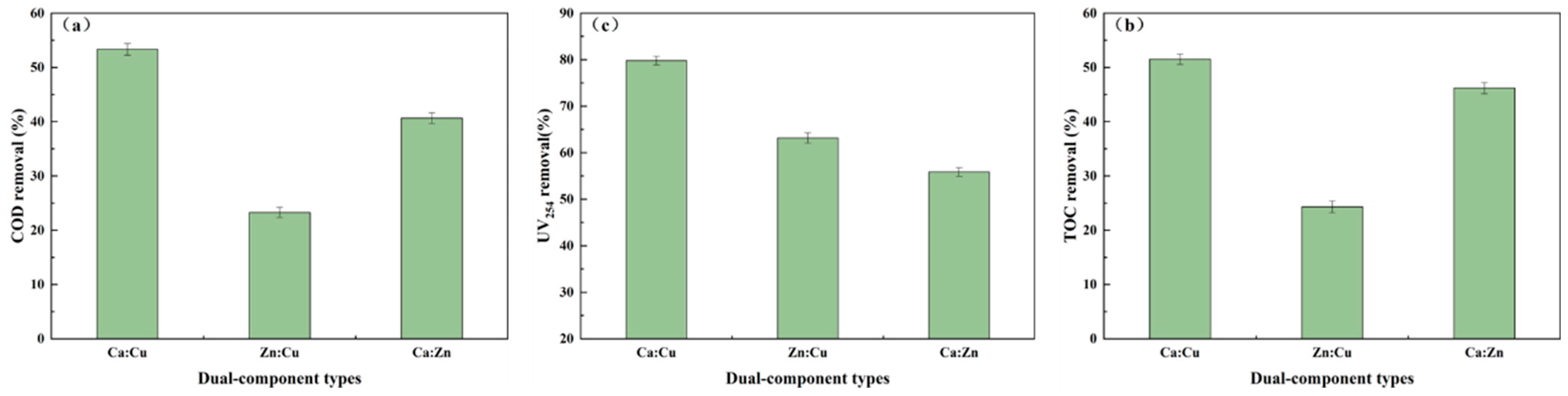
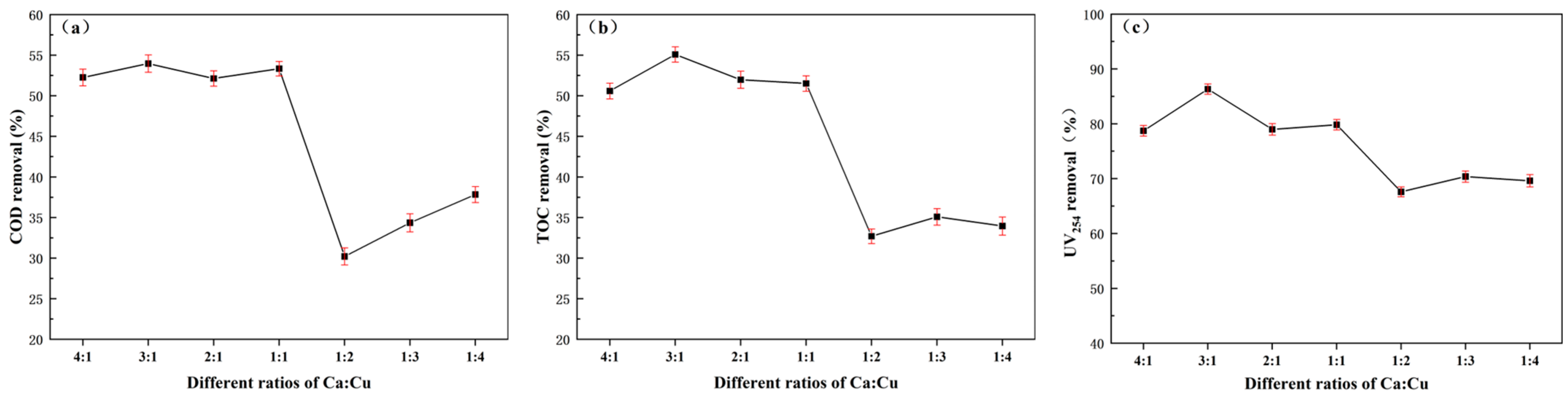
3.1.2. Dual-Component Screening of Active Components
3.2. Catalyst Characterization and Performance
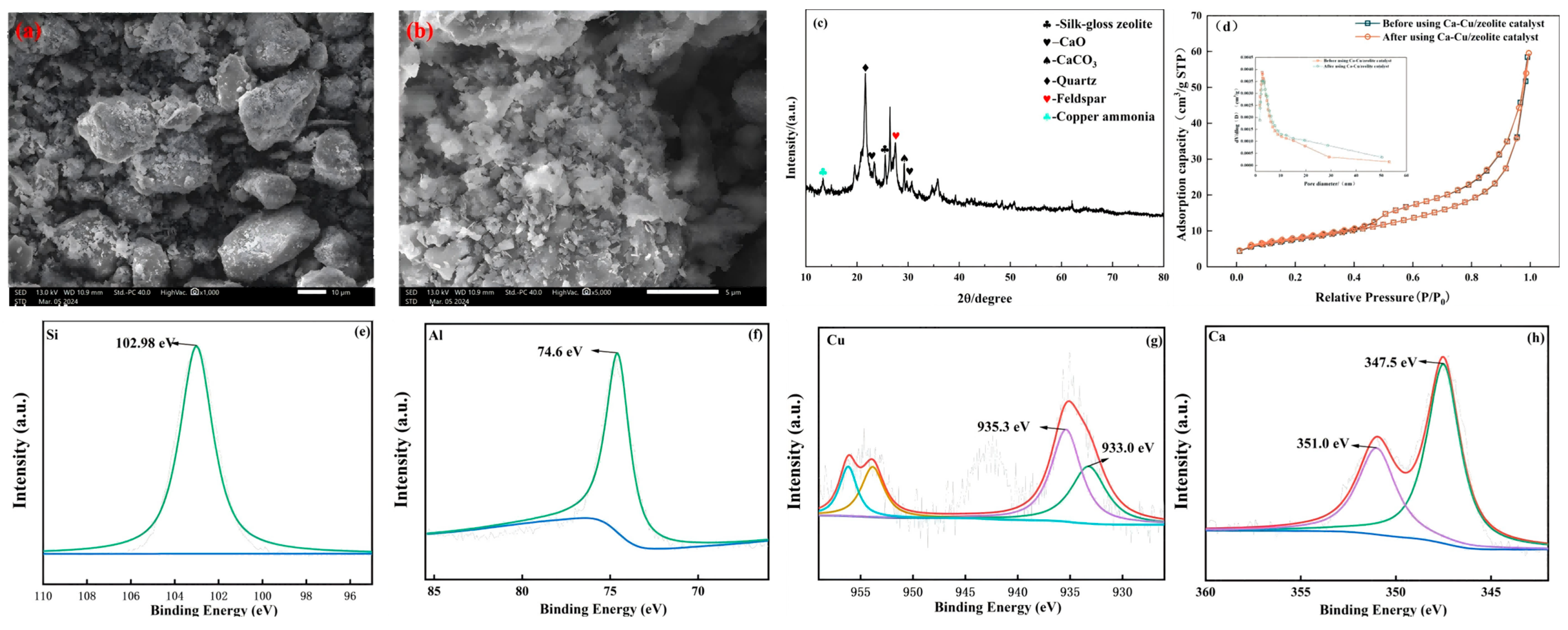
3.2.1. Catalyst Characterization
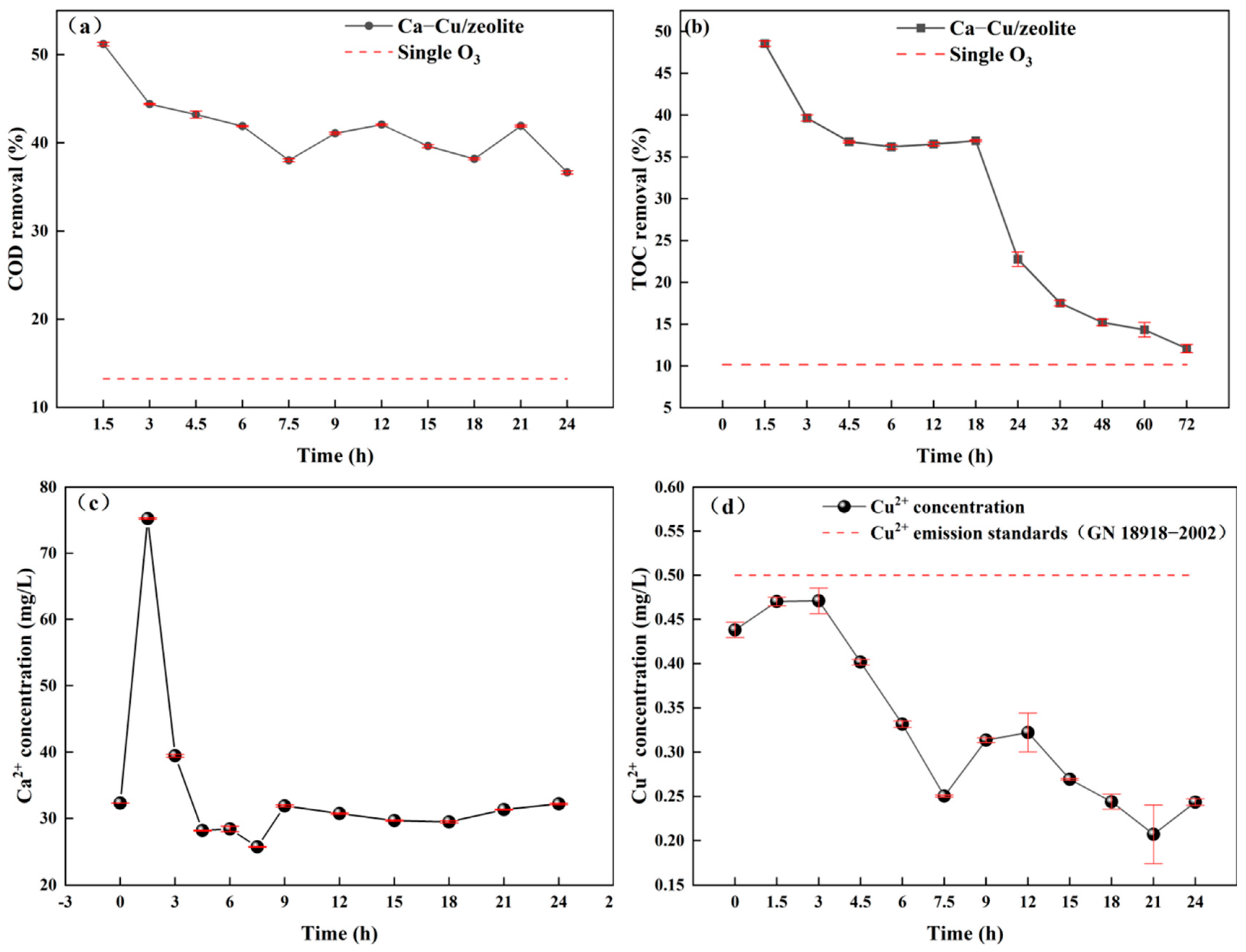
3.2.2. Catalytic Ozonation Performance
3.2.3. Catalyst Stability
3.3. Mechanism of Catalytic Ozonation of Petrochemical Wastewater
3.3.1. Ozone Catalytic Decomposition Kinetics
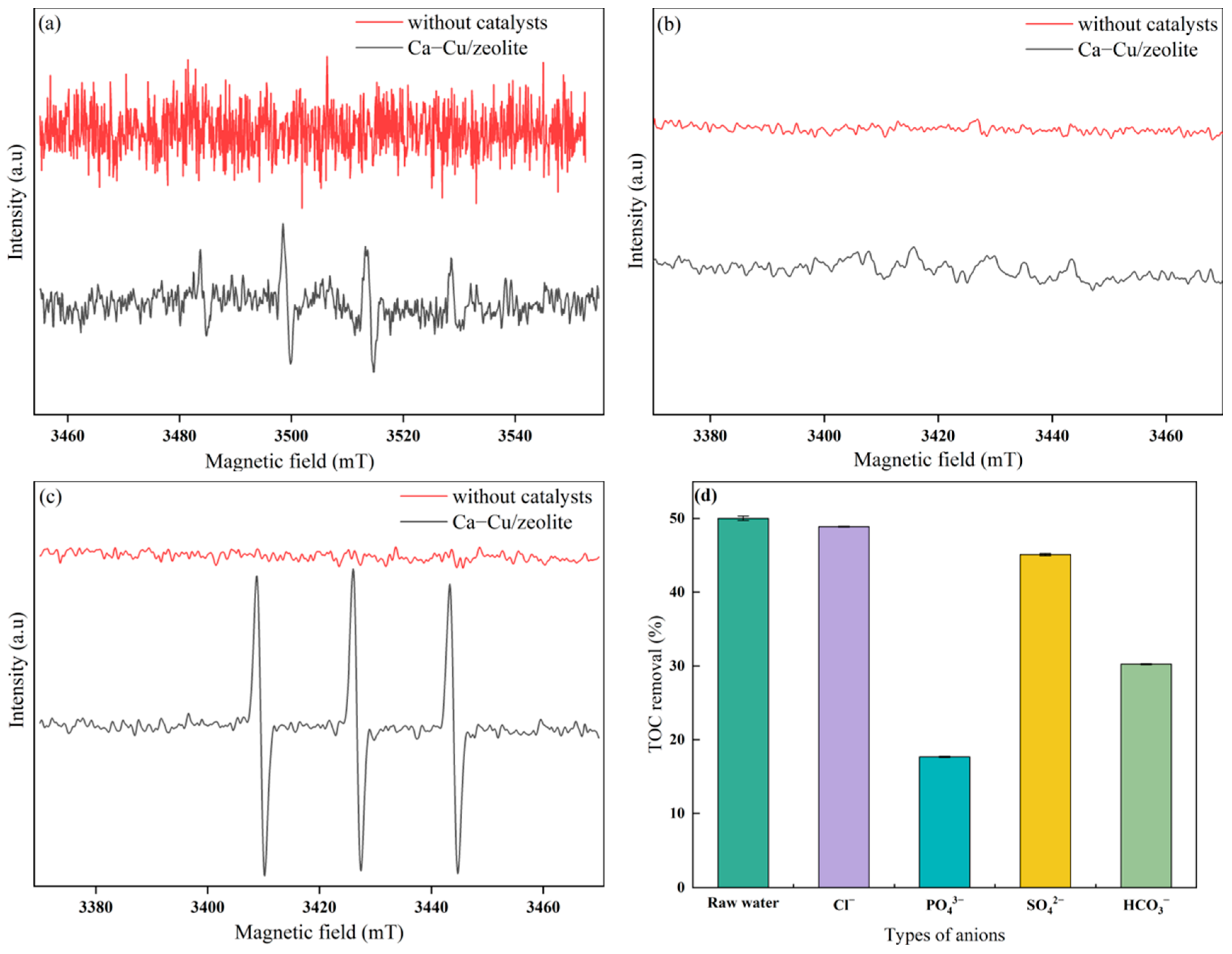
3.3.2. Identification of Reactive Oxygen Species
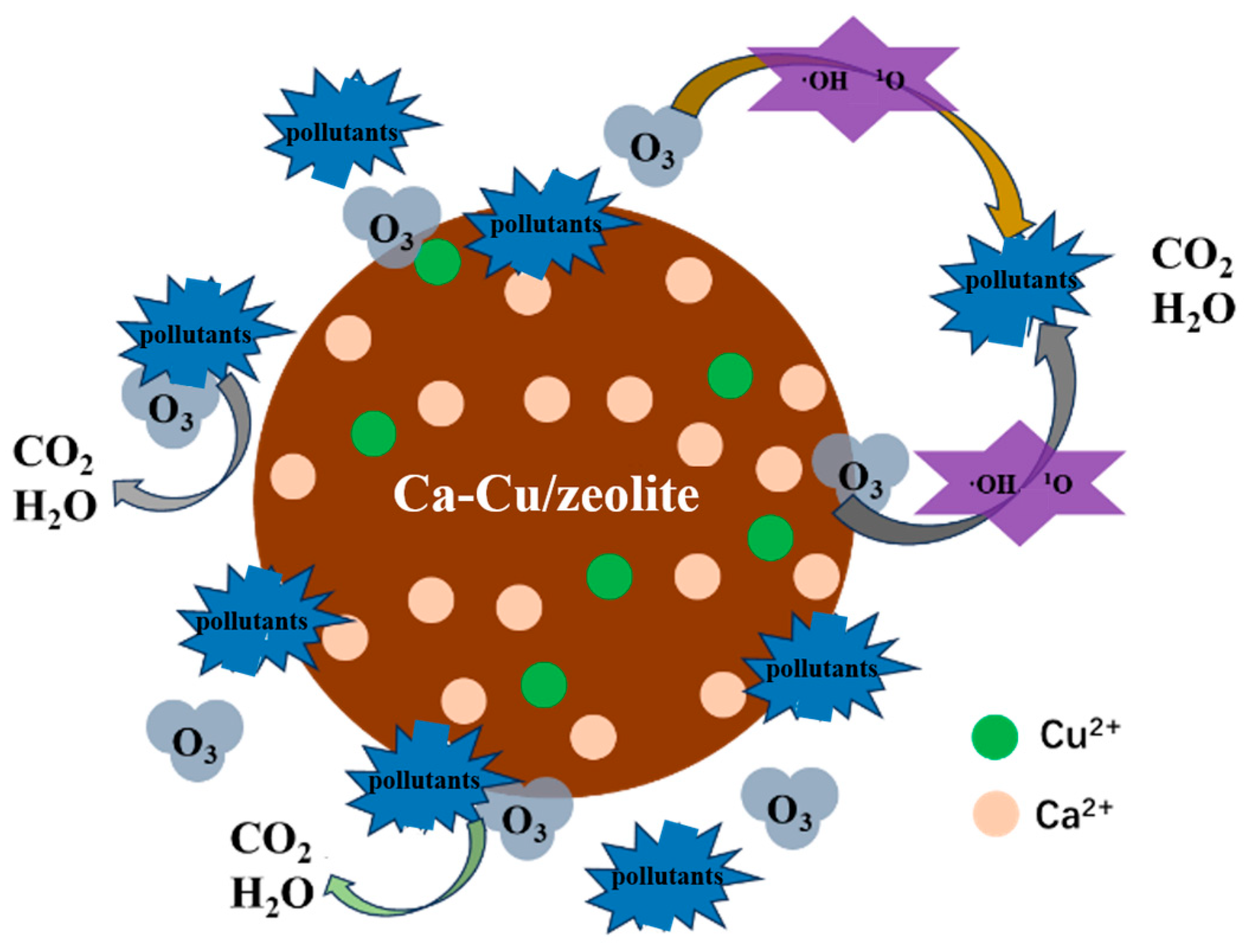
3.3.3. Mechanism of Pollutant Removal
3.4. Catalyst Preparation Costs and Carbon Emissions Accounting
3.4.1. Catalyst Preparation Cost Calculation
3.4.2. Catalyst Preparation Carbon Emissions Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonzalez, N.; Esplugas, R.; Marques, M.; Domingo, J.L. Concentrations of arsenic and vanadium in environmental and biological samples collected in the neighborhood of petrochemical industries: A review of the scientific literature. Sci. Total Environ. 2021, 771, 145149. [Google Scholar] [CrossRef]
- Jephcote, C.; Brown, D.; Verbeek, T.; Mah, A. A systematic review and meta-analysis of haematological malignancies in residents living near petrochemical facilities. Environ. Health 2020, 19, 53. [Google Scholar] [CrossRef]
- Ghazizade, M.J.; Koulivand, H.; Safari, E.; Heidari, L. Petrochemical waste characterization and management at Pars Special Economic Energy Zone in the south of Iran. Waste Manag. Res. 2021, 39, 199–208. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, S.; Han, Y.; Wolfe, F.A. Treatment of petrochemical wastewater and produced water from oil and gas. Water Environ. Res. 2019, 91, 1025–1033. [Google Scholar] [CrossRef]
- Bai, P.; Wu, P.; Yan, Z.; Zhao, X.S. Cation-anion double hydrolysis derived mesoporous γ-Al2O3 as an environmentally friendly and efficient aldol reaction catalyst. J. Mater. Chem. 2009, 19, 1554–1563. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, H.; Yan, Y. Catalytic oxidation of ethyl acetate over CuO/ZSM-5 catalysts: Effect of preparation method. J. Taiwan Inst. Chem. Eng. 2018, 84, 162–172. [Google Scholar] [CrossRef]
