Fatty Acids in Lumbricidae as Biomarkers of In Situ Metals Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Determination of Selected Metals in Waste Rock, Soil, Plant Roots and Lumbricidae
2.2.1. Analysis by Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
2.2.2. Analysis by Flame Atomic Absorption Spectrometry (FAAS)
2.3. Lipid Extraction and Recovery of Fatty Acids from Lumbricidae
2.4. Peroxidation and Unsaturation Indices
2.5. Analysis of Enzymatic Biomarkers of Oxidative Stress
2.6. Analysis of Non-Enzymatic Biomarkers of Oxidative Stress—TBARS (Thiobarbituric Acid Reactive Substances)
2.7. Statistical Analyses
3. Results
3.1. Metals: Waste Rock—Soil—Plant Roots—Earthworms
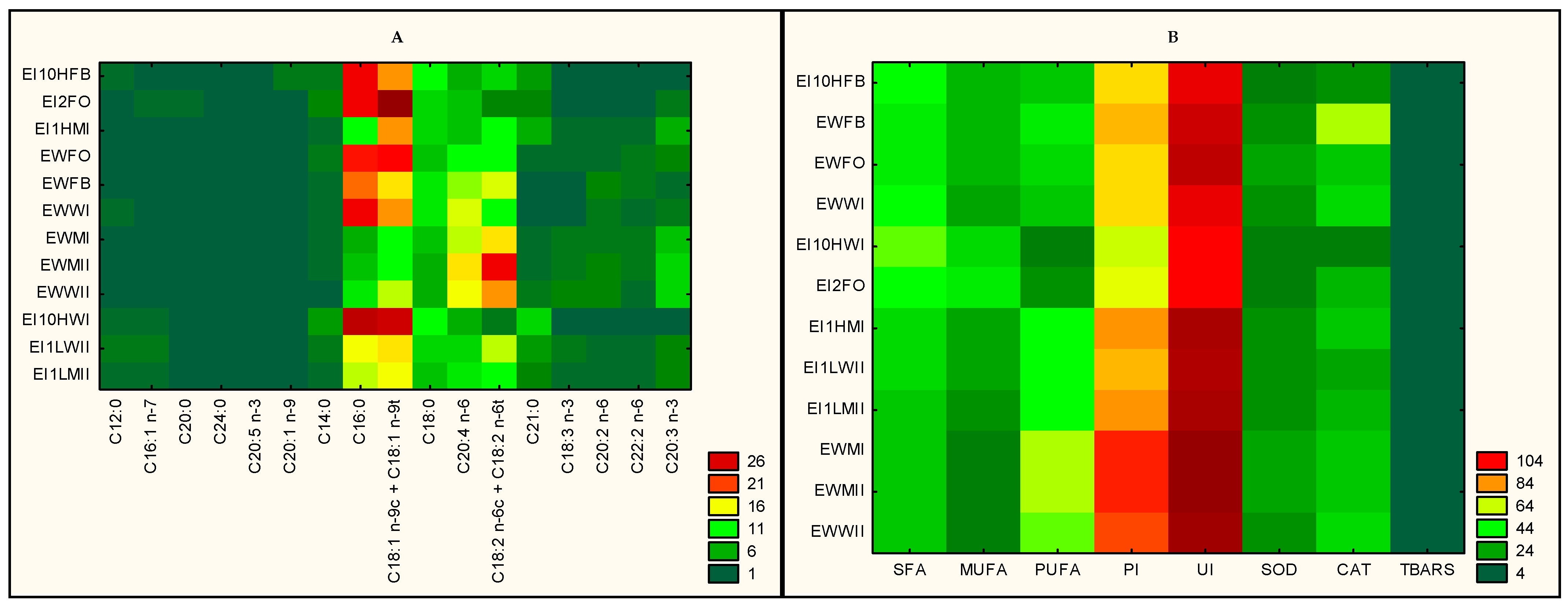
3.2. Biomarker FAs, Peroxidation (PI) and Unsaturation (UI) Indices, Enzymatic (SOD, CAT) and Non-Enzymatic (TBARS) Biomarkers of Oxidative Stress
3.3. Pearson’s Correlation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, Z.; Tang, Z.; Wang, C. A Brief Review and Evaluation of Earthworm Biomarkers in Soil Pollution Assessment. Environ. Sci. Pollut. Res. 2017, 24, 13284–13294. [Google Scholar] [CrossRef]
- Edwards, C.A. Earthworm Ecology; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Bartlett, M.D.; Briones, M.J.; Neilson, R.; Schmidt, O.; Spurgeon, D.; Creamer, R.E. A Critical Review of Current Methods in Earthworm Ecology: From Individuals to Populations. Eur. J. Soil Biol. 2010, 46, 67–73. [Google Scholar] [CrossRef]
- Capowiez, Y.; Lévèque, T.; Pelosi, C.; Capowiez, L.; Mazzia, C.; Schreck, E.; Dumat, C. Using the Ecosystem Engineer Concept to Test the Functional Effects of a Decrease in Earthworm Abundance Due to an Historic Metal Pollution Gradient. Appl. Soil Ecol. 2021, 158, 103816. [Google Scholar] [CrossRef]
- Sivakumar, S. Effects of Metals on Earthworm Life Cycles: A Review. Environ. Monit. Assess. 2015, 187, 530. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Kumar, R.; Gupta, R.K.; Kaur, T.; Kour, A.; Kaur, S.; Rajput, A. Heavy Metal Toxicity in Earthworms and Its Environmental Implications: A Review. Environ. Adv. 2023, 12, 100374. [Google Scholar] [CrossRef]
- Luo, W.; Verweij, R.A.; van Gestel, C.A. Determining the Bioavailability and Toxicity of Lead Contamination to Earthworms Requires Using a Combination of Physicochemical and Biological Methods. Environ. Pollut. 2014, 185, 1–9. [Google Scholar] [CrossRef]
- Jager, T. Mechanistic Approach for Estimating Bioconcentration of Organic Chemicals in Earthworms (Oligochaeta). Environ. Toxicol. Chem. 1998, 17, 2080–2090. [Google Scholar] [CrossRef]
- Barras, A.G.; Candolfi, I.; Arlettaz, R. Spatio-Temporal Patterns of Earthworm Abundance Suggest Time-Limited Food Availability for a Subalpine Bird Species. Pedobiologia 2022, 93–94, 150826. [Google Scholar] [CrossRef]
- Rhymer, C.M.; Devereux, C.L.; Denny, M.J.; Whittingham, M.J. Diet of Starling Sturnus vulgaris Nestlings on Farmland: The Importance of Tipulidae Larvae. Bird Study 2012, 59, 426–436. [Google Scholar] [CrossRef]
- Orłowski, G.; Książkiewicz-Parulska, Z.; Karg, J.; Bocheński, M.; Jerzak, L.; Zub, K. Using Soil from Pellets of White Storks Ciconia ciconia to Assess the Number of Earthworms (Lumbricidae) Consumed as Primary and Secondary Prey. Ibis 2016, 158, 587–897. [Google Scholar] [CrossRef]
- Navedo, J.G.; Gutiérrez, J.S.; Salmón, P.; Arranz, D.; Novo, M.; Díaz-Cosín, D.J.; Herrera, A.G.; Masero, J.A. Food Supply, Prey Selection and Estimated Consumption of Wintering Eurasian Curlews Feeding on Earthworms at Coastal Pastures. Ardea 2020, 107, 263–274. [Google Scholar] [CrossRef]
- Basedow, T. Changes in Agriculture in an Area in Northern Germany between the Years 1971 and 2000, and the Reactions of Populations of Predatory Carabids (Col., Carabidae), of Other Predators, and of Cereal Aphids, to These Changes. J. Plant Dis. Prot. 2002, 109, 1–14. [Google Scholar]
- Holland, J.M.; Hutchison, M.A.S.; Smith, B.; Aebischer, N.J. A Review of Invertebrates and Seed-bearing Plants as Food for Farmland Birds in Europe. Ann. Appl. Biol. 2006, 148, 49–71. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Zheng, D.M.; Wang, Q.C.; Lv, X.G. Bioaccumulation of Total and Methyl Mercury in Three Earthworm species (Drawida sp., Allolobophora sp., and Limnodrilus sp.). Bull. Environ. Contam. Toxicol. 2009, 83, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, B.; Georgescu, C.; Daraban, S. Use of Lumbricides Species as Biological Indicators of Environmental Pollution with Metalodisrupters. Lucr. Ştiinţifice 2011, 55, 63–67. [Google Scholar]
- Wang, X.; Cairang, S.; Du, J.; Wei, Z.; Wu, Q.; Hu, L.; Xu, M. A Large-Scale Assessment of Soil Heavy Metal Pollution Using Field-Collected Earthworms as Bio-Indicators in Shaoguan, South China. Environ. Heal. 2025, 3, 616–625. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 207: Earthworm, Acute Toxicity Tests. In OECD Guidelines for the Testing of Chemicals; OECD Publishing: Paris, France, 1984. [Google Scholar]
- OECD. Test No. 222: Earthworm Reproduction Test (Eisenia fetida/Eisenia andrei). In OECD Guidelines for the Testing of Chemicals; OECD Publishing: Paris, France, 2016. [Google Scholar]
- Velki, M.; Ečimović, S. Important Issues in Ecotoxicological Investigations Using Earthworms. Environ. Contam. Toxicol. 2016, 239, 157–184. [Google Scholar] [CrossRef]
- Van Gestel, C.A.; Koolhaas, J.E.; Hamers, T.; van Hoppe, M.; van Roovert, M.; Korsman, C.; Reinecke, S.A. Effects of Metal Pollution on Earthworm Communities in a Contaminated Floodplain Area: Linking Biomarker, Community and Functional Responses. Environ. Pollut. 2009, 157, 895–903. [Google Scholar] [CrossRef]
- Hobbelen, P.H.F.; Koolhaas, J.E.; van Gestel, C.A.M. Bioaccumulation of Heavy Metals in the Earthworms Lumbricus rubellus and Aporrectodea caliginosa in Relation to Total and Available Metal Concentrations in Field Soils. Environ. Pollut. 2006, 144, 639–646. [Google Scholar] [CrossRef]
- Ardestani, M.M.; van Straalen, N.M.; van Gestel, C.A. Uptake and Elimination Kinetics of Metals in Soil Invertebrates: A Review. Environ. Pollut. 2014, 193, 277–295. [Google Scholar] [CrossRef]
- Spurgeon, D.J.; Hopkin, S.P. Comparisons of Metal Accumulation and Excretion Kinetics in Earthworms (Eisenia fetida) Exposed to Contaminated Field and Laboratory Soils. Appl. Soil Ecol. 1999, 11, 227–243. [Google Scholar] [CrossRef]
- Sizmur, T.; Hodson, M.E. Do Earthworms Impact Metal Mobility and Availability in Soil?–A Review. Environ. Pollut. 2009, 157, 1981–1989. [Google Scholar] [CrossRef]
- Lev, S.M.; Matthies, N.; Snodgrass, J.W.; Casey, R.E.; Ownby, D.R. Effects of Zinc Exposure on Earthworms, Lumbricus terrestris, in an Artificial Soil. Bull. Environ. Contam. Toxicol. 2010, 84, 687–691. [Google Scholar] [CrossRef]
- Liu, X.; Hu, C.; Zhang, S. Effects of Earthworm Activity on Fertility and Heavy Metal Bioavailability in Sewage Sludge. Environ. Int. 2005, 31, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Yuvaraj, A.; Govarthanan, M.; Karmegam, N.; Biruntha, M.; Kumar, D.S.; Arthanari, M.; Govindarajan, R.K.; Tripathi, S.; Ghosh, S.; Kumar, P.; et al. Metallothionein Dependent-Detoxification of Heavy Metals in the Agricultural Field Soil of Industrial Area: Earthworm as Field Experimental Model System. Chemosphere 2021, 267, 129240. [Google Scholar] [CrossRef] [PubMed]
- Tirado-Ballestas, I.P.; Taylor, M.K.; Caballero-Gallardo, K.; Olivero-Verbel, J.; Callaham, M.A., Jr. Effects of Bituminous Coal Dust Exposure on Reproduction of Sinella curviseta (Collembola) and Eisenia fetida (Clitellata). Appl. Soil Ecol. 2025, 209, 106038. [Google Scholar] [CrossRef]
- Römbke, J.; Dorow, W.H.; Jänsch, S. Distribution and Diversity of Earthworms (Lumbricidae) in Hesse (Central Germany): Current Knowledge. Soil Org. 2018, 90, 171–185. [Google Scholar] [CrossRef]
- Garbacz, A.; Nowak, A.; Marzec-Grządziel, A.; Przybyś, M.; Gałązka, A.; Jaroszuk-Ściseł, J.; Grzywaczewski, G. Impact of Coal Waste Rock on Biological and Physicochemical Properties of Soils with Different Agricultural Uses. Sustainability 2025, 17, 2603. [Google Scholar] [CrossRef]
- Joniec, J.; Kwiatkowska, E.; Walkiewicz, A.; Grzywaczewski, G. Changes in Microbial Activity Associated with the Nitrogen Biogeochemical Cycle in Differently Managed Soils, Including Protected Areas and Those Reclaimed with Gangue. Sustainability 2025, 17, 4343. [Google Scholar] [CrossRef]
