Ecological Interactions and Climate-Driven Dynamics of Pine Wilt Disease: Implications for Sustainable Forest Management
Abstract
1. Introduction
- H1: Does PWN occur at different vertical positions within the tree (upper canopy, middle canopy, lower trunk) across different sites and years?
- H2: Is B. mucronatus density associated with the vertical position and year relative to PWN?
- H3: Does M. alternatus emergence increase with an increase in the daily mean temperature during the flight season?
2. Materials and Methods
2.1. Selection of Survey Sites
2.2. Selection of Tree Species for Investigation at Each Site
2.3. Distribution of PWN and B. mucronatus in Felled Trees
2.4. Monitoring the Emergence of M. Alternatus Using Aggregation Pheromone Traps
2.5. Investigation of PWN Presence in M. alternatus
2.6. Data Analysis
3. Results
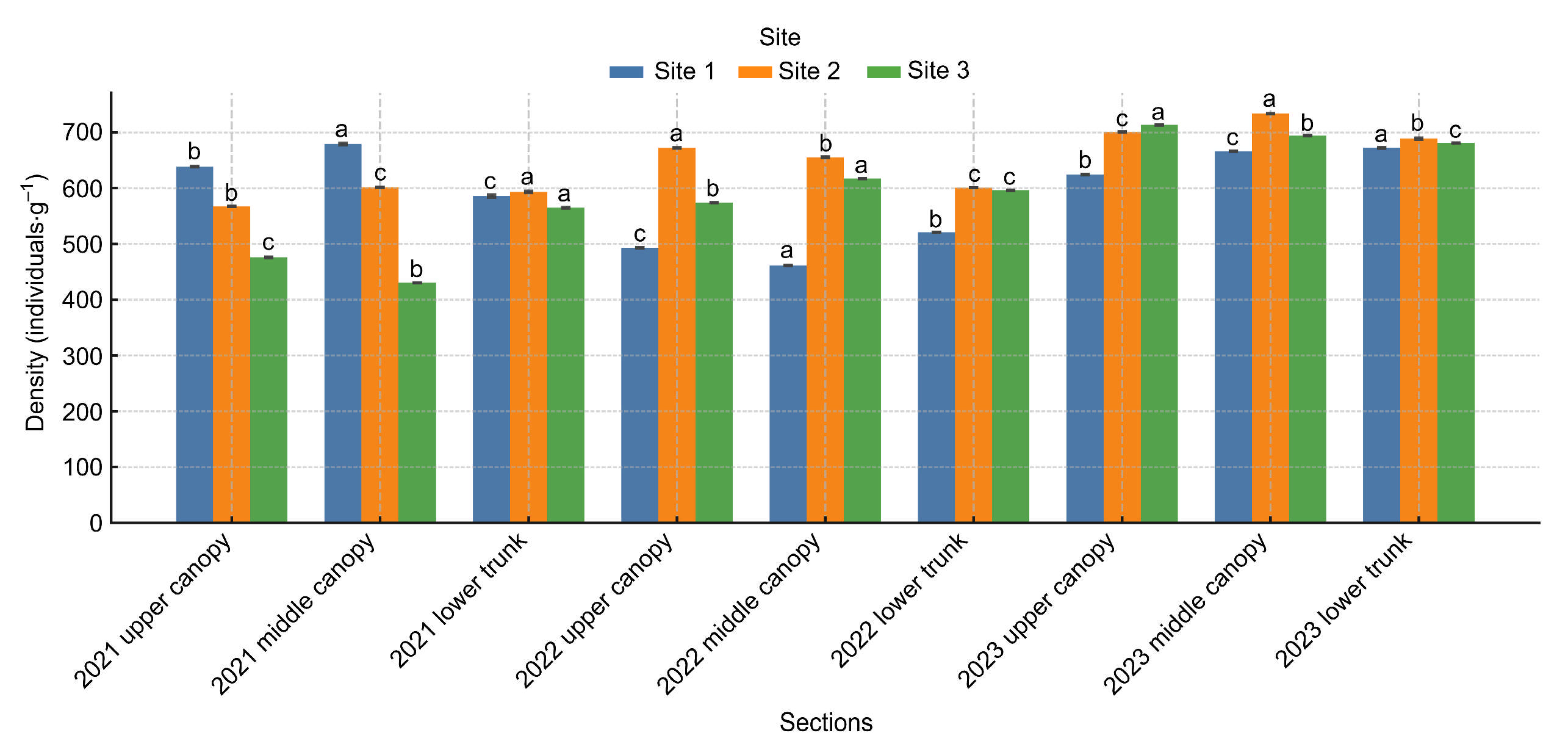
