Plant-Growth-Promoting Rhizobacteria as a Sustainable Strategy for Enhancing Quinoa Resilience to Salt Stress in Arid Regions
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Analysis
2.2. Growth Conditions, Plant Material, and Treatments
2.3. Crop Evapotranspiration (ETc) Determination
2.4. Analysis of the Nutrients and Components of Quinoa
2.5. Crop Water Production
2.6. Statistical Analyses
3. Results and Discussion
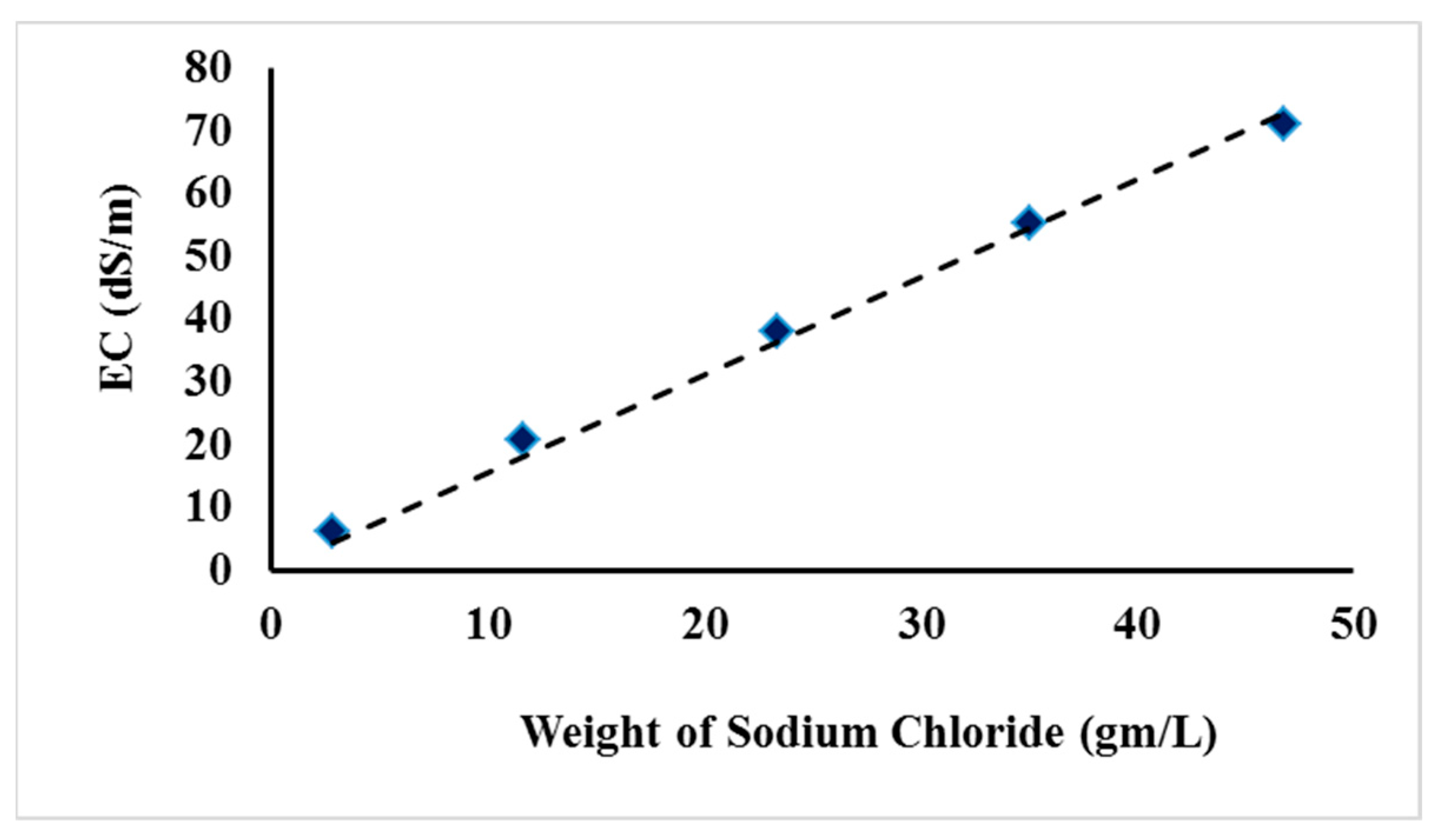
3.1. Soil Salinity Removal
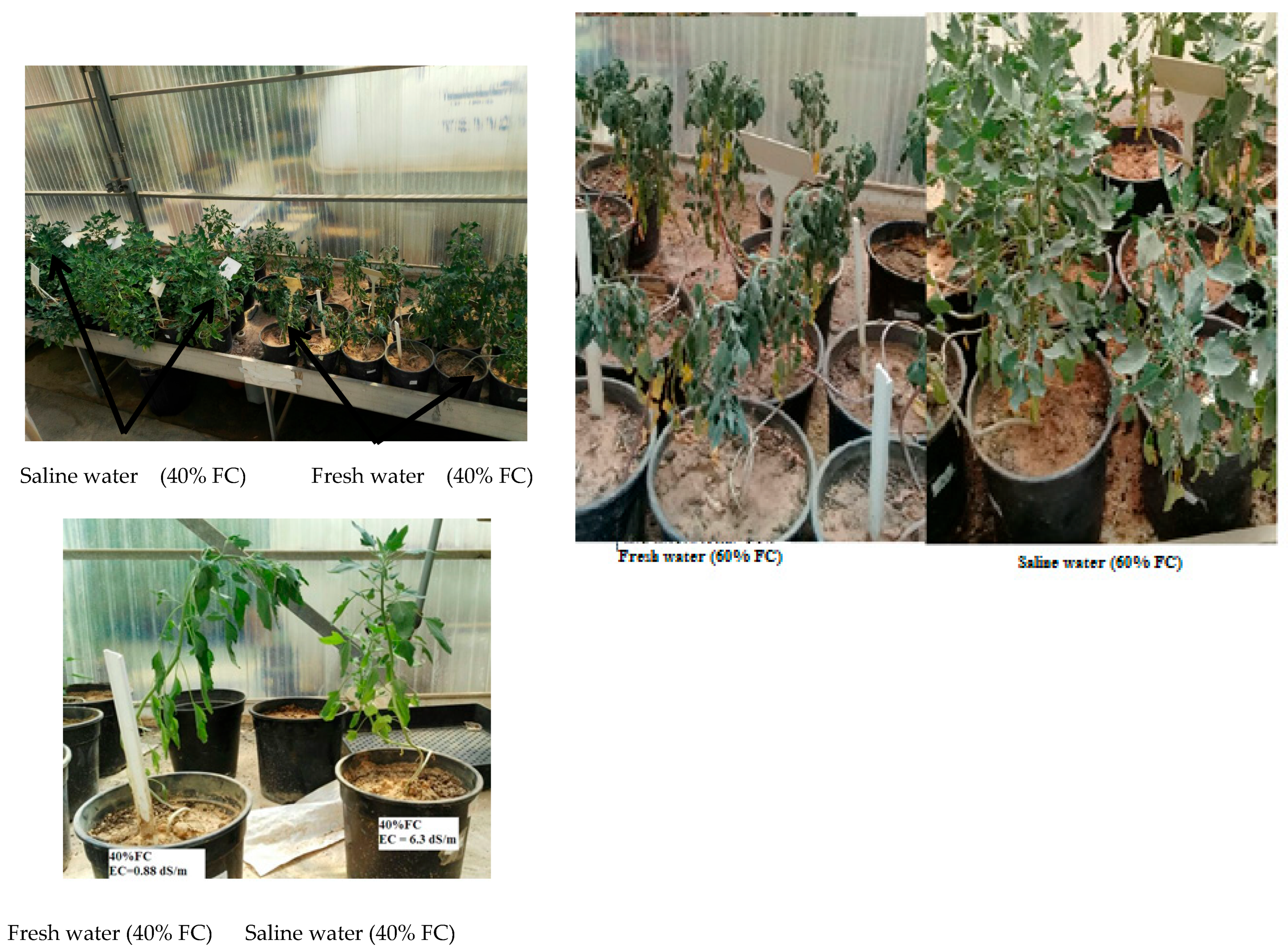
3.2. Drought Stress Application
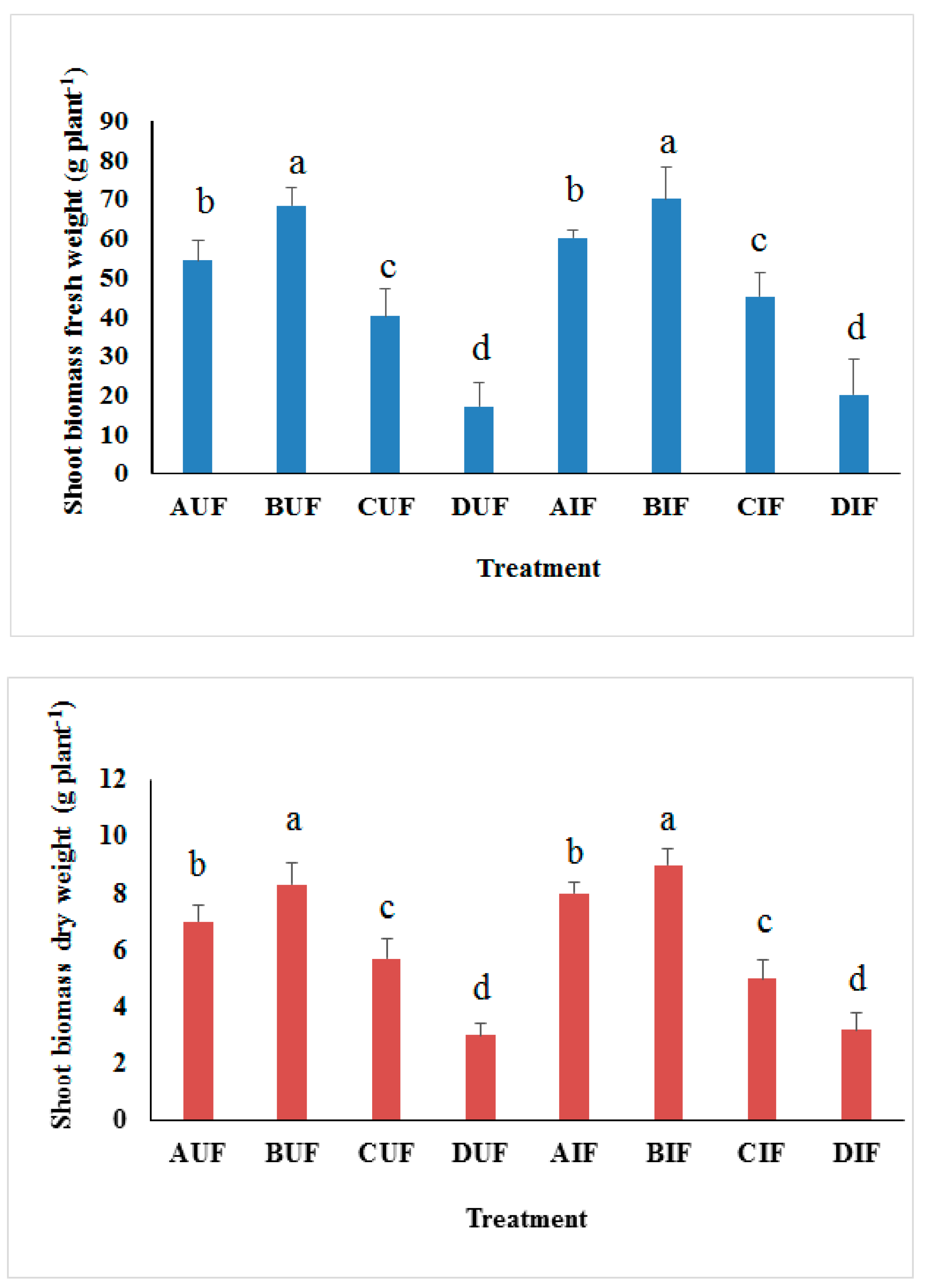
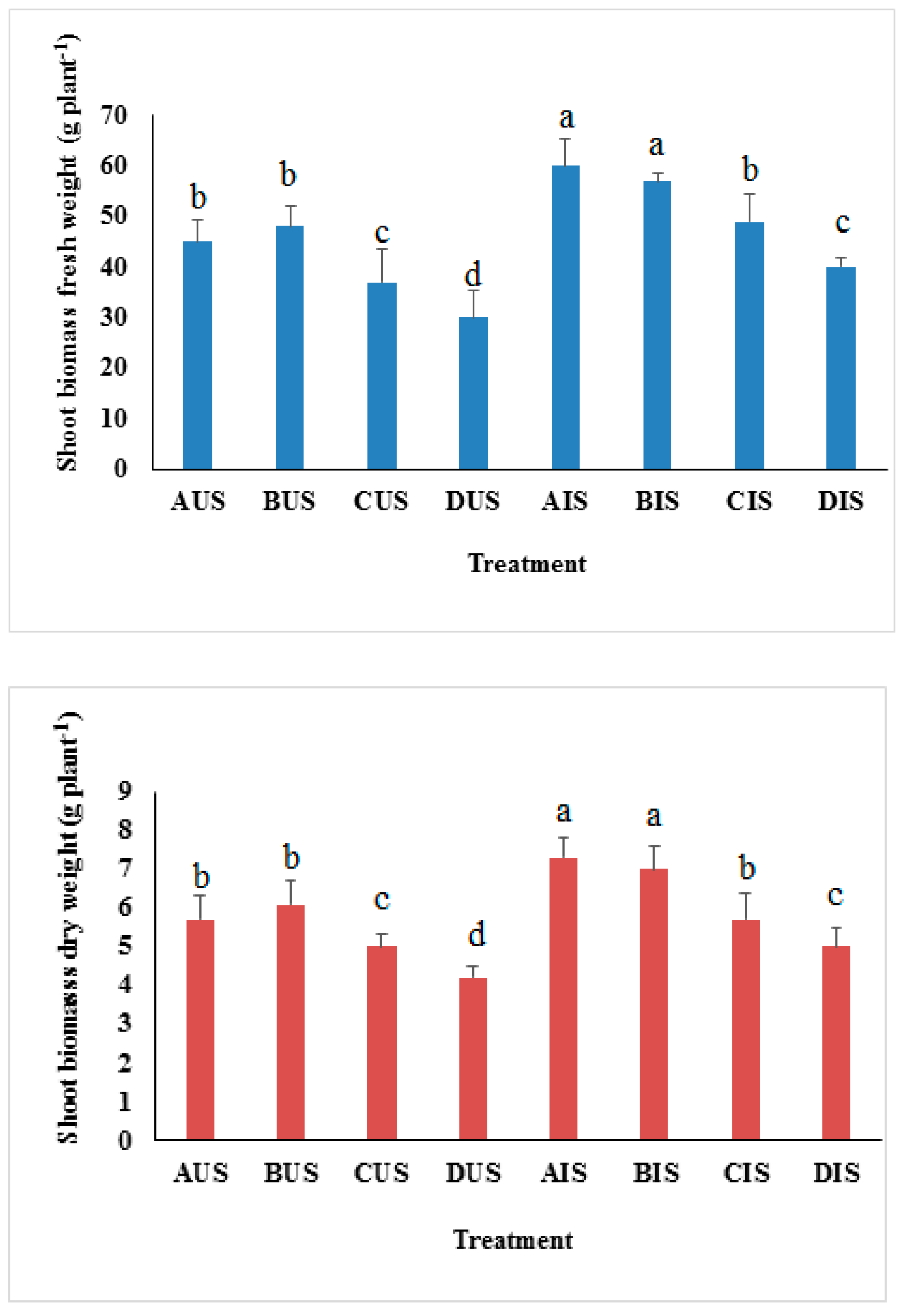
3.3. Biomass Production
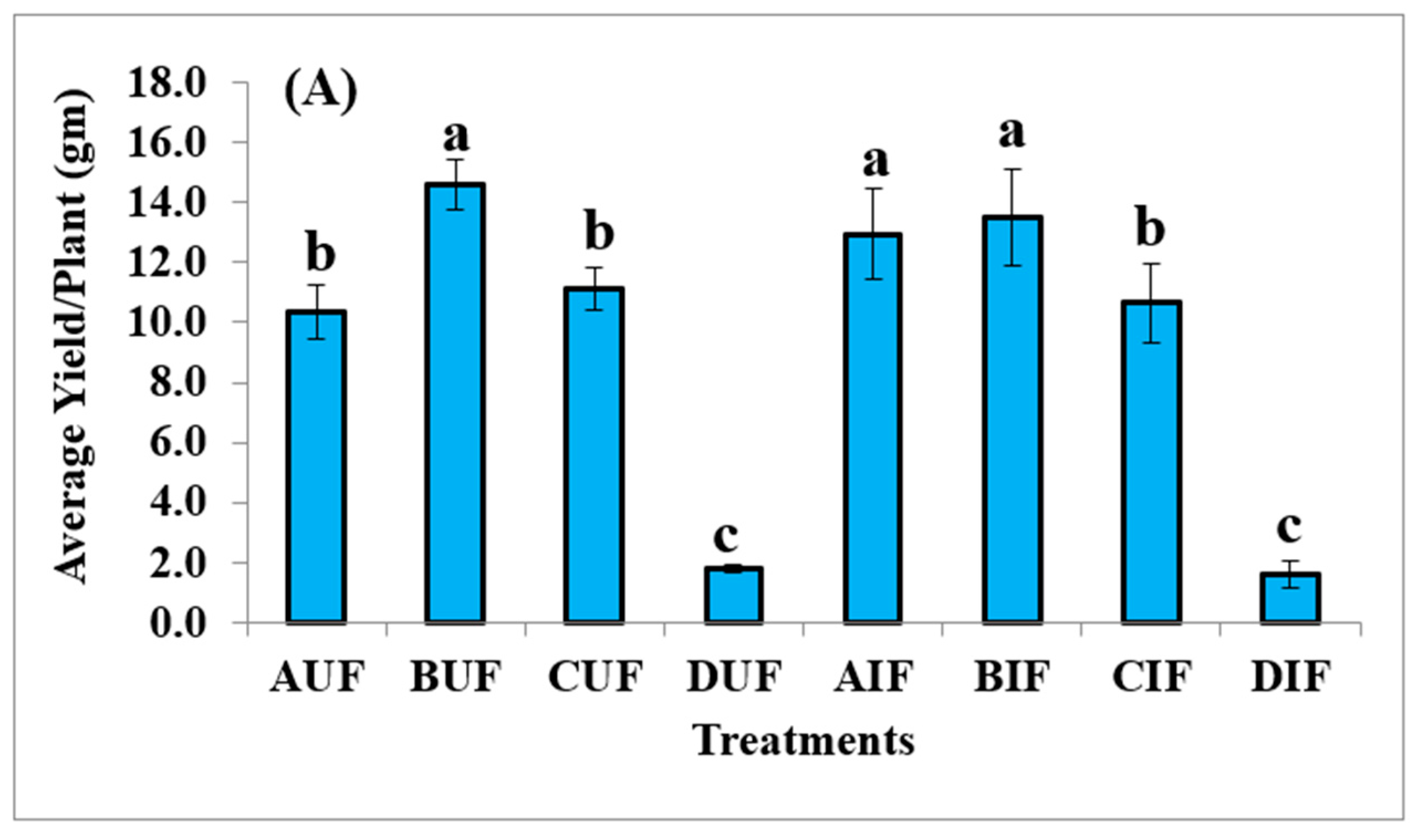
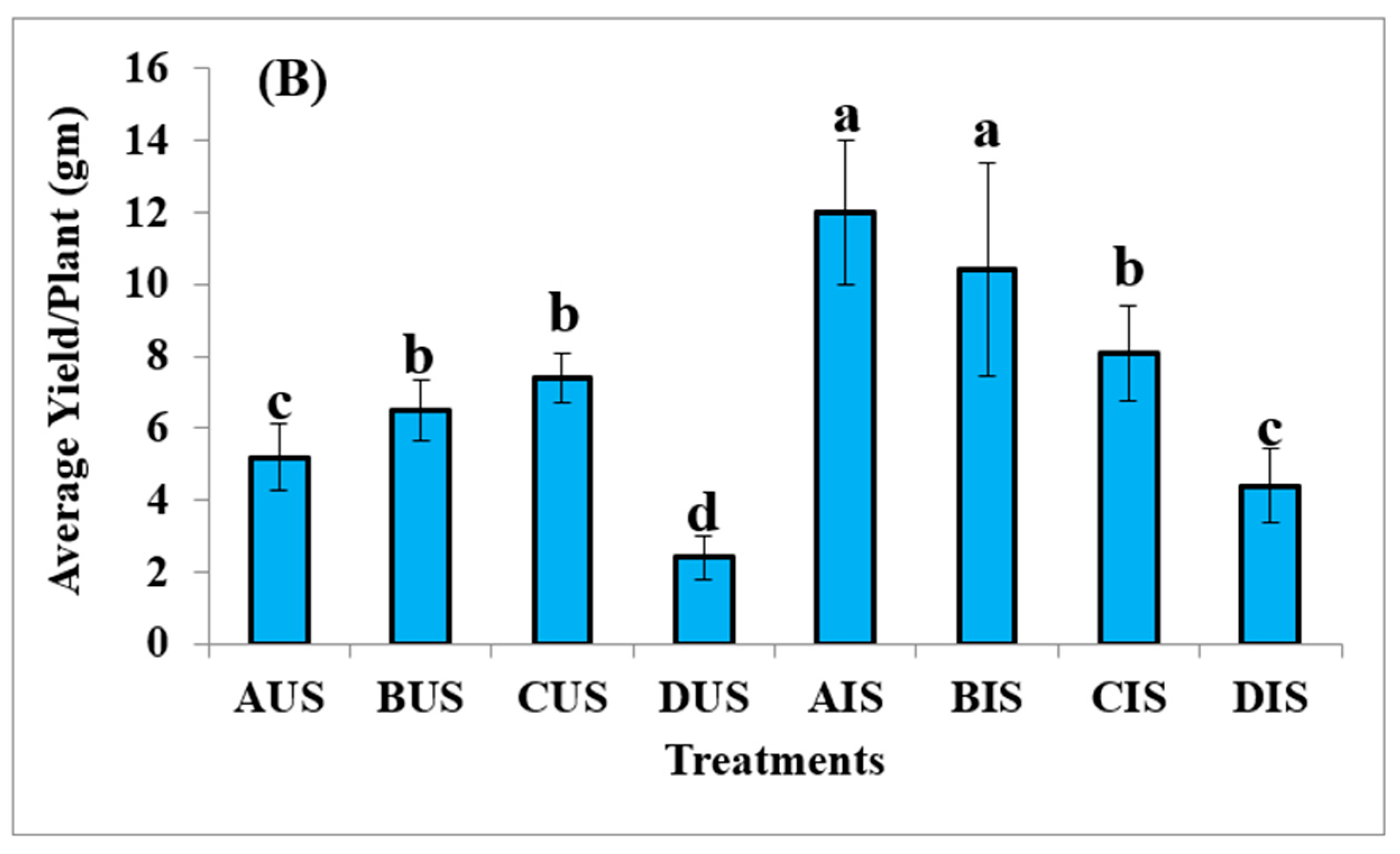
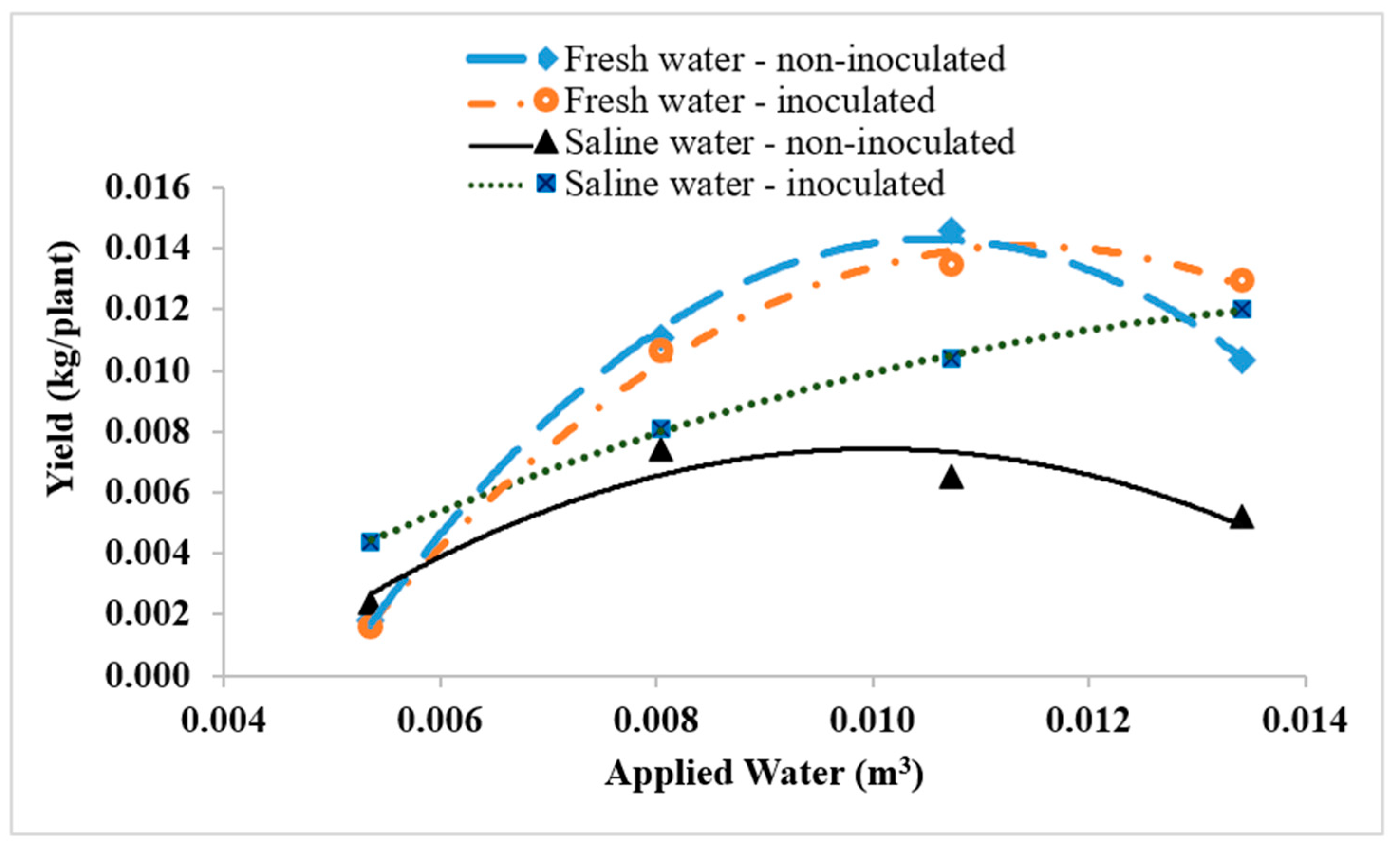
3.4. Yield of Quinoa Plants
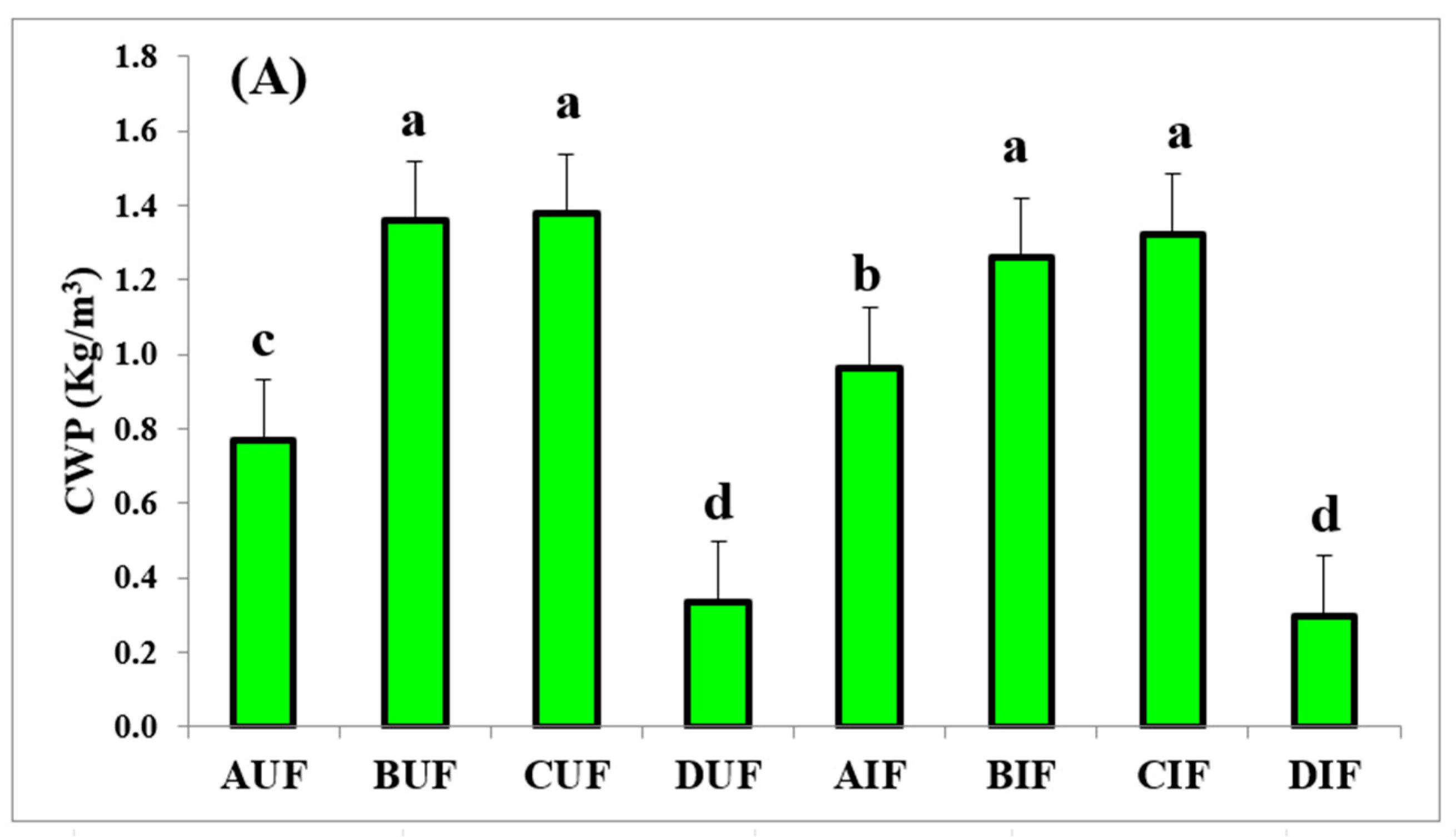
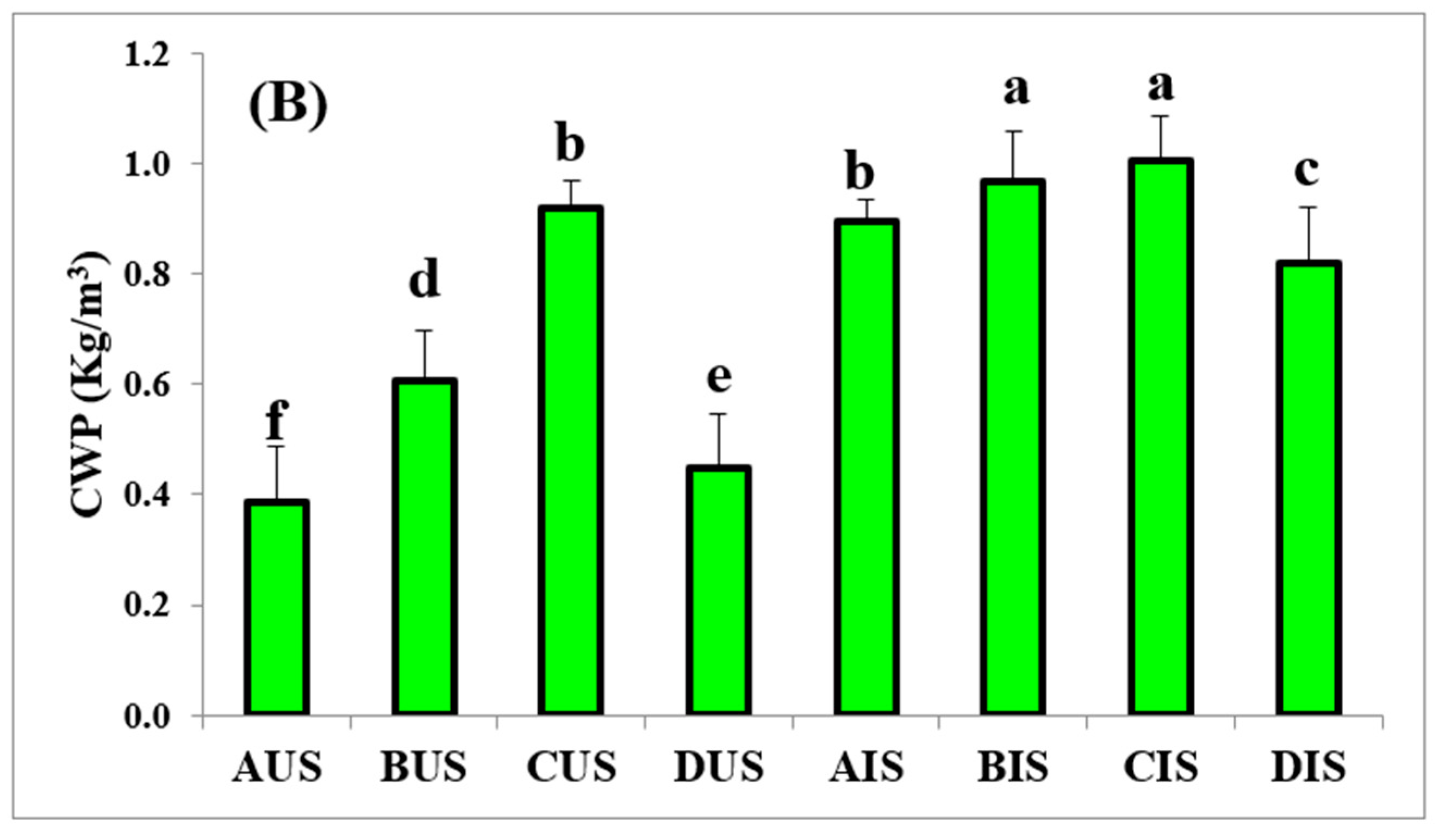
3.5. Crop Water Productivity (CWP)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| Abbreviation | Description |
| AIF | Soil irrigated with 100% of GWR using fresh water and inoculated with PGPR. |
| AUF | Soil irrigated with 100% of GWR using fresh water and non-inoculated with PGPR. |
| BIF | Soil irrigated with 80% of GWR using fresh water and inoculated with PGPR. |
| BUF | Soil irrigated with 80% of GWR using fresh water and non-inoculated with PGPR. |
| CIF | Soil irrigated with 60% of GWR using fresh water and inoculated with PGPR. |
| CUF | Soil irrigated with 60% of GWR using fresh water and non-inoculated with PGPR. |
| DIF | Soil irrigated with 40% of GWR using fresh water and inoculated with PGPR. |
| DUF | Soil irrigated with 40% of GWR using fresh water and non-inoculated with PGPR. |
| AIS | Soil irrigated with 100% of GWR using saline water and inoculated with PGPR. |
| AUS | Soil irrigated with 100% of GWR using saline water and non-inoculated with PGPR. |
| BIS | Soil irrigated with 80% of GWR using saline water and inoculated with PGPR. |
| BUS | Soil irrigated with 80% of GWR using saline water and non-inoculated with PGPR. |
