Steam-Assisted Semi-Carbonization Pretreatment of Corn Stalks: Effects on Physicochemical Properties for Enhanced Biomass Utilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
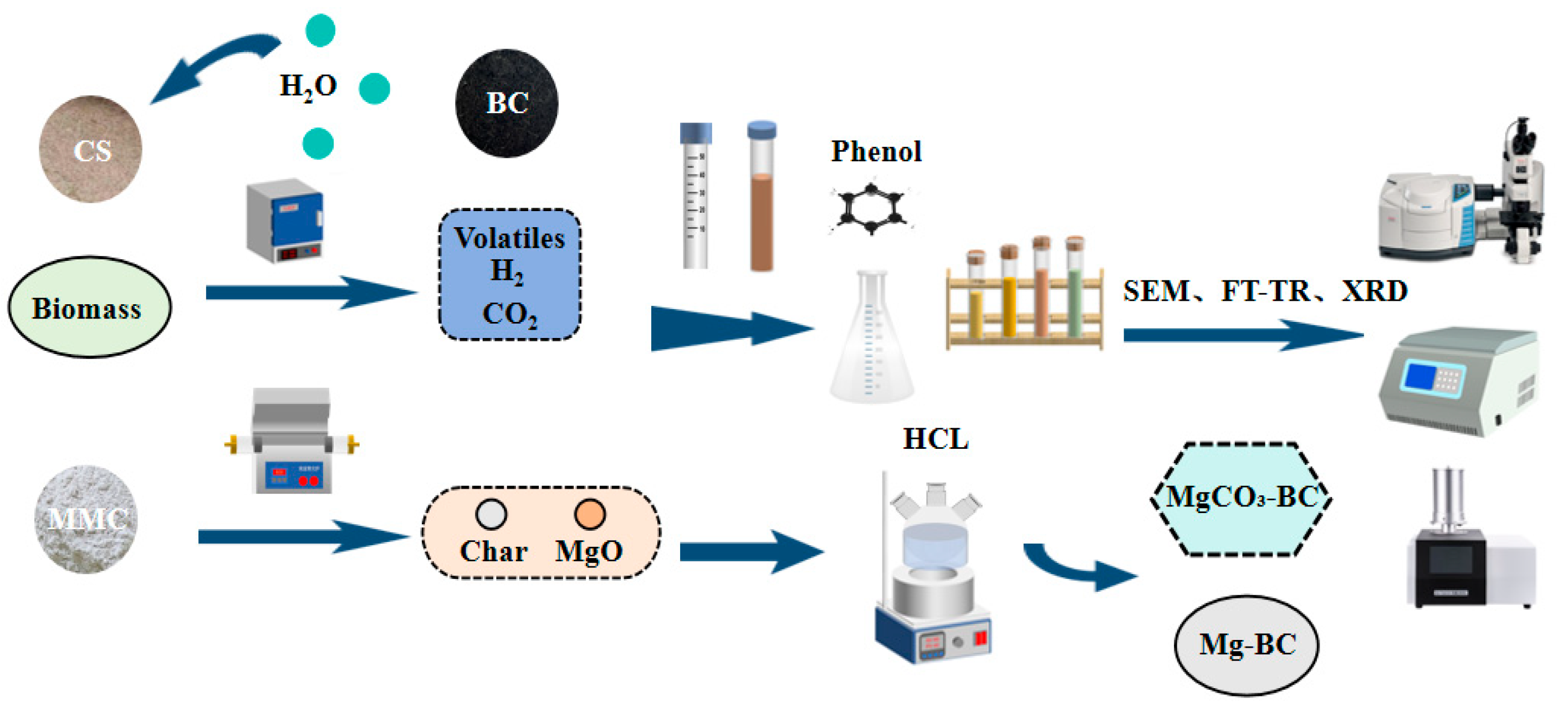
2.2. Semi-Carbonization Pretreatment Process
Pretreatment Process
- (1)
- Steam-assisted torrefaction pretreatment of corn stover
- (2)
- Direct co-torrefaction pretreatment of corn stover with magnesite
- (3)
- Acid-assisted loading and torrefaction pretreatment of corn stover with Magnesite
2.3. Analytical and Characterization Methods
2.3.1. Yield of Torrefied Corn Stover Biochar (CSBC)
2.3.2. Phenol Adsorption Experiments
- (1)
- Phenol measurement method
- (2)
- Phenol removal efficiency
- (3)
- Equilibrium adsorption capacity
2.3.3. SEM and Energy Spectrum Analysis
2.3.4. Determination of Calorific Value
2.3.5. FTIR Analysis
2.3.6. BET Surface Area Analysis
2.3.7. X-Ray Diffraction
2.3.8. Raman Analysis
2.4. Flowchart
3. Results and Discussion
3.1. Steam-Assisted Torrefaction of Corn Stalk
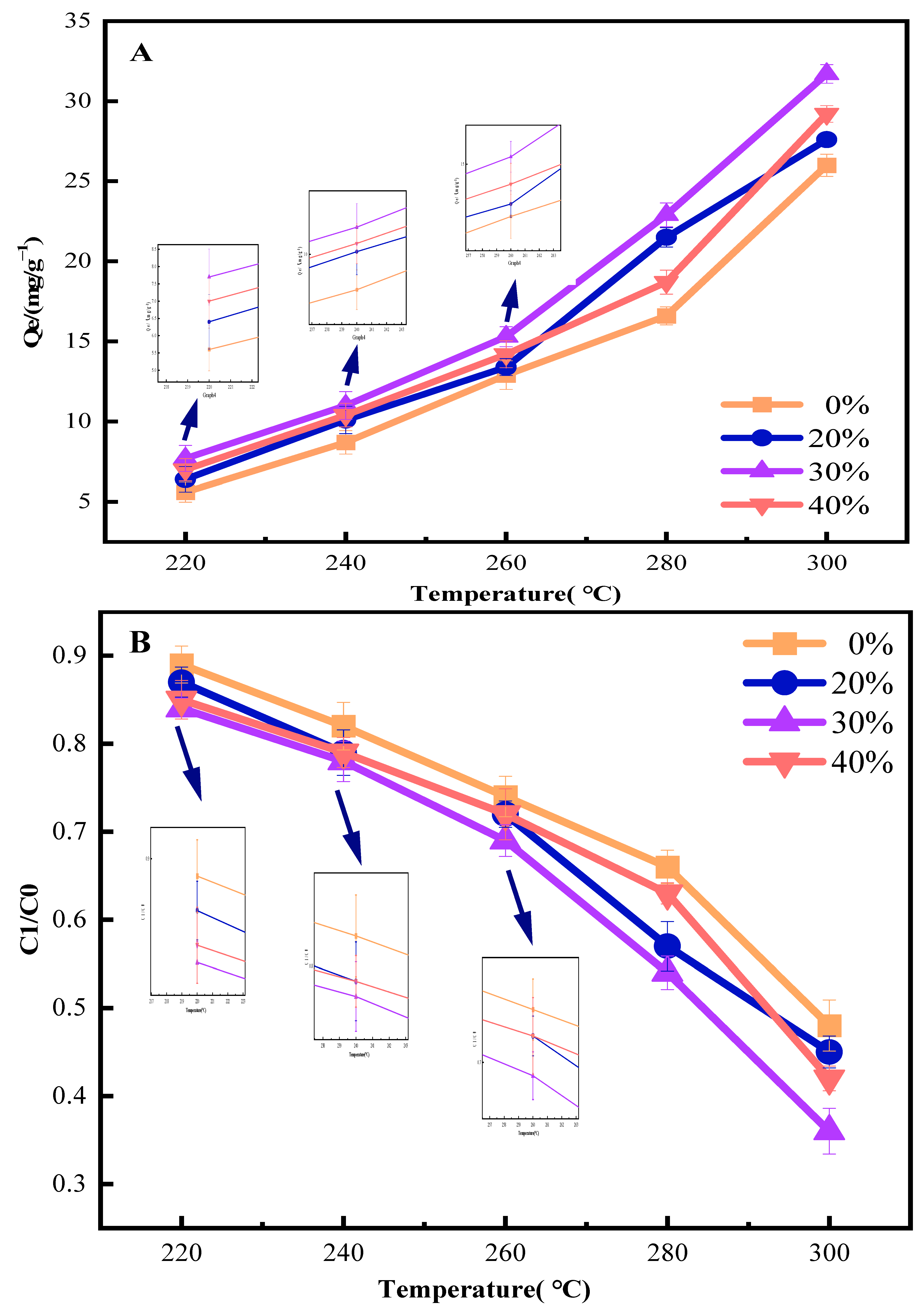
3.1.1. Influence of Torrefaction Conditions on Phenol Adsorption Performance of CSBC
3.1.2. Proximate and Ultimate Analyses of CSBC Under Different Torrefaction Treatment Conditions
- (1)
- Ultimate analysis of CSBC
- (2)
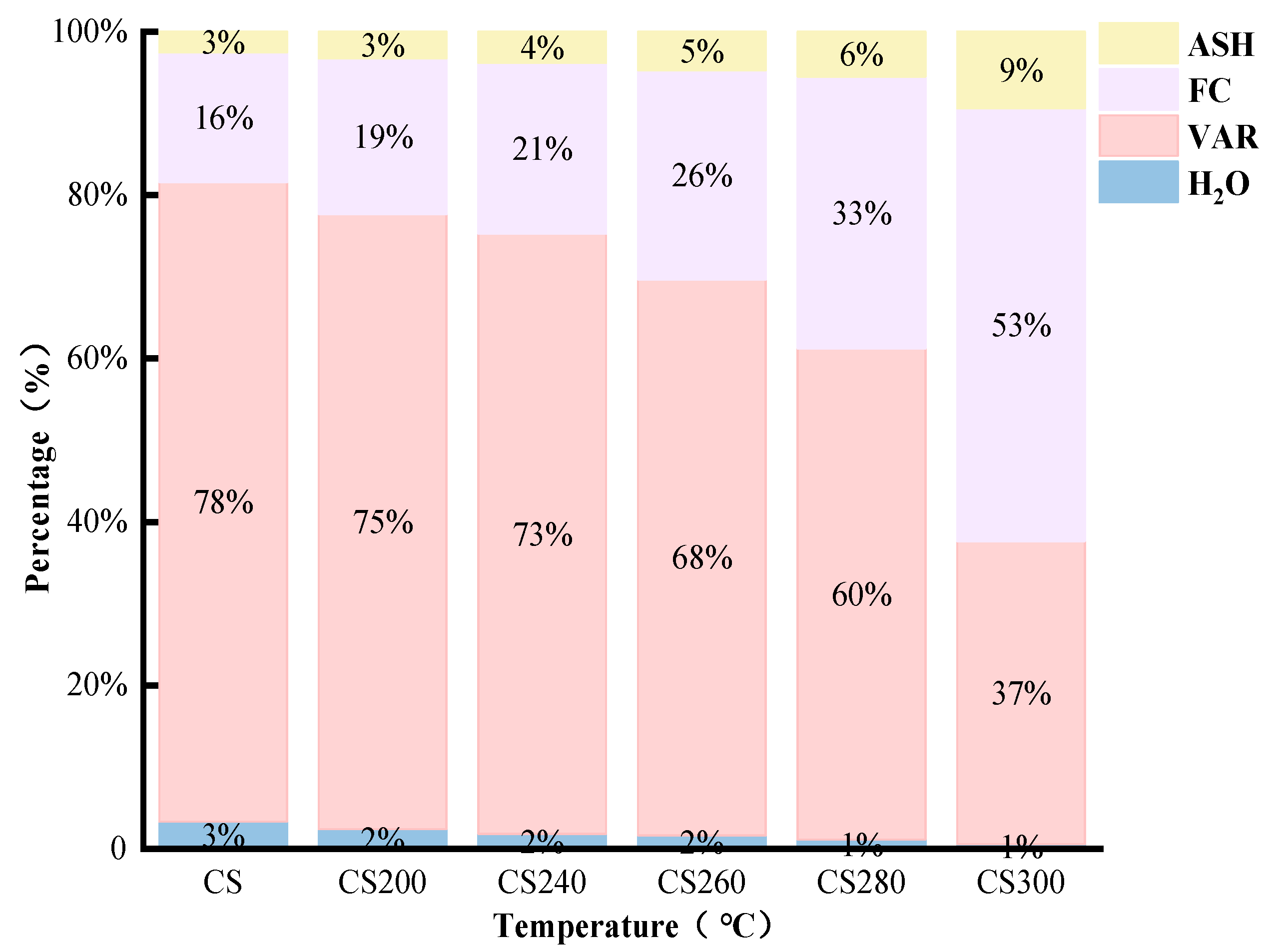
- Proximate analyses of CSBC
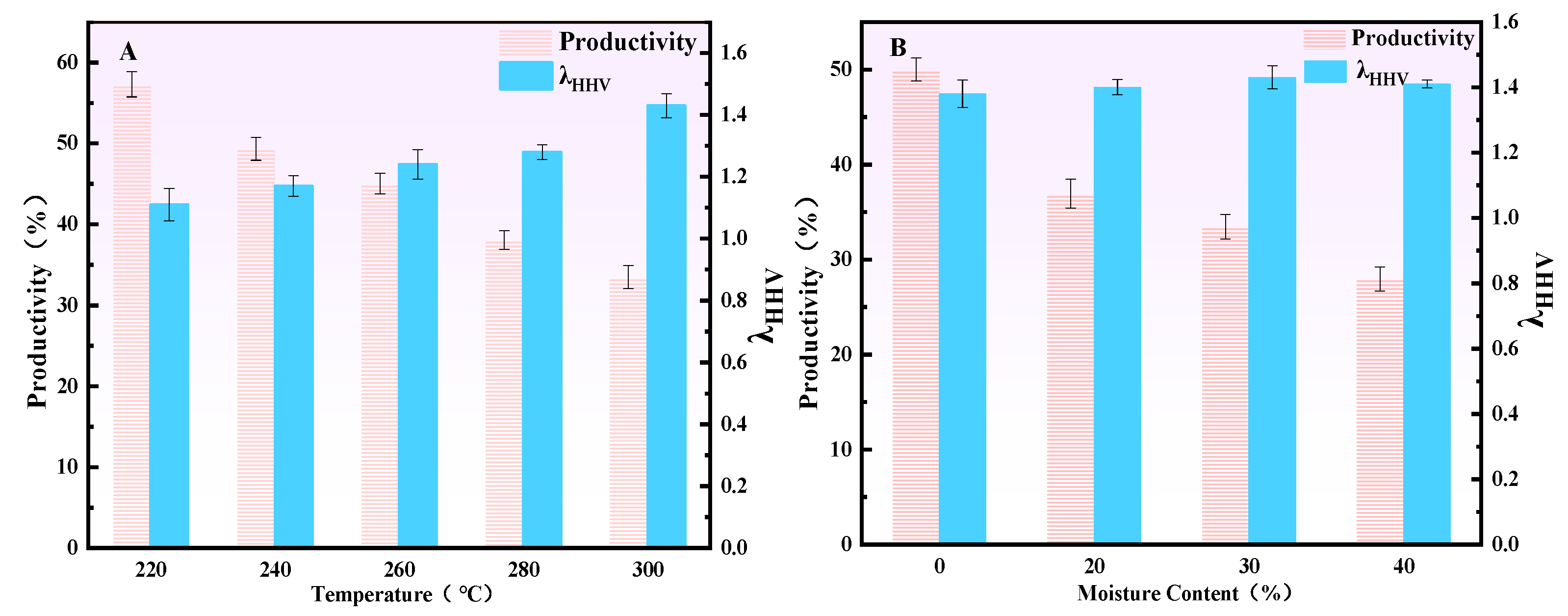
3.1.3. Influence of Torrefaction Treatment Conditions on the Energy Enhancement Coefficient of CSBC
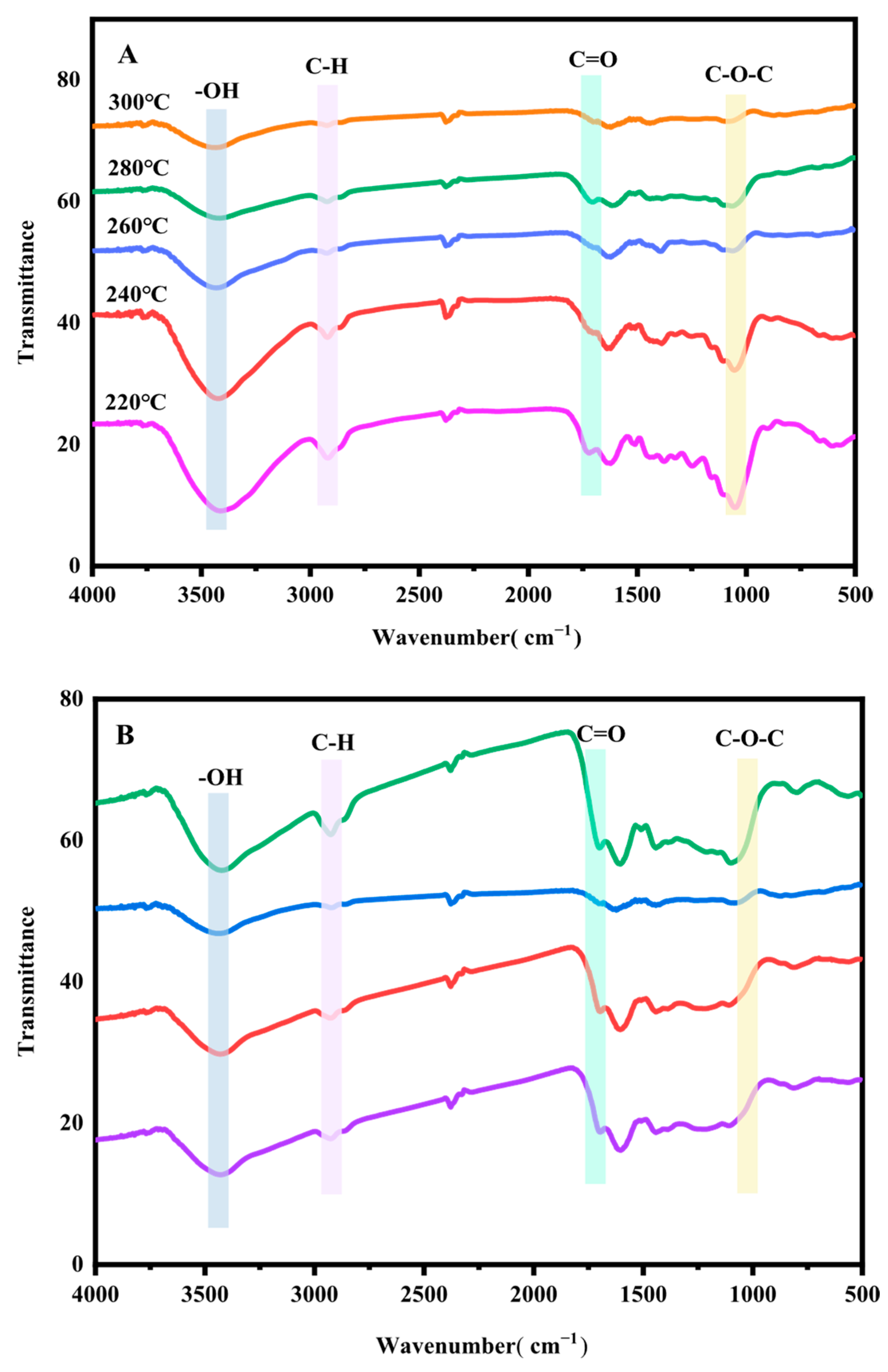
3.1.4. FTIR Analysis of CSBC Under Different Hydrothermal Carbonization Conditions
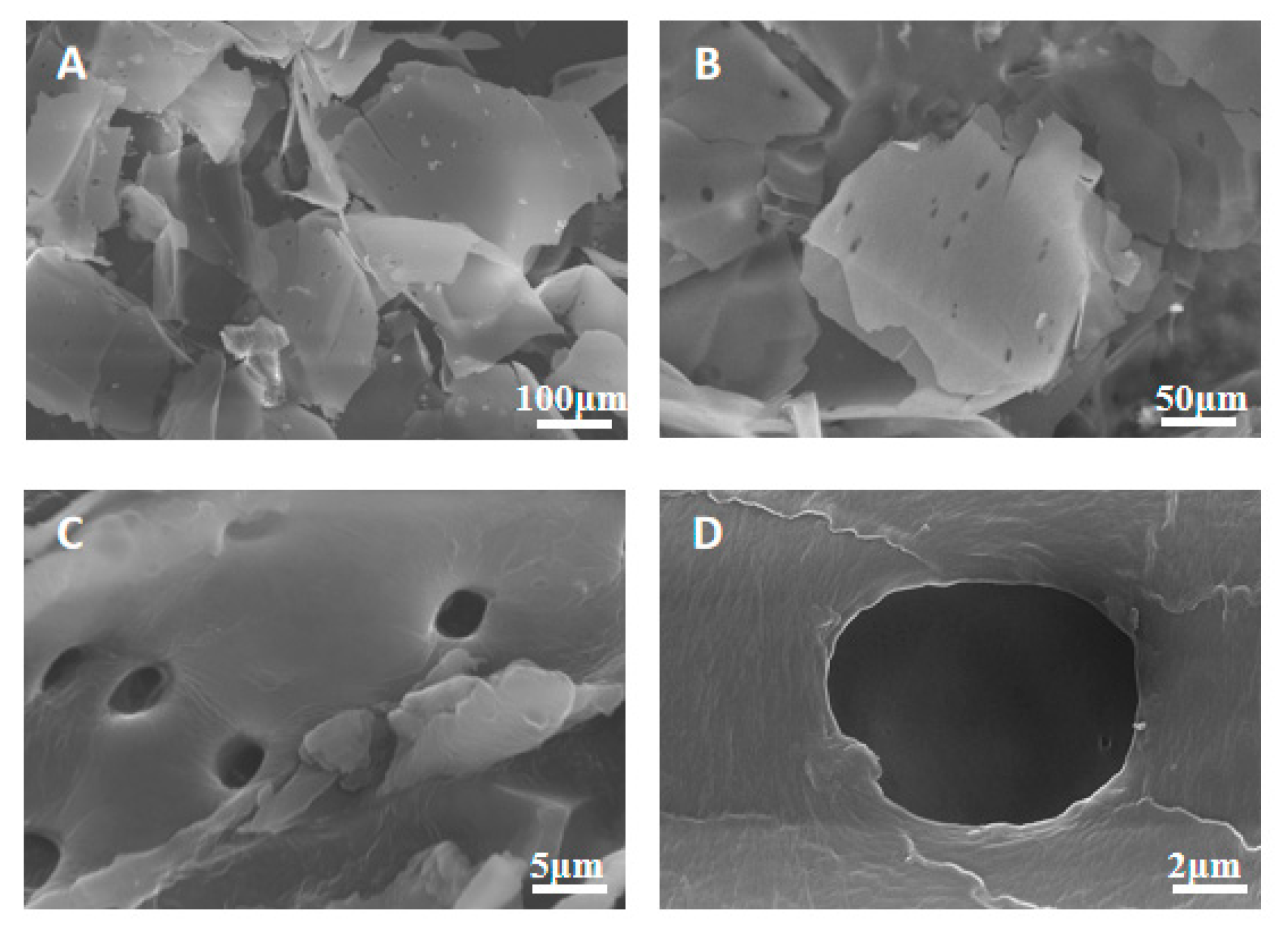
3.1.5. SEM Analysis Based on Torrefaction Treatment
3.2. Steam-Assisted Torrefaction Pretreatment of Corn Stalks and Magnesite
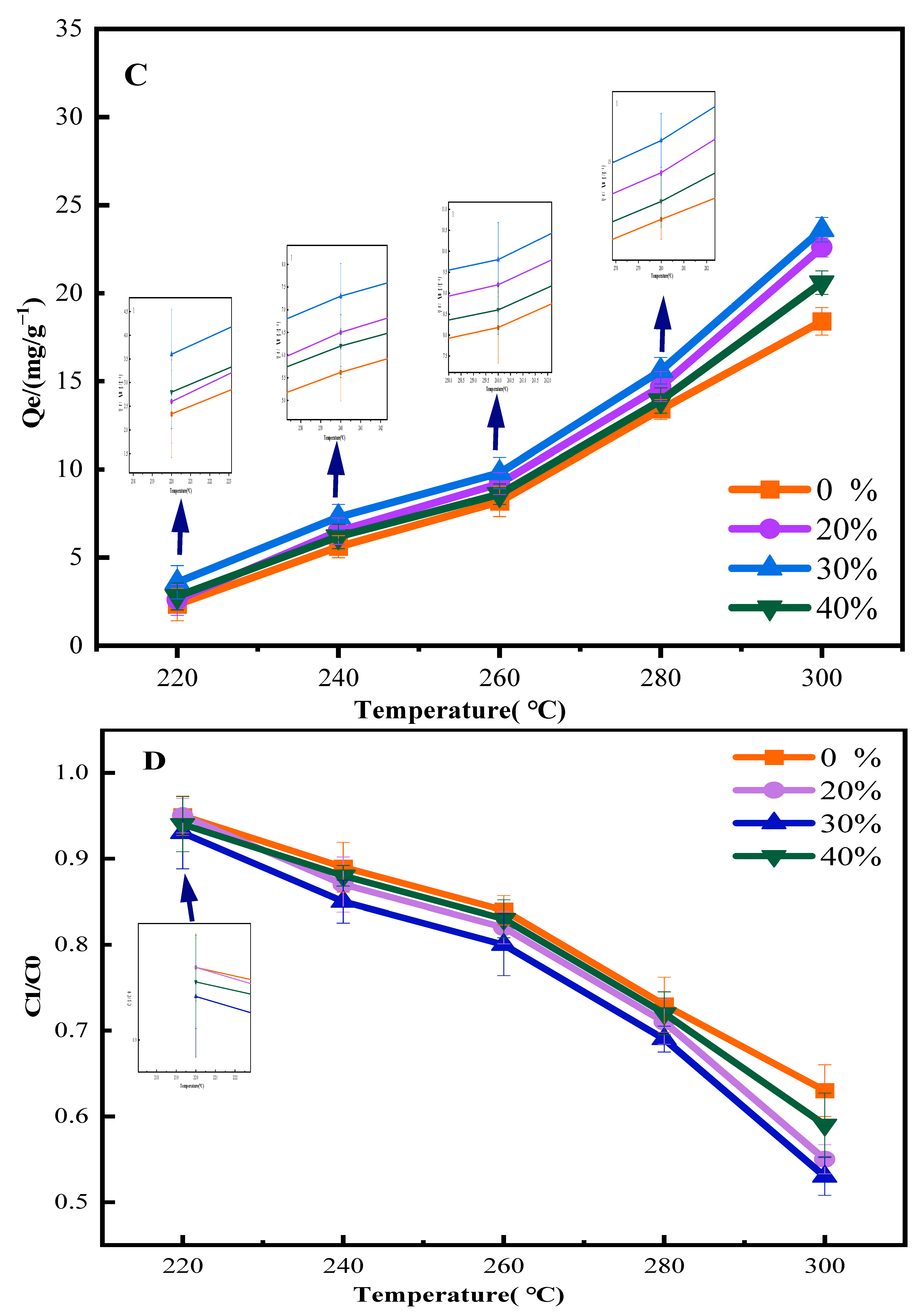
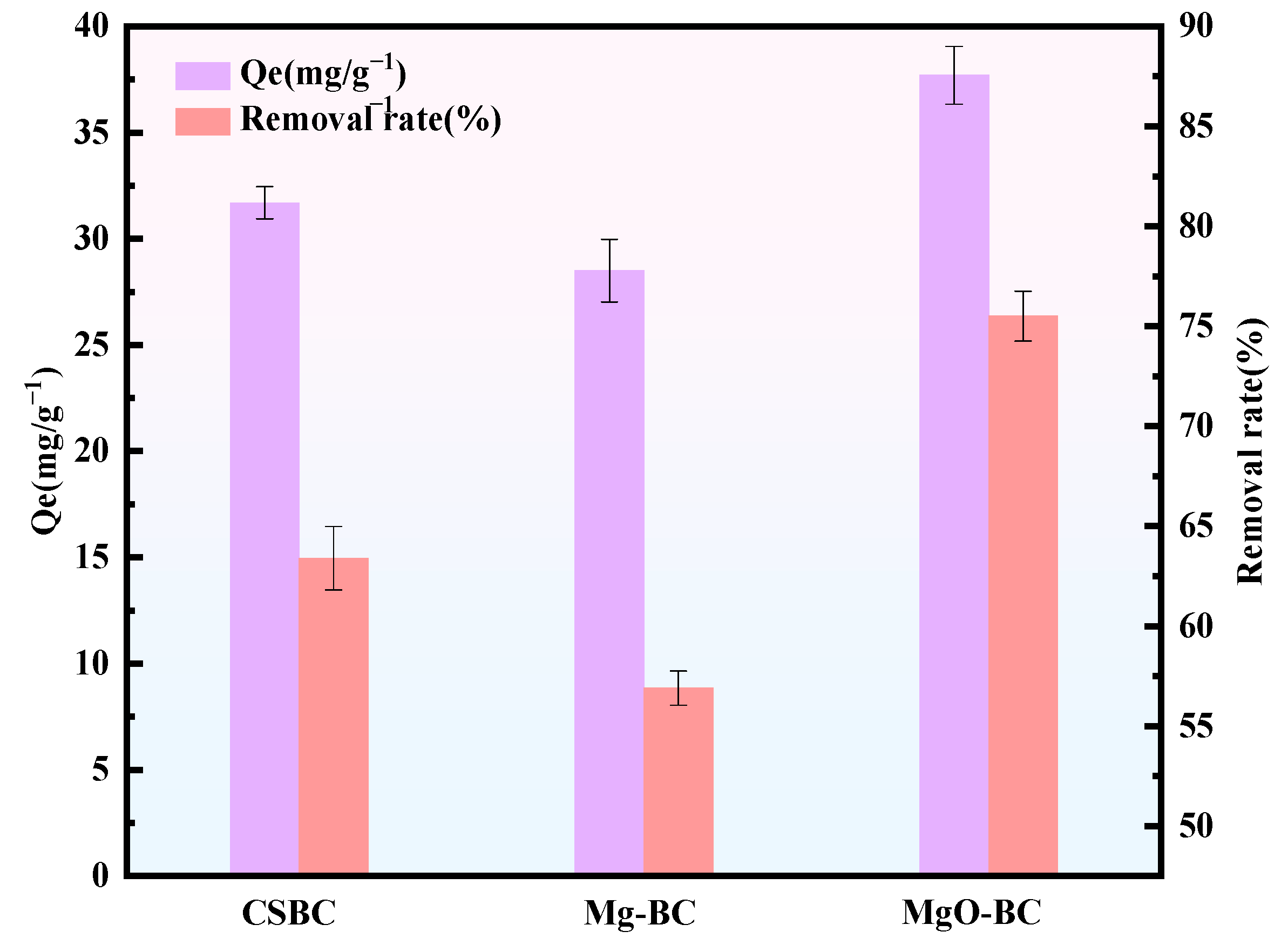
3.2.1. Effect of Different Pretreatment Methods on Phenol Adsorption Performance
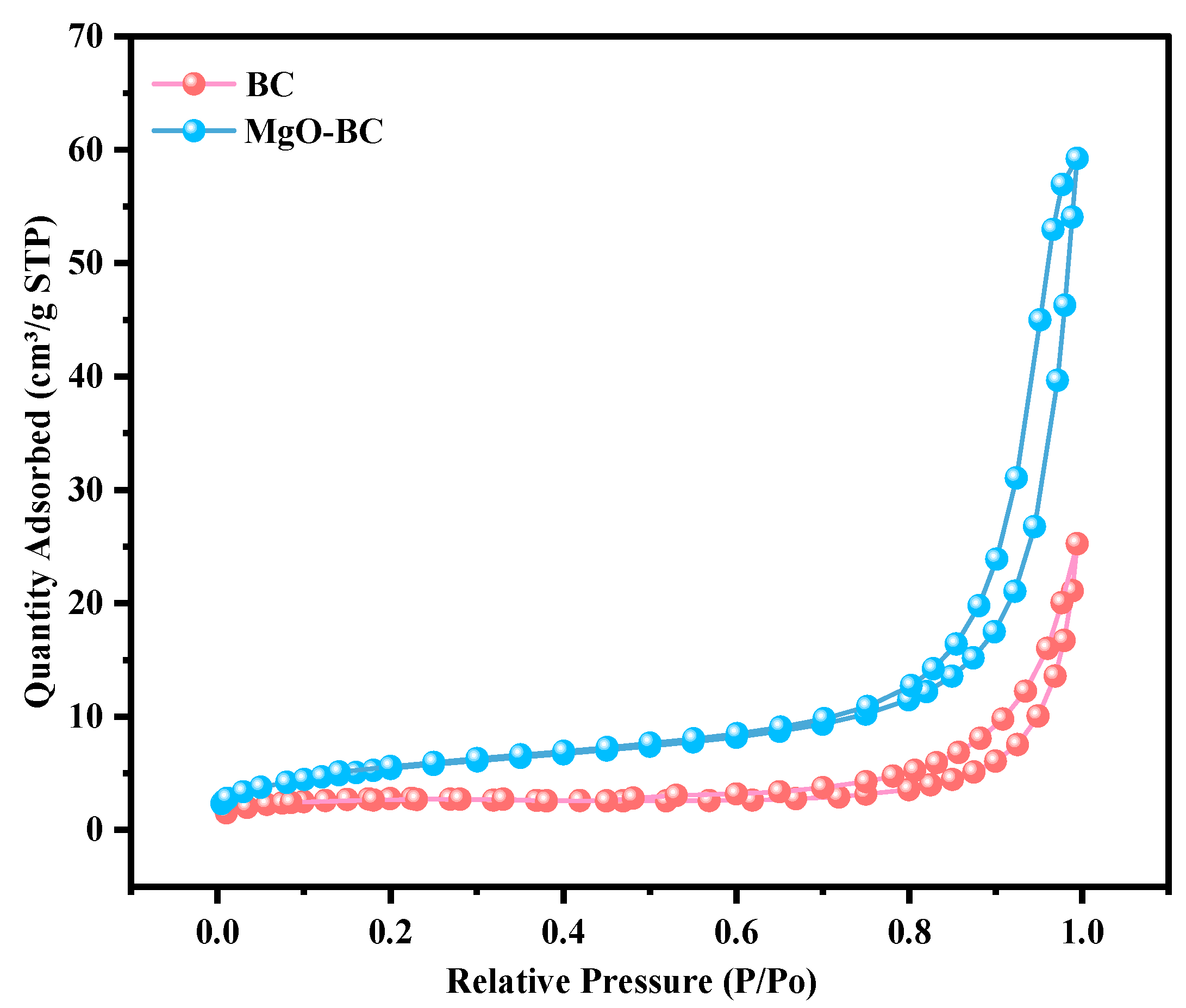
3.2.2. BET Surface Area Analysis
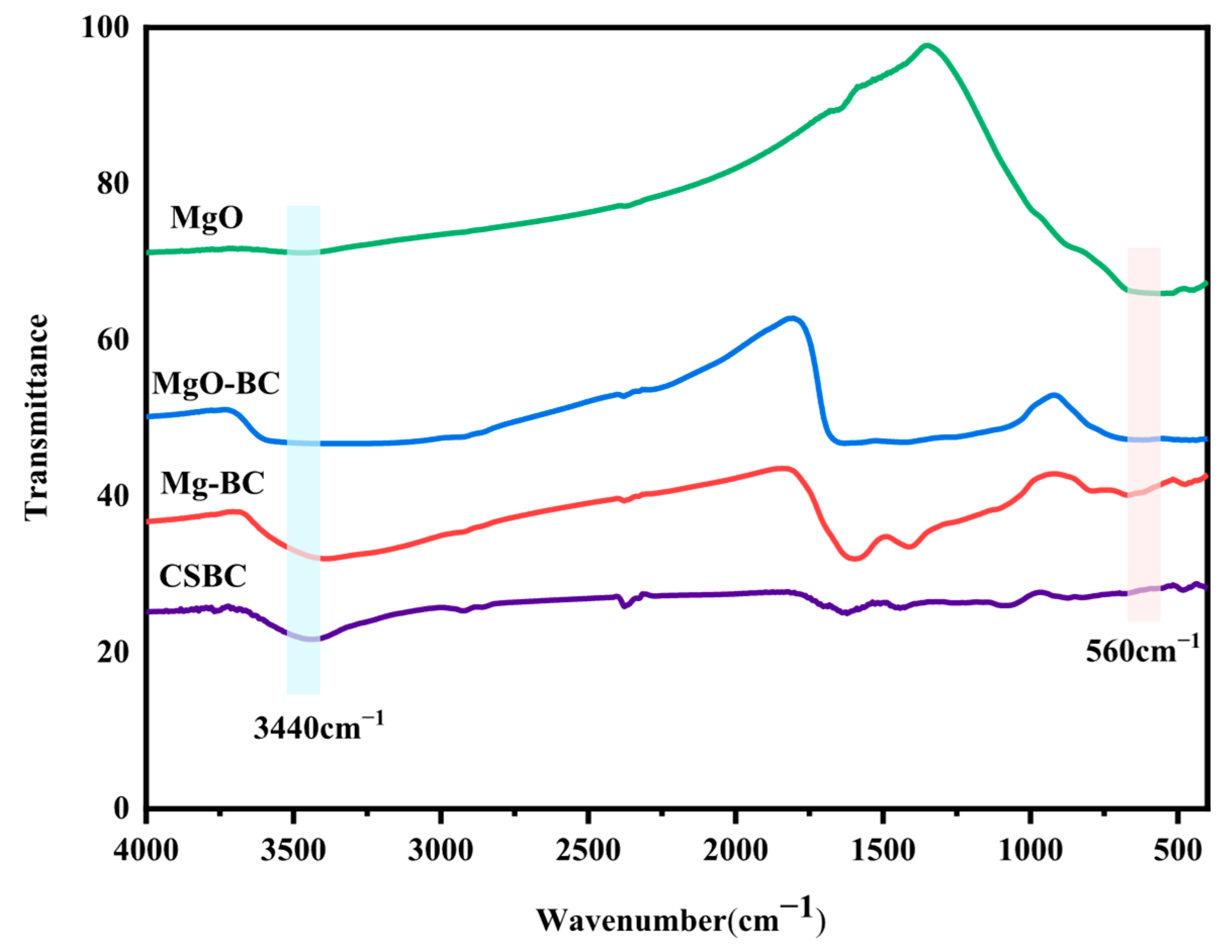
3.2.3. FTIR Spectra of Samples for Different Pretreatment Methods
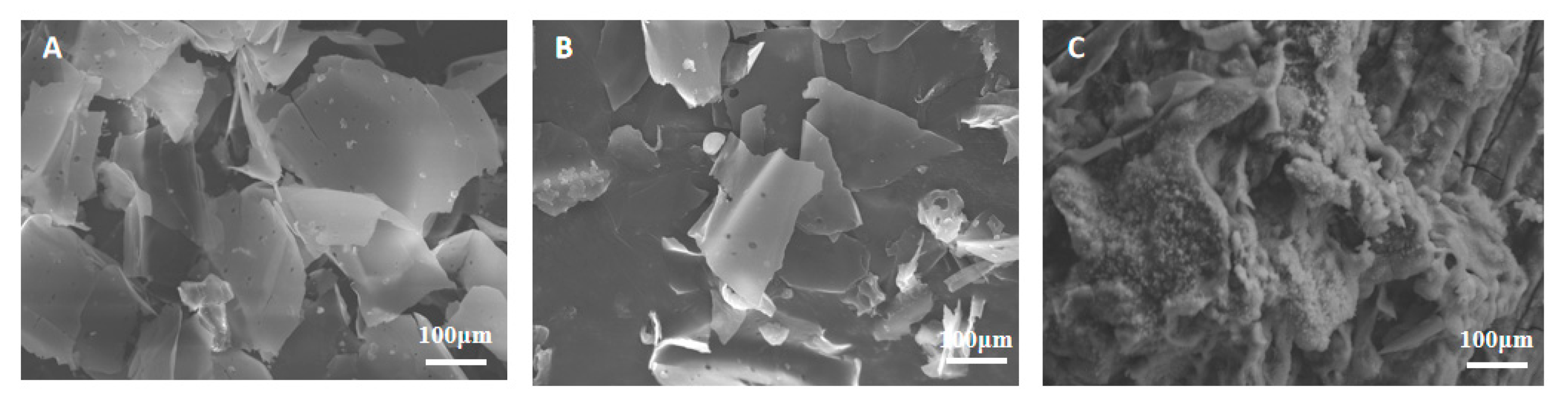
3.2.4. SEM-EDX Analysis for Different Pretreatment Methods
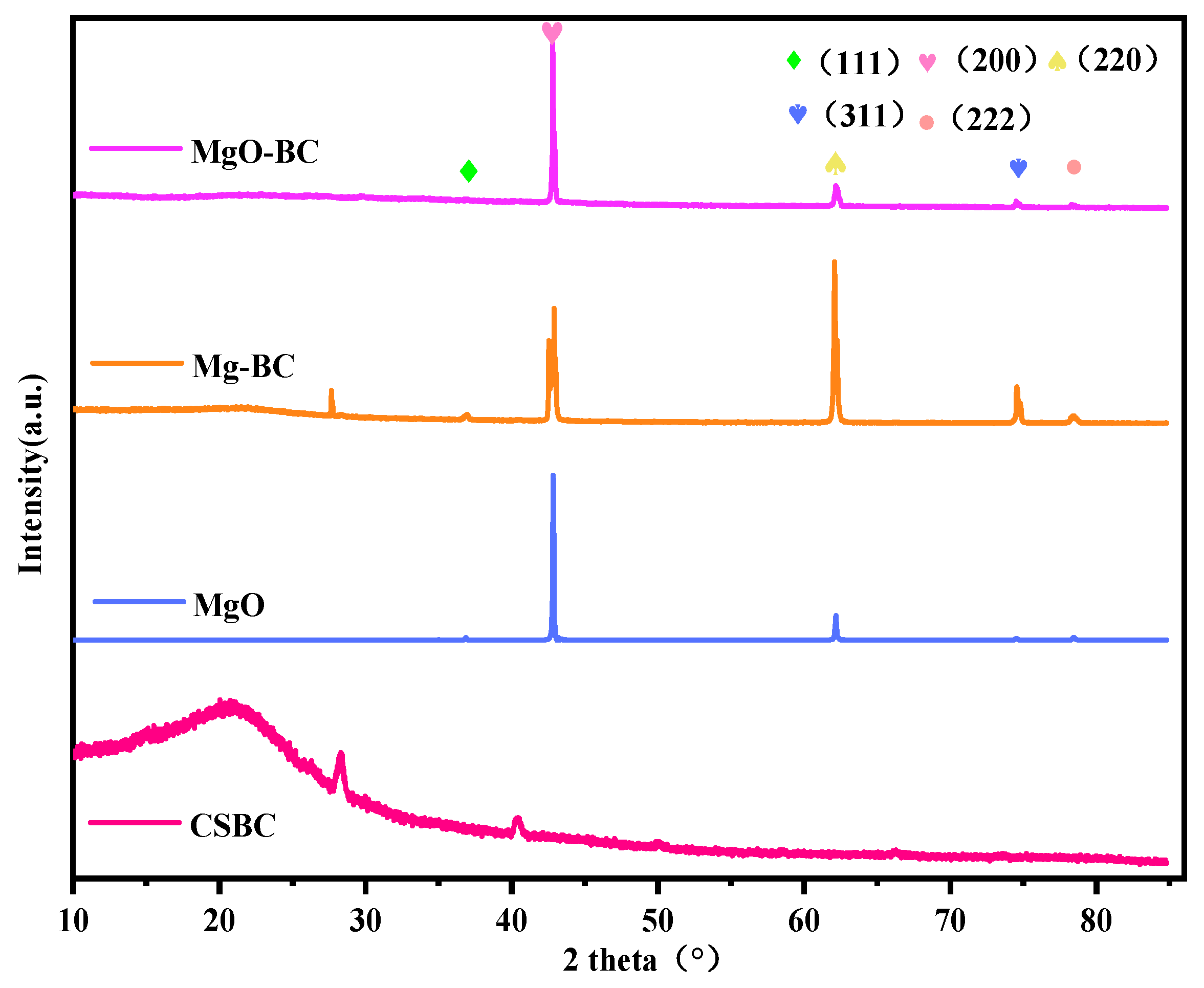
3.2.5. XRD Analysis for Different Pretreatment Methods
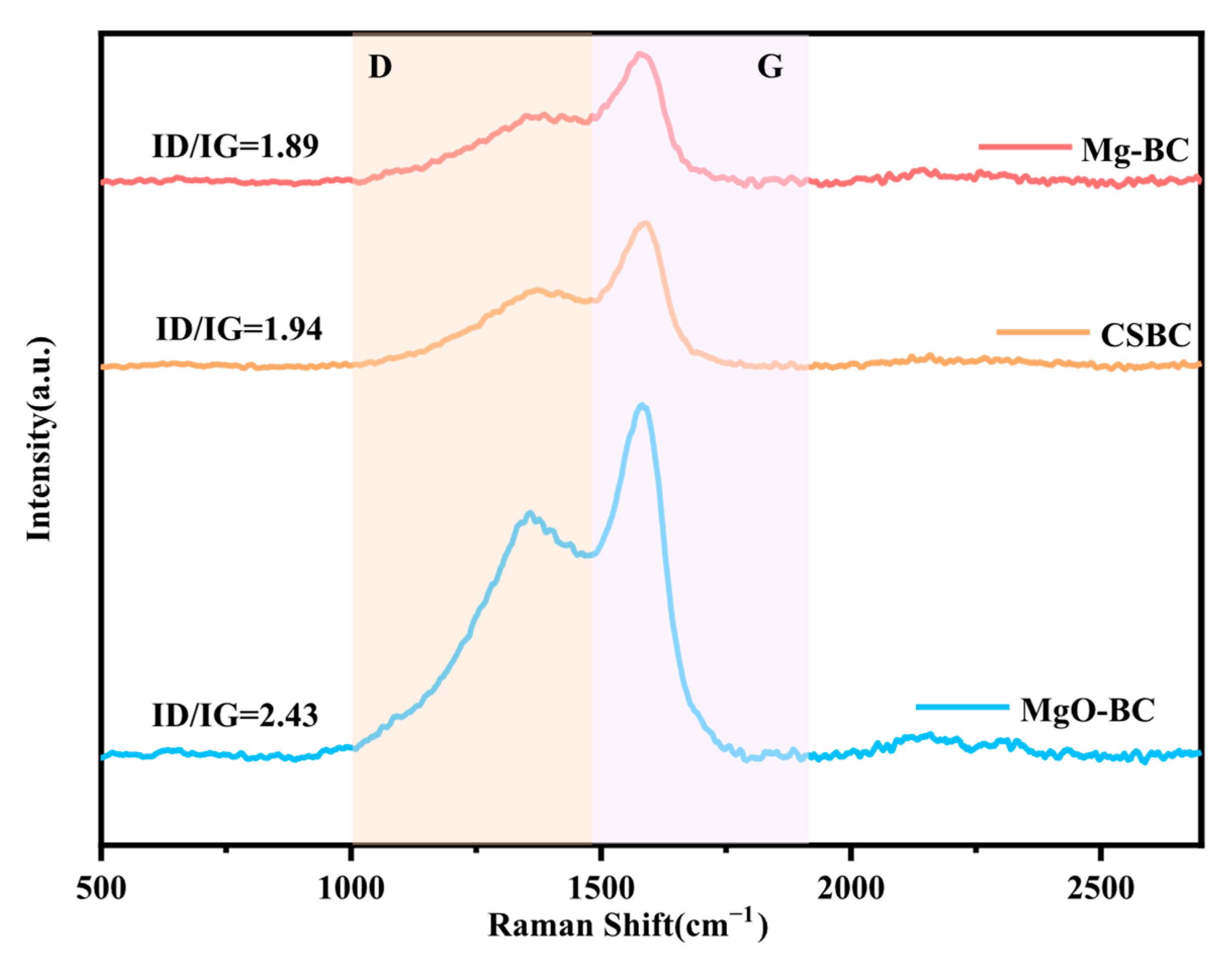