- Cui, Y.-H.; Xue, W.-J.; Yang, S.-Q.; Tu, J.-L.; Guo, X.-L.; Liu, Z.-Q. Electrochemical/peroxydisulfate/Fe3+ treatment of landfill leachate nanofiltration concentrate after ultrafiltration. Chem. Eng. J. 2018, 353, 208–217. [Google Scholar] [CrossRef]
- Pocostales, P.; Alvarez, P.; Beltran, F.J. Catalytic ozonation promoted by alumina-based catalysts for the removal of some pharmaceutical compounds from water. Chem. Eng. J. 2011, 168, 1289–1295. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, P.; Yu, Y.; Li, M.; Xi, H.; Fu, L.; Wu, C. Aluminum-based ozone catalysts prepared by mixing method: Characteristics, performance and carbon emissions. Chemosphere 2024, 349, 140842. [Google Scholar] [CrossRef]
- He, C.; Wang, J.; Wang, C.; Zhang, C.; Hou, P.; Xu, X. Catalytic ozonation of bio-treated coking wastewater in continuous pilot- and full-scale system: Efficiency, catalyst deactivation and in-situ regeneration. Water Res. 2020, 183, 116090. [Google Scholar] [CrossRef]
- Miao, F.; Cheng, T.; Wang, L.; Li, K.; Bao, M.; Ma, C.; Nie, K.; Liu, Y.; Jiao, W. Treatment of high-salt phenol wastewater by high-gravity technology intensified Co-Mn/γ-Al2O3 catalytic ozonation: Treatment efficiency, inhibition and catalytic mechanism. Chem. Eng. Sci. 2024, 292, 120019. [Google Scholar] [CrossRef]
- He, C.; Zhang, Z.; Han, J.; Gong, C.; Zhang, J.; Wang, L.; He, P.; Shan, Y.; Zhang, X. Advanced treatment of high-salinity wastewater by catalytic ozonation with pilot- and full-scale systems and the effects of Cu2+ in original wastewater on catalyst activity. Chemosphere 2023, 311, 136971. [Google Scholar] [CrossRef]
- Yuan, Y.; Garg, S.; Wang, Y.; Li, W.; Chen, G.; Gao, M.; Zhong, J.; Wang, J.; Waite, T.D. Influence of salinity on the heterogeneous catalytic ozonation process: Implications to treatment of high salinity wastewater. J. Hazard. Mater. 2022, 423, 127255. [Google Scholar] [CrossRef]
- Guo, L.; Xie, Y.; Xu, Y.; Zhou, J.; Sun, W.; Sun, Y. Pilot-scale study and biochemical verification of salt-tolerant catalyst Fe-Bi@γ-Al2O3 for catalytic ozonation of high-salinity wastewater. J. Environ. Chem. Eng. 2023, 11, 110031. [Google Scholar] [CrossRef]