| CIS | Soil irrigated with 60% of GWR using saline water and inoculated with PGPR. |
| CUS | Soil irrigated with 60% of GWR using saline water and non-inoculated with PGPR. |
| DIS | Soil irrigated with 40% of GWR using saline water and inoculated with PGPR. |
| DUS | Soil irrigated with 40% of GWR using saline water and non-inoculated with PGPR. |
References
- Alghamdi, A.G.; Aly, A.A.; Al-Omran, A.M.; Alkhasha, A.; Alharbi, A. Water conservation in tomato production using deficit irrigation and SALTMED model under greenhouse conditions. Sci. Afr. 2024, 24, e02185. [Google Scholar] [CrossRef]
- Alghamdi, A.G.; Alshami, A.K.; El-Shafei, A.; Alomran, A.M.; Alkhasha, A.; Aly, A.A.; Alharbi, A.R. Evaluating Tomato Performance: A Novel Approach of Combining Full and Deficit Irrigation with Saline Water. Agronomy 2024, 14, 559. [Google Scholar] [CrossRef]
- Aly, A.A.; Al-Barakah, F.N.; El-Mahrouky, M.A. Salinity Stress Promote Drought Tolerance of Chenopodium Quinoa Willd. Commun. Soil Sci. Plant Anal. 2018, 49, 1331–1343. [Google Scholar] [CrossRef]
- Alghamdi, A.G.; Aly, A.A.; Majrashi, M.A.; Ibrahim, H.M. Impact of climate change on hydrochemical properties and quality of groundwater for domestic and irrigation purposes in arid environment: A case study of Al Baha region, Saudi Arabia. Environ. Earth Sci. 2023, 82, 39. [Google Scholar] [CrossRef]
- United Nations (UN). World Population Prospects 2024: The Twenty Eighth Edition; UN Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2024; Available online: https://population.un.org/wpp/ (accessed on 1 April 2025).
- Zhao, Q.; Huang, J. Agricultural Science and Technology in China: A Roadmap to 2050; Springer: Beijing, China, 2011. [Google Scholar] [CrossRef]
- FAO. Quinoa for Future Food and Nutrition Security in Marginal Environments. In Proceedings of the International Quinoa Conference, Dubai, United Arab Emirates, 6–8 December 2016. [Google Scholar]
- Orcutt, D.M.; Nilsen, E.T. The Physiology of Plants Under Stress; John Wiley & Sons, Inc.: New York, NY, USA, 2000. [Google Scholar]
