Abstract
Engine oil condition critically affects vehicle performance, fuel efficiency, and engine durability. While conventional oil change strategies are based on fixed intervals or mileage thresholds, they often neglect real operating conditions and the actual state of lubricant degradation. This study investigates nine used engine oil samples collected from passenger vehicles operating in diverse environments, including city traffic, highway routes, hybrid systems, and diesel engines. The oils were assessed using kinematic viscosity measurements and Fourier transform infrared (FTIR) spectroscopy to monitor key degradation indicators—oxidation, nitration, sulfonation, fuel dilution, soot contamination, and additive depletion. Each case is fully documented with detailed operational histories, facilitating a nuanced, real-world understanding of oil aging. The results demonstrate that degradation levels vary considerably, even under similar mileage ranges, highlighting the influence of urban usage patterns and engine design. In several cases, premature or delayed oil changes were observed, confirming that standard service intervals may be suboptimal. FTIR proved effective in detecting subtle chemical transformations, particularly in samples affected by biofuel components or prolonged thermal stress. These findings emphasize the value of integrating laboratory diagnostics into oil change decision-making and support more tailored maintenance strategies. Such an approach can reduce unnecessary oil replacement, limit waste generation, and extend engine lifespan, contributing to both environmental and economic sustainability. This study supports the implementation of condition-based oil change strategies to minimize lubricant waste and promote maintenance practices aligned with sustainability principles.
1. Introduction
Monitoring the condition of engine oil during operation is crucial for maintaining engine performance and longevity. Regular analysis of engine oil helps to detect changes in its physical and chemical properties, which can indicate contamination, degradation, or wear of engine components [1,2]. Increased levels of trace metals in the oil can reveal excessive wear, while changes in viscosity and the presence of contaminants can signal oxidation or dilution issues [3]. Effective oil monitoring can prevent costly repairs by identifying potential problems early, reducing engine downtime, and optimizing oil change intervals [4,5]. Additionally, advanced methods such as the use of UV-visible spectrophotometry can distinguish oxidized from non-oxidized oil, providing a deeper understanding of oil degradation processes [6]. The integration of artificial intelligence techniques further enhances the predictive maintenance capabilities, allowing for more accurate forecasting of oil viscosity and overall engine health [7,8]. Therefore, maintaining a stringent engine oil monitoring regime is essential not only for engine health but also for cost management and environmental sustainability [9].
The significance of engine oil in vehicles cannot be overstated. It is a fundamental component that plays a crucial role in the operation and longevity of the engine. Engine oil is tasked with lubricating engine parts, reducing friction, and dissipating heat, which are essential for ensuring optimal engine performance [4,10]. Engine oil acts as a protective barrier between moving parts, preventing metal-to-metal contact that could lead to wear and damage. Additionally, it helps maintain proper engine temperature by absorbing excess heat generated during combustion [11,12]. Moreover, engine oil functions as a cleaning agent, capturing and suspending contaminants and particulates, preventing them from clogging vital engine components. Neglecting the importance of regular oil changes can lead to numerous problems, including increased engine wear, reduced fuel efficiency, and even engine failure. Therefore, understanding the significance of engine oil and knowing how frequently to change it is crucial for vehicle maintenance and ensuring its long-lasting and trouble-free operation [13].
Determining the optimal moment for an oil change in a vehicle presents a challenge that is not always straightforward. Numerous factors influence oil degradation, and their impact can vary depending on driving style, operating conditions, and the type of oil used. As a result, establishing a universal criterion suitable for every vehicle is difficult. Traditional methods, such as periodic oil changes based on a set time interval or a specific number of kilometers driven, are commonly used, but do not always account for the engine’s actual needs [14]. Many modern vehicles are equipped with systems that monitor oil condition and suggest the timing for an oil change based on real operating conditions [15]. However, such systems are not always available in all vehicles, leaving drivers without proper guidance. Even when available, their functionality is often questionable, because they are based on factors like the number of engine starts or kilometers driven rather than the physicochemical properties of the engine oil.
In recent years, laboratory analyses aimed at understanding the kinetics of changes in engine oil properties during actual operation have gained popularity. These tools allow for a more precise determination of the oil change interval by considering the engine’s real working conditions. Viscosity control and Fourier transform infrared (FTIR) spectroscopy analysis are some of key tools in assessing the condition of engine oil, especially used oil [16,17]. As oil degrades, its viscosity can either increase or decrease, leading to reduced lubrication efficiency and engine performance. FTIR is an advanced chemical analysis technique that enables the identification and quantitative assessment of chemical structures present in the oil. It is an extremely useful tool for detecting chemical changes that may occur in the oil due to degradation, exposure to high temperatures, or the presence of contaminants [8,18]. FTIR allows for the monitoring of oxidation levels, water presence, additive degradation, and various contaminants that can affect oil quality and engine performance.
