Investigating the Rooting of Stem Cuttings of Five Mediterranean Salvia spp., as a Means for Their Wider Exploitation in Sustainable Horticulture
Abstract
1. Introduction
1.1. Ornamental and Medicinal Importance of Salvia Species
1.2. Superiority of Vegetative over Sexual Propagation in Commercial Horticulture
1.3. Scientific Gap and Objective of the Study
2. Materials and Methods
2.1. Rooting of Cuttings
2.1.1. Cutting Origin (Salvia sp. and Mother Plant Type)
2.1.2. Season of Cutting Collection
2.1.3. IBA Application and Rooting Conditions
2.1.4. Recorded Data
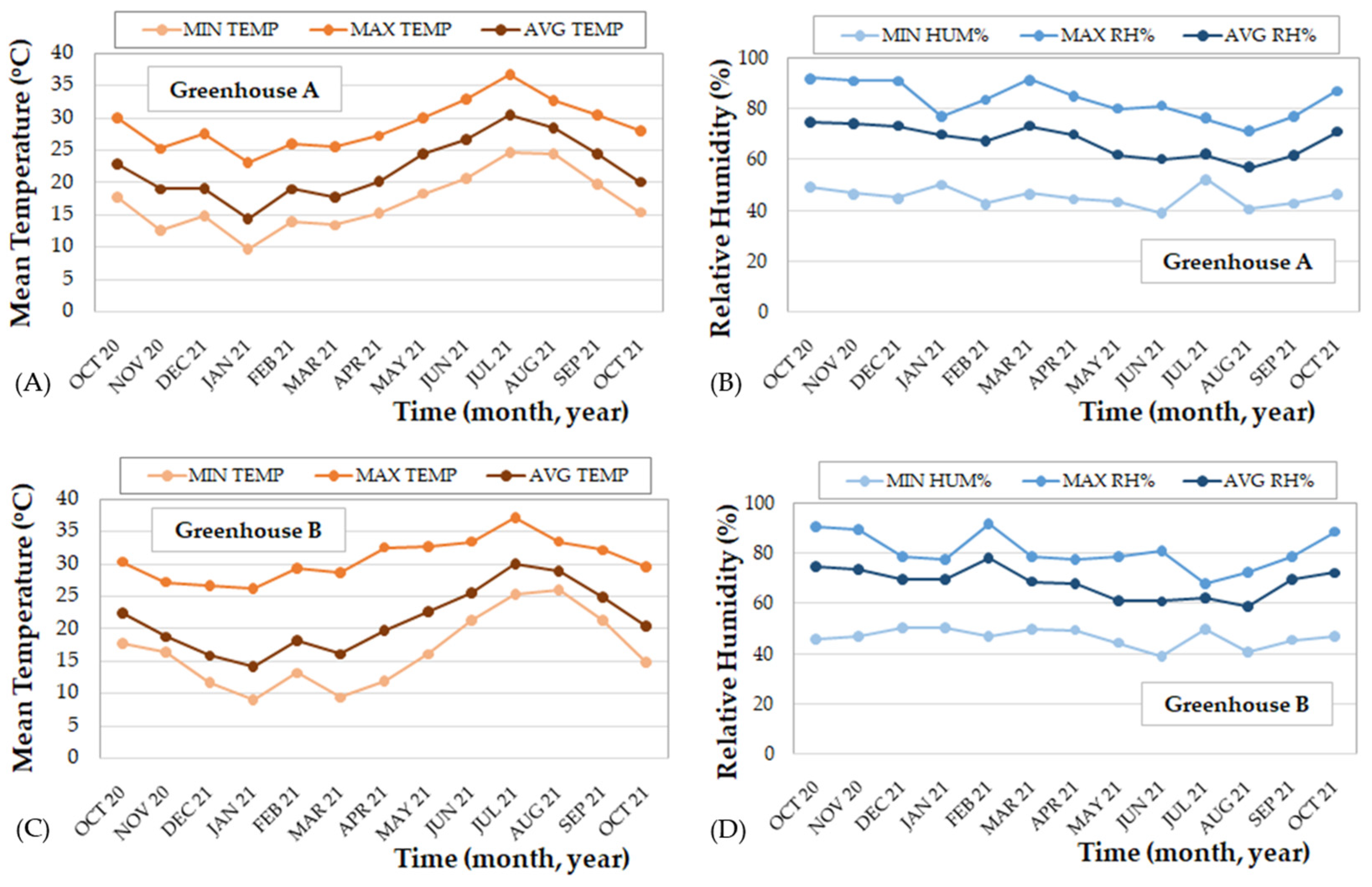
2.2. Climatic Data of Wild Cutting Collection Regions
2.3. Climatic Data from the Experimental Greenhouses A and B
2.4. Experimental Design and Statistical Analysis
3. Results
- (a)
- Regarding Salvia species, the highest rooting percentage was recorded for S. tomentosa (69.7%), followed by S. fruticosa (57.1%), whereas S. officinalis presented the lowest rooting percentage (38.7%). Fresh and dry weight of aboveground and underground parts of rooted cuttings were all higher in S. pomifera ssp. pomifera (Table 1).
- (b)
- As regards cutting origin, cuttings from greenhouse plants rooted at higher percentage (63.3%) than cuttings collected from wild plants (43.5%). However, fresh and dry weight of aboveground and underground parts of rooted cuttings were all higher in cuttings collected from wild plants (Table 1).
- (c)
- Regarding season of cutting collection, higher rooting percentage was achieved in autumn (62.9%), followed by spring (60.1%), while fresh and dry weight of aboveground and underground parts of rooted cuttings were also higher in autumn. Winter was excluded from this statistical analysis of fresh and dry weights, because of missing data, as some Salvia spp. did not root in this treatment (Table 1).
- (d)
- As regards rooting hormone treatment, the use of Rhizopon dusting powder produced the highest rooting percentage (64.4%), while the concentrations of alcoholic IBA solutions from 1500 to 6000 mg L−1 were equally effective among each other, but at a little lower percentage than Rhizopon. Fresh weights of aboveground and underground parts were higher in rooted cuttings treated with Rhizopon. In corresponding dry weights, apart from Rhizopon, IBA solutions from 1500 to 4500 mg L−1 also presented high values. The control (0 mg L−1 IBA) was excluded from this statistical analysis of fresh and dry weights, because of missing data, as some Salvia sp. did not root in this treatment (Table 1).