3.1. PWN Density in Felled Black Pine Trees
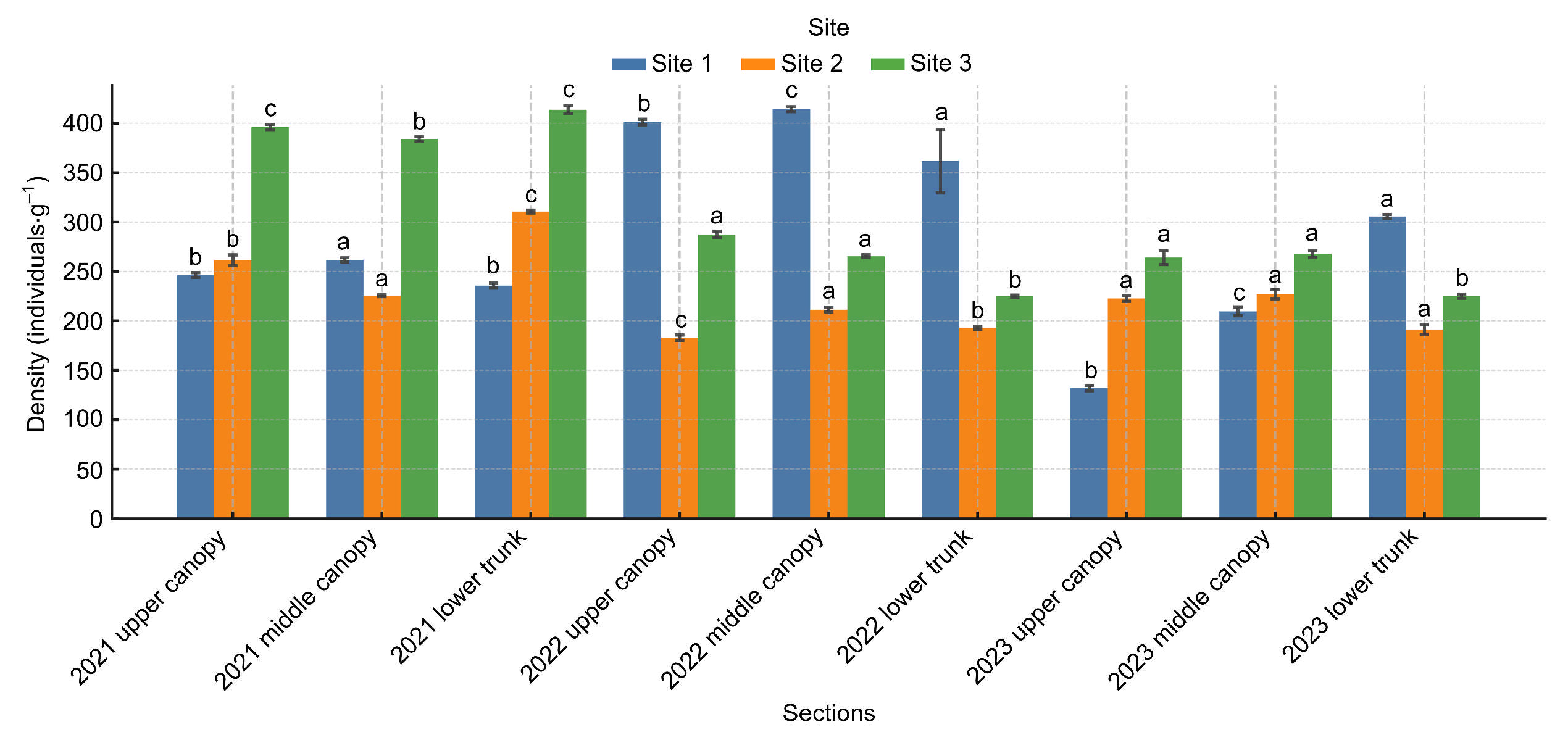
3.2. Distribution of B. mucronatus in Felled Trees
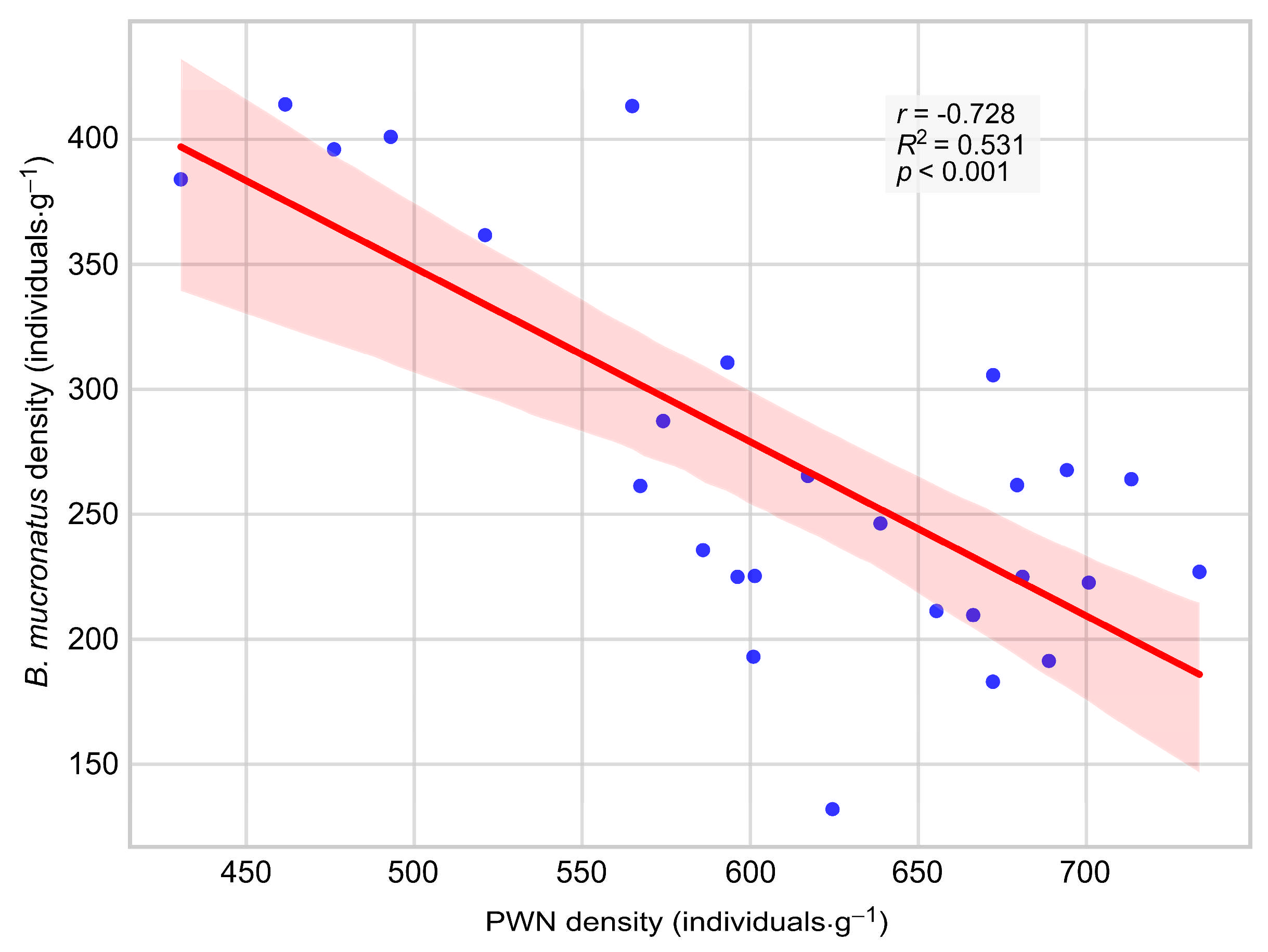
3.3. Comparison of PWN and B. mucronatus Densities in Felled Trees
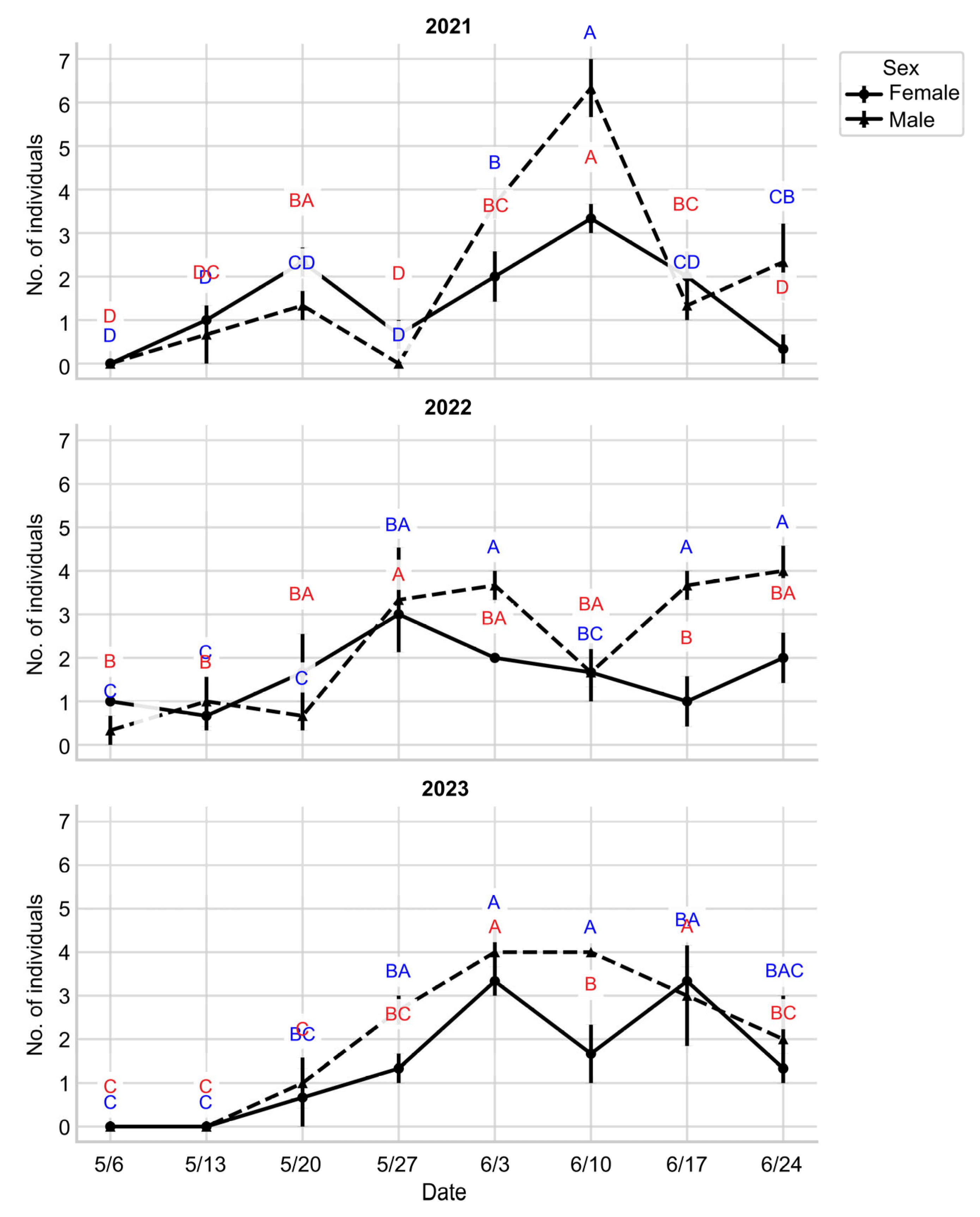
3.4. Density of M. alternatus, the PWN Vector
3.5. Density of PWN Hosted by M. alternatus
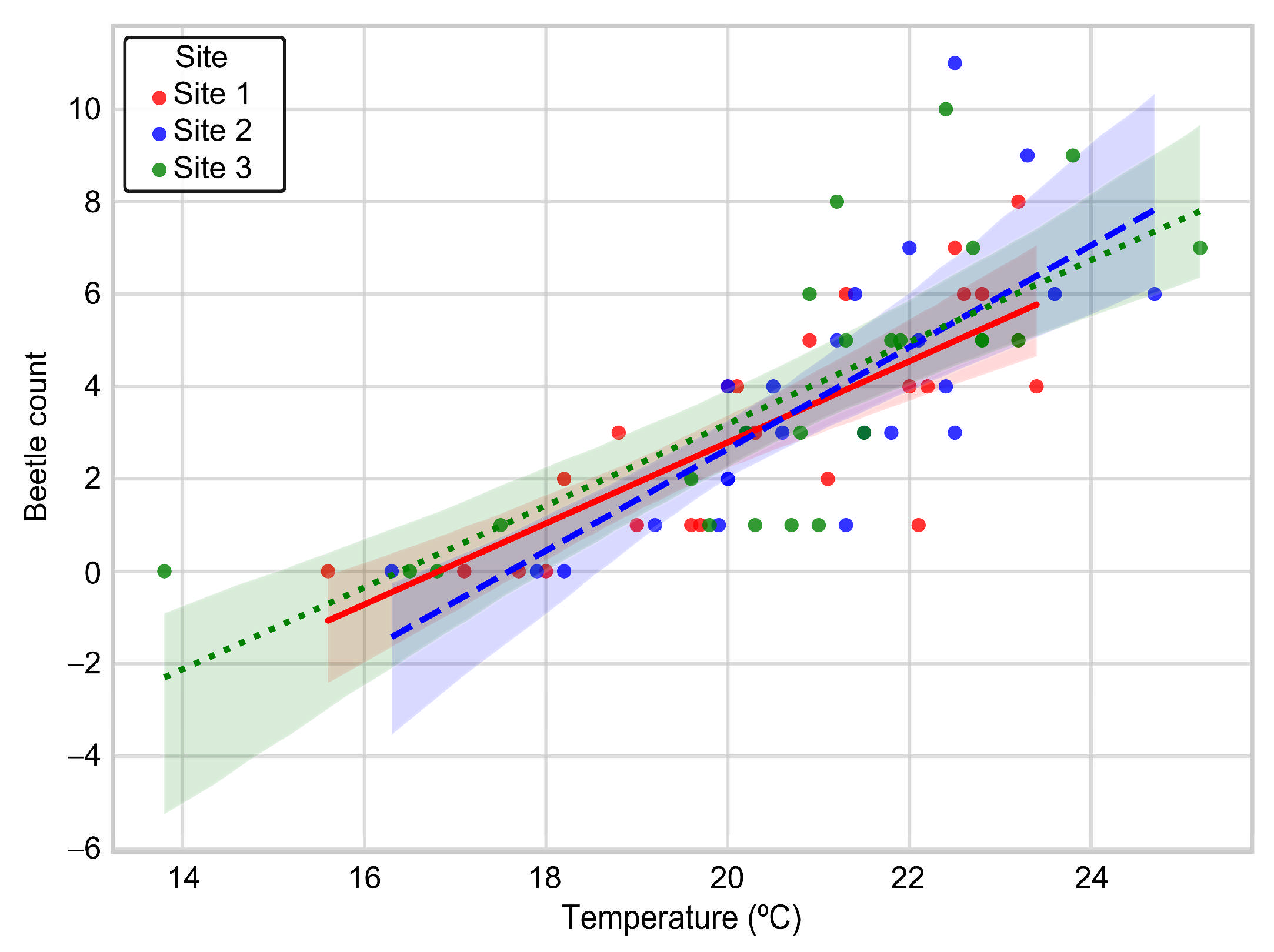
3.6. Comparison of Temperature-Dependent Vector Distribution Across the Survey Sites
4. Discussion
4.1. PWN Density Across Vertical Positions
4.2. Distribution Patterns of B. mucronatus
4.3. Co-Occurrence of PWN and B. mucronatus
4.4. Infestation of Vectors by PWN
4.5. Seasonal Emergence Dynamics of M. alternatus
4.6. Climatic Influences on M. alternatus Emergence
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DBH | diameter at breast height |
| DMRT | Duncan’s multiple range test |
