From Waste to Resource: Circular Economy Approaches to Valorize Fine Glass, Ceramic, and Plastic Residues in a Glass Recycling Plant
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
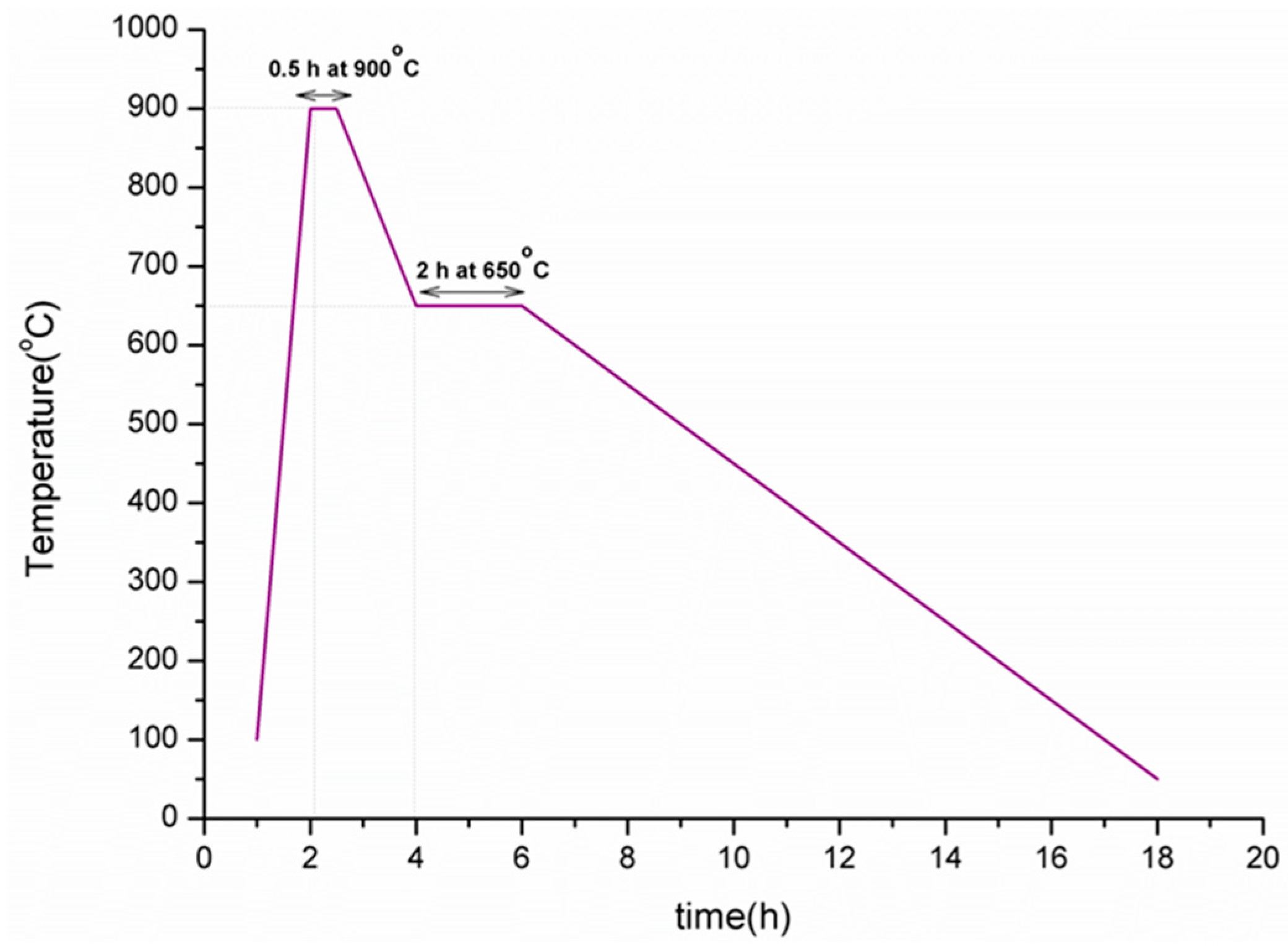
2.2.1. Foam Glass