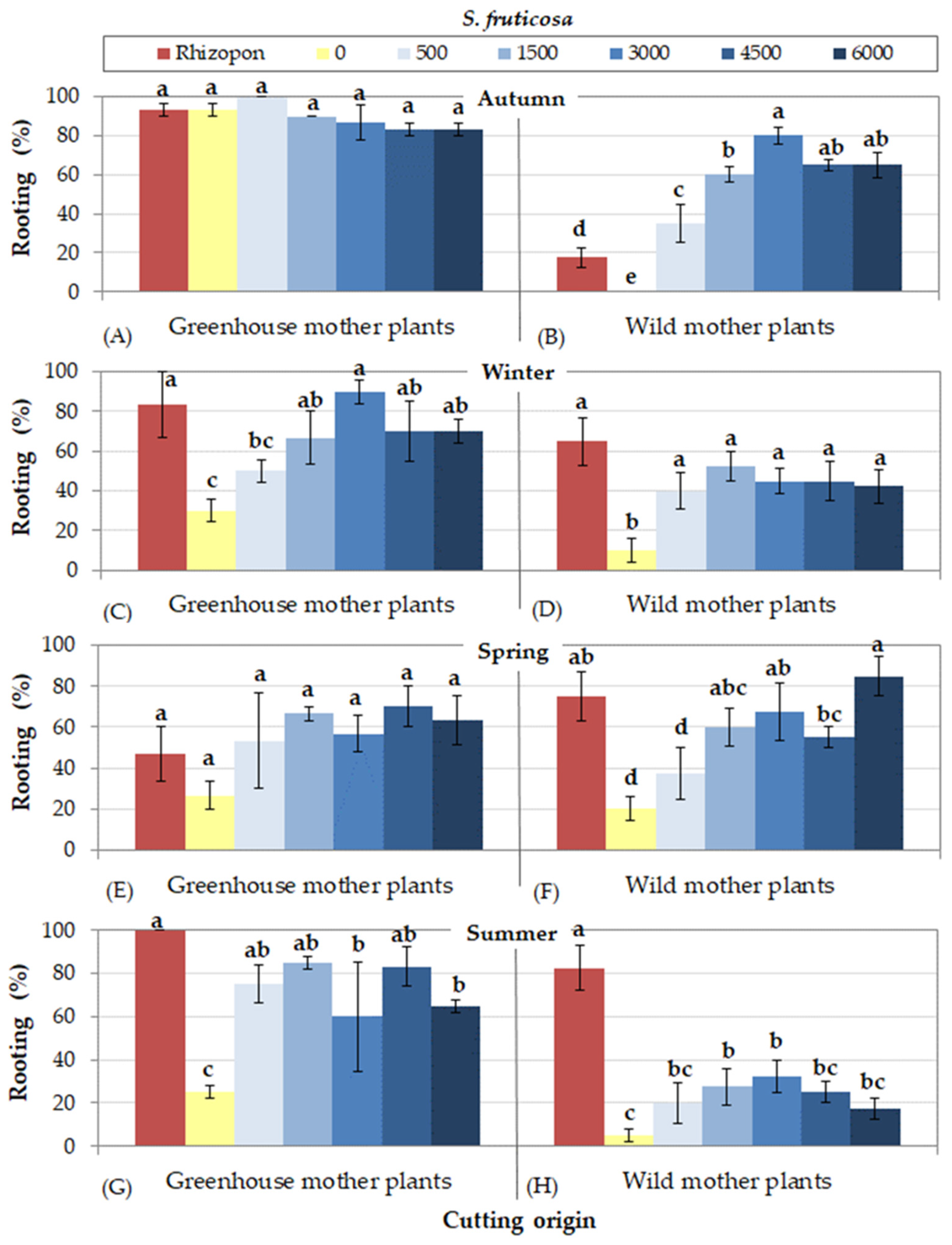
3.1. Rooting Cuttings of S. fruticosa
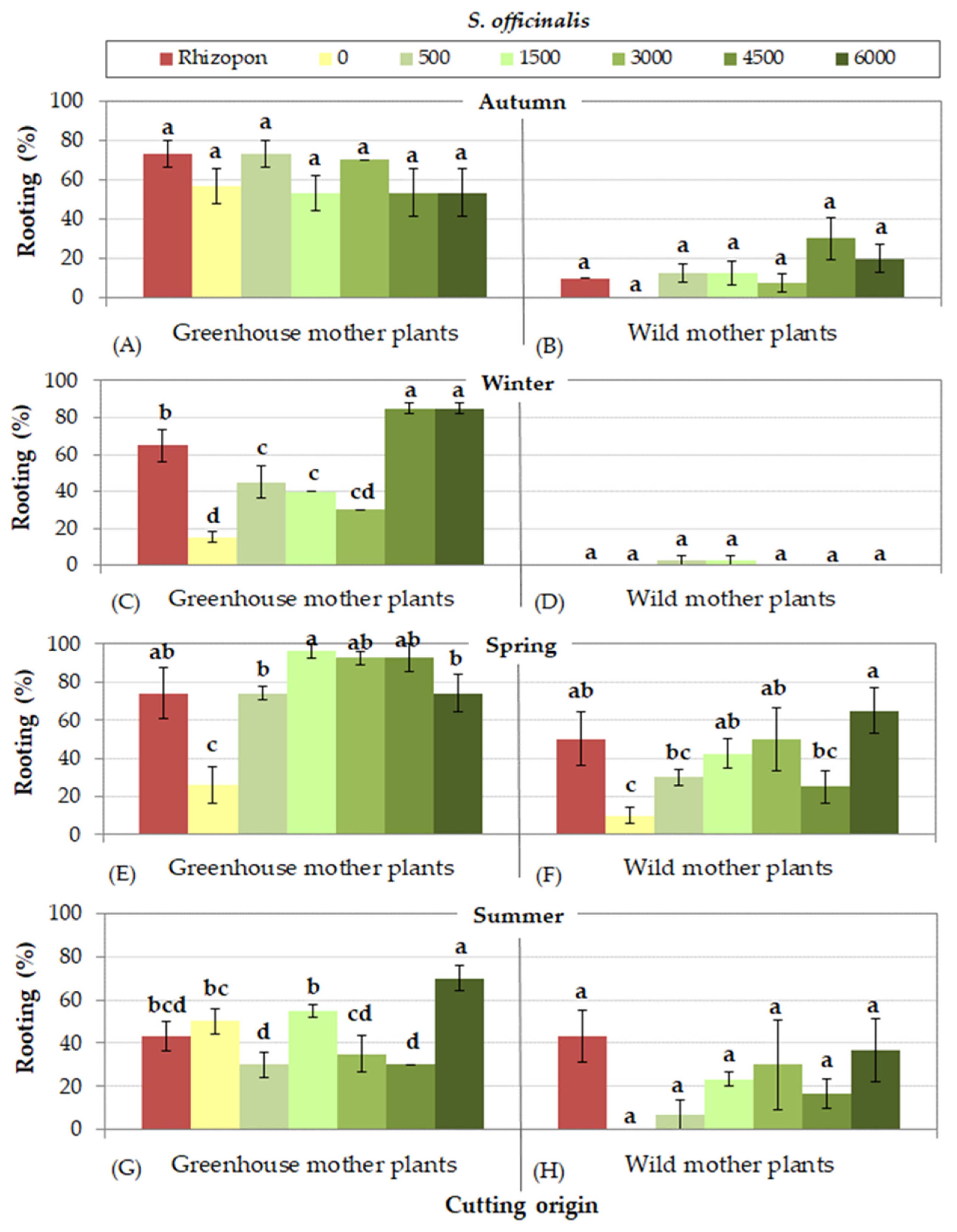
3.2. Rooting Cuttings of S. officinalis
3.3. Rooting Cuttings of S. pomifera ssp. pomifera
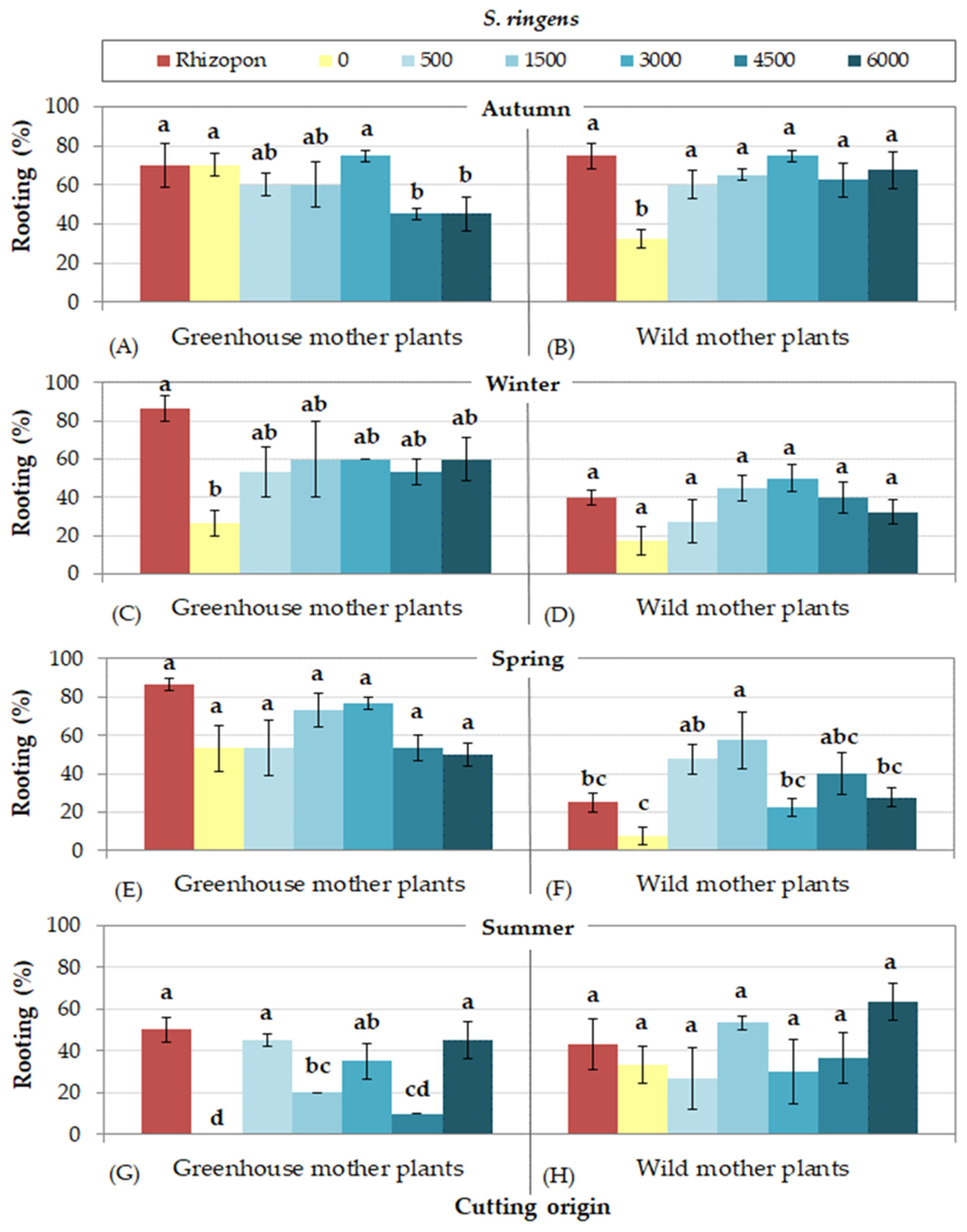
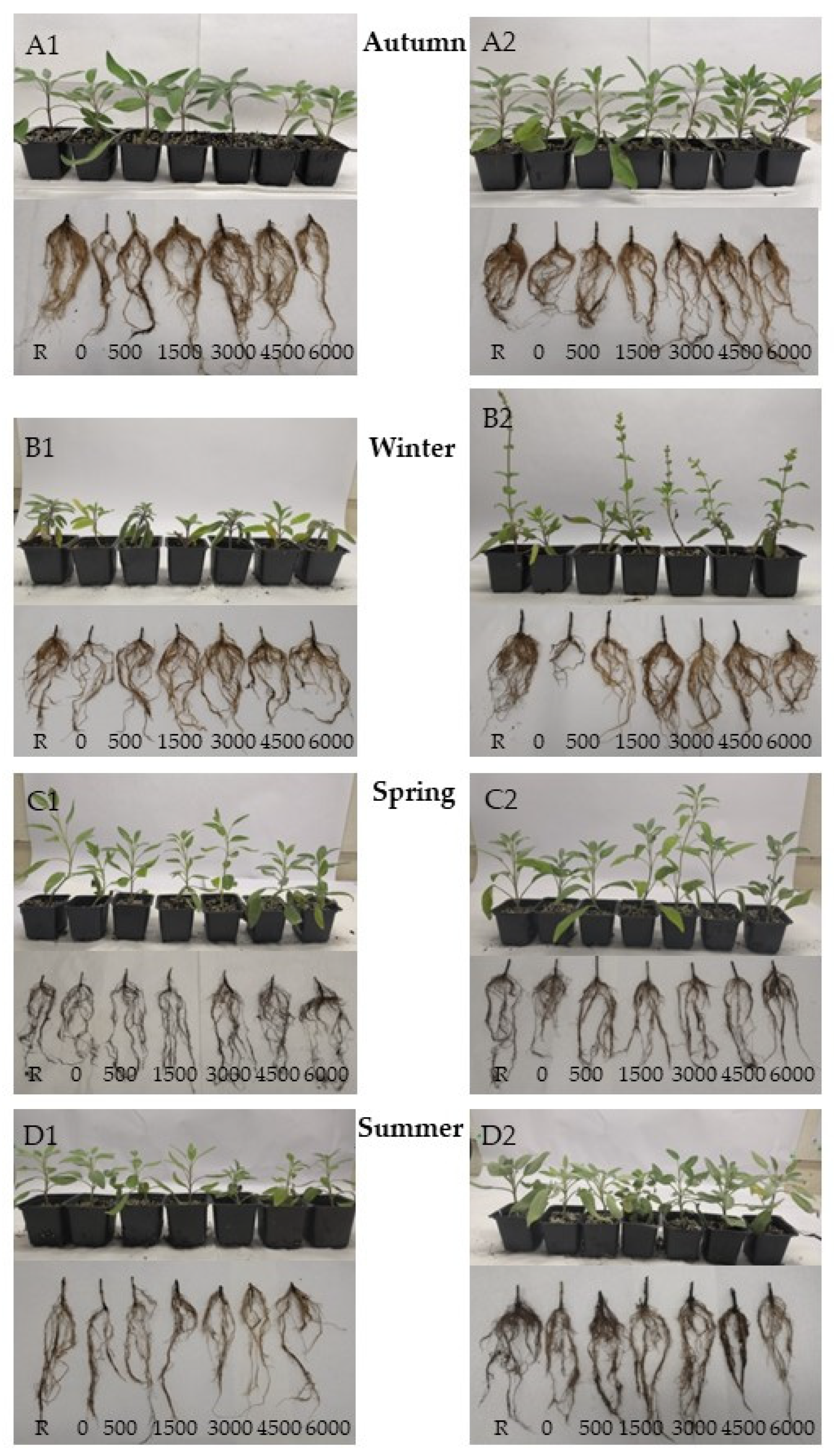
3.4. Rooting Cuttings of S. ringens
3.5. Rooting Cuttings of S. tomentosa
