Abstract
The galvanic industry requires considerable amounts of water and produces significant quantities of wastewater. Two types of wastewater are created in the processes of the galvanic application of metal coatings: used galvanic baths and wastewater generated while rinsing coated elements. The composition and amount of wastewater depend on the type of process, the plant’s operational system, and the quantity of water utilised to rinse the coated elements. In this article, the possibilities of using different techniques, such as chemical precipitation, coagulation and flocculation, ion exchange, adsorption, and membrane filtration, to remove heavy metals from galvanic wastewater were analysed and assessed. It was determined that the use of physicochemical methods (i.e., chemical precipitation, coagulation, and flocculation) to remove heavy metals has significant disadvantages, including operational costs connected with the purchase of chemical reagents and the emergence of metal complexes requiring management/utilisation. On the other hand, the processes of ion exchange and adsorption can be used only for wastewater characterised by a low heavy metal concentration, with organic matter preliminarily removed. In addition, waste polluted with heavy metals in the form of used regenerative baths and used sorbents is generated during these processes. In turn, the advanced techniques of membrane filtration allow for the removal of different types of organic pollutants and heavy metals. The processes of membrane wastewater treatment exhibit a range of advantages compared to traditional technologies, including the complete, environmentally friendly removal of permanent organic pollution, easy integration into conventional technologies, a limited amount of residue, a high level of separation, and a shorter process time. The efficiency of membrane wastewater treatment depends on many parameters, including, most of all, the composition, pH, and type of membrane, as well as process conditions. The possibility of using new types of membranes to remove heavy metals from spent galvanic baths was analysed, and the possibility of using the processes in wastewater treatment systems according to the circular economy model was assessed. The assessment of the efficiency of heavy metal removal in hybrid systems combining specific individual processes and the development of state-of-the-art material solutions to realise these processes may be an interesting direction of research in this field.
1. Introduction
The depletion of resources, their increasing prices, and the rising dependence on foreign suppliers constitute a serious danger to the further economic development of Poland and the European Union and a challenge in the context of environmental protection. Therefore, activities are undertaken with the aim of protecting against the challenges and, indirectly, improving the competitiveness of the markets in our country and the EU on the global market. The idea of the circular economy was created and presented in 2015 by the European Commission and has become one of the dominant development strategies in the EU. These matters are linked to the assumptions of the 2030 Agenda for Sustainable Development, established by all 193 UN member states, which contains 17 Sustainable Development Goals and 169 targets connected with the Goals to be realised by 2030. Among them, there are targets pertaining to wastewater treatment and the technologies of recycling and reuse of reclaimed water (Goal 6), the promotion of sustainable industrialisation and fostering innovation (Goal 9), and the struggle against climate change and sustainable use of land and water ecosystems (Goals 13–15) [1,2].
The sustainable management of resources enforces the development of state-of-the-art technologies that will allow for the management of filtered wastewater (water recycling and reclamation, closing water circuits), the limitation of environmental pollution (with, among others, heavy metals), the reclamation and energy savings on technological processes, and the efficient management of waste biomass. Such actions are the basis of eco-development, facilitating, according to worldwide trends, the transformation towards a circular economy. The optimisation of the current systems of industrial wastewater treatment encompasses a wide range of activities, allowing for the creation of technological solutions, which will make it possible to use treated wastewater for technical or production purposes. A rational approach to sustainable resource management is especially important in these industrial branches, where the considerable use of these resources and the generation of waste that may be potentially valorised are observed [3,4].
Two million tons of wastewater are discharged into the environment worldwide daily. Over 1200 plants connected with metal products manufacturing are currently registered in Poland, including around 330 that process and coat metals. According to the Statistics Poland data from 2017, only 3.6% have systems allowing for a completely closed circuit of technological water. Metal producers discharge 1.7 hm3 of wastewater a year, including 0.8 hm3 discharged directly to water or soil and 0.9 hm3 to wastewater [5].
In this article, the possibilities of using different techniques, such as chemical precipitation, coagulation and flocculation, ion exchange, adsorption, and membrane filtration, to remove heavy metals from galvanic wastewater were analysed. The literature review may be helpful in designing systems of galvanic wastewater treatment. According to the model of a closed-circuit economy, these systems will facilitate the retention of heavy metals and the limitation of water consumption by specific technological operations in galvanising plants.
2. Galvanic Wastewater
Spent electrolytic baths (so-called exhausted baths) and post-consumer baths created by rinsing coated elements (so-called rinsing water) are produced during the galvanic application of metallic coatings. The composition and amount of wastewater depend on the kind of coating application technology, the plant’s operating system, and the amount of water used to rinse the coated elements.
Wastewater produced in a galvanic process can be divided into four basic groups depending on the technology of the coating application:
- –
- Chromium wastewater containing Cr(VI) and other components of a bath for chrome plating, chromating, and etching of copper and alloys;
- –
- Cyanide wastewater containing simple cyanides, complex cyanides, metal ions (e.g., Zn, Cu), and other substances that compose plating baths;
- –
- Acidic–alkaline wastewater, which contains, depending on the technology and the composition of process baths, mineral acids (sulphuric, nitric, phosphorus, hydrofluoric, and others), alkali (sodium and potassium hydroxides, sodium carbonate), mineral salts (silicates, phosphates), metals (iron, nickel, copper, zinc), and surfactants (wetting agents);
- –
- Oily wastewater containing oils and fats produced in the processes of washing and degreasing, which are often emulsified [6,7].
The diversity of galvanic wastewater is the result of the numerous stages of coating processes and the variety of chemicals employed in the preparation of specific process baths. A simplified technological diagram of metal element galvanisation in electroplating barrels containing a chloride, low-acidic bath with organic brightening additives is presented in Figure 1. In this case, the stage of galvanisation comprises the application of a zinc layer on small, corrodible industrial parts. Special electrolytic solutions, in which the metal element is submerged, are used to this end. Next, the solution containing the element is connected to electricity. Steel elements should be free from grease, waxy substances, and oil, which prevent reactions between iron and zinc, before submergence in a zinc bath. The goal of the degreasing stage is to prepare a chemically clean surface on which an alloy of steel and zinc will be created. The second stage involves surface etching. It allows for the efficient removal of non-metallic substances (i.e., rust, mill scale, and other corrosion products) generated during the rolling and annealing of metal elements. After galvanisation, passivation is usually conducted to improve the resistance of metals to corrosion and the appearance of the metal product finishing [8].
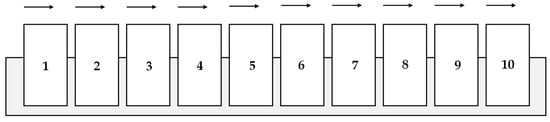
Figure 1.
A diagram of a technological line for galvanic metal element treatment in electroplating barrels: 1—degreasing, 2—warm rinsing, 3—cold rinsing, 4—etching, 5—rinsing, 6—galvanisation, 7—rinsing, 8—passivation, 9—rinsing, and 10—drying.
Galvanic wastewater is considered especially dangerous and burdensome for the environment due to its high content of heavy metals, such as Cr(VI), Zn(II), Cu(II), Cd(II), Fe(II), and Ni(II). Galvanic wastewater also contains dissolved nonorganic substances, such as mineral (e.g., sulphuric, hydrochloric, nitride, phosphorus, and hydrofluoric) acids, alkali (e.g., hydroxides: sodium, potassium, calcium, and calcium and sodium carbonates), and mineral salts (e.g., phosphates, silicates, and boranes) [9]. However, their colour depends on the coating operation. In the case of galvanisation, wastewater is brown-yellow; in the case of nickel plating, it is green, and in the case of copper plating, it is blue. The pH ranges and concentrations of specific galvanic wastewater components are presented in Table 1.

Table 1.
The characteristics of galvanic wastewater [8].
The challenge of disposing of galvanic wastewater is one of the most significant and urgent environmental challenges for industrial companies. The discharge of untreated spent galvanic baths into the environment causes the pollution of soil and water with heavy metals, and the wastewater passes into organisms, people, and animals. In turn, the accumulation of heavy metals in people and animals may cause dangerous poisoning, acute and chronic illnesses of the cardiovascular system, nervous system, and kidneys, and cancer. In addition, the presence of heavy metals in the water environment influences the water’s pH, the amount of oxygen dissolved in it, and its organoleptic qualities, thus limiting the process of water self-cleaning [10,11,12]. The maximum concentrations of selected heavy metals in the wastewater discharged into the environment are summarised in Table 2.

Table 2.
The maximal concentrations of selected heavy metals in wastewater discharged to the environment [13].
A range of techniques that ensure the proper cleaning of industrial wastewater (Figure 2) can be applied to remove heavy metals.