- Wang, J.; Lou, Y.; Zhuang, X.; Song, S.; Liu, W.; Xu, C. Magnetic Pr6O11/SiO2@Fe3O4 particles as the heterogeneous catalyst for the catalytic ozonation of acetochlor: Performance and aquatic toxicity. Sep. Purif. Technol. 2018, 197, 63–69. [Google Scholar] [CrossRef]
- Wang, W.-L.; Hu, H.-Y.; Liu, X.; Shi, H.-X.; Zhou, T.-H.; Wang, C.; Huo, Z.-Y.; Wu, Q.-Y. Combination of catalytic ozonation by regenerated granular activated carbon (rGAC) and biological activated carbon in the advanced treatment of textile wastewater for reclamation. Chemosphere 2019, 231, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, L.; Liu, C.; Ma, J.; Yan, C.; Mu, S.; Yu, M. Efficient degradation of tetracycline hydrochloride wastewater by microbubble catalytic ozonation with sludge biochar-loaded layered polymetallic hydroxide. Sep. Purif. Technol. 2024, 340, 126767. [Google Scholar] [CrossRef]
- Della Rocca, D.G.; De Noni Junior, A.; Rodriguez-Aguado, E.; Peralta, R.A.; Rodriguez-Castellon, E.; Li Puma, G.; Moreira, R.F.P.M. Mechanistic insights on the catalytic ozonation of trimethoprim in aqueous phase using geopolymer catalysts produced from mining waste. J. Environ. Chem. Eng. 2023, 11, 111163. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, Y.; Xie, S.; Zhou, J.; Sun, W. Catalytic ozonation of 2, 4-dichlorophenoxyacetic acid wastewater by Fe-La@ZE catalyst. J. Water Process Eng. 2024, 61, 105362. [Google Scholar] [CrossRef]
- Liu, D.; Wang, C.; Wang, Z.; Sun, Y.; Liu, X.; Xiao, S.; Li, L.; Zhou, J. Magnetically separable NiFe2O4/sepiolite catalyst for enhanced ozonation treatment of quinoline and bio-treated coking wastewater in a catalytic ozonation system. Process Saf. Environ. Prot. 2022, 159, 422–432. [Google Scholar] [CrossRef]
- Shao, S.; Li, Z.; Gao, K.; Zhang, J.; Liu, Y.; Jiao, W. Preparation of Cu-MnOx/γ-Al2O3 by high gravity-assisted impregnation method for heterogeneous catalytic ozonation of nitrobenzene. Sep. Purif. Technol. 2022, 280, 119896. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Bingre, R.; Louis, B.; Nguyen, P. An overview on zeolite shaping technology and solutions to overcome diffusion limitations. Catalysts 2018, 8, 163. [Google Scholar] [CrossRef]