3.2.6. Raman Analysis for Different Pretreatment Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mei, Y.; Gao, P.; Li, J.; He, X.-S.; Cui, L.-P.; Zhang, T.; Wang, Y.-J.; Xue, P. Enhanced Cadmium Sequestration from Animal Waste Using a Combined Modified Corn Straw Biochar: Unraveling the Mechanisms and Performance in Hazardous Metal Removal. Environ. Technol. Innov. 2025, 39, 104256. [Google Scholar] [CrossRef]
- Li, T.; Dai, Q.; Bi, X.; Wu, J.; Zhang, Y.; Feng, Y. Size Distribution and Chemical Characteristics of Particles from Crop Residue Open Burning in North China. J. Environ. Sci. 2021, 109, 66–76. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Z.; Fan, Q.; Yang, Y.; Zhang, Q. Fabricating Phosphorus-Doped Corn Straw Biochar for Efficient Adsorptive Removal of Imazethapyr from Aqueous Solutions. Surf. Interfaces 2025, 63, 106347. [Google Scholar] [CrossRef]
- Lugo-Arias, J.; González-Álvarez, J.; Maturana, A.; Villa-Parejo, J.; Barraza-Heras, C. Removal of Nitrate and Phosphate from Aqueous Solutions Using Bioadsorbents Derived from Agro-Industrial Wastes of Rice Husk and Corn Stalk. Biomass Convers. Biorefinery 2025, 15, 19453–19475. [Google Scholar] [CrossRef]
- Prado, E.R.L.; Rial, R.C. Advances in Conversion Technologies for Biofuels from Wheat and Corn Straws. Biocatal. Agric. Biotechnol. 2025, 63, 103481. [Google Scholar] [CrossRef]
- Shi, J.; Sheng, L.; Chen, M.; Wu, X.; Li, X.; Tan, Y.Q. pH-Responsive Collagen Nanocomposite Films Reinforced by Curcumin-Loaded Laponite Nanoplatelets for Dynamic Visualization of Shrimp Freshness. Food Hydrocoll. 2024, 157, 110447. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, Y.; Yang, N.; Guan, X.; Sheng, L.; Liu, L.; Zhong, W. Covalently Surface-Grafting A-zirconium Phosphate Nanoplatelets Enables Collagen Fiber Matrix with Ultraviolet Barrier, Antibacterial, and Flame-Retardant Properties. Int. J. Biol. Macromol. 2024, 254, 127999. [Google Scholar] [CrossRef]
- Shi, J.; Wu, X.; Sheng, L.; Chen, M.; Shi, L.; Zhang, Y.; Yang, J.; Mao, S.; Liu, Q.; Zhou, Z. Enabling Multifunctional Bio-Based Waterborne Polyurethane through Polyphenol-Mediated Assembly of Zirconium Phosphate Nanoplatelets for Patternable Coating. Compos. Sci. Technol. 2025, 266, 111178. [Google Scholar] [CrossRef]
- Bach, Q.-V.; Nguyen, D.D.; Lee, C.-J. Effect of Torrefaction on Steam Gasification of Biomass in Dual Fluidized Bed Reactor—A Process Simulation Study. Bioenergy Res. 2019, 12, 1042–1051. [Google Scholar] [CrossRef]
- Zheng, A.; Fan, Y.; Wei, G.; Zhao, K.; Huang, Z.; Zhao, Z.; Li, H. Chemical Looping Gasification of Torrefied Biomass Using NiFe2O4 as an Oxygen Carrier for Syngas Production and Tar Removal. Energy Fuels 2020, 34, 6008–6019. [Google Scholar] [CrossRef]