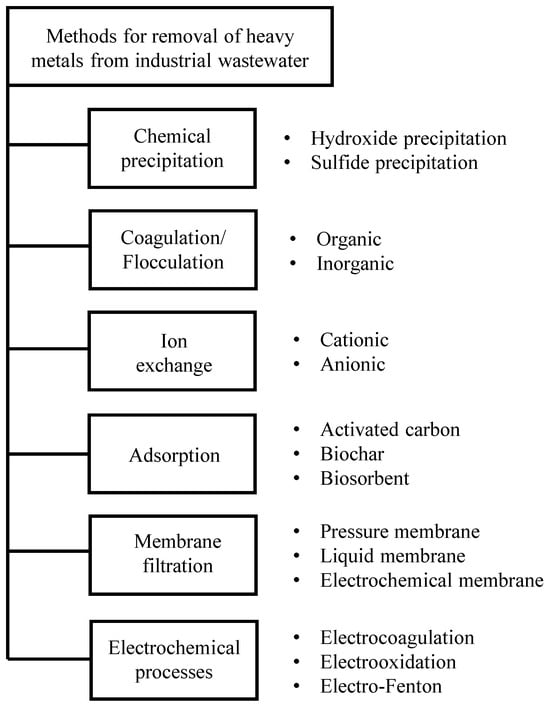
Figure 2.
The methods employed to remove heavy metals from industrial wastewater [8,14].
However, it needs to be kept in mind that, while choosing a method of wastewater treatment, one needs to take into account many aspects, including the concentration of specific pollutants, the expected efficiency of treatment in relation to the amount of wastewater discharged in a specific period of time, and the efficiency of removing specific components, as well as the safety of a specific method. Recent approaches have demonstrated the feasibility of recovering metallurgical by-products, such as electric arc furnace (FTP) dust, particularly for zinc recovery via thermochemical treatment. This type of recovery also supports circular economy principles in the treatment of industrial effluents [15].
3. Chemical Precipitation
The main task of the classical approach to wastewater treatment is removing, processing, or decreasing the concentration of pollutants to the level required by law. The diverse composition of galvanic wastewater, stemming from the utilisation of different technological processes, requires the employment of various methods of treatment. The most common method of treating wastewater generated in metalworking is neutralisation. It consists of the employment of chemical reactions that transform pollutants into compounds that are harmless to the wastewater receiver or, for example, precipitate heavy metal compounds that are difficult to dissolve (Figure 3).
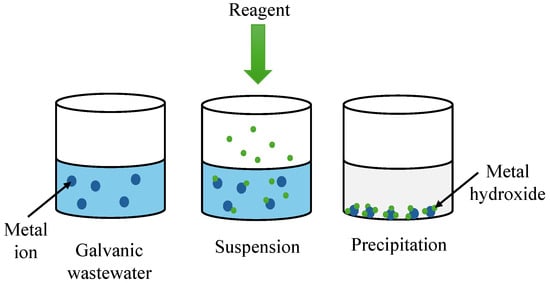
Figure 3.
A diagram showing chemical precipitation designed to remove heavy metal ions from galvanic wastewater.
Chemical precipitation is widely used to remove heavy metal ions from wastewater. The chemical reagent reacts with heavy metal ions in wastewater to form insoluble precipitates. These precipitates can then be removed from wastewater using sedimentation or filtration techniques [16,17]. Chemical precipitation methods can be divided into hydroxide and sulfide precipitation methods (Table 3).

Table 3.
Chemical precipitation methods for removing heavy metals from wastewater.
Chemical treatment consists of the reduction of chromium(VI) into chromium(III) in wastewater. Next, chromium(III) can be easily precipitated in the form of chromium(III) hydroxide—Cr(OH)3. In practice, chromium reduction is the basic method of chromium(VI) neutralisation. The reduction process is usually conducted with sodium pyrosulphite (Na2S2O5), sodium sulphite (Na2SO3), and sulphur dioxide (SO2) discharged into wastewater. As a result of these reactions, ion-reducing HSO3− is created. Chromium reduction with this ion occurs in an acidic environment. To achieve fast reduction, the pH should be lowered with sulphuric acid to ≤2.5 and, for example, sodium pyrosulphite in the amount of around 180 g per 100 g of CrO3 should be added. Under such conditions, the reduction occurs in around 2 min. After the reduction of chromium(VI) to chromium(III), the pH needs to be raised in order to precipitate chromium(III) hydroxide, Cr(OH)3, from the solution. The optimal pH for the abovementioned process ranges from 6.5 to 8.5 [23,24].
A very important stage in the chemical treatment of galvanic wastewater is the separation of suspensions produced during neutralisation from the cleaned wastewater. Suspension sedimentation is a long process (taking a few to a dozen or so hours) and often does not occur in full due to the partially colloidal nature of a suspension. Sedimentation can be considerably improved and accelerated by means of commonly available coagulants and flocculants. In the case of many suspensions that emerge after neutralisation, only a flocculant that aggregates particles from the suspension can be used. The application of flocculants considerably shortens the sedimentation time and facilitates the dehydration of the sediments produced. The dehydration of post-neutralisation sediments usually happens gravitationally (baggers) or under pressure (filter presses) [25].
This method’s relative simplicity, reliability, and ease of automatic pH control are counted among its advantages. However, the amphoteric properties of metals cause the near impossibility of defining a pH range in which all heavy metal ions could precipitate simultaneously. A serious flaw of such a solution is also the high use of chemicals and the generation of the reaction’s by-products, creating dangerous sediments. In addition, the presence of complexing factors complicates the possibility of metal extraction [26,27].
4. Coagulation/Flocculation
Coagulation and flocculation are key processes in galvanic wastewater treatment, whereby small particles are joined into larger agglomerates, which facilitates their removal. Coagulation destabilises suspended particles, and flocculation combines them into larger floccules, which can then be easily removed through sedimentation or filtration [28]. Coagulation and flocculation are efficient in removing heavy metals, such as chromium, nickel, and copper, commonly used in galvanic wastewater (Figure 4).
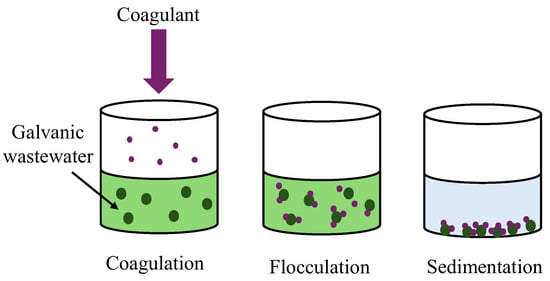
Figure 4.
A diagram of coagulation and flocculation of galvanic wastewater.
The advantage of an integrated coagulation/flocculation system is that it shortens the sedimentation time of the suspended particles of pollutants. On the other hand, sediment generation and high operational costs connected with the use of special coagulation and flotation factors are the disadvantages. The selection of conditions for these processes depends on the wastewater composition and the type of coagulants and flocculants used. The determination of optimal pH, the amount of coagulant and flocculant, and the time and intensity of mixing are crucial [29,30,31]. The following chemicals are most often employed in the coagulation/flocculation process of galvanic wastewater: aluminium sulphate—Al2(SO4)3·18H2O (powder) [32,33,34], iron chloride—FeCl3·6H2O (powder) [29,35], and polyaluminium chloride—PAC (powder) [36]. García-Ávila et al. [36] determined that aluminium sulphate is best at the removal of iron and aluminium from galvanic wastewater (in the form of baths used in the etching process). Using this 10% coagulant with wastewater at a pH adjusted to 5.5, the efficiency of iron and aluminium removal reached, respectively, ~98% and 93%. In turn, Hargreaves et al. [30] noted the removal of 77% of copper, 68% of lead, and 42% of zinc by means of iron chloride. Examples of other selected coagulation/flocculation agents are listed in Table 4.

Table 4.
Coagulation/flocculation methods for removing heavy metals from wastewater.
5. Ion Exchange
The ion exchange method consists of replacing unwanted ions in wastewater with environmentally neutral ions by means of special ion exchange resins. As a result of the interaction between wastewater and such a resin, heavy metals (copper, nickel, zinc, chromium), cyanide compounds, and radioactive substances can be removed. Depending on the type of functional group, ion exchange resins are divided into anion exchangers, with anion functional groups (e.g., sulphonic, carboxylic, aminodiacetate, phosphonic, hypophosphorous), and cation exchangers, with cation functional groups (e.g., quaternary ammonium, tertiary ammonium, phosphonic, sulphonic) [41]. A diagram of metal ion removal from galvanic wastewater is presented in Figure 5.
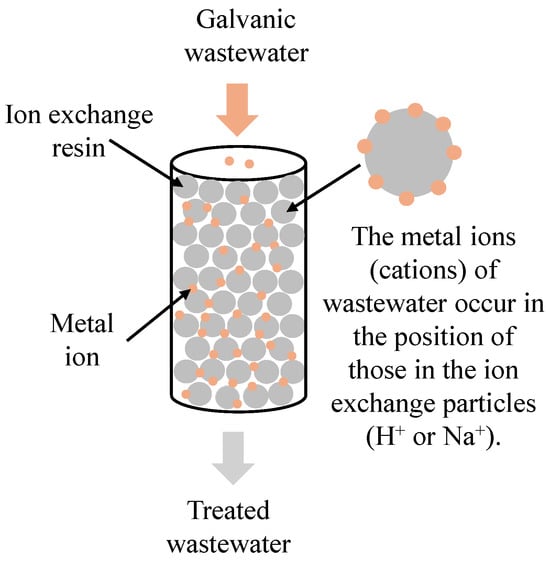
Figure 5.
