Analysis of the Efficiency and Environmental Impact of Municipal Solid Waste Incineration as a Tool for Sustainability Development in Kazakhstan
Abstract
1. Introduction
1.1. Literature Review
1.2. Theoretical Basis
- 1
- Landfill gases are gaseous products generated by the bacterial decomposition of the solid organic components of waste. These gases primarily consist of methane and carbon dioxide, with minor amounts of hydrogen sulfide and other gaseous compounds, many of which are greenhouse gases.
- 2
- Combustion flue gases are produced during the direct oxidation of MSW. They are characterized by high temperature, highly variable composition (due to the diverse range of materials combusted), and typically exhibit significant toxicity. This toxicity arises largely from the presence of inorganic substances of undefined chemical composition introduced into the incinerator or furnace, especially when unsorted waste undergoes thermal treatment.
- Sulfur oxides: This reduction is primarily due to the relatively low sulfur content of MSW, which ranges from 0.05% to 0.3% of the total mass. Additionally, a portion of the sulfur compounds present in MSW is converted into sulfates during combustion, which remain immobilized in the slag;
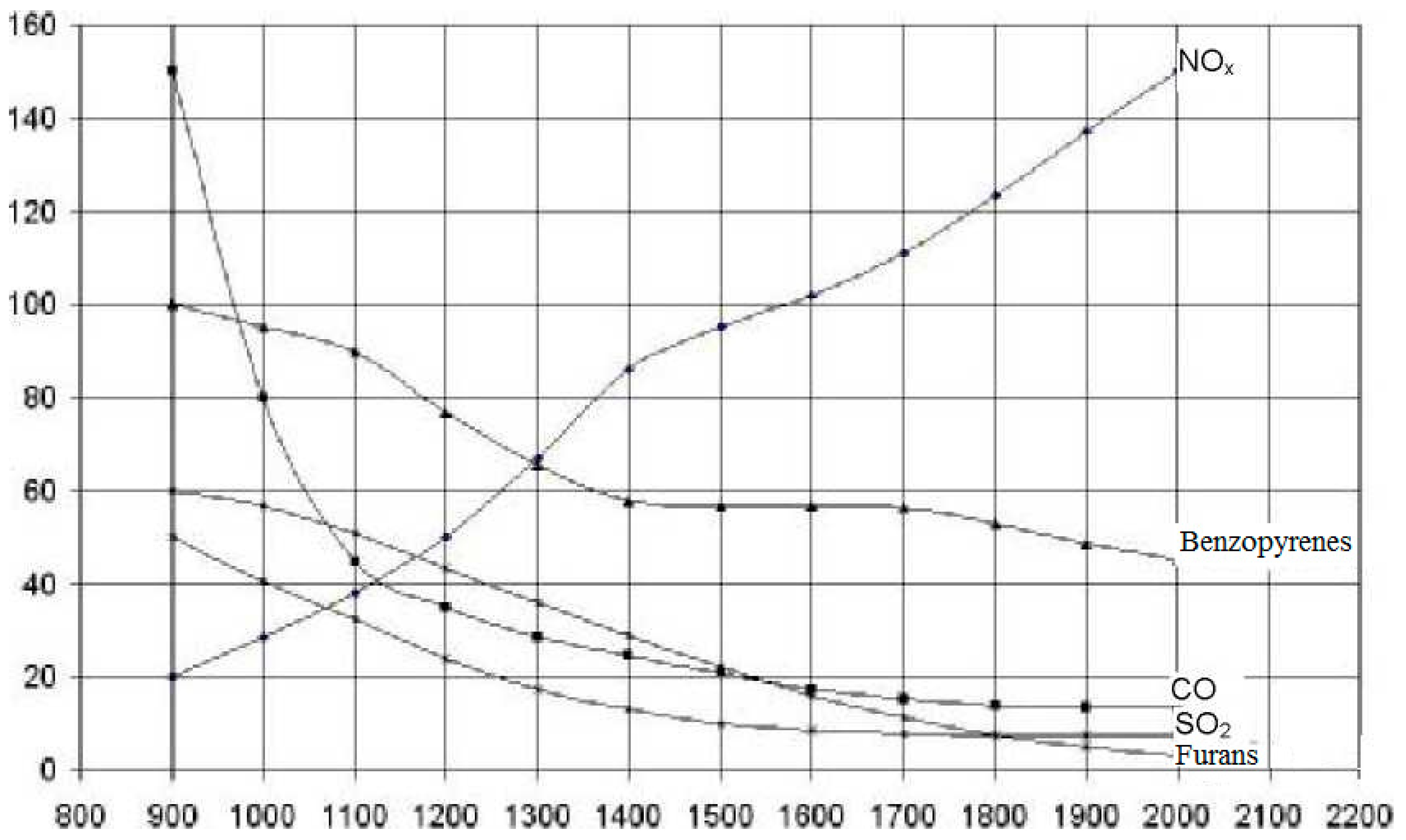
- Nitrogen oxides: The concentration of nitrogen oxides in flue gases depends largely on combustion temperature. In MSW incineration units, operating temperatures typically range between 850 and 1000 °C, whereas substantial nitrogen oxide formation generally occurs only at temperatures exceeding 1100 °C
- Primary formation, which occurs during the combustion of MSW at temperatures ranging from 300 to 600 °C;
- Secondary formation, which takes place during the cooling phase of flue gases containing hydrogen chloride (HCl), copper and iron compounds, and carbonaceous particulate