- Ghosh, S. Environmental Pollutants, Pathogens and Immune System in Earthworms. Environ. Sci. Pollut. Res. 2018, 25, 6196–6208. [Google Scholar] [CrossRef]
- Edwards, C.A.; Arancon, N.Q. The Influence of Environmental Factors on Earthworms. In Biology and Ecology of Earthworms; Springer: New York, NY, USA, 2022; pp. 191–232. [Google Scholar]
- Uwizeyimana, H.; Wang, M.; Chen, W.; Khan, K. The Eco-Toxic Effects of Pesticide and Heavy Metal Mixtures towards Earthworms in Soil. Environ. Toxicol. Pharmacol. 2017, 55, 20–29. [Google Scholar] [CrossRef]
- Musakhanian, J.; Rodier, J.D.; Dave, M. Oxidative Stability in Lipid Formulations: A Review of the Mechanisms, Drivers, and Inhibitors of Oxidation. AAPS PharmSciTech 2022, 23, 151. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Mathew, B.J.; Rai, R.; Chaurasiya, S.K. Phospholipase C in Bacterial Infections. In Phospholipases in Physiology and Pathology; Academic Press: Cambridge, MA, USA, 2023; pp. 217–234. [Google Scholar]
- Chakraborty, N.; Mitra, R.; Dasgupta, D.; Ganguly, R.; Acharya, K.; Minkina, T.; Popova, V.; Churyukina, E.; Keswani, C. Unraveling Lipid Peroxidation-Mediated Regulation of Redox Homeostasis for Sustaining Plant Health. Plant Physiol. Biochem. 2024, 206, 108272. [Google Scholar] [CrossRef] [PubMed]
- Sampedro, L.; Jeannotte, R.; Whalen, J.K. Trophic Transfer of Fatty Acids from Gut Microbiota to the Earthworm Lumbricus terrestris L. Soil. Biol. Biochem. 2006, 38, 2188–2198. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Shao, Y.; Xue, W.; Wang, N.; Xu, X.; Zhang, Z. Antioxidant Defense System Responses, Lysosomal Membrane Stability and DNA Damage in Earthworms (Eisenia fetida) Exposed to Perfluorooctanoic Acid: An Integrated Biomarker Approach to Evaluating Toxicity. RSC Adv. 2021, 11, 26481–26492. [Google Scholar] [CrossRef]
- Roubalová, R.; Procházková, P.; Dvořák, J.; Škanta, F.; Bilej, M. The Role of Earthworm Defense Mechanisms in Ecotoxicity Studies. Invertebr. Surviv. J. 2015, 12, 203–213. [Google Scholar]
- Chaitanya, R.K.; Shashank, K.; Sridevi, P. Oxidative Stress in Invertebrate Systems. In Free Radicals and Diseases; Ahmad, R., Ed.; IntechOpen: London, UK, 2016; Volume 19, pp. 51–68. [Google Scholar]
- Ahmad, S. Oxidative Stress and Antioxidant Defenses in Biology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- ISO 17034; General Requirements for the Competence of Reference Material Producers. ISO: Geneva, Switzerland, 2016.
- Hulbert, A.J.; Pamplona, R.; Buffenstein, R.; Buttemer, W.A. Life and Death: Metabolic Rate, Membrane Composition, and Life Span of Animals. Physiol. Rev. 2007, 87, 1175–1213. [Google Scholar] [CrossRef]
- Atli, G.; Grosell, M. Characterization and Response of Antioxidant Systems in the Tissues of the Freshwater Pond Snail (Lymnaea stagnalis) during Acute Copper Exposure. Aquat. Toxicol. 2016, 176, 38–44. [Google Scholar] [CrossRef]
- Radwan, M.A.; El-Gendy, K.S.; Gad, A.F. Biomarkers of Oxidative Stress in the Land Snail, Theba pisana for Assessing Ecotoxicological Effects of Urban Metal Pollution. Chemosphere 2010, 79, 40–46. [Google Scholar] [CrossRef]
- Radwan, M.A.; El-Gendy, K.S.; Gad, A.F. Oxidative Stress Biomarkers in the Digestive Gland of Theba pisana Exposed to Heavy Metals. Arch. Environ. Contam. Toxicol. 2010, 58, 828–835. [Google Scholar] [CrossRef]
- Matyja, M. Social Side of Agricultural Co-Operatives. The Case of Agricultural Production Co-Operatives in the Opole Voivodship. J. Agribus. Rural Dev. 2014, 33, 113–124. [Google Scholar]
- Benjamini, Y.; Drai, D.; Elmer, G.; Kafkafi, N.; Golani, I. Controlling the False Discovery Rate in Behavior Genetics Research. Behav. Brain Res. 2001, 125, 279–284. [Google Scholar] [CrossRef]
- Traczewska, T.M. Biologiczne Metody Oceny Skażenia Środowiska; Oficyna Wydawnicza Politechniki Wrocławskiej: Wroclaw, Poland, 2011; ISBN 978-83-7493-597-5. [Google Scholar]
- Gębski, M. Czynniki Glebowe Oraz Nawozowe Wpływające Na Przyswajanie Metali Ciężkich Przez Rośliny. Postępy Nauk. Rol. 1998, 45, 3–16. [Google Scholar]
- Bori, J.; Vallès, B.; Navarro, A.; Riva, M.C. Ecotoxicological Risks of the Abandoned F–Ba–Pb–Zn Mining Area of Osor (Spain). Environ. Geochem. Health 2017, 39, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Bouida, L.; Rafatullah, M.; Kerrouche, A.; Qutob, M.; Alosaimi, A.M.; Alorfi, H.S.; Hussein, M.A. A Review on Cadmium and Lead Contamination: Sources, Fate, Mechanism, Health Effects and Remediation Methods. Water 2022, 14, 3432. [Google Scholar] [CrossRef]
- Sizmur, T.; Tilston, E.L.; Charnock, J.; Palumbo-roe, B.; Watts, M.J.; Hodson, M.E. Impacts of Epigeic, Anecic and Endogeic Earthworms on Metal and Metalloid Mobility and Availability. J. Environ. Monit. 2011, 13, 266–273. [Google Scholar] [CrossRef]
- Hansda, A.; Kumar, V. A Comparative Review towards Potential of Microbial Cells for Heavy Metal Removal with Emphasis on Biosorption and Bioaccumulation. World J. Microbiol. Biotechnol. 2016, 32, 170. [Google Scholar] [CrossRef]
- Zhang, D.; Yin, C.; Abbas, N.; Mao, Z.; Zhang, Y. Multiple Heavy Metal Tolerance and Removal by an Earthworm Gut Fungus Trichoderma brevicompactum QYCD-6. Sci. Rep. 2020, 10, 6940. [Google Scholar] [CrossRef]
- Sturzenbaum, S.R.; Winters, C.; Galay, M.; Morgan, A.J.; Kille, P. Metal Ion Trafficking in Earthworms. J. Biol. Chem. 2001, 276, 34013–34018. [Google Scholar] [CrossRef]
- Stürzenbaum, S.R.; Kille, P.; Morgan, A.J. The Identification, Cloning and Characterization of Earthworm Metallothionein. Febs Lett. 1998, 431, 437–442. [Google Scholar] [CrossRef]
- Pauwels, M.; Frérot, H.; Souleman, D.; Vandenbulcke, F. Using Biomarkers in an Evolutionary Context: Lessons from the Analysis of Biological Responses of Oligochaete Annelids to Metal Exposure. Environ. Pollut. 2013, 179, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Terhivuo, J.; Pankokoski, E.; Hyvärinen, H.; Koivisto, I. Pb Uptake by Ecologically Dissimilar Earthworm (Lumbricidae) Species near a Lead Smelter in South Finland. Environ. Pollut. 1994, 85, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.M.; Mohammed, A.M. Effects of Zinc Accumulation on Earthworm Aporrectodea caliginosa (Haplotaxida: Lumbricidae). J. Ris. Biol. Dan. Apl. 2023, 5, 8–15. [Google Scholar] [CrossRef]
- Pelosi, C.; Gavinelli, F.; Petit-dit-Grezeriat, L.; Serbource, C.; Schoffer, J.T.; Ginocchio, R.; Yáñez, C.; Concheri, G.; Rault, M.; van Gestel, C.A.M. Copper Toxicity to Earthworms: A Comprehensive Review and Meta-Analysis. Chemosphere 2024, 362, 142765. [Google Scholar] [CrossRef]
- Yang, X.X.; Zhang, W.; Cao, X.F. Long-Term Effect of Copper with Sublethal Dose on Cytochrome P450 and Antioxidant Enzyme Activities of Earthworms. Acta Sci. Circumstantiae 2012, 32, 745–750. [Google Scholar]
- Bart, S.; Pelosi, C.; Nélieu, S.; Lamy, I.; Péry, A.R. An Energy-Based Model to Analyze Growth Data of Earthworms Exposed to Two Fungicides. Environ. Sci. Pollut. Res. 2020, 27, 741–750. [Google Scholar] [CrossRef]
- Marini, E.; De Bernardi, A.; Tagliabue, F.; Casucci, C.; Tiano, L.; Marcheggiani, F.; Vaccari, F.; Taskin, E.; Puglisi, E.; Brunetti, G.; et al. Copper Toxicity on Eisenia fetida in a Vineyard Soil: A Combined Study with Standard Tests, Genotoxicity Assessment and Gut Metagenomic Analysis. Environ. Sci. Pollut. Res. 2024, 31, 13141–13154. [Google Scholar] [CrossRef]
- Bundy, J.G.; Sidhu, J.K.; Rana, F.; Spurgeon, D.J.; Svendsen, C.; Wren, J.F.; Stürzenbaum, S.R.; Morgan, A.J.; Kille, P. “Systems Toxicology” Approach Identifies Coordinated Metabolic Responses to Copper in a Terrestrial Non-Model Invertebrate, the Earthworm Lumbricus rubellus. BMC Biol. 2008, 6, 25. [Google Scholar] [CrossRef]
- Šrut, M.; Menke, S.; Höckner, M.; Sommer, S. Earthworms and Cadmium–Heavy Metal Resistant Gut Bacteria as Indicators for Heavy Metal Pollution in Soils? Ecotoxicol. Environ. Saf. 2019, 171, 843–853. [Google Scholar] [CrossRef]
- Höckner, M.; Piechnik, C.A.; Fiechtner, B.; Weinberger, B.; Tomanek, L. Cadmium-Related Effects on Cellular Immunity Comprises Altered Metabolism in Earthworm Coelomocytes. Int. J. Mol. Sci. 2020, 21, 599. [Google Scholar] [CrossRef]