- Singh, R.K.; Sarkar, A.; Chakraborty, J.P. Effect of Torrefaction on the Physicochemical Properties of Pigeon Pea Stalk (Cajanus cajan) and Estimation of Kinetic Parameters. Renew. Energy 2019, 138, 805–819. [Google Scholar] [CrossRef]
- Tsalidis, G.A.; Di Marcello, M.; Spinelli, G.; De Jong, W.; Kiel, J.H.A. The Effect of Torrefaction on the Process Performance of Oxygen-Steam Blown CFB Gasification of Hardwood and Softwood. Biomass Bioenergy 2017, 106, 155–165. [Google Scholar] [CrossRef]
- Singh, R.K.; Sarkar, A.; Chakraborty, J.P. Effect of Torrefaction on the Physicochemical Properties of Eucalyptus Derived Biofuels: Estimation of Kinetic Parameters and Optimizing Torrefaction Using Response Surface Methodology (RSM). Energy 2020, 198, 117369. [Google Scholar] [CrossRef]
- Cvetinović, D.; Erić, A.; Cvetković, S.; Komatina, M.; Manić, N.; Janković, B. Performance Assessment and Techno-Economic Analysis of the Thermal Plasma Gasification of Biomass Using Air and Steam as Gasification Medium. Appl. Therm. Eng. 2025, 276, 126940. [Google Scholar] [CrossRef]
- Song, S.-H.; Kim, J.-K.; Kim, J.-W.; Park, C.-W.; Kim, J.-S. Low-Temperature Steam Gasification of Wood Using a Two-Stage Gasification Process to Avoid Bed Agglomeration in a Fluidized Bed Gasifier and Tar Problems. Energy 2025, 328, 136496. [Google Scholar] [CrossRef]
- Guo, J.; Foo, J.L.C.; Ge, L.; Chan, W.P.; Veksha, A.; Liu, G.; Hu, Z.; Guo, S.; Lisak, G. Steam-Assisted One-Step Fabrication of Ni-Ce Biochar Catalysts for Enhanced Biomass Pyrolysis-Steam Reforming. Chem. Eng. J. 2025, 509, 161302. [Google Scholar] [CrossRef]
- Zhou, Q.; Shen, Y.; Zhou, Q.; Zhang, C.; Gu, X. Torrefaction Integrated with Steam Gasification of Agricultural Biomass Wastes for Enhancing Tar Reduction and Hydrogen-Rich Syngas Production. Int. J. Hydrog. Energy 2024, 94, 474–484. [Google Scholar] [CrossRef]
- Şahin, Ö.; Saka, C. Preparation and Characterization of Activated Carbon from Acorn Shell by Physical Activation with H2O–CO2 in Two-Step Pretreatment. Bioresour. Technol. 2013, 136, 163–168. [Google Scholar] [CrossRef]
- Senthilkumar, V.; Prabhu, C. Development, Simulation and ANN Validation of Thermodynamic and Kinetic Models for Optimised Biomass Gasification for CO2 Reduction and Enhanced Syngas Yield. Int. J. Hydrog. Energy 2025, 163, 150681. [Google Scholar] [CrossRef]
- Vinodhkumar, S.; Balaji, S.; Kulanthaivel, P.; Deshmukh, R. Utilisation of Magnesite Mine Tailings as Sustainable Structural Fill Material: A Novel Approach to Mitigate Environmental Impact. Int. J. Environ. Sci. Technol. 2025, 22, 13661–13672. [Google Scholar] [CrossRef]