To strengthen the diagnostic framework of this research, FTIR spectroscopy and viscometry were selected as complementary methods, based on their proven diagnostic sensitivity. FTIR enables the identification of specific functional groups associated with lubricant degradation, such as carbonyls, nitrates, and sulfonyls, which cannot be reliably detected by onboard monitoring systems [15,19,20]. Viscosity, in turn, is a direct physical measure of the lubricant’s ability to maintain hydrodynamic film thickness and protect engine components, and its deviation from reference values has been consistently linked to wear risk and loss of efficiency [21,22]. Previous studies confirm that combining these two methods enhances the reliability of oil condition assessment and provides a robust foundation for condition-based maintenance strategies [23,24]. Viscosity control and FTIR analysis allow for real-time monitoring of oil condition and provide valuable information about its ability to effectively lubricate and protect the engine [25]. These tools enable more informed decisions regarding oil changes, contributing to extended engine longevity and minimizing potential risks associated with continuing to use degraded engine oil [26]. This study presents a detailed analysis of nine used oil samples. Importantly, this is part of a larger scientific endeavor conducted by Artur Wolak accessible to drivers across Poland. The history of each case is thoroughly documented and published on a dedicated YouTube channel. This approach allows other drivers to comment on, clarify, or inquire about any aspect of the study. Links to the case histories analyzed in this article are provided in the bibliography. The primary objective of this research is to address two key questions: “Are drivers making justified decisions regarding oil changes?” and “Do changes in engine oil properties indicate the need to shorten or extend the oil change interval?”
Although FTIR spectroscopy and viscosity measurements are well recognized in the literature as effective techniques for monitoring lubricant degradation, there remains a gap in studies that combine these methods with detailed, real-world documentation of vehicle operation. In particular, few investigations provide a systematic comparison between laboratory diagnostics and actual maintenance decisions made by drivers. To address this gap, the present study evaluates nine cases of used engine oils, linking FTIR and viscosity data with operational histories to determine whether oil replacement decisions were justified. The paper is structured as follows: Section 2 describes the materials and methods, Section 3 presents the results of viscosity and FTIR analyses, Section 4 discusses the findings with statistical evaluation of influencing factors, and Section 5 and Section 6 provide a summary and conclusions with implications for industry practice and future research.
2. Materials and Methods
For the analysis, 18 engine oil samples were utilized. Half of these samples were fresh oils from various manufacturers available on the market, while the other half consisted of their corresponding used oils after different operational mileages. Detailed information about the new oils is presented in Table 1. Notably, the history of each oil sample is documented in short films (~10 min) and published on the YouTube channel R2D (Research2Drivers) under the numbers indicated in Table 1 (from #65 to #74, excluding #66, which contained the engine oil authenticity test).

Table 1.
Specifications of the engine oils selected for the study (according to producer materials).
The engine oils were used in vehicles from various manufacturers, featuring different types of power units and varying levels of wear and tear. Six samples came from gasoline engines (G), including one with a liquid petroleum gas (LPG) system and one hybrid (H). The remaining three samples were from diesel engines (D). The specifications of the vehicles from which the samples were collected are presented in Table 2.

Table 2.
Specification of power units used with particular oils.
Table 3 contains information on the operational characteristics of each vehicle. Three vehicles were operated under typical urban conditions, involving short-distance driving and frequent engine starts. Such conditions are considered demanding because they promote the accumulation of contaminants, consequently accelerating the degradation of engine oil. The oil mileage varied between 5000 km and 11,000 km. Additionally, it is noteworthy that in two cases, fresh oil top-ups were necessary, and in two other cases, the entire oil system was flushed before using the respective oil. The duration of use, expressed in months, also varied significantly, ranging from 3 to 16 months. Detailed case histories of all investigated oils and vehicles are available in the Supplementary Materials (video documentation, YT channel).

Table 3.
Operation characteristics of engine oils used in the tests.
3. Methods
The analysis of the direction and intensity of the changes in the kinematic viscosity parameters at 40 °C and 100 °C of the tested samples was performed using an SVM Sta-binger model 3001 viscometer (Anton Paar GmbH, Graz, Austria) according to the ASTM D7042-21A standard.
The FTIR method was used to determine the degree of oxidation, nitration, sulfonation levels, and the soot content using a Thermo Nicolett IS5 apparatus (Thermo Fisher Scientific, Waltham, MA, USA) based on the ASTM E2412-23A standard. A number of parameters of the used engine oils were examined by analysis of differential FTIR spectra. Differences in the intensity of individual bands for used oils were determined in relation to the spectrum of fresh oil. A ZnSe cuvette with a 0.1 mm light-path was used.