- Sinkakarimi, M.H.; Solgi, E.; Hosseinzadeh, C.A. Interspecific Differences in Toxicological Response and Subcellular Partitioning of Cadmium and Lead in Three Earthworm species. Chemosphere 2020, 238, 124595. [Google Scholar] [CrossRef]
- Urionabarrenetxea, E.; Garcia-Velasco, N.; Marigómez, I.; Soto, M. Effects of Elevated Temperatures and Cadmium Exposure on Stress Biomarkers at Different Biological Complexity Levels in Eisenia fetida Earthworms. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 231, 108735. [Google Scholar] [CrossRef]
- Žaltauskaitė, J.; Sodienė, I. Effects of Cadmium and Lead on the Life-Cycle Parameters of Juvenile Earthworm Eisenia fetida. Ecotoxicol. Environ. Saf. 2014, 103, 9–16. [Google Scholar] [CrossRef]
- Du, Y.-L.; He, M.-M.; Xu, M.; Yan, Z.-G.; Zhou, Y.-Y.; Guo, G.-L.; Nie, J.; Wang, L.-Q.; Hou, H.; Li, F.-S. Interactive Effects between Earthworms and Maize Plants on the Accumulation and Toxicity of Soil Cadmium. Soil Biol. Biochem. 2014, 72, 193–202. [Google Scholar] [CrossRef]
- You, R.; Li, H.; Li, X.; Luo, L.; Wang, P.; Xia, H.; Zhou, Y. Ecotoxicological Impacts of Cadmium on Soil Microorganisms and Earthworms Eisenia foetida: From Gene Regulation to Physiological Processes. Front. Environ. Sci. 2024, 12, 1479500. [Google Scholar] [CrossRef]
- Wu, S.; Xu, X.; Zhao, S.; Shen, F.; Chen, J. Evaluation of Phenanthrene Toxicity on Earthworm (Eisenia fetida): An Ecotoxicoproteomics Approach. Chemosphere 2013, 93, 963–971. [Google Scholar] [CrossRef]
- Wijayawardena, M.A.; Megharaj, M.; Naidu, R. Bioaccumulation and Toxicity of Lead, Influenced by Edaphic Factors: Using Earthworms to Study the Effect of Pb on Ecological Health. J. Soils Sediments 2017, 17, 1064–1072. [Google Scholar] [CrossRef]
- Karpov, M.A.; Hobbs, C.; Jayasinghe, S.N.; Stürzenbaum, S.R. Metallomic Mapping of Gut and Brain in Heavy Metal Exposed Earthworms: A Novel Paradigm in Ecotoxicology. Biochem. Biophys. Res. Commun. 2024, 709, 149827. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Osborne, J.W. Enhanced Bioremoval of Lead by Earthworm–Lumbricus terrestris Co-Cultivated with Bacteria–Klebsiella variicola. J. Photochem. Photobiol. B Biol. 2017, 175, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Ding, C.; Ma, Y.; Wang, J.; Zhang, T.; Wang, X. Time-Dependent Responses of Earthworms to Soil Contaminated with Low Levels of Lead as Detected Using 1 H NMR Metabolomics. RSC Adv. 2017, 7, 34170–34181. [Google Scholar] [CrossRef]
- Žaltauskaitė, J.; Kniuipytė, I.; Kugelytė, R. Lead Impact on the Earthworm Eisenia fetida and Earthworm Recovery after Exposure. Water Air Soil Pollut. 2020, 231, 49. [Google Scholar] [CrossRef]
- Sharma, P.; Chouhan, R.; Bakshi, P.; Gandhi, S.G.; Kaur, R.; Sharma, A.; Bhardwaj, R. Amelioration of Chromium-Induced Oxidative Stress by Combined Treatment of Selected Plant-Growth-Promoting Rhizobacteria and Earthworms via Modulating the Expression of Genes Related to Reactive Oxygen Species Metabolism in Brassica juncea. Front. Microbiol. 2022, 13, 802512. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Xu, J.; Li, J.; Liu, Z. Determination of Metallothionein, Malondialdehyde, and Antioxidant Enzymes in Earthworms (Eisenia fetida) Following Exposure to Chromium. Anal. Lett. 2016, 49, 1748–1757. [Google Scholar] [CrossRef]
- Fernando, V.K.; Perera, I.C.; Dangalle, C.D.; Premawansa, S.; Wijesinghe, M.R. Histological Alterations in the Body Wall of the Tropical Earthworm Eudrilus eugeniae Exposed to Hexavalent Chromium. Bull. Environ. Contam. Toxicol. 2015, 94, 744–748. [Google Scholar] [CrossRef]
- Liu, P.; Song, Y.; Wei, J.; Mao, W.; Ju, J.; Zheng, S.; Zhao, H. Synergistic Effects of Earthworms and Plants on Chromium Removal from Acidic and Alkaline Soils: Biological Responses and Implications. Biology 2023, 12, 831. [Google Scholar] [CrossRef]
- Gupta, S.K.; Srivastava, R.; Mathur, N.; Saxena, P.N. The Comparative Effects of Metals on the Hatching of Earthworm Cocoons. Altern. Lab. Anim. 2006, 34, 491–498. [Google Scholar] [CrossRef]
- Tőzsér, D.; Mizser, S.; Karaffa, K.; Málik-Roffa, H.; Magura, T. A Meta-Analysis-Based Evaluation of Metallic Element Accumulation in Earthworms. Environ. Int. 2022, 169, 107546. [Google Scholar] [CrossRef]
- Gavrish, I.A.; Lebedev, S.V. Effect of Nanoparticles of Nickel on Morphobiochemical Parameters Eisenia fetida. IOP Conf. Ser. Earth Environ. Sci. 2019, 341, 012167. [Google Scholar] [CrossRef]
- Podolak, A.; Piotrowska, E.; Klimek, M.; Klimek, B.A.; Kruk, J.; Plytycz, B. Effects of Nickel, Zinc, and Lead-Contaminated Soil on Burrowing Rate and Coelomocytes of the Earthworm, Allolobophora chlorotica. Folia Biol. 2011, 59, 91–97. [Google Scholar] [CrossRef]
- Lock, K.; Janssen, C.R. Ecotoxicity of Nickel to Eisenia fetida, Enchytraeus albidus and Folsomia candida. Chemosphere 2002, 46, 197–200. [Google Scholar] [CrossRef]
- Hirano, T.; Tamae, K. Heavy Metal-induced Oxidative DNA Damage in Earthworms: A Review. Appl. Environ. Soil Sci. 2010, 2010, 726946. [Google Scholar] [CrossRef]
- Wang, G.; Xia, X.; Yang, J.; Tariq, M.; Zhao, J.; Zhang, M.; Huang, K.; Lin, K.; Zhang, W. Exploring the Bioavailability of Nickel in a Soil System: Physiological and Histopathological Toxicity Study to the Earthworms (Eisenia fetida). J. Hazard. Mater. 2020, 383, 121169. [Google Scholar] [CrossRef] [PubMed]
- Lamb, D.T.; Matanitobua, V.P.; Palanisami, T.; Megharaj, M.; Naidu, R. Bioavailability of Barium to Plants and Invertebrates in Soils Contaminated by Barite. Environ. Sci. Technol. 2013, 47, 4670–4676. [Google Scholar] [CrossRef] [PubMed]
- Menzie, C.A.; Southworth, B.; Stephenson, G.; Feisthauer, N. The Importance of Understanding the Chemical Form of a Metal in the Environment: The Case of Barium Sulfate (Barite). Hum. Ecol. Risk Assess. 2008, 14, 974–991. [Google Scholar] [CrossRef]
- Zhang, P.C.; Brady, P.V.; Arthur, S.E.; Zhou, W.Q.; Sawyer, D.; Hesterberg, D.A. Adsorption of Barium (II) on Montmorillonite: An EXAFS Study. Colloids Surf. A Physicochem. Eng. Asp. 2001, 190, 239–249. [Google Scholar] [CrossRef]
- Lasley, K.K.; Evanylo, G.K.; Kostyanovsky, K.I.; Shang, C.; Eick, M.; Lee Daniels, W. Chemistry and Transport of Metals from Entrenched Biosolids at a Reclaimed Mineral Sands Mining Site. J. Environ. Qual. 2010, 39, 1467–1477. [Google Scholar] [CrossRef]
- Hulbert, A.J.; Kelly, M.A.; Abbott, S.K. Polyunsaturated Fats, Membrane Lipids and Animal Longevity. J. Comp. Physiol. B 2014, 184, 149–166. [Google Scholar] [CrossRef]
- Nahmani, J.; Hodson, M.E.; Black, S. A Review of Studies Performed to Assess Metal Uptake by Earthworms. Environ. Pollut. 2007, 145, 402–424. [Google Scholar] [CrossRef]
- Kowalczyk-Pecka, D.; Kowalczuk-Vasilev, E.; Pecka, S. The Effect of Heterogeneous Copper Micro-Supplementation on Fatty Acid Profiles in the Tissues of Snails Helix pomatia (Gastropoda Pulmonata). Ecol. Indic. 2017, 76, 335–343. [Google Scholar] [CrossRef]
- Kowalczyk-Pecka, D.; Pecka, S.; Kowalczuk-Vasilev, E. Changes in Fatty Acid Metabolism Induced by Varied Micro-Supplementation with Zinc in Snails Helix pomatia (Gastropoda Pulmonata). Ecotoxicol. Environ. Saf. 2017, 138, 223–230. [Google Scholar] [CrossRef]
- Puca, A.A.; Chatgilialoglu, C.; Ferreri, C. Lipid Metabolism and Diet: Possible Mechanisms of Slow Aging. Int. J. Biochem. Cell Biol. 2008, 40, 324–333. [Google Scholar] [CrossRef]
- Kamińska, A.; Garbacz, A.; Sadurski, J. Katalaza i Dysmutaza Ponadtlenkowa Jako Wybrane Enzymatyczne Biomarkery Stresu Oksydacyjnego. In Wybrane Zagadnienia z Zakresu Ochrony i Zagrożeń Środowiska; Babicz, M., Nowakowicz-Dębek, B., Gawryluk, A., Eds.; Wydawnictwo Uniwersytetu Przyrodniczego w Lublinie: Lublin, Poland, 2024; pp. 85–91. ISBN 978-83-7259-448-8. [Google Scholar]
- Osowska, S.; Rogulska, J. Wskaźniki Peroksydacji Lipidów w Żywieniu Pozajelitowym. Prospect. Pharm. Sci. 2023, 21, 24–32. [Google Scholar] [CrossRef]
- Wang, Q.L.; Zhu, L.; Huang, B.J.; Li, Y. Toxicity Effects of Lead in Polluted Soil on Earthworm Coelomocyte Lysosome. J. Agro-Environ. Sci. 2007, 36, 1874–1878. [Google Scholar]
- Oves, M.; Saghir Khan, M.; Huda Qari, A.; Nadeen Felemban, M.; Almeelbi, T. Heavy Metals: Biological Importance and Detoxification Strategies. J. Bioremediation Biodegrad. 2016, 7, 334. [Google Scholar] [CrossRef]
 —material collection points; ▬▬—border of Polesie National Park).