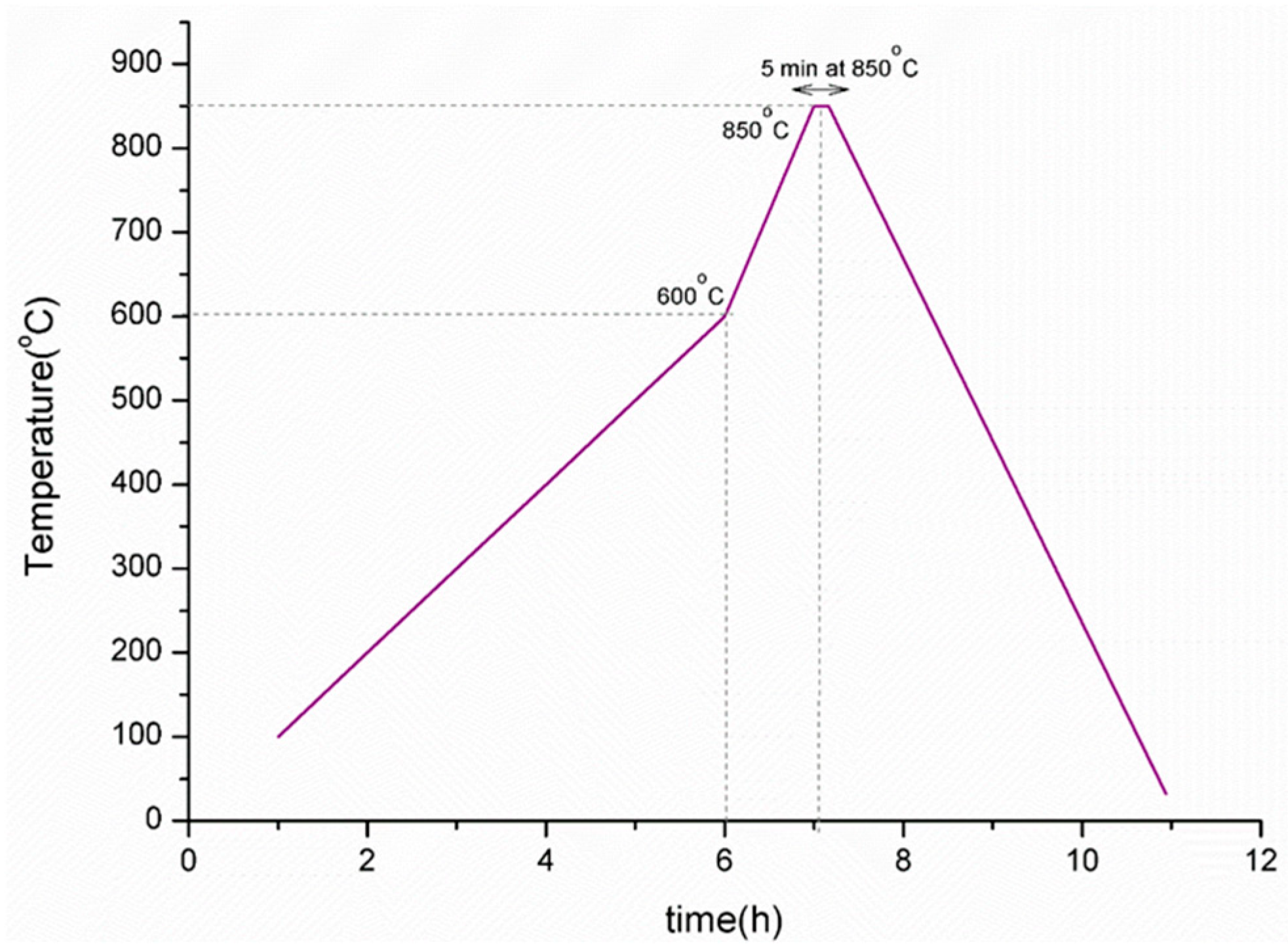
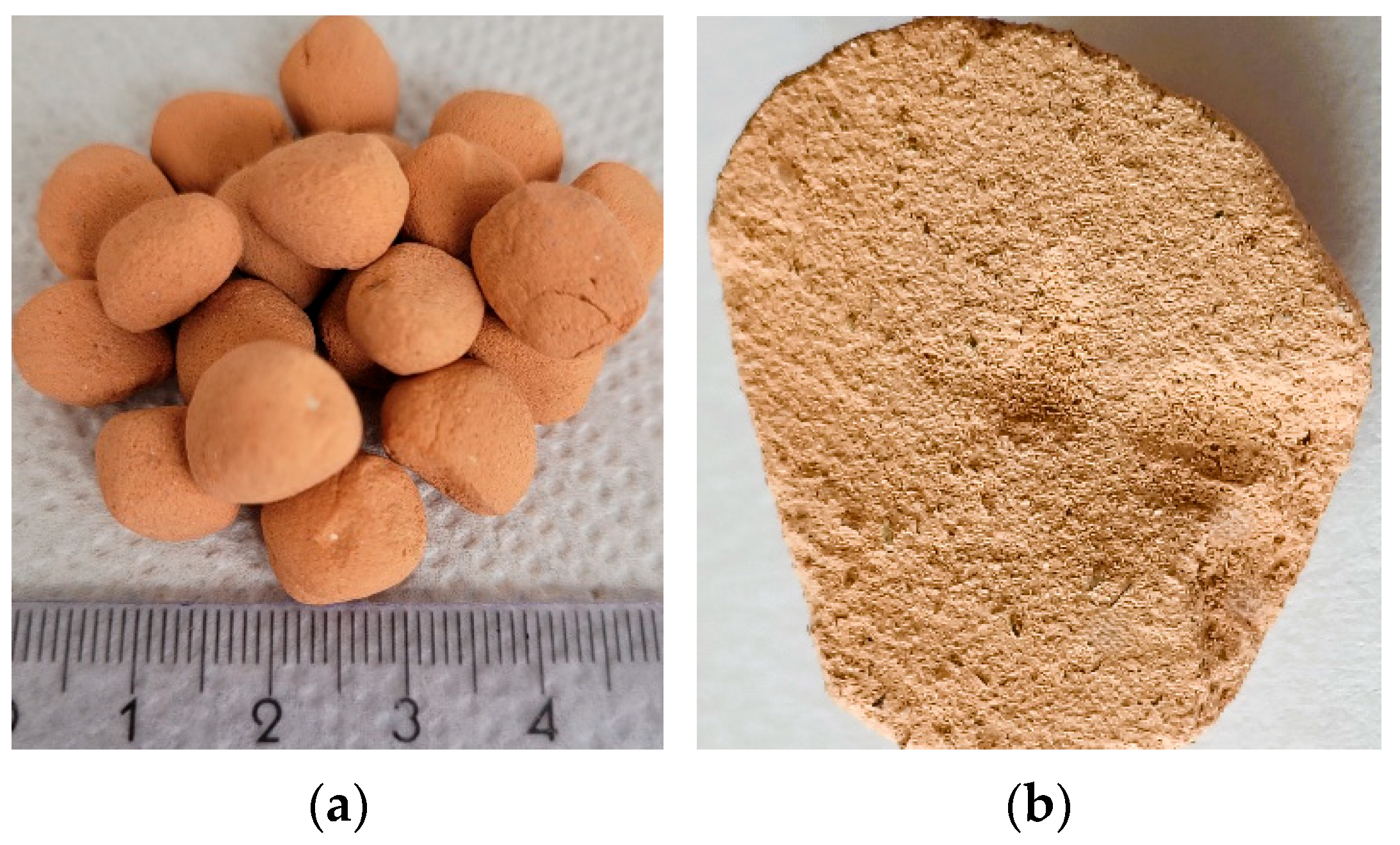
2.2.2. Ceramic–Glass Granules
2.2.3. Polyethylene Waxes
2.3. Methods
Statistical Analysis
- MnO2/SiC weight ratio—5 levels: 0.25, 0.33, 0.5, 0.8, and 1.0;
- MnO2 content—3 levels: 0.4 g, 0.8 g, and 1.0 g.