The primary spectral region where bands indicative of oxidation processes was observed is within the range of 1800–1670 cm−1. These bands are associated with the vibrations of carbonyl groups (C=O) in ketones and aldehydes, as well as carboxyl groups typical of organic acids and their derivatives, representing the oxidation level. In this region, negative bands related to the degradation of esters present in some oil bases or viscosity improvers can overlap with positive bands originating from fatty acid methyl esters (FAME) due to diesel fuel dilution. Negative bands related to the depletion of ester type additives, often around 1745 cm−1, are also noted in this region. Another analyzed region was around 1631 cm−1, characteristic of the vibrations of nitrate groups (-O-NO2) formed from the interaction of nitrogen oxides with partially oxidized hydrocarbons, such as radicals and alcohols, indicating the nitration level. The third analyzed region was 1300–1000 cm−1, where bands characteristic of C-O bonds appear in variously oxidized hydrocarbon structures, including alcohols, peroxides, ethers, acids, and their derivatives. Since bands typical of sulfonyl groups (-SO2-) also appear in this range, specifically of 1180–1120 cm−1, we refer to them as the sulfation level. The next region, 4000–3100 cm−1, is where the appearance of associated hydrogen bonds in compounds containing C-OH groups and water, both from oxidation and environmental contamination, can be observed. Negative bands related to the depletion of antioxidants, often around 3650 cm−1, are also noted in this region.
In the range of 1000–900 cm−1, negative bands related to the transformation of EP additives, such as zinc dithiophosphates, were observed. The range of 890–700 cm−1 showed bands associated with fuel dilution. Specifically, the bands in the 890–740 cm−1 range are positive, linked to aromatic structures introduced into the oil with the fuel, while the area around 720 cm−1, associated with aliphatic structures, is difficult to interpret due to overlapping effects of fuel dilution and partial evaporation of the oil base. Due to the high intensity of typical aliphatic C-H bands in the ranges (3000–2850 cm−1, 1510–1320 cm−1), their analysis is considered non-essential. In the range of 3100–3000 cm−1, overlapping bands of aromatic Ar-H (3100–3030 cm−1) related to fuel dilution and unsaturated C=C~H (3030–3010 cm−1) were observed. For assessing oil degradation parameters such as oxidation level and sulfation level, and evaluating the depletion of EP additives, a single-point baseline at 2000–1900 cm−1 was used. For the nitration level, a two-point linear baseline was defined by the adjacent minima of the ~1630 cm−1 band. For phenolic antioxidant depletion, a baseline was determined by the adjacent minima of the 3650 cm−1 band. Fuel dilution was assessed qualitatively. The presence of soot in the oil is concerning, as indicated by a uniform elevation of the differential spectrum baseline proportional to the wavenumber. The evaluation utilized the corrected absorbance value at the band extremum (maximum or minimum) converted to an optical path length of 0.1 mm.
The Mann–Whitney test was used for comparisons of quantitative variables between two groups. Spearman’s correlation coefficient was used to assess correlation between two quantitative variables. Nonparametric tests were used since low sample size results with inability to reasonably test the normality of distributions. Significance level was set to 0.05. All the analyses were conducted in R software, version 4.5.1 [27].
4. Results and Discussion
Table 4 presents the measured viscosity values for both fresh and used oils. As indicated in Table 1, the viscosity classes of the tested oils varied, resulting in significant differences in the viscosities of the fresh oils, as observed in Table 4. Oil sample #71 has the lowest viscosity, while oil sample #69 has the highest viscosity. Notably, the viscosity of the latter is nearly three times greater than that of oil sample #71.

Table 4.
The values of viscosity parameters for both new and used oils.
Table 5 shows the percentage changes in the viscosity of used oil samples. In this study, a change below 10% is considered a warning signal (warning range), while a change below 15% is deemed critical, indicating that the engine oil replacement interval has been exceeded.

Table 5.
The percentage change in viscosity parameters resulting from actual operation.
Analyzing the percentage changes aggregated in Table 5, several observations can be highlighted. Three oils (#70, #72, and #73) exhibited acceptable changes in viscosity, indicating no concerning signals. The engine oil in these cars performed its function properly. In two cases (#67 and #68), warning signals were observed. The viscosity changes measured at 40 °C were very close to the critical threshold of −15%. For these cars, the decision to change the engine oil was made at an appropriate time. In four cases (#65, #69, #71, and #74), the percentage change was within the critical range. Notably, in one instance, this change was −35%, which should be considered a highly alarming condition.
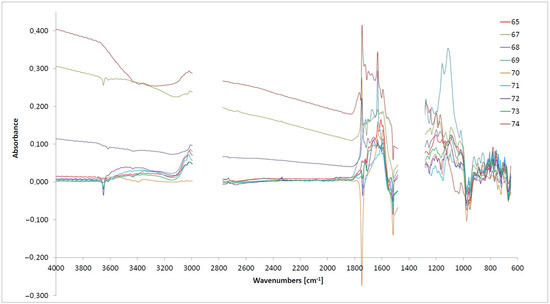
Figure 1.
The differential spectra for the examined oils cover the range of 600–4000 cm−1.
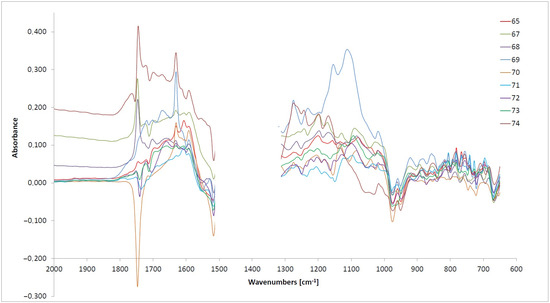
Figure 2.