—material collection points; ▬▬—border of Polesie National Park).
 —material collection points; ▬▬—border of Polesie National Park).
—material collection points; ▬▬—border of Polesie National Park).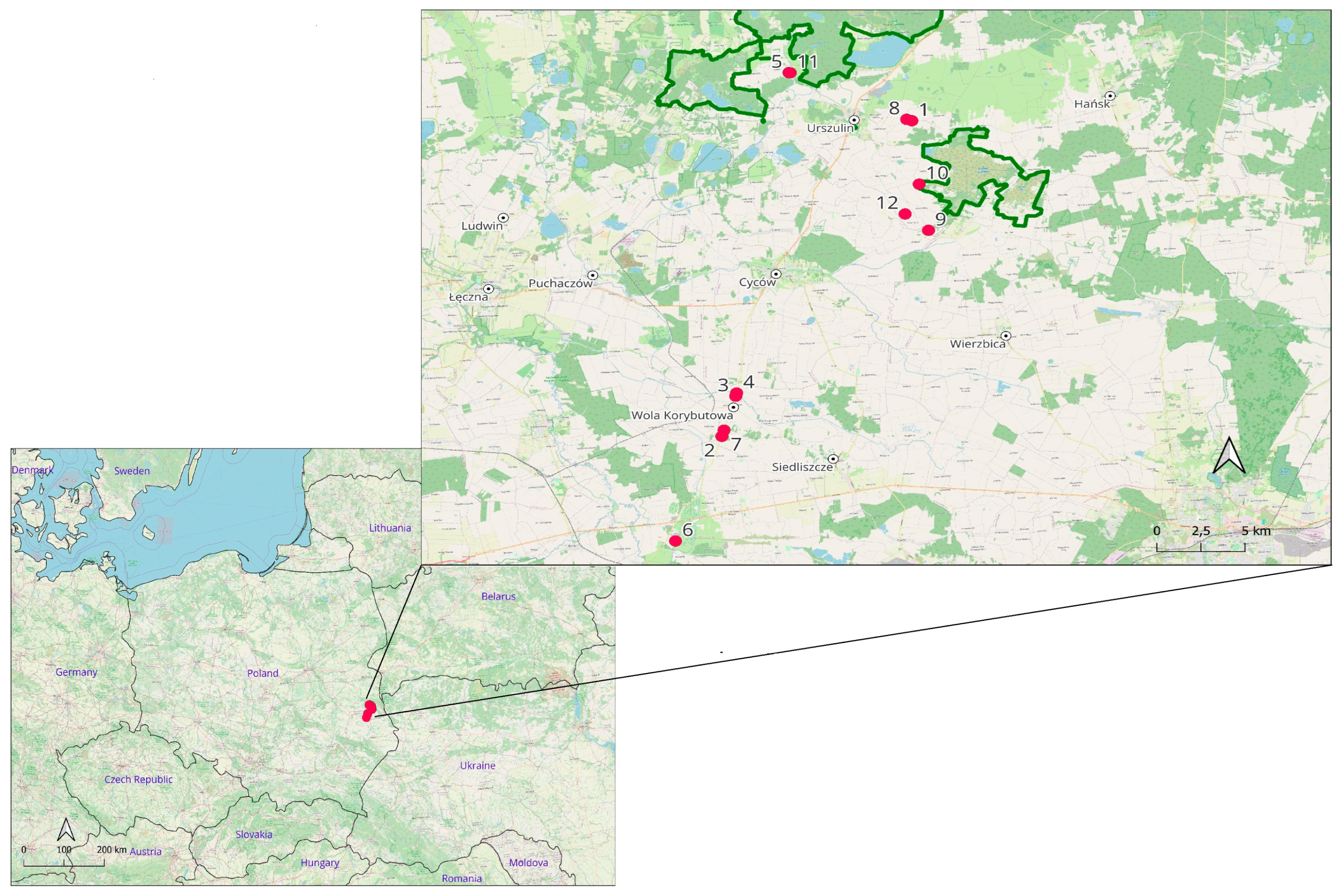
| Number of Maps | Sites | Geographic Coordinates | With or Without WR Deposit/ Time of WR Exposure | Research Material (Group) | |
|---|---|---|---|---|---|
| 1. | Buckwheat field (Fagopyrum esculentum) | N: 51°23′27.16″; E: 23°14′35.84″ | WR deposit > 10 years | Waste rock | WI10HFB |
| Soil | SI10HFB | ||||
| Plant roots | RI10HFB | ||||
| Earthworms | EI10HFB | ||||
| 2. | Oat field (Avena sativa) | N: 51°14′18.23″; E: 23°05′15.98″ | WR deposit 2 years | Waste rock | WI2FO |
| Soil | SI2FO | ||||
| Plant roots | RI2FO | ||||
| Earthworms | EI2FO | ||||
| 3. | Meadow I with Filipendulion ulmariae herb communities | N: 51°14′06.32″; E: 23°05′07.15″ | WR deposit > 1 year | Waste rock | WI1HMI |
| Soil | SI1HMI | ||||
| Plant roots | RI1HMI | ||||
| Earthworms | EI1HMI | ||||
| 4. | Meadow II with vegetation belonging to the Molinio-Arrhenatheretea class | N: 51°14′12.74″; E: 23°05′10.93″ | WR deposit < 1 year | Waste rock | WI1LMII |
| Soil | SI1LMII | ||||
| Plant roots | RI1LMII | ||||
| Earthworms | EI1LMII | ||||
| 5. | Wasteland I overgrown with pine and birch forest with dominant sand reed grass Calamagrostis epigejos and the presence of herb communities and clear-cut grasses Epilobion angustifolii | N: 51°25′22.12″; E: 23°08′44.81″ | WR deposit > 10 years | Waste rock | WI10HWI |
| Soil | SI10HWI | ||||
| Plant roots | RI10HWI | ||||
| Earthworms | EI10HWI | ||||
| 6. | Wasteland II with communities of ruderal thermophilic plants Onopordion acanthii | N: 51°08′52.15″; E: 23°01′32.98″ | WR deposit < 1 year | Waste rock | WI1LWII |
| Soil | SI1LWII | ||||
| Plant roots | RI1LWII | ||||
| Earthworms | EI1LWII | ||||
| 7. | Buckwheat field (Fagopyrum esculentum) | N: 51°23′29.93″; E: 23°14′31.36″ | Without WR deposit (control) | Soil | SWFB |
| Plant roots | RWFB | ||||
| Earthworms | EWFB | ||||
| 8. | Oat field (Avena sativa) | N: 51°14′34.98″; E: 23°05′23.72″ | Without WR deposit (control) | Soil | SWFO |
| Plant roots | RWFO | ||||
| Earthworms | EWFO | ||||
| 9. | Meadow I with vegetation from the Calthion association | N: 51°19′36.42″; E: 23°15′01.63″ | Without WR deposit (control) | Soil | SWMI |
| Plant roots | RWMI | ||||
| Earthworms | EWMI | ||||
| 10. | Meadow II with vegetation from the Molinion association | N: 51°21′16.57″; E: 23°14′49.31″ | Without WR deposit (control) | Soil | SWMII |
| Plant roots | RWMII | ||||
| Earthworms | EWMII | ||||
| 11. | Wasteland I overgrown with pine and birch forest with vegetation belonging to the Koelerio glaucae-Corynephoretea canescentis class | N: 51°25′21.63″; E: 23°08′47.66″ | Without WR deposit (control) | Soil | SWWI |
| Plant roots | RWWI | ||||
| Earthworms | EWWI | ||||
| 12. | Wasteland II with vegetation belonging to the Koelerio glaucae-Corynephoretea canescentis class | N: 51°20′12.81″; E: 23°13′57.74″ | Without WR deposit (control) | Soil | SWWII |
| Plant roots | RWWII | ||||
| Earthworms | EWWII | ||||
| Metals + CI [mg·kg−1] | Groups | SEM | |||||
|---|---|---|---|---|---|---|---|
| WI10HFB | WI10HWI | WI2FO | WI1HMI | WI1LWII | WI1LMII | ||
| Pb [CI] | 30 b 19.8–40.7 | 30 b 25.2–34.0 | 24 ab 20.0–28.2 | 20 ab 16.0–23.0 | 16 a 12.5–20.1 | 15 a 9.8–19.7 | 1.36 |
| Cd [CI] | 0 - | 0 - | 0 - | 0 - | 0 - | 0 - | - |
| Cr [CI] | 77 b 59.8–94.3 | 26 a 16.1–35.2 | 26 a 18.5–32.9 | 82 b 72.3–90.7 | 67 b 612.0–71.5 | 66 b 51.9–80.4 | 4.51 |
| Ba [CI] | 109 ab 97.1–120.7 | 101 a 88.3–112.5 | 91 a 82.1–99.5 | 124 b 112.5–136.3 | 158 c 145.8–169.3 | 226 d 209.5–241.6 | 8.68 |
| Ni [CI] | 29 b 25.3–33.3 | 19 a 16.0–21.9 | 26 ab 23.8–29.0 | 46 c 39.5–52.9 | 51 c 47.3–54.6 | 26 ab 22.2–30.4 | 2.21 |
| Zn [CI] | 101 c 81.2–120.3 | 43 ab 40.3–46.2 | 56 b 49.6–62.0 | 54 b 46.4–60.8 | 45 b 40.4–49.0 | 26 a 23.44–27.6 | 4.46 |
| Cu [CI] | 35 a 29.6–40.5 | 46 b 42.5–48.9 | 32 a 23.3–34.8 | 30 a 24.5–34.8 | 48 b 43.1–52.8 | 45 b 40.0–50.7 | 1.50 |
| Metals + CI [mg·kg−1] | Groups | SEM | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SI10HFB | SWFB | SI10HWI | SWWI | SI2FO | SWFO | SI1HMI | SWMI | SI1LWII | SWWII | SI1LMII | SWMII | |||
| Pb [CI] | 9.8 c 2.7–17.0 | 4.2 abc 1.9–6.5 | 16.2 d 13.3–19.1 | 3.0 ab 1.5–4.4 | 8.3 bc 4.7–11.7 | 3.7 abc 0.7–6.8 | 5.2 abc 1.3–9.0 | 0.9 a 0.3–1.6 | 2.1 a 0.4–3.7 | 1.1 a 0.3–1.8 | 1.1 a 0.3–2.0 | 0.9 a 0.3–1.4 | 0.20 | |
| Cd [CI] | 4.5 f 3.7–5.3 | 0.2 a 0.1–0.2 | 0.5 ab 0.1–0.9 | 0.1 a 0.1–0.1 | 0.4 ab 0.3–0.5 | 0.1 a 0.0–0.2 | 3.3 def 2.8–3.7 | 1.2 abc 0.8–1.6 | 3.5 ef 2.1–4.7 | 2.3 cde 0.8–3.8 | 2.9 de 1.8–4.1 | 1.7 bcd 1.3–2.2 | 0.16 | |
| Cr [CI] | 10.9 d 9.7–12.1 | 4.6 ab 4.0–5.1 | 8.4 e 7.2–9.6 | 3.8 a 3.4–4.3 | 4.5 ab 3.7–5.2 | 3.8 a 3.1–4.6 | 15.0 f 12.5–17.6 | 6.9 cd 6.3–7.6 | 5.7 abc 4.9–6.4 | 6.5 bcd 5.5–7.5 | 6.0 abc 5.6–6.3 | 5.4 abc 4.6–6.1 | 0.27 | |
| Ba [CI] | 19.5 b 16.9–22.2 | 0.2 a 0.1–0.2 | 1.2 a 1.0–1.4 | 0.2 a 0.1–0.2 | 1.6 a 1.3–1.9 | 0.3 a 0.3–0.4 | 27.5 c 23.9–31.1 | 2.4 a 2.0–2.7 | 19.5 b 17.4–21.6 | 18.8 b 17.3–20.3 | 25.6 c 23.7–27.5 | 1.5 a 1.3–1.7 | 0.08 | |
| Ni [CI] | 6.0 cd 5.2–6.8 | 1.9 a 1.6–2.1 | 9.2 e 7.9–10.4 | 1.9 a 1.5–2.3 | 3.1 ab 2.0–4.1 | 2.4 a 2.0–2.9 | 6.5 d 5.9–7.0 | 5.2 cd 4.7–5.7 | 4.8 cd 3.1–6.6 | 5.5 cd 4.76–6.2 | 4.5 bc 4.0–5.0 | 3.2 ab 2.8–3.5 | 0.13 | |