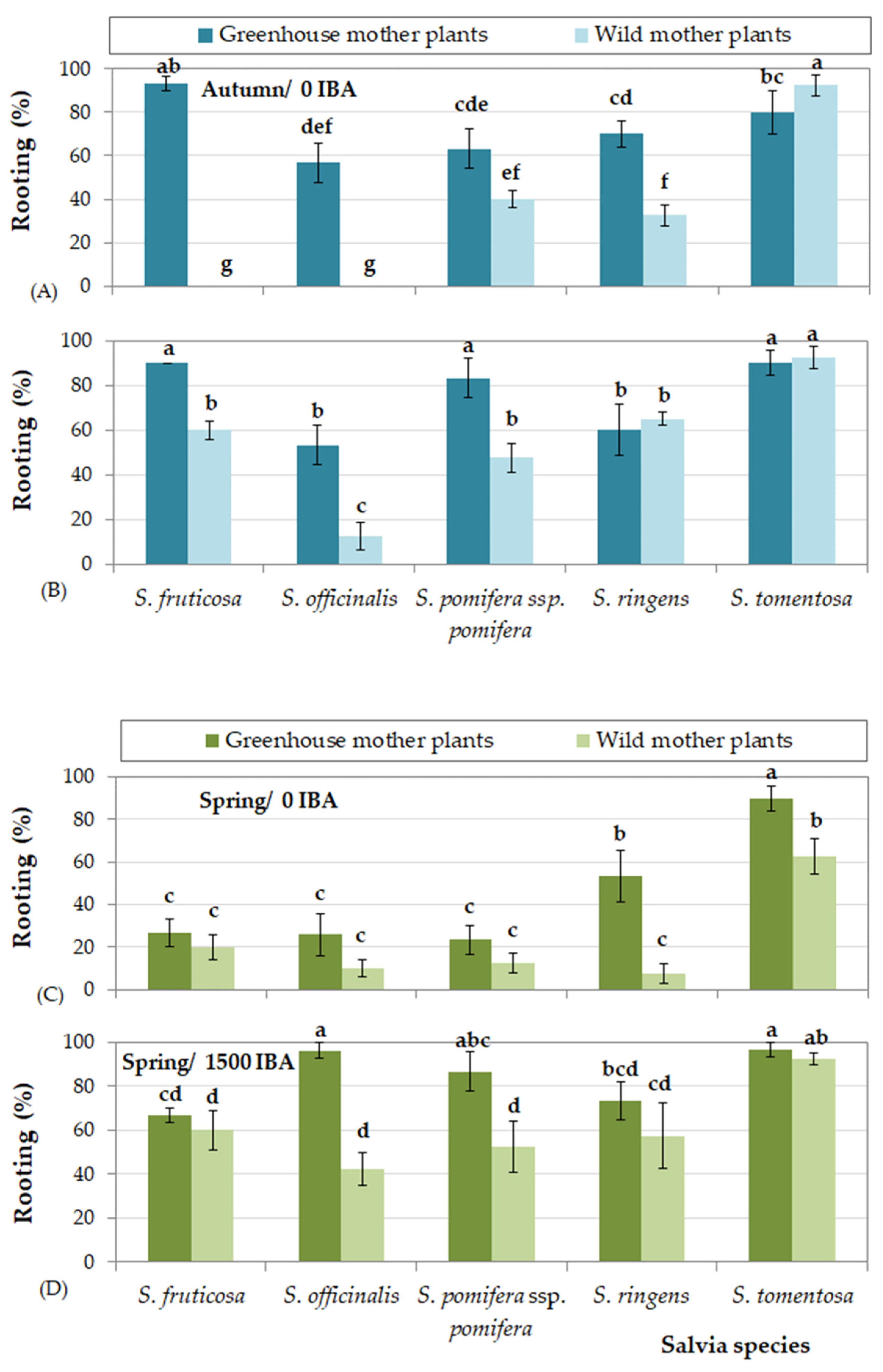
3.6. Comparative Evaluation of the Rooting Percentage of the Five Species and Two Types of Salvia Cuttings
- (a)
- Cuttings collected from greenhouse mother plants rooted at higher percentages than those collected from wild mother plants, excluding cuttings of S. tomentosa that rooted equally effectively, irrespective of cutting origin.
