Inhibition Mechanism of Calcium Hydroxide on Arsenic Volatilization During Sintering of Contaminated Excavated Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Sample Preparation
2.3. Characterization Methods
2.3.1. Compression Strength Test
2.3.2. ICP-MS
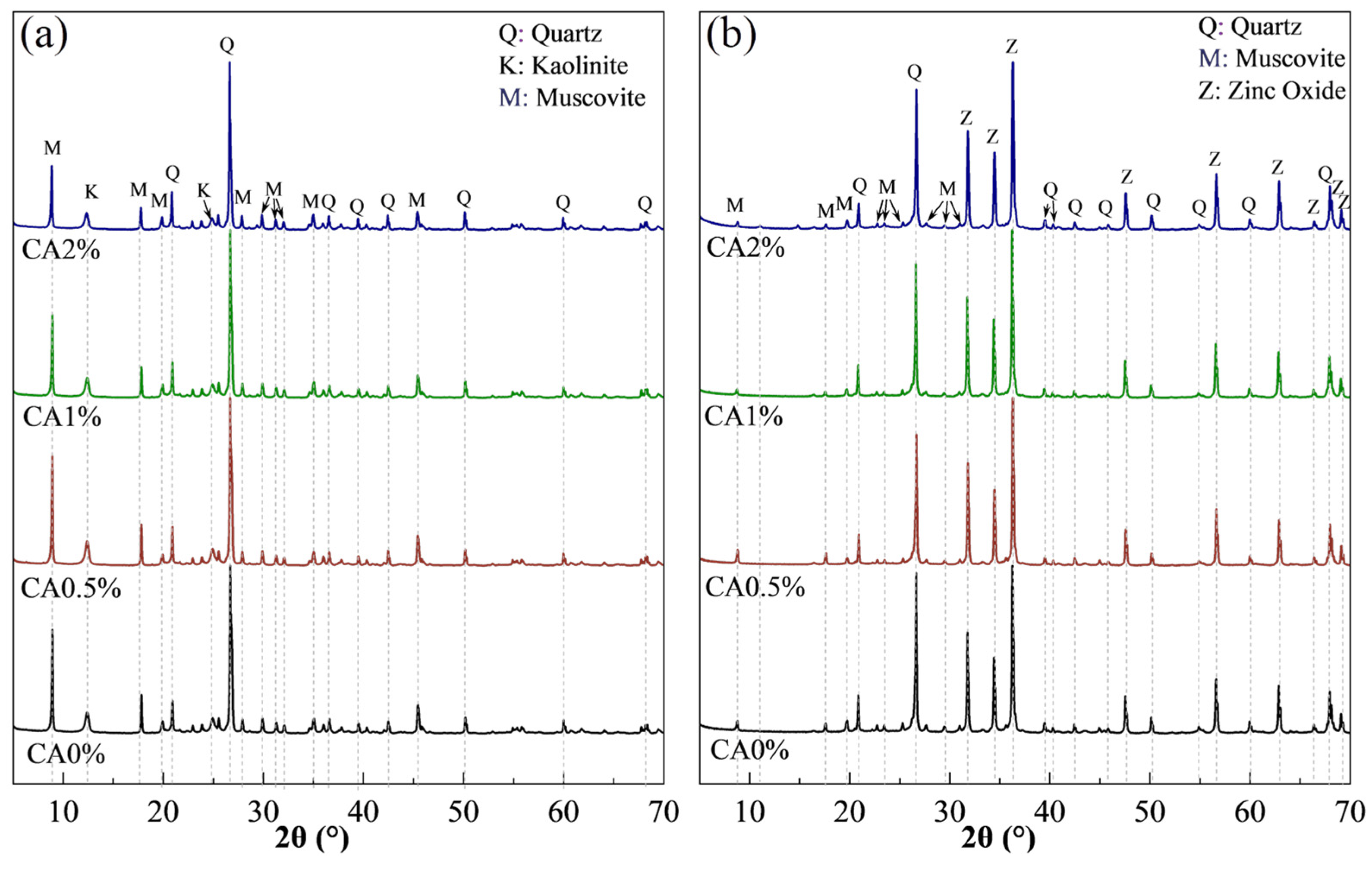
2.3.3. X-Ray Diffraction
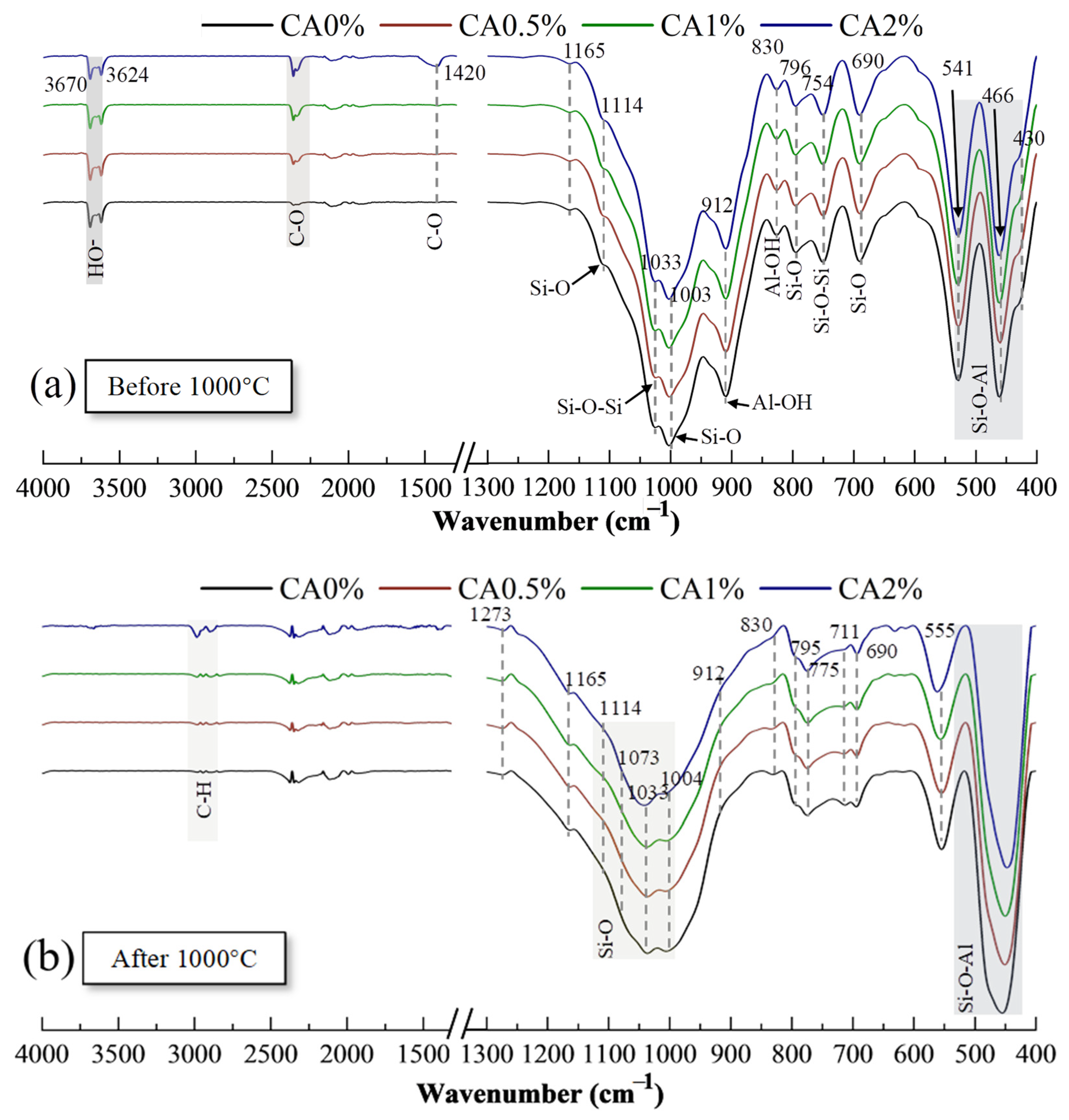
2.3.4. Fourier Transform Infrared Spectroscopy
