Analysis of Carbon Emissions and Carbon Reduction Benefits of Green Hydrogen and Its Derivatives Based on the Full Life Cycle
Abstract
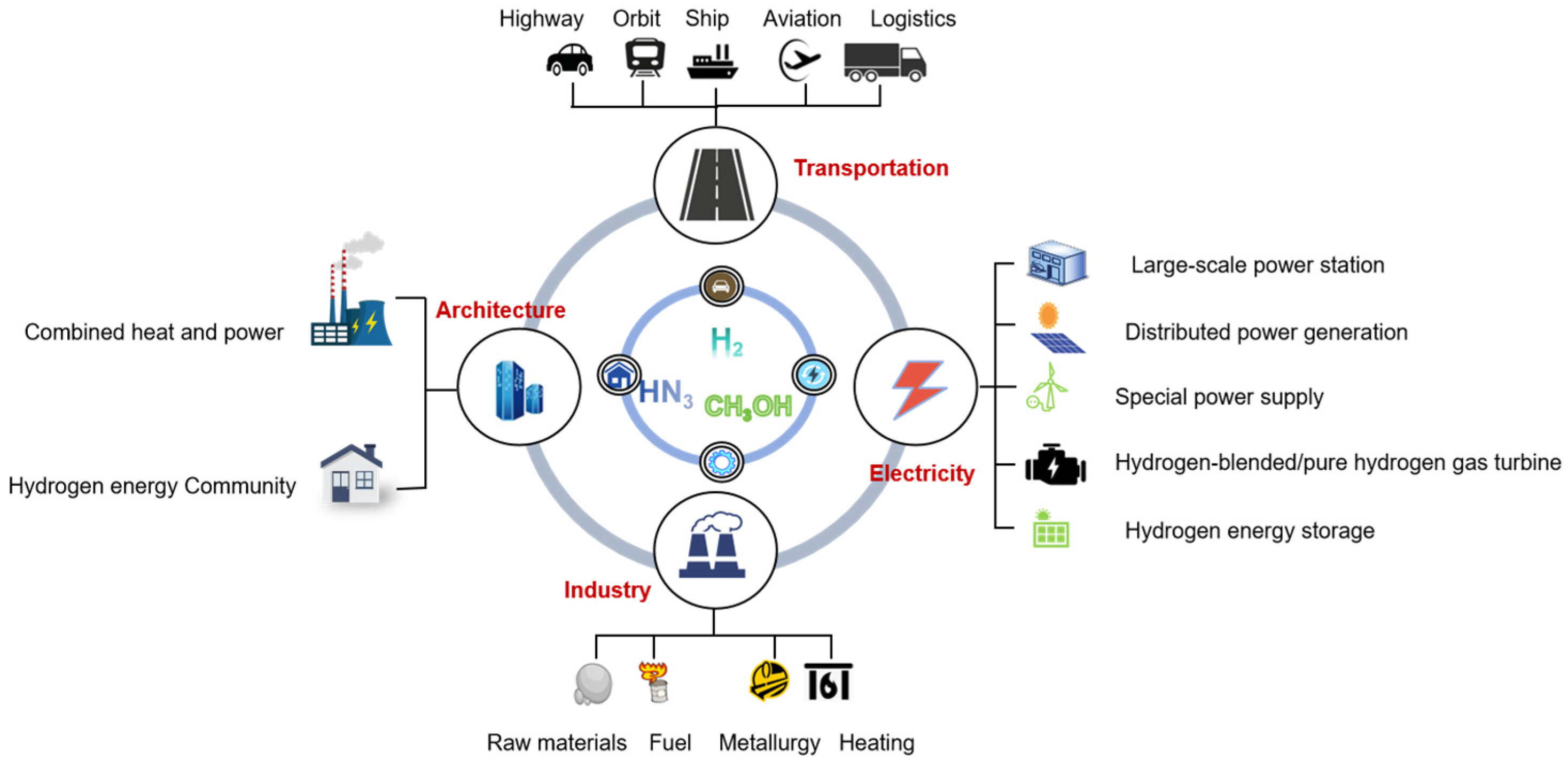
1. Introduction
2. Literature Review
2.1. Definition of “Green” for Green Hydrogen and Its Derivatives
2.1.1. Definition of Green Hydrogen
2.1.2. Definition of Green Methanol
2.1.3. Definition of Green Ammonia
2.2. Carbon Emission Accounting Methods and Scope for Green Hydrogen and Its Derivatives
2.3. Current Status of Carbon Emission Calculation for Green Hydrogen and Its Derivatives
3. Models and Data
3.1. Scenario Design for Carbon Emission Accounting of Green Hydrogen and Its Derivatives
3.1.1. Scenario Design for Carbon Emission Accounting of Green Hydrogen
- Hydrogen Production from Wind Power:
- Hydrogen Production from Photovoltaic Power:
3.1.2. Scenario Design for Carbon Emission Accounting of Green Hydrogen Derivatives
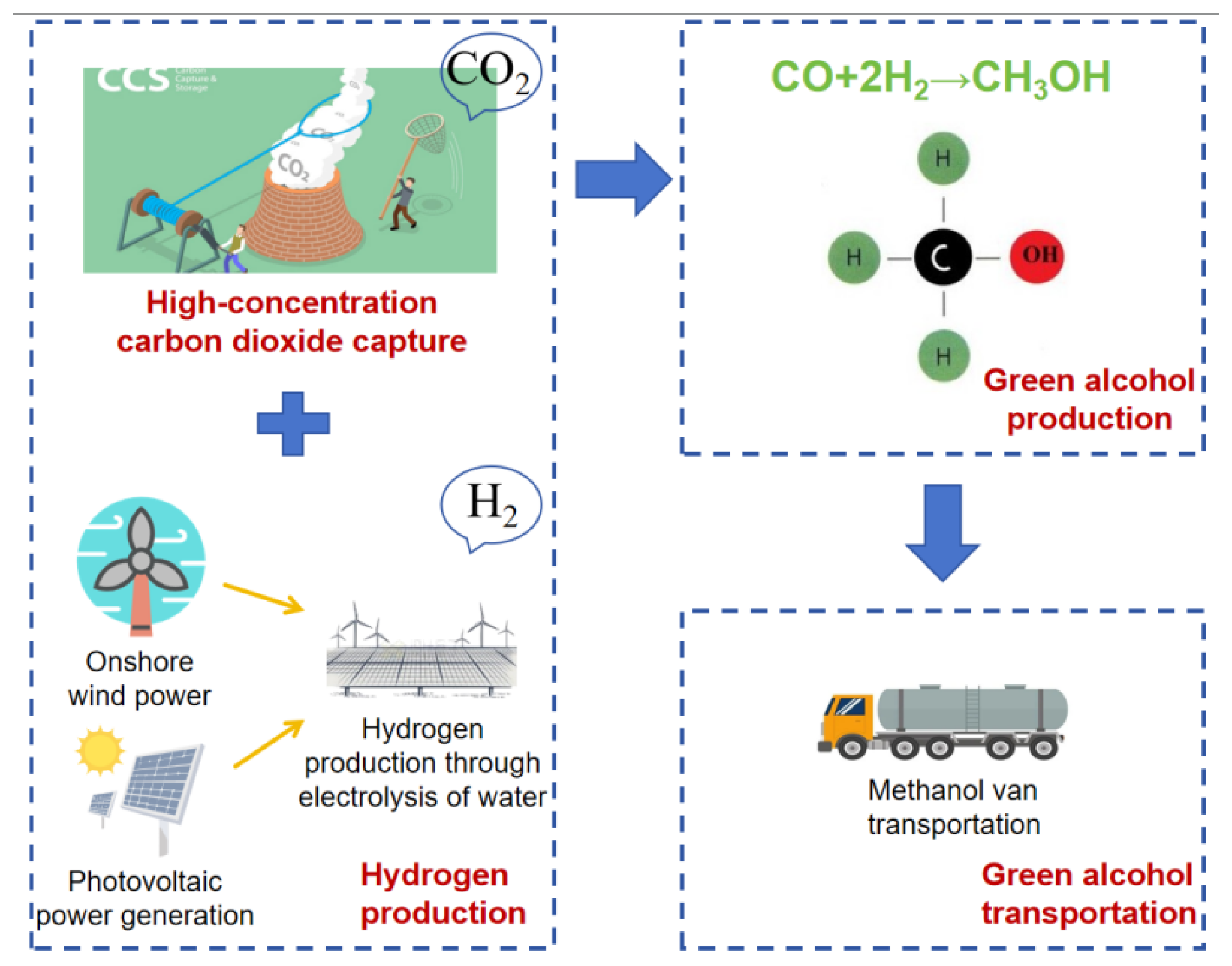
- CO2 Capture and Green Hydrogen Synthesis of Methanol:
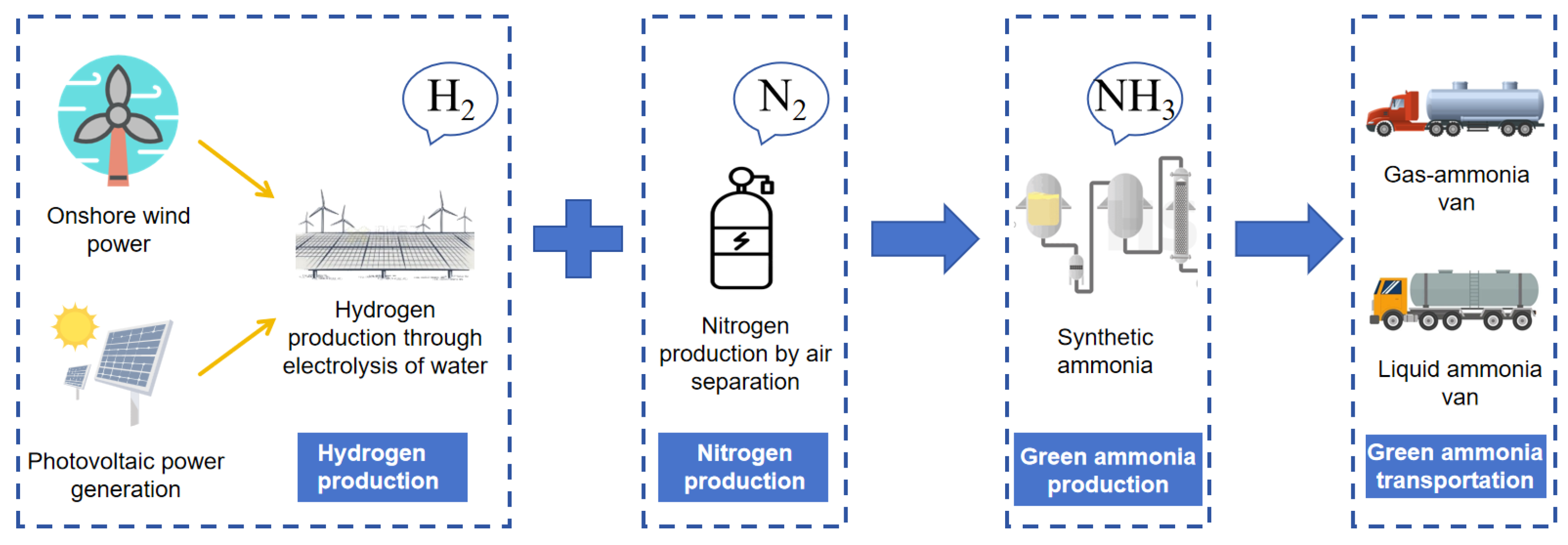
- Air Separation for Nitrogen Production and Green Hydrogen Synthesis of Ammonia:
3.2. Determination of the System Boundaries for Carbon Emission Accounting of Green Hydrogen and Its Derivatives
3.2.1. Determination of the System Boundaries for Carbon Emission Accounting of Green Hydrogen
- System Boundaries for Carbon Emission Accounting of Wind Power Hydrogen Production:
- System Boundaries for Carbon Emission Accounting of Photovoltaic Hydrogen Production:
3.2.2. Determination of the System Boundary for Carbon Emission Accounting of Green Hydrogen Derivatives
- Boundary of the carbon emission accounting system for synthetic methanol:
- Boundary of the Carbon Emission Accounting System for Ammonia Synthesis:
3.3. Carbon Emission Accounting Parameters for Green Hydrogen and Its Derivatives
3.3.1. Carbon Emission Accounting Parameters for Green Hydrogen
- Parameters for Hydrogen Production from Wind Power:
- Parameters for Hydrogen Production from Photovoltaic Power:
3.3.2. Carbon Emission Accounting Parameters for Green Hydrogen Derivatives
- Parameters for Methanol Synthesis:
- Parameters for Ammonia Synthesis:
3.4. Construction of Carbon Emission Accounting Model for Green Hydrogen and Its Derivative Products
3.4.1. Symbols and Indexes
3.4.2. Calculation Methods
3.5. Construction of Carbon Emission Reduction Model for Green Hydrogen and Its Derivative Products
4. Results and Discussion