- Lin, H.; Tian, L.; Liu, X.; Wang, G.; Xie, C.; Li, G.; Tian, C. Tailoring Intergranular Binding Phase by Pr6O11 in Mg2SiO4–MgO Refractories Prepared from Magnesite Flotation Tailings. Ceram. Int. 2025, 51, 14110–14117. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, W.; Jiang, J.; Li, R.; Gu, J.; Liang, W. Metagenomic Insights on Promoting the Removal of Resistome in Aerobic Composting Pig Manure by Lightly Burned Modified Magnesite. Sci. Total Environ. 2024, 957, 177101. [Google Scholar] [CrossRef]
- He, T.; Jin, B.; Pei, H.; Luo, Z.; Cai, Y. A Two-Stage Gasification System Achieving Clean Gas Production with Zero-Tar: 200 kWth Autothermal Pilot-Scale Experiment. Energy Convers. Manag. 2025, 345, 120335. [Google Scholar] [CrossRef]
- Oni, B.A.; Sanni, S.E.; Tomomewo, O.S.; Ibegbu, A.J. Gasification of Egusi Melon (Citrullus lanatus) Shell over a Novel Ni/Dolomite/MgO/γ–Al2O3 Catalyst for the Production of H2-Rich Gas. Process Saf. Environ. Prot. 2024, 183, 446–458. [Google Scholar] [CrossRef]
- Zan, W.; Ma, B.; Ma, Y.; Zhou, Z.; Cao, R.; Liu, G.; You, J. Synthesis and Properties of Porous MgAl2O4–Mg2SiO4 Refractory Aggregates from Magnesite Tailings and Bauxite Chamotte. Ceram. Int. 2025, 51, 14632–14643. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.-Y.; Liu, Y.; Yang, L.; Zheng, Y.; Kang, S.; Zhou, J. Preparation of Magnesium Potassium Phosphate Cement from Magnesite Tailings under Lower Calcination Temperature. Constr. Build. Mater. 2024, 451, 138869. [Google Scholar] [CrossRef]
- Ma, B.; Zan, W.; Liu, K.; Mu, X.; Deng, C.; Huang, A. Preparation and Properties of Porous MgO-Based Ceramics from Magnesite Tailings and Fused Magnesia. Ceram. Int. 2023, 49, 19072–19082. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Z.; Shan, J.; Wu, C.; Lichtfouse, E.; Liu, H. Efficient Recovery of Phosphate from Urine Using Magnesite Modified Corn Straw Biochar and Its Potential Application as Fertilizer. J. Environ. Chem. Eng. 2024, 12, 111925. [Google Scholar] [CrossRef]
- Liang, H.; Jiang, Y.; Wang, S.; Xue, Z.; Wang, C.; Wang, W.; Zuo, X.; Dai, Y.; Ruan, X. Facile and Scalable Synthesis of Long-Life Porous MgO-Nanofiber Functionalized Biochar Adsorbent from Magnesite for Phosphate Recycling. Sep. Purif. Technol. 2025, 354, 129234. [Google Scholar] [CrossRef]
- Artetxe, M.; Nahil, M.A.; Olazar, M.; Williams, P.T. Steam Reforming of Phenol as Biomass Tar Model Compound over Ni/Al2O3 Catalyst. Fuel 2016, 184, 629–636. [Google Scholar] [CrossRef]