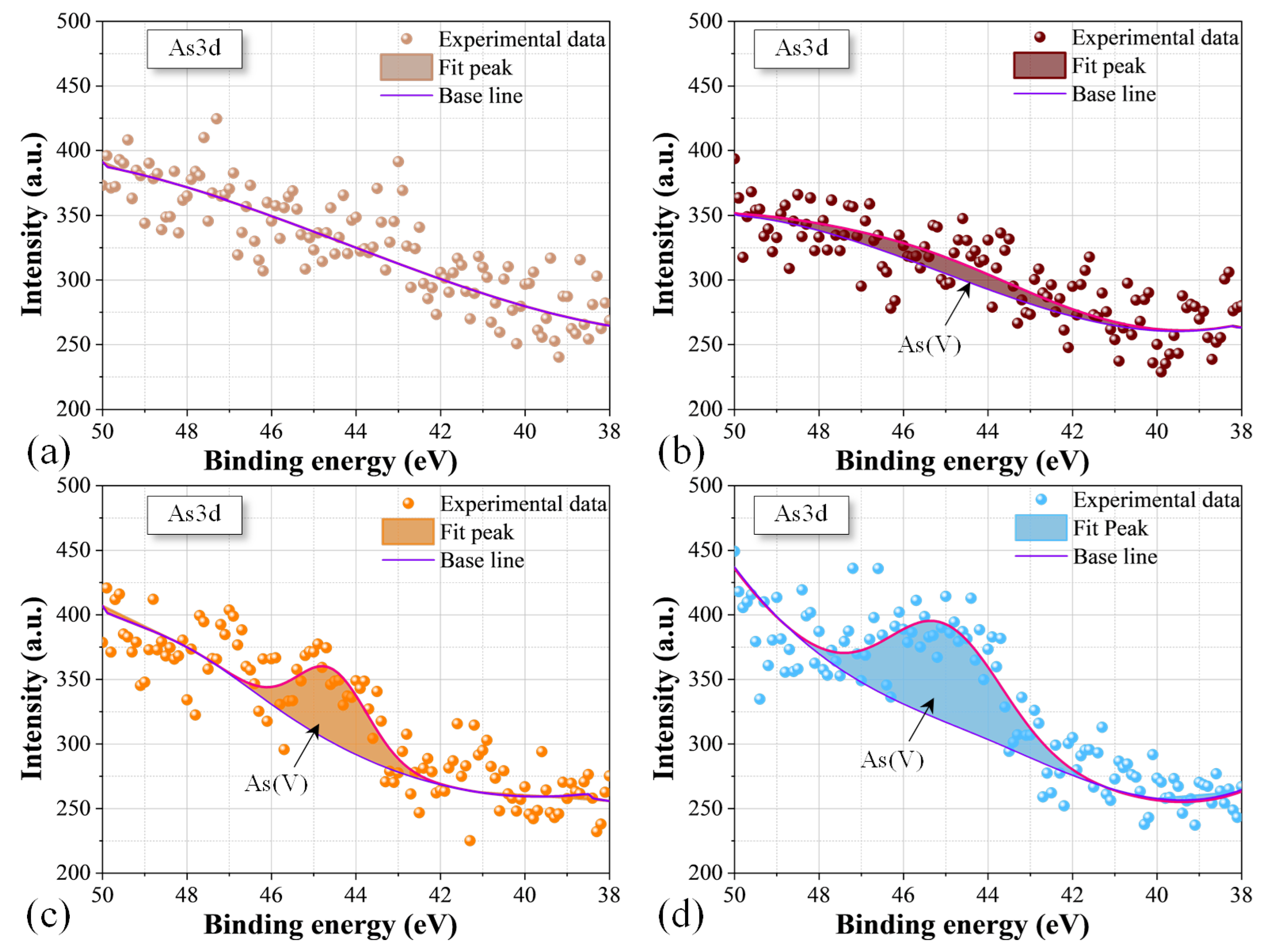
2.3.5. X-Ray Photoelectron Spectroscopy
2.3.6. Scanning Electron Microscope
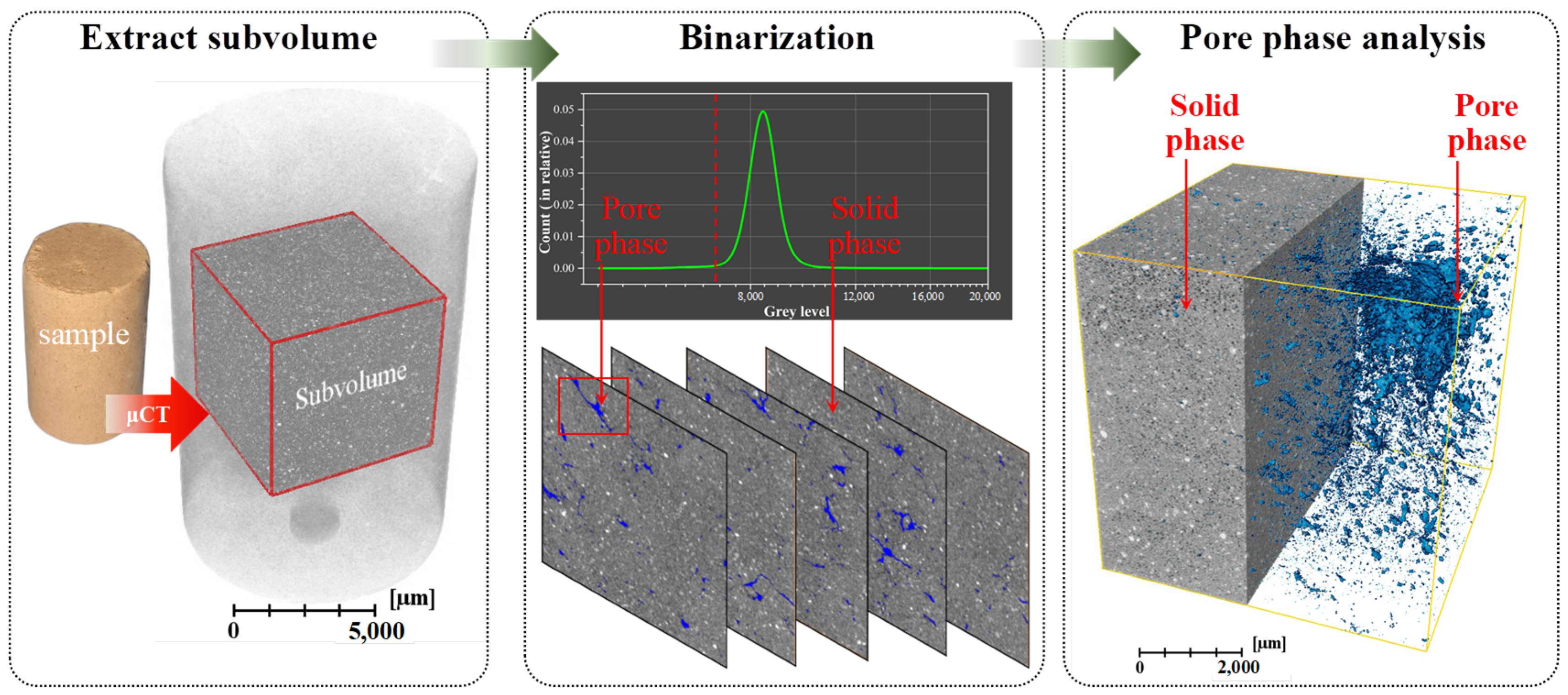
2.3.7. X-Ray Microtomography (μCT)
3. Results
3.1. Compressive Strength of Sintered Excavated Soils
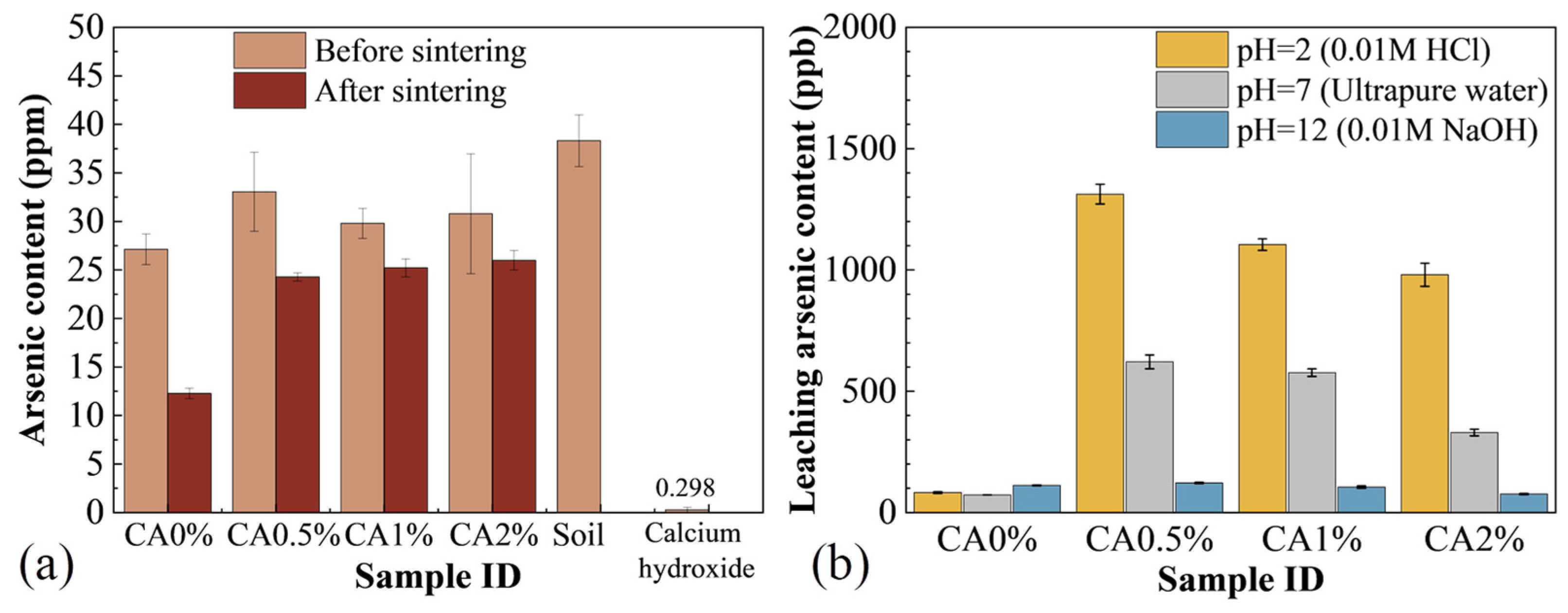
3.2. Arsenic Behavior Analysis
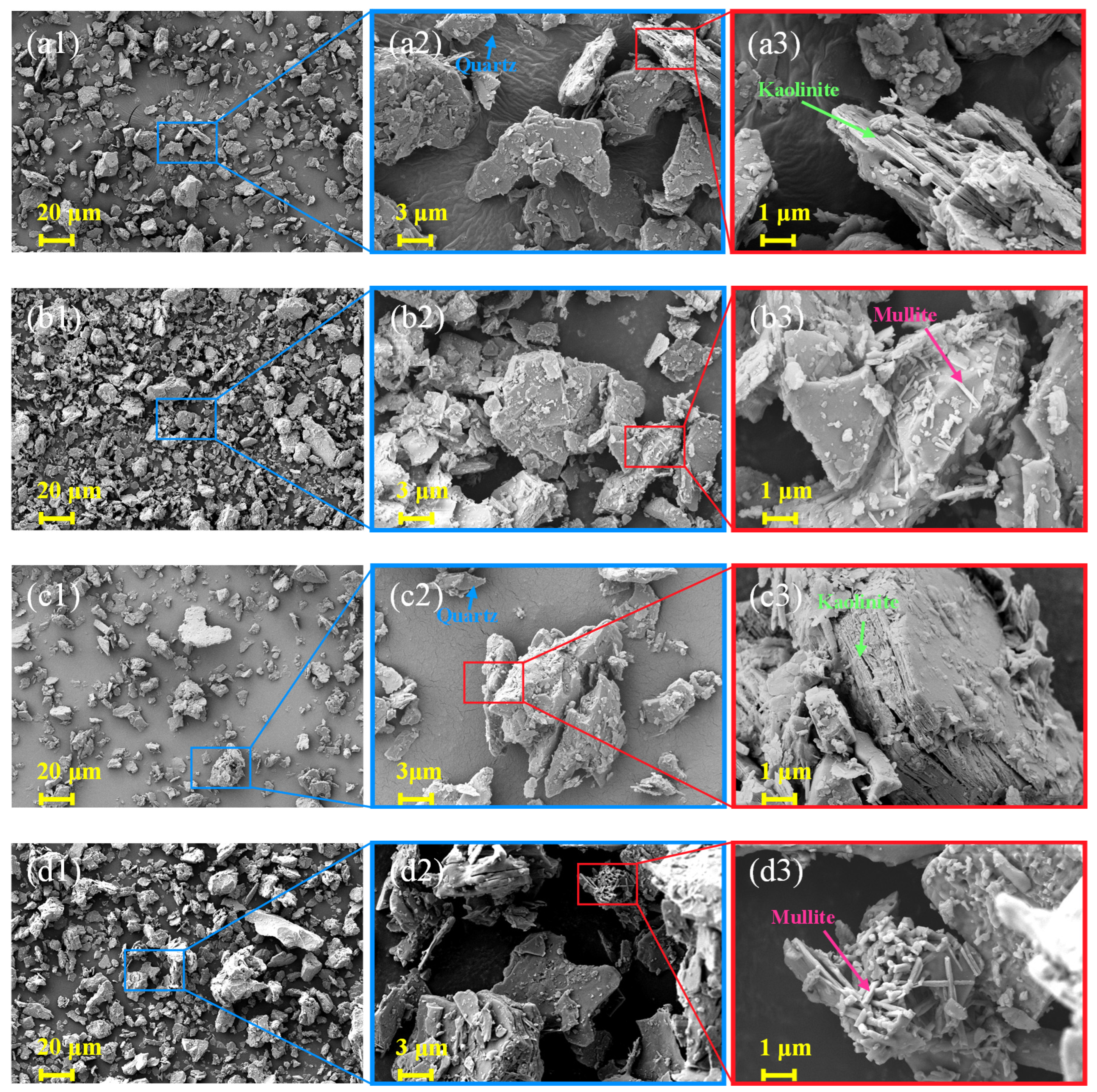
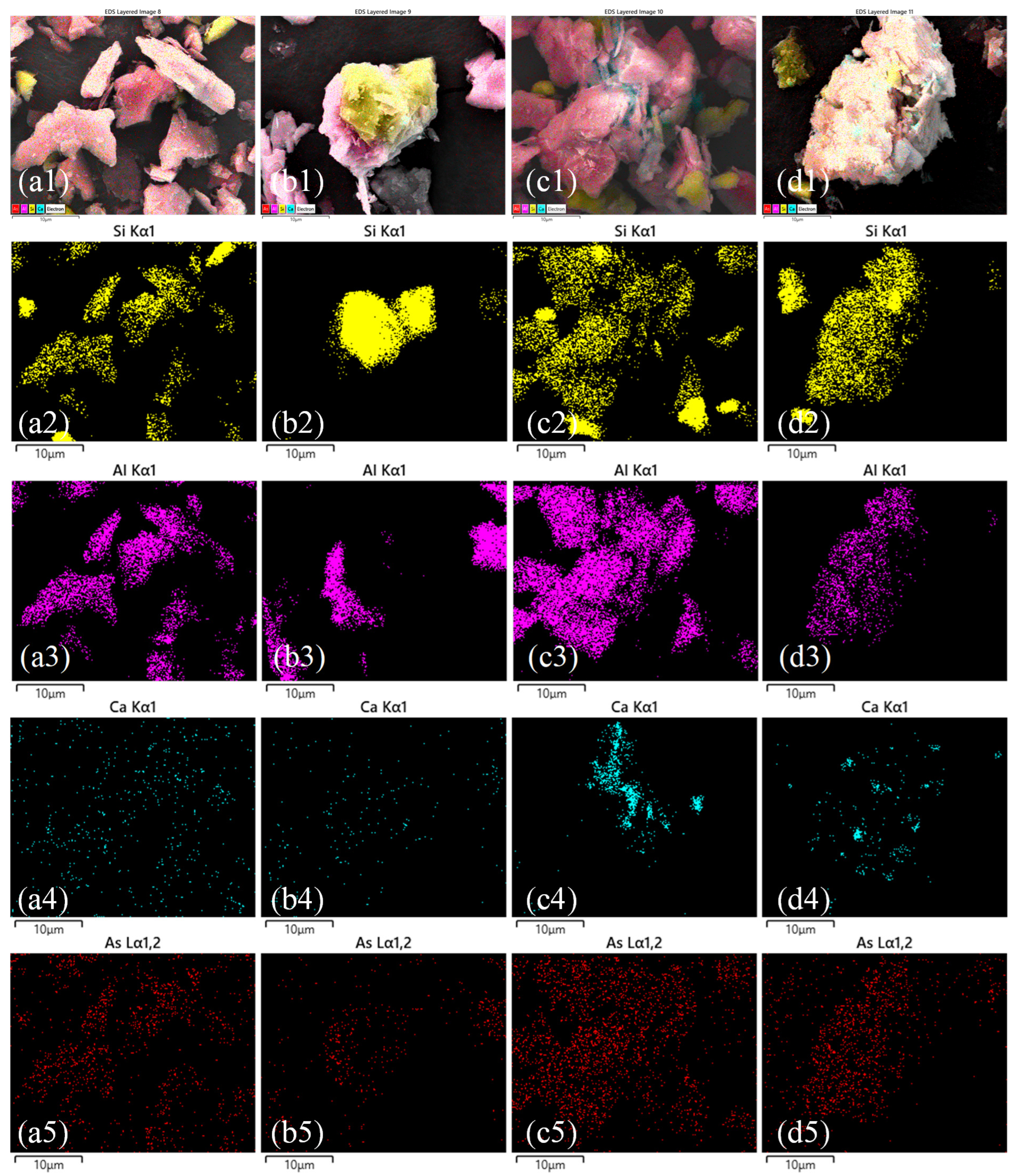