| UAV | unmanned aerial vehicle |
| KMA | Korea Meteorological Administration |
| EtOH | ethanol |
| PWD | pine wilt disease |
| PWN | pine wood nematode |
References
- Korea Forest Service. Statistical Yearbook of Forestry, No. 53; Korea Forest Service: Seoul, Republic of Korea, 2023; p. 36. [Google Scholar][Green Version]
- Abatzoglou, J.T.; Williams, A.P. Impact of anthropogenic climate change on wildfire across western US forests. Proc. Natl. Acad. Sci. USA 2016, 113, 11770–11775. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar] [CrossRef]
- Jolly, W.M.; Cochrane, M.A.; Freeborn, P.H.; Holden, Z.A.; Brown, T.J.; Williamson, G.J.; Bowman, D.M.J.S. Climate-induced variations in global wildfire danger from 1979 to 2013. Nat. Commun. 2015, 6, 7537. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, F.; Yamane, A.; Ikeda, T. The Japanese pine sawyer beetle as the vector of pine wilt disease. Annu. Rev. Entomol. 1984, 29, 115–135. [Google Scholar] [CrossRef]
- Myers, R.F. Pathogenesis in pine wilt caused by pinewood nematode, Bursaphelenchus xylophilus. J. Nematol. 1988, 20, 236–244. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2618809/ (accessed on 27 September 2025).
- SAS Institute Inc. SAS/STAT Statistical Software, Version 9.1; SAS Publishing: Cary, NC, USA, 2003.
- Mamiya, Y. Pathology of the pine wilt disease caused by Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 1983, 21, 201–220. [Google Scholar] [CrossRef]
- Dwinell, L.D.; Nickle, W.R. An Overview of the Pine Wood Nematode Ban in North America; Gen. Tech. Rep. SE-55; U.S. Department of Agriculture, Forest Service, Southeastern Forest Experiment Station: Asheville, NC, USA, 1989; 13p. Available online: https://www.srs.fs.usda.gov/pubs/gtr/uncaptured/gtr_se055.pdf (accessed on 27 September 2025).