4.1. Analysis of the Full Life Cycle Carbon Emissions of Green Hydrogen and Its Derivatives
4.1.1. Analysis of the Full Life Cycle Carbon Emissions of Green Hydrogen
- Hydrogen Production from Wind Power:
- Hydrogen Production from PV Power:
4.1.2. Analysis of the Full Life Cycle Carbon Emissions of Green Hydrogen Derivatives
- Methanol Synthesis:
- Ammonia Synthesis:
4.2. Analysis of the Green Attributes of Green Hydrogen and Its Derivatives
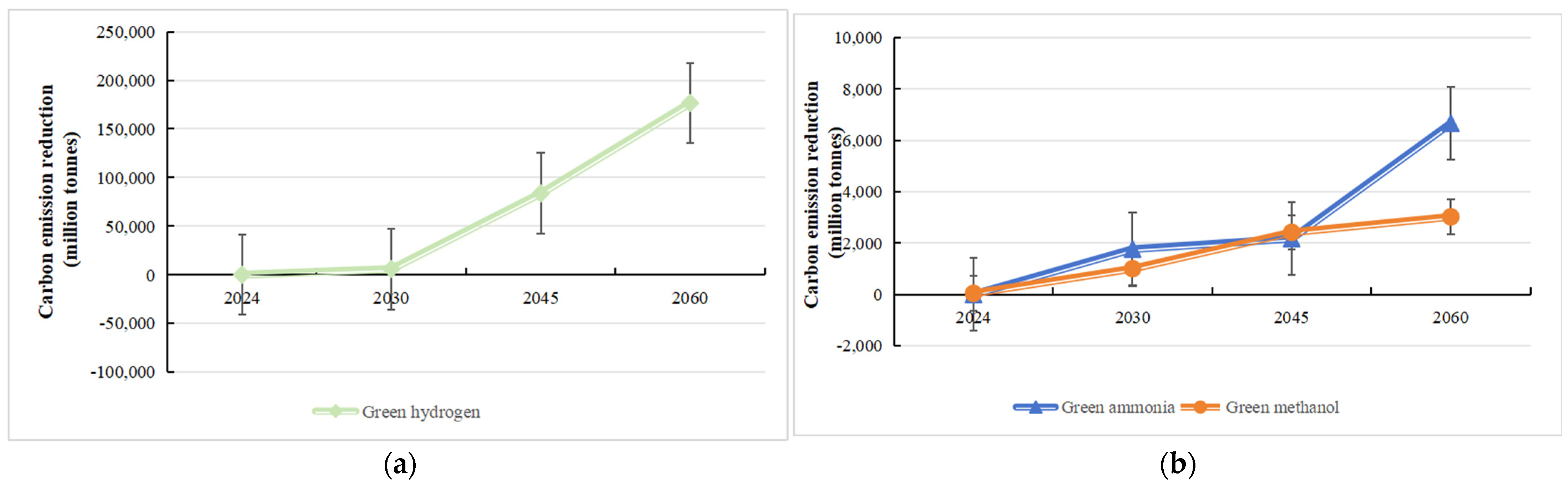
4.3. Analysis of Carbon Emission Reduction Benefits of Green Hydrogen and Its Derivatives
5. Conclusions
- Main Research Findings:
- Policy and Development Recommendations:
- Limitations and Future Research Directions:
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IRENA | International Renewable Energy Agency |
| AEA | Ammonia Energy Association |
| LCA | Life Cycle Assessment |
| PV | Photovoltaic |
References
- Abbasi, K.R.; Shahbaz, M.; Zhang, J.J.; Irgan, M.; Alvarado, R. Analyze the environmental sustainability factors of China: The role of fossil fuel energy and renewable energy. Renew. Energy 2022, 187, 390–402. [Google Scholar] [CrossRef]
- Zou, C.N.; Zhao, Q.; Zhang, G.S.; Xiong, B. Energy revolution: From a fossil energy era to a new energy era. Nat. Gas Ind. B 2016, 3, 1–11. [Google Scholar] [CrossRef]
- Wang, J.N.; Azam, W. Natural resource scarcity, fossil fuel energy consumption, and total greenhouse gas emissions in top emitting countries. Geosci. Front. 2024, 15, 101757. [Google Scholar] [CrossRef]
- Höök, M.; Tang, X. Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef]
- Feng, S.H.; Shafiei, M.W.M.; Ng, T.F.; Ren, J. The intersection of economic growth and environmental sustainability in China: Pathways to achieving SDG. Energy Strategy Rev. 2024, 55, 101530. [Google Scholar] [CrossRef]
- Izuchukwu, A.; Asomah, J.K.; Onoh, U.C. Assessing the Impact of Global Agreements on Combating Climate Change and Advancing Sustainable Development and Global Climate Change. Int. J. Geogr. Environ. Manag. 2024, 10, 2695–1878. [Google Scholar]
- Adanma, U.M.; Ogunbiyi, E.O. A comparative review of global environmental policies for promoting sustainable development and economic growth. Int. J. Appl. Res. Soc. Sci. 2024, 6, 954–977. [Google Scholar] [CrossRef]
- Dong, H.X.; Deng, Q.Y.; Li, C.J.; Liu, N.; Zhang, W.Z.; Hu, M.Y.; Xu, C.B. A comprehensive review on renewable power-to-green hydrogen-to-power systems: Green hydrogen production, transportation, storage, re-electrification and safety. Appl. Energy 2025, 390, 125821. [Google Scholar]