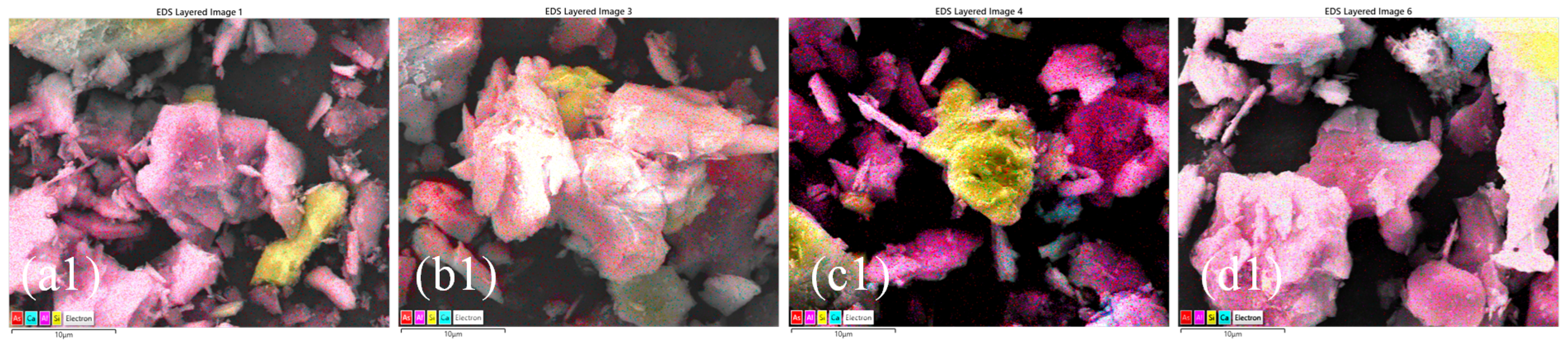
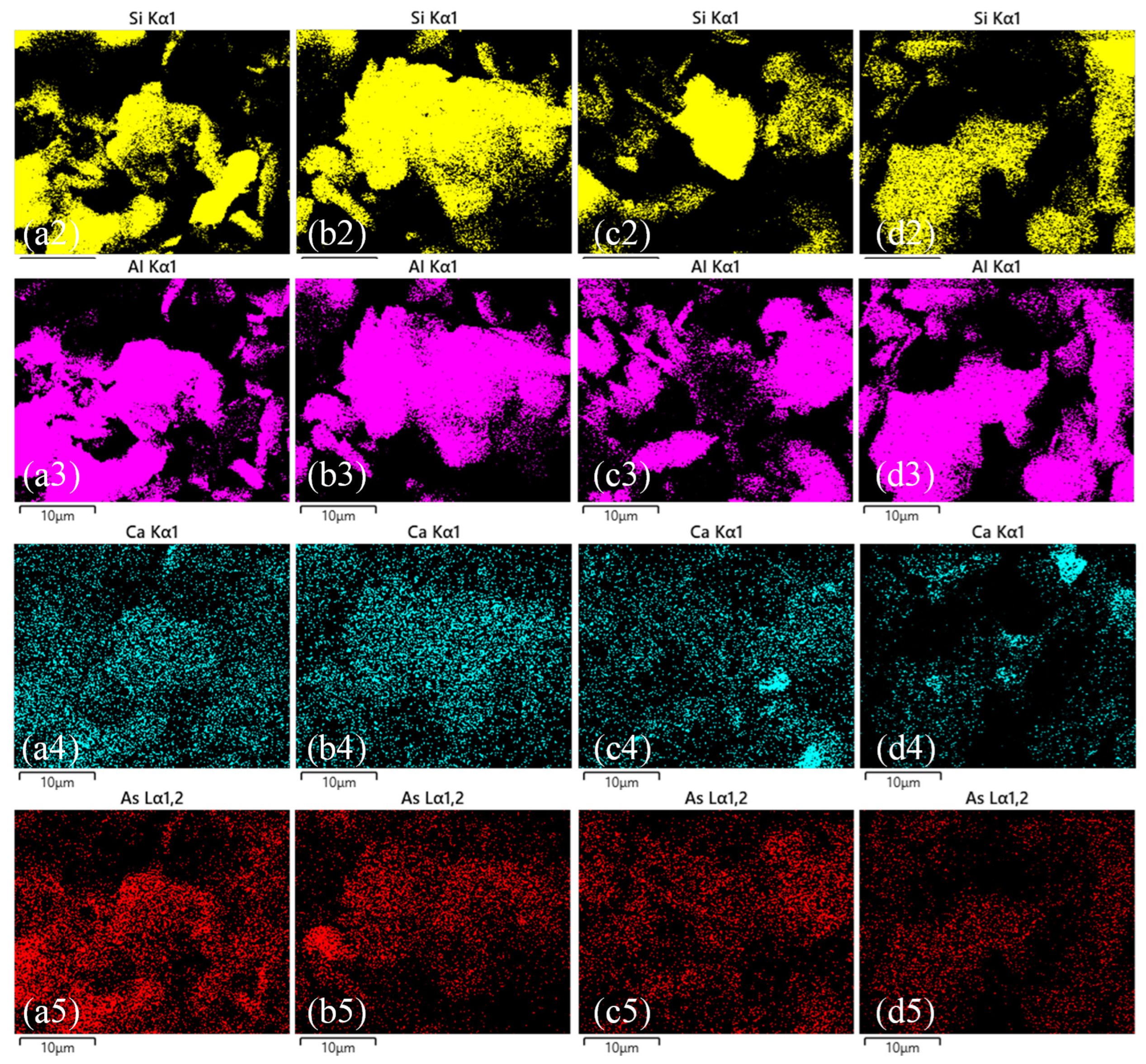
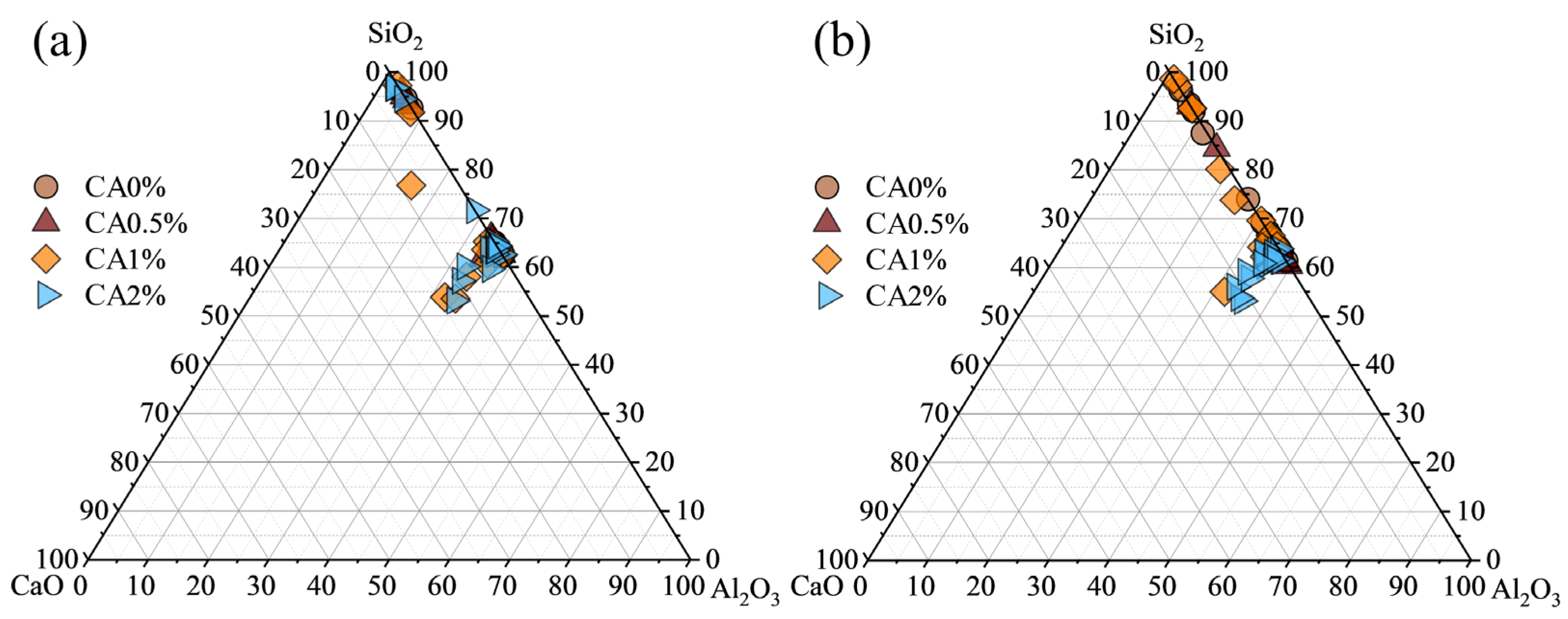
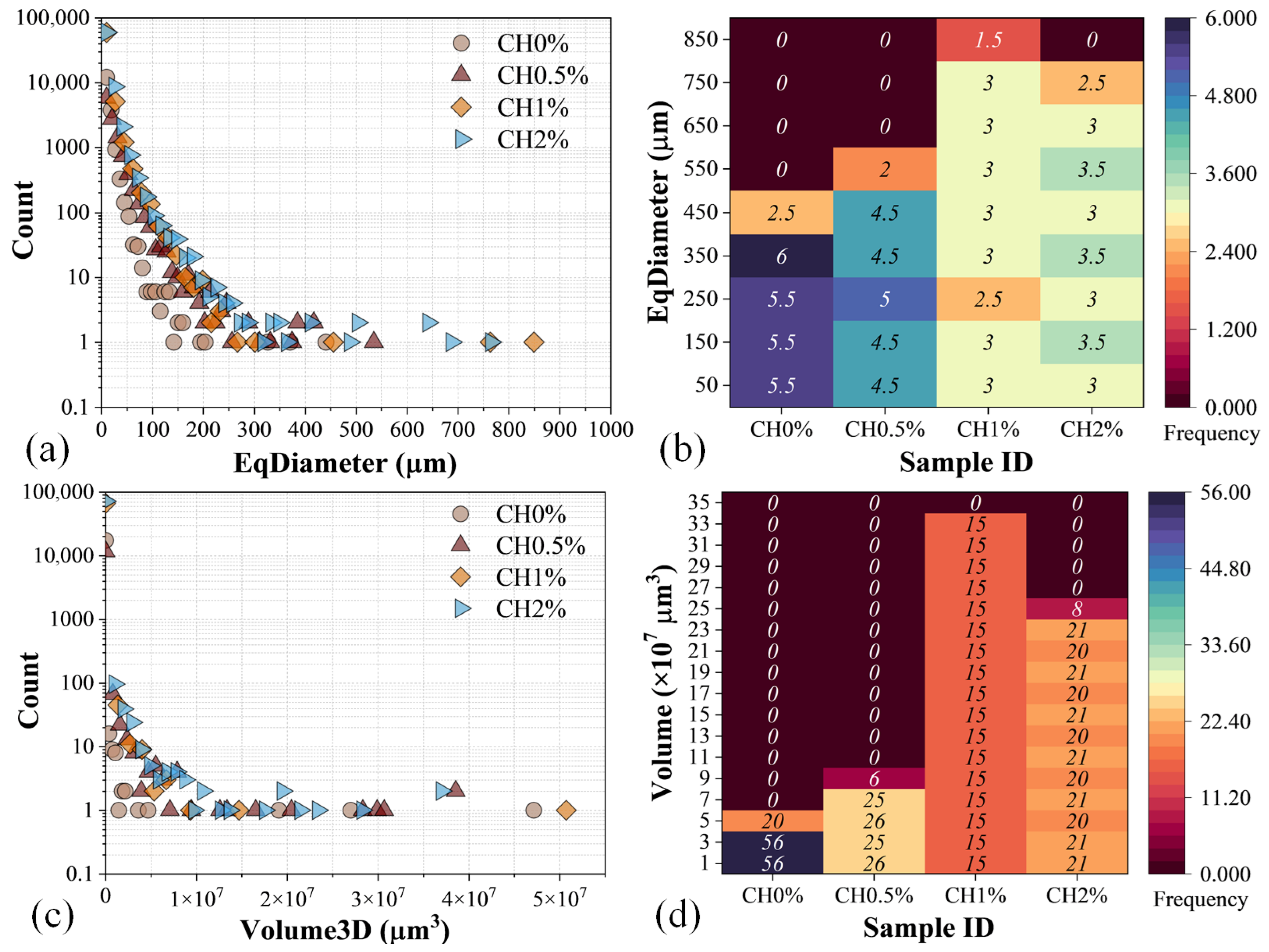
3.3. Microstructural and Elemental Analysis
3.3.1. SEM-EDS
3.3.2. μCT
3.4. Phase Composition and Chemical Speciation Analysis
3.4.1. X-Ray Diffraction
3.4.2. Fourier Transform Infrared Spectroscopy
3.4.3. X-Ray Photoelectron Spectroscopy
4. Discussion
5. Conclusions
- (1)
- Ca(OH)2 addition significantly reduced arsenic volatilization during sintering, with CA0.5% samples retaining 81% of the initial arsenic content (38.3 ppm) compared to only 51.8% in untreated (CA0%) samples. This stabilization occurs through the formation of thermally stable calcium arsenate (Ca3(AsO4)2) as confirmed by XPS analysis showing As(V) retention in CA1% and CA2% samples.