A diagram of ion exchange employed in order to remove heavy metals from galvanic wastewater.
Jasim and Ajjam [42] demonstrated the efficiency of using ion exchange resin to remove lead(II) and copper(II) from wastewater. The effects of the amount of resin, pH, time of contact, and metal ion concentration on the efficiency of the metals’ removal were analysed. The results provided proof that it was possible to remove as much as 94% of the lead(II) and 93% of the copper(II) under appropriate process conditions. Verbych et al. [43] and Li et al. [44] obtained equally good results using ion exchange resins to remove nickel(II) and copper(II). Examples of other selected resins are listed in Table 5.

Table 5.
Heavy metal removal using the ion exchange method.
A disadvantage of employing ion exchange to remove metals from industrial wastewater is the necessity of periodical ion exchanger regeneration with special solutions. As a consequence, wastewater in the form of used regenerative baths, which have to be cleaned or neutralised, is produced [48,49]. In addition, this method is effective only when the metal concentration is low and requires an earlier removal of organic substances from the wastewater [42,43,44,48].
6. Adsorption
Adsorption is another method that removes heavy metals from galvanic wastewater. Its main rule is the transfer of the ion mass from the liquid phase to the surface of the solid phase, limited by physical and/or chemical influences (Figure 6). The adsorption mechanism is defined by the physicochemical qualities of adsorbents and heavy metals and the conditions of the process (i.e., temperature, adsorbent amount, pH value, adsorption time, and initial concentration of metal ions) [50,51,52].
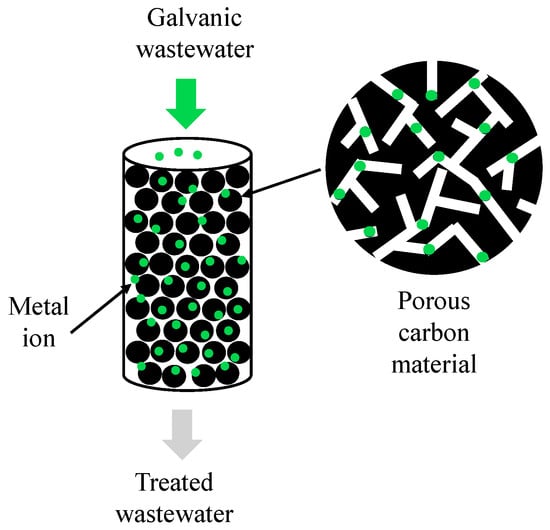
Figure 6.
A diagram of metal ion adsorption in carbon material pores used in order to treat galvanic wastewater.
Many different materials are considered to be efficient adsorbents of heavy metal ions from water solutions, including different types of activated carbons, carbon nanotubes and graphene-based materials [53,54,55,56,57,58,59,60], biochars [61,62,63], clays and their minerals [64,65], chitosan and its composites [66,67], nanomaterials based on metal oxides [68,69], biosorbents [70,71], and low-cost adsorbents, mostly natural waste products [72] but also industrial waste [73]. The adsorption of heavy metals can occur through a combination of physical adsorption, which utilises van der Waals forces and a large surface area to capture metal ions, and chemical mechanisms, such as electrostatic attraction, surface complexation, reduction, and precipitation. The effectiveness of these mechanisms is influenced by the properties of the adsorbent (such as surface area, pore size, and functional groups) and the specific properties of the metal ions (such as their chemical properties and their concentrations in solution). A summary of the removal capabilities of selected heavy metals using different types of adsorbents is presented in Table 6.

Table 6.
The removal rate of heavy metals using different adsorbents.
In the last few years, magnet iron oxides and different composite sorbents produced from them have become an attractive group of adsorbents. They are cheap, easy to prepare and modify, and, thanks to their magnetic qualities, can be successfully separated in a magnetic field. Magnetite (Fe3O4), maghemite (γ-Fe2O3), haematite (α-Fe2O3), and nanoparticles based on mixed iron oxides were identified as efficient adsorbents of different heavy metal ions from water solutions [67,68,69,70,74,75,76,77]. Korus et al. [52,74] created two types of iron oxide-based magnetic adsorbents, namely, unmodified magnetite (M NPs) and magnetite modified with poly-sodium-acrylate (PSA/M NPs), which can be used to remove heavy metals, i.e., Ni(II), Cu(II), Cr(VI), Zn(II), and Cr(III), from different types of galvanic wastewater. However, PSA/M NPs demonstrated a higher efficiency in the case of Ni(II), Cu(II), and Zn(II). Both adsorbents almost completely removed Cr(III) ions from galvanic wastewater (99% adsorption), but they removed Cr(VI) ions only partially (around 50%). Biosorbents in the form of various types of biomass are particularly useful for removing heavy metals from industrial wastewater: algae [78,79], bacteria [80,81], fungi [82,83], and plants [84,85,86], as well as agricultural [87,88,89] and industrial [90] wastes. The mechanism of biosorption of heavy metals from aqueous solutions depends on the type of biosorbent [89]. A summary of the mechanisms of heavy metal removal using various biosorbents is presented in Table 7.

Table 7.
Comparative analysis of the mechanisms of heavy metal biosorption from aqueous solutions.
In turn, the efficiency of removing heavy metals from aqueous solutions using biosorbents depends on their type and concentration [89]. A summary of the possibilities of removing selected heavy metals using various types of biosorbents is presented in Table 8.

Table 8.
The removal rate of heavy metals using different biosorbents.
Adsorption is characterised by low operational costs, high removal efficiency, adsorption material diversity, ease of introduction, and simple cleaning through the regeneration of adsorbed heavy metal ions [51]. However, one should also pay attention to the existing disadvantages of the adsorption process, such as the limited pH range, the prolonged time to achieve balance, the low selectivity, the necessity to regenerate adsorbents with chemical solutions, the decline in adsorbent quality after subsequent operation and regeneration cycles, and the need to dispose of the used adsorption bed [52].
7. Membrane Technologies
Pressure membrane processes (Figure 7) can constitute an effective alternative or a complement to the abovementioned methods of galvanic wastewater treatment [91,92,93,94]. According to the BAT—Best Available Techniques—idea, these technologies constitute one of the most important clean (waste-free) technologies, ensuring that up to 60% of the treated water is returned into circulation and that heavy metals are removed from the wastewater. As such, they are identified by the European Commission as a tool facilitating the introduction of the principles of the circular economy (CE) [94,95].
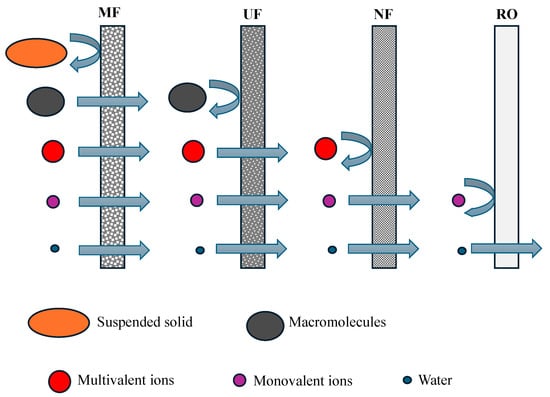
Figure 7.
Separation possibilities of pressure membrane processes: MF—microfiltration, UF—ultrafiltration, NF—nanofiltration, and RO—reverse osmosis.
Interesting prospects for the removal of heavy metal ions from galvanic wastewater are mostly related to the employment of such membrane processes, in which the pressure difference on both sides of a membrane is the driving force. Micellar-enhanced ultrafiltration (MEUF) [96], polymer-enhanced ultrafiltration (PEUF) [97], nanofiltration (NF) [93,98,99,100,101,102], and reverse osmosis (RO) [100,101] can be used to remove heavy metals (Table 9). Micelle-enhanced ultrafiltration (MEUF) removes heavy metals from wastewater by using surfactants (e.g., sodium dodecyl sulfate—SDS, linear alkylbenzene sulfonate—LAS) to form micelles that surround metal ions, which are then retained by the ultrafiltration membrane. Polymer-enhanced ultrafiltration (PEUF) removes heavy metals by using water-soluble polymers (e.g., poly(sodium 4-styrenesulfonate)—PSS), which bind metal ions to form larger macromolecular complexes that are then retained by the ultrafiltration membrane. High-pressure membrane processes (NF and RO) are used for the direct removal of heavy metals from wastewater [14,93,101,102,103]. However, the employment of RO and NF processes to remove galvanic wastewater should be preceded by preliminary treatment. The classical methods of chemical precipitation can be utilised to that end. However, their application entails the generation of sludge and sediments, which must be reprocessed. In turn, the effective separation of metal ions from galvanic wastewater in the NF process is strongly dependent on the concentration of salt mono- and multivalent ions [104,105,106]. In this respect, the employment of UF/RO for galvanic wastewater treatment is beneficial [107]. The advantage of such a combination of membrane processes is the possibility of recovering part of the treated water as cleaned water, which can be used again in the production cycles of a galvanising plant. Other advantages include less industrial wastewater polluted with heavy metals [108] and the recovery of zinc solutions for reuse in galvanic operations [49]. In addition to pressure-driven membrane processes, liquid membranes [109,110], diffusion dialysis [111], electrodialysis [112], and reverse electrodialysis [113] also serve to remove heavy metals from water solutions.