- Aly, A.A.; Al-Omran, A.M.; Sallam, A.S.; Al-Wabel, M.I.; Al-Shayaa, M.S. Vegetation cover change detection and assessment in arid environment using multi-temporal remote sensing images and ecosystem management approach. Solid Earth 2016, 7, 713–725. [Google Scholar] [CrossRef]
- Hariadi, Y.; Marandon, K.; Tian, Y.; Jacobsen, S.E.; Shabala, S. Ionic and osmotic relations in quinoa (Chenopodium quinoa Willd.) plants grown at various salinity levels. J. Exp. Bot. 2011, 62, 185–193. [Google Scholar] [CrossRef]
- Jacobsen, S.E.; Mujica, A.; Jensen, C.R. Resistance of quinoa (Chenopodium quinoa Willd.) to adverse, abiotic factors. J. Exp. Bot. 2003, 54, 21. [Google Scholar]
- Morales, A.J.; Bajgain, P.; Garver, Z.; Maughan, P.J.; Udall, J.A. Physiological responses of Chenopodium quinoa to salt stress. Int. J. Plant Physiol. Biochem. 2011, 3, 1–14. Available online: http://www.academicjournals.org/ijppb (accessed on 1 April 2025). [CrossRef]
- Cocozza1, C.; Pulvento, C.; Lavini, A.; Riccardi, M.; d’Andria, R.; Tognetti, R. Effects of Increasing Salinity Stress and Decreasing Water Availability on Ecophysiological Traits of Quinoa (Chenopodium quinoa Willd.) Grown in a Mediterranean Type Agroecosystem. J. Agron. Crop Sci. 2013, 199, 229–240. [Google Scholar] [CrossRef]
- Pulvento, C.; Riccardi, M.; Lavini, A.; Andria, R.D.; Ragab, R. Saltmed model to simulate yield and dry matter for quinoa crop and soil moisture content under different irrigation strategies in south Italy. Irrig. Drain. 2013, 62, 229–238. [Google Scholar] [CrossRef]
- Eisa, S.; Hussin, S.; Geissler, N.; Koyro, H.W. Effect of NaCl salinity on water relations, photosynthesis and chemical composition of Quinoa (Chenopodium quinoa Willd.) as a potential cash crop halophyte. Aust. J. Crop Sci. 2012, 6, 357–368. [Google Scholar]
- Long, N.V. Effects of salinity stress on growth and yield of quinoa (Chenopodium quinoa Willd.) at flower initiation stages. Vietnam J. Agri. Sci. 2016, 14, 321–327. [Google Scholar]
- Patel, P.R.; Sushil, S.; Kajal, S.S.; Patel, V.R.; Patel, V.J.; Khristi, S.M. Impact of salt stress on nutrient uptake and growth of cowpea. Braz. J. Plant Physiol. 2010, 22, 43–48. [Google Scholar] [CrossRef]
