Sustainable Biomass-Derived Photothermal Material for Solar-Driven Seawater Desalination and Wastewater Treatment
Abstract
1. Introduction
- (1)
- Surface carbonization selectively carbonizes the biomass’ surface while retaining its bulk natural structure (e.g., vascular bundles for water transport), offering the advantages of simplicity and low cost. For instance, Zhu et al. constructed a dual-layer solar evaporator by surface-carbonizing natural wood, leveraging wood’s longitudinal channels for water transport; it achieved 80.4% evaporation efficiency under 10 suns (10 kW·m−2) [25]. Zhang et al. used concentrated sulfuric acid to form a bowl-shaped carbonized layer on sorghum straw, enhancing light trapping (absorption > 90%); under 1 sun, it reached an evaporation rate of 1.96 kg·m−2·h−1 with 81.8% efficiency [26]. Jang et al. used a CO2 laser to carbonize the surface of balsa wood for sunlight absorption, with a water evaporation rate of 1.26 kg·m−2·h−1 and a photothermal conversion efficiency of 77% [27]. However, surface carbonization has limitations: the carbonized layer weakly adheres to the biomass substrate, risking detachment during long-term use. Additionally, performance heavily depends on the biomass’s inherent structure (e.g., straw’s hollow channels), restricting its applicability to biomass with pre-existing favorable macrostructures.
- (2)
- Complete carbonization involves full biomass conversion under vacuum or inert gas, producing a more stable carbon skeleton, but requiring stricter process control (e.g., temperature, gas atmosphere). Materials prepared this way often leverage natural porous structures: carbonized mushrooms, with their umbrella-like porous architecture, achieved 1.475 kg·m−2·h−1 evaporation rate and 78% efficiency under 1 kW·m−2 [28]. Zhu et al. reported that a carbonized daikon chip with highly developed honeycomb cellular structure was prepared by freeze-drying and carbonization. Under 1 sun, its water evaporation rate and solar steam efficiency are 1.57 kg·m−2·h−1 and 85.9%, respectively [29]. Carbonized jute sticks, utilizing natural central holes and microchannels, exhibited 1.52 kg·m−2·h−1 evaporation rate and 87.01% efficiency for seawater desalination under 1 sun [30]. Other fully carbonized biomass materials, such as lotus seedpods [31], sunflower stalks [32], corncobs [33], and durian rinds [34], have also demonstrated SSG potential, with their high performance attributed to