- (b)
- In autumn, higher rooting percentages were succeeded for all species, except of S. officinalis, in which cuttings rooted more efficiently in spring. Furthermore, cuttings of S. tomentosa rooted equally effectively in spring as well.
- (c)
- Cuttings collected from wild mother plants presented more difficulty in rooting in some seasons. To be more precise, the lowest rooting percentages were observed in autumn for S. fruticosa, in autumn and winher for S. officinalis, in summer for S. pomifera ssp. pomifera, and in all seasons excluding autumn for S. ringens. On the other hand, cuttings of S. tomentosa from wild mother plants rooted satisfactorily during all seasons.
- (d)
- The use of rooting hormone Rhizopon produced the highest rooting percentages for all species, although some IBA solution concentrations were equally effective depending on Salvia species and season. IBA solution at concentration 1500 mg L−1 was satisfactory for most species, excepting S. officinalis that responded better at the highest IBA concentration (6000 mg L−1).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papafotiou, M.; Martini, A.N.; Papanikolaou, E.; Stylias, E.G.; Kalantzis, A. Hybrids development between Greek Salvia species and their drought resistance evaluation along with Salvia fruticosa, under attapulgite-amended substrate. Agronomy 2021, 11, 2401. [Google Scholar] [CrossRef]
- Papafotiou, M.; Martini, A.N.; Tassoula, L.; Stylias, E.G.; Kalantzis, A.; Dariotis, E. Acclimatization of Mediterranean native sages (Salvia spp.) and interspecific hybrids in an urban green roof under regular and reduced irrigation. Sustainability 2022, 14, 4978. [Google Scholar] [CrossRef]
- Papafotiou, M.; Vlachou, G.; Martini, A.N. Investigation of the effects of the explant type and different plant growth regulators on micropropagation of five Mediterranean Salvia spp. native to Greece. Horticulturae 2023, 9, 96. [Google Scholar] [CrossRef]
- Varela-Stasinopoulou, D.S.; Nektarios, P.A.; Tsanakas, G.F.; Ntoulas, N.; Roukounakis, G.I.; Economou, A.S. Impact of substrate depth and irrigation regime on growth, flowering and physiological indices of Greek sage (Salvia fruticosa Mill.) grown on urban extensive green roof systems. Ecol. Eng. 2023, 186, 106816. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Viljoen, A.M.; Steenkamp, P. Antioxidant, antiinflammatory activities and HPLC analysis of South African Salvia species. Food Chem. 2010, 119, 684–688. [Google Scholar] [CrossRef]
- Uysal, I.; Koçer, O.; Mohammed, F.S.; Lekesiz, Ö.; Doğan, M.; Şabik, A.E.; Sevindik, E.; Gerçeker, F.Ö.; Sevindik, M. Pharmacological and nutritional properties: Genus Salvia. Adv. Pharmacol. Pharm. 2023, 11, 140–155. [Google Scholar] [CrossRef]
- Martini, A.N.; Stylias, E.G.; Papafotiou, M.; Kalantzis, A.; Bertsouklis, K.F. Interspecific crossability as a means to improve Mediterranean sage species with ornamental value. Acta Hortic. 2022, 1345, 425–430. [Google Scholar] [CrossRef]
- Papafotiou, M.; Martini, A.N.; Bertsouklis, K.F.; Vlachou, G.; Kanellou, E.; Stylias, E.G.; Kalantzis, A.; Tassoula, L.; Dariotis, E. A first approach for evaluation, breeding and promotion of native to Greece sage species for use as landscape plants. Acta Hortic. 2022, 1345, 359–366. [Google Scholar] [CrossRef]
- Blamey, M.; Grey-Wilson, C. Mediterranean Wild Flowers; Harper Collins Publishers: London, UK, 1993; pp. 401–402. [Google Scholar]
- Thanos, C.A.; Doussi, M.A. Ecophysiology of seed germination in endemic labiates of Crete. Isr. J. Plant Sci. 1995, 43, 227–237. [Google Scholar] [CrossRef]
- Schmiderer, C.; Novak, J. Salvia officinalis L. and Salvia fruticosa Mill.: Dalmatian and Three-Lobed Sage. In Medicinal, Aromatic and Stimulant Plants. Handbook of Plant Breeding; Novak, J., Blüthner, W.D., Eds.; Springer: Cham, Switzerland, 2020; Volume 12, pp. 523–537. [Google Scholar] [CrossRef]
- Karousou, R.; Vokou, D.; Kokkini, S. Distribution and essential oils of Salvia pomifera subsp. pomifera (Labiatae) on the island of Crete (S Greece). Biochem. Syst. Ecol. 1998, 26, 889–897. [Google Scholar] [CrossRef]
- Dweck, A.C. The Folklore and Cosmetic Use of Various Salvia Species. In Sage: The Genus Salvia; Kintzios, S.E., Ed.; Harwood Academic: Cleveland, OH, USA, 2000; pp. 1–26. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea, Diapenstaceae to Myoporaceae; Cambridge University Press: Cambridge, UK, 1972; Volume 3, pp. 188–190. [Google Scholar]
- Dinçer, C.; Tontul, I.; Çam, İ.B.; Özdemir, K.S.; Topuz, A.; Nadeem, H.Ş.; Ay, S.T.; Göktürk, R.S. Phenolic composition and antioxidant activity of Salvia tomentosa Miller: Effects of cultivation, harvesting year, and storage. Turk. J. Agric. For. 2013, 37, 561–567. [Google Scholar] [CrossRef]
- Stojanović, D.; Marčetić, M.; Ušjak, D.; Milenković, M. Composition and antimicrobial activity of essential oils of Salvia fruticosa and Salvia ringens (Lamiaceae). Vojnosanit. Pregl. 2022, 79, 62–68. [Google Scholar] [CrossRef]
- Bozyel, M.E.; Canli, K.; Benek, A.; Simsek, O.; Akata, I.; Altuner, E.M. Biochemical composition, and in vitro antimicrobial and antioxidant activities of Salvia fruticosa, an ethnomedicinal plant. Appl. Ecol. Environ. Res. 2023, 21, 3243–3256. [Google Scholar] [CrossRef]
- Dawra, M.; Bouajila, J.; El Beyrouthy, M.; Abi Rizk, A.; Taillandier, P.; Nehme, N.; El Rayess, Y. Chemical characterization and antioxidant, antibacterial, antiacetylcholinesterase and antiproliferation properties of Salvia fruticosa Miller extracts. Molecules 2023, 28, 2429. [Google Scholar] [CrossRef]
- Karneeb, S.; Baydoun, S.; Nasser, H.; Arnold-Apostolides, N.; El-Dakdouki, M.H. Chemical composition, antioxidant and hemolytic activities of sage (Salvia fruticosa Miller) cultivated in Lebanon. BAU J. Sci. Technol. 2023, 4, 3. [Google Scholar] [CrossRef]
- Aslan, K.; Kopar, E.E.; Kelle, K.; Karageçili, H.; Yilmaz, M.A.; Cakir, O.; Alwasel, S.; Gulcin, I. Phytochemical profile and bioactive properties of sage (Salvia fruticosa) and thyme (Thymus vulgaris) extracts. Int. J. Food Prop. 2025, 28, 2481148. [Google Scholar] [CrossRef]
- Alqudah, A.; Qnais, E.; Gammoh, O.; Bseiso, Y.; Wedyan, M. Potential anti-inflammatory activity of the Salvia fruticosa Mill. essential oil. J. Pharm. Pharmacogn. Res. 2024, 12, 14–26. [Google Scholar] [CrossRef]
- Koutsoulas, A.; Čarnecká, M.; Slanina, J.; Tóth, J.; Slaninová, I. Characterization of phenolic compounds and antiproliferative effects of Salvia pomifera and Salvia fruticosa extracts. Molecules 2019, 24, 2921. [Google Scholar] [CrossRef]
- Kyriakou, S.; Tragkola, V.; Plioukas, M.; Anestopoulos, I.; Chatzopoulou, P.S.; Sarrou, E.; Trafalis, D.T.; Deligiorgi, M.V.; Franco, R.; Pappa, A.; et al. Chemical and biological characterization of the anticancer potency of Salvia fruticosa in a model of human malignant melanoma. Plants 2021, 10, 2472. [Google Scholar] [CrossRef]
- Ververis, A.; Kyriakou, S.; Ioannou, K.; Chatzopoulou, P.S.; Panayiotidis, M.I.; Plioukas, M.; Christodoulou, K. Chemical profiling and antioxidant and anti-amyloid capacities of Salvia fruticosa extracts from Greece. Plants 2023, 12, 3191. [Google Scholar] [CrossRef]
- Grdiša, M.; Jug-Dujaković, M.; Lončarić, M.; Carović-Stanko, K.; Ninčević, T.; Liber, Z.; Radosavljević, I.; Šatović, Z. Dalmatian sage (Salvia officinalis L.): A review of biochemical contents, medical properties and genetic diversity. Agric. Conspec. Sci. 2015, 80, 69–78. Available online: https://hrcak.srce.hr/151149 (accessed on 16 April 2025).