The differential spectra for the examined oils cover the range of 2000–600 cm−1.
For oil sample #65, a minor level of engine oil degradation was observed. The nitration level is low 0.02 abs/0.1 mm (Table 6). The oxidation and sulfonation levels were near 0.1 abs/0.1 mm. Additionally, the spectrum did not indicate depletion of EP additives (Table 7). Differential spectrum analysis indicates the presence of fuel (gasoline) in the used engine oil, as evidenced by weak, positive bands in the range of 840–720 cm−1 and broadening around 3050 cm−1. The depletion of antioxidants does not seem significant; however, to assess antioxidants accurately, spectra should be recorded in a 1 mm cuvette within the 4000–3000 cm−1 range.

Table 6.
FTIR differential spectra analysis (oxidation, nitration, sulfonation level).

Table 7.
FTIR differential spectra analysis (EP degradation, antioxidant degradation).
For oil sample #67, a minor level of engine oil degradation was noted. The sample presents difficulties in assessing the degree of oxidation. Differential spectrum analysis reveals fuel (diesel) contamination in the used engine oil, indicated by positive bands around 1747 cm−1 (FAME) and depletion of additives containing carboxyl groups (negative band 1709 cm−1). These bands interfere with the oxidation assessment. Therefore, the band around 1695 cm−1 was used for evaluation. Degrees of oxidation and sulfonation are approximately 0.1 abs/0.1 mm. In the sulfonation assessment area, the typical sulfonic group band around 1150 cm−1 was not observed. Instead, bands typical for C-O bond vibrations around 1172 cm−1 and 1129 cm−1, originating from oxidized hydrocarbon structures or FAME contamination, were present. The degree of nitration was low, approximately 0.02 abs/0.1 mm. The initial stage of EP additive degradation was evident. At first glance, the depletion of antioxidants does not seem significant; however, to assess antioxidants accurately, spectra should be recorded in a 1 mm cuvette within the 4000–3000 cm−1 range. The presence of soot in the oil is concerning, as indicated by a uniform elevation of the differential spectrum baseline proportional to the wavenumber. It is unclear whether the soot results from the degradation of the current oil batch, as the oil does not appear to be severely degraded, or from contamination by remnants of previously used oils left in the system. Another probable source of soot is the degradation of FAME that entered the oil system, although the observed ester band does not indicate ongoing FAME oligomerization processes, which are the initial stage of deposit and soot formation by FAME.
For oil sample #68, a minor level of engine oil degradation was observed. The nitration level was low, 0.01 abs/0.1 mm. The degrees of oxidation, and sulfonation were below 0.1 abs/0.1 mm. The differential spectrum shows a band at 1747 cm−1 and bands in the range of 1250–1090 cm−1, indicating FAME contamination of the engine oil. The lack of distinct hydrocarbon bands from diesel dilution is difficult to explain. The initial stage of EP additive degradation was evident. At first glance, the depletion of antioxidants does not seem significant; however, to assess antioxidants accurately, spectra should be recorded in a 1 mm cuvette within the 4000–3000 cm−1 range. The spectrum also suggests soot contamination, consistent with the observations already described for sample #67.
For oil sample #69, a moderate level of engine oil degradation was observed. The oxidation, nitration, and sulfonation levels were above 0.1 abs/0.1 mm. These indicated considerable degradation of the oil. The assessment of oxidation bands was hindered by the negative bands, which indicates depletion of a carboxyl-containing components present in the oil. The degree of sulfonation could not be determined, as strong bands at 1154 and 1112 cm−1, typical for coolant contamination, were present. Additionally, the initial stage of EP additive degradation was evident. No changes were observed in the range typical for antioxidant bands.
For oil sample #70, a minor level of engine oil degradation was observed. The nitration level was about 0.05 abs/0.1 mm. The degrees of oxidation, and sulfonation were below 0.1 abs/0.1 mm. The assessment of oxidation degree was hindered by the depletion of an ester component present in the oil. Additionally, the initial stage of EP additive degradation was evident. No changes were observed in the range typical for antioxidant bands.
For oil sample #71, a minor level of engine oil degradation was observed. The nitration level was low 0.01 abs/0.1 mm. The degrees of oxidation, and sulfonation were below 0.05 abs/0.1 mm. Differential spectrum analysis indicates the presence of fuel (gasoline) in the used engine oil, as evidenced by weak, positive bands in the range of 840–720 cm−1. Additionally, the initial stage of EP additive degradation was evident. At first glance, the antioxidant was almost entirely depleted; however, to assess antioxidants accurately, spectra of the reference and tested oil should be recorded in a 1 mm cuvette within the 4000–3000 cm−1 range.