their intrinsic ability to absorb sunlight, facilitate water transport, and minimize heat loss. Notably, complete carbonization often causes biomass shrinkage and cracking due to dehydration [35]. Moreover, most carbonized biomass materials require integration with auxiliary components (such as foams, fabrics, papers, etc.) for thermal insulation and enhanced water transport [23]. Fang et al. fabricated a fully biomass-derived solar still with rice straw as the main component. The carbonized rice straw was vacuum-filtered to prepare a light-absorbing film. The rice straw was used as a water transmission channel, fixed by polystyrene foam (PS), and floated on the surface of the container, which achieved an evaporation rate of 1.2 kg·m−2·h−1 and a solar steam efficiency of 75.8% [36]. Tian et al. paired carbonized cattle manure with PS (insulation) and cotton cloth (water pathway), enabling the efficient treatment of high-salinity brine (≥15 wt%) [37]. Lv et al. used corn stalk biochar-coated polyurethane foam (PU) as a photothermal agent for interfacial solar water evaporation, and achieved a water evaporation rate of up to 1.38 kg·m−2·h−1 and solar-to-vapor conversion efficiency is 84% under 1 sun [38]. Mahjoub et al. used carbonized waste tea (photothermal layer) with reverse conical PU (insulation) to fabricate low-cost, self-cleaning evaporators [39].
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
2.2.1. Biomass-Derived Photothermal Material
2.2.2. Light-Absorbing Layer
2.3. Characterizations
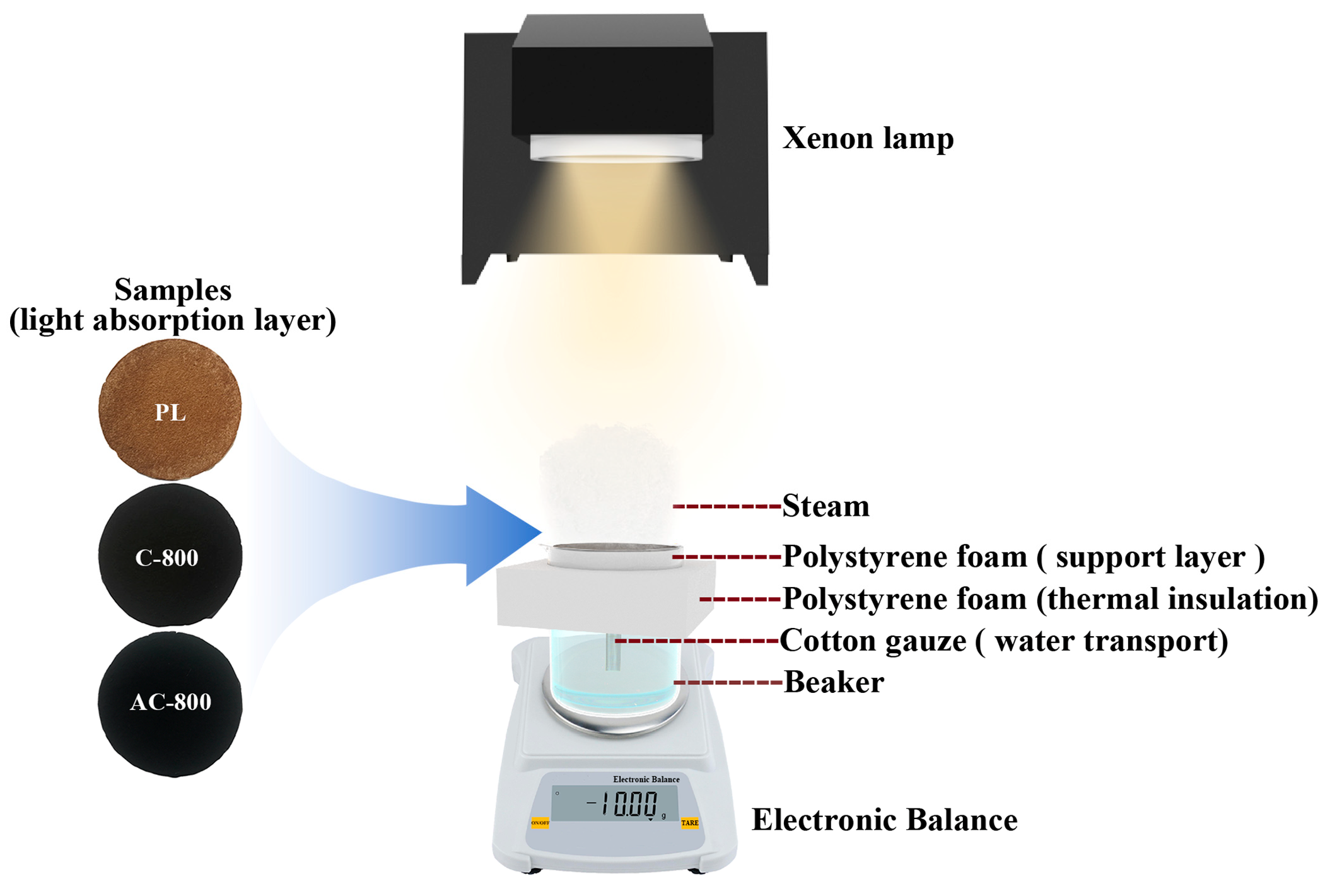
2.4. Solar-Driven Steam Generation Test
2.5. Purified Water Generation by a Solar Still System
2.6. Calculation of the Solar-to-Vapor Conversion Efficiency
2.7. Photocatalytic Experiments
3. Results
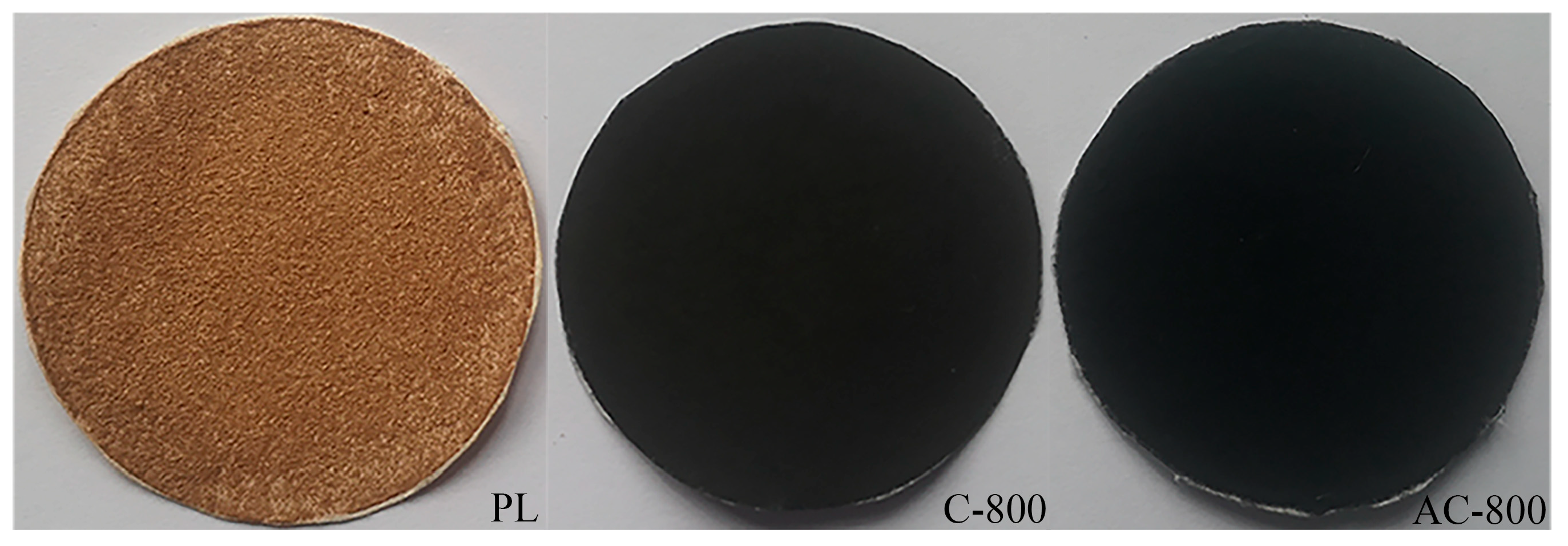
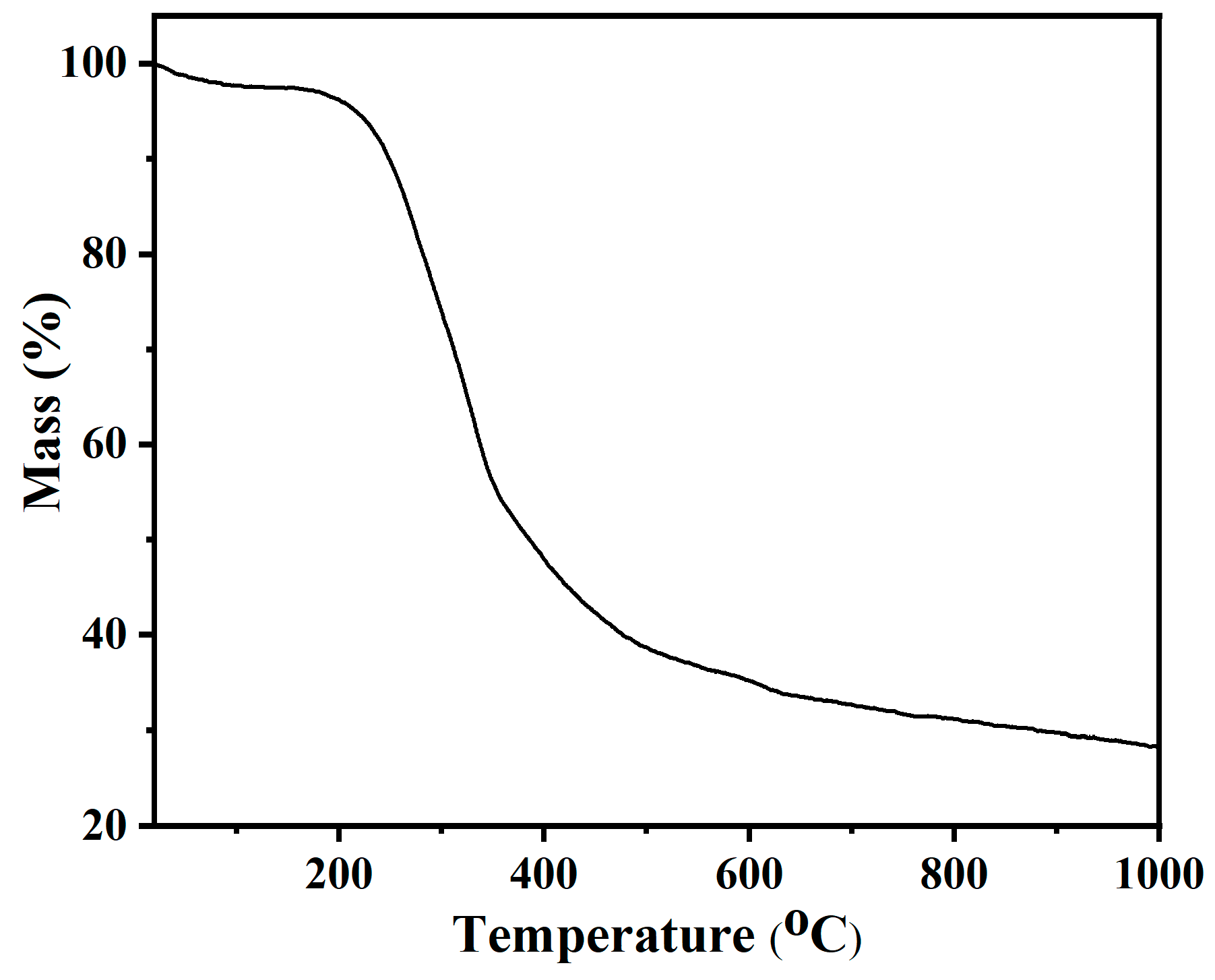
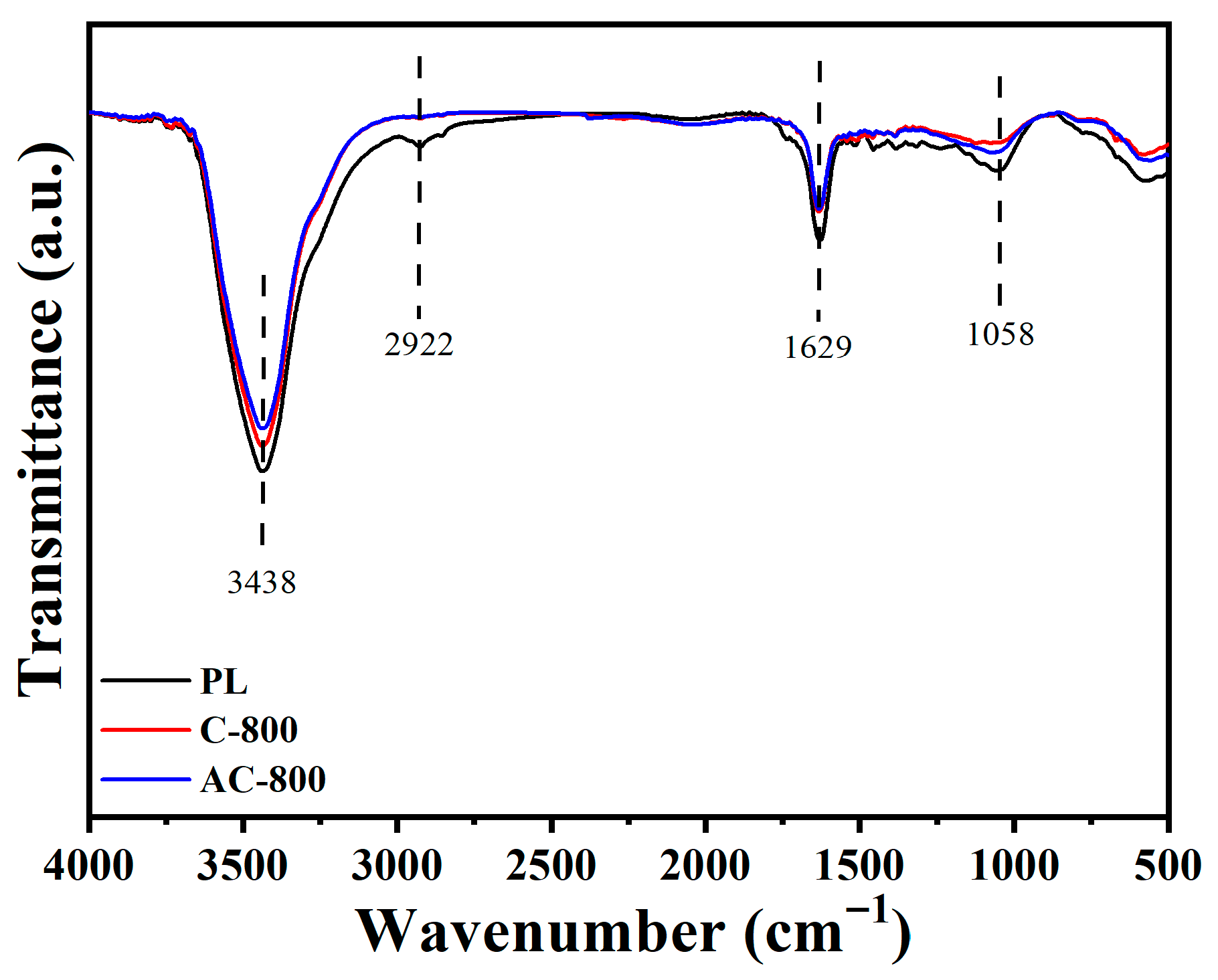
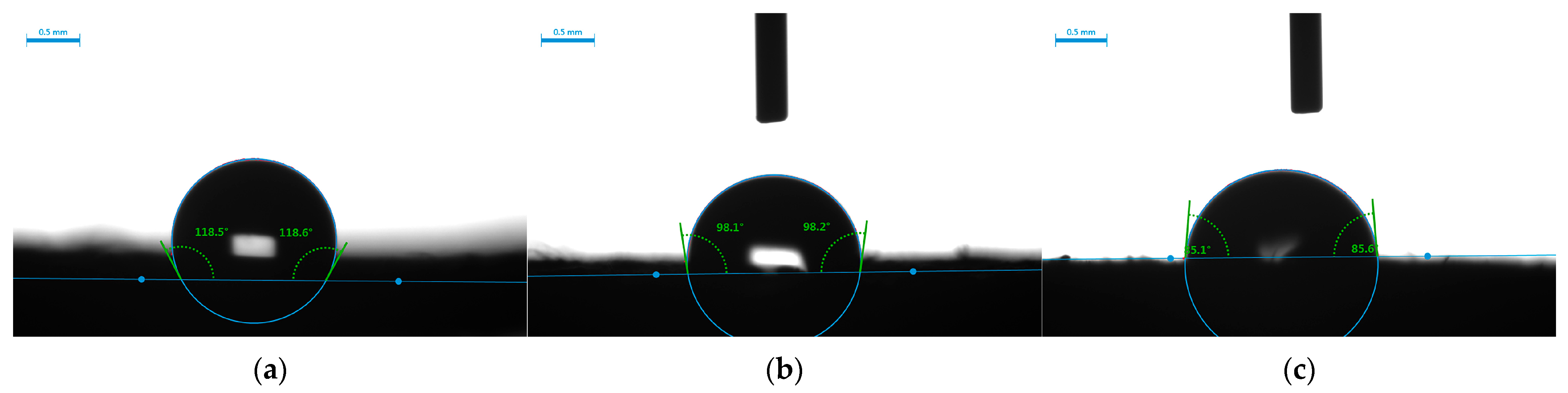
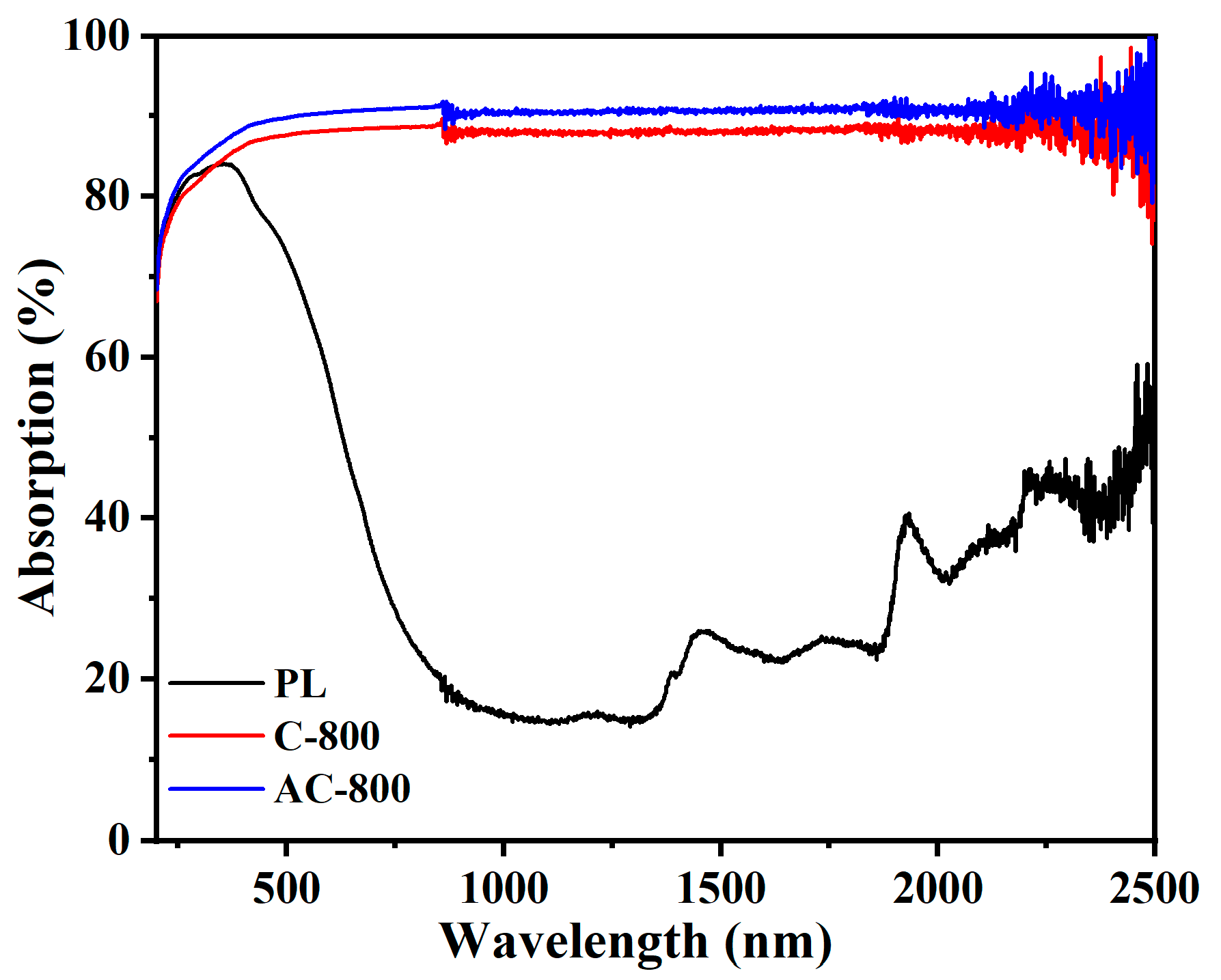
3.1. Characterization of Samples
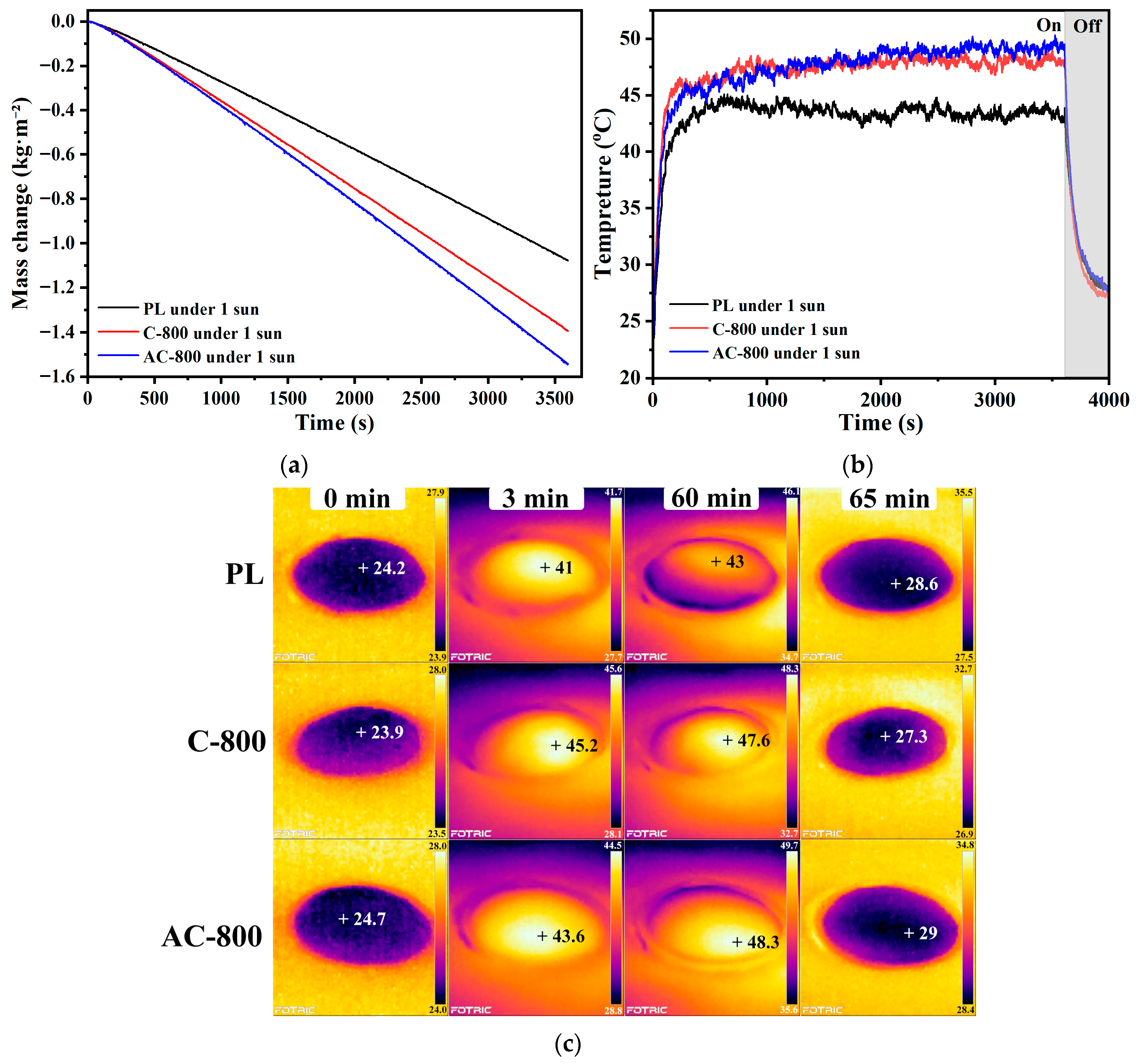
3.2. Solar-Driven Steam Generation Performance of Samples
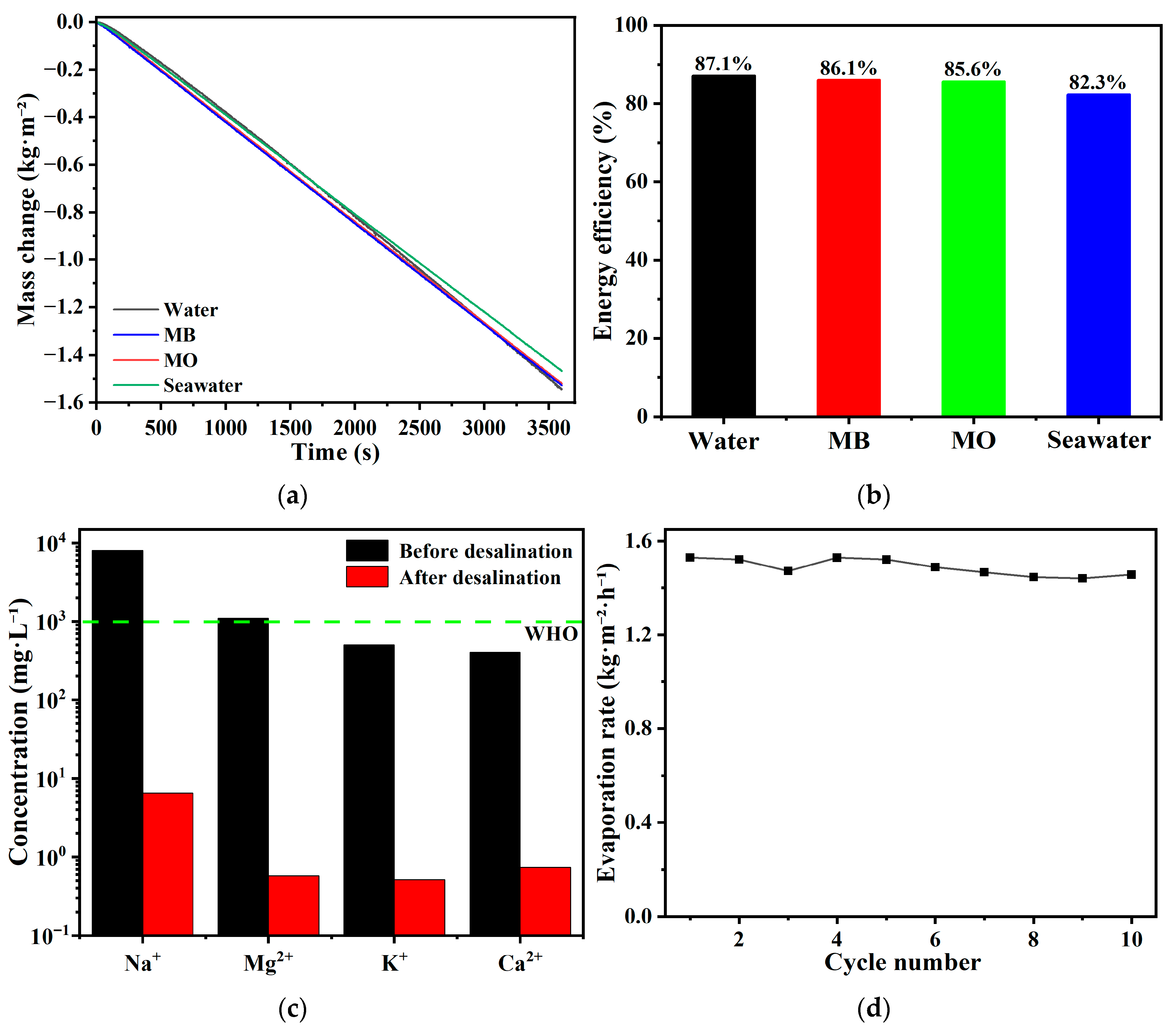
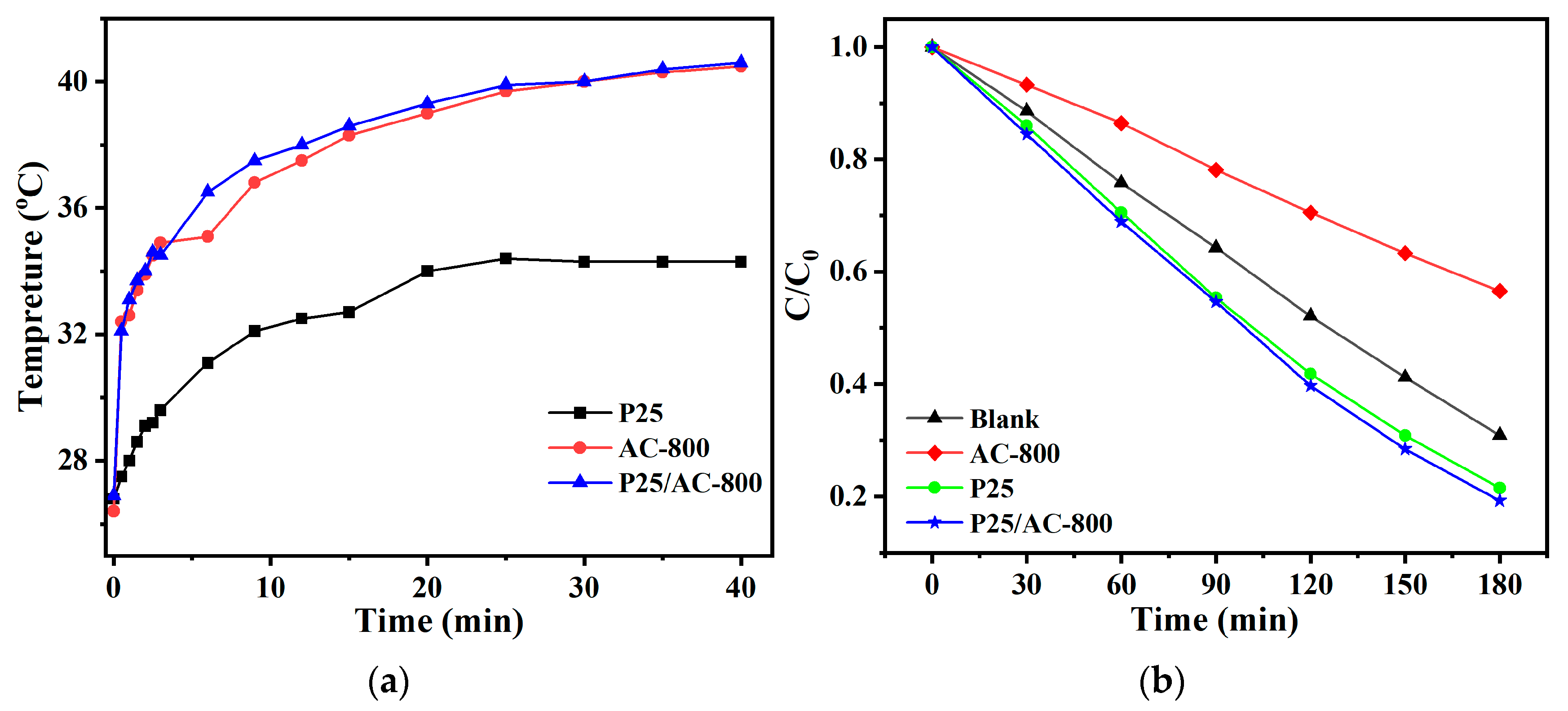
3.3. Photothermal–Photocatalytic Synergistic Performance in Wastewater Purification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Höök, M.; Tang, X. Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef]