3. Results
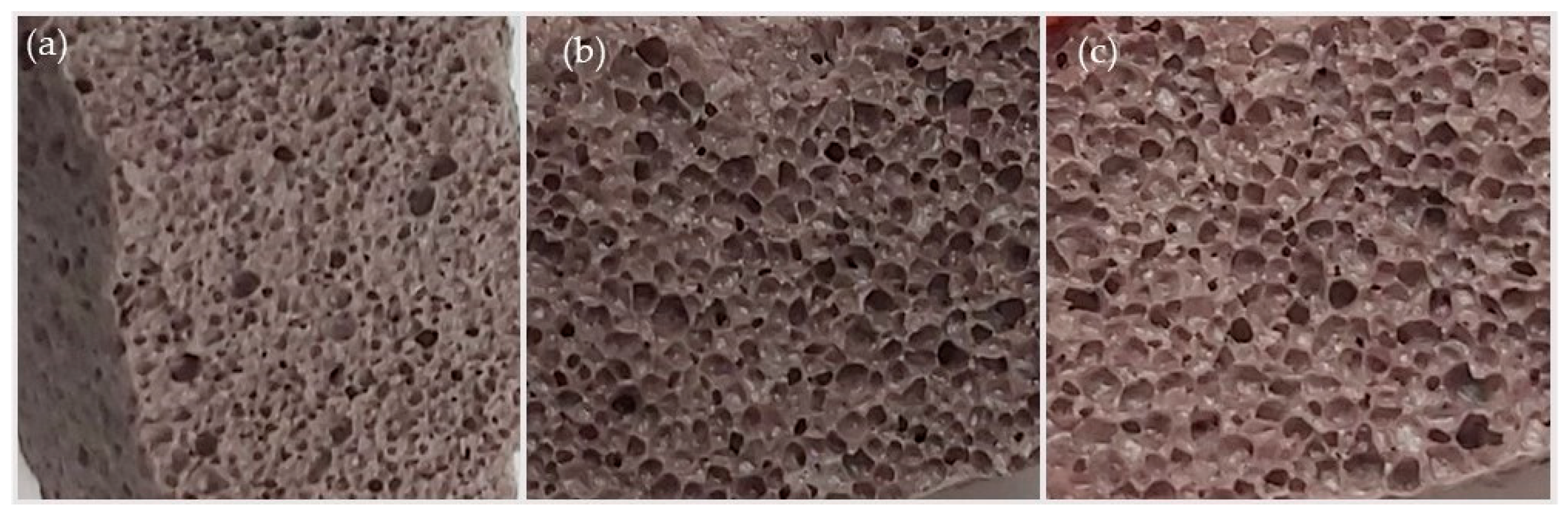
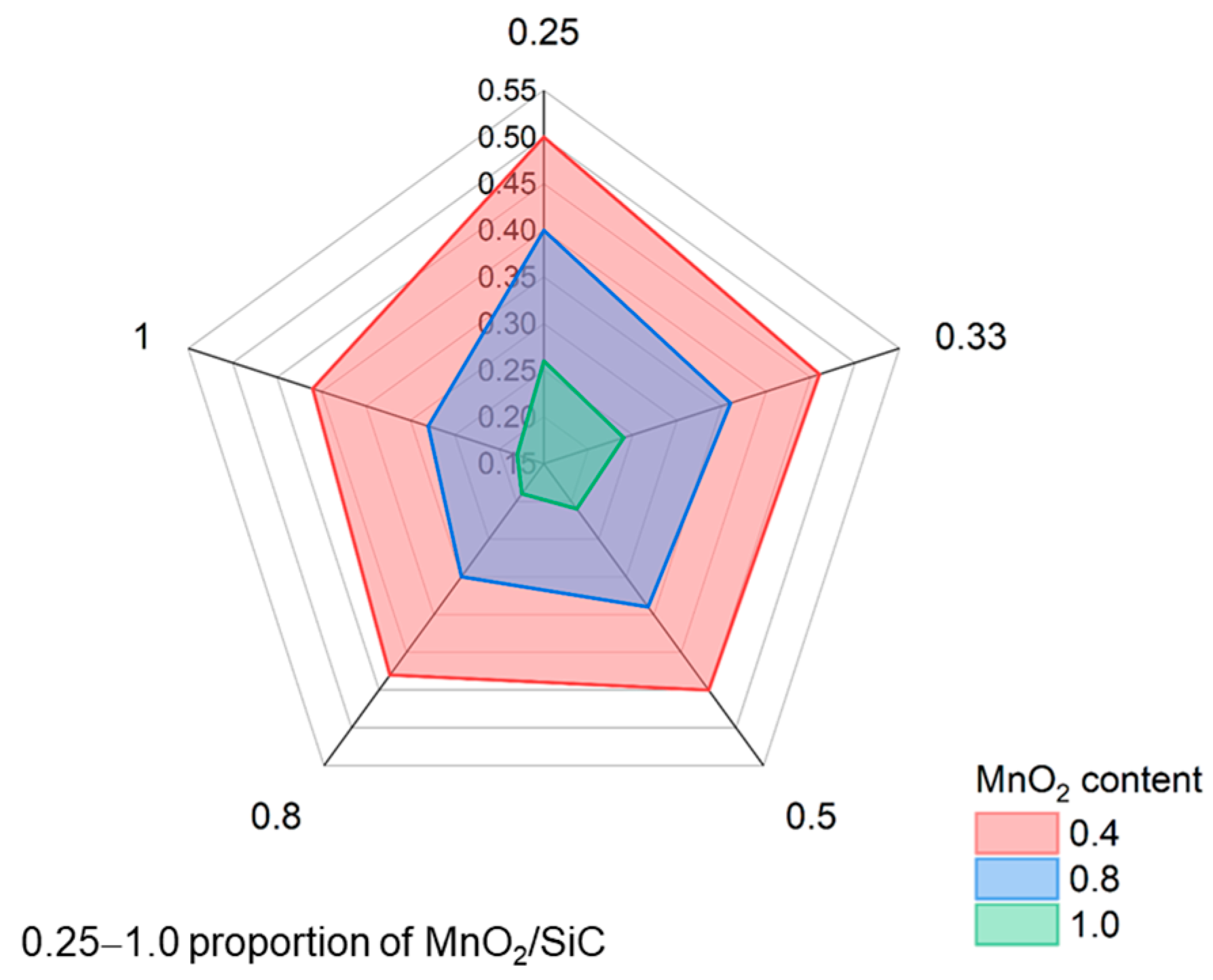
3.1. Foam Glass Characteristics
3.2. Ceramic–Glass Granules Characteristic
3.3. Polyethylene Waxes Characteristic
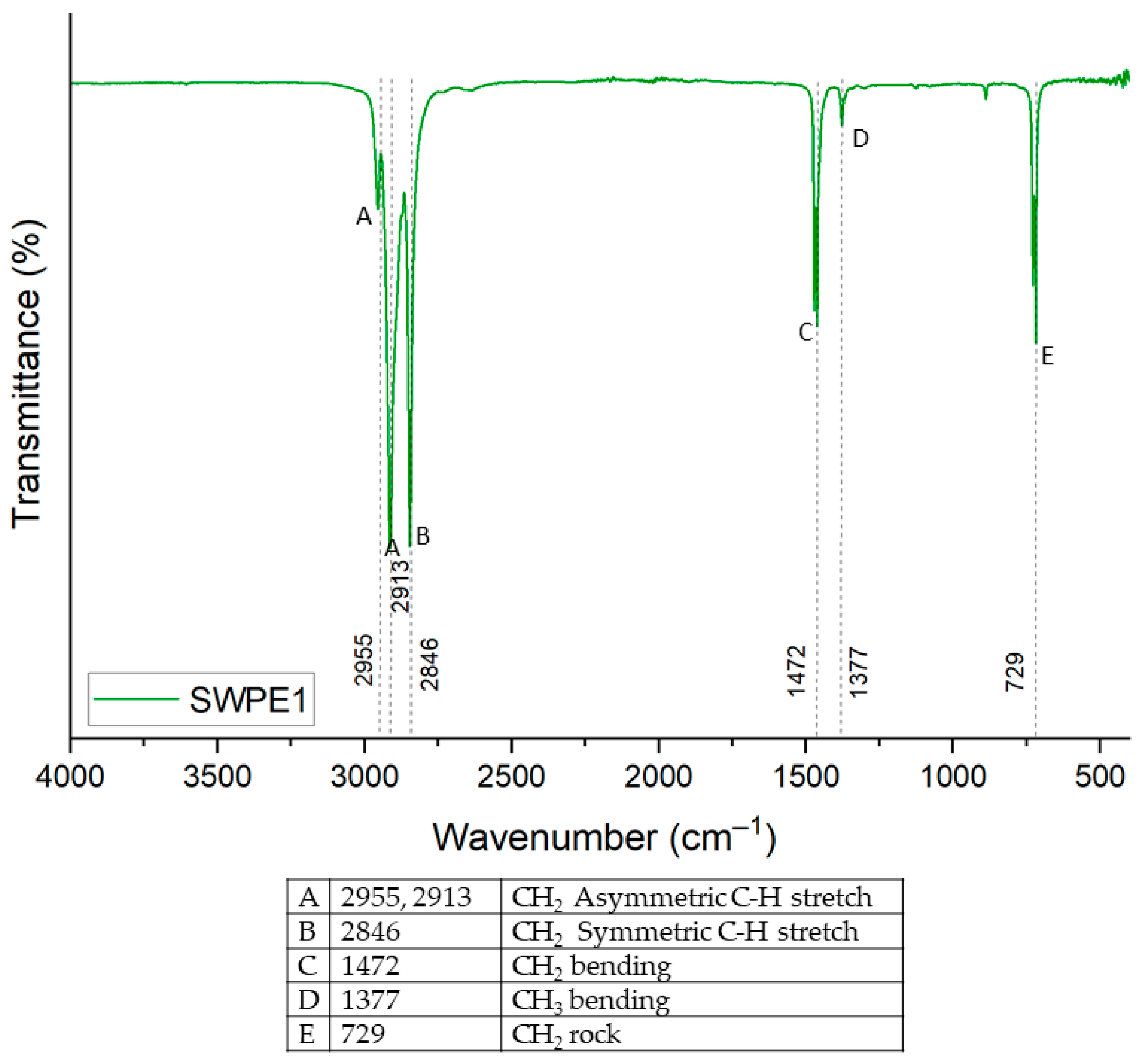
3.3.1. SWPE Wax
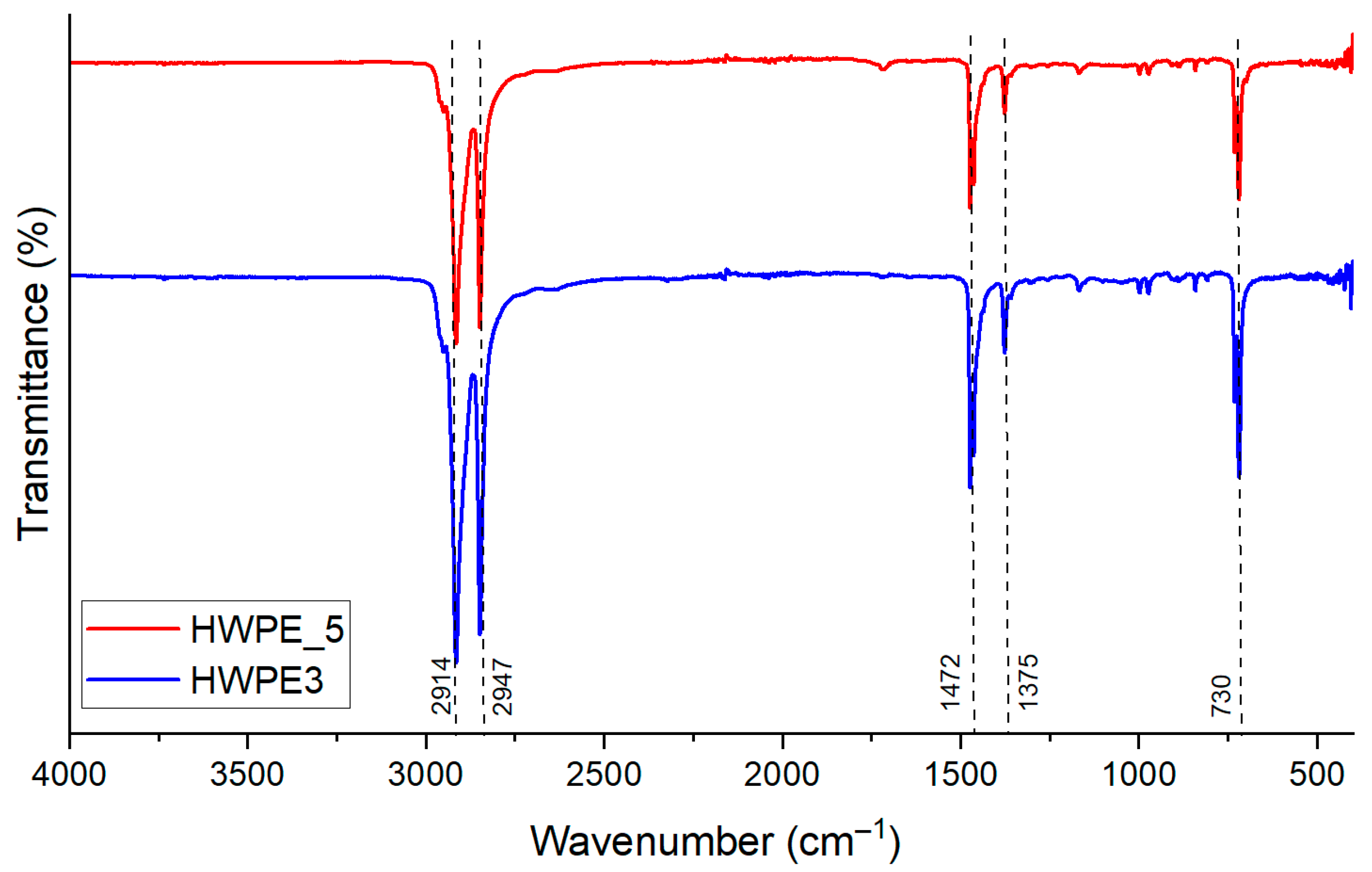
3.3.2. HWPE Wax
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FTIR | Fourier-transform infrared |
| LOI | Loss on ignition |
| SWPE | Soft polyethylene wax |
| HWPE | Hard polyethylene wax |
References
- Bjørnbet, M.; Vildåsen, S. Life cycle assessment to ensure sustainability of circular business models in manufacturing. Sustainability 2021, 13, 11014. [Google Scholar] [CrossRef]
- Emmanouil, C.; Roumeliotis, D.; Kostas, A.; Vagiona, D.G. Assessment of Circular Economy Implementation in Municipal Waste Management Through Performance Indicators and Citizens’ Opinion in a City in Western Greece. Sustainability 2025, 17, 2265. [Google Scholar] [CrossRef]
- Kirchherr, J.; Reike, D.; Hekkert, M. Conceptualizing the circular economy: An analysis of 114 definitions. Resour. Conserv. Recycl. 2018, 127, 221–232. [Google Scholar] [CrossRef]
- Bellopede, R.; Zichella, L.; Marini, P. Glass Waste3: A Preliminary Study for a New Industrial Recovery Processing. Sustainability 2020, 12, 1997. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, C.; Shen, Y.; Ruan, J. Pollution analysis of micro/nano meters glass particles and benzene produced from the friction cleaning process for the recovery of waste glass. Waste Manag. 2025, 193, 1–10. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Maldonado-Alameda, A.; Alfocea-Roig, A.; Mañosa, J.; Chimenos, J.; Formosa, J. Preliminary study of new sustainable, alkali-activated cements using the residual fraction of the glass cullet recycling as precursor. Appl. Sci. 2021, 11, 3528. [Google Scholar] [CrossRef]
