Integrating Species Richness, Distribution and Human Pressures to Assess Conservation Priorities in High Andean Salares
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
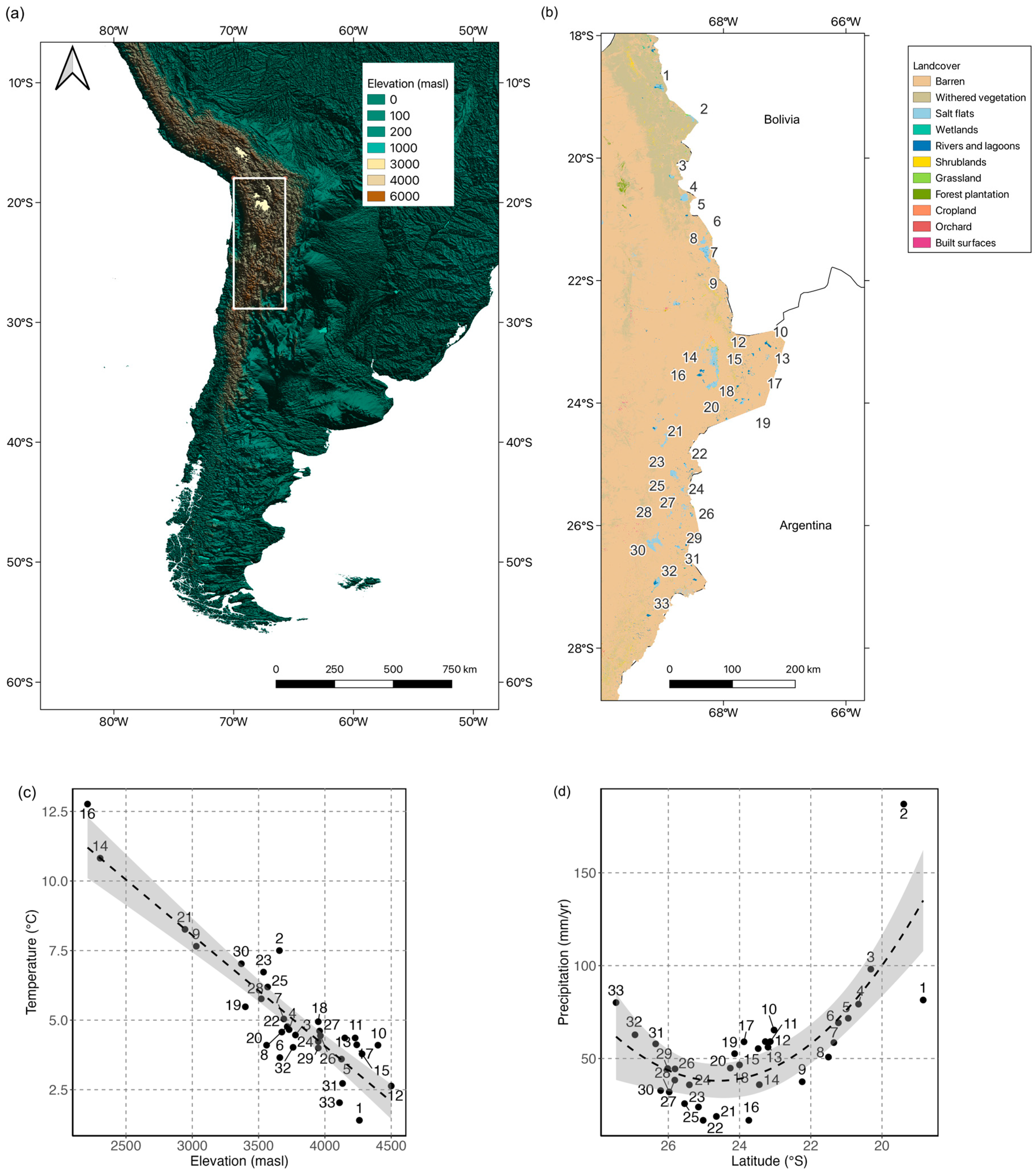
Location, Topography and Climate
2.2. Analysing Species Diversity and Distribution Across High Andean Salares
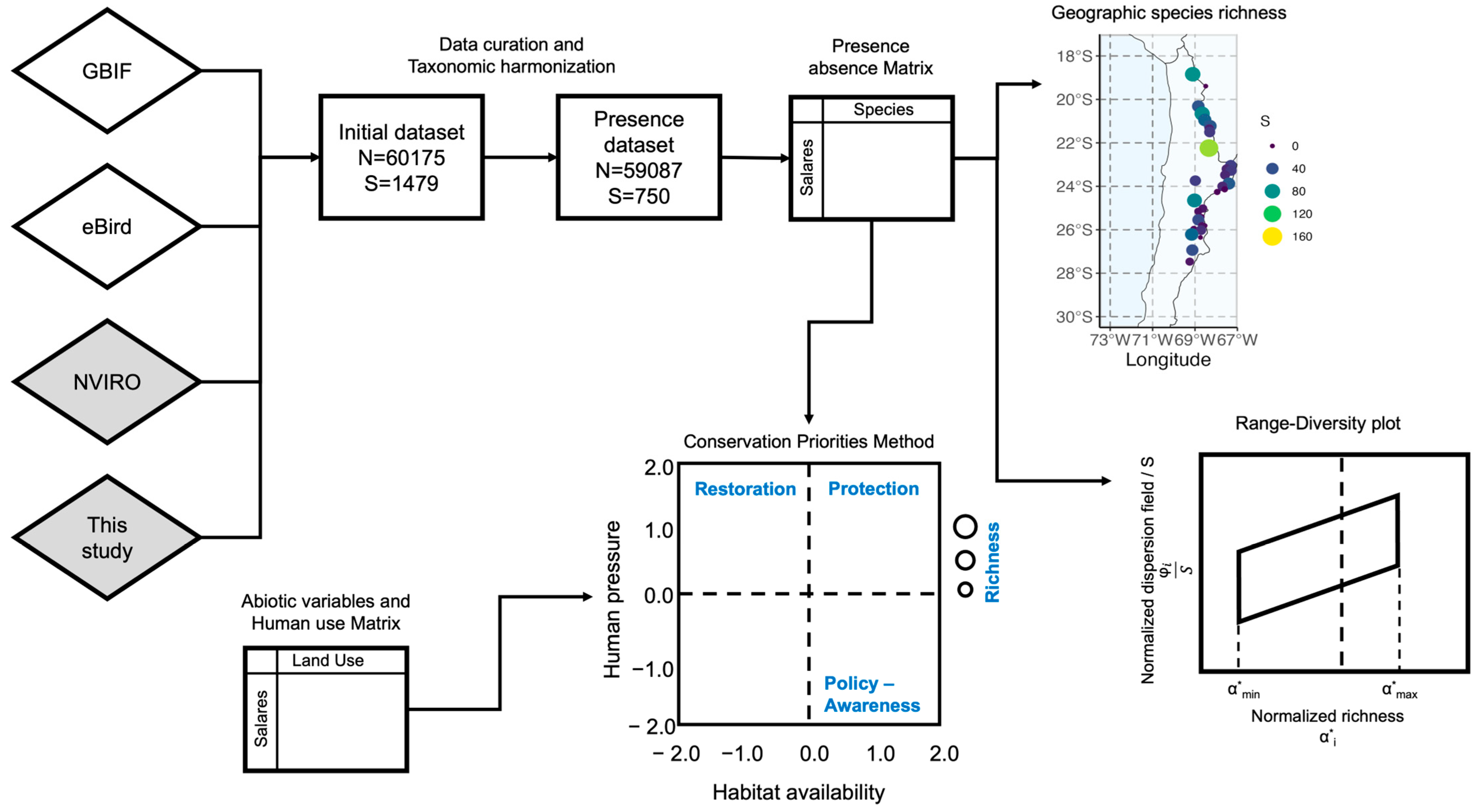
2.2.1. Analytical Framework
2.2.2. Biodiversity Geodatabase Curation and Processing
2.2.3. Construction of Presence–Absence Matrix (PAM)
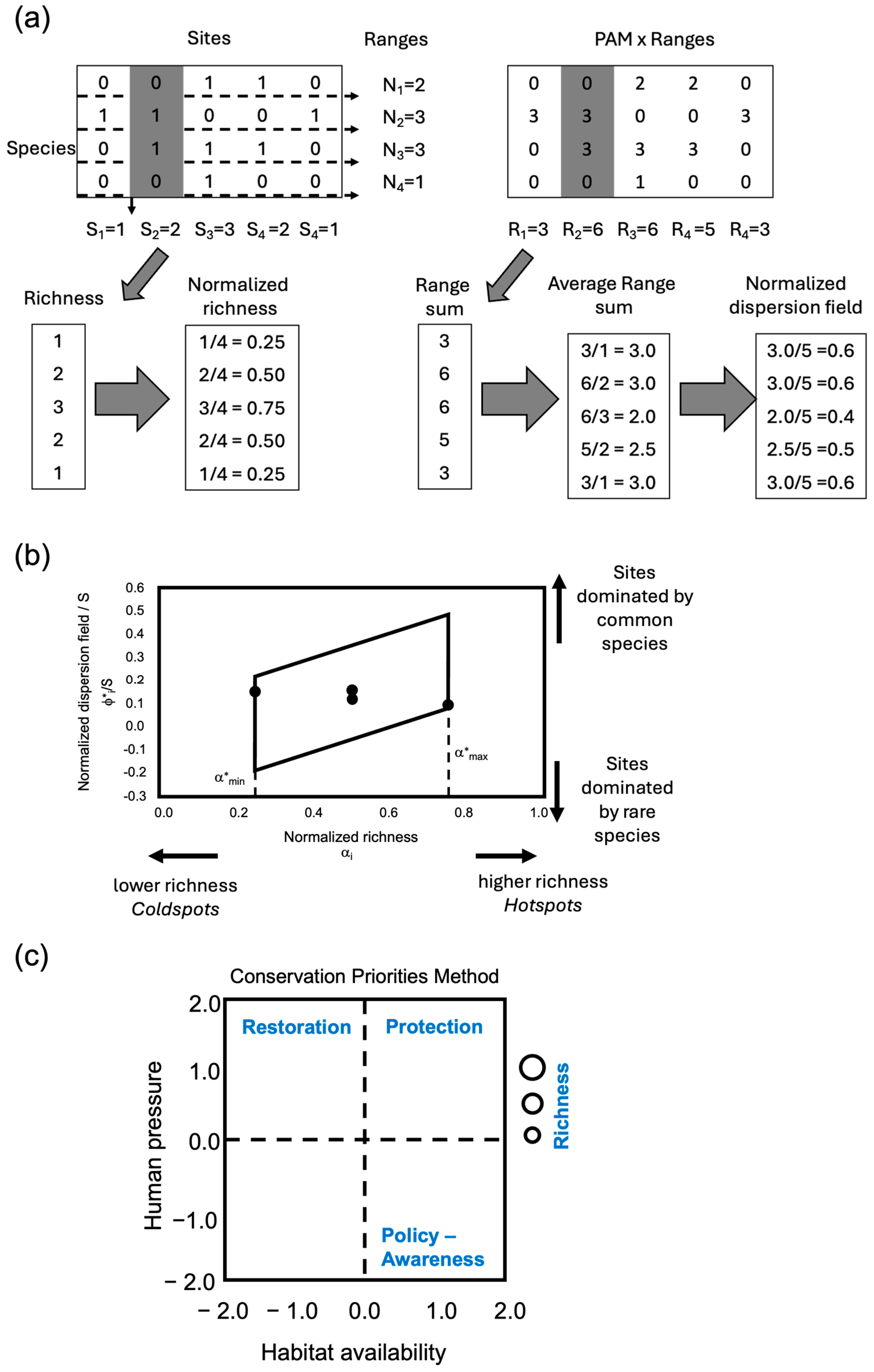
2.2.4. Analysis of Biodiversity Patterns: Range–Diversity Plots
2.3. Assessing the Role of Anthropic Pressures: Conservation Priorities Methods (CPM)
2.3.1. Habitat Availability
2.3.2. Assessment of Human Pressure Indicators
2.4. Comparative Assessment of Conservation Prioritization Schemes
3. Results
3.1. Geographic and Environmental Characteristics
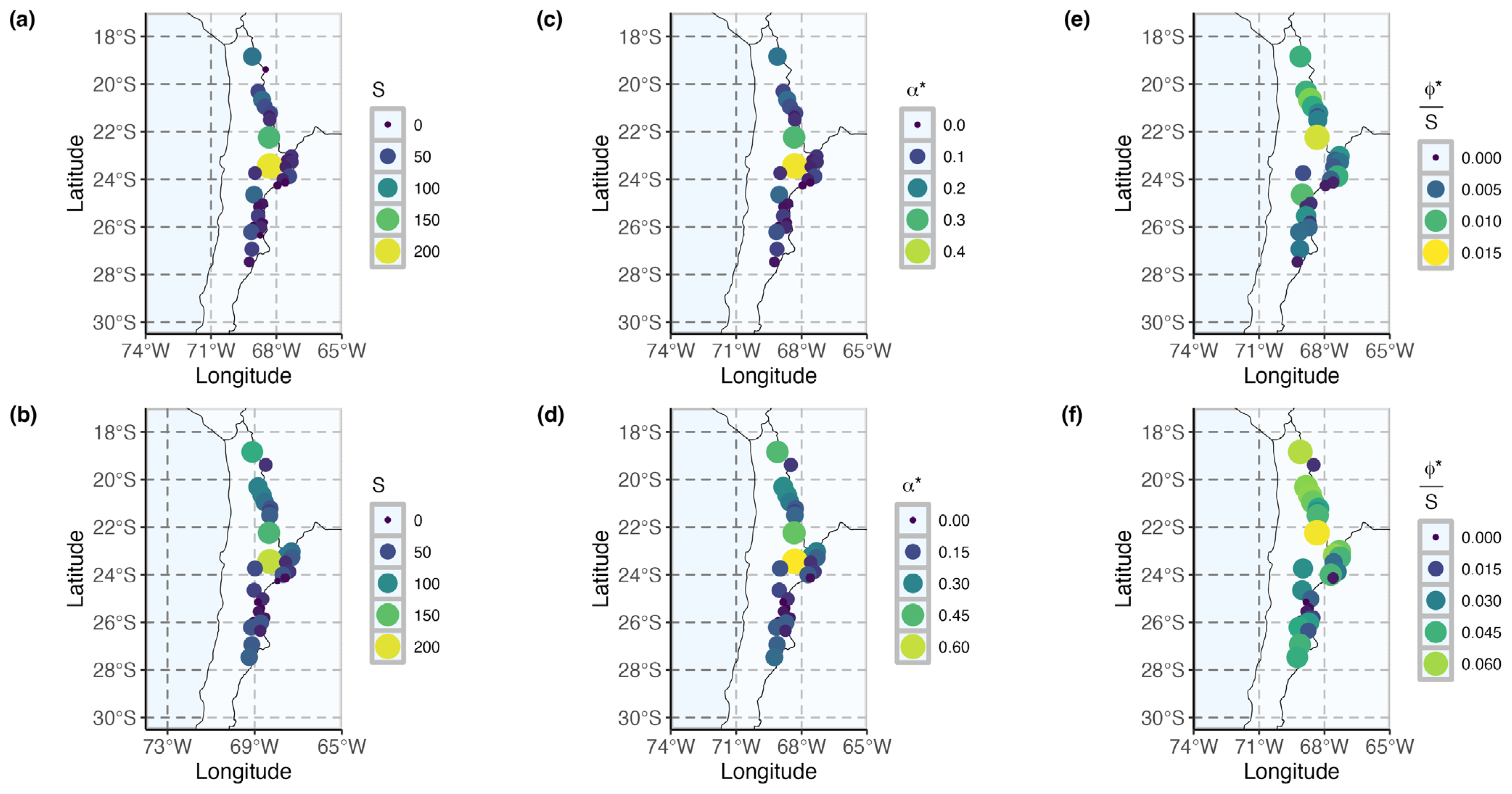
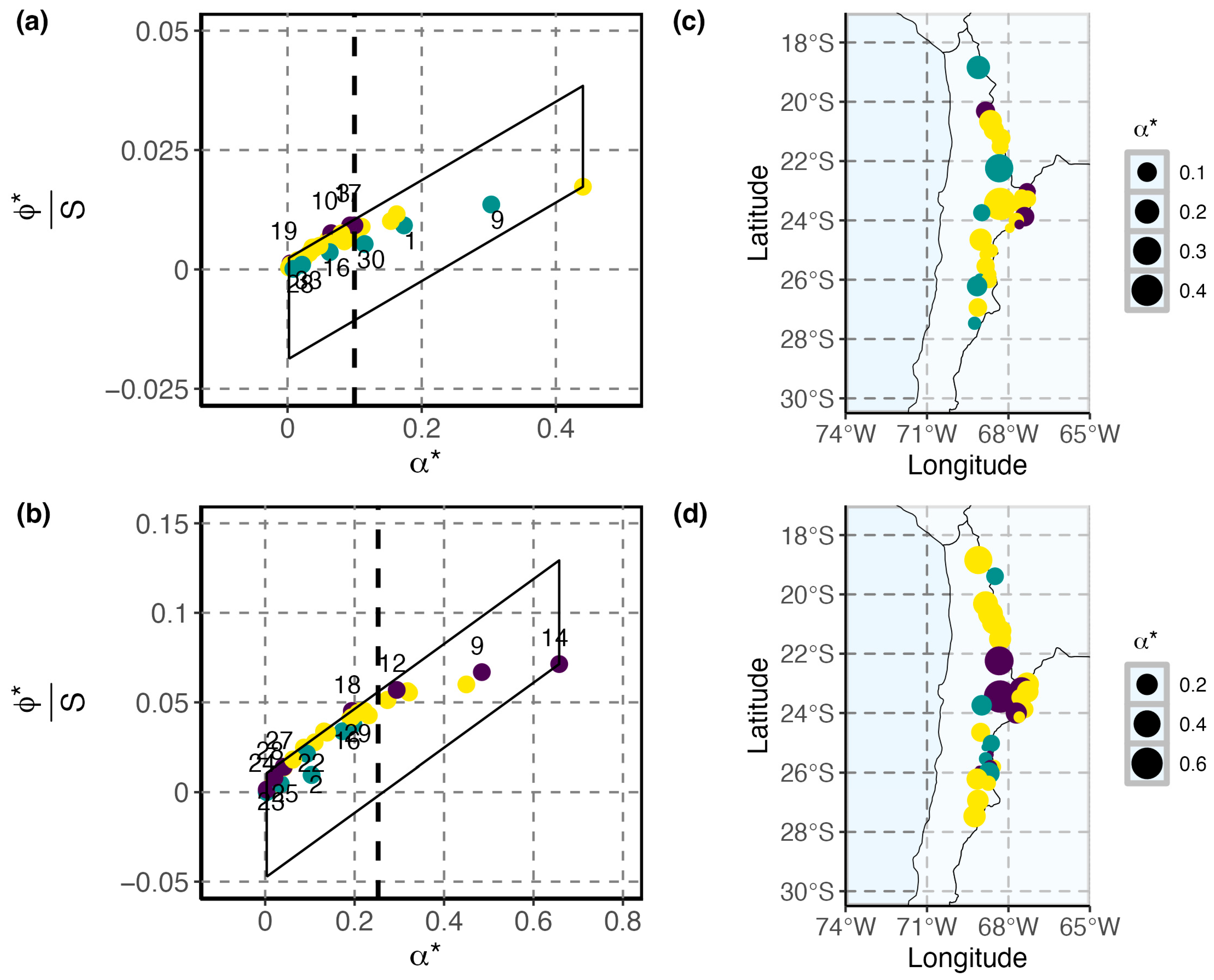
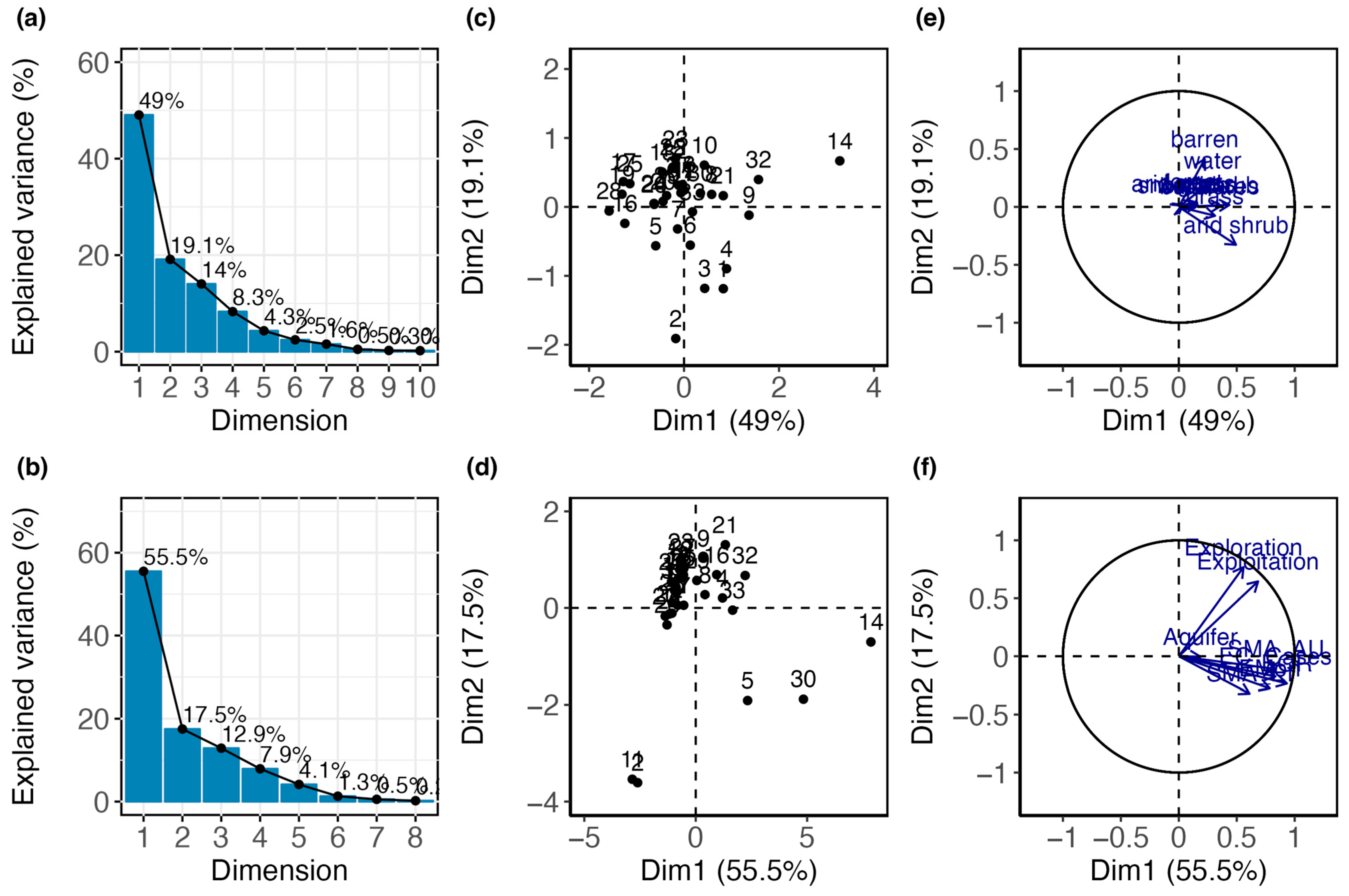
3.2. Patterns of Species Diversity and Distribution Across High Andean Salares
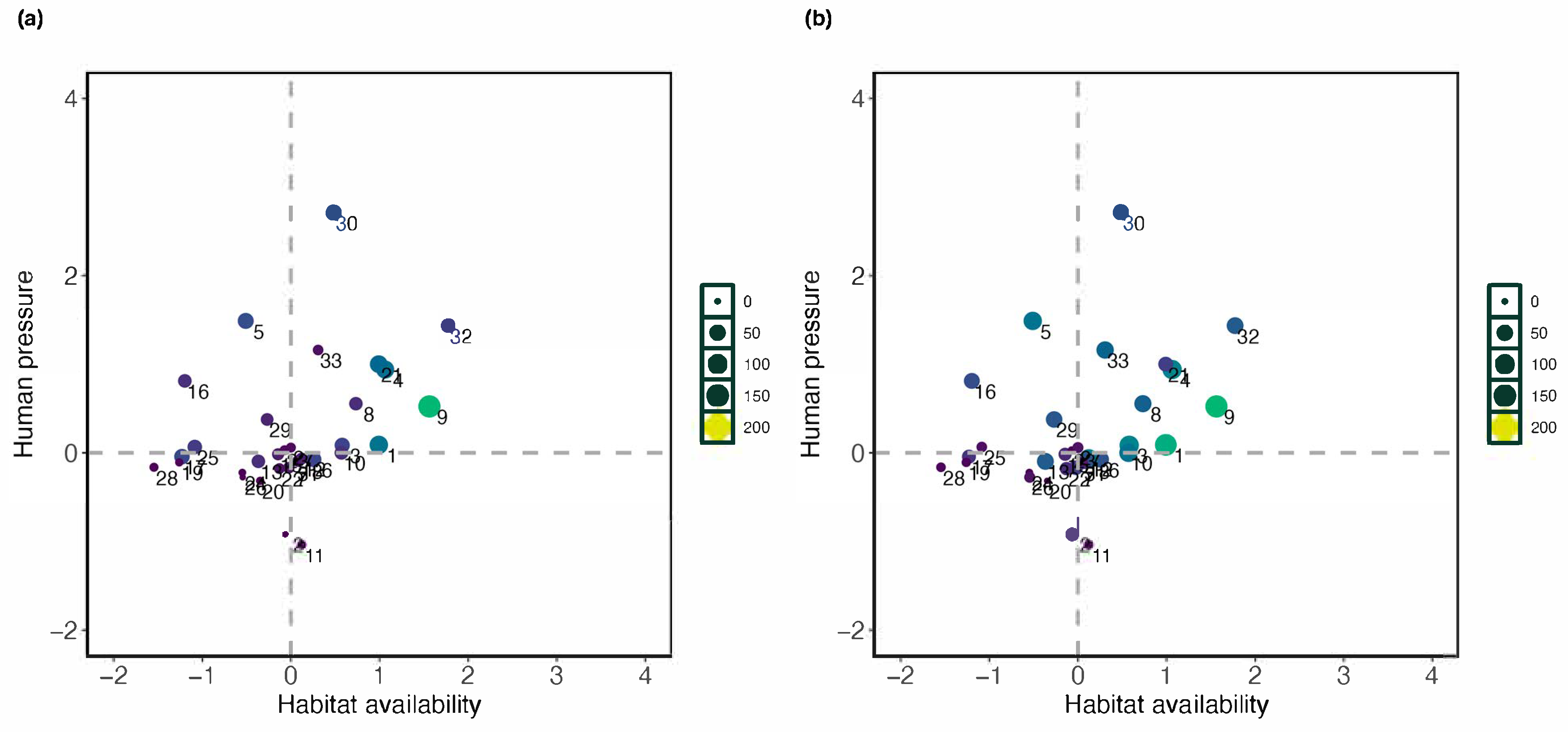
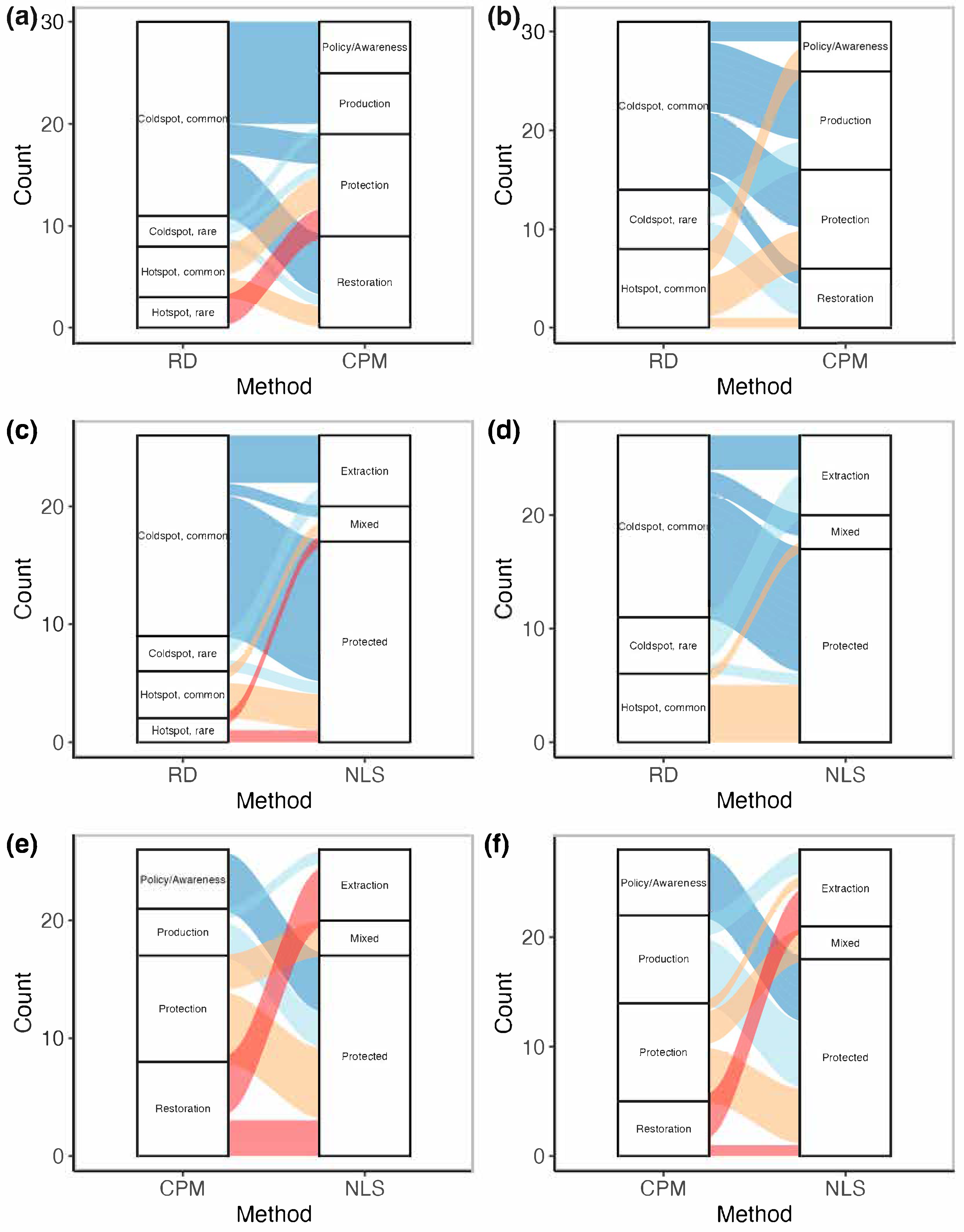
3.3. Conservation Priorities Assessment
4. Discussion
4.1. Drivers of Species Richness and Distribution
4.2. Conservation Prioritization Frameworks
4.3. Misalignment Between Ecological Conservation Priorities and Current Governance and Policy Instruments
4.4. Policy and Governance Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BV | Biodiversity Value |
| CPM | Conservation Priorities Method |
| DGA | Directorate General of Water |
| DEM | Digital Elevation Model |
| EIA | Environmental Impact Assessment |
| EQR | Environmental Qualification Resolution |
| FAO | Food and Agriculture Organization |
| GBIF | Global Biodiversity Information Facility |
| HA | Habitat Availability |
| HP | Human Pressure |
| IEB | Institute of Ecology and Biodiversity |
| masl | Meters above sea level |
| NLS | National Lithium Strategy |
| NVIRO | National Biodiversity Geospatial Platform derived from EIAs (defined in the study) |
| PAM | Presence–Absence Matrix |
| PCA | Principal Component Analysis |
| POWO | Plants of the World Online |
| RD | Range–Diversity |
| R2 | Coefficient of Statistical Determination (R-squared, used in regression) |
| SEA | Environmental Assessment Service |
| SEIA | Environmental Impact Assessment System |
| SMA | Environmental Superintendency |
| SSB | Sub-subbasin |
References