- Szymańska, S.; Lis, M.I.; Piernik, A.; Hrynkiewicz, K. Pseudomonas stutzeri and Kushneria marisflavi Alleviate Salinity Stress-Associated Damages in Barley, Lettuce, and Sunflower. Front. Microbiol. 2022, 13, 788893. [Google Scholar] [CrossRef]
- Lami, M.J.; Adler, C.; Caram-Di Santo, M.C.; Zenoff, A.M.; de Cristóbal, R.E.; Espinosa-Urgel, M.; Vincent, P.A. Pseudomonas stutzeri MJL19, a rhizosphere-colonizing bacterium that promotes plant growth under saline stress. J. Appl. Microbiol. 2020, 129, 1321–1336. [Google Scholar] [CrossRef] [PubMed]
- Slatni, T.; Ben Ahmed, H.; Belhaj Dali, A. Enhancing Quinoa Growth in Saline Environments through Salt-Tolerant Rhizobacteria from Halophyte Biotopes. Physiol. Plant. 2024, 176, e14466. [Google Scholar] [CrossRef] [PubMed]
- Zaki, R.M.; Afify, A.H.; Ashour, E.H.; El-Sawah, A.M. Salt-Tolerant Bacteria Support Salinity Stress Mitigating Impact of Arbuscular Mycorrhizal Fungi in Maize (Zea mays L.). Microorganisms 2025, 13, 1345. [Google Scholar] [CrossRef]
- Hillel, D. Soil and Water Physical Principles and Processes; Academic Press: New York, NY, USA, 1971. [Google Scholar]
- Sparks, D.L. Methods of Soil Analysis. Part 3. Chemical Methods; SSSA Book Ser. 5; ASA and SSSA: Madison, WI, USA, 1996. [Google Scholar]
- Paul, S.; Verma, O.P.; Rathi, M.S.; Tyagi, S.P. Effect of Azotobacter inoculation on seed germination and yield of onion (Allium cepa). J. Agric. Res. 2002, 23, 297–299. [Google Scholar]
- Kurrey, D.K.; Lahre, M.K.; Pagire, G.S. Effect of Azotobacter on growth and yield of onion (Allium cepa L.). J. Pharmacogn. Phytochem. 2018, 7, 1171–1175. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranpiration Guidelines for Computing Crop Water Requirements. Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998. [Google Scholar]
- Cuenca, R. HIrrigation System Design: An Engineering Approach; Prentice Hall, Inc.: Englewood Cliffs, NJ, USA, 1989; p. 552. [Google Scholar]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; FAO Irrigation and Drainage Paper, No. 29; FAO Irrigation and Drainage: Roma, Italy, 1985. [Google Scholar]