Table 9.
The retention rate of heavy metals using different membrane processes.
The main problem with the common utilisation of membrane processes in industrial wastewater treatment is the decline in membrane permeability as organic and/or inorganic components are deposited on the membrane surface and inner structure as part of the so-called fouling [114], scaling [115], or biofouling [116]. The improvement in the anti-fouling (anti-scaling, anti-biofouling) qualities of membranes through surface modification has been a key research trend of the last few years. Two types of membrane surface modification, including anchoring polymer chains [117,118] and applying a thin coating [119,120], are especially well analysed. Another approach to improving anti-fouling properties is the modification of the polymer membrane-making mix. Materials ranging from 1 to 100 nm in size, called nanomaterials, are usually employed to this end. These can be, e.g., nanoparticles, nanotubes, or two-dimensional layered materials. They are characterised by a well-developed surface, which ensures an exceptional permeability as well as extraordinary chemical and physical stabilities. New highly selective membranes with potential for water and wastewater treatment can be achieved using these nanomaterials’ functional properties, i.e., anti-bacterial, photocatalytic, and wettability [121]. Among the nanomaterials analysed with the potential to improve the qualities of membranes employed in water and wastewater treatment systems, one has to mention graphene [122], graphite oxide (GO) [123,124], carbon nanotubes (CNTs) [125], gold (Au) [126], copper (Cu) [127], silver (Ag) [128], zinc oxide (ZnO), and titanium oxide (TiO2) [129].
Adsorption membranes, which possess both filtration qualities and a high adsorptive capacity, constitute an interesting group of materials used to remove heavy metals from water solutions. Owing to that, they offer an effective method of separating heavy metals through the utilisation of functional groups on a membrane’s surface to selectively bind and remove metals [129,130,131,132]. One of the best approaches to heavy metal removal is the use of composite membranes incorporating selective metal–organic frameworks (MOFs), which are highly porous and have excellent surface properties. Mondal et al. [133] developed a polysulfone membrane modified with MOFs/graphene oxide, which allowed for the separation of heavy metals, including Pb(II), Cu(II), Zn(II), and Cd(II), at a level of 95–99%. Song et al. [134] developed a membrane based on a natural polymer (sodium alginate) modified with MOFs, which was used to effectively remove Cu(II) and Pb(II) from water. Another equally interesting approach is the removal of heavy metals using composite membranes containing adsorptive carbon structures (e.g., graphene, carbon nanotubes, biochars), which are highly porous and possess hydrophilic groups (hydroxyl, carboxyl) [135,136,137,138]. Mokubung et al. [135] developed PES membranes modified with a nanocomposite of biochar, iron(III) oxide, and graphene oxide. These membranes showed increased resistance to fouling and were characterised by high efficiency in heavy metal removal (Cr at the level of 92%, Cu—96%, Ni—92%, Zn—93%, Co—92%, Fe—90%).
If membranes serve to treat galvanic wastewater, it is important to manage used membranes, whose surfaces may be polluted with heavy metals. Depending on their type and state, such membranes can be subject to different kinds of management. Reuse after appropriate preparation and mechanical or chemical recycling are the most common, and membrane disposal is the worst-case scenario. Anyway, the pursuit to minimise waste and effectively use resources is key [139,140,141].
8. Treatment of Galvanic Wastewater in the CE Model
The possibilities of different technologies for removing heavy metals from galvanic industry wastewater have been studied. Some of the most commonly used treatments are chemical precipitation, coagulation/flocculation, ion exchange, adsorption, and membrane filtration. Their main advantages and limitations are presented in Table 10. Modern treatment of electroplating wastewater should enable the recovery of raw materials and water for reuse within the circular economy (CE) model.

Table 10.
Advantages and limitations of different technologies used for the removal of heavy metals from wastewater.
Membrane technologies offer promising prospects for galvanic wastewater treatment in the CE model. As they are effective at removing pollution, they facilitate the following:
- –
- Valuable raw material recovery from galvanic solutions through the separation of heavy metals (e.g., zinc, chromium, nickel, copper), facilitating their reuse in production processes, thus reducing the demand for primary raw materials and minimising waste;
- –
- Water recycling, leading to a considerable decrease in water use in galvanic processes and minimising the discharge of wastewater to the environment;
- –
- Reduction in cleaning costs through raw material recovery and lower costs connected with waste disposal;
- –
- Meeting the environmental requirements pertaining to the quality of wastewater discharged to receivers.
The membranes can also be used at different scales because they can be successfully employed both in large industrial plants and smaller galvanic plants, which makes them flexible and universal.
Taking into account the complexity and variability in galvanic wastewater composition, the preliminary treatment of galvanic wastewater by means of classical filtration, chemical processes (coagulation, flocculation), or low-pressure membrane processes (micro- or ultrafiltration) is often necessary. It is best to perform the basic separation of heavy metals in high-pressure membrane processes (nanofiltration and reverse osmosis), which allow for the recovery of concentrated heavy metal solutions for reuse. Membrane technologies should be supported by additional cleaning processes, e.g., adsorption methods on active deposits. The choice of specific operations depends on the composition of galvanic wastewater and the required level of its treatment. Planning appropriate management methods of the waste (e.g., used membranes, sediments, concentrated wastewater) generated during galvanic wastewater treatment is also indispensable.
9. Conclusions
This literature review determined that a range of methods, such as chemical precipitation, coagulation/flocculation, ion exchange, adsorption, and membrane filtration, can be used. The choice of an appropriate method depends on the composition and pH of the wastewater and the requirements pertaining to the level of its cleanliness. Under appropriate process conditions, the analysed methods may be characterised by a high efficiency in removing heavy metals from galvanic wastewater. However, there are still technological barriers that make the introduction of these methods on an industrial scale impossible. Therefore, future research into the possibility of galvanic wastewater treatment should concentrate on perfecting materials and the process optimisation of the technological solutions discussed in this paper. An interesting direction of research in this subject area can also include assessing the efficiency of heavy metal removal in hybrid systems created as a result of combining specific individual processes. Designing integrated galvanic wastewater treatment systems using modern materials and secondary waste management methods is the future for creating wastewater treatment plants that operate in a circular economy model.
Funding
Subvention was provided by the Łukasiewicz Research Network—Institute for Sustainable Technologies to the Minister of Science in Poland, under decision number DIR-WNO.705.3.8.2025.AJ.
Data Availability Statement
Data is contained within this article.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Smol, M.; Duda, J.; Czaplicka-Kotas, A.; Szołdrowska, D. Transformation towards Circular Economy (CE) in municipal waste management system: Model solutions for Poland. Sustainability 2020, 12, 4561. [Google Scholar] [CrossRef]
- Tomaszewska, J. Polish transition towards Circular Economy: Materials management and implications for the construction sector. Materials 2020, 13, 5228. [Google Scholar] [CrossRef]
- Ulusoy, A.; Atılgan, A.; Rolbiecki, R.; Jagosz, B.; Rolbiecki, S. Innovative approaches for sustainable wastewater resource management. Agriculture 2024, 14, 2111. [Google Scholar] [CrossRef]
- Pandey, A.K. Sustainable water management through integrated technologies and circular resource recovery. Environ. Sci. Water Res. Technol. 2025, 11, 1822–1846. [Google Scholar] [CrossRef]
- Rozkrut, D. Statistical Yearbook of Industry; Central Statistical Office: Warsaw, Poland, 2018. [Google Scholar]
- Zelinski, R.; Silvestre, W.P.; Duarte, J.; Livinalli, N.F.; Zeni, M.; Baldasso, C. Evaluation of the use of reverse osmosis in the treatment of galvanic effluents. J. Membr. Sci. Res. 2023, 9, 562616. [Google Scholar]
- Chavez Porras, Á.; Cristancho Montenegro, D.L.; Ospina Granados, É.A. A clean alternative for galvanic wastewater treatment: Literature review. Rev. Ing. Univ. Medellin 2009, 8, 39–50. [Google Scholar]
- Rajoria, S.; Vashishtha, M.; Sangal, V.K. Treatment of electroplating industry wastewater: A review on the various techniques. Environ. Sci. Pollut. Res. 2022, 29, 72196–72246. [Google Scholar] [CrossRef] [PubMed]
- Oladimeji, T.E.; Oyedemi, M.; Emetere, M.E.; Agboola, O.; Adeoye, J.B.; Odunlami, O.A. Review on the impact of heavy metals from industrial wastewater effluent and removal technologies. Heliyon 2024, 23, e40370. [Google Scholar] [CrossRef]
- Sodhi, K.K.; Mishra, L.C.; Singh, C.K.; Kumar, M. Perspective on the heavy metal pollution and recent remediation strategies. Curr. Res. Microb. Sci. 2022, 3, 100166. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Mala, J.; Maly, J. Effect of heavy metals on self-purification processes in rivers. Appl. Ecol. Environ. Res. 2009, 7, 333–340. [Google Scholar] [CrossRef]
- Directive 2010/75/EU of the European Parliament and of the Council of 24 November 2010 on Industrial Emissions (Integrated Pollution Prevention and Control). Available online: https://eur-lex.europa.eu/eli/dir/2010/75/oj/eng (accessed on 23 September 2025).