- IPBES Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Zenodo: Geneva, Switzerland, 2019.
- Díaz, S.; Settele, J.; Brondízio, E.S.; Ngo, H.T.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.A.; Butchart, S.H.M.; Chan, K.M.A.; et al. Pervasive Human-Driven Decline of Life on Earth Points to the Need for Transformative Change. Science 2019, 366, eaax3100. [Google Scholar] [CrossRef]
- Pörtner, H.-O.; Scholes, R.J.; Arneth, A.; Barnes, D.K.A.; Burrows, M.T.; Diamond, S.E.; Duarte, C.M.; Kiessling, W.; Leadley, P.; Managi, S.; et al. Overcoming the Coupled Climate and Biodiversity Crises and Their Societal Impacts. Science 2023, 380, eabl4881. [Google Scholar] [CrossRef] [PubMed]
- Pettorelli, N.; Graham, N.A.J.; Seddon, N.; Maria da Cunha Bustamante, M.; Lowton, M.J.; Sutherland, W.J.; Koldewey, H.J.; Prentice, H.C.; Barlow, J. Time to Integrate Global Climate Change and Biodiversity Science-Policy Agendas. J. Appl. Ecol. 2021, 58, 2384–2393. [Google Scholar] [CrossRef]
- Agusdinata, D.B.; Liu, W.; Eakin, H.; Romero, H. Socio-environmental impacts of lithium mineral extraction: Towards a research agenda. Environ. Res. Lett. 2018, 13, 123001. [Google Scholar] [CrossRef]
- Liu, W.; Agusdinata, D.B. Interdependencies of lithium mining and communities sustainability in Salar de Atacama, Chile. J. Clean. Prod. 2020, 260, 120838. [Google Scholar] [CrossRef]
- Ostapenko, O.; Alina, G.; Serikova, M.; Popp, L.; Kurbatova, T.; Bashu, Z. Towards Overcoming Energy Crisis and Energy Transition Acceleration: Evaluation of Economic and Environmental Perspectives of Renewable Energy Development. In Circular Economy for Renewable Energy; Koval, V., Olczak, P., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 109–128. ISBN 978-3-031-30800-0. [Google Scholar]
- Al-Jawad, J.; Ford, J.; Petavratzi, E.; Hughes, A. Understanding the Spatial Variation in Lithium Concentration of High Andean Salars Using Diagnostic Factors. Sci. Total Environ. 2024, 906, 167647. [Google Scholar] [CrossRef]
- Petavratzi, E.; Sanchez-Lopez, D.; Hughes, A.; Stacey, J.; Ford, J.; Butcher, A. The Impacts of Environmental, Social and Governance (ESG) Issues in Achieving Sustainable Lithium Supply in the Lithium Triangle. Miner. Econ. 2022, 35, 673–699. [Google Scholar] [CrossRef]
- Gutiérrez, J.S.; Moore, J.N.; Donnelly, J.P.; Dorador, C.; Navedo, J.G.; Senner, N.R. Climate Change and Lithium Mining Influence Flamingo Abundance in the Lithium Triangle. Proc. R. Soc. B 2022, 289, 20212388. [Google Scholar] [CrossRef]