- Knowles, K.; Beaubien, Y.; Wingfield, M.J.; French, D.W. The pinewood nematode is new in Canada. Can. J. Plant Pathol. 1983, 5, 40–41. [Google Scholar]
- Sutherland, J.R.; Ring, F.M.; Seed, J.E. Canadian conifers as hosts of the pinewood nematode (Bursaphelenchus xylophilus): Results of seedling inoculations. Scand. J. For. Res. 1991, 6, 209–216. [Google Scholar] [CrossRef]
- Abelleira, A.; Picoaga, A.; Mansilla, J.P.; Aguin, O. Detection of Bursaphelenchus xylophilus, causal agent of pine wilt disease, on Pinus pinaster in northwestern Spain. Plant Dis. 2011, 95, 776. [Google Scholar] [CrossRef]
- Cheng, H.R.; Lin, M.; Li, W.; Fang, Z. The occurrence of a pine wilting disease caused by a nematode found in Nanjing. For. Pest Dis. 1983, 4, 1–5. [Google Scholar]
- Enda, N. The status of pine wilt disease caused by Bursaphelenchus xylophilus (Steiner & Buhrer) Nickle and its control in Korea. J. Korean Soc. For. Sci. 1989, 78, 248–253. (In Korean) [Google Scholar]
- Mota, M.M.; Braasch, H.; Bravo, M.A.; Penas, P.C.; Burgermeister, W.; Metge, K.; Sousa, E. First report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology 1999, 1, 727–734. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, X.; Li, J.; Ren, J.; Ren, L.; Luo, Y. Pine wilt disease in Northeast and Northwest China: A comprehensive risk review. Forests 2023, 14, 174. [Google Scholar] [CrossRef]
- Yi, C.K.; Byun, B.H.; Park, J.D.; Yang, S.I.; Chang, K.H. First finding of the pine wood nematode, Bursaphelenchus xylophilus (Steiner & Buhrer) Nickle, and its insect vector in Korea. Res. Rep. For. Res. Inst. Seoul 1989, 38, 141–149. (In Korean) [Google Scholar]
- Mamiya, Y.; Enda, N. Transmission of Bursaphelenchus lignicolus (Nematoda: Aphelenchoididae) by Monochamus alternatus (Coleoptera: Cerambycidae). Nematologica 1972, 18, 159–162. [Google Scholar] [CrossRef]
- Kishi, Y. Pine Wood Nematode and the Japanese Pine Sawyer; Thomas Company Ltd.: Tokyo, Japan, 1995; p. 302. [Google Scholar]
- Futai, K. Pine wilt in Japan: From first incidence to the present. In Pine Wilt Disease; Zhao, B.G., Futai, K., Sutherland, J.R., Takeuchi, Y., Eds.; Springer: Tokyo, Japan, 2008; pp. 5–12. [Google Scholar]
- Park, Y.S.; Chung, Y.J.; Moon, Y.S. Hazard ratings of pine forests to a pine wilt disease at two spatial scales (individual trees and stands) using self-organizing map and random forest. Ecol. Inform. 2013, 13, 40–46. [Google Scholar] [CrossRef]
- Hirata, A.; Nakamura, K.; Nakao, K.; Kominami, Y.; Tanaka, N.; Ohashi, H.; Takano, K.T.; Takeuchi, W.; Matsui, T. Potential distribution of pine wilt disease under future climate change scenarios. PLoS ONE 2017, 12, e0182837. [Google Scholar] [CrossRef]
- Lu, Q.; Wang, W.; Liang, J.; Yan, D.; Jia, X.; Zhang, X. Potential suitability assessment of Bursaphelenchus xylophilus in China. For. Res. Chin. Acad. For. 2005, 18, 460–464. [Google Scholar]
- Rutherford, T.A.; Webster, J.M. Distribution of pine wilt disease with respect to temperature in North America, Japan, and Europe. Can. J. For. Res. 1987, 17, 1050–1059. [Google Scholar] [CrossRef]
- Kwon, T.S.; Lim, J.H.; Sim, S.J.; Kwon, Y.D.; Son, S.K.; Lee, K.Y.; Kim, Y.T.; Park, J.W.; Shin, C.H.; Ryu, S.B.; et al. Distribution patterns of Monochamus alternatus and M. saltuarius (Coleoptera: Cerambycidae) in Korea. J. Korean For. Soc. 2006, 95, 543–550. (In Korean) [Google Scholar]
- Jung, J.K.; Hong, E.J.; Kim, T.G.; Jeong, J.C. Ground beetle (Coleoptera: Carabidae) assemblage structure in Taeanhaean National Park: A comparison between coastal dune and windbreak forest. Korean J. Environ. Ecol. 2018, 32, 147–153. (In Korean) [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, Y.J.; Han, S.Y.; Park, C.G.; Choi, W.S. Detection of the infection stage of pine wilt disease and spread distance using UAV and remote sensing. Remote Sens. 2024, 16, 364. [Google Scholar] [CrossRef]
- Korea Forest Service. Pine Wilt Disease Control Guide; Korea Forest Service: Seoul, Republic of Korea, 2016; p. 20. [Google Scholar]
- Baermann, G. Eine einfache Methode zur Auffindung von Ancylostomum (Nematoden) Larven in Erdproben. Geneeskd Tijdschr Ned. Indië 1917, 57, 131–137. [Google Scholar]
- Korea Meteorological Administration. Climate Statistics Analysis. 2023. Available online: https://data.kma.go.kr/climate/RankState/selectRankStatisticsDivisionList.do?pgmNo=179 (accessed on 26 March 2024).
- Estorninho, M.; Lino-Neto, T.; Barbosa, P.; Fonseca, L. Differential impact of the pinewood nematode on Pinus pinaster under drought conditions. Front. Plant Sci. 2022, 13, 841707. [Google Scholar] [CrossRef] [PubMed]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Gause, G.F. The Struggle for Existence; Williams & Wilkins: Baltimore, MD, USA, 1934. [Google Scholar]
- Warton, D.I.; Blanchet, F.G.; O’Hara, R.B.; Ovaskainen, O.; Taskinen, S.; Walker, S.C.; Hui, F.K.C. Joint modelling in community ecology. Trends Ecol. Evol. 2015, 30, 766–779. [Google Scholar] [CrossRef] [PubMed]
- Mamiya, Y. History of pine wilt disease in Japan. J. Nematol. 1988, 20, 219–226. [Google Scholar]
- Lee, M.Y.; Lee, C.H.; Ha, M.L.; Kim, H. The dynamics of pine wilt nematode (B. xylophilus) in pine wilt disease-infected trees in Gyeongsangnam-do, Sacheon City. J. Korean Island 2023, 35, 213–227. (In Korean) [Google Scholar] [CrossRef]