matter, typically within the temperature range of 250 to 450 °C. This secondary formation is facilitated by a heterogeneous oxychlorination reaction involving carbon particles.
- Neutralization of hydrogen chloride (HCl) produced during combustion using alkaline reagents such as sodium bicarbonate (soda), calcium hydroxide (lime), or potassium hydroxide, thereby reducing the availability of HCl for dioxin synthesis;
- Inactivation of catalytically active transition metal ions, particularly copper and iron, by converting them into chemically inert forms–for example, through complexation of copper ions with amine-based ligands–thereby diminishing their catalytic role in dioxin formation
2. Materials and Methods
Equipment
- Heat output: 0.4 MW (0.34 Gcal/h);
- Grate area: 0.63 m2;
- Estimated fuel consumption at maximum power: 70 kg/h;
- Maximum working pressure: 6 kgf/cm2;
- Exhaust gas temperature: 170 °C;
- Inlet water temperature: 70 °C;
- Outlet water temperature: 150 °C.
- Oxygen (O2);
- Nitric oxide (NO);
- Total nitrogen oxides (NOx);
- Nitrogen dioxide (NO2);
- Carbon dioxide (CO2);
- Sulfur dioxide (SO2);
- Carbon monoxide (CO);
- Hydrogen sulfide (H2S);
- Ammonia (NH3);
- Hydrocarbons expressed as methane (CH4), propane (C3H8), or hexane (C6H14) equivalents;
- Additionally, measurements included:
- Differential pressure;
- Temperature and either excess pressure or vacuum of the gas flow at the sampling point;
- Excess air ratio and heat loss coefficients.
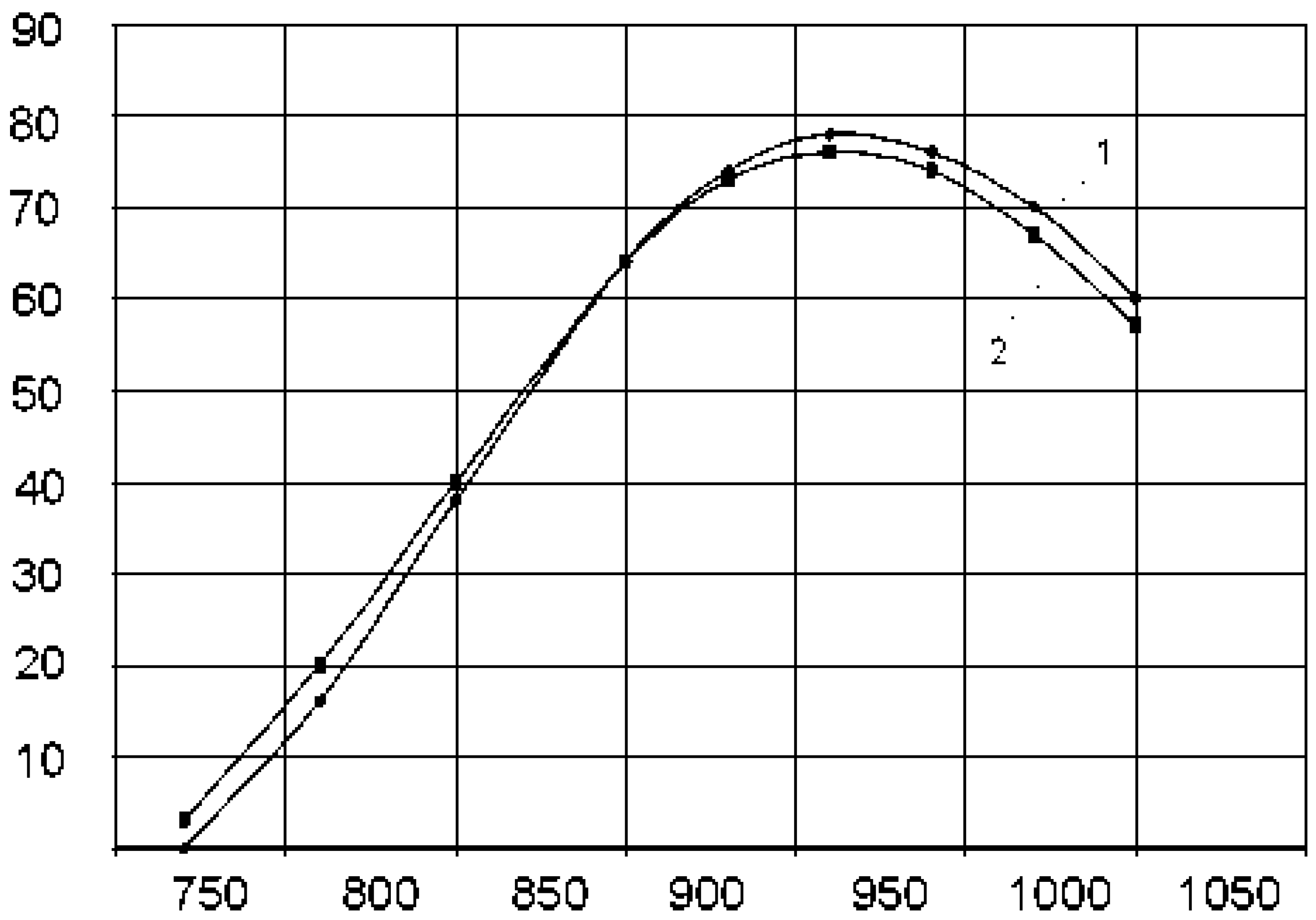
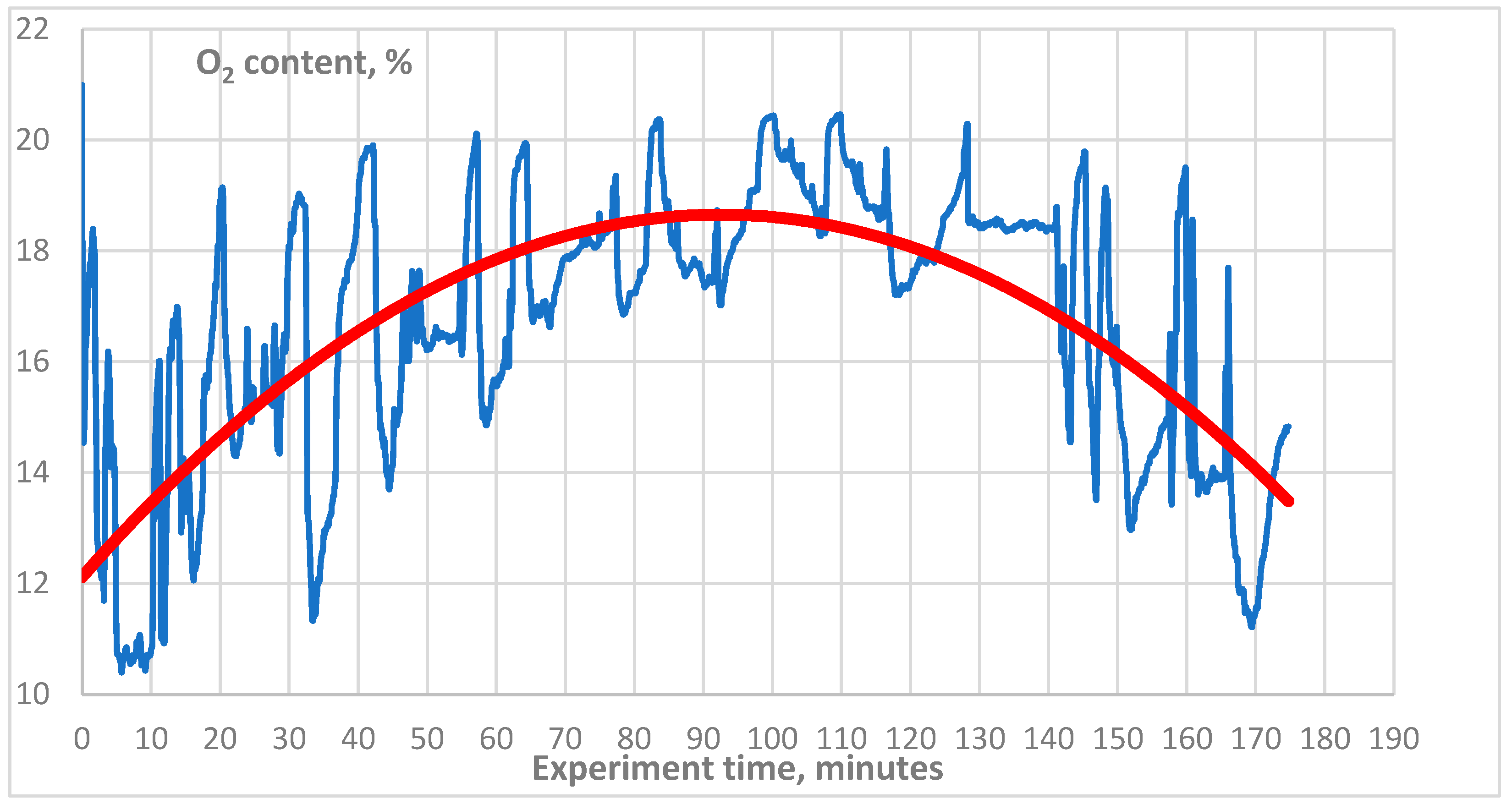
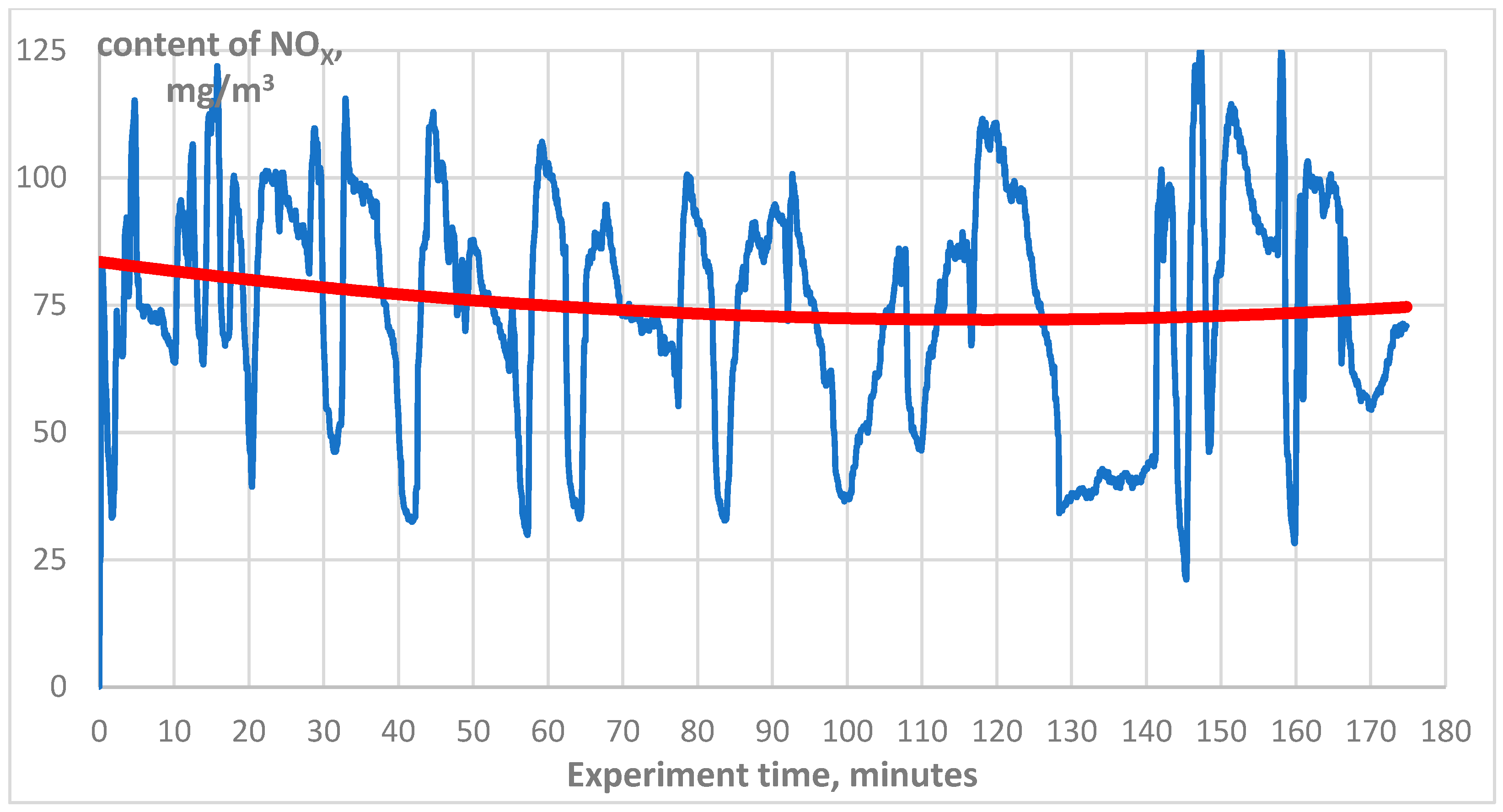
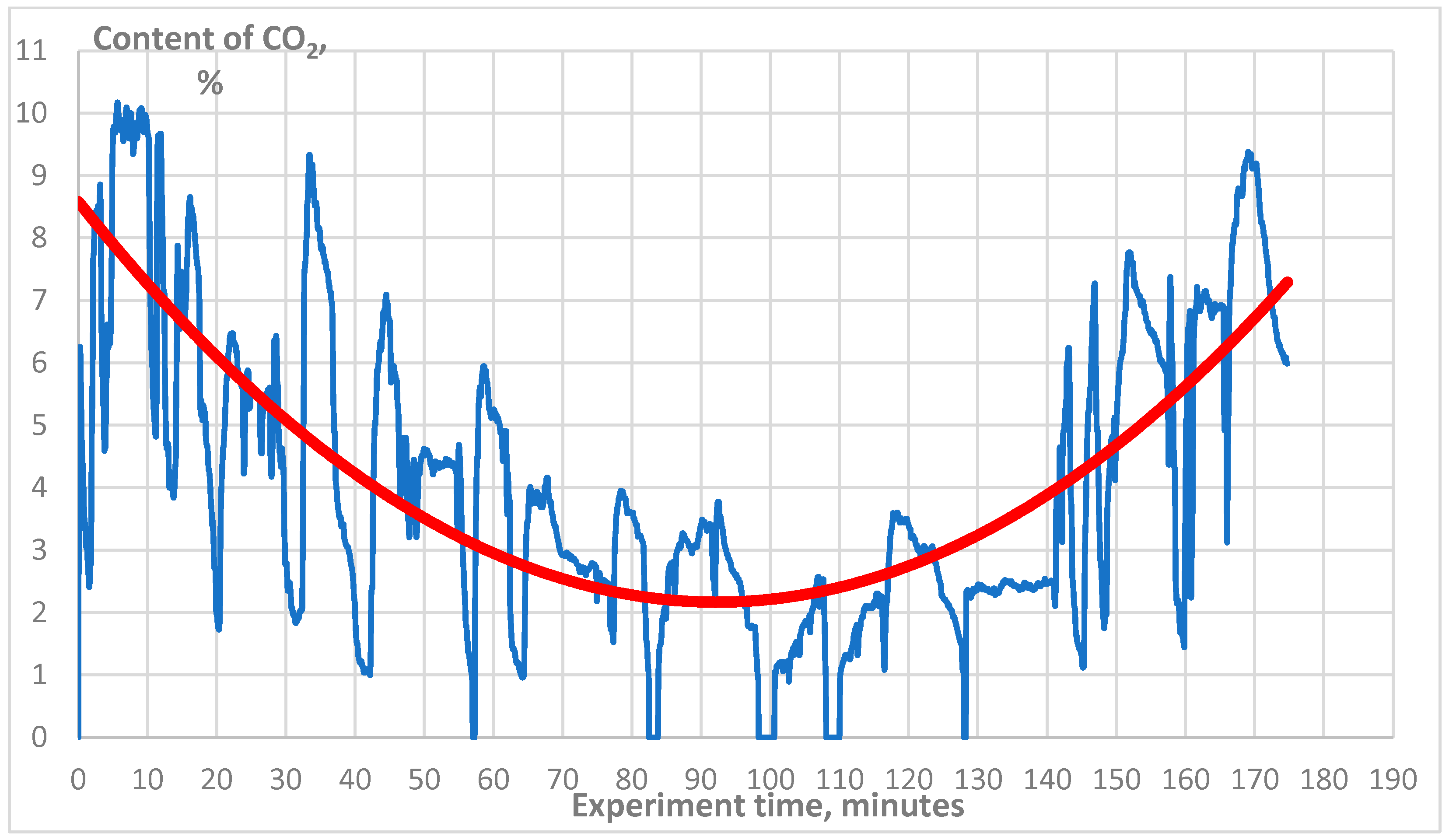
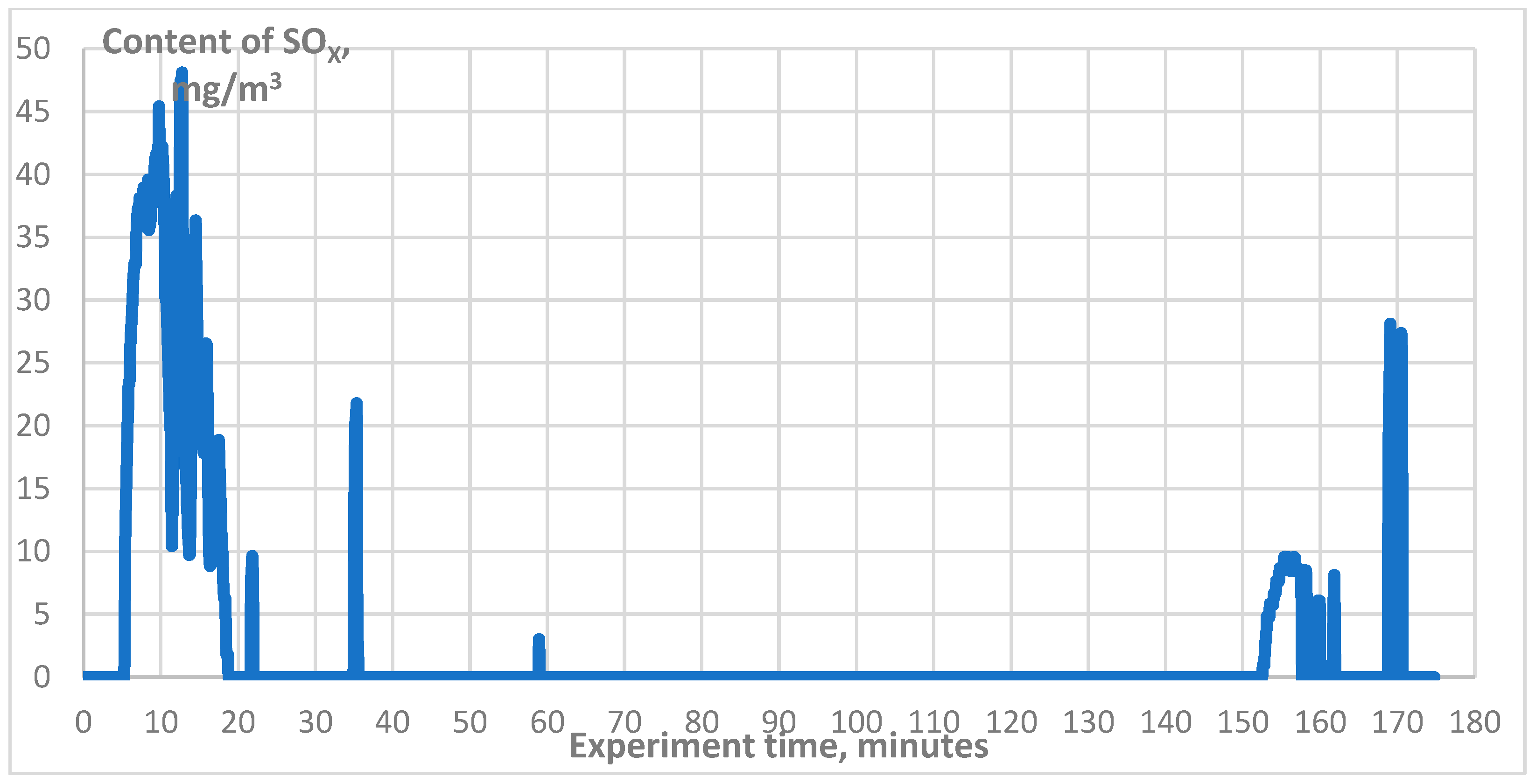
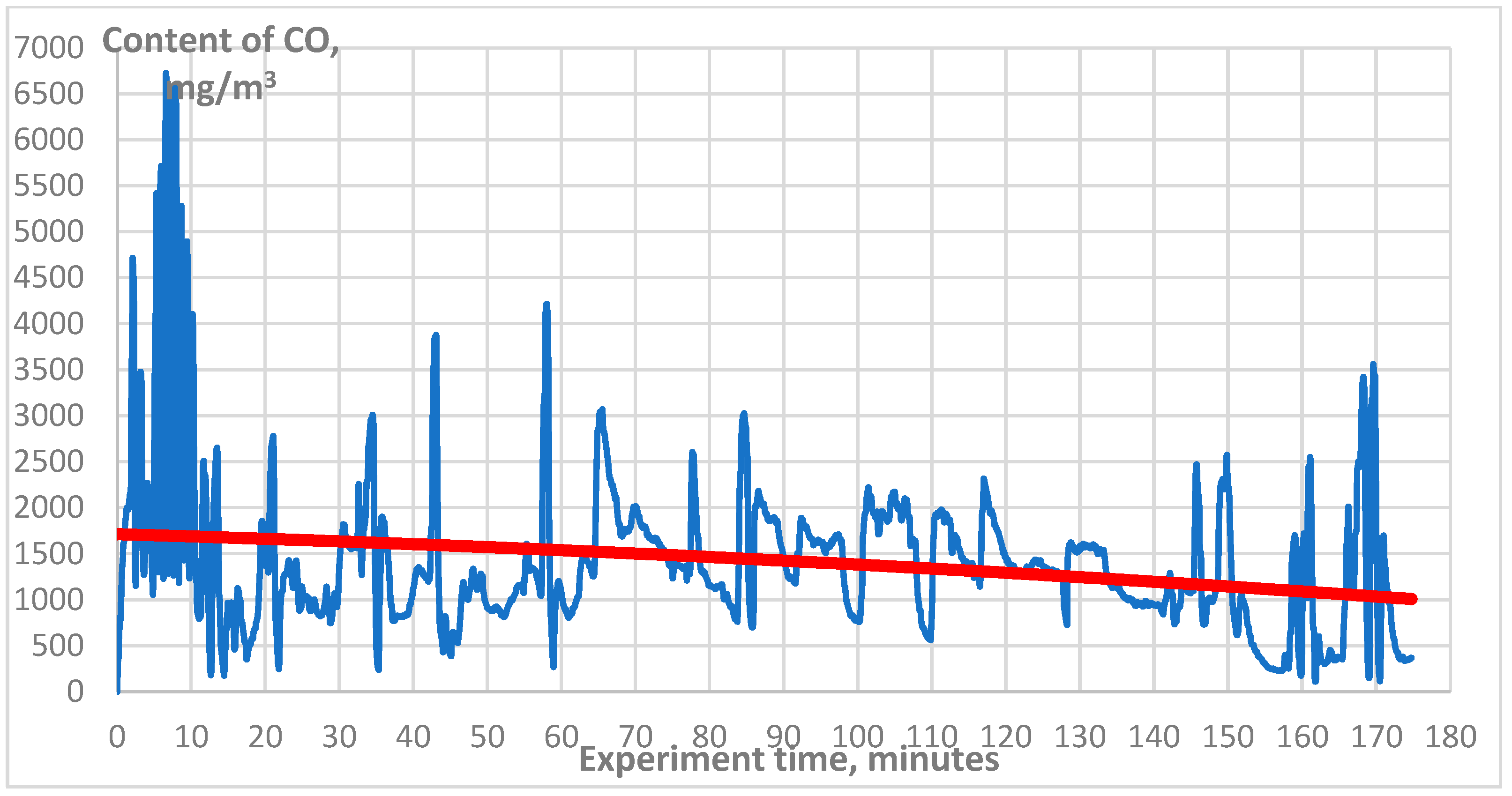
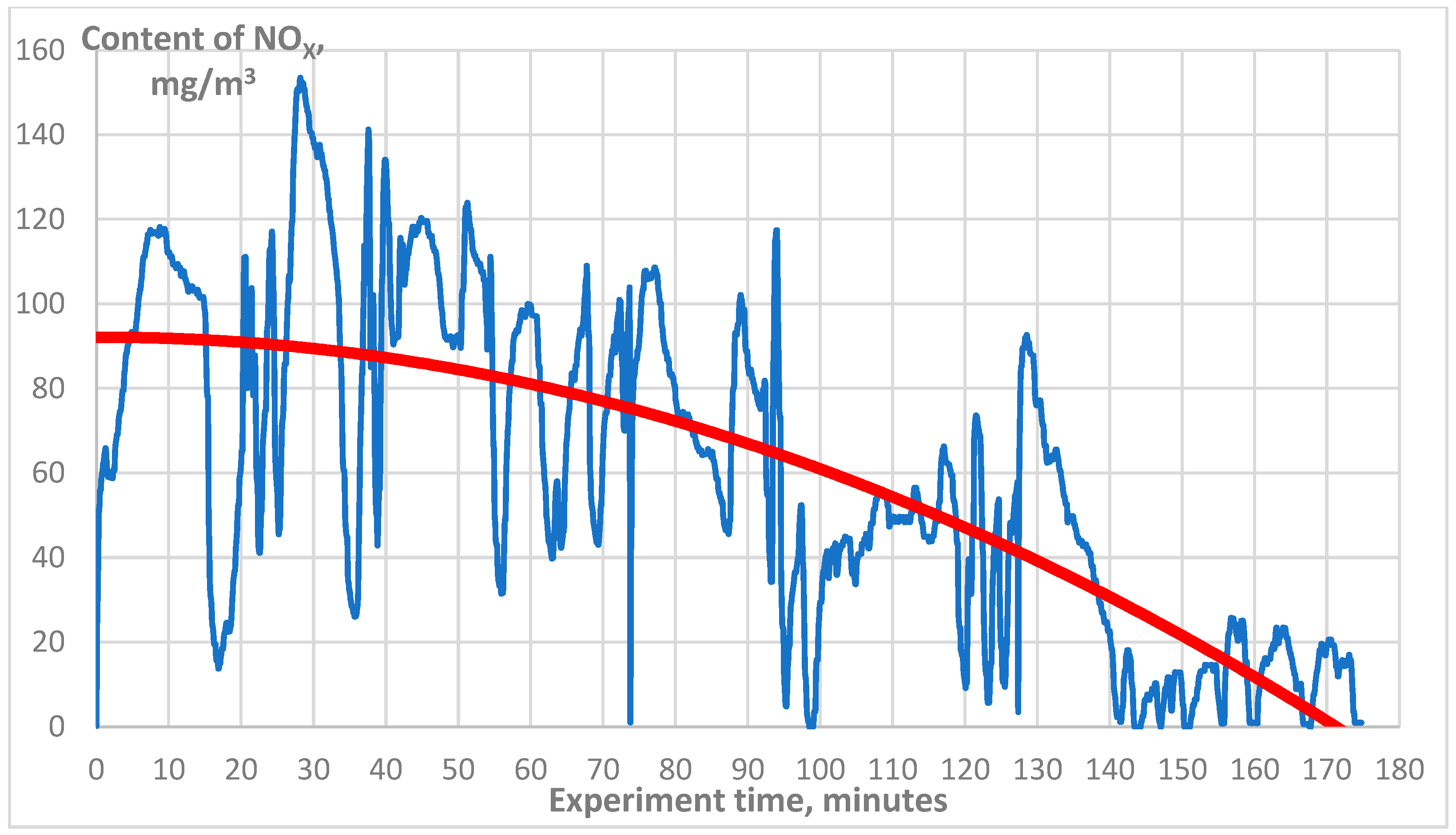
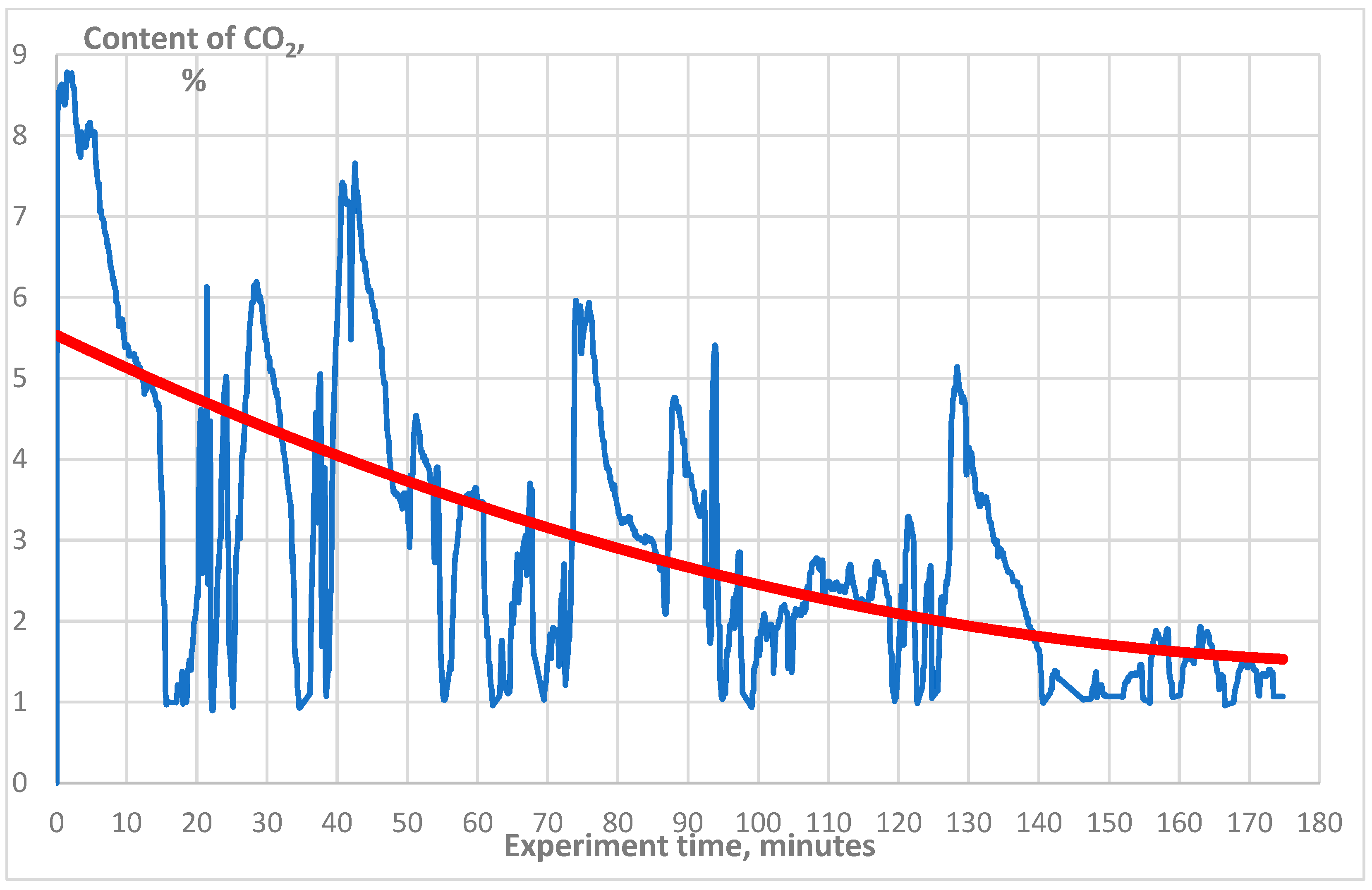
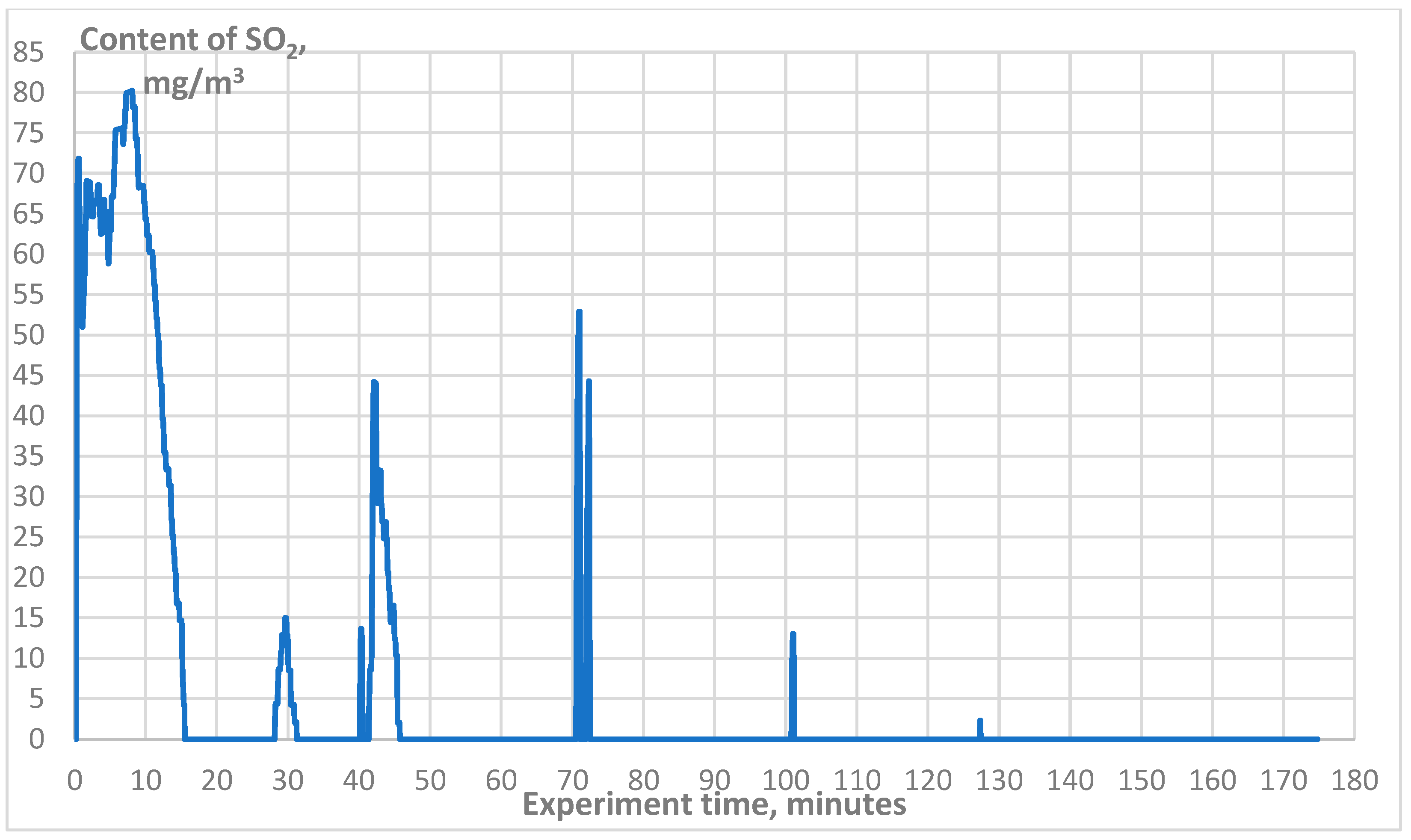