- Aqachmar, Z.; Ben Sassi, H.; Lahrech, K.; Barhdadi, A. Solar technologies for electricity production: An updated review. Int. J. Hydrogy Energy 2021, 46, 30790–30817. [Google Scholar] [CrossRef]
- Kabir, E.; Kumar, P.; Kumar, S.; Adelodun, A.A.; Kim, K.-H. Solar energy: Potential and future prospects. Renew. Sustain. Energy Rev. 2018, 82, 894–900. [Google Scholar] [CrossRef]
- Ghalambaz, M.; Sheremet, M.; Fauzi, M.A.; Fteiti, M.; Younis, O. A scientometrics review of solar thermal energy storage (STES) during the past forty years. J. Energy Storage 2023, 66, 107266. [Google Scholar] [CrossRef]
- Karami, S.; Roghabadi, F.A.; Maleki, M.; Ahmadi, V.; Sadrameli, S.M. Materials and structures engineering of sun-light absorbers for efficient direct solar steam generation. Sol. Energy 2021, 225, 747–772. [Google Scholar] [CrossRef]
- Dao, V.-D.; Vu, N.H.; Yun, S. Recent advances and challenges for solar-driven water evaporation system toward applications. Nano Energy 2020, 68, 104324. [Google Scholar] [CrossRef]
- Ghasemi, H.; Ni, G.; Marconnet, A.M.; Loomis, J.; Yerci, S.; Miljkovic, N.; Chen, G. Solar steam generation by heat localization. Nat. Commun. 2014, 5, 4449. [Google Scholar] [CrossRef]
- Mao, K.; Zhang, Y.; Tan, S.C. Functionalizing solar-driven steam generation towards water and energy sustainability. Nat. Water. 2025, 3, 144–156. [Google Scholar] [CrossRef]
- Singh, S.C.; ElKabbash, M.; Li, Z.; Li, X.; Regmi, B.; Madsen, M.; Jalil, S.A.; Zhan, Z.; Zhang, J.; Guo, C. Solar-trackable super-wicking black metal panel for photothermal water sanitation. Nat. Sustain. 2020, 3, 938–946. [Google Scholar] [CrossRef]
- Kashyap, V.; Ghasemi, H. Solar heat localization: Concept and emerging applications. J. Mater. Chem. A 2020, 8, 7035–7065. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; Li, S.; Lee, C.-S.; Zhang, X.-H. From Materials to Devices: Rationally Designing Solar Steam System for Advanced Applications. Small Methods 2022, 6, 2200835. [Google Scholar] [CrossRef]
- Farid, M.U.; Kharraz, J.A.; Wang, P.; An, A.K. High-efficiency solar-driven water desalination using a thermally isolated plasmonic membrane. J. Clean. Prod. 2020, 271, 122684. [Google Scholar] [CrossRef]
- Yang, B.; Li, C.; Wang, Z.; Dai, Q. Thermoplasmonics in Solar Energy Conversion: Materials, Nanostructured Designs, and Applications. Adv. Mater. 2022, 34, 2107351. [Google Scholar] [CrossRef]