| Zn [CI] | 16.8 c 14.6–19.0 | 1.6 ab 8.3–14.9 | 25.3 e 20.8–29.8 | 9.4 ab 7.3–11.5 | 10.8 ab 9.0–12.6 | 8.5 a 7.4–9.7 | 13.9 bc 13.0–14.9 | 10.3 ab 8.3–12.3 | 12.7 abc 10.8–14.6 | 11.5 ab 7.1–15.8 | 11.7 abc 10.4–13.1 | 8.1 a 7.5–8.8 | 0.24 | |
| Cu [CI] | 4.4 abc 3.7–5.2 | 2.2 a 1.7–2.6 | 25.6 e 22.2–29.1 | 5.9 c 4.2–7.6 | 3.6 abc 2.7–4.4 | 2.6 ab 2.1–3.2 | 5.3 bc 4.6–6.0 | 4.1 abc 3.0–5.2 | 4.5 abc 3.2–5.8 | 4.0 abc 3.3–4.7 | 4.4 abc 4.0–5.0 | 3.8 abc 3.4–4.2 | 0.15 | |
| Interactions | WR | * | * | * | * | * | * | * | * | * | * | * | * | |
| S | * | * | * | * | * | * | * | * | * | * | * | * | ||
| WRxS | * | * | * | * | * | * | * | * | * | * | * | * | ||
| Metals + CI [mg·kg−1] | Groups | SEM | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RI10HFB | RWFB | RI10HWI | RWWI | RI2FO | RWFO | RI1HMI | RWMI | RI1LWII | RWWII | RI1LMII | RWMII | |||
| Pb [CI] | 3.8 abc 1.7–5.8 | 1.9 ab 1.1–2.7 | 14.2 d 10.3–18.0 | 6.5 c 3.5–9.5 | 4.0 abc 2.9–5.0 | 1.7 a 1.2–2.3 | 3.9 abc 1.1–6.7 | 2.2 ab 1.0–3.4 | 5.8 bc 4.7–7.0 | 1.7 a 0.9–2.4 | 2.8 abc 1.0–4.7 | 2.0 ab 1.4–2.5 | 0.48 | |
| Cd [CI] | 0.9 ab 0.7–1.1 | 0.3 a 0.1–0.5 | 1.4 ab 0.8–2.0 | 0.5 ab 0.3–0.8 | 0.7 ab 0.5–1.0 | 0.5 ab 0.3–0.6 | 2.9 b 1.5–4.4 | 1.3 ab 0.9–3.6 | 0.9 ab 0.6–1.2 | 0.8 ab 0.1–2.0 | 3.1 b 0.6–5.5 | 2.3 ab 0.3–4.2 | 0.17 | |
| Cr [CI] | 4.5 bc 3.4–5.6 | 3.7 abc 2.4–5.1 | 37.0 f 34.1–40.0 | 20.9 e 17.8–24.0 | 6.5 c 5.7–7.3 | 4.8 bc 4.1–5.6 | 4.1 abc 3.6–4.6 | 1.5 a 0.9–2.2 | 3.3 ab 2.9–3.8 | 2.9 ab 2.2–3.7 | 10.5 d 9.7–11.3 | 1.5 a 1.5–1.6 | 1.31 | |
| Ba [CI] | 8.7 b 7.9–9.5 | 0.3 a 0.3–0.4 | 0.6 a 0.5–0.8 | 0.2 a 0.1–0.2 | 0.8 a 0.6–0.9 | 0.6 a 0.5–0.7 | 9.7 b 8.5–11.0 | 7.9 b 6.5–9.3 | 24.0 c 20.3–27.7 | 9.7 b 7.6–11.7 | 48.4 d 42.7–54.0 | 7.8 b 6.3–9.2 | 1.75 | |
| Ni [CI] | 13.3 c 12.4–14.3 | 8.6 ab 7.7–9.5 | 21.1 f 18.9–23.4 | 18.6 de 16.8–20.5 | 9.4 ab 7.1–11.8 | 7.3 a 6.2–8.3 | 11.4 bc 10.4–12.4 | 7.1 a 6.2–7.9 | 12.8 c 11.0–14.5 | 9.0 ab 7.8–10.3 | 17.2 d 15.6–18.8 | 7.4 a 6.3–8.5 | 0.61 | |
| Zn [CI] | 27.0 bcd 23.7–30.3 | 22.9 abc 19.3–26.4 | 28.3 cd 24.5–32.0 | 27.4 cd 23.6–31.1 | 18.3 a 16.6–20.1 | 18.3 a 17.0–19.6 | 20.3 ab 18.0–22.7 | 27.3 cd 20.9–33.7 | 52.6 e 47.9–57.2 | 24.5 abcd 22.0–27.0 | 28.9 cd 27.0–20.8 | 30.6 d 28.6–32.5 | 1.17 | |
| Cu [CI] | 10.4 ef 8.2–12.6 | 7.0 bcd 5.4–9.7 | 17.3 g 15.4–19.2 | 5.4 abc 4.4–6.5 | 4.9 ab 3.8–6.0 | 3.8 a 3.1–4.5 | 8.6 de 7.4–9.9 | 8.5 de 7.6–9.5 | 7.7 cd 7.0–8.5 | 4.5 ab 3.8–5.4 | 11.5 f 10.2–12.7 | 8.6 de 7.8–9.4 | 0.48 | |
| Interactions | WR | * | * | * | * | * | * | * | * | * | * | * | * | |
| S | * | * | * | * | * | * | * | * | * | * | * | * | ||
| WRxS | * | * | * | * | * | * | * | * | * | * | * | * | ||
| Metals + CI [mg·kg−1] | Groups | SEM | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EI10HFB | EWFB | EI10HWI | EWWI | EI2FO | EWFO | EI1HMI | EWMI | EI1LWII | EWWII | EI1LMII | EWMII | |||
| Pb [CI] | 3.5 cd 1.6–5.3 | 1.0 ab 0.6–1.4 | 5.3 e 4.1–6.5 | 2.3 abc 1.5–3.1 | 2.7 bc 2.0–3.4 | 1.0 ab 0.7–1.3 | 1.2 ab 0.5–1.8 | 0.6 a 0.4–0.8 | 3.5 cd 1.7–5.3 | 2.1 abc 1.8–2.4 | 1.0 ab 0.5–1.5 | 0.6 a 0.3–0.9 | 0.20 | |
| Cd [CI] | 0.9 ab 0.4–1.3 | 0.6 a 0.0–1.1 | 2.5 c 1.8–3.1 | 1.3 b 0.6–2.0 | 0.8 ab 0.7–0.9 | 0.5 a 0.4–0.7 | 0.5 a 0.4–0.6 | 0.3 a 0.3–0.4 | 0.4 a 0.2–0.6 | 0.4 a 0.1–0.6 | 0.3 a 0.3–0.4 | 0.2 a 0.1–0.3 | 0.09 | |
| Cr [CI] | 6.2 e 5.3–7.1 | 1.2 ab 0.8–1.5 | 6.0 de 5.0–7.0 | 2.5 abc 1.7–3.4 | 2.0 ab 1.4–2.6 | 0.6 a 0.5–0.6 | 12.1 f 10.5–13.7 | 5.2 de 3.4–7.1 | 2.8 bc 1.5–4.0 | 1.5 ab 0.9–2.1 | 4.2 cd 3.7–4.7 | 3.1 bc 2.7–3.6 | 0.41 | |
| Ba [CI] | 2.6 c 1.5–3.7 | 0.0 a 0.0–0.01 | 0.0 a 0.0–0.1 | 0.0 a 0.0–0.0 | 0.0 a 0.0–0.0 | 0.0 a 0.0–0.0 | 4.0 d 3.3–4.8 | 1.3 b 0.4–2.3 | 2.7 c 2.0–3.4 | 2.2 bc 2.0–2.4 | 1.8 bc 1.4–2.3 | 1.4 b 1.2–1.7 | 0.18 | |
| Ni [CI] | 1.9 c 1.3–2.5 | 0.8 ab 0.7–0.8 | 0.9 ab 0.8–0.9 | 0.2 a 0.1–0.2 | 0.1 a 0.1–0.1 | 0.1 a 0.1–0.1 | 3.1 d 2.3–4.0 | 2.1 c 1.6–2.5 | 1.6 bc 0.6–2.5 | 0.2 a 0.1–0.4 | 2.3 cd 1.8–2.8 | 1.8 c 1.6–2.0 | 0.14 | |
| Zn [CI] | 66.6 d 59.0–74.1 | 50.4 bc 46.5–54.4 | 115.9 f 107.4–124.3 | 95.5 e 88.1–102.9 | 57.9 cd 52.9–63.0 | 34.6 a 28.0–41.2 | 88.3 e 72.2–104.5 | 45.3 abc 37.0–53.7 | 36.7 ab 32.5–41.0 | 33.6 a 31.7–35.5 | 48.7 abc 44.2–53.2 | 41.1 ab 37.1–45.2 | 3.41 | |
| Cu [CI] | 3.8 de 2.9–4.6 | 1.7 ab 0.9–2.5 | 4.3 e 4.0–4.6 | 2.3 abc 1.6–3.1 | 1.7 ab 1.3–2.1 | 1.2 a 0.8–1.6 | 3.8 de 3.2–4.4 | 3.1 cd 2.9–3.2 | 2.6 bc 1.8–3.4 | 1.8 ab 1.5–2.0 | 2.8 bcd 2.5–3.1 | 2.6 bcd 2.3–3.0 | 0.13 | |
| Interactions | WR | * | * | * | * | * | * | * | * | * | * | * | * | |
| S | * | * | * | * | * | * | * | * | * | * | * | * | ||
| WRxS | * | * | * | * | * | * | * | * | * | * | * | * | ||
| Parameters + CI | Groups | SEM | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EI10HFB | EWFB | EI10HWI | EWWI | EI2FO | EWFO | EI1HMI | EWMI | EI1LWII | EWWII | EI1LMII | EWMII | |||
| C12:0 [CI] | 1.0 bcd 0.9–1.1 | 0.6 a 0.4–0.8 | 1.2 d 0.8–1.7 | 1.1 cd 0.9–1.2 | 0.8 abc 0.7–0.9 | 0.6 ab 0.6–0.7 | 0.8 abc 0.7–0.9 | 0.5 a 0.3–0.7 | 2.7 f 2.6–2.9 | 0.7 abc 0.5–1.0 | 2.0 e 1.8–2.1 | 0.5 a 0.4–0.6 | 0.09 | |
| C14:0 [CI] | 2.1 abc 1.9–2.3 | 1.8 ab 1.8–1.9 | 4.5 d 2.5–6.3 | 1.1 ab 0.3–3.3 | 3.9 cd 3.2–4.4 | 2.8 bcd 2.1–3.5 | 2.0 ab 1.7–2.3 | 1.4 ab 1.1–1.6 | 2.1 abc 1.9–2.2 | 0.9 a 0.7–1.1 | 1.5 ab 1.4–1.7 | 1.1 ab 0.9–1.4 | 0.16 | |
| C16:0 [CI] | 24.8 g 21.4–28.1 | 19.8 e 18.7–20.3 | 27.2 g 25.2–29.2 | 24.4 fg 23.6–25.3 | 24.8 g 23.2–26.4 | 21.1 ef 19.2–23.1 | 10.7 bc 8.4–13.0 | 5.7 a 4.8–6.7 | 16.0 d 14.5–17.4 | 8.2 ab 6.6–9.7 | 14.0 cd 12.9–15.0 | 6.0 a 5.1–6.9 | 0.99 | |