- Pequerul, A.; Perez, C.; Madero, P.; Val, J.; Monge, E. A rapid wet digestion method for plant analysis. In Optimization of Plant Nutrition. Developments in Plant and Soil Sciences; Fragoso, M.A.C., Van Beusichem, M.L., Houwers, A., Eds.; Springer: Dordrecht, The Netherlands, 1993; Volume 53. [Google Scholar] [CrossRef]
- Adams, M.A.; Richter, A.; Hill, A.K.; Colmer, T.C. Salt tolerance in Eucalyptus sp.: Identity and response of putative osmolytes. Plant Cell Environ. 2005, 28, 772–787. [Google Scholar] [CrossRef]
- Kijne, J.W.; Barker, R.; Molden, D. Improving Water Productivity in Agriculture: Editor’s Overview. In Water Productivity in Agriculture: Limits and Opportunities for Improvement; Kijne, J.W., Barker, R.M.D., Eds.; International Water Management Institute: Colombo, Sri Lanka, 2003; pp. xi–xix. [Google Scholar]
- Helweg, O.J. Functions of crop yield from applied water. Agron. J. 1991, 83, 769–773. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using IBM SPSS Statistics, 5th ed.; SAGE Publications: Newbury Park, CA, USA, 2018. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics: A Biometrical Approach, 2nd ed.; McGraw-Hill: New York, NY, USA, 1980. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Alomran, M.; Louki, I.I.; Aly, A.A.; Nadeem, M.E. Impact of Deficit Irrigation on Soil Salinity and Cucumber Yield under greenhouse condition in arid environment. J. Agric. Sci. Technol. 2013, 15, 1247–1259. [Google Scholar]
- Harmanto, V.; Babel, M.S.; Tantau, H.J. Water Requirement of Drip Irrigated Tomatoes Grown in Greenhouse in Tropical Environment. Agric. Water Manag. 2004, 71, 225–242. [Google Scholar]
- Kumar, A.; Verma, H.; Singh, V.K.; Singh, P.; Singh, S.; Ansari, W.; Yadav, A.; Singh, P.K.; Pandey, K. Role of Pseudomonas sp. in Sustainable Agriculture and Disease Management; Springer: Berlin, Germany, 2017; pp. 195–215. [Google Scholar] [CrossRef]