- (2)
- Ca(OH)2 acted both chemically, by forming stable As-bearing compounds, and physically, by promoting liquid-phase sintering that encapsulates arsenic. The decomposition of Ca(OH)2 into CaO enhances densification and reduces As mobility, while the alkaline environment further suppresses leaching, maintaining As concentrations below 110 ppb in all Ca(OH)2-modified samples under alkaline conditions.
- (3)
- An optimal Ca(OH)2 dosage of 0.5% achieves the best balance between arsenic retention and mechanical performance. This formulation yields a compressive strength of 9 MPa (29% higher than CA0%) while minimizing pore connectivity. Higher Ca(OH)2 contents lead to increased porosity and reduced strength, despite continued As immobilization benefits, indicating that excessive Ca(OH)2 addition can compromise structural properties while providing diminishing returns for As stabilization.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahman, S.; Rahman, I.M.M.; Hasegawa, H. Management of Arsenic-Contaminated Excavated Soils: A Review. J. Environ. Manag. 2023, 346, 118943. [Google Scholar] [CrossRef]
- Kishii, T. Utilization of Underground Space in Japan. Tunn. Undergr. Space Technol. 2016, 55, 320–323. [Google Scholar] [CrossRef]
- Huang, T.; Kou, S.; Liu, D.; Li, D.; Xing, F. Evaluation of the Techno-Economic Feasibility for Excavated Soil Recycling in Shenzhen, China. Sustainability 2022, 14, 3028. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Igarashi, T.; Villacorte-Tabelin, M.; Park, I.; Opiso, E.M.; Ito, M.; Hiroyoshi, N. Arsenic, Selenium, Boron, Lead, Cadmium, Copper, and Zinc in Naturally Contaminated Rocks: A Review of Their Sources, Modes of Enrichment, Mechanisms of Release, and Mitigation Strategies. Sci. Total Environ. 2018, 645, 1522–1553. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Wang, X.; Kua, H.; Geng, Y.; Bleischwitz, R.; Ren, J. Construction and Demolition Waste Management in China through the 3R Principle. Resour. Conserv. Recycl. 2018, 129, 36–44. [Google Scholar] [CrossRef]
- Lu, W.; Webster, C.; Chen, K.; Zhang, X.; Chen, X. Computational Building Information Modelling for Construction Waste Management: Moving from Rhetoric to Reality. Renew. Sustain. Energy Rev. 2017, 68, 587–595. [Google Scholar] [CrossRef]
- Yu, B.; Wang, J.; Wu, H.; Wong, A.B.; Liao, Y.; Zuo, J. Self-Fulfillment Degree of Construction and Demolition Waste Management Capability Based on the Triple-Balance Theory: A Case Study of Guangdong-Hong Kong-Macao Greater Bay Area. Waste Manag. 2021, 133, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Niu, L.; Liu, K.; Yin, S.; Liu, W. Arsenic in Agricultural Soils across China: Distribution Pattern, Accumulation Trend, Influencing Factors, and Risk Assessment. Sci. Total Environ. 2018, 616–617, 156–163. [Google Scholar] [CrossRef]
- Hettick, B.E.; Cañas-Carrell, J.E.; French, A.D.; Klein, D.M. Arsenic: A Review of the Element’s Toxicity, Plant Interactions, and Potential Methods of Remediation. J. Agric. Food Chem. 2015, 63, 7097–7107. [Google Scholar] [CrossRef]
- Mandal, B.K.; Suzuki, K.T. Arsenic Round the World: A Review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Li, J.; Kosugi, T.; Riya, S.; Hashimoto, Y.; Hou, H.; Terada, A.; Hosomi, M. Use of Batch Leaching Tests to Quantify Arsenic Release from Excavated Urban Soils with Relatively Low Levels of Arsenic. J. Soils Sediments 2017, 17, 2136–2143. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A Review of the Source, Behaviour and Distribution of Arsenic in Natural Waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Li, Y.; Yi, P.; Du, H.; Liu, W.; Mi, T.; Huang, L.; Gao, X.; Sun, X.; Xing, F. Activation of Locally Excavated Spoil for Utilization in Limestone Calcined Clay Cement (LC3). Constr. Build. Mater. 2024, 420, 135518. [Google Scholar] [CrossRef]
- Haas, M.; Galler, R.; Scibile, L.; Benedikt, M. Waste or Valuable Resource—A Critical European Review on Re-Using and Managing Tunnel Excavation Material. Resour. Conserv. Recycl. 2020, 162, 105048. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Zheng, W.; Cui, T.; Yang, Y. Study on the Properties and Process Parameters of Different Clays in Disc Granulation. Materials 2020, 13, 1714. [Google Scholar] [CrossRef]
- Li, H.; Yang, Y.; Zheng, W.; Chen, L.; Bai, Y. Immobilization of High Concentration Hexavalent Chromium via Core-Shell Structured Lightweight Aggregate: A Promising Soil Remediation Strategy. Chem. Eng. J. 2020, 401, 126044. [Google Scholar] [CrossRef]
- Gao, W.; Jian, S.; Li, B.; Li, X.; Tan, H.; Huang, W.; Yang, X. Solidification of Zinc in Lightweight Aggregate Produced from Contaminated Soil. J. Clean. Prod. 2020, 265, 121784. [Google Scholar] [CrossRef]
- Martin, R.T.; Bailey, S.W.; Eberl, D.D.; Fanning, D.S.; Guggenheim, S.; Kodama, H.; Pevear, D.R.; Środoń, J.; Wicks, F.J. Report of the Clay Minerals Society Nomenclature Committee: Revised Classification of Clay Materials. Clays Clay Miner. 1991, 39, 333–335. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, Q.; Yang, J.; Ma, S.; Frost, R.L. The Thermal Behavior of Kaolinite Intercalation Complexes-A Review. Thermochim. Acta 2012, 545, 1–13. [Google Scholar] [CrossRef]
- Ghorbel, A.; Fourati, M.; Bouaziz, J. Microstructural Evolution and Phase Transformation of Different Sintered Kaolins Powder Compacts. Mater. Chem. Phys. 2008, 112, 876–885. [Google Scholar] [CrossRef]
- Wang, S.; Gainey, L.; Mackinnon, I.D.R.; Allen, C.; Gu, Y.; Xi, Y. Thermal Behaviors of Clay Minerals as Key Components and Additives for Fired Brick Properties: A Review. J. Build. Eng. 2023, 66, 105802. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.; Zhang, Y.; Zou, C.; Anthony, E.J. Review of Arsenic Behavior during Coal Combustion: Volatilization, Transformation, Emission and Removal Technologies. Prog. Energy Combust. Sci. 2018, 68, 1–28. [Google Scholar] [CrossRef]
- Lim, K.T.; Shukor, M.Y.; Wasoh, H. Physical, Chemical, and Biological Methods for the Removal of Arsenic Compounds. BioMed Res. Int. 2014, 2014, 503784. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; Luch, A., Ed.; Experientia Supplementum; Springer: Basel, Switzerland, 2012; Volume 101, pp. 133–164. ISBN 978-3-7643-8339-8. [Google Scholar]
- Clarke, L.B. The Fate of Trace Elements during Coal Combustion and Gasification: An Overview. Fuel 1993, 72, 731–736. [Google Scholar] [CrossRef]
- Lu, H.; Chen, H.; Li, W.; Li, B. Transformation of Arsenic in Yima Coal during Fluidized-Bed Pyrolysis. Fuel 2004, 83, 645–650. [Google Scholar] [CrossRef]
- Liu, H.; Pan, W.-P.; Wang, C.; Zhang, Y. Volatilization of Arsenic During Coal Combustion Based on Isothermal Thermogravimetric Analysis at 600–1500 °C. Energy Fuels 2016, 30, 6790–6798. [Google Scholar] [CrossRef]
- Tang, Q.; Liu, G.; Yan, Z.; Sun, R. Distribution and Fate of Environmentally Sensitive Elements (Arsenic, Mercury, Stibium and Selenium) in Coal-Fired Power Plants at Huainan, Anhui, China. Fuel 2012, 95, 334–339. [Google Scholar] [CrossRef]
- Sterling, R.; Helble, J. Reaction of Arsenic Vapor Species with Fly Ash Compounds: Kinetics and Speciation of the Reaction with Calcium Silicates. Chemosphere 2003, 51, 1111–1119. [Google Scholar] [CrossRef]
- Jadhav, R.A.; Fan, L.S. Capture of Gas-Phase Arsenic Oxide by Lime: Kinetic and Mechanistic Studies. Environ. Sci. Technol. 2001, 35, 794–799. [Google Scholar] [CrossRef]
- Mahuli, S.; Agnihotri, R.; Chauk, S.; Ghosh-Dastidar, A.; Fan, L.-S. Mechanism of Arsenic Sorption by Hydrated Lime. Environ. Sci. Technol. 1997, 31, 3226–3231. [Google Scholar] [CrossRef]
- Wang, J.; Tomita, A. A Chemistry on the Volatility of Some Trace Elements during Coal Combustion and Pyrolysis. Energy Fuels 2003, 17, 954–960. [Google Scholar] [CrossRef]
- Li, Y.; Tang, J.X.; Zhang, T.; Lian, M.H.; Tian, X.Z. Study on Chemical Stabilization in Arsenic Contaminated Soil: A Review. In Progress in Environmental Protection and Processing of Resource; Trans Tech Publications Ltd.: Baech, Switzerland, April 2013; Volume 295, pp. 1089–1092. [Google Scholar]
- Tsang, D.C.W.; Yip, A.C.K.; Olds, W.E.; Weber, P.A. Arsenic and Copper Stabilisation in a Contaminated Soil by Coal Fly Ash and Green Waste Compost. Environ. Sci. Pollut. Res. Int. 2014, 21, 10194–10204. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.-H.; Moon, D.H.; Kim, K.-W.; Lee, K.-Y.; Lee, J.-H.; Kim, M.G. Mechanism for the Stabilization/Solidification of Arsenic-Contaminated Soils with Portland Cement and Cement Kiln Dust. J. Environ. Manag. 2010, 91, 2322–2328. [Google Scholar] [CrossRef]
- Kundu, S.; Gupta, A.K. Immobilization and Leaching Characteristics of Arsenic from Cement and/or Lime Solidified/Stabilized Spent Adsorbent Containing Arsenic. J. Hazard. Mater. 2008, 153, 434–443. [Google Scholar] [CrossRef]
- Coussy, S.; Paktunc, D.; Rose, J.; Benzaazoua, M. Arsenic Speciation in Cemented Paste Backfills and Synthetic Calcium–Silicate–Hydrates. Miner. Eng. 2012, 39, 51–61. [Google Scholar] [CrossRef]
- Hassan, K.M.; Fukushi, K.; Turikuzzaman, K.; Moniruzzaman, S.M. Effects of Using Arsenic–Iron Sludge Wastes in Brick Making. Waste Manag. 2014, 34, 1072–1078. [Google Scholar] [CrossRef]
- Liu, D.-G.; Min, X.-B.; Ke, Y.; Chai, L.-Y.; Liang, Y.; Li, Y.-C.; Yao, L.-W.; Wang, Z.-B. Co-Treatment of Flotation Waste, Neutralization Sludge, and Arsenic-Containing Gypsum Sludge from Copper Smelting: Solidification/Stabilization of Arsenic and Heavy Metals with Minimal Cement Clinker. Environ. Sci. Pollut. Res. 2018, 25, 7600–7607. [Google Scholar] [CrossRef]
- HJ 803-2016; Soil and Sediment-Determination of Aqua Regia Extracts of 12 Metal Elements-Inductively Coupled Plasma Mass Spectrometry. Chinese National Standard: Beijing, China, 2016.