- Polomski, J.; Rigling, D. Effect of watering regime on disease development in Pinus sylvestris seedlings inoculated with Bursaphelenchus vallesianus and B. mucronatus. Plant Dis. 2010, 94, 1055–1061. [Google Scholar] [CrossRef]
- Zhou, L.F.; Chen, F.M.; Wang, J.C.; Pan, H.Y.; Ye, J.R. Virulence of Bursaphelenchus mucronatus to pine seedlings and trees under field conditions. For. Pathol. 2016, 46, 563–570. [Google Scholar] [CrossRef]
- Park, S.C.; Moon, Y.S.; Kim, D.S. Low-pathogenic pinewood nematode found in dead trees and resistance of pines induced by its pre-inoculation. Korean J. Appl. Entomol. 2014, 53, 141–147. (In Korean) [Google Scholar] [CrossRef]
- Dormann, C.F.; Bobrowski, M.; Dehling, D.M.; Harris, D.J.; Hartig, F.; Lischke, H.; Moretti, M.; Pagel, J.; Pinkert, S.; Schleuning, M.; et al. Biotic interactions in species distribution modelling: Ten questions to guide interpretation and avoid false conclusions. Glob. Ecol. Biogeogr. 2018, 27, 1004–1022. [Google Scholar] [CrossRef]
- Gao, R.; Liu, L.; Fan, S.; Zheng, W.; Liu, R.; Zhang, Z.; Huang, R.; Zhao, L.; Shi, J. Occurrence and potential diffusion of pine wilt disease mediated by insect vectors in China under climate change. Pest Manag. Sci. 2024, 80, 6068–6081. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Lee, S.M.; Chung, Y.J.; Choi, K.S.; Moon, Y.S.; Park, C.G. Emergence ecology of Japanese pine sawyer, Monochamus alternatus (Coleoptera: Cerambycidae), a vector of pinewood nematode, Bursaphelenchus xylophilus. Korean J. Appl. Entomol. 2003, 42, 307–313. (In Korean) [Google Scholar]
- Lee, S.M.; Jung, Y.H.; Seo, S.T.; Kim, D.S.; Lee, D.W. Comparison of nematicidal effect and residual amount by injection time and number of holes using emamectin benzoate via tree injection against pine wood nematode, B. xylophilus. Korean J. Pestic. Sci. 2021, 25, 371–378. (In Korean) [Google Scholar]
- Lee, H.; Kim, J.; Park, S.; Choi, W.; Kang, J. Temperature-dependent oviposition models for Monochamus saltuarius (Coleoptera: Cerambycidae). Insects 2024, 15, 597. [Google Scholar] [CrossRef]
- Jung, C.S.; Kwon, H.J.; Kim, H.; Kim, J.H.; Nam, Y.W.; Kim, D.S. A Model for Predicting Spring Emergence of Monochamus saltuarius (Coleoptera: Cerambycidae) from Korean white pine, Pinus koraiensis. J. Econ. Entomol. 2015, 108, 1830–1838. [Google Scholar] [CrossRef]
- Pajares, J.A.; Alvarez, G.; Ibeas, F.; Gallego, D.; Hall, D.R.; Farman, D.I. Identification and field activity of a male-produced aggregation pheromone in the pine sawyer beetle, Monochamus galloprovincialis. J. Chem. Ecol. 2010, 36, 570–583. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.M.; Jung, Y.H.; Kwon, Y.D.; Kim, D.S.; Lee, D.W.; Park, C.G. Field evaluation on the synergistic attractiveness of 2-(1-undecyloxy)-1-ethanol and ipsenol to Monochamus saltuarius. Entomol. Res. 2016, 46, 31–35. [Google Scholar] [CrossRef]
- Lee, H.R.; Lee, S.C.; Lee, D.H.; Choi, W.S.; Jung, C.S.; Jeon, J.H.; Kim, J.E.; Park, I.K. Identification of the aggregation-sex pheromone produced by male Monochamus saltuarius, a major insect vector of the pine wood nematode. J. Chem. Ecol. 2017, 43, 670–678. [Google Scholar] [CrossRef]
- National Institute of Forest Science. A study on the establishment of a comprehensive management system for prevention of the spread of pine nematode damage. Natl. Inst. For. Sci. Res. Rep. 2023, 23, 14–22. (In Korean) [Google Scholar]
- Xiao, Y.; Li, S.; Zhang, X.; Chen, F.; Xu, J.; Zhao, L. Predicting the global potential distribution of Bursaphelenchus xylophilus under climate change scenarios using MaxEnt. BMC Ecol. Evol. 2024, 24, 48. [Google Scholar] [CrossRef]
- Gao, R.; Liu, L.; Fan, S.; Zhang, Z.; Zhao, L.; Shi, J. Relationship between pine wilt disease outbreaks and climatic factors in China. Forests 2019, 10, 816. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, S.M.; Huh, H.S.; Park, N.C.; Park, C.G. Escape of pine wood nematode, Bursaphelenchus xylophilus, through feeding and oviposition behavior of Monochamus alternatus and M. saltuarius adults. Korean J. Appl. Entomol. 2009, 48, 527–533. (In Korean) [Google Scholar] [CrossRef]
- Enda, N. Removing dauer larvae of Bursaphelenchus lignicolus from the body of Monochamus alternatus. Trans. Annu. Meet. Kanto Branch Jpn. For. Soc. 1972, 24, 32. (In Japanese) [Google Scholar]
- Hosoda, R.; Kobayashi, K. Drop-off procedures of the pine wood nematode from the pine sawyer (II). Trans. Annu. Meet. Kanto Branch Jpn. For. Soc. 1978, 29, 131–133. (In Japanese) [Google Scholar]
- Zou, Y.; Li, X.; Wang, Z.; Chen, J.; Xu, H.; Zhang, Y. Climate change may make pine wilt disease more prevalent by incorporating biotic interactions into risk projections. J. Appl. Ecol. 2024, 61, e14801. [Google Scholar] [CrossRef]
- Aierken, N.; Wang, Y.; Zhang, H.; Sun, J. Assessing global pine wilt disease risk based on ensemble modelling of climate and occurrences. Ecol. Indic. 2024, 164, 112089. [Google Scholar] [CrossRef]
- Barker, B.; Coop, L. Japanese Pine Sawyer Beetle (Monochamus alternatus) Phenology/Degree-Day and Climate Suitability Model White Paper, Version 1.0; Oregon IPM Center, Oregon State University: Corvallis, OR, USA, 2020.