- Galaz, V. Global Environmental Governance in Times of Turbulence. One Earth 2022, 5, 582–585. [Google Scholar] [CrossRef]
- Warren, J.K. Evaporites through Time: Tectonic, Climatic and Eustatic Controls in Marine and Nonmarine Deposits. Earth-Sci. Rev. 2010, 98, 217–268. [Google Scholar] [CrossRef]
- Risacher, F.; Alonso, H.; Salazar, C. The Origin of Brines and Salts in Chilean Salars: A Hydrochemical Review. Earth-Sci. Rev. 2003, 63, 249–293. [Google Scholar] [CrossRef]
- Marazuela, M. The Lithium Triangle: A Global Perspective. Miner. Econ. 2019, 32, 1–10. [Google Scholar]
- Marazuela, M.A.; Vázquez-Suñé, E.; Ayora, C.; García-Gil, A. Towards More Sustainable Brine Extraction in Salt Flats: Learning from the Salar de Atacama. Sci. Total Environ. 2020, 703, 135605. [Google Scholar] [CrossRef]
- Marazuela, M.A.; Vázquez-Suñé, E.; Ayora, C.; García-Gil, A.; Palma, T. Hydrodynamics of salt flat basins: The Salar de Atacama example. Sci. Total Environ. 2019, 651, 668–683. [Google Scholar] [CrossRef]
- Flores-Fernández, C.; Alba, R. Water or Mineral Resource? Legal Interpretations and Hydrosocial Configurations of Lithium Mining in Chile. Front. Water 2023, 5, 1075139. [Google Scholar] [CrossRef]
- Farías, M.E. Microbial Ecosystems in Central Andes Extreme Environments, Biofilms, Microbial Mats, Microbialites and Endoevaporites; Springer Nature: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Aguilar, P.; Acosta, E.; Dorador, C.; Sommaruga, R. Large Differences in Bacterial Community Composition among Three Nearby Extreme Waterbodies of the High Andean Plateau. Front. Microbiol. 2016, 7, 976. [Google Scholar] [CrossRef]
- Heine-Fuster, I.; López-Allendes, C.; Aránguiz-Acuña, A.; Véliz, D. Differentiation of Diatom Guilds in Extreme Environments in the Andean Altiplano. Front. Environ. Sci. 2021, 9, 701970. [Google Scholar] [CrossRef]
- Demergasso, C. Biodiversity in High-Altitude Ecosystems: Importance and Conservation. Biodivers. Conserv. 2008, 17, 1–15. [Google Scholar]
- Demergasso, C.; Escudero, L.; Casamayor, E.O.; Chong, G.; Balagué, V.; Pedrós-Alió, C. Novelty and spatio–temporal heterogeneity in the bacterial diversity of hypersaline Lake Tebenquiche (Salar de Atacama). Extremophiles 2008, 12, 491–504. [Google Scholar] [CrossRef]
- Dorador, C.; Vila, I.; Witzel, K.-P.; Imhoff, J.F. Bacterial and Archaeal Diversity in High Altitude Wetlands of the Chilean Altiplano. Fundam. Appl. Limnol. Arch. Hydrobiol. 2013, 182, 135–159. [Google Scholar] [CrossRef]