- Ciesla, J.; Franus, W.; Franus, M.; Kedziora, K.; Gluszczyk, J.; Szerement, J.; Jozefaciuk, G. Environmental-Friendly Modifications of Zeolite to Increase Its Sorption and Anion Exchange Properties, Physicochemical Studies of the Modified Materials. Materials 2019, 12, 3213. [Google Scholar] [CrossRef]
- Jozefaciuk, G.; Szatanik-Kloc, A.; Ambrozewicz-Nita, A. The surface area of zeolite-amended soils exceeds the sum of the inherent surface areas of soil and zeolite. Eur. J. Soil Sci. 2018, 69, 787–790. [Google Scholar] [CrossRef]
- Shekarchi, M.; Ahmadi, B.; Azarhomayun, F.; Shafei, B.; Kioumarsi, M. Natural zeolite as a supplementary cementitious material—A holistic review of main properties and applications. Constr. Build. Mater. 2023, 409, 133766. [Google Scholar] [CrossRef]
- Pansini, M. Natural zeolites as cation exchangers for environmental protection. Miner. Depos. 1996, 31, 563–575. [Google Scholar] [CrossRef]
- Shah, M.S.; Tsapatsis, M.; Siepmann, J.I. Identifying optimal zeolitic sorbents for sweetening of highly sour natural gas. Angew. Chem.-Int. Ed. 2016, 55, 5938–5942. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Azreena, I.N.; Peng, H.; Rabiee, H.; Ahmed, M.; Weng, Y.; Zhu, Z.; Kennedy, E.M.; Stockenhuber, M. Catalytic hydropyrolysis of biomass using natural zeolite-based catalysts. Chem. Eng. J. 2023, 476, 146630. [Google Scholar] [CrossRef]
- Munir, H.M.S.; Feroze, N.; Ramzan, N.; Sagir, M.; Babar, M.; Tahir, M.S.; Shamshad, J.; Mubashir, M.; Khoo, K.S. Fe-zeolite catalyst for ozonation of pulp and paper wastewater for sustainable water resources. Chemosphere 2022, 297, 134031. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhuo, Q.; Dai, L.; Guan, B. Mn anchored zeolite molecular nest for enhanced catalytic ozonation of cephalexin. Chemosphere 2023, 335, 139058. [Google Scholar] [CrossRef]