- Guban, D.; Muritala, I.K.; Roeb, M.; Sattler, C. Assessment of sustainable high temperature hydrogen production Technologies. Int. J. Hydrogen Energy 2020, 45, 26156–26165. [Google Scholar] [CrossRef]
- Cao, L.C.; Yu, I.K.M.; Xiong, X.X.; Tsang, D.C.W.; Zhang, S.C.; Clark, J.H.; Hu, C.W.; Ng, Y.H.; Shang, J.; Ok, Y.S. Biorenewable hydrogen production through biomass gasification: A review and future Prospects. Environ. Res. 2020, 186, 109547. [Google Scholar] [CrossRef]
- Dong, H.R.; Fu, Y.B.; Jia, Q.Q.; Zhang, T.; Meng, D.Q. Low carbon optimization of integrated energy microgrid based on life cycle analysis method and multi time scale energy Storage. Renew. Energy 2023, 206, 60–71. [Google Scholar] [CrossRef]
- Chang, M.; Thellufsen, J.Z.; Zakeri, B.; Pickering, B.; Pfenninger, S.; Lund, H.; Alberg Østergaard, P. Trends in tools and approaches for modelling the energy Transition. Appl. Energy 2021, 290, 116731. [Google Scholar] [CrossRef]
- Palmer, O.; Radet, H.; Camal, S.; Girard, R. Green and low-carbon hydrogen-the impact of classification rules and subsidies on asset sizing and energy sourcing for electrolytic hydrogen production. In Proceedings of the 2024 IEEE 34th Australasian Universities Power Engineering Conference (AUPEC), Sydney, Australia, 20–22 November 2024; pp. 1–6. [Google Scholar]
- Liu, D.; Wang, J.Y.; Rameezdeen, R.; Wang, H. Evolutionary dynamics of promoting green methanol industry based on Public-private partnership cooperation: A case study in China. Int. J. Hydrogen Energy 2025, 148, 149684. [Google Scholar] [CrossRef]
- Rodriguez-Pastor, D.A.; Soltero, V.M.; Chacartegui, R. Green methanol production from photovoltaics in Europe. Renew. Energy 2025, 254, 123751. [Google Scholar] [CrossRef]
- Schubert, T. Production routes of advanced renewable C1 to C4 alcohols as biofuel components–A review. Biofuels Bioprod. Biorefining 2020, 14, 845–878. [Google Scholar] [CrossRef]
- Gao, H.; Wang, X.K.; Wu, K.; Zheng, Y.R.; Wang, Q.Z.; Shi, W.; He, M. A Review of Building Carbon Emission Accounting and Prediction Models. Buildings 2023, 13, 1617. [Google Scholar] [CrossRef]
- Shi, W.; Li, W.; Qiao, F.W.; Wang, W.J.; An, Y.; Zhang, G.W. An Inter-provincial carbon quota study in China based on the contribution of clean energy to carbon Reduction. Energy Policy 2023, 182, 113770. [Google Scholar] [CrossRef]