- Yang, F.; Bao, Z.; Liang, Z.; He, G.; Li, J.; Liang, Q.; Li, J.; Luo, S.; Liu, Y. Black Silver-Decorated liquid metal nanofillers coupled with Glycerol-Modified hydrogel composites for high efficiency solar steam generation and thermoelectric conversion. Chem. Eng. J. 2024, 490, 151815. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Zhao, J.; Dai, Z.; Liu, W.; Chen, C.; Gao, S.; Golosov, D.A.; Zavadski, S.M.; Melnikov, S.N. Shape tailored Cu2ZnSnS4 nanosheet aggregates for high efficiency solar desalination. Mater. Res. Bull. 2019, 118, 110529. [Google Scholar] [CrossRef]
- Bai, Z.; Xu, H.; Li, G.; Yang, B.; Yao, J.; Guo, K.; Wang, N. MoS2 Nanosheets Decorated with Fe3O4 Nanoparticles for Highly Efficient Solar Steam Generation and Water Treatment. Molecules 2023, 28, 1719. [Google Scholar] [CrossRef]
- Vasquez, K.J.F.; Meireles, C.d.S.; Bacelos, M.S.; Andrade, G.R.S. Highly efficient interfacial solar steam generation using carbonized loofah-Cu2O composite. J. Alloys Compd. 2025, 1030, 180883. [Google Scholar] [CrossRef]
- Toyoda, M.; Inagaki, M. Carbon materials for solar steam-generation. Carbon 2023, 214, 118373. [Google Scholar] [CrossRef]
- Hu, S.; Qin, L.; Yi, H.; Lai, C.; Yang, Y.; Li, B.; Fu, Y.; Zhang, M.; Zhou, X. Carbonaceous Materials-Based Photothermal Process in Water Treatment: From Originals to Frontier Applications. Small 2024, 20, 2305579. [Google Scholar] [CrossRef] [PubMed]
- Ndagijimana, P.; Cui, B.; Zhang, X.; Nkinahamira, F.; Rong, H.; Guo, D.; Rugabirwa, B.; Hakizimana, J.C.; Ndokoye, P.; Nizeyimana, J.C. Advances in carbon-based materials for solar-driven steam generation, desalination and water treatment. Desalination 2025, 593, 118192. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, Y.; Zhao, F.; Yu, G. Hydrogels as an Emerging Material Platform for Solar Water Purification. Acc. Chem. Res. 2019, 52, 3244–3253. [Google Scholar] [CrossRef]
- Chen, J.; Xie, Y.; Tian, M.; Tian, J.; Liang, Z.; Xie, H.; Lv, M. Cellulose-derived hydrogel with low vaporization enthalpy enables highly efficient solar steam generation. Carbohydr. Polym. 2025, 356, 123385. [Google Scholar] [CrossRef]
- Li, Z.; Wei, S.; Ge, Y.; Zhang, Z.; Li, Z. Biomass-based materials for solar-powered seawater evaporation. Sci. Total Environ. 2023, 858, 160003. [Google Scholar] [CrossRef]
- Ivan, M.N.A.S.; Saha, S.; Saleque, A.M.; Ahmed, S.; Thakur, A.K.; Bai, G.; Miao, Z.; Saidur, R.; Tsang, Y.H. Progress in interfacial solar steam generation using low-dimensional and biomass-derived materials. Nano Energy 2024, 120, 109176. [Google Scholar] [CrossRef]