- Dorador, C.; Pardo, R.; Vila, I. Variaciones temporales de parmetros fsicos, qumicos y biolgicos de un lago de altura: El caso del lago Chungar. Rev. Chil. Hist. Nat. 2003, 76, 15–22. [Google Scholar] [CrossRef]
- Collado, G.A.; Torres-Díaz, C.; Vidal, M.A.; Valladares, M.A. Genetic Diversity, Morphometric Characterization, and Conservation Reassessment of the Critically Endangered Freshwater Snail, Heleobia Atacamensis, in the Atacama Saltpan, Northern Chile. Biology 2023, 12, 791. [Google Scholar] [CrossRef] [PubMed]
- Collado, G.A.; Méndez, M.A. Microgeographic Differentiation among Closely Related Species of Biomphalaria (Gastropoda: Planorbidae) from the Andean Altiplano. Zoöl. J. Linn. Soc. 2013, 169, 640–652. [Google Scholar] [CrossRef]
- Collado, G.A.; Valladares, M.A.; Méndez, M.A. A new species of Heleobia (Caenogastropoda: Cochliopidae) from the Chilean Altiplano. Zootaxa 2016, 4137, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Collado, G.A.; Méndez, M.A. Los taxa nominales de moluscos descritos por Courty del Salar de Ascotn, Altiplano chileno. Rev. Chil. Hist. Nat. 2012, 85, 233–235. [Google Scholar] [CrossRef]
- Collado, G.A. A New Freshwater Snail (Caenogastropoda: Cochliopidae) from the Atacama Desert, Northern Chile. Zootaxa 2015, 3925, 445–449. [Google Scholar] [CrossRef]
- Campos, J.C.; Rebolledo, N.; Sáez, P.; Fibla, P.; Méndez, M.; Lobos, G. Primeros registros de piscivoría y dermatofagia en Telmatobius philippii (telmatobiidae) desde el salar de ascotán. Norte de Chile. Rev. Latinoam. Herpetol. 2024, 7, 126–131. [Google Scholar] [CrossRef]
- Fibla, P.; Sáez, P.A.; Lobos, G.; Rebolledo, N.; Véliz, D.; Pastenes, L.; del Pozo, T.; Méndez, M.A. Delimitation of Endangered Telmatobius Species (Anura: Telmatobiidae) of the Chilean Salt Puna. Animals 2024, 14, 3612. [Google Scholar] [CrossRef]
- Lobos, G.; Rebolledo, N.; Sandoval, M.; Canales, C.; Perez-Quezada, J.F. Temporal Gap Between Knowledge and Conservation Needs in High Andean Anurans: The Case of the Ascotn Salt Flat Frog in Chile (Anura: Telmatobiidae: Telmatobius. S. Am. J. Herpetol. 2018, 13, 33–43. [Google Scholar] [CrossRef]
- Lobos, G.; Rebolledo, N.; Salinas, H.; Fibla, P.; Saez, P.A.; Mendez, M.A. Ecological Features of Telmatobius chusmisensis (Anura: Telmatobiidae), a Poorly Known Species from Northern Chile. S. Am. J. Herpetol. 2021, 20, 1–7. [Google Scholar] [CrossRef]