For oil sample #72, a minor level of engine oil degradation was observed. The nitration level was low 0.01 abs/0.1 mm. The degrees of oxidation, and sulfonation were near 0.05 abs/0.1 mm. Additionally, the initial stage of EP additive degradation was evident. At first glance, the antioxidant was almost entirely depleted; however, to assess antioxidants accurately, spectra of the reference and tested oil should be recorded in a 1 mm cuvette within the 4000–3000 cm−1 range.
For oil sample #73, a minor level of engine oil degradation was observed. The nitration level was low 0.01 abs/0.1 mm. The degrees of oxidation, and sulfonation were below 0.1 abs/0.1 mm. Additionally, the initial stage of EP additive degradation was evident. At first glance, the antioxidant was almost entirely depleted; however, to assess antioxidants accurately, spectra of the reference and tested oil should be recorded in a 1 mm cuvette within the 4000–3000 cm−1 range.
For oil sample #74, spectra of fresh Helix Ultra oil mixed with Archoil AR9100 were analyzed. Archoil AR9100 contains a significant amount of ester additives. The assessment used the corrected absorbance value at the band extremum (maximum or minimum) recalculated for an optical path length of 0.1 mm. A significant level of engine oil degradation was observed. The degrees of oxidation were above 0.1 abs/0.1 mm, nitration around 0.1 abs/0.1 mm, and sulfonation unexpectedly low at about 0.01 abs/0.1 mm. Additionally, the initial stage of EP additive degradation was evident. In the 4000–3000 cm−1 range, a broad negative band indicating dehydration reactions was observed. Similar to the observations for samples #67 i #68, the origin of this soot cannot be determined unambiguously: it may reflect degradation of the current oil batch, residues of previous oils left in the system, or partial decomposition of FAME, which is known to promote deposit and soot formation. The presence of soot in the oil suggests significant overheating during operation. It is likely that FAME from the significant amount of diesel fuel which entered to the oil, as indicated by the band around 1745 cm−1 in the differential spectrum. This may also contribute to increased oil degradation.
A statistical evaluation using nonparametric methods (Spearman’s correlation coefficient and Mann–Whitney test) revealed only a few significant relationships. Diesel engines exhibited a significantly greater increase in viscosity index compared to gasoline engines (p = 0.024, Mann–Whitney). The strongest correlation was observed between the time since the last oil change and the oxidation level (r = 0.705, p = 0.034), confirming that prolonged intervals promote oxidative degradation. No statistically significant dependencies were found for vehicle mileage, presence of a diesel particulate filter, dominant trip length, kilometers driven on the oil, or vehicle age when analyzed against percentage changes and degradation parameters. These negative findings are also informative, indicating that many operational characteristics commonly assumed to influence oil degradation did not show measurable effects in this limited dataset. Given the small sample size, the results should be regarded as preliminary, highlighting the need for larger studies to validate these trends.
Table 8 aggregates the exceedances of the limit values for the parameters of the analyzed oils. The limits and thresholds for each range, particularly for kinematic viscosity, have been provided previously. For EP degradation, the warning level is set at 0.05–0.1 abs/0.1 mm, and the alarm level is above 0.1 abs/0.1 mm. For phenolic antioxidant degradation, the warning level is 0.01–0.05 abs/0.1 mm, and the alarm level is above 0.05 abs/0.1 mm. For sulfonation, nitration, oxidation, and FAME contamination or ester additives degradation, the following levels have been set: warning at 0.1–0.2 abs/0.1 mm, and critical above 0.2 abs/0.1 mm.

Table 8.
Exceedances of the limit values for the analyzed engine oils.
When evaluating the appropriateness of the decision to change the engine oil, the following assumptions were made: If a sample showed no changes within the critical range, the decision to change the oil was made too early. If 1 to 2 critical changes were recorded, the decision to change the oil was justified and made at the right time. In any other case, the decision to change the oil was made too late.
Oil sample #65 exhibited a significant change in viscosity, but spectral analysis did not confirm severe engine oil degradation. In this case, it can be considered that the engine oil change was conducted at the appropriate time. It is worth noting that the mileage was not high—6300 km—but the usage was predominantly urban, providing a strong rationale not to wait until 10,000 km (as many drivers consider optimal). Oil sample #67 showed a warning-level change in one viscosity parameter (measured at 40 °C). However, infrared analysis indicated two critical changes (additive degradation and FAME dilution) plus a high soot content—nearly three times higher than in sample #68. In this situation, the decision to change the oil was fully justified. The mileage of 11,000 km, predominantly on non-urban routes with segments exceeding 20 km, is safe in this specific case. Oil sample #68 showed similar viscosity parameter changes to sample #67, but its infrared parameters were noticeably better. The difference may be due to a shorter mileage—specifically, 1100 km less. Operating conditions were very similar. Despite one critical change, it can be suggested that the oil change decision was slightly premature, by about 2000 km.