- Finnveden, G.; Hauschild, M.Z.; Ekvall, T.; Guinée, J.; Heijungs, R.; Hellweg, S.; Koehler, A.; Pennington, D.; Suh, S. Recent developments in Life Cycle Assessment. J. Environ. Manage 2009, 91, 1–21. [Google Scholar] [CrossRef]
- Qin, W.L.; Rong, N. Low-Carbon and Economical Optimal Scheduling of an Integrated Energy System with Hydrogen Energy Cascade Based on a Whole Life Cycle Approach. Power Syst. Clean Energy 2025, 41, 145–154. [Google Scholar]
- Zheng, L.X.; Zhao, D.Q.; Qi, X.L.; Chen, Y.S. Research on Energy Efficiency, Carbon Emission and Economy of Hydrogen Production Routes in China Based on Life Cycle Assessment Method. J. Eng. Thermophys. 2022, 43, 2305–2317. (In Chinese) [Google Scholar]
- de Kleijne, K.; Huijbregts, M.A.J.; Knobloch, F.; Zelm, R.V.; Hilbers, J.P.; de Coninck, H.; Hanssen, S.V. Worldwide greenhouse gas emissions of green hydrogen production and transport. Nat. Energy 2024, 9, 1139–1152. [Google Scholar] [CrossRef]
- Bai, Z.; Hao, W.J.; Li, Q.; Hao, H.L.; Wen, C.F.; Guo, S.; Huang, X.K. Capacity configuration optimization of wind-solar hydrogen production based on life cycle assessment. Integr. Intell. Energy 2024, 46, 1–11. [Google Scholar]
- Qian, J.J.; Xu, D.L.; Yuan, L.; Li, G.H.; Gao, R.Y.; Xu, B.S. Low-carbon Economic Operation Model of Integrated Energy System Considering the Whole Process Carbon Footprint and Flexible Output Models. Guangdong Electr. Power 2023, 36, 19–29. [Google Scholar]
- Huang, X.Y.; Xie, M.H.; Li, X.W.; Jiang, L.Y. Comparative Life Cycle Assessment and Carbon Footprint of Typical Hydrogen Energy Products. Environ. Sci. 2024, 45, 5641–5649. (In Chinese) [Google Scholar]
- Chu, Y.B.; Zhou, K.L.; Hu, R.; Yang, Z.W. Diversified hydrogen production methods can reduce carbon dioxide emissions and energy consumption across Chinese cities. Commun. Earth Environ. 2025, 6, 471. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Y.M.; Sun, K. Policy impact of cancellation of wind and photovoltaic subsidy on power generation companies in China. Renew. Energy 2021, 177, 134–147. [Google Scholar] [CrossRef]
- Bonacina, C.N.; Gaskare, N.B.; Valenti, G. Assessment of offshore liquid hydrogen production from wind power for ship refueling. Int. J. Hydrogen Energy 2021, 47, 1279–1291. [Google Scholar] [CrossRef]
- Tan, Z.F.; Tan, C.X.; Liang, Y. Techno-Economic Evaluation of Distributed Photovoltaic Hydrogen Production—Cost benet analysis based on the perspective of the entire lifecycle. Price Theory Pract. 2024, 3, 95–100. (In Chinese) [Google Scholar]