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). Chinese National Standard: Beijing, China, 2021.
- Carmignato, S.; Dewulf, W.; Leach, R. (Eds.) Industrial X-Ray Computed Tomography; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-59571-9. [Google Scholar]
- Hubler, M.H.; Gelb, J.; Ulm, F.-J. Microtexture Analysis of Gas Shale by XRM Imaging. J. Nanomech. Micromech. 2017, 7, 04017005. [Google Scholar] [CrossRef]
- Bullard, J.W.; Hagedorn, J.; Ley, M.T.; Hu, Q.; Griffin, W.; Terrill, J.E. A Critical Comparison of 3D Experiments and Simulations of Tricalcium Silicate Hydration. J. Am. Ceram. Soc. 2018, 101, 1453–1470. [Google Scholar] [CrossRef] [PubMed]
- Provis, J.L.; Myers, R.J.; White, C.E.; Rose, V.; van Deventer, J.S.J. X-Ray Microtomography Shows Pore Structure and Tortuosity in Alkali-Activated Binders. Cem. Concr. Res. 2012, 42, 855–864. [Google Scholar] [CrossRef]
- Kaestner, A.; Lehmann, E.; Stampanoni, M. Imaging and Image Processing in Porous Media Research. Adv. Water Resour. 2008, 31, 1174–1187. [Google Scholar] [CrossRef]
- Otsu, N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Hamano, K.; Muroyama, K.; Nakagawa, Z.; Saito, K. Effects of Addition of Calcium Compounds on Sintering of Magnesia. J. Ceram. Assoc. Jpn. 1979, 87, 474–482. [Google Scholar] [CrossRef]
- Fashina, B.; Deng, Y. Stacking Disorder and Reactivity of Kaolinites. Clays Clay Min. 2021, 69, 354–365. [Google Scholar] [CrossRef]
- Sang, Y.; Zhao, J.R. Reduction of Water Absorption Capacity of Cellulose Fibres for Its Application in Cementitious Materials. J. Compos. Mater. 2015, 49, 2757–2763. [Google Scholar] [CrossRef]
- Sanya, O.T.; Owoeye, S.S.; Isinkaye, O.E.; Simon, O.T. Chemical, Phase and Structural Change of Mullite Synthesized during Sintering of Kaolin. Int. J. Appl. Ceram. Tech. 2020, 17, 2259–2264. [Google Scholar] [CrossRef]
- Ding, D.; Jiang, W.; Xiao, G.; Fang, Y.; Zhu, X.; Chong, X.; Jin, E.; Luo, J.; Lei, C. Effects of CaO Addition on the Properties and Microstructure of Low-Grade Bauxite-Based Lightweight Ceramic Proppant. J. Aust. Ceram. Soc. 2023, 59, 957–967. [Google Scholar] [CrossRef]
- Newbury, D.E.; Leapman, R.D. Detection Limits for Analytical Electron Microscopy with Electron Energy Loss Spectrometry and Energy-Dispersive x-Ray Spectrometry. Proc. Annu. Meet. Electron. Microsc. Soc. Am. 1993, 51, 594–595. [Google Scholar] [CrossRef]
- Feng, W.; Jin, Y.; Zheng, D.; Fang, Y.; Dong, Z.; Cui, H. Study of Triethanolamine on Regulating Early Strength of Fly Ash-Based Chemically Foamed Geopolymer. Cem. Concr. Res. 2022, 162, 107005. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H. The Pore Characteristics of Geopolymer Foam Concrete and Their Impact on the Compressive Strength and Modulus. Front. Mater. 2016, 3, 38. [Google Scholar] [CrossRef]
- Deiters, M.; Rozanski, C.; Schack, T.; Haist, M. Influence of Calcined Clay on Micromechanical Properties and Creep of Hardened Cement Paste. ce papers 2023, 6, 431–440. [Google Scholar] [CrossRef]
- Chen, Y.; Furmann, A.; Mastalerz, M.; Schimmelmann, A. Quantitative Analysis of Shales by KBr-FTIR and Micro-FTIR. Fuel 2014, 116, 538–549. [Google Scholar] [CrossRef]
- Ho, G.D.; Tabelin, C.B.; Tangviroon, P.; Tamamura, S.; Igarashi, T. Effects of Cement Addition on Arsenic Leaching from Soils Excavated from Projects Employing Shield-Tunneling Method. Geoderma 2021, 385, 114896. [Google Scholar] [CrossRef]
- Amar, M.; Ladduri, B.; Alloul, A.; Benzerzour, M.; Abriak, N.-E. Microstructure and Mechanical Properties of Geopolymers Utilizing Excavated Soils, Metakaolin and Slags. J. Build. Eng. 2024, 86, 108755. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Sasaki, R.; Igarashi, T.; Park, I.; Tamoto, S.; Arima, T.; Ito, M.; Hiroyoshi, N. Simultaneous Leaching of Arsenite, Arsenate, Selenite and Selenate, and Their Migration in Tunnel-Excavated Sedimentary Rocks: I. Column Experiments under Intermittent and Unsaturated Flow. Chemosphere 2017, 186, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Andersen, F.A.; Brečević, L.; Beuter, G.; Dell’Amico, D.B.; Calderazzo, F.; Bjerrum, N.J.; Underhill, A.E. Infrared Spectra of Amorphous and Crystalline Calcium Carbonate. Acta Chem. Scand. 1991, 45, 1018–1024. [Google Scholar] [CrossRef]
- Castellano, M.; Turturro, A.; Riani, P.; Montanari, T.; Finocchio, E.; Ramis, G.; Busca, G. Bulk and Surface Properties of Commercial Kaolins. Appl. Clay Sci. 2010, 48, 446–454. [Google Scholar] [CrossRef]
- Vizcayno, C.; de Gutiérrez, R.M.; Castello, R.; Rodriguez, E.; Guerrero, C.E. Pozzolan Obtained by Mechanochemical and Thermal Treatments of Kaolin. Appl. Clay Sci. 2010, 49, 405–413. [Google Scholar] [CrossRef]
- González-García, D.M.; Téllez-Jurado, L.; Jiménez-Álvarez, F.J.; Zarazua-Villalobos, L.; Balmori-Ramírez, H. Evolution of a Natural Pozzolan-Based Geopolymer Alkalized in the Presence of Sodium or Potassium Silicate/Hydroxide Solution. Constr. Build. Mater. 2022, 321, 126305. [Google Scholar] [CrossRef]
- Cheng, S.; Ge, K.; Sun, T.; Shui, Z.; Chen, X.; Lu, J.-X. Pozzolanic Activity of Mechanochemically and Thermally Activated Coal-Series Kaolin in Cement-Based Materials. Constr. Build. Mater. 2021, 299, 123972. [Google Scholar] [CrossRef]
- Frost, R.L.; Makó, É.; Kristóf, J.; Kloprogge, J.T. Modification of Kaolinite Surfaces through Mechanochemical Treatment—A Mid-IR and near-IR Spectroscopic Study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2002, 58, 2849–2859. [Google Scholar] [CrossRef]
- Jin, B.; Chen, Y.; Pyles, H.; Baer, M.D.; Legg, B.A.; Wang, Z.; Washton, N.M.; Mueller, K.T.; Baker, D.; Schenter, G.K.; et al. Formation, Chemical Evolution and Solidification of the Dense Liquid Phase of Calcium (Bi)Carbonate. Nat. Mater. 2025, 24, 125–132. [Google Scholar] [CrossRef]
- Penke, Y.K.; Anantharaman, G.; Ramkumar, J.; Kar, K.K. A Synergistic Cu-Al-Fe Nano Adsorbent for Significant Arsenic Remediation and As(0) Supported Mitigation in Aqueous Systems. In Environmental Arsenic in a Changing World; CRC Press: London, UK, 2019; pp. 457–458. ISBN 978-1-351-04663-3. [Google Scholar]
- Puzio, B.; Manecki, M.; Kwaśniak-Kominek, M. Transition from Endothermic to Exothermic Dissolution of Hydroxyapatite Ca5(PO4)3OH–Johnbaumite Ca5(AsO4)3OH Solid Solution Series at Temperatures Ranging from 5 to 65 °C. Minerals 2018, 8, 281. [Google Scholar] [CrossRef]
- Li, J.-S.; Wang, L.; Cui, J.-L.; Poon, C.S.; Beiyuan, J.; Tsang, D.C.W.; Li, X.-D. Effects of Low-Alkalinity Binders on Stabilization/Solidification of Geogenic As-Containing Soils: Spectroscopic Investigation and Leaching Tests. Sci. Total Environ. 2018, 631–632, 1486–1494. [Google Scholar] [CrossRef]
- Xue, L.; Ren, J.; Wang, S.; Qu, D.; Wei, Z.; Yang, Q.; Li, Y. Preparation of Nanofiber Aerogels by Electrospinning and Studying of Its Adsorption Properties for Heavy-Metal and Dyes. J. Porous Mater. 2020, 27, 1589–1599. [Google Scholar] [CrossRef]
- Wang, L.; Cho, D.-W.; Tsang, D.C.W.; Cao, X.; Hou, D.; Shen, Z.; Alessi, D.S.; Ok, Y.S.; Poon, C.S. Green Remediation of As and Pb Contaminated Soil Using Cement-Free Clay-Based Stabilization/Solidification. Environ. Int. 2019, 126, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Ishizu, T.; Lee, S.K.; Hasatani, M. Kinetic Study of Ca(OH)2/CaO Reversible Thermochemical Reaction for Thermal Energy Storage by Means of Chemical Reaction. Kagaku Kogaku Ronbunshu 1985, 11, 542–548. [Google Scholar] [CrossRef]
- GB 5085.3-2007; Identification Standards for Hazardous Wastes—Identification for Extraction Toxicity. Chinese National Standard: Beijing, China, 2007.