- GB/T 21923-2008; General Rules for Inspection of Solid Biomass Fuels. Standard Press of China: Beijing, China, 2008.
- GB/T 28731-2012; Proximate Analysis of Solid Biofuels. Standard Press of China: Beijing, China, 2012.
- GB/T 30727-2014; Determination of Calorific Value of Solid Biofuels. Standard Press of China: Beijing, China, 2014.
- Chen, J.; Duan, Q.; Liu, J.; Zhang, S.; Zhang, J.; Lin, S. Adsorption of Fe-Modified Peanut Shell Biochar for Pb(II) in Mixed Pb(II), Cu(II), Ni(II) Solutions. Sci. Rep. 2025, 15, 13558. [Google Scholar] [CrossRef]
- Li, H.; Gui, Y.; Li, J.; Zhang, J.; Lü, J. Development of Lignin-Derived Hierarchically Porous Carbon by One-Step Pyrolysis in an Air/N2 Flow for Efficient Adsorption of Organic Pollutants. J. Anal. Appl. Pyrolysis 2024, 177, 106344. [Google Scholar] [CrossRef]
- Bentley, M.J.; Kearns, J.P.; Murphy, B.M.; Summers, R.S. Pre-Pyrolysis Metal and Base Addition Catalyzes Pore Development and Improves Organic Micropollutant Adsorption to Pine Biochar. Chemosphere 2022, 286, 131949. [Google Scholar] [CrossRef]
- Duan, H.; Liu, Q.; Mao, S.; He, Y.; Han, X.; Yu, J.; Xu, S. Effect of Tea Stalk Biochar Derived from Pyrolysis at Different Temperatures on Adsorption Capacity of Asphalt Fume. Constr. Build. Mater. 2025, 481, 141523. [Google Scholar] [CrossRef]
- Chungcharoen, T.; Limmun, W.; Srisang, S.; Phetpan, K.; Ruttanadech, N.; Youryon, P.; Kongtragoul, P.; Srisang, N. Enhanced Biodiesel Purification Using Coffee Husk Bioadsorbents: The Role of Pyrolysis Temperature, KOH Activation, and Adsorption Efficiency. Renew. Energy 2025, 244, 122700. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, K.; Yan, J.; Wang, Z.; Lei, Z.; Ren, S.; Kang, S.; Li, Z.; Shui, H. Sulfur Migration during Co-Pyrolysis of High Sulfur Coking Coal with Fat Coal and Lean Coal. Fuel 2024, 365, 131338. [Google Scholar] [CrossRef]
- Schmid, D.; Karlström, O.; Yrjas, P. Release of NH3, HCN and NO during Devolatilization and Combustion of Washed and Torrefied Biomass. Fuel 2020, 280, 118583. [Google Scholar] [CrossRef]
- Tumuluru, J.S.; Ghiasi, B.; Soelberg, N.R.; Sokhansanj, S. Biomass Torrefaction Process, Product Properties, Reactor Types, and Moving Bed Reactor Design Concepts. Front. Energy Res. 2021, 9, 728140. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, G.; Li, Q.; Zhao, Z.; Guo, Z.; Zhang, F.; Luan, Y.-N.; Liu, C. Optimizing Biochar Structure via Two-Step Pyrolysis for Uniform Magnesium Oxide Post-Doping with Enhanced Phosphate Removal Performance. Colloids Surf. Physicochem. Eng. Asp. 2025, 724, 137438. [Google Scholar] [CrossRef]