- Rinker, G. Minimum work associated with separating nitrogen from air: An exergy analysis. F1000Research 2024, 13, 158. [Google Scholar] [CrossRef]
- Adeli, K.; Nachtane, M.; Faik, A.; Saifaoui, D.; Boulezhar, A. How green hydrogen and ammonia are revolutionizing the future of energy production: A comprehensive review of the latest developments and future prospects. Appl. Sci. 2023, 13, 8711. [Google Scholar] [CrossRef]
- Du, Y.Y.; Huang, H.; Liu, H.B.; Zhao, J.Y.; Yang, Q.Z. Life Cycle Assessment of Abandonment of Onshore Wind Power for Hydrogen Production in China. Sustainability 2024, 16, 5772. [Google Scholar] [CrossRef]
- Yu, G.; Chen, Q.Q.; Wang, D.F.; Tang, Z.Y. Strategy-based optimization and life cycle environmental impact assessment of a stable photovoltaic power-to-hydrogen system. Energy Convers. Manag. 2024, 315, 118747. [Google Scholar]
- Estevez, R.; Aguado-Deblas, L.; Bautista, F.M.; López-Tenllado, F.J.; Romero, A.A.; Luna, D. A Review on Green Hydrogen Valorization by Heterogeneous Catalytic Hydrogenation of Captured CO2 into Value-added Products. Catalysts 2022, 12, 1555. [Google Scholar] [CrossRef]
- Li, G.; Ma, Z.R.; Zhao, J.; Zhou, J.L.; Peng, S.P.; Li, Y.L.; Wang, B.D. Research progress in green synthesis of ammonia as hydrogen-storage carrier under ‘hydrogen 2.0 economy’. Clean Energy 2023, 7, 116–131. [Google Scholar] [CrossRef]
- Qin, R.; Wang, P.Y.; Lin, C.; Cao, F.; Zhang, J.Y.; Chen, L.; Mu, S.C. Transition Metal Nitrides: Activity Origin, Synthesis and Electrocatalytic Applications. Acta Phys.-Chim. Sin. 2020, 37, 2009099. [Google Scholar] [CrossRef]
- Zhang, J.X.; Wang, Z.H.; He, Y.; Li, M.Y.; Wang, X.D.; Wang, B.; Zhu, Y.Q.; Cen, K.F. Comparison of onshore/offshore wind power hydrogen production through water electrolysis by life cycle assessment. Sustain. Energy Technol. Assess. 2023, 60, 103515. [Google Scholar] [CrossRef]
- Méndez, L.; Forniés, E.; Garrain, D.; Vázquez, A.P.; Souto, A.; Vlasenko, T. Upgraded metallurgical grade silicon and polysilicon for solar electricity production: A comparative life cycle assessment. Sci. Total Environ. 2021, 789, 147969. [Google Scholar] [CrossRef]
- Gerloff, N. Comparative Life-Cycle-Assessment analysis of three major water electrolysis technologies while applying various energy scenarios for a greener hydrogen production. J. Energy Storage 2021, 43, 102759. [Google Scholar] [CrossRef]