The remaining six oils can be divided into two groups. The first group includes oils with acceptable viscosity changes (samples #70, #72, #73). Their mileage was similar, ranging from 9000–10,000 km. However, oil sample #72 was used in a vehicle that operated 80% in urban conditions. The other two oils had significantly lower urban operation percentages (50% for sample #73 and 40% for sample #70). Infrared analysis confirmed high quality for only two oils from this group—samples #72 and #73. Oil sample #70 showed high ester degradation and high nitration level. In this situation, the engine oil change interval should be reduced by a few thousand kilometers (the exact amount needs to be verified). In the second group—oil samples #61, #71, and #74—at least one viscosity parameter and at least two infrared parameters were in the critical range. In this situation, the decision to change the engine oil was made too late. In summary, out of the nine analyzed cases, the decision was too early in three instances (#68, #72, #73), made at the appropriate time in three instances (#65, #67, #71), and made too late in three instances (#69, #70, #74).
The data indicate that variations in engine oil degradation are significantly influenced by vehicle type, engine operation conditions, and the inherent properties of the oil. Soot formation, particularly prominent in diesel engine samples (#67, #68, and #74), is attributed to the incomplete combustion processes typically associated with diesel engines. Furthermore, samples from vehicles used predominantly in urban conditions (e.g., #65 and #72) demonstrate accelerated soot formation due to short trips and frequent engine starts, which prevent optimal engine temperatures.
Trends in viscosity changes and oxidation levels will be highlighted, showing cases where oil change intervals were either appropriate or delayed. While some samples exhibited minimal changes, indicating well-timed oil changes (such as sample #65), others showed significant degradation (e.g., sample #69 with elevated nitration and additive depletion levels), suggesting that the intervals between oil changes were extended beyond the optimal period.
These findings aim to provide a comprehensive understanding of the degradation phenomena, ensuring that both broader patterns and specific details are clearly presented, enhancing the scientific value and practical implications of the study’s conclusions.
It is worth noting that several OEM vehicle handbooks classify frequent short-distance driving with numerous cold starts as “severe” operating conditions, recommending shorter oil drain intervals compared to standard service. In our dataset, oils from vehicles with predominantly urban usage (e.g., samples #65 and #72) showed signs of accelerated degradation, which is consistent with these OEM recommendations. This alignment supports the interpretation that condition-based diagnostics can complement OEM guidance by providing direct evidence of when “severe” service accelerates lubricant deterioration.
5. Summary
The findings indicated that while most oil samples exhibited low levels of overall degradation, specific issues such as additive depletion and fuel dilution were observed in some cases. All the oil samples from the diesel-fueled engines #67, #68, and #74 were contaminated by FAME. For instance, oil sample #65 showed significant changes in viscosity without severe degradation, suggesting that the oil change was appropriately timed. In contrast, oil sample #67 revealed critical changes in additives and FAME dilution, justifying the oil change after 11,000 km of predominantly non-urban driving. Similarly, oil sample #68, although showing better infrared parameters than sample #67, suggested that the oil change might have been slightly premature by about 2000 km.
The study also noted that oil samples #70, #72, and #73 exhibited acceptable viscosity changes, indicating proper performance, although sample #70 showed high ester degradation, suggesting a need to shorten the oil change interval. On the other hand, oil samples #69, #71, and #74 exhibited critical changes in both viscosity and infrared parameters, indicating that the oil change was conducted too late.
Overall, this research underscores the importance of regular oil condition monitoring using advanced techniques like FTIR and viscosity control. These methods provide valuable insights for making informed decisions about oil changes, contributing to prolonged engine life and reduced risks associated with using degraded oil. The study concludes that, of the nine analyzed cases, the decision to change the oil was too early in three instances (#68, #72, #73), made at the appropriate time in three instances (#65, #67, #71), and too late in three instances (#69, #70, #74).
The findings provide not only a set of parameters characterizing the condition of used engine oil but also propose a comprehensive evaluation procedure that can be integrated into maintenance schedules. By assessing engine oil in real-time and using these analytical methods to monitor critical indicators such as viscosity changes, oxidation, nitration, and soot contamination, the research enables more informed decision-making regarding oil change intervals. This approach is designed to minimize the risk of engine failure and extend the service life of automotive vehicles, thereby reducing operational costs and maximizing performance.
Furthermore, the inclusion of a method for expert evaluation (as detailed in Table 8) offers a practical framework for technicians and engineers, allowing them to interpret oil condition data more effectively and make proactive adjustments. By applying these techniques in routine monitoring, vehicle owners and professionals in the automotive industry can enhance vehicle longevity, optimize fuel efficiency, and minimize the likelihood of costly repairs, making the study not just scientifically relevant but also practically applicable for real-world scenarios.
6. Conclusions
The results of this study demonstrate that combining FTIR spectroscopy with viscosity measurements provides a robust basis for condition-based maintenance of automotive engines. Instead of relying solely on mileage-based intervals, this approach allows for more accurate identification of optimal oil replacement timing.
From an industrial perspective, implementing such diagnostics can reduce unnecessary oil waste, extend the service life of engines, and lower maintenance costs, while also contributing to sustainability by minimizing environmental impacts. Service workshops and fleet operators could benefit from adopting these methods as decision-support tools to improve operational efficiency.