- Fang, C.; Gao, X.; Zhang, X.; Zhu, J.; Sun, S.-P.; Wang, X.; Wu, W.D.; Wu, Z. Facile synthesis of alkaline-earth metal manganites for the efficient degradation of phenolic compounds via catalytic ozonation and evaluation of the reaction mechanism. J. Colloid Interface Sci. 2019, 551, 164–176. [Google Scholar] [CrossRef]
- Jothinathan, L.; Cai, Q.Q.; Ong, S.L.; Hu, J.Y. Fe-Mn doped powdered activated carbon pellet as ozone catalyst for cost-effective phenolic wastewater treatment: Mechanism studies and phenol by-products elimination. J. Hazard. Mater. 2022, 424, 127483. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, H. Catalytic ozonation for water and wastewater treatment: Recent advances and perspective. Sci. Total Environ. 2020, 704, 135249. [Google Scholar] [CrossRef]
- Shen, T.; Zhang, X.; Lin, K.-Y.A.; Tong, S. Solid base Mg-doped ZnO for heterogeneous catalytic ozonation of isoniazid: Performance and mechanism. Sci. Total Environ. 2020, 703, 134983. [Google Scholar] [CrossRef]
- Li, S.; Tang, Y.; Chen, W.; Hu, Z.; Li, X.; Li, L. Heterogeneous catalytic ozonation of clofibric acid using Ce/MCM-48: Preparation, reaction mechanism, comparison with Ce/MCM-41. J. Colloid Interface Sci. 2017, 504, 238–246. [Google Scholar] [CrossRef]
- Sun, W.; Zhu, H.; Xie, Y.; Zhou, J.; Sun, Y. Catalytic ozonation of coal chemical biochemical secondary effluent by a Cu-MnFe2O4/Bt ozone catalyst. J. Water Process Eng. 2025, 69, 106903. [Google Scholar]
- Yuan, X.; Xin, H.; Qin, X.; Li, X.; Liu, Y.; Guo, H. Self-assembly of SiO/Reduced Graphene Oxide composite as high-performance anode materials for Li-ion batteries. Electrochim. Acta 2015, 155, 251–256. [Google Scholar]
- Fang, R.; Sun, Q.-Q.; Zhou, P.; Yang, W.; Wang, P.-F.; Zhang, D. High-performance bilayer flexible resistive random access memory based on low-temperature thermal atomic layer deposition. Nanoscale Res. Lett. 2013, 8, 92. [Google Scholar] [CrossRef]
- Pauly, N.; Tougaard, S.; Yubero, F. Determination of the Cu 2p primary excitation spectra for Cu, Cu2O and CuO. Surf. Sci. 2014, 620, 17–22. [Google Scholar] [CrossRef]