- Petrova, M.; Nikolova, M.; Dimitrova, L.; Zayova, E. Micropropagation and evaluation of flavonoid content and antioxidant activity of Salvia officinalis L. Genet. Plant Physiol. 2015, 5, 48–60. Available online: https://www.ifrg-bg.com (accessed on 16 April 2025).
- Fu, Z.; Wang, H.; Hu, X.; Sun, Z.; Han, C. The pharmacological properties of Salvia essential oils. J. Appl. Pharm. Sci. 2013, 3, 122–127. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Akacha, B.B.; Kačániová, M.; Mekinić, I.G.; Kukula-Koch, W.; Koch, W.; Orhan, I.E.; Čmiková, N.; Taglieri, I.; Venturi, F.; Samartin, C.; et al. Sage (Salvia officinalis L.): A botanical marvel with versatile pharmacological properties and sustainable applications in functional foods. S. Afr. J. Bot. 2024, 169, 361–382. [Google Scholar] [CrossRef]
- Garg, D.; Kumar, V. A Review of therapeutic properties and uses of Salvia officinalis. J. Pharma Insights Res. 2024, 2, 146–154. [Google Scholar] [CrossRef]
- Purgato, G.A.; Píccolo, M.S.; Moreira, M.A.S.; Pizziolo, V.R.; Diaz-Muñoz, G.; Rossi, C.C.; Diaz, M.A.N. Isolation and identification of antimicrobial multicyclic terpenoids from the medicinal plant Salvia officinalis and development of a formulation against clinical Staphylococcus aureus strains. Lett. Appl. Microbiol. 2024, 77, ovae077. [Google Scholar] [CrossRef]
- Askari, Y.; Mohtashami, R. Evaluation of Thymus vulgaris, Salvia officinalis, Mentha piperita and Hyssopus officinalis plants with benefits on the respiratory organs. Int. J. Adv. Biol. Biomed. Res. 2022, 10, 173–184. [Google Scholar] [CrossRef]
- Jedidi, S.; Aloui, F.; Selmi, S.; Selmi, H.; Sammari, H.; Ayari, A.; Abbes, C.; Sebai, H. Antioxidant properties of Salvia officinalis decoction extract and mechanism of its protective effects on ethanol-induced liver and kidney injuries. J. Med. Food 2022, 25, 546–556. [Google Scholar] [CrossRef]
- Mot, M.-D.; Gavrilaș, S.; Lupitu, A.I.; Moisa, C.; Chambre, D.; Tit, D.M.; Bogdan, M.A.; Bodescu, A.-M.; Copolovici, L.; Copolovici, D.M.; et al. Salvia officinalis L. Essential oil: Characterization, antioxidant properties, and the effects of aromatherapy in adult patients. Antioxidants 2022, 11, 808. [Google Scholar] [CrossRef]
- Duletić-Laušević, S.N.; Alimpić Aradski, A.Z.; Kolarević, S.M.; Vuković-Gačić, B.S.; Oalđe, M.M.; Marin, P.D. Biological activities of Cretan Salvia pomifera extracts. Bot. Serbica 2018, 42, 209–216. [Google Scholar] [CrossRef]
- Šavikin, K.P.; Ristić, M.S.; Zdunić, G.M.; Stević, T.; Menković, N.R. Chemical composition and antimicrobial activity of essential oil of Salvia ringens Sibth. et Sm. var. baldacciana Briq. J. Essent. Oil Res. 2008, 20, 363–365. [Google Scholar] [CrossRef]
- Tzakou, O.; Pitarokili, D.; Chinou, I.B.; Harvala, C. Composition and antimicrobial activity of the essential oil of Salvia ringens. Planta Med. 2001, 67, 81–83. [Google Scholar] [CrossRef]
- Janicsák, G.; Hohmann, J.; Nikolova, M.; Genova, E.; Zupkó, I.; Forgo, P.; Máthé, I. Compounds from Salvia ringens Sibth. & Sm. with cytotoxic activity. Planta Med. 2007, 73, P371. [Google Scholar] [CrossRef]
- Tusevski, O.; Kostovska, A.; Iloska, A.; Trajkovska, L.J.; Gadzovska Simic, S. Phenolic production and antioxidant properties of some Macedonian medicinal plants. Cent. Eur. J. Biol. 2014, 9, 888–900. [Google Scholar] [CrossRef]
- Alimpić, A.; Pljevljakušić, D.; Šavikin, K.; Knežević, A.; Ćurčić, M.; Veličković, D.; Tatjana Stević, T.; Petrović, G.; Matevski, V.; Vukojević, J.; et al. Composition and biological effects of Salvia ringens (Lamiaceae) essential oil and extracts. Ind. Crops Prod. 2015, 76, 702–709. [Google Scholar] [CrossRef]
- Balkır, Ş.; Hazman, Ö.; Aksoy, L.; Yılmaz, M.A.; Cakir, O.; Kara, R.; Erol, İ. Phytochemical profile, antioxidant and antimicrobial potency of aerial parts of Salvia tomentosa Miller. Acta Chim. Slov. 2023, 70, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Yilar, M.; Kadioglu, I.; Telci, I. Chemical composition and antifungal activity of Salvia officinalis (L.), S. cryptantha (Montbret et Aucher ex Benth.), S. tomentosa (Mill.) plant essential oils and extracts. Fresenius Environ. Bull. 2018, 27, 1695–1706. [Google Scholar]
- Kahraman, H.A.; Usluer, M.S.; Kaya, M.M.; Tutun, S.; Tutun, H.; Demir, M.M.; Sevin, S. Antimicrobial potential and phenolic composition of Salvia tomentosa extract against some pathogenic bacteria. Fresenius Environ. Bull. 2022, 31, 9755–9761. [Google Scholar]
- Piątczak, E.; Kolniak-Ostek, J.; Gonciarz, W.; Lisiecki, P.; Kalinowska-Lis, U.; Szemraj, M.; Chmiela, M.; Zielińska, S. The effect of Salvia tomentosa Miller extracts, rich in rosmarinic, salvianolic and lithospermic acids, on bacteria causing opportunistic infections. Molecules 2024, 29, 590. [Google Scholar] [CrossRef]
- Ulukanli, Z.; Karabörklü, S.; Cenet, M.; Sagdic, O.; Ozturk, I.; Balcilar, M. Essential oil composition, insecticidal and antibacterial activities of Salvia tomentosa Miller. Med. Chem. Res. 2013, 22, 832–840. [Google Scholar] [CrossRef]
- Marchev, A.; Ivanov, I.; Denev, P.; Nikolova, M.; Gochev, V.; Stoyanova, A.; Pavlov, A.; Georgiev, V. Acetylcholinesterase inhibitory, antioxidant, and antimicrobial activities of Salvia tomentosa Mill. essential oil. J. BioSci. Biotechnol. 2015, 4, 219–229. [Google Scholar]
- Nicola, S.; Fontana, E.; Hoeberechts, J.; Saglietti, D. Rooting products and cutting timing on sage (Salvia officinalis L.) propagation. Acta Hortic. 2005, 676, 135–141. [Google Scholar] [CrossRef]
- Vlachou, G.; Martini, A.Ν.; Dariotis, Ε.; Papafotiou, Μ. Comparative evaluation of seed germination of five Mediterranean sage species (Salvia sp.) native to Greece. Acta Hortic. 2020, 1298, 593–598. [Google Scholar] [CrossRef]
- Kanellou, E.; Vlachou, G.; Martini, A.N.; Bertsouklis, K.F.; Papafotiou, M. Seed germination of five sage species (Salvia sp.) of populations native to Greece. Acta Hortic. 2022, 1345, 439–444. [Google Scholar] [CrossRef]
- Waman, A.A.; Smitha, G.R.; Bohra, P. A Review on clonal propagation of medicinal and aromatic plants through stem cuttings for promoting their cultivation and conservation. Curr. Agric. Res. J. 2019, 7, 122–138. [Google Scholar] [CrossRef]
- Kacar, O.; Azkan, N.; Çöplü, N. Effects of different rooting media and indole butyric acid on rooting of stem cuttings in sage (Salvia officinalis L. and Salvia triloba L.). J. Food Agric. Environ. 2009, 7, 349–352. [Google Scholar]
- Capecka, E. The effect of propagation term and method on the growth and fresh herb productivity of sage and balm cultivated in pots. Folia Hortic. 2012, 24, 67–71. [Google Scholar] [CrossRef]
- Parađiković, N.; Zeljković, S.; Tkalec, M.; Vinković, T.; Dervić, I.; Marić, M. Influence of rooting powder on propagation of sage (Salvia officinalis L.) and rosemary (Rosmarinus officinalis L.) with green cuttings. Poljoprivreda 2013, 19, 10–15. Available online: https://urn.nsk.hr/urn:nbn:hr:151:509773 (accessed on 14 April 2025).