- Cheng, Y.B. Lifecycle Carbon Emission Analysis of Low-Carbon Methanol Fuel. Green Pet. Petrochem. 2023, 8, 9–16. (In Chinese) [Google Scholar]
- Wang, M.H.; Chen, Z.Y.; Wang, W.; Ren, L.; Liu, J.Z.; Ou, X.M. Carbon footprint analysis of China’s ammonia energy production-storage-transportation-utilization full-chain for different application scenarios. Clean Coal Technol. 2024, 30, 1–12+187. (In Chinese) [Google Scholar]
- Liu, M.H.; Huang, X.; Li, S.; Shi, Y.X.; Yu, C.Q.; Cai, N.S. Carbon Emission and Energy Efficiency Analysis of Ammonia Production Routes in China from Life-cycle perspective and prospects. Sci. Sin. (Technol.) 2024, 54, 1329–1346. (In Chinese) [Google Scholar] [CrossRef]
- Tian, J.X.; Chen, W.; Sun, H.D.; Sun, K.Y.; Lv, Q.L. Current Status and Recommendations for Green Methanol Development. Pet. Refin. Chem. Eng. 2025, 56, 149–155. [Google Scholar]
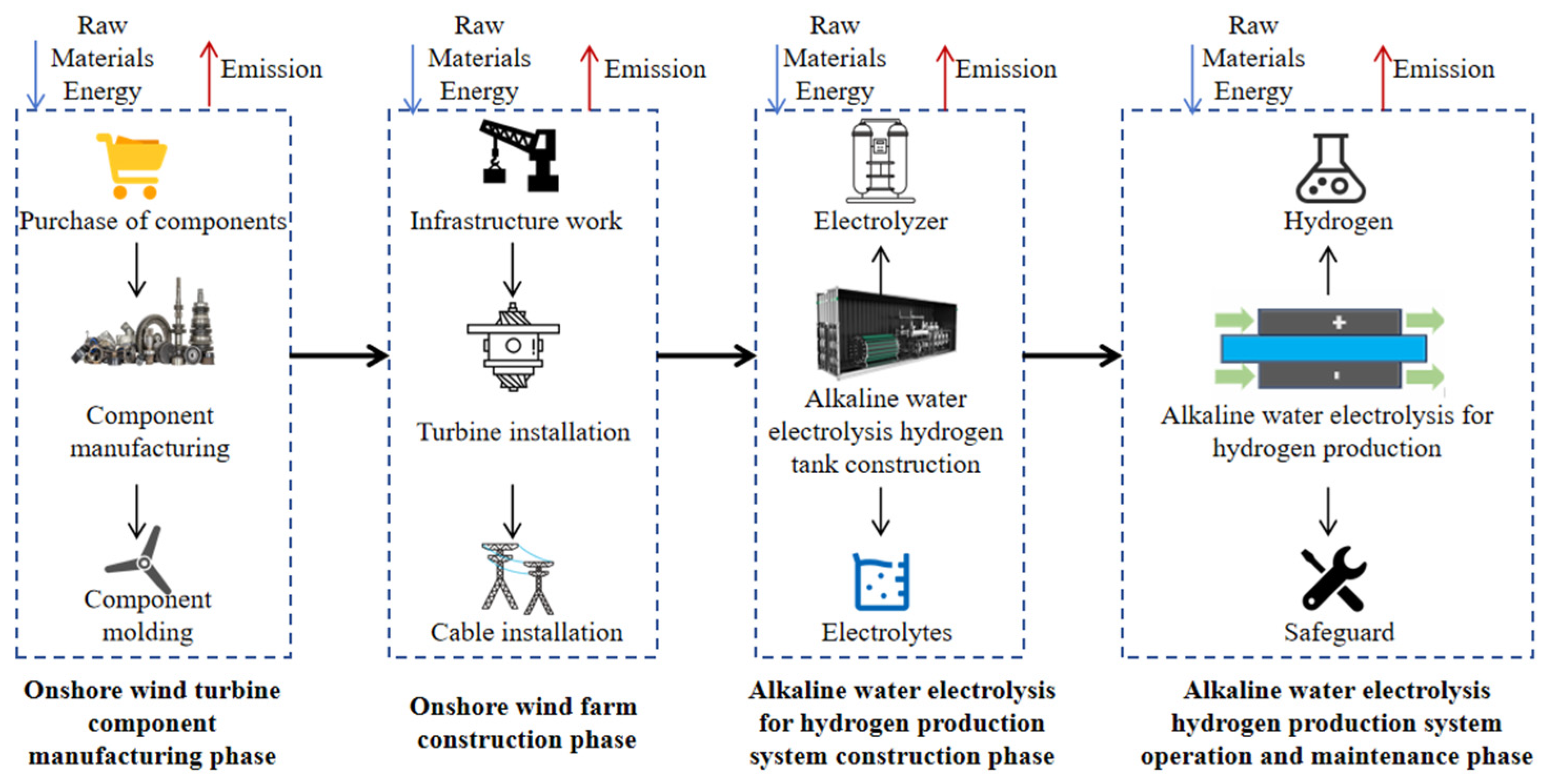
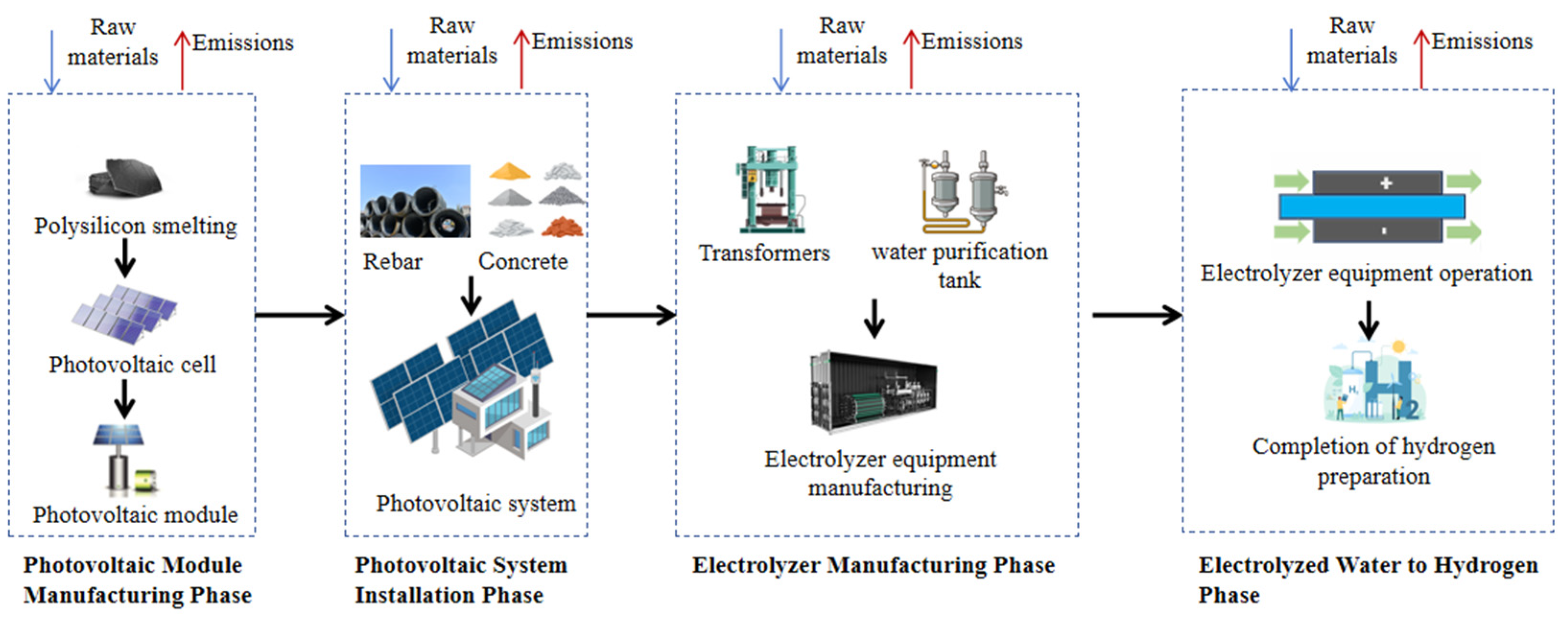

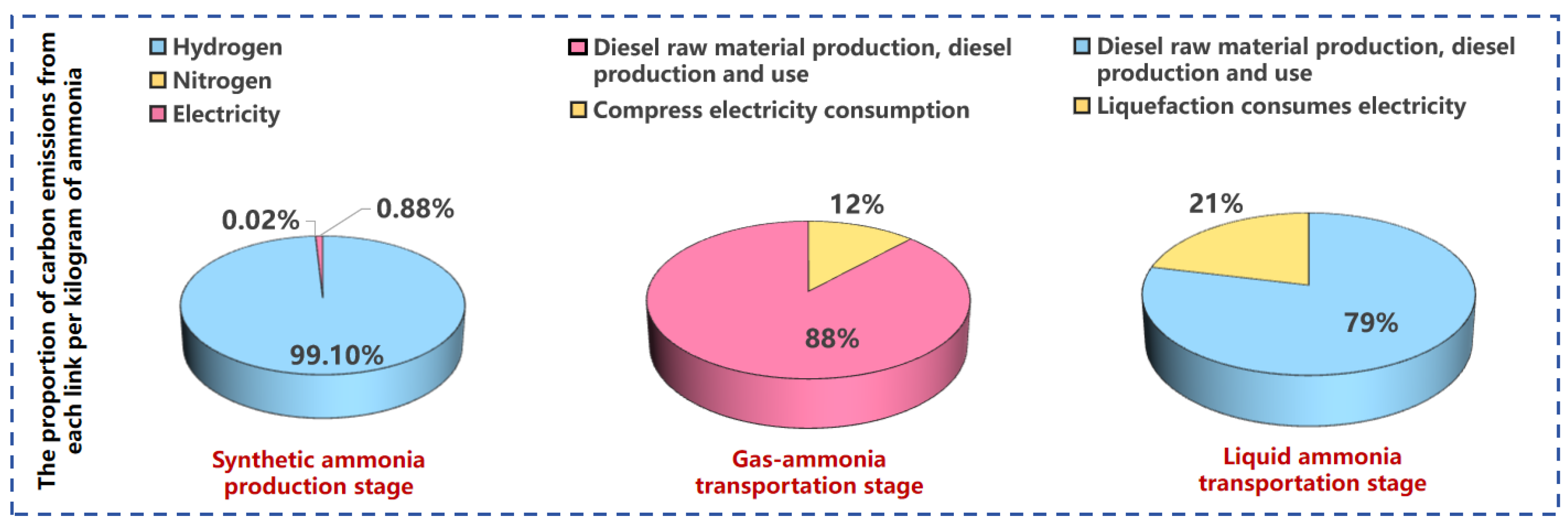

| Stage | List | |||
|---|---|---|---|---|
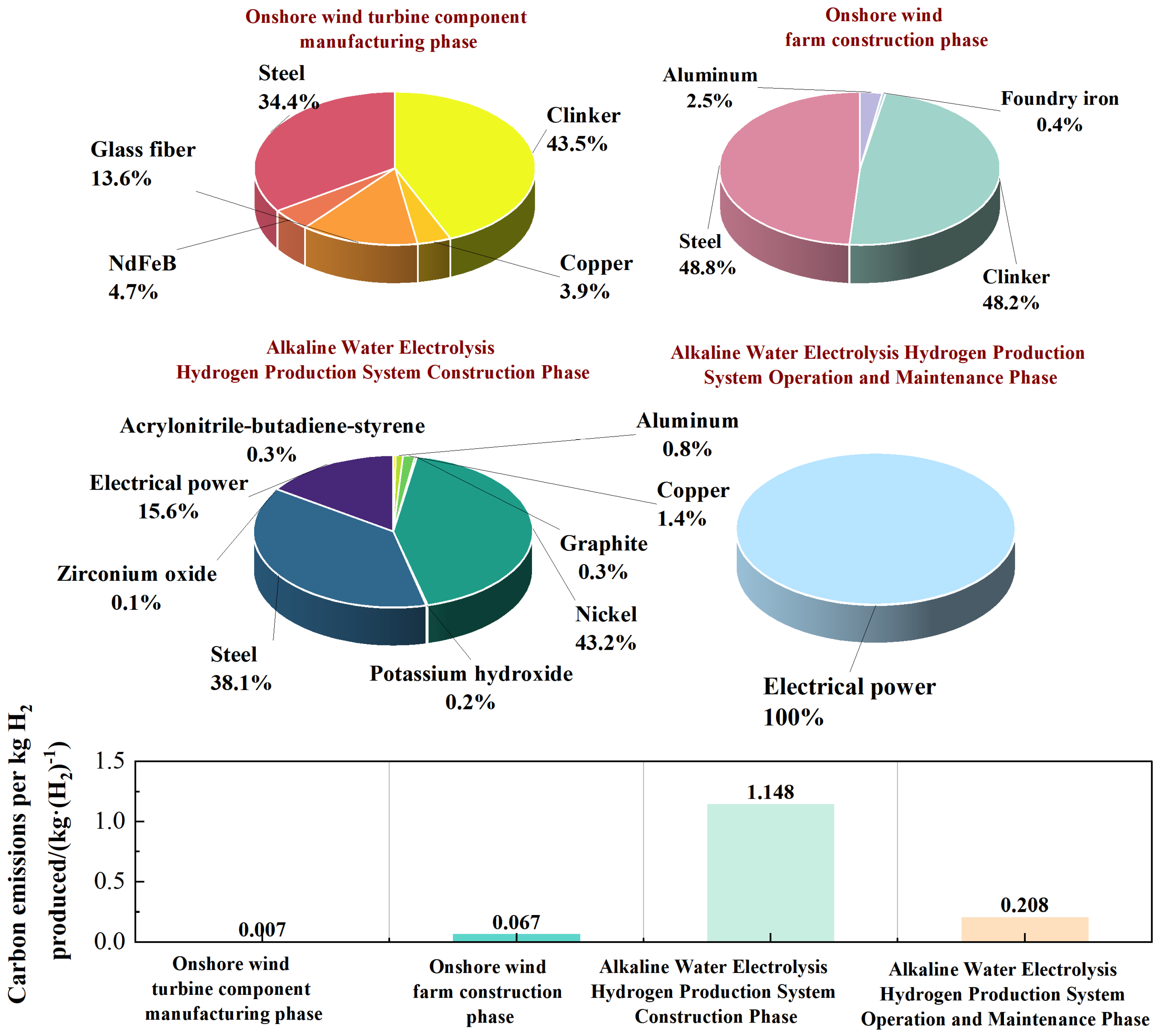
| Onshore Wind Turbine Component Manufacturing Phase | Cement: 3.42 × 10−3 kg | Copper: 4.6 × 10−5 kg | Glass fiber: 1.06 × 10−4 kg | Neodymium iron boron: 6 × 10−6 kg |
| Iron: 9.99 × 10−4 kg | ||||
| Onshore Wind Farm Construction Phase | Aluminum: 2.09 × 10−4 kg | Cast iron: 1.6 × 10−4 kg | Cement: 3.67 × 10−2 kg | Iron: 1.37 × 10−2 kg |
| Alkaline Water Electrolysis Hydrogen Production System Construction Phase | Acrylonitrile butadiene styrene: 1.07 × 10−4 kg | Aluminum: 6.02 × 10−4 kg | Copper: 2.68 × 10−3 kg | Graphite: 5.76 × 10−4 kg |
| Nickel: 2.01 × 10−2 kg | Potassium hydroxide: 1.46 × 10−2 kg | Iron: 1.84 × 10−1 kg | Zirconium oxide: 1.2 × 10−3 kg | |
| Electric power: 4.951 × 101 kWh | ||||
| Alkaline Water Electrolysis Hydrogen Production System Operation and Maintenance Phase | Electric power: 0.575 × 101 kWh | Water: 1 × 10−2 m3 | ||
| Stage | Data List | ||||
|---|---|---|---|---|---|
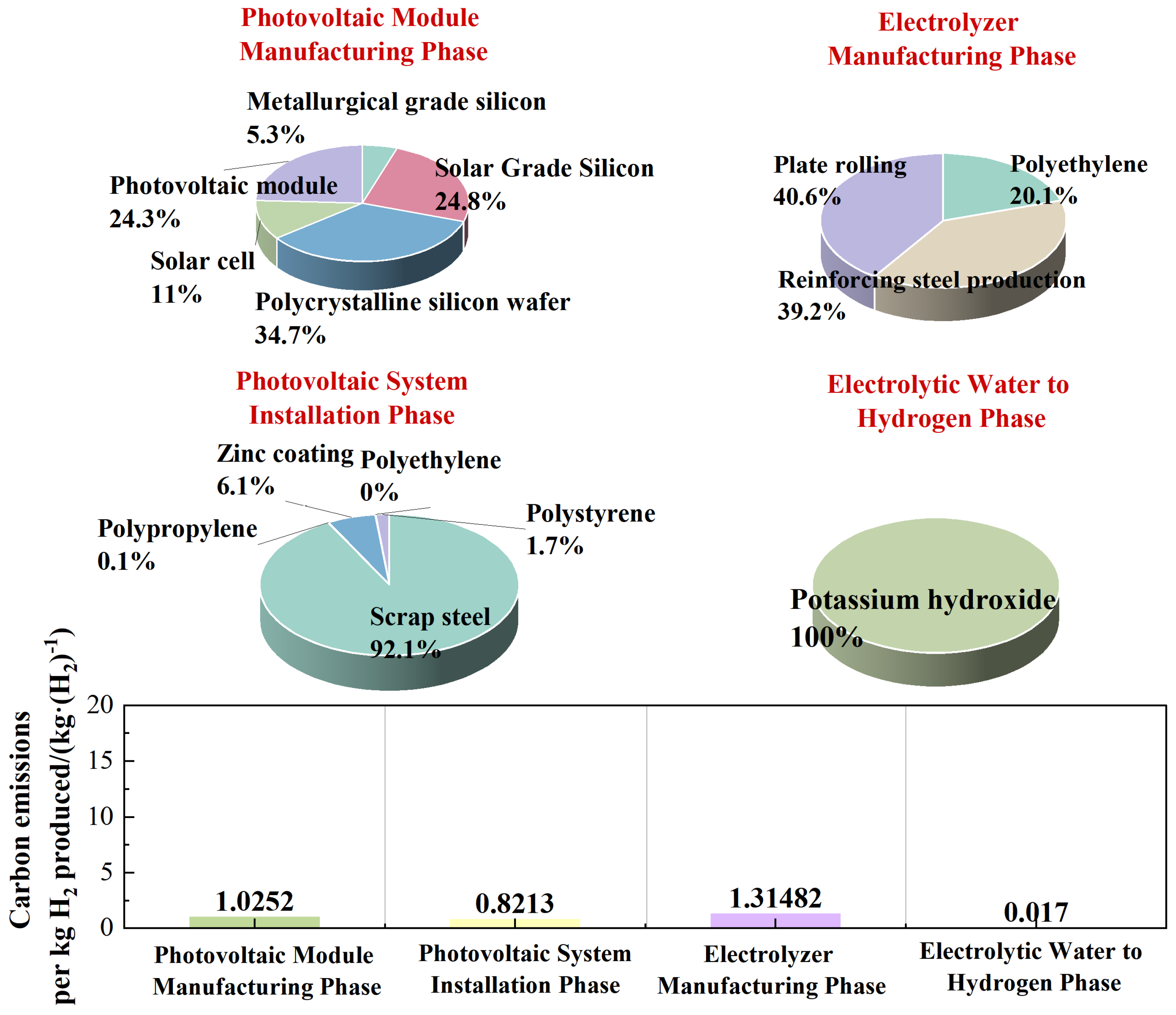
| PV Module Manufacturing Phase | Metallurgical grade silicon | Charcoal 1.7 × 10−3 kg | silica sand 2.7 × 10−2 kg | Petroleum: 5 × 10−3 kg | |
| Solar grade silicon | Lime: 5.8 × 10−3 kg | Graphite: 5.4 × 10−5 kg | |||
| Polycrystalline silicon wafer | Sodium hydroxide: 3.8 × 10−5 kg | Acetic acid: 5.6 × 10−4 kg | Acrylic acid: 2.8 × 10−5 kg | Brass: 7.45 × 10−5 kg | |
| Steel: 2.8 × 10−5 kg | Nickel: 1 × 10−5 kg | ||||