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Cherrat, A.; Drif, B.; Erradi, E.M.; Oubaouz, M.; Blaise, N.; El Ossmani, H.; Labjar, N.; El laoui-Belghiti, H.; Bettach, M.; Benzaazoua, M.; et al. Valorization of electric arc furnace FTP dust from Morocco steel industry for efficient recovery of refined zinc via coal treatment. Process Saf. Environ. Protect. 2025, 196, 106961. [Google Scholar] [CrossRef]
- Bharti, V. Hexavalent chromium reduction from real electroplating wastewater by chemical precipitation. Bull. Chem. Soc. Ethiop. 2020, 34, 67–74. [Google Scholar] [CrossRef]
- Meng, S.; Wen, S.; Han, G.; Wang, X.; Feng, Q. Wastewater treatment in mineral processing of non-ferrous metal resources: A review. Water 2022, 14, 726. [Google Scholar] [CrossRef]
- Ghirisan, A.L.; Dragan, S.; Pop, A. Heavy metal removal and neutralization of acid mine waste water-kinetic study. Can. J. Chem. Eng. 2007, 85, 900–905. [Google Scholar] [CrossRef]
- Chen, Q.; Luo, Z.; Hills, C. Precipitation of heavy metals from wastewater using simulated flue gas: Sequent additions of fly ash, lime and carbon dioxide. Water Res. 2009, 43, 2605–2614. [Google Scholar] [CrossRef]
- Giannopoulou, I.; Panias, D. Differential precipitation of copper and nickel from acidic polymetallic aqueous solutions. Hydrometallurgy 2008, 90, 137–146. [Google Scholar] [CrossRef]
- Guo, Z.R.; Zhang, G.; Fang, J. Enhanced chromium recovery from tanning wastewater. J. Clean. Prod. 2006, 14, 75–79. [Google Scholar] [CrossRef]
- Alvarez, M.T.; Crespo, C.; Mattiasson, B. Precipitation of Zn(II), Cu(II) and Pb(II) at bench-scale using biogenic hydrogen sulfidefrom the utilization of volatile fatty acids. Chemosphere 2007, 66, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Azis, M.Y.; Amedyan, N.N.; Hanefiatni; Suprabawati, A. Study of reducing chromium(VI) to chromium(III) ion using reduction and coagulation methods for electroplating industrial waste. J. Phys. Conf. Ser. 2021, 1763, 012042. [Google Scholar] [CrossRef]
- Yan, F.L.; Wang, Y.; Wang, W.H.; Zhao, J.X.; Feng, L.L.; Li, J.J.; Zhao, J.C. Application of biochars obtained through the pyrolysis of Lemna minor in the treatment of Ni-electroplating wastewater. J. Water Process Eng. 2020, 37, 101464. [Google Scholar] [CrossRef]
- Wu, B.; Dai, X.; Chai, X. Critical review on dewatering of sewage sludge: Influential mechanism, conditioning technologies and implications to sludge re-utilizations. Water Res. 2020, 180, 115912. [Google Scholar] [CrossRef]
- Arroub, H.; Hsissou, R.; Elharfi, A. Investigation of modified chitosan as potential polyelectrolyte polymer and eco-friendly for the treatment of galvanization wastewater using novel hybrid process. Results Chem. 2020, 2, 100047. [Google Scholar] [CrossRef]
- Zueva, S.B.; Ferella, F.; Innocenzi, V.; De Michelis, I.; Corradini, V.; Ippolito, N.M.; Vegliò, F. Recovery of zinc from treatment of spent acid solutions from the pickling stage of galvanizing plants. Sustainability 2021, 13, 407. [Google Scholar] [CrossRef]
- Lucas, M.S.; Teixeira, A.R.; Jorge, N.; Peres, J.A. Industrial wastewater treatment by coagulation–flocculation and advanced oxidation processes: A review. Water 2025, 17, 1934. [Google Scholar] [CrossRef]
- García-Ávila, F.; Mayancela-Santander, E.; Alvarado-Pacheco, B.; Valdiviezo-Gonzales, L.; Cadme-Galabay, M.; Zhindón-Arévalo, C.; Reynoso-Quispe, P. Comparative analysis of coagulants for selective removal of iron and aluminum from galvanic wastewater: A practical and effective approach. Ain Shams Eng. J. 2025, 16, 103393. [Google Scholar] [CrossRef]
- Hargreaves, A.J.; Vale, P.; Whelan, J.; Alibardi, L.; Constantino, C.; Dotro, G.; Cartmell, E.; Campo, P. Coagulation–flocculation process with metal salts, synthetic polymers and biopolymers for the removal of trace metals (Cu, Pb, Ni, Zn) from municipal wastewater. Clean Technol. Environ. Policy 2018, 20, 393–402. [Google Scholar] [CrossRef]
- Msemwa, G.G.; Nasr, M.; Abdelhaleem, A.; Fujii, M.; Ibrahim, M.G. Coagulation-flocculation/pyrolysis integrated system for dye-laden wastewater treatment: A techno-economic and sustainable approach. Water Air Soil Pollut. 2025, 236, 57. [Google Scholar] [CrossRef]
- Mensah-Akutteh, H.; Buamah, R.; Wiafe, S.; Nyarko, K.B. Optimization of coagulation-flocculation processes with aluminum coagulation using response surface methods. Appl. Water Sci. 2022, 12, 188. [Google Scholar] [CrossRef]
- Gandiwa, B.I.; Moyo, L.B.; Ncube, S.; Mamvura, T.A.; Mguni, L.L.; Hlabangana, N. Optimization of using a blend of plant based natural and synthetic coagulants for water treatment: Moringa Oleifera-Cactus Opuntia-alum blend. S. Afr. J. Chem. Eng. 2020, 34, 158–164. [Google Scholar]
- Daud, N.M.; Abdullah, S.R.S.; Hasan, H.A.; Othman, A.R.; Ismail, N. Coagulation-flocculation treatment for batik effluent as a baseline study for the upcoming application of green coagulants/flocculants towards sustainable batik industry. Heliyon 2023, 9, e17284. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.M.; Fawzy, M.E.; Nassar, H.F. Effective Chemical Coagulation Treatment Process for Cationic and Anionic Dyes Degradation. Egypt. J. Chem. 2022, 65, 299–307. [Google Scholar] [CrossRef]
- García-Ávila, F.; Criollo-Illescas, F.; Zhindón-Arévalo, C.; García-Uzca, C.; Donoso-Moscoso, S.; Alfaro-Paredes, E. Integration of rapid filters for the provision of drinking water at rural home level. Groundw. Sustain. Dev. 2024, 26, 101217. [Google Scholar] [CrossRef]
- Xiao, X.; Sun, Y.; Liu, J.; Zheng, H. Flocculation of heavy metal by functionalized starch-based bioflocculants: Characterization and process evaluation. Sep. Purif. Technol. 2021, 267, 118628. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, S.; Sun, W.; Zhu, S.; Zheng, H. Flocculation activity and evaluation of chitosan-based flocculant CMCTS-g-P(AM-CA) for heavy metal removal. Sep. Purif. Technol. 2020, 241, 116737. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, A.; Pan, S.Y.; Sun, W.; Zhu, C.; Shah, K.J.; Zheng, H. Novel chitosan-based flocculants for chromium and nickle removal in wastewater via integrated chelation and flocculation. J. Environ. Manag. 2019, 248, 109241. [Google Scholar] [CrossRef] [PubMed]
- López-Maldonado, E.A.; Zavala García, O.G.; Escobedo, K.C.; Oropeza-Guzman, M.T. Evaluation of the chelating performance of biopolyelectrolyte green complexes (NIBPEGCs) for wastewater treatment from the metal finishing industry. J. Hazard. Mater. 2017, 335, 18–27. [Google Scholar] [CrossRef]
- El Ouardi, Y.; Virolainen, S.; Mouele, E.S.M.; Laatikainen, M.; Repo, E.; Laatikainen, K. The recent progress of ion exchange for the separation of rare earths from secondary resources—A review. Hydrometallurgy 2023, 218, 106047. [Google Scholar] [CrossRef]
- Jasim, A.Q.; Ajjam, S.K. Removal of heavy metal ions from wastewater using ion exchange resin in a batch process with kinetic isotherm. S. Afr. J. Chem. Eng. 2024, 49, 43–54. [Google Scholar] [CrossRef]
- Verbych, S.; Hilal, N.; Sorokin, G.; Leaper, M. Ion exchange extraction of heavy metal ions from wastewater. Sep. Sci. Technol. 2005, 39, 2031–2040. [Google Scholar] [CrossRef]
- Li, T.; Xiao, K.; Yang, B.; Peng, G.; Liu, F.; Tao, L.; Chen, S.; Wei, H.; Yu, G.; Deng, S. Recovery of Ni(II) from real electroplating wastewater using fixed-bed resin adsorption and subsequent electrodeposition. Front. Environ. Sci. Eng. 2019, 13, 91. [Google Scholar] [CrossRef]