- Rapin, C.; Durand, E.; Skaper, M.A. Recycling and recovery of glass material. In Recycling, a Challenge for the Circular Economy; Cathelineau, M., Ed.; ISTE: London, UK, 2024; pp. 237–264. [Google Scholar] [CrossRef]
- Baek, C.R.; Kim, H.D.; Jang, Y.-C. Exploring glass recycling: Trends, technologies, and future trajectories. Environ. Eng. Res. 2025, 30, 240241. [Google Scholar] [CrossRef]
- Andreola, F.; Barbieri, L.; Giuranna, D.; Lancellotti, I.; Taurino, R. Technical solutions to improve recovery of scraps derived from treating glass packaging waste—Extended abstract. Environ. Eng. Manag. J. 2013, 12 (Suppl. S11), 57–60. [Google Scholar]
- Gimenez-Carbo, E.; Soriano, L.; Roig-Flores, M.; Serna, P. Characterization of Glass Powder from Glass Recycling Process Waste and Preliminary Testing. Materials 2021, 14, 2971. [Google Scholar] [CrossRef]
- Barbato, P.M.; Olsson, E.; Rigamonti, L. Quality degradation in glass recycling: Substitutability model proposal. Waste Manag. 2024, 182, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Cozzarini, L.; Fortuna, L.; Bevilacqua, P. Optimizing glass foam production from recycled sources: Influence of variation in cullet color and spent alkaline battery components. Adv. Eng. Mater. 2024, 26, 2401679. [Google Scholar] [CrossRef]
- König, J.; Petersen, R.R.; Iversen, N.; Yue, Y. Suppressing the effect of cullet composition on the formation and properties of foamed glass. Ceram. Int. 2018, 44, 11143–11150. [Google Scholar] [CrossRef]
- El Haggar, S. Sustainable Industrial Design and Waste Management: Cradle-to-Cradle for Sustainable Development; Elsevier Academic Press: Amsterdam, The Netherlands, 2010; pp. 173–179. [Google Scholar]
- Guo, Y.; Zhang, Y.; Huang, H.; Meng, X.; Liu, Y.; Tu, S.; Li, B. Novel glass ceramic foams materials based on polishing porcelain waste using the carbon ash waste as foaming agent. Constr. Build. Mater. 2016, 125, 1093–1100. [Google Scholar] [CrossRef]