| Solar cell | Nitric acid: 2.4 × 10−4 kg | Ammonia: 3.3 × 10−4 kg | Aluminum: 5.4 × 10−4 kg | Steel: 1.56 × 10−7 kg | |
| Polystyrene: 4.0722 × 10−6 kg | |||||
| PV module | Methanol: 2.1556 × 10−5 kg | Aluminum: 1.374 × 10−2 kg | Copper: 1.35 × 10−3 kg | Iron: 7.768 × 10−2 kg | |
| PV System Installation Phase | Concrete: 2 × 10−5 m3 | Polypropylene: 9 × 10−4 kg | Zinc coating: 3.12 × 10−2 m3 | Polyethylene: 9 × 10−4 kg | |
| Polystyrene: 4.54 × 10−3 kg | |||||
| Electrolyzer Manufacturing Phase | Polyethylene: 4.64 × 10−1 kg | Steel production: 2.323 × 10−1 kg | Steel plate rolling: 2.323 × 10−1 kg | ||
| Electrolyzed Water to Hydrogen Phase | Cooling water: 8.81 × 10−2 kg | Potassium hydroxide: 3.7 × 10−3 kg | |||
| Stage | Data List | |||
|---|---|---|---|---|
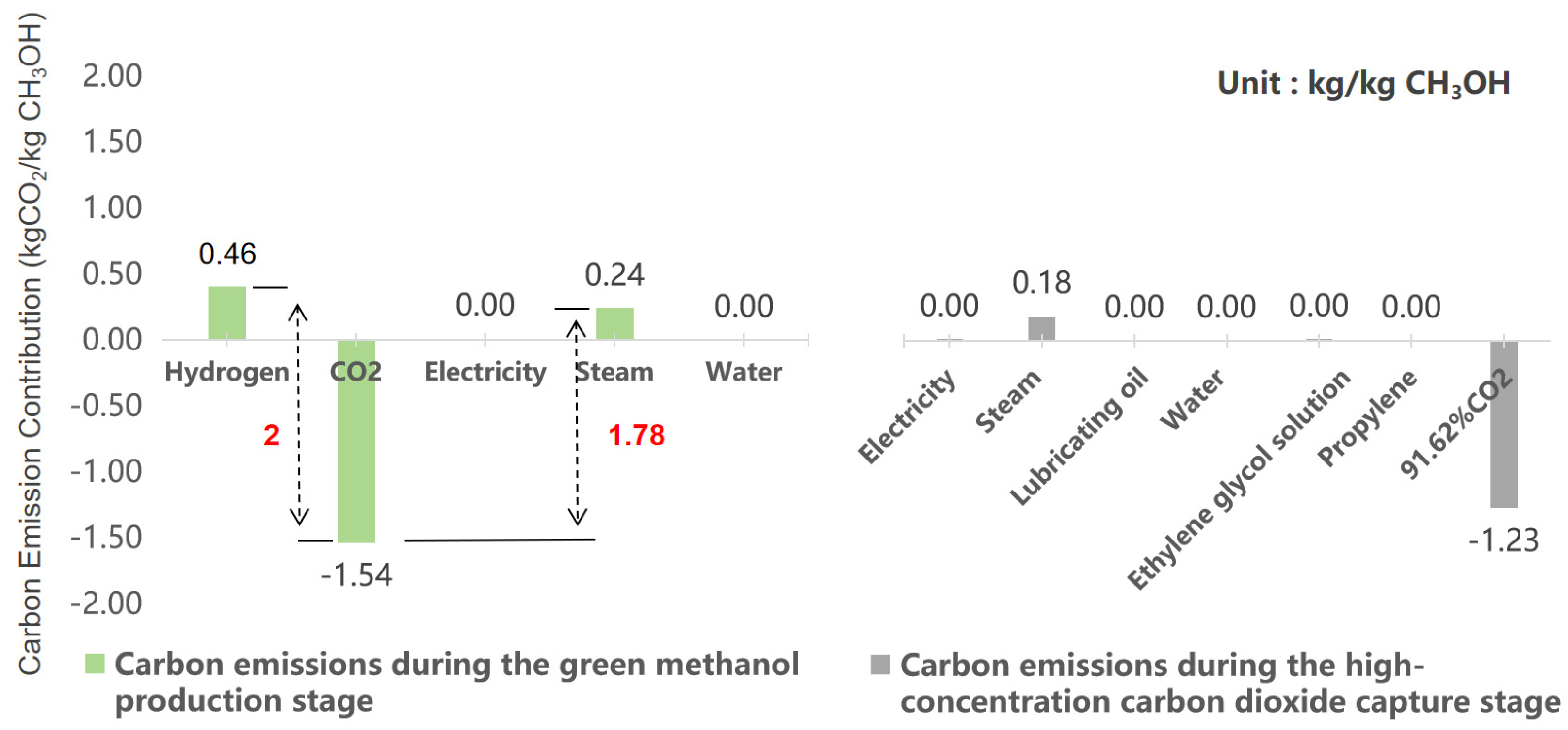
| High-concentration CO2 Capture [40] | 91.62% CO2: 1.23 kg | Propylene: 3.27 × 10−5 kg | Ethylene glycol solution: 2.42 × 10−6 kg | Water: 1.19 kg |
| Lubricating oil: 7.25 × 10−7 kg | Steam: 4.38 × 10−1 kg | Electricity: 7.99 × 10−2 kWh | ||
| Green Hydrogen Production | PV/wind power for green hydrogen: 2.305 × 10−1 kg | |||
| Green Methanol Production [40] | Green hydrogen (using hydrogen production calculation results): 1.99 × 10−1 kg | Captured CO2: 1.46 kg | Electricity: 1.69 × 10−1 kg | Steam: 5.91 × 10−1 kg |
| Recirculating water: 7.45 × 102 kg | ||||
| Green Methanol Transportation | Diesel feedstock production, diesel production, diesel use: 1.288 × 10−5 kg·km−1 | |||
| Stage | Data List | |||
| Air Separation Nitrogen Production | Electricity: 2.5 × 10−2 kWh | |||
| Green Hydrogen Production | PV/wind power for green hydrogen: 2.46 × 10−1 kg | |||
| Green Ammonia Synthesis Production | Green Hydrogen: 2.305 × 10−1 kg | Nitrogen: 1.1487 kg | Electricity: 1.3986 kWh | Circulating Water: 7.45 × 101 kg |
| Green Gaseous Ammonia Tanker Transportation [39] | Diesel feedstock production, diesel production, diesel use: 2.361 × 10−5 kg·km−1 | Compression Electricity Consumption: 1.8 × 10−1 kWh | ||
| Green Liquid Ammonia Tanker Transportation [39] | Diesel feedstock production, diesel production, diesel use: 2.125 × 10−5 kg·km−1 | Liquefaction Electricity Consumption: 6 × 10−1 kWh | ||
| Phase Meaning | Identifier | ||||
|---|---|---|---|---|---|
| Power generation equipment manufacturing | — | — | |||
| Power plant construction | — | — | |||
| Electrolyzer installation construction | — | — | |||
| Electrolyzer operation and maintenance | — | — | |||