- Zhu, M.; Li, Y.; Chen, G.; Jiang, F.; Yang, Z.; Luo, X.; Wang, Y.; Lacey, S.D.; Dai, J.; Wang, C.; et al. Tree-Inspired Design for High-Efficiency Water Extraction. Adv. Mater. 2017, 29, 1704107. [Google Scholar] [CrossRef]
- Zhang, F.; Qi, Z.; Wu, X.; Yang, K.; Cai, H.; Iqbal, W.; Zhang, J. Simple carbonization of sorghum straw: A Janus structure for highly efficient solar interface water evaporation. Mater. Today Chem. 2025, 45, 102618. [Google Scholar] [CrossRef]
- Jang, H.; Choi, J.; Lee, H.; Jeon, S. Corrugated Wood Fabricated Using Laser-Induced Graphitization for Salt-Resistant Solar Steam Generation. ACS Appl. Mater. Interfaces 2020, 12, 30320–30327. [Google Scholar] [CrossRef]
- Xu, N.; Hu, X.; Xu, W.; Li, X.; Zhou, L.; Zhu, S.; Zhu, J. Mushrooms as Efficient Solar Steam-Generation Devices. Adv. Mater. 2017, 29, 1606762. [Google Scholar] [CrossRef]
- Zhu, M.; Yu, J.; Ma, C.; Zhang, C.; Wu, D.; Zhu, H. Carbonized daikon for high efficient solar steam generation. Sol. Energy Mater. Sol. Cells 2019, 191, 83–90. [Google Scholar] [CrossRef]
- Ivan, M.N.A.S.; Saleque, A.M.; Ahmed, S.; Guo, Z.L.; Zu, D.; Xu, L.; Alam, T.I.; Hani, S.U.; Tsang, Y.H. Jute stick derived self-regenerating sustainable solar evaporators with different salt mitigation mechanisms for highly efficient solar desalination. J. Mater. Chem. A 2023, 11, 3961–3974. [Google Scholar] [CrossRef]
- Fang, J.; Liu, J.; Gu, J.; Liu, Q.; Zhang, W.; Su, H.; Zhang, D. Hierarchical Porous Carbonized Lotus Seedpods for Highly Efficient Solar Steam Generation. Chem. Mater. 2018, 30, 6217–6221. [Google Scholar] [CrossRef]
- Feng, Z.; OuYang, X.; Zhou, S.; Wang, J.; Lu, F.; Wang, S.; Deng, Q. Carbonized sunflower stalks with or without storage tissue for highly efficient water purification and desalination. J. Environ. Chem. Eng. 2023, 11, 110284. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Z.; Zhao, G.; Wang, L.; Jia, D.; Yang, Y.; Liu, X.; Wang, X.; Qiu, J. High performance carbonized corncob-based 3D solar vapor steam generator enhanced by environmental energy. Carbon 2021, 179, 337–347. [Google Scholar] [CrossRef]
- Zeng, L.; Deng, D.; Zhu, L.; Wang, H.; Zhang, Z.; Yao, Y. Biomass photothermal structures with carbonized durian for efficient solar-driven water evaporation. Energy 2023, 273, 127170. [Google Scholar] [CrossRef]