- Zhang, S.; Ning, S.; Liu, H.; Wang, X.; Wei, Y.; Yin, X. Preparation of ion-exchange resin via in-situ polymerization for highly selective separation and continuous removal of palladium from electroplating wastewater. Sep. Purif. Technol. 2021, 258, 117670. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, S.; Xia, L.; Wang, Z.; Suo, N.; Chen, H.; Long, Y.; Zhou, B.; Yu, Y. In-situ ion exchange electrocatalysis biological coupling (i-IEEBC) for simultaneously enhanced degradation of organic pollutants and heavy metals in electroplating wastewater. J. Hazard. Mater. 2019, 364, 562–570. [Google Scholar] [CrossRef]
- Ye, Z.; Yin, X.; Chen, L.; He, X.; Lin, Z.; Liu, C.; Ning, S.; Wang, X.; Wei, Y. An integrated process for removal and recovery of Cr(VI) from electroplating wastewater by ion exchange and reduction–precipitation based on a silica-supported pyridine resin. J. Clean. Prod. 2019, 236, 117631. [Google Scholar] [CrossRef]
- Bożęcka, A.M.; Sanak-Rydlewska, S. The use of ion exchangers for removing cobalt and nickel ions from water solutions. Arch. Min. Sci. 2018, 63, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Kowalik-Klimczak, A.; Gajewska-Midziałek, A.; Buczko, Z.; Łożyńska, M.; Życki, M.; Barszcz, W.; Ciciszwili, T.; Dąbrowski, A.; Kasierot, S.; Charasińska, J.; et al. Circular economy approach in treatment of galvanic wastewater employing membrane processes. Membranes 2023, 13, 325. [Google Scholar] [CrossRef]
- Raji, Z.; Karim, A.; Karam, A.; Khalloufi, S. Adsorption of heavy metals: Mechanisms, kinetics, and applications of various adsorbents in wastewater remediation—A review. Waste 2023, 1, 775–805. [Google Scholar] [CrossRef]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B.H. Adsorption of heavy metal ions by various low-cost adsorbents: A review. Int. J. Environ. Anal. Chem. 2020, 102, 342–379. [Google Scholar] [CrossRef]
- Korus, I. Separation of chosen heavy metals from multi-component mixtures and galvanic wastewater in adsorption on unmodified and modified magnetite. Desalination Water Treat. 2023, 301, 197–208. [Google Scholar] [CrossRef]
- Karnib, M.; Kabbani, A.; Holail, H.; Olama, Z. Heavy metals removal using activated carbon, silica and silica activated carbon composite. Energy Procedia 2014, 50, 113–120. [Google Scholar] [CrossRef]
- Ayub, S.; Siddique, A.A.; Khursheed, M.S.; Zarei, A.; Alam, I.; Asgari, E.; Changani, F. Removal of heavy metals (Cr, Cu, and Zn) from electroplating wastewater by electrocoagulation and adsorption processes. Desalination Water Treat. 2020, 179, 263–271. [Google Scholar] [CrossRef]
- Bankole, M.T.; Abdulkareem, A.S.; Mohammed, I.A.; Ochigbo, S.S.; Tijani, J.O.; Abubakre, O.K.; Roos, W.D. Selected heavy metals removal from electroplating wastewater by purified and polyhydroxylbutyrate functionalized carbon nanotubes adsorbents. Sci. Rep. 2019, 9, 4475. [Google Scholar] [CrossRef]
- Thajeel, A.S. Isotherm, kinetic and thermodynamic of adsorption of heavy metal ions onto local activated carbon. Aquat. Sci. Technol. 2013, 1, 53–77. [Google Scholar] [CrossRef]
- Aguayo-Villarreal, I.A.; Bonilla-Petriciolet, A.; Muñiz-Valencia, R. Preparation of activated carbons from pecan nutshell and their application in the antagonistic adsorption of heavy metal ions. J. Mol. Liq. 2017, 230, 686–695. [Google Scholar] [CrossRef]
- Yadav, V.B.; Gadi, R.; Kalra, S. Clay based nanocomposites for removal of heavy metals from water: A review. J. Environ. Manag. 2019, 232, 803–817. [Google Scholar] [CrossRef]
- Ahmed, S.; Fatema-Tuj-Zohra; Mahdi, M.M.; Mahmudunnabi, D.M.; Choudhury, T.R.; Zahangir Alam, M.; Nurnabi, M. Synthesis and characterization of graphene oxide for removal of Cr(III) from tannery effluent. Desalination Water Treat. 2021, 244, 201–211. [Google Scholar] [CrossRef]
- Abu-Nada, A.; McKay, G.; Abdala, A. Recent advances in applications of hybrid graphene materials for metals removal from wastewater. Nanomaterials 2020, 10, 595. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak, B.; Molenda, J.; Swat, M. The adsorption of chromium (III) ions from water solutions on biocarbons obtained from plant waste. Environ. Technol. Innov. 2021, 23, 101737. [Google Scholar] [CrossRef]
- Barszcz, W.; Łożyńska, M.; Molenda, J. Impact of pyrolysis process conditions on the structure of biochar obtained from apple waste. Sci. Rep. 2024, 14, 10501. [Google Scholar] [CrossRef]
- Syarifuddin, S.; Heryanto, H.; Suryani, S.; Tahir, D. Biochar from diverse wastes: A comprehensive bibliometric analysis of heavy metal adsorption in wastewater. Desalination Water Treat. 2024, 317, 100089. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Masindi, V.; Gitari, W.M. The potential of ball-milled South African bentonite clay for attenuation of heavy metals from acidic wastewaters: Simultaneous sorption of Co2+, Cu2+, Ni2+, Pb2+, and Zn2+ ions. J. Environ. Chem. Eng. 2015, 3, 2416–2425. [Google Scholar]
- .Wan Ngah, W.S.; Teong, L.C.; Hanafiah, M.A.K.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- He, Y.; Zhang, P.; Wang, L. Adsorption and removal of Cr6+, Cu2+, Pb2+, and Zn2+ from aqueous solution by magnetic nano-chitosan. Molecules 2023, 28, 2607. [Google Scholar] [CrossRef]
- Janani, R.; Gurunathan, B.; Sivakumar, K.; Varjani, S.; Ngo, H.H.; Gnansounou, E. Advancements in heavy metals removal from effluents employing nano-adsorbents: Way towards cleaner production. Environ. Res. 2022, 203, 111815. [Google Scholar] [CrossRef]
- Kumari, P.; Alam, M.; Siddiqi, W.A. Usage of nanoparticles as adsorbents for wastewater treatment: An emerging trend. Sustain. Mater. Technol. 2019, 22, e00128. [Google Scholar]
- Wang, Q.; Wang, Y.; Tang, J.; Yang, Z.; Zhang, L.; Huang, X. New insights into the interactions between Pb(II) and fruit waste biosorbent. Chemosphere 2022, 303, 135048. [Google Scholar] [CrossRef]
- Xie, S. Biosorption of heavy metal ions from contaminated wastewater: An eco-friendly approach. Green Chem. Lett. Rev. 2024, 17, 2357213. [Google Scholar] [CrossRef]
- Abas, S.N.A.; Ismail, M.H.S.; Kamal, L.M.; Izhar, S. Adsorption process of heavy metals by low-cost adsorbent: A review. World Appl. Sci. J. 2013, 28, 1518–1530. [Google Scholar]
- Ahmaruzzaman, M. Industrial wastes as low-cost potential adsorbents for the treatment of wastewater laden with heavy metals. Adv. Colloid Interface Sci. 2011, 166, 36–59. [Google Scholar] [CrossRef]
- Korus, I.; Bobik, M.; Bąk, K. Influence of ionic environment on the process of adsorption of heavy metal ions on magnetic iron oxides. Desalination Water Treat. 2020, 186, 224–233. [Google Scholar] [CrossRef]
- Phouthavong, V.; Yan, R.; Nijpanich, S.; Hagio, T.; Ichino, R.; Kong, L.; Li, L. Magnetic adsorbents for wastewater treatment: Advancements in their synthesis methods. Materials 2022, 15, 1053. [Google Scholar] [CrossRef] [PubMed]
- Aragaw, T.A.; Bogale, F.M.; Aragaw, B.A. Iron-based nanoparticles in wastewater treatment: A review on synthesis methods, applications, and removal mechanism. J. Saudi Chem. Soc. 2021, 25, 101280. [Google Scholar] [CrossRef]
- Wardani, D.A.P.; Rosmainar, L.; Iqbal, R.M.; Simarmat, S.N. Synthesis and characterization of magnetic adsorbent based on Fe2O3 -fly ash from Pulang Pisau’s power plant of Central Kalimantan. IOP Conf. Ser. Mater. Sci. Eng. 2020, 980, 012014. [Google Scholar] [CrossRef]
- Ordóñez, J.I.; Cortés, S.; Maluenda, P.; Soto, I. Biosorption of heavy metals with algae: Critical review of its application in real effluents. Sustainability 2023, 15, 5521. [Google Scholar] [CrossRef]
- Ciobanu, A.A.; Lucaci, A.R.; Bulgariu, L. Efficient metal ions biosorption on red and green algae biomass: Isotherm, kinetic and thermodynamic study. J. Appl. Phycol. 2024, 36, 3809–3827. [Google Scholar] [CrossRef]