- Zhu, M.; Ji, R.; Li, Z.; Wang, H.; Liu, L.; Zhang, Z. Preparation of glass ceramic foams for thermal insulation applications from coal fly ash and waste glass. Constr. Build. Mater. 2016, 112, 398–405. [Google Scholar] [CrossRef]
- Fang, X.; Li, Q.; Yang, T.; Li, Z.; Zhu, Y. Preparation and characterization of glass foams for artificial floating island from waste glass and Li2CO3. Constr. Build. Mater. 2017, 134, 358–363. [Google Scholar] [CrossRef]
- Vaisman, I.; Ketov, A.; Ketov, I. Cellular glass obtained from non-powder preforms by foaming with steam. Ceram. Int. 2016, 42, 15261–15268. [Google Scholar] [CrossRef]
- Wang, L.P.; Tseng, P.W.; Huang, K.J.; Chen, Y.J. Foam glass production from waste bottle glass using silicon cutting waste of loose abrasive slurry sawing as foaming agent. Constr. Build. Mater. 2023, 383, 131344. [Google Scholar] [CrossRef]
- Ji, R.; Zheng, Y.; Zou, Z.; Chen, Z.; Wei, S.; Jin, X.; Zhang, M. Utilization of mineral wool waste and waste glass for synthesis of foam glass at low temperature. Constr. Build. Mater. 2019, 215, 623–632. [Google Scholar] [CrossRef]
- Siddika, A.; Hajimohammadi, A.; Sahajwalla, V. A novel eco-friendly foaming technique for developing sustainable glass foams from the waste glass. Resour. Conserv. Recycl. 2023, 190, 106801. [Google Scholar] [CrossRef]
- Tokareva, A.; Kaassamani, S.; Waldmann, D. Using ceramic demolition wastes for CO2-reduced cement production. Constr. Build. Mater. 2024, 426, 135980. [Google Scholar] [CrossRef]
- Keshavarz, Z.; Mostofinejad, D. Porcelain and red ceramic wastes used as replacements for coarse aggregate in concrete. Constr. Build. Mater. 2019, 195, 218–230. [Google Scholar] [CrossRef]
- Tarhan, B.; Tarhan, M.; Aydin, T. Reusing sanitaryware waste products in glazed porcelain tile production. Ceram. Int. 2017, 43, 3107–3112. [Google Scholar] [CrossRef]