- Xu, Y.; Tang, C.; Ma, J.; Liu, D.; Qi, D.; You, S.; Cui, F.; Wei, Y.; Wang, W. Low-Tortuosity Water Microchannels Boosting Energy Utilization for High Water Flux Solar Distillation. Environ. Sci. Technol. 2020, 54, 5150–5158. [Google Scholar] [CrossRef]
- Fang, Q.; Li, T.; Chen, Z.; Lin, H.; Wang, P.; Liu, F. Full Biomass-Derived Solar Stills for Robust and Stable Evaporation To Collect Clean Water from Various Water-Bearing Media. ACS Appl. Mater. Interfaces 2019, 11, 10672–10679. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, X.; Wang, Z.; Caratenuto, A.; Chen, F.; Wan, Y.; Zheng, Y. Carbonized cattle manure-based photothermal evaporator with hierarchically bimodal pores for solar desalination in high-salinity brines. Desalination 2021, 520, 115345. [Google Scholar] [CrossRef]
- Lv, B.; Yang, S. Turning Corn Stalk Trashes into a Photothermal Agent for Interfacial Solar Water Evaporation for Sustainable Water Purification. Energy Technol. 2025, 13, 2401534. [Google Scholar] [CrossRef]
- Mahjoub, F.; Naghdi, B.; Roghabadi, F.A. Self-cleaning solar steam generator based on engineered carbonized waste tea photothermal layer. Clean. Mater. 2025, 17, 100327. [Google Scholar] [CrossRef]
- Padilla-Martínez, E.D.; Pérez-Buendía, S.K.; López-Sandoval, R.; Sánchez-Rodríguez, C.E. Electrochemical energy storage from spent coffee grounds-derived carbon by KOH activation. J. Energy Storage 2023, 71, 108115. [Google Scholar] [CrossRef]
- Mani, D.; Elango, D.; Priyadharsan, A.; Al-Humaid, L.A.; Al-Dahmash, N.D.; Ragupathy, S.; Jayanthi, P.; Ahn, Y.-H. Groundnut shell chemically treated with KOH to prepare inexpensive activated carbon: Methylene blue adsorption and equilibrium isotherm studies. Environ. Res. 2023, 231, 116026. [Google Scholar] [CrossRef]
- Sayed, D.M.; El-Deab, M.S.; Elshakre, M.E.; Allam, N.K. Nanocrystalline Cellulose Confined in Amorphous Carbon Fibers as Capacitor Material for Efficient Energy Storage. J. Phys. Chem. C 2020, 124, 7007–7015. [Google Scholar] [CrossRef]
- Ge, Y.; Su, Z.; Ivan, M.N.A.S.; Wang, C.; Tsang, Y.H.; Xu, S.; Bai, G. Bio-Derived Photothermal Materials and Evaporators for Sustainable Solar Energy-Driven Water Process. Langmuir 2022, 38, 13187–13194. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz Hedayati, M.; Elbahri, M. Antireflective Coatings: Conventional Stacking Layers and Ultrathin Plasmonic Metasurfaces, A Mini-Review. Materials 2016, 9, 497. [Google Scholar] [CrossRef] [PubMed]
- Wang, P. Emerging investigator series: The rise of nano-enabled photothermal materials for water evaporation and clean water production by sunlight. Environ. Sci. Nano 2018, 5, 1078–1089. [Google Scholar] [CrossRef]