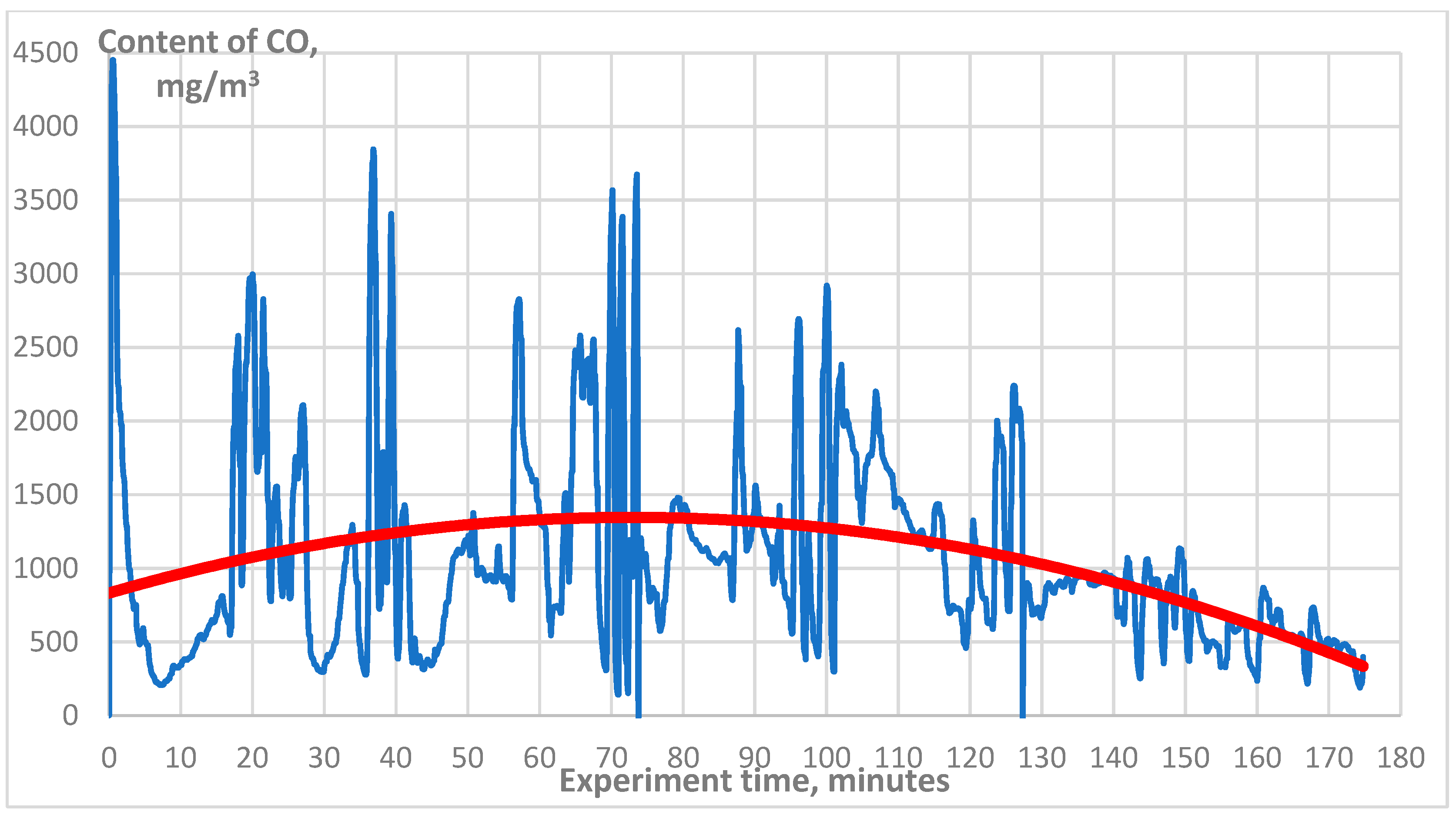
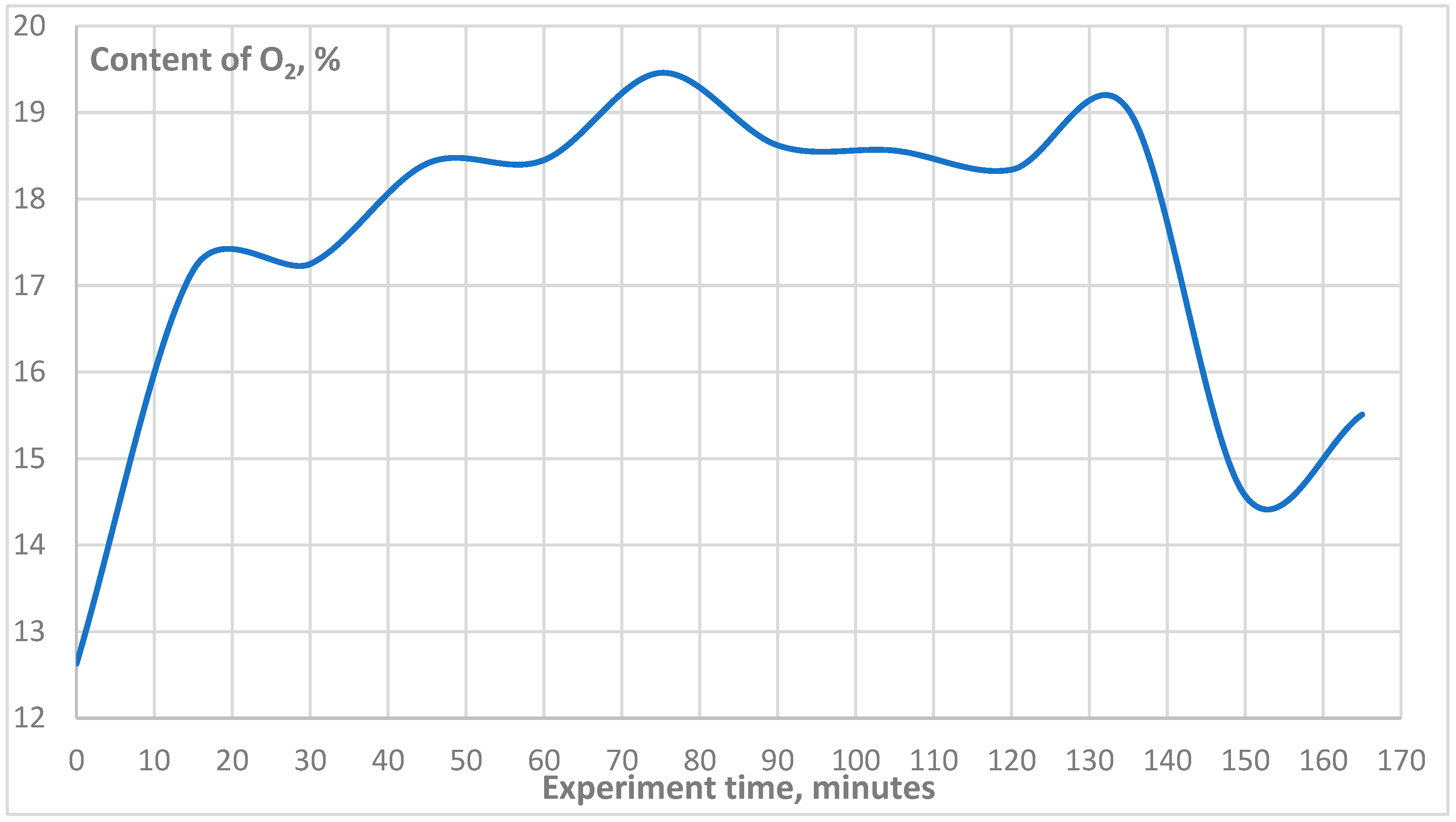
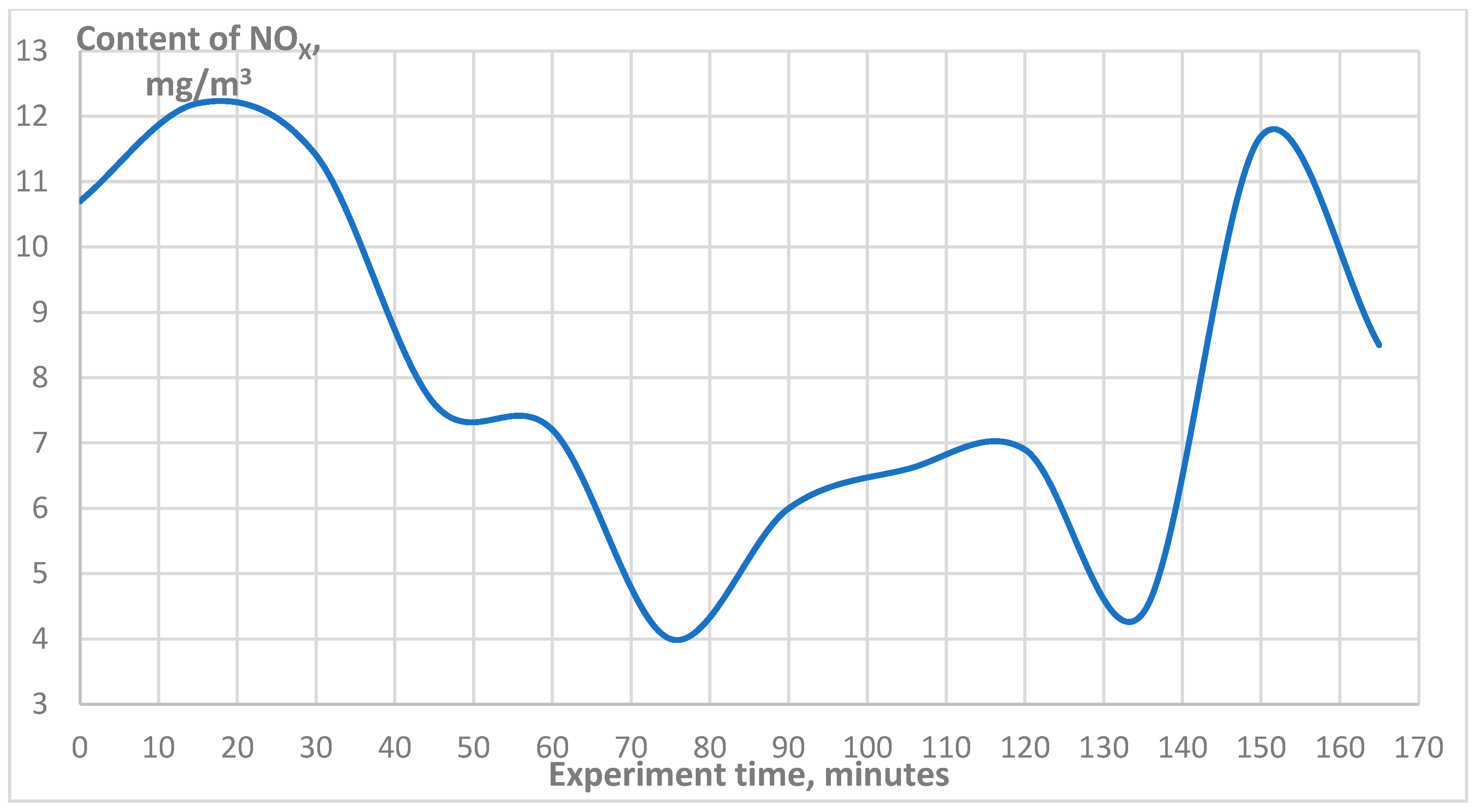
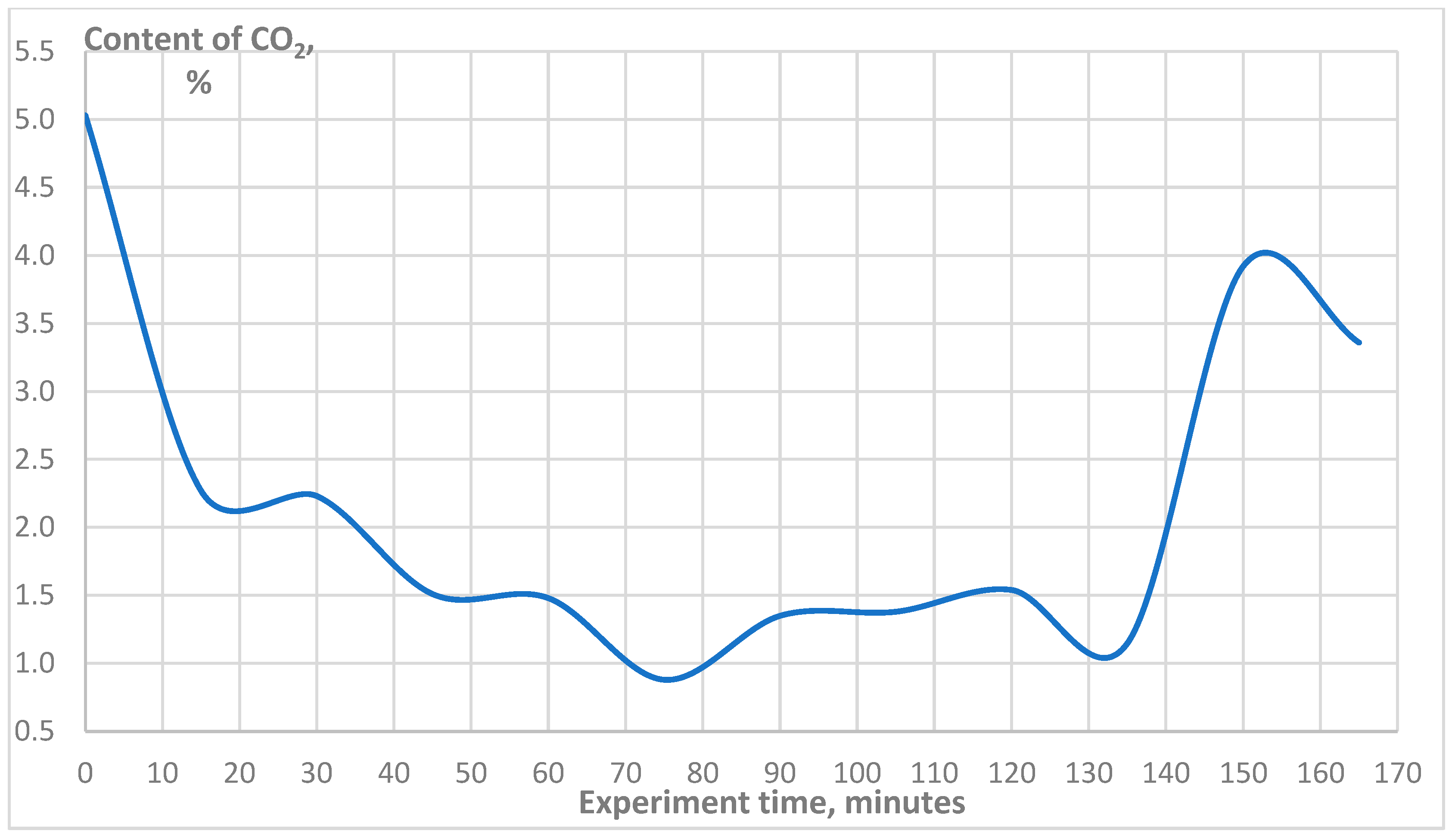
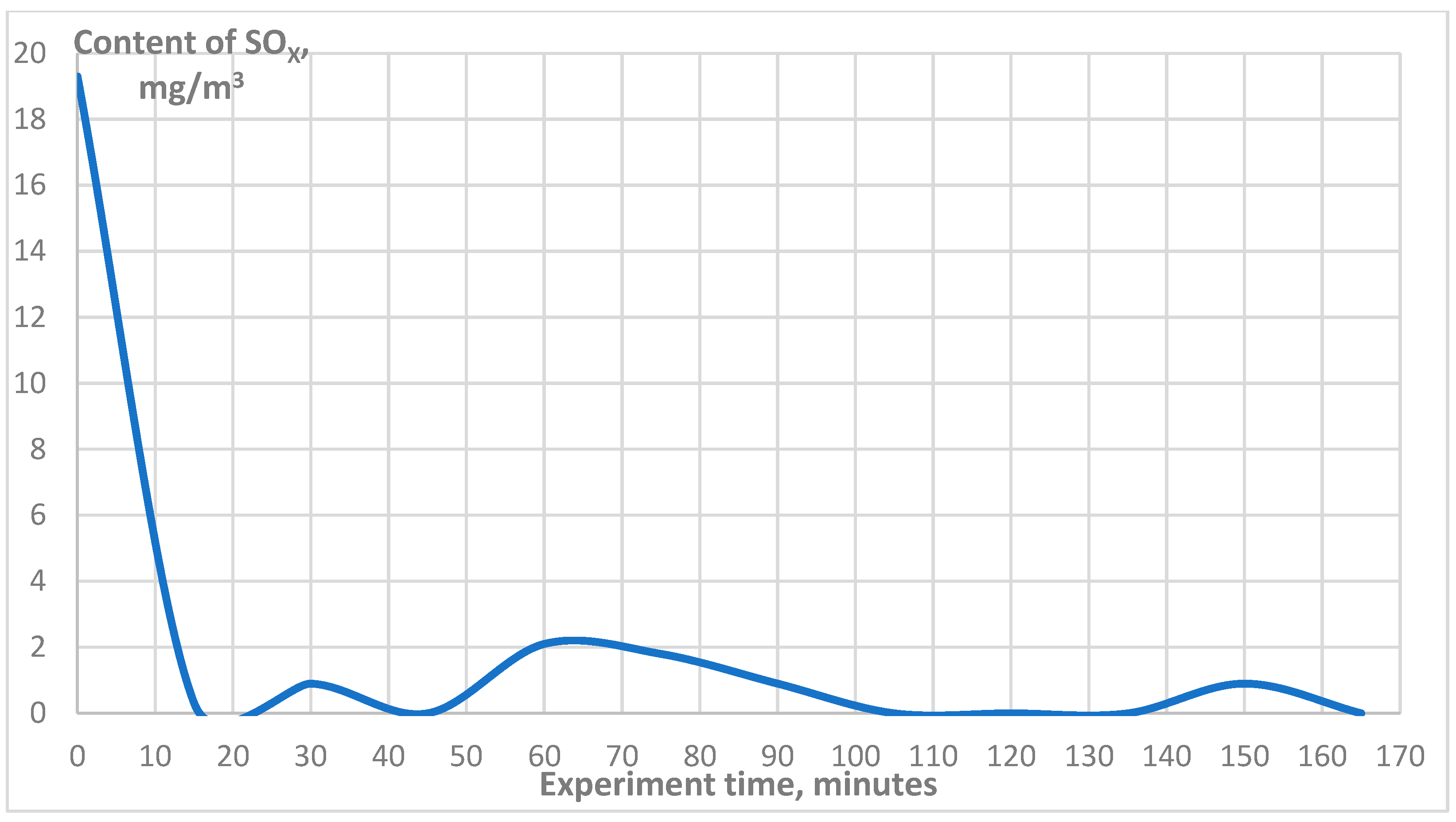
3. Results
4. Discussion
4.1. Technical Aspect
| NOx, mg/m3 | 75 |
| CO, mg/m3 | 1500 |
| SO2, mg/m3 | 0–48 |
| NOx, mg/m3 | 75 |
| CO, mg/m3 | 1000 |
| SO2, mg/m3 | 0–80 |
| NOx, mg/m3 | 8 |
| CO, mg/m3 | 160 |
| SO2, mg/m3 | 0–18 |
4.2. Economical Aspect
- Power plant in Astana with a capacity of 40 MW.
- Capital construction costs—58 million USD.
- Cost of sold electricity with government subsidies—0.061 USD/(kWh).
- Own needs—16.8%.
- Electricity generation—348 × 106 kWh/year.
- Operating costs—1.6 million USD.
- Annual income minus own needs and operating income—16.062 million USD.
- Annual profit minus income tax—12.85 million USD.
- Payback period of investments—4 years 6 months.
5. Conclusions
- The qualitative and quantitative compositions of flue gases produced during the combustion of both unsorted and sorted municipal solid waste (MSW) in Astana were identified;
- X-ray fluorescence analysis was performed using a wave-dispersive spectrometer “Axios” PANalytical.
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CIS | Commonwealth of Independent States (Former USSR) |
| MSW | Municipal solid waste |
| WIP | Waste incineration plant |
| EU | European Unit |
| WtE | Waste to energy |
| PVC | Polyvinyl chloride |
| PAH | Polycyclic aromatic hydrocarbon |
| MAC | Maximum allowable concentration |
| PCDD | Polychlorinated dibenzo-p-dioxin |
| PCDF | Polychlorinated dibenzofuran |
| JSC | Joint-stock company |
| LLP | Limited liability partnership |
References
- Strategy “Kazakhstan-2050”: A New Political Course for an Established State (Astana, December 14, 2012). Available online: https://adilet.zan.kz/rus/docs/K1200002050 (accessed on 29 August 2025).