| Hydrogen production (reuse) | — | — | √ | √ | |
| High-concentration CO2 capture | — | — | √ | — | |
| Air separation nitrogen production | — | — | — | √ | |
| Operation of the synthesis device | — | — | |||
| Product transportation | — | — |
| Green hydrogen carbon emissions (unit: kgCO2/kgH2) | |||||
| Country/Institution | CO2 equivalent threshold | Does PV green hydrogen meet the standard (Yes/No) | PV green hydrogen difference | Does WF green hydrogen meet the standard (Yes/No) | WF green hydrogen difference |
| European Union | 3.4 | Yes | 0.23 | Yes | 1.97 |
| Japan | 3.4 | Yes | 0.23 | Yes | 1.97 |
| United States | 4 | Yes | 0.83 | Yes | 2.57 |
| China | 4.9 | Yes | 1.73 | Yes | 3.47 |
| Green methanol carbon emissions (unit: kgCO2/kgCH3OH) | |||||
| Country/Institution | CO2 equivalent threshold | Does green methanol meet the standard (Yes/No) | Green methanol difference | ||
| European Union | 0.64 | Yes | 1.47 | ||
| Green ammonia carbon emissions (unit: kgCO2/kgNH3) | |||||
| Country/Institution | CO2 equivalent threshold | Does gaseous ammonia meet the standard (Yes/No) | Gaseous ammonia difference | Does liquid ammonia meet the standard (Yes/No) | Liquid ammonia difference |
| Japan | 0.84 | Yes | 0.27 | Yes | 0.26 |
| International Green Hydrogen Organization | 0.3 (green hydrogen to green ammonia) | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Qin, W.; Hu, M.; Zha, D.; Xuan, J.; Hou, K.; Feng, T. Analysis of Carbon Emissions and Carbon Reduction Benefits of Green Hydrogen and Its Derivatives Based on the Full Life Cycle. Sustainability 2025, 17, 9077. https://doi.org/10.3390/su17209077
Ma L, Qin W, Hu M, Zha D, Xuan J, Hou K, Feng T. Analysis of Carbon Emissions and Carbon Reduction Benefits of Green Hydrogen and Its Derivatives Based on the Full Life Cycle. Sustainability. 2025; 17(20):9077. https://doi.org/10.3390/su17209077
Chicago/Turabian StyleMa, Lili, Wenwen Qin, Mingyue Hu, Daoshun Zha, Jiadong Xuan, Kaixuan Hou, and Tiantian Feng. 2025. "Analysis of Carbon Emissions and Carbon Reduction Benefits of Green Hydrogen and Its Derivatives Based on the Full Life Cycle" Sustainability 17, no. 20: 9077. https://doi.org/10.3390/su17209077
APA StyleMa, L., Qin, W., Hu, M., Zha, D., Xuan, J., Hou, K., & Feng, T. (2025). Analysis of Carbon Emissions and Carbon Reduction Benefits of Green Hydrogen and Its Derivatives Based on the Full Life Cycle. Sustainability, 17(20), 9077. https://doi.org/10.3390/su17209077