- Pornrungroj, C.; Mohamad Annuar, A.B.; Wang, Q.; Rahaman, M.; Bhattacharjee, S.; Andrei, V.; Reisner, E. Hybrid photothermal–photocatalyst sheets for solar-driven overall water splitting coupled to water purification. Nat. Water 2023, 1, 952–960. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, X.; Fan, D.; Xu, X.; Lu, Y. Wood–hydrogel composites coated with C3N4 photocatalyst for synchronous solar steam generation and photocatalytic degradation. J. Mater. Sci. 2023, 58, 13154–13164. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, T.; Ren, S.; Feng, Y.; Zhang, Y.; Qian, S.; Tang, W.; Yin, X.; Wu, T.; Gao, S. Construction of evaporator based on the principle of three primary colors to achieve synchronous photothermal conversion water evaporation and photocatalytic degradation. Desalination 2025, 606, 118758. [Google Scholar] [CrossRef]
- Tang, Q.; Zhang, F.; Li, M.; Wang, Z.; Yang, Z.; Li, H. Preparations of polyvinyl alcohol/polyacrylamide/activated carbon@bismuth oxybromide (PVA/PAM/AC@BiOBr) bifunctional hydrogel and its photothermal evaporation and photocatalytic properties. Colloids Surf. A 2025, 725, 137532. [Google Scholar] [CrossRef]
- Joo, J.B.; Liu, H.; Lee, Y.J.; Dahl, M.; Yu, H.; Zaera, F.; Yin, Y. Tailored synthesis of C@TiO2 yolk–shell nanostructures for highly efficient photocatalysis. Catal. Today 2016, 264, 261–269. [Google Scholar] [CrossRef]
- Li, Y.; Shen, Q.; Guan, R.; Xue, J.; Liu, X.; Jia, H.; Xu, B.; Wu, Y. A C@TiO2 yolk–shell heterostructure for synchronous photothermal–photocatalytic degradation of organic pollutants. J. Mater. Chem. C 2020, 8, 1025–1040. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, X.; Zhang, G. TiO2-based catalysts for photothermal catalysis: Mechanisms, materials and applications. J. Cleaner Prod. 2022, 381, 135156. [Google Scholar] [CrossRef]
- Khalid, R.; Amin, H.M.A.; Shahid, M.; Kazuya, N.; Xing, R.; Liu, S.; Fujishima, A. Integrated photothermal and photocatalytic degradation of micro-/nanoplastics: A mini-review with mechanistic insights and future perspectives. J. Mater. Chem. A 2025, 13, 26110–26128. [Google Scholar] [CrossRef]
- Zhang, Y.; Ravi, S.K.; Tan, S.C. Food-derived carbonaceous materials for solar desalination and thermo-electric power generation. Nano Energy 2019, 65, 104006. [Google Scholar] [CrossRef]