- Inglezakis, V.J.; Moustakas, K.; Khamitova, G.; Tokmurzin, D.; Sarbassov, Y.; Rakhmatulina, R.; Serik, B.; Abikak, Y.; Poulopoulos, S.G. Current municipal solid waste management in the cities of Astana and Almaty of Kazakhstan and evaluation of alternative management scenarios. Clean Technol. Environ. Policy 2018, 20, 503–516. [Google Scholar] [CrossRef]
- Serikova, A.; Baidakov, A.; Syrlybayeva, N. The organization of municipal solid waste collection, disposal and recycling in Kazakhstan. In E3S Web of Conferences, Proceedings of the 1st International Conference on Business Technology for a Sustainable Environmental System, Almaty, Kazakhstan, 19–20 March 2020; EDP Sciences: Les Ulis, France, 2020; Volume 159, p. 01010. [Google Scholar] [CrossRef]
- Nukusheva, A.; Rustembekova, D.; Abdizhami, A.; Au, T.; Kozhantayeva, Z. Regulatory Obstacles in Municipal Solid Waste Management in Kazakhstan in Comparison with the EU. Sustainability 2023, 15, 1034. [Google Scholar] [CrossRef]
- Czekała, W.; Drozdowski, J.; Łabiak, P. Modern Technologies for Waste Management: A Review. Appl. Sci. 2023, 13, 8847. [Google Scholar] [CrossRef]
- Rumyantseva, A.; Berezyuk, M.; Savchenko, N.; Rumyantseva, E. Modern technologies of processing municipal solid waste: Investing in the future. In IOP Conference Series: Earth and Environmental Science, Proceedings of the International Conference on Sustainable, 19 May 2017, Yekaterinburg, Russia; IOP Publishing Ltd.: Bristol, UK, 2017; Volume 72, p. 012015. [Google Scholar] [CrossRef]
- Salem, K.S.; Clayson, K.; Salas, M.; Haque, N.; Rao, R.; Agate, S.; Singh, A.; Levis, J.W.; Mittal, A.; Yarbrough, J.M.; et al. A critical review of existing and emerging technologies and systems to optimize solid waste management for feedstocks and energy conversion. Matter 2023, 6, 3348–3377. [Google Scholar] [CrossRef]
- Glazyrin, S.A.; Aibuldinov, Y.K.; Kopishev, E.E.; Zhumagulov, M.G.; Bimurzina, Z.A. Analysis of the Composition and Properties of Municipal Solid Waste from Various Cities in Kazakhstan. Energies 2024, 17, 6426. [Google Scholar] [CrossRef]
- Committee for Environmental Regulation and Control of the Ministry of Ecology and Natural Resources of the Republic of Kazakhstan. The Law of the Republic of Kazakhstan. The Environmental Code of the Republic of Kazakhstan Dated 2 January 2021 No. 400-VI LRK; Akorda: Astana, Kazakhstan, 2023; 446p. Available online: https://www.gov.kz/memleket/entities/cerc/documents/details/113729?lang=kk (accessed on 2 October 2023). (In Kazakh)
- Zhuo, X.; Li, M.; Cheng, Q.; Luo, Z. Experimental Studies on the Combustion Characteristics of Multisource Organic Solid Waste for Collaborative Disposal Using Municipal Solid Waste Incinerators. ACS Omega 2024, 9, 2911–2919. [Google Scholar] [CrossRef]
- Sabiini, G.; Rishmany, J. Sorting and Miniaturization of Household Waste. Eur. J. Sci. Res. 2019, 153, 283–298. [Google Scholar]
- Domingo, J.L.; Marquès, M.; Mari, M.; Schuhmacher, M. Adverse health effects for populations living near waste incinerators with special attention to hazardous waste incinerators. A review of the scientific literature. Environ. Res. 2020, 187, 109631. [Google Scholar] [CrossRef]
- Levaggi, L.; Levaggi, R.; Marchiori, C.; Trecroci, C. Waste-to-Energy in the EU: The Effects of Plant Ownership, Waste Mobility, and Decentralization on Environmental Outcomes and Welfare. Sustainability 2020, 12, 5743. [Google Scholar] [CrossRef]
- Malinauskaite, J.; Jouhara, H.; Czajczyńska, D.; Stanchev, P.; Katsou, E.; Rostkowski, P.; Thorne, R.J.; Colón, J.; Ponsá, S.; Mansour, F.; et al. Municipal solid waste management and waste-to-energy in the context of a circular economy and energy recycling in Europe. Energy 2017, 141, 2013–2044. [Google Scholar] [CrossRef]
- Poretti, F.; Stengler, E. The Climate Roadmap of the European Waste-to-Energy Sector | The path to Carbon Negative. In Proceedings of the 16th Greenhouse Gas Control Technologies Conference (GHGT-16), Cheltenham, UK, 23–24 October 2022. [Google Scholar] [CrossRef]
- Tsekeris, G.; Anastassakis, G.N. Municipal Solid Waste-to-Energy in EU-27 towards a Circular Economy. Recycl. Sustain. Dev. 2022, 15, 83–96. [Google Scholar] [CrossRef]
- Malinauskaite, J.; Jouhara, H. The trilemma of waste-to-energy: A multi-purpose solution. Energy Policy 2019, 129, 636–645. [Google Scholar] [CrossRef]
- Brunner, P.H.; Morf, L.S. Waste to energy, indispensable cornerstone for circular economy: A mini-review. Waste Manag. Res. 2025, 43, 26–38. [Google Scholar] [CrossRef]
- Tauš, P.; Šimková, Z.; Cehlár, M.; Krajňáková, I.; Drozda, J. Fulfillment of EU Goals in the Field of Waste Management through Energy Recovery from Waste. Energies 2023, 16, 1913. [Google Scholar] [CrossRef]
- Scarlat, N.; Fahl, F.; Dallemand, J.F. Status and Opportunities for Energy Recovery from Municipal Solid Waste in Europe. Waste Biomass Valoris. 2019, 10, 2425–2444. [Google Scholar] [CrossRef]
- Taştan, M.; Uğural, M.N. Applicability Of Incineration Technology In Waste Management: Istanbul Case Study. Int. Sci. Vocat. Stud. J. 2022, 6, 121–137. [Google Scholar] [CrossRef]
- Themelis, N.J.; Ma, W. Waste to energy (WtE) in China: From latecomer to front runner. Waste Dispos. Sustain. Energy 2021, 3, 267–274. [Google Scholar] [CrossRef]
- Heeb, N.V.; Dolezal, I.S.; Bührer, T.; Mattrel, P.; Wolfensberger, M. Distribution of halogenated phenols including mixed brominated and chlorinated phenols in municipal waste incineration flue gas. Chemosphere 1995, 31, 3033–3041. [Google Scholar] [CrossRef]
- Liu, B.; Han, Z.; Liang, X. Dioxin emissions from municipal solid waste incineration in the context of waste classification policy. Atmos. Pollut. Res. 2023, 14, 101842. [Google Scholar] [CrossRef]
- Rasstegaev, A.N.; Gonopolsky, A.M.; Tarantsev, K.V.; Tarantseva, K. Assessment of the Efficiency and Safety of the Process for Utilization of Formaldehyde-Containing Solid Waste by the Method of Medium Temperature Dry Pyrolys. Ecol. Ind. Russ. 2021, 25, 46–51. [Google Scholar] [CrossRef]
- Olejarczyk, M.; Rykowska, I.; Urbaniak, W. Management of Solid Waste Containing Fluoride—A Review. Materials 2022, 15, 3461. [Google Scholar] [CrossRef]
- Shen, H.; Liu, B.; Lou, B.; Zhang, J.; Zhang, X.; Shen, H.; Liu, J.; Zhang, S. Separation of heavy metals from municipal solid waste incineration fly ash: A review. Ecotoxicol. Environ. Saf. 2025, 299, 118363. [Google Scholar] [CrossRef]
- Godyń, K.; Dutka, B. Preliminary Studies of Slag and Ash from Incinerated Municipal Waste for Prospective Applications. Energies 2023, 16, 117. [Google Scholar] [CrossRef]
- Hafid, H.S.; Rahman, N.A.A.; Abd-Aziz, S.; Hassan, M.A. Enhancement of organic acids production from model kitchen waste via anaerobic digestion. Afr. J. Biotechnol. 2011, 10, 14507–14515. [Google Scholar] [CrossRef]
- Aendo, P.; Netvichian, R.; Thiendedsakul, P.; Khaodhiar, S.; Tulayakul, P. Carcinogenic Risk of Pb, Cd, Ni, and Cr and Critical Ecological Risk of Cd and Cu in Soil and Groundwater around the Municipal Solid Waste Open Dump in Central Thailand. J. Environ. Public Health 2022, 2022, 3062215. [Google Scholar] [CrossRef]
- Hodson, E.L.; Martin, D.; Prinn, R.G. The municipal solid waste landfill as a source of ozone-depleting substances in the United States and United Kingdom. Atmos. Chem. Phys. 2010, 10, 1899–1910. [Google Scholar] [CrossRef]
- Glazyrin, S.A.; Bekin, C.E.; Varlamov, G.B.; Erzhanov, K.S.; Chebotarev, O.E. Problems of ash and slag waste disposal. In Proceedings of the VII International Scientific and Practical Conference: “Actual Problems of Transport and Power Engineering: Ways of Their Innovative Solution”, Astana, Kazakhstan, 15 March 2019; pp. 382–387. (In Russian). [Google Scholar]
- Meynendonckx, W.; Ishteva, M.; Verbeke, M.; Alderweireldt, N.; De Greef, J. Impact of furnace and waste layer control on HCl and SO2 in combustion gas from a grate-fired Waste-to-Energy boiler. Process Saf. Environ. Prot. 2025, 193, 710–720. [Google Scholar] [CrossRef]
- Umyshev, D.R.; Kibarin, A.A.; Seidaliyeva, A.B.; Iskakov, D.O.; Zhekenov, Y.L.; Jambayev, I.K.; Umysheva, M.M. Combustion Characteristics of Municipal Solid Waste in a Grate-Fired Solid-Fuel Hot Water Boiler. Energies 2025, 18, 3028. [Google Scholar] [CrossRef]
- Ns 28775-90; Gas Turbine Driven Gas Compressor Units. General Specifications. Moscow Standard Inform: Moscow, Russia, 2005.
- Lefebvre, A.H. Gas Turbine Combustion: Alternative Fuels and Emissions; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Usepa Federal Register. Review of national ambient air quality standards for carbon monoxide. Final Rule 2011, 76, 169. [Google Scholar]
- Directive 2010/75/EU of the European Parliament and of the Council of 24 November 2010 on industrial emissions (integrated pollution prevention and control). Off. J. Eur. Union 2010, 334, 14–119.
- GB/T 24021-2024/ISO 14021:2016; National Standard of the People’s Republic of China Environmental Management—Environmental Labels and Declarations—Self-Declared Environmental Claims (Type II Environmental Labelling). Standardization Administration of China: Beijing, China, 2024.