| C18:0 [CI] | 9.0 fg 8.0–10.0 | 8.1 defg 7.9–8.2 | 9.3 g 8.4–10.1 | 8.3 efg 7.9–8.7 | 7.7 bcdef 6.7–8.8 | 6.8 abcd 6.3–7.3 | 7.8 cdef 6.8–8.7 | 6.5 ab 6.0–7.0 | 7.1 abcde 6.9–7.2 | 6.0 a 5.7–6.2 | 6.8 abc 6.2–7.3 | 6.0 a 5.7–6.2 | 0.15 | |
| C20:0 [CI] | 0.6 bcd 0.4–0.7 | 0.4 abc 0.4–0.4 | 1.0 e 0.8–1.1 | 0.7 cde 0.6–0.8 | 1.0 e 0.6–1.4 | 0.8 de 0.7–1.0 | 0.7 cde 0.5–1.0 | 0.2 a 0.1–0.2 | 0.4 abc 0.4–0.5 | 0.3 ab 0.2–0.3 | 0.2 ab 0.2–0.4 | 0.1 a 0.1–0.2 | 0.04 | |
| C21:0 [CI] | 4.8 e 3.9–5.7 | 0.8 ab 0.7–0.9 | 7.6 f 6.3–9.0 | 0.7 a 0.6–0.9 | 3.3 d 2.7–3.9 | 1.4 abc 0.9–1.9 | 5.5 e 4.7–6.3 | 2.0 bc 1.7–2.3 | 4.8 e 4.5–5.2 | 2.3 cd 2.1–2.6 | 3.4 d 2.9–3.9 | 1.8 abc 1.5–2.0 | 0.27 | |
| C24:0 [CI] | 0.6 bc 0.3–1.0 | 0.3 a 0.2–0.3 | 0.8 c 0.7–0.9 | 0.4 ab 0.3–0.6 | 0.6 bc 0.4–0.8 | 0.3 a 0.2–0.4 | 0.4 ab 0.3–0.5 | 0.2 a 0.1–0.3 | 0.3 a 0.3–0.4 | 0.2 a 0.2–0.3 | 0.3 a 0.2–0.4 | 0.2 a 0.2–0.2 | 0.03 | |
| SFA [CI] | 42.2 bcd 34.9–49.5 | 37.0 abc 33.7–40.3 | 52.1 e 46.5–57.7 | 46.8 de 41.2–51.8 | 45.1 cde 41.3–48.9 | 36.8 abc 31.5–42.2 | 33.3 a 28.5–38.0 | 29.8 a 27.1–32.6 | 35.5 ab 32.8–38.1 | 28.5 a 24.9–32.1 | 31.1 a 28.4–33.9 | 28.4 a 26.1–30.1 | 1.05 | |
| C16:1 n−7 [CI] | 0.8 bc 0.6–1.0 | 0.3 a 1.2–1.4 | 1.6 d 1.4–1.8 | 1.0 bc 0.8–1.1 | 1.6 d 1.4–1.8 | 1.0 bc 0.7–1.2 | 1.0 bc 0.7–1.2 | 0.7 ab 0.5–0.8 | 2.2 e 1.9–2.5 | 0.8 bc 0.6–1.1 | 1.1 c 0.9–1.2 | 0.7 bc 0.6–0.9 | 0.07 | |
| C18:1 n−9c + C18:1 n−9t [CI] | 18.6 c 17.2–19.9 | 16.2 bc 15.4–17.0 | 26.8 d 23.0–30.5 | 18.4 c 16.1–20.9 | 31.5 e 28.3–34.8 | 23.7 d 20.6–26.8 | 18.5 c 15.5–21.6 | 11.1 a 9.0–13.1 | 16.8 bc 15.5–18.2 | 13.7 ab 13.7–14.7 | 15.4 abc 14.2–16.6 | 11.3 a 9.8–12.8 | 0.79 | |
| C20:1 n−9 [CI] | 2.4 d 1.7–3.1 | 0.4 ab 0.2–0.5 | 0.8 bc 0.4–1.0 | 0.2 a 0.2–0.3 | 0.7 abc 0.5–0.9 | 0.3 ab 0.2–0.5 | 0.8 abc 0.5–1.0 | 0.4 ab 0.2–0.5 | 0.9 c 0.7–1.1 | 0.3 ab 0.3–0.4 | 0.6 abc 0.5–0.8 | 0.4 ab 0.3–0.5 | 0.08 | |
| MUFA [CI] | 27.8 ef 26.8–28.8 | 26.7 de 19.7–33.6 | 33.8 fg 29.3–38.3 | 21.7 cde 17.8–25.5 | 36.8 g 32.9–40–7 | 27.8 ef 24.1–31.4 | 20.6 bcd 17.7–23.4 | 13.4 a 11.2–15.7 | 20.2 abcd 18.4–21.9 | 15.9 abc 14.7–17.2 | 17.3 abc 15.7–18.8 | 13.8 ab 12.5–15.1 | 1.00 | |
| C18:2 n−6c + C18:2 n−6t [CI] | 7.4 bc 5.9–8.9 | 14.9 efg 13.4–16.3 | 2.4 a 2.1–2.8 | 9.3 cd 7.9–10.6 | 3.2 ab 2.3–4.1 | 11.5 cde 9.3–13.8 | 11.8 ed 7.7–15.8 | 16.7 fg 14.8–18.5 | 14.0 ef 12.9–15.1 | 18.6 g 15.8–21.3 | 11.8 ed 9.5–14.0 | 24.4 h 21.7–27.1 | 0.80 | |
| C18:3 n−3 [CI] | 0.4 a 0.3–0.4 | 0.7 ab 0.6–0.7 | 0.4 a 0.3–0.6 | 0.8 ab 0.6–1.1 | 0.5 ab 0.3–0.8 | 1.9 c 1.5–2.2 | 1.1 b 0.8–1.4 | 2.7 d 2.1–3.2 | 2.3 cd 1.9–2.6 | 3.5 e 3.3–3.8 | 1.9 c 1.5–2.2 | 2.7 d 2.5–3.0 | 0.14 | |
| C20:2 n−6 [CI] | 0.4 a 0.3–0.5 | 3.2 d 2.4–4.1 | 0.3 a 0.2–0.5 | 2.6 d 2.6–3.0 | 0.7 ab 0.5–1.0 | 1.5 bc 1.3–1.7 | 1.1 abc 0.8–1.4 | 2.8 d 2.4–3.3 | 1.3 bc 1.1–1.5 | 3.2 d 2.9–3.5 | 1.8 c 1.4–2.2 | 3.0 d 2.7–3.4 | 0.14 | |
| C20:3 n−3 [CI] | 0.9 a 0.6–1.1 | 1.8 b 1.6–2.1 | 0.9 a 0.7–1.1 | 2.2 b 1.9–2.6 | 2.1 b 1.5–2.7 | 3.1 c 2.7–3.4 | 6.0 d 5.7–6.2 | 6.2 d 6.1–6.4 | 3.2 c 3.0–3.4 | 7.1 e 6.8–7.5 | 3.4 c 3.2–3.7 | 7.2 e 6.9–7.5 | 0.29 | |
| C20:4 n−6 [CI] | 5.9 a 4.7–7.1 | 12.8 cd 10.8–14.8 | 5.0 a 4.1–5.9 | 14.1 cde 11.6–16.6 | 6.1 a 5.7–6.5 | 11.0 bc 9.2–12.8 | 6.9 a 5.7–8.1 | 13.8 cde 11.8–15.9 | 7.8 ab 5.5–10.2 | 15.2 de 13.2–17.2 | 8.0 ab 6.1–10.0 | 16.9 e 13.8–20.0 | 0.55 | |
| C20:5 n−3 [CI] | 0.3 a 0.2–0.4 | 0.5 abc 0.4–0.6 | 0.3 a 0.1–0.4 | 0.7 bc 0.5–0.8 | 0.4 abc 0.3–0.6 | 0.7 bc 0.6–0.7 | 0.4 ab 0.2-–0.6 | 0.7 c 0.6–0.9 | 0.4 abc 0.2–0.6 | 0.6 bc 0.5–0.8 | 0.5 abc 0.4–0.7 | 0.7 c 0.6–0.9 | 0.02 | |
| C22:2 n−6 [CI] | 0.7 a 0.4–0.9 | 2.4 e 2.0–2.8 | 0.5 a 0.4–0.7 | 1.9 cde 1.6–2.2 | 0.8 ab 0.5–1.2 | 2.0 cde 1.6–2.4 | 1.0 ab 0.8–1.2 | 2.1 de 1.7–2.6 | 1.4 bc 1.1–1.7 | 1.9 cde 1.6–2.2 | 1.5 bcd 1.2–1.8 | 2.2 e 1.8–2.7 | 0.09 | |
| PUFA [CI] | 30.0 abc 24.4–35.7 | 36.3 abc 14.4–58.3 | 14.1 a 60.1–65.7 | 31.5 abc 12.1–51.0 | 18.1 ab 9.2–27.1 | 35.4 abc 18.7–52.1 | 46.2 abc 31.2–61.2 | 56.7 c 32.4–80.1 | 44.4 abc 25.2–53.5 | 55.4 c 37.1–73.6 | 51.6 bc 36.6–66.7 | 57.8 c 33.4–82.2 | 2.45 | |
| PI [CI] | 72.8 b 71.3–74.2 | 76.5 bc 75.1–78.0 | 62.9 a 93.1–99.7 | 73.8 b 71.8–75.7 | 64.9 a 62.5–67.3 | 75.9 bc 73.8–77.1 | 80.7 cd 77.9–83.5 | 92.2 e 90.0–94.6 | 79.0 cd 76.1–81.8 | 89.8 e 86.8–92.7 | 83.0 d 81.0–85.0 | 94.3 e 91.3–97.3 | 1.26 | |
| UI [CI] | 108.8 b 105.9–111.7 | 119.9 c 116.8–123.0 | 96.4 a 93.1–99.7 | 111.7 b 108.4–115.0 | 99.7 a 94.9–104.4 | 120.7 c 116.6–124.7 | 128.4 d 126.0–130.9 | 139.7 f 126.9–142.6 | 125.8 cd 123.5–128.1 | 135.2 ef 132–7–137.7 | 130.7 de 128.6–132.8 | 139.1 f 137.5–140.7 | 1.85 | |
| SOD [CI] | 13.3 a 12.4–14.1 | 19.6 c 17.9–21.2 | 13.8 a 13.014.7 | 18.8 c 17.2–20.3 | 15.5 ab 14.3–16.3 | 22.9 d 21.3–24.6 | 18.9 c 18.7–19.1 | 22.6 d 21.5–23.8 | 17.9 bc 17.1–18.8 | 18.4 c 16.6–202 | 19.8 c 18.5–21.2 | 23.4 d 22.2–24.6 | 0.43 | |
| CAT [CI] | 16.9 a 14.2–19.5 | 56.3 f 52.4–60.2 | 13.1 a 11.7–14.5 | 34.2 e 31.0–37.4 | 24.7 bc 23.2–26.1 | 31.7 de 30.1–33.4 | 29.3 cde 24.6–34.1 | 31.2 de 28.0–34.4 | 23.4 b 22.3–24.6 | 34.9 e 32.1–38.8 | 26.6 bcd 24.0–29.3 | 31.8 de 29.4–34.2 | 1.37 | |
| TBARS [CI] | 1.6 e 1.6–1.7 | 0.8 bc 0.7–0.8 | 1.7 e 1.6–1.9 | 0.8 bc 0.8–0.9 | 1.0 cd 0.8–1.1 | 0.6 ab 0.5–0.7 | 1.1 d 0.8–1.5 | 0.5 a 0.4–0.5 | 1.0 cd 0.8–1.7 | 0.8 bc 0.7–0.8 | 0.8 bc 0.8–0.8 | 0.5 a 0.4–0.5 | 0.05 | |