- Gudeva, L.; Trajkova, F.; Mihajlov, L.; Troicki, J. Influence of different auxins on rooting of rosemary, sage and elderberry. Annu. Res. Rev. Biol. 2017, 12, 1–8. [Google Scholar] [CrossRef]
- Nanos, C.; Tsoulpha, P.; Kostas, S.; Hatzilazarou, S.; Michail, I.; Anastasiadi, V.; Pipinis, E.; Gklavakis, E.; Kanellis, A.K.; Nianiou-Obeidat, I. Asexual propagation of Greek Salvia officinalis L. populations selected for ornamental use. Horticulturae 2023, 9, 847. [Google Scholar] [CrossRef]
- Bertsouklis, K.F.; Martini, A.N.; Papafotiou, M. Effect of cutting type and indole butyric acid on propagation of Salvia fruticosa with cuttings. Acta Hortic. 2022, 1345, 389–394. [Google Scholar] [CrossRef]
- Sağlam, A.N.; Yaver, S.; Başer, I.; Cinkiliç, L. The effects of different hormones and their doses on rooting of stem cuttings in anatolian sage (Salvia fruticosa Mill.). APCBEE Procedia 2014, 8, 348–353. [Google Scholar] [CrossRef][Green Version]
- Tan, U. Application of indole-3-butyric acid (IBA) enhances agronomic, physiological and antioxidant traits of Salvia fruticosa under saline conditions: A practical approach. PeerJ 2025, 13, e18846. [Google Scholar] [CrossRef]
- Martini, A.N.; Bertsouklis, K.; Vlachou, G.; Dariotis, E.; Papafotiou, M. Comparative evaluation of rooting cuttings of five Mediterranean sage species (Salvia sp.) native to Greece. Acta Hortic. 2020, 1298, 587–592. [Google Scholar] [CrossRef]
- Martini, A.N.; Bertsouklis, K.; Vlachou, G.; Papafotiou, M. Effect of season in rooting stem-tip cuttings of Mediterranean sages (Salvia spp.) native to Greece. AGROFOR Int. J. 2022, 7, 118–125. [Google Scholar] [CrossRef]
- Martini, A.N.; Bertsouklis, K.; Vlachou, G.; Papafotiou, M. Effect of cutting origin, season of collection, and treatment on rooting of shoot-tip cuttings of Salvia tomentosa Mill. Acta Hortic. 2025, 1417, 163–170. [Google Scholar] [CrossRef]
- Teklehaimanot, Z.; Mwang’ingo, P.L.; Mugasha, A.G.; Ruffo, C.K. Influence of the origin of stem cutting, season of collection and auxin application on the vegetative propagation of African Sandalwood (Osyris lanceolata) in Tanzania. South. Afr. For. J. 2004, 201, 13–24. [Google Scholar] [CrossRef]
- Salmi, M.S.; Hesami, M. Time of collection, cutting ages, auxin types and concentrations influence rooting Ficus religiosa L. stem cuttings. J. Appl. Environ. Biol. Sci. 2016, 6, 124–132. [Google Scholar]
- Yazar, M.; Erdoğan Genç, H.; Feyzioğlu, F.; Atar, F.; Turna, İ. Effects of auxins, medium, and collection season on the rooting of Caucasian Whortleberry (Vaccinium arctostaphylos L.) stem cuttings. J. Sustain. For. 2025, 1–18. [Google Scholar] [CrossRef]
- Kaushik, S.; Shukla, N. A review on effect of IBA and NAA and their combination on the rooting of stem cuttings of different ornamental crops. J. Pharmacogn. Phytochem. 2020, 9, 1881–1885. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, A.; Kaur, H.; Sangeetha, K.; Mehta, S.; Husen, A. Chapter 1-Physiological and environmental control of adventitious root formation in cuttings: An overview. In Environmental, Physiological and Chemical Controls of Adventitious Rooting in Cuttings; Husen, A., Ed.; Academic Press: London, UK, 2022; pp. 1–24. [Google Scholar] [CrossRef]
- de Klerk, G.J.; van der Krieken, W.; de Jong, J.C. Review the formation of adventitious roots: New concepts, new possibilities. Vitr. Cell. Dev. Biol.-Plant 1999, 35, 189–199. [Google Scholar] [CrossRef]
- Han, H.; Zhang, S.; Sun, X. A review on the molecular mechanism of plants rooting modulated by auxin. Afr. J. Biotechnol. 2009, 8, 348–353. Available online: https://academicjournals.org/journal/AJB/article-stat/3AE97F45526 (accessed on 17 April 2025).
- Guan, L.; Murphy, A.S.; Peer, W.A.; Gan, L.; Li, Y.; Cheng, Z.M. Physiological and molecular regulation of adventitious root formation. Crit. Rev. Plant Sci. 2015, 34, 506–521. [Google Scholar] [CrossRef]
- Owen, J.S., Jr.; Maynard, B.K. Environmental Effects on stem-Cutting Propagation: A Brief Review. In Combined Proceedings International Plant Propagators’ Society; International Plant Propagators’ Society, Ed.; IPPS: Carlisle, PA, USA, 2007; Volume 57, pp. 558–564. [Google Scholar]
- Inga Dirks, I.; Raviva, B.; Shelefa, O.; Hilla, A.; Eppel, A.; Aidoo, M.K.; Hoefgena, B.; Rapaport, T.; Gil, H.; Geta, E.; et al. Green roofs: What can we learn from desert plants? Isr. J. Ecol. Evol. 2016, 62, 58–67. [Google Scholar] [CrossRef]
- Davis, T.D.; Potter, J.R. Relations between carbohydrate, water status and adventitious root formation in leafy pea cuttings rooted under various levels of atmospheric CO2 and relative humidity. Physiol. Plant. 1989, 77, 185–190. [Google Scholar] [CrossRef]
- Druege, U.; Hilo, A.; Pérez-Pérez, J.M.; Klopotek, Y.; Acosta, M.; Shahinnia, F.; Zerche, S.; Franken, P.; Hajirezaei, M.R. Molecular and physiological control of adventitious rooting in cuttings: Phytohormone action meets resource allocation. Ann. Bot. 2019, 123, 929–949. [Google Scholar] [CrossRef]
- Husen, A.; Pal, M. Variation in shoot anatomy and rooting behaviour of stem cuttings in relation to age of donor plants in teak (Tectona grandis Linn. f.). New For. 2006, 31, 57–73. [Google Scholar] [CrossRef]
- Altamura, M.M. Root histogenesis in herbaceous and woody explants cultured in vitro. A critical review. Agronomie 1996, 16, 589–602. [Google Scholar] [CrossRef]
- Pignatti, G.; Crobeddu, S. Effects of rejuvenation on cutting propagation of Mediterranean shrub species. For. J. Silvic. For. Ecol. 2005, 2, 290–295. [Google Scholar] [CrossRef]
- Kostas, S.; Kaplani, A.; Koulaouzidou, E.; Kotoula, A.-A.; Gklavakis, E.; Tsoulpha, P.; Hatzilazarou, S.; Nianiou-Obeidat, I.; Kanellis, A.K.; Economou, A. Sustainable exploitation of Greek Rosmarinus officinalis L. populations for ornamental use through propagation by shoot cuttings and in vitro cultures. Sustainability 2022, 14, 4059. [Google Scholar] [CrossRef]