- Xiao, C.; Wang, S.; Guo, Y.; Zhang, Y.; Hasi, Q.-M.; Tian, Q.; Chen, L. Coffee Grounds-Doped Alginate Porous Materials for Efficient Solar Steam Generation. Langmuir 2022, 38, 1888–1896. [Google Scholar] [CrossRef]
- He, P.; Hao, L.; Liu, N.; Bai, H.; Niu, R.; Gong, J. Controllable synthesis of sea urchin-like carbon from metal-organic frameworks for advanced solar vapor generators. Chem. Eng. J. 2021, 423, 130268. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, X.; Qiu, Z.; Zhang, R.; Du, Q.; Zhao, Z.; Qiu, J. All-in-one photothermal/catalytic flexible membrane for highly efficient desalination and organic pollutant degradation. Nanoscale 2025, 17, 4721–4731. [Google Scholar] [CrossRef]




| Samples | SBET m2·g−1 | Vtotal cm3·g−1 | Vmeso cm3·g−1 | Vmicro cm3·g−1 |
|---|---|---|---|---|
| C-800 | 30.44 | 0.0056 | 0.0050 | 0.0014 |
| AC-800 | 1335.58 | 0.4005 | 0.2860 | 0.3384 |
| Entry | Photothermal Conversion Material | Surface Temperature (°C) | Evaporation Rate (kg·m−2·h−1) | Efficiency (%) | Reference |
|---|---|---|---|---|---|
| 1 | Carbonized lotus seedpods | 44.8 | 1.3 | 86.5 | [31] |
| 2 | Carbonized upper leaves of rice straw | 37.1 | 1.2 | 75.8 | [37] |
| 3 | Corn stalk biochar | 47.8 | 1.38 | 84 | [39] |
| 4 | Carbonized pasta | 38.1 | 1.3354 | 84.1 | [54] |
| 5 | Carbonized coffee grounds | 42.6 | 1.486 | 86.96 | [55] |
| 6 | AC-800 | 48.3 | 1.5441 | 87.1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.-B.; Guo, M.-X.; Fan, H.-L.; Li, F.-H.; Han, G.-P.; Guo, Q.-Q. Sustainable Biomass-Derived Photothermal Material for Solar-Driven Seawater Desalination and Wastewater Treatment. Sustainability 2025, 17, 8513. https://doi.org/10.3390/su17188513
Wu J-B, Guo M-X, Fan H-L, Li F-H, Han G-P, Guo Q-Q. Sustainable Biomass-Derived Photothermal Material for Solar-Driven Seawater Desalination and Wastewater Treatment. Sustainability. 2025; 17(18):8513. https://doi.org/10.3390/su17188513
Chicago/Turabian StyleWu, Jing-Bin, Ming-Xi Guo, Hong-Li Fan, Feng-Hai Li, Guo-Peng Han, and Qian-Qian Guo. 2025. "Sustainable Biomass-Derived Photothermal Material for Solar-Driven Seawater Desalination and Wastewater Treatment" Sustainability 17, no. 18: 8513. https://doi.org/10.3390/su17188513
APA StyleWu, J.-B., Guo, M.-X., Fan, H.-L., Li, F.-H., Han, G.-P., & Guo, Q.-Q. (2025). Sustainable Biomass-Derived Photothermal Material for Solar-Driven Seawater Desalination and Wastewater Treatment. Sustainability, 17(18), 8513. https://doi.org/10.3390/su17188513