| Interactions | WR | * | * | * | * | * | * | * | * | * | * | * | * | |
| S | * | * | * | * | * | * | * | * | * | * | * | * | ||
| WRxS | * | * | * | * | * | * | * | * | * | * | * | * | ||
| Pb | Cd | Cr | Ba | Ni | Zn | Cu | |
|---|---|---|---|---|---|---|---|
| C12:0 | 0.3890 * | 0.0663 | −0.0007 | 0.2654 | 0.1775 | −0.0193 | 0.1773 |
| moderate | negligible | negligible | weak | weak | negligible | weak | |
| C14:0 | 0.5423 * | 0.6091 * | 0.0577 | −0.3199 | −0.2122 | 0.3808 * | 0.1788 |
| strong | strong | negligible | moderate | weak | moderate | weak | |
| C16:0 | 0.6118 * | 0.6554 * | −0.1539 | −0.4808 * | −0.4707 * | 0.5083 * | 0.0297 |
| strong | strong | weak | moderate | moderate | strong | negligible | |
| C18:0 | 0.6005 * | 0.6693 * | 0.2994 | −0.1565 | −0.0531 | 0.7102 * | 0.4106 * |
| strong | strong | weak | weak | negligible | very strong | moderate | |
| C20:0 | 0.4632 * | 0.6007 * | 0.0943 | −0.3404 * | −0.3544 * | 0.5647 * | 0.0478 |
| moderate | strong | negligible | moderate | moderate | strong | negligible | |
| C21:0 | 0.6298 * | 0.4764 * | 0.6133 * | 0.3661 * | 0.3758 * | 0.5082 * | 0.6990 * |
| strong | moderate | strong | moderate | moderate | strong | strong | |
| C24:0 | 0.7112 * | 0.6797 * | 0.2858 | −0.1244 | −0.1379 | 0.6503 * | 0.4719 * |
| very strong | strong | weak | weak | weak | strong | moderate | |
| SFA | 0.6941 * | 0.8242 * | 0.0105 | −0.4497 * | −0.3921 * | 0.6662 * | 0.1997 |
| strong | very strong | negligible | moderate | moderate | strong | weak | |
| C16:1 n−7 | 0.5759 * | 0.2840 | 0.0355 | 0.0467 | −0.1015 | 0.1659 | 0.1615 |
| strong | weak | negligible | negligible | weak | weak | weak | |
| C18:1 n−9c + C18:1 n−9t | 0.4989 * | 0.5261 * | −0.0349 | −0.4112 * | −0.4117 * | 0.4000 * | −0.0488 |
| moderate | strong | negligible | moderate | moderate | moderate | negligible | |
| C20:1 n−9 | 0.4171 * | 0.1512 | 0.3584 * | 0.3497 * | 0.3384 * | 0.1553 | 0.4456 * |
| moderate | weak | moderate | moderate | moderate | weak | moderate | |
| MUFA | 0.5381 * | 0.5761 * | −0.0760 | −0.4405 * | −0.4311 * | 0.3948 * | −0.0090 |
| strong | strong | negligible | moderate | moderate | moderate | negligible | |
| C18:2 n−6c + C18:2 n−6t | −0.6062 * | −0.6551 * | −0.1763 | 0.2652 | 0.2318 | −0.6035 * | −0.2468 |
| strong | strong | weak | weak | weak | strong | weak | |
| C18:3 n−3 | −0.3961 * | −0.5514 * | −0.2366 | 0.2718 | 0.0695 | −0.6683 * | −0.2536 |
| moderate | strong | weak | weak | negligible | strong | weak | |
| C20:2 n−6 | −0.5832 * | −0.4221 * | −0.4061 * | −0.1546 | −0.1394 | −0.4235 * | −0.4340 * |
| strong | moderate | moderate | weak | weak | moderate | moderate | |
| C20:3 n−3 | −0.5459 * | −0.5682 * | 0.1359 | 0.4329 * | 0.3069 | −0.4344 * | −0.0928 |
| strong | strong | weak | moderate | moderate | moderate | negligible | |
| C20:4 n−6 | −0.5138 * | −0.3749 * | −0.4203 * | −0.1919 | −0.2052 | −0.3982 * | −0.4175 * |
| strong | moderate | moderate | weak | weak | moderate | moderate | |
| C20:5 n−3 | −0.5936 * | −0.4037 * | −0.3711 * | −0.1440 | −0.1846 | −0.4512 * | −0.4641 * |
| strong | moderate | moderate | weak | weak | moderate | moderate | |
| C22:2 n−6 | −0.6540 * | −0.4929 * | −0.4704 * | −0.2271 | −0.1448 | −0.5314 * | −0.5315 * |
| strong | moderate | moderate | weak | weak | strong | strong | |
| PUFA | −0.3862 * | −0.6185 * | 0.0257 | 0.3671 * | 0.3318 * | −0.4514 * | −0.0698 |
| moderate | strong | negligible | moderate | moderate | moderate | negligible | |
| PI | −0.6229 * | −0.6781 * | −0.0013 | 0.3990 * | 0.3757 * | −0.5845 * | −0.1122 |
| strong | strong | negligible | moderate | moderate | strong | weak | |
| UI | −0.6701 * | −0.7190 * | 0.0249 | 0.4405 * | 0.4342 * | −0.6093 * | −0.1342 |
| strong | very strong | negligible | moderate | moderate | strong | weak | |
| SOD | −0.7633 * | −0.5480 * | −0.2233 | −0.0612 | 0.1156 | −0.5072 * | −0.3934 * |
| very strong | strong | weak | negligible | weak | strong | moderate | |
| CAT | −0.5958 * | −0.4376 * | −0.3692 * | −0.1992 | −0.2099 | −0.3471 * | −0.5437 * |
| strong | moderate | moderate | weak | weak | moderate | strong | |
| TBARS | 0.7206 * | 0.6463 * | 0.4280 * | 0.1529 | 0.1107 | 0.6502 * | 0.5912 * |
| very strong | strong | moderate | weak | weak | strong | strong |
| Pb | Cd | Cr | Ba | Ni | Zn | Cu | |
|---|---|---|---|---|---|---|---|
| C12:0 | 0.7165 * | 0.6165 * | −0.1448 | −0.2304 | −0.5754 * | 0.6423 * | −0.0737 |
| very strong | strong | weak | weak | strong | strong | negligible | |
| C14:0 | −0.1872 | 0.1917 | −0.3305 | −0.4120 | −0.1974 | −0.1822 | −0.3154 |
| weak | weak | moderate | moderate | weak | weak | moderate | |
| C16:0 | 0.3720 | 0.6245 * | −0.5544 * | −0.8450 * | −0.7225 * | 0.5674 * | −0.5100 * |
| moderate | strong | strong | very strong | very strong | strong | strong | |
| C18:0 | 0.3083 | 0.6316 * | −0.2053 | −0.7266 * | −0.3816 | 0.7325 * | −0.1656 |
| moderate | strong | weak | very strong | moderate | very strong | weak | |
| C20:0 | 0.3583 | 0.5446 * | −0.5497 * | −0.7228 * | −0.7421 * | 0.3933 | −0.4963 * |
| moderate | strong | strong | very strong | very strong | moderate | moderate | |
| C21:0 | −0.1464 | −0.5509 * | 0.2691 | 0.7946 * | 0.2911 | −0.6538 * | 0.2014 |
| weak | strong | weak | very strong | weak | strong | weak | |
| C24:0 | 0.4410 | 0.5652 * | −0.0123 | −0.4781 * | −0.4734 * | 0.7543 * | −0.0628 |