- Cortés, A.; Miranda, E.; Rosenmann, M.; Rau, J.R. Thermal Biology of the Fossorial Rodent Ctenomys Fulvus from the Atacama Desert, Northern Chile. J. Therm. Biol. 2000, 25, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Jaksic, F.M.; Torres-Mura, J.C.; Cornelius, C.; Marquet, P.A. Small mammals of the Atacama Desert (Chile). J. Arid. Environ. 1999, 42, 129–135. [Google Scholar] [CrossRef]
- Storz, J.F.; Quiroga-Carmona, M.; Liphardt, S.; Herrera, N.D.; Bautista, N.M.; Opazo, J.C.; Rico-Cernohorska, A.; Salazar-Bravo, J.; Good, J.M.; D’Elía, G. Extreme High-Elevation Mammal Surveys Reveal Unexpectedly High Upper Range Limits of Andean Mice. Am. Nat. 2024, 203, 726–735. [Google Scholar] [CrossRef]
- Valladares, P. Mamferos terrestres de la Regin de Atacama, Chile: Comentarios sobre su distribucin y estado de conservacin. Gayana 2012, 76, 22–37. [Google Scholar] [CrossRef]
- Carrasco-Puga, G.; Díaz, F.P.; Soto, D.C.; Hernández-Castro, C.; Contreras-López, O.; Maldonado, A.; Latorre, C.; Gutiérrez, R.A. Revealing Hidden Plant Diversity in Arid Environments. Ecography 2021, 44, 98–111. [Google Scholar] [CrossRef]
- Revollo-Cadima, S.G.; Salazar-Bravo, J. Identifying Areas of Conservation Importance Based on Spatial Patterns of Evolutionary Diversity for Non-Volant Small Mammals in the Andean Puna. J. Arid. Environ. 2024, 224, 105230. [Google Scholar] [CrossRef]
- Broitman, B.; Sproles, E.; Weideman, C.; Salas, S.; Geldes, C.; Zambra, A.; González-Silvestre, L.; Bugueño, L. Building Consensus through Assessment Evidence from San Pedro de Atacama, Chile. In Mainstreaming Natural Capital and Ecosystem Services into Development Policy; Routledge: Oxfordshire, UK, 2019; pp. 121–148. [Google Scholar]
- Carrasco-Lagos, P.; Moreno, R.A.; Figueroa, A.; Espoz, C.; Maza, C.L. Sitios Ramsar de Chile; Universidad Santo Tomás: Santiago, Chile, 2015. [Google Scholar]
- Cubillos, C.F.; Paredes, A.; Yáñez, C.; Palma, J.; Severino, E.; Vejar, D.; Grágeda, M.; Dorador, C. Insights Into the Microbiology of the Chaotropic Brines of Salar de Atacama, Chile. Front. Microbiol. 2019, 10, 1611. [Google Scholar] [CrossRef]
- López-Steinmetz, R.L.; Salvi, S. Brine Grades in Andean Salars: When Basin Size Matters a Review of the Lithium Triangle. Earth-Sci. Rev. 2021, 217, 103615. [Google Scholar] [CrossRef]
- Salica, M.J.; Gastón, M.S.; Akmentins, M.S.; Vaira, M. Threatened Aquatic Andean Frogs and Mining Activity in the Lithium Triangle of South America: Can Both Coexist? Aquat. Conserv. Mar. Freshw. Ecosyst. 2024, 34, e4044. [Google Scholar] [CrossRef]
- U.S.G.S. Mineral Commodity Summaries 2024; U.S. Geological Survey: Reston, VA, USA, 2024.