- Wang, X.; Zhang, F.; Nong, Z. Mineral Phases and Release Behaviors of As in the Process of Sintering Residues Containing As at High Temperature. Sci. World J. 2014, 2014, 260504. [Google Scholar] [CrossRef] [PubMed]

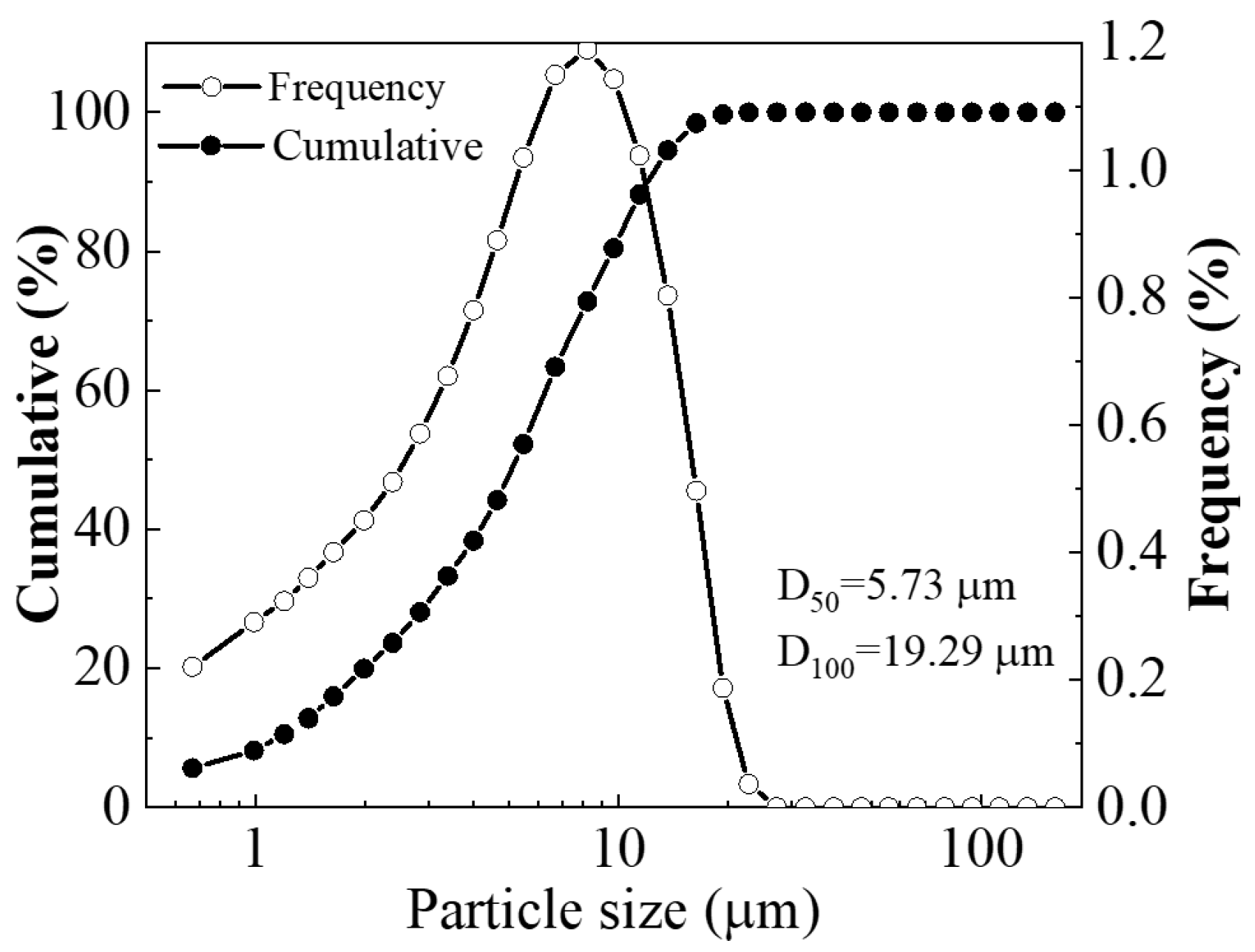
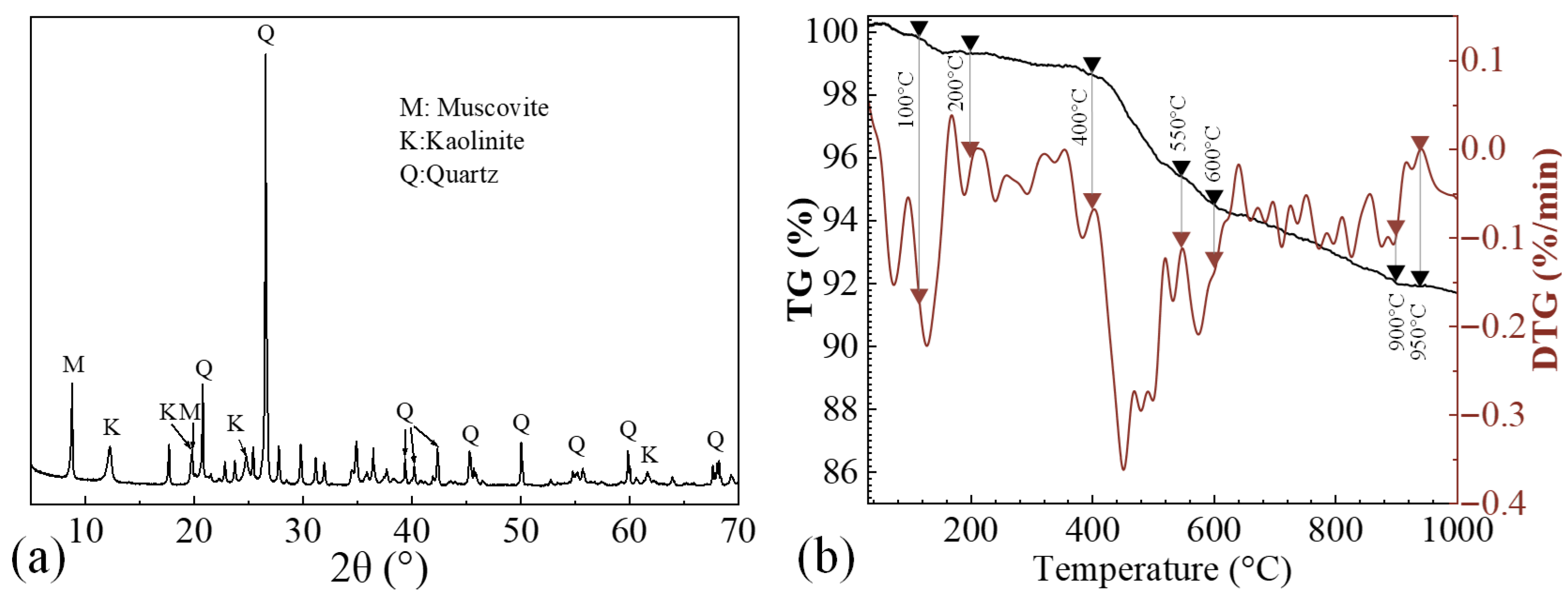
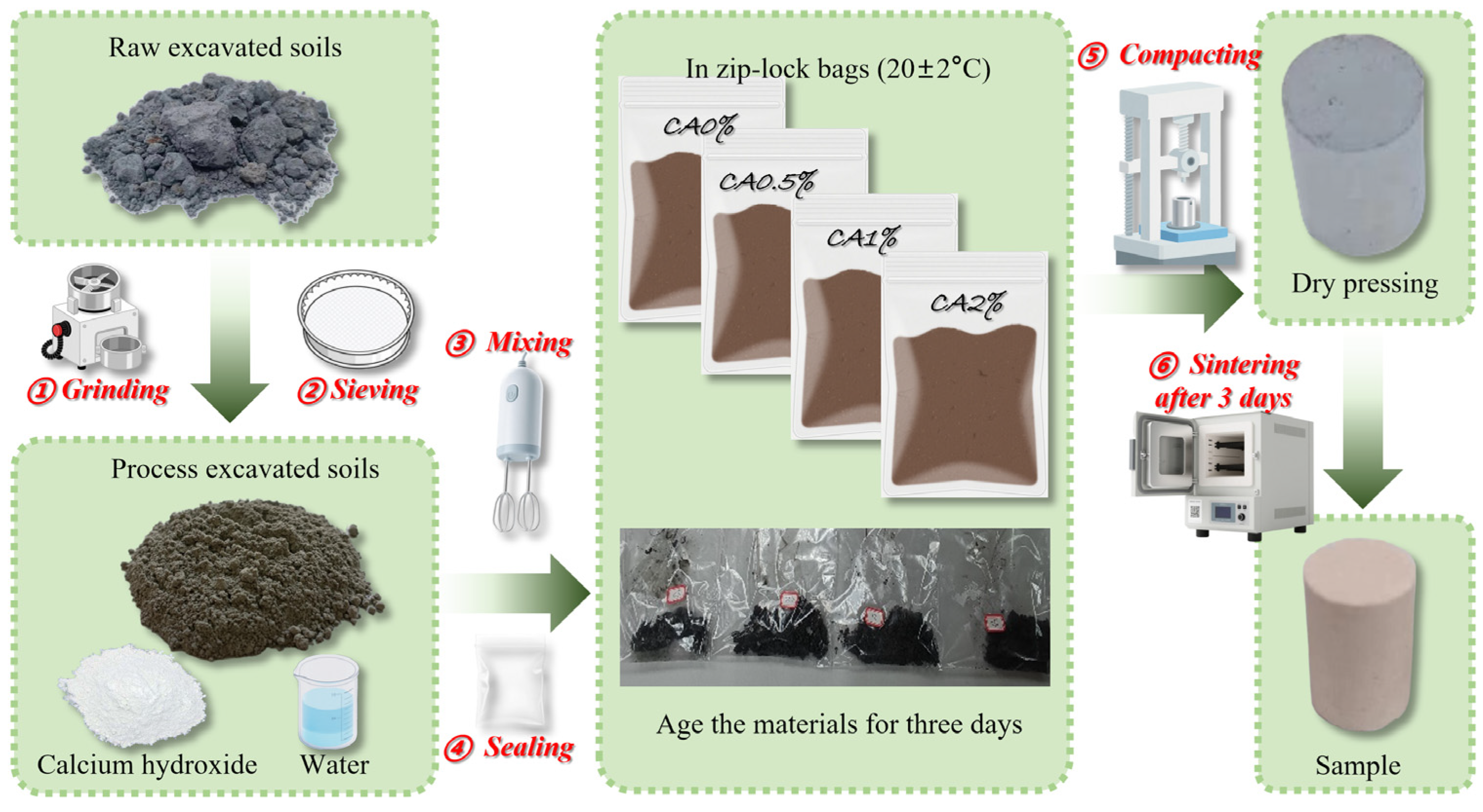
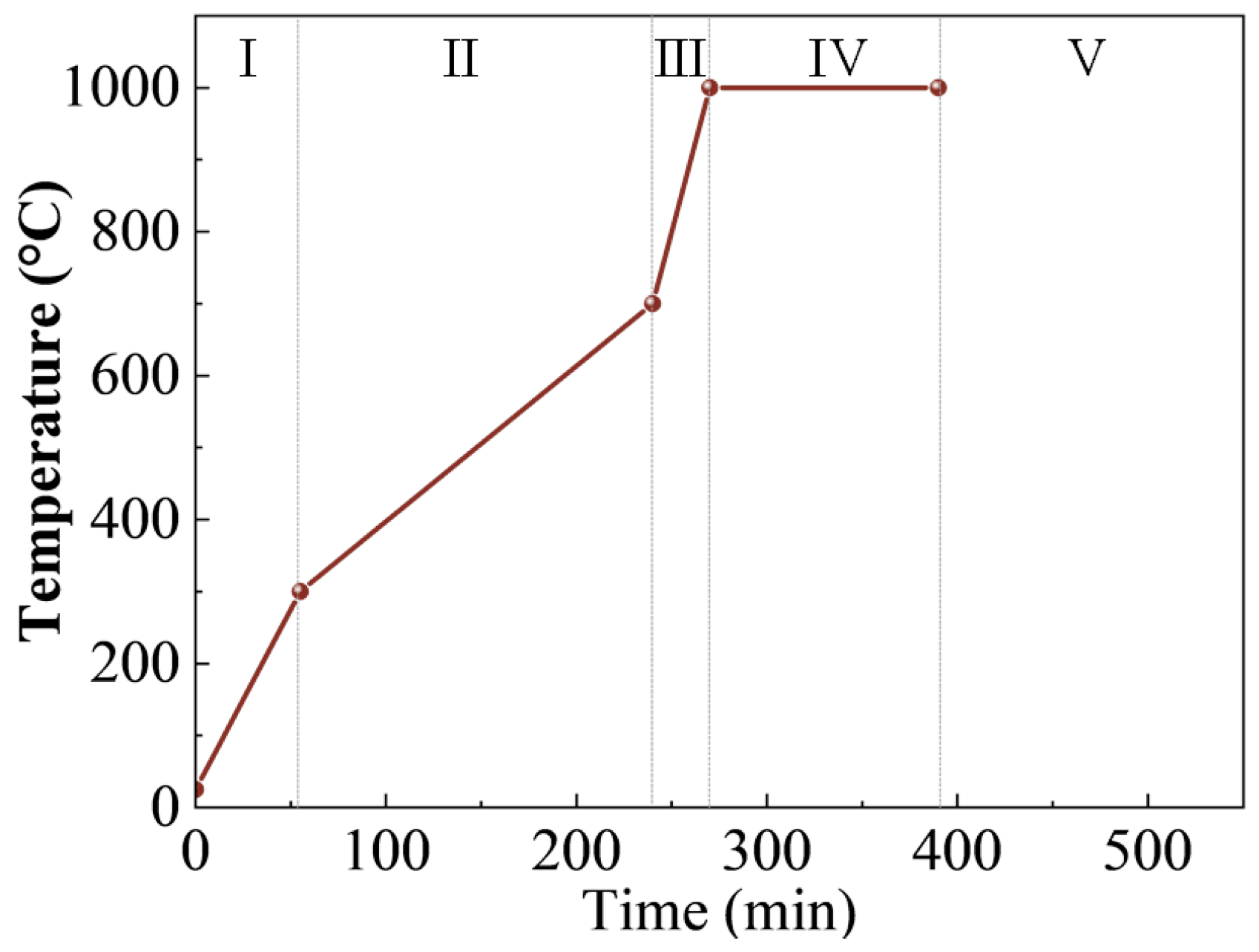


| SiO2 | Al2O3 | K2O | Fe2O3 | TiO2 | MgO | SO3 | P2O5 | CaO |
|---|---|---|---|---|---|---|---|---|
| 59.19 | 30.77 | 5.20 | 2.66 | 0.818 | 0.775 | 0.211 | 0.0939 | 0.74 |
| Sample ID | Soil (g) | Ca(OH)2 (g) | Water (g) | L/S |
|---|---|---|---|---|
| CA0% | 100 | 0 | 30 | 0.3 |
| CA0.5% | 100 | 0.5 | 30.15 | 0.3 |
| CA1% | 100 | 1 | 30.3 | 0.3 |
| CA2% | 100 | 2 | 30.6 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Jin, Y.; Wang, Y.; Dong, Z.; Feng, W. Inhibition Mechanism of Calcium Hydroxide on Arsenic Volatilization During Sintering of Contaminated Excavated Soils. Sustainability 2025, 17, 9027. https://doi.org/10.3390/su17209027
Li X, Jin Y, Wang Y, Dong Z, Feng W. Inhibition Mechanism of Calcium Hydroxide on Arsenic Volatilization During Sintering of Contaminated Excavated Soils. Sustainability. 2025; 17(20):9027. https://doi.org/10.3390/su17209027
Chicago/Turabian StyleLi, Xu, Yu Jin, Yaocheng Wang, Zhijun Dong, and Weipeng Feng. 2025. "Inhibition Mechanism of Calcium Hydroxide on Arsenic Volatilization During Sintering of Contaminated Excavated Soils" Sustainability 17, no. 20: 9027. https://doi.org/10.3390/su17209027
APA StyleLi, X., Jin, Y., Wang, Y., Dong, Z., & Feng, W. (2025). Inhibition Mechanism of Calcium Hydroxide on Arsenic Volatilization During Sintering of Contaminated Excavated Soils. Sustainability, 17(20), 9027. https://doi.org/10.3390/su17209027