- Baran, M.F.; Duz, Z.; Baran, A.; Keskin, C.; Aktepe, N. Removal of heavy metals in water by biosorption method using three different Bacillus sp- derived biosorbents. KSU J. Agric. Nat. 2022, 25, 449–458. [Google Scholar] [CrossRef]
- Pham, V.H.T.; Kim, J.; Chang, S.; Chung, W. Bacterial biosorbents, an efficient heavy metals green clean-up strategy: Prospects, challenges, and opportunities. Microorganisms 2022, 10, 610. [Google Scholar] [CrossRef]
- Legorreta-Castañeda, A.J.; Lucho-Constantino, C.A.; Beltrán-Hernández, R.I.; Coronel-Olivares, C.; Vázquez-Rodríguez, G.A. Biosorption of water pollutants by fungal pellets. Water 2020, 12, 1155. [Google Scholar] [CrossRef]
- El-Bondkly, A.M.A.; El-Gendy, M.M.A.A. Bioremoval of some heavy metals from aqueous solutions by two different indigenous fungi Aspergillus sp. AHM69 and Penicillium sp. AHM96 isolated from petroleum refining wastewater. Heliyon 2022, 8, e09854. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.H.; Shartooh, S.M.; Trigui, M. Biosorption and isotherm modeling of heavy metals using Phragmites australis. Sustainability 2025, 17, 5366. [Google Scholar] [CrossRef]
- Anis Mani, K.; Swarnalatha, A.P.; Naveen, K.N.; Suryanarayana Raju, J.N.S.; Shankramma, S.K.; Priyadharsini, K. Biosorption of heavy metals using plant-derived sorbents: An environmental solution for industrial wastewater. Glob. NEST J. 2025, 27, 06874. [Google Scholar]
- Karim, A.; Raji, Z.; Karam, A.; Khalloufi, S. Valorization of fibrous plant-based food waste as biosorbents for remediation of heavy metals from wastewater—A review. Molecules 2023, 28, 4205. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, H.A. Removal of heavy metals from wastewater using agricultural and industrial wastes as adsorbents. HBRC J. 2013, 9, 276–282. [Google Scholar] [CrossRef]
- Abdić, Š.; Memić, M.; Šabanović, E.; Sulejmanović, J.; Begić, S. Adsorptive removal of eight heavy metals from aqueous solution by unmodified and modified agricultural waste: Tangerine peel. Int. J. Environ. Sci. Technol. 2018, 15, 2511–2518. [Google Scholar] [CrossRef]
- Karnwal, A. Unveiling the promise of biosorption for heavy metal removal from water sources. Desalination Water Treat. 2024, 319, 100523. [Google Scholar] [CrossRef]
- Ghiaci, M.; Dorostkar, N.; Gil, A. Chicken bone ash as an efficient metal biosorbent for cadmium, lead, nickel, and zinc from aqueous solutions. Desalination Water Treat. 2014, 52, 3115–3121. [Google Scholar] [CrossRef]
- Pervov, A.G.; Adrianov, A.P.; Gorbunova, T.P.; Bagdasaryan, A.S. Membrane technologies in the solution of environmental problems. Pet. Chem. 2015, 55, 879–886. [Google Scholar] [CrossRef]
- Hegoburu, I.; Zedda, K.L.; Velizarov, S. Treatment of electroplating wastewater using NF pH-stable membranes: Characterization and application. Membranes 2020, 10, 399. [Google Scholar] [CrossRef]
- Kumar, J.; Joshi, H.; Malyan, S.K. Removal of copper, nickel, and zinc ions from an aqueous solution through electrochemical and nanofiltration membrane processes. Appl. Sci. 2022, 12, 280. [Google Scholar] [CrossRef]
- Rubel, E.; Tomassi, P.; Ziółkowski, J. Best Available Techniques (BAT) Guidelines for the Surface Treatment of Metals and Plastics; Institute of Precision Mechanics: Warsaw, Poland, January 2009. [Google Scholar]
- Brinkmann, T.; Giner, S.G.; Yükseler, H.; Roudier, S.; Delgado, S.L. Best Available Techniques (BAT) Reference Document for Common Waste Water and Waste Gas Treatment/Management Systems in the Chemical Sector; Industrial Emissions Directive 2010/75/EU (Integrated Pollution Prevention and Control): Warsaw, Poland, 2016. [Google Scholar]
- Ren, J.; Jiang, Y.; Ren, H.; Xue, X.; Yang, Z.; Yang, L.; Wang, J.; Tao, L. Micellar-enhanced ultrafiltration of heavy metal wastewater with palygorskite under various temperatures and pressures. J. Water Process Eng. 2024, 67, 106208. [Google Scholar] [CrossRef]
- Borbély, G.; Nagy, E. Removal of zinc and nickel ions by complexation–membrane filtration process from industrial wastewater. Desalination 2009, 240, 218–226. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, P.; Xu, D.; Zheng, J.; Zhan, Z.M.; Gao, Q.; Yuan, S.; Xu, Z.L.; Bruggen, B.V.D. Triethanolamine modification produces ultra-permeable nanofiltration membrane with enhanced removal efficiency of heavy metal ions. J. Membr. Sci. 2022, 644, 120127. [Google Scholar] [CrossRef]
- Mahmoud, A.E.D.; Mostafa, E. Nanofiltration membranes for the removal of heavy metals from aqueous solutions: Preparations and applications. Membranes 2023, 13, 789. [Google Scholar] [CrossRef]
- Dawam, M.; Gobara, M.; Oraby, H.; Zorainy, M.Y.; Nabil, I.M. Advances in membrane technologies for heavy metal removal from polluted water: A comprehensive review. Water Air Soil Pollut. 2025, 236, 461. [Google Scholar] [CrossRef]
- Castro, K.; Abejón, R. Removal of heavy metals from wastewaters and other aqueous streams by pressure-driven membrane technologies: An outlook on reverse osmosis, nanofiltration, ultrafiltration and microfiltration potential from a bibliometric analysis. Membranes 2024, 14, 180. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Min, X.; Tang, C.-J.; Sillanpää, M.A.; Zhao, F. Recent advances in membrane filtration for heavy metal removal from wastewater: A mini review. J. Water Process Eng. 2022, 49, 103023. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Othaman, R.; Hilal, N. Potential use of nanofiltration membranes in treatment of industrial wastewater from Ni-P electroless plating. Desalination 2004, 168, 241–252. [Google Scholar] [CrossRef]
- Figoli, A.; Cassano, A.; Criscuoli, A.; Mozumder, M.S.I.; Uddin, M.T.; Islam, M.A.; Drioli, E. Influence of operating parameters on the arsenic removal by nanofiltration. Water Res. 2010, 44, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Kowalik-Klimczak, A.; Zalewski, M.; Gierycz, P. Removal of Cr(III) ions from salt solution by nanofiltration: Experimental and modelling analysis. Pol. J. Chem. Technol. 2016, 18, 10–16. [Google Scholar] [CrossRef][Green Version]
- Petrinic, I.; Korenak, J.; Povodnik, D.; Hélix-Nielsen, C. A feasibility study of ultrafiltration/reverse osmosis (UF/RO)-based wastewater treatment and reuse in the metal finishing industry. J. Clean. Prod. 2015, 101, 292–300. [Google Scholar] [CrossRef]
- Innocenzi, V.; Cantarini, F.; Amato, A.; Morico, B.; Ippolito, N.M.; Beolchini, F.; Prisciandaro, M.; Vegliò, F. Case study on technical feasibility of galvanic wastewater treatment plant based on life cycle assessment and costing approach. J. Environ. Chem. Eng. 2020, 8, 104535. [Google Scholar] [CrossRef]
- Mendil, J.; Alalou, A.; Mazouz, H.; Al-Dahhan, M.H. Review of emulsion liquid membrane for heavy metals recovery from wastewater/water: Stability, efficiency, and optimization. Chem. Eng. Process. Process Intensif. 2024, 196, 109647. [Google Scholar] [CrossRef]
- Kaczorowska, M.A. The use of polymer inclusion membranes for the removal of metal ions from aqueous solutions—The latest achievements and potential industrial applications: A review. Membranes 2022, 12, 1135. [Google Scholar] [CrossRef]
- Kadłubowicz, A.; Janiszewska, M.; Baraniak, M.; Lota, G.; Staszak, K.; Regel-Rosocka, M. Diffusion dialysis and extraction integrated system for recovery of cobalt(II) from industrial effluent. J. Water Process. Eng. 2020, 39, 101754. [Google Scholar] [CrossRef]