- Gutiérrez, G.; Ruiz-León, D. Lithium in Chile: Present Status and Future Outlook. Mater. Adv. 2024, 5, 7850–7861. [Google Scholar] [CrossRef]
- López-Cubillos, S.; Muñoz-Ávila, L.; Roberson, L.A.; Suárez-Castro, A.F.; Ochoa-Quintero, J.M.; Crouzeilles, R.; Gallo-Cajiao, E.; Rhodes, J.; Dressler, W.; Martinez-Harms, M.J.; et al. The Landmark Escazú Agreement: An Opportunity to Integrate Democracy, Human Rights, and Transboundary Conservation. Conserv. Lett. 2022, 15, e12838. [Google Scholar] [CrossRef]
- Xu, H.; Cao, Y.; Yu, D.; Cao, M.; He, Y.; Gill, M.; Pereira, H.M. Ensuring Effective Implementation of the Post-2020 Global Biodiversity Targets. Nat. Ecol. Evol. 2021, 5, 411–418. [Google Scholar] [CrossRef]
- Ferrier, S. Mapping Spatial Pattern in Biodiversity for Regional Conservation Planning: Where to from Here? Syst. Biol. 2002, 51, 331–363. [Google Scholar] [CrossRef] [PubMed]
- Ferrier, S.; Powell, G.V.N.; Richardson, K.S.; Manion, G.; Overton, J.M.; Allnutt, T.F.; Cameron, S.E.; Mantle, K.; Burgess, N.D.; Faith, D.P.; et al. Mapping More of Terrestrial Biodiversity for Global Conservation Assessment. AIBS Bull. 2004, 54, 1101–1109. [Google Scholar] [CrossRef]
- Kacoliris, F.P.; Velasco, M.A.; Berkunsky, I.; Celsi, C.E.; Williams, J.D.; Di-Pietro, D.; Rosset, S. How to Prioritize Allocating Conservation Efforts: An Alternative Method Tested with Imperilled Herpetofauna. Anim. Conserv. 2016, 19, 46–52. [Google Scholar] [CrossRef]
- Vera, D.G.; Pietro, D.O.D.; Falasco, C.T.; Tettamanti, G.; Iriarte, L.; Harkes, M.; Kacoliris, F.P.; Berkunsky, I. Identifying Key Conservation Sites for the Reptiles of the Tandilia Mountains in Pampas Highlands. J. Nat. Conserv. 2023, 71, 126321. [Google Scholar] [CrossRef]
- Vilar, C.C.; Joyeux, J.-C.; Spach, H.L. Geographic Variation in Species Richness, Rarity, and the Selection of Areas for Conservation: An Integrative Approach with Brazilian Estuarine Fishes. Estuar. Coast. Shelf Sci. 2017, 196, 134–140. [Google Scholar] [CrossRef]
- Villalobos, F.; Dobrovolski, R.; Provete, D.B.; Gouveia, S.F. Is Rich and Rare the Common Share? Describing Biodiversity Patterns to Inform Conservation Practices for South American Anurans. PLoS ONE 2013, 8, 56073. [Google Scholar] [CrossRef]
- Villalobos, F.; Lira-Noriega, A.; Soberón, J.; Arita, H.T. Range–Diversity Plots for Conservation Assessments: Using Richness and Rarity in Priority Setting. Biol. Conserv. 2013, 158, 313–320. [Google Scholar] [CrossRef]
- Chao, A.; Chiu, C.; Hsieh, T.C.; Davis, T.; Nipperess, D.A.; Faith, D.P. Rarefaction and Extrapolation of Phylogenetic Diversity. Methods Ecol. Evol. 2015, 6, 380–388. [Google Scholar] [CrossRef]
- Chao, A.; Chiu, C.-H.; Jost, L. Unifying Species Diversity, Phylogenetic Diversity, Functional Diversity and Related Similarity/Differentiation Measures Through Hill Numbers. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 1–28. [Google Scholar] [CrossRef]
- Chao, A.; Jost, L. Estimating Diversity and Entropy Profiles via Discovery Rates of New Species. Methods Ecol. Evol. 2015, 6, 873–882. [Google Scholar] [CrossRef]
- Roswell, M.; Dushoff, J.; Winfree, R. A Conceptual Guide to Measuring Species Diversity. Oikos 2021, 130, 321–338. [Google Scholar] [CrossRef]
- Myers, N. Biodiversity hotspots revisited. BioScience 2003, 53, 916–917. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.R. Conservation Hotspots of Biodiversity and Endemism for Indo-Pacific Coral Reef Fishes. Aquat. Conserv. Mar. Freshw. Ecosyst. 2007, 18, 541–556. [Google Scholar] [CrossRef]
- Brooks, T.M.; Mittermeier, R.A.; Da Fonseca, G.A.B.; Gerlach, J.; Hoffmann, M.; Lamoreux, J.F.; Mittermeier, C.G.; Pilgrim, J.D.; Rodrigues, A.S.L. Global Biodiversity Conservation Priorities. Science 2006, 313, 58–61. [Google Scholar] [CrossRef]
- Trebilco, R.; Halpern, B.S.; Flemming, J.M.; Field, C.; Blanchard, W.; Worm, B. Mapping Species Richness and Human Impact Drivers to Inform Global Pelagic Conservation Prioritisation. Biol. Conserv. 2011, 144, 1758–1766. [Google Scholar] [CrossRef]
- Marchese, C. Biodiversity Hotspots: A Shortcut for a More Complicated Concept. Glob. Ecol. Conserv. 2015, 3, 297–309. [Google Scholar] [CrossRef]
- Orme, C.D.L.; Davies, R.G.; Burgess, M.; Eigenbrod, F.; Pickup, N.; Olson, V.A.; Webster, A.J.; Ding, T.-S.; Rasmussen, P.C.; Ridgely, R.S.; et al. Global Hotspots of Species Richness Are Not Congruent with Endemism or Threat. Nature 2005, 436, 1016–1019. [Google Scholar] [CrossRef]
- Roberts, C.M.; McClean, C.J.; Veron, J.E.N.; Hawkins, J.P.; Allen, G.R.; McAllister, D.E.; Mittermeier, C.G.; Schueler, F.W.; Spalding, M.; Wells, F.; et al. Marine Biodiversity Hotspots and Conservation Priorities for Tropical Reefs. Science 2002, 295, 1280–1284. [Google Scholar] [CrossRef] [PubMed]
- Arita, H.T.; Christen, J.A.; Rodríguez, P.; Soberón, J. Species Diversity and Distribution in Presence-Absence Matrices: Mathematical Relationships and Biological Implications. Am. Nat. 2008, 172, 519–532. [Google Scholar] [CrossRef]
- Arita, H.T.; Christen, A.; Rodríguez, P.; Soberón, J. The Presence–Absence Matrix Reloaded: The Use and Interpretation of Range–Diversity Plots. Glob. Ecol. Biogeogr. 2012, 21, 282–292. [Google Scholar] [CrossRef]
- Mendoza, A.M.; Arita, H.T. Priority Setting by Sites and by Species Using Rarity, Richness and Phylogenetic Diversity: The Case of Neotropical Glassfrogs (Anura: Centrolenidae). Biodivers. Conserv. 2014, 23, 909–926. [Google Scholar] [CrossRef]
- Cú-Vizcarra, J.D.; Villalobos, F.; Sosa, V.J.; Bolívar-Cimé, B. The Agony of Choice: Species Richness and Range Size in the Determination of Hotspots for the Conservation of Phyllostomid Bats. Perspect. Ecol. Conserv. 2022, 20, 360–368. [Google Scholar] [CrossRef]
- Borregaard, M.K.; Rahbek, C. Dispersion Fields, Diversity Fields and Null Models: Uniting Range Sizes and Species Richness. Ecography 2010, 33, 402–407. [Google Scholar] [CrossRef]
- Borregaard, M.K.; Graves, G.R.; Rahbek, C. Dispersion Fields Reveal the Compositional Structure of South American Vertebrate Assemblages. Nat. Commun. 2020, 11, 491. [Google Scholar] [CrossRef]
- Graves, G.R.; Rahbek, C. Source Pool Geometry and the Assembly of Continental Avifaunas. Proc. Natl. Acad. Sci. USA 2005, 102, 7871–7876. [Google Scholar] [CrossRef]
- Soberón, J.; Cobos, M.; Nuñez-Penichet, C. Visualizing Species Richness and Site Similarity from Presence-Absence Matrices. Biodivers. Inform. 2021, 16, 20–27. [Google Scholar] [CrossRef]
- García-Sanz, I.; Heine-Fuster, I.; Luque, J.A.; Pizarro, H.; Castillo, R.; Pailahual, M.; Prieto, M.; Pérez-Portilla, P.; Aránguiz-Acuña, A. Limnological Response from High-Altitude Wetlands to the Water Supply in the Andean Altiplano. Sci. Rep. 2021, 11, 7681. [Google Scholar] [CrossRef]
- Jacobsen, D.; Dangles, O. Ecology of High Altitude Waters; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Meixner, A.; Alonso, R.N.; Lucassen, F.; Korte, L.; Kasemann, S.A. Lithium and Sr Isotopic Composition of Salar Deposits in the Central Andes across Space and Time: The Salar de Pozuelos, Argentina. Miner. Depos. 2022, 57, 255–278. [Google Scholar] [CrossRef]
- Chávez, R.O.; Christie, D.A.; Olea, M.; Anderson, T.G. A Multiscale Productivity Assessment of High Andean Peatlands across the Chilean Altiplano Using 31 Years of Landsat Imagery. Remote Sens. 2019, 11, 2955. [Google Scholar] [CrossRef]
- Chávez, R.O.; Meseguer-Ruiz, O.; Olea, M.; Calderón-Seguel, M.; Yager, K.; Meneses, R.I.; Lastra, J.A.; Núñez-Hidalgo, I.; Sarricolea, P.; Serrano-Notivoli, R.; et al. Andean Peatlands at Risk? Spatiotemporal Patterns of Extreme NDVI Anomalies, Water Extraction and Drought Severity in a Large-Scale Mining Area of Atacama, Northern Chile. Int. J. Appl. Earth Obs. Geoinf. 2023, 116, 103138. [Google Scholar] [CrossRef]
- NOAA National Centers for Environmental Information. ETOPO 2022 15 Arc-Second Global Relief Model; NOAA National Centers for Environmental Information: Asheville, NC, USA, 2022. [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, D.; Yu, L.; Wang, X.; Chen, Y.; Bai, Y.; Hernández, H.J.; Galleguillos, M.; Estades, C.; Biging, G.S.; et al. Detailed Dynamic Land Cover Mapping of Chile: Accuracy Improvement by Integrating Multi-Temporal Data. Remote Sens. Environ. 2016, 183, 170–185. [Google Scholar] [CrossRef]
- Tapia, G.; Atán, J.S.A. Análisis Crítico de la Definición de Cuencas del Banco Nacional de Aguas. 2013. Available online: https://bibliotecadigital.ciren.cl/server/api/core/bitstreams/f7867b71-d4ba-43b0-b7af-66ab6a2b1b81/content (accessed on 18 July 2025).
- Leiva-Zelada, G.; Zelada-Muñoz, S. Gestión integrada de cuencas hidrográficas en Chile: Brechas y oportunidades en la propuesta constitucional. Sustain. Agri Food Environ. Res.-Discontin. 2024, 12, 1–13. [Google Scholar] [CrossRef]
- Budds, J. Gobernanza del agua y desarrollo bajo el mercado: Las relaciones sociales de control del agua en el marco del Código de Aguas de Chile. Investig. Geográficas Una Mirada Desde El Sur 2020, 59, 16–27. [Google Scholar] [CrossRef]
- Gregorio, A.D. Land Cover Classification System: Classification Concepts and User Manual; LCCS; Food & Agriculture Organization: Rome, Italy, 2005. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D.; van den Brand, T.; et al. Ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics. 2025. Available online: https://cran.r-project.org/web/packages/ggplot2/index.html (accessed on 10 October 2023).