Future research should focus on expanding the dataset, applying advanced statistical or machine-learning models to refine predictive capabilities, and validating the approach in large-scale fleet studies. Integrating laboratory diagnostics with real-time onboard monitoring represents a promising direction for bridging research with practical automotive applications.
7. Limitations
The primary limitation is the lack of assurance that the “fresh” oil originates from the same batch as the oil introduced into the engine. The second limitation is the concern that during the oil change, the oil may have been contaminated by residues of used oil or by the mixture of solvents (flush) used for its removal, which have unknown composition and properties. This results in limited ability to assess the changes occurring in the oil during operation due to engine work. The observed changes are a sum of the changes related to the mixing of fresh oil with residual oil and/or flush and the degradation of the oil during operation.
Supplementary Materials
The following document is available online at https://www.mdpi.com/article/10.3390/su17188214/s1, S1: Questionnaire in original language (Polish).
Author Contributions
Conceptualization, A.W. and W.K.; methodology, A.W. and W.K.; software, A.W. and W.K.; validation, A.W. and W.K.; formal analysis, A.W. and W.K.; investigation, A.W. and W.K.; resources, A.W.; data curation, A.W. and W.K.; writing—original draft preparation, A.W. and W.K.; writing—review and editing, A.W. and W.K.; visualization, A.W. and W.K.; supervision A.W. and W.K.; project administration, A.W., funding acquisition A.W. All authors have read and agreed to the published version of the manuscript.
Funding
The publication was financed from the subsidy granted to the Krakow University of Economics—Project nr 018/ZJB/2025/DOS.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Appendix. Additional raw data (FTIR spectra) are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement. This change does not affect the scientific content of the article.
Nomenclature
| API | American Petroleum Institute classification |
| ACEA | European Automobile Manufacturers’ Association classification |
| OEM | Original Equipment Manufacturer |
| ASTM | American Society for Testing and Materials |
| FTIR | Fourier Transform Infrared spectroscopy |
| FAME | Fatty Acid Methyl Esters |
| EP additives | Extreme Pressure additives |
| KV 40 °C | kinematic viscosity at 40 °C [mm2/s] |
| KV 100 °C | kinematic viscosity at 100 °C [mm2/s] |
| VI | Viscosity Index [-] |
| G | gasoline engine |
| D | diesel engine |
| G/LPG | gasoline engine with liquid petroleum gas |
| G/H | hybrid gasoline-electric engine |
References
- Urzędowska, W.; Stępień, Z. Wybrane Zagadnienia Dotyczące Zmian Właściwości Silnikowego Oleju Smarowego w Eksploatacji. Nafta-Gaz 2012, 12, 1102–1110. [Google Scholar]
- Cao, W.; Dong, G.; Xie, Y.B.; Peng, Z. Prediction of Wear Trend of Engines via On-Line Wear Debris Monitoring. Tribol. Int. 2018, 120, 510–519. [Google Scholar] [CrossRef]
- Srata, L.; Farres, S.; Chikri, M.; Addou, S.; Fethi, F. Detection of the Adulteration of Motor Oil by Laser Induced Fluorescence Spectroscopy and Chemometric Techniques. J. Fluoresc. 2023, 33, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Pourramezan, M.R.; Rohani, A.; Abbaspour-Fard, M.H. Unlocking the Potential of Soft Computing for Predicting Lubricant Elemental Spectroscopy. Lubricants 2023, 11, 382. [Google Scholar] [CrossRef]
- Mamgbi, R.; Cerny, J.; Barifaijo, E. Time of Exploitation and Detergency Properties of Low SAPS Engine Oil. Nafta-Gaz 2013, 1, 57–65. [Google Scholar]
- Holland, T.; Abdul-Munaim, A.M.; Mandrell, C.; Karunanithy, R.; Watson, D.G.; Sivakumar, P. Uv-Visible Spectrophotometer for Distinguishing Oxidation Time of Engine Oil. Lubricants 2021, 9, 37. [Google Scholar] [CrossRef]
- Chimeno-Trinchet, C.; Murru, C.; Díaz-García, M.E.; Fernández-González, A.; Badía-Laíño, R. Artificial Intelligence and Fourier-Transform Infrared Spectroscopy for Evaluating Water-Mediated Degradation of Lubricant Oils. Talanta 2020, 219, 121312. [Google Scholar] [CrossRef]
- Wakiru, J.M.; Pintelon, L.; Muchiri, P.N.; Chemweno, P.K. A Review on Lubricant Condition Monitoring Information Analysis for Maintenance Decision Support Mechanical Systems and Signal Processing. Mech. Syst. Signal Process. 2019, 118, 108–132. [Google Scholar] [CrossRef]