- Duongbia, N.; Kamopas, W.; Ruangrit, K.; Deethayat, T.; Asanakham, A.; Kiatsiriroat, T. Biochar from Co-Pyrolysis of Spent Coffee Ground with Leptolyngbya Sp. KC 45 Biomass and Residue for Energy and Agricultural Utilization. Energy Nexus 2025, 18, 100454. [Google Scholar] [CrossRef]
- Li, G.; Cheng, W.; Zhang, X.; Zhang, H.; Wang, W.; Shao, J.; Yang, H.; Chen, H.; Zhang, S. A Comprehensive Study on Fuel Property, Pyrolysis Kinetic and Product Characteristic of Torrefied Biomass for High-Value Utilization. Ind. Crops Prod. 2025, 230, 121052. [Google Scholar] [CrossRef]
- Wang, X.; Ma, D.; Jin, Q.; Deng, S.; Stančin, H.; Tan, H.; Mikulčić, H. Synergistic Effects of Biomass and Polyurethane Co-Pyrolysis on the Yield, Reactivity, and Heating Value of Biochar at High Temperatures. Fuel Process. Technol. 2019, 194, 106127. [Google Scholar] [CrossRef]
- Ren, H.; Li, B.; Zhou, X.; Chen, S.; Li, Y.; Hu, C.; Tian, J.; Zhang, G.; Pan, Y.; Zou, C. Wafer-Size VO2 Film Prepared by Water-Vapor Oxidant. Appl. Surf. Sci. 2020, 525, 146642. [Google Scholar] [CrossRef]
- Meng, X.; Xu, X.; Hu, S.; Chu, R.; Li, X.; Li, W.; Yu, S.; Sun, Y. Changes in the Pore Structure and Oxygen-Containing Functional Groups of Lignite under Steam Explosion and Their Influence on Water Molecule Resorption. Colloids Surf. Physicochem. Eng. Asp. 2025, 725, 137675. [Google Scholar] [CrossRef]
- Hassan, M.; Strezov, V. Wheat Straw Pyrolysis Fundamentals during Production of Biochar. J. Therm. Anal. Calorim. 2025. [Google Scholar] [CrossRef]
- Beljin, J.; Isakovski, M.K.; Agbaba, J.; Vujić, M.; Maletić, S.; Tubić, A. Adsorptive Behavior of Corn-Cob- and Straw-Derived Biochar for Polycyclic Aromatic Hydrocarbon Removal from Aqueous Systems. Processes 2025, 13, 1521. [Google Scholar] [CrossRef]
- Ahmed, M.W.; Singh, V.; Kamruzzaman, M. Near-Infrared Spectroscopy as a Green Analytical Tool for Sustainable Biomass Characterization for Biofuels and Bioproducts: An Overview. Bioresour. Technol. 2025, 433, 132722. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Wasim, M.; Ali, T.; Qureshi, R.N. Application of XRD, SEM-EDX and FTIR Techniques for Mineral Characterization of Geological Ores. Radiochim. Acta 2025, 113, 399–409. [Google Scholar] [CrossRef]
- Chen, T.; Xie, P.; Yu, Y.; Duan, X.; Sun, Y.; Guan, X. Metal Ions-Mediated Concerted Electron-Proton Transfer Enables Catalytic Oxidation of Phenolic Contaminants by Permanganate. Water Res. 2025, 268, 122622. [Google Scholar] [CrossRef]