- Abd El Hameed, N.S. Effect of indole butyric acid (IBA), cutting type and planting date on cuttings rooting of Myrtus communis. Middle East J. Agric. Res. 2018, 7, 1135–1145. [Google Scholar]
- Vlachou, G.; Martini, A.N.; Akoumianaki-Ioannidou, A.; Papafotiou, M. Seasonal variation of rooting of Ballota acetabulosa stem cuttings. Acta Hortic. 2021, 1327, 381–388. [Google Scholar] [CrossRef]
- Bartolini, G.; Petrucceli, R.; Pestelli, P. Preliminary study on in vivo rooting of two Olea europaea L. genotypes. Acta Hortic. 2008, 791, 191–195. [Google Scholar] [CrossRef]
- Delrio, C.; Rallo, L.; Caballero, J.M. Effects of carbohydrate content on the seasonal rooting of vegetative and reproductive cuttings of olive. J. Hortic. Sci. 1991, 66, 301–309. [Google Scholar] [CrossRef]
- Denaxa, N.-K.; Vemmos, S.N.; Roussos, P.A. The role of endogenous carbohydrates and seasonal variation in rooting ability of cuttings of an easy and a hard to root olive cultivars (Olea europaea L.). Sci. Hortic. 2012, 143, 19–28. [Google Scholar] [CrossRef]
- Blythe, E.K.; Sibley, J.L.; Tilt, K.M.; Ruter, J.M. Methods of auxin application in cutting propagation: A review of 70 years of scientific discovery and commercial practice. J. Environ. Hortic. 2007, 25, 166–185. [Google Scholar] [CrossRef]
- Loconsole, D.; Cristiano, G.; De Lucia, B. Image analysis of adventitious root quality in wild Sage and glossy Abelia cuttings after application of different Indole-3-butyric acid concentrations. Plants 2022, 11, 290. [Google Scholar] [CrossRef] [PubMed]
- Abu-Zahra, T.R.; Al-Shadaideh, A.N.; Abubaker, S.; Qrunfleh, I. Influence of auxin concentrations on different ornamental plants. Int. J. Bot. 2013, 9, 96–99. [Google Scholar] [CrossRef][Green Version]
- Lasheen, F.F.; Hewidy, M.; Emam, O.N.K. Impact of rooting promoters (PHOS ROOT-X) with or without using IBA on rooting of terminal and middle cuttings of rosemary (Rosmarinus officinalis L.). Sci. J. Flowers Ornam. Plants 2021, 8, 153–163. [Google Scholar] [CrossRef]
- Nicola, S.; Fontana, E.; Hoeberechts, J. Effects of rooting products on medicinal and aromatic plant cuttings. Acta Hortic. 2003, 614, 273–278. [Google Scholar] [CrossRef]







| Experimental Factor | Rooting (%) | Aboveground f.w. (g) | Underground f.w. (g) | Aboveground d.w. (g) | Underground d.w. (g) | |
|---|---|---|---|---|---|---|
| Salvia sp. | S. fruticosa | 57.1 b ɫ | 1.85 c | 1.22 c | 0.48 d | 0.17 c |
| S. officinalis | 38.7 e | 1.30 d | 1.08 d | 0.34 e | 0.16 c | |
| S. pomifera ssp. pomifera | 53.5 c | 3.39 a | 1.84 a | 0.92 a | 0.29 a | |
| S. ringens | 47.9 d | 1.96 bc | 0.97 e | 0.56 c | 0.12 d | |
| S. tomentosa | 69.7 a | 2.21 b | 1.31 b | 0.60 b | 0.18 b | |
| Cutting origin | Greenhouse mother plants | 63.3 a | 1.95 b | 1.18 b | 0.45 b | 0.14 b |
| Wild mother plants | 43.5 b | 2.34 a | 1.39 a | 0.71 a | 0.23 a | |
| Collection season | Autumn | 62.9 a | 2.71 a | 1.91 a | 0.72 a | 0.22 a |
| Winter ¥ | 45.3 c | - | - | - | - | |
| Spring | 60.1 b | 1.90 b | 1.05 b | 0.53 b | 0.20 b | |
| Summer | 45.1 c | 1.82 b | 0.90 c | 0.49 c | 0.13 c | |
| IBA treatment (mg L−1) | Rhizopon | 64.4 a | 2.57 a | 1.35 a | 0.62 a | 0.19 a |
| 0 ¥ | 33.1 e | - | - | - | - | |
| 500 | 49.2 d | 1.93 b | 1.11 b | 0.53 c | 0.19 a | |
| 1500 | 59.1 b | 2.17 b | 1.31 a | 0.59 ab | 0.18 ab | |
| 3000 | 56.6 bc | 2.12 b | 1.32 a | 0.58 b | 0.18 ab | |
| 4500 | 54.6 c | 2.05 b | 1.33 a | 0.62 a | 0.19 a | |
| 6000 | 56.6 bc | 2.02 b | 1.29 a | 0.53 c | 0.17 b | |
| Significance § | FSalvia species | ** | ** | ** | ** | ** |
| Fcutting origin | ** | * | ** | ** | ** | |
| Fcollection season | ** | ** | ** | ** | ** | |
| FIBA treatment | ** | * | ** | ** | * | |
| Fspecies×origin | ** | * | ** | ** | ** | |
| Fspecies×season | ** | NS | ** | ** | ** | |
| Forigin×season | * | ** | ** | ** | ** | |
| Fspecies×origin×season | ** | ** | ** | ** | ** | |
| Fspecies×IBA | ** | NS | NS | ** | * | |
| Forigin×IBA | ** | ΝS | NS | * | ** | |
| Fspecies×origin×IBA | ** | NS | NS | ** | ** | |
| Fseason×IBA | ** | NS | NS | ** | ** | |
| Fspecies×season×IBA | ** | NS | ** | ** | ** | |
| Forigin×season×IBA | ** | NS | * | ** | * | |
| Fsp.×origin×season×IBA | ** | NS | ** | ** | ** | |
| Experimental Factor | Rooting (%) | Aboveground f.w. (g) | Underground f.w. (g) | Aboveground d.w. (g) | Underground d.w. (g) | |
|---|---|---|---|---|---|---|
| Cutting origin | Greenhouse mother plants | 70.2 a ɫ | 1.74 b | 1.34 b | 0.39 b | 0.15 b |
| Wild mother plants | 44.0 b | 1.87 a | 1.45 a | 0.56 a | 0.24 a | |
| Collection season | Autumn | 68.0 a | 2.22 a | 1.83 a | 0.50 a | 0.19 b |
| Winter | 54.3 b | 1.68 b | 1.92 a | 0.46 b | 0.28 a | |
| Spring | 56.0 b | 1.62 b | 1.03 b | 0.47 ab | 0.18 b | |
| Summer | 50.2 b | 1.71 b | 0.80 c | 0.45 b | 0.14 c | |
| IBA treatment (mg L−1) | Rhizopon | 70.4 a | 1.98 a | 1.52 a | 0.53 a | 0.22 ab |
| 0 ¥ | 26.3 d | - | - | - | - | |
| 500 | 51.4 c | 1.66 c | 1.22 c | 0.44 c | 0.17 d | |
| 1500 | 63.5 ab | 1.85 ab | 1.26 c | 0.49 ab | 0.18 cd | |
| 3000 | 64.8 ab | 1.81 b | 1.38 b | 0.44 c | 0.19 bc | |
| 4500 | 62.1 ab | 1.77 bc | 1.44 ab | 0.46 bc | 0.19 cd | |
| 6000 | 61.5 b | 1.78 bc | 1.55 a | 0.47 bc | 0.23 a | |
| Significance § | Fcutting origin | ** | * | * | ** | ** |
| Fcollection season | ** | ** | ** | * | ** | |
| FIBA treatment | ** | ** | ** | ** | ** | |
| Forigin×season | ** | ** | ** | ** | ** | |
| Forigin×IBA | NS | ΝS | * | * | * | |
| Fseason×IBA | ** | ** | ** | * | ** | |
| Forigin×season×IBA | ** | * | ** | * | ** | |
| Experimental Factor | Rooting (%) | Aboveground f.w. (g) | Underground f.w. (g) | Aboveground d.w. (g) | Underground d.w. (g) | |
|---|---|---|---|---|---|---|
| Cutting origin | Greenhouse mother plants | 59.6 a ɫ | 1.46 a | 1.24 a | 0.35 a | 0.14 b |
| Wild mother plants | 18.8 b | 1.14 b | 0.93 b | 0.32 a | 0.19 a | |
| Collection season | Autumn | 37.6 b | 1.59 a | 1.64 a | 0.39 a | 0.19 a |
| Winter ¥ | 26.4 c | - | - | - | - | |
| Spring | 57.3 a | 0.97 c | 0.84 b | 0.32 b | 0.20 a | |