- Shaker, M.; Hamdani, S.S.; Muzata, T.S.; Rabnawaz, M. Driving selective upcycling of mixed polyethylene waste with table salt. Sci. Rep. 2024, 14, 14371. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Suh, S. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Alali, S.A.S.; Aldaihani, M.K.M.B.J.; Alanezi, K.M. Plant Design for the Conversion of Plastic Waste into Valuable Chemicals (Alkyl Aromatics). Appl. Sci. 2023, 13, 9221. [Google Scholar] [CrossRef]
- Sharuddin, S.D.A.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Ahmad, I.; Khan, M.I.; Khan, H.; Ishaq, M.; Tariq, R.; Gul, K.; Ahmad, W. Pyrolysis study of polypropylene and polyethylene into premium oil products. Int. J. Green Energy 2015, 12, 663–671. [Google Scholar] [CrossRef]
- Hou, J.; Lian, Y.; Luo, H.; Wang, H.; Sun, Y. Converting waste agricultural film to polyethylene waxes: A mechanism and whitening study. Polym. Degrad. Stab. 2023, 216, 110484. [Google Scholar] [CrossRef]
- Panda, A.K.; Mahapatra, P.M. Pyrolytic conversion of waste high-density polyethylene to wax: Temperature optimization and characterization of wax. Fine Chem. Eng. 2024, 5, 221–229. [Google Scholar] [CrossRef]
- PN-EN 1744-1+A1:2013-05; Tests for Chemical Properties of Aggregates—Part 1: Chemical Analysis. The Polish Committee for Standardization: Warszawa, Poland, 2025.
- PN-EN 772-21:2011; Determination of Water Absorption of Clay and Calcium Silicate Masonry Units by Cold Water Absorption. The Polish Committee for Standardization: Warszawa, Poland, 2025.
- PN-EN 12; Characterisation of Waste—Leaching—Compliance Test for Leaching of Granular Waste Materials and Sludges—Part 2: One Stage Batch Test at a Liquid to Solid Ratio of 10 L/kg for Materials with Particle Size Below 4 mm (Without or with Size Reduction). The Polish Committee for Standardization: Warszawa, Poland, 2025.