- GBIF.Org User Plantae Occurrence Download, Chile. 2023. Available online: https://doi.org/10.15468/dl.gbxkxy (accessed on 10 October 2023).
- GBIF.Org User Animalia Occurrence Download, Chile. 2023. Available online: https://doi.org/10.15468/dl.kq25mt (accessed on 10 October 2023).
- Kelling, S.; Fink, D.; Sorte, F.A.L.; Johnston, A.; Bruns, N.E.; Hochachka, W.M. Taking a ‘Big Data’ Approach to Data Quality in a Citizen Science Project. Ambio 2015, 44, 601–611. [Google Scholar] [CrossRef]
- Sullivan, B.L.; Aycrigg, J.L.; Barry, J.H.; Bonney, R.E.; Bruns, N.; Cooper, C.B.; Damoulas, T.; Dhondt, A.A.; Dietterich, T.; Farnsworth, A.; et al. The eBird Enterprise: An Integrated Approach to Development and Application of Citizen Science. Biol. Conserv. 2014, 169, 31–40. [Google Scholar] [CrossRef]
- Rodríguez-Luna, D.; Vela, N.; Alcalá, F.J.; Encina-Montoya, F. The Environmental Impact Assessment in Chile: Overview, Improvements, and Comparisons. Environ. Impact Assess. Rev. 2021, 86, 106502. [Google Scholar] [CrossRef]
- Grenié, M.; Berti, E.; Carvajal-Quintero, J.; Dädlow, G.M.L.; Sagouis, A.; Winter, M. Harmonizing Taxon Names in Biodiversity Data: A Review of Tools, Databases and Best Practices. Methods Ecol. Evol. 2023, 14, 12–25. [Google Scholar] [CrossRef]
- Govaerts, R. WCVP: World Checklist of Vascular Plants; Royal Botanic Gardens, Kew: Richmond, UK, 2024. [Google Scholar]
- Rodriguez, R.; Marticorena, C.; Alarcón, D.; Baeza, C.; Cavieres, L.; Finot, V.L.; Fuentes, N.; Kiessling, A.; Mihoc, M.; Pauchard, A.; et al. Catálogo de las plantas vasculares de Chile. Gayana. Botánica 2018, 75, 1–430. [Google Scholar] [CrossRef]
- D’Elía, G.; Canto, J.; Ossa, G.; Verde-Arregoitia, L.D.; Bostelmann, E.; Iriarte, A.; Amador, L.; Quiroga-Carmona, M.; Hurtado, N.; Cadenillas, R.; et al. Lista actualizada de los mamíferos vivientes de Chile. Boletín Mus. Nac. Hist. Nat. 2020, 69, 67–98. [Google Scholar] [CrossRef]
- Brown, J.H.; Stevens, G.C.; Kaufman, D.M. The Geographic Range: Size, Shape, Boundaries, and Internal Structure. Annu. Rev. Ecol. Syst. 1996, 27, 597–623. [Google Scholar] [CrossRef]
- Diniz-Filho, J.A.F. Structure and Dynamics of Geographic Ranges. In The Macroecological Perspective: Theories, Models and Methods; Springer: Berlin/Heidelberg, Germany, 2023; pp. 125–166. [Google Scholar]
- Gaston, K.J. The Structure and Dynamics of Geographic Ranges; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Gotelli, N.J. Null model analysis of species co-occurrence patterns. Ecology 2000, 81, 2606–2621. [Google Scholar] [CrossRef]
- Nuñez-Penichet, C.; Cobos, M.E.; Soberón, J.; Gueta, T.; Barve, N.; Barve, V.; Navarro-Sigüenza, A.G.; Peterson, A.T. Selection of Sampling Sites for Biodiversity Inventory: Effects of Environmental and Geographical Considerations. Methods Ecol. Evol. 2022, 13, 1595–1607. [Google Scholar] [CrossRef]
- Poveda Bonilla, R. Ingresos Fiscales por Litio en Chile; Natural Resource Governance Institute (NRGI): Lima, Peru, 2024. [Google Scholar]
- Brunson, J.C. Ggalluvial: Layered Grammar for Alluvial Plots. J. Open Source Softw. 2020, 5, 2017. [Google Scholar] [CrossRef] [PubMed]
- Brunson, J.C.; Read, Q. ggalluvial ggplot2 Extension. 2023. Available online: https://corybrunson.github.io/ggalluvial/ (accessed on 10 October 2023).
- Coca-Salazar, A.; Villca, H.; Torrico, M.; Alfaro, F.D. Plant Communities on the Islands of Two Altiplanic Salt Lakes in the Andean Region of Bolivia. Check List. 2016, 12, 1975. [Google Scholar] [CrossRef]
- La Sorte, F.A.; Fink, D.; Hochachka, W.M.; Kelling, S. Convergence of Broad-Scale Migration Strategies in Terrestrial Birds. Proc. R. Soc. B. 2016, 283, 20152588. [Google Scholar] [CrossRef]
- Moraga Sariego, P.; Ossandón Rosales, J.; Chahuán, F.; Sameshima, S. Memoria ambiental. La historia de la institucionalidad ambiental, a 50 años del golpe militar. Rev. Derecho Ambient. 2023, 20, 1–29. [Google Scholar] [CrossRef]
- Liu, W.; Agusdinata, D.B.; Myint, S.W. Spatiotemporal Patterns of Lithium Mining and Environmental Degradation in the Atacama Salt Flat, Chile. Int. J. Appl. Earth Obs. Geoinf. 2019, 80, 145–156. [Google Scholar] [CrossRef]
- Roy, B.A.; Zorrilla, M.; Endara, L.; Thomas, D.C.; Vandegrift, R.; Rubenstein, J.M.; Read, M. New Mining Concessions Could Severely Decrease Biodiversity and Ecosystem Services in Ecuador. Trop. Conserv. Sci. 2018, 11, 1940082918780427. [Google Scholar] [CrossRef]
- Seki, H.A.; Thorn, J.P.; Platts, P.J.; Shirima, D.D.; Marchant, R.A.; Abeid, Y.; Marshall, A.R. Indirect Impacts of Commercial Gold Mining on Adjacent Ecosystems. Biol. Conserv. 2022, 275, 109782. [Google Scholar] [CrossRef]
- Saenz-Agudelo, P.; Delrieu-Trottin, E.; DiBattista, J.D.; Martínez-Rincon, D.; Morales-González, S.; Pontigo, F.; Ramírez, P.; Silva, A.; Soto, M.; Correa, C. Monitoring Vertebrate Biodiversity of a Protected Coastal Wetland Using eDNA Metabarcoding. Environ. DNA 2022, 4, 77–92. [Google Scholar] [CrossRef]
- Wood, C.M.; Champion, J.; Brown, C.; Brommelsiek, W.; Laredo, I.; Rogers, R.; Chaopricha, P. Challenges and Opportunities for Bioacoustics in the Study of Rare Species in Remote Environments. Conserv. Sci. Pract. 2023, 5, e12941. [Google Scholar] [CrossRef]
- Mas-Carrió, E.; Schneider, J.; Nasanbat, B.; Ravchig, S.; Buxton, M.; Nyamukondiwa, C.; Stoffel, C.; Augugliaro, C.; Ceacero, F.; Taberlet, P.; et al. Assessing Environmental DNA Metabarcoding and Camera Trap Surveys as Complementary Tools for Biomonitoring of Remote Desert Water Bodies. Environ. DNA 2022, 4, 580–595. [Google Scholar] [CrossRef]
- Ritz, S.J.; Delgado, N.A. Discursive Strategies within Sustainability Trade-Offs: A Case on the Controversy over Transition Minerals. J. Clean. Prod. 2024, 469, 143196. [Google Scholar] [CrossRef]
| Variables | Source [Ref] | Layer Resolution/Scale | Access Link |
|---|---|---|---|
| Elevation (DEM) | ETOPO 2022 Global Relief Model) [81]. | 30 arc-s raster (~1 km) | https://www.ncei.noaa.gov/products/etopo-global-relief-model (accessed on 7 August 2025) |
| Mean annual temperature climatology | WorldClim v2.1 [82] | 30 arc-s raster (~1 km) | https://www.worldclim.org/data/worldclim21.html (accessed on 23 February 2025) |
| Mean annual precipitation climatology | |||
| Mean annual temperature weather station data | Dirección Meteorológica de Chile (DMC) | Point shapefile | https://climatologia.meteochile.gob.cl (accessed on 23 February 2025) |
| Mean annual precipitation weather station data | |||
| Hydrographic units (SSB boundaries) | Dirección General de Aguas (DGA), National Hydrographic Database | Polygon shapefile | https://dga.mop.gob.cl/uploads/sites/13/2024/07/Cuencas_BNA.zip (accessed on 23 February 2025) |