| Summer | 33.6 b | 1.34 b | 0.77 b | 0.31 b | 0.10 b | |
| IBA treatment (mg L−1) | Rhizopon | 44.9 ab | 1.39 a | 1.24 a | 0.33 a | 0.15 a |
| 0 ¥ | 19.7 d | - | - | - | - | |
| 500 | 34.3 c | 1.10 b | 0.96 a | 0.31 a | 0.17 a | |
| 1500 | 40.7 bc | 1.36 a | 1.03 a | 0.36 a | 0.15 a | |
| 3000 | 39.4 bc | 1.42 a | 1.05 a | 0.38 a | 0.16 a | |
| 4500 | 41.6 bc | 1.24 ab | 1.07 a | 0.34 a | 0.17 a | |
| 6000 | 50.5 a | 1.28 ab | 1.14 a | 0.30 a | 0.16 a | |
| Significance § | Fcutting origin | ** | ** | ** | NS | ** |
| Fcollection season | ** | ** | ** | * | ** | |
| FIBA treatment | ** | NS | NS | NS | NS | |
| Forigin×season | ** | * | ** | ** | ** | |
| Forigin×IBA | NS | ΝS | NS | NS | NS | |
| Fseason×IBA | ** | NS | NS | NS | NS | |
| Forigin×season×IBA | ** | NS | NS | NS | NS | |
| Experimental Factor | Rooting (%) | Aboveground f.w. (g) | Underground f.w. (g) | Aboveground d.w. (g) | Underground d.w. (g) | |
|---|---|---|---|---|---|---|
| Cutting origin | Greenhouse mother plants | 65.7 a ɫ | 2.17 b | 1.48 b | 0.53 b | 0.19 b |
| Wild mother plants | 41.3 b | 3.99 a | 2.27 a | 1.26 a | 0.38 a | |
| Collection season | Autumn | 66.2 a | 4.31 a | 2.63 a | 1.18 a | 0.35 a |
| Winter | 51.3 b | 3.19 b | 2.30 b | 0.92 b | 0.30 b | |
| Spring | 53.6 b | 2.25 d | 1.52 c | 0.70 c | 0.29 b | |
| Summer | 42.7 c | 2.58 c | 1.05 d | 0.78 c | 0.20 c | |
| IBA treatment (mg L−1) | Rhizopon | 64.2 ab | 3.38 a | 2.20 a | 1.00 a | 0.34 a |
| 0 | 29.2 d | 2.65 c | 0.99 c | 0.77 c | 0.22 c | |
| 500 | 42.2 c | 2.94 bc | 1.49 c | 0.88 bc | 0.22 c | |
| 1500 | 57.5 ab | 3.23 ab | 1.99 b | 0.93 ab | 0.28 b | |
| 3000 | 55.7 b | 3.01 ab | 2.01 ab | 0.89 abc | 0.28 b | |
| 4500 | 59.6 ab | 3.22 ab | 2.29 a | 0.92 ab | 0.33 a | |
| 6000 | 65.8 a | 3.14 ab | 2.16 ab | 0.87 bc | 0.29 b | |
| Significance § | Fcutting origin | ** | ** | ** | ** | ** |
| Fcollection season | ** | ** | ** | ** | ** | |
| FIBA treatment | ** | * | ** | * | ** | |
| Forigin×season | ** | ** | ** | ** | ** | |
| Forigin×IBA | * | ΝS | * | NS | * | |
| Fseason×IBA | ** | * | ** | NS | ** | |
| Forigin×season×IBA | * | NS | ** | NS | NS | |
| Experimental Factor | Rooting (%) | Aboveground f.w. (g) | Underground f.w. (g) | Aboveground d.w. (g) | Underground d.w. (g) | |
|---|---|---|---|---|---|---|
| Cutting origin | Greenhouse mother plants | 52.7 a ɫ | 1.56 b | 0.82 b | 0.41 b | 0.11 a |
| Wild mother plants | 43.0 b | 2.00 a | 1.07 a | 0.64 a | 0.11 a | |
| Collection season | Autumn | 61.6 a | 2.70 a | 1.31 a | 0.75 a | 0.19 a |
| Winter | 46.6 b | 1.25 c | 0.87 b | 0.43 b | 0.08 b | |
| Spring | 48.2 b | 1.57 b | 0.71 c | 0.48 b | 0.07 b | |
| Summer | 35.1 c | 1.61 b | 0.89 b | 0.43 b | 0.09 b | |
| IBA treatment (mg L−1) | Rhizopon | 59.6 a | 2.03 a | 1.04 a | 0.59 a | 0.13 a |
| 0 ¥ | 30.1 d | - | - | - | - | |
| 500 | 46.7 bc | 1.63 a | 0.78 b | 0.54 ab | 0.09 a | |
| 1500 | 54.3 ab | 1.80 a | 0.89 ab | 0.52 ab | 0.10 a | |
| 3000 | 53.0 ab | 1.80 a | 1.00 a | 0.51 ab | 0.12 a | |
| 4500 | 42.6 c | 1.76 a | 1.03 a | 0.50 ab | 0.13 a | |
| 6000 | 48.9 bc | 1.70 a | 0.93 ab | 0.48 b | 0.08 a | |
| Significance § | Fcutting origin | ** | ** | ** | ** | NS |
| Fcollection season | ** | ** | ** | ** | ** | |
| FIBA treatment | ** | NS | * | NS | NS | |
| Forigin×season | ** | ** | NS | ** | * | |
| Forigin×IBA | * | ΝS | NS | * | NS | |
| Fseason×IBA | NS | NS | NS | * | NS | |
| Forigin×season×IBA | * | NS | NS | * | NS | |
| Experimental Factor | Rooting (%) | Aboveground f.w. (g) | Underground f.w. (g) | Aboveground d.w. (g) | Underground d.w. (g) | |
|---|---|---|---|---|---|---|
| Cutting origin | Greenhouse mother plants | 69.1a ɫ | 1.69 b | 1.09 b | 0.46 b | 0.14 b |
| Wild mother plants | 70.3 a | 2.52 a | 1.38 a | 0.69 a | 0.21 a | |
| Collection season | Autumn | 81.3 a | 2.62 a | 1.86 a | 0.74 a | 0.20 a |
| Winter | 48.0 c | 1.88 c | 1.18 b | 0.54 c | 0.18 b | |
| Spring | 85.3 a | 2.18 b | 0.97 c | 0.60 b | 0.20 ab | |
| Summer | 64.1 b | 1.74 c | 0.94 c | 0.42 d | 0.13 c | |
| IBA treatment (mg L−1) | Rhizopon | 82.8 a | 2.30 a | 1.42 a | 0.75 a | 0.20 a |
| 0 | 60.5 de | 1.88 d | 0.83 e | 0.49 e | 0.16 cd | |
| 500 | 71.5 bc | 1.95 cd | 1.10 d | 0.52 de | 0.20 a | |
| 1500 | 79.5 ab | 2.30 a | 1.42 a | 0.60 b | 0.19 ab | |
| 3000 | 70.1 c | 2.15 ab | 1.40 ab | 0.55 cd | 0.18 abc | |
| 4500 | 67.2 cd | 2.04 bcd | 1.21 cd | 0.57 bc | 0.17 bcd | |
| 6000 | 56.3 e | 2.11 bc | 1.27 bc | 0.55 bcd | 0.14 d | |
| Significance § | Fcutting origin | NS | ** | ** | ** | ** |
| Fcollection season | ** | ** | ** | ** | ** | |
| FIBA treatment | ** | ** | ** | ** | ** | |
| Forigin×season | ** | ** | ** | ** | ** | |
| Forigin×IBA | ** | * | NS | ** | NS | |
| Fseason×IBA | ** | ** | ** | ** | * | |
| Forigin×season×IBA | ** | ** | ** | ** | NS | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martini, A.N.; Bertsouklis, K.; Vlachou, G.; Papafotiou, M. Investigating the Rooting of Stem Cuttings of Five Mediterranean Salvia spp., as a Means for Their Wider Exploitation in Sustainable Horticulture. Sustainability 2025, 17, 8999. https://doi.org/10.3390/su17208999
Martini AN, Bertsouklis K, Vlachou G, Papafotiou M. Investigating the Rooting of Stem Cuttings of Five Mediterranean Salvia spp., as a Means for Their Wider Exploitation in Sustainable Horticulture. Sustainability. 2025; 17(20):8999. https://doi.org/10.3390/su17208999
Chicago/Turabian StyleMartini, Aikaterini N., Konstantinos Bertsouklis, Georgia Vlachou, and Maria Papafotiou. 2025. "Investigating the Rooting of Stem Cuttings of Five Mediterranean Salvia spp., as a Means for Their Wider Exploitation in Sustainable Horticulture" Sustainability 17, no. 20: 8999. https://doi.org/10.3390/su17208999
APA StyleMartini, A. N., Bertsouklis, K., Vlachou, G., & Papafotiou, M. (2025). Investigating the Rooting of Stem Cuttings of Five Mediterranean Salvia spp., as a Means for Their Wider Exploitation in Sustainable Horticulture. Sustainability, 17(20), 8999. https://doi.org/10.3390/su17208999