- Glazyrin, S.A.; Aidymbaeva, Z.A.; Dostiyarov, A.M.; Zhumagulov, M.G.; Zlatov, N.; Strefanović, V.P. Method for Cleaning Flue Gases of a Drum-Type Power Boiler. Patent of Republic of Kazakhstan No. 5184, 23 April 2020. (In Russian). [Google Scholar]

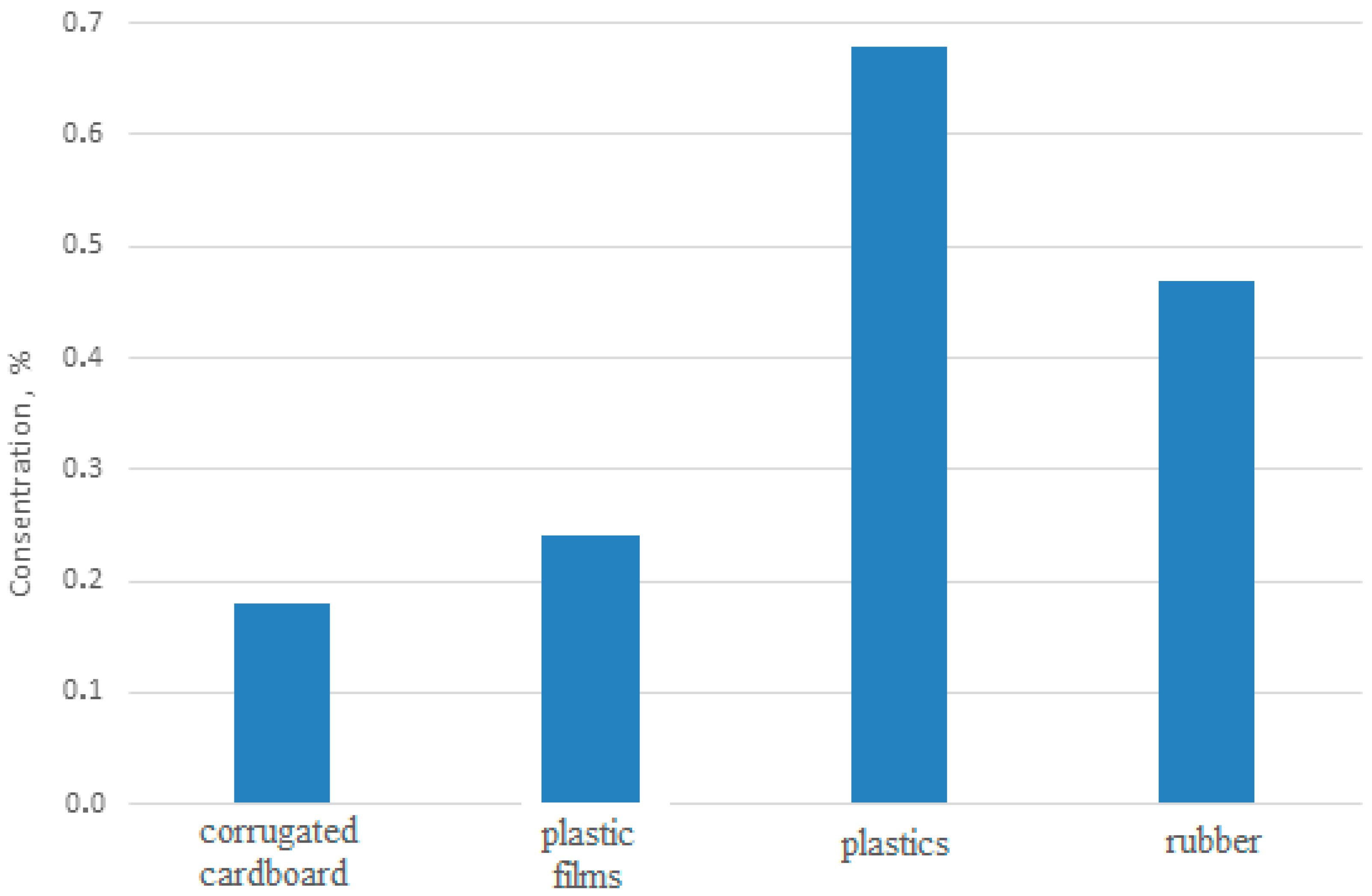
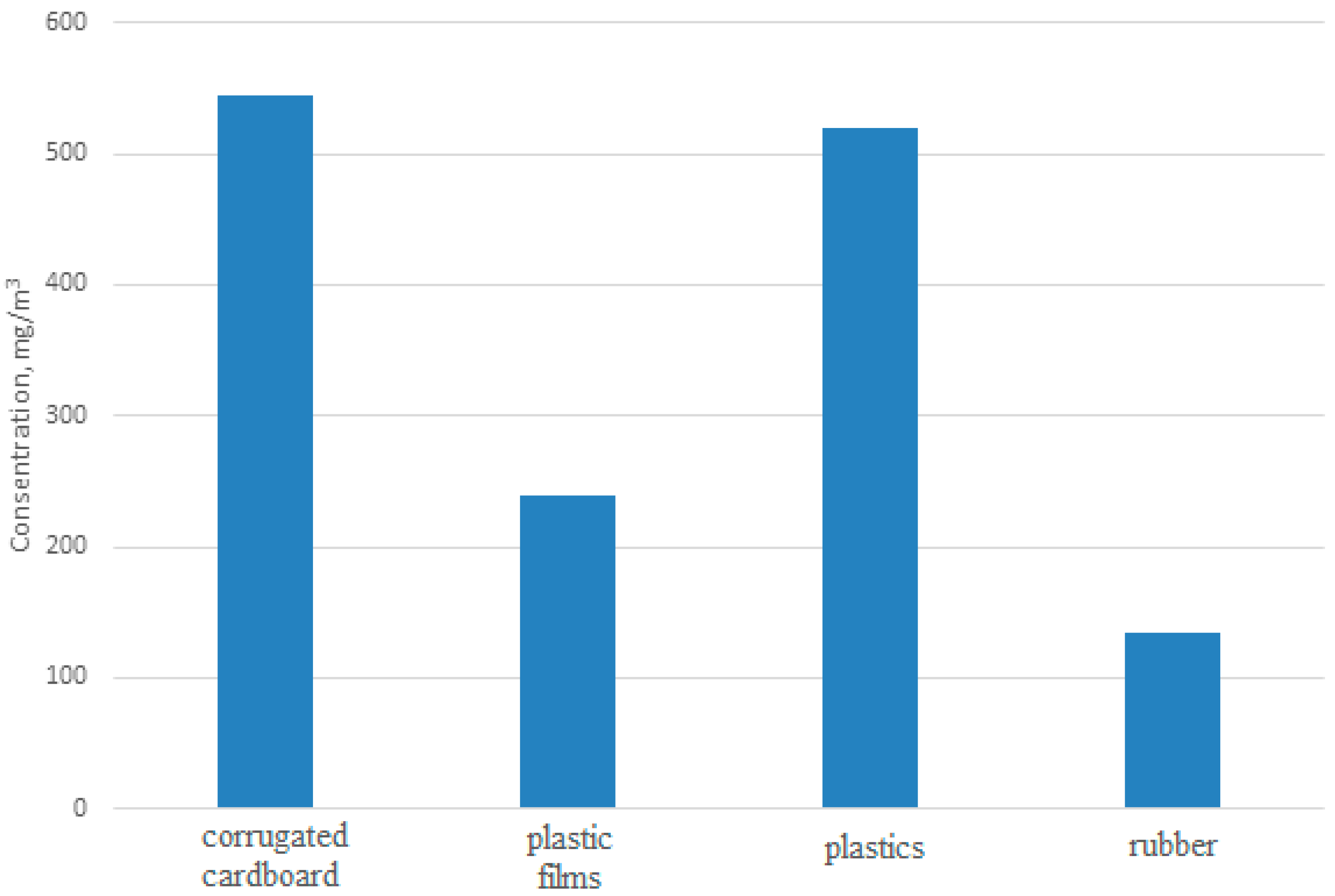



| Country | Method of Solid Waste Disposal and Share in Percentage | ||||
|---|---|---|---|---|---|
| Recycling | Backfill for Reclamation | Energy Recovery | Incineration Without Energy Recovery | Landfilling | |
| EU average | 37.9 | 10.7 | 6 | 0.7 | 44.7 |
| Italy | 79.3 | 0.1 | 5.7 | 2.5 | 12.4 |
| Belgium | 77.4 | 0 | 11.2 | 3.6 | 7.8 |
| Hungary | 63.2 | 5.4 | 6.4 | 0.5 | 24.5 |
| Latvia | 57.6 | 4.6 | 10 | 0 | 27.8 |
| Denmark | 56.7 | 18.4 | 18.9 | 0.1 | 5.9 |
| France | 55.8 | 10.4 | 5.5 | 1.3 | 27 |
| Croatia | 52.3 | 3.5 | 2 | 0 | 42.2 |
| Czech Republic | 50.9 | 34.6 | 3.5 | 0.3 | 10.7 |
| Poland | 49.3 | 20.3 | 3.6 | 0.4 | 26.4 |
| Portugal | 48.2 | 6.1 | 11.1 | 0.3 | 34.3 |
| Slovenia | 43.8 | 49.5 | 2.5 | 0.5 | 3.7 |
| Netherlands | 43 | 0 | 7.2 | 0.8 | 49 |
| Germany | 42.7 | 26.4 | 12 | 0.5 | 18.4 |
| Luxembourg | 41.1 | 32.6 | 2.6 | 0 | 23.7 |
| Spain | 38.7 | 10 | 2.9 | 0.2 | 48.2 |
| Slovakia | 38.2 | 14.7 | 6.7 | 0.5 | 39.9 |
| Austria | 36 | 12.7 | No data | No data | 45.6 |
| Lithuania | 34.1 | 2.8 | 6.3 | 0.1 | 56.7 |
| Estonia | 28.9 | 8.4 | 1.8 | 0 | 60.9 |
| Malta | 18.5 | 65.6 | 0 | 0.2 | 15.7 |
| Cyprus | 17 | 20.4 | 7.2 | 0 | 55.4 |
| Sweden | 13.1 | 2.7 | 6.8 | 0.1 | 77.3 |
| Ireland | 11.5 | 51.4 | 9.8 | 0.1 | 27.2 |
| Greece | 10.7 | 3.5 | 0.7 | 0 | 85.1 |
| Finland | 9.2 | 2.4 | 5 | 0.1 | 83.3 |
| Romania | 3.2 | 0.3 | 1 | 0 | 95.5 |
| Bulgaria | 2.9 | 0 | 0.5 | 0 | 96.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glazyrin, S.A.; Kopishev, E.E.; Zhumagulov, M.G.; Bimurzina, Z.A.; Aibuldinov, Y.K. Analysis of the Efficiency and Environmental Impact of Municipal Solid Waste Incineration as a Tool for Sustainability Development in Kazakhstan. Sustainability 2025, 17, 8696. https://doi.org/10.3390/su17198696
Glazyrin SA, Kopishev EE, Zhumagulov MG, Bimurzina ZA, Aibuldinov YK. Analysis of the Efficiency and Environmental Impact of Municipal Solid Waste Incineration as a Tool for Sustainability Development in Kazakhstan. Sustainability. 2025; 17(19):8696. https://doi.org/10.3390/su17198696
Chicago/Turabian StyleGlazyrin, Sergey A., Eldar E. Kopishev, Mikhail G. Zhumagulov, Zarina A. Bimurzina, and Yelaman K. Aibuldinov. 2025. "Analysis of the Efficiency and Environmental Impact of Municipal Solid Waste Incineration as a Tool for Sustainability Development in Kazakhstan" Sustainability 17, no. 19: 8696. https://doi.org/10.3390/su17198696
APA StyleGlazyrin, S. A., Kopishev, E. E., Zhumagulov, M. G., Bimurzina, Z. A., & Aibuldinov, Y. K. (2025). Analysis of the Efficiency and Environmental Impact of Municipal Solid Waste Incineration as a Tool for Sustainability Development in Kazakhstan. Sustainability, 17(19), 8696. https://doi.org/10.3390/su17198696