- Mao, X.; Liu, M.; Wang, X.; Hou, C.Z.; Shi, J. Effects of Deficit Irrigation on Yield and Water Use of Greenhouse Grown Cucumber in the North China Plain. Agric. Water Manag. 2003, 61, 219–228. [Google Scholar] [CrossRef]
- Aly, R.; El-Gazzar, N.; Abdel-Rahman, H. Screening of Quinoa Germplasms under High-SAR Saline Water Irrigation in Arid Regions. Agric. Water Manag. 2024, 275, 108041. [Google Scholar]
- Elhindi, K.M.; El-Din, A.S.; Elgorban, A.M. Quinoa Response to Application of Phosphogypsum and PGPR under Water Stress Associated with Salt-Affected Soil. Plants 2022, 11, 872. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Abdelgawad, G.; Hegazi, A.M. Effects of Irrigation Water Salinity on Yield and Physiological Performance of Quinoa in Egypt. Maghreb J. Agric. Sci. 2021, 8, 97–105. [Google Scholar]
- Sumbul, A.; Ansari, R.A.; Rizvi, R.; Mahmood, I. Azotobacter: A potential bio-fertilizer for soil and plant health management. Saudi J. Biol. Sci. 2020, 27, 3634–3640. [Google Scholar] [CrossRef]
- Coniglio, A.; Mora, V.; Puente, M.; Cassán, F. Azospirillum as Biofertilizer for Sustainable Agriculture: Azospirillum brasilense AZ39 as a Model of PGPR and Field Traceability. In Microbial Probiotics for Agricultural Systems: Advances in Agronomic Use; Zúñiga-Dávila, D., González-Andrés, F., Ormeño-Orrillo, E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 45–70. [Google Scholar]
- Oweis, T.; Hachum, A. Water Harvesting and Supplemental Irrigation for Improved Water Productivity of Dry Farming System in West Asia and North Africa. In Proceedings of the 4th International Crop Science Congress, Held at Brisbane, on the Theme “Crop Science for Diversified Planet”, Brisbane, Australia, 26 September–1 October 2004. [Google Scholar]
- Zhang, Y.; Kendy, E.; Yu, Q.; Liu, C.; Shen, Y.; Sun, H. Effect of Soil Water Deficit on Evapotranspiration, Crop Yield, and Water Use Efficiency in the North China Plain. Agric. Water Manag. 2004, 64, 107–122. [Google Scholar] [CrossRef]
- Geerts, S.; Raes, D. Deficit Irrigation as an On-farm Strategy to Maximize Crop Water Productivity in Dry Areas. Agric. Water Manag. 2009, 96, 1275–1284. [Google Scholar] [CrossRef]