- Lu, Q.; Yuan, S.; Chen, X.; Li, K.; Qian, T.; Zhao, Y.; Meng, L.; Xie, X.; Zhao, Y.; Zhou, Y. The effect of reaction condition on catalytic cracking of wheat straw pyrolysis volatiles over char-based Fe-Ni-Ca catalyst. Energy 2023, 263, 125722. [Google Scholar] [CrossRef]
- Fu, L.; Wang, P.; Wu, C.; Zhou, Y.; Song, Y.; Guo, S.; Li, Z.; Zhou, J. Upgrade of the biggest catalytic ozonation wastewater treatment plant in China: From pollution control to carbon reduction. J. Environ. Manag. 2024, 349, 119421. [Google Scholar] [CrossRef]
- GB 18918-2002; Discharge Standard of Pollutants for Municipal Wastewater Treatment Plant In State Environmental Protection Administration. General Administration of Quality Supervision, Inspection and Quarantine: Beijing, China, 2002.
- Li, B.; Zhao, X.; Sun, X.; Duan, Y.; Wan, C.; Fan, Y.; Wu, C. Regeneration of deactivated ozone catalysts in the treatment of high-alkalinity industrial wastewater. J. Environ. Chem. Eng. 2025, 13, 115327. [Google Scholar] [CrossRef]
- Kong, X.; Garg, S.; Chen, G.; Waite, T.D. Investigation of the deactivation and regeneration of an Fe2O3/Al2O3·SiO2 catalyst used in catalytic ozonation of coal chemical industry wastewater. J. Hazard. Mater. 2023, 451, 131194. [Google Scholar]
- Tan, X.; Wang, S.; Ma, L.; Xue, W.; Dong, S.; Li, M.; Wang, P.; Wei, H.; Sun, C. Catalytic ozone oxidation of m-cresol by spirulina biochar-modified perovskite: Catalyst deactivation and in situ regeneration. Sep. Purif. Technol. 2024, 329, 124994. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Brown, D.R.; Kasprzyk-Hordern, B. Catalytic ozonation for the removal of organic contaminants in water on ZSM-5 zeolites. Appl. Catal. B-Environ. 2014, 154, 110–122. [Google Scholar]
- Sun, W.; Cheng, Y.; Xiao, Z.; Zhou, J.; Shah, K.J.; Sun, Y. Catalytic ozonation of reverse osmosis membrane concentrates by catalytic ozonation: Properties and mechanisms. Water Environ. Res. 2024, 96, e11058. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Xiao, J.; Jiang, X.; Su, J.; Chu, S.; Ma, X.; Li, J. Three-dimensional electrode-enhanced ozone catalytic oxidation for thiamethoxam wastewater treatment: Performance, kinetics, and pathway. Catalysts 2024, 14, 245. [Google Scholar] [CrossRef]