- Centobelli, P.; Cerchione, R.; Esposito, E. Environmental Sustainability in the Service Industry of Transportation and Logistics Service Providers: Systematic Literature Review and Research Directions. Transp. Res. Part D Transp. Environ. 2017, 53, 454–470. [Google Scholar] [CrossRef]
- Rahmani, R.; Rahnejat, H.; Fitzsimons, B.; Dowson, D. The Effect of Cylinder Liner Operating Temperature on Frictional Loss and Engine Emissions in Piston Ring Conjunction. Appl. Energy 2017, 191, 568–581. [Google Scholar] [CrossRef]
- Srata, L.; Farres, S.; Fethi, F. Engine Oil Authentication Using near Infrared Spectroscopy and Chemometrics Methods. Vib. Spectrosc. 2019, 100, 99–106. [Google Scholar] [CrossRef]
- Will, F.; Boretti, A. A New Method to Warm Up Lubricating Oil to Improve the Fuel Efficiency During Cold Start. SAE Int. J. Engines 2011, 4, 175–187. [Google Scholar] [CrossRef]
- Du, Y.; Wu, T.; Makis, V. Parameter Estimation and Remaining Useful Life Prediction of Lubricating Oil with HMM. Wear 2017, 376–377, 1227–1233. [Google Scholar] [CrossRef]
- Adams, M.J.; Romeo, M.J.; Rawson, P. FTIR Analysis and Monitoring of Synthetic Aviation Engine Oils. Talanta 2007, 73, 629–634. [Google Scholar] [CrossRef]
- Wakiru, J.; Pintelon, L.; Muchiri, P.N.; Chemweno, P.K.; Mburu, S. Towards an Innovative Lubricant Condition Monitoring Strategy for Maintenance of Ageing Multi-Unit Systems. Reliab. Eng. Syst. Saf. 2020, 204, 107200. [Google Scholar] [CrossRef]
- Liu, Y.; Bao, K.; Wang, Q.; Zio, E. Application of FTIR Method to Monitor the Service Condition of Used Diesel Engine Lubricant Oil. In Proceedings of the 2019 4th International Conference on System Reliability and Safety, ICSRS 2019, Rome, Italy, 20–22 November 2019. [Google Scholar]
- Agoston, A.; Ötsch, C.; Jakoby, B. Viscosity Sensors for Engine Oil Condition Monitoring—Application and Interpretation of Results. Sens. Actuators A Phys. 2005, 121, 327–332. [Google Scholar] [CrossRef]
- Wang, Z.; Xue, X.; Yin, H.; Jiang, Z.; Li, Y. Review Article Research Progress on Monitoring and Separating Suspension Particles for Lubricating Oil. Complexity 2018, 2018, 9356451. [Google Scholar] [CrossRef]
- van de Voort, F.R.; Sedman, J.; Cocciardi, R.; Juneau, S. An Automated FTIR Method for the Routine Quantitative Determination of Moisture in Lubricants: An Alternative to Karl Fischer Titration. Talanta 2007, 72, 289–295. [Google Scholar] [CrossRef]
- Zzeyani, S.; Mikou, M.; Naja, J.; Elachhab, A. Spectroscopic Analysis of Synthetic Lubricating Oil. Tribol. Int. 2017, 114, 27–32. [Google Scholar] [CrossRef]
- Hirri, A.; Tagourmate, S.; Benamar, A.; Kzaiber, F.; Oussama, A. Prediction of Kinematic Viscosity in Motor Oil Using FTIR Coupled with Partial Least Squares Regression. Int. J. Chem. Mater. Environ. Res. 2017, 4, 102–107. [Google Scholar]
- Ibrahim, D.; Stapah, M.; Ruslan, M.A.A.; Yaakob, Y.; Budin, S.; Maideen, N.C.; Yusoff, H. Predicting the next Oil Change for Automotive Engine Oil. In Journal of Physics: Conference Series, Proceedings of the International Conference on Nanomaterials: Science, Engineering and Technology (ICoNSET) 2019, Penang Island, Malaysia, 5–6 August 2019; Institute of Physics Publishing: Bristol, UK, 2019; Volume 1349. [Google Scholar]
- Al-Ghouti, M.A.; Al-Degs, Y.S.; Amer, M. Application of Chemometrics and FTIR for Determination of Viscosity Index and Base Number of Motor Oils. Talanta 2010, 81, 1096–1101. [Google Scholar] [CrossRef]
- Sejkorová, M.; Kučera, M.; Hurtová, I.; Voltr, O. Application of FTIR-ATR Spectrometry in Conjunction with Multivariate Regression Methods for Viscosity Prediction of Worn-out Motor Oils. Appl. Sci. 2021, 11, 3842. [Google Scholar] [CrossRef]
- Wu, T.; Wu, H.; Du, Y.; Peng, Z. Progress and Trend of Sensor Technology for On-Line Oil Monitoring. Sci. China Technol. Sci. 2013, 56, 2914–2926. [Google Scholar] [CrossRef]
- Besser, C.; Agocs, A.; Ronai, B.; Ristic, A.; Repka, M.; Jankes, E.; McAleese, C.; Dörr, N. Generation of Engine Oils with Defined Degree of Degradation by Means of a Large Scale Artificial Alteration Method. Tribol. Int. 2019, 132, 39–49. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.r-project.org/ (accessed on 2 September 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).