| moderate | strong | negligible | moderate | moderate | very strong | negligible | |
| SFA | 0.4376 | 0.8136 * | −0.2776 | −0.7424 * | −0.5176 * | 0.7634 * | −0.2297 |
| moderate | very strong | weak | very strong | strong | very strong | weak | |
| C16:1 n−7 | 0.3165 | 0.1975 | 0.0262 | 0.0405 | −0.3385 | 0.2054 | 0.0266 |
| moderate | weak | negligible | negligible | moderate | weak | negligible | |
| C18:1 n−9c + C18:1 n−9t | 0.2199 | 0.3881 | −0.6156 * | −0.6950 * | −0.7230 * | 0.1711 | −0.6619 * |
| weak | moderate | strong | strong | very strong | weak | strong | |
| C20:1 n−9 | −0.3539 | −0.3852 | 0.1333 | 0.0803 | 0.2376 | −0.3860 | 0.0748 |
| moderate | moderate | weak | negligible | weak | moderate | negligible | |
| MUFA | 0.0993 | 0.4147 | −0.6381 * | −0.7510 * | −0.6142 * | 0.1113 | −0.6124 * |
| negligible | moderate | strong | very strong | strong | weak | strong | |
| C18:2 n−6c + C18:2 n−6t | −0.4229 | −0.6337 * | 0.2862 | 0.6775 * | 0.6158 * | −0.5369 * | 0.3185 |
| moderate | strong | weak | strong | strong | strong | moderate | |
| C18:3 n−3 | −0.1199 | −0.5308 * | 0.2854 | 0.8425 * | 0.2710 | −0.6458 * | 0.2193 |
| weak | strong | weak | very strong | weak | strong | weak | |
| C20:2 n−6 | 0.0262 | −0.0783 | 0.2798 | 0.3509 | 0.3531 | 0.0524 | 0.3344 |
| negligible | negligible | weak | moderate | moderate | negligible | moderate | |
| C20:3 n−3 | −0.1780 | −0.5483 * | 0.4454 | 0.8820 * | 0.5247 * | −0.5251 * | 0.4183 |
| weak | strong | moderate | very strong | strong | strong | moderate | |
| C20:4 n−6 | 0.1242 | −0.1593 | 0.2820 | 0.4563 | 0.3495 | 0.0341 | 0.3388 |
| weak | weak | weak | moderate | moderate | negligible | moderate | |
| C20:5 n−3 | −0.2069 | −0.0139 | 0.2735 | 0.3147 | 0.3039 | −0.0351 | 0.1591 |
| weak | negligible | weak | moderate | moderate | negligible | weak | |
| C22:2 n−6 | −0.4478 | −0.1844 | 0.0657 | −0.1406 | 0.2942 | −0.0890 | 0.0248 |
| moderate | weak | negligible | weak | weak | negligible | negligible | |
| PUFA | −0.1535 | −0.6050 * | 0.3274 | 0.4808 * | 0.3697 | −0.3284 | 0.2210 |
| weak | strong | moderate | moderate | moderate | moderate | weak | |
| PI | −0.3414 | −0.5888 * | 0.5556 * | 0.7966 * | 0.6996 * | −0.5128 * | 0.5202 * |
| moderate | strong | strong | very strong | strong | strong | strong | |
| UI | −0.4632 * | −0.6830 * | 0.5153 * | 0.7873 * | 0.7123 * | −0.6167 * | 0.4642 * |
| moderate | strong | strong | very strong | very strong | strong | moderate | |
| SOD | −0.7414 * | −0.3476 | 0.2964 | 0.0058 | 0.5492 * | −0.3516 | 0.2401 |
| very strong | moderate | weak | negligible | strong | moderate | weak | |
| CAT | −0.0609 | −0.0119 | −0.3603 | −0.3239 | −0.1371 | 0.0660 | −0.2586 |
| negligible | negligible | moderate | moderate | weak | negligible | weak | |
| TBARS | 0.7657 * | 0.5712 * | −0.5169 * | −0.2889 | −0.7934 * | 0.4795 * | −0.4378 |
| very strong | strong | strong | weak | very strong | moderate | moderate |
| Variable 1 | Variable 2 | r | p | FDR (BH) Thresholds | Significant |
|---|---|---|---|---|---|
| Pb | C12:0 | −0.7633 | 0.0000 * | 0.0003 | ** |
| Pb | C14:0 | 0.7206 | 0.0000 * | 0.0006 | ** |
| Pb | C16:0 | 0.7112 | 0.0000 * | 0.0010 | ** |
| Pb | C18:0 | 0.6941 | 0.0000 * | 0.0013 | ** |
| Pb | C20:0 | 0.6727 | 0.0000 * | 0.0016 | ** |
| Pb | C21:0 | −0.6701 | 0.0000 * | 0.0019 | ** |
| Pb | C24:0 | −0.6540 | 0.0000 * | 0.0023 | ** |
| Pb | SFA | 0.6298 | 0.0000 * | 0.0026 | ** |
| Pb | C16:1 n−7 | −0.6229 | 0.0000 * | 0.0029 | ** |
| Pb | C18:1 n−9c + C18:1 n−9t | 0.6118 | 0.0000 * | 0.0032 | ** |
| Pb | C20:1 n−9 | −0.6062 | 0.0000 * | 0.0035 | ** |
| Pb | MUFA | 0.6005 | 0.0000 * | 0.0039 | ** |
| Pb | C18:2 n−6c + C18:2 n−6t | −0.5958 | 0.0000 * | 0.0042 | ** |
| Pb | C18:3 n−3 | −0.5936 | 0.0000 * | 0.0045 | ** |
| Pb | C20:2 n−6 | −0.5832 | 0.0000 * | 0.0048 | ** |
| Pb | C20:3 n−3 | 0.5759 | 0.0000 * | 0.0052 | ** |
| Pb | C20:4 n−6 | −0.5459 | 0.0000 * | 0.0055 | ** |
| Pb | C20:5 n−3 | 0.5423 | 0.0000 * | 0.0058 | ** |
| Pb | C22:2 n−6 | 0.5381 | 0.0000 * | 0.0061 | ** |
| Pb | PUFA | −0.5138 | 0.0000 * | 0.0065 | ** |
| Pb | PI | 0.4989 | 0.0000 * | 0.0068 | ** |
| Pb | UI | 0.4768 | 0.0001 * | 0.0071 | ** |
| Pb | SOD | 0.4632 | 0.0002 * | 0.0074 | ** |
| Pb | CAT | 0.4171 | 0.0009 * | 0.0077 | ** |
| Pb | TBARS | 0.4052 | 0.0013 * | 0.0081 | ** |
| Pb | CD | −0.3961 | 0.0017 * | 0.0084 | ** |
| Pb | Cr | 0.3890 | 0.0021 * | 0.0087 | ** |
| Pb | Ba | −0.3862 | 0.0023 * | 0.0090 | ** |
| Pb | Ni | −0.1945 | 0.1363 | 0.0094 | - |
| Pb | Zn | −0.0772 | 0.5579 | 0.0097 | - |
| Pb | Cu | 0.0771 | 0.5582 | 0.0100 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garbacz, A.; Kowalczyk-Pecka, D.; Kursa, W. Fatty Acids in Lumbricidae as Biomarkers of In Situ Metals Exposure. Sustainability 2025, 17, 8076. https://doi.org/10.3390/su17178076
Garbacz A, Kowalczyk-Pecka D, Kursa W. Fatty Acids in Lumbricidae as Biomarkers of In Situ Metals Exposure. Sustainability. 2025; 17(17):8076. https://doi.org/10.3390/su17178076
Chicago/Turabian StyleGarbacz, Aleksandra, Danuta Kowalczyk-Pecka, and Weronika Kursa. 2025. "Fatty Acids in Lumbricidae as Biomarkers of In Situ Metals Exposure" Sustainability 17, no. 17: 8076. https://doi.org/10.3390/su17178076
APA StyleGarbacz, A., Kowalczyk-Pecka, D., & Kursa, W. (2025). Fatty Acids in Lumbricidae as Biomarkers of In Situ Metals Exposure. Sustainability, 17(17), 8076. https://doi.org/10.3390/su17178076