| Saline system area | Ministry of Mining, technical reports | Polygon shapefile | https://www.minmineria.cl (accessed on 23 February 2025) |
| Land cover | National Land Cover Dataset [83] | 30 m Raster (Landsat-based) | https://www.gep.uchile.cl/Landcover_CHILE.html (accessed on 23 February 2025) |
| ID | Salar | Commune (Region) | SSB Area (km2) | Saline System Surface (km2) | Elevation (masl) | Prec (mm/yr) | Temp (°C) |
|---|---|---|---|---|---|---|---|
| 1 | Salar de Surire | Putre (XV) | 562.30 | 130.18 | 4260 | 81.5 | 1.4 |
| 2 | Salar de Pisiga | Colchane (I) | 179.11 | 98.58 | 3657 | 187.0 | 7.5 |
| 3 | Salar del Huasco | Pica (I) | 600.45 | 52.28 | 3778 | 98.1 | 4.5 |
| 4 | Salar de Coposa | Pica (I) | 1111.03 | 86.85 | 3730 | 79.3 | 4.7 |
| 5 | Salar de Michincha | Pica (I) | 275.67 | 2.91 | 4125 | 71.7 | 3.6 |
| 6 | Salar de Ollague | Ollague(II) | 744.73 | 21.78 | 3660 | 69.2 | 3.7 |
| 7 | Salar de San Martín o Carcote | Ollague(II) | 524.43 | 110.54 | 3690 | 58.6 | 5.0 |
| 8 | Salar de Ascotan | Ollague(II) | 1425.77 | 242.47 | 3716 | 50.8 | 4.8 |
| 9 | Salar de Turi | Calama (II) | 2287.99 | 3030 | 37.5 | 7.7 | |
| 10 | Salar de Tara | San Pedro de Atacama (II) | 1506.86 | 61.54 | 4400 | 65.3 | 4.1 |
| 11 | Aguas Calientes I (Norte) | San Pedro de Atacama (II) | 908.76 | 15.70 | 4228 | 59.1 | 4.4 |
| 12 | Salar de Pujsa | San Pedro de Atacama (II) | 275.67 | 16.69 | 4500 | 56.1 | 2.6 |
| 13 | Salar de Loyoques o Quisquiro | San Pedro de Atacama (II) | 908.76 | 80.64 | 4150 | 59.1 | 4.4 |
| 14 | Salar de Atacama | San Pedro de Atacama (II) | 12,409.54 | 3417.6 | 2305 | 35.9 | 10.8 |
| 15 | Salar de Aguas Calientes II (Centro) | San Pedro de Atacama (II) | 1252.56 | 129.67 | 4280 | 55.3 | 3.8 |
| 16 | Salar de los Morros | Antofagasta (II) | 1158.87 | 59.52 | 2210 | 16.7 | 12.8 |
| 17 | Salar El Laco | San Pedro de Atacama (II) | 520.07 | 16.11 | 4240 | 59.0 | 4.1 |
| 18 | Salar Talar (Aguas Calientes III—Sur) | San Pedro de Atacama (II) | 671.58 | 45.04 | 3950 | 46.6 | 4.9 |
| 19 | Salar de Incahuasi | San Pedro de Atacama (II) | 495.42 | 19.69 | 3400 | 52.6 | 5.5 |
| 20 | Salar de Pular | San Pedro de Atacama (II) | 483.92 | 17.12 | 3560 | 44.8 | 4.1 |
| 21 | Salar de Punta Negra | Antofagasta (II) | 5230.59 | 242.5 | 2945 | 18.8 | 8.3 |
| 22 | Salar de Aguas Calientes IV (Sur Sur) | Antofagasta (II) | 1059.80 | 19.22 | 3675 | 16.7 | 4.6 |
| 23 | Salar de Pajonales | Antofagasta (II) | 1920.92 | 103.1 | 3537 | 23.9 | 6.7 |
| 24 | Salar de Gorbea | Diego de Almagro (III) | 352.91 | 29.08 | 3950 | 35.8 | 4.2 |
| 25 | Salar Agua Amarga | Diego de Almagro (III) | 855.03 | 23.21 | 3568 | 25.7 | 6.2 |
| 26 | Salar de Las Parinas | Diego de Almagro (III) | 385.27 | 41.29 | 3966 | 44.4 | 4.4 |
| 27 | Salar de La Isla | Diego de Almagro (III) | 871.59 | 158.33 | 3960 | 38.4 | 4.6 |
| 28 | Salar de los Infieles | Diego de Almagro (III) | 252.22 | 6.8 | 3520 | 32.0 | 5.8 |
| 29 | Salar Grande | Diego de Almagro (III) | 832.41 | 31.98 | 3950 | 44.6 | 4.0 |
| 30 | Salar de Pedernales | Diego de Almagro (III) | 2099.10 | 327.71 | 3370 | 32.7 | 7.0 |
| 31 | Salar de Piedra Parada | Diego de Almagro (III) | 656.90 | 28.52 | 4133 | 57.9 | 2.7 |
| 32 | Salar de Maricunga | Copiapó (III) | 2071.23 | 144.03 | 3760 | 62.8 | 4.0 |
| 33 | Laguna del Negro Francisco | Tierra Amarilla (III) | 636.43 | 25.41 | 4110 | 80.1 | 2.0 |
| Kingdom | Phylum | Class | Native | Endemic | Errant | Exotic | Total |
|---|---|---|---|---|---|---|---|
| Animalia | Chordata | Amphibia | 4 | 3 | — | — | 7 |
| Animalia | Chordata | Aves | 198 | 2 | 5 | 4 | 209 |
| Animalia | Chordata | Mammalia | 39 | 1 | — | 1 | 41 |
| Animalia | Chordata | Reptilia | 29 | 3 | — | — | 32 |
| Total Animal Kingdom | 270 | 9 | 5 | 5 | 289 | ||
| Plantae | Bryophyta | Bryopsida | 7 | — | — | — | 7 |
| Plantae | Tracheophyta | Equisetopsida | 237 | 15 | — | 15 | 267 |
| Plantae | Tracheophyta | Gnetopsida | 4 | — | — | 1 | 5 |
| Plantae | Tracheophyta | Liliopsida | 71 | — | — | 6 | 77 |
| Plantae | Tracheophyta | Magnoliopsida | 94 | 9 | — | 2 | 105 |
| Total Plantae Kingdom | 413 | 24 | 0 | 24 | 461 | ||
| Total | 683 | 33 | 5 | 29 | 750 | ||
| Salar | Plantae | Animalia | ||||||
|---|---|---|---|---|---|---|---|---|
| Coldspots | Hotspots | Coldspots | Hotspots | |||||
| Common | Rare | Common | Rare | Common | Rare | Common | Rare | |
| Salar de Surire | 1 | 1 | ||||||
| Salar de Tara | 1 | 1 | ||||||
| Aguas Calientes I (Norte) | 1 | |||||||
| Salar de Pujsa | 1 | 1 | ||||||
| Salar de Loyoques o Quisquiro | 1 | 1 | ||||||
| Salar de Atacama | 1 | 1 | ||||||
| Salar de Aguas Calientes II (Centro) | 1 | 1 | ||||||
| Salar de los Morros | 1 | 1 | ||||||
| Salar El Laco | 1 | 1 | ||||||
| Salar Talar (Aguas Calientes III—Sur) | 1 | 1 | ||||||
| Salar de Pisiga | 1 | |||||||
| Salar de Incahuasi | 1 | 1 | ||||||
| Salar de Pular | 1 | |||||||
| Salar de Punta Negra | 1 | 1 | ||||||
| Salar de Aguas Calientes IV (Sur Sur) | 1 | 1 | ||||||
| Salar de Pajonales | 1 | 1 | ||||||
| Salar de Gorbea | 1 | 1 | ||||||
| Salar Agua Amarga | 1 | 1 | ||||||
| Salar de Las Parinas | 1 | |||||||
| Salar de La Isla | 1 | 1 | ||||||
| Salar del Huasco | 1 | 1 | ||||||
| Salar de los Infieles | 1 | 1 | ||||||
| Salar Grande | 1 | 1 | ||||||
| Salar de Pedernales | 1 | 1 | ||||||
| Salar de Piedra Parada | 1 | |||||||
| Salar de Maricunga | 1 | 1 | ||||||
| Laguna del Negro Francisco | 1 | 1 | ||||||
| Salar de Coposa | 1 | 1 | ||||||
| Salar de Michincha | 1 | 1 | ||||||
| Salar de Ollague | 1 | 1 | ||||||
| Salar de San Martín o Carcote | 1 | 1 | ||||||
| Salar de Ascotan | 1 | 1 | ||||||
| Salar de Turi | 1 | 1 | ||||||
| Total | 19 | 3 | 5 | 3 | 17 | 6 | 8 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Rojas, M.; Estévez, R.A.; Romero, C.; Pérez, S.; Labra, F.A. Integrating Species Richness, Distribution and Human Pressures to Assess Conservation Priorities in High Andean Salares. Sustainability 2025, 17, 8139. https://doi.org/10.3390/su17188139
Hernández-Rojas M, Estévez RA, Romero C, Pérez S, Labra FA. Integrating Species Richness, Distribution and Human Pressures to Assess Conservation Priorities in High Andean Salares. Sustainability. 2025; 17(18):8139. https://doi.org/10.3390/su17188139
Chicago/Turabian StyleHernández-Rojas, Marcelo, Rodrigo A. Estévez, Cristian Romero, Sebastián Pérez, and Fabio A. Labra. 2025. "Integrating Species Richness, Distribution and Human Pressures to Assess Conservation Priorities in High Andean Salares" Sustainability 17, no. 18: 8139. https://doi.org/10.3390/su17188139
APA StyleHernández-Rojas, M., Estévez, R. A., Romero, C., Pérez, S., & Labra, F. A. (2025). Integrating Species Richness, Distribution and Human Pressures to Assess Conservation Priorities in High Andean Salares. Sustainability, 17(18), 8139. https://doi.org/10.3390/su17188139