- Zhang, S.; Quan, X.; Zheng, J.-F.; Wang, D. Probing the interphase “HO·zone” originated by carbon nanotube during catalytic ozonation. Water Res. 2017, 122, 86–95. [Google Scholar] [PubMed]
- Nawaz, F.; Xie, Y.; Xiao, J.; Cao, H.; Ghazi, Z.A.; Guo, Z.; Chen, Y. The influence of the substituent on the phenol oxidation rate and reactive species in cubic MnO2 catalytic ozonation. Catal. Sci. Technol. 2016, 6, 7875–7884. [Google Scholar]
- Qu, J.; Liu, J.; Al-Dhabi, N.A.; Leng, Y.; Yi, J.; Jiang, K.; Xing, W.; Yin, D.; Tang, W. Ni-EDTA decomplexation and Ni removal from wastewater by electrooxidation coupled with electrocoagulation: Optimization, mechanism and biotoxicity assessment. Sep. Purif. Technol. 2025, 376, 133980. [Google Scholar] [CrossRef]
- Li, P.; Miao, R.; Wang, P.; Sun, F.; Li, X.-Y. Bi-metal oxide-modified flat-sheet ceramic membranes for catalytic ozonation of organic pollutants in wastewater treatment. Chem. Eng. J. 2021, 426, 131263. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Brown, D.R.; Kasprzyk-Hordern, B. Mechanisms of catalytic ozonation on alumina and zeolites in water: Formation of hydroxyl radicals. Appl. Catal. B-Environ. 2012, 123, 94–106. [Google Scholar] [CrossRef]
- Nawrocki, J.; Kasprzyk-Hordern, B. The efficiency and mechanisms of catalytic ozonation. Appl. Catal. B-Environ. 2010, 99, 27–42. [Google Scholar] [CrossRef]
- Liu, D.; Wang, C.; Song, Y.; Wei, Y.; He, L.; Lan, B.; He, X.; Wang, J. Effective mineralization of quinoline and bio-treated coking wastewater by catalytic ozonation using CuFe2O4/Sepiolite catalyst: Efficiency and mechanism. Chemosphere 2019, 227, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sun, Z.; Ma, J.; Liu, H. Influencing mechanism of bicarbonate on the catalytic ozonation of nitrobenzene in aqueous solution by ceramic honeycomb supported manganese. J. Mol. Catal. A-Chem. 2010, 322, 26–32. [Google Scholar] [CrossRef]
- Shan, C.; Xu, Y.; Hua, M.; Gu, M.; Yang, Z.; Wang, P.; Lu, Z.; Zhang, W.; Pan, B. Mesoporous Ce-Ti-Zr ternary oxide millispheres for efficient catalytic ozonation in bubble column. Chem. Eng. J. 2018, 338, 261–270. [Google Scholar] [CrossRef]