- Darla, U.R.; Lataye, D.H.; Kumar, A.; Pandit, B.; Ubaidullah, M. Adsorption of Phenol Using Adsorbent Derived from Saccharum officinarum Biomass: Optimization, Isotherms, Kinetics, and Thermodynamic Study. Sci. Rep. 2023, 13, 18356. [Google Scholar] [CrossRef] [PubMed]
- Eboibi, B.E.; Ogbue, M.C.; Udochukwu, E.C.; Umukoro, J.E.; Okan, L.O.; Agarry, S.E. Maize Cob (Zea mays) as Natural Biomass Sorbent for Crude Oil Biosorptive Removal from Contaminated Seawater: Taguchi Process Optimization and Biosorptive Removal Mechanism. Environ. Monit. Assess. 2023, 195, 1145. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, C.; Scherdel, C.; Kriesten, M.; Leicht, P.; Keilbach, A.; Ehmann, H.; Kotnik, P.; Reichenauer, G.; Thommes, M. Reliable Surface Area Determination of Powders and Meso/Macroporous Materials: Small-Angle X-Ray Scattering and Gas Physisorption. Microporous Mesoporous Mater. 2022, 329, 111554. [Google Scholar] [CrossRef]
- Goodarzi, F.; Kock, M.; Mielby, J.; Kegnæs, S. CO2 Methanation Using Metals Nanoparticles Supported on High Surface Area MgO. J. CO2 Util. 2023, 69, 102396. [Google Scholar] [CrossRef]
- Sreekanth, R.; Pattar, J.; Anupama, A.V.; Mallikarjunaswamy, A.M.M. Synthesis of High Surface Area and Plate-like Magnesium Oxide Nanoparticles by pH-Controlled Precipitation Method. Appl. Phys. A 2021, 127, 797. [Google Scholar] [CrossRef]
- Ehiro, T. Characterization of Ibuprofen-Loaded MgO by One-Step Mid- and Far-FT-IR Spectroscopy. Chem. Pharm. Bull. 2021, 69, 693–697. [Google Scholar] [CrossRef]
- Rajagopalachar, S.; Pattar, J.; Mulla, S. Synthesis and Characterization of Plate-like High Surface Area MgO Nanoparticles for Their Antibacterial Activity against Bacillus cereus (MTCC 430) and Pseudomonas aeruginosa (MTCC 424) Bacterias. Inorg. Chem. Commun. 2022, 144, 109907. [Google Scholar] [CrossRef]
- Anbazhagi, S.; Murugesan, A.; Muthukrishnan, P.; Paramanantham, M.; Rajasekar, S.; Pazhanisamy, T.; Arivazhagan, S.; Karuppiah, C. Synthesis and Characterization of ZnO/MgO Nanocomposites: Targeted Drug Delivery Strategies for Cancer Treatment. Inorg. Chem. Commun. 2025, 180, 115014. [Google Scholar] [CrossRef]
- Jing, F.; Guan, J.; Tang, W.; Chen, J. Mechanistic Insight into Adsorptive Removal of Ionic NOR and Nonionic DEP Organic Contaminates by Clay-Biochar Composites. Environ. Pollut. 2022, 310, 119881. [Google Scholar] [CrossRef]
- Chen, S.; Xia, Y.; Zhang, B.; Chen, H.; Chen, G.; Tang, S. Disassembly of Lignocellulose into Cellulose, Hemicellulose, and Lignin for Preparation of Porous Carbon Materials with Enhanced Performances. J. Hazard. Mater. 2021, 408, 124956. [Google Scholar] [CrossRef]