- Cerrillo-Gonzalez, M.D.M.; Villen-Guzman, M.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Metal recovery from wastewater using electrodialysis separation. Metals 2024, 14, 38. [Google Scholar] [CrossRef]
- Siekierka, A.; Yalcinkaya, F.; Bryjak, M. Recovery of transition metal ions with simultaneous power generation by reverse electrodialysis. J. Environ. Chem. Eng. 2023, 11, 110145. [Google Scholar] [CrossRef]
- AlSawaftah, N.; Abuwatfa, W.; Darwish, N.; Husseini, G. A Comprehensive review on membrane fouling: Mathematical modelling, prediction, diagnosis, and mitigation. Water 2021, 13, 1327. [Google Scholar] [CrossRef]
- Matin, A.; Rahman, F.; Shafi, H.Z.; Zubair, S.M. Scaling of reverse osmosis membranes used in water desalination: Phenomena, impact, and control; future directions. Desalination 2019, 455, 135–157. [Google Scholar] [CrossRef]
- AlSawaftah, N.; Abuwatfa, W.; Darwish, N.; Husseini, G.A. A Review on membrane biofouling: Prediction, characterization, and mitigation. Membranes 2022, 12, 1271. [Google Scholar] [CrossRef]
- Pinem, J.A.; Wardani, A.K.; Aryanti, P.T.P.; Khoiruddin, K.; Wenten, I.G. Hydrophilic modification of polymeric membrane using graft polymerization method: A mini review. IOP Conf. Ser. Mater. Sci. Eng. 2019, 547, 012054. [Google Scholar] [CrossRef]
- Suresh, D.; Goh, P.S.; Ismail, A.F.; Hilal, N. Surface design of liquid separation membrane through graft polymerization: A state of the art review. Membranes 2021, 11, 832. [Google Scholar] [CrossRef]
- Numaan, M.M.; Jasim, A.M.; Xing, Y.; Fidalgo, M.M. Thin-layer TiO2 membrane fabrication by condensed layer deposition. Materials 2024, 17, 4436. [Google Scholar] [CrossRef]
- Kacprzyńska-Gołacka, J.; Kowalik-Klimczak, A.; Woskowicz, E.; Wieciński, P.; Łożyńska, M.; Sowa, S.; Barszcz, W.; Kaźmierczak, B. Microfiltration membranes modified with silver oxide by plasma treatment. Membranes 2020, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Nain, A.; Sangili, A.; Hu, S.-R.; Chen, C.-H.; Chen, Y.-L.; Chang, H.-T. Recent progress in nanomaterial-functionalized membranes for removal of pollutants. IScience 2022, 25, 104616. [Google Scholar] [CrossRef] [PubMed]
- Polak, D.; Szwast, M.; Fabianowski, W.; Rosiński, M. Ceramic membranes modified by carbon used for laundry wastewater treatment. Chem. Eng. Trans. 2019, 74, 931–936. [Google Scholar]
- Tiwary, S.K.; Singh, M.; Chavan, S.V.; Karim, A. Graphene oxide-based membranes for water desalination and purification. npj 2D Mater. Appl. 2024, 8, 27. [Google Scholar] [CrossRef]
- Kowalik-Klimczak, A.; Woskowicz, E.; Kacprzyńska-Gołacka, J. The surface modification of polyamide membranes using graphene oxide. Colloids Surf. A 2020, 587, 124281. [Google Scholar] [CrossRef]
- Lee, B.; Kim, C. Innovative membrane technology for water treatment solutions: Current status and future prospects of carbon nanotube membranes. Environ. Eng. Res. 2024, 29, 240104. [Google Scholar] [CrossRef]
- Mergola, L.; Carbone, L.; Bloise, E.; Lazzoi, M.R.; Del Sole, R. Sustainable and reusable modified membrane based on green gold nanoparticles for efficient methylene blue water decontamination by a photocatalytic process. Nanomaterials 2024, 14, 1611. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Rodríguez, B.; Giraldo, H.; Quintero, Y.; Quezada, R.; Hassan, N.; Estay, H. Copper-modified polymeric membranes for water treatment: A comprehensive review. Membranes 2021, 11, 93. [Google Scholar] [CrossRef]
- Mozia, S.; Jose, M.; Sienkiewicz, P.; Szymański, K.; Darowna, D.; Zgrzebnicki, M.; Markowska-Szczupa, A. Polyethersulfone ultrafiltration membranes modified with hybrid Ag/titanate nanotubes: Physicochemical characteristics, antimicrobial properties, and fouling resistance. Desalination Water Treat. 2018, 128, 106–118. [Google Scholar] [CrossRef]
- Kowalik-Klimczak, A.; Stanisławek, E.; Kacprzyńska-Gołacka, J.; Bednarska, A.; Osuch-Słomka, E.; Skowroński, J. The polyamide membranes functionalized by nanoparticles for biofouling control. Desalination Water Treat. 2018, 128, 243–252. [Google Scholar] [CrossRef]
- Buonomenna, M.G.; Mousavi, S.M.; Hashemi, S.A.; Lai, C.W. Water cleaning adsorptive membranes for efficient removal of heavy metals and metalloids. Water 2022, 14, 2718. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T. Removal of heavy metals and pollutants by membrane adsorption techniques. Appl. Water Sci. 2018, 8, 19. [Google Scholar] [CrossRef]
- Vo, T.S.; Hossain, M.M.; Jeong, H.M.; Kim, K. Heavy metal removal applications using adsorptive membranes. Nano Converg. 2020, 7, 36. [Google Scholar] [CrossRef]
- Mondal, M.; Indurkar, P.D. Heavy metals remediation using MOF5@ GO composite incorporated mixed matrix ultrafiltration membrane. Chem. Eng. J. 2024, 494, 153155. [Google Scholar] [CrossRef]
- Song, J.; Chen, L.; Zhou, Y.; Yuan, Z.; Niu, Y.; Wei, Z. Efficient adsorptive separation of Cu(II) from Ph by ZIF-8 constructed with sodium alginate polymer backbone. Int. J. Biol. Macromol. 2024, 279, 135501. [Google Scholar] [CrossRef]
- Mokubung, K.E.; Madzivha, N.N.; Lau, W.J.; Nxumalo, E.N. Polyethersulfone/biochar-Fe3O4/GO mixed matrix membranes with enhanced antifouling properties for heavy metals removal from acid mine drainage. Inorg. Chem. Commun. 2025, 176, 114190. [Google Scholar] [CrossRef]
- Mukherjee, R.; Bhunia, P.; De, S. Impact of graphene oxide on removal of heavy metals using mixed matrix membrane. Chem. Eng. J. 2016, 292, 284–297. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, F.; Xue, J.; Chen, S.; Wang, J.; Yang, Y. Enhanced removal of heavy metal ions from aqueous solution using manganese dioxide-loaded biochar: Behavior and mechanism. Sci. Rep. 2020, 10, 6067. [Google Scholar] [CrossRef]
- Hlihor, R.M.; Gavrilescu, M. Removal of some environmentally relevant heavy metals using low-cost natural sorbents. Environ. Eng. Manag. J. 2009, 8, 353–372. [Google Scholar] [CrossRef]
- Tian, C.; Chen, J.; Bai, Z.; Wang, X.; Dai, R.; Wang, Z. Recycling of end-of-life polymeric membranes for water treatment: Closing the loop. J. Membr. Sci. Lett. 2023, 3, 100063. [Google Scholar] [CrossRef]
- Somrani, A.; Abohelal, K.; Pontié, M. A Mini Review of Reused End-of-Life Reverse Osmosis (EoL RO) Membranes. Membranes 2025, 15, 217. [Google Scholar] [CrossRef]
- Neelamegam, P.; Muthusubramanian, B. A sustainable novel approach of recycling end-of-life reverse osmosis (RO) membrane as additives in concrete and mathematical model with response surface methodology (RSM). Environ. Sci. Pollut. Res. 2024, 31, 19304–19328. [Google Scholar] [CrossRef]
- Benalia, M.C.; Youcef, L.; Bouaziz, M.G.; Achour, S.; Menasra, H. Removal of heavy metals from industrial wastewater by chemical precipitation: Mechanisms and sludge characterization. Arab. J. Sci. Eng. 2022, 47, 5587–5599. [Google Scholar] [CrossRef]
- Macena, M.; Pereira, H.; Cruz-Lopes, L.; Grosche, L.; Esteves, B. Competitive Adsorption of Metal Ions by Lignocellulosic Materials: A Review of Applications, Mechanisms and Influencing Factors. Separations 2025, 12, 70. [Google Scholar] [CrossRef]
- Sgreccia, E.; Rogalska, C.; Gallardo Gonzalez, F.S.; Prosposito, P.; Burratti, L.; Knauth, P.; Vona, M.L.B. Heavy metal decontamination by ion exchange polymers for water purification: Counterintuitive cation removal by an anion exchange polymer. J. Mater. Sci. 2024, 59, 2776–2787. [Google Scholar] [CrossRef]
- Zhu, Y.; Fan, W.; Zhou, T.; Li, X. Removal of chelated heavy metals from aqueous solution: A review of current methods and mechanisms. Sci. Total Environ. 2019, 678, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).