- Steduto, P.; Albrizio, R. Resource Use Efficiency of Field-grown Sunflower, Sorghum, Wheat and Chickpea. II. Water Use Efficiency and Comparison with Radiation Use Efficiency. Agric. Forest Meteorol. 2005, 130, 269–281. [Google Scholar] [CrossRef]
- Zhang, H.; Oweis, T. Water-yield Relations and Optimal Irrigation Scheduling of Wheat in the Mediterranean Region. Agric. Water Manag. 1999, 38, 195–211. [Google Scholar] [CrossRef]
- Al-Harbi, A.R.; Al-Omran, A.M.; El-Adgham, F.I. Effect of Drip Irrigation Levels and Emitters Depth on (Abelmoschus esculentus) Growth. J. Appl. Sci. 2008, 8, 2764–2769. [Google Scholar] [CrossRef][Green Version]
| Main Factor | Percentage of GWR | Inoculation Status | Abbreviations |
|---|---|---|---|
| Fresh water | 100% of GWR | With inoculation | AIF |
| Without | AUF | ||
| 80% of GWR | With inoculation | BIF | |
| Without | BUF | ||
| 60% of GWR | With inoculation | CIF | |
| Without | CUF | ||
| 40% of GWR | With inoculation | DIF | |
| Without | DUF | ||
| Saline water | 100% of GWR | With inoculation | AIS |
| Without | AUS | ||
| 80% of GWR | With inoculation | BIS | |
| Without | BUS | ||
| 60% of GWR | With inoculation | CIS | |
| Without | CUS | ||
| 40% of GWR | With inoculation | DIS | |
| Without | DUS |
| Treatments | Cd | Co | Cr | Cu | Fe | Mn | Ni | Pb | Zn | P | Ca | Mg | Na | K | Cl | K/Na |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/100 g Plant | g/kg | % | Ratio | |||||||||||||
| AUF | 1.31 d * | ND | 0.31 c | 0.17 c | 11.9 a | 4.2 c | ND | ND | 1.6 c | 3.4 e | 15.15 c | 4.75 c | 1.8 e | 2.3 e | 7.3 c | 1.28 b |
| DUF | 2.11 c | ND | 0.39 b | 0.09 d | 10.1 b | 4.1 d | ND | ND | 0.7 d | 2.2 f | 16.61 b | 3.90 e | 2.4 c | 2.6 b | 9.4 b | 1.08 d |
| AIF | 1.34 d | ND | 0.29 c | 0.18 c | 12.0 a | 4.7 c | ND | ND | 1.8 b | 3.5 c | 18.91 a | 4.77 c | 1.6 e | 2.8 b | 6.4 e | 1.75 a |
| DIF | 2.21 c | ND | 0.41 b | 0.19 c | 10.2 b | 4.2 d | ND | ND | 0.9 d | 2.3 f | 19.02 a | 4.55 c | 2.1 c | 2.4 e | 9.1 b | 1.14 c |
| AUS | 3.3 b | ND | 0.51 a | 0.27 b | 9.6 c | 7.1 b | ND | ND | 2.1 b | 3.6 c | 11.02 e | 5.55 b | 3.1 b | 3.2 a | 7.8 c | 1.03 e |