- König, J.; Petersen, R.R.; Yue, Y. Influence of the glass particle size on the foaming process and physical characteristics of foam glasses. J. Non-Cryst. Solids 2016, 447, 190–197. [Google Scholar] [CrossRef]
- Deng, F.; Wang, F.; Shi, X.; Liu, L.; Liao, Q. Synthesis and properties of foam glass-ceramics from granite tailings by using SiC and MnO2 as the mixed foaming agent. Ceram. Int. 2023, 49, 34647–34656. [Google Scholar] [CrossRef]
- Jwaida, Z.; Dulaimi, A.; Bernardo, L.F.A. The use of waste ceramic in concrete: A review. CivilEng 2024, 5, 482–500. [Google Scholar] [CrossRef]
- Ray, S.; Haque, M.; Sakib, M.N.; Mita, A.F.; Rahman, M.M.; Tanmoy, B.B. Use of ceramic wastes as aggregates in concrete production: A review. J. Build. Eng. 2021, 43, 102567. [Google Scholar] [CrossRef]
- Meena, R.V.; Jain, J.K.; Chouhan, H.S.; Beniwal, A.S. Use of waste ceramics to produce sustainable concrete: A review. Clean. Mater. 2022, 4, 100085. [Google Scholar] [CrossRef]
- Kicińska, A.; Pomykała, R.; Izquierdo-Diaz, M. Changes in soil pH and mobility of heavy metals in contaminated soils. Eur. J. Soil Sci. 2022, 73, e13203. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, Z.; Zhan, Y.; Zheng, X.; Zhou, M.; Yan, G.; Butterbach-Bahl, K. Potential benefits of liming to acid soils on climate change mitigation and food security. Glob. Change Biol. 2021, 27, 2807–2821. [Google Scholar] [CrossRef]
- Xu, F.; Zhao, Y.; Li, K. Using waste plastics as asphalt modifier: A review. Materials 2022, 15, 110. [Google Scholar] [CrossRef]
- Shivasharana, C.T.; Kesti, S.S. Physical and chemical characterization of low density polyethylene and high density polyethylene. J. Adv. Sci. Res. 2019, 10, 30–34. [Google Scholar]
- Mochane, M.J.; Mokhena, T.C.; Motaung, T.E.; Linganiso, L.Z. Shape-stabilized phase change materials of polyolefin/wax blends and their composites. J. Therm. Anal. Calorim. 2020, 139, 2951–2963. [Google Scholar] [CrossRef]
- ATR-FT-IR Spectrum of Polyethylene Wax (4000–225 cm−1). Available online: https://spectra.chem.ut.ee/paint/binders/polyethylene-wax/ (accessed on 29 May 2025).
- Antosz, A.; Ptak, S. Studies on the possibilities of reusing waste materials generated during candle production. Nafta-Gaz 2022, 78, 760–775. [Google Scholar] [CrossRef]
- Blengini, G.A.; Busto, M.; Fantoni, M.; Fino, D. Eco-efficient waste glass recycling: Integrated waste management and green product development through LCA. Waste Manag. 2012, 32, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, J.; Siedlecka, E. The Method of Silicon Carbon Recovery from Post-Lier Wastewater. PL Patent 227000, 31 October 2017. [Google Scholar]
- Kawai, F.; Watanabe, M.; Shibata, M.; Yokoyama, S.; Sudate, Y.; Hayashi, S. Comparative study on biodegradability of polyethylene wax by bacteria and fungi. Polym. Degrad. Stab. 2004, 86, 105–114. [Google Scholar] [CrossRef]
- IndexBox. EU: Artificial and Prepared Waxes of Polyethylene Glycol Market Analysis, Forecast, Size, Trends and Insights. Available online: https://www.indexbox.io/store/eu-artificial-and-prepared-waxes-of-polyethylene-glycol-market-analysis-forecast-size-trends-and-insights/ (accessed on 12 August 2025).




| Grain Size, mm | Share, % |
|---|---|
| >4 | 0 |
| 4–2 | 7.0 |
| 2–1 | 31.0 |
| 1–0.5 | 29.0 |
| 0.5–0.25 | 18.8 |
| 0.25–0.125 | 8.0 |
| 0.125–0.063 | 2.0 |
| <0.063 | 4.2 |
| Sample No. | MnO2, g | MnO2/SiC | Density g/cm3 | Sample Composition wt% | ||
|---|---|---|---|---|---|---|
| Glass | MnO2 | SiC | ||||
| 1 | 0.4 | 0.25 | 0.5 | 96.15 | 0.77 | 3.08 |
| 2 | 0.33 | 0.46 | 96.88 | 0.78 | 2.34 | |
| 3 | 0.5 | 0.45 | 97.66 | 0.78 | 1.56 | |
| 4 | 0.8 | 0.43 | 98.23 | 0.79 | 0.98 | |
| 5 | 1.0 | 0.41 | 98.42 | 0.79 | 0.79 | |
| 6 | 0.8 | 0.25 | 0.4 | 92.59 | 1.48 | 5.93 |
| 7 | 0.33 | 0.36 | 93.95 | 1.50 | 4.55 | |
| 8 | 0.5 | 0.34 | 95.42 | 1.53 | 3.05 | |
| 9 | 0.8 | 0.30 | 96.53 | 1.54 | 1.93 | |
| 10 | 1.0 | 0.28 | 96.90 | 1.55 | 1.55 | |