- Kun, F.; Shao, Q.Z. Accounting Method and Its Application of Provincial Electricity CO2 Emissions Responsibility. China Popul. Resour. Environ. 2014, 24, 27–34. (In Chinese) [Google Scholar]

| Parameter | Unit | Value |
|---|---|---|
| Total organic carbon (TOC) | mg/L | 19.0 ± 5.0 |
| Chemical oxygen demand (COD) | mg/L | 75.0 ± 20.0 |
| Suspended solid (SS) | mg/L | 23.0 ± 4.0 |
| pH | — | 7.5 ± 0.5 |
| Total nitrogen | mg/L | 12.5 ± 2.0 |
| Total phosphate | mg/L | 0.8 ± 0.4 |
| SO42− | mg/L | 580 ± 50 |
| Cl− | mg/L | 350 ± 30 |
| HCO3− | mg/L | 65 ± 10 |
| Serial Number | A | B | C | TOC Removal |
|---|---|---|---|---|
| mg/(L·h) | g/L | min | % | |
| 1 | 60 | 100 | 60 | 15.29 |
| 2 | 120 | 100 | 60 | 18.98 |
| 3 | 60 | 500 | 60 | 35.36 |
| 4 | 120 | 500 | 60 | 35.91 |
| 5 | 60 | 300 | 30 | 22.25 |
| 6 | 120 | 300 | 30 | 24.45 |
| 7 | 60 | 300 | 90 | 37.62 |
| 8 | 120 | 300 | 90 | 44.12 |
| 9 | 90 | 100 | 30 | 13.26 |
| 10 | 90 | 500 | 30 | 31.71 |
| 11 | 90 | 100 | 90 | 25.51 |
| 12 | 90 | 500 | 90 | 42.39 |
| 13 | 90 | 300 | 60 | 33.92 |
| 14 | 90 | 300 | 60 | 35.15 |
| 15 | 90 | 300 | 60 | 33.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Wang, F.; Ma, G.; Qin, Z.; Xi, H.; Yu, Y.; Wu, C. Sustainable Ozonation Using Natural Zeolite-Based Catalysts for Petrochemical Wastewater Treatment. Sustainability 2025, 17, 9110. https://doi.org/10.3390/su17209110
Yuan Y, Wang F, Ma G, Qin Z, Xi H, Yu Y, Wu C. Sustainable Ozonation Using Natural Zeolite-Based Catalysts for Petrochemical Wastewater Treatment. Sustainability. 2025; 17(20):9110. https://doi.org/10.3390/su17209110
Chicago/Turabian StyleYuan, Yue, Fang Wang, Guoxin Ma, Zhikai Qin, Hongbo Xi, Yin Yu, and Changyong Wu. 2025. "Sustainable Ozonation Using Natural Zeolite-Based Catalysts for Petrochemical Wastewater Treatment" Sustainability 17, no. 20: 9110. https://doi.org/10.3390/su17209110
APA StyleYuan, Y., Wang, F., Ma, G., Qin, Z., Xi, H., Yu, Y., & Wu, C. (2025). Sustainable Ozonation Using Natural Zeolite-Based Catalysts for Petrochemical Wastewater Treatment. Sustainability, 17(20), 9110. https://doi.org/10.3390/su17209110