- Khalil, A.M.; Msaadi, R.; Sassi, W.; Ghanmi, I.; Pires, R.; Michely, L.; Snoussi, Y.; Chevillot-Biraud, A.; Lau-Truong, S.; Chehimi, M.M. Facile Diazonium Modification of Pomegranate Peel Biochar: A Stupendous Derived Relationship between Thermal and Raman Analyses. Carbon Lett. 2022, 32, 1519–1529. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; An, X.; Wang, Y.; Gao, M.; Wang, R.; Ke, P.; Cheng, X. Defects and Oxygen Functional Groups on Sludge Biochar Synergistic Activation of PMS for Degradation of Sulfamethoxazole and Practical Application. J. Environ. Chem. Eng. 2024, 12, 114958. [Google Scholar] [CrossRef]
- Yang, H.; Ding, Z.; Liu, Y.; Zhang, S.; Zou, Y.; Bai, G.; Zhang, Y.; Xia, S. Biomass-Derived Carbon Aerogel for Peroxymonosulfate Activation to Remove Tetracycline: Carbonization Temperature, Oxygen-Containing Functional Group Content, and Defect Degree. Ind. Crops Prod. 2022, 177, 114437. [Google Scholar] [CrossRef]

| Sample | CS | CS220 | CS240 | CS260 | CS280 | CS300 |
| ultimate analysis (wt%) | ||||||
| C | 52.51 | 53.42 | 56.50 | 59.72 | 61.92 | 68.63 |
| H | 6.08 | 5.92 | 5.83 | 5.72 | 5.14 | 4.68 |
| O | 36.61 | 36.27 | 32.55 | 28.58 | 26.1 | 16.91 |
| N | 1.60 | 1.20 | 0.96 | 0.83 | 0.72 | 0.68 |
| S | 0.20 | 0.19 | 0.16 | 0.15 | 0.12 | 0.10 |
| O/C | 0.70 | 0.68 | 0.58 | 0.48 | 0.42 | 0.25 |
| H/C | 0.12 | 0.11 | 0.10 | 0.09 | 0.08 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, S.; Li, Q.; Kou, W.; Sun, Z.; Li, X.; Wang, Y.; Zhao, H.; Gao, P. Steam-Assisted Semi-Carbonization Pretreatment of Corn Stalks: Effects on Physicochemical Properties for Enhanced Biomass Utilization. Sustainability 2025, 17, 9091. https://doi.org/10.3390/su17209091
Gu S, Li Q, Kou W, Sun Z, Li X, Wang Y, Zhao H, Gao P. Steam-Assisted Semi-Carbonization Pretreatment of Corn Stalks: Effects on Physicochemical Properties for Enhanced Biomass Utilization. Sustainability. 2025; 17(20):9091. https://doi.org/10.3390/su17209091
Chicago/Turabian StyleGu, Shiyan, Qi Li, Wei Kou, Zhaonan Sun, Xiaoxia Li, Yitong Wang, Haiqiao Zhao, and Peng Gao. 2025. "Steam-Assisted Semi-Carbonization Pretreatment of Corn Stalks: Effects on Physicochemical Properties for Enhanced Biomass Utilization" Sustainability 17, no. 20: 9091. https://doi.org/10.3390/su17209091
APA StyleGu, S., Li, Q., Kou, W., Sun, Z., Li, X., Wang, Y., Zhao, H., & Gao, P. (2025). Steam-Assisted Semi-Carbonization Pretreatment of Corn Stalks: Effects on Physicochemical Properties for Enhanced Biomass Utilization. Sustainability, 17(20), 9091. https://doi.org/10.3390/su17209091