- Gao, R.; Zhang, Z.; Zhao, L.; Shi, J. The Potentially Suitable Geographical Area for Monochamus alternatus under Current and Future Climates. Insects 2023, 14, 182. [Google Scholar] [CrossRef]
- Chen, L.; Lu, W.; Lamont, B.B.; Liu, Y.; Wei, P.; Xue, W.; Xiong, Z.; Tang, L.; Wang, Y.; Wang, P.; et al. Modeling the Distribution of Pine Wilt Disease in China under Climate Change. Ecol. Evol. 2024, 14, e70277. [Google Scholar] [CrossRef]


| Site | Area | Location (GPS) | PWD Damage Level |
|---|---|---|---|
| Site 1 | Geoje-si, Jangseungpo-dong, Irun-myeon | 35°24′50.00″ N 129°13′02.00″ E 35°22′53.00″ N 129°11′56.00″ E | Severe |
| Site 2 | Sacheon-si, Seopo-myeon | 35°39′34.00″ N 128°34′51.00″ E | Severe |
| Site 3 | Jinju-si, Jangjae-dong, Hachon-dong | 35°20′36.00″ N 128°10′00.00″ E 35°22′38.00″ N 128°07′47.00″ E | Moderate |
| Area | Year | Month/Day | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 5/6 | 5/13 | 5/19 | 5/27 | 6/2 | 6/10 | 6/17 | 6/24 | ||
| Site 1 | 2021 | 18.0 | 19.7 | 20.1 | 17.7 | 21.3 | 23.2 | 20.0 | 21.1 |
| 2022 | 19.0 | 18.8 | 19.6 | 22.2 | 20.9 | 20.3 | 22.6 | 23.2 | |
| 2023 | 15.6 | 17.1 | 18.2 | 22.0 | 22.8 | 22.5 | 23.4 | 22.1 | |
| Site 2 | 2021 | 17.9 | 20.6 | 20.0 | 19.2 | 21.2 | 22.5 | 21.5 | 21.3 |
| 2022 | 20.0 | 19.9 | 20.5 | 22.0 | 21.4 | 20.2 | 22.5 | 24.7 | |
| 2023 | 16.3 | 18.2 | 20.0 | 21.8 | 23.3 | 22.1 | 23.6 | 22.4 | |
| Site 3 | 2021 | 13.8 | 21.0 | 20.8 | 17.5 | 20.9 | 22.4 | 21.5 | 21.8 |
| 2022 | 19.8 | 20.7 | 19.6 | 21.2 | 21.3 | 20.2 | 22.8 | 25.2 | |
| 2023 | 16.5 | 16.8 | 20.3 | 21.9 | 22.7 | 22.8 | 23.8 | 23.2 | |
| Year | Site | Section | Mean ± SD | F(df) | p-Value |
|---|---|---|---|---|---|
| 2021 | Site 1 | Upper canopy | 585.9 ± 1.0 | F(2,6) = 8282.0 | p < 0.001 |
| Middle canopy | 679.4 ± 0.2 | ||||
| Lower trunk | 638.7 ± 1.1 | ||||
| 2021 | Site 2 | Upper canopy | 567.3 ± 2.9 | F(2,6) = 325.7 | p < 0.001 |
| Middle canopy | 601.3 ± 0.5 | ||||
| Lower trunk | 593.2 ± 0.0 | ||||
| 2021 | Site 3 | Upper canopy | 430.5 ± 2.5 | F(2,6) = 1807.1 | p < 0.001 |
| Middle canopy | 476.1 ± 3.9 | ||||
| Lower trunk | 564.9 ± 1.3 | ||||
| 2022 | Site 1 | Upper canopy | 461.6 ± 1.4 | F(2,6) = 2204.3 | p < 0.001 |
| Middle canopy | 493.0 ± 1.2 | ||||
| Lower trunk | 521.1 ± 0.5 | ||||
| 2022 | Site 2 | Upper canopy | 574.1 ± 0.6 | F(2,6) = 1589.2 | p < 0.001 |
| Middle canopy | 617.1 ± 1.3 | ||||
| Lower trunk | 596.2 ± 0.7 | ||||
| 2022 | Site 3 | Upper canopy | 600.9 ± 0.6 | F(2,6) = 11,859.2 | p < 0.001 |
| Middle canopy | 655.4 ± 0.4 | ||||
| Lower trunk | 672.2 ± 0.7 | ||||
| 2023 | Site 1 | Upper canopy | 624.5 ± 1.7 | F(2,6) = 771.4 | p < 0.001 |
| Middle canopy | 666.3 ± 2.2 | ||||
| Lower trunk | 672.3 ± 0.5 | ||||
| 2023 | Site 2 | Upper canopy | 688.9 ± 0.6 | F(2,6) = 5050.7 | p < 0.001 |
| Middle canopy | 733.7 ± 0.5 | ||||
| Lower trunk | 700.7 ± 0.6 | ||||