| DUS | 3.9 a | ND | 0.42 b | 0.28 b | 8.1 e | 9.2 a | ND | ND | 1.8 b | 3.8 b | 12.03 d | 4.70 c | 3.62 a | 3.61 a | 11.0 a | 1.00 f |
| AIS | 3.1 b | ND | 0.53 a | 0.39 a | 9.8 c | 7.7 b | ND | ND | 2.5 a | 4.6 a | 17.14 b | 6.40 a | 2.6 c | 2.9 b | 7.3 c | 1.12 c |
| DIS | 3.3 b | ND | 0.41 b | 0.41 a | 8.5 e | 9.5 a | ND | ND | 1.9 b | 3.9 b | 15.01 c | 4.81 c | 3.2 b | 3.5 a | 9.2 b | 1.09 d |
| Treatment | Crop Water Production Function | R2 | Maximum Yield (kg/Plant) | Applied Water (m3) |
|---|---|---|---|---|
| Fresh irrigation water (Inoculated) | Y (Inoculated) = −334.14(AW)2 + 7.6478(AW) − 0.0297 | 0.996 | 0.0141 | 0.0114 |
| Fresh irrigation water (Non-inoculated) | Y (Non-inoculated) = −470.23(AW)2 + 9.9129(AW) − 0.0379 | 0.998 | 0.0143 | 0.0105 |
| Saline irrigation water (Inoculated) | Y (Inoculated) = −72.966(AW)2 + 2.3058(AW) − 0.0058 | 0.9992 | 0.0124 | 0.0158 |
| Saline irrigation water (Non-inoculated) | Y (Non-inoculated) = −218.9(AW)2 + 4.3898(AW) − 0.0146 | 0.8938 | 0.0074 | 0.0100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Barakaha, F.N.; Alghamdi, A.G. Plant-Growth-Promoting Rhizobacteria as a Sustainable Strategy for Enhancing Quinoa Resilience to Salt Stress in Arid Regions. Sustainability 2025, 17, 9048. https://doi.org/10.3390/su17209048
Al-Barakaha FN, Alghamdi AG. Plant-Growth-Promoting Rhizobacteria as a Sustainable Strategy for Enhancing Quinoa Resilience to Salt Stress in Arid Regions. Sustainability. 2025; 17(20):9048. https://doi.org/10.3390/su17209048
Chicago/Turabian StyleAl-Barakaha, Fahad N., and Abdulaziz G. Alghamdi. 2025. "Plant-Growth-Promoting Rhizobacteria as a Sustainable Strategy for Enhancing Quinoa Resilience to Salt Stress in Arid Regions" Sustainability 17, no. 20: 9048. https://doi.org/10.3390/su17209048
APA StyleAl-Barakaha, F. N., & Alghamdi, A. G. (2025). Plant-Growth-Promoting Rhizobacteria as a Sustainable Strategy for Enhancing Quinoa Resilience to Salt Stress in Arid Regions. Sustainability, 17(20), 9048. https://doi.org/10.3390/su17209048