| 11 | 1.0 | 0.25 | 0.26 | 90.91 | 1.81 | 7.27 |
| 12 | 0.33 | 0.24 | 92.59 | 1.85 | 5.56 | |
| 13 | 0.5 | 0.21 | 94.34 | 1.89 | 3.77 | |
| 14 | 0.8 | 0.19 | 95.69 | 1.92 | 2.39 | |
| 15 | 1.0 | 0.18 | 96.16 | 1.92 | 1.92 | |
| Sample Description | Density of Foam Glass, g/cm3 | Group |
|---|---|---|
| MnO2 = 1.0 g, MnO2/SiC = 1.0 | 0.180 | a |
| MnO2 = 1.0 g, MnO2/SiC = 0.8 | 0.190 | a |
| MnO2 = 1.0 g, MnO2/SiC = 0.5 | 0.210 | ab |
| MnO2 = 1.0 g, MnO2/SiC = 0.33 | 0.240 | abc |
| MnO2 = 1.0 g, MnO2/SiC = 0.25 | 0.260 | abcd |
| MnO2 = 0.8 g, MnO2/SiC = 1.0 | 0.280 | abcde |
| MnO2 = 0.8 g, MnO2/SiC = 0.8 | 0.300 | bcde |
| MnO2 = 0.8 g, MnO2/SiC = 0.5 | 0.340 | bcdef |
| MnO2 = 0.8 g, MnO2/SiC = 0.33 | 0.360 | cdef |
| MnO2 = 0.8 g, MnO2/SiC = 0.25 | 0.400 | defg |
| MnO2 = 0.4 g, MnO2/SiC = 1.0 | 0.410 | efgh |
| MnO2 = 0.4 g, MnO2/SiC = 0.8 | 0.430 | fgh |
| MnO2 = 0.4 g, MnO2/SiC = 0.5 | 0.450 | gh |
| MnO2 = 0.4 g, MnO2/SiC = 0.33 | 0.460 | gh |
| MnO2 = 0.4 g, MnO2/SiC = 0.25 | 0.500 | h |
| Grain Size, µm | Share, % |
|---|---|
| 0.1–5 | 28.2 |
| 6–10 | 19.0 |
| 11–30 | 32.0 |
| 31–50 | 10.5 |
| 51–100 | 1.5 |
| 101–300 | 8.8 |
| >300 | 0.0 |
| Component | Granules 1 | Granules 2 | Granules 3 |
|---|---|---|---|
| Ceramic | 5.9 | 25.0 | 24.7 |
| Glass | 35.2 | 37.5 | 37.0 |
| Clay | 29.4 | 12.5 | 12.4 |
| Water | 29.4 | 25.0 | 24.7 |
| Ca(OH)2 | 0.1 | 0 | 1.2 |
| Type of Granules | Metal Content in Water Extracts, ppm | pH After 24 h | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zn | Cr | Pb | Fe | Na | Cd | P | Al | Mg | Ca | K | Cu | Ni | ||
| Granules 1 | <0.001 | <0.001 | 4.2 | 8.24 | 47.8 | <0.001 | 13.6 | 75.8 | 7.98 | 64.4 | 73.9 | <0.001 | <0.001 | 8.9 |
| Granules 2 | <0.001 | <0.001 | 4.4 | 6.1 | 48.3 | <0.001 | 13.8 | 84.5 | 8.0 | 61.2 | 90.0 | <0.001 | <0.001 | 8.7 |
| Granules 3 | <0.001 | <0.001 | 4.3 | 8.1 | 46.0 | <0.001 | 14.0 | 84.5 | 7.8 | 58.4 | 73.4 | <0.001 | <0.001 | 9.0 |
| Sample | Substitution, % | Temperature, °C | Time, min | Melting Point, °C |
|---|---|---|---|---|
| SWPE1 | 30.0 | 155–175 | 30 | 55 |
| SWPE2 | 27.0 | 155–175 | 30 | 60 |
| Sample | Pyrolysis Temperature, °C | Pyrolysis Time, min | Melting Point of Wax, °C | Yield, % | ||
|---|---|---|---|---|---|---|
| Wax | Gas | Liquid | ||||
| HWPE1 | 375 | 20 | - | 0.00 * | 0.77 | 0.00 |
| HWPE2 | 385 | 20 | 105 | 97.08 | 2.92 | 0.31 |
| HWPE3 | 395 | 20 | 104 | 93.56 | 6.44 | 0.30 |
| HWPE4 | 400 | 20 | 103 | 89.24 | 10.76 | 0.33 |
| HWPE5 | 410 | 20 | 100 | 85.90 | 14.10 | 0.26 |
| HWPE6 | 420 | 20 | 98 | 58.30 | 32.90 | 8.80 |
| HWPE7 | 430 | 20 | 95 | 34.63 | 42.11 | 23.19 |
| HWPE8 | 440 | 20 | 95 | 14.41 | 60.10 | 25.39 |
| HWPE3_15 | 395 | 15 | 104 | 94.41 | 5.30 | 0.29 |
| HWPE3_10 | 395 | 10 | 103 | 95.62 | 4.10 | 0.28 |
| HWPE3_5 | 395 | 5 | 103 | 96.15 | 3.60 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siedlecka, E.; Siedlecki, J.; Bednarski, B.; Białek, S. From Waste to Resource: Circular Economy Approaches to Valorize Fine Glass, Ceramic, and Plastic Residues in a Glass Recycling Plant. Sustainability 2025, 17, 7966. https://doi.org/10.3390/su17177966
Siedlecka E, Siedlecki J, Bednarski B, Białek S. From Waste to Resource: Circular Economy Approaches to Valorize Fine Glass, Ceramic, and Plastic Residues in a Glass Recycling Plant. Sustainability. 2025; 17(17):7966. https://doi.org/10.3390/su17177966
Chicago/Turabian StyleSiedlecka, Ewa, Jarosław Siedlecki, Beniamin Bednarski, and Szymon Białek. 2025. "From Waste to Resource: Circular Economy Approaches to Valorize Fine Glass, Ceramic, and Plastic Residues in a Glass Recycling Plant" Sustainability 17, no. 17: 7966. https://doi.org/10.3390/su17177966
APA StyleSiedlecka, E., Siedlecki, J., Bednarski, B., & Białek, S. (2025). From Waste to Resource: Circular Economy Approaches to Valorize Fine Glass, Ceramic, and Plastic Residues in a Glass Recycling Plant. Sustainability, 17(17), 7966. https://doi.org/10.3390/su17177966