| 2023 | Site 3 | Upper canopy | 681.0 ± 0.5 | F(2,6) = 1531.4 | p < 0.001 |
| Middle canopy | 694.2 ± 0.7 | ||||
| Lower trunk | 713.4 ± 0.9 |
| Year | Site | Section | Mean ± SD | F(df) | p-Value |
|---|---|---|---|---|---|
| 2021 | Site 1 | Upper canopy | 246.3 ± 4.2 | F(2,6) = 11.65 | p = 0.009 |
| Middle canopy | 261.7 ± 9.5 | ||||
| Lower trunk | 235.7 ± 5.0 | ||||
| 2021 | Site 2 | Upper canopy | 261.3 ± 3.5 | F(2,6) = 471.9 | p < 0.001 |
| Middle canopy | 310.7 ± 4.5 | ||||
| Lower trunk | 225.3 ± 1.5 | ||||
| 2021 | Site 3 | Upper canopy | 384.0 ± 2.7 | F(2,6) = 27.1 | p = 0.001 |
| Middle canopy | 396.0 ± 4.4 | ||||
| Lower trunk | 413.3 ± 6.8 | ||||
| 2022 | Site 1 | Upper canopy | 401.0 ± 5.0 | F(2,6) = 87.5 | p < 0.001 |
| Middle canopy | 414.0 ± 4.6 | ||||
| Lower trunk | 361.7 ± 5.0 | ||||
| 2022 | Site 2 | Upper canopy | 193.0 ± 2.7 | F(2,6) = 43.9 | p < 0.001 |
| Middle canopy | 211.3 ± 4.0 | ||||
| Lower trunk | 183.0 ± 4.4 | ||||
| 2022 | Site 3 | Upper canopy | 287.3 ± 2.5 | F(2,6) = 2.89 | p = 0.132 |
| Middle canopy | 265.3 ± 55.7 | ||||
| Lower trunk | 225.0 ± 1.7 | ||||
| 2023 | Site 1 | Upper canopy | 132.0 ± 4.6 | F(2,6) = 357.2 | p < 0.001 |
| Middle canopy | 209.7 ± 5.0 | ||||
| Lower trunk | 305.7 ± 12.0 | ||||
| 2023 | Site 2 | Upper canopy | 227.0 ± 7.9 | F(2,6) = 21.4 | p = 0.002 |
| Middle canopy | 222.7 ± 7.8 | ||||
| Lower trunk | 191.3 ± 6.0 | ||||
| 2023 | Site 3 | Upper canopy | 267.7 ± 8.5 | F(2,6) = 51.2 | p < 0.001 |
| Middle canopy | 264.0 ± 3.6 | ||||
| Lower trunk | 225.0 ± 3.6 |
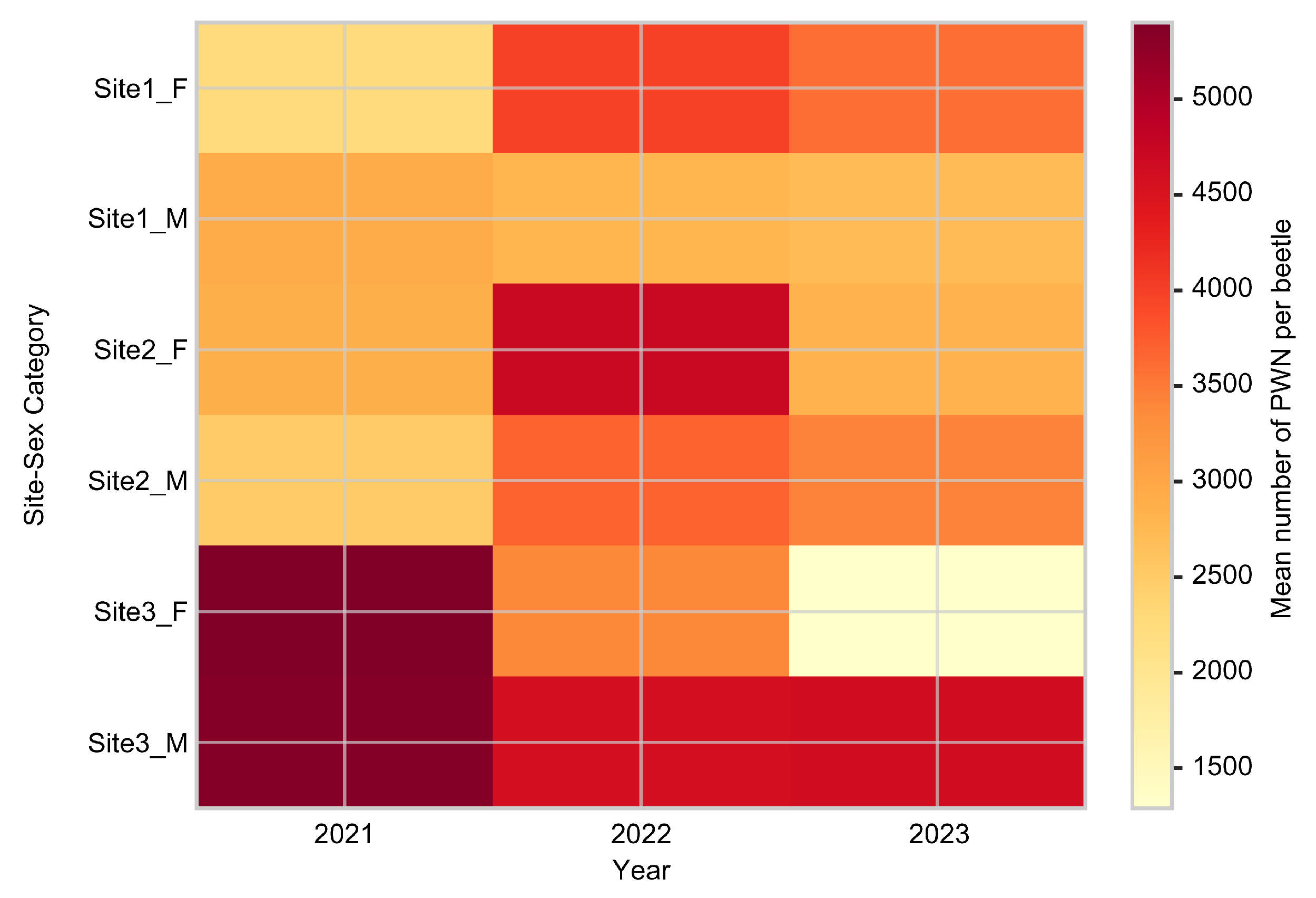
| Year | Site 1 ♀ | Site 1 ♂ | Site 2 ♀ | Site 2 ♂ | Site 3 ♀ | Site 3 ♂ | Infection Rate (%) |
|---|---|---|---|---|---|---|---|
| 2021 | 2083.6 | 3041.9 | 2902.9 | 2741.6 | 5804.4 | 4014.4 | 57 |
| 2022 | 3504.4 | 2754.3 | 4629.7 | 3422.8 | 3589.7 | 4100.0 | 55 |
| 2023 | 2646.1 | 2576.6 | 2949.4 | 2797.6 | 1226.3 | 4554.3 | 54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.K.; Kim, H.; Ha, M.-L. Ecological Interactions and Climate-Driven Dynamics of Pine Wilt Disease: Implications for Sustainable Forest Management. Sustainability 2025, 17, 8796. https://doi.org/10.3390/su17198796
Lee CK, Kim H, Ha M-L. Ecological Interactions and Climate-Driven Dynamics of Pine Wilt Disease: Implications for Sustainable Forest Management. Sustainability. 2025; 17(19):8796. https://doi.org/10.3390/su17198796
Chicago/Turabian StyleLee, Chong Kyu, Hyun Kim, and Man-Leung Ha. 2025. "Ecological Interactions and Climate-Driven Dynamics of Pine Wilt Disease: Implications for Sustainable Forest Management" Sustainability 17, no. 19: 8796. https://doi.org/10.3390/su17198796
APA StyleLee, C. K., Kim, H., & Ha, M.-L. (2025). Ecological Interactions and Climate-Driven Dynamics of Pine Wilt Disease: Implications for Sustainable Forest Management. Sustainability, 17(19), 8796. https://doi.org/10.3390/su17198796






